Introduction
Chronic inflammatory diseases represent a major
global health burden, encompassing conditions such as
atherosclerosis, type 2 diabetes mellitus, inflammatory bowel
disease and neuroinflammatory disorders (1,2).
These pathologies share common molecular mechanisms involving
dysregulated inflammatory responses, altered cellular metabolism
and compromised vascular integrity. The identification of
regulatory networks that coordinate these seemingly disparate
processes has emerged as a critical priority for developing more
effective therapeutic interventions.
Contemporary advances in molecular and cellular
biology have identified a previously uncharacterized regulatory
network linking two functionally distinct classes of signaling
molecules: G protein-coupled receptor 124 (GPR124), which is an
adhesion G protein-coupled receptor, and peroxisome
proliferator-activated receptor γ (PPARγ), which is a
ligand-activated nuclear receptor (3,4).
Converging experimental evidence has demonstrated their mechanistic
interconnection through differential regulation of the canonical
Wnt/β-catenin signaling cascade, establishing the 'GPR124-Wnt-PPARγ
regulatory axis'. This molecular framework exhibits notable
implications for understanding and therapeutically targeting
chronic inflammatory pathologies characterized by dysregulated
tissue homeostasis and aberrant inflammatory resolution. GPR124,
also known as tumor endothelial marker 5 (TEM5) and adhesion G
protein-coupled receptor A2, was initially reported to serve an
essential role in central nervous system (CNS) vascularization and
blood-brain barrier (BBB) formation (5,6).
Subsequent research has revealed broader expression patterns and
functions for GPR124, including roles in peripheral angiogenesis,
diabetic complications and inflammatory responses (7). The receptor functions as a highly
specific co-activator for Wnt7a and Wnt7b ligands, forming a
ternary complex with the GPI-anchored protein RECK to potentiate
canonical Wnt/β-catenin signaling (8,9).
PPARγ, conversely, represents one of the most
extensively studied nuclear receptors, renowned for its role as a
master regulator of adipogenesis, glucose homeostasis and
anti-inflammatory responses (10,11). The therapeutic importance of
PPARγ is underscored by the clinical success of thiazolidinediones,
a class of PPARγ agonists, in treating type 2 diabetes and the
ongoing development of selective PPARγ modulators (SPPARγMs) for
primary biliary cholangitis, nonalcoholic steatohepatitis (NASH)
and other inflammatory conditions (12,13). Notably, PPARγ functions as a
potent antagonist of Wnt/β-catenin signaling through direct
protein-protein interactions that promote β-catenin degradation
(14).
The opposing effects of GPR124 and PPARγ on Wnt
signaling create a molecular switch that governs cellular responses
to inflammatory stimuli, metabolic stress and vascular injury
(15). This regulatory mechanism
has particular relevance for chronic inflammatory diseases, where
the balance between pro-angiogenic, proliferative responses
mediated by Wnt activation, and anti-inflammatory, metabolic
regulatory responses mediated by PPARγ determines disease
progression and therapeutic responsiveness.
The present comprehensive review examines the
molecular mechanisms underlying GPR124-PPARγ axis function,
analyzes its pathophysiological importance in major chronic
inflammatory diseases, and evaluates emerging therapeutic
strategies that target this regulatory network. In addition, a
critical assessment of current knowledge gaps is provided and
future research directions that are needed to translate mechanistic
insights into clinical applications are outlined.
Molecular architecture of the
GPR124-Wnt-PPARγ axis
GPR124: Structure, function and signaling
mechanisms
GPR124 is classified within the adhesion subfamily
of G protein-coupled receptors, and is distinguished by its complex
modular architecture comprising an exceptionally large N-terminal
extracellular domain containing multiple leucine-rich repeats,
immunoglobulin-like domains and hormone receptor motifs, coupled to
a canonical seven-transmembrane spanning region (16). This distinctive structural
organization confers dual functionality, enabling the receptor to
simultaneously mediate cell-cell adhesive interactions and
transduce intracellular signaling cascades, thereby reflecting its
bifunctional roles in both intercellular communication and signal
transduction processes (9).
The physiological significance of GPR124 was first
established through genetic studies demonstrating its absolute
requirement for CNS angiogenesis and BBB formation (9,17). Global or endothelial-specific
deletion of the GPR124 gene results in embryonic lethality
associated with profound CNS-specific vascular defects, including
delayed vascular penetration into neural tissue, formation of
pathological glomeruloid structures and widespread cerebral
hemorrhage (5). These defects
are accompanied by failure to establish BBB properties, as
evidenced by loss of glucose transporter 1 (Glut1) expression and
increased vascular permeability (18).
The molecular mechanism underlying GPR124 function
involves its role as a ligand-specific co-receptor for canonical
Wnt signaling (19). GPR124
forms a cell-surface complex with the GPI-anchored protein RECK,
and this GPR124/RECK complex serves as a highly specific
co-receptor for Wnt7a and Wnt7b ligands (20). The complex facilitates the
binding and presentation of Wnt7a/b to the primary Wnt receptor
complex consisting of Frizzled family receptors and low-density
lipoprotein receptor-related protein (LRP)5/6 co-receptors, thereby
potentiating canonical Wnt/β-catenin signaling (21).
Notably, GPR124 function exhibits marked context
dependency. While essential for developmental angiogenesis,
conditional knockout studies have revealed that GPR124 is largely
dispensable for maintaining adult vascular homeostasis under
physiological conditions (17,22). However, it is markedly activated
in response to pathological stress, including ischemic injury,
tumor growth and inflammatory challenges (17). This stress-responsive activation
pattern positions GPR124 as a critical mediator of adaptive
vascular responses in disease states.
PPARγ: Nuclear receptor function and
anti-inflammatory mechanisms
PPARγ represents a ligand-activated transcription
factor belonging to the nuclear receptor superfamily (23). This receptor exists in multiple
isoforms generated through alternative promoter usage and splicing,
with PPARγ1 showing broad tissue distribution and PPARγ2 being
predominantly expressed in adipose tissue (24). Upon ligand binding, PPARγ
undergoes conformational changes that promote heterodimerization
with retinoid X receptor and subsequent binding to PPAR response
elements in target gene promoters (25).
The anti-inflammatory repertoire of PPARγ
encompasses diverse mechanistic modalities that transcend
conventional ligand-dependent transcriptional activation (26). These include direct
transrepression mechanisms involving physical protein-protein
interactions between ligand-activated PPARγ and pro-inflammatory
transcription factors, including NF-κB and activator protein-1,
resulting in context-dependent inhibition of inflammatory gene
expression programs (27).
Additionally, PPARγ orchestrates the transcriptional upregulation
of anti-inflammatory mediators and facilitates the biosynthesis of
specialized pro-resolving mediators, thereby promoting active
resolution of inflammatory responses rather than mere inflammatory
suppression (28).
In immune cells, PPARγ activation promotes
anti-inflammatory macrophage polarization, enhances regulatory
T-cell function and suppresses dendritic cell activation (29). These effects are mediated through
metabolic reprogramming that shifts cellular energy production from
glycolysis toward oxidative phosphorylation and fatty acid
oxidation, metabolic changes that are incompatible with sustained
inflammatory activation (30).
Wnt/β-catenin signaling: The central hub
of GPR124-PPARγ crosstalk
The canonical Wnt/β-catenin signaling pathway
functions as the principal molecular convergence point mediating
the functional crosstalk between GPR124 and PPARγ signaling
networks (31,32). Under basal conditions,
cytoplasmic β-catenin undergoes constitutive phosphorylation by a
multiprotein destruction complex containing adenomatous polyposis
coli (APC), Axin scaffolding proteins and glycogen synthase
kinase-3β (GSK-3β), subsequently targeting β-catenin for
ubiquitin-mediated proteasomal degradation and maintaining low
steady-state levels (33). Wnt
ligand engagement with cognate Frizzled/LRP receptor complexes
disrupts this cytoplasmic destruction machinery, permitting
β-catenin stabilization, nuclear translocation and the formation of
transcriptionally active complexes with T-cell factor/lymphoid
enhancer factor transcription factors (34).
GPR124-mediated potentiation of Wnt7a/b signaling
results in robust β-catenin stabilization and nuclear accumulation,
particularly in endothelial cells during angiogenic responses
(19). This activation drives
the expression of genes essential for vascular development, barrier
function and angiogenic sprouting, including Glut1,
Claudin-5 and VE-cadherin (18).
In contrast, PPARγ activation leads to potent
inhibition of Wnt/β-catenin signaling through direct
protein-protein interactions (35). Ligand-activated PPARγ physically
binds to β-catenin through a specific catenin-binding domain,
facilitating the interaction of β-catenin with the destruction
complex, and promoting its phosphorylation and degradation
(3,36). This mechanism effectively reduces
cellular β-catenin levels and suppresses Wnt target gene
expression.
The opposing effects of GPR124 and PPARγ on
β-catenin stability create a molecular rheostat that fine-tunes
cellular responses to environmental stimuli. The relative activity
of these pathways determines whether cells adopt pro-angiogenic,
proliferative phenotypes associated with Wnt activation, or
anti-inflammatory, metabolically quiescent states associated with
PPARγ signaling (Fig. 1).
