Ear and nose diseases are often neglected; however,
for most patients, the symptoms persist and recur. The low cure
rates and high recurrence rates inevitably influence the quality of
learning, living and work of patients, particularly of children and
adolescents, resulting in significant healthcare costs as diseases
progress (1,2). The upper respiratory tract is the
first stop in the fight against viral infections and
otolaryngological disorders have attracted wide attention,
especially after the sudden outbreak of the severe acute
respiratory syndrome coronavirus 2 (SARS-CoV-2) in December 2019
(3). Numerous investigations
have reported the etiology, pathogenesis, progression and prognosis
of ear and nose diseases, in which air pollution and meteorological
factors play key roles (4,5).
Over the past decade, climate change and weather
events have gained widespread attention (6). Recent research has established that
air pollution has an effect on ear and nose diseases, specifically
aggravating these diseases by evoking oxidative stress,
exacerbating inflammatory responses and inducing autoimmunity
(7,8). Air pollution is currently one of
the greatest risk factors for diseases and premature death.
Specifically, air pollution (both household and ambient air
pollution) caused 6.7 million deaths in 2019, of which ambient air
pollution contributed to 4.5 million deaths, an increase from 4.2
million deaths in 2015 and 2.9 million deaths in 2000 (9). Ambient air pollution is primarily
caused by particulate matter (PM) and toxic gases. Based on the
aerodynamic diameter, PM can be further classified into coarse
particles (≤10 μm, PM10), fine particles (≤2.5
μm, PM2.5) and ultrafine particles (≤1 μm,
PM1) (10). Toxic
gases include CO, black carbon (BC), nitrogen oxides, sulfur oxides
and ozone (O3). The impact of indoor air pollution on
human health has gained increasing attention (11,12). A study has shown that indoor dust
and bio-super particles are closely linked to the onset of various
diseases, with this effect being independent of environmental air
pollution (13). Additionally,
human activities also significantly influence indoor air quality
(14). Research indicates that
indoor microbial communities may pose potential health risks
(15). Therefore, indoor air
pollution is treated as an independent topic of discussion in the
present review. Household air pollutants include environmental
tobacco smoke (ETS), volatile organic compounds (VOCs) and indoor
allergens.
In addition to air pollution, the role of
meteorological factors has become increasingly important.
Meteorological factors, including temperature, relative humidity
(RH) and wind speed increase not only the activity of pathogens but
also the susceptibility of the population (16,17). Conversely, meteorological factors
and environmental pollution are deeply interrelated. For example,
drier and cooler conditions can boost O3 pollution by
enhancing the rate of photochemical production (18). In addition, bacterial communities
in indoor air are affected by environmental pollution and
meteorological factors. Studies have indicated that airborne
bacterial populations may be altered due to the influence of
household activities, such as burning scented candles, which leads
to significant changes in bacterial diversity (14,15). The interaction between
environmental pollution and meteorological factors promotes the
onset and exacerbation of ear and nose diseases, particularly
allergic rhinitis (AR), otitis media (OM) and sudden sensorineural
hearing loss (SSNHL) (19,20). This emphasizes the importance of
improving indoor ventilation and surface hygiene to reduce health
risks.
To thoroughly analyze the impact of air pollution
and meteorological factors on the onset and progression of ear and
nose diseases, while considering the comorbidity and holistic
characteristics of ear and nose diseases as well as the mechanisms
of known environmental factors, the present study provides, to the
best of our knowledge, the first review of the interactions between
air pollution, meteorological factors and SARS-CoV-2 on ear and
nose diseases. The present review focuses on ear and nose diseases
that are closely associated with inflammatory responses or
autoimmunity, including AR, OM and SSNHL. We propose that
chemosensory dysfunction (olfactory and auditory impairment) may
serve as an early indicator of environmental neurotoxicity,
offering a new perspective for assessing the impact of
environmental pollution on neurological health. Additionally, the
present review explores the effects of environmental factors on
sensory organ function from both the olfactory and auditory
dimensions with the aim of implementing disease prevention and
control through environmental management. The coronavirus disease
2019 (COVID-19) pandemic, as an environmental factor, has imposed a
significant economic burden on healthcare systems and exacerbated
environmental and climate crises (3,21). The respiratory system is the
primary target of SARS-CoV-2 infection. As direct portals for viral
infection, the ear and nose may be repeatedly attacked, leading to
symptoms such as nasal congestion, runny nose and hearing loss.
Therefore, the present review discusses the impact of COVID-19 as a
unique environmental factor in ear and nose diseases. The detailed
search strategies to identify relevant literature can be found in
Tables SI-SIV.
The ear and nose are closely interconnected
functionally, with the eustachian tube (ET) playing a key role in
this relationship. The ET, located in the petrous bone of the
temporal lobe, extends from the anterior wall of the middle ear to
the nasopharynx and connects the two structures. The main functions
of the ET include ventilation of the middle ear, clearance of
secretions and protection against direct sound transmission and
pathogenic microorganisms (22).
Environmental factors such as air pollution and meteorological
conditions can influence the physiological functions of ET and
contribute to its dysfunction. For example, exposure to air
pollutants can lead to oxidative stress, inflammation and immune
response changes, which may impair the ability of the ET to
maintain middle ear pressure and clear secretions, creating
conditions conducive to infection and inflammation. This
dysfunction eventually leads to otological symptoms (23). Therefore, understanding the
impact of environmental factors on the physiological systems of the
ear and nose is crucial for improving the prevention and management
of related diseases such as OM and other middle ear disorders.
Tables I and II present the results of population
and laboratory studies on the impact of environmental factors on
the ear and nose physiological systems.
AR, an inflammatory disease, is an IgE-mediated type
1 hypersensitivity response to inhaled allergens characterized by
rhinorrhea, nasal congestion, sneezing and nasal itching (24). AR primarily consists of
sensitization and effector phases. In the sensitization phase, the
allergen induces a shift in the T helper (Th)1/Th2 balance towards
a predominant Th2 response, ultimately stimulating B cells to
produce IgE. In the effector phase, IgE mediates the degranulation
of basophils and mast cells, releasing various bioactive substances
that ultimately trigger allergic symptoms (4).
The effect of ambient air pollution on the incidence
of AR in humans has garnered widespread attention. In 2016, a
multicenter epidemiological study of 18 major cities in mainland
China found a significant increase in self-reported adult AR in
2011 compared with 2005 and reported that the prevalence of AR was
positively associated with short-term outdoor air pollution
exposure, especially the concentration of SO2 (25). A recent retrospective registry
study in the Guangdong-Hong Kong-Macao Greater Bay Area (China)
observed that each 10 μg/m3 increment in the
concentration of SO2, NO2, PM2.5,
PM10 and O3 corresponded to a significant
increase in the daily number of hospital outpatients with AR by
7.69, 2.43, 1.84, 1.55 and 0.34%, respectively (26). Two additional studies based on
the European Community Respiratory Health Survey and the
Epidemiological Study on the Genetics and Environment on Asthma
revealed that higher PM2.5 exposure is related to an
increased severity of AR. However, no association was found between
air pollution exposure and the incidence of AR (27,28). Possible reasons for this include
population heterogeneity and the duration of exposure. Therefore,
future studies should gather more comprehensive data and focus on
diverse ethnicities and different exposure durations.
Recently, the role of ambient air pollution on human
growth and development has attracted considerable attention. A
retrospective observational study surveyed 3,177 preschoolers in
five districts of Shanghai (China) and indicated that prenatal and
postnatal exposure to NO2 led to a higher prevalence of
AR in childhood in a single-pollutant model, an association that
remained significant in a multi-pollutant model (29). Similarly, another study that
adopted machine learning approaches based on a 14-year follow-up
birth cohort revealed that both the dose and duration of prenatal
exposure to NO2 were significant predictors of AR
incidence until adolescence (30). These studies suggest that younger
individuals require more attention when considering the impact of
air pollution on AR onset.
In addition to outdoor air pollution, the home
environment is also closely related to the occurrence of AR. Since
children spend most of their time indoors, the impact of indoor
pollution on the incidence of AR in children has become a growing
concern (31). In children,
whose immune systems are not fully developed, early exposure to
mold may increase the risk of immune responses to inhaled allergens
and irritants (32).
Specifically, a study on indoor mold exposure has shown that
children living in environments with high mold concentrations have
a significantly increased risk of developing AR (33). Several epidemiological studies
have also indicated that children raised in environments with
elevated mold levels are at a higher risk of AR, particularly in
those with prolonged mold exposure (34-36). Furthermore, early exposure to
mold may affect the development of the immune system, making
children more susceptible to allergic reactions, which is closely
related to the maturity of their immune systems (37). Additionally, exposure to ETS has
been positively correlated with an increased prevalence of AR in
children (38-40). Harmful substances in ETS,
especially inflammatory factors and oxidative stress reaction
products, can damage the upper respiratory tract barrier in
children, leading to the development of allergic diseases (40). A study has found that inhaling
environmental smoke not only increases the risk of AR in children
but may also exacerbate pre-existing allergic symptoms (41). Specifically, a questionnaire
study conducted in China showed that behaviors such as home
renovation, purchasing new furniture, cooking with natural gas and
burning mosquito coils were associated with an increased incidence
of AR in children, with a particularly significant impact observed
in girls (11). Moreover,
another study across seven cities in northeastern China found that
girls exposed to ETS had a higher likelihood of developing AR
compared with those not exposed (2.33 vs. 1.61%) (42). These findings suggest that the
effect of environmental smoke on AR in children involves a complex
mechanism that requires further investigation to improve the
understanding of its specific pathways.
VOCs, such as chemicals commonly found in paints and
furniture, have also been found to increase the risk of allergies
in children. These VOCs include harmful substances such as benzene,
toluene and xylene, and prolonged exposure may trigger immune
responses in children, leading to the development of AR (43). This is particularly true during
home renovation or when purchasing new furniture, as the
concentration of VOCs significantly increases, thereby raising the
risk of children being exposed to these chemicals (44). The impact of these household
exposure sources on the health of children may be related to the
incomplete development of their immune and respiratory systems.
Children's immune systems are more sensitive compared with adults,
making them more susceptible to exposure to indoor pollutants,
which increases the likelihood of developing allergic diseases.
Particularly in indoor environments, household pollution sources
such as mold, ETS and VOCs have a more notable impact on children's
health, as they spend a substantial amount of time indoors and are
thus exposed to these pollutants more frequently. Therefore,
improving the home environment and controlling air quality are
crucial for the prevention of allergic diseases in children.
PM from urban air pollution consists primarily of a
mixture of carbonaceous cores, organic compounds and metallic
compounds and is considered to affect AR through three major
biological pathways: Allergy, oxidative stress and inflammation
(45,46). PM can enhance allergen
sensitization and induce a local inflammatory response in the nasal
passages (47). Bowatte et
al (48) demonstrated that
early childhood exposure to traffic-related PM enhances allergic
sensitization and the degree of this association increases with
increasing age. Castañeda et al (49) found that PM possesses
adjuvant-like properties and can synergize with allergens to
promote the allergic inflammatory response. A recent experimental
study in an ovalbumin (OVA)-induced combined AR and asthma syndrome
mouse model demonstrated that exposure to PM2.5 may
increase the levels of GATA binding protein 3, retinoic acid
receptor-related orphan receptor γ, IL-4, IL-5, IL-13 and IL-17 and
decrease the production of Th1-associated cytokines, IL-12 and
IFN-γ, in nasal lavage fluid by activating the nuclear factor κβ
signaling pathway, thereby exacerbating nasal inflammatory response
(7). Notably, a growing body of
research has shown that PM can also induce inflammatory cell
infiltration in the nasal cavity, independent of allergens
(47,50,51). A previous in vitro study
suggested that urban PM (UPM) alone stimulates the generation of
Th2, Th1 and Th17 effector phenotypes as a source of antigens.
Moreover, the study observed a decrease in allergen-specific memory
T cell cytokine responses in dendritic cells (DCs) loaded with both
the UPM and house dust mites compared with DCs loaded with UPM
alone (52). These findings
reveal the complex mechanisms of PM in AR development and imply
that future studies should consider other coexisting factors.
In addition to air pollution, meteorological factors
also affect the number of patients with AR. For instance a
low-latitude multi-city study in China revealed that low
temperatures, low humidity and high wind speeds can lead to
increased outpatient visits for AR. Notably, in a stratified
analysis, the study found that adolescents and younger adults were
more sensitive to low humidity than children and older adults
(26). Similarly, another
retrospective study in an area with a humid subtropical monsoon
climate found an increased incidence of AR during childhood for
children exposed to low RH, wind speed and mean air pressure
(62). However, the results of
investigations on the effects of RH on AR are inconsistent. For
instance, a hospital-based study in Beijing (China) reported more
outpatient visits for AR at higher RH levels (16). A study focusing on classroom
humidity level in ten North Carolina schools showed that both high
RH (>50%) and low RH (<30%) increased the risk of allergic
diseases (63). These
inconsistencies may arise from various factors, such as differences
in study design, regional allergen distribution, environmental
confounders and different biological mechanisms by which high and
low RH may affect AR.
Environmental pollution and meteorological factors
may play a role in the development of AR. High temperature, low
humidity and environmental pollution increase the risk of AR caused
by airborne pollen (64-66). A study by Wu et al
(16) indicated that low
temperatures and high RH enhanced the effects of air pollution on
AR. The study further revealed that each 10 mg/m3
increase in PM2.5 concentration led to an increase in AR
outpatient visits by 2.34% at low temperature and 1.82% at high
RH.
