Copper is an essential trace mineral required for
numerous physiological processes, including aerobic respiration,
oxidative stress regulation and biosynthesis (1-4).
Despite its key role in biological activities, intracellular copper
levels are tightly regulated because both copper deficiency, such
as in Menkes disease, anemia, and neurodegeneration, and copper
excess, as seen in Wilson disease, liver injury, neurodegenerative
disorder and several types of cancer, can lead to severe
pathological conditions (5-9).
When intracellular copper accumulates excessively, the organism
initiates specific regulatory programs to decrease the copper
content (10,11). Programmed cell death (PCD) refers
to an orderly, gene-regulated process that maintains cell and
systemic homeostasis and is key for both physiological and
pathological cell turnover (12). The classical forms of PCD include
apoptosis, necroptosis, pyroptosis, ferroptosis and autophagy
(13). Apoptosis is
characterized primarily by caspase activation and the release of
cytochrome C from mitochondria under genetic control (14). Necroptosis involves both necrosis
and apoptosis and is typically triggered by the binding of death
receptors (such as tumor necrosis factor receptor 1 and the Fas
receptor) to their respective ligands (15). Pyroptosis is mediated by
pore-forming gasdermin proteins, which are activated by caspase-1
and the inflammasome complex, leading to cell membrane rupture
(16). Ferroptosis is an
iron-dependent process driven by lipid peroxidation of unsaturated
fatty acids under the action of ferrous ions or lipoxygenase
(17). Autophagy promotes the
degradation and recycling of intracellular components under stress
conditions to maintain cell homeostasis (18). In recent years, as the mechanisms
of cell death have been elucidated, cuproptosis has emerged as a
newly discovered form of PCD (19). Unlike traditional pathways such
as apoptosis, necroptosis, autophagy or ferroptosis, cuproptosis
depends on the intracellular accumulation of copper, which directly
binds lipoylated proteins in the tricarboxylic acid (TCA) cycle,
causing protein aggregation and iron-sulfur cluster degradation.
This results in proteotoxic stress, mitochondrial dysfunction and
cell death (19). In addition to
direct copper accumulation, copper also induces other forms of cell
death. For example, copper-induced apoptosis occurs via the
catalytic generation of reactive oxygen species (ROS), leading to
oxidative stress, DNA damage and the activation of apoptotic
pathways (20). Copper can also
directly or indirectly modulate apoptosis-associated proteins, such
as by activating p53 and enhancing its pro-death function (21). Copper-induced ferroptosis may
occur via promotion of iron absorption and utilization, increasing
intracellular iron levels and exacerbating lipid peroxidation,
thereby increasing ferroptotic death (22). Copper also produces hydroxyl
radicals through Fenton-like reactions, triggering lipid
peroxidation and ferroptosis (23). In addition, copper may induce the
autophagic degradation of GPX4, facilitating ferroptosis (24). Copper-induced autophagy is
triggered by the activation of AMPK, inhibition of mTOR or direct
interaction with UNC-51-like kinase ½ (25). This process also occurs via
upregulation of autophagy-related genes and activation of the
transcription factor (TF)EB, promoting autophagosome and
autolysosome formation, which may lead to autophagy-dependent cell
death (26). Moreover,
copper-induced ROS generation and endoplasmic reticulum stress
promote NLRP3 inflammasome assembly and gasdermin D activation,
thus triggering pyroptosis (6).
Recent research has revealed an association between copper
metabolism dysregulation and tumor initiation and progression
(6). Copper serves a dual role
in cancer: Imbalanced copper metabolism promotes tumor cell
proliferation and survival by activating the receptor tyrosine
kinase, PI3K/Akt/mTOR, and MAPK/ERK signaling pathways (6,27) and modulating the tumor
microenvironment (TME) through angiogenesis and immune evasion
(28), while cuproptosis
suppresses tumor growth by inducing cell death and activating
immune responses (6). Copper
stimulates apoptosis, necrosis, autophagy, ferroptosis and
cuproptosis and enhances antitumor immunity by activating immune
cells (11). As copper
dysregulation is common in cancer cells, targeting copper levels or
metabolic pathways can trigger cuproptosis, thereby inhibiting
tumor growth and progression. Cuproptosis may thus represent a
promising anticancer strategy. Furthermore, the potential effects
of cuproptosis and other forms of cell death, such as ferroptosis
and apoptosis, provide a theoretical basis for combination therapy
(22). In recent years,
therapeutic strategies for gastrointestinal cancer have shifted
from local surgical resection to systemic, multimodal therapy
(29). Although progress has
been made in terms of conventional chemotherapy and targeted
therapies in certain patients, drug resistance and relapse remain
challenges. With the advent of precision medicine and
immunotherapy, gastrointestinal cancer management has evolved from
single-modality chemotherapy to integrated metabolic regulation,
immune remodeling, and microenvironmental intervention. Immune
checkpoint inhibitors (PD-1/PD-L1 and CTLA-4 antibodies) and
antiangiogenic agents have demonstrated promising efficacy and
potential for application in patients with gastrointestinal and
hepatocellular tumors (30,31). Concurrently, metabolism-immune
integrated therapy has been proposed to enhance immune responses by
modulating tumor energy metabolism and redox balance (32). The regulation of metal ion
homeostasis has been recognized as a key component of systemic
therapy, with copper serving a pivotal role in the regulation of
mitochondrial respiration and oxidative stress (19). As a copper-dependent PCD pathway,
cuproptosis may serve as a link between metabolism and immunity.
Studies (30,31,33) have highlighted the interplay
between ion metabolism, oxidative stress and immune signaling as a
central axis in systemic therapy for gastrointestinal tumors,
thereby providing a theoretical foundation for incorporating
cuproptosis into therapeutic frameworks. Accordingly, the present
review systematically summarizes the mechanisms of copper
metabolism and cuproptosis to understand metabolic and immune
microenvironmental remodeling in gastrointestinal cancer, offering
conceptual and theoretical support for metabolic targeting and
systemic treatment strategies.
Copper, a widely distributed metallic element in
nature, is an essential trace element for the human body. Most
dietary copper exists in the form of Cu2+ and is
absorbed primarily in the duodenum and small intestine (34). After absorption through the
gastrointestinal tract into the bloodstream, ~90% of copper binds
to ceruloplasmin in the plasma, while the remaining portion is
associated with albumin, transcuprein and histidine. These copper
complexes are transported via the portal vein to the liver and
other organs where copper exerts physiological effects (35). The liver serves as the primary
storage organ for copper (5).
Mitochondria are the notable sites of copper utilization due to the
presence of copper-dependent enzymes such as cytochrome c oxidase,
which is involved in oxidative phosphorylation, and 1-5% of total
cellular superoxide dismutase 1 (SOD1), which serves a critical
role in mitigating oxidative stress within the mitochondrial
matrix. These enzymes highlight the essential role of copper in
maintaining mitochondrial function and cellular health (36). Excess copper is secreted into the
bile and blood, excreted via the intestine or delivered to
peripheral tissues to catalyze physiological reactions (37). A small portion is excreted in
urine, sweat and menstrual fluid (37). Extracellular copper exists
predominantly in the divalent Cu2+ state and cannot be
directly used by cells (38,39). At the cellular level,
Cu2+ enters through divalent metal transporter 1 or is
reduced to Cu+ by six-transmembrane epithelial antigens
such as Six-Transmembrane Epithelial Antigen of the Prostate 1
(STEAP1) or duodenal cytochrome b, after which Cu+ is
imported into the cytoplasm via the high-affinity copper
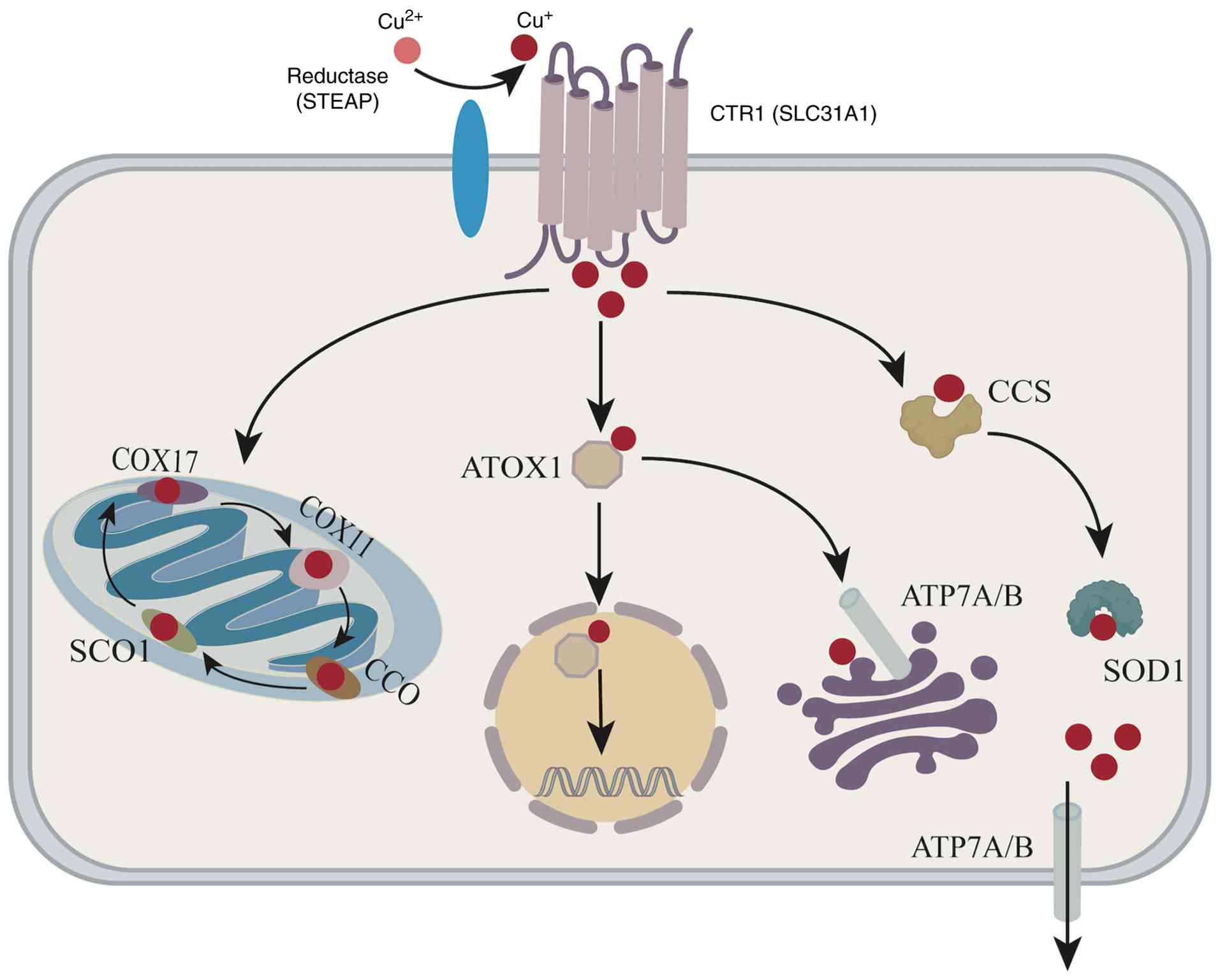
transporter 1 (CTR1) (40,41). Once inside the cytosol, excess
copper is sequestered by metallothioneins (34) or delivered to specific organelles
by copper chaperone proteins. For example, the copper chaperone for
SOD (CCS) transfers copper to SOD1, enabling copper insertion,
disulfide bond formation and the localization of SOD1 to the
cytosol or mitochondria (42).
