Musculoskeletal crosstalk is a fundamental
physiological process that maintains human health, involving the
dynamic interaction and regulation between bone and muscle through
intricate molecular signaling mechanisms (1). Myokines such as insulin-like growth
factor-1 (IGF-1) and fibroblast growth factor-2 (FGF-2) are pivotal
in mediating this crosstalk (2).
IGF-1 regulates muscle growth and metabolism by promoting myocyte
proliferation and repair, while FGF-2 plays a critical role in
muscle regeneration and repair (3). Notably, both IGF-1 and FGF-2 not
only regulate muscle growth but also markedly influence bone
metabolism, bone density maintenance and the repair and
regeneration of bone tissue (4).
This inter-system regulatory mechanism highlights the importance of
musculoskeletal crosstalk as a critical area of research (5). Recent developments in intercellular
communication have identified macrophage-derived exosomes as
emerging signaling mediators, gaining increasing attention in the
scientific community (6).
Exosomes are small extracellular vesicles secreted by cells that
carry a range of bioactive molecules, including proteins, RNAs, and
lipids, facilitating intercellular communication and the regulation
of cellular functions and tissue physiology (7).
Macrophages regulate musculoskeletal interactions
through the secretion of exosomes (8). Exosomes derived from M1 and M2
macrophages exhibit distinct effects on musculoskeletal metabolism
(Tables I and II) (9-13). M1 macrophages, which are
pro-inflammatory, release exosomes enriched with pro-inflammatory
cytokines that can stimulate osteoclastic bone resorption,
potentially contributing to osteoporosis and bone damage. By
contrast, M2 macrophages, which possess anti-inflammatory
properties, secrete exosomes containing anti-inflammatory factors
that promote bone formation and repair. Macrophages modulate
muscle-bone signaling through the secretion of specific exosome
subtypes. Exosome-associated factors like IGF-1 and FGF-2 regulate
bone metabolic homeostasis, either promoting or inhibiting bone
remodeling.
The present review aimed to synthesize recent
insights into macrophage-derived exosomes and their role in
regulating musculoskeletal crosstalk, with a focus on their
involvement in IGF-1- and FGF-2-mediated signaling pathways. It
examined how exosomes influence the functions of these key factors
in bone metabolism, elucidated their roles in muscle and bone
homeostasis and suggested future research avenues. Additionally, it
sought to provide a theoretical framework for the development of
therapeutic strategies targeting exosomes, particularly for the
treatment of bone metabolic disorders and musculoskeletal diseases
(14).
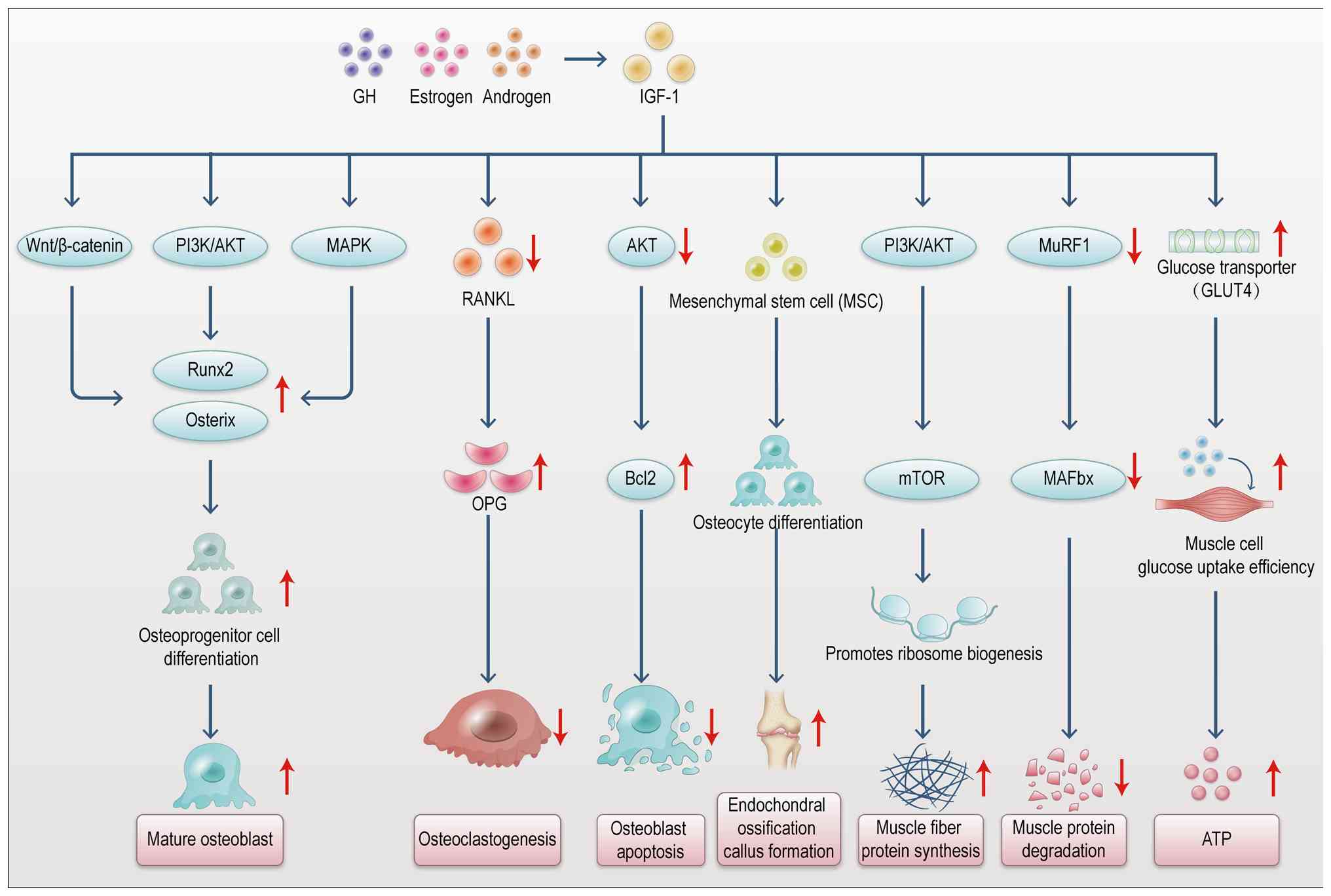
Within the musculoskeletal system, muscle-derived
factors such as IGF-1 and FGF-2 are essential in mediating tissue
crosstalk (Table III)
(15-18). These factors coordinate the
development and repair of both bone and muscle by regulating their
bidirectional interactions. IGF-1 is a pivotal growth factor that
promotes muscle cell proliferation and differentiation while also
exerting significant regulatory effects on the skeletal system
(19). Binding to its receptor
activates the PI3K/Akt pathway, fostering muscle growth and
enhancing bone mineralization and density, thereby serving as a
critical link between bone and muscle (20). FGF-2 is a key regulator of
fibroblasts and bone marrow-derived cells, playing an indispensable
role in bone repair and muscle regeneration (21). Upon binding to fibroblast growth
factor receptors (FGFRs), FGF-2 activates downstream pathways,
including MAPK/ERK and PI3K/Akt, that promote the growth and
differentiation of osteoblasts and myocytes (22). Notably, FGF-2 supports effective
repair of muscle and bone tissues following exercise or trauma.
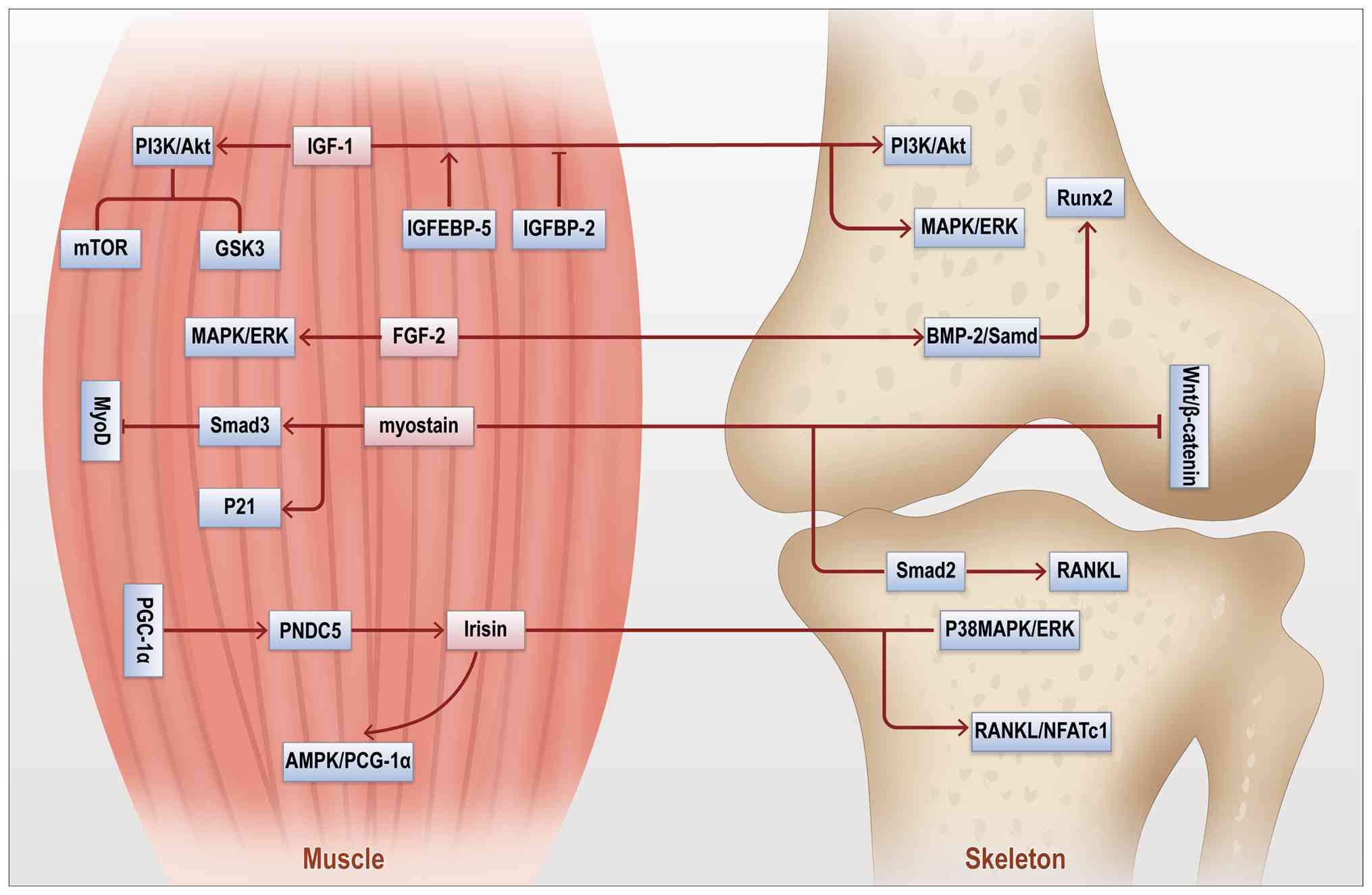
Beyond mechanical signals, endocrine factors also
play a vital role in musculoskeletal crosstalk (31). Muscles and bones mutually
regulate and support each other via various endocrine factors,
including IGF-1, growth hormone, sex hormones and FGF-2 (Fig. 2) (32). During growth, IGF-1 secreted by
muscles promotes muscle development and, via circulation, also
affects the skeletal system, facilitating bone formation (33). Binding to the IGF-1 receptor
(IGF-1R) in bone activates the PI3K-Akt and MAPK pathways,
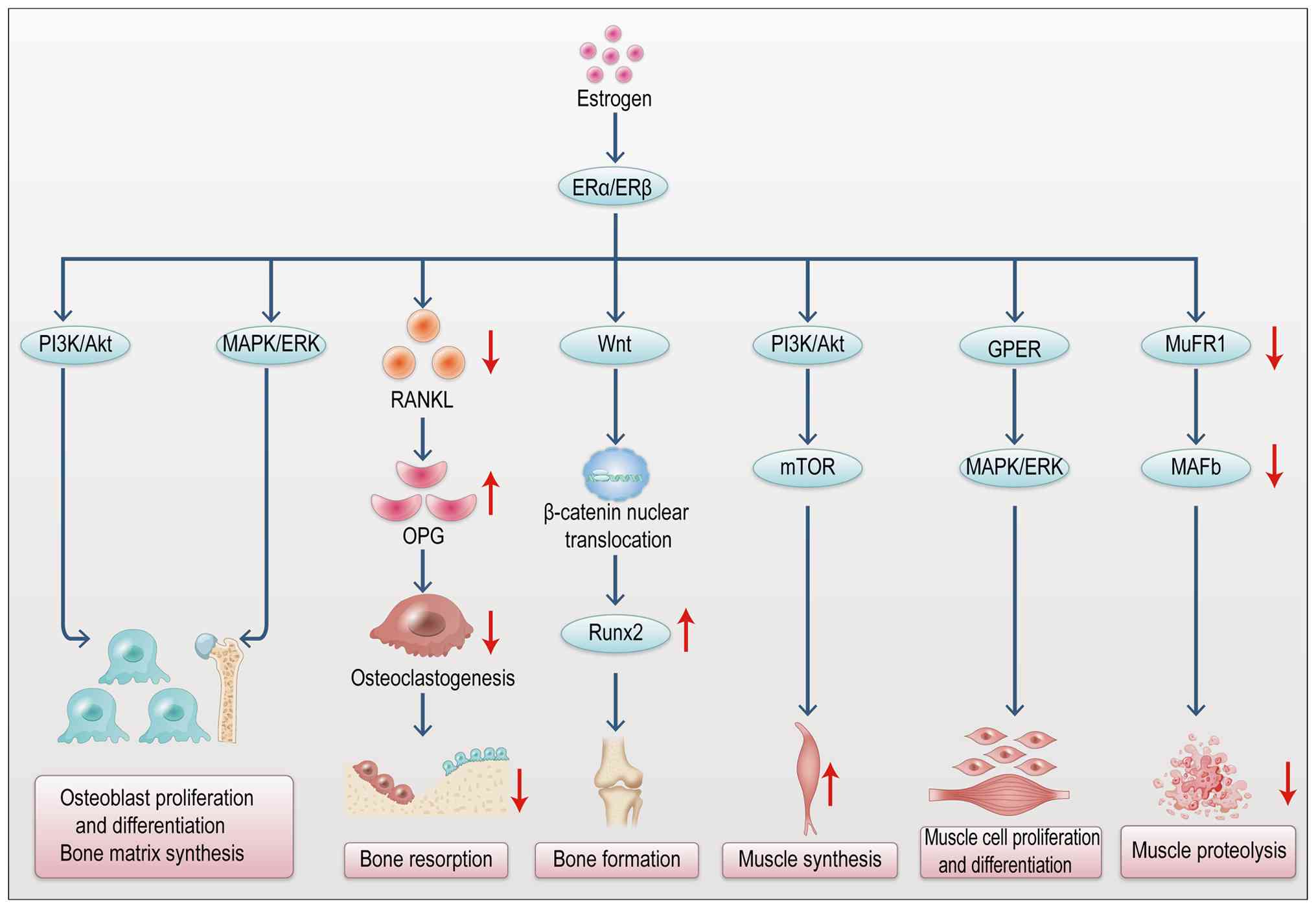
regulating osteoblast proliferation and differentiation (34). Sex hormones, particularly
estrogen, are critical in regulating muscle-bone interactions
(35). Estrogen maintains bone
density and mass by promoting bone formation and inhibiting
resorption, while also enhancing musculoskeletal synergy by
regulating muscle strength and endurance (Fig. 3) (35). FGF-2, as an endocrine factor in
bone, contributes to bone repair and regulates growth and
mineralization by binding to FGFRs (Fig. 2) (36). Particularly during aging or
following fracture, FGF-2 supports bone repair by stimulating
osteoblast proliferation and differentiation (37).
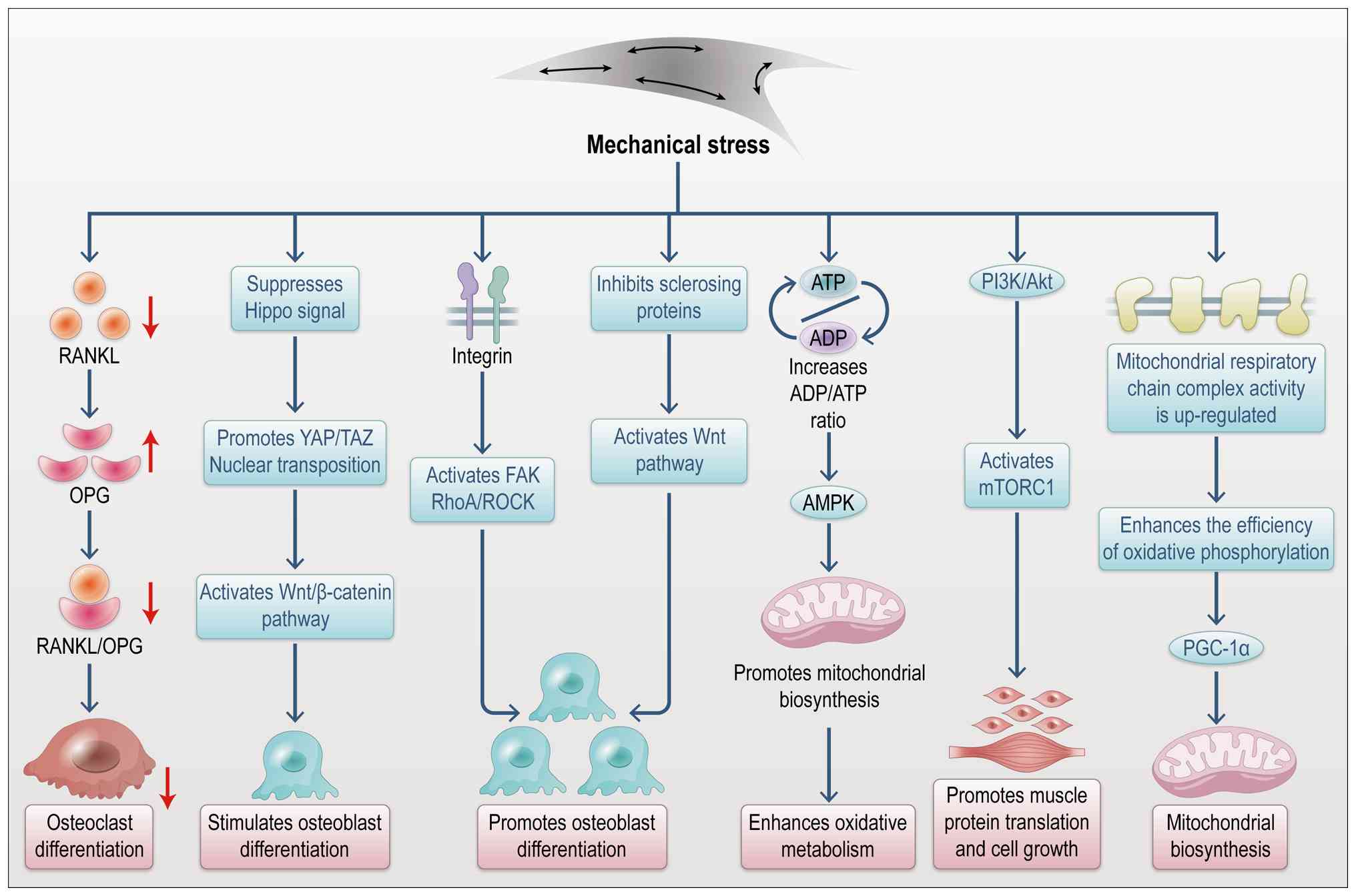
As a pivotal growth factor in muscle-bone
communication, IGF-1 regulates musculoskeletal crosstalk through
multiple signaling pathways (Fig.