As aforementioned, high-humidity environments
facilitate the growth of allergens such as mold (67). Aspergillus fumigatus can
stimulate human basophils to express B-cell activating factor,
which promotes the proliferation and differentiation of B cells,
further inducing IgE production and intensifying the immune
response to allergens in patients with AR (68). Furthermore, both air humidity and
cloud cover thickness can affect the amount of ultraviolet B (UVB)
exposure received by an individual. Narrow-band UVB therapy, based
on UVB, has become a routine clinical treatment for AR (69). An in vivo study revealed
that rats treated with narrowband UVB exhibit significant
downregulation of H1R gene expression in the nasal mucosa (70). H1R is associated with allergic
diseases, and H1R antagonists have become the first-line treatment
for AR (71,72). Thus, the inhibitory effect of UVB
on AR may primarily result from modulation of H1R expression in the
nasal mucosa. High temperatures can also accelerate the spread and
range of pollen in the air. Pollen is a common allergen widely
studied for its effects on allergic diseases. Previous studies have
found that in the context of AR, pollen can induce DCs to secrete
immune regulatory factors, such as IL-5 and IL-13, thereby
enhancing the differentiation of Th2 cells (73-75). Additionally, IL-5, IL-4 and IL-13
promote B cell activation and IgE secretion, thereby exacerbating
the onset of AR (76).
Results from evidence-based medicine suggest that
low levels of vitamin D may increase susceptibility to AR (77). A meta-analysis revealed that
in vitro vitamin D supplementation alleviated the symptoms
of AR (78). Although direct
evidence is lacking, a study on allergic diseases found a positive
correlation between increased exposure to solar radiation and
decreased incidence of allergic diseases in children. Furthermore,
providing vitamin D supplements to mothers during pregnancy can
modify the association between meteorological exposure patterns and
allergen sensitization of children (79). Although few intervention studies
are available, it is reasonable to conclude from existing research
that a potential link between 'environmental factors-vitamin D-AR'
is possible and that supplementation of vitamin D could serve as a
potential preventive strategy for AR.
OM is a common infection in early childhood and is
particularly prevalent among children under the age of 3 years
(80). OM not only affects
children's hearing but may also be associated with hearing loss in
older adults. A study has shown that hearing loss is linked to
age-related cognitive decline and prolonged hearing problems may
exacerbate the health burden in the elderly (81). A major consequence of recurrent
OM is conductive hearing loss, which affects the development of
speech, language, balance and learning abilities, while imposing a
notable economic burden on healthcare systems (82,83).
Increasing evidence suggests that environmental
pollution plays a notable role in OM onset. A 2024 European
birth-cohort study observed a dose-response relationship between
prenatal and early-postnatal exposure to traffic-related air
pollution (such as NO2) and the risk of ear infections
(including OM) in infants (84).
Another retrospective cohort study conducted in Changsha (China)
revealed a correlation between prenatal exposure to SO2
and the occurrence of OM (85).
Additionally, the effect of PM on OM has attracted increasing
attention. A retrospective study based on data from the Korean
National Health Insurance Service indicated that exposure to
PM2.5/PM10 was associated with the incidence
of acute OM (AOM) in children under the age of 2 years, with every
10 μg/m3 increase in PM2.5
concentration corresponding to a 4.5% increase in the relative risk
of AOM (86). Notably, the study
found that PM2.5 and PM10 had the most
significant negative effects on children under 2 years, typically
occurring on the day of exposure. Besides, a combined
cross-sectional and retrospective cohort study in Changsha (China)
indicated that prenatal and postnatal exposure to PM increased the
lifetime risk of OM in childhood and further revealed a cumulative
effect of PM2.5 exposure during the 9 gestational months
and PM10 exposure during the early post-natal period on
OM development (87). Similarly,
a retrospective study using time-series analysis showed that
exposure to PM2.5 within 5 days led to an increase in
the incidence of AOM in children aged 0 to 3 years, this
association was more pronounced during the warm seasons and in
children with a history of upper respiratory infections (47).
Compared with outdoor air pollution, indoor air
pollution may have a greater impact on the incidence of OM,
particularly given that children spend most of their time indoors.
A recent national cross-sectional study on Australian children
found a positive correlation between indoor environmental factors
(such as the use of gas heating, reverse-cycle air conditioning and
pet ownership) and the lifetime risk of OM (88). Another retrospective cohort study
in China indicated that postnatal exposure to indoor renovations
(such as new furniture and redecoration) significantly increased
the lifetime risk of OM in preschool children; this association was
particularly pronounced in girls (85). ETS, which is a major indoor
pollutant, is a critical factor. It is estimated that ~40% of
children are exposed to ETS globally (89). Numerous studies have confirmed
that ETS is a significant risk factor for OM in children (90,91). Despite the growing body of
literature, data is lacking on the control of indoor pollutants.
Therefore, proper monitoring of indoor environmental pollution is
crucial for preventing OM in children.
Extensive research has been conducted on the
pathogenic mechanisms by which PM affects OM. Recent mechanistic
research indicates that fine PM influences the onset and
progression of OM through several biological pathways such as
inflammation, oxidative stress, mucin-gene upregulation and
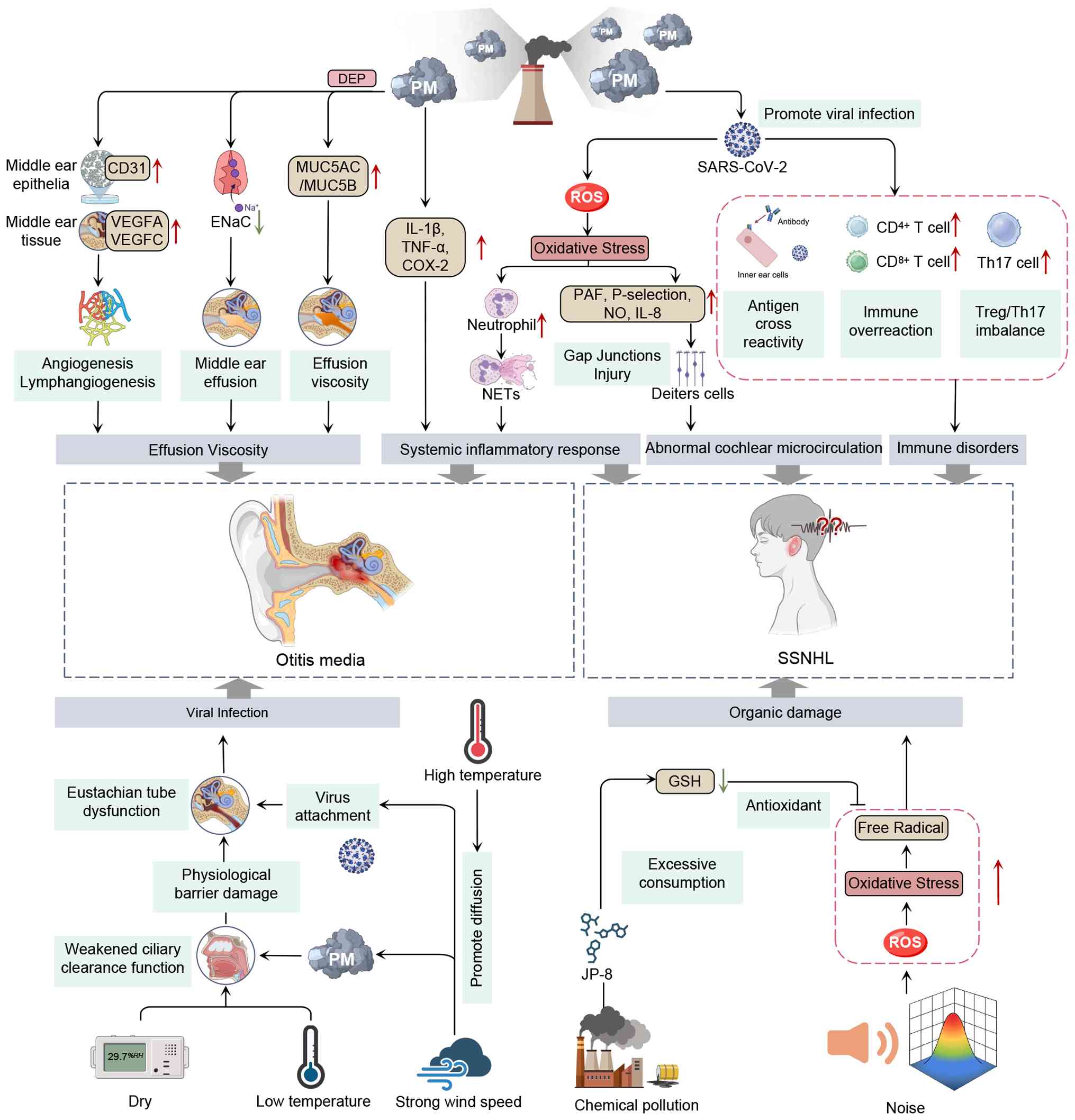
angiogenesis/lymphangiogenesis (92,93). For instance, ultrafine
combustion-derived particles (such as DEPs) constitute a
significant fraction of fine and ultrafine PM capable of traversing
the alveolar-capillary barrier and eliciting systemic inflammatory
responses. In vitro studies have shown that PM exposure
activates inflammatory cytokines (such as TNF-α and IL-1β) and
upregulates mucin genes such as MUC5AC and MUC5B in human middle
ear epithelial cell lines (HMEECs), resulting in viscous middle-ear
effusions that hinder fluid clearance and contribute to both acute
and chronic OM (94,95). Using in vivo animal models
(rats), it has been demonstrated that PM exposure leads to
goblet-cell hyperplasia in the ET and middle-ear mucosa, thickens
sub-epithelial layers, increases capillary density and
angiogenic/lymphangiogenic factor expression (such as VEGF, VEGFC
and CD31) and disrupts epithelial sodium channel (ENaC) expression,
which is essential for perimucosal fluid absorption and middle-ear
homeostasis, thereby suggesting that early ENaC-targeted
intervention may have therapeutic potential (5). In addition, a previous in
vitro study indicated that potential involvement of ROS could
be induced by PM in the progression of OM, as it was found that ROS
may promote mitochondrial dysfunction and inflammatory responses,
thereby leading to HMEEC apoptosis (96). However, clinical evidence
supporting the use of MUC5AC and MUC5B as diagnostic or prognostic
biomarkers in OM remains very limited. Although mucin expression
has been evaluated in respiratory and airway diseases (such as
chronic obstructive pulmonary disease and bronchitis) and shown to
be associated with disease progression, ear-specific, large-scale
clinical studies validating these biomarkers in patients with OM
are lacking (97). Consequently,
despite their mechanistic importance, these biomarkers have not yet
been adopted in routine clinical practice, mainly due to small
sample sizes, heterogeneity in design and the absence of
standardized assays. Large-scale, standardized clinical studies are
therefore required to assess whether inflammatory,
oxidative-stress, mucin- and angiogenesis/lymphangiogenesis-related
biomarkers (including MUC5AC and MUC5B) can serve as reliable
diagnostic or prognostic indicators in OM management.
In addition to air pollution, meteorological factors
are associated with the onset and progression of OM. A
retrospective observational study conducted in Cuneo (Italy)
revealed a distinct seasonal pattern in the incidence of AOM in
children, with more emergency visits (EVs) in winter and fewer
visits in summer. Moreover, this seasonal pattern was closely
related to upper respiratory tract infections (19). Similarly, a recent retrospective
study in Vienna found that EVs related to AOM were more frequent in
the winter. The study also observed that a 3-day period of cold
weather could increase the risk of AOM-related EVs within 1 day of
the temperature event (98).
Notably, this study and another study found that high atmospheric
pressure (AP), low wind speed and high humidity contributed to an
increased incidence of AOM (19,98). However, Vienna, which has a
temperate continental climate, typically experiences higher AP and
humidity during winter; therefore, further statistical analysis is
needed to establish causal relationships while controlling for
potential confounding effects among meteorological factors. A
retrospective cross-sectional study conducted in Shanghai confirmed
that RH has a significant impact on the incidence of AOM in
preschool children. The study reported that for every 1% increase
in RH, the number of AOM-related visits by preschool children
increased by 10.84% (99). Given
the increasing frequency of weather events due to climate change,
further research in other countries and regions is necessary.
In addition to air pollution, meteorological
factors, including low temperatures and dry air, can increase the
risk of AOM. Viral upper respiratory tract infections have been
proposed as a possible bridge linking meteorological factors and
AOM since AOM is often secondary to acute upper respiratory tract
infections (19,100). Cold and dry weather conditions
have been shown to reduce nasal mucociliary clearance and lead to
fluid loss in the nasal passages and ET, thereby weakening the
upper respiratory defense and heightening susceptibility to viral
infection (98). Moreover,
viruses can trigger nasopharyngeal inflammation and ET dysfunction,
which in turn facilitates further invasion by viruses and bacteria
into the middle ear, enhances epithelial cell bacterial adherence
and colonization and thus promotes the onset and development of AOM
(101). Notably, influenza
incidence also peaks in the cold, dry winter months, which provides
an additional explanation for the high winter incidence of AOM.
Notably, PM has been shown to compromise the barrier function of
nasal mucosal epithelial cells (100), thereby increasing the risk and
severity of upper respiratory tract infections, which inevitably
increases the incidence of AOM (102). Cold and dry air further amplify
this effect, underscoring the combined influence of air pollution
and meteorological factors on OM (103). Beyond temperature and humidity,
other meteorological elements such as AP and wind speed may also
contribute to OM. For example, high AP may exacerbate negative
middle ear pressure under ET dysfunction, facilitating pathogen
ingress, whereas strong winds can enhance the spread of PM and
viruses (104). Children, due
to their higher respiratory rate and anatomical features (notably,
a shorter and flatter ET), are particularly susceptible to
environmental pollutants and meteorological stresses. This
anatomical disadvantage also makes them more prone to developing
OM, especially during upper respiratory tract infections (105).
