The concept of autism spectrum disorder and
recent progress
Core symptoms and current epidemiological
status
Autism spectrum disorder (ASD) encompasses a group
of pervasive neurodevelopmental conditions characterized by a broad
spectrum of symptoms, broadly categorized into core and associated
symptoms. Core symptoms include persistent deficits in social
interaction and communication, as well as restricted and repetitive
patterns of behavior and interests that significantly impair daily
functioning. By contrast, associated symptoms encompass attention
deficits, mood disturbances, sensory processing abnormalities,
sleep disorders, language impairment, anxiety, epilepsy, mania and
self-injurious behaviors. Core symptoms typically emerge in early
childhood and persist throughout life (1-3).
In recent years, increasing public awareness of ASD has coincided
with a steady increase in its rate of diagnosis, rendering it a
significant global public health concern. According to 2022 data
from the US Centers for Disease Control and Prevention (CDC), the
incidence of ASD in the USA reached ~1 in 31 children. Moreover,
the prevalence of ASD among boys is notably higher than that among
girls, with a male-to-female ratio of ~3.44:1 (4). Furthermore, advances in early
screening and diagnostic tools have enabled the identification of a
greater number of individuals with milder ASD phenotypes, thereby
enhancing epidemiological research in this field.
Large-scale genome-wide association studies (GWAS)
(5) and family-based genetic
analyses (6) have revealed that
ASD possesses a complex and highly heterogeneous genetic
background. Genetic factors are estimated to account for ~81% of
the risk of developing ASD, whereas environmental factors
contribute to a ~14-22% risk. Of note, >100 ASD-associated risk
genes have been identified, including neurexin 1 (NRXN1),
Src homology 3 and multiple ankyrin repeat domains 2/3 (SHANK2,
SHANK3) and chromodomain helicase DNA binding protein 8
(CHD8). The principal mutation types include de novo
truncating mutations, missense mutations and copy number variations
(CNVs). These risk-associated genes influence gene expression,
neurogenesis, synaptic function and chromatin regulatory networks
(6-8).
By contrast, environmental factors primarily
influence early fetal development through maternal health
conditions and external exposures. A previous large-scale study
involving 22,156 cases of ASD across three Nordic countries
underscored the crucial role of perinatal and prenatal factors.
Maternal gestational hypertension (odds ratio, 1.4), preeclampsia
[relative risk (RR), 1.3], overweight or obesity (RR, 1.3) and an
advanced maternal age (≥35 years; RR, 1.3) were all shown to be
significantly associated with an increased risk of offspring
developing ASD. Moreover, an advanced paternal age (each 10-year
increase corresponding to a 21% rise in the risk of ASD),
interpregnancy intervals that are either too short (<12 months)
or too long (≥72 months) and exposure to specific medications, such
as valproic acid (VPA) during pregnancy, were also closely linked
to elevated risk of developing ASD (5). A previous study demonstrated that
the absolute risk of developing ASD reached 4.4% in the VPA-exposed
group as compared to the unexposed group with 1.5%. Thus, beyond
genetic predisposition, aberrations in the intrauterine environment
and maternal metabolic dysregulation may play a critical role in
disrupting fetal neurodevelopment and represent important triggers
for ASD (9).
Current treatment strategies and key
challenges
Early diagnosis and intervention for ASD are deemed
pivotal for improving the quality of life of affected children.
Such early interventions can enhance language abilities, social
communication skills and behavioral regulation, particularly when
administered during the rapid phase of neurodevelopment (<3
years of age). This timing is critical for optimizing long-term
outcomes, helping to maximize the cognitive, linguistic and social
capacities of children (8,10). The American Academy of Pediatrics
recommends autism screening at 18 and 24 months of age (https://www.aap.org/en/patient-care/autism).
Currently, the primary diagnostic tools include the Modified
Checklist for Autism in Toddlers Revised (M-CHAT-R) (11), the Autism Diagnostic Observation
Schedule, Second Edition (ADOS-2) and the Autism Diagnostic
Interview-Revised (ADI-R) (12).
Children who screen positive require comprehensive evaluations,
including behavioral observation and an in-depth analysis of
developmental history (3,8).
However, as the symptoms of ASD are heterogeneous and vary greatly
among individuals, early diagnosis remains challenging,
particularly in cases with atypical or mild presentations.
At present, treatment for ASD primarily focuses on
behavioral interventions and symptom management, as there is no
universally accepted cure (3).
The behavioral intervention remains the first-line approach,
commonly implemented through Early Intensive Behavioral
Intervention (EIBI) and Naturalistic Developmental Behavioral
Interventions (NDBI) (13).
EIBI is grounded in the principles of Applied
Behavior Analysis (ABA) and is typically designed for children
<5 years of age. It involves intensive therapy, usually at least
25 h per week over a minimum period of 1 year, to strengthen
social, language, cognitive and adaptive behaviors. Among NDBI, the
Early Start Denver Model (ESDM) (14) is the most representative
approach. It integrates multiple developmental domains, including
cognition, language, social interaction, motor skills and
imitation, into behavioral interventions. ESDM emphasizes creating
meaningful activities for children, fostering interactive
relationships and emotional engagement, and promoting the
generalization of learning. Motivating activities are incorporated
into daily routines to help children acquire new skills, improve
language, social and cognitive functioning, and reduce disruptive
behaviors (15). These
behavioral strategies currently constitute the cornerstone of ASD
therapy.
Pharmacological interventions are mainly used to
address comorbid symptoms, such as risperidone and aripiprazole,
which effectively reduce irritability and aggressive behaviors
(16). Methylphenidate,
atomoxetine and guanfacine may alleviate hyperactivity and
attention deficits. For sleep disturbances, melatonin is commonly
prescribed (8).
These interventions mainly aim to alleviate symptoms
and minimize the effect on daily life and quality of life, rather
than addressing the underlying causes of ASD. To date, to the best
of our knowledge, no medication has been shown to effectively treat
the core symptoms of ASD, and treatment outcomes vary widely among
individuals. Consequently, there is an urgent need to explore novel
treatment strategies, particularly those targeting the underlying
pathophysiological mechanisms, which have become a central focus of
contemporary ASD research.
Future directions for early diagnosis and
intervention
The future of early ASD diagnosis and intervention
hinges on precision, personalization and enhanced accessibility.
First, personalized intervention plans are crucial for addressing
the heterogeneity of ASD (17).
By integrating assessments of the behavioral, cognitive and
language abilities of a child, along with data-driven analytical
methods, more tailored interventions can be designed to meet
individual needs (18). In
addition, using biomarkers (e.g., miRNAs or exosomal components) to
aid in diagnosis and treatment offers a potential avenue for
refining interventions (19),
such as identifying neuroinflammatory features, which may enable
more targeted immunomodulatory therapies (20).
The integration of emerging technologies presents
new opportunities for the treatment of ASD. For instance,
mesenchymal stem cells (MSCs) exhibit tremendous promise owing to
their multilineage differentiation potential, immunomodulatory
properties, and capacity to secrete various neuroprotective factors
(21,22). Beyond enhancing the
neuroinflammatory microenvironment and supporting neuroplasticity
in vivo, MSCs also release exosomes that transport essential
proteins, nucleic acids, and miRNAs to repair damaged neurons and
glial cells (23). These
molecules, in turn, modulate gene expression and cell function,
mitigate neuroinflammation and repair synaptic structures (24). Exosomes possess low
immunogenicity, the ability to cross the blood-brain barrier, and
the capacity for targeted delivery of specific bioactive molecules
(25,26). A number of studies have focused
on the use of MSC-derived exosomes (MSC-Exos) in ASD interventions,
exploring their potential mechanisms and translational applications
for improving social, communication and cognitive functions
(22,27-29).
Moreover, developing novel evaluation metrics is
crucial for assessing the efficacy of interventions. Researchers
can more comprehensively track the progress of a child in detection
and intervention by integrating behavioral data, neuroimaging
findings (30,31) and biomarker changes (32). Long-term follow-up assessments
further elucidate how specific strategies affect social adaptation
and overall quality of life in adulthood (33).
Lastly, enhancing the social awareness of ASD and
strengthening policy support are also required. Public education
campaigns can help reduce societal bias, and training programs for
parents and educators can improve the effectiveness of early
diagnosis and intervention (34). In this regard, additional funding
from government research agencies and related organizations can
expand the reach of innovative research and healthcare services for
individuals with ASD.
Overall, the advancements in ASD diagnosis and
intervention hinge on technological progress, cross-disciplinary
collaboration, and strategic resource allocation. By harmonizing
scientific breakthroughs with robust policy support, current
treatment barriers can be addressed and this may help foster
renewed optimism for children with ASD and their families.
Molecular genetics and neuropathology of
ASD
With deepening insight into ASD, research on its
etiology and pathogenesis has expanded from early, straightforward
genetic associations to a comprehensive framework encompassing
molecular pathology, neural network disruptions and
interdisciplinary approaches. In recent years, emerging
technologies, such as high-throughput single-cell sequencing
(35) and brain organoid models
(36), have rapidly evolved,
enabling a more in-depth analysis of gene-environment interactions
and neurodevelopmental abnormalities in ASD. These advances also
provide novel approaches to potential interventions and therapies.
Against this backdrop, the following sections provide a systematic
review of current research on ASD, focusing on genetic landscapes,
synaptic imbalances, inflammatory regulation, glial cell activation
and immune dysregulation (37).
Genetic characteristics and polygenic
complexity of ASD
Epidemiological studies in recent years have
underscored the dominant role of genetic factors in ASD, which is
recognized as a complex disorder driven largely by heritability.
Multiple twin studies consistently demonstrate much higher
concordance for ASD in monozygotic (MZ) than dizygotic (DZ) twins.
In a population-based study using standardized ADI-R/ADOS
assessments, probandwise concordance ranged from ~58-77% for MZ
twins and ~21-36% for DZ twins across strict-autism and broader ASD
definitions; rare sex-specific DZ subgroups can be lower (38). A previous meta-analysis further
indicates near-perfect MZ correlations (~98%) with DZ correlations
that vary by the assumed prevalence threshold, yielding substantial
heritability (~64-91%) and showing that estimates of the shared
environmental component are sensitive to modelled prevalence
(39). Through GWAS and familial
genetic analyses comparing large-scale datasets of individuals with
ASD and healthy controls, numerous ASD-associated susceptibility
genes and variations have been identified, including CHD8,
NRXN1, SHANK2, SHANK3 and contactin associated
protein 2 (CNTNAP2) (6-8,40). Among these genes, CHD8
encodes a chromatin remodeler that regulates a wide array of
neurodevelopmental genes by binding to their promoters and
enhancers. Previous studies have reported that CHD8
haploinsufficiency results in dysregulated transcriptional
programs, aberrant neurogenesis, and epigenetic abnormalities
(41). NRXN1 encodes the
presynaptic adhesion molecule neurexin-1, which interacts with
postsynaptic ligands such as neuroligins to form trans-synaptic
connections and is essential for synapse formation and homeostasis.
In ASD mouse models carrying NRXN1 mutations, CNVs have been
shown to cause synaptic connectivity defects, impairments in neural
circuit development and synaptic transmission, ultimately leading
to deficits in social interaction and communication (42). The SHANK gene family,
particularly SHANK3, functions as a core scaffolding and
signaling component of the postsynaptic density (PSD) at excitatory
synapses, orchestrating the organization of
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid and
N-methyl-D-aspartate receptors, cytoskeletal structures, and
intracellular signaling pathways. SHANK deficiency leads to
PSD disassembly, impaired synaptic plasticity, altered dendritic
spine morphology and excitatory/inhibitory imbalance, thereby
contributing to ASD-related behavioral abnormalities. Both animal
models and patient-derived induced pluripotent stem cells studies
have provided strong evidence supporting these mechanisms (43,44). The CNTNAP2, a member of
the neurexin superfamily, is involved in axon-myelin interactions,
synaptic function and neuronal positioning. Early candidate gene
studies identified CNTNAP2 as being associated with ASD,
language impairment and epilepsy. The loss or mutation of
CNTNAP2 has been linked to abnormal neuronal migration
during development, defects in axonal and myelin function, and
disrupted excitatory neuronal activity (45).
The analysis of the Simons Simplex Collection (SSC)
has identified six key risk loci (1q21.1, 3q29, 7q11.23, 16p11.2,
15q11.2-13 and 22q11.2). These large CNVs likely harbor multiple
risk genes of moderate effect, primarily involving upstream
regulators of gene expression during neural development, as well as
synapse-related genes that directly impact neuronal structure and
function (6).
Exome sequencing data, when integrated with
functional annotations of different types of genetic variants
(e.g., rare single-nucleotide variants, insertions/deletions and
CNVs) and selection-pressure metrics, e.g., Loss of Function
Observed and Expected Upper Bound Fraction (LOEUF) scores, indicate
that the risk of developing ASD is strongly associated with rare
coding variants and de novo mutations. Among the genes
significantly linked to ASD (false discovery rate ≤0.001), de
novo protein-truncating variants, damaging missense variants
and CNVs account for 57.5, 21.1 and 8.44% of the genetic risk,
respectively. Notably, CNVs carry a markedly high relative risk
since they alter the copy number of large genomic segments,
potentially affecting multiple genes and leading to cumulative
effects (7).
CNVs frequently occur in gene-dense, functionally
important regions critical for neurodevelopment, synaptic
plasticity and chromatin regulation. Abnormalities in these regions
may lead to extensive functional disruption (46). In addition, CNVs can alter gene
dosage (e.g., an increase or decrease in gene copies), and
dosage-sensitive genes can exhibit significant biological effects
when copy numbers deviate from the normal (47,48). A number of CNVs also exhibit
pleiotropy, where a single gene may influence multiple biological
processes or phenotypes, so its dysregulation can affect various
systems concurrently (49).