4) (38). Upon binding to
its receptor, IGF-1 activates the PI3K/Akt pathway, promoting
muscle fiber proliferation and repair while modulating bone cell
metabolism (39). In the
skeletal system, the PI3K/Akt pathway influences osteoblast and
osteoclast activity via downstream mTOR signaling, thus regulating
bone formation and resorption (40). Activation of PI3K/Akt leads to
Akt kinase phosphorylation, which in turn activates proteins
involved in cell growth and metabolism, promoting proliferation,
survival and differentiation (41). In bone, the PI3K/Akt pathway is
essential for bone formation by stimulating osteoblast
proliferation and differentiation, while enhancing bone matrix
mineralization (42). IGF-1
activation of this pathway improves bone density and accelerates
fracture healing, highlighting its role in bone repair (43).
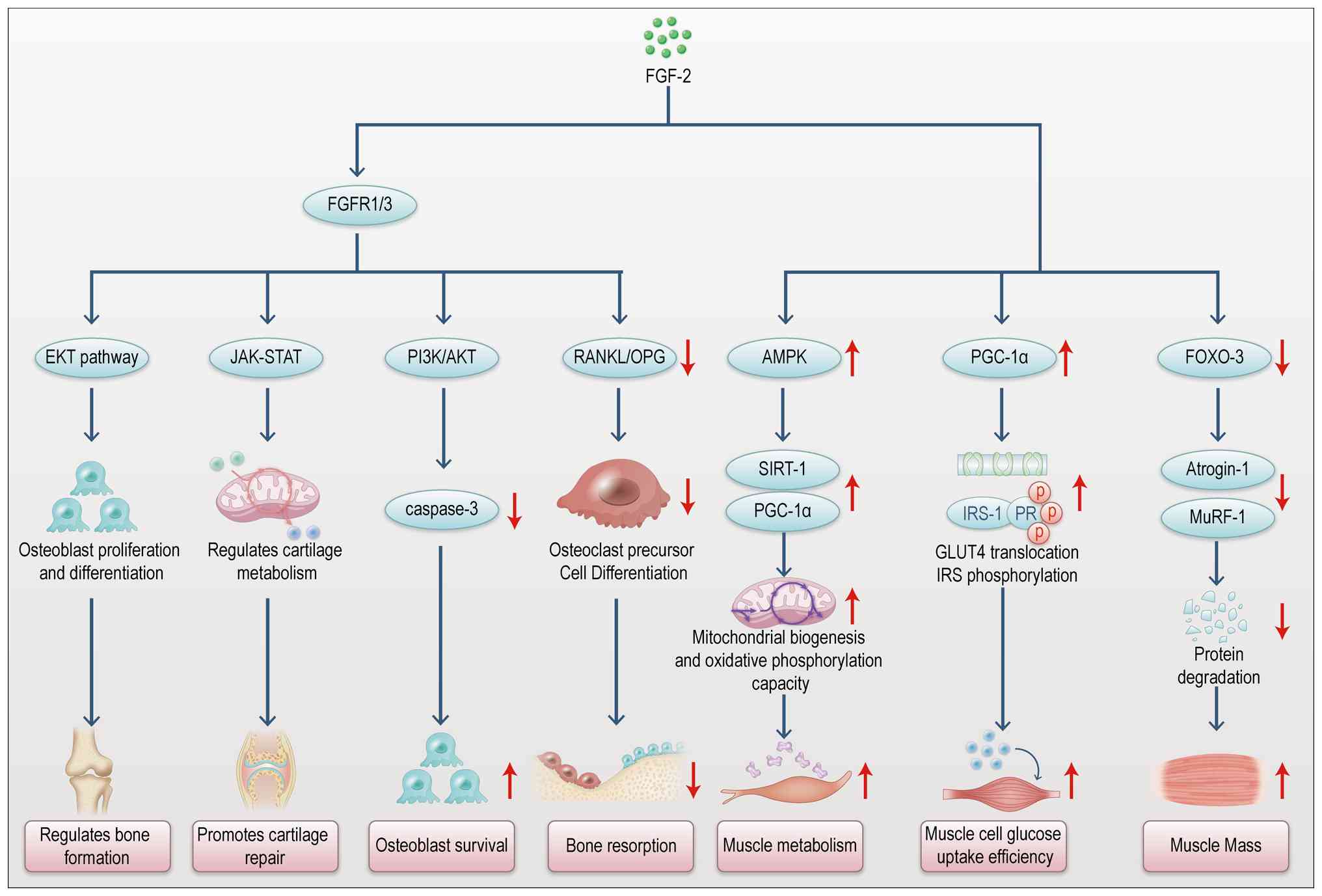
FGF-2 contributes to musculoskeletal crosstalk by
promoting muscle growth and regulating bone formation and repair
through the FGFR (Fig. 5)
(44,45). In bone metabolism, FGF-2
modulates osteoblast and osteoclast functions, influencing bone
formation and resorption via the MAPK/ERK signaling pathway
(46). Research shows that FGF-2
enhances bone repair by stimulating osteoblast proliferation and
migration, accelerating new bone formation during fracture healing
(47). Additionally, FGF-2 helps
maintain bone density by balancing osteoblast and osteoclast
activity, thus reducing bone resorption (48). FGF-2 exerts a bidirectional
regulatory effect in musculoskeletal crosstalk, promoting bone
formation while inhibiting excessive bone resorption. In summary,
both IGF-1 and FGF-2 are crucial in musculoskeletal interactions,
regulating bone and muscle growth and repair through their
respective signaling pathways and coordinating the development and
repair of the musculoskeletal system through their interplay.
Exosomes are small, bilayer lipid-enclosed vesicles
secreted by cells, encompassing exosomes, microvesicles and
apoptotic bodies (7). Among
these, exosomes are the most extensively studied subtype (49). They carry a variety of cargo,
including proteins, metabolites, nucleic acids and lipids, derived
from their parent cells, exerting biological effects similar to
those of the original cells (Table
IV) (50-53). Exosomes can bind to recipient
cells through surface ligands or be internalized via paracrine
signaling and membrane fusion (such as endocytosis) (54), facilitating the transfer of
bioactive molecules and modulating recipient cell functions.
Macrophage-derived exosomes play critical roles in regulating
inflammation and influencing the progression of bone diseases, such
as osteoporosis, fractures and osteoarthritis, markedly affecting
bone metabolism and homeostasis (55-57). Research indicates that both the
composition and quantity of exosomes vary depending on the
macrophage polarization state (58-60). In recent years,
macrophage-derived exosomes have gained growing research interest,
following extensive earlier studies on exosomes from mesenchymal
stem cells (MSCs).
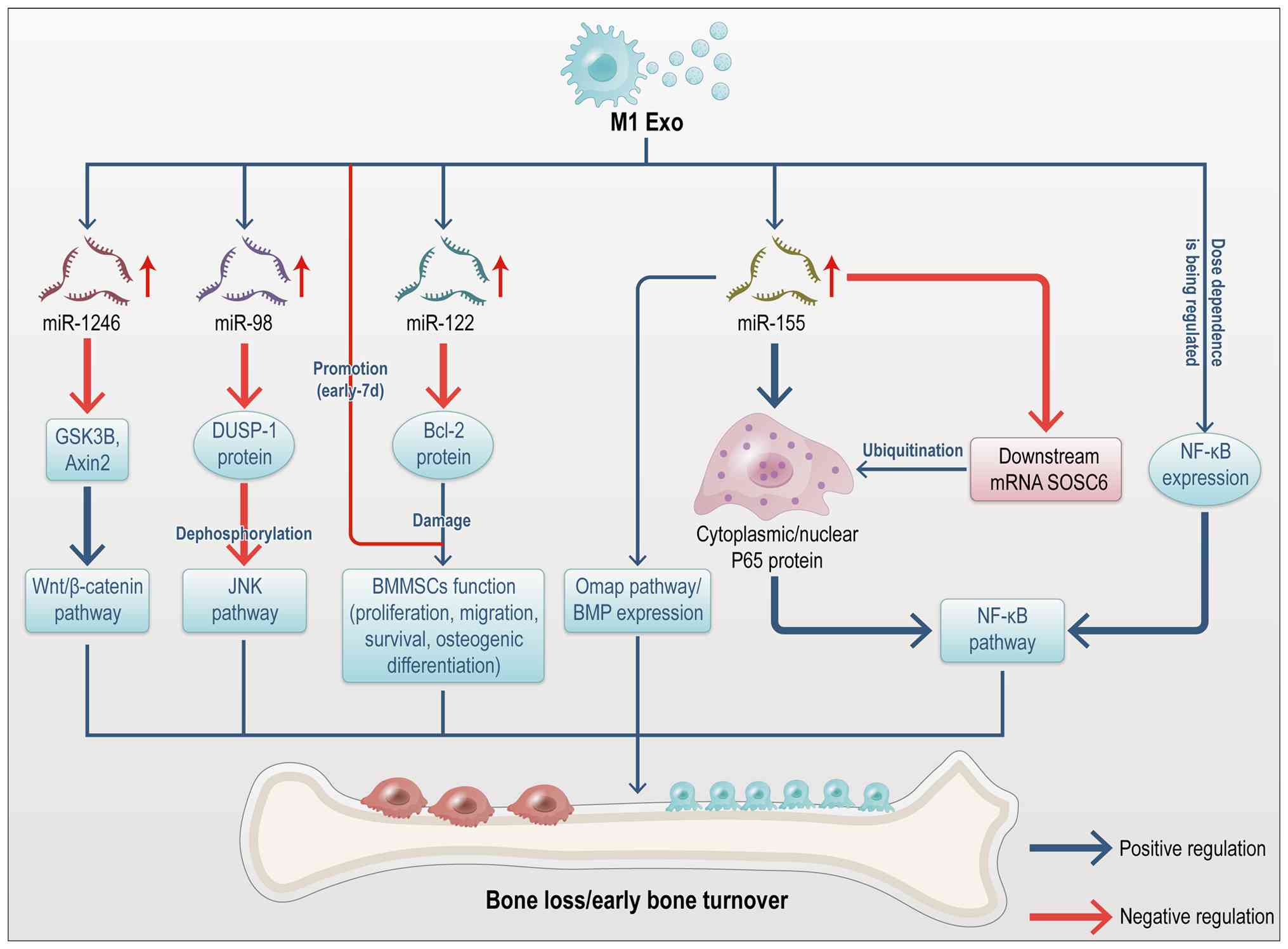
M1-polarized macrophages promote the release of
chemotactic and inflammatory mediators, which facilitate osteoclast
activation and debris clearance at fracture sites (61-63). MicroRNAs (miRNAs), highly
conserved non-coding RNAs, post-transcriptionally regulate gene
expression and play a key role in intercellular communication as
integral components of exosomes (64). The miRNA profiles of M1 and M2
polarized macrophages exhibit significant differences. Kang et
al (59) demonstrated that
M1 exosomes are enriched with miR-155, which inhibits the bone
morphogenetic protein (BMP) signaling pathway by downregulating
BMP2, BMP9, and Runt-related transcription factor 2 (Runx2). Given
that BMP2 is critical for osteoblast differentiation (65), its downregulation impedes bone
regeneration. Ge et al (60) reported that exosomal miR-155 from
M1 macrophages targets SOCS6, resulting in elevated p65 protein
levels. This activation of the nuclear factor
kappa-light-chain-enhancer of activated B cells (NF-κB) pathway
exacerbates inflammation. Additionally, miR-222 is highly expressed
in M1 macrophages (66). Studies
have shown that exosomal miR-222 from M1 macrophages induces
apoptosis in bone marrow mesenchymal stem cells (BMMSCs) by
inhibiting Bcl-2 expression (67-69). Yu et al (70) demonstrated that exosomal miR-98
from M1 macrophages targets DUSP1 in MC3T3-E1 cells and a
postmenopausal osteoporosis murine model, inhibiting osteogenic
differentiation. Another study showed that M1 exosomes are enriched
with miR-1246, which activates the Wnt pathway by downregulating
GSK3β and Axin2, thereby promoting cartilage inflammation and
degradation (71). These
findings were primarily validated using bioinformatics analyses,
sequencing and related methodologies. In summary, M1
macrophage-derived exosomes, which are enriched with miRNAs such as
miR-155, miR-222, miR-98 and miR-1246, modulate downstream
signaling pathways to promote inflammation and impair bone repair
both in vitro and in vivo. The specific miRNA cargo
determines the downstream signaling effects by targeting key nodes
within signaling networks. Exosomes serve as specific carriers,
enabling macrophage-derived miRNAs to function as promising
biomarkers for monitoring and regulating bone remodeling. However,
the complete repertoire of miRNAs and other cargo within macrophage
exosomes remains incompletely characterized. Further investigation
is needed to understand how macrophage polarization influences
exosomal miRNA cargo and the precise mechanisms through which these
miRNAs regulate downstream pathways. The effects of macrophage
exosomes on BMMSC differentiation have been extensively studied. He
et al (72) collected
conditioned medium (CM) from M1 macrophages and observed enhanced
BMMSC proliferation, adipogenic differentiation and extracellular
matrix deposition. Upon isolating exosomes from this CM, it was
found that M1 exosomes, but not M2 exosomes, promoted stem cell
proliferation, osteogenesis and adipogenesis. This finding was
corroborated by Xia et al (73). By contrast, Kang et al
(59) reported that co-culture
with M1 macrophages markedly reduced BMP2 and BMP9 expression in
mouse MSCs, suggesting that M1 macrophage-derived exosomes impair
the osteoinductive effects of BMPs and suppress osteogenic gene
expression in MSCs. Through exosomal communication, M1 macrophages
primarily enhance early and mid-stage osteogenesis, exerting
stronger effects on BMMSC proliferation, osteogenesis and
adipogenesis compared with M2 macrophages. These differing effects
on osteogenesis may be attributed to variations in recipient cell
sources. Experimental discrepancies could stem from multiple
factors, including macrophage maturity, co-culture conditions, the
presence of other cytokines in the CM, recipient cell lineage, and
technical differences in exosome isolation from specific
conditioned media (62,73). The balance between osteogenic and
adipogenic differentiation in BMMSCs is essential for maintaining
bone mass. Investigating the mechanisms by which M1
macrophage-derived exosomes regulate this balance in BMMSCs may
offer insights into clinical disease pathogenesis and aid in the
development of targeted therapies. Fig. 6 summarizes the regulation of bone
remodeling signaling pathways by M1 macrophage-derived
exosomes.
