Introduction
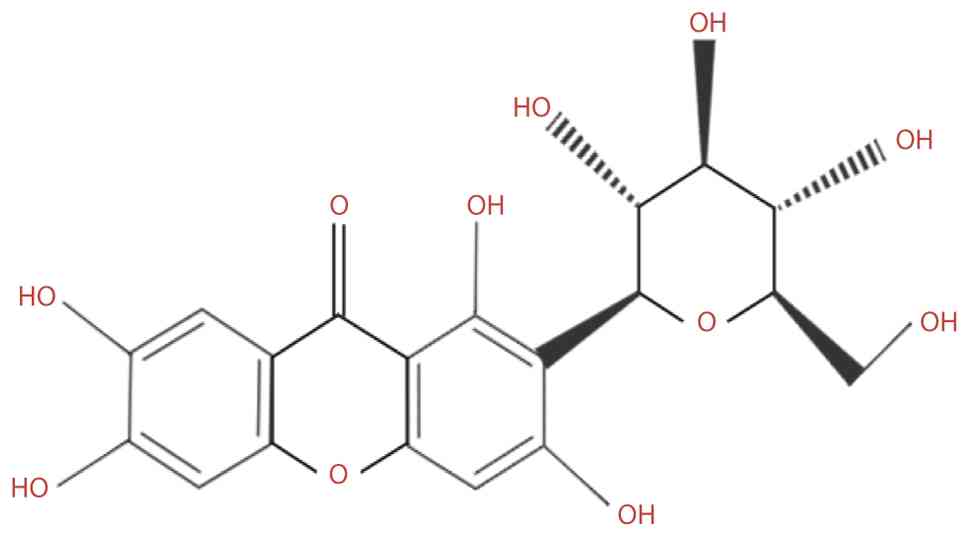
Mangiferin (MGF) is a yellow crystalline flavonoid
with a molecular weight of 422.34 g/mol (Fig. 1). Despite its low water
solubility (0.111 mg/ml) (1) and
poor oral bioavailability (~1.2%) (2,3),
due to its limited lipophilicity and intestinal permeability
(4), MGF has been identified in
96 species, 28 genera and 19 families of angiosperms (5). Its unique structure, featuring a
catechol moiety and a C-glucosidic linkage, contributes to its
interactions with diverse biological targets and underpins its
broad pharmacological activities. Moreover, MGF complies with
Lipinski's rule of five, suggesting favorable drug-like properties
despite its absorption limitations (6). MGF isolated from indica rice leaves
has various pharmacological benefits; notably, it exhibits potent,
broad-spectrum antimicrobial activity against diverse fungal and
bacterial pathogens, with minimum inhibitory concentration (MIC)
values of 1.95-62.5 and 1.95-31.25 μg/ml, respectively
(7). In addition, it exerts
wide-ranging pharmacological effects by modulating key metabolic
pathways, including glycolysis, the tricarboxylic acid (TCA) cycle,
and lipid and amino acid metabolism, supporting its potential use
in treating metabolic disorders such as hyperlipidemia and
hyperglycemia (8). Additionally,
MGF has neuroprotective, anti-inflammatory and anticancer effects
by reducing oxidative stress, inhibiting proinflammatory signaling,
and suppressing tumor proliferation and metastasis. Advances in
formulation and drug delivery strategies are expected to further
improve its clinical applicability across various diseases
(4,9).
The present review has three objectives. First, the
current evidence on MGF was organized by mechanism, focusing on
AMP-activated protein kinase (AMPK), NF-κB, PI3K/Akt, MAPK and NLR
family pyrin domain-containing 3 (NLRP3) pathways, and their
relation to metabolic regulation, oxidative stress, apoptosis,
angiogenesis and immune modulation. Second, the preclinical
efficacy of MGF across metabolic, inflammatory and cancer models
was evaluated using reproducible endpoints, such as insulin
sensitivity, fibrotic markers, tumor growth, invasion and MMP-9
activity. Third, key barriers to translation, including low
solubility, poor oral bioavailability and rapid metabolism, were
examined, and delivery and structural optimization strategies that
may improve exposure were assessed. Evidence selection prioritized
primary studies and systematic analyses with prespecified methods.
By integrating data on molecular mechanisms, pharmacology and
drug-delivery strategies, the present review aims to identify
consistent efficacy signals, highlight areas of uncertainty and
propose priorities for future human studies.
Literature search strategy
To ensure the comprehensiveness and reliability of
the present review, a structured literature search was conducted in
the PubMed (https://pubmed.ncbi.nlm.nih.gov/), Web of Science
(http://www.webofscience.com/), Scopus
(http://www.scopus.com/) and Google Scholar
(http://scholar.google.com/) databases.
The search included studies published between January 2000 and June
2025. The following key words and their combinations were searched:
'mangiferin', 'pharmacological activity', 'bioavailability',
'metabolism', 'toxicity', 'mechanism of action' and 'therapeutic
potential'. The inclusion criteria were as follows: i) Original
research or review articles investigating the pharmacological,
biochemical or clinical properties of MGF; ii) studies providing
mechanistic, pharmacokinetic (PK) or toxicological data; and iii)
publications written in English and indexed in peer-reviewed
journals. The exclusion criteria were as follows: i)
Non-peer-reviewed materials, conference abstracts or duplicated
data; ii) studies using complex herbal mixtures without
quantifiable MGF content; and iii) articles lacking sufficient
methodological detail or outcome data. References were screened by
title, abstract and full text, and only those meeting the inclusion
criteria were included in the final review.
Historical discovery, botanical sources and
early pharmacological insights into MGF
The chemical structure of MGF, identified as a
1,3,6,7-tetrahydroxyxanthone C-glucoside, was first elucidated by
Aritomi and Kawasaki in 1969 (10). However, detailed structural
characterization via nuclear magnetic resonance spectroscopy and
X-ray diffraction was not achieved until the early 2000s (11). Between the 1960s and 1980s, MGF
and its isomers were isolated from several botanical sources,
including Anemarrhena asphodeloides Bunge. A subsequent
study broadened its known distribution: In 1995, MGF was detected
in ferns from New Zealand, and in 2008, markedly high
concentrations (up to 6.12%) were reported in the leaves of wild
coffee plants (7). MGF is most
abundant in members of the Anacardiaceae family, particularly
Mangifera indica L., but it has also been isolated from
other medicinal plants, such as Prunus dulcis, Gentiana
manshurica, Swertia mussotii and Pyrrosia calvata
(12). Advances in phytochemical
techniques, including high-performance liquid chromatography and
mass spectrometry, have notably improved the precision of MGF
identification and quantification across diverse plant
matrices.
An early pharmacological study explored the
physiological and therapeutic effects of MGF (10). A previous study demonstrated that
the substance possesses a spectrum of biological activities beyond
its initial inotropic effect on frog hearts, including
immunomodulatory, reproductive, antitumor and antiviral properties
(13). Metabolic investigations
have indicated that MGF is biotransformed by the human intestinal
microbiota, with deglycosylation to norathyriol a key step
(8). In preclinical PK studies,
oral MGF has been shown to exhibit low systemic exposure, which is
reflected by its limited detection in the plasma and urine of
rodent models (14). These
observations have motivated formulation strategies to improve
exposure. In 2012, nanostructured lipid carriers were applied to
MGF, providing a basis for subsequent nanotechnology-oriented
approaches that aim to increase its solubility, stability and oral
bioavailability (15).
Absorption and metabolism of MGF
Absorption of MGF
Despite its wide pharmacological spectrum, MGF has
low oral bioavailability, largely due to its poor solubility and
limited membrane permeability. The stability of its C-glycosidic
bond prevents enzymatic cleavage and reduces transmembrane
transport, thereby restricting passive absorption across the
intestinal epithelium (16). A
previous in vivo study demonstrated that only 1.15% of
orally administered MGF (30 mg/kg) is bioavailable in mice, whereas
intraperitoneal administration results in markedly increased
systemic levels. Additionally, extensive first-pass hepatic
metabolism further decreases the amount of active MGF entering
systemic circulation (17).
Regional differences in intestinal absorption have
been reported in rats. In situ perfusion experiments have
indicated greater apparent permeability in the duodenum compared
with that in the jejunum, and greater permeability in the jejunum
than in the ileum, which suggests that the upper small intestine is
the principal site of uptake (18). After absorption, MGF is
distributed via the circulation to multiple organs, including the
heart, liver, spleen, lungs, kidneys, testes and prostate. In rats
administered oral MGF, peak concentrations in the plasma, lungs and
kidneys have been reported to occur 4-6 h after dosing. Under the
reported analytical conditions, MGF is not detected in brain
tissue, which is consistent with limited penetration across the
blood-brain barrier in this model (19).
Metabolism of MGF
MGF undergoes biotransformation through both
microbial and host enzymatic systems. A frequently observed pathway
is microbial or enzymatic deglycosylation of C-glucoside,
generating the aglycone norathyriol (metabolite M1), which has
greater membrane permeability than the parent compound (20). Subsequent phase I reactions (for
example, dehydroxylation and O-methylation) and phase II
conjugations (including glucuronidation and sulfation) yield
additional metabolites with distinct physicochemical properties
(21). These steps can occur
sequentially or in parallel within the gut lumen and microbiota,
the intestinal epithelium and the liver, and the relative
contribution of each route depends on the species, dose and
formulation (21). A schematic
overview of these metabolic routes and representative metabolites
is provided in Fig. 2. Thus,
while the formation of M1 is well documented (20,21), it should be considered a
predominant rather than obligatory initial event in the overall
metabolic scheme.
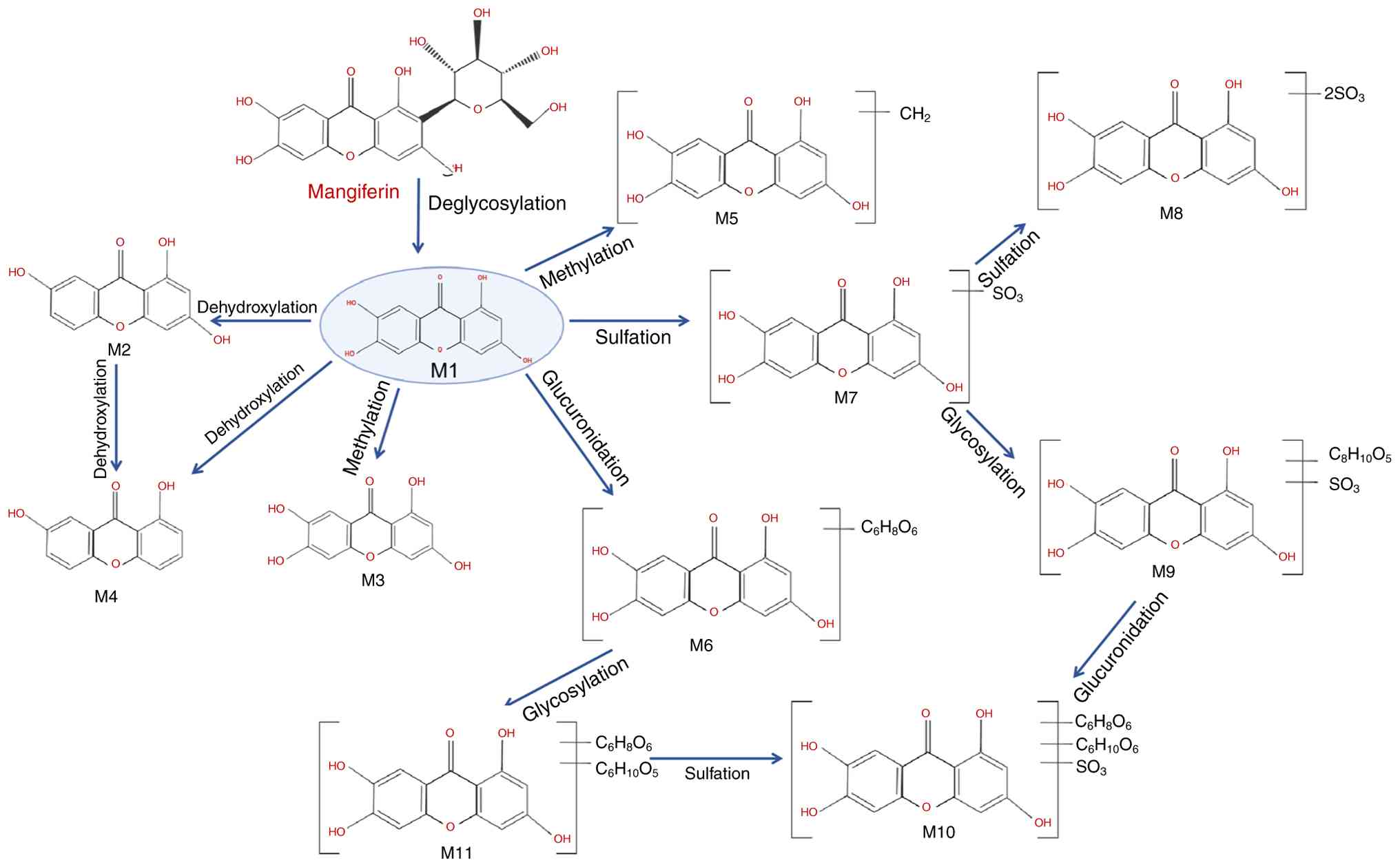 | Figure 2Diagram of the relevant
pharmacological properties of mangiferin and its potential
mechanism of action. The metabolic pathways include the following:
M1, deglycosylation to norathyriol; M2, 1,3,7-trihydroxyxanthone;
M3, methylation to 1,3,6-trihydroxy-7-methoxyxanthone; M4,
dehydroxylation to 1,7-dihydroxyxanthone; M5, further methylation
to methoxy dimethyl xanthone; M6, glucuronidation products; M7,
sulfated derivatives; M8, dithio-sulfate norathyriol; M9, sulfate
norathyriol glucoside; M10, norathyriol glucuronide sulfate; and
M11, norathyriol glucuronide. |
Specifically, M1 is dehydroxylated to form
1,3,7-trihydroxyxanthone (M2) and 1,7-dihydroxyxanthone (M4), with
M2 being the predominant urinary metabolite in rodents (22). Methylation of M1 yields compounds
such as 1,3,6-trihydroxy-7-methoxyxanthone (M3) and methoxy
dimethyl xanthone (M5). Conjugation reactions generate glucuronide
(M6), sulfate (M7) and mixed derivatives, including dithio-sulfate
norathyriol (M8), sulfate norathyriol glucoside (M9), norathyriol
glucuronide (M11) and its sulfated form (M10) (11,23).
The gut microbiota serves a critical role in the
deglycosylation and subsequent metabolism of MGF, representing a
key determinant of its systemic availability and pharmacological
effects (24). Although
microbial metabolism enables the formation of bioactive
metabolites, it remains unclear whether MGF itself modulates the
composition and function of the gut microbiota. Further research is
needed to clarify this bidirectional interaction, and to
characterize the full spectrum of bioactive metabolites relevant to
disease prevention and therapy (25).