Moreover, CNVs can disrupt regulatory elements (e.g., enhancers and
promoters) in non-coding regions, thereby altering gene expression
and regulatory networks (48).
The Genes associated with ASD exhibit a higher
expression in mature neurons, aligning with their roles in neuronal
maturation and synaptic connectivity. By contrast, genes linked to
developmental delay are predominantly active in immature neurons
and progenitor cells. This suggests that different gene networks
affect the nervous system at specific developmental stages
(7).
Core neuropathological mechanisms of
ASD
A primary pathological hallmark of ASD is abnormal
synaptic development and plasticity, primarily manifested as
disruptions in synapse formation and pruning. This leads to
excessive or insufficient synaptic connections, compromising the
precise establishment of neuronal networks (50-52). The excitatory and inhibitory
ratio, a core mechanism in synaptic signaling, is critically
disrupted in a number of cases, typically manifesting as excessive
excitatory activity [e.g., elevated glutamate receptor function
(53)] or diminished inhibitory
signaling (e.g., reduced gamma-aminobutyric acid receptor levels),
both of which may lead to neuronal circuit instability, driving
hyperexcitability or network dysregulation (54). Either scenario can result in
hyperactive or imbalanced neuronal networks. Furthermore, mutations
in ASD-associated genes, such as neuroligin 4 (NLGN4)
(55) and SHANK3
(56) can impair presynaptic
neurotransmitter release and postsynaptic receptor responsiveness,
thereby reducing synaptic transmission efficiency. Abnormalities in
synaptic plasticity processes, including long-term potentiation
(LTP) and long-term depression, further exacerbate these
dysfunctions, as reflected by impaired modulation of synaptic
strength in key brain regions, such as the hippocampus, prefrontal
cortex and amygdala (57).
Additionally, synaptic protein misfolding and the
activation of endoplasmic reticulum stress can lead not only to a
loss of synaptic function, but also to heightened neuroinflammation
via the unfolded protein response, thereby aggravating ASD
pathophysiology (58).
Collectively, these disruptions in synaptic development and
plasticity underlie prominent deficits in social interaction,
cognitive function, and behavioral patterns in ASD, providing
essential clues for understanding its molecular mechanisms and for
developing potential therapeutic approaches (59).
Developmental abnormalities in specific brain
regions are a crucial aspect of the neurobiological features of
ASD. Research indicates that both structure and function are
significantly affected in the prefrontal cortex (60), hippocampus (61) and basal ganglia (62). Alterations in causal connectivity
within the prefrontal cortex are closely linked to deficits in
social behaviors and cognitive functions, typified by an
excitatory/inhibitory signaling imbalance and diminished synaptic
plasticity (60). In the central
region of the hippocampus, which is critical for learning and
memory, reduced synaptic density and impaired LTP are commonly
observed in ASD and directly correlate with decreases in spatial
memory (61). Moreover,
abnormalities in basal ganglia circuitry, particularly those
involved in gating motor output, have been implicated in repetitive
and stereotypic behaviors; changes in synaptic transmission within
these pathways may affect behavior by modulating motor control and
reward mechanisms (62).
Such developmental anomalies manifest at the
micro-level of neuronal connections and alter interregional network
communication, thereby contributing to the diverse and complex
behavioral phenotypes of ASD. In-depth research into these brain
regions' developmental perturbations is instrumental in elucidating
the pathological mechanisms of ASD and lays the groundwork for
precision-targeted therapies.
Cytological basis of neuroinflammation in
ASD
Neuroinflammation and aberrant immune regulation
play pivotal roles in the pathogenesis of ASD, with the activation
of microglia and astrocytes constituting a core cellular foundation
of this neuroinflammatory process. Microglia and astrocytes exhibit
distinct polarization states in the neuroinflammatory
microenvironment of ASD, with M1/A1 subtypes promoting injury and
M2/A2 subtypes supporting repair. The interplay of these brain
cells is summarized below and illustrated in Fig. 1.
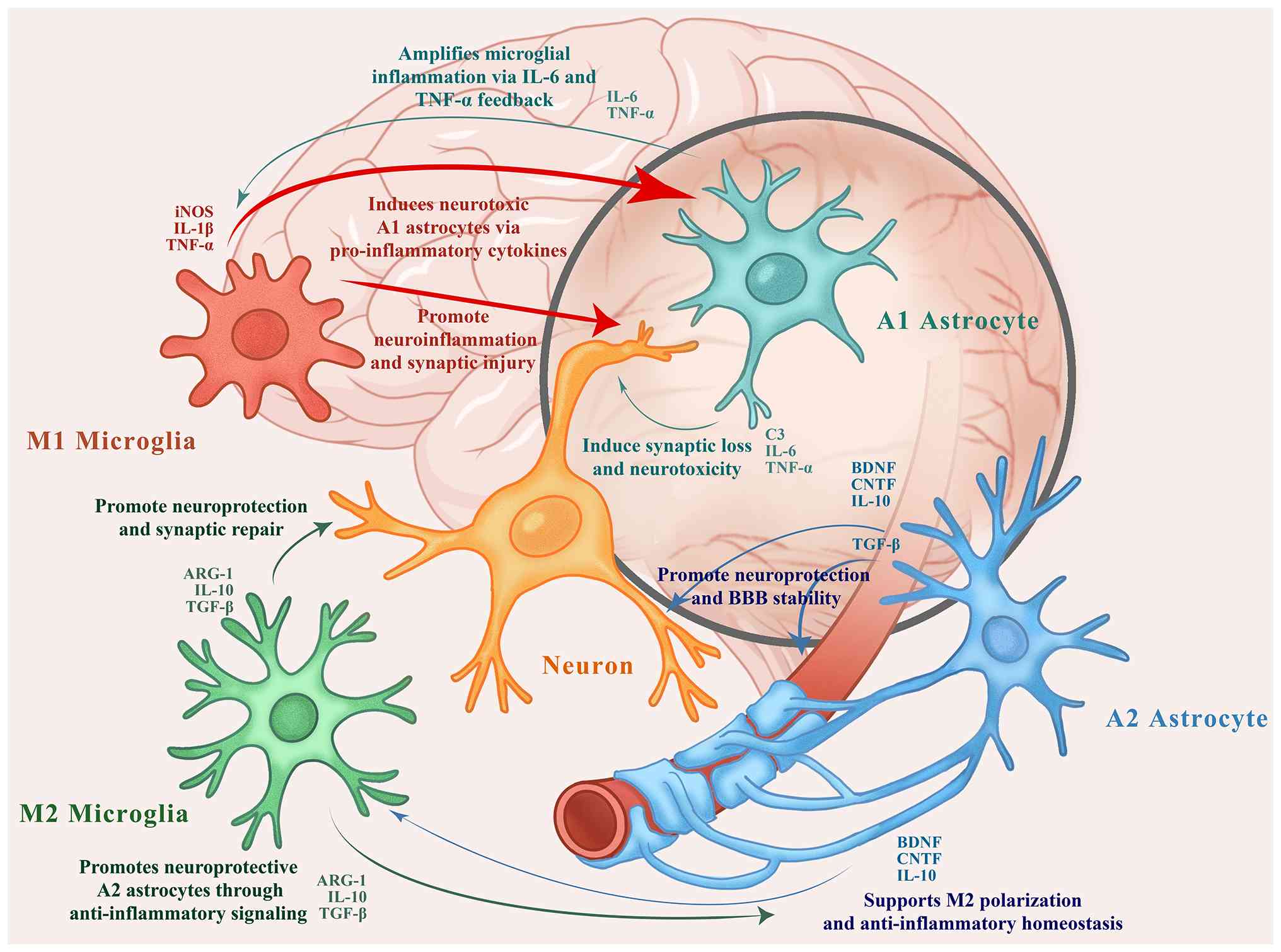 | Figure 1Interactions between microglia,
astrocytes, and neurons in the autism spectrum disorder-affected
brain. M1 microglia and A1 astrocytes release pro-inflammatory
cytokines (e.g., TNF-α, IL-1β and IL-6) that amplify
neuroinflammation and induce synaptic damage. In contrast, M2
microglia and A2 astrocytes secrete anti-inflammatory and
neurotrophic factors (e.g., IL-10, TGF-β, BDNF and CNTF), promoting
synaptic repair, BBB stability and homeostasis. Arrows indicate
intercellular regulatory pathways. The authors created this figure
referencing multiple sources (40-56,62-68,74-82). TNF-α, tumor necrosis factor-α;
IL, interleukin; TGF-β, transforming growth factor β; BMP, bone
morphogenetic protein; CNTF, ciliary neurotrophic factor; iNOS,
inducible nitric oxide synthase; BBB, blood brain barrier. |
Microglia: Dual polarization and
synaptic phagocytosis
Microglia are the primary immune cells of the
central nervous system (CNS) and exhibit dual polarization states,
i.e., M1 (classical activation) and M2 (alternative activation). In
response to inflammatory stimuli, M1-type microglia secrete
proinflammatory factors, such as tumor necrosis factor-α (TNF-α),
interleukin (IL)-1β and IL-6 (63-65) and reactive oxygen species
(66), driving neuroinflammation
and pathological damage. They can also enhance phagocytic activity,
causing direct harm to neurons and synapses (67). Conversely, M2-type microglia are
induced by anti-inflammatory factors, such as IL-4 and IL-13
(68), and promote the release
of neuroprotective mediators, including IL-10 and transforming
growth factor-β (TGF-β) (68-70). This balance between M1 and M2
polarization is crucial for regulating neuronal activity, synaptic
plasticity, and the clearance of apoptotic cells, thus helping
maintain CNS homeostasis under normal conditions.
Microglial synaptic phagocytosis plays a critical
role in shaping neural circuits; however, its dysregulation can
occur in either direction, either through excessive elimination of
synapses or insufficient pruning. This bidirectional imbalance
contributes to abnormal neural connectivity, underscoring the
complex and multifaceted role of microglia in the pathophysiology
of ASD. During normal development, microglia refine neural circuits
by pruning superfluous synapses. However, the hyperactivation of
microglia in ASD may lead to aberrant over-pruning of synapses,
thereby reducing synaptic density and impairing neural circuits,
particularly in brain regions linked to social behavior and
cognition. This phenomenon may underlie the synaptic deficits
observed in specific ASD subtypes (71). By contrast, a dysregulated
microglial function can result in insufficient synaptic pruning,
leading to excessive synaptic connections and abnormal network
synchronization, as well as reduced information-processing
efficiency. This effect may be more pronounced in other ASD
subgroups. This imbalance in synaptic pruning is influenced by
pro-inflammatory cytokines, microglial signaling pathways [e.g.,
TREM2 (72,73) and STING (74)] and region-specific factors.
Consequently, the dual role of microglial phagocytosis highlights
the complexity and heterogeneity of the pathogenesis of ASD,
supporting the rationale for therapeutic strategies that target
microglial modulation.
Astrocytes: Reactive transformation
and neuroinflammatory regulation
Astrocytes are instrumental in maintaining CNS
homeostasis and supporting neuronal function. However, in ASD,
astrocyte activation is likewise closely tied to the progression of
neuroinflammation (75).
Activated astrocytes can release both pro-inflammatory and
anti-inflammatory mediators, such as IL-6 (76) and IL-10 (77), exerting a bidirectional influence
on the inflammatory process. Moreover, intermediate filament
proteins in astrocytes, including glial fibrillary acidic protein
(78) and vimentin (79), exhibit an altered expression and
distribution. These proteins regulate the degradation of
extracellular matrix components (80). In the brains of patients with
ASD, astrocytes often display morphological changes characteristic
of reactive astrocytes, i.e., hypertrophic, with thicker and
shorter processes, particularly the neurotoxic A1 subtype. These
alterations are linked to astrocyte dysfunction, disrupted
neurogenesis and neuroinflammation, thereby exacerbating
neurological abnormalities in individuals with ASD (81-83).
Pro-inflammatory cytokines and
neuroinflammatory signaling
Pro-inflammatory cytokines are key factors driving
neuroinflammation in ASD. Their dysregulated expression signifies
aberrant immune activation and may directly or indirectly inflict
neuronal damage. Elevated levels of IL-1β and TNF-α have been
strongly associated with compromised synaptic function and reduced
neuronal viability (84).
Furthermore, cytokines can modify neurotransmitter metabolism and
release, further destabilizing neural networks. For example, IL-6
has been shown to regulate glutamate transporter expression,
thereby exacerbating excitotoxicity (85). These inflammatory mediators also
function through signaling cascades, such as nuclear factor
κ-light-chain-enhancer of activated B cells (NF-κB) and JAK-STAT to
regulate the intensity and duration of the immune response, thereby
exacerbating neuronal dysfunction and cell death (86).
In summary, the activation of microglia and
astrocytes significantly modulates neuroinflammation and immune
responses in ASD, profoundly influencing pathological progression.
By releasing pro-inflammatory cytokines and altering synaptic
function, aberrant glial activation not only promotes neuronal
damage, but also undermines the stability of neuronal networks. A
more precise understanding of the atypical inflammatory and immune
mechanisms in ASD is therefore pivotal for illuminating its
pathophysiology and developing effective therapeutic
strategies.
Mesenchymal stem cells and their derived
exosomes in ASD: Research and applications
Biological characteristics and
immunoregulatory mechanisms of action of MSCs
MSCs are adult stem cells characterized by
multipotent differentiation and self-renewal capacities, initially
identified in bone marrow by Friedenstein et al (87). These cells adhere to plastic
culture flasks and exhibit robust biological functions. They are
widely distributed in various tissues, such as bone marrow, adipose
tissue, umbilical cord, placenta, and dental pulp (88). A schematic overview of these MSC
sources is illustrated in Fig.