A critical event in bone healing is the transition
from the pro-inflammatory M1 macrophage phenotype to the
anti-inflammatory M2 phenotype (74). In the later stages of bone
healing, M2 macrophages establish an anti-inflammatory environment
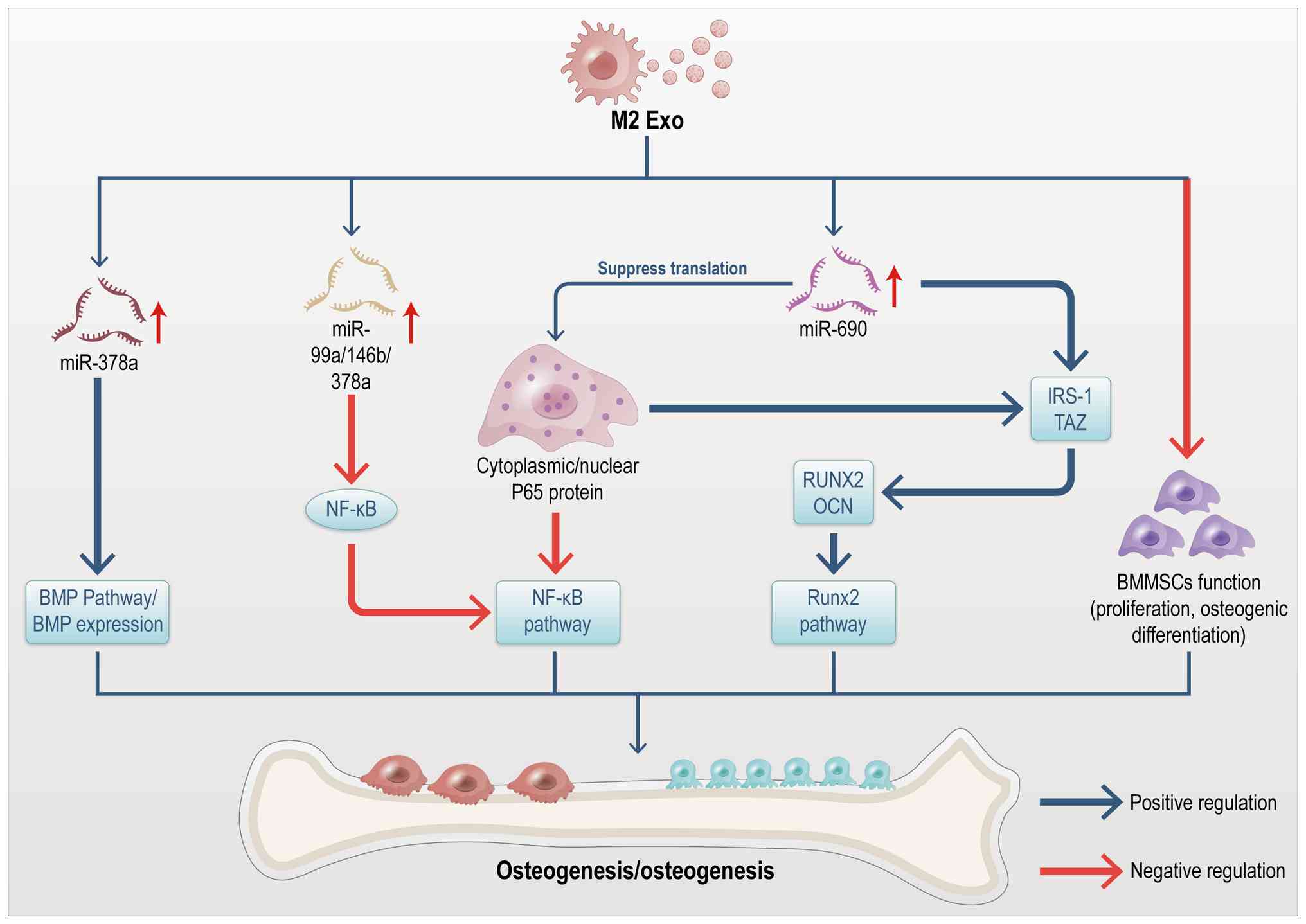
that promotes osteogenesis in BMMSCs. Li et al (56) identified exosomes as key
mediators of the osteogenic process induced by M2 macrophages. BMPs
are key endogenous regulators of osteogenesis, with the loss of
BMP2 or BMP4 function leading to significant impairments in
osteogenesis. BMPs activate Smad proteins and upregulate Runx2
expression, driving MSC differentiation into osteoblasts (75,76). Using an in vivo bone
defect model, Kang et al (59) demonstrated that M2
macrophage-derived exosomes promote bone regeneration. Further
analysis revealed that M2 exosomes are enriched with miR-378a,
which enhances osteoinduction by modulating the BMP signaling
pathway, thus promoting cranial bone regeneration in mice. Studies
also indicate that exosomes from IL-4-stimulated M2 macrophages,
enriched with miRNAs such as miR-99a-5p, miR-146b-5p and
miR-378-3p, suppress inflammation by downregulating the TNF-α and
NF-κB signaling pathways in Apoe−/− mice (77). Moreover, exosomes derived from M2
macrophages promote osteogenic differentiation of BMMSCs through
miR-690, IRS-1 and TAZ, while inhibiting adipogenic differentiation
(56). Yu et al (78) reported that miR-690, enriched in
M2 macrophage-derived exosomes, upregulates osteogenic
differentiation in C2C12 myogenic progenitor cells by inhibiting
the translation of the NF-κB p65 protein. M2 macrophage-derived
exosomes are also shown to carry high levels of miR-221-5p, which
promotes tissue repair and regeneration by binding to the 3'
untranslated region (UTR) of E2F2 mRNA and negatively regulating
its expression in pancreatic ductal adenocarcinoma (79). Collectively, these studies
suggest that M2 macrophage-derived exosomes infiltrate the bone
marrow microenvironment and transfer specific miRNAs (such as
miR-378a, miR-690, miR-99a-5p) to BMMSCs, facilitating osteogenic
differentiation and fracture healing (80,81). These miRNAs likely function as
pro-osteogenic agents by modulating key pathways such as BMP
signaling to enhance osteogenesis. However, the precise roles of M2
exosome-derived miRNAs in bone formation and osteoclastogenesis
remain to be fully elucidated (82). Emerging evidence suggests that
interactions between BMMSCs and extracellular signals from M2
macrophages markedly contribute to bone formation. However, the
exact mechanisms underlying these interactions remain debated,
possibly due to differences in cell sources, co-culture conditions,
and macrophage polarization protocols. Some studies propose that CM
from M0 or M2 macrophages promotes the mineralization of BMMSCs in
the later stages of osteogenesis (83-86). By contrast, other studies report
opposing findings. For instance, Xia et al (73) found that exosomes derived from M2
macrophages inhibit BMMSC proliferation, with no significant effect
on the expression of osteogenic or adipogenic genes in BMMSCs, and
possibly even suppress chondrogenic differentiation. The regulatory
effects of M2 macrophage-derived exosomes on bone remodeling
signaling pathways are summarized in Fig. 7.
Exosomes play a pivotal role in regulating protein
synthesis and muscle regeneration by promoting satellite cell
proliferation and myofiber formation. This regulation occurs
through the delivery of myogenic growth factors such as IGF, FGF-2,
and hepatocyte growth factor (HGF) (87). Forterre et al (88) employed proteomic analysis to
identify numerous proteins associated with muscle growth and
metabolism in exosomes secreted by skeletal muscle. For example,
exosomes extracted from C2C12-derived myotubes contained key
components, such as integrin subunit β1, CD9, CD81, neural cell
adhesion molecule (NCAM), CD44 and myosin, that are potentially
crucial for myocyte differentiation. Similarly, Mobley et al
(89) demonstrated that exosomes
derived from whey protein enhance muscle protein synthesis in
vitro. Autophagy plays a critical role in maintaining muscle
mass and function; its inhibition exacerbates muscle atrophy
(90). AMPK, a central
intracellular energy sensor, alleviates sarcopenia (SP) symptoms
and mitochondrial dysfunction (91). Chen et al (92) discovered that exosomes from MSCs
ameliorate muscle atrophy by enhancing AMPK/ULK1-mediated
autophagy. Guescini et al (93) observed that exosomal miR-133b and
miR-181a-5p are rapidly released into the bloodstream
post-exercise, promoting muscle regeneration. This mechanism may
partially explain how exercise mitigates SP. Furthermore,
Chaturvedi et al (94)
demonstrated in animal models that exercise-induced exosomes
promote skeletal muscle regeneration through the upregulation of
miR-9b and miR-29.
Additionally, extracellular vesicles from M1
macrophages can polarize recipient macrophages toward an M2-like
phenotype, thereby altering skeletal muscle homeostasis under
high-glucose conditions. Collectively, these findings highlight the
roles of both M1- and M2-derived macrophage exosomes in regulating
SP.
Exosomes serve as critical messengers in muscle-bone
crosstalk, enabling skeletal muscles to influence bone activity
through EV-mediated communication. This exosome-mediated
communication between skeletal muscle cells occurs via autocrine,
paracrine, or endocrine pathways (95-98). Exosomes derived from C2C12
myoblasts promote the osteogenic differentiation of MC3T3-E1
preosteoblasts, likely mediated by the upregulation of miR-27a-3p
in recipient cells. This miRNA suppresses specific target genes and
activates β-catenin signaling, thereby enhancing osteogenesis
(99). Additionally, exosomes
from C2C12 myoblasts enhance Wnt/β-catenin signaling in
TOPflash-MLOY4 osteocyte-like cells, promoting cell survival and
providing protection against apoptosis and oxidative stress. This
protective effect is likely due to exosomes-mediated inactivation
of Wnt inhibitors, such as SOST, DKK2, and SFRP2 (100). Li et al (101) showed that myoblast-derived
exosomes carry Prrx2, which activates miR22HG transcription,
promoting osteogenic differentiation of BMMSCs via the YAP pathway
and miR-128. Furthermore, CXCR4, present on exosomes produced by
mouse fibroblasts, targets these vesicles to the bone marrow, where
miR-188-containing exosomes fuse with lipids to form hybrid
nanoparticles. These nanoparticles then release miR-188 in a
targeted manner, inhibiting adipogenesis and promoting BMMSC
differentiation into osteoblasts (102). Regarding the influence of bone
cell-derived exosomes on skeletal muscle, long non-coding RNAs
(lncRNAs) are critical. For example, Zheng et al (103) found that osteoblasts induce
myogenic differentiation of C2C12 myoblasts via exosomal lncRNAs,
specifically TUG1 and DANCR. Toita et al (104) demonstrated that collagen
patches releasing phosphatidylserine-containing liposomes promote
M1-to-M2 macrophage polarization, facilitating concurrent bone and
muscle tissue healing. Transcriptome analysis via next-generation
sequencing revealed that M1 macrophage secretory products inhibit
the differentiation of preosteoblasts and myoblasts, while M2
macrophage secretory products promote it. This highlights the
importance of timely M1-to-M2 polarization for effective tissue
regeneration. As shown in Fig.
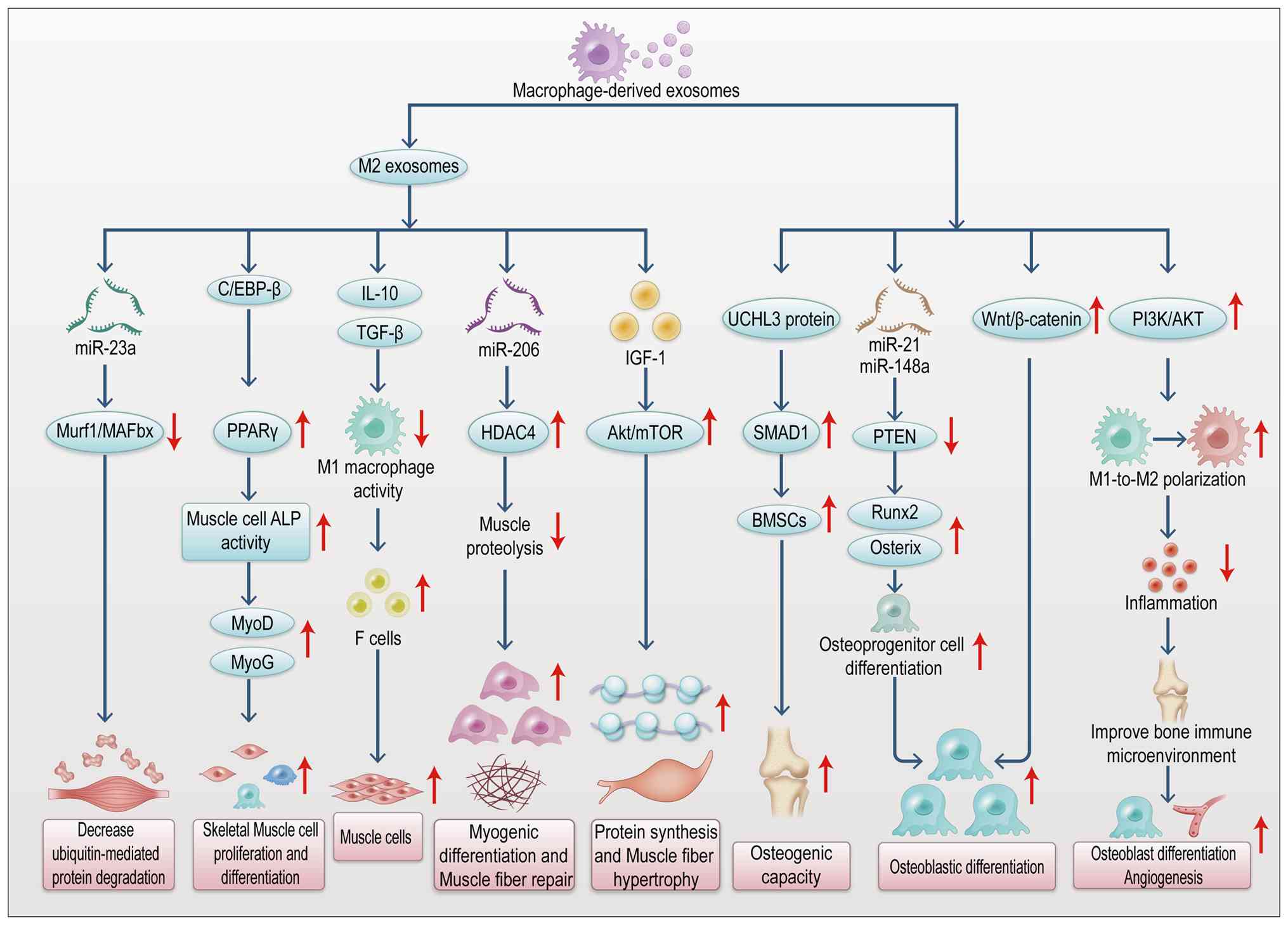
8, macrophages, particularly the M2 phenotype, release exosomes
carrying the transcription factor C/EBPβ. This factor promotes
skeletal muscle cell proliferation and differentiation via the
PPARγ signaling pathway (105).
Depletion of macrophage exosomes containing C/EBPβ markedly
inhibits ALP activity in muscle cells and reduces the expression of
myogenic markers (such as MyoD and MyoG), delaying muscle injury
repair (105). M2
macrophage-derived exosomes suppress M1 macrophage activity by
delivering anti-inflammatory factors, such as IL-10 and TGF-β,
while mitigating the damaging effects of pro-inflammatory factors
like TNF-α and IL-6 on muscle fibers. They also promote the
directed differentiation of stem cells into myocytes (106). Additionally, M2 macrophage
exosomes activate the PI3K/AKT pathway, facilitating the shift from
M1 to M2 polarization. This process leads to reduced inflammatory
cytokine levels, improved bone immune microenvironment and enhanced
osteoblast differentiation and angiogenesis (107). Under mechanical stimulation,
macrophages increase exosome secretion. These exosomes carry the
UCHL3 protein, which enhances the osteogenic potential of BMMSCs
and drives callus formation through the SMAD1 signaling pathway
(108). Exosome-encapsulated
miRNAs, including miR-21 and miR-148a, inhibit osteogenic
inhibitors like PTEN and upregulate RUNX2 and osterix (106). In osteosarcoma models, exosomes
coordinate osteoclast and osteoblast activity by transmitting
Wnt/β-catenin signals, contributing to the maintenance of bone
homeostasis (106). These
findings highlight the critical role of macrophage-derived exosomes
in regulating the proliferation and repair of both bone and muscle
tissues, modulating muscle-bone crosstalk and enhancing tissue
regeneration.
Exosomes function as essential intercellular
signaling vehicles, transporting a variety of biologically active
molecules, including miRNAs, mRNAs, proteins and lipids (109). These components regulate bone
metabolism by modulating signaling pathways in target cells
(110). Notably, miRNAs within
macrophage-derived exosomes are recognized as key regulatory
factors (111). Research
indicates that macrophage-derived exosomal miRNAs influence bone
metabolism indirectly by regulating key signaling molecules such as
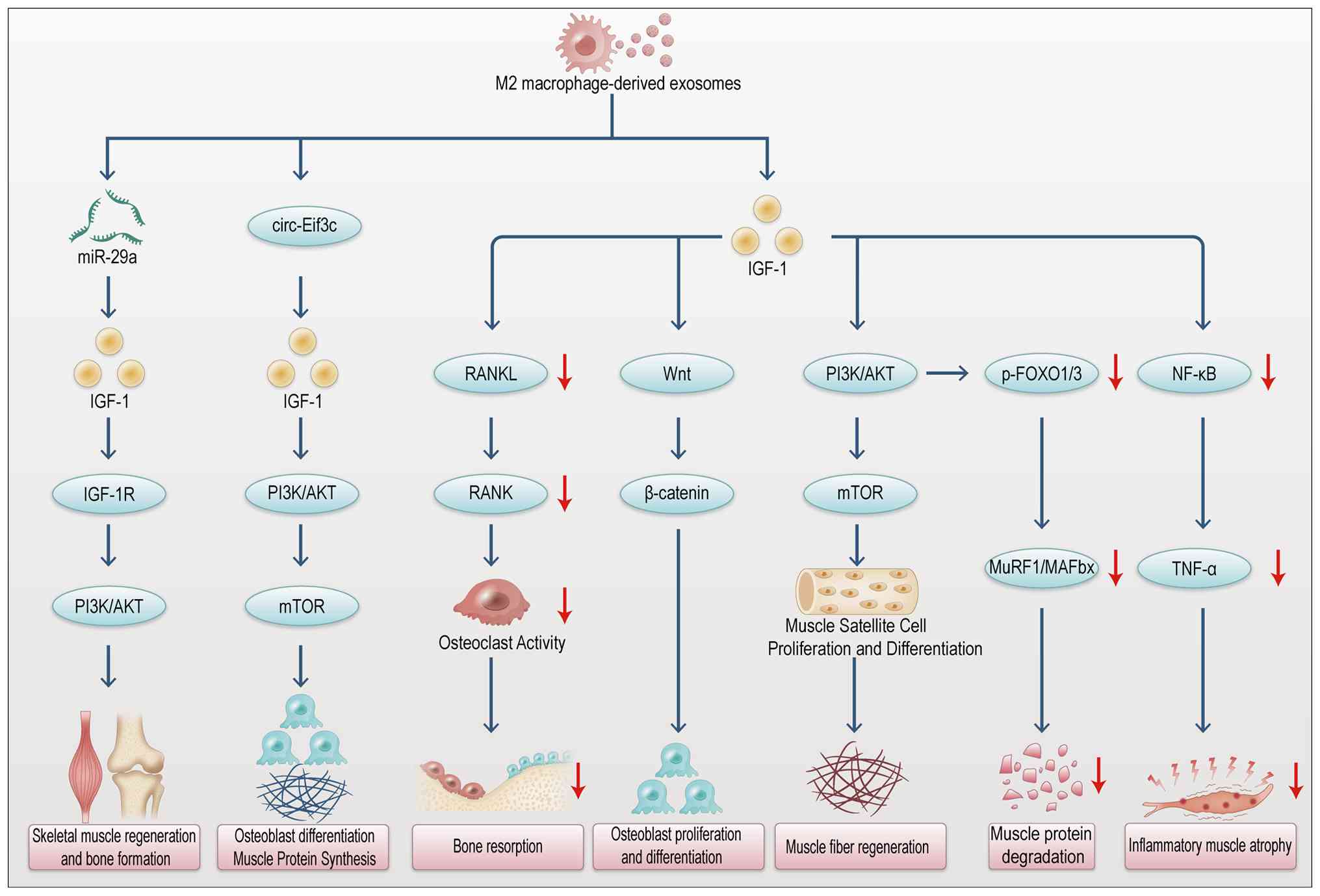
IGF-1 and FGF-2 (Figs. 9 and
10) (112). miRNAs modulate gene expression
by binding to target mRNAs, resulting in translational repression
or mRNA degradation (113). In
musculoskeletal crosstalk, exosomal miRNAs from macrophages play
pivotal roles in the regulation of IGF-1, FGF-2 and other signaling
pathways (Figs. 9 and 10) (114). For instance, macrophage-derived
exosomal miR-21 promotes IGF-1 signaling by targeting inhibitory
molecules within the PI3K-Akt pathway, thereby enhancing bone
formation (115). Similarly,
macrophage-derived exosomal miR-29 boosts osteoblast function,
promoting bone matrix synthesis and mineralization through the
regulation of FGF-2 expression (116). By contrast, M1 exosomal miR-155
directly inhibits IGF-1R and downstream PI3K/Akt signaling,
diminishing osteoblast differentiation (117). Additionally, M1 exosomal
miR-143 exacerbates insulin-resistant muscle atrophy by inhibiting
IRS-1 and obstructing the pro-muscle protein synthesis effect of
IGF-1 (118). Thus,
macrophage-derived exosomes regulate musculoskeletal crosstalk via
exosomal miRNAs during IGF-1 and FGF-2 signaling, revealing a novel
molecular mechanism (Figs. 9 and
10).