Cytochrome c oxidase 17 (COX17) transfers copper to the
mitochondrial inner membrane subunits COX1 and COX2 (38), which participate in the assembly
of the respiratory chain. In addition, copper is transported
through the secretory pathway via antioxidant-1 copper chaperone
(ATOX1), which delivers copper to the trans-Golgi network (TGN) and
copper-transporting ATPases (ATP7A and ATP7B) located in the Golgi
or plasma membrane, maintaining cell copper homeostasis (26). The overall process of copper
metabolism is illustrated in Fig.
1.
In addition to the aforementioned transporters, CTR1
is the primary plasma membrane protein responsible for cellular
copper uptake. CTR1, which has the highest copper-binding affinity
among known transporters, specifically binds extracellular
Cu2+ and transports it into the cytoplasm, where it
serves as a major copper chaperone for enzymes such as SOD
(5,43) (Table I). Following exposure to
extracellular copper, CTR1 recognizes and binds copper, undergoes
conformational changes and transports copper into cells to sustain
normal physiological function (44). Once internalized, copper binds to
chaperones such as ATOX1 and COX17, which deliver copper to target
enzymes, mitochondria or metalloproteins in the endoplasmic
reticulum, ensuring proper copper distribution (45). The upregulation of CTR1 leads to
cellular copper overload (46).
Additionally, solute carrier family 25 member 3 (SLC25A3), a
mitochondrial phosphate carrier, is capable of copper transport,
and its upregulation results in mitochondrial matrix copper
overload (47).
ATPase copper-transporting α (ATP7A) and β (ATP7B)
are P-type copper-transporting ATPases that regulate copper efflux
and intracellular distribution (48). They use the energy derived from
ATP hydrolysis to actively transport copper across membranes
against their concentration gradients, thereby maintaining copper
homeostasis (48,49). ATP7A is expressed ubiquitously,
whereas ATP7B is expressed primarily in the liver (50). In hepatocytes, ATP7B mediates the
excretion of excess copper into bile, while unabsorbed copper is
eliminated through feces (51).
Copper efflux is essential for preventing copper-induced
cytotoxicity. When the intracellular Cu+ concentration
increases above a threshold, ATOX1 mediates Cu+ transfer
to ATP7A/B in the TGN, after which these proteins relocate to the
plasma membrane to export Cu+ (26). ATP7A and ATP7B thus serve central
roles in maintaining systemic copper balance. Dysfunction of these
ATPases leads to severe multisystem disorders such as Menkes
(52) and Wilson's disease
(53). Copper imbalance
contributes to cardiovascular disease (54), retinal disorders (55) and tumorigenesis (43) and also perturbs mitochondrial
respiration, glycolysis, insulin resistance and lipid metabolism
(56-58).
As a heavy metal element, copper is typically
associated with toxicity, however, copper also has essential
physiological functions as a cofactor for numerous proteins and
enzymes (5). For example,
cytochrome c oxidase is a copper-dependent enzyme that is key for
the final step in the electron transport chain, where it
facilitates the transfer of electrons to oxygen during oxidative
phosphorylation (59). SOD1),
another copper-containing enzyme, protects cells from oxidative
damage by converting superoxide radicals to hydrogen peroxide and
oxygen (60). Its versatile
redox activity, which involves cycling between Cu+ and
Cu2+, enables copper to serve as a crucial catalytic
cofactor in biochemical reactions (61), including oxidative stress
regulation (1,2), cellular respiration (56), neurotransmitter synthesis and
metabolism (62) and epigenetic
modification (63). In addition
to redox functions, copper contributes to hematopoiesis, immune
regulation, melanin and connective tissue formation and central
nervous system protection (8,64,65). Copper has also been implicated in
cancer diagnosis and therapy. Advances in metal-based medicine have
focused on achieving targeted toxicity through chemical
coordination and controlled drug delivery (19,66). Conversely, copper-depleting
therapy [tetrathiomolybdate (TTM)] exerts antitumor effects by
chelating copper in the serum, decreasing vascular endothelial
growth factor expression and modulating the immunosuppressive TME.
These mechanisms demonstrate antimetastatic and antiangiogenic
effects in models of breast, colorectal and hepatocellular
carcinoma (HCC) (67-69). Although other metals, such as
platinum and technetium, have been applied in chemotherapy, imaging
and radiotherapy (70,71), the dual nature of copper, in
which it is both physiologically essential and potentially toxic,
links it with energy metabolism and immune regulation.
Consequently, copper homeostasis has emerged as a frontier topic in
the study of metal-based anticancer therapeutics (72,73).
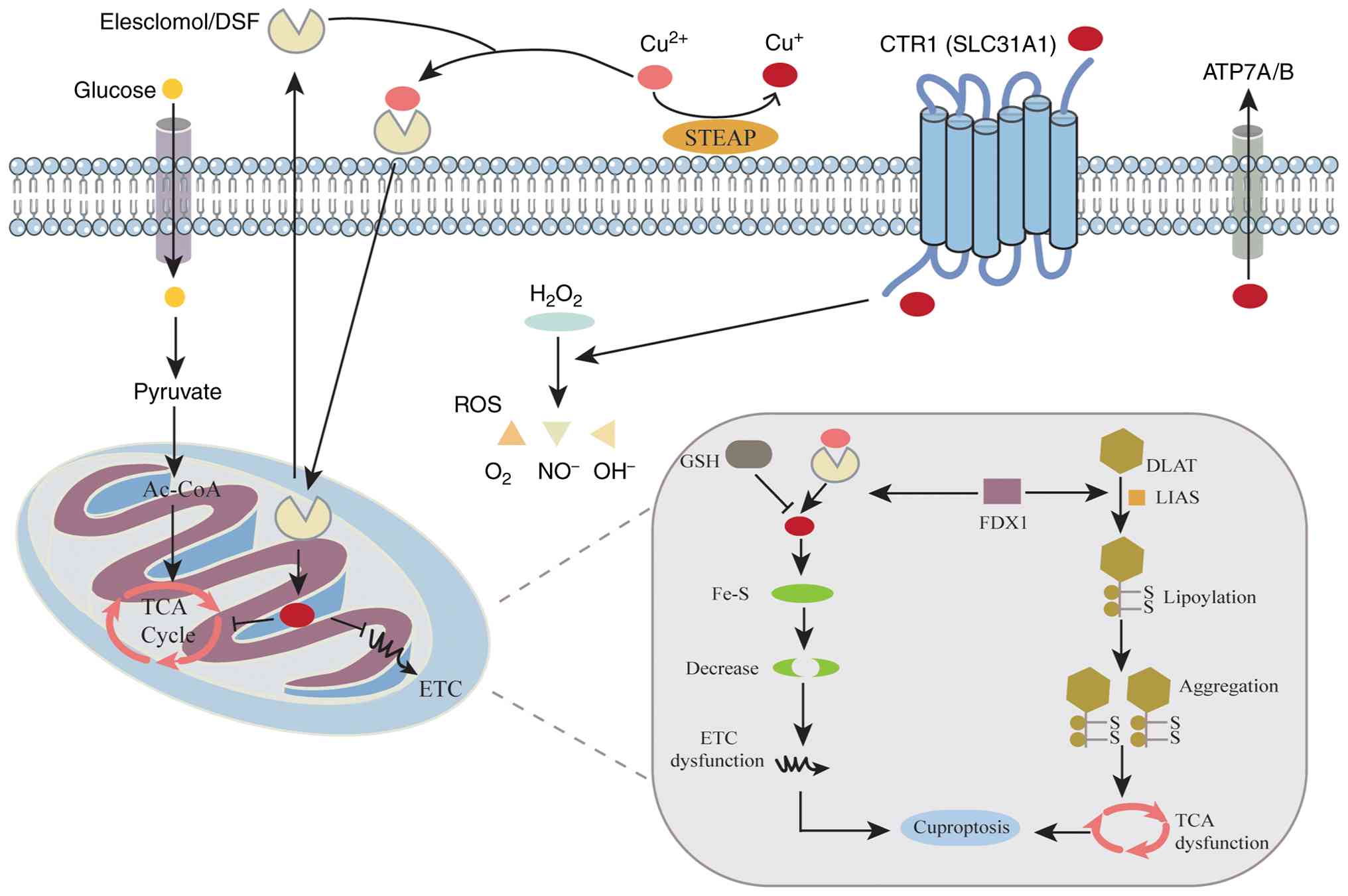
Copper can trigger multiple forms of cell death,
including apoptosis, oxidative stress-induced necrosis, autophagy
and ferroptosis (74). Recent
study have revealed that under the action of copper ionophores such
as elesclomol (ES), copper induces cuproptosis, a distinct form of
PCD, through unique mechanisms involving the disruption of
iron-sulfur (Fe-S) cluster proteins and the induction of lipid
peroxidation (19). The key
hallmark of cuproptosis is the abnormal aggregation of lipoylated
proteins and depletion of Fe-S cluster proteins, leading to
mitochondrial contraction, chromatin fragmentation and cell
membrane rupture (75).
Mechanistically, ES transports Cu2+ into the cytoplasm,
where it is reduced to Cu+ by ferredoxin 1 (FDX1). The
reduced Cu+ enters mitochondria and binds directly to
key lipoylated enzymes in the TCA cycle, such as dihydrolipoamide
S-acetyltransferase (DLAT) and dihydrolipoamide
S-succinyltransferase (DLST). This interaction induces protein
aggregation and the loss of Fe-S clusters (19). Fe-S clusters are key cofactors
for numerous mitochondrial enzymes and respiratory chain complexes,
such as succinate dehydrogenase subunit A and NADH:ubiquinone
oxidoreductase subunit S1. Their disruption impairs the electron
transport chain and decreases ATP synthesis and mitochondrial
membrane potential (76).