2. Owing to their diverse sources and distinct functional
properties, have emerged as a significant research focus in
regenerative medicine and cell therapy.
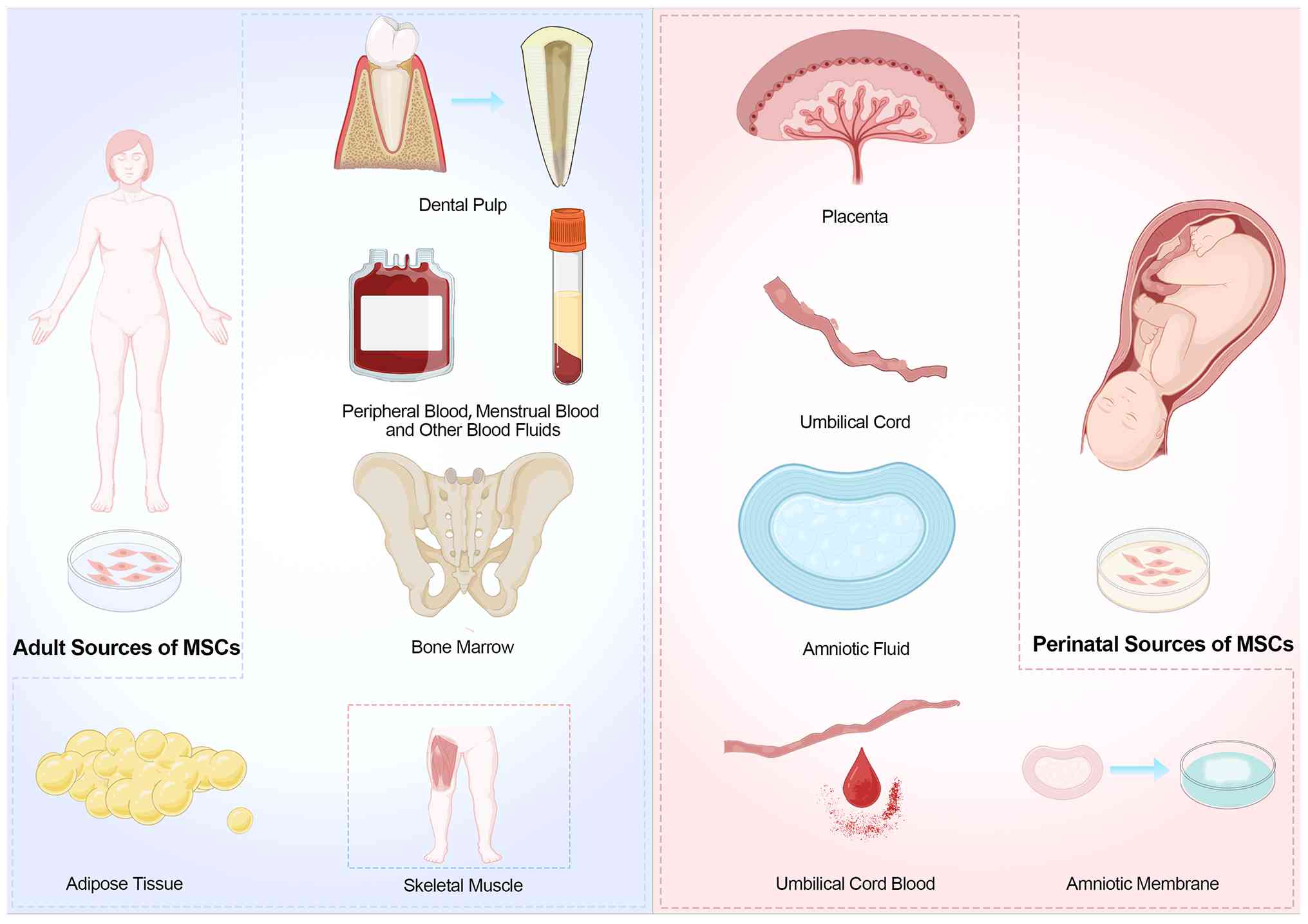 | Figure 2Sources of MSCs. Adult MSCs can be
derived from bone marrow, adipose tissue, peripheral blood,
skeletal muscle, and dental pulp. Perinatal MSCs are typically
harvested from the placenta, umbilical cord, cord blood, amniotic
fluid, and amniotic membrane. This figure was created based on data
or concepts adapted from the study by Brown et al (88), but was drawn and designed by the
authors. MSCs, mesenchymal stem cells. |
Firstly, MSCs possess notable multipotent
differentiation potential under specific conditions; they can
differentiate into various cell types, including osteoblasts,
chondrocytes, myocytes, adipocytes, and neuron-like cells. This
property is under precise genetic regulation (89). For instance, RUNX family
transcription factor 2 (RUNX2) plays a pivotal role in
osteogenic differentiation (90); peroxisome proliferator-activated
receptor γ (PPAR-γ) controls adipogenic differentiation
(91); SRY-Box transcription
factor 9 (SOX9) is crucial for chondrogenesis (92); and the differentiation of
neuron-like cells relies on the expression of proteins, such as
Nestin and neuronal differentiation 1 (NeuroD1) under the induction
of neurotrophic factors (93).
These genes, which fine-tune the Wnt/β-catenin TGF-β/bone
morphogenetic protein (BMP) and Notch 1 signaling pathways, provide
the molecular underpinnings for the applications of MSCs in
regenerative medicine.
Secondly, MSCs play a prominent role in
immunomodulation. Research suggests that MSCs can secrete multiple
immunoregulatory factors and interact with immune cells, thus
exerting immunosuppressive and immunobalancing effects. For
example, PGE2, TGF-β, IL-10 and HLA-G secreted by MSCs can
effectively inhibit T-cell proliferation and induce the generation
of regulatory T-cells (Tregs), thereby promoting immune tolerance
(94). Furthermore, by causing
the polarization of macrophages toward the M2 phenotype, MSCs can
significantly reduce the release of pro-inflammatory cytokines,
such as IL-6 and TNF-α (95),
thereby improving the pathological environment associated with
inflammation. These properties render MSCs promising therapeutic
candidates for various immune-related diseases (96).
Additionally, the low immunogenicity of MSCs aids in
their long-term survival in the host. This advantage arises from
their low expression of Major Histocompatibility Complex (MHC)
class I molecules and the lack of MHC class II and costimulatory
molecules (e.g., CD80 and CD86) (97). Such features markedly reduce
T-cell activation and diminish strong host immune rejection.
Moreover, MSCs secrete multiple immunosuppressive factors (e.g.,
PGE2, IDO1 and HLA-G) to attenuate immune responses further
(98). These combined mechanisms
enable MSCs to survive and exert therapeutic effects in allogeneic
transplantation settings, with minimal reliance on
immunosuppressants.
Research advances of MSCs in ASD
MSCs promote neuroprotection and functional recovery
in various neurological disorders through multiple pathways. For
instance, MSCs secrete brain-derived neurotrophic factor (BDNF),
vascular endothelial growth factor (VEGF) and neurotrophin-3
(NT-3), which can enhance neuronal survival, axonal regeneration,
and synaptic function by activating the phosphatidylinositol 3
kinase (PI3K)/protein kinase B (Akt) and MAPK/ERK signaling
pathways (99,100). Through reducing
neuroinflammation and oxidative stress, MSCs have been shown to
attenuate disease progression in Alzheimer's disease and
Parkinson's disease, while improving neurological and cognitive
functions (101,102). In spinal cord injury models,
MSCs facilitate axonal regeneration and functional recovery
(103).
Notably, relevant studies on ASD also demonstrate
promising results. In a rat model of VPA-induced ASD, MSC
transplantation was shown to markedly improve social interaction,
mitigate repetitive behaviors and alleviate anxiety-like behaviors
(104). These effects may be
attributed to the immunomodulatory and neuroprotective functions of
MSCs which reduce neuroinflammation and enhance synaptic
plasticity, ultimately improving core behavioral symptoms.
Despite the considerable therapeutic potential of
MSCs in ASD, challenges remain regarding clinical translation,
including immunorejection, diminished proliferative capacity over
time, and concerns over transplantation safety. In this regard,
exosomes secreted by MSCs have emerged as a research hotspot in
recent years due to their critical roles in immune regulation and
tissue repair (105). Compared
with conventional cell transplantation, MSC-derived exosomes offer
a 'cell-free therapy' strategy characterized by small size, ease of
storage, low immunogenicity, and suitability for standardized
production and quality control. These features not only provide new
perspectives and directions for the precision treatment of
neurodevelopmental disorders such as ASD, but may also enhance the
feasibility of clinical translation (106).
Structure and biological functions of
exosomes
Exosomes are extracellular vesicles measuring
~30-150 nm in diameter, originating from endosomal pathways and
released into bodily fluids through the fusion of multivesicular
bodies with the cell membrane (107,108). A lipid bilayer encases exosomes
enriched with various biologically active components, including
proteins, nucleic acids (mRNAs, miRNAs and lncRNAs, etc.) and
lipids. Their stable structure enables exosomes to serve as unique
mediators of intercellular communication and regulation, thus
providing a theoretical basis for potential therapeutic
applications (109). The
composition and biogenesis pathways of MSC-Exos, as well as their
downstream effects on neuronal and glial cells in ASD models, are
summarized in Fig. 3. Exosomes
exert their therapeutic effects primarily through interacting with
their cargo in recipient cells. Exosomes deliver their contents
directly into target cells, either by fusing with the plasma
membrane or undergoing receptor-mediated endocytosis, and thereby
modulate gene expression and signaling pathways.
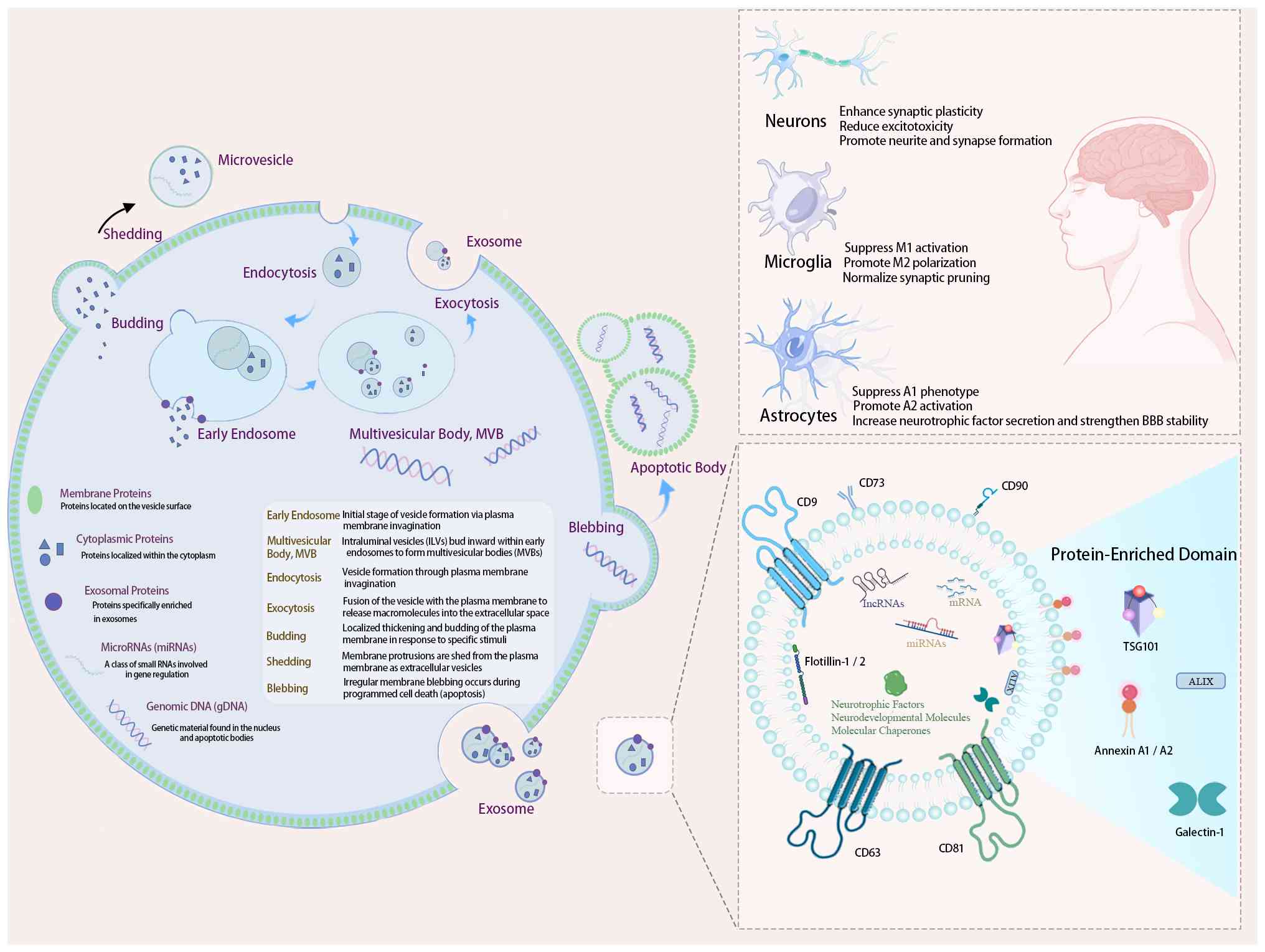 | Figure 3Biogenesis, molecular contents and
mechanisms of MSC-derived exosomes in ASD therapy. (Left panel)
MSC-derived exosomes originate from endosomal pathways. They
encapsulate proteins, mRNAs and miRNAs. (Top right panel) These
exosomes interact with neurons, microglia and astrocytes,
modulating synaptic plasticity, microglial polarization, and
astrocyte phenotype to alleviate neuroinflammation associated with
ASD. (Bottom right panel) Exosomal surface and cargo proteins
include CD63, CD81, CD9, CD73, flotillin-1/2, TSG101, Annexin
A1/A2, galectin-1, neurotrophic factors and molecular chaperones.
