Cell death is a fundamental biological process that
governs cell fate, tissue regeneration and host immune responses
(1). Among the various forms of
cell death, ferroptosis has recently emerged as a unique,
iron-dependent modality of programmed cell death that has gained
significant scientific interest (2). Morphologically, biochemically and
genetically, ferroptosis is distinct from other well-established
cell death modalities, including apoptosis, necrosis and autophagy
(3). Rather than being a
stochastic or uncontrolled process, ferroptosis is precisely
orchestrated by complex signaling pathways and metabolic networks;
its core mechanism involves the dysregulation and interplay of
three pivotal modules: Iron, lipid and amino acid metabolism
(4,5).
Cognitive impairment represents a major global
public health challenge, encompassing a clinical spectrum from mild
cognitive impairment to severe dementia. This condition profoundly
affects the quality of life of patients and imposes a significant
socioeconomic burden (6). This
dysfunction serves as a common pathological endpoint for a
multitude of neurological disorders. The present review focuses on
the following key categories of disorders closely associated with
cognitive impairment: Neurodegenerative diseases including
Alzheimer's disease (AD) and Parkinson's disease (PD), which are
pathologically characterized by the progressive loss of neurons in
specific brain regions and the abnormal aggregation of functional
proteins (7), and
diabetes-associated cognitive impairment (DACI), which is caused
mainly by factors such as insulin resistance and neuroinflammation
(8).
Accumulating evidence indicates that ferroptosis
plays a pivotal role in the pathophysiology of the aforementioned
cognitive disorders. However, despite the rapid expansion of
research into ferroptosis within the field of neuroscience, the
existing body of knowledge remains relatively fragmented and
unsystematized (9). Therefore,
the present review aims to systematically dissect the core
molecular mechanisms of ferroptosis, elucidate its specific
pathogenic roles in cognitive disorders including AD, PD and DACI
as well as provide a comprehensive overview of emerging diagnostic
biomarkers and therapeutic strategies targeting this cell death
pathway. By synthesizing current research advancements, the present
review aims to provide new perspectives on the common pathological
pathways underlying these diseases and to establish a theoretical
foundation to guide the development of effective clinical
interventions.
Although several reviews have discussed the role of
ferroptosis in neurodegenerative diseases, most have focused on a
single disorder, isolated pathways or specific mechanistic aspects
(7,10,11). By contrast, the novelty of the
present review is its cross-disease perspective. The present review
summarizes ferroptosis-related mechanisms across AD, PD and DACI,
emphasizing both the common molecular features and the unique
triggers in each disease. Moreover, the present review integrates
recent progress in ferroptosis-related biomarkers and evaluates the
translational potential of ferroptosis-targeted therapeutic
strategies. By discussing the practical challenges, clinical
feasibility and workflow integration of these biomarkers, the
present review aims to bridge the gap between basic mechanistic
research and clinical application.
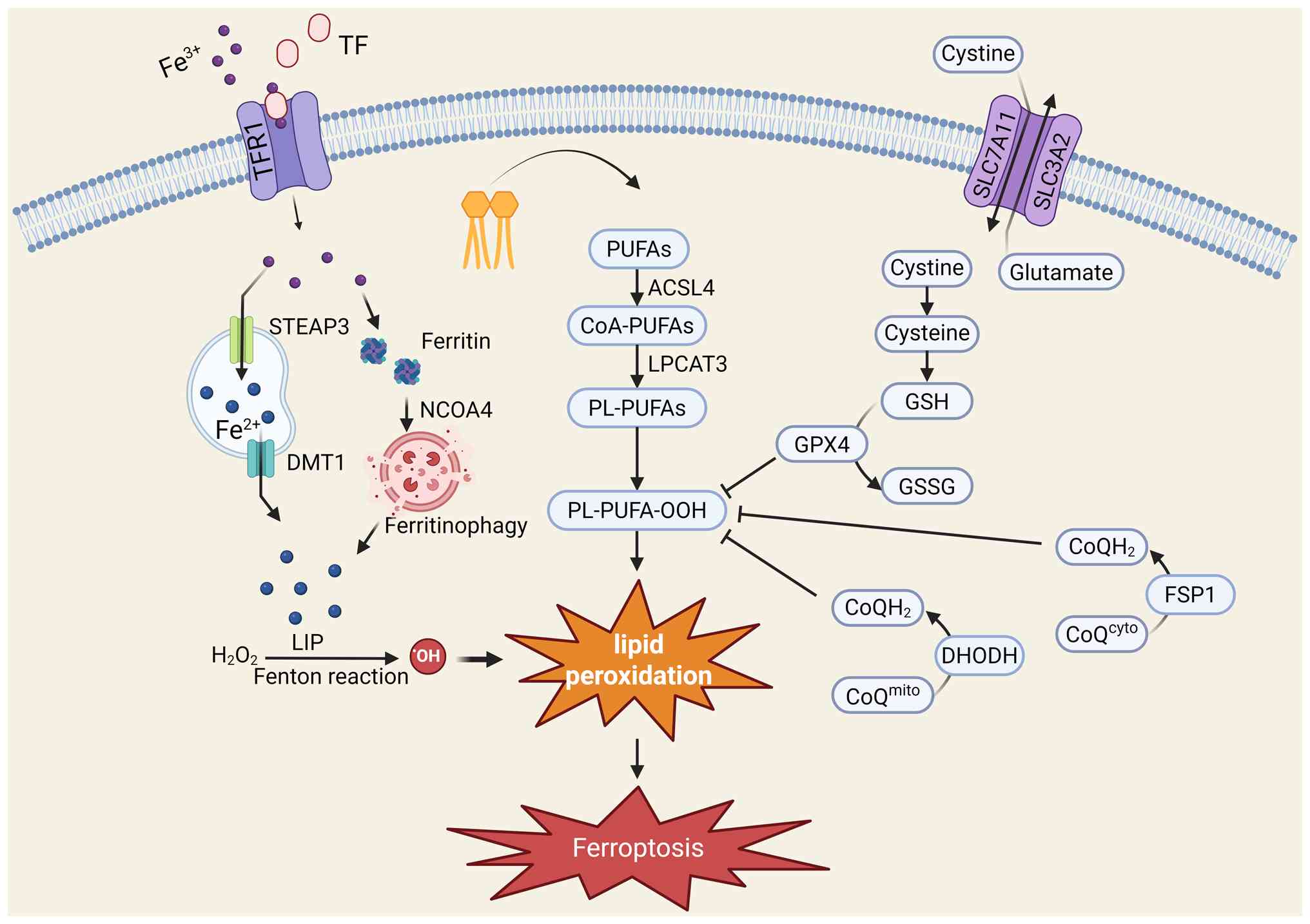
Ferroptosis is a complex biological process
regulated by the interplay of multiple metabolic pathways (Fig. 1); its hallmark feature is the
uncontrolled, iron-dependent accumulation of lipid peroxides on
cellular membranes (12),
ultimately leading to the destruction of membrane integrity and
cell death. Understanding the interplay among lipid metabolism,
iron metabolism and the antioxidant defense system is fundamental
to elucidating the role of ferroptosis in disease.
Lipid peroxidation constitutes the final step of
ferroptosis execution, the occurrence of which depends on specific
lipid substrates and catalytic enzymes. Owing to its high lipid
content, particularly its enrichment in polyunsaturated fatty acids
(PUFAs), the brain is especially vulnerable to ferroptosis
(13).
PUFAs, especially arachidonic acid and adrenic acid,
are the primary substrates for lipid peroxidation during
ferroptosis. The bis-allylic hydrogen atoms within the molecular
chains of these fatty acids are chemically labile and highly
susceptible to abstraction, thereby initiating the chain reaction
of lipid peroxidation (14). The
content and composition of PUFAs within the cell membrane directly
determine a cell's sensitivity to ferroptosis.
PUFAs cannot be directly peroxidized; they must
first be integrated into membrane phospholipids. This process is
accomplished through the synergistic actions of two key enzymes:
ACSL4 and LPCAT3. ACSL4 is responsible for esterifying PUFAs into
their corresponding acyl-CoA derivatives, after which LPCAT3
catalyzes the incorporation of these activated PUFAs into membrane
phospholipids to form phospholipid-containing polyunsaturated fatty
acids (PL-PUFAs) (14).
Consequently, ACSL4 is considered a critical 'gatekeeper' enzyme
for ferroptosis, as its expression level and activity cellular
sensitivity to ferroptosis; its high expression enriches the cell
membrane with oxidizable PUFAs, effectively 'priming' the cell for
ferroptosis (15).
Once incorporated into the membrane, PL-PUFAs are
oxidized to phospholipid hydroperoxides (PL-PUFA-OOH) through
enzymatic or non-enzymatic pathways. The enzymatic pathway is
primarily catalyzed by members of the lipoxygenase family (16). The non-enzymatic pathway is
driven by the Fenton reaction, which is catalyzed by intracellular
labile Fe2+ (17).
The accumulation of these PL-PUFA-OOH molecules disrupts the
integrity and fluidity of the cell membrane, ultimately leading to
membrane rupture, the release of the cellular contents and cell
death (18).
Although iron is an essential trace element for
life, its dyshomeostasis is a key catalyst for ferroptosis. Cells
possess an intricate system to regulate iron uptake, storage,
utilization and efflux; disruptions at any point can lead to
ferroptosis.
Cells store excess iron within ferritin, a spherical
protein shell composed of 24 subunits, to mitigate the toxicity of
free iron in the LIP (21,22). However, upon exposure to specific
signaling cues, cells can degrade ferritin through a selective
autophagic process known as ferritinophagy (23). This process is mediated by a
specific cargo receptor, nuclear receptor coactivator 4, which
recognizes and binds to ferritin, targeting it for lysosomal
degradation (24). This process
releases large amounts of stored iron into the LIP (23). Ferritinophagy is a key mechanism
for rapidly increasing the iron content of the LIP when needed and
is important for increasing cellular sensitivity to ferroptosis
(25).
Cells have evolved a complex, multilayered
antioxidant defense network to counteract the onset of ferroptosis.
This network maintains cellular redox homeostasis by eliminating
lipid peroxides or inhibiting their formation. These defense
mechanisms can be broadly categorized into the canonical
glutathione peroxidase 4 (GPX4)-dependent pathway and
non-canonical, independent pathways.
The canonical axis defending against ferroptosis is
centered on the GPX4/GSH system (26). This pathway begins with the cell
membrane-bound cystine/glutamate antiporter, System Xc−
(27). Composed of the light
chain subunit solute carrier family 7 member 11 (SLC7A11) and the
heavy chain subunit SLC3A2, System Xc− imports
extracellular cystine, which is then rapidly reduced to cysteine
intracellularly (28). Cysteine
serves as the rate-limiting substrate for the synthesis of GSH
(29). As the core effector
molecule of this pathway, GPX4 is a unique selenoprotein and the
only member of the GPX family capable of directly catalyzing the
reduction of membrane phospholipid hydroperoxides (30). GPX4 utilizes GSH as a cofactor to
efficiently reduce toxic lipid hydroperoxides into non-toxic
phospholipid alcohols, thereby halting the lipid peroxidation chain
reaction and effectively suppressing ferroptosis (31). Consequently, the inactivation of
GPX4, either through the inhibition of System Xc− or the
depletion of intracellular GSH, represents a classic experimental
method for inducing ferroptosis.
In addition to the canonical GPX4/GSH axis, cells
possess other parallel, GPX4-independent anti-ferroptotic pathways
that provide additional layers of protection. One key non-canonical
system is the ferroptosis suppressor protein 1 (FSP1)/coenzyme Q10
(CoQ10) pathway (32). Localized
to the plasma membrane, FSP1 utilizes NADPH as an electron donor to
reduce CoQ10 to its antioxidant form, ubiquinol. Ubiquinol acts as
a lipophilic radical-trapping antioxidant that can effectively
scavenge lipid peroxyl radicals, thus providing a critical
alternative line of defense when GPX4 function is compromised.
Another important independent anti-ferroptotic axis is the GTP
cyclohydrolase-1/tetrahydrobiopterin (BH4) pathway (33). BH4 is not only a crucial enzyme
cofactor but also a potent radical scavenger. Studies have shown
that it exerts powerful anti-ferroptotic effects through two
mechanisms: Protecting CoQ10 from depletion and directly inhibiting
lipid peroxidation (34,35). In parallel to the plasma
membrane-localized FSP1 system, a distinct, mitochondria-specific
antioxidant axis that counteracts ferroptosis centered on the
enzyme dihydroorotate dehydrogenase (DHODH) has been identified
(36). DHODH is a flavoprotein
of the electron transport chain that is anchored to the inner
mitochondrial membrane, where it performs its canonical function in
de novo pyrimidine synthesis (37). Crucially, this enzymatic process
reduces CoQ10 to its active antioxidant form, ubiquinol (36). This dedicated mitochondrial pool
of ubiquinol acts as a potent, radical-trapping antioxidant,
directly neutralizing lipid peroxyl radicals within the inner
mitochondrial membrane and thereby shielding the organelle from
lipid peroxidation and subsequent ferroptotic collapse (38). Given that mitochondria are both a
primary source of endogenous ROS and a central hub for iron
metabolism, their membranes are uniquely vulnerable to ferroptotic
insults. This defense system thus provides a critical layer of
localized protection specifically within this susceptible
organelle, offering new insights for understanding cellular
resilience and identifying potential therapeutic targets in
neurodegenerative disorders where mitochondrial dysfunction is a
core pathological feature.