SSNHL is a subset of sudden hearing loss, which is
sensorineural in nature and typically defined as a drop of at least
30 decibels (dB) across at least three consecutive audiometric
frequencies occurring within a 72-h window (106). Although its incidence remains
relatively low (estimated at 5 to 27 cases per 100,000 individuals
annually), SSNHL remains a worrying otological emergency that can
lead to persistent hearing impairment and tinnitus, imposing
notable psychological distress and financial burden on patients
(107).
Despite considerable research, the complex etiology
of SSNHL remains unclear. Recent epidemiological evidence
implicates air pollution as a risk factor for SSNHL (108). Additionally, a cross-sectional
study in Taiwan observed an increased incidence of SSNHL from 2000
to 2015 and further revealed that SSNHL episodes occur more
frequently in Southern Taiwan, which possesses a higher mean
PM2.5 annual concentration compared with Norther Taiwan
(109). Additionally, a
case-control study in South Korea examining short-term exposure to
various air pollutants, including SO2, NO2,
O3, CO and PM10, found that SSNHL occurrence
is significantly associated with elevated NO2
concentrations (110). By
contrast, a long-term cohort study combining two large datasets
provided strong evidence that chronic exposure to PM2.5,
CO, NO and NO2 increases the risk of SSNHL (111). These contradictory findings may
be explained by the cumulative time-dependent effect of air
pollution on promoting SSNHL. Additionally, recent data indicate
that individuals >30 years are particularly sensitive to
NO2 exposure (112).
A large epidemiological study conducted in Taiwan revealed a
dose-dependent relationship between NO2 and SSNHL
(113). In addition to PM and
gaseous air pollutants, the roles of ETS and heavy-metal exposure
in SSNHL have also been investigated (114-116). Zinc, as an effective
supplement, has been shown to significantly aid in the hearing
recovery of patients with SSNHL when used in combination with other
treatments (117). However,
current research primarily focuses on a limited number of countries
and large-scale studies involving diverse ethnic groups and regions
are needed to further validate these effects.
The exact pathological mechanisms of SSNHL remain
unclear; however, possible mechanisms include viral infections,
immune-mediated cellular stress responses and vascular occlusion
(118,119). PM exposure has been shown to
increase susceptibility to viral infections by allowing viruses to
reach the inner ear via the bloodstream or other routes, ultimately
inducing cochleitis or neuritis (120). Recent experimental evidence
demonstrates that PM2.5 exposure significantly alters
airway and systemic immune responses, such as impaired innate
immunity, disrupted epithelial barriers and skewing of adaptive
immunity, thereby facilitating viral infection and propagation
(121). After viral invasion,
the adaptive immune system is activated, triggering processes
including antigenic cross-reactivity, T cell-mediated cellular
immunity and regulatory T cell (Treg)/Th17 imbalance in the inner
ear; these immune alterations exacerbate inner-ear damage and
contribute to the onset of SSNHL (122,123). Notably, large-scale
epidemiological data from the COVID-19 era show increased rates of
SSNHL and vestibular neuritis following systemic viral infections
(21).
The role of PM in neurological diseases has also
attracted much attention. Several studies have demonstrated that PM
can induce the expression of inflammatory mediators and the
generation of ROS and reactive nitrogen species (NOS) in the
central nervous system (CNS), resulting in neuroinflammation, lipid
denaturation, microglial dysfunction and even blood-brain barrier
dysfunction, which may be associated with SSNHL (8,124,125). Notably, under inflammatory
conditions, inducible NOS released by inflammatory cells may
produce higher and prolonged NO concentrations. Elevated NO levels
in the cochlea have been reported to accelerate the uncoupling of
gap junctions in Deiters' cells and delay synaptic transmission,
both of which can lead to hearing impairment or even deafness
(126,127). Endothelial dysfunction-driven
microthrombosis is increasingly being recognized as a key feature
of SSNHL (128). Exposure to
fine PM induces excessive oxidative stress, depletes cellular NO
bioavailability and promotes the upregulation of adhesion molecules
(such as P-selectin), platelet-activating factors, leukotriene B4
and cytokines, including IL-8, thereby driving endothelial injury
and microvascular compromise (129). Notably, blood supply to the
cochlea is provided by the labyrinthine artery and lacks collateral
circulation. Once thrombosis disrupts microcirculation, it leads to
edema, ischemia and hypoxia in the inner ear tissues; this damage
might be one reason for hearing loss. In addition to air pollution,
strong wind speeds and extreme heat cause viral transmission, which
may contribute to the pathophysiology of SSNHL (20) (Fig. 2).
Meteorological factors are considered to be involved
in multiple neurological diseases (130); however, the role of
meteorological conditions as risk factors for SSNHL remains
controversial. A retrospective study in Busan (Republic of Korea)
reported that increased mean wind speed, maximum wind speed and a
wider daily AP range were weakly associated with a higher incidence
of SSNHL. The study also found a weak negative association between
mean temperature and SSNHL admissions (17). However, a 2024 time-series study
from Hefei (China) examining the mean temperature (T-mean), diurnal
temperature range (DTR), AP and RH, found that a lower T-mean,
higher DTR and all levels of AP were significantly associated with
increased SSNHL admissions, whereas wind speed did not emerge as a
strong independent predictor (131). Similarly, another
hospital-based study observed that SSNHL episodes occurred more
frequently on or immediately after days with higher wind speeds
(20). However, several
investigations have failed to confirm a consistent correlation
between meteorological variables and SSNHL (132,133). Furthermore, the seasonal
pattern of SSNHL onset remains controversial; some authors have
observed a higher incidence in autumn (134,135), whereas others found no clear
seasonal imbalances (20,136).
These conflicting epidemiological findings do not support any
definitive conclusions. According to our analysis of the relevant
literature, several factors may have accounted for these results.
First, the duration of several studies was too short to evaluate
the influence of meteorological factors on SSNHL onset (131). Second, some studies had small
samples and some were limited to specific regions, making it easier
to draw conflicting conclusions (17). Finally, given the low prevalence
of SSNHL, common weather conditions do not allow for significant
impacts (20); therefore, it may
be more appropriate to focus on extreme weather conditions.
Although population studies have revealed
associations between various climatic factors and the incidence of
SSNHL, current research has yet to clearly elucidate the specific
molecular mechanisms by which climatic factors alter the incidence
of SSNHL. Notably, existing studies have suggested that climatic
factors significantly regulate the chemical sensory functions of
the ear at the molecular level (137,138). Therefore, analyzing and
exploring the impact of environmental factors on ear sensory
receptors will provide valuable insights for future research on the
relationship between environmental factors and ear disease.
In recent years, the complex interactions between
environmental pollution and climate factors have received
increasing attention (139-141). A 2020 study found significant
seasonal variations in air pollutants in Beijing. Specifically, the
concentrations of PM2.5, PM10,
SO2, NO2 and CO decreased in summer, whereas
O3 concentrations increased. Furthermore, the incidence
of nasal bleeding significantly correlated with these pollutant
indicators (P<0.05) (142).
Further research indicated that although air pollutants negatively
affect ear and nose health in other seasons, the concentrations of
PM10, NO2 and SO2 are positively
correlated with the number of ear and nose outpatient visits in
winter (143). Therefore,
analyzing the interaction between meteorological conditions and
environmental exposure can help uncover potential pathogenic
mechanisms and identify vulnerable populations. Additionally,
further research based on this perspective can provide important
theoretical support for the development of precise preventive
strategies and optimization of public health policies in the
context of climate change.
Climate change, due to rising temperatures, changes
in precipitation patterns and an increase in extreme weather
events, may exacerbate the generation and diffusion of air
pollutants. For example, higher temperatures can promote the
formation of O3 (144) and O3, as an air
pollutant, is associated with an increased incidence of OM in
children following exposure in pregnant women (145). In addition, climate change may
alter the diffusion pathways and concentrations of air pollutants
(146,147). Strong winds can rapidly spread
pollutants and alter their deposition patterns. These changes are
particularly significant in areas where urbanization is
accelerating. High-rise buildings and narrow streets often create
the 'street canyon effect', restricting air circulation and leading
to the accumulation of pollutants at localized points (148,149). High concentrations of PM and
volatile organic gases directly affect the human ear and nose,
increasing the risk of chemical sensory damage (150-152). At the same time, the
accumulated high concentrations of PM may also reduce the UVB
radiation that reaches the skin surface. UVB has been shown to
promote the synthesis of 25-hydroxyvitamin D [25(OH)D3]
in the human body, thereby protecting ear and nose chemical sensory
functions (153,154). Strong winds can extend the
range of viral transmission and exacerbate ear and nose diseases.
However, environmental pollution, particularly air pollution,
industrial emissions and traffic emissions, may affect the rate and
pattern of climate change by altering local climate systems. The
emission of greenhouse gases, such as carbon dioxide, nitrogen
oxides and sulfur oxides is one of the main drivers of global
warming (155). The effects of
temperature on ear and nose diseases have been reported previously
(20,136). Moreover, rising temperatures
can exacerbate fungal growth and promote the spread of allergens
such as pollen, increasing the risk of allergic diseases. An
increased airborne concentration of allergens contributes
significantly to the incidence of nasal diseases (32).
Atmospheric pollution and meteorological
variability are emerging global environmental issues that involve
complex interactions between specific substances, chemicals and
pathogens. Given that olfactory organs are directly exposed to the
external environment, various environmental xenobiotics, including
chemicals, dust and viruses, constitute important risk factors.
Consequently, the olfactory system is a primary biological target
of air-pollution exposure (156). A key question is whether
pollution exacerbates olfactory decline independently or
accelerates age-related degenerative processes. Existing evidence
suggests that pollutants may independently affect olfactory
function by triggering chronic inflammation, oxidative stress and
other mechanisms, and this effect is independent of the aging
process (157). However, a
study has indicated that the effects of pollution may be more
pronounced due to accelerated age-related degeneration,
particularly in older populations (158).
Although there are few direct reports on the
association between meteorological factors and olfactory
dysfunction (159,160), it is not difficult to determine
whether meteorological factors, including temperature, sunlight,
wind speed, heavy rainfall and humidity, play an important role in
olfactory dysfunction. AR can cause swelling of the nasal mucosa,
chronic inflammation of the nasal cavity as well as damage to the
olfactory bulb and the olfactory nerve, which is an important
bridge between meteorological conditions and olfactory dysfunction
(161,162). The previous section elucidated
the close connection between meteorological factors and AR. In
addition, Shin et al (153) found that serum
25[OH]D3 deficiency is significantly associated with
olfactory dysfunction in children and that this association is
independent of olfactory dysfunction caused by AR. The mechanism
can be explained by the following two aspects: First, receptors for
25[OH]D3 are present in nerve cells and its deficiency
can cause olfactory nerve dysfunction, resulting in olfactory
decline (154). Second,
25[OH]D3 reduces inflammation by downregulating the
production of the Th17 cell signature, cytokine IL-17, and
upregulating the number of IL-10+ and Foxp3+
Treg cells (163,164). Serum 25[OH]D3
deficiency may contribute to chronic inflammation in the olfactory
neuroepithelium and, thus, olfactory dysfunction. As a precursor of
25[OH]D3, vitamin D is primarily derived from sunlight
(165) (Fig. 1).
Air pollution is categorized into physical
(including PM and nanoparticles) and chemical (including DEPs,
heavy metals, pesticides and herbicides) pollutants. Andersson
et al (156) found a
statistically significant association between long-term exposure to
PM2.5 and olfactory identification. When the established
model was corrected for age, the association was stronger in older
populations. Therefore, the interaction between PM2.5
and age significantly affects olfactory discrimination. However,
the study did not find an association between short-term
PM2.5 exposure and olfactory function. The cumulative
effects of air pollutants on the olfactory system may result in
olfactory loss during aging even at relatively low levels of
pollution exposure. Numerous studies have shown that long-term
exposure to PM10, SO2, NO2, CO,
cadmium and ammonia can induce and exacerbate inflammatory
responses in the nasal cavity, leading to olfactory dysfunction
(166,167). A study by Bernal-Meléndez et
al (168) showed that
repeated exposure to diesel engine exhaust fumes during gestation
not only affects fetal olfactory tissues and systems but also
influences monoaminergic neurotransmission in the fetal olfactory
bulb, leading to altered olfactory behavior at birth. During
breathing, due to reduced filtration and clearance by the nasal
mucosa, poorer mucosal cilia transport rates prolong PM retention
time, which in turn may increase the risk of carcinogenic effects
(169). Airborne PM and other
pollutants not only contact the olfactory epithelium (OE) and bind
to olfactory neurons but can also pass through various abundantly
expressed transporters and reach the olfactory bulb via the
olfactory nerve. When chemical pollutants attach to ultrafine
particles, the resulting complexes are transported across cell
membranes via endocytosis. At the same time, the binding of
PM2.5 to chemical pollutants may put additional stress
on the physical structure of the OE, allowing the mixture to reach
the CNS through the paracellular pathway as some viruses do, and
this binding may also affect the transformation of chemicals in the
body (170).