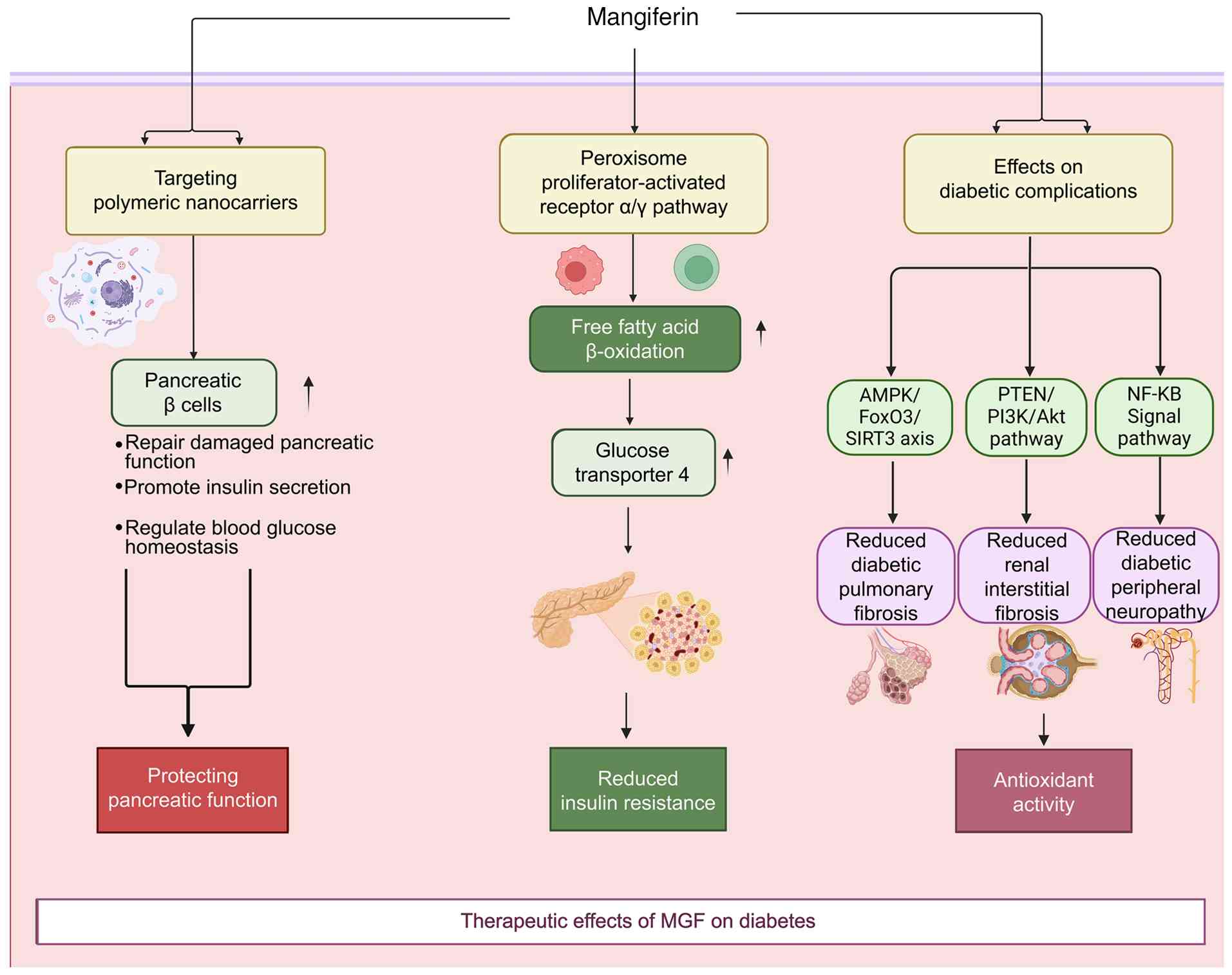
Pharmacological effects of MGF on
diabetes
Diabetes mellitus results from impaired pancreatic
β-cell function and/or peripheral insulin resistance, ultimately
disrupting glucose homeostasis. MGF has notable antidiabetic
potential due to its insulinotropic and insulin-sensitizing effects
(Fig. 3).
β-cell protection and pancreatic
regeneration
MGF supports pancreatic function by preserving and
regenerating β cells. In animal models of type 1 and type 2
diabetes, tail vein administration of MGF-loaded polymeric
nanocarriers (5-10 mg/kg) have been reported to increase β-cell
proliferation, inhibit apoptosis and restored insulin secretion,
thereby improving glycemic control (26). Similarly, repeated oral
administration of MGF (30 or 90 mg/kg for 14 days) in partially
pancreatectomized mice may promote islet regeneration through the
upregulation of key cell-cycle regulators (such as cyclin D1 and
Ki-67) and pancreatic progenitor markers, including Pdx1 and Ngn3
(27).
Insulin sensitization and glucose
metabolism
MGF also alleviates insulin resistance by modulating
metabolic, inflammatory and oxidative stress pathways. Activation
of the peroxisome proliferator-activated receptor (PPAR)α/γ axis
enhances fatty acid β-oxidation and upregulates glucose transporter
4 (GLUT4), thereby improving insulin sensitivity (28). Given that chronic inflammation
and oxidative stress are central to the development of diabetes
(29), MGF mitigates these
processes through the inhibition of HIF-1α and NF-κB signaling, the
suppression of JNK phosphorylation, and the reduction in
endoplasmic reticulum stress, collectively restoring insulin
signaling (30).
In addition, MGF directly regulates glucose
metabolism. It promotes glycolysis and glycogen synthesis while
inhibiting gluconeogenesis by modulating key enzymes such as
glycogen synthase kinase 3β (GSK3β), glucose-6-phosphatase and
phosphoenolpyruvate carboxykinase. Furthermore, MGF reverses high
glucose-induced impairment of GLUT4 translocation and glucose
uptake, partly via AMPK activation and Akt phosphorylation, thereby
improving hepatic energy metabolism and overall insulin
responsiveness (31).
Structural derivatives and therapeutic
effects on diabetic complications
Structural modification of MGF has produced more
potent derivatives. For example, compound X-3, a semi-synthetic
derivative prepared from MGF, has been reported to markedly reduce
hyperglycemia and obesity in mice after 8 weeks of oral treatment
(40-120 mg/kg), primarily through AMPK pathway activation (32).
In addition to its ability to control glycemia, MGF
has shown activity against diabetic complications in preclinical
models. In a mouse model of diabetic pulmonary fibrosis, oral
administration of 60 mg/kg MGF every 3 days for 4 weeks has been
shown to reduce the progression of fibrosis. Reported effects
include the inhibition of endothelial-mesenchymal transition and
modulation of the AMPK/FoxO3/sirtuin (SIRT)3 pathway, as evidenced
by changes in pathway proteins and fibrosis markers (33). In streptozotocin-induced diabetic
mice, oral administration of 15-60 mg/kg/day MGF for 4 weeks may
attenuate renal injury, which is consistent with diabetic
nephropathy. This previous study reported downregulation of
phosphatase and tensin homolog (PTEN)/PI3K/Akt signaling, and
reduced expression of fibronectin, collagen I and α-smooth muscle
actin (α-SMA), along with decreases in renal fibrosis, inflammation
and oxidative stress endpoints (34). Taken together, these data support
a potential role for MGF in mitigating pulmonary and renal
complications of diabetes under experimental conditions, while
confirming that the proposed mechanisms, including inhibition of
endothelial-mesenchymal transition and modulation of
AMPK/FoxO3/SIRT3 signaling in diabetic pulmonary fibrosis, and
downregulation of PTEN/PI3K/Akt signaling with reduced
fibronectin/collagen I/α-SMA expression in diabetic nephropathy,
are supported by molecular endpoints measured in these models
(33,34).
In models of diabetic peripheral neuropathy, MGF
alleviates neuroinflammation by reducing TNF-α, TGF-β1, IL-1β and
IL-6 levels in the sciatic nerve. Concurrently, it enhances
antioxidant defenses and upregulates nerve growth factor, thereby
promoting nerve regeneration and functional recovery (35).
Anticancer mechanisms of MGF
MGF exerts potent anticancer activity through
multiple mechanisms that target key cellular processes underlying
tumor initiation and progression. These include the induction of
cell cycle arrest, the promotion of apoptosis through various
intracellular pathways, the inhibition of tumor invasion and
metastasis, the modulation of immune responses, and the enhancement
of radiosensitivity in cancer such as glioblastoma (GBM) (36,37). Fig. 4 schematically summarizes the
major signaling pathways [such as nuclear factor erythroid
2-related factor 2 (Nrf2)/reactive oxygen species (ROS), ATR/Chk1,
CDK1/cyclin B1, NF-κB, PI3K/Akt, MAPK, caspases and microRNAs
(miRNAs/miRs)] through which MGF induces apoptosis of tumor
cells.
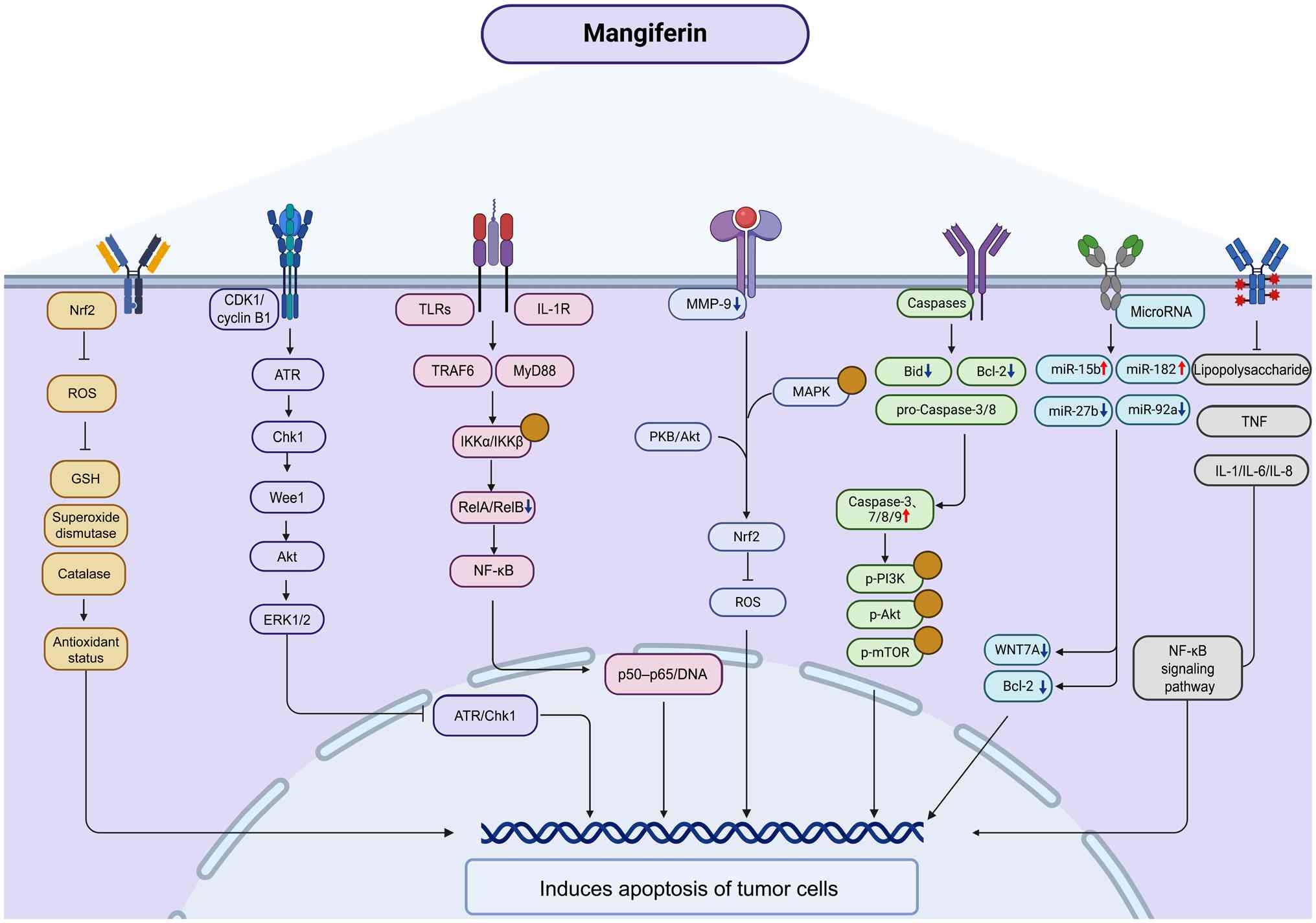 | Figure 4Molecular mechanism of the anticancer
effect of MGF. MGF suppresses tumor growth by promoting apoptosis,
exerting anti-inflammatory effects, regulating mitochondrial
function, modulating autophagy and enhancing immune responses.
Upward and downward arrows indicate activation/upregulation or
inhibition/downregulation by MGF, respectively. Yellow circles
indicate activation of the indicated signaling nodes. GSH,
glutathione; IL-1R, IL-1 receptor; MGF, mangiferin; miR, microRNA;
Nrf2, nuclear factor erythroid 2-related factor 2; p-,
phosphorylated proteins; ROS, reactive oxygen species; TLRs,
Toll-like receptors; TRAF6, TNF receptor-associated factor 6. |
According to previous cancer-related experiments,
MGF most consistently modulates four signaling axes that share
common upstream triggers and downstream responses (38,39). For NF-κB, typical engagement is
inferred from reduced IKKα/IKKβ phosphorylation, stabilization of
IκBα, and reduced nuclear p65 DNA binding, with downstream
decreases in pro-survival and invasive effectors, such as BCL2L1,
BIRC5 and MMP-9 (40-43). For Nrf2, evidence includes
diminished Kelch-like ECH-associated protein 1-mediated
ubiquitination, increased nuclear Nrf2, and the induction of heme
oxygenase (HO)-1, NAD(P)H quinone dehydrogenase 1 (NQO1) and other
phase II enzymes, aligning with decreased ROS and lipid
peroxidation (44,45). MAPK modulation is context
dependent; a number of models show attenuation of ERK1/2, JNK and
p38 phosphorylation, reduced activator protein (AP)-1 activity, and
consequent decreases in proliferation and cytokine output (38,46). For PI3K/Akt, changes in Akt
Thr308/Ser473, and downstream GSK3β and mTOR complex 1 lead to
reduced protein synthesis and survival signaling in tumor cells,
whereas in metabolic tissues, they support GLUT4 translocation and
glycogen synthesis (16,47-54). Notably, crosstalk is evident:
AMPK activation can suppress NF-κB priming and MAPK activity,
whereas redox shifts through Nrf2 dampen inflammasome priming,
which converges on MMP-9 and invasion programs. To aid
interpretation, each subsequent subsection in the present review
reports on the model, exposure, phosphorylation status of key
signaling proteins and a disease-relevant functional endpoint (for
example, apoptosis markers, invasion or tumor growth), and provides
a brief appraisal of sample size and risks of bias.
Cell cycle arrest via CDK1/cyclin B1
In HL-60 cells, some studies have reported increased
CDK1 and cyclin B1 mRNA following MGF exposure, indicating
regulation at the transcriptional level in this model (55). However, across tumor cell
systems, the functional consequence remains G2/M arrest,
with reduced proliferation and increased susceptibility to
apoptosis. This outcome is consistent with reports of decreased
CDK1/cyclin B1 activity, altered phosphorylation of CDK1 with
reduced activating phosphorylation and increased inhibitory
phosphorylation, and engagement of checkpoint signaling, including
ATR-dependent Chk1 activation, in other tumor cell lines exposed to
MGF (56,57). This evidence supports multilevel
regulation of the CDK1/cyclin B1 axis by MGF. Differences at the
mRNA level are plausibly attributable to the assay, timing, dose
and cellular context, whereas the consistent phenotype is
G2/M blockade with growth suppression (55-57).
Apoptosis pathways
Caspase activation
The induction of apoptosis via caspase activation is
a well-established anticancer mechanism. Caspases are a family of
cysteine proteases that execute programmed cell death through a
proteolytic cascade. Initiator caspases (such as caspase-8 and -9)
activate effector caspases (including caspase-3, -6 and -7),
leading to degradation of intracellular components and apoptotic
cell death (58,59). MGF has been shown to promote this
cascade through multiple upstream regulators, including the Bcl-2
protein family and cytochrome c-mediated pathways (60).
MGF induces apoptosis in nasopharyngeal carcinoma
cells by modulating the mitochondrial pathway, decreasing
antiapoptotic Bcl-2 and increasing proapoptotic Bax expression
(61). In HeLa cervical cancer
cells, ethanolic mango peel extract enriched with MGF downregulates
Bid, Bcl-2 and pro-caspase-3 and -8, while activating caspases-3,
-7, -8 and -9, and cleaving poly(ADP-ribose) polymerase
(PARP)-hallmarks of caspase-mediated apoptosis (62).
In SGC-7901 gastric cancer cells, MGF suppresses
Bcl-2, Bcl-xL and Mcl-1 expression, and increases Bax, Bad, and
cleaved caspase-3 and -9 levels. Additionally, it inhibits the
PI3K/Akt/mTOR signaling axis, further promoting apoptosis and
suppressing cell survival (47).
Modulation of miRNAs
miRNAs are short, noncoding RNAs that
post-transcriptionally regulate gene expression and serve dual
roles in cancer as oncogenes or tumor suppressors (63). MGF influences the expression of
several miRNAs associated with tumor suppression and apoptosis.
In glioma cells, MGF upregulates miR-15b, which
induces apoptosis by downregulating the expression of oncogenic
targets such as WNT7A (37,64). Similarly, in prostate cancer PC3
cells, MGF elevates miR-182 levels, leading to decreased Bcl-2
expression and increased apoptotic activity (65). In lung adenocarcinoma models, MGF
reduces the expression of miR-27b and miR-92a, two oncogenic miRNAs
highly expressed in tumor tissues, thereby inhibiting tumor cell
proliferation and promoting apoptosis (66).