Nuclear factor erythroid 2-related factor 2 (NRF2)
acts as the 'master regulator' of the cellular response to
oxidative stress. Under oxidative or electrophilic stress, NRF2 is
activated and translocates to the nucleus, where it binds to
antioxidant response elements to initiate the transcription of a
number of downstream genes. The proteins encoded by these genes
have diverse functions, including promoting GSH synthesis,
regenerating NADPH and upregulating iron storage proteins
(ferritin), thereby constructing a robust, multilayered defense
against ferroptosis (32).
The classic tumor suppressor p53 plays a complex,
dual role in the regulation of ferroptosis. On the one hand, p53
can promote ferroptosis by inhibiting the expression of SLC7A11
(39). On the other hand, it can
also suppress ferroptosis by regulating other metabolic pathways
(such as activating CDKN1A/p21) (40). The ultimate effect of p53 is
highly dependent on the cell type and the specific stress context
(41).
The regulatory mechanisms of ferroptosis are not
isolated but are intimately linked to the core metabolic activities
of cells. The initiation of ferroptosis is deeply integrated with
amino acid metabolism (glutamate/cystine balance), lipid metabolism
(PUFA availability) and metal homeostasis (iron regulation). For
instance, the activity of System Xc− is directly linked
to the levels of the neurotransmitter glutamate and the synthesis
of the antioxidant GSH (42).
The execution phase depends on the biological properties of the
cell membrane and the activity of lipid-modifying enzymes (ACSL4
and LPCAT3) (20). Moreover,
iron is a critical cofactor for core bioenergetic processes such as
mitochondrial respiration and oxygen transport and the
Fe2+ required for ferroptosis (32). This intrinsic connectivity
implies that ferroptosis is not merely a simple 'death switch' but
rather a composite reflection of the overall metabolic state of the
cell. Any pathological process that can disrupt these
interconnected metabolic hubs, such as mitochondrial dysfunction in
PD or oxidative stress in AD, may inadvertently lower the cellular
threshold for resistance to ferroptosis. This explains why
ferroptosis has emerged as a common pathological pathway in
multiple, seemingly unrelated diseases; it represents a shared
downstream execution point where diverse upstream damage signals
converge.
The fundamental molecular mechanisms of ferroptosis
are specifically activated and regulated in different disease
contexts and are emerging as key factors driving the pathological
progression of cognitive impairment (Table I). This section describes the
specific mechanisms of ferroptosis in AD, PD and DACI.
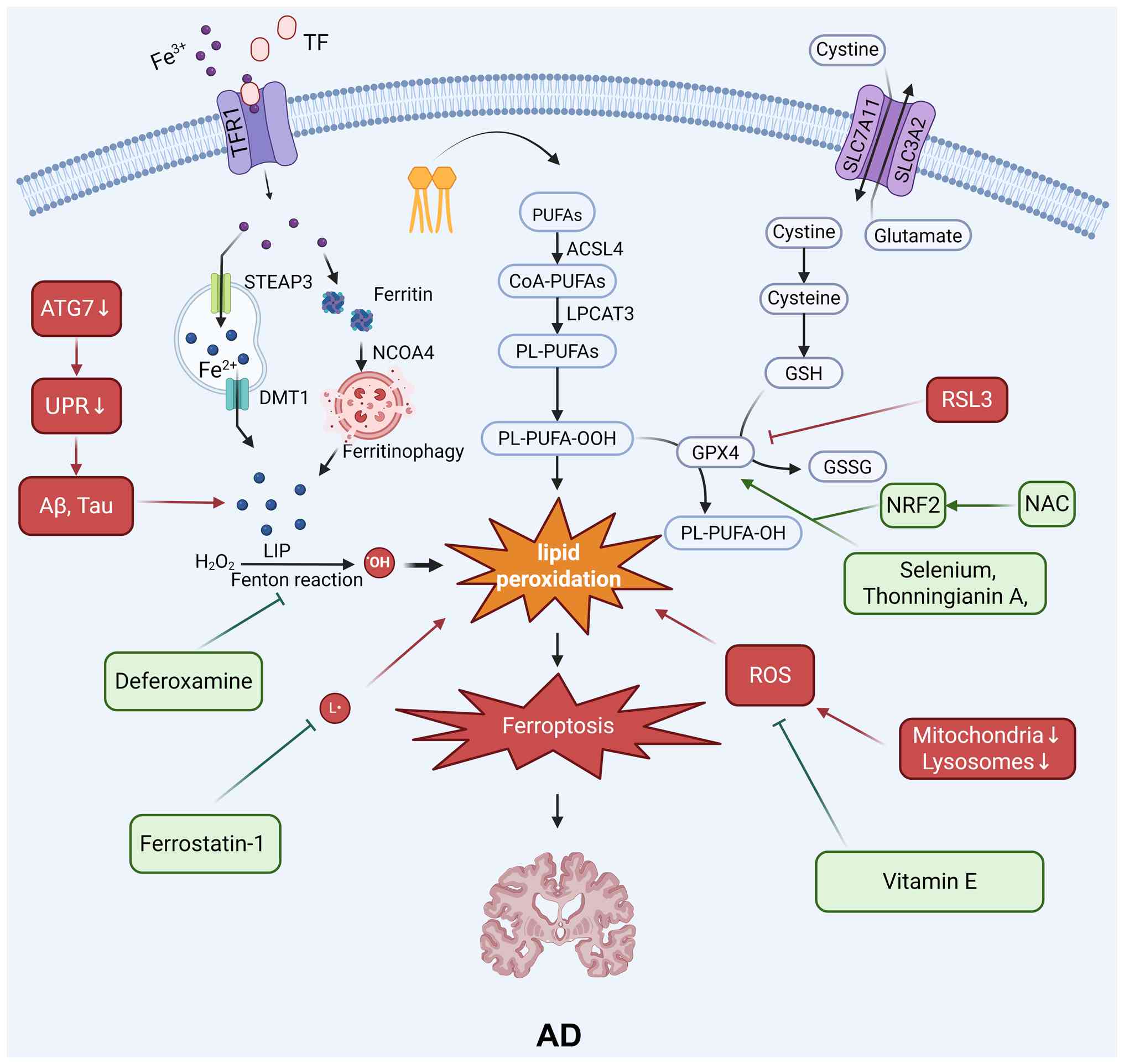
The regulation of ferroptosis can impact AD through
multiple mechanisms, including the regulation of GSH levels, the
Fenton reaction, amyloid-β (Aβ), lipid metabolism, iron metabolism
and the NRF2 signaling pathway (Fig.
2).
An abnormal increase in iron levels in the AD brain
is a well-documented phenomenon, especially in the regions most
severely affected by neurodegeneration (6). Iron dyshomeostasis is not only an
epiphenomenon but is also strongly implicated in the core
pathological processes of AD. On the one hand, excess iron ions can
directly catalyze ROS production, exacerbating oxidative stress,
while also promoting the generation and aggregation of Aβ and
catalyzing the hyperphosphorylation of the tau protein. On the
other hand, Aβ and tau aggregates can disrupt iron homeostasis, for
instance, by interfering with the function of iron-efflux proteins,
thereby further intensifying iron accumulation. This positive
feedback loop, or 'vicious cycle', between iron and Aβ/tau
significantly lowers the threshold for neuronal resistance to
ferroptosis, rendering them exceptionally vulnerable (43).
The AD brain is in a state of chronic, sustained
oxidative stress. Aβ oligomers and fibrils are themselves sources
of ROS and can directly induce lipid peroxidation. Critically, a
study has shown that the levels of GSH, a key antioxidant, are
significantly reduced in the AD brain and that the extent of this
depletion correlates positively with the severity of cognitive
impairment (43). The depletion
of GSH leads directly to the failure of the GPX4 antioxidant
system, resulting in cells being unable to effectively neutralize
lipid peroxides and thereby allowing the onset of ferroptosis.
In recent years, a new paradigm in AD pathology has
emerged: The ferroptosis of microglia, the resident immune cells of
the brain. In the pathological environment of AD, microglia are
activated to clear Aβ plaques, damaged myelin, and cellular debris.
However, this debris (particularly myelin) is rich in iron. During
the phagocytosis and degradation of this iron-rich material, the
microglia themselves can undergo ferroptosis due to iron overload
(44). This process not only
results in the loss of protective immune cells but also the release
of proinflammatory cytokines and damage-associated molecular
patterns during their death, further exacerbating the
neuroinflammatory response (45). This process creates a
self-amplifying cycle of damage that promotes neurodegeneration
(46).
Genetic studies have provided direct evidence for
the involvement of ferroptosis in AD (47,48). Transcriptomic analyses of brain
tissue samples from patients with AD have revealed significant
alterations in the expression of a range of ferroptosis-related
genes (FRGs). For example, the genes encoding transferrin receptor
(TFRC), glucose transporters and the master antioxidant regulator
NRF2 are closely associated with the pathological progression of AD
(49).
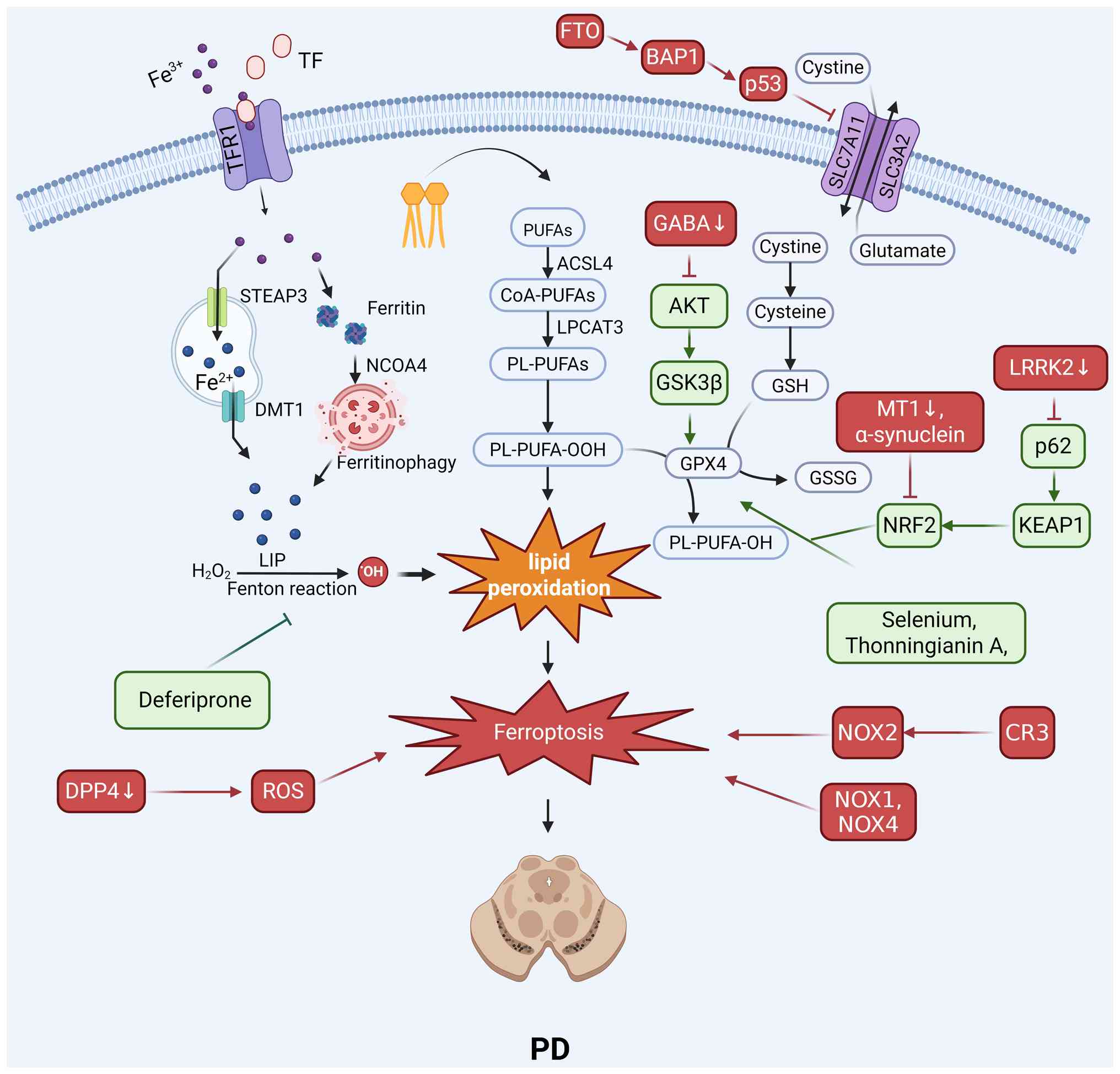
Ferroptosis is regulated by multiple interconnected
pathways, including the GPX4/GSH system, iron and lipid metabolism,
the NRF2 signaling pathway and mitochondrial function, in PD
(Fig. 3).