Consequently, mitochondrial energy production decreases,
accompanied by increased inner membrane permeability, elevated
Ca2+ concentration and the accumulation of ROS (77). The aggregation of lipoylated
proteins further induces proteotoxic stress, disrupting
proteostasis and exacerbating mitochondrial injury. Together, these
molecular events define the mitochondrial mechanism of cuproptosis
(Fig. 2).
Increasing evidence has demonstrated that the
dysregulation of copper metabolism is associated with the onset and
progression of various diseases, particularly malignancy (6,7,103). As a key signaling metal, copper
participates in cancer development by promoting cell proliferation,
angiogenesis and metastasis (104). Copper serves dual roles in
cancer biology; it is indispensable for cellular metabolism, but
its dysregulation is associated with oncogenesis. Elevated copper
levels have been detected in tumor tissue or serum in patients with
multiple types of cancer, including breast (105-109), lung (110-112) and gastrointestinal cancer
(113-116), oral (117), thyroid (118) and gallbladder carcinoma
(119) and gynecological
(115,116) and prostate cancer (120). These findings indicate that
copper is not only a key factor in tumor growth and metastasis but
also a necessary micronutrient for tumor cells (73,121). Mechanistically, copper promotes
tumor progression through multiple pathways. First, copper
stimulates angiogenesis by activating angiogenic factors and
enhancing the proliferation and migration of vascular endothelial
cells (122), thereby
supporting tumor initiation, growth and metastasis (73,74,123,124). Newly formed vasculature
provides key nutrients and serves as a conduit for tumor cell
dissemination. Second, copper serves as a cofactor for several
metalloenzymes, including MMP-9, SOD1, vascular adhesion protein-1
and lysyl oxidase (LOX), all of which are key for cancer invasion
and metastasis (125-128). The ATOX1-ATP7A-LOX axis
promotes metastatic dissemination by facilitating the
copper-dependent activation of LOX and LOX-like enzymes, which
remodel the extracellular matrix and increase tumor invasiveness
(73,129). Third, copper activates the
MAPK/ERK signaling pathway, thereby promoting tumor cell
proliferation (130). In
addition to these direct mechanisms, copper also modulates the TME
to promote cancer progression. Copper influences tumor metabolic
reprogramming, enhancing cell survival under hypoxic conditions
(6). Moreover, copper
contributes to immune evasion by regulating immune cell activity or
promoting the expansion of immunosuppressive cell populations,
allowing tumor cells to escape host immune surveillance (131). For example, copper alters
macrophage polarization, shifting tumor-associated macrophages
toward the M2 phenotype, which enhances immune suppression and
facilitates tumor invasion and metastasis (11,132). Collectively, these findings
suggest that copper serves multiple roles in tumorigenesis and
disease progression, including roles in cell proliferation,
angiogenesis, metastasis, metabolic reprogramming and immune
escape, positioning copper as both a potential biomarker and
therapeutic target in cancer biology.
The global incidence of ESCA in 2020 was ~604,000
new cases, and about 544,000 people died from ESCA; ESCA ranks 11th
in terms of global cancer incidence and 7th in terms of
cancer-associated mortality (133). Despite notable advances in
diagnosis and therapy, the 5-year survival rate of patients with
esophageal squamous cell carcinoma remains 20% owing to its late
detection and rapid progression (134). Therefore, identifying novel
diagnostic biomarkers and therapeutic strategies is key for
improving prognosis and survival outcomes. Methyltransferase-like 3
(METTL3) expression is markedly elevated in ESCA tissue compared
with that in normal esophageal epithelium, particularly in highly
malignant tumors, and is associated with disease progression
(135). Liu et al
(136) investigated the role of
METTL3 in ESCA and revealed its association with glycolysis,
cuproptosis and the competing endogenous (ce) RNA regulatory
network. These findings demonstrated that METTL3 serves as a
critical mediator of ESCA progression by modulating
glycolysis-associated gene expression. Upregulation of METTL3 in
esophageal carcinoma enhances glycolytic flux, increases glucose
uptake and lactate production and promotes tumor growth (128). Moreover, METTL3 expression is
associated with the expression of genes associated with
cuproptosis. Mechanistically, METTL3 serves dual regulatory roles
in glycolysis and cuproptosis via N6-methyladenosine (m6A) RNA
modification. Specifically, METTL3 alters and stabilizes
glycolysis-associated mRNAs, thereby altering tumor cell energy
metabolism and proliferation. Concurrently, METTL3 may regulate
genes associated with copper homeostasis, affecting intracellular
copper levels and inducing cell death. Additionally, METTL3 is
involved in the ceRNA network, in which long non-coding (lnc) and
circular RNAs compete for shared microRNAs (miRNAs or miRs),
influencing posttranscriptional regulation. By modulating the
stability or function of ceRNA components, METTL3 indirectly
affects gene expression and tumor progression. These findings
indicate that targeting METTL3 and its associated pathways may
represent a promising therapeutic strategy for ESCA, especially in
highly glycolytic and copper-enriched subtypes. The aforementioned
study provides valuable insights into the epigenetic and metabolic
regulation of ESCA, underscoring the potential of METTL3 as a
therapeutic target that links epigenetic modification, metabolic
reprogramming and copper-induced cell death. Further study have
identified the cuproptosis-associated genes Centromere Protein E
and SHC SH2 Domain-Binding Protein 1 as key biomarkers of Barrett's
esophagus (BE) progression to esophageal adenocarcinoma (EAC)
(137). These genes may
influence the immune microenvironment and promote the
transformation from BE to EAC, offering molecular targets for early
diagnosis and treatment. Metabolic abnormalities (lactate
accumulation and mitochondrial dysfunction) within tumor cells and
the surrounding microenvironment impair immune responses and
promote immune evasion (33).
Moreover, disruption of metal ion homeostasis, particularly copper
imbalance, alters oxidative stress and mitochondrial metabolism,
further influencing tumor survival and therapeutic resistance
(32,138). Therefore, integrating
cuproptosis pathways into chemoradiotherapy or immunotherapy
regimens may enhance radiosensitivity and remodel the tumor immune
microenvironment, offering a rational framework for combination
treatment strategies in ESCA.
GC is an epithelial malignancy originating in the
stomach. In 2022, there were ~968,000 new cases of GC worldwide,
accounting for 4.9% of all new cancer cases, making it the fifth
most common cancer by incidence globally (139). At the same time, gastric cancer
accounted for approximately 6.8% of cancer-related deaths globally,
with around 660,000 deaths, ranking fifth among the leading causes
of cancer death worldwide (139). Disruptions in PCD serve a
crucial role in GC pathogenesis (140,141). Copper levels are notably
elevated in gastric tumor tissue compared with normal gastric
mucosa, particularly in high-grade malignancy. Moreover, the copper
content is positively associated with the TNM stage of GC (142). In a recent study, Sun et
al (79) investigated the
mechanism of cuproptosis in GC, identifying its association with
METTL16, an atypical m6A RNA METTL involved in m6A modification.
METTL16 serves as a key mediator of cuproptosis by regulating FDX1
mRNA through m6A modification (79). Elevated copper levels in GC
tissue promote the lactylation of METTL16 at lysine residue K229,
which enhances its enzymatic activity and upregulates FDX1
expression, inducing cell death. Mechanistically, SIRT2 serves as a
critical delactylase, removing lactyl groups from METTL16-K229 and
inhibiting METTL16 activity. Treatment with
(Z)-2-cyano-3-[5-(2,5-dichlorophenyl)
furan-2-yl]-N-quinolin-5-ylprop-2-enamide, a selective SIRT2
inhibitor, increases METTL16 lactylation and FDX1 expression,
thereby promoting cuproptosis. These findings suggest that the
combined use of copper ionophores (such as ES) and SIRT2 inhibitors
may represent a promising therapeutic strategy for GC, particularly
in aggressive, high-copper and high-lactate subtypes such as
mucinous adenocarcinoma. Sun et al provided valuable
mechanistic insight into the role of METTL16-mediated RNA
modification and lactylation in copper-induced cell death,
highlighting the therapeutic potential of targeting the
METTL16-SIRT2-FDX1 axis in GC treatment. Polypyrimidine
tract-binding protein 3 (PTBP3) is markedly upregulated in
peritoneal metastases of GC and is associated with poor prognosis
(143). Single-cell RNA
sequencing and transcriptome analysis reveal that PTBP3 regulates
its downstream target COX11, impairing its function and decreasing
the mitochondrial copper content, which enables tumor cells to
evade cuproptosis. Researchers have developed an antisense
oligonucleotide (ASO) targeting the short isoform of COX11 pre-mRNA
exon 4 (136), effectively
degrading COX11 mRNA and disrupting copper homeostasis. In a
patient-derived organoid xenograft model combination therapy using
exogenous copper ionophores and ASO drugs leads to excessive
mitochondrial copper accumulation, proteotoxic stress and the
induction of cuproptosis, thereby suppressing peritoneal metastasis
(143). This provides a new
therapeutic strategy targeting PTBP3-mediated COX11 splicing to
restore copper-dependent cell death in metastatic GC. In stomach
adenocarcinoma (STAD), FDX1, lipoic Acid Synthase (LIAS), Metal
Regulatory Transcription Factor 1 (MTF1) and Pyruvate Dehydrogenase
E1 Subunit Alpha 1 have been identified as key genes associated
with cuproptosis (144). FDX1
is highly expressed in STAD tumor tissues, associated with poor
prognosis and increased chemosensitivity to cisplatin and
5-fluorouracil, making FDX1 a potential predictive biomarker for
chemotherapy response (145).
LIAS and MTF1 exhibit notable prognostic value, where higher
expression levels are associated with improved survival.
Collectively, these molecular markers not only contribute to
prognostic evaluation but also provide a foundation for the
development of copper-targeted anticancer drugs. Aberrant copper
homeostasis in GC is associated with mitochondrial dysfunction and
ROS accumulation, whereas cuproptosis promotes energy metabolism
collapse and cell death (146,147). Future studies should explore
the potential synergy between copper-modulating drugs (such as ES)
and immunotherapy or antiangiogenic therapy with the aim of
achieving a coordinated antitumor effect through the simultaneous
regulation of metabolic, immune and redox networks.
Based on GLOBOCAN 2020, HCC ranks sixth in incidence
and third in cancer-associated mortality worldwide (148). Although notable therapeutic
progress has been made over the past decade, the prognosis of HCC
remains poor, largely because most patients are diagnosed at
advanced stages, precluding surgical or localized treatment
(149,150). Hence, identifying effective
molecular targets and therapeutic strategies for HCC is key.
Clinical studies have reported significantly elevated serum copper
levels (151,152), increased copper-protein
complexes and enhanced expression of copper-binding proteins in
patients with HCC (153,154),
as well as the downregulation of copper transporters such as
ATP7A/B and SLC31A1/2 (155).