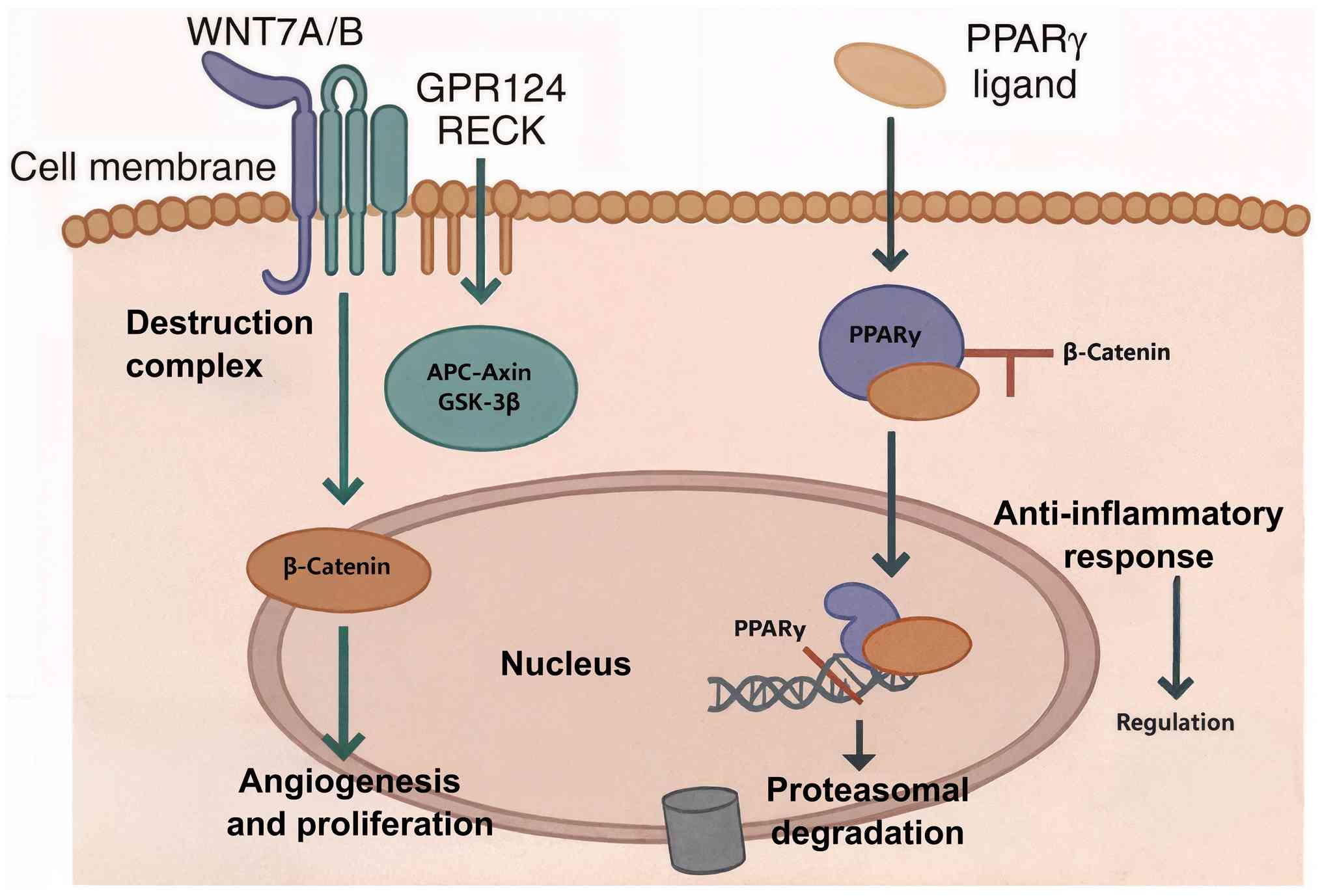 | Figure 1Molecular rheostat of the
GPR124-Wnt-PPARγ axis. This diagram illustrates the antagonistic
regulation of the canonical Wnt/β-catenin pathway by GPR124 and
PPARγ. GPR124 functions as a co-activator of Wnt7a/b ligands,
potentiating Wnt signaling. This leads to the inhibition of the
cytoplasmic destruction complex (composed of APC, Axin and GSK-3β),
resulting in the stabilization and nuclear translocation of
β-catenin. In the nucleus, β-catenin activates target genes that
promote angiogenesis and proliferation. By contrast,
ligand-activated PPARγ exerts potent antagonistic effects. It
directly binds to β-catenin, facilitating its interaction with the
destruction complex, and promoting its subsequent phosphorylation
and proteasomal degradation. This action suppresses Wnt target gene
expression, leading to anti-inflammatory responses and metabolic
regulation. APC, adenomatous polyposis coli; GSK-3β, glycogen
synthase kinase-3β; GPR124, G protein-coupled receptor 124; PPARγ,
peroxisome proliferator-activated receptor γ. |
Context-dependent pathway
interactions
The functional relationship between GPR124 and PPARγ
exhibits marked context dependency that reflects the complex
regulatory mechanisms governing their expression and activity
(37,38). While the canonical Wnt/β-catenin
pathway serves as the primary signaling nexus for this crosstalk,
it is important to recognize that GPR124 can also engage other
signaling pathways. This expands the functional repertoire of this
receptor beyond its role as a Wnt-specific co-receptor. For
example, a recent study in trophoblast cells demonstrated that
GPR124 can regulate cell proliferation, migration, invasion and
inflammation by modulating the JNK and P38 MAPK pathways (39). This finding not only highlights
the mechanistic diversity of GPR124 signaling but also suggests
that its biological functions may be broader than previously
appreciated, opening new avenues for understanding its
pathophysiological significance.
During normal physiological conditions, both
receptors maintain low basal activity, with GPR124 showing minimal
expression in quiescent endothelium and PPARγ primarily active in
metabolic tissues (17).
Inflammatory stimuli trigger coordinated but opposing changes in
pathway activity. Pro-inflammatory cytokines, such as TNF-α and
IL-1β, upregulate GPR124 expression in endothelial cells while
simultaneously suppressing PPARγ activity through
post-translational modifications and coactivator sequestration
(40). This creates a
pro-inflammatory state characterized by enhanced Wnt signaling,
increased angiogenic potential and reduced anti-inflammatory
capacity. Conversely, anti-inflammatory mediators and metabolic
signals promote PPARγ activation while suppressing GPR124-mediated
Wnt signaling (15). This
regulatory pattern enables dynamic switching between inflammatory
and resolution phases, with the GPR124-Wnt-PPARγ axis serving as a
central coordinator of these transitions.
Studies have also revealed tissue-specific
variations in pathway interactions. In adipose tissue, GPR124
appears to facilitate early stages of adipogenesis that enable
subsequent PPARγ-driven terminal differentiation, suggesting
cooperative rather than purely antagonistic relationships in
certain contexts (41).
Similarly, in certain types OF cancer, aberrant Wnt signaling can
paradoxically activate rather than inhibit PPARγ, reflecting the
complex nature of pathway crosstalk in transformed cells (4).
Pathophysiological implications in chronic
inflammatory diseases
Atherosclerosis: Opposing roles in
vascular inflammation
Atherosclerosis represents a paradigmatic chronic
inflammatory disease where the GPR124-Wnt-PPARγ axis serves crucial
but opposing roles in disease progression (42). The condition is characterized by
the formation of lipid-rich plaques within arterial walls,
accompanied by chronic inflammation, endothelial dysfunction and
progressive vascular remodeling (43).
GPR124 facilitates atherosclerotic progression
through a coordinated array of pro-inflammatory and pro-atherogenic
mechanisms that collectively promote vascular wall pathology
(44). Enhanced GPR124
expression in atherosclerotic vessels potentiates NLR family pyrin
domain containing 3 inflammasome assembly and activation,
culminating in the proteolytic maturation and secretion of the
inflammatory cytokines IL-1β and IL-18 (45). This inflammatory cascade
orchestrates the recruitment and activation of circulating
monocytes and their differentiation into pro-inflammatory
macrophages within the arterial intima, thereby perpetuating
chronic inflammatory responses that drive plaque formation and
destabilization. Furthermore, GPR124-mediated Wnt signaling
promotes the phenotypic modulation of vascular smooth muscle cells
from a contractile to a synthetic, proliferative phenotype,
facilitating their migration into the intimal space, and
contributing to neointimal hyperplasia and pathological arterial
remodeling (44).
The pro-angiogenic effects of GPR124 also contribute
to plaque progression by promoting the formation of intraplaque
neovascularization (46). These
newly formed vessels are typically immature and leaky, facilitating
the infiltration of inflammatory cells and lipoproteins into the
plaque core. The resulting increase in plaque inflammation and
lipid accumulation promotes plaque vulnerability, and increases the
risk of rupture and thrombotic complications (47).
By contrast, PPARγ provides robust protection
against atherosclerotic development through multiple
anti-inflammatory and metabolic mechanisms (42). PPARγ activation in macrophages
promotes the alternative M2 polarization state, which is
characterized by enhanced phagocytic capacity, increased production
of anti-inflammatory factors, such as IL-10, TGF-β and IL-1
receptor antagonist, and improved cholesterol efflux (48). These effects facilitate the
clearance of apoptotic cells and lipid debris from atherosclerotic
lesions, promoting plaque stabilization and resolution of
inflammation (49).
PPARγ also exerts direct protective effects on
endothelial cells, enhancing nitric oxide production, reducing
oxidative stress and improving endothelial barrier function
(42). These effects help
maintain vascular homeostasis and prevent the endothelial
dysfunction that initiates atherosclerotic development (50). Furthermore, PPARγ activation
promotes the expression of genes, such as ABCA1,
ABCG1 and SR-B1, involved in reverse cholesterol
transport, facilitating the removal of excess cholesterol from
arterial walls (51).
The opposing roles of GPR124 and PPARγ in
atherosclerosis suggest that the balance between these pathways
critically determines disease progression. Therapeutic strategies
that simultaneously inhibit GPR124-mediated pro-inflammatory
signaling while enhancing PPARγ-mediated anti-inflammatory
responses may provide synergistic benefits for atherosclerosis
prevention and treatment (Fig.
2).