The integrity of the GPX4/GSH antioxidant axis is
critical for dopaminergic neuron survival. The oxidation product of
dopamine can induce ferroptosis by covalently modifying GPX4 and
mediating its ubiquitination and degradation (50). Conversely, experimental GPX4
overexpression exerts neuroprotective effects as it reduces
neuronal death (50). This
pathway is also targeted by other PD-related factors; the
leucine-rich repeat kinase 2 (LRRK2) protein induces ferroptosis by
acting on the System Xc−/GSH/GPX4 pathway (51) and the m6A demethylase fat mass
and obesity-associated protein can promote ferroptosis via the
p53/SLC7A11 axis (39).
Given the central role of iron, therapeutic
strategies have focused on its chelation. The drug deferiprone has
been shown to inhibit ferroptosis by chelating excess iron within
the substantia nigra in vivo (52). This result is compounded by the
fact that α-synuclein aggregation, a hallmark of PD, can also
induce ferroptosis through mechanisms such as membrane disruption
and altered calcium influx (53).
The regulation of lipid metabolism, particularly
through the enzyme ACSL4, is a key control point. The PD-relevant
neurotoxin salsolinol induces ferroptosis by upregulating ACSL4
expression (54). By contrast,
the natural compound acteoside exerts a protective effect by
downregulating ACSL4 (54).
Downstream lipid peroxidation can be directly inhibited by
radical-trapping antioxidants such as ferrostatin-1 (55).
The NRF2 antioxidant response pathway is a major
defensive system whose impairment contributes to PD. Pathological
α-synuclein upregulation induces ferroptosis by reducing NRF2
protein levels and impairing antioxidant defenses (56). The therapeutic activation of this
pathway with compounds such as dimethyl fumarate,
tert-butylhydroquinone and sulforaphane can inhibit ferroptosis by
upregulating the expression of downstream antioxidant genes
(57). Additionally, the
activation of melatonin MT1 receptors can suppress ferroptosis
through the sirtuin 1/NRF2/heme oxygenase 1 (HO-1)/GPX4 signaling
cascade (58).
Mitochondrial dysfunction acts as a core driver of
ferroptosis through a combination of iron homeostasis imbalance and
oxidative stress (59). This
oxidative environment is further exacerbated by neuroinflammatory
processes. Microglial complement receptor 3 can induce ferroptosis
via NADPH oxidase 2 (NOX2) (60), and other NADPH oxidases,
including NOX1 (61) and NOX4
(62), are also key contributors
to the ferroptotic process.
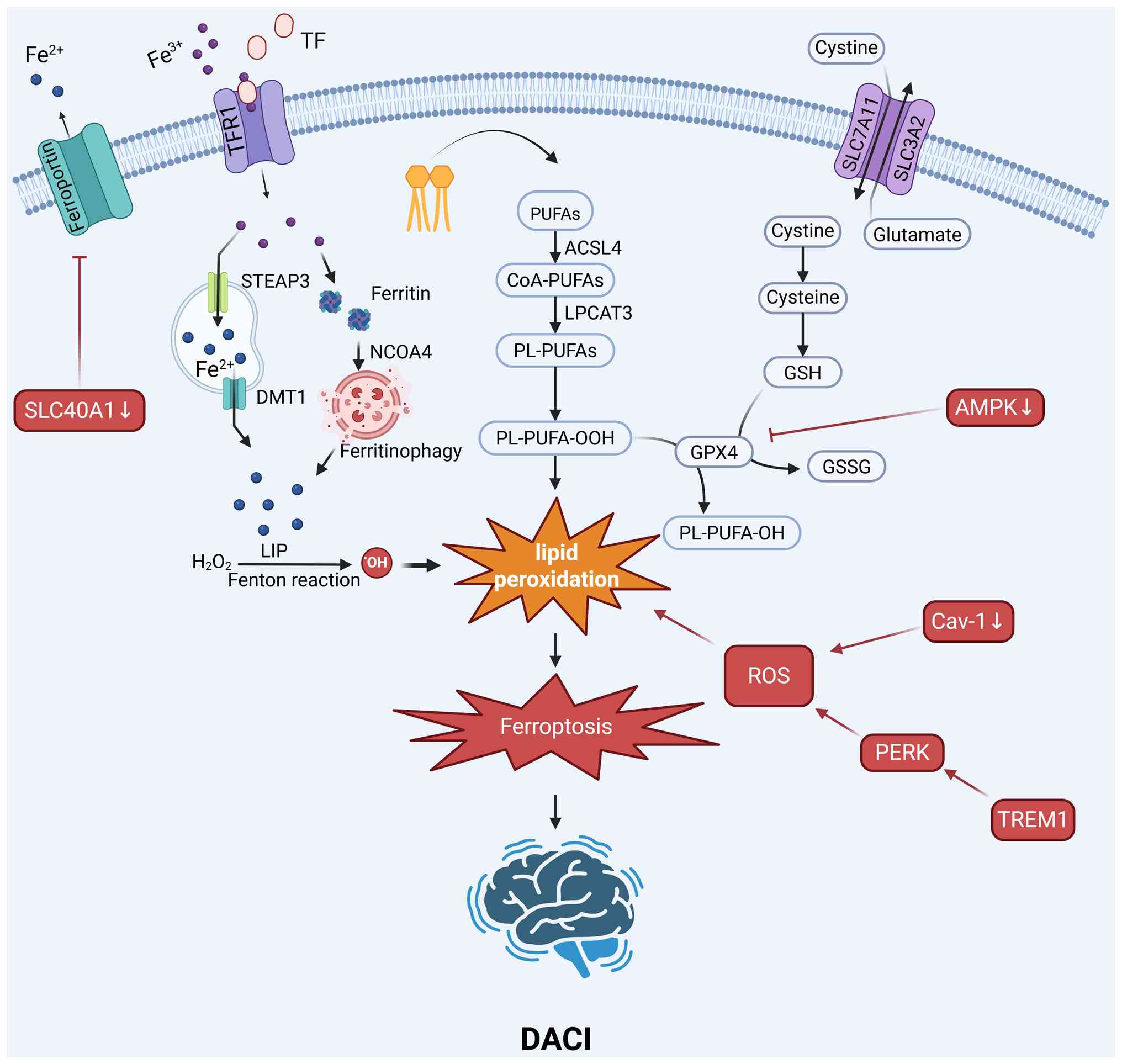
The regulation of ferroptosis can affect DACI
through multiple mechanisms, including the modulation of the
GPX4/GSH system, iron and ROS metabolism, mitochondrial homeostasis
and the NRF2 and PKR-like endoplasmic reticulum kinase (PERK)
signaling pathways (Fig. 4).
The metabolic state in DACI significantly affects
the activity of the GPX4 antioxidant axis and the sensitivity of
cells to ferroptosis. The AMP-activated protein kinase (AMPK)
pathway, a key sensor of cellular energy, plays a protective role.
AMPK agonists inhibit ferroptosis by activating AMPK, which
subsequently upregulates GPX4 expression and downregulates
lipocalin-2 expression in vivo (63). Additionally, berberine has been
shown to protect against high glucose-induced damage to glomerular
podocytes by activating the NRF2/HO-1/GPX4 pathway, thereby
inhibiting ferroptosis (64).
The diabetic state creates a pro-ferroptotic
environment characterized by iron dyshomeostasis (65). Extensive iron deposition is
observed in the hippocampus of diabetic models and elevated plasma
Fe2+ levels correlate with cognitive decline in patients
(66,67). The increased iron levels can
catalyze the Fenton reaction, leading to lipid peroxidation.
Therapeutic interventions aim to mitigate this iron-induced stress.
Erythropoietin has been shown to inhibit ferroptosis by reducing
iron overload and lipid peroxidation both in vitro and in
vivo (68). Similarly, the
antioxidant liproxstatin-1 can effectively inhibit ferroptosis by
attenuating iron accumulation and oxidative stress in vivo
(63).
Triggering receptor expressed on myeloid cells 1
(TREM1) is a key driver of microglial-mediated ferroptosis in
individuals with DACI (69).
Under high-glucose conditions, the expression of TREM1 is
upregulated, activating the PERK pathway (69). This process leads to the
inactivation of antioxidant systems and increased lipid
peroxidation and ROS levels, ultimately inducing ferroptosis in
microglia and exacerbating neuroinflammation.
The transcription factor NRF2 is a master regulator
of the antioxidant response and a key target for inhibiting
ferroptosis in individuals with DACI. Several compounds have been
shown to confer protection by modulating this pathway. Quercetin
inhibits ferroptosis by activating the NRF2/HO-1 pathway in models
of diabetic nephropathy, a mechanism that is potentially relevant
to DACI (72). Berberine also
leverages this pathway, activating the NRF2/HO-1/GPX4 axis to
suppress ferroptosis (64).
Furthermore, vitamin D can inhibit ferroptosis by activating the
vitamin D receptor, which in turn upregulates the NRF2/HO-1
signaling cascade, as shown in in vitro and in vivo
models (73).
Although the initial etiologies of AD, PD and DACI
are distinct, they all ultimately converge on the common cell death
pathway of ferroptosis. These diseases share several core
pathological features: Oxidative stress, neuroinflammation and
mitochondrial dysfunction (74).
Oxidative stress directly generates ROS to initiate lipid
peroxidation (32);
neuroinflammation, particularly the activation of microglia, alters
iron metabolism and promotes ROS release (44); and mitochondrial dysfunction is a
major source of both ROS and the LIP (75). The specific upstream triggers of
each disease (such as Aβ, ischemia and hemorrhage) activate these
common downstream pathological processes, which in turn are the
direct upstream activators of the core ferroptosis machinery (iron
overload, lipid peroxidation and antioxidant system depletion).
Therefore, ferroptosis is not merely another form of cell death, it
acts as a 'central executioner', a common endpoint where diverse
neural injury signals converge. This recognition highlights the
potential of ferroptosis as a therapeutic target. Theoretically, an
effective antiferroptotic agent could possess broad value as a
treatment for multiple mechanistically distinct cognitive
disorders.
Given the central role of ferroptosis in the
pathological processes of various cognitive disorders, its
associated molecules and metabolites hold immense potential as
novel diagnostic and prognostic biomarkers. At present, diagnostic
tools for neurodegenerative diseases primarily rely on the
assessment of late-stage clinical symptoms or costly neuroimaging
examinations and lack pathology-based biomarkers that would enable
intervention in the early stages of the disease (76). The development of biomarkers that
can reflect the state of ferroptosis in the brain is crucial for
enabling early diagnosis, assessing disease progression and
monitoring therapeutic responses (Table II).
Cerebrospinal fluid (CSF) and blood represent two
vital biofluids for accessing pathological information regarding
the central nervous system. Detecting changes in
ferroptosis-related molecules within these biofluids holds promise
for the development of minimally invasive diagnostic methods.
Measuring the levels of iron homeostasis-related
proteins in CSF or serum is a direct strategy. For example,
multiple studies have shown that the levels of ferritin in the CSF
of patients with AD are elevated and this increase is associated
with faster rates of cognitive decline and brain atrophy,
suggesting that CSF ferritin levels could be a valuable prognostic
biomarker (77,78). Urbano et al (79) demonstrated that CSF ferritin
levels are associated with the conversion from mild cognitive
impairment to dementia. Furthermore, CSF iron levels correlate
positively with tau protein levels (80). In the BioFINDER cohort study, CSF
ferritin levels reaching a threshold value of 12.47 ng/ml predicted
cognitive deterioration in patients with mild cognitive impairment
(81). Moreover, Apolipoprotein
E4 carrier status strengthens the diagnostic association between
brain iron content and AD pathology (81). A study also found that patients
with PD with substantia nigra hyperechogenicity exhibit
significantly lower cognitive function than those without
hyperechogenicity; among patients with PD and poorer cognitive
function, CSF iron levels are significantly elevated while
transferrin levels are significantly reduced (82). Additionally, the levels of
transferrin and non-transferrin-bound iron may also reflect the
status of iron load in the brain (77).
Lipid peroxidation is the key step in the execution
of ferroptosis and its metabolites are direct indicators of
ferroptotic activity. Malondialdehyde and 4-hydroxynonenal are two
major end-products of lipid peroxidation and their elevated levels
in CSF or blood have been shown to be correlated with the
pathological severity of AD and PD (33,83).
The state of the antioxidant system reflects the
capacity of the cell to resist ferroptosis. GSH is central to the
GPX4 system and its reduced levels in CSF are closely associated
with severe cognitive impairment in patients with AD, suggesting
that GSH could serve as a marker for the failure of the brain's
antioxidant capacity and the risk of ferroptosis (43).
Using high-throughput sequencing technologies,
researchers can identify ferroptosis-related molecular markers at
the genetic and transcriptomic levels.
Through bioinformatics methods, the expression
profile of a set of FRGs can be analyzed in patient tissues or
peripheral blood cells to construct prognostic models. For example,
in AD research, an FRG signature composed of genes such as DNA
damage inducible transcript 4, mucin 1 and CD44 was found to not
only distinguish patients with AD from healthy controls but also to
predict disease severity and immune cell infiltration in the brain
(43). Such multigene signatures
offer greater robustness and predictive power than single-gene
markers.
Certain genes that play key roles in the ferroptosis
pathway may also serve as independent prognostic indicators based
on their expression levels. Measuring the expression levels of
genes such as TFRC or SLC7A11 in brain tissue or blood from
patients with neurodegenerative diseases could provide clues for
assessing ferroptotic activity and disease progression (49).