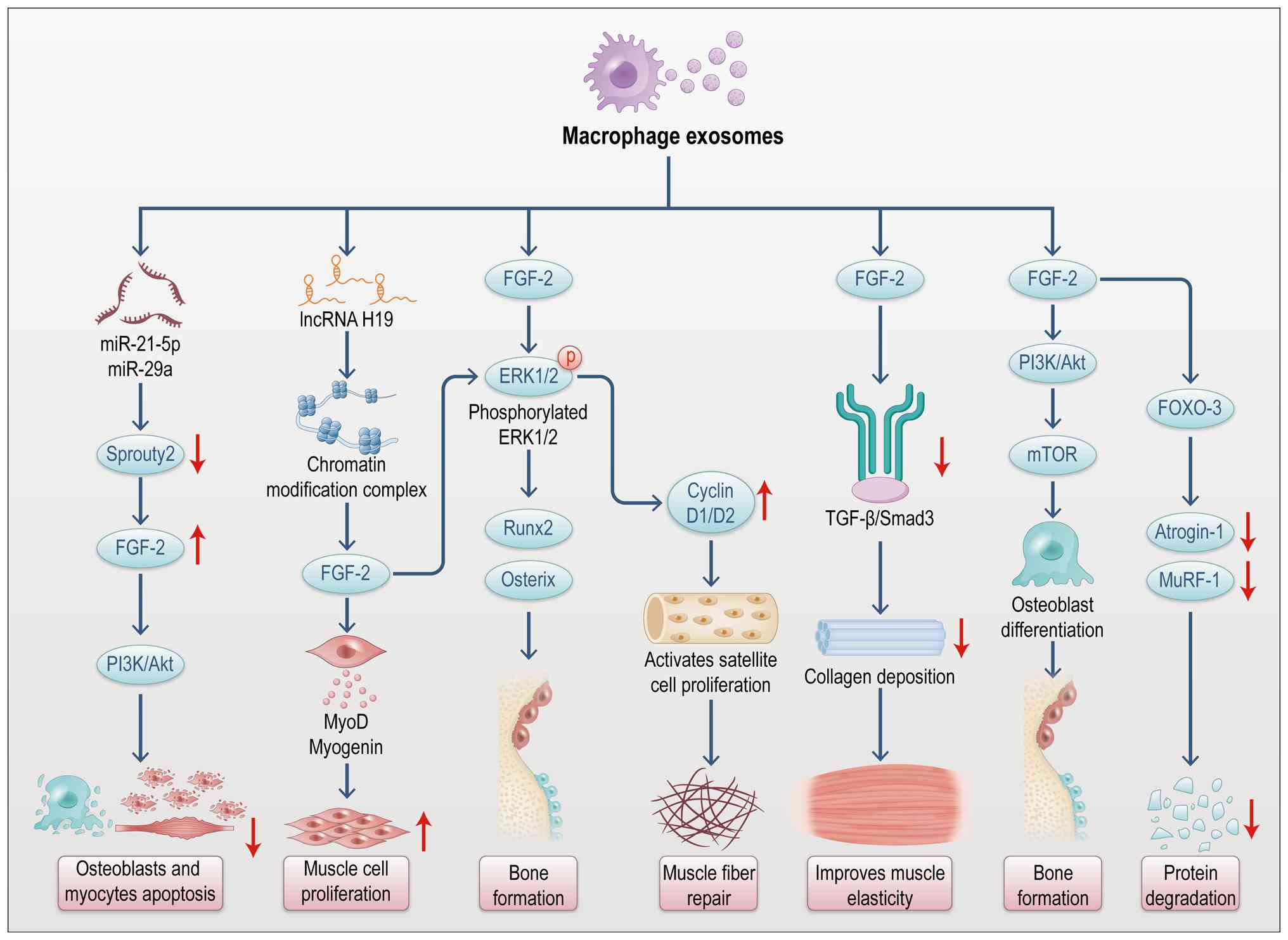
Exosomes carry a diverse array of cargo molecules
that regulate bone and muscle metabolism through interactions with
receptors on the surface of musculoskeletal cells (119). Signal transduction by IGF-1 and
FGF-2 in bone metabolism is initiated when these factors bind to
their specific cell surface receptors (120). Macrophage-derived exosomes can
modulate bone metabolism by transporting receptors or receptor
ligands that facilitate the binding of signaling factors to their
target cells (121). Exosomal
ligands for IGF-1R and FGFR directly bind to their corresponding
receptors on bone or muscle cells, triggering signal transduction
(122). Upon IGF-1 binding,
IGF-1R activates intracellular signaling cascades, primarily via
the PI3K/Akt and MAPK pathways, to regulate bone and muscle growth
and repair (123,124). Macrophage-derived exosomes can
deliver IGF-1R or its ligands, thereby enhancing IGF-1 signaling to
promote bone formation and muscle repair (Fig. 9) (125). Similarly, FGF-2 binding to FGFR
initiates signaling cascades that regulate the proliferation and
differentiation of bone marrow stromal cells and osteoblasts
(126). FGF-2 targets
sclerostin in bone and myostatin in skeletal muscle to counteract
the harmful effects of glucocorticoids on musculoskeletal
degradation (127). The
exosome-mediated interaction between FGF-2 and FGFR enhances bone
repair and plays a pivotal role in musculoskeletal crosstalk
(Fig. 10) (128). In summary, macrophage-derived
exosomes facilitate signal transduction and regulate bone
metabolism by transporting receptors (such as IGF-1R, FGFR) and
their ligands, strengthening bone formation and repair, and
promoting musculoskeletal crosstalk.
Osteoblast proliferation, differentiation and
mineralization are essential processes in bone metabolism,
regulated by macrophage-derived exosomes through various mechanisms
(129). During osteogenesis,
active molecules such as miRNAs and proteins facilitate osteoblast
proliferation, differentiation, and mineralization (130). Exosomal miRNAs secreted by
macrophages, including miR-21 and miR-29, can activate the PI3K/Akt
and Wnt/β-catenin signaling pathways in osteoblasts, promoting
their proliferation and differentiation (131); M1 macrophage-derived exosomes
aggravate bone loss in postmenopausal osteoporosis via a
miR-98/dual specificity phosphatase 1 (Dusp1)/c-Jun N-terminal
kinase (JNK) axis (132); M2
macrophagy-derived exosomal miRNA-26a-5p induces osteogenic
differentiation of bone mesenchymal stem cells (133); Exosomal miR-486-5p secreted by
M2 macrophage influences the differentiation potential of bone
marrow mesenchymal stem cells and osteoporosis (134). These miRNAs enhance osteoblast
differentiation and bone matrix deposition by inhibiting negative
regulatory factors and activating key transcription factors, such
as Runx2 and Osterix (135,136). Additionally, exosomal proteins
such as TGF-β and BMPs support mineralization by regulating
osteoblast proliferation and differentiation (137). TGF-β activates the Smad
signaling pathway through receptor binding, enhancing osteoblast
mineralization (138). BMPs, on
the other hand, activate the Smad1/5/8 pathway, which promotes
osteoblast differentiation and stimulates bone matrix synthesis and
mineralization (139), as
summarized in Table V (140-143).
The function of osteoblasts is further regulated by
a series of transcription factors, with Runx2 and Osterix being two
critical regulators of osteogenic differentiation (144). Macrophage-derived exosomes
influence osteoblast function by modulating the expression of these
transcription factors (145,146). Exosomes secreted by macrophages
carry specific miRNAs, such as miR-124-3p and miR-146a, which
regulate Runx2 and Osterix expression (147,148). For example, miR-224-5p targets
the 3' UTR of Runx2, preventing its degradation and thereby
promoting osteoblast differentiation (149); miR-6879-5p carried by M2
macrophage-derived exosomes increases Runx2 expression and promotes
osteogenic differentiation and aerobic glycolysis in human
periodontal ligament stem cells (hPDLSCs) via modulating
TRIM26-mediated ubiquitination of pyruvate kinase M (PKM) (150); M2 macrophage exosomes carrying
miRNA-26a-5p can induce osteogenic differentiation of bone
marrow-derived stem cells to inhibit lipogenic differentiation by
promoting the expression of RUNX-2 (151). Additionally, miR-664-3p
promotes osteoblast differentiation and mineralization by
regulating Osterix expression (152). As a key transcription factor
downstream of Runx2, Osterix drives bone matrix formation and
mineralization (147). As well
as miRNAs, proteins such as TGF-β and BMP in macrophage-derived
exosomes also promote osteoblast differentiation by modulating
Runx2 and Osterix expression (153,154). TGF-β enhances Runx2 expression
through the activation of the Smad2/3 signaling pathway, promoting
osteoblast differentiation (136). BMPs stimulate Osterix
expression and enhance bone matrix deposition and mineralization
via the Smad1/5/8 signaling pathway (155).
The formation and activation of osteoclasts, the
primary cells responsible for bone resorption, are essential
processes in bone metabolism (156). Macrophage-derived exosomes
contribute to bone resorption and regulate bone metabolism by
modulating osteoclast formation and activation (157). Osteoclast formation is governed
by various factors, including Receptor Activator of Nuclear Factor
κ-B Ligand (RANKL) and Macrophage Colony-Stimulating Factor (M-CSF)
(158,159). Macrophage-derived exosomes
promote osteoclast formation by carrying RANKL and M-CSF (160,161). Specifically, exosomal RANKL
binds to the RANK receptor on osteoclasts, activating the NF-κB and
MAPK signaling pathways to drive osteoclast formation and
activation (162,163). By binding to its receptor
c-Fms, M-CSF activates the PI3K/Akt signaling pathway, accelerating
osteoclast differentiation (164). Additionally, macrophage-derived
exosomes can further promote osteoclast formation by modulating
other cytokines, such as IL-1 and TNF-α, which enhance RANKL
expression through the activation of the NF-κB signaling pathway,
thereby promoting osteoclast activation (57). Consequently, macrophage-derived
exosomes facilitate bone resorption by carrying RANKL, M-CSF, and
other factors that regulate osteoclast formation and
activation.
IGF-1 and FGF-2 play pivotal roles in both bone and
muscle metabolism. Osteoporosis, often accompanied by SP, is a
condition that involves diminished bone density and function,
alongside muscle degradation. Macrophage-derived extracellular
vesicles have been shown to improve both osteoporosis and SP by
regulating IGF-1 and FGF-2 signaling.
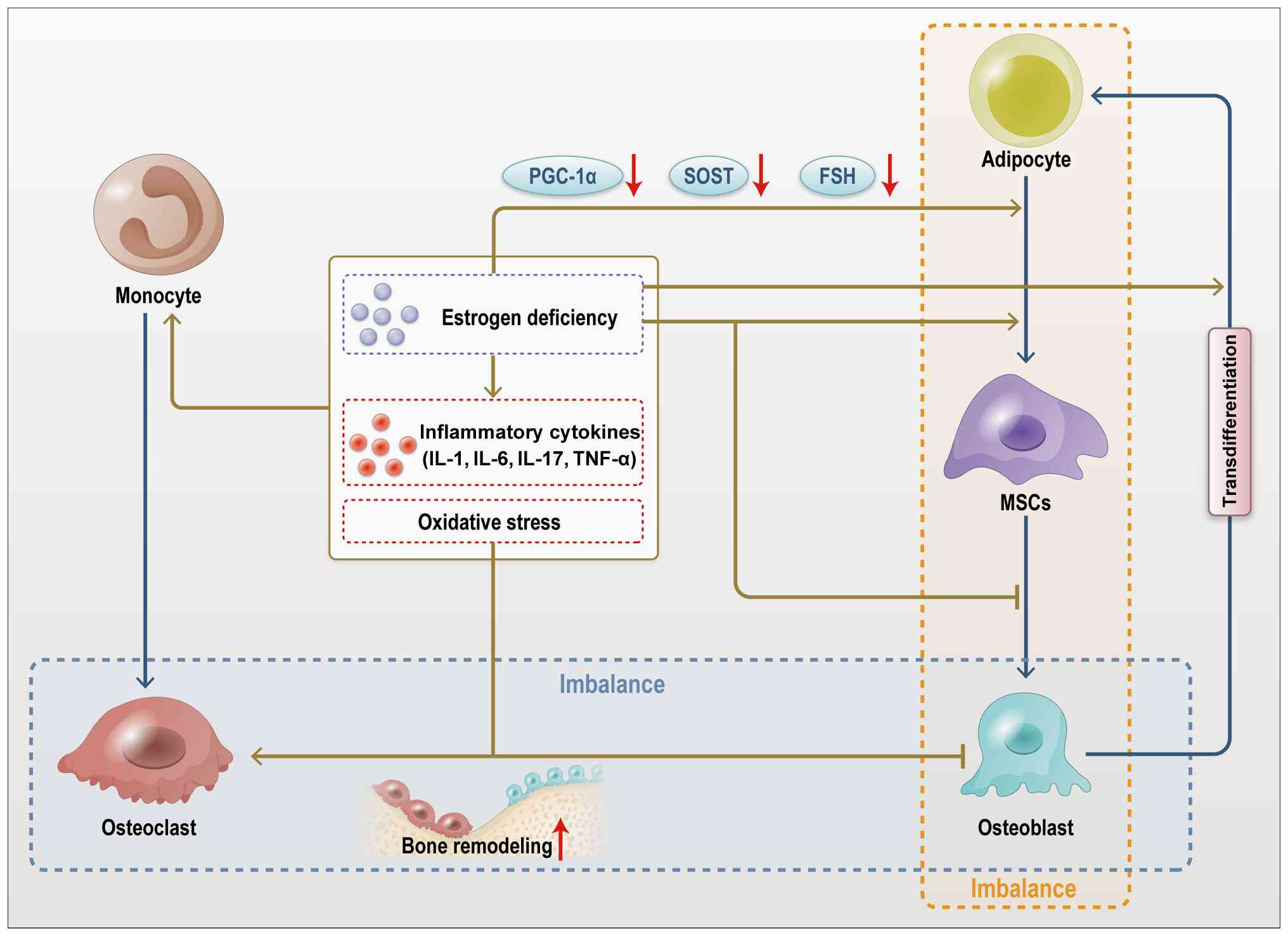
Osteoporosis is characterized by reduced bone
density, deterioration of bone microarchitecture and increased
fracture risk, primarily affecting older adults (172). Recent studies suggest that
osteoporosis is not only associated with abnormal bone metabolism
but also with impaired muscle function (Figs. 11 and 12), highlighting the significance of
musculoskeletal crosstalk in the pathogenesis of osteoporosis
(Figs. 11 and 12) (172,173). In osteoporosis, the functions
of macrophage-derived exosomes, IGF-1, and FGF-2 are markedly
altered. Macrophages, as key immune cells (174,175), contribute to bone metabolism
through exosomes that transport a variety of signaling molecules,
including cytokines, miRNAs, and proteins (176). Impaired macrophage function in
patients with osteoporosis alters the signaling molecule profile
within exosomes (177). These
changes may accelerate bone loss by disrupting the balance between
bone resorption and formation through various signaling pathways.
IGF-1 plays a central role in bone metabolism by regulating bone
formation, primarily through the PI3K/Akt and MAPK signaling
pathways (178,179).
The primary manifestations of osteoporosis, bone
loss and reduced bone strength, are influenced by the interaction
of macrophage-derived exosomes, IGF-1 and FGF-2 (180). These components synergistically
regulate bone formation and resorption, thereby affecting bone mass
(181). Exosomal miRNAs, such
as miR-21 and miR-146a, derived from macrophages, can promote
osteoclast differentiation and activity by modulating the
RANKL/RANK pathway, leading to excessive bone resorption (182). Additionally, exosomal protein
factors such as TGF-β and BMP further inhibit bone formation by
regulating osteoblast function (183). In osteoporosis, IGF-1, a key
osteogenic factor, is often underexpressed, impairing osteogenesis
and leading to reduced bone formation (184). Similarly, FGF-2 signaling is
suppressed in osteoporosis, contributing to further bone density
loss (185). This disruption in
signaling between IGF-1 and FGF-2 exacerbates bone loss. Exosomes
carrying RANKL and TGF-β promote osteoclastogenesis and bone
resorption (186), while the
deficiency of IGF-1 and FGF-2 signaling further impairs bone
formation. Disruption of this delicate musculoskeletal crosstalk
and the associated molecular pathways plays a pivotal role in the
pathogenesis of osteoporosis (187).