The authors created a figure referencing multiple sources (119-123,133-139,141-143). ASD, autism spectrum disorder;
MSCs, mesenchymal stem cells. |
Nucleic acid cargo and gene
regulation
The miRNAs in exosomes play vital roles in
regulating gene expression, as they can modulate inflammation,
neuronal survival, and synaptic plasticity by targeting specific
signaling cascades (110,111). Previous studies have
demonstrated that miRNAs encapsulated in exosomes, such as miR-124
packaged within exosomes, have been demonstrated to enhance
neuronal regeneration and reduce neuroinflammation (112-115). Other miRNAs, such as miR-21,
miR-155 and miR-146a, can suppress the NF-κB pathway, thereby
reducing the secretion of pro-inflammatory factors (e.g., IL-6 and
TNF-α) and increasing the levels of anti-inflammatory IL-10,
ultimately mitigating neuroinflammation. These miRNAs may also
inhibit excessive activation of the mTOR pathway, reducing aberrant
neuronal activity (115-118).
Additionally, miR-873a-5p within exosomes can promote microglial
polarization toward the anti-inflammatory M2 phenotype (120). Apart from miRNAs, mRNAs and
lncRNAs, exosomes can also contain these molecules, which can
affect recipient cell functions through translation or
transcriptional regulation, influencing tissue repair and immune
modulation (121-123).
Protein cargo and neural repair
Exosomes also harbor proteins, such as heat shock
proteins (HSPs) (124),
neuronal adhesion molecules [e.g., intercellular adhesion molecule
1 (125)] and cytokines [e.g.,
TGF-β (126)] that can directly
modulate axonal growth, promote synaptic remodeling, and strengthen
neural network connectivity, are considered critical processes in
immune regulation, neuroprotection, and tissue repair. Furthermore,
exosomal membrane lipids (e.g., sphingomyelin and
phosphatidylserine) can enhance synapse formation and stability,
thereby influencing the functional properties of recipient cells
(127). These molecular
mechanisms provide a basis for improving cognitive and behavioral
deficits in ASD.
Neurotrophic factors and signaling
pathways
Exosomes are often enriched with neurotrophic
factors and neurodevelopmental molecules, such as Nestin, NeuroD1,
BDNF and VEGF. These factors act through signaling pathways,
including the Wnt/β-catenin, TGF-β/BMP and PI3K/Akt pathways,
thereby supporting neuronal proliferation, migration and
differentiation (128-130).
Gut microbiota and the gut-brain
axis
ASD has been closely linked to gut microbiome
dysbiosis. Exosomes can modulate gut microbiota diversity by
increasing the abundance of bifidobacteria and lactobacilli and
improving gut barrier function (131). Exosomes containing miR-155,
miR-181a and TGF-β can reduce intestinal inflammation and
permeability. Exosomes containing miR-155, miR-181a and TGF-β can
reduce intestinal inflammation and permeability, ultimately
influencing the gut-brain axis and indirectly affecting the central
nervous system function (132-134).
Exosomes carry a versatile repertoire of bioactive
molecules that can modulate inflammatory responses, promote neural
repair and regulate the gut-brain axis. These properties underscore
the potential of exosome-based, cell-free therapy for ASD and other
neurodevelopmental disorders.
Potential value of exosomes in the
treatment of ASD
In recent years, MSC-Exos have exhibited immense
promise in ASD research and interventions due to their unique
composition and diverse functionalities (29,129). Compared with direct cell
transplantation, exosomes provide greater safety, controllability
and ease of storage. By carrying various miRNAs, proteins and
bioactive molecules, MSC-Exos can exert immunomodulatory,
neuroprotective and synaptic repair effects in the CNS (135,136). Their ability to regulate
neuroinflammation, restore synaptic plasticity, and rebalance the
gut-brain axis underscores the potential of exosome-based
'cell-free therapy' (129),
paving the way for new avenues of research and clinical
applications in ASD.
Studies using animal models of ASD have demonstrated
that the intranasal administration of MSC-Exos markedly improves
social interaction, cognition and repetitive behaviors (22,29,135,136). These effects may be attributed
to miRNAs and anti-inflammatory agents delivered by exosomes, which
modulate immune responses and neuronal network functionality. For
example, exosomal miRNAs [such as miR-21-5p (137), miR-17-92 (138) and miR-146a (139)] and multiple bioactive proteins
[including HSP70 and TGF-β (140)] help regulate neuroinflammation,
synaptic plasticity, and neurogenesis via intercellular signaling.
Additionally, research suggests that neurotrophic factors present
in exosomes can enhance neuronal survival and differentiation,
thereby improving behavioral outcomes. These factors also enhance
synaptic plasticity, promote network-level functional integration,
and reduce CNS inflammation by increasing anti-inflammatory
cytokines (22,29,135,141).
In summary, MSC-Exos, owing to their diverse
bioactive molecules and stable delivery system repertoire, have
shown comprehensive effects on immunoregulation, neural repair and
synaptic regeneration in ASD. They are thus emerging as a promising
therapeutic alternative to conventional cell-based treatments.
Ongoing research will further elucidate the underlying mechanisms
of exosomes in ASD, optimize exosome preparation and administration
strategies, and, through multidisciplinary collaboration, develop
safe, efficacious and scalable exosome-based therapeutics. These
efforts hold the potential to propel the field toward precision
interventions and clinical translation for ASD.
Clinical progress of stem cells and exosomes
in ASD
In recent years, stem cell-based therapy has emerged
as one of the key areas of interest for the treatment of ASD. By
employing stem cells or their exosomes, researchers aim to modulate
immune responses, improve cerebral blood flow, and enhance neuronal
repair in individuals with ASD (21). Early clinical trials have
documented varying degrees of improvement in behavior, language
skills and social abilities among ASD patients receiving MSC
therapy (142) with minimal
adverse events (143).
Additionally, these trials noted decreases in inflammatory markers
and improvements in brain metabolic activity, further supporting
the potential role of MSCs in the treatment of ASD (142).
Current status of clinical research and
key findings
Several completed clinical trials worldwide have
investigated the use of stem cell therapy for the treatment of ASD.
The following paragrpahs summarizes the primary clinical studies
and their principal outcomes:
Use of umbilical cord blood-derived
stem cells
Lv et al (144) conducted a study combining cord
blood mononuclear cells (CBMNCs) with umbilical cord-derived
mesenchymal stem cells, enrolling 37 patients with ASD. Their
results revealed that the combination treatment group had
significantly greater improvements in the Childhood Autism Rating
Scale (CARS) and Aberrant Behavior Checklist (ABC) scores compared
to either the CBMNC-only group or the control group (which received
only rehabilitative interventions). No severe adverse events were
reported, underscoring both the safety and the superior efficacy of
combination therapy (144).
Preliminary attempts with human
embryonic stem cells
Shroff (145)
reported outcomes for 3 patients with ASD treated with human
embryonic stem cells. Improvements were observed in motor skills,
cognition, social interaction and sensory sensitivities, with no
adverse events noted. Although the sample size was small, this
study highlights the potential of stem cell therapies for
alleviating core symptoms of ASD (145).
Potential of autologous umbilical cord
blood therapy
Chez et al (146) conducted a randomized,
double-masked, placebo-controlled crossover study involving 29
children with ASD aged 2 to 6 years, evaluating the safety and
efficacy of autologous umbilical cord blood transfusions. Although
treatment did not significantly improve its primary endpoints, some
participants exhibited trends toward improved socialization and
language skills. No severe adverse events occurred, suggesting that
stem cell therapy may hold promise for enhancing social functioning
(146).
Changes in brain network
connectivity
Simhal et al (147) used diffusion tensor imaging to
assess the effect of umbilical cord blood stem cell therapy on
white matter networks in patients with ASD. Their study revealed
that post-treatment, the robustness of the white matter network was
markedly increased, especially in regions involved in social and
communication functions. These findings may be related to the
ability of stem cells to regulate neuroinflammation and promote
neuroplasticity (147).
Stem cells combined with behavioral
interventions
Thanh et al (148) conducted an open-label clinical
trial in Vietnam, enrolling 30 children with ASD aged 3 to 7 years.
The intervention combined autologous bone marrow mononuclear cell
transplantation with the ESDM, an early behavioral intervention.
The results revealed a reduction in median CARS scores from 50 to
46.5 (P<0.05) and an increase in Vineland Adaptive Behavior
Scale scores from 53.5 to 60.5, indicating substantial improvements
in social interaction, language abilities, and daily living skills,
with no severe adverse events reported (148).
Collectively, the aforementioned clinical studies
suggest that MSC-based and MSC exosome therapies hold promise for
improving social communication, language skills, behavioral
challenges and cognition in individuals with ASD. Notably, combined
treatments, including the incorporation of behavioral interventions
or personalized therapeutic regimens that appear effective, may be
due to their multi-target mechanisms of action. The majority of
studies did not report severe adverse events, reflecting an overall
favorable safety profile. However, a number of existing studies
involve small sample sizes and open-label designs, lacking the
robust evidence from large-scale randomized controlled trials
(RCTs). Additionally, the precise mechanisms underlying the
observed therapeutic effects remain incompletely understood. Future
investigations should further refine treatment protocols, clarify
the role of exosomes in cell therapy, and establish the long-term
safety and efficacy of these interventions. Expanding multicenter,
large-sample RCTs is crucial for evaluating the performance of
different stem cell types and administration strategies. Moreover,
exploring synergistic effects between stem cells and behavioral or
pharmacological interventions will help optimize individualized
treatment plans. Finally, integrating gene-editing technologies and
engineered exosomes could enhance their clinical utility. Such
efforts will provide a more robust theoretical and practical
foundation for applying stem cell and exosome-based therapies in
ASD.
Potential for clinical translation and
existing barriers
Due to pronounced immunomodulatory properties,
tissue repair potential and favorable safety profile, MSCs have
attracted widespread attention in recent years, gradually becoming
an essential focus of stem cell-based therapies. As MSC research
advances, the exosomes they secrete (MSC-Exos) have been identified
as a key functional mediator and are expected to play a potential
role in ASD interventions (149). The majority of clinical studies
on MSCs and MSC-Exos remain in phase I/II trials (150). Early data suggest that patients
with ASD treated with these therapies experience improved social
interaction and language skills, as well as decreased inflammatory
markers, such as TNF-α and IL-6 (151,152). Nonetheless, the clinical
translation of MSCs and their exosomes depends on the efficacy of
preclinical studies, as well as substantial practical and
regulatory challenges. Although preliminary findings are
encouraging, limitations in sample size, follow-up duration, and
study design mean that large-scale, multicenter clinical data are
still required to confirm their long-term efficacy and safety. A
discussion of the advantages and challenges inherent in clinical
translation is presented below:
Core advantages and multifaceted
mechanisms
Multifunctionality: Immunomodulation and
neuroprotection
The immunomodulatory and neuroprotective properties
of MSCs render them attractive for addressing multiple pathological
mechanisms underlying ASD. Research indicates that MSC-Exos can
regulate T- and B-cell activation, induce immune tolerance, and
suppress the secretion of inflammatory cytokines, thus improving
neuroinflammatory conditions (22). Growth factors, miRNAs, and other
bioactive substances in MSC-Exos also promote neuronal survival and
synaptic plasticity (111).
Owing to their differentiation potential, MSCs and their exosomes
may also serve as tools for neural repair, providing potential
therapeutic value for ASD-related neurodevelopmental anomalies.
Low immunogenicity and favorable safety
profile
MSCs exhibit relatively low immunogenicity in
allogeneic transplantation (153). Their exosomes, which lack
complete cell-surface antigen expression (154) and do not carry a nucleus or
full genomic content (155),
further minimize risks of immune rejection and tumorigenesis.
Consequently, MSC-Exos do not require stringent donor-recipient
matching to the same extent as allogeneic MSCs, reducing potential
complications and ethical concerns.
Targeted action and personalized therapy
Exosomes can enter target cells through
receptor-mediated endocytosis or membrane fusion, delivering a
diverse array of active molecules that influence cell
proliferation, differentiation, apoptosis, and immune responses.
Owing to their nanoscale size and lipid bilayer structure, exosomes
demonstrate relatively high transmembrane delivery efficiency;
research suggests that exosomes can traverse the blood-brain
barrier (156). As the
pathogenesis of ASD involves abnormal neural networks and central
neuroinflammation, MSC-Exos capable of penetrating the CNS may help
restructure the brain microenvironment and enhance therapeutic
outcomes. Moreover, engineering MSCs or modifying exosome cargo
based on individual patient pathology (e.g., through gene editing)
may enable more precise, personalized treatments.
Large-scale production, transport and
storage
Under specific culture conditions, exosomes can be
produced and purified in large quantities. Compared to cell
transplantation, which requires highly viable, well-characterized
cells in sufficient numbers, exosomes are easier to standardize and
mass-produce, rendering clinical deployment more feasible.