Neuroimaging techniques, particularly magnetic
resonance imaging (MRI), represent a non-invasive, in vivo
method for assessing iron deposition in the brain and can serve as
an indirect means of evaluating ferroptosis risk. Advanced MRI
sequences, such as T2* relaxometry, are highly sensitive to iron
deposition. Compared with elderly individuals negative for AD
biomarkers, cognitively normal elderly individuals positive for AD
biomarkers show significantly higher R2* levels in the caudate
nucleus, putamen and globus pallidus, suggesting increased iron
content (84). In PD research,
this technique can be used to precisely quantify the iron content
in the substantia nigra region and has been found to be closely
correlated with disease severity and motor deficits (85). This method is useful not only for
diagnosis but also for tracking disease progression and evaluating
the efficacy of iron-targeted therapies.
Although numerous potential biomarkers have been
identified, the vast majority are still in the preclinical or early
clinical research stages (11).
Transforming these research findings into stable, reliable and
easily accessible clinical diagnostic tools remains a significant
challenge.
Given the insidious and progressive nature of
neurodegenerative diseases, the utility of a single biomarker
measurement is questionable. Early in the disease course,
ferroptotic processes may be subtle and localized, potentially
yielding false-negative results. Longitudinal monitoring,
therefore, represents a more clinically meaningful approach.
Tracking the trajectory of changes in a panel of
ferroptosis-related markers within accessible biofluids offers
greater prognostic value than a single snapshot measurement. This
dynamic monitoring is essential for identifying high-risk
individuals, tracking disease progression and evaluating
therapeutic responses.
Biomarker sources profoundly impact clinical
feasibility. While analysis of postmortem brain tissue is the gold
standard for research, it is not relevant for clinical diagnosis. A
study on human postmortem brain tissue has revealed that the
pathological progression of AD is accompanied by decreased
expression of nuclear receptor coactivator 4 and GPX4 in gray
matter (86). Meanwhile,
research using human brain organoid models has demonstrated that
ferroptosis inhibitors (Ferrostatin-1) can block Aβ pathology,
reduce lipid peroxidation and restore iron storage function in AD
organoids (86).
By contrast, CSF provides a more direct window into
central nervous system pathology, yet the invasiveness of lumbar
puncture limits its utility for routine screening or frequent
monitoring. Consequently, developing biomarkers in easily
accessible peripheral biofluids, such as blood, is a key priority.
However, this approach faces several challenges, including the
potential for the blood-brain barrier (BBB) to mask or dilute
CNS-specific signals and the difficulty in distinguishing
brain-derived markers from those originating systemically (87,88). In particular, the development of
peripheral blood biomarkers that can accurately reflect the state
of local ferroptosis within the brain is key and represents a major
hurdle for future research. Future research must therefore focus on
identifying markers capable of specifically crossing the BBB and on
developing ultrasensitive technologies for reliable peripheral
detection.
A major challenge in translating ferroptosis
research from the laboratory to clinical practice is how to
integrate ferroptosis biomarkers into current diagnostic pathways
for cognitive impairment. A stepwise clinical workflow may provide
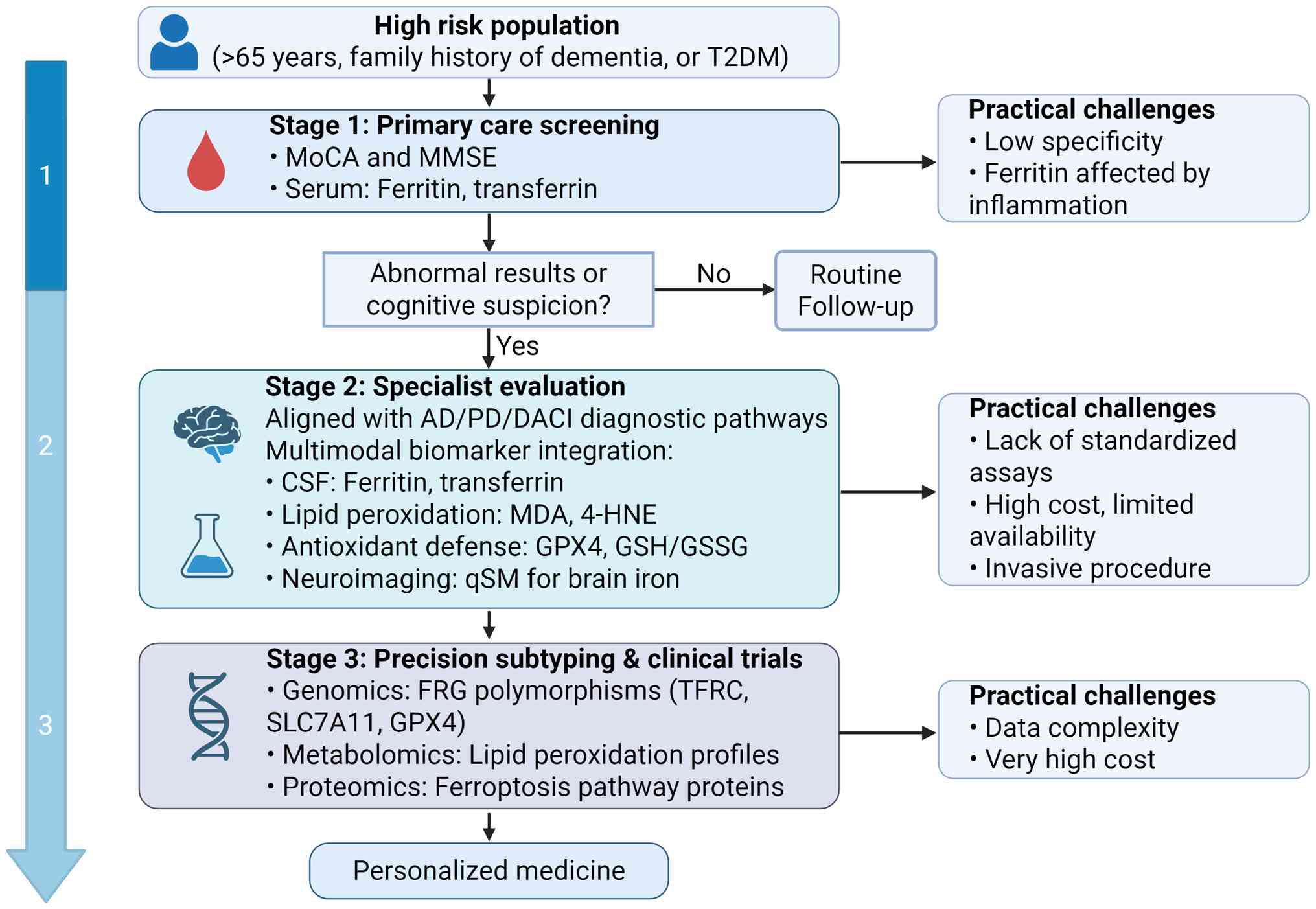
a practical framework for this translation (Fig. 5).
At the first stage, ferroptosis-related tests could
be added to primary care as part of low-cost, population-wide
screening. For individuals at increased risk, such as those >65
years of age, with a family history of dementia or with comorbid
type 2 diabetes mellitus (T2DM) (89-91), routine blood tests may be
expanded to include serum ferritin and transferrin levels. These
tests could be combined with simple cognitive assessments, such as
the Montreal Cognitive Assessment or the Mini-Mental State
Examination (92,93). Individuals who show both mild
cognitive changes and abnormal iron metabolism could be flagged as
high-risk and referred for specialist evaluation. This approach is
affordable and easy to implement, but its specificity is low as
ferritin and related markers are affected by inflammation and liver
disease (94).
At the specialist evaluation stage, diagnosis could
be refined in memory clinics or neurology departments using more
specific ferroptosis biomarkers. Key measurements include lipid
peroxidation markers such as malondialdehyde and 4-hydroxynonenal,
antioxidant defense markers such as GPX4 activity and the
GSH/oxidized GSH ratio and advanced MRI-based assessments such as
quantitative susceptibility mapping to quantify brain iron
deposition (95,96). These ferroptosis markers should
be interpreted together with established diagnostic tools,
including CSF Aβ42, phosphorylated tau, total tau and Aβ PET
imaging (97). Signs of
increased ferroptosis activity, when combined with these
biomarkers, may improve diagnostic accuracy and help with
differential diagnosis. The main barriers at this stage are the
lack of standardized laboratory assays and the high cost and
limited availability of advanced imaging.
The advanced research stage focuses on precision
subtyping and clinical trial applications. Multi-omics approaches,
such as metabolomics to identify lipid peroxidation profiles and
genomics to characterize polymorphisms in ferroptosis-regulating
genes, may allow more accurate pathological classification.
Although these approaches are currently limited by high cost and
complex data processing, they show strong potential for identifying
patient groups that may benefit from ferroptosis-targeted
treatments.
Advanced age is the most significant non-modifiable
risk factor for major cognitive disorders, including AD, PD and
DACI (98-100). A compelling body of evidence
suggests that the aging process itself fosters a 'pro-ferroptotic'
state in the brain, thereby lowering the threshold for
neurodegeneration (101,102).
This connection is rooted in several age-related physiological
changes that directly impinge on the core regulatory axes of
ferroptosis.
Physiological aging systematically increases
neuronal susceptibility to ferroptosis through the synergistic
action of four interconnected mechanisms. First, normal aging is
associated with the progressive dysregulation of iron homeostasis,
often leading to iron accumulation in vulnerable brain regions such
as the hippocampus and substantia nigra (52). This expands the labile,
catalytically active iron pool, providing ample 'fuel' to initiate
destructive lipid peroxidation via the Fenton reaction. Second, the
efficiency of endogenous antioxidant systems decreases with age, a
core feature of which is the reduction in the levels of the master
intracellular antioxidant, GSH (103,104). As GPX4 is entirely dependent on
GSH to neutralize toxic lipid peroxides, the depletion of GSH
directly weakens the cell's most critical line of defense against
ferroptosis (105). Third,
age-related mitochondrial dysfunction is another key contributor.
Senescent mitochondria not only produce more ROS due to inefficient
electron transport but also exhibit impaired iron-sulfur cluster
biogenesis, leading to improper iron handling and accumulation
within the mitochondria, thus creating a potent internal source of
oxidative stress (106,107). Finally, although the total
brain lipid content may decrease with age, the enzymes responsible
for incorporating PUFAs into membrane phospholipids remain active,
ensuring the continued presence of the necessary substrates for the
final execution step of ferroptosis (75).
In summary, age-dependent changes such as increased
catalytic iron levels, weakened antioxidant defenses, elevated
mitochondrial-derived oxidative stress and sustained membrane
vulnerability to peroxidation, collectively place neurons in the
aging brain in a fragile state. This underlying vulnerability
explains why the pathological triggers of various neurodegenerative
diseases can converge on ferroptosis in the aging brain. Therefore,
ferroptosis emerges as a core mechanism that intimately links age,
the most universal risk factor, to cognitive decline, the shared
pathological outcome.
Given the core pathogenic role of ferroptosis in
various cognitive disorders, targeting this pathway has emerged as
a highly attractive and novel therapeutic strategy. To this end,
researchers have developed or screened a range of drugs and
compounds capable of inhibiting ferroptosis through various
approaches and have validated their neuroprotective effects using
various disease models (Table
III).
Cognitive impairment in AD is closely associated
with disrupted iron homeostasis, excessive oxidative stress and the
accumulation of Aβ and tau proteins, which collectively promote
ferroptosis. Several natural compounds have been found to be
effective at modulating ferroptosis-related pathways to counteract
these processes.
A common strategy involves activating the NRF2
signaling pathway, a master regulator of antioxidant responses. For
example, ganoderic acid A activates the NRF2/SLC7A11/GPX4 axis
(108), whereas saponins
derived from Astragalus membranaceus act on the NOX4/NRF2
pathway to restore iron balance (109). Lignans from Schisandra
chinensis activate the NRF2/FPN1 pathway, reducing
phosphorylated tau levels and improving cognitive function
(110). Similarly, avicularin
enhances cognition by modulating NOX4/NRF2 signaling (111) and artemisinin derivatives
increase NRF2 activity by targeting its inhibitor kelch-like
ECH-associated protein 1 (Keap1) (112).
Some compounds work by directly supporting the
GPX4/GSH antioxidant defense system. Thonningianin A, a newly
identified ferroptosis inhibitor, activates GPX4 through the
AMPK/NRF2 pathway (113). Total
lignans from Schisandra increase the activity of the
NADK/NADPH/GSH system to reduce Aβ deposition (114) and schisandrin B downregulates
glycogen synthase kinase 3β (GSK3β) expression to promote NRF2/GPX4
signaling (115). Clinical
studies suggest that selenium may help slow cognitive decline
(116,117). Reduced selenium levels in the
brains of patients with AD are associated with disease progression.
In addition, selenium-loaded nanospheres can deliver selenium, an
essential cofactor for GPX4, and markedly improve cognition in AD
models (118).
Other strategies target molecules other than NRF2
and GPX4. Berberine reduces oxidative damage by inhibiting the
JNK/p38 MAPK pathway (119).
Neuritin protects against cognitive impairment by activating
PI3K/Akt (120), whereas
ghrelin mitigates neuroinflammation by acting on the bone
morphogenetic protein 6/SMAD1 pathway (121). In addition, tetrahedral
framework nucleic acids have been developed to directly neutralize
Aβ toxicity and restore synaptic integrity (122).