Copper concentrations in HCC tissue are markedly higher than those
in normal liver tissue and elevated serum copper levels are
associated with tumor progression (156). In a recent study, Li et
al (157) investigated the
mechanism of cuproptosis in HCC, focusing on DLAT, a key gene
associated with copper-induced cell death. The aforementioned study
reported that maternal embryonic leucine zipper kinase (MELK)
serves as a key mediator of cuproptosis by activating the PI3K/mTOR
signaling pathway. Elevated copper levels in HCC promote MELK
expression and activity, which upregulate DLAT expression and
support mitochondrial function, thus facilitating HCC progression.
Mechanistically, treatment with the copper ionophore ES decreases
the expression of translocase of outer mitochondrial membrane 20
and enhances DLAT oligomerization, thereby suppressing MELK
activity and triggering cuproptosis. These findings indicate that
the combination of copper ionophores and PI3K/mTOR pathway
inhibitors may represent a promising therapeutic approach for
treating HCC. Li et al (157) provided key mechanistic insight
into the MELK-DLAT regulatory axis, highlighting its potential as a
therapeutic target in liver cancer. Exposure of Hep3B hepatoma
cells to Cu2+ suppresses histone acetyltransferase
activity, leading to hypoacetylation of histones H3 and H4, which
promotes cell proliferation (19). Copper also binds pyruvate
dehydrogenase kinase 1 (PDK1), enhancing its interaction with AKT,
thereby activating the PDK1-AKT oncogenic signaling cascade and
promoting HCC progression (158). Conversely, copper chelators or
CTR1 inhibitors downregulate the CTR1-AKT axis, thus inhibiting
tumor growth (158). CTR1 is
aberrantly upregulated in breast cancer and is negatively regulated
by NEDD4-like E3 ubiquitin ligase (NEDD4l), which promotes CTR1
ubiquitination and degradation. NEDD4l exerts tumor-suppressive
effects by inhibiting CTR1-mediated AKT signaling. These findings
reveal an association between the CTR1/Cu pathway and PDK1-AKT
oncogenic signaling, underscoring the therapeutic potential of
targeting the CTR1-Cu axis in cancers driven by AKT
hyperactivation. By contrast with copper accumulation, FDX1
expression is notably lower in HCC tissues than in normal liver
tissue, and its expression is positively associated with patient
survival, with higher FDX1 expression predicting longer overall
survival. Moreover, FDX1 levels are positively associated with
oxaliplatin sensitivity. In a recent study, Quan et al
(159) elucidated the molecular
mechanism of cuproptosis in HCC, focusing on the FDX1-associated
lncRNA/miRNA regulatory axis. Through bioinformatic analyses, the
aforementioned study identified LINC02362 as a ceRNA that modulates
miR-18a-5p, which directly targets FDX1. Upregulation of the
LINC02362/miR-18a-5p/FDX1 pathway signaling suppresses HCC cell
proliferation. Conversely, LINC02362 knockdown decreases
intracellular copper concentrations and induces resistance to
ES-Cu-mediated cell death. Additionally, upregulation of this axis
enhances the sensitivity of HCC to oxaliplatin by promoting
cuproptosis. These findings suggest that LINC02362/miR-18a-5p/FDX1
is a novel regulatory pathway capable of overcoming oxaliplatin
resistance in HCC through cuproptotic mechanisms. Recent
preclinical studies have shown that copper chelators, including
triethylenetetramine and D-penicillamine, significantly decrease
copper levels and inhibit tumor growth in HCC (160). By restoring copper homeostasis,
these agents may also overcome resistance to conventional therapy,
supporting their potential clinical use in HCC treatment. Emerging
research indicates that metabolic reprogramming and copper
imbalance are common features of HCC (161). Excess copper disrupts
mitochondrial oxidative phosphorylation and lipoyl enzyme complex
activity, leading to lipid metabolic disorder and energy collapse
(19). Moreover, the expression
of genes associated with cuproptosis, such as FDX1, LIAS and DLAT,
is downregulated in HCC tissue and the expression of these genes is
significantly associated with immune phenotypes and overall
survival (162). In conclusion,
copper serves as both a metabolic cofactor and a potential
therapeutic target in HCC. Regulation of copper levels or
exploitation of its bioactive properties offers a promising avenue
for novel anticancer strategies. Future studies should elucidate
the mechanisms of copper-targeted therapy, optimize therapeutic
regimens and conduct rigorous clinical trials to validate their
safety and efficacy.
According to GLOBOCAN 2020, pancreatic cancer is a
highly aggressive malignancy, ranking twelfth in incidence but
sixth in overall cancer-associated mortality worldwide (163). Despite advances in treatment,
the 5-year overall survival rate remains ~ 10% (164). Pancreatic cancer remains among
the most common types of treatment-refractory malignancy. Elevated
serum copper levels may contribute to pancreatic cancer development
(165). Novel
nanomaterial-based strategies to exploit cuproptosis for
therapeutic benefit (166). For
example, tussah silk fibroin (TSF)-based nanoparticles (NPs) use
TME-responsive release mechanisms to deliver copper and the
cuproptosis-inducing drug ES directly to pancreatic cancer cells.
Upon targeted delivery, TSF@ ES-Cu NPs induce cuproptosis,
releasing damage-associated molecular patterns that activate
antitumor immunity (166). This
promotes dendritic cell maturation and macrophage M1 polarization,
thereby reshaping the TME and enhancing immune responses.
Collectively, these findings demonstrate that TSF@ ES-Cu NPs
suppress pancreatic cancer growth through a dual mechanism of
cuproptosis induction and immune microenvironment remodeling,
offering a promising avenue for clinical translation. In addition,
other research have explored the prognostic and therapeutic roles
of cuproptosis-associated lncRNAs in pancreatic ductal
adenocarcinoma (PAAD) (167).
Researchers have developed a cuproptosis-immune-related (CIR) score
to characterize the interaction between cuproptosis and the tumor
immune microenvironment (168).
By integrating single-cell sequencing and transcriptomic data,
researchers have identified immune- and cuproptosis-related genes
associated with PAAD and constructed a CIR score model (168). This score not only predicts
prognosis in patients and the immune landscape but also reflects
the tumor mutational burden (TMB), immune checkpoint sensitivity,
and drug responsiveness. Patients in the high CIR score group
exhibit higher TMB and poorer survival, whereas those in the low
CIR score group exhibit stronger immune activation and greater
potential responsiveness to immunotherapy. Mouse model experiments
have validated the predictive power of the CIR score in guiding
combination therapy involving immunotherapy, targeted therapy and
chemotherapy (168). Such
combined regimens significantly inhibit PAAD progression,
highlighting the translational potential of cuproptosis-based
prognostic markers in personalized immunotherapy. Mechanistically,
these findings indicate that the cuproptosis-metabolic
reprogramming-immune activation axis represents a novel paradigm
for the systemic treatment of pancreatic cancer. The integration of
copper homeostasis regulation into individualized therapeutic
frameworks provides both theoretical and practical foundations for
precision medicine in PAAD.
CRC, one of the most common malignant tumors of the
digestive system, is the third most commonly diagnosed cancer
worldwide, with approximately 1.9 million new cases annually
(169). Cuproptosis-related
genes, such as FDX1, SDHB, DLAT and DLST, are expressed at higher
levels in normal compared with tumor tissues (170). Moreover, higher expression of
these genes in tumor tissue is associated with better prognosis
(170). When ES, a copper
carrier, combines with copper, the proliferation of CRC cells is
significantly inhibited and apoptosis is promoted. This effect is
markedly suppressed by the copper chelator TTM, further confirming
the mechanism of copper-induced cell death. Furthermore,
2-deoxy-D-glucose, a glycolysis inhibitor, can significantly
enhance cuproptosis (170). In
addition, galactose promotes oxidative phosphorylation by
inhibiting the glycolytic pathway in tumor cells, thereby enhancing
copper-induced cell death (170). These results indicate that the
inhibition of glycolysis can increase the sensitivity of tumor
cells to cuproptosis. Studies have also shown that 4-octyl
itaconate (4-OI), a cell-permeable derivative of itaconic acid,
inhibits glycolysis by targeting the key glycolytic enzyme GAPDH,
thereby enhancing cuproptosis. 4-OI suppresses GAPDH activity,
decreases lactate production and subsequently promotes
copper-induced cell death. Furthermore, in vivo experiments
demonstrated that 4-OI has significant antitumor effects and that
its combination with ES markedly decreases tumor volume. Yang et
al (170) provided new
insights into the role of cuproptosis in CRC and revealed that 4-OI
enhances copper-induced cell death by inhibiting glycolysis. These
findings not only provide a novel therapeutic strategy for CRC but
also establish theoretical support for cuproptosis as a potential
anticancer approach. Cuproptosis-associated genes demonstrate
important prognostic value in colon adenocarcinoma because their
expression levels are associated with patient survival, TME
characteristics and drug sensitivity (171). To the best of our knowledge,
the aforementioned study was the first to systematically analyze
the roles of genes associated with cuproptosis in colon
adenocarcinoma and to elucidate their potential mechanisms in tumor
progression and immune microenvironment regulation. Similarly,
genes associated with cuproptosis serve a significant role in the
prognosis and treatment of rectal adenocarcinoma (172). Copper chelators or carriers may
synergize with chemotherapy or immunotherapy, thereby improving the
prognosis of liver-metastatic CRC (173).
Cuproptosis-associated drugs have demonstrated
antitumor potential in clinical trials for gastrointestinal cancer
(174,175). For example, the
disulfiram/copper complex inhibits the proliferation of GC cells by
inducing oxidative stress and DNA damage while increasing the
sensitivity of tumor cells to chemotherapeutic agents (174). In addition, TTM, a copper
chelator, exerts antiangiogenic and chemosensitizing effects in
metastatic CRC (175). The
potential clinical applications of these drugs include combining
them with chemotherapy to enhance therapeutic efficacy, targeting
copper metabolism-associated proteins to improve treatment
precision and using biomarkers such as serum copper levels to guide
medication strategies. Nevertheless, further research is needed to
optimize the design of cuproptosis-associated drugs to improve
their efficacy and decrease side effects. However, future in-depth
studies on the molecular mechanisms of these drugs and additional
clinical trials to verify their safety and effectiveness are
needed.
As a newly identified form of regulated cell death,
cuproptosis has become a prominent focus in tumor biology,
particularly in gastrointestinal cancer. The present review
summarizes the mechanistic pathways and therapeutic potential of
cuproptosis, emphasizing key molecular events such as mitochondrial
damage, oxidative stress and protein lipoylation, as well as copper
imbalance and its association with tumor initiation and
development. Current evidence indicates that disruption of copper
metabolism is associated with tumor growth and progression. By
modulating intracellular copper levels, cuproptosis inhibits tumor
cell proliferation through various mechanisms, including the
induction of PCD and the enhancement of antitumor immune responses.