Induction of the mitochondrial
pathway
MGF also initiates intrinsic apoptosis via the
mitochondrial pathway (67).
Disruption of mitochondrial membrane potential is a critical early
event in the apoptotic cascade and is often regulated by
intracellular calcium homeostasis and endoplasmic reticulum stress
(68). MGF reduces the
mitochondrial membrane potential in HT29 colon cancer cells,
leading to mitochondrial outer membrane permeabilization and
apoptosis (69). In lung cancer
mouse models, MGF alters mitochondrial metabolism by increasing
lipid peroxidation and suppressing key TCA cycle and electron
transport enzymes, including isocitrate dehydrogenase, succinate
dehydrogenase, malate dehydrogenase and α-ketoglutarate
dehydrogenase (70). Notably, in
models of fluoride-induced neurotoxicity, MGF restores
mitochondrial integrity by inhibiting proapoptotic proteins (Bax,
caspase-3 and caspase-9) and upregulating Bcl-2. These protective
effects are mediated via JNK inhibition and activation of the
Nrf2/HO-1 pathway (71). Thus,
the ability of MGF to induce mitochondrial dysfunction in cancer
cells and preserve mitochondrial health in normal cells reflects
its selective cytotoxic potential.
Induction of oxidative stress
ROS serve a paradoxical role in cancer, contributing
to tumor initiation while also serving as apoptotic triggers when
in excess (72). MGF modulates
oxidative stress pathways by interacting with both pro-oxidant and
antioxidant systems. MGF activates the Nrf2 pathway, stabilizing
Nrf2 protein levels by inhibiting its ubiquitination, which leads
to increased transcription of antioxidant enzymes, such as NQO1
(73,74). This activity suppresses
intracellular ROS accumulation and induces apoptosis in leukemia
HL-60 cells (75). In
neuroblastoma SK-N-SH cells, MGF reduces ROS production and the
activity of key antioxidants, such as glutathione (GSH), superoxide
dismutase (SOD), catalase and GSH peroxidase (GSH-Px), pushing the
redox balance toward apoptosis (9,76). Additionally, MGF enhances phase
II detoxifying enzymes, including GSH S-transferase, quinone
reductase and UDP-glucuronosyltransferase, while reducing lipid
peroxidation in lung cancer models (77,78).
In a diethylnitrosamine-induced hepatocellular
carcinoma rat model, treatment with MGF has been shown to improve
hepatic oxidative stress markers, as follows: Malondialdehyde (MDA)
content was reduced, whereas SOD activity and GSH levels were
elevated, and tumor formation was attenuated (79). In colorectal cancer, an
azoxymethane-induced rat model treated with MGF has been shown to
exhibit improved antioxidant enzyme activity, reduced aberrant
crypt foci formation and modulation of apoptotic proteins, such as
Bax, Bcl-2 and caspase-3, have been reported, linking redox control
to tumor suppression in colon tissue (80).
In malignant contexts where antioxidant defenses
become critically depleted, MGF-mediated redox modulation may serve
as an initiating event that dictates cell fate. Sustained ROS
accumulation disrupts mitochondrial integrity, diminishes the
membrane potential and promotes apoptotic signaling, as observed in
tumor and myoblast models (81).
In addition to this proapoptotic cascade, accumulating evidence has
suggested that redox regulation itself constitutes a primary
mechanistic axis rather than a subordinate branch of apoptosis,
reflecting broader control of mitochondrial and metabolic
homeostasis (82,83).
In malignant settings with severely depleted
antioxidant defenses, persistent ROS elevation can damage
mitochondria, impair membrane potential and promote apoptotic
signaling. However, available data suggest that MGF-driven
restoration of redox homeostasis represents a central mechanistic
axis of its antitumor activity, rather than merely a downstream
branch of apoptosis (84,85).
Inhibition of tumor invasion via MMP-9
suppression
MMPs, particularly MMP-2, MMP-9 and MMP-11, are
critical enzymes involved in tumor invasion, metastasis and
angiogenesis (86). Among these
proteins, MMP-9 serves a central role in degrading extracellular
matrix components, facilitating tumor cell dissemination. MGF has
been demonstrated to have a selective inhibitory effect on MMP-9,
making it a promising candidate for antimetastatic therapy
(87).
MGF inhibits tumor invasion in part by selectively
suppressing MMP-9 expression and activity. In human glioma models,
MGF reduces phorbol 12-myristate 13-acetate-induced MMP-9 without
materially affecting other MMP family members, which is consistent
with decreased NF-κB and AP-1 binding to the MMP-9 promoter, and
with upstream attenuation of Akt and MAPK signaling (41). A Computational study has provided
support for direct interactions between MGF derivatives and the
MMP-9 catalytic site, which may contribute to enzymatic inhibition
(88).
MGF has also been shown to sensitize glioma cells by
modulating signaling pathways involved in MMP-9 regulation,
including the NF-κB and PKB/Akt pathways. In metastatic melanoma
models, low-dose MGF enhances the antitumor activity of
citrus-derived glycosides, partly by increasing TNF-α expression
and inhibiting metastasis (89).
Immunomodulatory activity
Suppression of NF-κB signaling
In addition to its downstream effects on apoptosis,
NF-κB signaling represents a key immunomodulatory axis. Chronic
inflammation drives tumorigenesis in ~20% of cancers (90), largely through activation of the
NF-κB pathway (91,92). MGF interferes with multiple nodes
of NF-κB signaling, thereby limiting inflammation-driven
carcinogenesis (93). NF-κB
transcription factors, particularly RelA (p65) and RelB, promote
the expression of pro-survival and proinflammatory genes (94). MGF suppresses RelA and RelB
expression, reduces NF-κB transcriptional activity, and induces
apoptosis in malignancies such as multiple myeloma (95).
In the canonical NF-κB pathway, extracellular
stimuli such as pathogen-associated molecular patterns or
proinflammatory cytokines (such as IL-1 and TNF-α) activate
Toll-like receptors and IL-1 receptor (IL-1R), leading to
recruitment of the MyD88-IL-1R-associated kinase 1/4-TNF
receptor-associated factor 6 complex (96,97). This initiates downstream
signaling via TAK1-TAB1/2 and the IKK complex (IKKα, IKKβ and
NEMO), ultimately phosphorylating IκB and releasing the NF-κB
p50/p65 dimer for nuclear translocation (98).
MGF effectively interferes with this cascade. In
vitro studies have shown that pretreatment with MGF or its
enriched extract Vimang reduces the phosphorylation of IKKα and
IKKβ without altering their total protein levels (99). Furthermore, MGF impairs the
nuclear translocation and DNA-binding activity of p65 in response
to TNF-α stimulation (100),
thereby disrupting NF-κB-driven transcription and weakening the
survival response in tumor cells (101). This NF-κB inhibition is
accompanied by classical apoptotic hallmarks. MGF upregulates
proapoptotic markers, including cleaved caspase-3, cleaved PARP-1,
p53, phosphorylated-p53 and p53-upregulated modulator of apoptosis,
while downregulating antiapoptotic proteins, such as survivin and
Bcl-xL (40). Additionally, MGF
suppresses NF-κB-inducing kinase, further reinforcing the blockade
of both the canonical and noncanonical NF-κB pathways, ultimately
limiting cancer cell proliferation, metastasis and survival.
MGF also modulates stress and survival pathways that
converge on NF-κB. In HL-60 leukemia cells, MGF activates Nrf2 and
lowers intracellular ROS, which is consistent with the attenuation
of oxidative stress (75). In
epithelial cancer models, MGF enhances the cytotoxic effects of
chemotherapeutics while suppressing NF-κB activation. In HeLa and
HT29 cells, this suppression coincides with improved responses to
oxaliplatin and reduced drug resistance. In A549 lung cancer cells,
MGF increases the antiproliferative impact of chemotherapy in
association with G2/M arrest and inhibition of the
PKC-NF-κB axis (56). These
apoptotic outcomes are considered secondary to the primary
immunomodulatory role of MGF through NF-κB inhibition.
Modulation of immune cells and
cytokine responses
In addition to its direct cytotoxicity, MGF
modulates host immune responses, enhancing antitumor immunity. It
influences various immune cell populations, including macrophages,
neutrophils, splenocytes and natural killer (NK) cells, which are
critical components of the tumor microenvironment (78). MGF induces cytoskeletal
rearrangement in macrophages, alters their morphology and enhances
their phagocytic capacity (102). It also modulates immune
responses triggered by lipopolysaccharides (LPS), TNF-α and IL-1,
potentially limiting tumor-induced DNA damage in normal tissues and
mitigating tumor-promoting inflammation (103).
In MDA-MB-231 breast cancer cells, MGF disrupts
Rac1/WAVE2 signaling and inhibits cytokine secretion (for example,
IL-6 and IL-8), thereby reducing invasive capacity and inflammatory
crosstalk (104).
Dose-dependent immunostimulatory effects have also been observed in
murine models; low concentrations (5-40 mg/ml) enhance the
proliferation of splenocytes and thymocytes, whereas higher doses
inhibit tumor cell proliferation. MGF activates splenocytes,
facilitates plant lectin agglutination and stimulates NK-resistant
tumor cell killing by macrophages (105,106).
Radiosensitization in GBM
GBM is a highly aggressive brain tumor with a poor
prognosis and a limited response to conventional radiotherapy.
While radiotherapy remains a cornerstone of GBM treatment, tumor
recurrence due to radioresistance remains a major challenge.
Radiosensitizers are pharmacological agents that increase tumor
sensitivity to radiation while minimizing harm to normal tissues,
representing an attractive adjunct strategy in GBM management.
Recent evidence has suggested that MGF enhances
radiosensitivity in GBM through multiple mechanisms. It
synergistically inhibits the proliferation of U87 and U118 GBM
cells in a dose- and time-dependent manner when combined with
radiation. MGF exerts these effects by downregulating MMP-9
expression and modulating miR-15b, thereby disrupting tumor cell
survival and invasion (35).
Notably, MGF selectively inhibits the nonhomologous end joining
pathway, a major DNA repair mechanism in GBM cells, thereby
impairing DNA repair following radiation and enhancing
radiotherapeutic efficacy. By sensitizing tumor cells to
radiation-induced DNA damage, MGF improves therapeutic outcomes
without necessitating an increase in radiation dose (36). A comprehensive summary of the
targets, dosages, study models, signaling pathways and mechanistic
insights associated with MGF across different cancer-related
contexts is presented in Table I
(4,5,7,8,13-17,55,56,107-114).
 | Table ISummary of representative studies on
MGF and the underlying molecular mechanisms. |
Table I
Summary of representative studies on
MGF and the underlying molecular mechanisms.
| Target/pathway | Dose and
duration | Experimental
model/sample size | Type of study | Major signaling
pathways | Principal
findings/cellular products |
Limitations/remarks | (Refs.) |
|---|
| Apoptosis | 12.5-200
μM | CNE2, SGC-7901
gastric carcinoma cells | In
vitro | Caspase;
PI3K-Akt | Bax (up), Bad (up),
Bcl-2 (down); activation of caspase-3/9; | Limited to gastric
cell lines | (4,5) |
| miRNA
modulation | 12.5-100 mg/ml | H2030/H1299/A549
lung cancer cells | In
vitro | miR-92a,
miR-27b | Downregulated
oncogenic miRNAs; suppressed proliferation and induced apoptosis in
LUAD cells | No animal or human
data | (7) |
| miRNA
modulation | 25-100
μM | Human U87 glioma
cells | In
vitro | MMP-9/miR-15b | MMP-9 (down),
miR-15b (down); attenuated invasion | Mechanism indirect;
requires in vivo validation | (8) |
| Mitochondrial
pathway | 1/50/400
μM | HT-29 colon
adenocarcinoma cells (ATCC HTB-38) | In
vitro | PPAR, SIRT, NF-κB,
STAT3, HIF, Wnt | Multitargeting of
mitochondrial oxidoreductases and β-oxidation; inhibited
proliferation, metastasis, angiogenesis | In vitro
only; pharmacokinetic data were not available | (13) |
| Mitochondrial
pathway | 10/25/50
μM | SH-SY5Y
neuroblastoma cells | In
vitro | JNK; Nrf2/HO-1 | Normalized
mitochondrial dynamics, reduced oxidative stress | Limited neuronal
model | (14) |
| Lipid
peroxidation | 10
μg/ml | U-937
macrophages | In
vitro | NF-κB | Inhibited
TNF-induced lipid peroxidation; catalase (up); apoptosis
protection | Preclinical
only | (15) |
| Lipid
peroxidation | 50 mg/kg |
Benzo(a)pyrene-induced lung cancer in
Swiss albino mice (n=6/group) | In vivo | NF-κB;
mitochondrial genes | Reduced
mitochondrial lipid peroxidation; altered TCA/ETC enzymes; induced
apoptosis | No chronic safety
data | (16) |
| Oxidative-stress
pathway | 50/100/200 mg/kg
×14 days | Wistar rats
(n=8/group) | In vivo | Nrf2 | Breg levels,
activated Nrf2 antioxidant pathway; proinflammatory mediators
(down) | Rodent model; needs
clinical validation | (17) |
| Cell-cycle
proteins | 0.5/0.75/1
μM | HL-60 leukemia
cells | In
vitro | CDK1/cyclin B1 | Increased cyclin
B1; triggered G2/M arrest | Short-term
exposure; lacks mechanistic protein validation | (55) |
| Cell-cycle
proteins | 10/50/100
mg/kg | A549 lung carcinoma
cells | In
vitro | CDK1/cyclin B1 | Induced
G2/M-phase arrest; apoptosis via NF-κB inhibition | Only cell-line
data; no in vivo validation | (56) |
|
Pharmacokinetics/absorption | 30 mg/kg (single
oral dose) | Wistar rats
(n=6) | In vivo | - | Peak tissue level
in small intestine at 0.5 h (754 ng/ml); nonlinear absorption | No human PK
association | (107) |
|
Anti-invasion/anti-metastasis | 1 mg/ml; 50
μl/well | U87MG/U373MG/CRT-MG
glioma cells | In
vitro | MMP-9; PI3K-Akt;
MAPK | Suppressed MMP-9
via inhibition of NF-κB and AP-1; Akt/MAPK phosphorylation
(down) | No in vivo
validation | (108) |
|
Anti-inflammatory | 4.2 mg | RAW 264.7
macrophages | In
vitro | NF-κB | IL-12 (down), TNF-α
(up), IL-1 (up), IL-6 (down); anti-inflammatory shift | Lacks time-course
data | (109) |
| miRNA
modulation | 10/20/40
μM | PC3 human prostate
cancer cells | In
vitro | Bcl-2/miR-182 | Bcl-2 (down),
miR-182 (up); promoted apoptosis | Single-cell
model | (110) |
| Mitochondrial
pathway | 100 mg/kg × 21
days | ER−
breast-cancer xenograft mice (n=8) | In vivo | NF-κB;
caspase-3 | Tumor volume
reduced by ~89%, comparable to cisplatin (91.5%) | Short duration; no
toxicity endpoints | (111) |
| Inflammatory
models | 20-40
μM/5-20 mg/kg | RAW264.7 cells and
mouse air-pouch model (n=6/group) | In vitro and
in vivo | ROS; NF-κB | Reduced ROS;
blocked NF-κB nuclear translocation; suppressed cytokines | Dose-dependent
effects only short term | (112) |
|
Safety/toxicity | ≤1,000 mg/kg × 28
days | Sprague-Dawley rats
(n=10/group) | In vivo | - | No abnormal
hematology; LD50 >2,000 mg/kg | Limited to 28
days | (113) |
| Clinical cognition
study | 300 mg/day × 7
days | 60 healthy adults,
randomized double-blind | Clinical |
Cognitive/antioxidant | Improved attention
and working memory; no adverse events | Short duration;
healthy subjects only | (114) |
Applications in other diseases
In addition to its well-characterized roles in
diabetes and cancer, MGF exerts protective effects across a broad
spectrum of pathological conditions. These include inflammatory,
neurodegenerative, ocular, renal, cardiovascular, dermatological
and infectious diseases (7,36,106,115-118). Although the mechanistic depth
of investigation in these fields is comparatively limited,
accumulating preclinical and early clinical evidence has
underscored the versatile pharmacodynamic (PD) profile of MGF. Its
therapeutic actions converge on several core molecular pathways,
particularly the suppression of oxidative stress and NF-κB-mediated
inflammation, and the activation of AMPK and SIRT1 signaling
(119-121). Fig. 5 provides an overview of these
shared mechanisms and representative disease contexts, illustrating
the diverse yet interconnected pharmacological potential of MGF
beyond metabolic and oncological disorders.