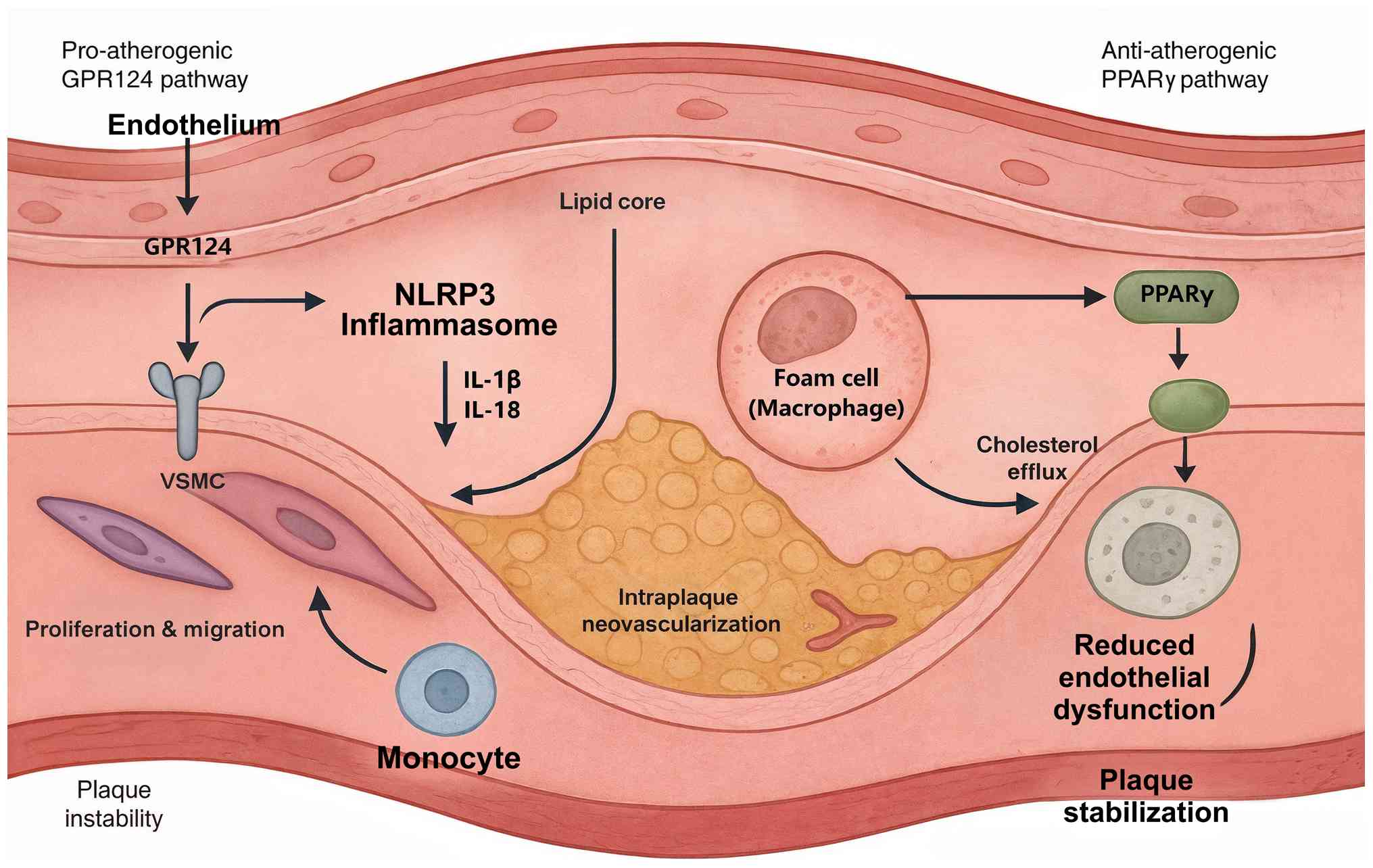 | Figure 2Opposing roles of the GPR124-PPARγ
axis in atherosclerosis. The conflicting functions of GPR124 and
PPARγ in the progression of atherosclerosis are presented. The
pro-atherogenic GPR124 pathway shows that enhanced GPR124
expression promotes NLRP3 inflammasome activation, leading to the
secretion of pro-inflammatory cytokines IL-1β and IL-18. This
inflammatory cascade facilitates monocyte recruitment, while
GPR124-mediated Wnt signaling also drives the proliferation and
migration of VSMCs and stimulates intraplaque neovascularization,
all contributing to plaque instability. Conversely, the
anti-atherogenic PPARγ pathway illustrates that PPARγ activation
promotes M2 macrophage polarization, which enhances cholesterol
efflux from foam cells. PPARγ also exerts direct protective effects
on the endothelium, reducing dysfunction, ultimately leading to
plaque stabilization. GPR124, G protein-coupled receptor 124;
NLRP3, NLR family pyrin domain containing 3; PPARγ, peroxisome
proliferator-activated receptor γ; VSMCs, vascular smooth muscle
cells. |
Diabetic complications: Vascular
protection and metabolic regulation
Type 2 diabetes mellitus and its associated
complications represent another major area where the
GPR124-Wnt-PPARγ axis exerts notable pathophysiological influence
(21). Diabetic complications,
including diabetic nephropathy, retinopathy and neuropathy, involve
complex interactions between metabolic dysfunction, chronic
inflammation and microvascular injury (52).
Recent investigations have established GPR124 as a
pivotal cytoprotective mediator in the pathogenesis of diabetic
nephropathy, revealing novel mechanistic insights into podocyte
preservation strategies (7,53). GPR124 expression in glomerular
podocytes confers protection against diabetes-induced cellular
senescence and structural injury through direct molecular
interactions with the cytoskeletal protein vinculin and negative
regulatory modulation of focal adhesion kinase signaling cascades
(54). This cytoprotective
mechanism preserves podocyte structural integrity and maintains
glomerular filtration barrier selectivity, with clinical studies
demonstrating positive associations between GPR124 expression
levels and estimated glomerular filtration rate, alongside inverse
associations with proteinuria severity, establishing GPR124 as a
potential biomarker for nephroprotective therapeutic responses.
The protective effects of GPR124 in diabetic
nephropathy appear to involve both direct cellular mechanisms and
indirect effects mediated through Wnt signaling modulation
(15). GPR124-mediated Wnt
activation promotes the expression of genes involved in podocyte
survival and barrier function, including NPHS1 and
Bcl-2, while also enhancing the regenerative capacity of
injured glomerular cells (55).
These effects help maintain kidney function and slow the
progression of diabetic nephropathy.
In diabetic retinopathy, GPR124 serves a more
complex role that reflects the dual nature of angiogenic responses
in this condition (56). While
pathological angiogenesis contributes to vision-threatening
complications, appropriate vascular responses are necessary for
maintaining retinal function and preventing ischemic injury
(57). GPR124-mediated
regulation of retinal angiogenesis may therefore represent a
therapeutic target for achieving the delicate balance between
preventing pathological neovascularization while preserving
physiological vascular function.
PPARγ provides complementary protection against
diabetic complications through its master regulatory role in
glucose homeostasis and insulin sensitivity (10). PPARγ activation improves systemic
metabolic control, reducing hyperglycemia and associated oxidative
stress that drives diabetic complications (58). The receptor also exerts direct
protective effects on the vascular system, brain/CNS, kidneys,
eyes, peripheral nerves, liver, lungs and components of the immune
system, promoting anti-inflammatory responses and enhancing
cellular stress resistance. Evidence includes neuroprotection and
cognitive benefits in the CNS, renoprotection in diabetic kidney
disease, retinal protection in the eye, improvement of diabetic
peripheral neuropathy, mitigation of hepatic steatosis/NASH,
reduction of respiratory infection/inflammation and pulmonary
vascular remodeling in the lung, and modulation of innate immunity
(59-63).
The clinical success of the PPARγ agonists
thiazolidinediones in reducing diabetic complications demonstrates
the therapeutic potential of targeting this pathway (64). These agents not only improve
glycemic control, but also provide direct organ protection through
anti-inflammatory and cytoprotective mechanisms (65). The combination of GPR124-mediated
vascular protection with PPARγ-mediated metabolic improvement
suggests potential synergistic benefits for preventing and treating
diabetic complications.
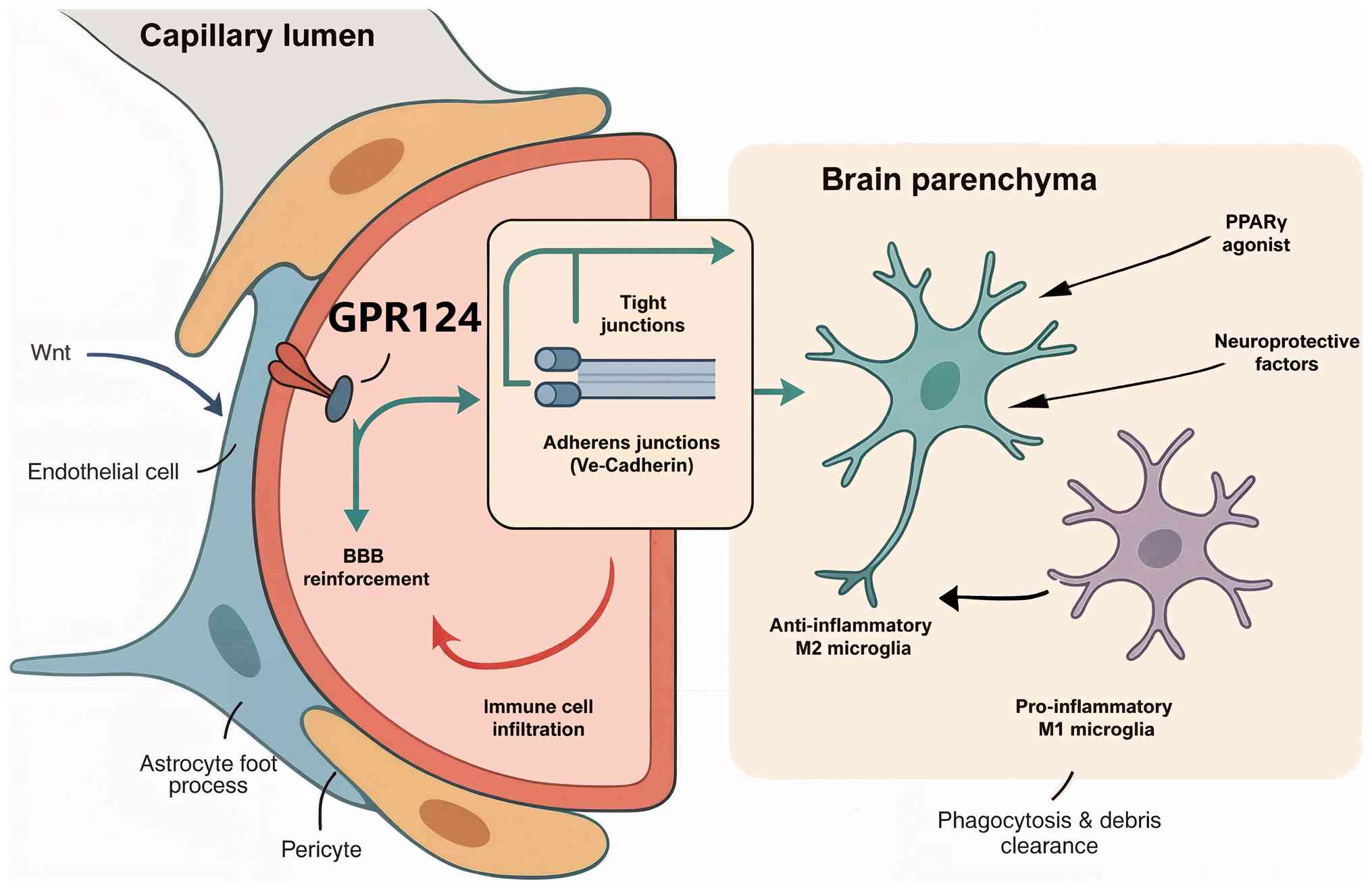
Neuroinflammation: BBB integrity and
neuroprotection
Chronic neuroinflammatory diseases, including
Alzheimer's disease, multiple sclerosis and Parkinson's disease,
involve complex interactions between BBB dysfunction, microglial
activation and neuronal injury (66). The GPR124-Wnt-PPARγ axis serves
key roles in regulating these pathological processes through
distinct but complementary mechanisms (9).