Moreover, cuproptosis-associated drugs, such as disulfiram/copper
complexes and TTM, have demonstrated promising anticancer potential
in preclinical and clinical studies because they induce oxidative
stress, increase chemosensitivity and inhibit angiogenesis.
However, the clinical application of these drugs faces several
challenges, including limited efficacy, toxicity management and a
lack of large-scale validation. Future studies should further
clarify the molecular mechanisms of cuproptosis, develop novel
copper-dependent therapeutic agents with improved selectivity and
safety and conduct clinical trials to assess their translational
value. In conclusion, cuproptosis offers a novel conceptual and
therapeutic direction for gastrointestinal oncology, holding
promise for treatment options that could improve prognosis and
survival outcomes.
Not applicable.
LZ and YC conceived the study. LT, JZ and BT edited
the manuscript. Data authentication is not applicable. All authors
have read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by grants from the National
Natural Science Foundation of China (grant no. 82073087) and
Medical Research Union Fund for High-quality Health Development of
Guizhou Province (grant no. 2024GZYXKYJJXM0019).
|
1
|
Lutsenko S, Roy S and Tsvetkov P:
Mammalian copper homeostasis: Physiological roles and molecular
mechanisms. Physiol Rev. 105:441–491. 2025. View Article : Google Scholar :
|
|
2
|
Locatelli M and Farina C: Role of copper
in central nervous system physiology and pathology. Neural Regen
Res. 20:1058–1068. 2025. View Article : Google Scholar
|
|
3
|
Chen G, Li J, Han H, Du R and Wang X:
Physiological and molecular mechanisms of plant responses to copper
stress. Int J Mol Sci. 23:129502022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Verdejo-Torres O, Klein DC, Novoa-Aponte
L, Carrazco-Carrillo J, Bonilla-Pinto D, Rivera A, Bakhshian A,
Fitisemanu FM, Jiménez-González ML, Flinn L, et al: Cysteine rich
intestinal protein 2 is a copper-responsive regulator of skeletal
muscle differentiation and metal homeostasis. PLoS Genet.
20:e10114952024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen L, Min J and Wang F: Copper
homeostasis and cuproptosis in health and disease. Signal Transduct
Target Ther. 7:3782022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo Z, Chen D, Yao L, Sun Y, Li D, Le J,
Dian Y, Zeng F, Chen X and Deng G: The molecular mechanism and
therapeutic landscape of copper and cuproptosis in cancer. Signal
Transduct Target Ther. 10:1492025. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang Y, Wu J, Wang L, Ji G and Dang Y:
Copper homeostasis and cuproptosis in health and disease. MedComm
(2020). 5:e7242024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Myint ZW, Oo TH, Thein KZ, Tun AM and
Saeed H: Copper deficiency anemia: Review article. Ann Hematol.
97:1527–1534. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gromadzka G, Tarnacka B, Flaga A and
Adamczyk A: Copper dyshomeostasis in neurodegenerative
diseases-therapeutic implications. Int J Mol Sci. 21:92592020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sailer J, Nagel J, Akdogan B, Jauch AT,
Engler J, Knolle PA and Zischka H: Deadly excess copper. Redox
Biol. 75:1032562024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang S, Huang Q, Ji T, Li Q and Hu C:
Copper homeostasis and copper-induced cell death in tumor immunity:
Implications for therapeutic strategies in cancer immunotherapy.
Biomark Res. 12:1302024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang XR and Cull B: Apoptosis and
autophagy: Current understanding in tick-pathogen interactions.
Front Cell Infect Microbiol. 12:7844302022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hong Y, He J, Deng D, Liu Q, Zu X and Shen
Y: Targeting kinases that regulate programmed cell death: A new
therapeutic strategy for breast cancer. J Transl Med. 23:4392025.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bertheloot D, Latz E and Franklin BS:
Necroptosis, pyroptosis and apoptosis: An intricate game of cell
death. Cell Mol Immunol. 18:1106–1121. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao W, Wang X, Zhou Y, Wang X and Yu Y:
Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor
immunotherapy. Signal Transduct Target Ther. 7:1962022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rao Z, Zhu Y, Yang P, Chen Z, Xia Y, Qiao
C, Liu W, Deng H, Li J, Ning P and Wang Z: Pyroptosis in
inflammatory diseases and cancer. Theranostics. 12:4310–4329. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang X, Stockwell BR and Conrad M:
Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol
Cell Biol. 22:266–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Debnath J, Gammoh N and Ryan KM: Autophagy
and autophagy-related pathways in cancer. Nat Rev Mol Cell Biol.
24:560–575. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsvetkov P, Coy S, Petrova B, Dreishpoon
M, Verma A, Abdusamad M, Rossen J, Joesch-Cohen L, Humeidi R,
Spangler RD, et al: Copper induces cell death by targeting
lipoylated TCA cycle proteins. Science. 375:1254–1261. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao G, Sun H, Zhang T and Liu JX: Copper
induce zebrafish retinal developmental defects via triggering
stresses and apoptosis. Cell Commun Signal. 18:452020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ostrakhovitch EA and Cherian MG: Role of
p53 and reactive oxygen species in apoptotic response to copper and
zinc in epithelial breast cancer cells. Apoptosis. 10:111–121.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, Du Y, Zhou Y, Chen Q, Luo Z, Ren Y,
Chen X and Chen G: Iron and copper: Critical executioners of
ferroptosis, cuproptosis and other forms of cell death. Cell Commun
Signal. 21:3272023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vana F, Szabo Z, Masarik M and
Kratochvilova M: The interplay of transition metals in ferroptosis
and pyroptosis. Cell Div. 19:242024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xue Q, Yan D, Chen X, Li X, Kang R,
Klionsky DJ, Kroemer G, Chen X, Tang D and Liu J: Copper-dependent
autophagic degradation of GPX4 drives ferroptosis. Autophagy.
19:1982–1996. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fu Y, Zeng S, Wang Z, Huang H, Zhao X and
Li M: Mechanisms of copper-induced autophagy and links with human
diseases. Pharmaceuticals (Basel). 18:992025. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xue Q, Kang R, Klionsky DJ, Tang D, Liu J
and Chen X: Copper metabolism in cell death and autophagy.
Autophagy. 19:2175–2195. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Y, Qiao S, Wang P, Li M, Ma X, Wang H
and Dong J: Copper's new role in cancer: How cuproptosis-related
genes could revolutionize glioma treatment. BMC Cancer. 25:8592025.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tang X, Yan Z, Miao Y, Ha W, Li Z, Yang L
and Mi D: Copper in cancer: From limiting nutrient to therapeutic
target. Front Oncol. 13:12091562023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shoda K, Kawaguchi Y, Maruyama S and
Ichikawa D: Essential updates 2023/2024: Recent advances of
multimodal approach in patients for gastric cancer. Ann
Gastroenterol Surg. 9:1119–1127. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ricci AD, Rizzo A and Brandi G: DNA damage
response alterations in gastric cancer: knocking down a new wall.
Future Oncol. 17:865–868. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rizzo A and Ricci AD: Challenges and
future trends of hepatocellular carcinoma immunotherapy. Int J Mol
Sci. 23:113632022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vitale E, Rizzo A, Santa K and Jirillo E:
Associations between 'Cancer Risk', 'Inflammation' and 'Metabolic
Syndrome': A scoping review. Biology (Basel). 13:3522024.
|
|
33
|
Brandi G, Ricci AD, Rizzo A, Zanfi C,
Tavolari S, Palloni A, De Lorenzo S, Ravaioli M and Cescon M: Is
post-transplant chemotherapy feasible in liver transplantation for
colorectal cancer liver metastases? Cancer Commun (Lond).
40:461–464. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mason KE: A conspectus of research on
copper metabolism and requirements of man. J Nutr. 109:1979–2066.
1979. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Eisses JF and Kaplan JH: The mechanism of
copper uptake mediated by human CTR1: A mutational analysis. J Biol
Chem. 280:37159–37168. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zischka H and Einer C: Mitochondrial
copper homeostasis and its derailment in Wilson disease. Int J
Biochem Cell Biol. 102:71–75. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jiayi H, Ziyuan T, Tianhua X, Mingyu Z,
Yutong M, Jingyu W, Hongli Z and Li S: Copper homeostasis in
chronic kidney disease and its crosstalk with ferroptosis.
Pharmacol Res. 202:1071392024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen J, Jiang Y, Shi H, Peng Y, Fan X and
Li C: The molecular mechanisms of copper metabolism and its roles
in human diseases. Pflugers Arch. 472:1415–1429. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lutsenko S: Dynamic and cell-specific
transport networks for intracellular copper ions. J Cell Sci.
134:jcs2405232021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
McKie AT, Barrow D, Latunde-Dada GO, Rolfs
A, Sager G, Mudaly E, Mudaly M, Richardson C, Barlow D, Bomford A,
et al: An iron-regulated ferric reductase associated with the
absorption of dietary iron. Science. 291:1755–1759. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mastrogiannaki M, Matak P, Keith B, Simon
MC, Vaulont S and Peyssonnaux C: HIF-2alpha, but not HIF-1alpha,
promotes iron absorption in mice. J Clin Invest. 119:1159–1166.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kawamata H and Manfredi G: Import,
maturation, and function of SOD1 and its copper chaperone CCS in
the mitochondrial intermembrane space. Antioxid Redox Signal.
13:1375–1384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang Z, Jin D, Zhou S, Dong N, Ji Y, An P,
Wang J, Luo Y and Luo J: Regulatory roles of copper metabolism and
cuproptosis in human cancers. Front Oncol. 13:11234202023.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ohrvik H and Thiele DJ: How copper
traverses cellular membranes through the mammalian copper
transporter 1, Ctr1. Ann N Y Acad Sci. 1314:32–41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yu Q, Xiao Y, Guan M, Zhang X, Yu J, Han M
and Li Z: Copper metabolism in osteoarthritis and its relation to
oxidative stress and ferroptosis in chondrocytes. Front Mol Biosci.
11:14724922024. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim H, Wu X and Lee J: SLC31 (CTR) family
of copper transporters in health and disease. Mol Aspects Med.
34:561–570. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cobine PA, Moore SA and Leary SC: Getting
out what you put in: Copper in mitochondria and its impacts on
human disease. Biochim Biophys Acta Mol Cell Res. 1868:1188672021.