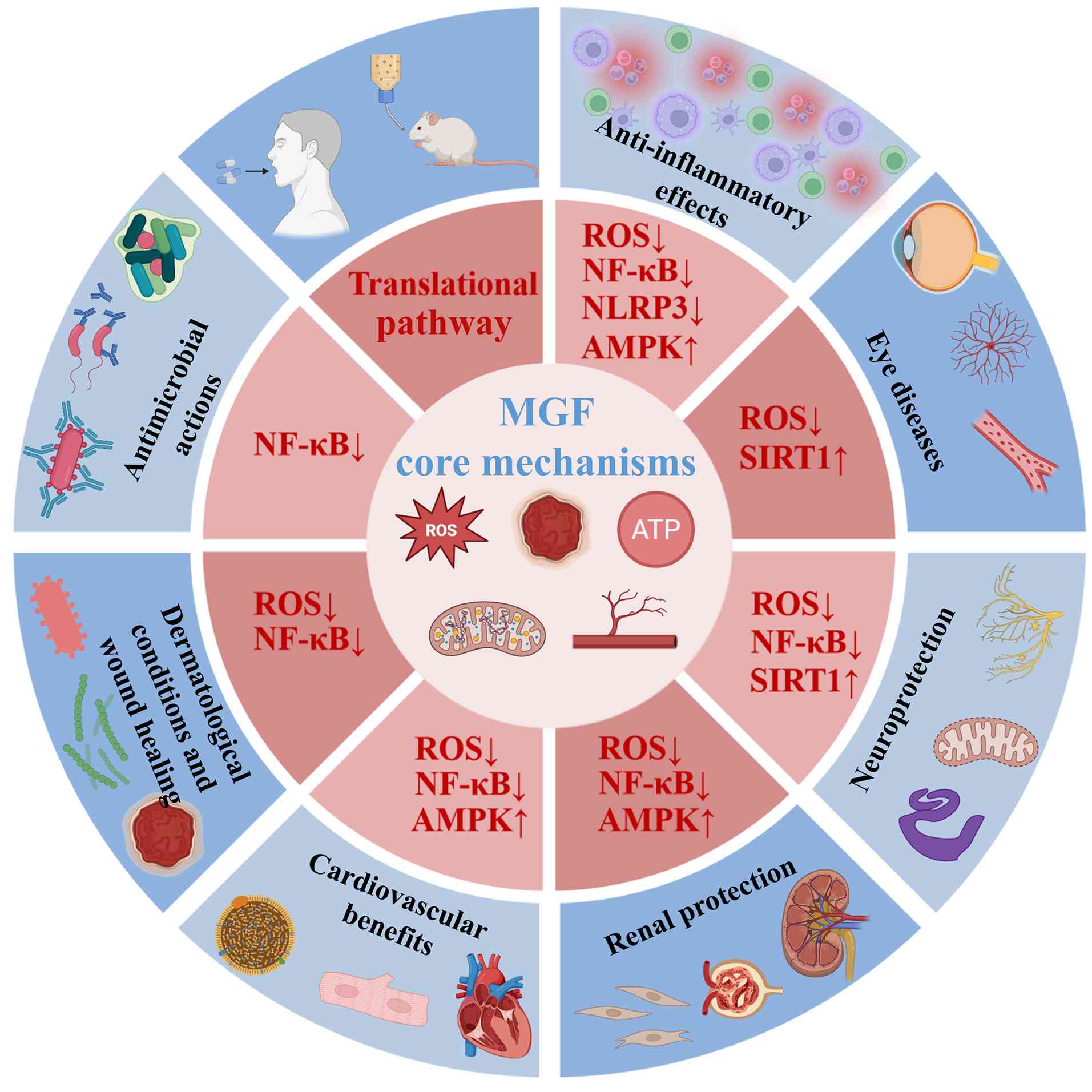 | Figure 5Representative therapeutic
applications of MGF and its core molecular mechanisms. MGF exhibits
broad pharmacological activity across multiple organ systems, which
is primarily mediated through the attenuation of oxidative stress
and inflammatory signaling. The core mechanisms include a reduction
in ROS levels, suppression of NF-κB and NLRP3 inflammasome
activation, and activation of the AMPK and SIRT1 pathways. These
convergent regulatory effects underpin the diverse protective
actions of MGF in inflammatory, neurodegenerative, ocular, renal,
cardiovascular, dermatological and infectious diseases. The listed
conditions represent key examples rather than an exhaustive account
of the therapeutic potential of MGF, highlighting its versatility
as a pleiotropic bioactive compound. AMPK, AMP-activated protein
kinase; MGF, mangiferin; NLRP3, NLR family pyrin domain-containing
3; ROS, reactive oxygen species; SIRT, sirtuin. |
Anti-inflammatory effects
Chronic inflammation is a key driver of various
pathological conditions, including degenerative, autoimmune and
metabolic diseases. MGF, characterized by its C-glucosyl and
polyhydroxylated xanthone structure, exhibits potent antioxidant
and anti-inflammatory activities by scavenging free radicals and
modulating key signaling pathways.
In intervertebral disc degeneration, MGF has been
shown to attenuate mitochondrial dysfunction and reduce ROS in
human nucleus pulposus cells. It suppresses TNF-α-induced NF-κB
activation and downregulates the expression of apoptosis-related
markers, including pro-apoptotic caspases and Bcl-2 family
proteins, indicating its therapeutic potential for treating
degenerative disc disorders (122).
In a rat model of rheumatoid arthritis, MGF (15-45
mg/kg, 2-week administration) inhibits the MAPK and NF-κB pathways,
thereby reducing inflammatory cell proliferation, migration and
cytokine secretion. MGF also promotes inflammatory apoptosis and
attenuates the pathogenic activity of fibroblast-like synoviocytes
in arthritic rats (123).
In metabolic inflammation, MGF improves insulin
sensitivity and lipid metabolism. In a rodent model of high-fat
diet-induced nonalcoholic fatty liver disease (NAFLD), MGF (25-100
mg/kg, 12 weeks) activates AMPK and suppresses NLRP3 inflammasome
signaling, reducing hepatic inflammation and steatosis (124). An additional study has
confirmed the ability of MGF to inhibit the NF-κB and JNK pathways,
with reduced body weight and plasma triglyceride and cholesterol
levels in NAFLD mouse models (125).
In models of acute lung injury, pre-administration
of MGF (100 mg/kg) suppresses LPS-induced NLRP3 inflammasome
activation in macrophages via NF-κB inhibition. This results in
reduced inflammatory cytokine production and amelioration of
pulmonary pathology (126).
Eye diseases
Oxidative stress serves a central role in the
pathogenesis of ocular disorders such as diabetic retinopathy and
macular degeneration. MGF has demonstrated therapeutic benefits in
preclinical models of retinal and lens injury through antioxidant
and antiangiogenic mechanisms (127).
In ischemic retinal injury, MGF downregulates HIF-1α
and glial fibrillary acidic protein while upregulating SIRT1,
thereby protecting retinal ganglion cells from oxidative damage in
mouse models (128). In
diabetic retinopathy, MGF inhibits angiogenesis and endothelial
cell migration by targeting the PI3K/Akt/mTOR pathway, suggesting
potential for clinical translation (129).
Notably, the capacity of MGF to cross the
blood-retinal barrier enhances its therapeutic efficacy (130). In streptozotocin-induced
diabetic rats, biochemical analysis has revealed that MGF increases
antioxidant enzyme activity (SOD, GSH-Px), reduces lipid
peroxidation (MDA), and decreases advanced glycation end-products
(AGEs) and sorbitol levels in the lens and serum, thereby
contributing to the prevention of diabetic ocular complications
(131).
Neuroprotection
MGF exhibits robust neuroprotective properties
across multiple animal models of neurodegeneration, ischemia and
cognitive impairment. Its mechanisms include the modulation of
oxidative stress, mitochondrial protection and anti-inflammatory
signaling (132).
In
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse models
and α-synuclein overexpression transgenic mouse models of
Parkinson's disease, MGF reduces neuroinflammation and oxidative
stress by inhibiting NF-κB signaling, resulting in improved motor
coordination and neuronal survival in the substantia nigra
(133). It also improves memory
by enhancing cholinergic transmission through acetylcholinesterase
inhibition and NF-κB suppression (134).
In hypoxia/reoxygenation-induced N2a neuroblastoma
cell models of ischemic injury, MGF activates the SIRT1/PPARγ
coactivator-1α axis to preserve mitochondrial function and reduce
neuronal damage (135). A
placebo-controlled human study demonstrated that acute MGF
administration enhances attention, long-term memory and cognitive
performance in healthy adults (136).
MGF also protects against β-amyloid-induced
oxidative damage, restoring redox balance and preserving
mitochondrial integrity in Alzheimer's disease models, such as
amyloid β-treated neuronal cultures and APP/PS1 transgenic mice
(137). In aging mice, MGF
improves memory, reduces hippocampal pathology, and decreases the
β-amyloid burden and lipid peroxidation (138). Furthermore, it enhances
hippocampal neuronal excitability and cognitive processing,
supporting its potential as a neurocognitive enhancer (139).
Renal protection
Renal dysfunction, often driven by oxidative stress
and inflammation, contributes to the pathogenesis of acute and
chronic kidney diseases. MGF has demonstrated renoprotective
effects across a range of kidney injury models by modulating
inflammation, oxidative stress and fibrotic signaling pathways
(140).
In vitro studies have shown that MGF
suppresses IL-6 and IL-8 production by inhibiting the MAPK and
NF-κB pathways, thereby attenuating inflammatory responses in renal
epithelial cells (141). In a
mouse model of ischemia-reperfusion injury, MGF (10, 30 or 100
mg/kg) protects renal tubular cells by reducing inflammation and
apoptosis through activation of the adenosine-CD73 pathway
(141). Similarly, in
D(+)-galactosamine-induced nephrotoxicity, MGF exhibits antioxidant
properties by inhibiting inducible nitric oxide synthase and
downregulating NF-κB signaling, leading to improved renal function
and histology (142).
MGF (20, 50 or 100 mg/kg) also protects against
sepsis-induced acute kidney injury in an LPS-induced sepsis mouse
model by preserving the endothelial glycocalyx component
syndecan-1, reducing ROS and MDA production, enhancing tight
junction integrity, and limiting vascular leakage and inflammation
(142).
Diabetic nephropathy is a common microvascular
complication of type 1 and type 2 diabetes (143). MGF (50 mg/kg) mitigates renal
injury in streptozotocin-induced diabetic mice by modulating
hexokinase II-mitochondrial interactions and inhibiting NADPH
oxidase 4, thereby reducing oxidative stress (144). Moreover, MGF (12.5, 25 or 50
mg/kg) enhances autophagic flux via AMPK-mTOR signaling, delaying
the progression of diabetic nephropathy (145).
MGF also attenuates renal interstitial fibrosis, a
hallmark of diabetic kidney disease. In streptozotocin-induced
diabetic mice, MGF (15, 30 or 60 mg/kg) suppresses inflammation and
oxidative stress by modulating the PTEN/PI3K/Akt pathway, leading
to reduced expression of fibrotic markers such as collagen I,
fibronectin and α-SMA (34).
Cardiovascular benefits
MGF has lipid-lowering, antioxidant and
anti-inflammatory properties that contribute to cardiovascular
protection. A randomized, double-blind clinical trial demonstrated
that MGF supplementation can markedly decrease serum triglyceride
and free fatty acid levels in overweight individuals with
hyperlipidemia, highlighting its clinical potential (146).
In high-fat diet-induced hyperlipidemic rats, MGF
(50, 100 or 150 mg/kg, orally administered for 6 weeks) has been
shown to activate hepatic AMPK, resulting in improved lipid
metabolism and reduced circulating free fatty acids (147). Furthermore, in high-fat
diet-induced hyperlipidemic rats, MGF-loaded
N-succinyl-chitosan-grafted alginate nanoparticles enhance
therapeutic efficacy by increasing the hepatic expression of PPARα,
carnitine palmitoyltransferase I and FAT/CD36, while suppressing
lipogenic genes, such as SREBP-1c, ACC, DGAT2 and MTP (148).
In rat models of diabetic cardiomyopathy, MGF (20
mg/kg for 16 weeks) has been shown to notably decrease ROS
production and AGE accumulation, partly through the inhibition of
NF-κB signaling. This preserves myocardial function and delays
cardiomyopathic remodeling (149).
Dermatological benefits and wound
healing
In murine models of contact dermatitis induced by
oxazolone, MGF suppresses TNF-α-stimulated macrophage activation
and inhibits NF-κB pathway activation, resulting in decreased
production of inflammatory mediators and oxidative stress markers
(150). Its C-glucosylxanthone
structure contributes to its efficacy as a skin-targeted
anti-inflammatory compound.
Oxygenated anthraquinone derivatives of MGF,
including isobavachalcone, gartanin and glucosylated xanthone, have
demonstrated antimicrobial activity against acne-causing bacteria
such as Staphylococcus epidermidis. These compounds also
inhibit inflammatory pathways, including PPAR and prostaglandin
cascades, suggesting their therapeutic potential in both acne and
inflammatory dermatoses (151).
In wound healing, MGF-loaded hydrogels or liposomes
(10, 20 and 40 μM) have been reported to accelerate flap
regeneration in rat models of skin injury. This treatment may
inhibit oxidative stress-induced apoptosis, increase the Bcl-2/Bax
ratio and decrease cleaved caspase-3 expression (29). MGF also enhances PPARγ expression
and suppresses NF-κB activity, further promoting anti-inflammatory
and cytoprotective effects in dermal tissues (29).
Antimicrobial actions
MGF possesses broad-spectrum antimicrobial activity
against both fungal and bacterial pathogens, including strains
involved in drug resistance and biofilm formation. Purified MGF has
MIC values ranging from 1.95 to 62.5 μg/ml for fungi and
from 1.95 to 31.25 μg/ml for bacteria, with a time-kill
study confirming its bactericidal and fungicidal efficacy (1). Extracts from mango leaves, which
are rich in MGF, have shown inhibitory activity against
gram-positive bacteria (Staphylococcus aureus, Bacillus
subtilis, Streptococcus pneumoniae) and gram-negative pathogens
(Escherichia coli, Klebsiella pneumoniae, Pseudomonas
aeruginosa, Shigella spp.) (114,152,153). Furthermore, MGF derivatives
have demonstrated efficacy against K. pneumoniae by
disrupting biofilms, impairing virulence factor expression and
increasing antibiotic susceptibility (154).
In a murine periodontitis model, oral
administration of MGF (50 mg/kg for 8 weeks) has been reported to
suppress Porphyromonas gingivalis-induced alveolar bone
loss, downregulate TNF-α production and inhibit NF-κB and
JAK1/STAT1/3 phosphorylation in the gingival epithelium (155). In Caenorhabditis elegans
models infected with P. aeruginosa, MGF compounds regulate
quorum sensing pathways and reduce bacterial virulence in both
dauer larvae and adult stages, further supporting their
anti-infective potential (156).
Translational aspects
PK, and absorption, distribution,
metabolism and excretion overview
Orally administered MGF shows low aqueous
solubility and limited intestinal permeability, resulting in poor
systemic exposure at conventional doses (157-160). After uptake in the proximal
small intestine, extensive first-pass metabolism and gut
microbiota-mediated deglycosylation (yielding norathyriol and
downstream conjugates) further reduce parent drug levels (21,160-162). The tissue distribution in
rodents indicates measurable concentrations in the liver, kidney
and lung with delayed plasma peaks, whereas brain penetration
appears limited under standard conditions (107,163). These properties underscore the
need to link PD readouts to exposures achievable with clinically
realistic regimens, and to report unbound concentrations where
possible.
Key barriers to translation
The main obstacles include: i) Low solubility and
permeability [constraining Cmax/area under the curve
(AUC) after oral dosing] ((164-166), ii) metabolic lability and
conjugation that curtails exposure to the parent compound MGF
(21,167,168), iii) variability introduced by
microbiome-dependent deglycosylation (165,169), and iv) inconsistent reporting
of dose-exposure relationships across preclinical studies (170,171). These issues complicate
cross-study comparisons and dose selection for human studies.