The degeneration of dopaminergic neurons in PD is
fueled by iron overload, mitochondrial dysfunction and oxidative
stress, all of which are tightly linked to ferroptosis. Therapeutic
strategies have therefore focused on disrupting these pathological
cycles.
Iron chelation remains a straightforward
intervention. Deferoxamine (DFO) has been shown to preserve
dopaminergic neurons in animal models (123). However, the clinical outcomes
associated with deferiprone, an oral iron chelator, have been
inconsistent. For instance, one trial reported worsening motor
symptoms in patients with early-stage PD, highlighting the
complexity of iron-targeted therapy (52,60,124).
Several natural compounds activate endogenous
antioxidant pathways to increase the resilience of the brain to
oxidative stress. Acteoside inhibits ACSL4 (54) while simultaneously activating
NRF2 (125). Granulathiazole A
reduces α-synuclein accumulation through NRF2/HO-1 activation
(126) and betulinic acid
improves behavioral outcomes by reducing ROS levels (127). Dingzhen pills, a traditional
herbal formulation, inhibit the cyclic GMP-AMP synthase/stimulator
of interferon genes pathway to protect dopaminergic neurons
(128).
Efforts have also been directed at modulating key
proteins in the ferroptosis cascade. γ-aminobutyric acid from
Lactobacillus reuteri alleviates pathology by activating the
Akt/GSK3β/GPX4 axis (129),
emodin upregulates the mitochondrial protein ubiquitin-cytochrome C
reductase core protein 1 to suppress ferroptosis (130) and corilagin protects neurons by
regulating the Toll-like receptor 4/Src/NOX2 pathway (131). Additionally, the inhibition of
LRRK2 has been linked to reduced ferroptotic damage via the
p62/Keap1/NRF2 axis (132) and
teneligliptin, a dipeptidyl peptidase-4 inhibitor, decreases ROS
levels and prevents ferroptosis (133).
Therapeutic interventions for DACI often target the
metabolic dysregulation that creates a pro-ferroptotic environment.
Several repurposed diabetes drugs and natural compounds have been
shown to exert neuroprotective effects by inhibiting ferroptosis.
The GLP-1 receptor agonist liraglutide improves cognition by
repairing mitochondria and modulating the GPX4/SLC7A11 pathway
(134).
Activation of the NRF2 antioxidant pathway is a
common strategy. The natural product sinomenine improves cognition
by regulating the EGF/NRF2/HO-1 pathway (135). Artemisinin has also been shown
to ameliorate cognitive impairment in T2DM mice by activating NRF2
(136). Other compounds work by
inhibiting inflammatory signaling; dihydromyricetin alleviates
hippocampal ferroptosis by inhibiting the JNK pathway (137).
Other approaches aim to correct the iron and ROS
imbalance directly. Erythropoietin has been shown to improve
cognition by reducing iron overload and lipid peroxidation in
diabetic models (68).
Resveratrol inhibits the ability of microRNA-9-3p to upregulate
SLC7A11 expression, thereby improving cognition in the context of
DACI (138).
Ferroptosis, a distinct form of iron-dependent cell
death driven by lipid peroxidation, has emerged as a key
pathological mechanism underlying multiple cognitive disorders,
including AD, PD and DACI. Preclinical studies have consistently
shown that targeting ferroptosis with drugs such as iron chelators,
lipid peroxidation inhibitors or NRF2 activators can produce
significant neuroprotective effects. However, translating these
promising findings into clinical success remains a formidable
challenge.
One of the major pharmacological barriers is the
BBB, which restricts the entry of most therapeutic agents into the
central nervous system. A number of compounds with robust in
vitro efficacy, such as DFO and certain natural products, fail
to exert therapeutic effects in vivo due to their poor BBB
permeability (139). Thus,
improving drug delivery across the BBB (through molecular
optimization or nanotechnology-based delivery systems) is a
critical priority.
Target specificity and off-target effects represent
other challenges. Iron homeostasis plays dual roles in maintaining
normal brain function and contributes to pathology. Broad-spectrum
iron chelation, as highlighted by the 'Deferiprone Paradox', risks
impairing physiological processes while attempting to block
ferroptosis. Similarly, electrophilic NRF2 activators may
non-specifically react with other thiol-containing proteins,
causing cytotoxicity (140).
Future therapeutics must achieve high specificity and ideally be
selectively activated under pathological conditions.
A number of currently available ferroptosis
modulators also face limitations in drug-like properties, such as
poor water solubility, instability and suboptimal pharmacokinetics,
which hinder their development into clinically viable agents
(141). Improving these
characteristics through medicinal chemistry is essential.
Compounding these pharmacological issues is the
lack of reliable biomarkers for monitoring ferroptosis in the human
brain. In the absence of validated protein, metabolite or genetic
markers in CSF or blood, identifying patients most likely to
benefit from ferroptosis-targeting therapies, assessing the
treatment response or dose adjustment are difficult (9). Most proposed biomarkers are still
in the exploratory stage and require rigorous clinical
validation.
Several innovative strategies should be prioritized
to overcome these obstacles. Nanodelivery systems can facilitate
BBB penetration, improve drug solubility and stability, and enable
targeted delivery to pathological brain regions (142,143). Combination therapies that
integrate ferroptosis inhibitors with other modalities, such as
dopamine replacement therapy for PD, anti-Aβ or anti-tau agents for
AD and anti-inflammatory drugs for chronic neuroinflammation, may
yield synergistic benefits. Personalized medicine approaches that
incorporate individual disease stages, genetic profiles and
biomarker status are critical for optimizing therapeutic outcomes.
Furthermore, mechanistic research is essential to elucidate the
unresolved aspects of ferroptosis, including the final molecular
executors and the crosstalk between ferroptosis and other cell
death pathways, such as apoptosis and autophagy. These insights
will inform the discovery of more precise and effective drug
targets. At present, the field of ferroptosis is at a critical
inflection point, shifting from mechanistic discovery to
translational application. While preclinical data highlight its
therapeutic potential (74),
clinical progress has been slow due to unresolved delivery and
diagnostic issues (144).
Animal models are indispensable for mechanistic exploration but
cannot fully replicate the long-term, multifactorial progression of
human neurodegenerative diseases.
Therefore, the future of ferroptosis-targeted
therapy depends not only on discovering new inhibitors or inducers
but also on solving the core issues of how to deliver the right
drug to the right brain region in the right patient and how to
reliably monitor ferroptosis in clinical settings.
Interdisciplinary collaboration among researchers from the
neuroscience, pharmacology, medicinal chemistry and biomarker
fields is essential. Without such concerted efforts, the enormous
therapeutic promise of ferroptosis modulation may remain confined
to the preclinical realm.
Not applicable.
This work was funded by the National Natural Science Foundation
of China (grant no. 82271967), the Nanjing Medical Science and
Technology Development Fund (grant no. ZKX22038) and the China
Postdoctoral Science Foundation (grant no. 2024M751466).
Not applicable.
JM and WX provided supervision and assisted with
writing and editing. SG conducted the literature search and drafted
and compiled the manuscript and created the figures. DK, SF, YL, XY
and ZJ provided constructive suggestions for the article and
contributed to its design and structuring. JM and WX modified and
adjusted the figures, ensuring their accuracy and clarity. All
authors have read and approved the final version of the manuscript.
Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Chen X, Comish PB, Tang D and Kang R:
Characteristics and biomarkers of ferroptosis. Front Cell Dev Biol.
9:6371622021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang H and Xie Y: Advances in ferroptosis
research: A comprehensive review of mechanism exploration, drug
development, and disease treatment. Pharmaceuticals (Basel).
18:3342025. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ma W, Jiang X, Jia R and Li Y: Mechanisms
of ferroptosis and targeted therapeutic approaches in urological
malignancies. Cell Death Discov. 10:4322024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yan HF, Zou T, Tuo QZ, Xu S, Li H, Belaidi
AA and Lei P: Ferroptosis: Mechanisms and links with diseases.
Signal Transduct Target Ther. 6:492021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zheng J and Conrad M: Ferroptosis: When
metabolism meets cell death. Physiol Rev. 105:651–706. 2025.
View Article : Google Scholar
|
|
6
|
Huang X: A concise review on oxidative
stress-mediated ferroptosis and cuproptosis in Alzheimer's disease.
Cells. 12:13692023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Naderi S, Khodagholi F, Janahmadi M,
Motamedi F, Torabi A, Batool Z, Heydarabadi MF and Pourbadie HG:
Ferroptosis and cognitive impairment: Unraveling the link and
potential therapeutic targets. Neuropharmacology. 263:1102102025.
View Article : Google Scholar
|
|
8
|
Zhang Y, Wang Y, Li Y, Pang J, Höhn A,
Dong W, Gao R, Liu Y, Wang D, She Y, et al: Methionine restriction
alleviates Diabetes-associated cognitive impairment via activation
of FGF21. Redox Biol. 77:1033902024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ryan SK, Ugalde CL, Rolland AS, Skidmore
J, Devos D and Hammond TR: Therapeutic inhibition of ferroptosis in
neurodegenerative disease. Trends Pharmacol Sci. 44:674–688. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Khan G, Hussain MS, Khan Y, Fatima R,
Ahmad S, Sultana A and Alam P: Ferroptosis and its contribution to
cognitive impairment in Alzheimer's disease: Mechanisms and
therapeutic potential. Brain Res. 1864:1497762025. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma M, Jing G, Tian Y, Yin R and Zhang M:
Ferroptosis in cognitive impairment associated with diabetes and
Alzheimer's disease: Mechanistic insights and new therapeutic
opportunities. Mol Neurobiol. 62:2435–2449. 2025. View Article : Google Scholar
|
|
12
|
Jacquemyn J, Ralhan I and Ioannou MS:
Driving factors of neuronal ferroptosis. Trends Cell Biol.
34:535–546. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yan N and Zhang JJ: The emerging roles of
ferroptosis in vascular cognitive impairment. Front Neurosci.
13:8112019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Y, Li Z, Ran Q and Wang P: Sterols in
ferroptosis: From molecular mechanisms to therapeutic strategies.
Trends Mol Med. 31:36–49. 2025. View Article : Google Scholar
|
|
15
|
Li QS and Jia YJ: Ferroptosis: A critical
player and potential therapeutic target in traumatic brain injury
and spinal cord injury. Neural Regen Res. 18:506–512. 2023.
View Article : Google Scholar
|
|
16
|
Shui S, Zhao Z, Wang H, Conrad M and Liu
G: Non-enzymatic lipid peroxidation initiated by photodynamic
therapy drives a distinct ferroptosis-like cell death pathway.
Redox Biol. 45:1020562021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang S, Zhang M, Liu C, Li M, Lou Y and
Tan H: Redox mechanism of glycerophospholipids and relevant
targeted therapy in ferroptosis. Cell Death Discov. 11:3582025.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fei W, Chen D, Tang H, Li C, Zheng W, Chen
F, Song Q, Zhao Y, Zou Y and Zheng C: Targeted GSH-exhausting and
hydroxyl radical self-producing manganese-silica nanomissiles for
MRI guided ferroptotic cancer therapy. Nanoscale. 12:16738–16754.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee J and Hyun DH: The interplay between
intracellular iron homeostasis and neuroinflammation in
neurodegenerative diseases. Antioxidants (Basel). 12:9182023.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang XD, Liu ZY, Wang MS, Guo YX, Wang
XK, Luo K, Huang S and Li RF: Mechanisms and regulations of
ferroptosis. Front Immunol. 14:12694512023. View Article : Google Scholar :
|
|
21
|
Kakhlon O and Cabantchik ZI: The labile
iron pool: Characterization, measurement, and participation in
cellular processes(1). Free Radic Biol Med. 33:1037–1046. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arosio P, Elia L and Poli M: Ferritin,
cellular iron storage and regulation. IUBMB Life. 69:414–422. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mancias JD, Wang X, Gygi SP, Harper JW and
Kimmelman AC: Quantitative proteomics identifies NCOA4 as the cargo
receptor mediating ferritinophagy. Nature. 509:105–109. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mancias JD, Pontano Vaites L, Nissim S,
Biancur DE, Kim AJ, Wang X, Liu Y, Goessling W, Kimmelman AC and
Harper JW: Ferritinophagy via NCOA4 is required for erythropoiesis
and is regulated by iron dependent HERC2-mediated proteolysis.
Elife. 4:e103082015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ajoolabady A, Aslkhodapasandhokmabad H,
Libby P, Tuomilehto J, Lip GYH, Penninger JM, Richardson DR, Tang
D, Zhou H, Wang S, et al: Ferritinophagy and ferroptosis in the
management of metabolic diseases. Trends Endocrinol Metab.
32:444–462. 2021. View Article : Google Scholar
|
|
26
|
Forcina GC and Dixon SJ: GPX4 at the
crossroads of lipid homeostasis and ferroptosis. Proteomics.
19:e18003112019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bridges RJ, Natale NR and Patel SA: System
xc− Cystine/glutamate antiporter: An update on molecular
pharmacology and roles within the CNS. Br J Pharmacol. 165:20–34.
2012. View Article : Google Scholar :
|
|
28
|
Conrad M and Sato H: The oxidative
stress-inducible cystine/glutamate antiporter, system x (c) (-):
Cystine supplier and beyond. Amino Acids. 42:231–246. 2012.