During the COVID-19 pandemic, several studies
emphasized the integral role of chemosensory systems in the
airborne airways of viruses entering the human body, a pathway that
may also be exploited by environmental contaminants (171,172). SARS-CoV-2 virulence may be
altered in contaminated areas (169). COVID-19 elicits strong systemic
and localized immune responses, leading to the release of cytokines
and other inflammatory molecules. These molecules can cross the
blood-brain barrier and affect the olfactory system, directly or
indirectly causing and exacerbating inflammation and damage to the
olfactory neurons (173). A
cohort study conducted among young adults in Sweden indicated that
long-term exposure to air pollution in living environments was
associated with an increased risk of COVID-19 following SARS-CoV-2
infection. The association between exposure to PM2.5 and
COVID-19 was significantly stronger than that of PM10,
BC and nitrogen oxides (174).
A recent study has shown that ~80% of patients with long-term
COVID-19 still experience olfactory dysfunction 2 years after
infection, with some patients continuing to experience complete
anosmia, whereas those without apparent anosmia still commonly
report a reduction in olfactory function (175). These symptoms are closely
related to changes in the angiotensin-converting enzyme 2 (ACE2)
receptor and oxidative stress, supporting the possibility that air
pollution and SARS-CoV-2 infection synergistically promote
long-term ear and nose sensory dysfunctions (176). ACE2 is widely expressed in the
upper respiratory tract and olfactory epithelial cells and acts as
the primary receptor for SARS-CoV-2. ACE2 facilitates the entry of
the virus and the infection of epithelial cells by interacting with
viral spike proteins (177).
Population studies have found that individual differences in the
response to SARS-CoV-2 are closely related to molecular differences
in ACE2 expression (172,178). Furthermore, air pollutants,
especially fine PM2.5, upregulate ACE2 expression
through oxidative stress pathways, significantly increasing the
susceptibility of the host to SARS-CoV-2 infection, causing
epithelial cell damage and further exacerbating olfactory and
gustatory dysfunction (171,179,180). Air pollution may alter the
distribution of ACE2 receptors in the olfactory system, making it a
more vulnerable target for viral transmission, thereby increasing
the risk of olfactory disorders (181). Oxidative stress plays a crucial
role in the pathological process of SARS-CoV-2 infection, leading
to the production of ROS, which directly damages cells and
activates inflammatory responses (182). Air pollutants, such as
PM2.5, further damage the OE and neurons by inducing
oxidative stress, worsening the recovery of olfactory function.
Therefore, oxidative stress is considered a key mechanism linking
environmental pollution and the sensory dysfunction of the ear and
nose caused by SARS-CoV-2. Susceptible factors (including genetic,
epigenetic and immune factors), the combined effects of past and
current air pollution exposure and SARS-CoV-2 infection may lead to
long-term COVID symptoms. Long-term olfactory training can help
patients recover their sense of smell to pre-infection levels
(173).
As a serious public health problem, hearing loss
has resulted in growing disease burden, especially among the older
population (183,184). Air pollutants and extreme
meteorological factors can cause peripheral and central auditory
dysfunction and appear to be associated with noise exposure and
viral infections (such as SARS-CoV-2), which increase
susceptibility to hearing loss through superimposed or synergistic
mechanisms.
The potential synergistic effects of noise and air
pollution on hearing function have been proposed in multiple
studies, and quantitative tests have been conducted to analyze this
interaction. For example, a prospective cohort study of 1,179
oilfield workers found that both air pollution and noise exposure
significantly increased the risk of occupational hearing loss, both
independently and in combination (185). Studies suggest that noise and
air pollution may jointly affect hearing function through common
biological pathways, such as oxidative stress, inflammation and
endothelial dysfunction (186,187). For instance, PM2.5,
which induces oxidative stress by generating ROS, may cause
endothelial dysfunction, which affects the cochlear blood supply
and increases the risk of hearing loss (188).
The global climate crisis is worsening and the
frequency and severity of extreme meteorological conditions are
increasing. The relationship between meteorological conditions and
auditory health has become a major epidemiological concern;
however, a study has argued that there is no direct relationship
(189). We hypothesize that the
direct mechanism of auditory impairment may not be meteorological
factors, but rather interaction with ototoxic environmental
confounders (including environmental pollutants, viruses and
bacteria) or exacerbation of pre-existing otological diseases.
Specifically, high wind speeds facilitate viral transmission,
inducing a systemic immune response that causes SSNHL and central
auditory dysfunction. Moreover, inflammation of the upper
respiratory tract due to infection can lead to ET dysfunction,
which in turn can cause inflammatory lesions in the otological
region. As the disease progresses, the tympanic membrane mobility
decreases, ultimately leading to conductive hearing loss (190). Under high APs, bacteria and
viruses can diffuse further and exacerbate hearing loss. Future
studies may need to collect more data, incorporate factors such as
upper respiratory infections and explore interactions between
multiple weather factors (Fig.
2).
Hydrocarbon fuels contain long-chain and
short-chain aromatic and aliphatic hydrocarbons. Epidemiological
evidence and animal studies have demonstrated that exposure to jet
fuel causes lethality in presynaptic sensory cells, which in turn
exacerbates noise-induced hearing loss (NIHL) in air force
personnel. This fuel has recently been shown to increase
susceptibility to NIHL. For example, the levels of the distortion
product otoacoustic emission, a measure of non-linear transduction
from outer hair cells, have been shown to be reduced after exposure
to Jet Propellant 8 (JP-8), with a no-damage level of 97 dB (no
hearing loss or cell death) noise (191). In addition, cytocochleograms
plotting the percentage of sensory cell death revealed a
significant loss of outer hair cells. Compound action potentials
recorded from the peripheral auditory nerve showed that loss of
outer hair cells was responsible for permanent hearing loss. A
possible mechanism is that JP-8 depletes glutathione both in
vitro and in vivo and the depletion of this important
antioxidant increases the likelihood of noise-induced oxidative
stress (192). Notably, as
reported in a study by Guthrie et al (193) that investigated the effect of
JP-8 on the development of hearing loss, this fuel might cause
central auditory processing dysfunction (CAPD) in normal-hearing
Long Evans rats without detectable sensory cell damage, suggesting
that CAPD might exist in the absence of hearing loss (194). Therefore, it is recommended
that individuals at risk of hydrocarbon fuel exposure undergo an
audiological assessment, which should include a conventional
audiological assessment in addition to neurophysiological and/or
psychoacoustic assessments of the central auditory function.
Notably, in a study by Fechter et al (195), male rats appeared to be more
susceptible to enhanced NIHL from JP-8 exposure. The enhanced
sensitivity of male rats to JP-8 and noise may reflect true sex
differences in noise susceptibility. However, this might also
reflect toxicodynamic factors related to body lipid storage, rather
than sexual dimorphism, as male and female F344 rats exhibit
significantly different weight gain patterns. Differences in body
fat levels between sexes may lead to greater JP-8 fuel stores in
male rats, thereby prolonging the duration of the elevated JP-8
body burden (196).
COVID-19 is thought to cause CNS and peripheral
nervous system dysfunction. Growing evidence suggests that patients
infected with SARS-CoV-2 are at an increased risk for hearing
impairment, particularly SSNHL (197,198). A study based on data from
visits to tertiary hospitals in China reported an increase in the
incidence of SSNHL and tinnitus during the COVID-19 pandemic
(3). Another hospital-based
study in Eastern India included 452 patients with COVID-19, of whom
28 developed hearing impairment and 24 developed SSNHL (199). Possible mechanisms of hearing
loss due to SARS-CoV-2 infection include induction of cochlear
microcirculatory dysfunction (200,201), inflammation of the nervous
system (including the CNS, peripheral nervous system and auditory
centers in the temporal lobe) (202,203) and activation of systemic immune
responses due to viral infection (204,205). Furthermore, SARS-CoV-2 causes
organ ischemia, tissue inflammation and a hypercoagulable state by
inducing endothelial cell inflammation, which may lead to cochlear
microcirculatory dysfunction and hearing loss (206). Degen et al (207) observed a cochlear inflammatory
response on magnetic resonance imaging in patients with hearing
impairment complicated by COVID-19 and hypothesized that hearing
loss was due to the spread of meningitis to the cochlea as a result
of the SARS-CoV-2 infection. In addition, hearing loss increases
the risk of depression and anxiety in infected patients (208,209). Early screening of patients with
SARS-CoV-2 infection plays a key role in promoting hearing recovery
and psychological well-being (207). As aforementioned, pollutants
and meteorological conditions increase the risk of OM and SSNHL,
thereby causing hearing loss. PM enhances human susceptibility to
the virus by damaging the respiratory mucosa and may carry viral
particles in conjunction with strong wind speeds to facilitate
transmission of SARS-CoV-2. Epidemiological evidence demonstrates
that exposure to environmental pollutants increases the incidence
of, and mortality from, COVID-19 (210,211). In conclusion, we consider that
environmental pollution and meteorological factors may be
associated with SARS-CoV-2 infection through synergistic and/or
superimposed effects that cause hearing loss (Fig. 2).
Although the present review provides an in-depth
exploration of the relationship between environmental factors,
SARS-CoV-2 infection and ear and nose diseases, several limitations
remain in the current research, particularly regarding population
heterogeneity and exposure assessment methods. Individuals of
different ethnicities, ages, sexes and socioeconomic backgrounds
may have different sensitivities to environmental pollution, which
affects the generalizability of the research findings. Moreover, a
number of studies rely on environmental air quality monitoring data
or self-reported exposure, which may not accurately reflect the
actual exposure level of individuals, particularly when pollution
sources are complex or exposure varies over time and space.
Therefore, future studies should adopt more precise exposure
assessment methods, such as personal air quality monitoring and
biomarker analysis, to improve the accuracy of assessments and
account for the effects of long-term exposure. This will help
provide an improved understanding of the relationship between
environmental factors and diseases.
To the best of knowledge, the present review
provides the first systematic comprehensive examination of the
interactions among air pollution, meteorological factors and
SARS-CoV-2 in ear and nose diseases, filling a critical gap in the
literature. We propose that chemosensory dysfunction (olfactory and
auditory impairments) may serve as an early indicator of
environmental neurotoxicity, offering a novel perspective on how
environmental pollution can affect the nervous system. The present
review highlights the complex roles of environmental factors in
diseases such as RH, OM and SSNHL, emphasizing the need for further
research on these interactions. Despite existing research, several
unknowns remain that warrant future studies focusing on: i)
Longitudinal research, to explore the cumulative effects of
long-term exposure to air pollution and meteorological changes; ii)
molecular mechanisms, to elucidate how these factors induce
diseases through immune and inflammatory pathways; and iii)
mitigation strategies, to reduce the impact of these environmental
factors through environmental management, personal protective
measures and policy interventions.
Not applicable.
YCZ and PTZ made significant intellectual and
technical contributions, including conceptualizing the study,
conducting the initial literature review, refining the core content
and designing the manuscript structure. In addition, YCZ and PTZ
created the figures and formatted the references using appropriate
software. LZ, ZHX, WJZ and MF contributed to data curation and
writing the original draft. YXH and YCL contributed to organizing
tables, conducting preliminary literature reviews and investigating
of the innovative positioning of this manuscript. YHL contributed
to conception, supervision and writing/revising the manuscript.
Data authentication is not applicable. All authors read and
approved the final version of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The study was supported by funding National Natural Science
Foundation (grant no. 82171127).
|
1
|
Wu S, Yu Y, Zheng Z and Cheng Q: High
mobility group box-1: A potential therapeutic target for allergic
rhinitis. Eur J Med Res. 28:4302023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu ZB, Zhu WY, Fei B and Lv LY: Effects
of oral steroids combined with postauricular steroid injection on
patients with sudden sensorineural hearing loss with delaying
intervention: A retrospective analysis. Niger J Clin Pract.
26:760–764. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jin L, Fan K, Tan S, Liu S, Wang Y and Yu
S: Analysis of the characteristics of outpatient and emergency
diseases in the department of otolaryngology during the 'COVID-19'
pandemic. Sci Prog. 104:3685042110363192021. View Article : Google Scholar
|
|
4
|
Zhu Y, Yu J, Zhu X, Yuan J, Dai M, Bao Y
and Jiang Y: Experimental observation of the effect of
immunotherapy on CD4+ T cells and Th1/Th2 cytokines in mice with
allergic rhinitis. Sci Rep. 13:52732023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park MK, Chae SW, Kim HB, Cho JG and Song
JJ: Middle ear inflammation of rat induced by urban particles. Int
J Pediatr Otorhinolaryngol. 78:2193–2197. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang S, Zhang C, Cai W, Bai Y, Callaghan
M, Chang N, Chen B, Chen H, Cheng L, Dai H, et al: The 2023 China
report of the lancet countdown on health and climate change: Taking
stock for a thriving future. Lancet Public Health. 8:e978–e995.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Piao CH, Fan Y, Nguyen TV, Song CH, Kim HT
and Chai OH: PM2.5 exposure regulates Th1/Th2/Th17 cytokine
production through NF-κB signaling in combined allergic rhinitis
and asthma syndrome. Int Immunopharmacol. 119:1102542023.
View Article : Google Scholar
|
|
8
|
Lamorie-Foote K, Liu Q, Shkirkova K, Ge B,
He S, Morgan TE and Mack WJ, Sioutas C, Finch CE and Mack WJ:
Particulate matter exposure and chronic cerebral hypoperfusion
promote oxidative stress and induce neuronal and oligodendrocyte
apoptosis in male mice. J Neurosci Res. 101:384–402. 2023.
View Article : Google Scholar :
|
|
9
|
Fuller R, Landrigan PJ, Balakrishnan K,
Bathan G, Bose-O'Reilly S, Brauer M, Caravanos J, Chiles T, Cohen
A, Corra L, et al: Pollution and health: A progress update. Lancet
Planet Health. 6:e535–e547. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Verhoeven JI, Allach Y, Vaartjes ICH,
Klijn CJM and de Leeuw FE: Ambient air pollution and the risk of
ischaemic and haemorrhagic stroke. Lancet Planet Health.