Delivery/formulation strategies
Multiple approaches have improved exposure in
preclinical systems, including nanostructured lipid carriers,
polymeric and chitosan-based nanogels, phospholipid complexes
(2,172,173), and targeted nanoparticles
designed to enhance intestinal uptake or lymphatic transport and to
stabilize MGF against rapid metabolism (172,174-176). Early structure-led strategies
(for example, monosodium salts/derivatives) have also reported
increased apparent potency or improved tissue delivery in disease
models (166,177,178). In the future, head-to-head
comparisons using common dose units, matched controls and
standardized PK endpoints (AUC, bioavailability, tissue-plasma
ratios) will be essential for identifying clinically scalable
options (170,179).
Safety/toxicity signals
A nonclinical study has previously reported
favorable tolerability at pharmacologically relevant exposures,
with adverse findings emerging at substantially higher doses
(180). However, several safety
uncertainties remain: i) Limited chronic toxicity datasets for
purified MGF (as opposed to plant extracts) (108); ii) sparse evaluation of
drug-drug interaction risks given pathway cross-talk (for example,
with PI3K/Akt, AMPK or NF-κB modulators) (181-183); and iii) incomplete reporting of
standardized laboratory safety assessments and histopathology in a
number of efficacy-focused studies (184). Extract-based toxicology (such
as that assessing mango leaf preparations) should not be
overextrapolated to the purified compound without compositional
bridging.
Clinical evidence and next steps
Early human data are limited, heterogeneous (often
extract-based) and not yet definitive for purified MGF. Small
studies suggest metabolic or performance signals in select
contexts, but they seldom include rigorous PK/PD integration,
dose-ranging or long-term safety monitoring (139,166,185). The next steps include: i) Phase
I studies with single- and multiple-ascending doses of purified MGF
and exposure-enhancing formulations; ii) assessment of the effects
of food intake on MGF PKs and bioequivalence for leading delivery
platforms, including nanostructured lipid carriers, phospholipid
complexes and chitosan-based nanoparticles; iii) PK/PD modeling
anchored to validated mechanistic biomarkers (such as p65 nuclear
translocation, Nrf2 target induction) and disease-relevant
functional endpoints; and iv) randomized, controlled phase II
trials using clinically translatable doses and standardized safety
surveillance.
Evidence gaps
Preclinical findings regarding MGF are promising;
however, the evidence base shows important gaps that limit
translation. Notably, the results vary across cell lines and animal
strains, and several mechanisms that are frequently cited (for
example, NF-κB or MMP-9 modulation, G2/M arrest and AMPK
activation) appear to be highly context dependent, with
inconsistent replication under different stimuli or dosing
conditions (4,13,117,124,186,187). A number of studies rely on
single models, small or unreported sample sizes, and pretreatment
designs rather than therapeutic intervention after disease onset,
which reduces external validity (117,188). PK constraints remain
under-characterized, including oral exposure at clinically relevant
doses, the relative contribution of metabolites such as
norathyriol, tissue penetration in target organs and the
variability introduced by different formulations (21,159,162). Material standardization is
uneven, with frequent use of extracts or nanoparticles without full
reporting of purity, content uniformity or release profiles, making
cross-study comparisons difficult and weakening conclusions
regarding the active entity (161,181). Mechanistic claims often rely on
surrogate protein readouts, such as changes in pathway marker
proteins (for example, NF-κB p65 nuclear translocation or AMPK
phosphorylation), without orthogonal validation or causal rescue
experiments, and dose-response and time-course data are incomplete
for several indications (13,186,189). Comparative efficacy vs.
standard of care is rarely tested, and combination studies seldom
include PK or PD interaction assessments. Furthermore, safety
reporting is inconsistent, with limited long-term toxicity,
immunomodulatory risk and drug-drug interaction data, and with few
studies that stratify patients by sex or age (184,185,190). Human evidence remains sparse,
with a lack of standardized endpoints, validated translational
biomarkers, and prospective trials that incorporate randomization,
blinding and predefined primary outcomes (184,191). Addressing these gaps will
require well-powered, rigorously controlled studies that integrate
exposure-response relationships, prespecified functional end points
and reproducible materials, together with head-to-head and
combination designs that reflect real clinical use.
Conclusion and future prospects
MGF, a natural C-glucosylxanthone, has broad
pharmacological effects, including antidiabetic, anticancer,
anti-inflammatory, antioxidant and neuroprotective activities, with
low toxicity and good biocompatibility, making it a promising
therapeutic candidate.
Notably, the clinical use of MGF is limited by poor
solubility and low oral bioavailability. Advances such as nanogels,
lipid carriers and phospholipid complexes have improved absorption
and stability (23,192,193). Structural modifications and
derivatives such as monosodium MGF also enhance efficacy, partly
via the modulation of oxidative stress pathways (194). Future work should focus on
optimized delivery systems, rational structural designs, and
rigorous clinical trials to confirm safety and dosing (170). Building on the growing
preclinical base, next-step studies should prioritize clinically
scalable formulations, such as polymeric or lipid nanocarriers,
self-microemulsifying drug delivery systems (forming fine
oil-in-water microemulsions in the gastrointestinal tract) and
solid lipid nanoparticles (lipid-based colloidal carriers),
cyclodextrin inclusions and phospholipid complexes, supported by
standardized chemistry, manufacturing and controls characterization
and direct PK comparisons with conventional extracts (195,196). This approach is justified by
consistent improvements in solubility, uptake and systemic exposure
demonstrated in nanodelivery research, as well as by early human PK
findings that MGF monosodium salt substantially increases
bioavailability compared with the parent compound (166,195). These PK advantages make dose
finding and scheduling practically feasible in phase I/II trials.
To ensure translational credibility, forthcoming clinical studies
should integrate exposure-response analysis and biomarker
verification, quantifying indicators such as Nrf2 target induction,
NF-κB suppression, and oxidative or DNA damage markers in
accessible tissues or circulating immune cells. Consistent with the
development principles proposed by previous scholars, these
strategies effectively integrate formulation, dosage and PDs into a
coherent translational framework (166,195,197).
Deeper investigations into signaling pathways,
including the NF-κB, Nrf2 and AMPK pathways, will further clarify
the underlying mechanisms of MGF and support its clinical
translation (184,198,199). Mechanistically enriched studies
should define, for each indication discussed in this review (for
example, cancer, inflammatory disorders and liver disease), the
predominant signaling axis, distinguishing between redox-regulated
Nrf2 signaling, inflammatory NF-κB activation and metabolic AMPK
modulation, as summarized in reviews of the molecular mechanisms of
MGF in cancer/inflammation and liver disease, including the NF-κB,
Nrf2 and AMPK pathways (198,199). Such resolution will guide
rational combinations, for example, pairing MGF with radiotherapy
or cytotoxic drugs where redox priming or DNA-repair interference
is desired or combining with immunomodulators where NF-κB/innate
signaling is dominant (200-202). This pathway-specific
combination strategy, which pairs MGF with radiotherapy or
cytotoxic agents for redox priming or DNA-repair interference, or
with immunomodulators that act on the dominant NF-κB/innate
signaling pathway, is inspired by prior 'redox-directed'
therapeutic approaches, and is further supported by MGF studies
showing Nrf2-centred and AMPK-linked anti-inflammatory effects
(75,120). Together, a biomarker-driven,
pathway-specific strategy may result in MGF progressing from a
broadly-acting pleiotropic lead compound to a precisely targeted
therapeutic candidate for defined patient subgroups.
In conclusion, MGF represents a compelling natural
compound with substantial therapeutic potential. With continued
advancements in formulation science, structural modification and
clinical validation, MGF may emerge as a clinically valuable agent
in modern pharmacotherapy. Overcoming its current limitations
through interdisciplinary innovation will be essential to
determining its full potential in the prevention and treatment of
metabolic, inflammatory, infectious, degenerative and neoplastic
diseases.
Availability of data and materials
Not applicable.
Authors' contributions
YD, QH and MT contributed equally to the conception
and design of the review, performed the literature search, screened
the articles, extracted and synthesized the data, and drafted the
initial manuscript. SZ and ZW participated in the literature search
and data extraction, performed extensive analysis and
interpretation of the data, summarized and organized the extracted
findings, and assisted with figure and table preparation. CJ
contributed to the organization and interpretation of the extracted
data, and critically revised the manuscript for important
intellectual content. ZL and SS conceived and supervised the study,
resolved discrepancies in study selection and data interpretation,
provided critical revision of the manuscript, and are the
corresponding authors. Data authentication is not applicable. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
AGE
|
advanced glycation end-products
|
|
AMPK
|
AMP-activated protein kinase
|
|
PPAR
|
peroxisome proliferator-activated
receptor
|
|
PTEN
|
phosphatase and tensin homolog
|
|
ROS
|
reactive oxygen species
|
|
SIRT
|
sirtuin
|
|
SOD
|
superoxide dismutase
|
Acknowledgements
The authors would like to thank Professor Yi Wang
(University of Electronic Science and Technology of China, Chengdu,
China), for her valuable guidance and assistance in preparing this
manuscript.
Funding
This work was supported by the Fujian Provincial Natural Science
Foundation (grant no. 2025J08106), the National Natural Science
Foundation of China (grant no. 82505722) and the National Natural
Science Foundation of China (grant no. 82474616).
References
|
1
|
Wang M, Liang Y, Chen K, Wang M, Long X,
Liu H, Sun Y and He B: The management of diabetes mellitus by
mangiferin: Advances and prospects. Nanoscale. 14:2119–2135. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bhattacharyya S, Ahmmed SM, Saha BP and
Mukherjee PK: Soya phospholipid complex of mangiferin enhances its
hepatoprotectivity by improving its bioavailability and
pharmacokinetics. J Sci Food Agric. 94:1380–1388. 2014. View Article : Google Scholar
|
|
3
|
Mei S, Perumal M, Battino M, Kitts DD,
Xiao J, Ma H and Chen X: Mangiferin: A review of dietary sources,
absorption, metabolism, bioavailability, and safety. Crit Rev Food
Sci Nutr. 63:3046–3064. 2023. View Article : Google Scholar
|
|
4
|
Morozkina SN, Nhung Vu TH, Generalova YE,
Snetkov PP and Uspenskaya MV: Mangiferin as new potential
anti-cancer agent and mangiferin-integrated polymer systems-a novel
research direction. Biomolecules. 11:792021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang M, Zhang Z, Huo Q, Wang M, Sun Y, Liu
H, Chang J, He B and Liang Y: Targeted polymeric nanoparticles
based on mangiferin for enhanced protection of pancreatic β-cells
and type 1 diabetes mellitus efficacy. ACS Appl Mater Interfaces.
14:11092–11103. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Akkewar AS, Mishra KA and Sethi KK:
Mangiferin: A natural bioactive immunomodulating glucosylxanthone
with potential against cancer and rheumatoid arthritis. J Biochem
Mol Toxicol. 38:e237652024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yehia RS and Altwaim SA: An insight into
in vitro antioxidant, antimicrobial, cytotoxic, and apoptosis
induction potential of mangiferin, a bioactive compound derived
from mangifera indica. Plants (Basel). 12:15392023.PubMed/NCBI
|
|
8
|
Xiang G, Guo S, Xing N, Du Q, Qin J, Gao
H, Zhang Y and Wang S: Mangiferin, a potential supplement to
improve metabolic syndrome: Current status and future
opportunities. Am J Chin Med. 52:355–386. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li HW, Lan TJ, Yun CX, Yang KD, Du ZC, Luo
XF, Hao EW and Deng JG: Mangiferin exerts neuroprotective activity
against lead-induced toxicity and oxidative stress via Nrf2
pathway. Chin Herb Med. 12:36–46. 2020.
|
|
10
|
Aritomi M and Kawasaki T: A new xanthone
C-glucoside, position isomer of mangiferin, from anemarrhena
asphodeloides bunge. Tetrahedron Lett. 12:941–944. 1969. View Article : Google Scholar
|
|
11
|
Talamond P, Conejero GV, Verdeil JL and
Poëssel JL: Isolation of C-glycosyl xanthones from coffea
pseudozanguebariae and their location. Nat Prod Commun.
6:1885–1888. 2011.
|
|
12
|
Vyas A, Syeda K, Ahmad A, Padhye S and
Sarkar FH: Perspectives on medicinal properties of mangiferin. Mini
Rev Med Chem. 12:412–425. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Du S, Liu H, Lei T, Xie X, Wang H, He X,
Tong R and Wang Y: Mangiferin: An effective therapeutic agent
against several disorders (review). Mol Med Rep. 18:4775–4786.
2018.PubMed/NCBI
|
|
14
|
Kavitha M, Nataraj J, Essa MM, Memon MA
and Manivasagam T: Mangiferin attenuates MPTP induced dopaminergic
neurodegeneration and improves motor impairment, redox balance and
bcl-2/bax expression in experimental Parkinson's disease mice. Chem
Biol Interact. 206:239–247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hudecová A, Kusznierewicz B, Hašplová K,
Huk A, Magdolenová Z, Miadoková E, Gálová E and Dušinská M:
Gentiana asclepiadea exerts antioxidant activity and enhances DNA
repair of hydrogen peroxide- and silver nanoparticles-induced DNA
damage. Food Chem Toxicol. 50:3352–3359. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Andreu GP, Delgado R, Velho JA, Curti C
and Vercesi AE: Iron complexing activity of mangiferin, a naturally
occurring glucosylxanthone, inhibits mitochondrial lipid
peroxidation induced by Fe2+-citrate. Eur J Pharmacol. 513:47–55.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gu PC, Wang L, Han MN, Peng J, Shang JC,
Pan YQ and Han WL: Comparative pharmacokinetic study of mangiferin
in normal and alloxan-induced diabetic rats after oral and
intravenous administration by UPLC-MS/MS. Pharmacology. 103:30–37.
2019. View Article : Google Scholar
|
|
18
|
Liu H, Wu B, Pan G, He L, Li Z, Fan M,
Jian L, Chen M, Wang K and Huang C: Metabolism and pharmacokinetics
of mangiferin in conventional rats, pseudo-germ-free rats, and
streptozotocin-induced diabetic rats. Drug Metab Dispos.
40:2109–2118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gelabert-Rebato M, Wiebe JC, Martin-Rincon
M, Gericke N, Perez-Valera M, Curtelin D, Galvan-Alvarez V,
Lopez-Rios L, Morales-Alamo D and Calbet JAL: Mangifera indica l.
Leaf extract in combination with luteolin or quercetin enhances
VO2peak and peak power output, and preserves skeletal muscle
function during ischemia-reperfusion in humans. Front Physiol.
9:7402018. View Article : Google Scholar :
|
|
20
|
Ehianeta TS, Laval SP and Yu B: Bio- and
chemical syntheses of mangiferin and congeners. Biofactors.
42:445–458. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tian X, Gao Y, Xu Z, Lian S, Ma Y, Guo X,
Hu P, Li Z and Huang C: Pharmacokinetics of mangiferin and its
metabolite-norathyriol, part 1: Systemic evaluation of hepatic
first-pass effect in vitro and in vivo. Biofactors. 42:533–544.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hasanah U, Miki K, Nitoda T and Kanzaki H:
Aerobic bioconversion of c-glycoside mangiferin into its aglycone
norathyriol by an isolated mouse intestinal bacterium. Biosci
Biotechnol Biochem. 85:989–997. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li X, Jafari SM, Zhou F, Hong H, Jia X,
Mei X, Hou G, Yuan Y, Liu B, Chen S, et al: The intracellular fate
and transport mechanism of shape, size and rigidity varied
nanocarriers for understanding their oral delivery efficiency.
Biomaterials. 294:1219952023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Furlanetto V, Kalyani DC, Kostelac A, Puc
J, Haltrich D, Hällberg BM and Divne C: Structural and functional
characterization of a gene cluster responsible for deglycosylation
of C-glucosyl flavonoids and xanthonoids by deinococcus aerius. J
Mol Biol. 436:1685472024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pereira QC, Fortunato IM, Oliveira FS,
Alvarez MC, Santos TWD and Ribeiro ML: Polyphenolic compounds:
Orchestrating intestinal microbiota harmony during aging.
Nutrients. 16:10662024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kaur P, Gupta RC, Dey A, Malik T and
Pandey DK: Optimization of harvest and extraction factors by full
factorial design for the improved yield of C-glucosyl xanthone
mangiferin from swertia chirata. Sci Rep. 11:163462021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Suman RK, Borde MK, Mohanty IR and Singh
HK: Mechanism of action of natural dipeptidyl peptidase-IV
inhibitors (berberine and mangiferin) in experimentally induced
diabetes with metabolic syndrome. Int J Appl Basic Med Res.