View Article : Google Scholar
|
|
48
|
Chen Z, Li YY and Liu X: Copper
homeostasis and copper-induced cell death: Novel targeting for
intervention in the pathogenesis of vascular aging. Biomed
Pharmacother. 169:1158392023. View Article : Google Scholar
|
|
49
|
Andersson M, Mattle D, Sitsel O, Klymchuk
T, Nielsen AM, Moller LB, White SH, Nissen P and Gourdon P:
Copper-transporting P-type ATPases use a unique ion-release
pathway. Nat Struct Mol Biol. 21:43–48. 2014. View Article : Google Scholar :
|
|
50
|
Telianidis J, Hung YH, Materia S and
Fontaine SL: Role of the P-Type ATPases, ATP7A and ATP7B in brain
copper homeostasis. Front Aging Neurosci. 5:442013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fanni D, Pilloni L, Orru S, Coni P,
Liguori C, Serra S, Lai ML, Uccheddu A, Contu L, Van Eyken P and
Faa G: Expression of ATP7B in normal human liver. Eur J Histochem.
49:371–378. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kaler SG: ATP7A-related copper transport
diseases-emerging concepts and future trends. Nat Rev Neurol.
7:15–29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yang GM, Xu L, Wang RM, Tao X, Zheng ZW,
Chang S, Ma D, Zhao C, Dong Y, Wu S, et al: Structures of the human
Wilson disease copper transporter ATP7B. Cell Rep. 42:1124172023.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Fukai T, Ushio-Fukai M and Kaplan JH:
Copper transporters and copper chaperones: Roles in cardiovascular
physiology and disease. Am J Physiol Cell Physiol. 315:C186–C201.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ugarte M, Osborne NN, Brown LA and Bishop
PN: Iron, zinc, and copper in retinal physiology and disease. Surv
Ophthalmol. 58:585–609. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ishida S, Andreux P, Poitry-Yamate C,
Auwerx J and Hanahan D: Bioavailable copper modulates oxidative
phosphorylation and growth of tumors. Proc Natl Acad Sci USA.
110:19507–19512. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wooton-Kee CR, Robertson M, Zhou Y, Dong
B, Sun Z, Kim KH, Liu H, Xu Y, Putluri N, Saha P, et al: Metabolic
dysregulation in the Atp7b(-/-) Wilson's disease mouse model. Proc
Natl Acad Sci USA. 117:2076–2083. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yang H, Ralle M, Wolfgang MJ, Dhawan N,
Burkhead JL, Rodriguez S, Kaplan JH, Wong GW, Haughey N and
Lutsenko S: Copper-dependent amino oxidase 3 governs selection of
metabolic fuels in adipocytes. PLoS Biol. 16:e20065192018.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kim E, Chufan EE, Kamaraj K and Karlin KD:
Synthetic models for heme-copper oxidases. Chem Rev. 104:1077–1133.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Anwar S, Sarwar T, Khan AA and Rahmani AH:
Therapeutic applications and mechanisms of superoxide dismutase
(SOD) in different pathogenesis. Biomolecules. 15:11302025.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Grubman A and White AR: Copper as a key
regulator of cell signalling pathways. Expert Rev Mol Med.
16:e112014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Heffern MC, Park HM, Au-Yeung HY, Van de
Bittner GC, Ackerman CM, Stahl A and Chang CJ: In vivo
bioluminescence imaging reveals copper deficiency in a murine model
of nonalcoholic fatty liver disease. Proc Natl Acad Sci USA.
113:14219–14224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Herranz N, Dave N, Millanes-Romero A,
Morey L, Diaz VM, Lorenz-Fonfria V, Gutierrez-Gallego R, Jerónimo
C, Di Croce L, García de Herreros A and Peiró S: Lysyl oxidase-like
2 deaminates lysine 4 in histone H3. Mol Cell. 46:369–376. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Percival SS: Copper and immunity. Am J
Clin Nutr. 67(5 Suppl): 1064S–1068S. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
An Y, Li S, Huang X, Chen X, Shan H and
Zhang M: The role of copper homeostasis in brain disease. Int J Mol
Sci. 23:138502022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cheng YF, Zhao YJ, Chen C and Zhang F:
Heavy metals toxicity: Mechanism, health effects, and therapeutic
interventions. MedComm (2020). 6:e702412025. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zheng P, Zhou C, Lu L, Liu B and Ding Y:
Elesclomol: A copper ionophore targeting mitochondrial metabolism
for cancer therapy. J Exp Clin Cancer Res. 41:2712022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kong R and Sun G: Targeting copper
metabolism: A promising strategy for cancer treatment. Front
Pharmacol. 14:12034472023. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Nan L, Yuan W, Guodong C and Yonghui H:
Multitargeting strategy using tetrathiomolybdate and lenvatinib:
Maximizing antiangiogenesis activity in a preclinical liver cancer
model. Anticancer Agents Med Chem. 23:786–793. 2023. View Article : Google Scholar
|
|
70
|
Cohen R, Raeisi M, Chibaudel B, Yothers G,
Goldberg RM, Bachet JB, Wolmark N, Yoshino T, Schmoll HJ, Haller
DG, et al: Impact of tumor and node stages on the efficacy of
adjuvant oxaliplatin-based chemotherapy in stage III colon cancer
patients: An ACCENT pooled analysis. ESMO Open. 10:1044812025.
View Article : Google Scholar
|
|
71
|
Nawar MF and Turler A: New strategies for
a sustainable (99m) Tc supply to meet increasing medical demands:
Promising solutions for current problems. Front Chem.
10:9262582022. View Article : Google Scholar
|
|
72
|
Wang W, Mo W, Hang Z, Huang Y, Yi H, Sun Z
and Lei A: Cuproptosis: Harnessing transition metal for cancer
therapy. ACS Nano. 17:19581–19599. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ge EJ, Bush AI, Casini A, Cobine PA, Cross
JR, DeNicola GM, Dou QP, Franz KJ, Gohil VM, Gupta S, et al:
Connecting copper and cancer: From transition metal signalling to
metalloplasia. Nat Rev Cancer. 22:102–113. 2022. View Article : Google Scholar :
|
|
74
|
Li Y: Copper homeostasis: Emerging target
for cancer treatment. IUBMB Life. 72:1900–1908. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Cong Y, Li N, Zhang Z, Shang Y and Zhao H:
Cuproptosis: Molecular mechanisms, cancer prognosis, and
therapeutic applications. J Transl Med. 23:1042025. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Shim D and Han J: Coordination chemistry
of mitochondrial copper metalloenzymes: Exploring implications for
copper dyshomeostasis in cell death. BMB Rep. 56:575–583. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Cheng H, Yang B, Ke T, Li S, Yang X,
Aschner M and Chen P: Mechanisms of metal-induced mitochondrial
dysfunction in neurological disorders. Toxics. 9:1422021.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Tsvetkov P, Detappe A, Cai K, Keys HR,
Brune Z, Ying W, Thiru P, Reidy M, Kugener G, Rossen J, et al:
Mitochondrial metabolism promotes adaptation to proteotoxic stress.
Nat Chem Biol. 15:681–689. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Sun L, Zhang Y, Yang B, Sun S, Zhang P,
Luo Z, Feng T, Cui Z, Zhu T, Li Y, et al: Lactylation of METTL16
promotes cuproptosis via m(6)A-modification on FDX1 mRNA in gastric
cancer. Nat Commun. 14:65232023. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Polishchuk EV, Concilli M, Iacobacci S,
Chesi G, Pastore N, Piccolo P, Paladino S, Baldantoni D, van
IJzendoorn SC, Chan J, et al: Wilson disease protein ATP7B utilizes
lysosomal exocytosis to maintain copper homeostasis. Dev Cell.
29:686–700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Yang L, Zhang Y, Wang Y, Jiang P, Liu F
and Feng N: Ferredoxin 1 is a cuproptosis-key gene responsible for
tumor immunity and drug sensitivity: A pan-cancer analysis. Front
Pharmacol. 13:9381342022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Sheftel AD, Stehling O, Pierik AJ,
Elsasser HP, Muhlenhoff U, Webert H, Hobler A, Hannemann F,
Bernhardt R and Lill R: Humans possess two mitochondrial
ferredoxins, Fdx1 and Fdx2, with distinct roles in steroidogenesis,
heme, and Fe/S cluster biosynthesis. Proc Natl Acad Sci USA.
107:11775–11780. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Huang X, Wang T, Ye J, Feng H and Zhang X,
Ma X, Wang B, Huang Y and Zhang X: FDX1 expression predicts
favourable prognosis in clear cell renal cell carcinoma identified
by bioinformatics and tissue microarray analysis. Front Genet.
13:9947412022. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Mayr JA, Zimmermann FA, Fauth C, Bergheim
C, Meierhofer D, Radmayr D, Zschocke J, Koch J and Sperl W: Lipoic
acid synthetase deficiency causes neonatal-onset epilepsy,
defective mitochondrial energy metabolism, and glycine elevation.
Am J Hum Genet. 89:792–797. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Cai Y, He Q, Liu W, Liang Q, Peng B, Li J,
Zhang W, Kang F, Hong Q, Yan Y, et al: Comprehensive analysis of
the potential cuproptosis-related biomarker LIAS that regulates
prognosis and immunotherapy of pan-cancers. Front Oncol.
12:9521292022. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Casteel J, Miernyk JA and Thelen JJ:
Mapping the lipoylation site of Arabidopsis thaliana plastidial
dihydrolipoamide S-acetyltransferase using mass spectrometry and
site-directed mutagenesis. Plant Physiol Biochem. 49:1355–1361.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Rumping L, Tessadori F, Pouwels PJW,
Vringer E, Wijnen JP, Bhogal AA, Savelberg SMC, Duran KJ, Bakkers
MJG, Ramos RJJ, et al: GLS hyperactivity causes glutamate excess,
infantile cataract and profound developmental delay. Hum Mol Genet.
28:96–104. 2019. View Article : Google Scholar
|
|
88
|
Rumping L, Buttner B, Maier O, Rehmann H,
Lequin M, Schlump JU, Schmitt B, Schiebergen-Bronkhorst B, Prinsen
HCMT, Losa M, et al: Identification of a loss-of-function mutation
in the context of glutaminase deficiency and neonatal epileptic
encephalopathy. JAMA Neurol. 76:342–350. 2019. View Article : Google Scholar :
|
|
89
|
Agarwal P, Sandey M, DeInnocentes P and
Bird RC: Tumor suppressor gene p16/INK4A/CDKN2A-dependent
regulation into and out of the cell cycle in a spontaneous canine
model of breast cancer. J Cell Biochem. 114:1355–1363. 2013.