Additionally, exosomes can be stored for extended periods under
appropriate conditions while retaining their bioactivity, thereby
circumventing many of the stability challenges encountered with
live MSCs. This facilitates large-scale manufacturing, clinical
stockpiling, and broader geographic distribution.
Real-world challenges and
bottlenecks
Production techniques and quality control
Variations in MSC culture conditions and tissue
sources can produce substantial differences in exosome yield and
quality. Standard protocols for extracting, purifying and
characterizing exosomes have yet to be universally adopted.
Reagents, equipment and technical approaches vary greatly among
laboratories, which can potentially affect the reproducibility of
results. To achieve industrial-scale production and clinical
adoption of MSC-Exos, international or national-level
standardization is needed, encompassing MSC culture conditions,
exosome extraction, purification, and the establishment of quality
metrics. Key parameters include identifying and quantifying cargo
molecules, particle size distribution and surface markers. Without
such standards, ensuring the stability and consistency of MSC-Exos
across different batches remains difficult.
Heterogeneity in sources and cell states
MSCs can be derived from various sources, including
bone marrow, adipose tissue, umbilical cord, and other tissues,
resulting in variability based on tissue origin, donor
characteristics and passage number. For instance, adipose-derived
MSCs generally have more substantial adipogenic potential, whereas
bone marrow-derived MSCs may be more adept at osteogenic
differentiation (157). In the
context of ASD therapy, MSC-Exos from different sources or
differing cell expansion histories may yield divergent
immunomodulatory and neuroprotective effects. A systematic
investigation is required to determine an optimal source or a
combination strategy for clinical use.
Complexity of mechanisms
Although a number of studies have documented the
neuroprotective, immunoregulatory and neural network repair
functions of MSC-Exos, the precise molecular mechanisms underlying
these functions remain incompletely understood (22,136,149). It remains to be determined
which miRNAs or proteins in MSC-Exos are essential for regulating
neuronal repair and immune balance in ASD. In addition, the
mechanisms through which exosomes interact with target cells and
influence downstream signaling pathways remain to be elucidated.
Further research using in vitro and in vivo models is
required to clarify these mechanisms, improving the specificity and
reliability of future clinical applications.
Safety and ethical oversight
While MSC-Exos are associated with a lower
immunogenicity and tumorigenic risk than direct cell
transplantation, comprehensive preclinical safety evaluations and
multicenter clinical trials are necessary to assess long-term
risks. The clinical use of stem cells and their derivatives is
strictly regulated in many countries, requiring compliance with
guidelines for pharmaceutical and biological products, as well as
adherence to ethical considerations, including donor sourcing and
traceability during preparation and application (158).
Large-scale manufacturing and
cost-effectiveness
Generating MSC-Exos on a large scale requires
efficient isolation and concentration protocols for MSC culture.
Once translated to clinical practice, cost and patient
accessibility become critical factors. In the event that production
remains prohibitively costly without relevant policy support, the
widespread adoption of MSC-Exos in the treatment of ASD may be
limited.
Delivery efficiency and long-term safety
Achieving the efficient, targeted delivery of
exosomes to the CNS and specific lesion sites to enhance
therapeutic specificity poses a significant challenge. Moreover,
current exosome-related clinical trials typically feature short
follow-up periods (144,146,147).
Exosomes hold potential as a long-term intervention strategy;
however, their extended use may carry unknown risks, including
effects on the immune system and neural function. Rigorous
long-term monitoring and evaluation are therefore necessary.
Individual differences and clinical
applicability
ASD is highly heterogeneous, with variability in
age, subtype and comorbid conditions affecting treatment responses
to MSCs or MSC-Exos. Personalized therapies tailored to individual
disease stages and pathological profiles remain a significant
challenge. Biomarker analysis and molecular subtyping may enhance
individualized treatment strategies. Systematic clinical trials are
necessary to establish optimal dosing and administration protocols
for diverse patient populations.
Conclusion
In conclusion, MSCs and MSC-Exos have demonstrated
considerable therapeutic potential in the treatment of ASD. MSCs
and MSC-Exos can alleviate neuroinflammation, enhance synaptic
plasticity, and promote neural network repair through their
immunomodulatory and neuroprotective effects. MSC-Exos offers
substantial advantages for ASD treatment, including multifaceted
therapeutic mechanisms, low immunogenicity and potential for
large-scale production. Nevertheless, critical hurdles, such as
manufacturing protocols, quality control, elucidation of the
mechanism, long-term safety, and personalized application, need to
be overcome before MSC-Exos can be widely integrated into ASD
therapies. Addressing these challenges through multidisciplinary
collaboration and further research will pave the way for more
precise, safe, and effective interventions. With their low
immunogenicity, ability to cross the blood-brain barrier, and
efficient delivery of bioactive molecules, exosomes are promising
candidates for treating ASD and other neurodevelopmental disorders.
However, several critical challenges remain, including issues
related to large-scale production, quality control, and elucidation
of underlying mechanisms. Moreover, rigorous clinical trials under
strict regulatory and ethical frameworks are required to establish
the safety and efficacy of MSC-Exos. Therefore, it is urgent to
clarify their mechanisms of action, develop standardized
manufacturing and quality control protocols, and optimize long-term
safety and personalized treatment strategies. With the integration
of high-throughput omics technologies, interdisciplinary
collaboration, and continuous innovation, MSC-Exos are expected to
advance in basic research and clinical application, offering
greater precision and broader therapeutic potential in ASD.
Ultimately, only by overcoming key scientific and technical hurdles
can MSC-Exos become a viable strategy for individualized
interventions.
Future perspectives and application
directions
Advancing the therapeutic efficacy and clinical
translation of MSC-derived exosomes in ASD will require sustained
innovation across multiple dimensions. To improve in vivo
delivery efficiency, various delivery platforms, such as magnetic
nanoparticles (159) and
hydrogels (160), have been
developed to enhance targeting and stability in the central nervous
system. Additionally, chemical modifications (161) and genetic engineering
techniques (162) have been
employed to increase the therapeutic specificity and prolong the
half-life of exosomes. Engineered MSCs that overexpress functional
proteins and miRNAs, such as neurotrophic factors or
anti-inflammatory molecules (163), have shown promise in enhancing
the neuroprotective and immunoregulatory effects of their exosomes,
thereby broadening their application potential in ASD and other
neurological disorders. To meet the demands of personalized
medicine, customized exosome formulations and treatment protocols
tailored to individual patient profiles may improve therapeutic
outcomes and reduce adverse effects. The application of
high-throughput omics technologies, such as genomics, proteomics
and metabolomics continues to unveil the key molecular pathways and
networks through which MSCs and their exosomes modulate neurorepair
and immune responses. In particular, in-depth investigations into
the roles and interactions of miRNAs, proteins, and other bioactive
cargos will provide a robust foundation for evaluating therapeutic
efficacy and guiding the engineering of exosomes. For clinical
translation, it is essential to establish standardized and
efficient protocols for MSC cultivation and exosome purification,
along with internationally recognized quality control standards to
ensure batch-to-batch and inter-institutional consistency.
Large-scale, multicenter randomized controlled trials will be
crucial for validating the safety and efficacy of MSC-Exos,
necessitating refined trial designs that address patient selection,
dosage regimens, outcome measures, and long-term follow-up. Given
the complexity of ASD pathogenesis and its clinical heterogeneity,
future breakthroughs in MSC-Exos therapy will depend on
interdisciplinary collaboration across neuroscience, molecular
biology, immunology, and materials science, combined with
pharmacological and behavioral interventions. Continuous
technological innovation and integrative strategies will ultimately
pave the way for more effective and safer treatment options for
individuals with ASD.
Availability of data and materials
Not applicable.
Authors' contributions
ZS was involved in the conceptualization of the
study. ZS and NA were involved in the collection and curation data
from the literature, and in the writing and preparation of the
original draft of the manuscript. MM was involved in visualization
and literature analysis. ZL supervised the study. ZS, NA and ZL
reviewed and edited the final manuscript. All authors have read and
approved the final manuscript. Data authentications is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were
used to improve the readability and language of the manuscript or
to generate images, and subsequently, the authors revised and
edited the content produced by the AI tools as necessary, taking
full responsibility for the ultimate content of the present
manuscript.
Abbreviations:
|
ASD
|
autism spectrum disorder
|
|
CNV
|
copy number variation
|
|
MSC
|
mesenchymal stem cell
|
|
MSC-Exos
|
mesenchymal stem cell-derived
exosomes
|
|
NF-κB
|
nuclear factor κ-light-chain-enhancer
of activated B cells
|
|
PSD
|
postsynaptic density
|
|
PI3K
|
phosphatidylinositol 3 kinase
|
|
Akt
|
protein kinase B
|
|
RR
|
relative risk
|
|
TGF-β
|
transforming growth factor β
|
|
BMP
|
bone morphogenetic protein
|
|
VPA
|
valproic acid
|
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the Enterprise
Joint Fund Project of Hunan Provincial Natural Science Foundation
(grant no. 2024JJ9097).
References
|
1
|
Hung LY and Margolis KG: Autism spectrum
disorders and the gastrointestinal tract: Insights into mechanisms
and clinical relevance. Nat Rev Gastroenterol Hepatol. 21:142–163.
2024. View Article : Google Scholar
|
|
2
|
Kanner L: Autistic disturbances of
affective contact. Nerv Child. 2:217–250. 1943.
|
|
3
|
Association AP: Diagnostic and Statistical
Manual of Mental Disorders. 2022.
|
|
4
|
Shaw KA, Williams S, Patrick ME,
Valencia-Prado M, Durkin MS, Howerton EM, Ladd-Acosta CM, Pas ET,
Bakian AV, Bartholomew P, et al: Prevalence and early
identification of autism spectrum disorder among children aged 4
and 8 years-autism and developmental disabilities monitoring
network, 16 sites, United States, 2022. MMWR Surveill Summ.
74:1–22. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bai D, Yip BHK, Windham GC, Sourander A,
Francis R, Yoffe R, Glasson E, Mahjani B, Suominen A, Leonard H, et
al: Association of genetic and environmental factors with autism in
a 5-country cohort. JAMA Psychiatry. 76:1035–1043. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sanders SJ, He X, Willsey AJ,
Ercan-Sencicek AG, Samocha KE, Cicek AE, Murtha MT, Bal VH, Bishop
SL, Dong S, et al: Insights into autism spectrum disorder genomic
architecture and biology from 71 risk loci. Neuron. 87:1215–1233.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fu JM, Satterstrom FK, Peng M, Brand H,
Collins RL, Dong S, Wamsley B, Klei L, Wang L, Hao SP, et al: Rare
coding variation provides insight into the genetic architecture and
phenotypic context of autism. Nat Genet. 54:1320–1331. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hirota T and King BH: Autism spectrum
disorder: A review. JAMA. 329:157–168. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Christensen J, Grønborg TK, Sørensen MJ,
Schendel D, Parner ET, Pedersen LH and Vestergaard M: Prenatal
valproate exposure and risk of autism spectrum disorders and
childhood autism. JAMA. 309:1696–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zwaigenbaum L, Brian J and Ip A: Early
detection for autism spectrum disorder in young children. Paediatr
Child Health. 24:424–443. 2019.In English, French. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Robins DL, Casagrande K, Barton M, Chen
CMA, Dumont-Mathieu T and Fein D: Validation of the modified
checklist for Autism in toddlers, revised with follow-up
(M-CHAT-R/F). Pediatrics. 133:37–45. 2014. View Article : Google Scholar :
|
|
12
|
Volkmar F, Siegel M, Woodbury-Smith M,
King B, McCracken J and State M; American Academy of Child and
Adolescent Psychiatry (AACAP) Committee on Quality Issues (CQI):
Practice parameter for the assessment and treatment of children and
adolescents with autism spectrum disorder. J Am Acad Child Adolesc
Psychiatry. 53:237–257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song J, Reilly M and Reichow B: Overview
of meta-analyses on naturalistic developmental behavioral
interventions for children with autism spectrum disorder. J Autism
Dev Disord. 55:1–13. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dawson G, Rogers S, Munson J, Smith M,
Winter J, Greenson J, Donaldson A and Varley J: Randomized,
controlled trial of an intervention for toddlers with autism: The
early start denver model. Pediatrics. 125:e17–e23. 2010. View Article : Google Scholar
|
|
15
|
Wang Z, Loh S, Tian J and Chen QJ: A
meta-analysis of the effect of the early start denver model in
children with autism spectrum disorder. Int J Dev Disabil.
68:587–597. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fieiras C, Chen M, Liquitay CE, Meza N,
Rojas V, Franco J and Madrid E: Risperidone and aripiprazole for
autism spectrum disorder in children: an overview of systematic
reviews. BMJ Evid Based Med. 28:7–14. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aglinskas A, Hartshorne J and Anzellotti
S: Contrastive machine learning reveals the structure of
neuroanatomical variation within autism. Science. 376:1070–1074.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pizzano M, Shire S, Shih W, Levato L,
Landa R, Lord C, Smith T and Kasari C: Profiles of minimally verbal
autistic children: Illuminating the neglected end of the spectrum.
Autism Res. 17:1218–1229. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Konečná B, Radošinská J, Keményová P and
Repiská G: Detection of disease-associated microRNAs-application
for autism spectrum disorders. Rev Neurosci. 31:757–769. 2020.
View Article : Google Scholar
|
|
20
|
Yao TT, Chen L, Du Y, Jiang ZY and Cheng
Y: MicroRNAs as regulators, biomarkers, and therapeutic targets in
autism spectrum disorder. Mol Neurobiol. 62:5039–5056. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen W, Ren Q, Zhou J and Liu W:
Mesenchymal stem cell-induced neuroprotection in pediatric
neurological diseases: Recent update of underlying mechanisms and
clinical utility. Appl Biochem Biotechnol. 196:5843–5858. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liang Y, Duan L, Xu X, Li X, Liu M, Chen
H, Lu J and Xia J: Mesenchymal stem cell-derived exosomes for
treatment of autism spectrum disorder. ACS Appl Bio Mater.