View Article : Google Scholar
|
|
29
|
Xue Z, Nuerrula Y, Sitiwaerdi Y and Eli M:
Nuclear factor erythroid 2-related factor 2 promotes
radioresistance by regulating glutamate-cysteine ligase modifier
subunit and its unique immunoinvasive pattern. Biomol Biomed.
24:545–559. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ursini F and Maiorino M: Lipid
peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic
Biol Med. 152:175–185. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang WS, SriRamaratnam R, Welsch ME,
Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji
AF, Clish CB and Brown LM: Regulation of ferroptotic cancer cell
death by GPX4. Cell. 156:317–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Benarroch E: What is the role of
ferroptosis in neurodegeneration? Neurology. 101:312–319. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou M, Xu K, Ge J, Luo X, Wu M, Wang N
and Zeng J: Targeting ferroptosis in Parkinson's disease:
Mechanisms and emerging therapeutic strategies. Int J Mol Sci.
25:130422024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kraft VAN, Bezjian CT, Pfeiffer S,
Ringelstetter L, Müller C, Zandkarimi F, Merl-Pham J, Bao X,
Anastasov N, Kössl J, et al: GTP cyclohydrolase
1/tetrahydrobiopterin counteract ferroptosis through lipid
remodeling. ACS Cent Sci. 6:41–53. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Soula M, Weber RA, Zilka O, Alwaseem H, La
K, Yen F, Molina H, Garcia-Bermudez J, Pratt DA, Birsoy K, et al:
Metabolic determinants of cancer cell sensitivity to canonical
ferroptosis inducers. Nat Chem Biol. 16:1351–1360. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee
H, Koppula P, Wu S, Zhuang L, Fang B, et al: DHODH-mediated
ferroptosis defence is a targetable vulnerability in cancer.
Nature. 593:586–590. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Teng D, Swanson KD, Wang R, Zhuang A, Wu
H, Niu Z, Cai L, Avritt FR, Gu L, Asara JM, et al: DHODH modulates
immune evasion of cancer cells via CDP-choline dependent regulation
of phospholipid metabolism and ferroptosis. Nat Commun.
16:38672025. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cao J, Chen X, Chen L, Lu Y, Wu Y, Deng A,
Pan F, Huang H, Liu Y, Li Y, et al: DHODH-mediated mitochondrial
redox homeostasis: A novel ferroptosis regulator and promising
therapeutic target. Redox Biol. 85:1037882025. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li Z, Chen X, Xiang W, Tang T and Gan L:
m6A demethylase FTO-mediated upregulation of BAP1 induces neuronal
ferroptosis via the p53/SLC7A11 axis in the MPP+/MPTP-induced
Parkinson's disease model. ACS Chem Neurosci. 16:405–416. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tarangelo A, Magtanong L, Bieging-Rolett
KT, Li Y, Ye J, Attardi LD and Dixon SJ: p53 suppresses metabolic
stress-induced ferroptosis in cancer cells. Cell Rep. 22:569–575.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jiang M, Qiao M, Zhao C, Deng J, Li X and
Zhou C: Targeting ferroptosis for cancer therapy: Exploring novel
strategies from its mechanisms and role in cancers. Transl Lung
Cancer Res. 9:1569–1584. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lewerenz J, Hewett SJ, Huang Y, Lambros M,
Gout PW, Kalivas PW, Massie A, Smolders I, Methner A, Pergande M,
et al: The cystine/glutamate antiporter system x(c)(-) in health
and disease: From molecular mechanisms to novel therapeutic
opportunities. Antioxid Redox Signal. 18:522–555. 2013. View Article : Google Scholar :
|
|
43
|
Sun Y, Xiao Y, Tang Q, Chen W and Lin L:
Genetic markers associated with ferroptosis in Alzheimer's disease.
Front Aging Neurosci. 16:13646052024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Streit WJ, Phan L and Bechmann I:
Ferroptosis and pathogenesis of neuritic plaques in Alzheimer
disease. Pharmacol Rev. 77:1000052025. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Proneth B and Conrad M: Ferroptosis and
necroinflammation, a yet poorly explored link. Cell Death Differ.
26:14–24. 2019. View Article : Google Scholar
|
|
46
|
McIntosh A, Mela V, Harty C, Minogue AM,
Costello DA, Kerskens C and Lynch MA: Iron accumulation in
microglia triggers a cascade of events that leads to altered
metabolism and compromised function in APP/PS1 mice. Brain Pathol.
29:606–621. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhao H, Wang J, Li Z, Wang S, Yu G and
Wang L: Identification Ferroptosis-related hub genes and diagnostic
model in Alzheimer's disease. Front Mol Neurosci. 16:12806392023.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tian M, Shen J, Qi Z, Feng Y and Fang P:
Bioinformatics analysis and prediction of Alzheimer's disease and
alcohol dependence based on Ferroptosis-related genes. Front Aging
Neurosci. 15:12011422023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang Y, Wang M and Chang W: Iron
dyshomeostasis and ferroptosis in Alzheimer's disease: Molecular
mechanisms of cell death and novel therapeutic drugs and targets
for AD. Front Pharmacol. 13:9836232022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sun J, Lin XM, Lu DH, Wang M, Li K, Li SR,
Li ZQ, Zhu CJ, Zhang ZM, Yan CY, et al: Midbrain dopamine oxidation
links ubiquitination of glutathione peroxidase 4 to ferroptosis of
dopaminergic neurons. J Clin Invest. 133:e1731102023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zheng Z, Zhang S, Liu X, Wang X, Xue C, Wu
X, Zhang X, Xu X, Liu Z, Yao L and Lu G: LRRK2 regulates
ferroptosis through the system xc-GSH-GPX4 pathway in the
neuroinflammatory mechanism of Parkinson's disease. J Cell Physiol.
239:e312502024. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Negida A, Hassan NM, Aboeldahab H, Zain
YE, Negida Y, Cadri S, Cadri N, Cloud LJ, Barrett MJ and Berman B:
Efficacy of the Iron-chelating agent, deferiprone, in patients with
Parkinson's disease: A systematic review and Meta-analysis. CNS
Neurosci Ther. 30:e146072024. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Angelova PR, Choi ML, Berezhnov AV,
Horrocks MH, Hughes CD, De S, Rodrigues M, Yapom R, Little D, Dolt
KS, et al: Alpha synuclein aggregation drives ferroptosis: An
Interplay of iron, calcium and lipid peroxidation. Cell Death
Differ. 27:2781–2796. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang H, Wu S, Li Q, Sun H and Wang Y:
Targeting ferroptosis: Acteoside as a neuroprotective agent in
salsolinol-induced Parkinson's disease models. Front Biosci
(Landmark Ed). 30:266792025. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yan J, Bao L, Liang H, Zhao L, Liu M, Kong
L, Fan X, Liang C, Liu T, Han X, et al: A druglike ferrostatin-1
analogue as a ferroptosis inhibitor and photoluminescent indicator.
Angew Chem Int Ed Engl. 64:e2025021952025. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
A A, W C, N N, L M, M D and Zhang DD:
α-Syn overexpression, NRF2 suppression, and enhanced ferroptosis
create a vicious cycle of neuronal loss in Parkinson's disease.
Free Radic Biol Med. 192:130–140. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Gong K, Zhou S, Xiao L, Xu M, Zhou Y, Lu
K, Yu X, Zhu J, Liu C and Zhu Q: Danggui shaoyao san ameliorates
Alzheimer's disease by regulating lipid metabolism and inhibiting
neuronal ferroptosis through the AMPK/Sp1/ACSL4 signaling pathway.
Front Pharmacol. 16:15883752025. View Article : Google Scholar
|
|
58
|
Lv QK, Tao KX, Yao XY, Pang MZ, Cao BE,
Liu CF and Wang F: Melatonin MT1 receptors regulate the
Sirt1/Nrf2/ho-1/Gpx4 pathway to prevent α-synuclein-induced
ferroptosis in Parkinson's disease. J Pineal Res. 76:e129482024.
View Article : Google Scholar
|
|
59
|
Wang W, Thomas ER, Xiao R, Chen T, Guo Q,
Liu K, Yang Y and Li X: Targeting mitochondria-regulated
ferroptosis: A new frontier in Parkinson's disease therapy.
Neuropharmacology. 274:1104392025. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wang Q, Liu J, Zhang Y, Li Z, Zhao Z,
Jiang W, Zhao J, Hou L and Wang Q: Microglial CR3 promotes neuron
ferroptosis via NOX2-mediated iron deposition in rotenone-induced
experimental models of Parkinson's disease. Redox Biol.
77:1033692024. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang H, Mao W, Zhang Y, Feng W, Bai B, Ji
B, Chen J, Cheng B and Yan F: NOX1 triggers ferroptosis and
ferritinophagy, contributes to Parkinson's disease. Free Radic Biol
Med. 222:331–343. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lin Z, Ying C, Si X, Xue N, Liu Y, Zheng
R, Chen Y, Pu J and Zhang B: NOX4 exacerbates Parkinson's disease
pathology by promoting neuronal ferroptosis and neuroinflammation.
Neural Regen Res. 20:2038–2052. 2025. View Article : Google Scholar
|
|
63
|
Xie Z, Wang X, Luo X, Yan J, Zhang J, Sun
R, Luo A and Li S: Activated AMPK mitigates Diabetes-related
cognitive dysfunction by inhibiting hippocampal ferroptosis.
Biochem Pharmacol. 207:1153742023. View Article : Google Scholar
|
|
64
|
Wei M, Liu X, Tan Z, Tian X, Li M and Wei
J: Ferroptosis: A new strategy for Chinese herbal medicine
treatment of diabetic nephropathy. Front Endocrinol (Lausanne).
14:11880032023. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sha W, Hu F, Xi Y, Chu Y and Bu S:
Mechanism of ferroptosis and its role in type 2 diabetes mellitus.
J Diabetes Res. 2021:99996122021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Liu J, Hu X, Xue Y, Liu C, Liu D, Shang Y,
Shi Y, Cheng L, Zhang J, Chen A and Wang J: Targeting hepcidin
improves cognitive impairment and reduces iron deposition in a
diabetic rat model. Am J Transl Res. 12:4830–4839. 2020.PubMed/NCBI
|
|
67
|
Tang W, Li Y, He S, Jiang T, Wang N, Du M,
Cheng B, Gao W, Li Y and Wang Q: Caveolin-1 alleviates
diabetes-associated cognitive dysfunction through modulating
neuronal ferroptosis-mediated mitochondrial homeostasis. Antioxid
Redox Signal. 37:867–886. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Guo T, Yu Y, Yan W, Zhang M, Yi X, Liu N,
Cui X, Wei X, Sun Y, Wang Z, et al: Erythropoietin ameliorates
cognitive dysfunction in mice with type 2 diabetes mellitus via
inhibiting iron overload and ferroptosis. Exp Neurol.
365:1144142023. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhao Y, Guo H and Li Q, Wang N, Yan C,
Zhang S, Dong Y, Liu C, Gao W, Zhu Y and Li Q: TREM1 induces
microglial ferroptosis through the PERK pathway in
diabetic-associated cognitive impairment. Exp Neurol.
383:1150312025. View Article : Google Scholar
|
|
70
|
Ma H, Jiang T, Tang W, Ma Z, Pu K, Xu F,
Chang H, Zhao G, Gao W, Li Y and Wang Q: Transplantation of
platelet-derived mitochondria alleviates cognitive impairment and
mitochondrial dysfunction in db/db mice. Clin Sci (Lond).
134:2161–2175. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Asterholm IW, Mundy DI, Weng J, Anderson
RGW and Scherer PE: Altered mitochondrial function and metabolic
inflexibility associated with loss of caveolin-1. Cell Metab.
15:171–185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Feng Q, Yang Y, Qiao Y, Zheng Y, Yu X, Liu
F, Wang H, Zheng B, Pan S, Ren K, et al: Quercetin ameliorates
diabetic kidney injury by inhibiting ferroptosis via activating
Nrf2/HO-1 signaling pathway. Am J Chin Med. 51:997–1018. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Li J, Cao Y, Xu J, Li J, Lv C, Gao Q,
Zhang C, Jin C, Wang R, Jiao R and Zhu H: Vitamin D improves
cognitive impairment and alleviates ferroptosis via the Nrf2
signaling pathway in aging mice. Int J Mol Sci. 24:153152023.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wei Z, Yu H, Zhao H, Wei M, Xing H, Pei J,
Yang Y and Ren K: Broadening horizons: Ferroptosis as a new target
for traumatic brain injury. Burns Trauma. 12:tkad0512024.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Abdukarimov N, Kokabi K and Kunz J:
Ferroptosis and iron homeostasis: Molecular mechanisms and
neurodegenerative disease implications. Antioxidants (Basel).
14:5272025. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wang B, Fu C, Wei Y, Xu B, Yang R, Li C,
Qiu M, Yin Y and Qin D: Ferroptosis-related biomarkers for
alzheimer's disease: Identification by bioinformatic analysis in
hippocampus. Front Cell Neurosci. 16:10239472022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ficiarà E, Munir Z, Boschi S, Caligiuri ME
and Guiot C: Alteration of iron concentration in Alzheimer's
disease as a possible diagnostic biomarker unveiling ferroptosis.