View Article : Google Scholar
|
|
90
|
Serrano M, Hannon GJ and Beach D: A new
regulatory motif in cell-cycle control causing specific inhibition
of cyclin D/CDK4. Nature. 366:704–407. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Bian Z, Yu Y, Yang T, Quan C, Sun W and Fu
S: Effect of tumor suppressor gene cyclin-dependent kinase
inhibitor 2A wild-type and A148T mutant on the cell cycle of human
ovarian cancer cells. Oncol Lett. 7:1229–1232. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Samimi G, Safaei R, Katano K, Holzer AK,
Rochdi M, Tomioka M, Goodman M and Howell SB: Increased expression
of the copper efflux transporter ATP7A mediates resistance to
cisplatin, carboplatin, and oxaliplatin in ovarian cancer cells.
Clin Cancer Res. 10:4661–4669. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Tian Z, Jiang S, Zhou J and Zhang W:
Copper homeostasis and cuproptosis in mitochondria. Life Sci.
334:1222232023. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Mangala LS, Zuzel V, Schmandt R, Leshane
ES, Halder JB, Armaiz-Pena GN, Spannuth WA, Tanaka T, Shahzad MM,
Lin YG, et al: Therapeutic targeting of ATP7B in ovarian carcinoma.
Clin Cancer Res. 15:3770–3780. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Yun Y, Wang Y, Yang E and Jing X:
Cuproptosis-related gene - SLC31A1, FDX1 and ATP7B-polymorphisms
are associated with risk of lung cancer. Pharmgenomics Pers Med.
15:733–742. 2022.
|
|
96
|
Wang D, Tian Z, Zhang P, Zhen L, Meng Q,
Sun B, Xu X, Jia T and Li S: The molecular mechanisms of
cuproptosis and its relevance to cardiovascular disease. Biomed
Pharmacother. 163:1148302023. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Halliwell B, Adhikary A, Dingfelder M and
Dizdaroglu M: Hydroxyl radical is a significant player in oxidative
DNA damage in vivo. Chem Soc Rev. 50:8355–8360. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Chandimali N, Bak SG, Park EH, Lim HJ, Won
YS, Kim EK, Park SI and Lee SJ: Free radicals and their impact on
health and antioxidant defenses: A review. Cell Death Discov.
11:192025. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Wong-Ekkabut J, Xu Z, Triampo W, Tang IM,
Tieleman DP and Monticelli L: Effect of lipid peroxidation on the
properties of lipid bilayers: A molecular dynamics study. Biophys
J. 93:4225–4236. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Kehm R, Baldensperger T, Raupbach J and
Höhn A: Protein oxidation - Formation mechanisms, detection and
relevance as biomarkers in human diseases. Redox Biol.
42:1019012021. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Vo TTT, Peng TY, Nguyen TH, Bui TNH, Wang
CS, Lee WJ, Chen YL, Wu YC and Lee IT: The crosstalk between
copper-induced oxidative stress and cuproptosis: A novel potential
anticancer paradigm. Cell Commun Signal. 22:3532024. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Chen W, Jiang T, Wang H, Tao S, Lau A,
Fang D and Zhang DD: Does Nrf2 contribute to p53-mediated control
of cell survival and death? Antioxid Redox Signal. 17:1670–1675.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Shan D, Song J, Ren Y, Zhang Y, Ba Y, Luo
P, Cheng Q, Xu H, Weng S, Zuo A, et al: Copper in cancer: Friend or
foe? Metabolism, dysregulation, and therapeutic opportunities.
Cancer Commun (Lond). 45:577–607. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Dow JA: The essential roles of metal ions
in insect homeostasis and physiology. Curr Opin Insect Sci.
23:43–50. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Ding X, Jiang M, Jing H, Sheng W, Wang X,
Han J and Wang L: Analysis of serum levels of 15 trace elements in
breast cancer patients in Shandong, China. Environ Sci Pollut Res
Int. 22:7930–7935. 2015. View Article : Google Scholar
|
|
106
|
Kuo HW, Chen SF, Wu CC, Chen DR and Lee
JH: Serum and tissue trace elements in patients with breast cancer
in Taiwan. Biol Trace Elem Res. 89:1–11. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Pavithra V, Sathisha TG, Kasturi K,
Mallika DS, Amos SJ and Ragunatha S: Serum levels of metal ions in
female patients with breast cancer. J Clin Diagn Res. 9:BC25–c27.
2015.PubMed/NCBI
|
|
108
|
Feng JF, Lu L, Zeng P, Yang YH, Luo J,
Yang YW and Wang D: Serum total oxidant/antioxidant status and
trace element levels in breast cancer patients. Int J Clin Oncol.
17:575–583. 2012. View Article : Google Scholar
|
|
109
|
Zowczak M, Iskra M, Torlinski L and Cofta
S: Analysis of serum copper and zinc concentrations in cancer
patients. Biol Trace Elem Res. 82:1–8. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Diez M, Cerdan FJ, Arroyo M and Balibrea
JL: Use of the copper/zinc ratio in the diagnosis of lung cancer.
Cancer. 63:726–730. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Jin Y, Zhang C, Xu H, Xue S, Wang Y, Hou
Y, Kong Y and Xu Y: Combined effects of serum trace metals and
polymorphisms of CYP1A1 or GSTM1 on non-small cell lung cancer: A
hospital based case-control study in China. Cancer Epidemiol.
35:182–187. 2011. View Article : Google Scholar
|
|
112
|
Oyama T, Matsuno K, Kawamoto T, Mitsudomi
T, Shirakusa T and Kodama Y: Efficiency of serum copper/zinc ratio
for differential diagnosis of patients with and without lung
cancer. Biol Trace Elem Res. 42:115–127. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Stepien M, Jenab M, Freisling H, Becker
NP, Czuban M, Tjonneland A, Olsen A, Overvad K, Boutron-Ruault MC,
Mancini FR, et al: Pre-diagnostic copper and zinc biomarkers and
colorectal cancer risk in the European Prospective Investigation
into Cancer and Nutrition cohort. Carcinogenesis. 38:699–707. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Sohrabi M, Gholami A, Azar MH, Yaghoobi M,
Shahi MM, Shirmardi S, Nikkhah M, Kohi Z, Salehpour D, Khoonsari
MR, et al: Trace element and heavy metal levels in colorectal
cancer: Comparison between cancerous and non-cancerous tissues.
Biol Trace Elem Res. 183:1–8. 2018. View Article : Google Scholar
|
|
115
|
Margalioth EJ, Schenker JG and Chevion M:
Copper and zinc levels in normal and malignant tissues. Cancer.
52:868–872. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Yaman M, Kaya G and Yekeler H:
Distribution of trace metal concentrations in paired cancerous and
non-cancerous human stomach tissues. World J Gastroenterol.
13:612–618. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Khanna SS and Karjodkar FR: Circulating
immune complexes and trace elements (Copper, Iron and Selenium) as
markers in oral precancer and cancer: A randomised, controlled
clinical trial. Head Face Med. 2:332006. View Article : Google Scholar
|
|
118
|
Baltaci AK, Dundar TK, Aksoy F and
Mogulkoc R: Changes in the serum levels of trace elements before
and after the operation in thyroid cancer patients. Biol Trace Elem
Res. 175:57–64. 2017. View Article : Google Scholar
|
|
119
|
Basu S, Singh MK, Singh TB, Bhartiya SK,
Singh SP and Shukla VK: Heavy and trace metals in carcinoma of the
gallbladder. World J Surg. 37:2641–2646. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Saleh SAK, Adly HM, Abdelkhaliq AA and
Nassir AM: Serum levels of selenium, zinc, copper, manganese, and
iron in prostate cancer patients. Curr Urol. 14:44–49. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Blockhuys S, Celauro E, Hildesjo C, Feizi
A, Stal O, Fierro-Gonzalez JC and Wittung-Stafshede P: Defining the
human copper proteome and analysis of its expression variation in
cancers. Metallomics. 9:112–123. 2017. View Article : Google Scholar
|
|
122
|
Wang W, Lu K, Jiang X, Wei Q, Zhu L, Wang
X, Jin H and Feng L: Ferroptosis inducers enhanced cuproptosis
induced by copper ionophores in primary liver cancer. J Exp Clin
Cancer Res. 42:1422023. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Harris ED: A Requirement for Copper in
Angiogenesis. Nutr Rev. 62:60–64. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
De Luca A, Barile A, Arciello M and Rossi
L: Copper homeostasis as target of both consolidated and innovative
strategies of anti-tumor therapy. J Trace Elem Med Biol.
55:204–213. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Barker HE, Cox TR and Erler JT: The
rationale for targeting the LOX family in cancer. Nat Rev Cancer.
12:540–552. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Groleau J, Dussault S, Haddad P, Turgeon
J, Menard C, Chan JS and Rivard A: Essential role of copper-zinc
superoxide dismutase for ischemia-induced neovascularization via
modulation of bone marrow-derived endothelial progenitor cells.
Arterioscler Thromb Vasc Biol. 30:2173–2181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Lowndes SA and Harris AL: Copper chelation
as an antiangiogenic therapy. Oncol Res. 14:529–539. 2004.
View Article : Google Scholar
|
|
128
|
Pannecoeck R, Serruys D, Benmeridja L,
Delanghe JR, van Geel N, Speeckaert R and Speeckaert MM: Vascular
adhesion protein-1: Role in human pathology and application as a
biomarker. Crit Rev Clin Lab Sci. 52:284–300. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Wong CC, Tse AP, Huang YP, Zhu YT, Chiu
DK, Lai RK, Au SL, Kai AK, Lee JM, Wei LL, et al: Lysyl
oxidase-like 2 is critical to tumor microenvironment and metastatic
niche formation in hepatocellular carcinoma. Hepatology.
60:1645–1658. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Salvador F, Martin A, Lopez-Menendez C,
Moreno-Bueno G, Santos V, Vazquez-Naharro A, Santamaria PG, Morales
S, Dubus PR, Muinelo-Romay L, et al: Lysyl oxidase-like protein
LOXL2 promotes lung metastasis of breast cancer. Cancer Res.
77:5846–5859. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Cheng F, Peng G, Lu Y, Wang K, Ju Q, Ju Y
and Ouyang M: Relationship between copper and immunity: The
potential role of copper in tumor immunity. Front Oncol.
12:10191532022. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Kang J, Lin C, Chen J and Liu Q: Copper
induces histone hypoacetylation through directly inhibiting histone
acetyltransferase activity. Chem Biol Interact. 148:115–123. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
International Agency for Research on
Cancer: Oesophageal cancer. GLOBOCAN. 2022, https://www.iarc.who.int/cancer-type/oesophageal-cancer/.
Accessed Dec 2, 2025
|
|
134
|
Zhang Y, Liu S, Zhou S, Yu D, Gu J, Qin Q,
Cheng Y and Sun X: Focal adhesion kinase: Insight into its roles
and therapeutic potential in oesophageal cancer. Cancer Lett.