3:6384–6393. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hedayat M, Ahmadi M, Shoaran M and Rezaie
J: Therapeutic application of mesenchymal stem cells derived
exosomes in neurodegenerative diseases: A focus on non-coding RNAs
cargo, drug delivery potential, perspective. Life Sci.
320:1215662023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Su R: Mesenchymal stem cell exosomes as
nanotherapeutic agents for neurodegenerative diseases. Highl Sci
Eng Technol. 2:7–14. 2022. View Article : Google Scholar
|
|
25
|
Tian MS and Yi XN: The role of mesenchymal
stem cell exosomes in the onset and progression of Alzheimer's
Disease. Biomed Sci. 10:6–13. 2024.
|
|
26
|
Ge Y, Wu J, Zhang L, Huang N and Luo Y: A
new strategy for the regulation of neuroinflammation: Exosomes
derived from mesenchymal stem cells. Cell Mol Neurobiol. 44:242024.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Elsherif R, Abdel-Hafez AM, Hussein O,
Sabry D, Abdelzaher L and Bayoumy AA: The potential ameliorative
effect of mesenchymal stem cells-derived exosomes on cerebellar
histopathology and their modifying role on PI3k-mTOR signaling in
rat model of autism spectrum disorder. J Mol Histol. 56:652025.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao S, Zhong Y, Shen F, Cheng X, Qing X
and Liu J: Comprehensive exosomal microRNA profile and construction
of competing endogenous RNA network in autism spectrum disorder: A
pilot study. Biomol Biomed. 24:292–301. 2024. View Article : Google Scholar :
|
|
29
|
Perets N, Oron O, Herman S, Elliott E and
Offen D: Exosomes derived from mesenchymal stem cells improved core
symptoms of genetically modified mouse model of autism Shank3B. Mol
Autism. 11:1–13. 2020. View Article : Google Scholar
|
|
30
|
Ressa HJ, Newman BT, Jacokes Z, McPartland
JC, Kleinhans NM, Druzgal TJ, Pelphrey KA and Van Horn JD:
Widespread associations between behavioral metrics and brain
microstructure in ASD suggest age mediates subtypes of ASD.
BioRxiv. 28:2024.09.04.611183. 2024.
|
|
31
|
Almuqhim F and Saeed F: ASD-GResTM: Deep
learning framework for ASD classification using Gramian angular
field. Proceedings (IEEE Int Conf Bioinforma Biomed).
2023:2837–2843. 2023.
|
|
32
|
Tang X, Ran X, Liang Z, Zhuang H, Yan X,
Feng C, Qureshi A, Gao Y and Shen L: Screening biomarkers for
autism spectrum disorder using plasma proteomics combined with
machine learning methods. Clin Chim Acta. 565:1200182025.
View Article : Google Scholar
|
|
33
|
Herbrecht E, Lazari O, Notter M, Kievit E,
Schmeck K and Spiegel R: Short-term and highly intensive early
intervention FIAS: Two-year outcome results and factors of
influence. Front Psychiatry. 11:6872020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Manohar H and Kandasamy P: Clinical
outcomes of children with ASD-preliminary findings from a 18 month
follow up study. Asian J Psychiatry. 64:1028162021. View Article : Google Scholar
|
|
35
|
Yuan B, Wang M, Wu X, Cheng P, Zhang R,
Zhang R, Yu S, Zhang J, Du Y, Wang X and Qiu Z: Identification of
de novo Mutations in the Chinese autism spectrum disorder cohort
via whole-exome sequencing unveils brain regions implicated in
autism. Neurosci Bull. 39:1469–1480. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Levy RJ and Paşca SP: What have organoids
and assembloids taught us about the pathophysiology of
neuropsychiatric disorders? Biol Psychiatry. 93:632–641. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Al-Beltagi M, Saeed NK, Bediwy AS, Bediwy
EA and Elbeltagi R: Decoding the genetic landscape of autism: A
comprehensive review. World J Clin Pediatr. 13:984682024.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hallmayer J, Cleveland S, Torres A,
Phillips J, Cohen B, Torigoe T, Miller J, Fedele A, Collins J,
Smith K, et al: Genetic heritability and shared environmental
factors among twin pairs with autism. Arch Gen Psychiatry.
68:1095–1102. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tick B, Bolton P, Happé F, Rutter M and
Rijsdijk F: Heritability of autism spectrum disorders: A
meta-analysis of twin studies. J Child Psychol Psychiatry.
57:585–595. 2016. View Article : Google Scholar
|
|
40
|
Shaw KA, Bilder DA, McArthur D, Williams
AR, Amoakohene E, Bakian AV, Durkin MS, Fitzgerald RT, Furnier SM,
Hughes MM, et al: Early identification of autism spectrum disorder
among children aged 4 years-autism and developmental disabilities
monitoring network, 11 sites, United States, 2020. MMWR Surveill
Summ. 72:1–15. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Barnard RA, Pomaville MB and O'Roak BJ:
Mutations and modeling of the chromatin remodeler CHD8 define an
emerging autism etiology. Front Neurosci. 9:4772015. View Article : Google Scholar
|
|
42
|
Xu B, Ho Y, Fasolino M, Medina J, O'Brien
WT, Lamonica JM, Nugent E, Brodkin ES, Fuccillo MV, Bucan M and
Zhou Z: Allelic contribution of Nrxn1α to autism-relevant
behavioral phenotypes in mice. PLoS Genet. 19:e10106592023.
View Article : Google Scholar
|
|
43
|
Chiola S, Napan KL, Wang Y, Lazarenko RM,
Armstrong CJ, Cui J and Shcheglovitov A: Defective AMPA-mediated
synaptic transmission and morphology in human neurons with
hemizygous SHANK3 deletion engrafted in mouse prefrontal cortex.
Mol Psychiatry. 26:4670–4686. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Guo B, Chen J, Chen Q, Ren K, Feng D, Mao
H, Yao H, Yang J, Liu H, Liu Y, et al: Anterior cingulate cortex
dysfunction underlies social deficits in Shank3 mutant mice. Nat
Neurosci. 22:1223–1234. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vogt D, Cho KKA, Shelton SM, Paul A, Huang
ZJ, Sohal VS and Rubenstein JLR: Mouse Cntnap2 and human CNTNAP2
ASD alleles cell autonomously regulate PV+ cortical interneurons.
Cereb Cortex. 28:3868–3879. 2018. View Article : Google Scholar
|
|
46
|
Kushima I, Nakatochi M, Aleksic B, Okada
T, Kimura H, Kato H, Morikawa M, Inada T, Ishizuka K, Torii Y, et
al: Cross-Disorder analysis of genic and regulatory copy number
variations in bipolar disorder, schizophrenia, and autism spectrum
disorder. Biol Psychiatry. 92:362–374. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rein B and Yan Z: 16p11.2 copy number
variations and neurodevelopmental disorders. Trends Neurosci.
43:886–901. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zarrei M, Burton C, Engchuan W, Young EJ,
Higginbotham EJ, MacDonald JR, Trost B, Chan AJS, Walker S,
Lamoureux S, et al: A large data resource of genomic copy number
variation across neurodevelopmental disorders. NPJ Genomic Med.
4:262019. View Article : Google Scholar
|
|
49
|
Costain G, Walker S, Argiropoulos B,
Baribeau DA, Bassett AS, Boot E, Devriendt K, Kellam B, Marshall
CR, Prasad A, et al: Rare copy number variations affecting the
synaptic gene DMXL2 in neurodevelopmental disorders. J Neurodev
Disord. 11:32019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hauw JJ, Hausser-Hauw C and Barthélémy C:
Synapse and primary cilia dysfunctions in autism spectrum
disorders. Avenues to normalize these functions. Rev Neurol
(Paris). 180:1059–1070. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ferrucci L, Cantando I, Cordella F, Di
Angelantonio S, Ragozzino D and Bezzi P: Microglia at the
tripartite synapse during postnatal development: implications for
autism spectrum disorders and schizophrenia. Cells. 12:28272023.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Pagani M, Barsotti N, Bertero A, Trakoshis
S, Ulysse L, Locarno A, Miseviciute I, De Felice A, Canella C,
Supekar K, et al: mTOR-related synaptic pathology causes autism
spectrum disorder-associated functional hyperconnectivity. Nat
Commun. 12:60842021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Giansante G, Marte A, Romei A, Prestigio
C, Onofri F, Benfenati F, Baldelli P and Valente P: Presynaptic
L-type Ca2+ channels increase glutamate release probability and
excitatory strength in the hippocampus during chronic
neuroinflammation. J Neurosci. 40:6825–6841. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Riekki R, Pavlov I, Tornberg J, Lauri S,
Airaksinen M and Taira T: Altered synaptic dynamics and hippocampal
excitability but normal long-term plasticity in mice lacking
hyperpolarizing GABA A receptor-mediated inhibition in CA1
pyramidal neurons. J Neurophysiol. 99:3075–3089. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cast T, Boesch D, Smyth K, Shaw A,
Ghebrial M and Chanda S: An autism-associated mutation impairs
neuroligin-4 glycosylation and enhances excitatory synaptic
transmission in human neurons. J Neurosci. 41:392–407. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Amal H, Barak B, Bhat V, Gong G, Joughin
BA, Wang X, Wishnok JS, Feng G and Tannenbaum S: Shank3 mutation in
a mouse model of autism leads to changes in the S-nitroso-proteome
and affects key proteins involved in vesicle release and synaptic
function. Mol Psychiatry. 25:1835–1848. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Higa GSV, Viana FJC, Francis-Oliveira J,
Cruvinel E, Franchin TS, Marcourakis T, Ulrich H and De Pasquale R:
Serotonergic neuromodulation of synaptic plasticity.
Neuropharmacology. 257:1100362024. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liu W, Chen QY, Li XH, Zhou Z and Zhuo M:
Cortical tagged synaptic long-term depression in the anterior
cingulate cortex of adult mice. J Neurosci. 44:e00282420242024.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hagena H and Manahan-Vaughan D: Interplay
of hippocampal long-term potentiation and long-term depression in
enabling memory representations. Philos Trans R Soc Lond B Biol
Sci. 379:202302292024. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cong J, Zhuang W, Liu Y, Yin S, Jia H, Yi
C, Chen K, Xue K, Li F, Yao D, et al: Altered default mode network
causal connectivity patterns in autism spectrum disorder revealed
by Liang information flow analysis. Hum Brain Mapp. 44:2279–2293.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kember J, Patenaude P, Sweatman H, Van
Schaik L, Tabuenca Z and Chai X: Specialization of anterior and
posterior hippocampal functional connectivity differs in autism.
Autism Res. 17:1126–1139. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Grossberg S and Kishnan D: Neural dynamics
of autistic repetitive behaviors and fragile X syndrome: Basal
ganglia movement gating and mGluR-modulated adaptively timed
learning. Front Psychol. 9:2692018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Xiao T, Wan J, Qu H and Li Y:
Tripartite-motif protein 21 knockdown extenuates LPS-triggered
neurotoxicity by inhibiting microglial M1 polarization via
suppressing NF-κB-mediated NLRP3 inflammasome activation. Arch
Biochem Biophys. 30:1089182021. View Article : Google Scholar
|
|
64
|
Zhou L, Wang D, Qiu X, Zhang W, Gong Z,
Wang Y and Xu X: DHZCP Modulates microglial M1/M2 polarization via
the p38 and TLR4/NF-κB signaling pathways in LPS-stimulated
microglial Cells. Front Pharmacol. 11:11262020. View Article : Google Scholar
|
|
65
|
Tao W, Hu Y, Chen Z, Dai Y, Hu Y and Qi M:
Magnolol attenuates depressive-like behaviors by polarizing
microglia towards the M2 phenotype through the regulation of
Nrf2/HO-1/NLRP3 signaling pathway. Phytomedicine. 91:1536922021.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Tsai C, Chen G, Chen YC, Shen CK, Lu DY,
Yang LY, Chen JH and Yeh WL: Regulatory effects of quercetin on
M1/M2 macrophage polarization and oxidative/antioxidative balance.
Nutrients. 14:672021. View Article : Google Scholar
|
|
67
|
Lehrman EK, Wilton DK, Litvina EY, Welsh
CA, Chang ST, Frouin A, Walker AJ, Heller MD, Umemori H, Chen C and
Stevens B: CD47 protects synapses from excess microglia-mediated
pruning during development. Neuron. 100:120–134.e6. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kobashi S, Terashima T, Katagi M, Nakae Y,
Okano J, Suzuki Y, Urushitani M and Kojima H: Transplantation of
M2-Deviated microglia promotes recovery of motor function after
spinal cord injury in mice. Mol Ther. 28:254–265. 2020. View Article : Google Scholar :
|
|
69
|
Li Y, Liu Z, Song Y, Pan JJ, Jiang Y, Shi
X, Liu C, Ma Y, Luo L, Mamtilahun M, et al: M2 microglia-derived
extracellular vesicles promote white matter repair and functional
recovery via miR-23a-5p after cerebral ischemia in mice.
Theranostics. 12:3553–3573. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Li Z, Xiao J, Xu X, Li W, Zhong R, Qi L,
Chen J, Cui G, Wang S, Zheng Y, et al: M-CSF, IL-6, and TGF-β
promote generation of a new subset of tissue repair macrophage for
traumatic brain injury recovery. Sci Adv. 7:eabb62602021.