GPR124 expression and activity are indispensable for
preserving BBB structural and functional integrity during acute and
chronic neuroinflammatory states, representing a critical
neurovascular protective mechanism (17). This protective function reflects
both the fundamental developmental role of this receptor in CNS
vascularization and its stress-responsive reactivation during
pathological conditions. In experimental models of ischemic
cerebrovascular injury, endothelial-specific genetic ablation of
GPR124 precipitates a severe BBB compromise, which is characterized
by enhanced microvascular permeability, increased susceptibility to
hemorrhagic transformation and markedly worse neurological
functional outcomes. The underlying molecular mechanisms involve
GPR124-mediated preservation of intercellular junctional complexes,
including both tight junction proteins (claudin-5, occludin and
zonula occludens-1) and adherens junction components (VE-cadherin
and β-catenin), which collectively regulate paracellular
permeability and maintain the selective barrier properties
essential for CNS homeostasis (20).
The protective effects of GPR124 on BBB function
involve the maintenance of tight junction proteins and adherens
junction complexes that regulate paracellular permeability
(67). GPR124-mediated Wnt
signaling promotes the expression of claudin-5, occludin and
VE-cadherin, essential components of the BBB structure (68,69). Additionally, GPR124 activation
enhances the expression of specific transporters such as Glut1 that
are required for BBB function (17).
During chronic neuroinflammation, BBB dysfunction
facilitates the infiltration of peripheral immune cells and
inflammatory mediators into the CNS, exacerbating neuronal injury
and disease progression (70).
GPR124-mediated preservation of barrier integrity thus provides a
critical protective mechanism against neuroinflammatory progression
(71). Beyond its direct
structural effects on endothelial tight junctions, this
barrier-protective function indirectly regulates neuroinflammation
by physically limiting the infiltration of peripheral immune cells
into the CNS parenchyma (17).
Whether GPR124 on endothelial or perivascular cells also directly
modulates the expression of adhesion molecules or chemokines
involved in immune cell trafficking remains an important area for
future investigation.
PPARγ contributes to neuroprotection through
powerful anti-inflammatory and antioxidant mechanisms (72). PPARγ activation in microglia
promotes the anti-inflammatory M2 polarization state, reducing the
production of neurotoxic cytokines, and enhancing the clearance of
protein aggregates and cellular debris (73). These effects help resolve
neuroinflammation and create a supportive environment for neuronal
survival and repair (66).
PPARγ also exerts direct neuroprotective effects on
neurons and oligodendrocytes, enhancing cellular stress resistance
and promoting survival signaling pathways (74). The role of this receptor in
regulating mitochondrial function and oxidative metabolism provides
additional protection against the energetic stress that
characterizes numerous neurodegenerative diseases (75).
The combination of GPR124-mediated BBB protection
and PPARγ-mediated neuroinflammation resolution represents a
promising therapeutic approach for chronic neuroinflammatory
diseases. This dual targeting strategy could address both the
vascular and inflammatory components of neurodegeneration,
potentially providing superior neuroprotective outcomes compared
with single-pathway interventions (Fig. 3).
Cancer-associated inflammation: Tumor
angiogenesis and immune evasion
Cancer-associated inflammation represents a complex
pathophysiological process where the GPR124-Wnt-PPARγ axis exerts
context-dependent effects on tumor progression, angiogenesis and
immune responses (76). The
chronic inflammatory microenvironment within tumors involves
dynamic interactions between cancer cells, stromal cells, immune
cells and blood vessels, which collectively determine tumor growth
and metastatic potential (77).
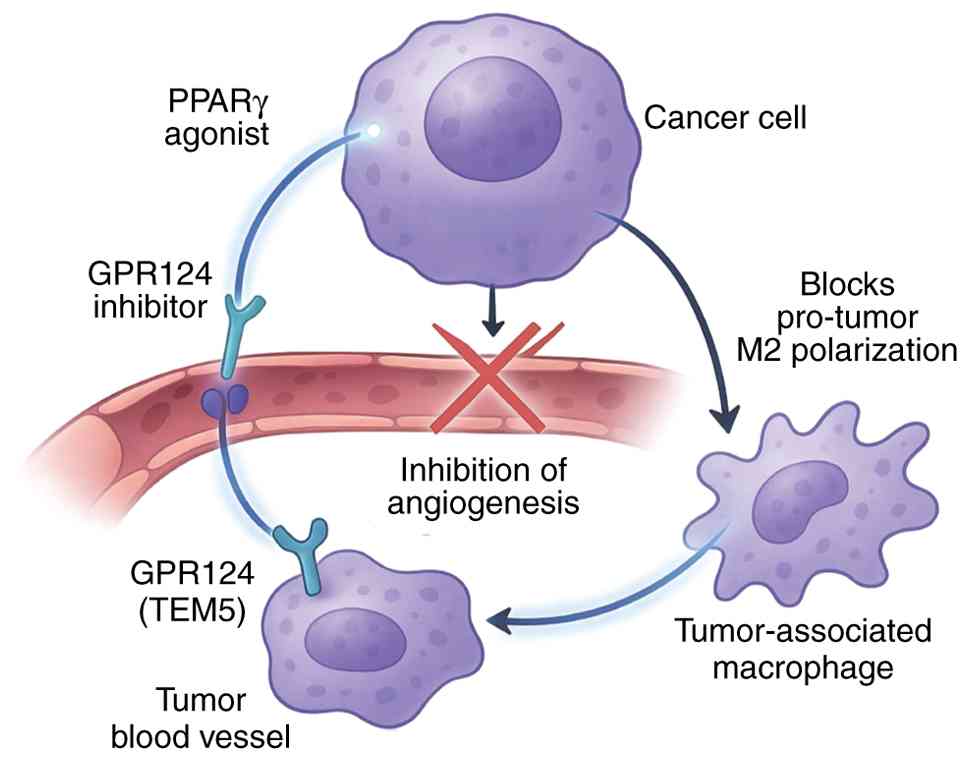
GPR124 contributes to tumor progression primarily
through its pro-angiogenic functions in tumor endothelium (22). Originally identified as TEM5,
GPR124 shows elevated expression in tumor vasculature compared with
in normal tissues (78). This
enhanced expression facilitates tumor angiogenesis by promoting
endothelial cell proliferation, migration and tube formation in
response to Wnt7a/b ligands secreted by tumor cells (79).
Previous studies have revealed additional roles for
GPR124 in cancer cell biology beyond its endothelial functions
(20). In glioblastoma, GPR124
regulates cancer cell proliferation through its effects on
microtubule assembly and mitotic progression (80). The interaction of the receptor
with centrosome proteins affects chromosome segregation and cell
cycle progression, contributing to the rapid proliferation that is
characteristic of aggressive brain tumors (81).
GPR124 also influences tumor-associated inflammation
through effects on immune cell recruitment and activation (82). Enhanced GPR124 signaling in
tumor-associated macrophages promotes the pro-tumorigenic M2
polarization state, facilitating tumor immune evasion and creating
a supportive microenvironment for cancer progression (83,84). This influence on TAMs suggests a
broader role for GPR124 in shaping the tumor immune
microenvironment beyond angiogenesis (21). Its potential involvement in
stromal remodeling or the recruitment of other immunosuppressive
cell populations, such as myeloid-derived suppressor cells (MDSCs),
remains an underexplored area that warrants further investigation
to fully understand its contribution to cancer-associated
inflammation and immune evasion.
PPARγ demonstrates multifaceted and
context-dependent regulatory functions in cancer-associated
inflammatory processes (13,85), exhibiting both tumor-suppressive
and, paradoxically, tumor-promoting activities depending on the
specific cellular context, tumor microenvironment and disease
stage. In solid malignancies, such as endometrial cancer, melanoma
and breast cancer, PPARγ functions as a bona fide tumor suppressor
through its coordinated anti-proliferative, pro-apoptotic and
pro-differentiation effects on transformed cells (13). PPARγ activation induces cell
cycle arrest at critical checkpoints, enhances apoptotic
susceptibility through both intrinsic and extrinsic pathways, and
promotes terminal cellular differentiation programs that antagonize
the dedifferentiated phenotype characteristic of aggressive
malignancies (86).
Concurrently, the anti-inflammatory properties of PPARγ contribute
to the resolution of tumor-promoting chronic inflammation by
suppressing the production of growth-stimulatory cytokines,
angiogenic factors and tissue remodeling enzymes that collectively
support neoplastic progression and metastatic dissemination
(87). However, research has
revealed that PPARγ can also contribute to cancer progression under
certain conditions (88). In
established tumors, PPARγ activation may promote metabolic
reprogramming that supports cancer cell survival and drug
resistance (89). Furthermore,
PPARγ can mediate tumor adaptation to immunotherapy through the
regulation of immunosuppressive factors such as vascular
endothelial growth factor-A and the expansion of MDSCs (90).
The dual roles of PPARγ in cancer highlight the
importance of understanding context-dependent pathway functions
when developing therapeutic strategies. The interaction between
GPR124-mediated pro-angiogenic signaling and PPARγ-mediated
metabolic regulation may determine whether tumors adopt aggressive,
highly vascularized phenotypes or more indolent, metabolically
adapted states.
Therapeutic implications and clinical
translation
Single-pathway targeting strategies
The distinct roles of GPR124 and PPARγ in chronic
inflammatory diseases provide multiple opportunities for
therapeutic intervention through single-pathway targeting
approaches. These strategies leverage the specific functions of
each receptor to address particular aspects of disease pathology
while minimizing off-target effects.
GPR124 modulation
GPR124 represents an attractive therapeutic target
due to its context-dependent essentiality and restricted expression
pattern (17). The requirement
for this receptor primarily during developmental or pathological
stress conditions suggests that therapeutic modulation could
provide notable benefits while minimizing effects on normal
physiological processes.
For conditions requiring enhanced vascular
integrity, such as stroke or diabetic complications, GPR124
activation represents a logical therapeutic strategy (17). Engineered Wnt surrogate molecules
designed to specifically activate the GPR124/RECK receptor complex
are currently under development for treating vascular retinopathies
(91). These agents could
potentially be adapted for other conditions requiring BBB
protection or enhanced vascular stability.
The development of GPR124 agonists faces several
technical challenges, including the complexity of the signaling
mechanisms of the receptor and the need for tissue-specific
targeting (92). Current
approaches focus on small molecules that enhance GPR124/RECK
complex formation or peptide-based agents derived from the
extracellular domain of the receptor (44).