13:133–142. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shimada T, Nagai E, Harasawa Y, Watanabe
M, Negishi K, Akase T, Sai Y, Miyamoto K and Aburada M: Salacia
reticulata inhibits differentiation of 3T3-l1 adipocytes. J
Ethnopharmacol. 136:67–74. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mao X, Liu L, Cheng L, Cheng R, Zhang L,
Deng L, Sun X, Zhang Y, Sarmento B and Cui W: Adhesive
nanoparticles with inflammation regulation for promoting skin flap
regeneration. J Control Release. 297:91–101. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang CQ, Xu JH, Yan DD, Liu BL, Liu K and
Huang F: Mangiferin ameliorates insulin resistance by inhibiting
inflammation and regulatiing adipokine expression in adipocytes
under hypoxic condition. Chin J Nat Med. 15:664–673.
2017.PubMed/NCBI
|
|
31
|
Fan X, Jiao G, Pang T, Wen T, He Z, Han J,
Zhang F and Chen W: Ameliorative effects of mangiferin derivative
TPX on insulin resistance via PI3k/AKT and AMPK signaling pathways
in human HepG2 and HL-7702 hepatocytes. Phytomedicine.
114:1547402023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Han J, Yi J, Liang F, Jiang B, Xiao Y, Gao
S, Yang N, Hu H, Xie WF and Chen W: X-3, a mangiferin derivative,
stimulates AMP-activated protein kinase and reduces hyperglycemia
and obesity in db/db mice. Mol Cell Endocrinol. 405:63–73. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fu TL, Li GR, Li DH, He RY, Liu BH, Xiong
R, Xu CZ, Lu ZL, Song CK, Qiu HL, et al: Mangiferin alleviates
diabetic pulmonary fibrosis in mice via inhibiting
endothelial-mesenchymal transition through AMPK/FoxO3/SIRT3 axis.
Acta Pharmacol Sin. 45:1002–1018. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Song Y, Liu W, Tang K, Zang J, Li D and
Gao H: Mangiferin alleviates renal interstitial fibrosis in
streptozotocin-induced diabetic mice through regulating the
PTEN/PI3k/Akt signaling pathway. J Diabetes Res. 2020:94817202020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shivam and Gupta AK: Neuroprotective
effects of isolated mangiferin from swertia chirayita leaves
regulating oxidative pathway on streptozotocin-induced diabetic
neuropathy in experimental rats. Cent Nerv Syst Agents Med Chem.
24:182–195. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Iqbal H, Inam-Ur-Raheem M, Munir S, Rabail
R, Kafeel S, Shahid A, Mousavi Khaneghah A and Aadil RM:
Therapeutic potential of mangiferin in cancer: unveiling regulatory
pathways, mechanisms of action, and bioavailability enhancements-an
updated review. Food Sci Nutr. 12:1413–1429. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xiao J, Liu L, Zhong Z, Xiao C and Zhang
J: Mangiferin regulates proliferation and apoptosis in glioma cells
by induction of microRNA-15b and inhibition of MMP-9 expression.
Oncol Rep. 33:2815–2820. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gold-Smith F, Fernandez A and Bishop K:
Mangiferin and cancer: Mechanisms of action. Nutrients. 8:3962016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rahmani AH, Almatroudi A, Allemailem KS,
Alharbi HOA, Alwanian WM, Alhunayhani BA, Algahtani M, Theyab A,
Almansour NM, Algefary AN, et al: Role of mangiferin in management
of cancers through modulation of signal transduction pathways.
Biomedicines. 11:32052023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Takeda T, Tsubaki M, Sakamoto K, Ichimura
E, Enomoto A, Suzuki Y, Itoh T, Imano M, Tanabe G, Muraoka O, et
al: Mangiferin, a novel nuclear factor kappa b-inducing kinase
inhibitor, suppresses metastasis and tumor growth in a mouse
metastatic melanoma model. Toxicol Appl Pharmacol. 306:105–112.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jung JS, Jung K, Kim DH and Kim HES:
Selective inhibition of MMP-9 gene expression by mangiferin in
PMA-stimulated human astroglioma cells: involvement of PI3k/akt and
MAPK signaling pathways. Pharmacol Res. 66:95–103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dilshara MG, Kang CH, Choi YH and Kim GY:
Mangiferin inhibits tumor necrosis factor-α-induced matrix
metalloproteinase-9 expression and cellular invasion by suppressing
nuclear factor-κB activity. BMB Rep. 48:559–564. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shoji K, Tsubaki M, Yamazoe Y, Satou T,
Itoh T, Kidera Y, Tanimori Y, Yanae M, Matsuda H, Taga A, et al:
Mangiferin induces apoptosis by suppressing Bcl-xL and XIAP
expressions and nuclear entry of NF-κB in HL-60 cells. Arch Pharm
Res. 34:469–475. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gong M, Li Y, Ye X, Zhang L, Wang Z, Xu X,
Shen Y and Zheng C: Loss-of-function mutations in KEAP1 drive lung
cancer progression via KEAP1/NRF2 pathway activation. Cell Commun
Signal. 18:982020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
DeBlasi JM, Falzone A, Caldwell S,
Prieto-Farigua N, Prigge JR, Schmidt EE, Chio IIC, Karreth FA and
DeNicola GM: Distinct nrf2 signaling thresholds mediate lung tumor
initiation and progression. Cancer Res. 83:1953–1967. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pal PB, Sinha K and Sil PC: Mangiferin
attenuates diabetic nephropathy by inhibiting oxidative stress
mediated signaling cascade, TNFα related and mitochondrial
dependent apoptotic pathways in streptozotocin-induced diabetic
rats. PLoS One. 9:e1072202014. View Article : Google Scholar
|
|
47
|
Du M, Wen G, Jin J, Chen Y, Cao J and Xu
A: Mangiferin prevents the growth of gastric carcinoma by blocking
the PI3K-Akt signalling pathway. Anticancer Drugs. 29:167–175.
2018. View Article : Google Scholar
|
|
48
|
Zou B, Wang H, Liu Y, Qi P, Lei T, Sun M
and Wang Y: Mangiferin induces apoptosis in human ovarian
adenocarcinoma OVCAR3 cells via the regulation of Notch3. Oncol
Rep. 38:1431–1441. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xia J, Li X, Yao J, Song D, Gu Z, Zheng G
and Tu C: Mangiferin targets PFKFB3 to inhibit glioblastoma
progression by suppressing glycolysis and PI3k/AKT/mTOR signaling.
Brain Res Bull. 230:1115202025. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Girón MD, Sevillano N, Salto R, Haidour A,
Manzano M, Jiménez ML, Rueda R and López-Pedrosa JM: Salacia
oblonga extract increases glucose transporter 4-mediated glucose
uptake in L6 rat myotubes: Role of mangiferin. Clin Nutr.
28:565–574. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Apontes P, Liu Z, Su K, Benard O, Youn DY,
Li X, Li W, Mirza RH, Bastie CC, Jelicks LA, et al: Mangiferin
stimulates carbohydrate oxidation and protects against metabolic
disorders induced by high-fat diets. Diabetes. 63:3626–3636. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Singh AK, Raj V, Keshari AK, Rai A, Kumar
P, Rawat A, Maity B, Kumar D, Prakash A, De A, et al: Isolated
mangiferin and naringenin exert antidiabetic effect via
PPAR(γ)/GLUT4 dual agonistic action with strong metabolic
regulation. Chem Biol Interact. 280:33–44. 2018. View Article : Google Scholar
|
|
53
|
Mistry J, Biswas M, Sarkar S and Ghosh S:
Antidiabetic activity of mango peel extract and mangiferin in
alloxan-induced diabetic rats. Future J Pharm. 9:222023. View Article : Google Scholar
|
|
54
|
Alsaedi AQ, Nader MA, El-Kashef DH and
Abdelmageed ME: Mangiferin mitigates dexamethasone-induced insulin
resistance in rats: Insight into vascular dysfunction and hepatic
steatosis. Front Pharmacol. 16:15727582025. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Żuryń A, Krajewski A, Szulc D, Litwiniec A
and Grzanka A: Activity of cyclin B1 in HL-60 cells treated with
etoposide. Acta Histochem. 118:537–543. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Shi W, Deng J, Tong R, Yang Y, He X, Lv J,
Wang H, Deng S, Qi P, Zhang D and Wang Y: Molecular mechanisms
underlying mangiferin-induced apoptosis and cell cycle arrest in
A549 human lung carcinoma cells. Mol Med Rep. 13:3423–3432. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Pal-Ghosh R, Xue D, Warburton R, Hill N,
Polgar P and Wilson JL: CDC2 is an important driver of vascular
smooth muscle cell proliferation via FOXM1 and PLK1 in pulmonary
arterial hypertension. Int J Mol Sci. 22:69432021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Gonnella R, Zarrella R, Di Crosta M,
Benedetti R, Arena A, Santarelli R, Gilardini Montani MS, D'Orazi G
and Cirone M: HSP110 inhibition in primary effusion lymphoma cells:
One molecule, many pro-survival targets. Cancers (Basel).
15:56512023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Huysentruyt J, Steels W, Ruiz Perez M,
Verstraeten B, Vadi M, Divert T, Flies K, Takahashi N, Lambrecht
BN, Declercq W, et al: RIPK1 protects naive and regulatory T cells
from TNFR1-induced apoptosis. Cell Death Differ. 31:820–832. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liu Z, Li Y, Zhu Y, Li N, Li W, Shang C,
Song G, Li S, Cong J, Li T, et al: Apoptin induces pyroptosis of
colorectal cancer cells via the GSDME-dependent pathway. Int J Biol
Sci. 18:717–730. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Pan LL, Wang AY, Huang YQ, Luo Y and Ling
M: Mangiferin induces apoptosis by regulating Bcl-2 and bax
expression in the CNE2 nasopharyngeal carcinoma cell line. Asian
Pac J Cancer Prev. 15:7065–7068. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kim H, Kim H, Mosaddik A, Gyawali R, Ahn
KS and Cho SK: Induction of apoptosis by ethanolic extract of mango
peel and comparative analysis of the chemical constitutes of mango
peel and flesh. Food Chem. 133:416–422. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Budakoti M, Panwar AS, Molpa D, Singh RK,
Busselberg D, Mishra AP, Coutinho HDM and Nigam M: Micro-RNA: The
darkhorse of cancer. Cell Signal. 83:1099952021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Maclean JA II, King ML, Okuda H and
Hayashi K: WNT7a regulation by mir-15b in ovarian cancer. PLoS One.
11:e01561092016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Li M, Ma H, Yang L and Li P: Mangiferin
inhibition of proliferation and induction of apoptosis in human
prostate cancer cells is correlated with downregulation of b-cell
lymphoma-2 and upregulation of microRNA-182. Oncol Lett.
11:817–822. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Chi XJ, Meng JJ, Lin CY, Su QS, Qin YY,
Wei RH, Lan D and Huang C: Mangiferin inhibits human lung
adenocarcinoma by suppressing MiR-27b and MiR-92a. Evid Based
Complement Alternat Med. 2021:28229502021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Green DR: The mitochondrial pathway of
apoptosis: Part I: MOMP and beyond. Cold Spring Harb Perspect Biol.
14:a0410382022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Bhat TA, Chaudhary AK, Kumar S, O'Malley
J, Inigo JR, Kumar R, Yadav N and Chandra D: Endoplasmic
reticulum-mediated unfolded protein response and mitochondrial
apoptosis in cancer. Biochim Biophys Acta Rev Cancer. 1867:58–66.
2017. View Article : Google Scholar
|
|
69
|
Rodriguez-Gonzalez JC, Hernández-Balmaseda
I, Declerck K, Pérez-Novo C, Logie E, Theys C, Jakubek P,
Quiñones-Maza OL, Dantas-Cassali G, Carlos Dos Reis D, et al:
Antiproliferative, antiangiogenic, and antimetastatic therapy
response by mangiferin in a syngeneic immunocompetent colorectal
cancer mouse model involves changes in mitochondrial energy
metabolism. Front Pharmacol. 12:6701672021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Rajendran P, Ekambaram G and Sakthisekaran
D: Effect of mangiferin on benzo(a)pyrene induced lung
carcinogenesis in experimental swiss albino mice. Nat Prod Res.
22:672–680. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Tang Z, Lai CC, Luo J, Ding YT, Chen Q and
Guan ZZ: Mangiferin prevents the impairment of mitochondrial
dynamics and an increase in oxidative stress caused by excessive
fluoride in SH-SY5Y cells. J Biochem Mol Toxicol. 35:e227052021.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hajam YA, Rani R, Ganie SY, Sheikh TA,
Javaid D, Qadri SS, Pramodh S, Alsulimani A, Alkhanani MF, Harakeh
S, et al: Oxidative stress in human pathology and aging: molecular
mechanisms and perspectives. Cells. 11:5522022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ren J, Su D, Li L, Cai H, Zhang M, Zhai J,
Li M, Wu X and Hu K: Anti-inflammatory effects of aureusidin in
LPS-stimulated RAW264.7 macrophages via suppressing NF-κB and
activating ROS- and MAPKs-dependent Nrf2/HO-1 signaling pathways.
Toxicol Appl Pharmacol. 387:1148462020. View Article : Google Scholar
|
|
74
|
Qin ZZ, Ruan J, Lee MR, Sun K, Chen P,
Chen Y, Hong M, Xia LH, Fang J and Tang H: Mangiferin promotes
bregs level, activates Nrf2 antioxidant signaling, and inhibits
proinflammatory cytokine expression in murine splenic mononuclear
cells in vitro. Curr Med Sci. 41:454–464. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhao J, Zhang B, Li S, Zeng L, Chen Y and
Fang J: Mangiferin increases nrf2 protein stability by inhibiting
its ubiquitination and degradation in human HL60 myeloid leukemia
cells. Int J Mol Med. 33:1348–1354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kavitha M, Manivasagam T, Essa MM,
Tamilselvam K, Selvakumar GP, Karthikeyan S, Thenmozhi JA and
Subash S: Mangiferin antagonizes rotenone: induced apoptosis
through attenuating mitochondrial dysfunction and oxidative stress
in SK-N-SH neuroblastoma cells. Neurochem Res. 39:668–676. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Walia V, Chaudhary SK and Kumar Sethiya N:
Therapeutic potential of mangiferin in the treatment of various
neuropsychiatric and neurodegenerative disorders. Neurochem Int.
143:1049392021. View Article : Google Scholar
|
|
78
|
Rui R, Zhou L and He S: Cancer
immunotherapies: Advances and bottlenecks. Front Immunol.
14:12124762023. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Yang G, Shang X, Cui G, Zhao L, Zhao H and
Wang N: Mangiferin attenuated diethynitrosamine-induced
hepatocellular carcinoma in sprague-dawley rats via alteration of
oxidative stress and apoptotic pathway. J Environ Pathol Toxicol
Oncol. 38:1–12. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Abdul-Aziz Ahmed K, Jabbar AAJ, Abdulla
MA, Zuhair Alamri Z, Ain Salehen N, Abdel Aziz Ibrahim I, Almaimani
G, Bamagous GA, Almaimani RA, Almasmoum HA, et al: Mangiferin
(mango) attenuates AOM-induced colorectal cancer in rat's colon by
augmentation of apoptotic proteins and antioxidant mechanisms. Sci
Rep. 14:8132024. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Padma VV, Kalaiselvi P, Yuvaraj R and
Rabeeth M: Mangiferin induces cell death against rhabdomyosarcoma
through sustained oxidative stress. Integr Med Res. 4:66–75. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Song J, Li Y, Song J, Hou F, Liu B and Li
A: Mangiferin protects mitochondrial function by preserving
mitochondrial hexokinase-II in vessel endothelial cells. Biochim
Biophys Acta Mol Basis Dis. 1863:1829–1839. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Imran M, Arshad MS, Butt MS, Kwon JH,
Arshad MU and Sultan MT: Mangiferin: A natural miracle bioactive
compound against lifestyle related disorders. Lipids Health Dis.
16:842017. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Trachootham D, Alexandre J and Huang P:
Targeting cancer cells by ROS-mediated mechanisms: A radical
therapeutic approach? Nat Rev Drug Discov. 8:579–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Ismail MB, Rajendran P, Abuzahra HM and
Veeraraghavan VP: Mangiferin inhibits apoptosis in
doxorubicin-induced vascular endothelial cells via the Nrf2
signaling pathway. Int J Mol Sci. 22:42592021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Kucharczyk K, Florczak A, Kaminska A,
Guzniczak N, Sikorska A, Deptuch T and Dams-Kozlowska H:
MMPs-responsive silk spheres for controlled drug release within
tumor microenvironment. Int J Biol Macromol. 269:1320162024.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Chen J, Liu Z, Fang H, Su Q, Fan Y, Song L
and He S: Therapeutic efficacy of a novel self-assembled
immunostimulatory siRNA combining apoptosis promotion with RIG-I
activation in gliomas. J Transl Med. 22:3952024. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Gálvez-Rodríguez A, Ferino-Pérez A,
Rodríguez-Riera Z, Guerra IR and Jáuregui-Haza UJ: In silico
evaluation of new mangiferin-based positron emission tomography
radiopharmaceuticals through the inhibition of metalloproteinase-9.