Int J Mol Sci. 22:44792021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ayton S, Faux NG and Bush AI; Alzheimer's
disease neuroimaging initiative: Ferritin levels in the
cerebrospinal fluid predict Alzheimer's disease outcomes and are
regulated by APOE. Nat Commun. 6:67602015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Urbano T, Michalke B, Chiari A, Malagoli
C, Bedin R, Tondelli M, Vinceti M and Filippini T: Iron species in
cerebrospinal fluid and dementia risk in subjects with mild
cognitive impairment: A cohort study. Neurotoxicology. 110:1–9.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Urbano T, Vinceti M, Carbone C, Wise LA,
Malavolti M, Tondelli M, Bedin R, Vinceti G, Marti A, Chiari A, et
al: Exposure to cadmium and other trace elements among individuals
with mild cognitive impairment. Toxics. 12:9332024. View Article : Google Scholar :
|
|
81
|
Ayton S, Janelidze S, Kalinowski P,
Palmqvist S, Belaidi AA, Stomrud E, Roberts A, Roberts B, Hansson O
and Bush AI: CSF ferritin in the clinicopathological progression of
Alzheimer's disease and associations with APOE and inflammation
biomarkers. J Neurol Neurosurg Psychiatry. 94:211–219. 2023.
View Article : Google Scholar
|
|
82
|
Yu SY, Cao CJ, Zuo LJ, Chen ZJ, Lian TH,
Wang F, Hu Y, Piao YS, Li LX, Guo P, et al: Clinical features and
dysfunctions of iron metabolism in Parkinson disease patients with
hyper echogenicity in Substantia nigra: A Cross-sectional study.
BMC Neurol. 18:92018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Padurariu M, Ciobica A, Hritcu L, Stoica
B, Bild W and Stefanescu C: Changes of some oxidative stress
markers in the serum of patients with mild cognitive impairment and
Alzheimer's disease. Neurosci Lett. 469:6–10. 2010. View Article : Google Scholar
|
|
84
|
Lin Q, Shahid S, Hone-Blanchet A, Huang S,
Wu J, Bisht A, Loring D, Goldstein F, Levey A, Crosson B, et al:
Magnetic resonance evidence of increased iron content in
subcortical brain regions in asymptomatic Alzheimer's disease. Hum
Brain Mapp. 44:3072–3083. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Dusek P, Hofer T, Alexander J, Roos PM and
Aaseth JO: Cerebral iron deposition in neurodegeneration.
Biomolecules. 12:7142022. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Majerníková N, Marmolejo-Garza A, Salinas
CS, Luu MDA, Zhang Y, Trombetta-Lima M, Tomin T, Birner-Gruenberger
R, Lehtonen Š, Koistinaho J, et al: The link between amyloid β and
ferroptosis pathway in Alzheimer's disease progression. Cell Death
Dis. 15:7822024. View Article : Google Scholar
|
|
87
|
Kang JH, Korecka M, Lee EB, Cousins KAQ,
Tropea TF, Chen-Plotkin AA, Irwin DJ, Wolk D, Brylska M, Wan Y and
Shaw LM: Alzheimer disease biomarkers: Moving from CSF to plasma
for reliable detection of amyloid and tau pathology. Clin Chem.
69:1247–1259. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Hampel H, O'Bryant SE, Molinuevo JL,
Zetterberg H, Masters CL, Lista S, Kiddle SJ, Batrla R and Blennow
K: Blood-based biomarkers for Alzheimer disease: Mapping the road
to the clinic. Nat Rev Neurol. 14:639–652. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Livingston G, Huntley J, Sommerlad A, Ames
D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J,
Cooper C, et al: Dementia prevention, intervention, and care: 2020
report of the lancet commission. Lancet. 396:413–446. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Biessels GJ and Despa F: Cognitive decline
and dementia in diabetes mellitus: Mechanisms and clinical
implications. Nat Rev Endocrinol. 14:591–604. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Andrews SJ, Tolosa-Tort P, Jonson C,
Fulton-Howard B, Renton AE, Yokoyama JS and Yaffe K; Alzheimer's
Disease Neuroimaging Initiative: The role of genomic-informed risk
assessments in predicting dementia outcomes. Alzheimers Dement.
21:e708262025. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Nasreddine ZS, Phillips NA, Bédirian V,
Charbonneau S, Whitehead V, Collin I, Cummings JL and Chertkow H:
The Montreal cognitive assessment, MoCA: A brief screening tool for
mild cognitive impairment. J Am Geriatr Soc. 53:695–699. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Folstein MF, Folstein SE and McHugh PR:
'Mini-mental state'. A practical method for grading the cognitive
state of patients for the clinician J Psychiatr Res. 12:189–198.
1975.
|
|
94
|
Cullis JO, Fitzsimons EJ, Griffiths WJ,
Tsochatzis E and Thomas DW; British Society for Haematology:
Investigation and management of a raised serum ferritin. Br J
Haematol. 181:331–340. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Stockwell BR, Friedmann Angeli JP, Bayir
H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK,
Kagan VE, et al: Ferroptosis: A regulated cell death nexus linking
metabolism, redox biology, and disease. Cell. 171:273–285. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Wang Y and Liu T: Quantitative
susceptibility mapping (QSM): Decoding MRI data for a tissue
magnetic biomarker. Magn Reson Med. 73:82–101. 2015. View Article : Google Scholar :
|
|
97
|
Jack CR, Bennett DA, Blennow K, Carrillo
MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F,
Karlawish J, et al: NIA-AA research framework: Toward a biological
definition of Alzheimer's disease. Alzheimers Dement. 14:535–562.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Chen HJ, Dong X, Wang Y, Wang K, Feng G,
Bai T, Zhang M, Gan K, Peng JJ, Huang W, et al: Polygenic risk for
Alzheimer's disease in healthy aging: Age-related and APOE-driven
effects on brain structures and cognition. Genome Med. 17:1262025.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Liu Y, Chen M, Chen P, Lin X, Chen S, Liu
C, Wang D, Deng H, Li Q and Wu Y: Machine learning-based
stratification of mild cognitive impairment in Parkinson's disease:
A multicenter cross-sectional analysis. BMC Med Inform Decis Mak.
25:3842025. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Ren Q, Niu S, Zhi L and Liu H: Risk factor
analysis of cognitive frailty in older adults with type 2 diabetes
mellitus: A cross-sectional study. Front Aging Neurosci.
17:16674052025. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Hambright WS, Fonseca RS, Chen L, Na R and
Ran Q: Ablation of ferroptosis regulator glutathione peroxidase 4
in forebrain neurons promotes cognitive impairment and
neurodegeneration. Redox Biol. 12:8–17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Masaldan S, Bush AI, Devos D, Rolland AS
and Moreau C: Striking while the iron is hot: Iron metabolism and
ferroptosis in neurodegeneration. Free Radic Biol Med. 133:221–233.
2019. View Article : Google Scholar
|
|
103
|
Thorwald MA, Godoy-Lugo JA, Kerstiens E,
Garcia G, Kim M, Shemtov SJ, Silva J, Durra S, O'Day PA, Mack WJ,
et al: Down syndrome with Alzheimer's disease brains have increased
iron and associated lipid peroxidation consistent with ferroptosis.
Alzheimers Dement. 21:e703222025. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Ra F and K H: Oxidative stress in brain
aging. Implications for therapeutics of neurodegenerative diseases.
Neurobiol Aging. 23:795–807. 2002. View Article : Google Scholar
|
|
105
|
Ji Y, Zheng K, Li S, Ren C, Shen Y, Tian
L, Zhu H, Zhou Z and Jiang Y: Insight into the potential role of
ferroptosis in neurodegenerative diseases. Front Cell Neurosci.
16:10051822022. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Tian Y, Tian Y, Yuan Z, Zeng Y, Wang S,
Fan X, Yang D and Yang M: Iron metabolism in aging and age-related
diseases. Int J Mol Sci. 23:36122022. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Baier MP, Sharum SI, Wilson JL, Narasimhan
S, Salim C and Logan S: Enhancing mitochondrial matrix antioxidant
SOD2 in astrocytes mitigates cellular senescence and cognitive
impairment in aging. Aging Dis. Oct 9–2025.Epub ahead of print.
PubMed/NCBI
|
|
108
|
Lu Q, Shao N, Fang Z, Ouyang Z, Shen Y,
Yang R, Liu H, Cai B and Wei T: The anti-Alzheimer's disease
effects of ganoderic acid a by inhibiting Ferroptosis-lipid
peroxidation via activation of the NRF2/SLC7A11/GPX4 signaling
pathway. Chem Biol Interact. 412:1114592025. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Wang M, Li M, Jiang Y, Wang S, Yang X,
Naseem A, Algradi AM, Hao Z, Guan W, Chen Q, et al: Saponins from
Astragalus membranaceus(Fisch.) Bge alleviated neuronal ferroptosis
in Alzheimer's disease by regulating the NOX4/Nrf2 signaling
pathway. J Agric Food Chem. 73:7725–7740. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Meng X, Zhao W, Yang R, Xu SQ, Wang SY, Li
MM, Jiang YK, Hao ZC, Guan W, Kuang HX, et al: Lignans from
Schisandra chinensis(Turcz.) Baill ameliorates cognitive impairment
in Alzheimer's disease and alleviates ferroptosis by activating the
Nrf2/FPN1 signaling pathway and regulating iron levels. J
Ethnopharmacol. 341:1193352025. View Article : Google Scholar
|
|
111
|
Li Z, Lu Y, Zhen Y, Jin W, Ma X, Yuan Z,
Liu B, Zhou XL and Zhang L: Avicularin inhibits ferroptosis and
improves cognitive impairments in Alzheimer's disease by modulating
the NOX4/Nrf2 axis. Phytomedicine. 135:1562092024. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Deng PX, Silva M, Yang N, Wang Q, Meng X,
Ye KQ, Gao HC and Zheng WH: Artemisinin inhibits neuronal
ferroptosis in Alzheimer's disease models by targeting KEAP1. Acta
Pharmacol Sin. 46:326–337. 2025. View Article : Google Scholar
|
|
113
|
Yong Y, Yan L, Wei J, Feng C, Yu L, Wu J,
Guo M, Fan D, Yu C, Qin D, et al: A novel ferroptosis inhibitor,
thonningianin a, improves Alzheimer's disease by activating GPX4.
Theranostics. 14:6161–6184. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Wu Y, Wei M, Wang M, Guo M, Yu H, Chen Y,
Xu T and Zhou Y: Schisandra total lignans ameliorate neuronal
ferroptosis in 3xTg-AD mice via regulating NADK/NADPH/GSH pathway.
Phytomedicine. 140:1566122025. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Ding T, Song M, Wu Y, Li Z, Zhang S and
Fan X: Schisandrin B ameliorates Alzheimer's disease by suppressing
neuronal ferroptosis and ensuing microglia M1 polarization.
Phytomedicine. 142:1567802025. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zhang ZH, Chen C, Jia SZ, Cao XC, Liu M,
Tian J, Hoffmann PR, Xu HX, Ni JZ and Song GL: Selenium restores
synaptic deficits by modulating NMDA receptors and selenoprotein K
in an Alzheimer's disease model. Antioxid Redox Signal. 35:863–884.
2021. View Article : Google Scholar
|
|
117
|
Zeng H and Jin Z: The role of ferroptosis
in Alzheimer's disease: Mechanisms and therapeutic potential
(review). Mol Med Rep. 32:1922025. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Wang J, Wang Z, Li Y, Hou Y, Yin C, Yang
E, Liao Z, Fan C, Martin LL and Sun D: Blood brain Barrier-targeted
delivery of double selenium nanospheres ameliorates neural
ferroptosis in Alzheimer's disease. Biomaterials. 302:1223592023.
View Article : Google Scholar
|
|
119
|
Sun C, Gao X, Sha S, Wang S, Shan Y, Li L,
Xing C, Guan H and Du H: Berberine alleviates Alzheimer's disease
by activating autophagy and inhibiting ferroptosis through the
JNK-p38MAPK signaling pathway. Int Immunopharmacol. 155:1145502025.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Song D, Gui F, Li G, Zhuang S, Sun J, Tan
X, Hong C and Huang J: Neuritin improves cognitive impairments in
APP/PS1 Alzheimer's disease mice model by mitigating neuronal
ferroptosis via PI3K/Akt activation. Int J Biol Macromol.
303:1406622025. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Guo Y, Zhao J, Liu X, Lu P, Liang F, Wang
X, Wu J and Hai Y: Ghrelin induces ferroptosis resistance and M2
polarization of microglia to alleviate neuroinflammation and
cognitive impairment in Alzheimer's disease. J Neuroimmune
Pharmacol. 20:62025. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Tan L, Xie J, Liao C, Li X, Zhang W, Cai
C, Cheng L and Wang X: Tetrahedral framework nucleic acids inhibit
aβ-mediated ferroptosis and ameliorate cognitive and synaptic
impairments in Alzheimer's disease. J Nanobiotechnology.