View Article : Google Scholar
|
|
71
|
Ding X, Wang J, Huang M, Chen Z, Liu J,
Zhang Q, Zhang C, Xiang Y, Zen K and Li L: Loss of microglial SIRPα
promotes synaptic pruning in preclinical models of
neurodegeneration. Nat Commun. 12:20302021. View Article : Google Scholar
|
|
72
|
Penney J, Ralvenius WT, Loon A, Cerit O,
Dileep V, Milo B, Pao PC, Woolf H and Tsai LH: iPSC-derived
microglia carrying the TREM2 R47H/+ mutation are proinflammatory
and promote synapse loss. Glia. 72:452–469. 2024. View Article : Google Scholar
|
|
73
|
Nugent A, Lin K, Lengerich B, Lianoglou S,
Przybyla L, Davis SS, Llapashtica C, Wang J, Kim DJ, Xia D, et al:
TREM2 regulates microglial cholesterol metabolism upon chronic
phagocytic challenge. Neuron. 105:837–854. 2019. View Article : Google Scholar
|
|
74
|
Kong L, Li W, Chang E, Wang W, Shen N, Xu
X, Wang X, Zhang Y, Sun W, Hu W, et al: mtDNA-STING axis mediates
microglial polarization via IRF3/NF-κB signaling after ischemic
stroke. Front Immunol. 13:8609772022. View Article : Google Scholar
|
|
75
|
Diaz-Castro B, Bernstein AM, Coppola G,
Sofroniew MV and Khakh BS: Molecular and functional properties of
cortical astrocytes during peripherally induced neuroinflammation.
Cell Rep. 36:1095082021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Chistyakov D, Gavrish G, Goriainov S,
Chistyakov V, Astakhova A, Azbukina N and Sergeeva M: Oxylipin
profiles as functional characteristics of acute inflammatory
responses in astrocytes pre-treated with IL-4, IL-10, or LPS. Int J
Mol Sci. 21:17802020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Burmeister A, Johnson M, Yaemmongkol J and
Marriott I: Murine astrocytes produce IL-24 and are susceptible to
the immunosuppressive effects of this cytokine. J
Neuroinflammation. 16:552019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Li D, Liu X, Liu T, Liu H, Tong L, Jia SW
and Wang YF: Neurochemical regulation of the expression and
function of glial fibrillary acidic protein in astrocytes. Glia.
68:878–897. 2020. View Article : Google Scholar
|
|
79
|
Wilhelmsson U, Pozo-Rodrigalvarez A, Kalm
M, de Pablo Y, Widestrand Å, Pekna M and Pekny M: The role of GFAP
and vimentin in learning and memory. Biol Chem. 400:1147–1156.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ribot J, Breton R, Calvo C, Moulard J,
Ezan P, Zapata J, Samama K, Moreau M, Bemelmans AP, Sabatet V, et
al: Astrocytes close the mouse critical period for visual
plasticity. Science. 373:77–81. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Hasel P, Rose I, Sadick J, Kim R and
Liddelow S: Neuroinflammatory astrocyte subtypes in the mouse
brain. Nat Neurosci. 24:1475–1487. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Ziff O, Clarke B, Taha D, Crerar H,
Luscombe N and Patani R: Meta-analysis of human and mouse ALS
astrocytes reveals multi-omic signatures of inflammatory reactive
states. Genome Res. 32:71–84. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Liddelow SA, Guttenplan KA, Clarke LE,
Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS,
Peterson TC, et al: Neurotoxic reactive astrocytes are induced by
activated microglia. Nature. 541:481–487. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Versele R, Sevin E, Gosselet F, Fenart L
and Candela P: TNF-α and IL-1β modulate blood-brain barrier
permeability and decrease amyloid-β peptide efflux in a human
blood-brain barrier model. Int J Mol Sci. 23:102352022. View Article : Google Scholar
|
|
85
|
Takahashi K, Ishibashi Y, Chujo K, Suzuki
I and Sato K: Neuroprotective potential of L-glutamate transporters
in human induced pluripotent stem cell-derived neural cells against
excitotoxicity. Int J Mol Sci. 24:126052023. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Luo Y, Yu Y, He H and Fan N: Acute
ketamine induces neuronal hyperexcitability and deficits in
prepulse inhibition by upregulating IL-6. Prog Neuropsychopharmacol
Biol Psychiatry. 130:1109132024. View Article : Google Scholar
|
|
87
|
Friedenstein AJ, Chailakhjan RK and
Lalykina KS: The development of fibroblast colonies in monolayer
cultures of guinea-pig bone marrow and spleen cells. Cell Tissue
Kinet. 3:393–403. 1970.PubMed/NCBI
|
|
88
|
Brown SE, Tong W and Krebsbach PH: The
derivation of mesenchymal stem cells from human embryonic stem
cells. Cells Tissues Organs. 189:256–260. 2009. View Article : Google Scholar :
|
|
89
|
Stefańska K, Němcová L, Blatkiewicz M, Zok
A, Kaczmarek M, Pieńkowski W, Mozdziak P, Piotrowska-Kempisty H and
Kempisty B: Expression profile of new marker genes involved in
differentiation of human Wharton's Jelly-derived mesenchymal stem
cells into chondrocytes, osteoblasts, adipocytes and neural-like
cells. Int J Mol Sci. 24:129392023. View Article : Google Scholar
|
|
90
|
Lee H, Kim SHL, Yoon H, Ryu J, Park HH,
Hwang NS and Park TH: Intracellular delivery of recombinant RUNX2
facilitated by cell-penetrating protein for the osteogenic
differentiation of hMSCs. ACS Biomater Sci Eng. 6:5202–5214. 2020.
View Article : Google Scholar
|
|
91
|
Zhang X, Liu L, Dou C, Cheng P, Liu L, Liu
H, Ren S, Wang C, Jia S, Chen L, et al: PPAR gamma-regulated
MicroRNA 199a-5p underlies bone marrow adiposity in aplastic
anemia. Mol Ther Nucleic Acids. 17:678–687. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Caron M, Eveque M, Cillero-Pastor B,
Heeren RMA, Housmans B, Derks K, Cremers A, Peffers MJ, van Rhijn
LW, van den Akker G and Welting TJM: Sox9 determines translational
capacity during early chondrogenic differentiation of ATDC5 cells
by regulating expression of ribosome biogenesis factors and
ribosomal proteins. Front Cell Dev Biol. 9:6860962021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Yan Z, Shi X, Wang H, Si C, Liu Q and Du
Y: Neurotrophin-3 promotes the neuronal differentiation of BMSCs
and improves cognitive function in a rat model of Alzheimer's
disease. Front Cell Neurosci. 15:6293562021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Chen QH, Wu F, Liu L, Chen HB, Zheng R,
Wang HL and Yu LN: Mesenchymal stem cells regulate the Th17/Treg
cell balance partly through hepatocyte growth factor in vitro. Stem
Cell Res Ther. 11:912020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Chen L, Zhang Q, Chen QH, Ran FY, Yu LM,
Liu X, Fu Q, Song GY, Tang JM and Zhang T: Combination of G-CSF and
AMD3100 improves the anti-inflammatory effect of mesenchymal stem
cells on inducing M2 polarization of macrophages through
NF-κB-IL1RA signaling pathway. Front Pharmacol. 10:5792019.
View Article : Google Scholar
|
|
96
|
Soufihasanabad S, Mahmoudi M,
Taghavi-Farahabadi M, Mirsanei Z, Mahmoudi R, Abdallah JK, Babaei E
and Hashemi SM: In vivo polarization of M2 macrophages by
mesenchymal stem cell-derived extracellular vesicles: A novel
approach to macrophage polarization and its potential in treating
inflammatory diseases. Med Hypotheses. 187:1113532024. View Article : Google Scholar
|
|
97
|
Huaman O, Bahamonde J, Cahuascanco B,
Jervis M, Palomino J, Torres C and Peralta O: Immunomodulatory and
immunogenic properties of mesenchymal stem cells derived from
bovine fetal bone marrow and adipose tissue. Res Vet Sci.
124:212–222. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Yang S, Wei Y, Sun R, Lu W, Lv H, Xiao X,
Cao Y, Jin X and Zhao M: Umbilical cord blood-derived mesenchymal
stromal cells promote myeloid-derived suppressor cell proliferation
by secreting HLA-G to reduce acute graft-versus-host disease after
hematopoietic stem cell transplantation. Cytotherapy. 22:718–733.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
He J, Zhang N, Zhu Y, Jin R and Wu F: MSC
spheroids-loaded collagen hydrogels simultaneously promote neuronal
differentiation and suppress inflammatory reaction through PI3K-Akt
signaling pathway. Biomaterials. 265:1204482020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Huang W, Wang C, Xie L, Wang X, Zhang L,
Chen C and Jiang B: Traditional two-dimensional mesenchymal stem
cells (MSCs) are better than spheroid MSCs on promoting retinal
ganglion cells survival and axon regeneration. Exp Eye Res.
185:1076992019. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zavatti M, Gatti M, Beretti F, Palumbo C
and Maraldi T: Exosomes derived from human amniotic fluid
mesenchymal stem cells preserve microglia and neuron cells from Aβ.
Int J Mol Sci. 23:49672022. View Article : Google Scholar
|
|
102
|
Angeloni C, Gatti M, Prata C, Hrelia S and
Maraldi T: Role of mesenchymal stem cells in counteracting
oxidative stress-related neurodegeneration. Int J Mol Sci.
21:32992020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zeng CW: Multipotent mesenchymal stem
cell-based therapies for spinal cord injury: Current progress and
future prospects. Biology (Basel). 12:6532023.PubMed/NCBI
|
|
104
|
Sun S, Luo S, Chen J, Zhang O, Wu Q, Zeng
N, Bi J, Zheng C, Yan T, Li Z, et al: Human umbilical cord-derived
mesenchymal stem cells alleviate valproate-induced immune stress
and social deficiency in rats. Front Psychiatry. 15:14316892024.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Yang Y, Peng Y, Li Y, Shi T, Luan Y and
Yin C: Role of stem cell derivatives in inflammatory diseases.
Front Immunol. 14:11539012023. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Moghadasi S, Elveny M, Rahman H, Suksatan
W, Jalil AT, Abdelbasset WK, Yumashev AV, Shariatzadeh S, Motavalli
R, Behzad F, et al: A paradigm shift in cell-free approach: The
emerging role of MSCs-derived exosomes in regenerative medicine. J
Transl Med. 19:3022021. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Krylova S and Feng D: The machinery of
exosomes: Biogenesis, release, and uptake. Int J Mol Sci.
24:13372023. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Zheng D, Huo M, Li B, Wang W, Piao H, Wang
Y, Zhu Z, Li D, Wang T and Liu K: The role of exosomes and exosomal
MicroRNA in cardiovascular disease. Front Cell Dev Biol.
8:6161612021. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Tenchov R, Sasso J, Wang X, Liaw W, Chen
CA and Zhou Q: Exosomes-Nature's lipid nanoparticles, a rising star
in drug delivery and diagnostics. ACS Nano. 16:17802–17846. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Wu J, Li H, He J, Tian X, Luo S, Li J, Li
W, Zhong J, Zhang H, Huang Z, et al: Downregulation of
microRNA-9-5p promotes synaptic remodeling in the chronic phase
after traumatic brain injury. Cell Death Dis. 12:92021. View Article : Google Scholar :
|
|
111
|
Corradi E, Costa ID, Gavoci A, Iyer A,
Roccuzzo M, Otto TA, Oliani E, Bridi S, Strohbuecker S,
Santos-Rodriguez G, et al: Axonal precursor miRNAs hitchhike on
endosomes and locally regulate the development of neural circuits.
EMBO J. 39:e1025132020. View Article : Google Scholar
|
|
112
|
Li R, Zhao K, Ruan Q, Meng C and Yin F:
Bone marrow mesenchymal stem cell-derived exosomal microRNA-124-3p
attenuates neurological damage in spinal cord ischemia-reperfusion
injury by downregulating Ern1 and promoting M2 macrophage
polarization. Arthritis Res Ther. 22:752020. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Jiang D, Gong F, Ge X, Lv C, Huang C, Feng
S, Zhou Z, Rong Y, Wang J, Ji C, et al: Neuron-derived
exosomes-transmitted miR-124-3p protect traumatically injured
spinal cord by suppressing the activation of neurotoxic microglia
and astrocytes. J Nanobiotechnology. 18:1052020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Song Y, Li Z, He T, Qu M, Jiang L, Li W,
Shi X, Pan J, Zhang L, Wang Y, et al: M2 microglia-derived exosomes
protect the mouse brain from ischemia-reperfusion injury via
exosomal miR-124. Theranostics. 9:2910–2923. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Cui Y, Yin Y, Xiao Z, Zhao Y, Chen B, Yang
B, Xu B, Song H, Zou Y, Ma X and Dai J: LncRNA Neat1 mediates
miR-124-induced activation of Wnt/β-catenin signaling in spinal
cord neural progenitor cells. Stem Cell Res Ther. 10:4002019.