J Mol Graph Model. 124:1085692023. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Delgado-Hernández R, Hernández-Balmaseda
I, Rodeiro-Guerra I, Cesar Rodriguez Gonzalez J, De Wever O, Logie
E, Declerck K, Pérez-Novo C and Vanden Berghe W: Anti-angiogenic
effects of mangiferin and mechanism of action in metastatic
melanoma. Melanoma Res. 30:39–51. 2020. View Article : Google Scholar
|
|
90
|
Prata C, Angeloni C and Maraldi T:
Strategies to counteract oxidative stress and inflammation in
chronic-degenerative diseases 2.0. Int J Mol Sci. 25:50262024.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ghodsi A, Hidalgo A and Libreros S: Lipid
mediators in neutrophil biology: Inflammation, resolution and
beyond. Curr Opin Hematol. 31:175–192. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Lu C, Deng S, Liu Y, Yang S, Qin D, Zhang
L, Wang RR and Zhang Y: Inhibition of macrophage MAPK/NF-κB pathway
and th2 axis by mangiferin ameliorates MC903-induced atopic
dermatitis. Int Immunopharmacol. 133:1120382024. View Article : Google Scholar
|
|
93
|
Nilkhet S, Mongkolpobsin K,
Sillapachaiyaporn C, Wongsirojkul N, Tencomnao T and Chuchawankul
S: M1 macrophages polarized by crude polysaccharides isolated from
auricularia polytricha exhibit anti-tumor effect on human breast
cancer cells. Sci Rep. 14:81792024. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Li SZ, Shu QP, Zhou HM, Liu YY, Fan MQ,
Liang XY, Qi LZ, He YN, Liu XY, Du XH, et al: CLK2 mediates
IκBα-independent early termination of NF-κB activation by inducing
cytoplasmic redistribution and degradation. Nat Commun.
15:39012024. View Article : Google Scholar
|
|
95
|
Wu Y, Yao X, Shi X, Xu Z, Ren J, Shi M, Li
M, Liu J and Du X: Myeloma extracellular vesicle-derived RAGE
increases inflammatory responses and myotube atrophy in multiple
myeloma through activation of the TLR4/NF-κB p65 pathway.
Apoptosis. 29:849–864. 2024. View Article : Google Scholar
|
|
96
|
Cui Y, Li Z, Ni L, Yu S, Shan X, Hu P, Ji
Z, Jing W, Zhou Y, Wang B, et al: Induction of MTHFD2 in
macrophages inhibits reactive oxygen species-mediated NF-κB
activation and protects against inflammatory responses. J Immunol.
212:1345–1356. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zheng M, Liu W, Zhang R, Jiang D, Shi Y,
Wu Y, Ge F and Chen C: E3 ubiquitin ligase BCA2 promotes breast
cancer stemness by up-regulation of SOX9 by LPS. Int J Biol Sci.
20:2686–2697. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Eisa NH, Helmy SA, El-Kashef DH,
El-Sherbiny M and Elsherbiny NM: Pramipexole protects against
diabetic neuropathy: effect on oxidative stress,
TLR4/IRAK-1/TRAF-6/NF-κB and downstream inflammatory mediators. Int
Immunopharmacol. 128:1115142024. View Article : Google Scholar
|
|
99
|
García-Rivera D, Delgado R, Bougarne N,
Haegeman G and Berghe WV: Gallic acid indanone and mangiferin
xanthone are strong determinants of immunosuppressive anti-tumour
effects of mangifera indica l. Bark in MDA-MB231 breast cancer
cells. Cancer Lett. 305:21–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Dai C, Yu L, Wang Z, Deng P, Li L, Gu Z,
He X, Wang J and Yuan J: Mangiferin and taurine ameliorate MSRV
infection by suppressing NF-κB signaling. Microbiol Spectr.
11:e05146222023. View Article : Google Scholar
|
|
101
|
Gálvez-Rodríguez A, Ferino-Pérez A,
Rodríguez-Riera Z, Rodeiro Guerra I, řeha D, Minofar B and
Jáuregui-Haza UJ: Explaining the interaction of mangiferin with
MMP-9 and NF-κβ: A computational study. J Mol Model. 28:2662022.
View Article : Google Scholar
|
|
102
|
Chen Q, Wang S, Bao R, Wang D, Wu Y, Zhang
Y, Liu M and Wang T: Combination of mangiferin and T0901317
targeting autophagy promotes cholesterol efflux from macrophage
foam cell in atherosclerosis. Chin Med. 19:52024. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zhu P, Liu C, Li B, Zhao C, Zhou T, Xue X
and Zhang B: Mangiferin attenuates IL-1β-induced chondrocytes
apoptosis. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 46:25–31. 2021.In
Chinese, English. PubMed/NCBI
|
|
104
|
Deng Q, Tian YX and Liang J: Mangiferin
inhibits cell migration and invasion through Rac1/WAVE2 signalling
in breast cancer. Cytotechnology. 70:593–601. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Shang HS, Chen CJ, Shih YL, Peng SF, Chen
YL, Liu KC, Huang HC, Hsueh SC, Chen KW, Lu HF, et al: Mangiferin
induces immune responses and evaluates the survival rate in WEHI-3
cell generated mouse leukemia in vivo. Environ Toxicol. 36:77–85.
2021. View Article : Google Scholar
|
|
106
|
Jeong JJ, Jang SE, Hyam SR, Han MJ and Kim
DH: Mangiferin ameliorates colitis by inhibiting IRAK1
phosphorylation in NF-κB and MAPK pathways. Eur J Pharmacol.
740:652–661. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Kammalla AK, Ramasamy MK, Inampudi J,
Dubey GP, Agrawal A and Kaliappan I: Comparative pharmacokinetic
study of mangiferin after oral administration of pure mangiferin
and US patented polyherbal formulation to rats. AAPS PharmSciTech.
16:250–258. 2015. View Article : Google Scholar :
|
|
108
|
Reddeman RA, Glávits R, Endres JR, Clewell
AE, Hirka GB, Vértesi A, Béres E and Szakonyiné IP: A toxicological
evaluation of mango leaf extract (mangifera indica) containing 60%
mangiferin. J Toxicol. 2019:47630152019. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zhang Q, Kong X, Yuan H, Guan H, Li Y and
Niu Y: Mangiferin improved palmitate-induced-insulin resistance by
promoting free fatty acid metabolism in HepG2 and c2c12 cells via
PPARα: mangiferin improved insulin resistance. J Diabetes Res.
2019:20526752019. View Article : Google Scholar
|
|
110
|
Jeyakodi S, Krishnakumar A, Dalal M and
Shetty BS: Assessment of efficacy and safety of mangifera indica
extract (stadice®) for cognitive function: A randomized,
double-blind, placebo-controlled study. Cureus. 16:e657512024.
|
|
111
|
Bulugonda RK, Kumar KA, Gangappa D, Beeda
H, Philip GH, Muralidhara Rao D and Faisal SM: Mangiferin from
pueraria tuberosa reduces inflammation via inactivation of NLRP3
inflammasome. Sci Rep. 7:426832017. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Jyotshna, Khare P and Shanker K:
Mangiferin: A review of sources and interventions for biological
activities. Biofactors. 42:504–514. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Irshad N, Naeem H, Shahbaz M, Imran M,
Mujtaba A, Hussain M, Al Abdulmonem W, Alsagaby SA, Yehuala TF,
Abdelgawad MA, et al: Mangiferin: An effective agent against human
malignancies. Food Sci Nutr. 12:7137–7157. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Prado Y, Merino N, Acosta J, Herrera JA,
Luque Y, Hernández I, Prado E, Garrido G, Delgado R and Rodeiro I:
Acute and 28-day subchronic toxicity studies of mangiferin, a
glucosyl xanthone isolated from mangifera indica L. Stem bark. J
Pharm Pharmacogn Res. 3:13–23. 2015. View Article : Google Scholar
|
|
115
|
Feng ST, Wang ZZ, Yuan YH, Sun HM, Chen NH
and Zhang Y: Mangiferin: a multipotent natural product preventing
neurodegeneration in Alzheimer's and Parkinson's disease models.
Pharmacol Res. 146:1043362019. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Santonocito D, Vivero-Lopez M, Lauro MR,
Torrisi C, Castelli F, Sarpietro MG and Puglia C: Design of
nanotechnological carriers for ocular delivery of mangiferin:
Preformulation study. Molecules. 27:13282022. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Suchal K, Malik S, Khan SI, Malhotra RK,
Goyal SN, Bhatia J, Kumari S, Ojha S and Arya DS: Protective effect
of mangiferin on myocardial ischemia-reperfusion injury in
streptozotocin-induced diabetic rats: Role of AGE-RAGE/MAPK
pathways. Sci Rep. 7:420272017. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Espinosa-Espinosa L, Garduño-Siciliano L,
Rodriguez-Canales M, Hernandez-Portilla LB, Canales-Martinez MM and
Rodriguez-Monroy MA: The wound-healing effect of mango peel extract
on incision wounds in a murine model. Molecules. 27:2592022.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Pal PB, Sinha K and Sil PC: Mangiferin, a
natural xanthone, protects murine liver in Pb(II) induced hepatic
damage and cell death via MAP kinase, NF-κB and mitochondria
dependent pathways. PLoS One. 8:e568942013. View Article : Google Scholar
|
|
120
|
Chen X and Huang J: Mangiferin inhibits
hypoxia/reoxygenation-induced alveolar epithelial cell injury via
the SIRT1/AMPK signaling pathway. Exp Ther Med. 22:12202021.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Sadhukhan P, Saha S, Dutta S and Sil PC:
Mangiferin ameliorates cisplatin induced acute kidney injury by
upregulating Nrf-2 via the activation of PI3K and exhibits
synergistic anticancer activity with cisplatin. Front Pharmacol.
9:6382018. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Li W, Wang K, Liu Y, Wu H, He Y, Li C,
Wang Q, Su X, Yan S, Su W, et al: A novel drug combination of
mangiferin and cinnamic acid alleviates rheumatoid arthritis by
inhibiting TLR4/NFκB/NLRP3 activation-induced pyroptosis. Front
Immunol. 13:9129332022. View Article : Google Scholar
|
|
123
|
Radu AF and Bungau SG: Management of
rheumatoid arthritis: An overview. Cells. 10:28572021. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Yong Z, Ruiqi W, Hongji Y, Ning M, Chenzuo
J, Yu Z, Zhixuan X, Qiang L, Qibing L, Weiying L and Xiaopo Z:
Mangiferin ameliorates HFD-induced NAFLD through regulation of the
AMPK and NLRP3 inflammasome signal pathways. J Immunol Res.
2021:40845662021. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Wang H, Zhu YY, Wang L, Teng T, Zhou M,
Wang SG, Tian YZ, Du L, Yin XX and Sun Y: Mangiferin ameliorates
fatty liver via modulation of autophagy and inflammation in
high-fat-diet induced mice. Biomed Pharmacother. 96:328–335. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Li N, Xiong R, He R, Liu B, Wang B and
Geng Q: Mangiferin mitigates lipopolysaccharide-induced lung injury
by inhibiting NLRP3 inflammasome activation. J Inflamm Res.
14:2289–2300. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Saccà SC, Cutolo CA, Ferrari D, Corazza P
and Traverso CE: The eye, oxidative damage and polyunsaturated
fatty acids. Nutrients. 10:6682018. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Kim SJ, Sung MS, Heo H, Lee JH and Park
SWO: Mangiferin protects retinal ganglion cells in ischemic mouse
retina via SIRT1. Curr Eye Res. 41:844–855. 2016. View Article : Google Scholar
|
|
129
|
Fung TH, Patel B, Wilmot EG and Amoaku WM:
Diabetic retinopathy for the non-ophthalmologist. Clin Med (Lond).
22:112–116. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Shi J, Lv H, Tang C, Li Y, Huang J and
Zhang H: Mangiferin inhibits cell migration and angiogenesis via
PI3k/AKT/mTOR signaling in high glucose- and hypoxia-induced
RRCECs. Mol Med Rep. 23:4732021. View Article : Google Scholar :
|
|
131
|
Li X, Cui X, Wang J, Yang J, Sun X, Li X,
Zhu Q and Li W: Rhizome of Anemarrhena asphodeloides counteracts
diabetic ophthalmopathy progression in streptozotocin-induced
diabetic rats. Phytother Res. 27:1243–1250. 2013. View Article : Google Scholar
|
|
132
|
Hayes MT: Parkinson's disease and
parkinsonism. Am J Med. 132:802–807. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Li Y, Xia Y, Yin S, Wan F, Hu J, Kou L,
Sun Y, Wu J, Zhou Q, Huang J, et al: Targeting microglial
α-synuclein/TLRs/NF-kappaB/NLRP3 inflammasome axis in Parkinson's
disease. Front Immunol. 12:7198072021. View Article : Google Scholar
|
|
134
|
Chu YC, Yang CS, Cheng MJ, Fu SL and Chen
JJ: Comparison of various solvent extracts and major bioactive
components from unsalt-fried and salt-fried rhizomes of anemarrhena
asphodeloides for antioxidant, anti-α-glucosidase, and
anti-acetylcholinesterase activities. Antioxidants (Basel).
11:3852022. View Article : Google Scholar
|
|
135
|
Chen M, Wang Z, Zhou W, Lu C, Ji T, Yang
W, Jin Z, Tian Y, Lei W, Wu S, et al: SIRT1/PGC-1α signaling
activation by mangiferin attenuates cerebral hypoxia/reoxygenation
injury in neuroblastoma cells. Eur J Pharmacol. 907:1742362021.
View Article : Google Scholar
|
|
136
|
Arora MK, Kisku A and Jangra A: Mangiferin
ameliorates intracerebroventricular-quinolinic acid-induced
cognitive deficits, oxidative stress, and neuroinflammation in
wistar rats. Indian J Pharmacol. 52:296–305. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Liu T, Song Y and Hu A: Neuroprotective
mechanisms of mangiferin in neurodegenerative diseases. Drug Dev
Res. 82:494–502. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Du Z, Fanshi F, Lai YH, Chen JR, Hao E,
Deng J and Hsiao CD: Mechanism of anti-dementia effects of
mangiferin in a senescence accelerated mouse (SAMP8) model. Biosci
Rep. 39:BSR201904882019. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
López-Ríos L, Wiebe JC, Vega-Morales T and
Gericke N: Central nervous system activities of extract mangifera
indica L. J Ethnopharmacol. 260:1129962020. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Ho HJ and Shirakawa H: Oxidative stress
and mitochondrial dysfunction in chronic kidney disease. Cells.
12:882022. View Article : Google Scholar
|
|
141
|
Wang B, Wan J, Gong X, Kuang G, Cheng X
and Min S: Mangiferin attenuates renal ischemia-reperfusion injury
by inhibiting inflammation and inducing adenosine production. Int
Immunopharmacol. 25:148–154. 2015. View Article : Google Scholar
|
|
142
|
Zhang D, Han S, Zhou Y, Qi B and Wang X:
Therapeutic effects of mangiferin on sepsis-associated acute lung
and kidney injuries via the downregulation of vascular permeability
and protection of inflammatory and oxidative damages. Eur J Pharm
Sci. 152:1054002020. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Samsu N: Diabetic nephropathy: Challenges
in pathogenesis, diagnosis, and treatment. Biomed Res Int.
2021:14974492021. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Xu GK, Sun CY, Qin XY, Han Y, Li Y, Xie GY
and Min-Jian Q: Effects of ethanol extract of bombax ceiba leaves
and its main constituent mangiferin on diabetic nephropathy in
mice. Chin J Nat Med. 15:597–605. 2017.PubMed/NCBI
|
|
145
|
Wang X, Gao L, Lin H, Song J, Wang J, Yin
Y, Zhao J, Xu X, Li Z and Li L: Mangiferin prevents diabetic
nephropathy progression and protects podocyte function via
autophagy in diabetic rat glomeruli. Eur J Pharmacol. 824:170–178.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Chen L, Li S, Zhu J, You A, Huang X, Yi X
and Xue M: Mangiferin prevents myocardial infarction-induced
apoptosis and heart failure in mice by activating the Sirt1/FoxO3a
pathway. J Cell Mol Med. 25:2944–2955. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Han J, Yang N, Zhang F, Zhang C, Liang F,
Xie W and Chen W: Rhizoma anemarrhenae extract ameliorates
hyperglycemia and insulin resistance via activation of
AMP-activated protein kinase in diabetic rodents. J Ethnopharmacol.