Conversely, GPR124 inhibition may be beneficial in
conditions where excessive angiogenesis or Wnt signaling
contributes to pathology, such as certain types of cancer or
proliferative retinopathies (93). Therapeutic antibodies targeting
GPR124 or small molecule inhibitors that disrupt receptor function
are being investigated for anti-angiogenic applications (94).
PPARγ targeting
PPARγ represents one of the most clinically
validated therapeutic targets in metabolism and inflammation, with
multiple approved drugs and an active development pipeline
(10). The clinical success of
thiazolidinediones in treating type 2 diabetes demonstrates the
therapeutic potential of PPARγ activation, while ongoing research
explores applications in cancer, neurodegeneration and inflammatory
diseases (95).
Advances in PPARγ drug development focus on
selective modulators that retain beneficial anti-inflammatory and
insulin-sensitizing effects while minimizing adverse metabolic
consequences such as weight gain and fluid retention (96). These SPPARγMs achieve improved
therapeutic profiles through differential coactivator recruitment
and tissue-specific gene expression patterns (97).
Notable recent approvals include seladelpar for
primary biliary cholangitis and the advanced development of
lanifibranor for NASH (98).
These successes demonstrate the continued clinical relevance of
PPARγ targeting and the potential for expansion into additional
inflammatory conditions.
For chronic inflammatory diseases, PPARγ agonists
provide broad anti-inflammatory effects that address multiple
aspects of disease pathology (99). The ability of the receptor to
promote inflammation resolution, enhance tissue repair and improve
metabolic function makes it particularly attractive for conditions
involving chronic inflammation and metabolic dysfunction (100).
Despite these pharmacological advances, achieving
optimal tissue-specific targeting and maintaining an ideal
therapeutic index that maximizes beneficial effects while
minimizing adverse consequences remains a formidable challenge in
contemporary PPARγ modulator development. Addressing these
limitations necessitates innovative approaches encompassing both
rational ligand design strategies and sophisticated drug delivery
methodologies. Promising avenues include the development of
covalent modulators designed to selectively engage tissue-resident
co-regulatory proteins, implementation of targeted delivery systems
utilizing antibody-drug conjugates or engineered nanoparticle
carriers, and exploitation of tissue-specific promoter elements for
localized therapeutic agent expression (101,102). Such progressive strategies
represent essential innovations required to identify the complete
therapeutic potential of PPARγ modulation while achieving superior
safety profiles and enhanced patient tolerability across diverse
clinical applications.
Combination targeting strategies
The opposing regulation of Wnt signaling by GPR124
and PPARγ provides a compelling rationale for combination
therapeutic approaches that target both pathways simultaneously
(37). Such strategies could
achieve synergistic benefits by addressing multiple aspects of
disease pathology while potentially reducing the required doses of
individual agents.
Dual pathway targeting
In diseases characterized by excessive Wnt signaling
and inadequate anti-inflammatory responses, such as certain types
of cancer or atherosclerosis, combination strategies involving
GPR124 inhibition and PPARγ activation could provide synergistic
benefits (44). This therapeutic
paradigm is predicated upon the mechanistically sound hypothesis
that concurrent suppression of pro-angiogenic and pro-proliferative
Wnt signaling cascades, combined with pharmacological enhancement
of anti-inflammatory and pro-resolution PPARγ pathways, will
generate synergistic therapeutic benefits that exceed the additive
effects of individual pathway modulation. The theoretical
advantages of such dual-targeting approaches encompass enhanced
comprehensive pathway modulation, the reduced likelihood of the
development of compensatory resistance mechanisms, and the
potential for dose reduction strategies that minimize individual
agent-associated toxicities while maintaining therapeutic efficacy.
Additionally, this combinatorial targeting strategy addresses the
temporal dynamics of disease progression, wherein GPR124 inhibition
provides immediate effects on pathological angiogenesis and
cellular proliferation, while PPARγ activation delivers sustained
anti-inflammatory benefits and promotes active resolution of
chronic inflammatory states (Fig.
4).
Sequential pathway modulation
For conditions involving phases of tissue injury
followed by repair and resolution, sequential modulation of the
GPR124-Wnt-PPARγ axis may provide optimal therapeutic outcomes
(15). This approach would
involve initial GPR124 activation to promote vascular integrity and
tissue survival during acute injury phases, followed by PPARγ
activation to enhance inflammation resolution and tissue remodeling
during recovery phases.
Stroke represents a paradigmatic example where
sequential targeting could be beneficial (103). Early GPR124 activation could
help maintain BBB integrity and prevent hemorrhagic transformation,
while delayed PPARγ activation could promote neuroinflammation
resolution and enhance long-term recovery (19).
Precision medicine approaches
The inherently context-dependent and
patient-specific variability in GPR124-PPARγ axis functionality
strongly supports the implementation of precision medicine
strategies that incorporate comprehensive individual patient
phenotyping to optimize therapeutic selection and clinical outcomes
(22). Such personalized
therapeutic approaches should integrate multiple determinants of
treatment responsiveness, including genetic polymorphism profiles,
epigenetic modifications, biomarker signatures reflecting pathway
activity states and disease-specific pathophysiological
considerations. The convergence of pharmacogenomic insights,
advanced biomarker discovery platforms and real-time therapeutic
monitoring technologies creates unprecedented opportunities for
implementing truly individualized treatment paradigms that maximize
therapeutic efficacy while minimizing adverse effects across
diverse patient populations with chronic inflammatory diseases.
Genetic variants and
pharmacogenomics
Genetic variants in GPR124 and PPARγ
markedly influence receptor function and therapeutic responsiveness
(104). PPARγ
polymorphisms, particularly the Pro12Ala variant, affect insulin
sensitivity, cardiovascular risk and response to thiazolidinedione
therapy (105). Similarly,
GPR124 variants may influence vascular development, BBB
function and susceptibility to neurovascular diseases (106).
Future therapeutic approaches could incorporate
genetic testing to guide treatment selection and dosing strategies.
Patients with specific genetic profiles might benefit from
particular combinations of GPR124 and PPARγ modulators, while
others might require alternative therapeutic approaches (107).
Biomarker-guided therapy
The development of biomarkers reflecting GPR124 and
PPARγ pathway activity could enable real-time monitoring of
therapeutic responses and guide treatment adjustments (107). Potential biomarkers include
circulating levels of Wnt signaling components, PPARγ target
gene expression profiles and imaging-based assessments of vascular
function (15).
Advanced imaging techniques could provide
non-invasive assessment of BBB integrity, tissue perfusion and
inflammatory activity, enabling personalized treatment monitoring
and optimization. These approaches could help identify patients
most likely to benefit from specific therapeutic interventions and
guide treatment duration and intensity.
Challenges, safety and tolerability
considerations
Despite the promising therapeutic potential of
targeting the GPR124-Wnt-PPARγ axis, several challenges,
particularly regarding drug development, safety and tolerability,
must be addressed for successful clinical translation.
Drug development challenges
GPR124 represents a relatively novel therapeutic
target with limited pharmaceutical investment and few available
chemical tools (92). The
development of selective GPR124 modulators requires notable
investment in drug discovery and optimization, including
structure-activity relationship studies, selectivity profiling and
pharmacokinetic optimization. The complex signaling mechanisms of
GPR124, involving interactions with RECK and specific Wnt ligands,
present additional challenges for designing drugs that achieve
appropriate selectivity for GPR124/RECK-mediated signaling while
avoiding unintended effects on other Wnt pathway components
(108).
Safety, tolerability and off-target
effects
The pharmacological modulation of these fundamental
homeostatic pathways necessitates comprehensive safety assessment
frameworks that thoroughly evaluate potential on-target adverse
effects, off-target toxicities and complex drug-drug interactions
inherent to combination therapeutic regimens.
For PPARγ-targeting agents, the clinical utility of
full receptor agonists, exemplified by the thiazolidinedione class,
remains constrained by notable mechanism-based adverse effects,
including excessive adipogenesis-mediated weight gain, fluid
retention leading to peripheral edema and potential heart failure
exacerbation, and increased fracture risk attributed to osteoblast
function impairment (109).
While SPPARγMs represent a pharmacological advancement designed to
mitigate these dose-limiting toxicities, complete dissociation of
therapeutic benefits from adverse metabolic consequences has not
been fully achieved in clinical practice, highlighting the need for
continued drug development innovations.
GPR124 modulation also presents theoretical safety
concerns that require careful evaluation. Given the essential role
of the receptor in CNS vascularization, systemic or long-term
inhibition could have unintended consequences on vascular function
(19) and tissue perfusion,
particularly in patients with underlying vascular comorbidities
(17). Conversely, therapeutic
activation of GPR124 must be carefully controlled to avoid
promoting pathological angiogenesis, a concern in conditions such
as cancer-associated inflammation or proliferative retinopathies
(22,110).
Finally, combination therapies, while theoretically
synergistic, present additional complexity for safety assessment.
Rigorous preclinical evaluation is required to investigate
potential drug-drug interactions, additive toxicities and the
emergence of novel adverse effects that may not be apparent in
single-agent studies.
Future directions and research
priorities
Mechanistic understanding
Despite notable advances in understanding GPR124 and
PPARγ functions, several critical knowledge gaps remain that limit
therapeutic development. Priority areas for future research include
detailed characterization of tissue-specific signaling mechanisms,
identification of additional pathway components and elucidation of
disease-specific regulatory networks.
The molecular basis for context-dependent pathway
interactions requires further investigation. Understanding how the
cellular environment, inflammatory stimuli and metabolic status
influence GPR124-PPARγ crosstalk will be essential for predicting
therapeutic responses and optimizing treatment strategies.
Advanced methodological approaches, including
single-cell RNA sequencing (scRNA-seq), spatial transcriptomics and
real-time pathway monitoring, may provide novel insights into
pathway dynamics and regulatory mechanisms (111). These technologies will enable
detailed characterization of cell-type-specific pathway functions
and temporal patterns of pathway activation during disease
progression.
Furthermore, comprehensive preclinical validation
studies are critically required to substantiate the theoretical
synergistic potential of dual GPR124/PPARγ modulation strategies
and to establish their translational feasibility for clinical
development. These investigations should employ state-of-the-art
methodological approaches, including scRNA-seq for cellular
heterogeneity analysis, spatial transcriptomics for tissue
architecture-function relationships, and functional genomics assays
in disease-relevant organoid models and genetically modified animal
systems. Such systematic preclinical evaluation is essential to
confirm mechanistic plausibility, quantify therapeutic synergy
indices, identify optimal dosing combinations, characterize
pharmacokinetic and pharmacodynamic interactions, and proactively
identify potential safety signals before clinical translation can
be responsibly pursued.