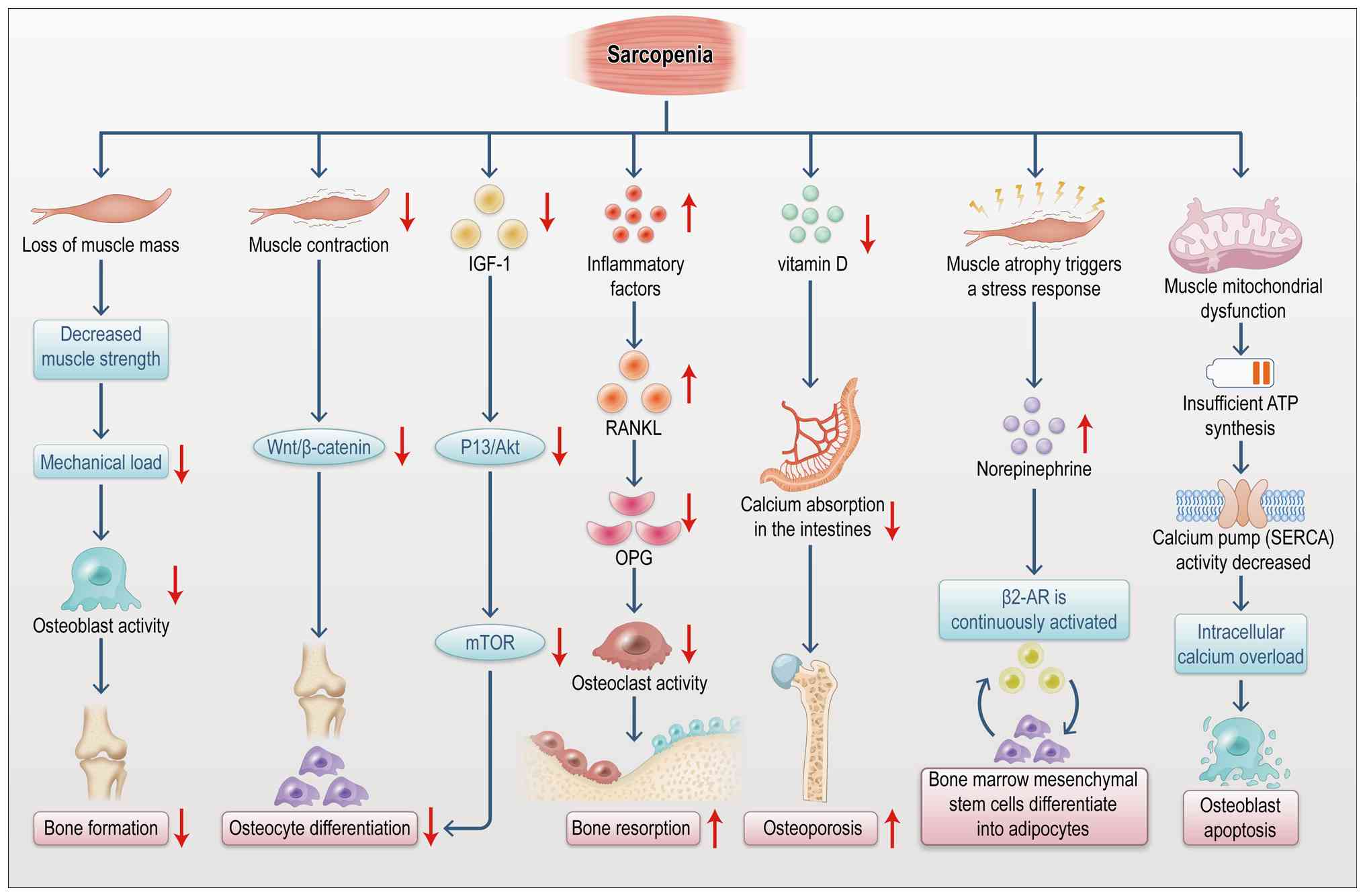
SP refers to the age-related progressive loss of
skeletal muscle mass and function (188). It is not only a natural part of
aging but is also closely linked to various diseases and
pathological conditions. The relationship between SP and abnormal
bone metabolism has gained considerable attention in recent years.
Muscle atrophy and bone metabolism interact bidirectionally,
forming a critical regulatory mechanism for maintaining bone health
(189). As depicted in Fig. 13, the onset of SP is often
accompanied by decreased bone density and altered bone metabolism.
Muscle atrophy influences bone metabolism through multiple
mechanisms (190). It reduces
muscle strength and load-bearing capacity, thereby diminishing
mechanical stress on bones. This reduction in mechanical
stimulation leads to increased bone resorption and decreased bone
formation (190). Additionally,
SP affects bone metabolism through the secretion of inflammatory
factors, such as IL-6 and TNF-α (191). These inflammatory mediators
activate osteoclast signaling pathways, enhancing bone resorption
and consequently reducing bone density (192). Moreover, endocrine dysfunction
linked to SP plays a significant role in abnormal bone metabolism.
Muscle atrophy leads to reduced secretion of myokines, such as
IGF-1 and FGF-2, impairing bone formation.
Macrophage-derived exosomes, along with IGF-1 and
FGF-2, play pivotal roles in the bone metabolism abnormalities
associated with SP (193,194). Macrophages, as key immune
cells, secrete exosomes carrying a range of cytokines and miRNAs
that are essential for musculoskeletal crosstalk (195). The miRNAs, cytokines and growth
factors transported by macrophage exosomes affect all aspects of
bone metabolism (196). For
example, macrophage-derived exosomes promote osteoclast formation
and activity by delivering miRNAs (such as miR-146a and miR-21),
thus enhancing bone resorption (197). These exosomal miRNAs can
activate specific signaling pathways by binding to receptors on
bone cells, influencing the pathogenesis of conditions such as
osteoporosis and bone loss (197). Moreover, the roles of IGF-1 and
FGF-2 in SP are particularly important (198). IGF-1 is well-established as
essential for muscle tissue, promoting not only muscle growth and
repair but also bone matrix formation by enhancing osteoblast
function (199). However,
circulating IGF-1 levels are often reduced in patients with SP,
contributing to bone metabolism disorders. Similarly, FGF-2 is
another key osteogenic factor involved in SP.
Exosome isolation from macrophages typically
involves techniques such as ultracentrifugation, size exclusion
chromatography, immunoaffinity capture, and kit-based methods.
Identification techniques include transmission electron microscopy
(TEM), nanoparticle tracking analysis (NTA), western blotting (WB)
and nanoflow cytometry. Each method offers distinct advantages and
limitations. In muscle and bone metabolism experiments, in
vitro methods include osteoblast differentiation assays,
osteoclast inhibition tests, muscle cell differentiation
experiments, and muscle metabolism studies. In vivo
approaches include osteoporosis models, fracture healing models,
and SP models.
Exosomes are nanoscale extracellular vesicles
(30-150 nm in diameter) secreted by cells and are widely
distributed in various body fluids (200). The isolation and purification
of exosomes from macrophages is a critical step in studying
musculoskeletal crosstalk and its impact on bone metabolism
(201). Conventional methods
for exosome isolation primarily include ultracentrifugation and
commercial kit-based approaches (201). Ultracentrifugation is regarded
as the gold-standard technique for exosome separation, relying on
density-based differentiation to isolate exosomes from other
cellular components (202).
This process involves removing cellular debris through low-speed
centrifugation, followed by high-speed ultracentrifugation to
pellet exosomes (202).
Although this method is widely adopted, straightforward and
cost-effective, it is time-consuming and requires significant
technical expertise (202).
Despite these limitations, ultracentrifugation remains the most
commonly used method due to its high yield and efficiency in
exosome collection (203). By
contrast, commercial kit-based methods use reagents for exosome
separation, often employing immunomagnetic beads or affinity
capture technologies (204).
These kits are convenient, easy to use, and improve exosome purity
markedly (204). However, they
tend to be expensive and may exhibit lower extraction efficiency,
especially in samples with high levels of contaminating cellular
material (204). A comparative
summary of these techniques is provided in Tables VI and VII (205-216).
Investigating the impact of macrophage-derived
exosomes, IGF-1, and FGF-2 on musculoskeletal crosstalk requires
the establishment of a co-culture system that incorporates both
muscle cells and osteocytes. Commonly used bone cell models include
the MC3T3-E1 murine pre-osteoblastic cell line and the RAW264.7
murine monocytic cell line (222). These cell lines exhibit high
proliferative and differentiation capacities, making them ideal for
in vitro investigations (222). For muscle cell models, the
C2C12 mouse myoblast cell line and the L6 rat skeletal muscle cell
line are frequently used (223). The C2C12 cell line, in
particular, differentiates into myotubes under appropriate
conditions and is widely employed in muscle biology research
(224). This co-culture model
simulates the in vivo physiological environment and serves
as a robust platform for studying muscle-bone interactions.
Co-culture systems are typically established using either Transwell
inserts or direct contact methods. In the Transwell system, a
porous membrane (typically 0.4 μm) separates the cell types,
enabling paracrine signaling via soluble factors while preventing
direct cell-cell contact (225). This setup allows for precise
control over the transfer of soluble factors between cell
compartments (225). However,
direct contact co-culture more accurately replicates the physical
interactions and signaling processes, including mechanical
stimulation, between muscle and bone cells (226).
Experimental designs investigating the interactions
between macrophage-derived exosomes, IGF-1, and FGF-2 typically
involve four key components: Exosome isolation and processing, gene
interference, protein expression analysis and functional assays. A
critical step in this experimental design is the processing of
exosomes, typically secreted by macrophage cell lines such as
RAW264.7. Exosomes from M1 macrophages are enriched with
pro-inflammatory factors, while exosomes derived from M2
macrophages carry anti-inflammatory and tissue-repair factors
(227). In experiments,
exosomes isolated from macrophage cell lines (such as RAW264.7) are
introduced into muscle and bone cell co-culture systems to assess
their effects on cellular proliferation, differentiation, and
mineralization (228). To
determine the specific influence of IGF-1 and FGF-2, their
functions can be inhibited using specific antagonists or gene
interference techniques, such as siRNA, to confirm their roles in
mediating musculoskeletal crosstalk. Additionally, techniques such
as WB and qPCR can be employed to analyze how M1 and M2 exosomes
regulate IGF-1 and FGF-2 expression, as well as the activity of
their downstream signaling pathways, including PI3K/Akt and MAPK
(229). Functional assays, such
as osteogenic evaluation via Alizarin Red staining and osteoclastic
activity assessment using TRAP staining, can be used to verify the
effects of exosomes on bone cell function (230). Collectively, this experimental
approach provides a framework for elucidating the complex
interactions and regulatory mechanisms involving M1- and
M2-macrophage-derived exosomes, IGF-1, and FGF-2 in bone
metabolism.
In animal studies, various intervention strategies
are employed to investigate the effects of macrophage-derived
exosomes (from M1 or M2 phenotypes), IGF-1, FGF-2 and related
factors on bone metabolism. These agents, such as M1- or M2-derived
exosomes, IGF-1 and FGF-2, are typically administered through local
injection or systemic intravenous delivery. Researchers establish
multiple experimental groups, including those treated with
M1-derived exosomes, M2-derived exosomes, IGF-1, FGF-2, and vehicle
controls, to enable direct comparison of the specific effects of
each intervention on bone metabolism. Standard detection endpoints
include bone densitometry such as dual-energy X-ray absorptiometry,
histological analysis of bone tissue (such as hematoxylin and eosin
[H&E] staining, Alizarin Red staining) and serum biomarkers of
bone metabolism (such as osteocalcin, C-terminal telopeptide of
type I collagen, bone-specific alkaline phosphatase) (246). These parameters offer a
comprehensive evaluation of bone metabolism, allowing for an
in-depth assessment of the effects mediated by M1 and M2
macrophage-derived exosomes. Collectively, these animal experiments
shed light on how M1 and M2 macrophage-derived exosomes modulate
IGF-1 and FGF-2 signaling within the musculoskeletal crosstalk
network, influencing osteocyte proliferation, differentiation and
mineralization. These findings provide a foundation for developing
novel therapeutic strategies.
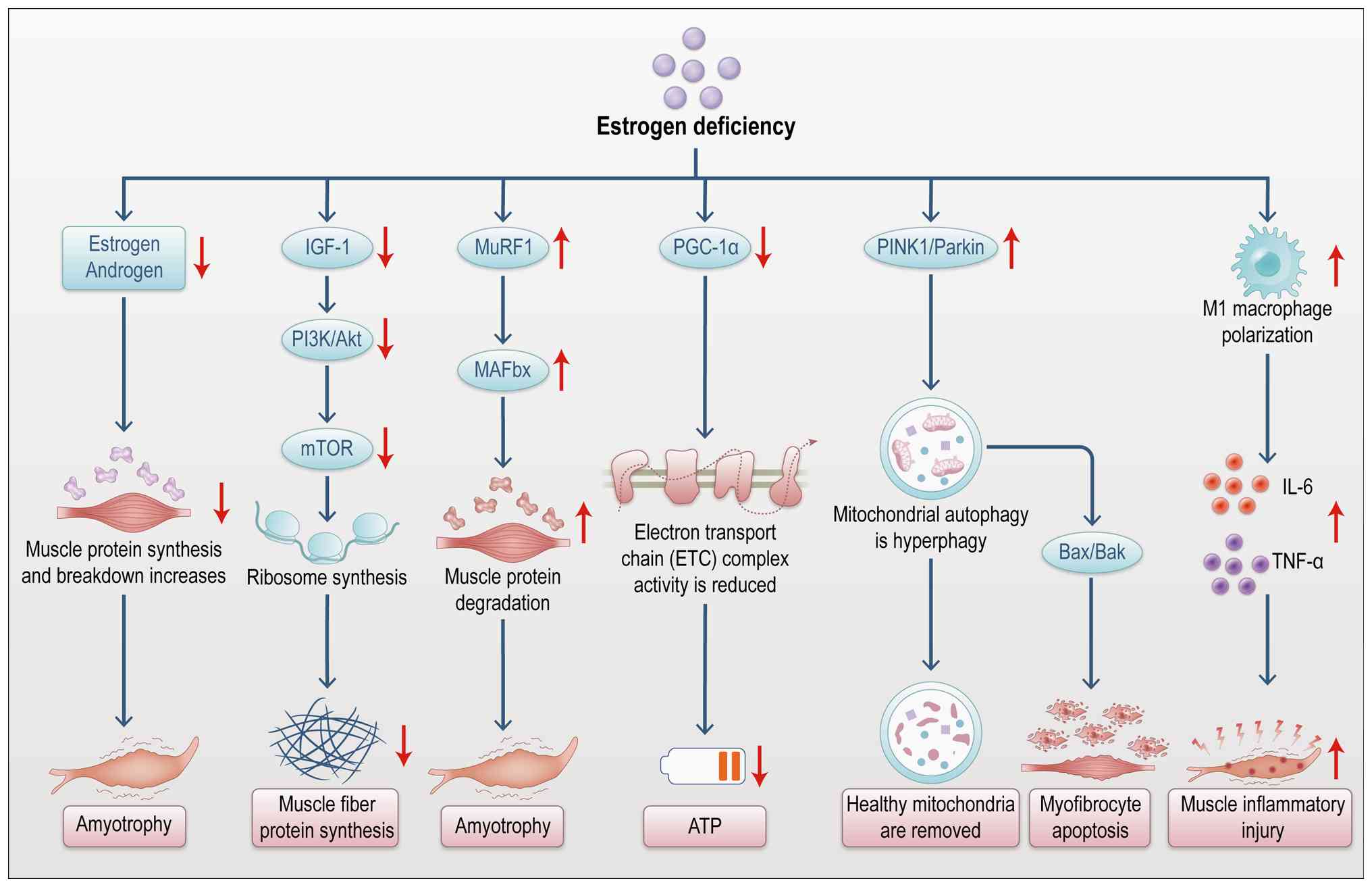
Estrogen influences bone and muscle metabolism
differently in males and females (Table IX) (261-271). The decline in estrogen levels
is the primary cause of postmenopausal osteoporosis in women
(240), leading to reduced bone
mass, muscle atrophy and an increased risk of falls and fractures
(240). SP often coexists with
osteoporosis, resulting in osteosarcopenia (272). While male androgens
predominantly regulate muscle and bone metabolism, estrogen remains
crucial for maintaining bone strength and muscle metabolic
adaptability (267). Further
studies indicate that in obesity or infection models, male
macrophages tend to be M1 polarized, with their exosomes rich in
pro-inflammatory miRNAs (such as miR-155) that inhibit the insulin
signaling pathway (273). By
contrast, macrophage extracellular vesicles in female models carry
higher levels of anti-inflammatory factors (such as miR-125a-5p),
enhancing tissue repair (274).
The decrease in estrogen levels in postmenopausal women leads to an
M1/M2 imbalance, accelerating bone loss (with osteoporosis rates
twice as high as in men) (274). In males, high testosterone
levels are positively associated with muscle mass (275). However, obese males exhibit
higher expression of miR-155 in macrophage exosomes from adipose
tissue, which increases the risk of insulin resistance (276). M2 macrophage exosomes mediate
the polarization of macrophages into anti-inflammatory phenotypes
in female models, accelerating muscle regeneration (277). These findings suggest that
estrogen exerts distinct effects on bone and muscle metabolism
across sexes, leading to different regulatory mechanisms of
macrophage exosomes in estrogen-induced muscle-bone metabolism
abnormalities, influenced by sex-specific factors.