496:93–103. 2021. View Article : Google Scholar
|
|
135
|
Liu XS, Yuan LL, Gao Y, Zhou LM, Yang JW
and Pei ZJ: Overexpression of METTL3 associated with the metabolic
status on (18)F-FDG PET/CT in patients with esophageal carcinoma. J
Cancer. 11:4851–4860. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Liu XS, Zhang Y, Liu ZY, Gao Y, Yuan LL,
Zeng DB, Tan F, Wan HB and Pei ZJ: METTL3 as a novel diagnosis and
treatment biomarker and its association with glycolysis,
cuproptosis and ceRNA in oesophageal carcinoma. J Cell Mol Med.
28:e181952024. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Lin K, Hu K, Chen Q and Wu J: The function
and immune role of cuproptosis associated hub gene in Barrett's
esophagus and esophageal adenocarcinoma. Biosci Trends. 17:381–392.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Bas O, Sahin TK, Karahan L, Rizzo A and
Guven DC: Prognostic significance of the cachexia index (CXI) in
patients with cancer: A systematic review and meta-analysis. Clin
Nutr ESPEN. 68:240–247. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
International Agency for Research on
Cancer: Stomach cancer. GLOBOCAN. 2022, https://www.iarc.who.int/cancer-type/stomach-cancer/.
Accessed Dec 2, 2025
|
|
140
|
Wang H, Liu M, Zeng X, Zheng Y, Wang Y and
Zhou Y: Cell death affecting the progression of gastric cancer.
Cell Death Discov. 8:3772022. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Song Q, Liu S, Wu D and Cai A: Multiple
programmed cell death patterns predict the prognosis and drug
sensitivity in gastric cancer. Front Immunol. 16:15114532025.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Li Y, Sun W, Yuan S, Liu X, Zhang Z, Gu R,
Li P and Gu X: The role of cuproptosis in gastric cancer. Front
Immunol. 15:14356512024. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Zhou Y, Dong C, Shen X, Wang P, Chen T, Li
W, Sun X, Li P, Xu C, Duan K, et al: Targeting PTBP3-Mediated
alternative splicing of COX11 induces cuproptosis for inhibiting
gastric cancer peritoneal metastasis. Adv Sci (Weinh).
12:e24159832025. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Zuo X, Lei Y, Ou S, Yuan X, Shi P, Li Q
and Xu Y: Integration of cuproptosis-related gene signatures in
stomach adenocarcinoma: Implications for prognostic prediction and
therapeutic strategies in cancer drug resistance. Discov Oncol.
16:8852025. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Xie XZ, Zuo L, Huang W, Fan QM, Weng YY,
Yao WD, Jiang JL and Jin JQ: FDX1 as a novel biomarker and
treatment target for stomach adenocarcinoma. World J Gastrointest
Surg. 16:1803–1824. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Lu L, Yang W, Gu Y, Jin L and Liang Z: The
role of cuproptosis in the occurrence and development of gastric
cancer. Front Pharmacol. 16:16642002025. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Chen Y, Liao Y, Huang L and Luo Z:
Exploring copper metabolism-induced cell death in gastric cancer: A
single-cell RNA sequencing study and prognostic model development.
Discov Oncol. 15:4822024. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
International Agency for Research on
Cancer: Liver cancer. GLOBOCAN. 2022, https://www.iarc.who.int/cancer-type/liver-cancer/.
Accessed Dec 2, 2025
|
|
149
|
Yu M, Chen Z, Zhou Q, Zhang B, Huang J,
Jin L, Zhou B, Liu S, Yan J, Li X, et al: PARG inhibition limits
HCC progression and potentiates the efficacy of immune checkpoint
therapy. J Hepatol. 77:140–151. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Huang H, Tsui YM and Ng IO: Fueling HCC
dynamics: Interplay between tumor microenvironment and tumor
initiating cells. Cell Mol Gastroenterol Hepatol. 15:1105–1116.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Fang AP, Chen PY, Wang XY, Liu ZY, Zhang
DM, Luo Y, Liao GC, Long JA, Zhong RH, Zhou ZG, et al: Serum copper
and zinc levels at diagnosis and hepatocellular carcinoma survival
in the Guangdong liver cancer cohort. Int J Cancer. 144:2823–2832.
2019. View Article : Google Scholar
|
|
152
|
Gunjan D, Shalimar, Nadda N, Kedia S,
Nayak B, Paul SB, Gamanagatti SR and Acharya SK: Hepatocellular
carcinoma: An unusual complication of longstanding wilson disease.
J Clin Exp Hepatol. 7:152–154. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Haratake J, Horie A and Takeda S:
Histochemical and ultrastructural study of copper-binding protein
in hepatocellular carcinoma. Cancer. 60:1269–1274. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Lefkowitch JH, Muschel R, Price JB, Marboe
C and Braunhut S: Copper and copper-binding protein in
fibrolamellar liver cell carcinoma. Cancer. 51:97–100. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Davis CI, Gu X, Kiefer RM, Ralle M, Gade
TP and Brady DC: Altered copper homeostasis underlies sensitivity
of hepatocellular carcinoma to copper chelation. Metallomics.
12:1995–2008. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Poznanski J, Soldacki D, Czarkowska-Paczek
B, Bonna A, Kornasiewicz O, Krawczyk M, Bal W and Pączek L:
Cirrhotic liver of liver transplant recipients accumulate silver
and co-accumulate copper. Int J Mol Sci. 22:17822021. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Li Z, Zhou H, Zhai X, Gao L, Yang M, An B,
Xia T, Du G, Li X, Wang W and Jin B: MELK promotes HCC
carcinogenesis through modulating cuproptosis-related gene
DLAT-mediated mitochondrial function. Cell Death Dis. 14:7332023.
View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Guo J, Cheng J, Zheng N, Zhang X, Dai X,
Zhang L, Hu C, Wu X, Jiang Q, Wu D, et al: Copper promotes
tumorigenesis by activating the PDK1-AKT oncogenic pathway in a
copper transporter 1 dependent manner. Adv Sci (Weinh).
8:e20043032021. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Quan B, Liu W, Yao F, Li M, Tang B, Li J,
Ren Z and Yin X: LINC02362/hsa-miR-18a-5p/FDX1 axis suppresses
proliferation and drives cuproptosis and oxaliplatin sensitivity of
hepatocellular carcinoma. Am J Cancer Res. 13:5590–5609.
2023.PubMed/NCBI
|
|
160
|
Oliveri V: Selective targeting of cancer
cells by copper ionophores: An overview. Front Mol Biosci.
9:8418142022. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Zhou C, Yang J, Liu T, Jia R, Yang L, Sun
P and Zhao W: Copper metabolism and hepatocellular carcinoma:
Current insights. Front Oncol. 13:11866592023. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Zhao X, Chen J, Yin S, Shi J, Zheng M, He
C, Meng H, Han Y, Han J, Guo J, et al: The expression of
cuproptosis-related genes in hepatocellular carcinoma and their
relationships with prognosis. Front Oncol. 12:9924682022.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
International Agency for Research on
Cancer: Pancreatic cancer. GLOBOCAN. 2022, https://www.iarc.who.int/cancer-type/pancreatic-cancer/.
Accessed Dec 2, 2025
|
|
164
|
Leiphrakpam PD, Chowdhury S, Zhang M,
Bajaj V, Dhir M and Are C: Trends in the global incidence of
pancreatic cancer and a brief review of its histologic and
molecular subtypes. J Gastrointest Cancer. 56:712025. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Lener MR, Scott RJ, Wiechowska-Kozlowska
A, Serrano-Fernandez P, Baszuk P, Jaworska-Bieniek K, Sukiennicki
G, Marciniak W, Muszyńska M, Kładny J, et al: Serum concentrations
of selenium and copper in patients diagnosed with pancreatic
cancer. Cancer Res Treat. 48:1056–1064. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Gao S, Ge H, Gao L, Gao Y, Tang S, Li Y,
Yuan Z and Chen W: Silk fibroin nanoparticles for enhanced
cuproptosis and immunotherapy in pancreatic cancer treatment. Adv
Sci (Weinh). 12:e24176762025. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Zhou C, Yi C, Yi Y, Qin W, Yan Y, Dong X,
Zhang X, Huang Y, Zhang R, Wei J, et al: LncRNA PVT1 promotes
gemcitabine resistance of pancreatic cancer via activating
Wnt/β-catenin and autophagy pathway through modulating the
miR-619-5p/Pygo2 and miR-619-5p/ATG14 axes. Mol Cancer. 19:1182020.
View Article : Google Scholar
|
|
168
|
Sun Y, Yao L, Man C, Gao Z, He R and Fan
Y: Development and validation of cuproptosis-related lncRNAs
associated with pancreatic cancer immune microenvironment based on
single-cell. Front Immunol. 14:12207602023. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
International Agency for Research on
Cancer: Colorectal cancer. GLOBOCAN. 2022, https://www.iarc.who.int/cancer-type/colorectal-cancer/.
Accessed Dec 2, 2025
|
|
170
|
Yang W, Wang Y, Huang Y, Yu J, Wang T, Li
C, Yang L, Zhang P, Shi L, Yin Y, et al: 4-Octyl itaconate inhibits
aerobic glycolysis by targeting GAPDH to promote cuproptosis in
colorectal cancer. Biomed Pharmacother. 159:1143012023. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Gu Y, Li C, Yan Y, Ming J, Li Y, Chao X
and Wang T: Comprehensive analysis and verification of the
prognostic significance of cuproptosis-related genes in colon
adenocarcinoma. Int J Mol Sci. 25:118302024. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Ma J, Lin H, Wang Y, Zhang Y, Zhou C, Tang
D, Kagawa Y, Hou D and Jiang G: The unique role of cuproptosis in
the prognosis and treatment of rectum adenocarcinoma. J
Gastrointest Oncol. 16:367–385. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Wang Y, Pei P, Yang K, Guo L and Li Y:
Copper in colorectal cancer: From copper-related mechanisms to
clinical cancer therapies. Clin Transl Med. 14:e17242024.
View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Li Y, Chen F, Chen J, Chan S, He Y, Liu W
and Zhang G: Disulfiram/Copper induces antitumor activity against
both nasopharyngeal cancer cells and cancer-associated fibroblasts
through ROS/MAPK and ferroptosis pathways. Cancers (Basel).
12:1382020. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Chan N, Willis A, Kornhauser N, Ward MM,
Lee SB, Nackos E, Seo BR, Chuang E, Cigler T, Moore A, et al:
Correction: Influencing the tumor microenvironment: A phase II
study of copper depletion using tetrathiomolybdate in patients with
breast cancer at high risk for recurrence and in preclinical models
of lung metastases. Clin Cancer Res. 26:50512020. View Article : Google Scholar : PubMed/NCBI
|