Therapeutic development
The translation of mechanistic insights into
clinical therapies requires coordinated efforts in drug discovery,
preclinical validation and clinical development. Priority areas
include the development of selective GPR124 modulators,
optimization of PPARγ-targeting strategies and validation of
combination therapeutic approaches.
Collaborative efforts between academic institutions,
pharmaceutical companies and regulatory agencies will be essential
for advancing GPR124-targeting therapeutics through clinical
development. The establishment of standardized assays, validated
animal models and clinical biomarkers will facilitate therapeutic
development and regulatory approval.
Clinical translation
The successful clinical translation of GPR124-PPARγ
targeting strategies will require careful patient selection,
biomarker development and clinical trial design. Initial clinical
studies should focus on diseases with a clear mechanistic rationale
and measurable clinical endpoints, such as diabetic complications
or stroke prevention.
The development of companion diagnostics and
biomarker-guided treatment algorithms will be essential for
implementing precision medicine approaches. These tools will enable
the identification of patients most likely to benefit from specific
therapeutic interventions and provide objective measures of
treatment response.
Conclusion
The GPR124-Wnt-PPARγ regulatory axis constitutes a
fundamental molecular rheostat that governs the dynamic equilibrium
between pro-inflammatory, pro-angiogenic cellular responses, and
anti-inflammatory, metabolic homeostatic programs in chronic
inflammatory disease pathogenesis. This comprehensive review has
systematically examined the complex molecular architecture
underlying this regulatory network, elucidated its
pathophysiological importance across multiple disease paradigms and
critically evaluated emerging therapeutic opportunities for
clinical translation. The antagonistic regulation of Wnt/β-catenin
signaling by GPR124 and PPARγ establishes a molecular switch
mechanism that determines cellular fate decisions in response to
inflammatory stimuli, metabolic perturbations and tissue injury
signals, thereby providing novel mechanistic insights into the
pathogenesis of atherosclerosis, diabetic complications,
neuroinflammatory disorders and cancer-associated inflammatory
processes.
The opposing effects of GPR124 and PPARγ on
Wnt/β-catenin signaling create a molecular switch that determines
cellular responses to inflammatory stimuli, metabolic stress and
tissue injury. Understanding this regulatory mechanism provides
novel insights into the pathogenesis of atherosclerosis, diabetic
complications, neuroinflammation and cancer-associated
inflammation. The context-dependent nature of pathway interactions
explains how the same molecular components can contribute to both
protective and pathological responses depending on the cellular
environment and disease stage.
The therapeutic implications of targeting the
GPR124-Wnt-PPARγ axis are substantial, encompassing both
single-pathway and combination targeting strategies. While PPARγ
represents a clinically validated target with multiple approved
therapies, GPR124 remains an emerging target with significant
untapped potential. The development of selective GPR124 modulators
and optimized combination therapies could provide new treatment
options for chronic inflammatory diseases that inadequately respond
to current interventions.
Future research priorities include detailed
mechanistic characterization of pathway interactions, development
of selective therapeutic agents, and validation of combination
targeting strategies in preclinical and clinical studies. The
successful translation of these research advances into clinical
applications will require coordinated efforts across multiple
disciplines and stakeholders.
The GPR124-Wnt-PPARγ axis represents a promising
frontier for understanding and treating chronic inflammatory
diseases. As the mechanistic understanding regarding this axis
advances and therapeutic tools improve, this regulatory network may
provide the foundation for next-generation precision medicine
approaches that address the complex, multifactorial nature of
chronic inflammatory pathology.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science
Foundation of China (grant no. 82201080) and the Academic
Enhancement Support Program of Hainan Medical University (grant no.
XSTS2025027).
Availability of data and materials
Not applicable.
Authors' contributions
MWC wrote the original draft of the manuscript,
conceptualized the study, conducted the investigation (i.e., the
review and analysis of literature and data), designed the
methodology and created the visualizations (figures). SYT reviewed
and edited the manuscript, contributed to the use of software tools
(Endnote reference management), helped with the creation of
visualizations (including figure design), and performed the
validation of the findings by cross-checking the literature and
data. TW reviewed and edited the manuscript, contributed to the
conceptualization of the study, and assisted with the investigation
by contributing to literature review and analysis. ZLG reviewed and
edited the manuscript, supervised the project, acquired funding for
the study, provided resources (including access to databases,
literature or other study materials), and was responsible for
project administration (e.g., coordinating contributions from all
authors and ensuring project timelines were met). Data
authentication is not applicable. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
BBB
|
blood-brain barrier
|
|
CNS
|
central nervous system
|
|
GPR124
|
G protein-coupled receptor 124
|
|
PPARγ
|
peroxisome proliferator-activated
receptor γ
|
References
|
1
|
Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng
J, Li Y, Wang X and Zhao L: Inflammatory responses and
inflammation-associated diseases in organs. Oncotarget.
9:7204–7218. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hotamisligil GS: Inflammation,
metaflammation and immunometabolic disorders. Nature. 542:177–185.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lecarpentier Y, Claes V, Vallée A and
Hébert JL: Interactions between PPAR gamma and the canonical
Wnt/Beta-catenin pathway in type 2 diabetes and colon cancer. PPAR
Res. 2017:58790902017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vallée A, Lecarpentier Y, Guillevin R and
Vallée JN: Opposite interplay between the canonical WNT/β-Catenin
pathway and PPAR Gamma: A potential therapeutic target in gliomas.
Neurosci Bull. 34:573–588. 2018. View Article : Google Scholar
|
|
5
|
Anderson KD, Pan L, Yang XM, Hughes VC,
Walls JR, Dominguez MG, Simmons MV, Burfeind P, Xue Y, Wei Y, et
al: Angiogenic sprouting into neural tissue requires Gpr124, an
orphan G protein-coupled receptor. Proc Natl Acad Sci USA.
108:2807–2812. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kuhnert F, Mancuso MR, Shamloo A, Wang HT,
Choksi V, Florek M, Su H, Fruttiger M, Young WL, Heilshorn SC and
Kuo CJ: Essential regulation of CNS angiogenesis by the orphan G
protein-coupled receptor GPR124. Science. 330:985–989. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Duan Y, Chu Q, Lv H, Li J, Guo X,
Gao Y, Liu M, Tang W, Hu H, et al: G-protein coupled receptor
GPR124 protects against podocyte senescence and injury in diabetic
kidney disease. Kidney Int. 107:652–665. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou Y, Wang Y, Tischfield M, Williams J,
Smallwood PM, Rattner A, Taketo MM and Nathans J: Canonical WNT
signaling components in vascular development and barrier formation.
J Clin Invest. 124:3825–3846. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Posokhova E, Shukla A, Seaman S, Volate S,
Hilton MB, Wu B, Morris H, Swing DA, Zhou M, Zudaire E, et al:
GPR124 functions as a WNT7-specific coactivator of canonical
β-catenin signaling. Cell Rep. 10:123–130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mal S, Dwivedi AR and Kumar V, Kumar N,
Kumar B and Kumar V: Role of peroxisome Proliferator-activated
receptor gamma (PPARγ) in different disease states: Recent updates.
Curr Med Chem. 28:3193–3215. 2021. View Article : Google Scholar
|
|
11
|
Tu CC, Hsieh TH, Chu CY, Lin YC, Lin BJ
and Chen CH: Targeting PPARγ via SIAH1/2-mediated
ubiquitin-proteasomal degradation as a new therapeutic approach in
luminal-type bladder cancer. Cell Death Dis. 15:9082024. View Article : Google Scholar
|
|
12
|
Jangra A, Babu B, Divakar S, Gowramma B,
Rajan S, Jangra S and Malakar V: An in-depth review of PPARγ
modulators as anti-diabetes therapeutics. Drug Metab Rev.
57:311–337. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chi T, Wang M, Wang X, Yang K, Xie F, Liao
Z and Wei P: PPAR-γ Modulators as current and potential cancer
treatments. Front Oncol. 11:7377762021. View Article : Google Scholar
|
|
14
|
Jansson EA, Are A, Greicius G, Kuo IC,
Kelly D, Arulampalam V and Pettersson S: The Wnt/beta-catenin
signaling pathway targets PPARgamma activity in colon cancer cells.
Proc Natl Acad Sci USA. 102:1460–1465. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xue C, Chu Q, Shi Q, Zeng Y, Lu J and Li
L: Wnt signaling pathways in biology and disease: Mechanisms and
therapeutic advances. Signal Transduct Target Ther. 10:1062025.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bostaille N, Gauquier A, Twyffels L and
Vanhollebeke B: Molecular insights into Adgra2/Gpr124 and Reck
intracellular trafficking. Biol Open. 5:1874–1881. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang J, Mancuso MR, Maier C, Liang X,
Yuki K, Yang L, Kwong JW, Wang J, Rao V, Vallon M, et al: Gpr124 is
essential for blood-brain barrier integrity in central nervous
system disease. Nat Med. 23:450–460. 2017. View Article : Google Scholar :
|
|
18
|
Gastfriend BD, Nishihara H, Canfield SG,
Foreman KL, Engelhardt B, Palecek SP and Shusta EV: Wnt signaling
mediates acquisition of blood-brain barrier properties in naïve
endothelium derived from human pluripotent stem cells. ELife.
10:e709922021. View Article : Google Scholar
|
|
19
|
Zhou Y and Nathans J: Gpr124 controls CNS
angiogenesis and blood-brain barrier integrity by promoting
ligand-specific canonical wnt signaling. Dev Cell. 31:248–256.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu H, Kohno S, Voon DC, Hussein NH, Zhang
Y, Nakayama J, Takegami Y and Takahashi C: RECK/GPR124-driven WNT
signaling in pancreatic and gastric cancer cells. Cancer Sci.
115:3013–3025. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
America M, Bostaille N, Eubelen M, Martin
M, Stainier DYR and Vanhollebeke B: An integrated model for Gpr124
function in Wnt7a/b signaling among vertebrates. Cell Rep.
39:1109022022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Cho SG, Wu X, Siwko S and Liu M:
G-protein coupled receptor 124 (GPR124) in endothelial cells
regulates vascular endothelial growth factor (VEGF)-induced tumor
angiogenesis. Curr Mol Med. 14:543–554. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kroker AJ and Bruning JB: Review of the
structural and dynamic mechanisms of PPARγ partial agonism. PPAR
Res. 2015:8168562015. View Article : Google Scholar
|
|
24
|
Lefterova MI, Haakonsson AK, Lazar MA and
Mandrup S: PPARγ and the global map of adipogenesis and beyond.