View Article : Google Scholar
|
|
116
|
Han P, Sunada-Nara K, Kawashima N, Fujii
M, Wang S, Kieu TQ, Yu Z and Okiji T: MicroRNA-146b-5p suppresses
pro-inflammatory mediator synthesis via targeting TRAF6, IRAK1, and
RELA in lipopolysaccharide-stimulated human dental pulp cells. Int
J Mol Sci. 24:74332023. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Ho D, Lynd TO, Jun C, Shin J, Millican RC,
Estep BK, Chen J, Zhang X, Brott BC, Kim DW, et al: MiR-146a
encapsulated liposomes reduce vascular inflammatory responses
through decrease of ICAM-1 expression, macrophage activation, and
foam cell formation. Nanoscale. 15:3461–3474. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Venuti A, Musarra-Pizzo M, Pennisi R,
Tankov S, Medici MA, Mastino A, Rebane A and Sciortino M:
HSV-1\EGFP stimulates miR-146a expression in a NF-κB-dependent
manner in monocytic THP-1 cells. Sci Rep. 9:51572019. View Article : Google Scholar
|
|
119
|
Riazifar M, Mohammadi M, Pone E, Yeri A,
Lässer C, Segaliny AI, McIntyre LL, Shelke GV, Hutchins E, Hamamoto
A, et al: Stem cell-derived exosomes as nanotherapeutics for
autoimmune and neurodegenerative disorders. ACS Nano. 13:6670–668.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Long X, Yao X, Jiang QQ, Yang Y, He X,
Tian W, Zhao K and Zhang H: Astrocyte-derived exosomes enriched
with miR-873a-5p inhibit neuroinflammation via microglia phenotype
modulation after traumatic brain injury. J Neuroinflammation.
17:892020. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Wang QM, Lian GY, Sheng SM, Xu J, Ye LL,
Min C and Guo SF: Exosomal lncRNA NEAT1 inhibits NK-Cell activity
to promote multiple myeloma cell immune escape via an EZH2/PBX1
axis. Mol Cancer Res. 22:125–136. 2024. View Article : Google Scholar
|
|
122
|
Zhao F, Li Z, Dong Z, Wang Z, Guo P, Zhang
D and Li S: Exploring the potential of exosome-related LncRNA pairs
as predictors for immune microenvironment, survival outcome, and
microbiotain landscape in esophageal squamous cell carcinoma. Front
Immunol. 13:9181542022. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Zhang W, Yan Y, Peng J, Thakur A, Bai N,
Yang K and Xu Z: Decoding roles of exosomal lncRNAs in tumor-immune
regulation and therapeutic potential. Cancers (Basel). 15:2862022.
View Article : Google Scholar
|
|
124
|
Taha E, Ono K and Eguchi T: Roles of
extracellular HSPs as biomarkers in immune surveillance and immune
evasion. Int J Mol Sci. 20:45882019. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Montesinos JJ, López-García L,
Cortés-Morales VA, Arriaga-Pizano L, Valle-Ríos R, Fajardo-Orduña
GR and Castro-Manrreza ME: Human bone marrow mesenchymal
stem/stromal cells exposed to an inflammatory environment increase
the expression of ICAM-1 and release microvesicles enriched in this
adhesive molecule: Analysis of the participation of TNF-α and
IFN-γ. J Immunol Res. 2020:88396252020. View Article : Google Scholar
|
|
126
|
Wu D, Deng S, Li L, Liu T, Zhang T, Li J,
Yu Y and Xu Y: TGF-β1-mediated exosomal lnc-MMP2-2 increases
blood-brain barrier permeability via the miRNA-1207-5p/EPB41L5 axis
to promote non-small cell lung cancer brain metastasis. Cell Death
Dis. 12:7212021. View Article : Google Scholar
|
|
127
|
Cauvi D, Hawisher D, Derunes J, Rodriguez
E and De Maio A: Membrane phospholipids activate the inflammatory
response in macrophages by various mechanisms. FASEB J.
38:e236192024. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Zhou W, Silva M, Feng C, Zhao S, Liu L, Li
S, Zhong J and Zheng W: Exosomes derived from human placental
mesenchymal stem cells enhanced the recovery of spinal cord injury
by activating endogenous neurogenesis. Stem Cell Res Ther.
12:1742021. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Harrell CR, Volarevic A, Djonov V and
Volarevic V: Mesenchymal stem cell-derived exosomes as new remedy
for the treatment of neurocognitive disorders. Int J Mol Sci.
22:14332021. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Xun C, Ge L, Tang F, Wang L, Zhuo Y, Long
L, Qi J, Hu L, Duan D, Chen P and Lu M: Insight into the proteomic
profiling of exosomes secreted by human OM-MSCs reveals a new
potential therapy. Biomed Pharmacother. 131:1105842020. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Liu A, Li C, Wang C, Liang X and Zhang X:
Impact of mesenchymal stem cells on the gut microbiota and
microbiota associated functions in inflammatory bowel disease: A
systematic review of preclinical evidence on animal models. Curr
Stem Cell Res Ther. 19:981–992. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Ocansey D, Zhang Z, Xu X, Liu L, Amoah S,
Chen X, Wang B, Zhang X and Mao F: Mesenchymal stem cell-derived
exosome mitigates colitis via the modulation of the gut
metagenomics-metabolomics-farnesoid X receptor axis. Biomater Sci.
10:4822–4836. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Gu L, Ren F, Fang X, Yuan L, Liu G and
Wang S: Exosomal MicroRNA-181a derived from mesenchymal stem cells
improves gut microbiota composition, barrier function, and
inflammatory status in an experimental colitis model. Front Med
(Lausanne). 8:6606142021. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Alexandrov P, Zhai Y, Li W and Lukiw W:
Lipopolysaccharide-stimulated, NF-kB-, miRNA-146a- and
miRNA-155-mediated molecular-genetic communication between the
human gastrointestinal tract microbiome and the brain. Folia
Neuropathol. 57:211–219. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Geffen Y, Perets N, Horev R, Yudin D, Oron
O, Elliott E, Marom E, Danon U and Offen D: Exosomes derived from
adipose mesenchymal stem cells: A potential non-invasive intranasal
treatment for autism. Cytotherapy. 22:S492020.
|
|
136
|
Geffen Y, Horev R, Perets N, Marom E,
Danon U and Offen D: Immuno-modulation and neuroprotection mediate
the therapeutic effect of exosomes in mice model of autism.
Cytotherapy. 22:S49–S50. 2020.
|
|
137
|
Garcia G, Pinto S, Ferreira S, Lopes D,
Serrador MJ, Fernandes A, Vaz AR, Mendonça A, Edenhofer F, Malm T,
et al: Emerging role of miR-21-5p in neuron-glia dysregulation and
exosome transfer using multiple models of Alzheimer's disease.
Cells. 11:33772022. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Zhang Y, Zhang Y, Chopp M, Pang H, Zhang
Z, Mahmood A and Xiong Y: MiR-17-92 cluster-enriched exosomes
derived from human bone marrow mesenchymal stromal cells improve
tissue and functional recovery in rats after traumatic brain
injury. J Neurotrauma. 38:1535–1550. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Fan B, Chopp M, Zhang Z and Liu X:
Treatment of diabetic peripheral neuropathy with engineered
mesenchymal stromal cell-derived exosomes enriched with
microRNA-146a provide amplified therapeutic efficacy. Exp Neurol.
341:1136942021. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Chen Y, Tian Z, He L, Liu C, Wang N, Rong
L and Liu B: Exosomes derived from miR-26a-modified MSCs promote
axonal regeneration via the PTEN/AKT/mTOR pathway following spinal
cord injury. Stem Cell Res Ther. 12:2242021. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Perets N, Hertz S, London M and Offen D:
Intranasal administration of exosomes derived from mesenchymal stem
cells ameliorates autistic-like behaviors of BTBR mice. Mol Autism.
9:1–12. 2018. View Article : Google Scholar
|
|
142
|
Kabataş S, Civelek E, Savrunlu E,
Karaaslan U, Yıldız Ö and Karaöz E: Advances in the treatment of
autism spectrum disorder: Wharton jelly mesenchymal stem cell
transplantation. World J Methodol. 15:958572025. View Article : Google Scholar
|
|
143
|
Barmada A, Sharan J, Band N and Prodromos
C: Serious adverse events have not been reported with spinal
intrathecal injection of mesenchymal stem cells: A systematic
review. Curr Stem Cell Res Ther. 18:829–833. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Lv YT, Zhang Y, Liu M, Qiuwaxi JN, Ashwood
P, Cho SC, Huan Y, Ge RC, Chen XW, Wang ZJ, et al: Transplantation
of human cord blood mononuclear cells and umbilical cord-derived
mesenchymal stem cells in autism. J Transl Med. 11:1962013.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Shroff G: Human embryonic stem cells in
the treatment of autism: A case series. Innov Clin Neurosci.
14:12–16. 2017.PubMed/NCBI
|
|
146
|
Chez M, Lepage C, Parise C, Dang-Chu A,
Hankins A and Carroll M: Safety and observations from a
placebo-controlled, crossover study to assess use of autologous
umbilical cord blood stem cells to improve symptoms in children
with Autism. Stem Cells Transl Med. 7:333–341. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Simhal AK, Carpenter KLH, Nadeem S,
Kurtzberg J, Song A, Tannenbaum A, Sapiro G and Dawson G: Measuring
robustness of brain networks in autism spectrum disorder with Ricci
curvature. Sci Rep. 10:108192020. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Thanh LN, Nguyen HP, Ngo MD, Bui VA, Dam
PTM, Bui HTP, Ngo DV, Tran KT, Dang TTT, Duong BD, et al: Outcomes
of bone marrow mononuclear cell transplantation combined with
interventional education for autism spectrum disorder. Stem Cells
Transl Med. 10:14–26. 2021. View Article : Google Scholar
|
|
149
|
Al-Dhalimy A, Salim HM, Shather A, Naser
IH, Hizam M and Alshujery MK: The pathological and therapeutically
role of mesenchymal stem cell (MSC)-derived exosome in degenerative
diseases; Particular focus on LncRNA and microRNA. Pathol Res
Prract. 250:1547782023. View Article : Google Scholar
|
|
150
|
Dilsiz N: Mesenchymal Stem Cell-Derived
Exosomes in Clinical Trial. Pak BioMed J. 8:12025.
|
|
151
|
Hadizadeh A, Akbari-Asbagh R,
Heirani-Tabasi A, Soleimani M, Gorovanchi P, Daryani NE, Vahedi A,
Nazari H, Banikarimi SP, Dibavar MA, et al: Localized
administration of mesenchymal stem cell-derived exosomes for the
treatment of refractory perianal fistula in Crohn's disease
patients: A phase II clinical trial. Dis Colon Rectum.
67:1564–1575. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Fahlevie F, Apriningsih H, Sutanto Y,
Reviono R, Adhiputri A, Aphridasari J and Prasetyo W: Effects of
secretome supplementation on interleukin-6, tumor necrosis
factor-α, procalcitonin, and the length of stay in acute
exacerbation COPD patients. Narra J. 3:e1712023. View Article : Google Scholar
|
|
153
|
Xie M, Tao L, Zhang Z and Wang W:
Mesenchymal stem cells mediated drug delivery in tumor-targeted
therapy. Curr Drug Deliv. 18:876–891. 2020.PubMed/NCBI
|
|
154
|
Xu S, Liu B, Fan J, Xue C, Lu Y, Li C and
Cui D: Engineered mesenchymal stem cell-derived exosomes with high
CXCR4 levels for targeted siRNA gene therapy against cancer.
Nanoscale. 14:4098–4113. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Tang Y, Zhou Y and Li HJ: Advances in
mesenchymal stem cell exosomes: A review. Stem Cell Res Ther.
12:712021. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Abdelsalam M, Ahmed M, Osaid Z, Hamoudi R
and Harati R: Insights into exosome transport through the
blood-brain barrier and the potential therapeutical applications in
brain diseases. Pharmaceuticals (Basel). 16:5712023. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Mohamed-Ahmed S, Fristad I, Lie S, Suliman
S, Mustafa K, Vindenes H and Idris S: Adipose-derived and bone
marrow mesenchymal stem cells: A donor-matched comparison. Stem
Cell Res Ther. 9:1682018. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Gowen A, Shahjin F, Chand S, Odegaard KE
and Yelamanchili SV: Mesenchymal stem cell-derived extracellular
vesicles: Challenges in clinical applications. Front Cell Dev Biol.
8:1492020. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Li B, Chen X, Qiu W, Zhao R, Duan J, Zhang
S, Pan Z, Zhao S, Guo Q, Qi Y, et al: Synchronous disintegration of
ferroptosis defense axis via engineered exosome-conjugated magnetic
nanoparticles for glioblastoma therapy. Adv Sci (Weinh).
9:e21054512022. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Zhang M, Zhang R, Chen H, Zhang X, Zhang
Y, Liu H, Li C, Chen Y, Zeng Q and Huang G: Injectable
supramolecular hybrid hydrogel delivers IL-1β-stimulated exosomes
to target neuroinflammation. ACS Appl Mater Interfaces.
15:6486–6498. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Feng C, Xiong Z, Wang C, Xiao W, Xiao H,
Xie K, Chen K, Liang H, Zhang X and Yang H: Folic acid-modified
Exosome-PH20 enhances the efficiency of therapy via modulation of
the tumor microenvironment and directly inhibits tumor cell
metastasis. Bioact Mater. 6:963–974. 2021.
|
|
162
|
Zhan Q, Yi K, Qi H, Li S, Li X, Wang Q,
Wang Y, Liu C, Qiu M, Yuan X, et al: Engineering blood exosomes for
tumor-targeting efficient gene/chemo combination therapy.
Theranostics. 10:7889–7905. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Zhao Y, Gan YM, Xu G, Hua K and Liu D:
Exosomes from MSCs overexpressing microRNA-223-3p attenuate
cerebral ischemia through inhibiting microglial M1 polarization
mediated inflammation. Life Sci. 260:1184032020. View Article : Google Scholar : PubMed/NCBI
|

