172:368–376. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Wang Y, Karmakar T, Ghosh N, Basak S and
Gopal Sahoo N: Targeting mangiferin loaded n-succinyl
chitosan-alginate grafted nanoparticles against atherosclerosis-a
case study against diabetes mediated hyperlipidemia in rat. Food
Chem. 370:1313762022. View Article : Google Scholar
|
|
149
|
Hou J, Zheng D, Fung G, Deng H, Chen L,
Liang J, Jiang Y and Hu Y: Mangiferin suppressed advanced glycation
end products (AGEs) through NF-κB deactivation and displayed
anti-inflammatory effects in streptozotocin and high fat
diet-diabetic cardiomyopathy rats. Can J Physiol Pharmacol.
94:332–340. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Zhao Y, Wang W, Wu X, Ma X, Qu R, Chen X,
Liu C, Liu Y, Wang X, Yan P, et al: Mangiferin antagonizes
TNF-α-mediated inflammatory reaction and protects against
dermatitis in a mice model. Int Immunopharmacol. 45:174–179. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Gunter NV, Teh SS, Lim YM and Mah SH:
Natural xanthones and skin inflammatory diseases: Multitargeting
mechanisms of action and potential application. Front Pharmacol.
11:5942022020. View Article : Google Scholar
|
|
152
|
Kumar M, Saurabh V, Tomar M, Hasan M,
Changan S, Sasi M, Maheshwari C, Prajapati U, Singh S, Prajapat RK,
et al: Mango (mangifera indica l.) Leaves: nutritional composition,
phytochemical profile, and health-promoting bioactivities.
Antioxidants (Basel). 10:2292021.
|
|
153
|
Ouf SA, Galal AMF, Ibrahim HS, Hassan AZ,
Mekhael MKG, El-Yasergy KF, El-Ghany MNA, Rizk MA and Hanna AG:
Phytochemical and antimicrobial investigation of the leaves of five
egyptian mango cultivars and evaluation of their essential oils as
preservatives materials. J Food Sci Technol. 58:3130–3142. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Saifi S, Ashraf A, Hasan GM, Shamsi A and
Hassan MI: Insights into the preventive actions of natural
compounds against klebsiella pneumoniae infections and drug
resistance. Fitoterapia. 173:1058112024. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Li H, Wang Q, Ding Y, Bao C and Li W:
Mangiferin ameliorates porphyromonas gingivalis-induced
experimental periodontitis by inhibiting phosphorylation of nuclear
factor-κB and janus kinase 1-signal transducer and activator of
transcription signaling pathways. J Periodontal Res. 52:1–7. 2017.
View Article : Google Scholar
|
|
156
|
Angusamy A, Balasubramanian V, Arunmurugan
B, Arunachalam K, Issac Abraham SVP, Murugesan S, Krishnasamy B,
Sundaram J and Arumugam VR: Anti-infective potential of
plant-derived quorum sensing inhibitors against multi-drug
resistant human and aquatic bacterial pathogens. World J Microbiol
Biotechnol. 39:1472023. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Ma H, Chen H, Sun L, Tong L and Zhang T:
Improving permeability and oral absorption of mangiferin by
phospholipid complexation. Fitoterapia. 93:54–61. 2014. View Article : Google Scholar
|
|
158
|
Pacheco-Ordaz RN, Antunes-Ricardo M,
Gutiérrez-Uribe JA and González-Aguilar GA: Intestinal permeability
and cellular antioxidant activity of phenolic compounds from mango
(mangifera indica cv. Ataulfo) peels. Int J Mol Sci. 19:5142018.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Hou S, Wang F, Li Y, Li Y, Wang M, Sun D
and Sun C: Pharmacokinetic study of mangiferin in human plasma
after oral administration. Food Chem. 132:289–294. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Bunt D, Schwalbe M, Hayeeawaema F and El
Aidy S: Gut microbiota-mediated conversion of mangiferin to
norathyriol alters short chain fatty acid and urate metabolism. Gut
Microbes. 17:25084222025. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Barakat S, Nasr M, Ahmed RF, Badawy S and
Mortada N: Recent formulation advances of mangiferin. Rev Bras
Farmacogn. 32:871–882. 2022. View Article : Google Scholar
|
|
162
|
Guo X, Cheng M, Hu P, Shi Z, Chen S, Liu
H, Shi H, Xu Z, Tian X and Huang C: Absorption, metabolism, and
pharmacokinetics profiles of norathyriol, an aglycone of
mangiferin, in rats by HPLC-MS/MS. J Agric Food Chem.
66:12227–12235. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Lin A, Li J, Li D, Jin H and Liu Y: Tissue
distribution study of mangiferin after intragastric administration
of mangiferin monomer, rhizoma anemarrhenae, and rhizoma
anemarrhenae-phellodendron decoctions in normal or type 2 diabetic
rats by LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci.
1122-1123:18–28. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Al-Madhagi H: From nature to
nanotechnology: The bioactivities of mangiferin explored.
Nanotechnol Sci Appl. 18:277–294. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Shaikh I and Tatapudi HK: A review on
formulation and analytical aspect of mangiferin a natural phenolic
xanthonoid. Discov Chem. 2:1152025. View Article : Google Scholar
|
|
166
|
Fuentes-Rios D, Sanchez-Rodriguez A,
Lopez-Rios L, Garcia-Gonzalez E, Martinez-Canton M, Galvan-Alvarez
V, Gallego-Selles A, Martin-Rincon M, Calbet JAL and Vega-Morales
T: Human pharmacokinetic profiling and comparative analysis of
mangiferin and its monosodium derivative from mangifera indica
extracts using UHPLC-MS/MS with (1)h NMR and MALDI-TOF
confirmation. Molecules. 30:4612025. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Zhou H, Song S, Lan X, Li Y, Yuan X, Yang
J, Li M, Cao T and Zhang J: Comprehensive profiling of mangiferin
metabolites in vivo and in vitro based on the 'drug metabolite
clusters' analytical strategy. ACS Omega. 8:9934–9946. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
168
|
van der Merwe JD, Joubert E, Manley M, de
Beer D, Malherbe CJ and Gelderblom WCA: Mangiferin glucuronidation:
Important hepatic modulation of antioxidant activity. Food Chem
Toxicol. 50:808–815. 2012. View Article : Google Scholar
|
|
169
|
Souza JR, Trevisan MTS, Feitosa JP,
Ricardo NGM, Hull WE, Erben G, Würtele G, Breuer A, Frei E and
Ulrich CM: Transformation of mangiferin to norathyriol by human
fecal matrix in anaerobic conditions: Comprehensive NMR of the
xanthone metabolites, antioxidant capacity, and comparative
cytotoxicity against cancer cell lines. Nat Prod Commun. Mar
5–2020.Epub ahead of print.
|
|
170
|
Sarfraz M, Khan A, Batiha GE, Akhtar MF,
Saleem A, Ajiboye BO, Kamal M, Ali A, Alotaibi NM, Aaghaz S, et al:
Nanotechnology-based drug delivery approaches of mangiferin:
Promises, reality and challenges in cancer chemotherapy. Cancers
(Basel). 15:41942023. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Shaikenov RO, Klimshina VI, Morozkina SN
and Snetkov PP: Polymeric matrices for mangiferin delivery: Ways to
enhance bioavailability and therapeutic effect. Eng Proc.
87:782025.
|
|
172
|
Razura-Carmona FF, Pérez-Larios A,
González-Silva N, Herrera-Martínez M, Medina-Torres L,
Sáyago-Ayerdi SG and Sánchez-Burgos JA: Mangiferin-loaded polymeric
nanoparticles: Optical characterization, effect of
anti-topoisomerase I, and cytotoxicity. Cancers (Basel).
11:19652019. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Samadarsi R and Dutta D: Anti-oxidative
effect of mangiferin-chitosan nanoparticles on oxidative
stress-induced renal cells. Int J Biol Macromol. 151:36–46. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Zhang Y, Wang Y, Li X, Nie D, Liu C and
Gan Y: Ligand-modified nanocarriers for oral drug delivery:
Challenges, rational design, and applications. J Control Release.
352:813–832. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Ejazi SA, Louisthelmy R and Maisel K:
Mechanisms of nanoparticle transport across intestinal tissue: An
oral delivery perspective. ACS Nano. 17:13044–13061. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Wang H, Shao W, Lu X, Gao C, Fang L, Yang
X and Zhu P: Synthesis, characterization, and in vitro anti-tumor
activity studies of the hyaluronic acid-mangiferin-methotrexate
nanodrug targeted delivery system. Int J Biol Macromol.
239:1242082023. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Guo H, Chen M, Li M, Hu M, Chen B and Zhou
C: Pharmacokinetic comparisons of mangiferin and mangiferin
monosodium salt in rat plasma by UPLC-MS/MS. J Chem. Nov
14–2019.Epub ahead of print. View Article : Google Scholar
|
|
178
|
Lin H, Teng H, Wu W, Li Y, Lv G, Huang X,
Yan W and Lin Z: Pharmacokinetic and metabolomic analyses of
mangiferin calcium salt in rat models of type 2 diabetes and
non-alcoholic fatty liver disease. BMC Pharmacol Toxicol.
21:592020. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Faria M, Björnmalm M, Thurecht KJ, Kent
SJ, Parton RG, Kavallaris M, Johnston APR, Gooding JJ, Corrie SR,
Boyd BJ, et al: Minimum information reporting in bio-nano
experimental literature. Nat Nanotechnol. 13:777–785. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Sellamuthu PS, Muniappan BP, Perumal SM
and Kandasamy M: Antihyperglycemic effect of mangiferin in
streptozotocin induced diabetic rats. J Health Sci. 55:206–214.
2009. View Article : Google Scholar
|
|
181
|
Rodeiro I, José Gómez-Lechón M, Perez G,
Hernandez I, Herrera JA, Delgado R, Castell JV and Teresa Donato M:
Mangifera indica L. Extract and mangiferin modulate cytochrome p450
and UDP-glucuronosyltransferase enzymes in primary cultures of
human hepatocytes. Phytother Res. 27:745–752. 2013. View Article : Google Scholar
|
|
182
|
Sun D, Zhang CZ, Ran RX, Cao YF, Du Z, Fu
ZW, Huang CT, Zhao ZY, Zhang WH and Fang ZZ: In vitro comparative
study of the inhibitory effects of mangiferin and its aglycone
norathyriol towards UDP-glucuronosyl transferase (UGT) isoforms.
Molecules. 22:10082017. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Chieli E, Romiti N, Rodeiro I and Garrido
G: In vitro effects of mangifera indica and polyphenols derived on
ABCB1/p-glycoprotein activity. Food Chem Toxicol. 47:2703–2710.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Shang Y, Tian J, Zhang Z, Luo L, Saeheng
S, Qiu S, Zhou R, Chen J and Li L: Mangiferin: Sources,
anti-inflammatory activities, and molecular mechanisms. J Agric
Food Chem. 73:27145–27160. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Na L, Zhang Q, Jiang S, Du S, Zhang W, Li
Y, Sun C and Niu Y: Mangiferin supplementation improves serum lipid
profiles in overweight patients with hyperlipidemia: A double-blind
randomized controlled trial. Sci Rep. 5:103442015. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Peng ZG, Yao YB, Yang J, Tang YL and Huang
X: Mangiferin induces cell cycle arrest at G2/M phase through
ATR-Chk1 pathway in HL-60 leukemia cells. Genet Mol Res.
14:4989–5002. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Li J, Liu M, Yu H, Wang W, Han L, Chen Q,
Ruan J, Wen S, Zhang Y and Wang T: Mangiferin improves hepatic
lipid metabolism mainly through its metabolite-norathyriol by
modulating SIRT-1/AMPK/SREBP-1c signaling. Front Pharmacol.
9:2012018. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Bhatia J, Suchal K, Bhargava P, Malik S
and Arya D: Evaluation of the cardioprotective effect of mangiferin
in an experimental model of ischemia reperfusion injury. J
Hypertens. 39:e2802021. View Article : Google Scholar
|
|
189
|
Zivković J, Kumar KA, Rushendran R, Ilango
K, Fahmy NM, El-Nashar HAS, El-Shazly M, Ezzat SM, Melgar-Lalanne
G, Romero-Montero A, et al: Pharmacological properties of
mangiferin: Bioavailability, mechanisms of action and clinical
perspectives. Naunyn Schmiedebergs Arch Pharmacol. 397:763–781.
2024. View Article : Google Scholar
|
|
190
|
Ren K, Li H, Zhou HF, Liang Y, Tong M,
Chen L, Zheng XL and Zhao GJ: Mangiferin promotes macrophage
cholesterol efflux and protects against atherosclerosis by
augmenting the expression of ABCA1 and ABCG1. Aging (Albany NY).
11:10992–11009. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Minniti G, Laurindo LF, Machado NM, Duarte
LG, Guiguer EL, Araujo AC, Dias JA, Lamas CB, Nunes YC, Bechara MD,
et al: Mangifera indica L., By-products, and mangiferin on
cardio-metabolic and other health conditions: A systematic review.
Life (Basel). 13:22702023.PubMed/NCBI
|
|
192
|
Shen JM, Xu L, Lu Y, Cao HM, Xu ZG, Chen T
and Zhang HX: Chitosan-based luminescent/magnetic hybrid nanogels
for insulin delivery, cell imaging, and antidiabetic research of
dietary supplements. Int J Pharm. 427:400–409. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Khurana RK, Bansal AK, Beg S, Burrow AJ,
Katare OP, Singh KK and Singh B: Enhancing biopharmaceutical
attributes of phospholipid complex-loaded nanostructured lipidic
carriers of mangiferin: Systematic development, characterization
and evaluation. Int J Pharm. 518:289–306. 2017. View Article : Google Scholar
|
|
194
|
Zhao C, Pu Z, Gao J, Liu C, Xing J, Lang
W, Chen J, Yuan C and Zhou C: 'Multiomics' analyses combined with
systems pharmacology reveal the renoprotection of mangiferin
monosodium salt in rats with diabetic nephropathy: Focus on
improvements in renal ferroptosis, renal inflammation, and podocyte
insulin resistance. J Agric Food Chem. 71:358–381. 2023. View Article : Google Scholar
|
|
195
|
Vishwakarma KK, Hafeez A, Usmani SA, Noor
L and Khan IR: Nanocarrier-based delivery approaches of mangiferin:
an updated review on leveraging biopharmaceutical characteristics
of the bioactive. Curr Pharm Biotechnol. Sep 23–2024.Epub ahead of
print. PubMed/NCBI
|
|
196
|
Melo-Betances E, Rodríguez-Bautista CC and
Núñez-Sellés AJ: Synthesis of mangiferin derivatives, complexes,
and carriers as potential therapeutic candidates for cancer
treatment: An update. Front Pharmacol. 16:15987192025. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Wondrak GT: Redox-directed cancer
therapeutics: Molecular mechanisms and opportunities. Antioxid
Redox Signal. 11:3013–3069. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Li L, Dong Y, Liu X and Wang M: Mangiferin
for the management of liver diseases: A review. Foods. 12:24692023.
View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Mei S, Ma H and Chen X: Anticancer and
anti-inflammatory properties of mangiferin: A review of its
molecular mechanisms. Food Chem Toxicol. 149:1119972021. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
du Plessis-Stoman D, du Preez J and van de
Venter M: Combination treatment with oxaliplatin and mangiferin
causes increased apoptosis and downregulation of NFκB in cancer
cell lines. Afr J Tradit Complement Altern Med. 8:177–184.
2011.
|
|
201
|
Mu F, Liu T, Zheng H, Xie X, Lei T, He X,
Du S, Tong R and Wang Y: Mangiferin induces radiosensitization in
glioblastoma cells by inhibiting nonhomologous end joining. Oncol
Rep. 40:3663–3673. 2018.PubMed/NCBI
|
|
202
|
Wei Z, Yan L, Chen Y, Bao C and Deng J and
Deng J: Mangiferin inhibits macrophage classical activation via
downregulating interferon regulatory factor 5 expression. Mol Med
Rep. 14:1091–1098. 2016. View Article : Google Scholar : PubMed/NCBI
|