Trends Endocrinol Metab. 25:293–302. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Z, Luo L, Yu W, Li P, Ou D, Liu J, Ma
H, Sun Q, Liang A, Huang C, et al: PPARγ phase separates with RXRα
at PPREs to regulate target gene expression. Cell Discov. 8:372022.
View Article : Google Scholar
|
|
26
|
Ahmadian M, Suh JM, Hah N, Liddle C,
Atkins AR, Downes M and Evans RM: PPARγ signaling and metabolism:
The good, the bad and the future. Nat Med. 19:557–566. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bougarne N, Paumelle R, Caron S, Hennuyer
N, Mansouri R, Gervois P, Staels B, Haegeman G and De Bosscher K:
PPARalpha blocks glucocorticoid receptor alpha-mediated
transactivation but cooperates with the activated glucocorticoid
receptor alpha for transrepression on NF-kappaB. Proc Natl Acad Sci
USA. 106:7397–7402. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Basil MC and Levy BD: Specialized
pro-resolving mediators: Endogenous regulators of infection and
inflammation. Nat Rev Immunol. 16:51–67. 2016. View Article : Google Scholar
|
|
29
|
Gao Z, Xu X, Li Y, Sun K, Yang M, Zhang Q,
Wang S, Lin Y, Lou L, Wu A, et al: Mechanistic insight into PPARγ
and tregs in atherosclerotic immune inflammation. Front Pharmacol.
12:7500782021. View Article : Google Scholar
|
|
30
|
O'Neill LA and Pearce EJ: Immunometabolism
governs dendritic cell and macrophage function. J Exp Med.
213:15–23. 2016. View Article : Google Scholar :
|
|
31
|
Kasprzak A: Angiogenesis-related functions
of wnt signaling in colorectal carcinogenesis. Cancers (Basel).
12:36012020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sidrat T, Rehman ZU, Joo MD, Lee KL and
Kong IK: Wnt/β-catenin Pathway-mediated PPARδ expression during
embryonic development differentiation and disease. Int J Mol Scie.
22:18542021. View Article : Google Scholar
|
|
33
|
van Kappel EC and Maurice MM: Molecular
regulation and pharmacological targeting of the β-catenin
destruction complex. Br J Pharmacol. 174:4575–4588. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lecarpentier Y, Schussler O, Hébert JL and
Vallée A: Multiple targets of the canonical WNT/β-Catenin signaling
in cancers. Front Oncol. 9:12482019. View Article : Google Scholar
|
|
35
|
Sabatino L, Pancione M, Votino C,
Colangelo T, Lupo A, Novellino E, Lavecchia A and Colantuoni V:
Emerging role of the β-catenin-PPARγ axis in the pathogenesis of
colorectal cancer. World J Gastroenterol. 20:7137–7151. 2014.
View Article : Google Scholar :
|
|
36
|
Liu J, Wang H, Zuo Y and Farmer SR:
Functional interaction between peroxisome proliferator-activated
receptor gamma and beta-catenin. Mol Cell Biol. 26:5827–5837. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vallée A, Lecarpentier Y and Vallée JN:
Interplay of opposing effects of the WNT/β-Catenin pathway and
PPARγ and implications for SARS-CoV2 treatment. Front Immunol.
12:6666932021. View Article : Google Scholar
|
|
38
|
Cui H, Wang Y, Huang H, Yu W, Bai M, Zhang
L, Bryan BA, Wang Y, Luo J, Li D, et al: GPR126 protein regulates
developmental and pathological angiogenesis through modulation of
VEGFR2 receptor signaling. J Biol Chem. 289:34871–34885. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shen Y, Lian Y, Xiao L, Miu Y, Niu J and
Cui Q: GPR124 promotes trophoblast proliferation, migration, and
invasion and inhibits trophoblast cell apoptosis and inflammation
via JNK and P38 MAPK pathways. J Cell Physiol. 239:e312982024.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Westerweel PE and Verhaar MC: Protective
actions of PPAR-gamma activation in renal endothelium. PPAR Res.
2008:6356802008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Audano M, Pedretti S, Caruso D, Crestani
M, De Fabiani E and Mitro N: Regulatory mechanisms of the early
phase of white adipocyte differentiation: An overview. Cell Mol
Life Sci. 79:1392022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Luan J, Ji X and Liu L: PPARγ in
atherosclerotic endothelial dysfunction: Regulatory compounds and
PTMs. Int J Mol Sci. 24:144942023. View Article : Google Scholar
|
|
43
|
Patial S, Sharma A, Raj K and Shukla G:
Atherosclerosis: Progression, risk factors, diagnosis, treatment,
probiotics and synbiotics as a new prophylactic hope. The Microbe.
5:1002122024. View Article : Google Scholar
|
|
44
|
Lin WY, Dong YL, Lin Y, Sunchuri D and Guo
ZL: Potential role of G protein-coupled receptor 124 in
cardiovascular and cerebrovascular disease (review). Exp Ther Med.
29:22025. View Article : Google Scholar
|
|
45
|
Lu N, Cheng W, Liu D, Liu G, Cui C, Feng C
and Wang X: NLRP3-Mediated inflammation in atherosclerosis and
associated therapeutics. Front Cell Dev Biol. 10:8233872022.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chai Q, Guo C, Li L, Cao J, Liu H and Lu
Z: Association of angiogenesis-associated genes with
atherosclerotic plaque progression, intraplaque hemorrhage, and
immune infiltration. Heliyon. 10:e326922024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kolodgie FD, Gold HK, Burke AP, Fowler DR,
Kruth HS, Weber DK, Farb A, Guerrero LJ, Hayase M, Kutys R, et al:
Intraplaque hemorrhage and progression of coronary atheroma. N Engl
J Med. 349:2316–2325. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yu L, Gao Y, Aaron N and Qiang L: A
glimpse of the connection between PPARγ and macrophage. Front
Pharmacol. 14:12543172023. View Article : Google Scholar
|
|
49
|
Bäck M, Yurdagul A Jr, Tabas I, Öörni K
and Kovanen PT: Inflammation and its resolution in atherosclerosis:
Mediators and therapeutic opportunities. Nat Rev Cardiol.
16:389–406. 2019.PubMed/NCBI
|
|
50
|
Beyer AM, Baumbach GL, Halabi CM, Modrick
ML, Lynch CM, Gerhold TD, Ghoneim SM, de Lange WJ, Keen HL, Tsai
YS, et al: Interference with PPARgamma signaling causes cerebral
vascular dysfunction, hypertrophy, and remodeling. Hypertension.
51:867–871. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chawla A, Boisvert WA, Lee CH, Laffitte
BA, Barak Y, Joseph SB, Liao D, Nagy L, Edwards PA, Curtiss LK, et
al: A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in
cholesterol efflux and atherogenesis. Mol Cell. 7:161–171. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Iwasaki H, Yagyu H and Shimano H: A
Comprehensive analysis of diabetic complications and advances in
management strategies. J Atheroscler Thromb. 32:550–559. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li X, Zhang Y, Xing X, Li M, Liu Y, Xu A
and Zhang J: Podocyte injury of diabetic nephropathy: Novel
mechanism discovery and therapeutic prospects. Biomed Pharmacother.
168:1156702023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li L and Liu Y: Podocyte aging and
diabetic kidney disease. Kidney Int. 107:596–598. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wan J, Hou X, Zhou Z, Geng J, Tian J, Bai
X and Nie J: WT1 ameliorates podocyte injury via repression of
EZH2/β-catenin pathway in diabetic nephropathy. Free Radic Biol
Med. 108:280–299. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang Y, Yang M, Wang X, Zou H, Chen X and
Yuan R: Role of Gpr124 in the migration and proliferation of
retinal microvascular endothelial cells and microangiopathies in
diabetic retinopathy. Mol Biotechnol. 67:2467–2480. 2025.
View Article : Google Scholar
|
|
57
|
Al-Latayfeh M, Silva PS, Sun JK and Aiello
LP: Antiangiogenic therapy for ischemic retinopathies. Cold Spring
Harb Perspect Med. 2:a0064112012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Villacorta L, Schopfer FJ, Zhang J,
Freeman BA and Chen YE: PPARgamma and its ligands: Therapeutic
implications in cardiovascular disease. Clin Sci (Lond).
116:205–218. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ding Y, Kang J, Liu S, Xu Y and Shao B:
The protective effects of peroxisome proliferator-activated
receptor gamma in cerebral Ischemia-Reperfusion injury. Front
Neurol. 11:5885162020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chaudhari N, Talwar P, Parimisetty A,
Lefebvre d'Hellencourt C and Ravanan P: A molecular web:
Endoplasmic reticulum stress, inflammation, and oxidative stress.
Front Cell Neurosci. 8:2132014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Dantzer R: Neuroimmune interactions: From
the brain to the immune system and vice versa. Physiol Rev.
98:477–504. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wong CK, McLean BA, Baggio LL, Koehler JA,
Hammoud R, Rittig N, Yabut JM, Seeley RJ, Brown TJ and Drucker DJ:
Central glucagon-like peptide 1 receptor activation inhibits
Toll-like receptor agonist-induced inflammation. Cell Metab.
36:130–143.e5. 2024. View Article : Google Scholar
|
|
63
|
Ho LT, Fang YW, Hsu PS, Wang JT and Tsai
MH: Association between glucagon-like peptide-1 receptor agonist
therapy and respiratory illness in patients with type 2 diabetes: A
retrospective observational cohort study. Sci Rep. 15:356252025.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Thangavel N, Al Bratty M, Akhtar Javed S,
Ahsan W and Alhazmi HA: Targeting peroxisome Proliferator-activated
receptors using thiazolidinediones: Strategy for design of novel
antidiabetic drugs. Int J Med Chem. 2017:10697182017.PubMed/NCBI
|
|
65
|
Kapadia R, Yi JH and Vemuganti R:
Mechanisms of anti-inflammatory and neuroprotective actions of
PPAR-gamma agonists. Front Biosci. 13:1813–1826. 2008. View Article : Google Scholar
|
|
66
|
Adamu A, Li S, Gao F and Xue G: The role
of neuroinflammation in neurodegenerative diseases: Current
understanding and future therapeutic targets. Front Aging Neurosci.
16:13479872024. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lochhead JJ, Yang J, Ronaldson PT and
Davis TP: Structure, function, and regulation of the Blood-brain
barrier tight junction in central nervous system disorders. Front
Physiol. 11:9142020. View Article : Google Scholar :
|
|
68
|
Hashimoto Y, Greene C, Munnich A and
Campbell M: The CLDN5 gene at the blood-brain barrier in health and
disease. Fluids Barriers CNS. 20:222023. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Liebner S, Dijkhuizen RM, Reiss Y, Plate
KH, Agalliu D and Constantin G: Functional morphology of the
blood-brain barrier in health and disease. Acta Neuropathol.