Although RAB-GTPase-modified exosomes (RAB-EXOs),
engineered via click chemistry, can target bone tissue in complex
in vivo environments, their targeting and enrichment
efficiencies remain suboptimal (278). Drug concentrations at sites of
deep-seated bone infections, such as osteomyelitis, are often
insufficient and systemic administration can result in off-target
effects and potential adverse reactions. Studies show that
intravenously injected exosomes are primarily taken up by the
liver, skeletal muscle and adipose tissue (279-281). Enhancing exosome enrichment at
specific bone lesion sites remains a critical challenge that needs
urgent resolution (282).
Exosomes derived from macrophages of different sources and
polarization states exhibit significant differences in composition
and biological function. Exosomes from M1 and M2 macrophages can
exert opposing biological effects, and this heterogeneity presents
a considerable challenge for developing standardized therapies. One
study demonstrated that exosomes secreted by adipose tissue
macrophages in obese mice promote insulin resistance (283), whereas those from M2
macrophages improve insulin sensitivity (284). Ensuring batch-to-batch
consistency and functional stability of therapeutic exosomes is a
major challenge, as is overcoming technical bottlenecks in their
large-scale production. Exosomes isolated from 20-25 lean mice are
needed to treat one obese mouse, yielding too little for clinical
translation. Although in vitro induction of M2 macrophages
can partially address source limitations, optimization of culture
conditions, purification protocols and storage stability remains
necessary. The use of composite technologies with carrier materials
(such as hydrogels) also faces challenges in large-scale
production. While specific miRNAs, such as miR-690, mediate the
insulin-sensitizing effects of M2 macrophage-derived exosomes
(284) and the PI3K/AKT pathway
regulates macrophage polarization, significant knowledge gaps
remain regarding their application in treating musculoskeletal
tissues. The full molecular network underlying bone metabolic
diseases is not yet fully understood (285). Key questions concerning
exosomal release kinetics, interactions with host cells, and
long-term effects of exosomal cargo require further in-depth
investigation. This lack of knowledge hinders the precise design
and optimization of therapeutic regimens.
Exosomes are nanoscale extracellular vesicles with
diverse bioactivities; however, their long-term safety profile
remains incompletely characterized. Although animal studies have
shown that RAB-EXOs induce no adverse reactions within a 28-day
period in osteomyelitis treatment (286), their immunogenicity, potential
toxicity and the risk of off-target effects in human applications
require systematic evaluation. This is especially important for
patients with metabolic diseases, as exosome-based therapies may
have complex and unpredictable effects on systemic metabolic
networks. Consequently, a comprehensive safety evaluation framework
and robust risk mitigation strategies must be established.
In conclusion, exosomes derived from M1 and M2
macrophages play pivotal roles in muscle-bone crosstalk,
particularly within molecular signaling pathways mediated by
myokines such as IGF-1 and FGF-2. These factors are essential
regulators of muscle growth and bone metabolism, promoting muscle
cell proliferation and differentiation while influencing bone cell
function through various signaling pathways. Their roles extend
beyond direct regulation of local cellular behavior, modulating
bone metabolic homeostasis via macrophage-derived exosomes. Ongoing
research continues to uncover the complex mechanisms through which
M1 and M2 macrophage-derived exosomes affect bone metabolism.
Exosomes facilitate intercellular communication by transporting
bioactive molecules (such as proteins, RNAs and lipids), regulating
bone tissue formation and remodeling. Specifically, exosomes
secreted by M1 and M2 macrophages can modulate bone density and
strength by influencing osteoblast and osteoclast activity,
promoting bone health and aiding in repair. These findings provide
valuable insights into the intricate mechanisms governing bone
metabolism and lay the groundwork for developing new therapeutic
strategies.
To address current challenges, researchers are
exploring several strategies, including engineering targeted
modification technologies to enhance exosomal tissue specificity,
establishing standardized production and quality control protocols,
applying multi-omics approaches to clarify mechanisms of action and
developing smart, responsive carrier systems to improve delivery
efficiency. Innovative solutions, such as M2 macrophage
exosome-hydrogel composites, show promise for bone regeneration
therapy, but further preclinical and clinical studies are essential
to evaluate their safety and efficacy. Interdisciplinary
collaboration will be vital in advancing this field. Further
investigation into the mechanisms of M1 and M2 macrophage-derived
exosomes is expected to lead to more effective interventions for
bone-related diseases, including osteoporosis and fracture
repair.
Not applicable.
Conceptualization was by RMC, MZ, and JBH. Data
curation was by ZXW and YFC. Funding acquisition was secured by
MWL. Investigation was by SJG and YLZ. Project administration was
performed by YLZ. Software management was overseen by ZBY. Figures 1-13 were prepared by MWL and RMC.
Supervision was led by MWL. Validation was performed by RMC and
visualization was by MZ. The original draft of the manuscript was
written by MWL, who contributed to the review and editing. Data
authentication is not applicable. All authors read and approved the
final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by the Union Foundation of
Yunnan Provincial Science and Technology Department and Kunming
Medical University (grant no. 202201AY070001-091), Yunnan Province
Clinical Center for Skin Immune Diseases (grant no.
YWLCYXZX2023300076) and the Natural Science Foundation of China
(grant no. 81960350).
|
1
|
Kirk B, Lombardi G and Duque G: Bone and
muscle crosstalk in ageing and disease. Nat Rev Endocrinol.
21:375–390. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shirvani H, Shamsoddini A, Bazgir B,
McAinch AJ, Najjari A and Arabzadeh E: Metabolic crosstalk between
skeletal muscle and cartilage tissue: Insights into myokines in
osteoarthritis. Mol Biol Rep. 52:9572025. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chuang YH, Chuang WL, Huang SP and Huang
CH: Expression of epidermal growth factor, basic fibroblast growth
factor and insulin growth factor-1 and relation to myocyte
regeneration of obstructed ureters in rats. Scand J Urol Nephrol.
39:7–14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cecerska-Heryć E, Goszka M, Serwin N,
Roszak M, Grygorcewicz B, Heryć R and Dołęgowska B: Applications of
the regenerative capacity of platelets in modern medicine. Cytokine
Growth Factor Rev. 64:84–94. 2022. View Article : Google Scholar
|
|
5
|
Gries KJ, Zysik VS, Jobe TK, Griffin N,
Leeds BP and Lowery JW: Muscle-derived factors influencing bone
metabolism. Semin Cell Dev Biol. 123:57–63. 2022. View Article : Google Scholar
|
|
6
|
Pieters BCH, Cappariello A, van den Bosch
MHJ, van Lent PLEM, Teti A and van de Loo FAJ: Macrophage-derived
extracellular vesicles as carriers of alarmins and their potential
involvement in bone homeostasis. Front Immunol. 10:19012019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen Y, Liu H, He Y, Yang B, Lu W and Dai
Z: Roles for exosomes in the pathogenesis, drug delivery and
therapy of psoriasis. Pharmaceutics. 17:512025. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yue Y, Cao S, Cao F, Wei Y, Li A, Wang D,
Liu P, Zeng H and Lin J: Unveiling research hotspots: A
bibliometric study on macrophages in musculoskeletal diseases.
Front Immunol. 16:15193212025. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng A, Liu H, Yin G and Xie Q:
Macrophage-derived exosomes in autoimmune diseases: Mechanistic
insights and therapeutic implications. Immunol Res. 73:1712025.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fan C, Wang W, Yu Z, Wang J, Xu W, Ji Z,
He W, Hua D, Wang W, Yao L, et al: M1 macrophage-derived exosomes
promote intervertebral disc degeneration by enhancing nucleus
pulposus cell senescence through LCN2/NF-κB signaling axis. J
Nanobiotechnology. 22:3012024. View Article : Google Scholar
|
|
11
|
Wen Z, Li S, Liu Y, Liu X, Qiu H, Che Y,
Bian L and Zhou M: An engineered M2 macrophage-derived
exosomes-loaded electrospun biomimetic periosteum promotes cell
recruitment, immunoregulation, and angiogenesis in bone
regeneration. Bioact Mater. 50:95–115. 2025.PubMed/NCBI
|
|
12
|
Liu M, Ng M, Phu T, Bouchareychas L,
Feeley BT, Kim HT, Raffai RL and Liu X: Polarized macrophages
regulate fibro/adipogenic progenitor (FAP) adipogenesis through
exosomes. Stem Cell Res Ther. 14:3212023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou M, Li B, Liu C, Hu M, Tang J, Min J,
Cheng J and Hong L: M2 Macrophage-derived exosomal miR-501
contributes to pubococcygeal muscle regeneration. Int
Immunopharmacol. 101(Pt B): 1082232021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yuan Z, Jiang D, Yang M, Tao J, Hu X, Yang
X and Zeng Y: Emerging roles of macrophage polarization in
osteoarthritis: Mechanisms and therapeutic strategies. Orthop Surg.
16:532–550. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ascenzi F, Barberi L, Dobrowolny G, Villa
Nova Bacurau A, Nicoletti C, Rizzuto E, Rosenthal N, Scicchitano BM
and Musarò A: Effects of IGF-1 isoforms on muscle growth and
sarcopenia. Aging Cell. 18:e129542019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park SH, Park J, Yoo JY, Kim HS, Lee M and
Kim OK: Humulus japonicus enhances bone growth and
microarchitecture in rats: Potential Involvement of IGF-1
signaling. J Med Food. 28:542–552. 2025.PubMed/NCBI
|
|
17
|
Qiu Y, Yu B, Jiang C, Yin H, Meng J, Wang
H, Chen L, Cai Y, Ren T, Qin Q, et al: Bone marrow mesenchymal stem
cells overexpressing FGF-2 loaded onto a decellularized
extracellular matrix hydrogel for the treatment of osteoarthritis.
Biomater Sci. 14:9–30. 2026. View Article : Google Scholar
|
|
18
|
Jaśkiewicz Ł, Romaszko-Wojtowicz A,
Chmielewski G, Kuna J and Krajewska-Włodarczyk M: Effect of
myokines on bone tissue metabolism: a systematic review. Bone.
201:1176542025. View Article : Google Scholar
|
|
19
|
Zhao Z, Yan K, Guan Q, Guo Q and Zhao C:
Mechanism and physical activities in bone-skeletal muscle
crosstalk. Front Endocrinol (Lausanne). 14:12879722024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yin L, Lu L, Lin X and Wang X: Crucial
role of androgen receptor in resistance and endurance
trainings-induced muscle hypertrophy through
IGF-1/IGF-1R-PI3K/Akt-mTOR pathway. NutrMetab (Lond). 17:262020.
View Article : Google Scholar
|
|
21
|
Fu S, Yin L, Lin X, Lu J and Wang X:
Effects of cyclic mechanical stretch on the proliferation of L6
myoblasts and its mechanisms: PI3K/Akt and MAPK signal pathways
regulated by IGF-1 receptor. Int J Mol Sci. 19:16492018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shen L, Li Y and Zhao H: Fibroblast growth
factor signaling in macrophage polarization: Impact on health and
diseases. Front Immunol. 15:13904532024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Z, Cao F, Liang D, Pan M, Lu WW, Lyu
H, Xie Y, Zhang L and Tang P: Mechanical effects in aging of the
musculoskeletal system: Molecular signaling and spatial scale
alterations. J Orthop Translat. 52:464–477. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mao Y, Jin Z, Yang J, Xu D, Zhao L, Kiram
A, Yin Y, Zhou D, Sun Z, Xiao L, et al: Muscle-bone cross-talk
through the FNIP1-TFEB-IGF2 axis is associated with bone metabolism
in human and mouse. Sci Transl Med. 16:eadk98112024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yin P, Chen M, Rao M, Lin Y, Zhang M, Xu
R, Hu X, Chen R, Chai W, Huang X, et al: Deciphering immune
landscape remodeling unravels the underlying mechanism for
synchronized muscle and bone aging. Adv Sci (Weinh).
11:e23040842024. View Article : Google Scholar
|
|
26
|
Gómez-Bruton A, Matute-Llorente Á,
González-Agüero A, Casajús JA and Vicente-Rodríguez G: Plyometric
exercise and bone health in children and adolescents: A systematic
review. World J Pediatr. 13:112–121. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Han J, Zhang J, Zhang X, Luo W, Liu L, Zhu
Y, Liu Q and Zhang XA: Emerging role and function of Hippo-YAP/TAZ
signaling pathway in musculoskeletal disorders. Stem Cell Res Ther.
15:3862024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schiaffino S, Reggiani C, Akimoto T and
Blaauw B: molecular mechanisms of skeletal muscle hypertrophy. J
Neuromuscul Dis. 8:169–183. 2021. View Article : Google Scholar :
|
|
29
|
Von den Hoff JW, Carvajal Monroy PL,
Ongkosuwito EM, van Kuppevelt TH and Daamen WF: Muscle fibrosis in
the soft palate: Delivery of cells, growth factors and
anti-fibrotics. Adv Drug Deliv Rev. 146:60–76. 2019. View Article : Google Scholar
|
|
30
|
Savadipour A, Palmer D, Ely EV, Collins
KH, Garcia-Castorena JM, Harissa Z, Kim YS, Oestrich A, Qu F,
Rashidi N and Guilak F: The role of PIEZO ion channels in the
musculoskeletal system. Am J Physiol Cell Physiol. 324:C728–C740.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kirk B, Feehan J, Lombardi G and Duque G:
Muscle, bone, and fat crosstalk: The biological role of myokines,
osteokines, and adipokines. Curr Osteoporos Rep. 18:388–400. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo L, Quan M, Pang W, Yin Y and Li F:
Cytokines and exosomal miRNAs in skeletal muscle-adipose crosstalk.
Trends Endocrinol Metab. 34:666–681. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Calejo I, Costa-Almeida R, Reis RL and
Gomes ME: A physiology-inspired multifactorial toolbox in
soft-to-hard musculoskeletal interface tissue engineering. Trends
Biotechnol. 38:83–98. 2020. View Article : Google Scholar
|
|
34
|
Adamičková A, Chomaničová N, Gažová A,
Maďarič J, Červenák Z, Valášková S, Adamička M and Kyselovic J:
Effect of atorvastatin on angiogenesis-related genes VEGF-A, HGF
and IGF-1 and the modulation of PI3K/AKT/mTOR transcripts in
bone-marrow-derived mesenchymal stem cells. Curr Issues Mol Biol.
45:2326–2337. 2023. View Article : Google Scholar
|
|
35
|
Xu L, Zhao Q, Li K, Zhang Y, Wang C, Hind
K, Wang L, Liu Y and Cheng X: The role of sex hormones on bone
mineral density, marrow adiposity, and muscle adiposity in
middle-aged and older men. Front Endocrinol (Lausanne).
13:8174182022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang Z, Wang Y, Tang Y, Guo X, Gao Q, Shao
Y, Wang J, Tian R and Shi Y: Sodium benzoate inhibits osteoblast
differentiation and accelerates bone loss by regulating the
FGF2/p38/RUNX2 pathway. J Agric Food Chem. 73:13891–13901. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ogura H, Nakamura T, Ishii T, Saito A,
Onodera S, Yamaguchi A, Nishii Y and Azuma T: Mechanical
stress-induced FGF-2 promotes proliferation and consequently
induces osteoblast differentiation in mesenchymal stem cells.
Biochem Biophys Res Commun. 684:1491452023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bakker AD and Jaspers RT: IL-6 and IGF-1
signaling within and between muscle and bone: How important is the
mTOR pathway for bone metabolism? Curr Osteoporos Rep. 13:131–139.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yoshida T and Delafontaine P: Mechanisms
of IGF-1-mediated regulation of skeletal muscle hypertrophy and
atrophy. Cells. 9:19702020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chai J, Xu L and Liu N: miR-23b-3p
regulates differentiation of osteoclasts by targeting PTEN via the
PI3k/AKT pathway. Arch Med Sci. 18:1542–1557. 2019.PubMed/NCBI
|
|
41
|
Liu X, Liu L, Chen K, Sun L, Li W and
Zhang S: Huaier shows anti-cancer activities by inhibition of cell
growth, migration and energy metabolism in lung cancer through
PI3K/AKT/HIF-1α pathway. J Cell Mol Med. 25:2228–2237. 2021.
View Article : Google Scholar
|
|
42
|
Hang K, Wang Y, Bai J, Wang Z, Wu W, Zhu
W, Liu S, Pan Z, Chen J and Chen W: Chaperone-mediated autophagy
protects the bone formation from excessive inflammation through
PI3K/AKT/GSK3β/β-catenin pathway. FASEB J. 38:e236462024.
View Article : Google Scholar
|
|
43
|
Peifer C, Oláh T, Venkatesan JK, Goebel L,
Orth P, Schmitt G, Zurakowski D, Menger MD, Laschke MW, Cucchiarini
M and Madry H: locally directed recombinant adeno-associated
virus-mediated igf-1 gene therapy enhances osteochondral repair and
counteracts early osteoarthritis in vivo. Am J Sports Med.
52:1336–1349. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang C, Wang J, Xie Y, Wang L, Yang L, Yu
J, Miyamoto A and Sun F: Development of FGF-2-loaded electrospun
waterborne polyurethane fibrous membranes for bone regeneration.
Regen Biomater. 8:rbaa0462020. View Article : Google Scholar
|
|
45
|
Kogure K, Hasuike A, Kurachi R, Igarashi
Y, Idesawa M and Sato S: Effect of a recombinant human basic
fibroblast growth factor 2 (rhFGF-2)-Impregnated atelocollagen
sponge on vertical guided bone regeneration in a rat calvarial
model. Dent J (Basel). 13:1772025. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yu X, Qi Y, Zhao T, Fang J, Liu X, Xu T,
Yang Q and Dai X: NGF increases FGF2 expression and promotes
endothelial cell migration and tube formation through PI3K/Akt and
ERK/MAPK pathways in human chondrocytes. Osteoarthritis Cartilage.
27:526–534. 2019. View Article : Google Scholar
|
|
47
|
Yin J, Qiu S, Shi B, Xu X, Zhao Y, Gao J,
Zhao S and Min S: Controlled release of FGF-2 and BMP-2 in tissue
engineered periosteum promotes bone repair in rats. Biomed Mater.