5:e542–e552. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang J, Zhang Y, Li B, Zhao Z, Huang C,
Zhang X, Deng Q, Lu C, Qian H, Yang X, et al: Asthma and allergic
rhinitis among young parents in China in relation to outdoor air
pollution, climate and home environment. Sci Total Environ.
751:1417342021. View Article : Google Scholar
|
|
12
|
Yang J, Seo JH, Jeong NN and Sohn JR:
Effects of legal regulation on indoor air quality in facilities for
sensitive populations-A field study in Seoul, Korea. Environ
Manage. 64:344–352. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang J, Kim YK, Kang TS, Jee YK and Kim
YY: Importance of indoor dust biological ultrafine particles in the
pathogenesis of chronic inflammatory lung diseases. Environ Health
Toxicol. 32:e20170212017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yun H, Seo JH, Kim YG and Yang J: Impact
of scented candle use on indoor air quality and airborne
microbiome. Sci Rep. 15:101812025. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang J, Kim JS, Jeon HW, Lee J and Seo JH:
Integrated culture-based and metagenomic profiling of airborne and
surface-deposited bacterial communities in residential
environments. Environ Pollut. 382:1267032025. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu R, Guo Q, Fan J, Guo C, Wang G, Wu W
and Xu J: Association between air pollution and outpatient visits
for allergic rhinitis: Effect modification by ambient temperature
and relative humidity. Sci Total Environ. 821:1529602022.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee HM, Kim MS, Kim DJ, Uhm TW, Yi SB, Han
JH and Lee IW: Effects of meteorological factor and air pollution
on sudden sensorineural hearing loss using the health claims data
in Busan, Republic of Korea. Am J Otolaryngol. 40:393–399. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu K, Fuchs H, Hofzumahaus A, Tan Z, Wang
H, Zhang L, Schmitt SH, Rohrer F, Bohn B, Broch S, et al: Fast
photochemistry in wintertime haze: Consequences for pollution
mitigation strategies. Environ Sci Technol. 53:10676–10684. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gestro M, Condemi V, Bardi L, Fantino C
and Solimene U: Meteorological factors, air pollutants, and
emergency department visits for otitis media: a time series study.
Int J Biometeorol. 61:1749–1764. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Seo JH, Jeon EJ, Park YS, Kim J, Chang KH
and Yeo SW: Meteorological conditions related to the onset of
idiopathic sudden sensorineural hearing loss. Yonsei Med J.
55:1678–1682. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ko HY and Kim MH: A nationwide
population-based study for audio-vestibular disorders following
COVID-19 infection. Audiol Neurootol. 30:245–251. 2025. View Article : Google Scholar
|
|
22
|
Janzen-Senn I, Schuon RA, Tavassol F,
Lenarz T and Paasche G: Dimensions and position of the eustachian
tube in humans. PLoS One. 15:e02326552020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Juszczak H, Aubin-Pouliot A, Sharon JD and
Loftus PA: Sinonasal risk factors for eustachian tube dysfunction:
Cross-sectional findings from NHANES 2011-2012. Int Forum Allergy
Rhinol. 9:466–472. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liva GA, Karatzanis AD and Prokopakis EP:
Review of rhinitis: Classification, types, pathophysiology. J Clin
Med. 10:31832021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang XD, Zheng M, Lou HF, Wang CS, Zhang
Y, Bo MY, Ge SQ, Zhang N, Zhang L and Bachert C: An increased
prevalence of self-reported allergic rhinitis in major Chinese
cities from 2005 to 2011. Allergy. 71:1170–1180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luo X, Hong H, Lu Y, Deng S, Wu N, Zhou Q,
Chen Z, Feng P, Zhou Y, Tao J, et al: Impact of air pollution and
meteorological factors on incidence of allergic rhinitis: A
low-latitude multi-city study in China. Allergy. 78:1656–1659.
2023. View Article : Google Scholar
|
|
27
|
Burte E, Leynaert B, Marcon A, Bousquet J,
Benmerad M, Bono R, Carsin AE, de Hoogh K, Forsberg B, Gormand F,
et al: Long-term air pollution exposure is associated with
increased severity of rhinitis in 2 European cohorts. J Allergy
Clin Immunol. 145:834–842.e6. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Burte E, Leynaert B, Bono R, Brunekreef B,
Bousquet J, Carsin AE, De Hoogh K, Forsberg B, Gormand F, Heinrich
J, et al: Association between air pollution and rhinitis incidence
in two European cohorts. Environ Int. 115:257–266. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu W, Huang C, Cai J, Fu Q, Zou Z, Sun C
and Zhang J: Prenatal and postnatal exposures to ambient air
pollutants associated with allergies and airway diseases in
childhood: A retrospective observational study. Environ Int.
142:1058532020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang Y, Wen HJ, Guo YL, Wei TY, Wang WC,
Tsai SF, Tseng VS and Wang SJ: Prenatal exposure to air pollutants
and childhood atopic dermatitis and allergic rhinitis adopting
machine learning approaches: 14-Year follow-up birth cohort study.
Sci Total Environ. 777:1459822021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bornehag CG, Sundell J and Sigsgaard T:
Dampness in buildings and health (DBH): Report from an ongoing
epidemiological investigation on the association between indoor
environmental factors and health effects among children in Sweden.
Indoor Air. 14(Suppl 7): S59–S66. 2004. View Article : Google Scholar
|
|
32
|
Kidon MI, See Y, Goh A, Chay OM and
Balakrishnan A: Aeroallergen sensitization in pediatric allergic
rhinitis in Singapore: Is air-conditioning a factor in the tropics?
Pediatr Allergy Immunol. 15:340–343. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li S, Cao S, Duan X, Zhang Y, Gong J, Xu
X, Guo Q, Meng X and Zhang J: Household mold exposure in
association with childhood asthma and allergic rhinitis in a
northwestern city and a southern city of China. J Thorac Dis.
14:1725–1737. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Thacher JD, Gruzieva O, Pershagen G, Melén
E, Lorentzen JC, Kull I and Bergström A: Mold and dampness exposure
and allergic outcomes from birth to adolescence: Data from the
BAMSE cohort. Allergy. 72:967–974. 2017. View Article : Google Scholar
|
|
35
|
Weber A, Fuchs N, Kutzora S, Hendrowarsito
L, Nennstiel-Ratzel U, von Mutius E, Herr C and Heinze S; GME Study
Group: Exploring the associations between parent-reported
biological indoor environment and airway-related symptoms and
allergic diseases in children. Int J Hyg Environ Health.
220:1333–1339. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen HI, Lin YT, Jung CR and Hwang BF:
Interaction between catalase gene promoter polymorphisms and indoor
environmental exposure in childhood allergic rhinitis.
Epidemiology. 28(Suppl 1): S126–S132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pirker AL and Vogl T: Development of
systemic and mucosal immune responses against gut microbiota in
early life and implications for the onset of allergies. Front
Allergy. 5:14393032024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shargorodsky J, Garcia-Esquinas E,
Navas-Acien A and Lin SY: Allergic sensitization, rhinitis, and
tobacco smoke exposure in U.S. children and adolescents. Int Forum
Allergy Rhinol. 5:471–476. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang HJ: Impact of perinatal environmental
tobacco smoke on the development of childhood allergic diseases.
Korean J Pediatr. 59:319–327. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yigit E, Yuksel H, Ulman C and Yilmaz O:
Nasal effects of environmental tobacco smoke exposure in children
with allergic rhinitis. Respir Med. 236:1078862025. View Article : Google Scholar
|
|
41
|
Choi MJ, Park J and Kim SY: Association
between secondhand smoke and allergic diseases in Korean
adolescents: Cross-sectional analysis of the 2019 KYRBS. Healthcare
(Basel). 11:8512023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dong GH, Qian ZM, Wang J, Trevathan E, Ma
W, Chen W, Xaverius PK, Buckner-Petty S, Ray A, Liu MM, et al:
Residential characteristics and household risk factors and
respiratory diseases in Chinese women: The seven northeast cities
(SNEC) study. Sci Total Environ. 463-464:389–394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhou L, Huang C, Lu R, Wang X, Sun C and
Zou Z: Volatile organic compounds in children's bedrooms, Shanghai,
China: Sources and influential factors. Atmos Pollut Res.
14:1017512023. View Article : Google Scholar
|
|
44
|
Ridolo E, Pederzani A, Barone A, Ottoni M,
Crivellaro M and Nicoletta F: Indoor air pollution and atopic
diseases: A comprehensive framework. Explor Asthma Allergy.
2:170–185. 2024. View Article : Google Scholar
|
|
45
|
Bell ML, Dominici F, Ebisu K, Zeger SL and
Samet JM: Spatial and temporal variation in PM(2.5) chemical
composition in the United States for health effects studies.
Environ Health Perspect. 115:989–995. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rumelhard M, Ramgolam K, Hamel R, Marano F
and Baeza-Squiban A: Expression and role of EGFR ligands induced in
airway cells by PM2.5 and its components. Eur Respir J.
30:1064–1073. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Joubert IA, Geppert M, Johnson L,
Mills-Goodlet R, Michelini S, Korotchenko E, Duschl A, Weiss R,
Horejs-Höck J and Himly M: Mechanisms of particles in
sensitization, effector function and therapy of allergic disease.
Front Immunol. 11:13342020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bowatte G, Lodge C, Lowe AJ, Erbas B,
Perret J, Abramson MJ, Matheson M and Dharmage SC: The influence of
childhood traffic-related air pollution exposure on asthma, allergy
and sensitization: A systematic review and a meta-analysis of birth
cohort studies. Allergy. 70:245–256. 2015. View Article : Google Scholar
|
|
49
|
Castañeda AR, Bein KJ, Smiley-Jewell S and
Pinkerton KE: Fine particulate matter (PM2.5) enhances
allergic sensitization in BALB/c mice. J Toxicol Environ Health A.
80:197–207. 2017. View Article : Google Scholar
|
|
50
|
Lubitz S, Schober W, Pusch G, Effner R,
Klopp N, Behrendt H and Buters JT: Polycyclic aromatic hydrocarbons
from diesel emissions exert proallergic effects in birch pollen
allergic individuals through enhanced mediator release from
basophils. Environ Toxicol. 25:188–197. 2010. View Article : Google Scholar
|
|
51
|
Lorenz G, Ernst S and Probst J:
Significance of ultrasound study in accident surgery. Aktuelle
Traumatol. 15:187–194. 1985.In German. PubMed/NCBI
|
|
52
|
Matthews NC, Pfeffer PE, Mann EH, Kelly
FJ, Corrigan CJ, Hawrylowicz CM and Lee TH: urban particulate
matter-activated human dendritic cells induce the expansion of
potent inflammatory Th1, Th2, and Th17 effector cells. Am J Respir
Cell Mol Biol. 54:250–262. 2016. View Article : Google Scholar :
|
|
53
|
Xia M, Harb H, Saffari A, Sioutas C and
Chatila TA: A Jagged 1-Notch 4 molecular switch mediates airway
inflammation induced by ultrafine particles. J Allergy Clin
Immunol. 142:1243–1256.e17. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Xia M, Viera-Hutchins L, Garcia-Lloret M,
Noval Rivas M, Wise P, McGhee SA, Chatila ZK, Daher N, Sioutas C
and Chatila TA: Vehicular exhaust particles promote allergic airway
inflammation through an aryl hydrocarbon receptor-notch signaling
cascade. J Allergy Clin Immunol. 136:441–453. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Rouadi PW, Idriss SA, Naclerio RM, Peden
DB, Ansotegui IJ, Canonica GW, Gonzalez-Diaz SN, Rosario Filho NA,
Ivancevich JC, Hellings PW, et al: Immunopathological features of
air pollution and its impact on inflammatory airway diseases (IAD).
World Allergy Organ J. 13:1004672020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
He X, Zhang L, Xiong A, Ran Q, Wang J, Wu
D, Niu B, Liu S and Li G: PM2.5 aggravates NQO1-induced mucus
hyper-secretion through release of neutrophil extracellular traps
in an asthma model. Ecotoxicol Environ Saf. 218:1122722021.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Fujii T, Hayashi S, Hogg JC, Mukae H, Suwa
T, Goto Y, Vincent R and van Eeden SF: Interaction of alveolar
macrophages and airway epithelial cells following exposure to
particulate matter produces mediators that stimulate the bone
marrow. Am J Respir Cell Mol Biol. 27:34–41. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Beeh KM, Kornmann O, Buhl R, Culpitt SV,
Giembycz MA and Barnes PJ: Neutrophil chemotactic activity of
sputum from patients with COPD: Role of interleukin 8 and
leukotriene B4. Chest. 123:1240–1247. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lavinskiene S, Jeroch J, Malakauskas K,
Bajoriuniene I, Jackute J and Sakalauskas R: Peripheral blood
neutrophil activity during Dermatophagoides pteronyssinus-induced
late-phase airway inflammation in patients with allergic rhinitis
and asthma. Inflammation. 35:1600–1609. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wei T and Tang M: Biological effects of
airborne fine particulate matter (PM2.5) exposure on
pulmonary immune system. Environ Toxicol Pharmacol. 60:195–201.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zawrotniak M, Juszczak M, Mosio-Wójcik J
and Rapala-Kozik M: Neutrophil extracellular traps in upper
respiratory tract secretions: Insights into infectious and allergic
rhinitis. Front Immunol. 14:12959212023. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hu Y, Xu Z, Jiang F, Li S, Liu S, Wu M,
Yan C, Tan J, Yu G, Hu Y, et al: Relative impact of meteorological
factors and air pollutants on childhood allergic diseases in
Shanghai, China. Sci Total Environ. 706:1359752020. View Article : Google Scholar
|
|
63
|
Angelon-Gaetz KA, Richardson DB, Marshall
SW and Hernandez ML: Exploration of the effects of classroom
humidity levels on teachers' respiratory symptoms. Int Arch Occup
Environ Health. 89:729–737. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Damialis A, Häring F, Gökkaya M, Rauer D,
Reiger M, Bezold S, Bounas-Pyrros N, Eyerich K, Todorova A, Hammel
G, et al: Human exposure to airborne pollen and relationships with
symptoms and immune responses: Indoors versus outdoors, circadian
patterns and meteorological effects in alpine and urban
environments. Sci Total Environ. 653:190–199. 2019. View Article : Google Scholar
|
|
65
|
Upperman CR, Parker JD, Akinbami LJ, Jiang
C, He X, Murtugudde R, Curriero FC, Ziska L and Sapkota A: Exposure
to extreme heat events is associated with increased hay fever
prevalence among nationally representative sample of US adults:
1997-2013. J Allergy Clin Immunol Pract. 5:435–441.e2. 2017.