135:311–336. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhang S, Gao Y, Zhao Y, Huang TY, Zheng Q
and Wang X: Peripheral and central neuroimmune mechanisms in
Alzheimer's disease pathogenesis. Mol Neurodegener. 20:222025.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Chen DY, Sun NH, Lu YP, Hong LJ, Cui TT,
Wang CK, Chen XH, Wang SS, Feng LL, Shi WX, et al: GPR124
facilitates pericyte polarization and migration by regulating the
formation of filopodia during ischemic injury. Theranostics.
9:5937–5955. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Nicolakakis N and Hamel E: The nuclear
receptor PPARgamma as a therapeutic target for cerebrovascular and
brain dysfunction in Alzheimer's disease. Front Aging Neurosci.
2:212021.
|
|
73
|
Shao F, Wang X, Wu H, Wu Q and Zhang J:
Microglia and neuroinflammation: Crucial pathological mechanisms in
traumatic brain Injury-Induced Neurodegeneration. Front Aging
Neurosci. 14:8250862022. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Corona JC and Duchen MR: PPARγ as a
therapeutic target to rescue mitochondrial function in neurological
disease. Free Radic Biol Med. 100:153–163. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Bhatti JS, Bhatti GK and Reddy PH:
Mitochondrial dysfunction and oxidative stress in metabolic
disorders-A step towards mitochondria based therapeutic strategies.
Biochim Biophys Acta Mol Basis Dis. 1863:1066–1077. 2017.
View Article : Google Scholar
|
|
76
|
Greten FR and Grivennikov SI: Inflammation
and cancer: Triggers, mechanisms, and consequences. Immunity.
51:27–41. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
de Visser KE and Joyce JA: The evolving
tumor microenvironment: From cancer initiation to metastatic
outgrowth. Cancer Cell. 41:374–403. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Cullen M, Elzarrad MK, Seaman S, Zudaire
E, Stevens J, Yang MY, Li X, Chaudhary A, Xu L, Hilton MB, et al:
GPR124, an orphan G protein-coupled receptor, is required for
CNS-specific vascularization and establishment of the blood-brain
barrier. Proc Natl Acad Sci USA. 108:5759–5764. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Olsen JJ, Pohl S, Deshmukh A, Visweswaran
M, Ward NC, Arfuso F, Agostino M and Dharmarajan A: The role of wnt
signalling in angiogenesis. Clin Biochem Rev. 38:131–142. 2017.
|
|
80
|
Cherry AE, Vicente JJ, Xu C, Morrison RS,
Ong SE, Wordeman L and Stella N: GPR124 regulates microtubule
assembly, mitotic progression, and glioblastoma cell proliferation.
Glia. 67:1558–1570. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Hosea R, Hillary S, Naqvi S, Wu S and
Kasim V: The two sides of chromosomal instability: Drivers and
brakes in cancer. Signal Transduct Target Ther. 9:752024.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Hernández-Vásquez MN, Adame-García SR,
Hamoud N, Chidiac R, Reyes-Cruz G, Gratton JP, Côté JF and
Vázquez-Prado J: Cell adhesion controlled by adhesion G
protein-coupled receptor GPR124/ADGRA2 is mediated by a protein
complex comprising intersectins and Elmo-Dock. J Biol Chem.
292:12178–12191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zhao W, Zhang Z, Xie M, Ding F, Zheng X,
Sun S and Du J: Exploring tumor-associated macrophages in
glioblastoma: From diversity to therapy. NPJ Precis Oncol.
9:1262025. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Peters JJ, Teng C, Peng K and Li X:
Deciphering the blood-brain barrier paradox in brain metastasis
development and therapy. Cancers (Basel). 17:2982025. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Hernandez-Quiles M, Broekema MF and
Kalkhoven E: PPARgamma in metabolism, immunity, and cancer: Unified
and diverse mechanisms of action. Front Endocrinol (Lausanne).
12:6241122021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Sainis I, Vareli K, Karavasilis V and
Briasoulis E: PPARgamma: The portrait of a target ally to cancer
chemopreventive agents. PPAR Res. 2008:4364892008. View Article : Google Scholar
|
|
87
|
Yang XY, Wang LH and Farrar WL: A role for
PPARgamma in the regulation of cytokines in immune cells and
cancer. PPAR Res. 2008:9617532008. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Li H, Weiser-Evans MC and Nemenoff R:
Anti- and protumorigenic effects of PPARγ in lung cancer
progression: A Double-edged sword. PPAR Res. 2012:3620852012.
View Article : Google Scholar
|
|
89
|
Sun J, Yu L, Qu X and Huang T: The role of
peroxisome proliferator-activated receptors in the tumor
microenvironment, tumor cell metabolism, and anticancer therapy.
Front Pharmacol. 14:11847942023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Liu X, Kang X, Kang H and Yan H: The
immunosuppressive role of MDSCs in HCC: Mechanisms and therapeutic
opportunities. Cell Commun Signal. 23:1552025. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Post Y, Lu C, Fletcher RB, Yeh WC, Nguyen
H, Lee SJ and Li Y: Design principles and therapeutic applications
of novel synthetic WNT signaling agonists. iScience. 27:1099382024.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Cho C, Smallwood PM and Nathans J: Reck
and Gpr124 are essential receptor cofactors for
Wnt7a/Wnt7b-Specific signaling in mammalian CNS angiogenesis and
Blood-brain barrier regulation. Neuron. 95:1056–1073.e5. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Nguyen H, Lee SJ and Li Y: Selective
activation of the Wnt-Signaling pathway as a novel therapy for the
treatment of diabetic retinopathy and other retinal vascular
diseases. Pharmaceutics. 14:24762022. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Liu GH, Chen T, Zhang X, Ma XL and Shi HS:
Small molecule inhibitors targeting the cancers. MedComm (2020).
3:e1812022. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Wei W and Wan Y: Thiazolidinediones on
PPARγ: The roles in bone remodeling. PPAR Res. 2011:8671802011.
View Article : Google Scholar
|
|
96
|
Blackburn GL: From bench to bedside: Novel
mechanisms and therapeutic advances through the development of
selective peroxisome proliferator-activated receptor gamma
modulators. PPAR Res. 91(Suppl): 251S–253S. 2010.
|
|
97
|
Xie X, Chen W, Zhang N, Yuan M, Xu C,
Zheng Z, Li H and Wang L: Selective tissue distribution mediates
Tissue-Dependent PPARγ activation and insulin sensitization by
INT131, a selective PPARγ modulator. Front Pharmacol. 8:3172017.
View Article : Google Scholar
|
|
98
|
Hirschfield GM, Shiffman ML, Gulamhusein
A, Kowdley KV, Vierling JM, Levy C, Kremer AE, Zigmond E, Andreone
P, Gordon SC, et al: Seladelpar efficacy and safety at 3 months in
patients with primary biliary cholangitis: ENHANCE, a phase 3,
randomized, Placebo-controlled study. Hepatology. 78:397–415. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Landreth G, Jiang Q, Mandrekar S and
Heneka M: PPARgamma agonists as therapeutics for the treatment of
Alzheimer's disease. Neurotherapeutics. 5:481–489. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Spite M, Clària J and Serhan CN:
Resolvins, specialized proresolving lipid mediators, and their
potential roles in metabolic diseases. Cell Metab. 19:21–36. 2014.
View Article : Google Scholar
|
|
101
|
Xie X, Yu T, Li X, Zhang N, Foster LJ,
Peng C, Huang W and He G: Recent advances in targeting the
'undruggable' proteins: From drug discovery to clinical trials.
Signal Transduct Target Ther. 8:3352023. View Article : Google Scholar
|
|
102
|
Wang R, Hu B, Pan Z, Mo C, Zhao X, Liu G,
Hou P, Cui Q, Xu Z, Wang W, et al: Antibody-drug conjugates (ADCs):
Current and future biopharmaceuticals. J Hematol Oncol. 18:512025.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Gregor S, Saumur TM, Crosby LD, Powers J
and Patterson KK: Study paradigms and principles investigated in
motor learning research after stroke: A scoping review. Arch
Rehabil Res Clin Transl. 3:1001112021.PubMed/NCBI
|
|
104
|
Soccio RE, Chen ER, Rajapurkar SR,
Safabakhsh P, Marinis JM, Dispirito JR, Emmett MJ, Briggs ER, Fang
B, Everett LJ, et al: Genetic variation determines PPARγ function
and Anti-diabetic drug response in vivo. Cell. 162:33–44. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Priya SS, Sankaran R, Ramalingam S, Sairam
T and Somasundaram LS: Genotype phenotype correlation of genetic
polymorphism of PPAR gamma gene and therapeutic response to
pioglitazone in type 2 diabetes Mellitus-A pilot study. J Clin
Diagn Res. 10:FC11–FC14. 2016.PubMed/NCBI
|
|
106
|
Konig S, Jayarajan V, Wray S, Kamm R and
Moeendarbary E: Mechanobiology of the blood-brain barrier during
development, disease and ageing. Nat Commun. 16:72332025.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Suchý T, Zieschang C, Popkova Y, Kaczmarek
I, Weiner J, Liebing AD, Çakir MV, Landgraf K, Gericke M,
Pospisilik JA, et al: The repertoire of Adhesion G protein-coupled
receptors in adipocytes and their functional relevance. Int J Obes
(Lond). 44:2124–2136. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Vanhollebeke B, Stone OA, Bostaille N, Cho
C, Zhou Y, Maquet E, Gauquier A, Cabochette P, Fukuhara S,
Mochizuki N, et al: Tip cell-specific requirement for an atypical
Gpr124- and Reck-dependent Wnt/β-catenin pathway during brain
angiogenesis. ELife. 4:e064892015. View Article : Google Scholar
|
|
109
|
Amato AA and de Assis Rocha Neves F:
Idealized PPARγ-Based therapies: Lessons from bench and bedside.
PPAR Res. 2012:9786872012. View Article : Google Scholar
|
|
110
|
St Croix B, Rago C, Velculescu V, Traverso
G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C,
Vogelstein B and Kinzler KW: Genes expressed in human tumor
endothelium. Science. 289:1197–1202. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Wang M, Zhang S, Li R and Zhao Q:
Unraveling the specialized metabolic pathways in medicinal plant
genomes: A review. Front Plant Sci. 15:14595332024. View Article : Google Scholar
|