22:6822024. View Article : Google Scholar
|
|
123
|
Lei L, Yuan J, Dai Z, Xiang S, Tu Q, Cui
X, Zhai S, Chen X, He Z, Fang B, et al: Targeting the labile iron
pool with engineered DFO nanosheets to inhibit ferroptosis for
Parkinson's disease therapy. Adv Mater. 36:e24093292024. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Ko CJ, Gao SL, Lin TK, Chu PY and Lin HY:
Ferroptosis as a major factor and therapeutic target for
neuroinflammation in Parkinson's disease. Biomedicines. 9:16792021.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Han Z, Wang B, Wen YQ, Li YN, Feng CX,
Ding XS, Shen Y, Yang Q and Gao L: Acteoside alleviates lipid
peroxidation by enhancing Nrf2-mediated mitophagy to inhibit
ferroptosis for neuroprotection in Parkinson's disease. Free Radic
Biol Med. 223:493–505. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Kong L, Wang Y, Tong Z, Dai R, Yusuf A, Du
L, Liu B, Huang Z and Hu L: Granulathiazole a protects
6-OHDA-induced Parkinson's disease from ferroptosis via activating
Nrf2/HO-1 pathway. Bioorg Chem. 147:1073992024. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Pandey SK, Nanda A, Gautam AS and Singh
RK: Betulinic acid protects against lipopolysaccharide and ferrous
sulfate-induced oxidative stress, ferroptosis, apoptosis, and
neuroinflammation signaling relevant to Parkinson's disease. Free
Radic Biol Med. 233:340–354. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Fu G, Li T, Zhao Y, Zhang S, Xue X, Yang
Y, Li T, Luo S, Yue G and Lei T: Dingzhen pills inhibit neuronal
ferroptosis and neuroinflammation by inhibiting the cGAS-STING
pathway for Parkinson's disease mice. Chin Med. 20:872025.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Dong X, Yang T and Jin Z: Lactobacillus
Reuteri-derived γ-amino butyric acid alleviates MPTP-induced
Parkinson's disease through inhibiting Ferroptosis via the
AKT-GSK3β-GPX4 axis. NPJ Parkinsons Dis. 11:1492025. View Article : Google Scholar
|
|
130
|
Yusun A, Wan HM, Chen HX, Sun M, Zhang CN
and Ding XD: Emodin ameliorates dopaminergic neuron loss in the
MPP+ induced Parkinson's disease model: Significant inhibition of
Ferroptosis by activating UQCRC1 protein. Nat Prod Res. 1–12. 2025.
View Article : Google Scholar
|
|
131
|
Lei Y, Zhou J, Xu D, Chai S and Xiong N:
Corilagin attenuates neuronal apoptosis and ferroptosis of
Parkinson's disease through regulating the TLR4/src/NOX2 signaling
pathway. ACS Chem Neurosci. 16:968–980. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Liu X, Zheng Z, Xue C, Wang X, Li J, Liu
Z, Xin W, Xu X, Zhou D, Yao L and Lu G: LRRK2 mediates
α-synuclein-induced neuroinflammation and ferroptosis through the
p62-Keap1-Nrf2 pathway in Parkinson's disease. Inflammation.
48:3666–3691. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Huang L, Pi J, Gu L, Liao Z and Wang W:
Teneligliptin, a DPP4 inhibitor protects dopaminergic neurons in PD
models via inhibiting of oxidative stress and ferroptosis. Eur J
Pharmacol. 1002:1777822025. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
An JR, Su JN, Sun GY, Wang QF, Fan YD,
Jiang N, Yang YF and Shi Y: Liraglutide alleviates cognitive
deficit in db/db mice: Involvement in oxidative stress, iron
overload, and ferroptosis. Neurochem Res. 47:279–294. 2022.
View Article : Google Scholar
|
|
135
|
Chen J, Guo P, Han M, Chen K, Qin J and
Yang F: Cognitive protection of sinomenine in type 2 diabetes
mellitus through regulating the EGF/Nrf2/HO-1 signaling, the
microbiota-gut-brain axis, and hippocampal neuron ferroptosis.
Phytother Res. 37:3323–3341. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Wang B, Zhu S, Guo M, Ma RD, Tang YL, Nie
YX and Gu HF: Artemisinin ameliorates cognitive decline by
inhibiting hippocampal neuronal ferroptosis via Nrf2 activation in
T2DM mice. Mol Med. 30:352024. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Wang Z, Feng S, Li Q, Song Z, He J, Yang
S, Yan C and Ling H: Dihydromyricetin alleviates hippocampal
ferroptosis in type 2 diabetic cognitive impairment rats via
inhibiting the JNK-inflammatory factor pathway. Neurosci Lett.
812:1374042023. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Hu Y, Xu J, Wang J, Wang J, Li Y, Chen W
and Zhang Q: Resveratrol alleviates diabetic adipose Tissue-derived
extracellular vesicles-induced hippocampal ferroptosis and
cognitive dysfunction via inhibiting miR-9-3p/SLC7A11 axis. Mol
Neurobiol. 62:12307–12330. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Chen X, Pang X, Yeo AJ, Xie S, Xiang M,
Shi B, Yu G and Li C: The molecular mechanisms of ferroptosis and
its role in blood-brain barrier dysfunction. Front Cell Neurosci.
16:8897652022. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Wang J, Cao Y, Lu Y, Zhu H, Zhang J, Che
J, Zhuang R and Shao J: Recent progress and applications of small
molecule inhibitors of Keap1-Nrf2 axis for neurodegenerative
diseases. Eur J Med Chem. 264:1159982024. View Article : Google Scholar
|
|
141
|
Liu Q, Zhao Y, Zhou H and Chen C:
Ferroptosis: Challenges and opportunities for nanomaterials in
cancer therapy. Regen Biomater. 10:rbad0042023. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Delila L, Nebie O, Le NTN, Timmerman K,
Lee DY, Wu YW, Chou ML, Buée L, Chou SY, Blum D, et al:
Neuroprotective effects of intranasal extracellular vesicles from
human platelet concentrates supernatants in traumatic brain injury
and Parkinson's disease models. J Biomed Sci. 31:872024. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Liu J, Huang J, Zhang Z, Zhang R, Sun Q,
Zhang Z, Liu Y and Ma B: Mesenchymal stem cell-derived exosomes
ameliorate delayed neurocognitive recovery in aged mice by
inhibiting hippocampus ferroptosis via activating SIRT1/Nrf2/HO-1
signaling pathway. Oxid Med Cell Longev. 2022:35932942022.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Xu Y, Jia B, Li J, Li Q and Luo C: The
interplay between ferroptosis and neuroinflammation in central
neurological disorders. Antioxidants (Basel). 13:3952024.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Cai Z, Wang S, Cao S, Chen Y, Penati S,
Peng V, Yuede CM, Beatty WL, Lin K, Zhu Y, et al: Loss of ATG7 in
microglia impairs UPR, triggers ferroptosis, and weakens amyloid
pathology control. J Exp Med. 222:e202301732025. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Li Y, Sun M, Cao F, Chen Y, Zhang L, Li H,
Cao J, Song J, Ma Y, Mi W and Zhang X: The ferroptosis inhibitor
liproxstatin-1 ameliorates LPS-induced cognitive impairment in
mice. Nutrients. 14:45992022. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Long HZ, Cheng Y, Zhou ZW, Luo HY, Wen DD
and Gao LC: The key roles of organelles and ferroptosis in
alzheimer's disease. J Neurosci Res. 100:1257–1280. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Conrad M and Proneth B: Selenium: Tracing
another essential element of ferroptotic cell death. Cell Chem
Biol. 27:409–419. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Kalyanaraman B: NAC, NAC, Knockin' on
Heaven's door: Interpreting the mechanism of action of
N-acetylcysteine in tumor and immune cells. Redox Biol.
57:1024972022. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Xu J, Shen R, Qian M, Zhou Z, Xie B, Jiang
Y, Yu Y and Dong W: Ferroptosis in Alzheimer's disease: The
regulatory role of glial cells. J Integr Neurosci. 24:258452025.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Li Y, Zhang E, Yang H, Chen Y, Tao L, Xu
Y, Chen T and Shen X: Gastrodin ameliorates cognitive dysfunction
in vascular dementia rats by suppressing ferroptosis via the
regulation of the Nrf2/Keap1-GPx4 signaling pathway. Molecules.
27:63112022. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Yang L and Nao J: Ferroptosis: A potential
therapeutic target for Alzheimer's disease. Rev Neurosci.
34:573–598. 2023. View Article : Google Scholar
|
|
154
|
Mesa-Herrera F, Marín R, Torrealba E and
Díaz M: Multivariate assessment of lipoxidative metabolites, trace
biometals, and antioxidant and detoxifying activities in the
cerebrospinal fluid define a fingerprint of preclinical stages of
Alzheimer's disease. J Alzheimers Dis. 86:387–402. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Mayr E, Rotter J, Kuhrt H, Winter K,
Stassart RM, Streit WJ and Bechmann I: Detection of molecular
markers of ferroptosis in human Alzheimer's disease brains. J
Alzheimers Dis. 102:1133–1154. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Ayton S, Wang Y, Diouf I, Schneider JA,
Brockman J, Morris MC and Bush AI: Brain iron is associated with
accelerated cognitive decline in people with Alzheimer pathology.
Mol Psychiatry. 25:2932–2941. 2020. View Article : Google Scholar
|
|
157
|
Shi YS, Chen JC, Lin L, Cheng YZ, Zhao Y,
Zhang Y and Pan XD: Dendrobine rescues cognitive dysfunction in
diabetic encephalopathy by inhibiting ferroptosis via activating
Nrf2/GPX4 axis. Phytomedicine. 119:1549932023. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Yang Q, Zhou L, Liu C, Liu D, Zhang Y, Li
C, Shang Y, Wei X, Li C and Wang J: Brain iron deposition in type 2
diabetes mellitus with and without mild cognitive impairment-an in
vivo susceptibility mapping study. Brain Imaging Behav.
12:1479–1487. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Hao L, Mi J, Song L, Guo Y, Li Y, Yin Y
and Zhang C: SLC40A1 mediates ferroptosis and cognitive dysfunction
in type 1 diabetes. Neuroscience. 463:216–226. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Clarke NC, McCabe E, Jensen LD, Creaven BS
and Costello DA: Salicylaldehyde benzoylhydrazone protects against
ferroptosis in models of neurotoxicity and behavioural dysfunction,
in vitro and in vivo. J Mol Neurosci. 75:772025. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Wang Y, Li P, Liang Y and Wang D: ANO6
targets TMEM30A to regulate endoplasmic reticulum Stress-induced
lipid peroxidation and ferroptosis in Alzheimer's cells. Cell
Biochem Biophys. 83:3707–3715. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Lu Z, Jiang Z, Huang X, Chen Y, Feng L,
Mai J, Lao L, Li L, Chen WH and Hu J: Anti-Alzheimer effects of an
HDAC6 inhibitor, WY118, alone and in combination of lithium
chloride: Synergistic suppression of ferroptosis via the modulation
of tau phosphorylation and MAPK signaling. Eur J Pharmacol.
997:1776052025. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Ramesh M and Govindaraju T: MiR-7a-Klf4
axis as a regulator and therapeutic target of neuroinflammation and
ferroptosis in Alzheimer's disease. bioRxiv. Mar 25–2025.Epub ahead
of print. View Article : Google Scholar
|
|
164
|
Zheng N, Zhang Z, Liu H, Zong S, Zhang L,
Cui X, Liu Y, Wang C, Chen R and Lu Z: MK886 ameliorates
Alzheimer's disease by activating the PRKCI/AKT signaling pathway.
Eur J Pharmacol. 993:1773592025. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Feng Y, Wang H, Hu Y, Zhang X, Miao X, Li
Z and Jia J: Hederagenin ameliorates ferroptosis-induced damage by
regulating PPARα/Nrf2/GPX4 signaling pathway in HT22 cells: An in
vitro and in silico study. Bioorg Chem. 155:1081192025. View Article : Google Scholar
|
|
166
|
Tian L, Li H, Xiong W, Li X, Duan S, Yang
C and Shi C: Proteomic alteration in catalpol treatment of
Alzheimer's disease by regulating HSPA5/GPX4. Eur J Pharmacol.
987:1770752025. View Article : Google Scholar
|
|
167
|
Gugliandolo A, Bramanti P and Mazzon E:
Role of vitamin E in the treatment of Alzheimer's disease: Evidence
from animal models. Int J Mol Sci. 18:25042017. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Xiao S, Liu L, Qin X, Xu L, Chai Z and Li
Z: Astragenol alleviates neuroinflammation and improves Parkinson's
symptoms through amino acid metabolism pathway and inhibition of
ferroptosis. J Ethnopharmacol. 348:1198962025. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Wang J, Geng T, Yao X and Liu Y: GDF15
attenuates Parkinson's disease progression via suppressing the
activation of cGAS-STING pathway. Mol Cell Biochem. 480:4449–4466.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Zhao X, Kang Z, Han R, Wang M, Wang Y, Sun
X, Wang C, Zhou J, Cao L and Lu M: JWA binding to NCOA4 alleviates
degeneration in dopaminergic neurons through suppression of
ferritinophagy in Parkinson's disease. Redox Biol. 73:1031902024.
View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Fu X, Qu L, Xu H and Xie J: Ndfip1
protected dopaminergic neurons via regulating mitochondrial
function and ferroptosis in Parkinson's disease. Exp Neurol.
375:1147242024. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Wang T, Sobue A, Watanabe S, Komine O,
Saido TC, Saito T and Yamanaka K: Dimethyl fumarate improves
cognitive impairment and neuroinflammation in mice with Alzheimer's
disease. J Neuroinflammation. 21:552024. View Article : Google Scholar : PubMed/NCBI
|