View Article : Google Scholar
|
|
66
|
Konishi S, Ng CF, Stickley A, Nishihata S,
Shinsugi C, Ueda K, Takami A and Watanabe C: Particulate matter
modifies the association between airborne pollen and daily medical
consultations for pollinosis in Tokyo. Sci Total Environ.
499:125–132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Mendell MJ, Mirer AG, Cheung K, Tong M and
Douwes J: Respiratory and allergic health effects of dampness,
mold, and dampness-related agents: A review of the epidemiologic
evidence. Environ Health Perspect. 119:748–756. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Boita M, Heffler E, Pizzimenti S, Raie A,
Saraci E, Omedè P, Bussolino C, Bucca C and Rolla G: Regulation of
B-cell-activating factor expression on the basophil membrane of
allergic patients. Int Arch Allergy Immunol. 166:208–212. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Bella Z, Kiricsi Á, Viharosné ÉD, Dallos
A, Perényi Á, Kiss M, Koreck A, Kemény L, Jóri J, Rovó L and
Kadocsa E: Rhinophototherapy in persistent allergic rhinitis. Eur
Arch Otorhinolaryngol. 274:1543–1550. 2017. View Article : Google Scholar
|
|
70
|
Kamimura S, Kitamura Y, Fujii T, Okamoto
K, Sanada N, Okajima N, Wakugawa T, Fukui H, Mizuguchi H and Takeda
N: Effects of narrow-band UVB on nasal symptom and upregulation of
histamine H1 receptor mRNA in allergic rhinitis model
rats. Laryngoscope Investig Otolaryngol. 6:34–41. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wang W, Yu H, Pan Y and Shao S: Combined
treatment with H1 and H4 receptor antagonists improves Th2
inflammatory responses in the nasal mucosa of allergic rhinitis
rats. Am J Rhinol Allergy. 35:809–816. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Shaha A, Mizuguchi H, Kitamura Y, Fujino
H, Yabumoto M, Takeda N and Fukui H: Effect of royal jelly and
Brazilian green propolis on the signaling for histamine
H1 receptor and interleukin-9 gene expressions
responsible for the pathogenesis of the allergic rhinitis. Biol
Pharm Bull. 41:1440–1447. 2018. View Article : Google Scholar
|
|
73
|
Gilles S, Fekete A, Zhang X, Beck I, Blume
C, Ring J, Schmidt-Weber C, Behrendt H, Schmitt-Kopplin P and
Traidl-Hoffmann C: Pollen metabolome analysis reveals adenosine as
a major regulator of dendritic cell-primed T(H) cell responses. J
Allergy Clin Immunol. 127:454–461. e1–e9. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Gueguen C, Bouley J, Moussu H, Luce S,
Duchateau M, Chamot-Rooke J, Pallardy M, Lombardi V, Nony E,
Baron-Bodo V, et al: Changes in markers associated with dendritic
cells driving the differentiation of either TH2 cells or regulatory
T cells correlate with clinical benefit during allergen
immunotherapy. J Allergy Clin Immunol. 137:545–558. 2016.
View Article : Google Scholar
|
|
75
|
Heinl PV, Graulich E, Weigmann B,
Wangorsch A, Ose R, Bellinghausen I, Khatri R, Raker VK, Scheurer
S, Vieths S, et al: IL-10-modulated dendritic cells from birch
pollen- and hazelnut-allergic patients facilitate Treg-mediated
allergen-specific and cross-reactive tolerance. Allergy.
79:2826–2839. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ogulur I, Mitamura Y, Yazici D, Pat Y,
Ardicli S, Li M, D'Avino P, Beha C, Babayev H, Zhao B, et al: Type
2 immunity in allergic diseases. Cell Mol Immunol. 22:211–242.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Alnori H, Alassaf FA, Alfahad M, Qazzaz
ME, Jasim M and Abed MN: Vitamin D and immunoglobulin E status in
allergic rhinitis patients compared to healthy people. J Med Life.
13:463–468. 2020. View Article : Google Scholar
|
|
78
|
Kawada K, Sato C, Ishida T, Nagao Y,
Yamamoto T, Jobu K, Hamada Y, Izawa Ishizawa Y, Ishizawa K and Abe
S: Vitamin D supplementation and allergic rhinitis: A systematic
review and meta-analysis. Medicina (Kaunas). 61:3552025. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Lefebvre L, Amazouz H, Rancière F and
Momas I: Early exposure to sunlight and allergic morbidity: The
PARIS birth cohort. Sci Total Environ. 930:1725432024. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Vergison A, Dagan R, Arguedas A,
Bonhoeffer J, Cohen R, Dhooge I, Hoberman A, Liese J, Marchisio P,
Palmu AA, et al: Otitis media and its consequences: Beyond the
earache. Lancet Infect Dis. 10:195–203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Li FF, Wang JP, Zhang WJ, Zhou PT, Fan M,
Cai NN, Cai YF, Han K, Yang YP, Fu ZY, et al: Trends and mechanisms
of Alzheimer's disease and hearing impairment: A 20-year
perspective. Ageing Res Rev. 110:1027992025. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Monasta L, Ronfani L, Marchetti F, Montico
M, Vecchi Brumatti L, Bavcar A, Grasso D, Barbiero C and Tamburlini
G: Burden of disease caused by otitis media: Systematic review and
global estimates. PLoS One. 7:e362262012. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
GBD 2019 Hearing Loss Collaborators:
Hearing loss prevalence and years lived with disability, 1990-2019:
Findings from the global burden of disease study 2019. Lancet.
397:996–1009. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Belachew AB, Rantala AK, Jaakkola MS, Hugg
TT, Sofiev M, Kukkonen J and Jaakkola JJK: Prenatal and early life
exposure to air pollution and the risk of severe lower respiratory
tract infections during early childhood: The espoo cohort study.
Occup Environ Med. 81:209–216. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Deng Q, Lu C, Jiang W, Zhao J, Deng L and
Xiang Y: Association of outdoor air pollution and indoor renovation
with early childhood ear infection in China. Chemosphere.
169:288–296. 2017. View Article : Google Scholar
|
|
86
|
Park M, Han J, Park J, Jang MJ and Park
MK: Particular matter influences the incidence of acute otitis
media in children. Sci Rep. 11:197302021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Lu C, Li Q, Qiao Z, Liu Q and Wang F:
Effects of pre-natal and post-natal exposures to air pollution on
onset and recurrence of childhood otitis media. J Hazard Mater.
459:1322542023. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Veivers D, Williams GM, Toelle BG,
Waterman AMC, Guo Y, Denison L, Yang BY, Dong GH, Jalaludin B,
Marks GB and Knibbs LD: The indoor environment and otitis media
among australian children: A national cross-sectional study. Int J
Environ Res Public Health. 19:15512022. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Livingston G, Huntley J, Sommerlad A, Ames
D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J,
Cooper C, et al: Dementia prevention, intervention, and care: 2020
Report of the lancet commission. Lancet. 396:413–446. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Flor LS, Anderson JA, Ahmad N, Aravkin A,
Carr S, Dai X, Gil GF, Hay SI, Malloy MJ, McLaughlin SA, et al:
Health effects associated with exposure to secondhand smoke: A
burden of proof study. Nat Med. 30:149–167. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Adamolekun G, Adedoyin FF and Siganos A:
Firm-level pollution and membership of emission trading schemes. J
Environ Manage. 351:1199702024. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Kayalar Ö, Rajabi H, Konyalilar N,
Mortazavi D, Aksoy GT, Wang J and Bayram H: Impact of particulate
air pollution on airway injury and epithelial plasticity;
underlying mechanisms. Front Immunol. 15:13245522024. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Kim BG, Choi DY, Kim MG, Jang AS, Suh MW,
Lee JH, Oh SH and Park MK: Effect of angiogenesis and
lymphangiogenesis in diesel exhaust particles inhalation in mouse
model of LPS induced acute otitis media. Front Cell Infect
Microbiol. 12:8245752022. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Song JJ, Lee JD, Lee BD, Chae SW and Park
MK: Effect of diesel exhaust particles on human middle ear
epithelial cells. Int J Pediatr Otorhinolaryngol. 76:334–338. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Kwak HH, Park JH, Kim HS, Lee HM, Kim SD,
Mun SJ and Cho KS: Inflammatory effects of particulate matter
exposure on the nasal and paranasal sinus mucosa in rats. Int J Mol
Sci. 26:58852025. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Lee SH, Ha SM, Jeong MJ, Park DJ, Polo CN,
Seo YJ and Kim SH: Effects of reactive oxygen species generation
induced by Wonju City particulate matter on mitochondrial
dysfunction in human middle ear cell. Environ Sci Pollut Res Int.
28:49244–49257. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Nahas G, Chen Y, Ningundi A, Tercyak S and
Preciado D: Middle ear microRNAs drive mucin gene response.
Laryngoscope. 135:1815–1820. 2025. View Article : Google Scholar
|
|
98
|
Nieratschker M, Haas M, Lucic M, Pichler
F, Brkic FF, Parzefall T, Riss D and Liu DT: Fluctuations in
emergency department visits related to acute otitis media are
associated with extreme meteorological conditions. Front Public
Health. 11:11531112023. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Jiang YF, Luo WW, Zhang X, Ren DD and
Huang YB: Relative humidity affects acute otitis media visits of
preschool children to the emergency department. Ear Nose Throat J.
102:467–472. 2023. View Article : Google Scholar
|
|
100
|
Montgomery MT, Sajuthi SP, Cho SH, Everman
JL, Rios CL, Goldfarbmuren KC, Jackson ND, Saef B, Cromie M, Eng C,
et al: Genome-wide analysis reveals mucociliary remodeling of the
nasal airway epithelium induced by urban PM2.5. Am J
Respir Cell Mol Biol. 63:172–184. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Li C, Jiang X, Wei Y, Wang Y, Lao X, Yue Q
and Chong KC: Air pollutants, seasonal influenza, and acute otitis
media in children: A population-based analysis using 22-year
hospitalization data. BMC Public Health. 24:15812024. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Wang Y, Zheng B, Xiong P, Liu Y, Shu L,
Shen Y, Lu T and Yang Y: PM2.5 induced nasal mucosal barrier
dysfunction and epithelial-mesenchymal transition to promote
chronic rhinosinusitis through IL4I1-AhR signaling pathway. Toxics.
13:4882025. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Lu HF, Zhou YC, Yang LT, Zhou Q, Wang XJ,
Qiu SQ, Cheng BH and Zeng XH: Involvement and repair of epithelial
barrier dysfunction in allergic diseases. Front Immunol.
15:13482722024. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Tian H, Zhang H, Chen Y and Zhong C: A
study of the influence of meteorological and environmental factors
on otitis media with effusion in Lanzhou. Indian J Otolaryngol Head
Neck Surg. 76:5234–5247. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Klimek L, Brough HA, Arasi S,
Toppila-Salmi S, Bergmann C, Jutel M, Bousquet J, Hox V, Gevaert P,
Tomazic PV, et al: Otitis media with effusion (OME) and eustachian
tube dysfunction: The role of allergy and immunity-An EAACI
position paper. Allergy. 80:2429–2441. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Yang J, Liu Y, Yu L and Duan M: Editorial:
Sudden deafness. Front Neurol. 15:15200182024. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Chamoun J, Larson P, Altaye M, Tabangin M,
Sun DQ and Gordon SA: Contribution of tinnitus burden and hearing
loss to geriatric depression. Laryngoscope. Oct 29–2025.Epub ahead
of print. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Ranjdoost F, Ghaffari ME, Azimi F,
Mohammadi A, Fouladi-Fard R and Fiore M: Association between air
pollution and sudden sensorineural hearing loss (SSHL): A
systematic review and meta-analysis. Environ Res. 239:1173922023.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Tang SE, Wu SY, Jhou FY, Chung CH, Chien
WC and Wang CH: Comparison of the incidence of sudden sensorineural
hearing loss in Northern Taiwan and Southern Taiwan (2000-2015). J
Med Sci. 42:228–235. 2022. View Article : Google Scholar
|
|
110
|
Choi HG, Min C and Kim SY: Air pollution
increases the risk of SSNHL: A nested case-control study using
meteorological data and national sample cohort data. Sci Rep.