13:0250012018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wu H, Yin G, Pu X, Wang J, Liao X and
Huang Z: Inhibitory effects of combined bone morphogenetic protein
2, vascular endothelial growth factor, and basic fibroblast growth
factor on osteoclast differentiation and activity. Tissue Eng Part
A. 27:1387–1398. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lötvall J, Hill AF, Hochberg F, Buzás EI,
Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S,
Quesenberry P, et al: Minimal experimental requirements for
definition of extracellular vesicles and their functions: A
position statement from the International Society for Extracellular
Vesicles. J Extracell Vesicles. 3:269132014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Akbar N, Paget D and Choudhury RP:
Extracellular vesicles in innate immune cell programming.
Biomedicines. 9:7132021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Garzetti L, Menon R, Finardi A, Bergami A,
Sica A, Martino G, Comi G, Verderio C, Farina C and Furlan R:
Activated macrophages release microvesicles containing polarized M1
or M2 mRNAs. J Leukoc Biol. 95:817–825. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Guan X, Li C, Wang X, Yang L, Lu Y and Guo
Z: Engineered M2 macrophage-derived exosomes: mechanisms and
therapeutic potential in inflammation regulation and regenerative
medicine. Acta Biomater. 203:38–58. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mäki-Mantila K, Niskanen EA, Kainulainen
K, Pardas LP, Aaltonen N, Wahbi W, Takabe P, Rönkä A, Rilla K and
Pasonen-Seppänen S: Extracellular vesicles derived from
pro-inflammatory M1 macrophages induce an inflammatory and invasive
phenotype in melanoma cells. Cell Commun Signal. 24:102025.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Vizoso FJ, Eiro N, Cid S, Schneider J and
Perez-Fernandez R: Mesenchymal stem cell secretome: Toward
cell-free therapeutic strategies in regenerative medicine. Int J
Mol Sci. 18:18522017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liu S, Chen J, Shi J, Zhou W, Wang L, Fang
W, Zhong Y, Chen X, Chen Y, Sabri A and Liu S: M1-like
macrophage-derived exosomes suppress angiogenesis and exacerbate
cardiac dysfunction in a myocardial infarction microenvironment.
Basic Res Cardiol. 115:222020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Li Z, Wang Y, Li S and Li Y: Exosomes
derived from M2 macrophages facilitate osteogenesis and reduce
adipogenesis of BMSCs. Front Endocrinol (Lausanne). 12:6803282021.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhang Y, Liang Y and Zhou Y: M2
polarization of RAW264.7-derived exosomes inhibits osteoclast
differentiation and inflammation via PKM2/HIF-1α axis. Immunol
Invest. 54:1195–1209. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Huang R, Wang X, Zhou Y and Xiao Y:
RANKL-induced M1 macrophages are involved in bone formation. Bone
Res. 5:170192017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kang M, Huang CC, Lu Y, Shirazi S,
Gajendrareddy P, Ravindran S and Cooper LF: Bone regeneration is
mediated by macrophage extracellular vesicles. Bone.
141:1156272020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ge X, Tang P, Rong Y, Jiang D, Lu X, Ji C,
Wang J, Huang C, Duan A, Liu Y, et al: Exosomal miR-155 from
M1-polarized macrophages promotes Endo MT and impairs mitochondrial
function via activating NF-κB signaling pathway in vascular
endothelial cells after traumatic spinal cord injury. Redox Biol.
41:1019322021. View Article : Google Scholar
|
|
61
|
Hu Y, Wang Y, Chen T, Hao Z, Cai L and Li
J: Exosome: Function and application in inflammatory bone diseases.
Oxid Med Cell Longev. 2021:63249122021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Pajarinen J, Lin T, Gibon E, Kohno Y,
Maruyama M, Nathan K, Lu L, Yao Z and Goodman SB: Mesenchymal stem
cell-macrophage crosstalk and bone healing. Biomaterials.
196:80–89. 2019. View Article : Google Scholar
|
|
63
|
Paschalidi P, Gkouveris I, Soundia A,
Kalfarentzos E, Vardas E, Georgaki M, Kostakis G, Erovic BM,
Tetradis S, Perisanidis C and Nikitakis NG: The role of M1 and M2
macrophage polarization in progression of medication-related
osteonecrosis of the jaw. Clin Oral Investig. 25:2845–2857. 2021.
View Article : Google Scholar :
|
|
64
|
Boldin MP and Baltimore D: MicroRNAs, new
effectors and regulators of NF-κB. Immunol Rev. 246:205–220. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Salhotra A, Shah HN, Levi B and Longaker
MT: Mechanisms of bone development and repair. Nat Rev Mol Cell
Biol. 21:696–711. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wang Z, Zhu H, Shi H, Zhao H, Gao R, Weng
X, Liu R, Li X, Zou Y, Hu K, et al: Exosomes derived from M1
macrophages aggravate neointimal hyperplasia following carotid
artery injuries in mice through miR-222/CDKN1B/CDKN1C pathway. Cell
Death Dis. 10:4222019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Qi Y, Zhu T, Zhang T, Wang X, Li W, Chen
D, Meng H and An S: M1 macrophage-derived exosomes transfer miR-222
to induce bone marrow mesenchymal stem cell apoptosis. Lab Invest.
101:1318–1326. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Li L, Zheng B, Zhang F, Luo X, Li F, Xu T,
Zhao H, Shi G, Guo Y, Shi J and Sun J: LINC00370 modulates
miR-222-3p-RGS4 axis to protect against osteoporosis progression.
Arch Gerontol Geriatr. 97:1045052021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Jiang C, Xia W, Wu T, Pan C, Shan H, Wang
F, Zhou Z and Yu X: Inhibition of microRNA-222 up-regulates TIMP3
to promotes osteogenic differentiation of MSCs from fracture rats
with type 2 diabetes mellitus. J Cell Mol Med. 24:686–694. 2020.
View Article : Google Scholar
|
|
70
|
Yu L, Hu M, Cui X, Bao D, Luo Z, Li D, Li
L, Liu N, Wu Y, Luo X and Ma Y: M1 macrophage-derived exosomes
aggravate bone loss in postmenopausal osteoporosis via a
microRNA-98/DUSP1/JNK axis. Cell Biol Int. 45:2452–2463. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Peng S, Yan Y, Li R, Dai H and Xu J:
Extracellular vesicles from M1-polarized macrophages promote
inflammation in the temporomandibular joint via miR-1246 activation
of the Wnt/β-catenin pathway. Ann N Y Acad Sci. 1503:48–59. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
He XT, Li X, Yin Y, Wu RX, Xu XY and Chen
FM: The effects of conditioned media generated by polarized
macrophages on the cellular behaviours of bone marrow mesenchymal
stem cells. J Cell Mol Med. 22:1302–1315. 2018. View Article : Google Scholar :
|
|
73
|
Xia Y, He XT, Xu XY, Tian BM, An Y and
Chen FM: Exosomes derived from M0, M1 and M2 macrophages exert
distinct influences on the proliferation and differentiation of
mesenchymal stem cells. PeerJ. 8:e89702020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Schlundt C, Fischer H, Bucher CH,
Rendenbach C, Duda GN and Schmidt-Bleek K: The multifaceted roles
of macrophages in bone regeneration: A story of polarization,
activation and time. Acta Biomater. 133:46–57. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wei F, Zhou Y, Wang J, Liu C and Xiao Y:
The immunomodulatory role of BMP-2 on macrophages to accelerate
osteogenesis. Tissue Eng Part A. 24:584–594. 2018. View Article : Google Scholar
|
|
76
|
Wang J, Xue Y, Wang Y, Liu C, Hu S, Zhao
H, Gu Q, Yang H, Huang L, Zhou X and Shi Q: BMP-2 functional
polypeptides relieve osteolysis via bi-regulating bone formation
and resorption coupled with macrophage polarization. NPJ Regen Med.
8:62023. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Bouchareychas L, Duong P, Covarrubias S,
Alsop E, Phu TA, Chung A, Gomes M, Wong D, Meechoovet B, Capili A,
et al: Macrophage exosomes resolve atherosclerosis by regulating
hematopoiesis and inflammation via microRNA cargo. Cell Rep.
32:1078812020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Yu S, Geng Q, Pan Q, Liu Z, Ding S, Xiang
Q, Sun F, Wang C, Huang Y and Hong A: miR-690, a Runx2-targeted
miRNA, regulates osteogenic differentiation of C2C12 myogenic
progenitor cells by targeting NF-kappaB p65. Cell Biosci. 6:102016.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Yang Y, Guo Z, Chen W, Wang X, Cao M, Han
X, Zhang K, Teng B, Cao J, Wu W, et al: M2 macrophage-derived
exosomes promote angiogenesis and growth of pancreatic ductal
adenocarcinoma by targeting E2F2. Mol Ther. 29:1226–1238. 2021.
View Article : Google Scholar :
|
|
80
|
Xu T, Luo Y, Wang J, Zhang N, Gu C, Li L,
Qian D, Cai W, Fan J and Yin G: Exosomal miRNA128-3pfrom
mesenchymal stem cells of aged rats regulates osteogenesis and bone
fracture healing by targeting Smad5. J Nanobiotechnology.
18:472020. View Article : Google Scholar
|
|
81
|
Zhang D, Wu Y, Li Z, Chen H, Huang S, Jian
C and Yu A: miR-144-5p, an exosomal miRNA from bone marrow-derived
macrophage in type 2 diabetes, impairs bone fracture healing via
targeting Smad1. J Nanobiotechnology. 19:2262021. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Huang X, Xiong X, Liu J, Zhao Z and Cen X:
MicroRNAs-containing extracellular vesicles in bone remodeling:
anemerging frontier. Life Sci. 254:1178092020. View Article : Google Scholar
|
|
83
|
Luo ML, Jiao Y, Gong WP, Li Y, Niu LN, Tay
FR and Chen JH: Macrophages enhance mesenchymal stem cell
osteogenesis via down-regulation of reactive oxygen species. J
Dent. 94:1032972020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ren Q, Xing W, Jiang B, Feng H, Hu X, Suo
J, Wang L and Zou W: Tenascin-C promotes bone regeneration via
inflammatory macrophages. Cell Death Differ. 32:763–775. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Halper J, Dolfi B, Ivanov S, Madel MB and
Blin-Wakkach C: Macrophages and osteoclasts: Similarity and
divergence between bone phagocytes. Front Immunol. 16:16838722025.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Hao W, Chen S, Chao H, Li Z, Yang H, Chen
D, Li S, Zhang S, Zhang J, Wang J, et al: IL-33-Induced TREM2(+)
macrophages promote pathological new bone formation through
CREG1-IGF2R axis in ankylosing spondylitis. Adv Sci (Weinh).
12:e25009522025. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Choi JS, Yoon HI, Lee KS, Choi YC, Yang
SH, Kim IS and Cho YW: Exosomes from differentiating human skeletal
muscle cells trigger myogenesis of stem cells and provide
biochemical cues for skeletal muscle regeneration. J Control
Release. 222:107–115. 2016. View Article : Google Scholar
|
|
88
|
Forterre A, Jalabert A, Berger E, Baudet
M, Chikh K, Errazuriz E, De Larichaudy J, Chanon S, Weiss-Gayet M,
Hesse AM, et al: Proteomic analysis of C2C12 myoblast and myotube
exosome-like vesicles: A new paradigm for myoblast-myotube cross
talk? PLoS One. 9:e841532014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Mobley CB, Mumford PW, McCarthy JJ, Miller
ME, Young KC, Martin JS, Beck DT, Lockwood CM and Roberts MD: Whey
protein-derived exosomes increase protein synthesis and hypertrophy
in C2-C12 myotubes. J Dairy Sci. 100:48–64. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yin J, Qian Z, Chen Y, Li Y and Zhou X:
MicroRNA regulatory networks in the pathogenesis of sarcopenia. J
Cell Mol Med. 24:4900–4912. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Gopal Krishnan PD, Lee WX, Goh KY, Choy
SM, Turqueza LRR, Lim ZH and Tang HW: Transcriptional regulation of
autophagy in skeletal muscle stem cells. Dis Model Mech.
18:DMM0520072025. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Chen W, Chen Y, Liu Y and Wang X:
Autophagy in muscle regeneration: Potential therapies for
myopathies. J Cachexia Sarcopenia Muscle. 13:1673–1685. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Guescini M, Canonico B, Lucertini F,
Maggio S, Annibalini G, Barbieri E, Luchetti F, Papa S and Stocchi
V: Muscle releases alpha-sarcoglycan positive extracellular
vesicles carrying miRNAs in the bloodstream. PLoS One.
10:e01250942015. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Chaturvedi P, Kalani A, Medina I,
Familtseva A and Tyagi SC: Cardiosome mediated regulation of MMP9
in diabetic heart: Role of mir29b and mir455 in exercise. J Cell
Mol Med. 19:2153–2161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Kalluri R and LeBleu VS: The biology,
function, and biomedical applications of exosomes. Science.
367:eaau69772020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Qin W and Dallas SL: Exosomes and
extracellular RNA in muscle and bone aging and crosstalk. Curr
Osteoporos Rep. 17:548–559. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Herrmann M, Engelke K, Ebert R,
Müller-Deubert S, Rudert M, Ziouti F, Jundt F, Felsenberg D and
Jakob F: Interactions between muscle and bone-where physics meets
biology. Biomolecules. 10:4322020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Li G, Zhang L, Wang D, AIQudsy L, Jiang
JX, Xu H and Shang P: Muscle-bone crosstalk and potential therapies
for sarco-osteoporosis. J Cell Biochem. 120:14262–14273. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Xu Q, Cui Y, Luan J, Zhou X, Li H and Han
J: Exosomes from C2C12 myoblasts enhance osteogenic differentiation
of MC3T3-E1 pre-osteoblasts by delivering miR-27a-3p. Biochem
Biophys Res Commun. 498:32–37. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Kitase Y, Vallejo JA, Gutheil W, Vemula H,
Jähn K, Yi J, Zhou J, Brotto M and Bonewald LF: β-aminoiso-butyric
acid, 1-BAIBA, is a muscle-derived osteocyte survival factor. Cell
Rep. 22:1531–1544. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Li Y, Wang X, Pan C, Yuan H, Li X, Chen Z
and He H: Myoblast-derived exosomal Prrx2 attenuates osteoporosis
via transcriptional regulation of lncRNA-MIR22HG to activate Hippo
pathway. Mol Med. 29:542023. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Li H, Lin X, Yang D, Chen Z, Wang X, Re F,
Wei J and Chen J: Cancer-associated fibroblasts support bone tropic
metastasis by acting as coordinators between the tumor
microenvironment and bone matrix in breast cancer. Neoplasma.
68:10–22. 2021. View Article : Google Scholar
|
|
103
|
Zheng YL, Song G, Guo JB, Su X, Chen YM,
Yang Z, Chen PJ and Wang XQ: Interactions among lncRNA/circRNA,
miRNA, and mRNA in musculoskeletal degenerative diseases. Front
Cell Dev Biol. 9:7539312021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Toita R, Shimizu Y, Shimizu E, Deguchi T,
Tsuchiya A, Kang JH, Kitamura M, Kato A, Yamada H, Yamaguchi S and
Kasahara S: Collagen patches releasing phosphatidylserine liposomes
guide M1-to-M2 macrophage polarization and accelerate simultaneous
bone and muscle healing. Acta Biomater. 187:51–65. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Wehbe Z, Wehbe M, Al Khatib A, Dakroub AH,
Pintus G, Kobeissy F and Eid AH: Emerging understandings of the
role of exosomes in atherosclerosis. J Cell Physiol.
240:e314542025. View Article : Google Scholar :
|
|
106
|
An F, Wang X, Wang C, Liu Y, Sun B, Zhang
J, Gao P and Yan C: Research progress on the role of lncRNA-miRNA
networks in regulating adipogenic and osteogenic differentiation of
bone marrow mesenchymal stem cells in osteoporosis. Front
Endocrinol (Lausanne). 14:12106272023. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Liu K, Luo X, Lv ZY, Zhang YJ, Meng Z, Li
J, Meng CX, Qiang HF, Hou CY, Hou L, et al: Macrophage-derived
exosomes promote bone mesenchymal stem cells towards osteoblastic
fate through microRNA-21a-5p. Front Bioeng Biotechnol.
9:8014322022. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Xiong Y, Tang Y, Fan F, Zeng Y, Li C, Zhou
G, Hu Z, Zhang L and Liu Z: Exosomal hsa-miR-21-5p derived from
growth hormone-secreting pituitary adenoma promotes abnormal bone
formation in acromegaly. Transl Res. 215:1–16. 2020. View Article : Google Scholar
|
|
109
|
Choi SH, Chung KY, Johnson BJ, Go GW, Kim
KH, Choi CW and Smith SB: Co-culture of bovine muscle satellite
cells with preadipocytes increases PPARү and C/EBPβ gene expression
in differentiated myoblasts and increases GPR43 gene expression in
adipocytes. J Nutr Biochem. 24:539–543. 2013. View Article : Google Scholar
|
|
110
|
Yuan R and Li J: Role of macrophages
andtheir exosomes in orthopedic diseases. Peer J.
12:e171462024.
|
|
111
|
Li K, Yan G, Huang H, Zheng M, Ma K, Cui
X, Lu D, Zheng L, Zhu B, Cheng J and Zhao J: Anti-inflammatory and
immunomodulatory effects of the extracellular vesicles derived from
human umbilical cord mesenchymal stem cells on osteoarthritis via
M2 macrophages. J Nanobiotechnology. 20:382022. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Pu P, Wu S, Zhang K, Xu H, Guan J, Jin Z,
Sun W, Zhang H and Yan B: Mechanical force induces
macrophage-derived exosomal UCHL3 promoting bone marrow mesenchymal
stem cell osteogenesis by targeting SMAD1. J Nanobiotechnology.
21:882023. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Zhdanov VP: Interplay of cellular mRNA,
miRNA and Viral miRNA during infection of a cell. Int J Mol Sci.
24:1222022. View Article : Google Scholar
|
|
114
|
Bin-Bin Z, Da-Wa ZX, Chao L, Lan-Tao Z,
Tao W, Chuan L, Chao-Zheng L, De-Chun L, Chang F, Shu-Qing W, et
al: M2 macrophagy-derived exosomal miRNA-26a-5p induces osteogenic
differentiation of bone mesenchymal stem cells. J Orthop Surg Res.