9:82702019. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Tsai SC, Hsu YC, Lai JN, Chou RH, Fan HC,
Lin FC, Zhang R, Lin CL and Chang KH: Long-term exposure to air
pollution and the risk of developing sudden sensorineural hearing
loss. J Transl Med. 19:4242021. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Luo X, Zuo W, Ren Q, Wang L, Wu D, Xiang Y
and Zhong S: Correlation of air pollution and risk of sudden
sensorineural hearing loss: A Mendelian randomization study. Sci
Rep. 15:289212025. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Chang KH, Tsai SC, Lee CY, Chou RH, Fan
HC, Lin FC, Lin CL and Hsu YC: Increased risk of sensorineural
hearing loss as a result of exposure to air pollution. Int J
Environ Res Public Health. 17:19692020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Umesawa M, Kobashi G, Kitoh R, Nishio SY,
Ogawa K, Hato N, Sone M, Fukuda S, Hara A, Ikezono T, et al:
Relationships among drinking and smoking habits, history of
diseases, body mass index and idiopathic sudden sensorineural
hearing loss in Japanese patients. Acta Otolaryngol. 137(Suppl 1):
S17–S23. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Lalwani AK, Liu YH and Weitzman M:
Secondhand smoke and sensorineural hearing loss in adolescents.
Arch Otolaryngol Head Neck Surg. 137:655–662. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Wang DH, Xu H, Zheng YH, Gu DS, Zhu YJ,
Ren Y, Wang SC, Yang L and Xu LW: Environmental exposure to lead
and cadmium and hearing loss in Chinese adults: A case-control
study. PLoS One. 15:e02331652020. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Yang CH, Ko MT, Peng JP and Hwang CF: Zinc
in the treatment of idiopathic sudden sensorineural hearing loss.
Laryngoscope. 121:617–621. 2011. View Article : Google Scholar
|
|
118
|
Harenberg J, Jonas JB and Trecca EMC: A
liaison between sudden sensorineural hearing loss and SARS-CoV-2
infection. Thromb Haemost. 120:1237–1239. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Trecca EMC, Gelardi M and Cassano M:
COVID-19 and hearing difficulties. Am J Otolaryngol. 41:1024962020.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Sung CYW, Seleme MC, Payne S, Jonjic S,
Hirose K and Britt W: Virus-induced cochlear inflammation in
newborn mice alters auditory function. JCI Insight. 4:e1288782019.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Ma J, Chiu YF, Kao CC, Chuang CN, Chen CY,
Lai CH and Kuo ML: Fine particulate matter manipulates immune
response to exacerbate microbial pathogenesis in the respiratory
tract. Eur Respir Rev. 33:2302592024. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Schraff SA, Schleiss MR, Brown DK,
Meinzen-Derr J, Choi KY, Greinwald JH and Choo DI: Macrophage
inflammatory proteins in cytomegalovirus-related inner ear injury.
Otolaryngol Head Neck Surg. 137:612–618. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Xie W, Karpeta N, Tong B, Liu Y, Zhang Z
and Duan M: Comorbidities and laboratory changes of sudden
sensorineural hearing loss: A review. Front Neurol. 14:11424592023.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Choi D, Lee G, Kim KH and Bae H:
Particulate matter exacerbates the death of dopaminergic neurons in
parkinson's disease through an inflammatory response. Int J Mol
Sci. 23:64872022. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Tanaka M, Okuda T, Itoh K, Ishihara N,
Oguro A, Fujii-Kuriyama Y, Nabetani Y, Yamamoto M, Vogel CFA and
Ishihara Y: Polycyclic aromatic hydrocarbons in urban particle
matter exacerbate movement disorder after ischemic stroke via
potentiation of neuroinflammation. Part Fibre Toxicol. 20:62023.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Blasits S, Maune S and Santos-Sacchi J:
Nitric oxide uncouples gap junctions of supporting Deiters cells
from Corti's organ. Pflugers Arch. 440:710–712. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Bauer L, Laksono BM, de Vrij FMS, Kushner
SA, Harschnitz O and van Riel D: The neuroinvasiveness,
neurotropism, and neurovirulence of SARS-CoV-2. Trends Neurosci.
45:358–368. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Kim JS and Lee H: Inner ear dysfunction
due to vertebrobasilar ischemic stroke. Semin Neurol. 29:534–540.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Kuntic M, Hahad O, Al-Kindi S, Oelze M,
Lelieveld J, Daiber A and Münzel T: Pathomechanistic synergy
between particulate matter and traffic noise-induced cardiovascular
damage and the classical risk factor hypertension. Antioxid Redox
Signal. 42:827–847. 2025. View Article : Google Scholar
|
|
130
|
Bongioanni P, Del Carratore R, Corbianco
S, Diana A, Cavallini G, Masciandaro SM, Dini M and Buizza R:
Climate change and neurodegenerative diseases. Environ Res.
201:1115112021. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Li XB, Han YX, Fu ZY, Zhang YC, Fan M,
Sang SJ, Chen XX, Liang BY, Liu YC, Lu PC, et al: Association of
sudden sensorineural hearing loss with meteorological factors: A
time series study in Hefei, China, and a literature review. Environ
Sci Pollut Res Int. 31:42970–42990. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Ryu IY, Park SH, Park EB, Kim HJ, Kim SH
and Yeo SG: Factors prognostic of season-associated sudden
sensorineural hearing loss: A retrospective observational study. J
Audiol Otol. 21:44–48. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Danielides V, Nousia CS, Bartzokas A,
Lolis CJ, Kateri M and Skevas A: Weather conditions and sudden
sensorineural hearing loss. BMC Ear Nose Throat Disord. 2:22002.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Kim SH, Kim SJ, Im H, Kim TH, Song JJ and
Chae SW: A trend in sudden sensorineural hearing loss: Data from a
population-based study. Audiol Neurootol. 22:311–316. 2017.
View Article : Google Scholar
|
|
135
|
Wu CS, Lin HC and Chao PZ: Sudden
sensorineural hearing loss: Evidence from Taiwan. Audiol Neurootol.
11:151–156. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Jourdy DN, Donatelli LA, Victor JD and
Selesnick SH: Assessment of variation throughout the year in the
incidence of idiopathic sudden sensorineural hearing loss. Otol
Neurotol. 31:53–57. 2010. View Article : Google Scholar
|
|
137
|
Kuzmenko NV, Tsyrlin VA, Pliss MG and
Galagudza MM: Health effects of atmospheric pressure fluctuations:
A review of biometeorological research. Int J Biometeorol.
69:2171–2187. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Martinac B, Nomura T, Chi G, Petrov E,
Rohde PR, Battle AR, Foo A, Constantine M, Rothnagel R, Carne S, et
al: Bacterial mechanosensitive channels: Models for studying
mechanosensory transduction. Antioxid Redox Signal. 20:952–969.
2014. View Article : Google Scholar
|
|
139
|
He YS, Cao F, Hu X, Liu YC, Tao SS, Wang
P, Hou S and Pan HF: Time trends in the burden of environmental
heat and cold exposure among children and adolescents. JAMA
Pediatr. 179:55–64. 2025. View Article : Google Scholar
|
|
140
|
Wang JP, Xie ZH, Zhou PT, Liang BY, Han K,
Fu ZY, Li FF, Liu YH, Pan HF and Liu YC: Epidemiological and
experimental evidence of environmental factor-related autoimmune
thyroid disease: A systematic review. Ecotoxicol Environ Saf.
305:1192562025. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Cao F, Liu ZR, Ni QY, Zha CK, Zhang SJ, Lu
JM, Xu YY, Tao LM, Jiang ZX and Pan HF: Emerging roles of air
pollution and meteorological factors in autoimmune eye diseases.
Environ Res. 231:1161162023. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Lu YX, Liang JQ, Gu QL, Pang C and Huang
CL: Pediatric epistaxis and its correlation between air pollutants
in beijing from 2014 to 2017. Ear Nose Throat J. 99:513–517. 2020.
View Article : Google Scholar
|
|
143
|
Zhang F, Xu J, Zhang Z, Meng H, Wang L, Lu
J, Wang W and Krafft T: Ambient air quality and the effects of air
pollutants on otolaryngology in Beijing. Environ Monit Assess.
187:4952015. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Wang J, Li J, Li X, Wang D and Fang C:
Relationship between ozone and air temperature in future
conditions: A case study in sichuan basin, China. Environ Pollut.
343:1232762024. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Yang W, Qiao Z, Li Q, Jia X, Liu Y, Zeng
Z, Wang F and Lu C: Early-life ozone exposure and childhood otitis
media: Unveiling critical windows of risk. Sci Total Environ.
953:1761242024. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Tian Y, Jia B, Zhao P, Song D, Huang F and
Feng Y: Size distribution, meteorological influence and uncertainty
for source-specific risks: PM2.5 and
PM10-bound PAHs and heavy metals in a Chinese megacity
during 2011-2021. Environ Pollut. 312:1200042022. View Article : Google Scholar
|
|
147
|
Chuang MT, Lee CT and Hsu HC: Quantifying
PM2.5 from long-range transport and local pollution in
Taiwan during winter monsoon: An efficient estimation method. J
Environ Manage. 227:10–22. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Fry JL, Ooms P, Krol M, Kerckhoffs J,
Vermeulen R, Wesseling J and van den Elshout S: Effect of street
trees on local air pollutant concentrations (NO2, BC,
UFP, PM2.5) in rotterdam, the Netherlands. Environ Sci
Atmos. 5:394–404. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Yan J, Sun N, Zheng J, Zhang Y and Yin S:
Uneven PM2.5 dispersion pattern across an open-road
vegetation barrier: Effects of planting combination and wind
condition. Sci Total Environ. 917:1704792024. View Article : Google Scholar
|
|
150
|
Bi J, Belle JH, Wang Y, Lyapustin AI,
Wildani A and Liu Y: Impacts of snow and cloud covers on
satellite-derived PM2.5 levels. Remote Sens Environ.
221:665–674. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Yang M, Wang Y, Li H, Li T, Nie X, Cao F,
Yang F, Wang Z, Wang T, Qie G, et al: Polycyclic aromatic
hydrocarbons (PAHs) associated with PM2.5 within
boundary layer: Cloud/fog and regional transport. Sci Total
Environ. 627:613–621. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Hu J, Zhao T, Liu J, Cao L, Xia J, Wang C,
Zhao X, Gao Z, Shu Z and Li Y: Nocturnal surface radiation cooling
modulated by cloud cover change reinforces PM2.5
accumulation: Observational study of heavy air pollution in the
Sichuan Basin, Southwest China. Sci Total Environ. 794:1486242021.
View Article : Google Scholar
|
|
153
|
Shin YH, Ha EK, Kim JH, Yon DK, Lee SW,
Sim HJ, Sung M, Jee HM and Han MY: Serum vitamin D level is
associated with smell dysfunction independently of aeroallergen
sensitization, nasal obstruction, and the presence of allergic
rhinitis in children. Pediatr Allergy Immunol. 32:116–123. 2021.
View Article : Google Scholar
|
|
154
|
Kalueff AV and Tuohimaa P: Neurosteroid
hormone vitamin D and its utility in clinical nutrition. Curr Opin
Clin Nutr Metab Care. 10:12–19. 2007.
|
|
155
|
Montzka SA, Dlugokencky EJ and Butler JH:
Non-CO2 greenhouse gases and climate change. Nature. 476:43–50.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Andersson J, Oudin A, Nordin S, Forsberg B
and Nordin M: PM2.5 exposure and olfactory functions.
Int J Environ Health Res. 32:2484–2495. 2022. View Article : Google Scholar
|
|
157
|
Molot J, Sears M, Marshall LM and Bray RI:
Neurological susceptibility to environmental exposures:
Pathophysiological mechanisms in neurodegeneration and multiple
chemical sensitivity. Rev Environ Health. 37:509–530. 2022.
View Article : Google Scholar
|
|
158
|
Jahedi F, Goudarzi G, Ahmadi M and Safdari
F: A bibliometric and systematic review of the global impact of air
pollution on Alzheimer's disease: Insights from cohort studies. J
Alzheimers Dis Rep. 9:254248232513688832025. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Lotsch J and Hummel T: A data
science-based analysis of seasonal patterns in outpatient
presentations due to olfactory dysfunction. Rhinology. 58:151–157.
2020. View Article : Google Scholar
|
|
160
|
Potter MR, Chen JH, Lobban NS and Doty RL:
Olfactory dysfunction from acute upper respiratory infections:
Relationship to season of onset. Int Forum Allergy Rhinol.
10:706–712. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Chen H, Cheng Y, Du H, Zhang C, Zhou Y,
Zhao Z, Li Y, Friedemann T, Mei J, Schröder S and Chen M: Shufeng
Jiedu capsule ameliorates olfactory dysfunction via the AMPK/mTOR
autophagy pathway in a mouse model of allergic rhinitis.
Phytomedicine. 107:1544262022. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Stuck BA and Hummel T: Olfaction in
allergic rhinitis: A systematic review. J Allergy Clin Immunol.
136:1460–1470. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Urry Z, Chambers ES, Xystrakis E, Dimeloe
S, Richards DF, Gabryšová L, Christensen J, Gupta A, Saglani S,
Bush A, et al: The role of 1α,25-dihydroxyvitamin D3 and cytokines
in the promotion of distinct Foxp3+ and IL-10+ CD4+ T cells. Eur J
Immunol. 42:2697–2708. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Joshi S, Pantalena LC, Liu XK, Gaffen SL,
Liu H, Rohowsky-Kochan C, Ichiyama K, Yoshimura A, Steinman L,
Christakos S and Youssef S: 1,25-Dihydroxyvitamin D(3) ameliorates
Th17 autoimmunity via transcriptional modulation of
interleukin-17A. Mol Cell Biol. 31:3653–3669. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Holick MF: Resurrection of vitamin D
deficiency and rickets. J Clin Invest. 116:2062–2072. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Shi Z, Xi L, Wang Y and Zhao X: Chronic
exposure to environmental pollutant ammonia causes damage to the
olfactory system and behavioral abnormalities in mice. Environ Sci
Technol. 57:15412–15421. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Choi YS, Jeong BS, Lee YK and Kim YD:
Effects of air pollution on chemosensory dysfunction in COVID-19
patients. J Korean Med Sci. 37:e2902022. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Bernal-Meléndez E, Lacroix MC, Bouillaud
P, Callebert J, Olivier B, Persuy MA, Durieux D, Rousseau-Ralliard
D, Aioun J, Cassee F, et al: Repeated gestational exposure to
diesel engine exhaust affects the fetal olfactory system and alters
olfactory-based behavior in rabbit offspring. Part Fibre Toxicol.
16:52019. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Calderón-Garcidueñas L, Franco-Lira M,
Torres-Jardón R, Henriquez-Roldán C, Barragán-Mejía G,
Valencia-Salazar G, González-Maciel A, Reynoso-Robles R,
Villarreal-Calderón R and Reed W: Pediatric respiratory and
systemic effects of chronic air pollution exposure: Nose, lung,
heart, and brain pathology. Toxicol Pathol. 35:154–162. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Wei S, Xu T, Jiang T and Yin D:
Chemosensory dysfunction induced by environmental pollutants and
its potential as a novel neurotoxicological indicator: A review.
Environ Sci Technol. 55:10911–10922. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Paital B and Agrawal PK: Air pollution by
NO2 and PM2.5 explains COVID-19 infection
severity by overexpression of angiotensin-converting enzyme 2 in
respiratory cells: A review. Environ Chem Lett. 19:25–42. 2021.
View Article : Google Scholar
|
|
172
|
Miyashita L, Foley G, Semple S, Gibbons
JM, Pade C, McKnight Á and Grigg J: Curbside particulate matter and
susceptibility to SARS-CoV-2 infection. J Allergy Clin Immunol
Glob. 2:1001412023. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Alarfaj AA, Aldrweesh AK, Aldoughan AF,
Alarfaj SM, Alabdulqader FK and Alyahya KA: Olfactory dysfunction
following COVID-19 and the potential benefits of olfactory
training. J Clin Med. 12:47612023. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Yu Z, Ekström S, Bellander T, Ljungman P,
Pershagen G, Eneroth K, Kull I, Bergström A, Georgelis A, Stafoggia
M, et al: Ambient air pollution exposure linked to long COVID among
young adults: A nested survey in a population-based cohort in
Sweden. Lancet Reg Health Eur. 28:1006082023. View Article : Google Scholar
|
|
175
|
McWilliams MP, Coelho DH, Reiter ER and
Costanzo RM: Recovery from Covid-19 smell loss: Two-years of follow
up. Am J Otolaryngol. 43:1036072022. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Hu B, Gong M, Xiang Y, Qu S, Zhu H and Ye
D: Mechanism and treatment of olfactory dysfunction caused by
coronavirus disease 2019. J Transl Med. 21:8292023. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Raghuvamsi PV, Tulsian NK, Samsudin F,
Qian X, Purushotorman K, Yue G, Kozma MM, Hwa WY, Lescar J, Bond
PJ, et al: SARS-CoV-2 S protein: ACE2 interaction reveals novel
allosteric targets. Elife. 10:e636462021. View Article : Google Scholar
|
|
178
|
Antony P and Vijayan R: Role of SARS-CoV-2
and ACE2 variations in COVID-19. Biomed J. 44:235–244. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Li HH, Liu CC, Hsu TW, Lin JH, Hsu JW, Li
AF, Yeh YC, Hung SC and Hsu HS: Upregulation of ACE2 and TMPRSS2 by
particulate matter and idiopathic pulmonary fibrosis: A potential
role in severe COVID-19. Part Fibre Toxicol. 18:112021. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Yadav R, Chaudhary JK, Jain N, Chaudhary
PK, Khanra S, Dhamija P, Sharma A, Kumar A and Handu S: Role of
structural and non-structural proteins and therapeutic targets of
SARS-CoV-2 for COVID-19. Cells. 10:8212021. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Botto L, Bulbarelli A, Lonati E, Cazzaniga
E and Palestini P: Correlation between exposure to UFP and ACE/ACE2
pathway: Looking for possible involvement in COVID-19 pandemic.
Toxics. 12:5602024. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Wieczfinska J, Kleniewska P and Pawliczak
R: Oxidative stress-related mechanisms in SARS-CoV-2 infections.
Oxid Med Cell Longev. 2022:55890892022. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Jiang CY, Han K, Yang F, Yin SY, Zhang L,
Liang BY, Wang TB, Jiang T, Chen YR, Shi TY, et al: Global,
regional, and national prevalence of hearing loss from 1990 to
2019: A trend and health inequality analyses based on the global
burden of disease study 2019. Ageing Res Rev. 92:1021242023.
View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Li FF, Fu ZY, Han K, Liang BY, Han YX, Liu
YH, Tong BS and Liu YC: Trends and driving factors of age-related
hearing loss and severity over 30 years: A cross-sectional study.
BMC Geriatr. 25:3872025. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Li Z, Zhang H, Wang N, Zhang S, Luo Z,
Xuan X, Liu M, Chen X, Li X, Xue L and Wu J: Effects of air
pollution and noise exposure on occupational hearing loss in oil
workers: A prospective cohort study. BMC Public Health.
25:25272025. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Ju MJ, Park SK, Kim SY and Choi YH:
Long-term exposure to ambient air pollutants and hearing loss in
Korean adults. Sci Total Environ. 820:1531242022. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Manukyan AL: Noise as a cause of
neurodegenerative disorders: Molecular and cellular mechanisms.
Neurol Sci. 43:2983–2993. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Liu T, Cao L, Lv P and Bai S: Associations
between household solid fuel use and hearing loss in a Chinese
population: A population-based prospective cohort study. Ecotoxicol
Environ Saf. 236:1135062022. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Poindexter AN III, Thompson DJ, Gibbons
WE, Findley WE, Dodson MG and Young RL: Residual embryos in failed
embryo transfer. Fertil Steril. 46:262–267. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Kim HY: Eustachian tube dysfunction in
hearing loss: Mechanistic pathways to targeted interventions.
Biomedicines. 13:26862025. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Fechter LD, Gearhart C, Fulton S, Campbell
J, Fisher J, Na K, Cocker D, Nelson-Miller A, Moon P and Pouyatos
B: Promotion of noise-induced cochlear injury by toluene and
ethylbenzene in the rat. Toxicol Sci. 98:542–551. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Stoica BA, Boulares AH, Rosenthal DS, Iyer
S, Hamilton ID and Smulson ME: Mechanisms of JP-8 jet fuel
toxicity. I. Induction of apoptosis in rat lung epithelial cells.
Toxicol Appl Pharmacol. 171:94–106. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Guthrie OW, Xu H, Wong BA, McInturf SM,
Reboulet JE, Ortiz PA and Mattie DR: Exposure to low levels of
jet-propulsion fuel impairs brainstem encoding of stimulus
intensity. J Toxicol Environ Health A. 77:261–280. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Guthrie OW, Wong BA, McInturf SM, Reboulet
JE, Ortiz PA and Mattie DR: Inhalation of hydrocarbon jet fuel
suppress central auditory nervous system function. J Toxicol
Environ Health A. 78:1154–1169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Fechter LD, Fisher JW, Chapman GD, Mokashi
VP, Ortiz PA, Reboulet JE, Stubbs JE, Lear AM, McInturf SM, Prues
SL, et al: Subchronic JP-8 jet fuel exposure enhances vulnerability
to noise-induced hearing loss in rats. J Toxicol Environ Health A.
75:299–317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Grobe N, Narayanan L, Brown DN, Law ST,
Sibomana I, Shiyanov P, Reo NV, Hack CE, Sterner TR and Mattie DR:
Lipid, water, and protein composition to facilitate kinetic
modeling of the auditory pathway. Toxicol Mech Methods. 29:53–59.
2019. View Article : Google Scholar
|
|
197
|
Kilic O, Kalcioglu MT, Cag Y, Tuysuz O,
Pektas E, Caskurlu H and Cetın F: Could sudden sensorineural
hearing loss be the sole manifestation of COVID-19? An
investigation into SARS-COV-2 in the etiology of sudden
sensorineural hearing loss. Int J Infect Dis. 97:208–211. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Yamada S, Kita J, Shinmura D, Nakamura Y,
Sahara S, Misawa K and Nakanishi H: Update on findings about sudden
sensorineural hearing loss and insight into its pathogenesis. J
Clin Med. 11:63872022. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Hatim KS, Narayankar S and Mulla T:
Patterns and prevalence of benign breast disease in Western India.
Int J Res Med Sci. 5:6842017. View Article : Google Scholar
|
|
200
|
Zhai Z, Li C, Chen Y, Gerotziafas G, Zhang
Z, Wan J, Liu P, Elalamy I and Wang C; Prevention Treatment of VTE
Associated with COVID-19 Infection Consensus Statement Group:
Prevention and treatment of venous thromboembolism associated with
coronavirus disease 2019 infection: A consensus statement before
guidelines. Thromb Haemost. 120:937–948. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Spiezia L, Boscolo A, Poletto F, Cerruti
L, Tiberio I, Campello E, Navalesi P and Simioni P:
COVID-19-related severe hypercoagulability in patients admitted to
intensive care unit for acute respiratory failure. Thromb Haemost.
120:998–1000. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Wan D, Du T, Hong W, Chen L, Que H, Lu S
and Peng X: Neurological complications and infection mechanism of
SARS-COV-2. Signal Transduct Target Ther. 6:4062021. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Mao L, Jin H, Wang M, Hu Y, Chen S, He Q,
Chang J, Hong C, Zhou Y, Wang D, et al: Neurologic manifestations
of hospitalized patients with coronavirus disease 2019 in Wuhan,
China. JAMA Neurol. 77:683–690. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Chandrasekhar SS, Tsai Do BS, Schwartz SR,
Bontempo LJ, Faucett EA, Finestone SA, Hollingsworth DB, Kelley DM,
Kmucha ST, Moonis G, et al: Clinical practice guideline: sudden
hearing loss (update) executive summary. Otolaryngol Head Neck
Surg. 161:195–210. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao
Y, Xie C, Ma K, Shang K, Wang W and Tian DS: Dysregulation of
immune response in patients with coronavirus 2019 (COVID-19) in
Wuhan, China. Clin Infect Dis. 71:762–768. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Varga Z, Flammer AJ, Steiger P, Haberecker
M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka
F and Moch H: Endothelial cell infection and endotheliitis in
COVID-19. Lancet. 395:1417–1418. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Degen C, Lenarz T and Willenborg K: Acute
profound sensorineural hearing loss after COVID-19 pneumonia. Mayo
Clin Proc. 95:1801–1803. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
de Beer EL, Gründeman RL, Wilhelm AJ,
Caljouw CJ, Klepper D and Schiereck P: Caffeine suppresses length
dependency of Ca2+ sensitivity of skinned striated muscle. Am J
Physiol. 254:C491–C497. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Nicolini H: Depression and anxiety during
COVID-19 pandemic. Cir Cir. 88:542–547. 2020.PubMed/NCBI
|
|
210
|
Yu Z, Bellander T, Bergström A, Dillner J,
Eneroth K, Engardt M, Georgelis A, Kull I, Ljungman P, Pershagen G,
et al: Association of short-term air pollution exposure with
SARS-CoV-2 infection among young adults in Sweden. JAMA Netw Open.
5:e2281092022. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Li Z, Tao B, Hu Z, Yi Y and Wang J:
Effects of short-term ambient particulate matter exposure on the
risk of severe COVID-19. J Infect. 84:684–691. 2022. View Article : Google Scholar : PubMed/NCBI
|















![Impact of air pollution and
meteorological factors on olfactory function and AR. PM induces
Th1/Th2 immune imbalance, enhances systemic oxidative stress and
inflammatory responses, thereby leading to olfactory dysfunction
and the onset of AR. High temperatures exacerbate the airborne
transmission of pollen, further intensifying Th1/Th2 imbalance and
allergic reactions. Weather conditions and air humidity affect
fungal proliferation, promoting B cell activation and triggering
allergic reactions. Furthermore, weather changes influence UVB
radiation, thus regulating the Th1/Th2 immune balance. Viruses
induce systemic inflammation, further exacerbating olfactory
dysfunction and AR. Additionally, viruses can bind to the ACE2
receptors on nasal epithelial cells, leading to olfactory damage,
while PM exacerbates this damage by promoting ACE2 expression.
25[OH]D3, 25-hydroxyvitamin D; ACE2,
angiotensin-converting enzyme 2; AR, allergic rhinitis; DCs,
dendritic cells; MUC5AC, mucin 5AC; NETs, neutrophil extracellular
traps; NQO1, quinone oxidoreductase 1; PAHs, polycyclic aromatic
hydrocarbons; PM, particulate matter; ROS, reactive oxygen species;
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; Th,
T-helper; UVB, ultraviolet B; GATA3, GATA binding protein 3; RORγ,
retinoic acid receptor-related orphan receptor γ; BAFF, B-cell
activating factor.](/article_images/ijmm/57/3/ijmm-57-03-05726-g00.jpg)