17:1372022. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Qi L, Hong S, Zhao T, Yan J, Ge W, Wang J,
Fang X, Jiang W, Shen SG and Zhang L: DNA Tetrahedron Delivering
miR-21-5p promotes senescent bone defects repair through
synergistic regulation of osteogenesis and angiogenesis. Adv
Healthc Mater. 13:e24012752024. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Hrdlicka HC, Pereira RC, Shin B, Yee SP,
Deymier AC, Lee SK and Delany AM: Inhibition of miR-29-3p isoforms
via tough decoy suppresses osteoblast function in homeostasis but
promotes intermittent parathyroid hormone-induced bone anabolism.
Bone. 143:1157792021. View Article : Google Scholar
|
|
117
|
Gu Y, Ma L, Song L, Li X, Chen D and Bai
X: miR-155 inhibits mouse Osteoblast differentiation by suppressing
SMAD5 expression. Biomed Res Int. 2017:18935202017. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Lan S and Albinsson S: Regulation of
IRS-1, insulin signaling and glucose uptake by miR-143/145 in
vascular smooth muscle cells. Biochem Biophys Res Commun.
529:119–125. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Hade MD, Suire CN and Suo Z: Mesenchymal
stem cell-derived exosomes: Applications in regenerative medicine.
Cells. 10:19592021. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Mezil Y, Obeid J, Raha S, Hawke TJ and
Timmons BW: The Systemic Effects of Exercise on Regulators of
Muscle and Bone in Girls and Women. Pediatr Exerc Sci. 32:117–123.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Zhang J, Rong Y, Luo C and Cui W: Bone
marrow mesenchymal stem cell-derived exosomes prevent
osteoarthritis by regulating synovial macrophage polarization.
Aging (Albany NY). 12:25138–25152. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Qin M, Zhu J, Xing L, Fan Y, Luo J, Sun J,
Chen T, Zhang Y and Xi Q: Adipose-derived exosomes ameliorate
skeletal muscle atrophy via miR-146a-5p/IGF-1R signaling. J
Nanobiotechnology. 22:7542024. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Hu GF, Wang C, Hu GX, Wu G, Zhang C, Zhu
W, Chen C, Gu Y, Zhang H and Yang Z: AZD3463, an IGF-1R inhibitor,
suppresses breast cancer metastasis to bone via modulation of the
PI3K-Akt pathway. Ann Transl Med. 8:3362020. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Cao D, Lei Y, Ye Z, Zhao L, Wang H, Zhang
J, He F, Huang L, Shi D, Liu Q, et al: Blockade of IGF/IGF-1R
signaling axis with soluble IGF-1R mutants suppresses the cell
proliferation and tumor growth of human osteosarcoma. Am J Cancer
Res. 10:3248–3266. 2020.PubMed/NCBI
|
|
125
|
Song X, Xue Y, Fan S, Hao J and Deng R:
Lipopolysaccharide-activated macrophages regulate the osteogenic
differentiation of bone marrow mesenchymal stem cells through
exosomes. PeerJ. 10:e134422022. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Shu C, Smith SM, Little CB and Melrose J:
Use of FGF-2 and FGF-18 to direct bone marrow stromal stem cells to
chondrogenic and osteogenic lineages. Future Sci OA. 2:FSO1422016.
View Article : Google Scholar
|
|
127
|
Adhikary S, Choudhary D, Tripathi AK,
Karvande A, Ahmad N, Kothari P and Trivedi R: FGF-2 targets
sclerostin in bone and myostatin in skeletal muscle to mitigate the
deleterious effects of glucocorticoid on musculoskeletal
degradation. Life Sci. 229:261–276. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Le Blanc S, Simann M, Jakob F, Schütze N
and Schilling T: Fibroblast growth factors 1 and 2 inhibit
adipogenesis of human bone marrow stromal cells in 3D collagen
gels. Exp Cell Res. 338:136–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Yang F, Chen C, Yang C, Chen R, Liu Z, Wen
L, Xiao H, Zhou L, Geng B and Xia Y: Exosome-mediated perturbation
of the immune-bone metabolism axis: A mechanistic investigation
into bone loss in a simulated microgravity environment. Artif Cells
Nanomed Biotechnol. 53:494–513. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Hu K and Olsen BR: Osteoblast-derived VEGF
regulates osteoblast differentiation and bone formation during bone
repair. J Clin Invest. 126:509–526. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Jiménez-Ortega RF, Ortega-Meléndez AI,
Patiño N, Rivera-Paredez B, Hidalgo-Bravo A and Velázquez-Cruz R:
The involvement of microRNAs in bone remodeling signaling pathways
and their role in the development of osteoporosis. Biology (Basel).
13:5052024.PubMed/NCBI
|
|
132
|
Geng Z, Sun T, Yu J, Wang N, Jiang Q, Wang
P, Yang G, Li Y, Ding Y, Zhang J, et al: cinobufagin suppresses
lipid peroxidation and inflammation in osteoporotic mice by
promoting the delivery of miR-3102-5p by macrophage-derived
exosomes. Int J Nanomedicine. 19:10497–10512. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Hosseinpour S, Dai H, Walsh LJ and Xu C:
Mesoporous core-cone silica nanoparticles can deliver mirna-26a to
macrophages to exert immunomodulatory effects on osteogenesis in
vitro. Nanomaterials (Basel). 13:17552023. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Liu J, Sun Z, You Y, Zhang L, Hou D, Gu G,
Chen Y and Jiao G: M2 macrophage-derived exosomal miR-486-5p
influences the differentiation potential of bone marrow mesenchymal
stem cells and osteoporosis. Aging (Albany NY). 15:9499–9520. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Zhao W, Zhang S, Wang B, Huang J, Lu WW
and Chen D: Runx2 and microRNA regulation in bone and cartilage
diseases. Ann N Y Acad Sci. 1383:80–87. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Faraldi M, Sansoni V and Lombardi G:
Recent advances in the role of miRNAs in bone disease. Curr Opin
Endocrinol Diabetes Obes. 32:149–155. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Chen G, Deng C and Li YP: TGF-β and BMP
signaling in osteoblast differentiation and bone formation. Int J
Biol Sci. 8:272–288. 2012. View Article : Google Scholar :
|
|
138
|
Kuroyanagi G, Tokuda H, Fujita K, Kawabata
T, Sakai G, Kim W, Hioki T, Tachi J, Matsushima-Nishiwaki R, Otsuka
T, et al: Upregulation of TGF-β-induced HSP27 by HSP90 inhibitors
in osteoblasts. BMC Musculoskelet Disord. 23:4952022. View Article : Google Scholar
|
|
139
|
Wu Y, Zhang C and Lv C: Direct and
indirect regulation of bone metabolism by lactoferrin. Front
Endocrinol (Lausanne). 16:16603122025. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Méndez-Mancilla A, Turiján-Espinoza E,
Vega-Cárdenas M, Hernández-Hernández GE, Uresti-Rivera EE,
Vargas-Morales JM and Portales-Pérez DP: miR-21, miR-221, miR-29
and miR-34 are distinguishable molecular features of a
metabolically unhealthy phenotype in young adults. PLoS One.
19:e03004202024. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Fu X, Li Y, Huang T, Yu Z, Ma K, Yang M,
Liu Q, Pan H, Wang H, Wang J and Guan M: Runx2/Osterix and zinc
uptake synergize to orchestrate osteogenic differentiation and
citrate containing bone apatite formation. Adv Sci (Weinh).
5:17007552018.PubMed/NCBI
|
|
142
|
Luo K: Signaling cross talk between
TGF-β/Smad and other signaling pathways. Cold Spring Harb Perspect
Biol. 9:a0221372017.
|
|
143
|
Zou ML, Chen ZH, Teng YY, Liu SY, Jia Y,
Zhang KW, Sun ZL, Wu JJ, Yuan ZD, Feng Y, et al: The Smad Dependent
TGF-beta and BMP signaling pathway in bone remodeling and
therapies. Front Mol Biosci. 8:5933102021.
|
|
144
|
Zhang B, Zhang X, Xiao J, Zhou X, Chen Y
and Gao C: Neuropeptide Y upregulates Runx2 and osterix and
enhances osteogenesis in mouse MC3T3-E1 cells via an autocrine
mechanism. Mol Med Rep. 22:4376–4382. 2020.PubMed/NCBI
|
|
145
|
Chen M, Li Y, Yang Z, Chen M, Li J, Bao G,
Ma L and Hu J: M2-exo promote orthodontic bone remodeling via the
MeCP2-TCF20-HDAC1 axis. Stem Cell Res Ther. 16:5692025.PubMed/NCBI
|
|
146
|
Fu XH, Li JP, Li XY, Tan Y, Zhao M, Zhang
SF, Wu XD and Xu JG: M2-Macrophage-Derived exosomes promote
meningioma progression through TGF-β signaling pathway. J Immunol
Res. 2022:83265912022.
|
|
147
|
Li H, Yang Y, Gao Y, Li B, Yang J, Liu P,
Zhang M and Ning G: Exosomes derived from hypoxia-preconditioned M2
macrophages alleviate degeneration in knee osteoarthritis through
the miR-124-3p/STAT3 axis. J Transl Med. 23:7722025.
|
|
148
|
Xie Q, Wei W, Ruan J, Ding Y, Zhuang A, Bi
X, Sun H, Gu P, Wang Z and Fan X: Effects of miR-146a on the
osteogenesis of adipose-derived mesenchymal stem cells and bone
regeneration. Sci Rep. 7:428402017.PubMed/NCBI
|
|
149
|
Ding S, Ma Y, Yang J, Tang Y, Jin Y, Li L
and Ma C: MiR-224-5p inhibits osteoblast differentiation and
impairs bone formation by targeting Runx2 and Sp7. Cytotechnology.
75:505–516. 2023.PubMed/NCBI
|
|
150
|
Liao X, Yang Z, Li Y, Cui Y, Ma L, Liang
C, Guan Z and Hu J: M2 macrophage-derived exosome facilitates
aerobic glycolysis and osteogenic differentiation of hPDLSCs by
regulating TRIM26-induced PKM ubiquitination. Free Radic Biol Med.
237:88–100. 2025.PubMed/NCBI
|
|
151
|
Le G, Wen R, Fang H, Huang Z, Wang Y and
Luo H: Exosomal miR-122 derived from M2 macrophages induces
osteogenic differentiation of bone marrow mesenchymal stem cells in
the treatment of alcoholic osteonecrosis of the femoral head. J
Orthop Surg Res. 20:1072025. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Xu Y, Jin Y, Hong F, Ma Y, Yang J, Tang Y,
Zhu Z, Wu J, Bao Q, Li L, et al: MiR-664-3p suppresses osteoblast
differentiation and impairs bone formation via targeting Smad4 and
Osterix. J Cell Mol Med. 25:5025–5037. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Sun WL, Wang N and Xu Y: Impact of
miR-302b on calcium-phosphorus metabolism and vascular
calcification of rats with chronic renal failure by regulating
BMP-2/Runx2/Osterix signaling pathway. Arch Med Res. 49:164–171.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Ling F, Bai J, Xie J, Liu J, Lu Q, Yuan L,
Li H and Qian Z: Biomimetic periosteum combining BMP-2-loaded M2
macrophage-derived exosomes for enhanced bone defect repair. Front
Bioeng Biotechnol. 13:16393942025. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Yan CP, Wang XK, Jiang K, Yin C, Xiang C,
Wang Y, Pu C, Chen L and Li YL: β-Ecdysterone enhanced bone
regeneration through the BMP-2/SMAD/RUNX2/Osterix signaling
pathway. Front Cell Dev Biol. 10:8832282022. View Article : Google Scholar
|
|
156
|
Zhang Y, Yu T, Xiang Q, van den Tillaart
F, Ma J, Zhuang Z, Stessuk T, Wang H and van den Beucken JJJP:
Osteoclasts drive bone formation in ectopic and orthotopic
environments. Biomaterials. 322:1233772025. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Chen X, Wan Z, Yang L, Song S, Fu Z, Tang
K, Chen L and Song Y: Exosomes derived from reparative M2-like
macrophages prevent bone loss in murine periodontitis models via
IL-10 mRNA. J Nanobiotechnology. 20:1102022. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Udagawa N, Koide M, Nakamura M, Nakamichi
Y, Yamashita T, Uehara S, Kobayashi Y, Furuya Y, Yasuda H, Fukuda C
and Tsuda E: Osteoclast differentiation by RANKL and OPG signaling
pathways. J Bone Miner Metab. 39:19–26. 2021. View Article : Google Scholar
|
|
159
|
Kim HJ, Kang WY, Seong SJ, Kim SY, Lim MS
and Yoon YR: Follistatin-like 1 promotes osteoclast formation via
RANKL-mediated NF-ĸB activation and M-CSF-induced precursor
proliferation. Cell Signal. 28:1137–1144. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Nakao Y, Fukuda T, Zhang Q, Sanui T,
Shinjo T, Kou X, Chen C, Liu D, Watanabe Y, Hayashi C, et al:
Exosomes from TNF-alpha-treated human gingiva-derived MSCs enhance
M2 macrophage polarization and inhibit periodontal bone loss. Acta
Biomater. 122:306–324. 2021. View Article : Google Scholar
|
|
161
|
Chen S, Liu J and Zhu L: M2-like
macrophage-derived exosomes inhibit osteoclastogenesis via
releasing miR-1227-5p. Immunobiology. 230:1528612025. View Article : Google Scholar
|
|
162
|
Takeda T, Tsubaki M, Genno S, Tomita K and
Nishida S: RANK/RANKL axis promotes migration, invasion, and
metastasis of osteosarcoma via activating NF-ĸB pathway. Exp Cell
Res. 436:1139782024. View Article : Google Scholar
|
|
163
|
Guo W, Li H, Lou Y, Zhang Y, Wang J, Qian
M, Wei H, Xiao J and Xu Y: Tyloxapol inhibits RANKL-stimulated
osteoclastogenesis and ovariectomized-induced bone loss by
restraining NF-ĸB and MAPK activation. J OrthopTranslat.
28:148–158. 2021.
|
|
164
|
Fan YS, Li Q, Hamdan N, Bian YF, Zhuang S,
Fan K and Liu ZJ: Tetrahydroxystilbene glucoside regulates
proliferation, differentiation, and OPG/RANKL/M-CSF expression in
MC3T3-E1 Cells via the PI3K/Akt pathway. Molecules. 23:23062018.
View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Drissi H and Sanjay A: The multifaceted
osteoclast; far and beyond bone resorption. J Cell Biochem.
117:1753–1756. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Guo ZY, Yin NN, Li XF, Wang MM, Sui XN,
Jiang CD, Xu MH, Jia XE, Fu CJ, Chen TL and Liu X: Exosomes
secreted from M2-polarized macrophages inhibit osteoclast
differentiation via CYLD. Tissue Cell. 93:1026452025. View Article : Google Scholar
|
|
167
|
Yasuda H: Discovery of the RANKL/RANK/OPG
system. J Bone Miner Metab. 39:2–11. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Hakki SS, Batoon L, Koh AJ, Kannan R,
Mendoza-Reinoso V, Rubin J, Mccauley LK and Roca H: The effects
ofpreosteoblast-derived exosomes on macrophages and bone in mice. J
Cell Mol Med. 28:e180292024. View Article : Google Scholar
|
|
169
|
Gebraad A, Kornilov R, Kaur S, Miettinen
S, Haimi S, Peltoniemi H, Mannerström B and Seppänen-Kaijansinkko
R: Monocyte-derived extracellular vesicles stimulate cytokine
secretion and gene expression of matrix metalloproteinases by
mesenchymal stem/stromal cells. FEBS J. 285:2337–2359. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Zhu S, Yao F, Qiu H, Zhang G, Xu H and Xu
J: Coupling factors and exosomal packaging microRNAs involved in
the regulation of bone remodelling. Biol Rev Camb Philos Soc.
93:469–480. 2018. View Article : Google Scholar
|
|
171
|
Liu S, Yan X, Guo J, An H, Li X, Yang L,
Yu X and Li S: Periodontal ligament-associated protein-1 knockout
mice regulate the differentiation of osteoclasts and osteoblasts
through TGF-β1/Smad signaling pathway. J Cell Physiol.
239:e310622024. View Article : Google Scholar
|
|
172
|
Stevenson J; medical advisory council of
the British Menopause Society: Prevention and treatment of
osteoporosis in women. Post Reprod Health. 29:11–14. 2023.
View Article : Google Scholar :
|
|
173
|
Händel MN, Cardoso I, von Bülow C, Rohde
JF, Ussing A, Nielsen SM, Christensen R, Body JJ, Brandi ML,
Diez-Perez A, et al: Fracture risk reduction and safety by
osteoporosis treatment compared with placebo or active comparator
in postmenopausal women: systematic review, network meta-analysis,
and meta-regression analysis of randomised clinical trials. BMJ.
381:e0680332023. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Tagliaferri C, Wittrant Y, Davicco MJ,
Walrand S and Coxam V: Muscle and bone, two interconnected tissues.
Ageing Res Rev. 21:55–70. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Chen K, Jiao Y, Liu L, Huang M, He C, He
W, Hou J, Yang M, Luo X and Li C: communications between bone
marrow macrophages and bone cells in bone remodeling. Front Cell
Dev Biol. 8:5982632020. View Article : Google Scholar
|
|
176
|
Li Q, Gao H, Ma X, Wang Z, Zhao L and Xiao
W: Exosome-mediated crosstalk between the cardiovascular and
musculoskeletal systems: Mechanisms and therapeutic potential
(Review). Int J Mol Med. 56:1292025. View Article : Google Scholar :
|
|
177
|
Hou C, Zhang Y, Lv Z, Luan Y, Li J, Meng
C, Liu K, Luo X, Chen L and Liu F: Macrophage exosomes modified by
miR-365-2-5p promoted osteoblast osteogenic differentiation by
targeting OLFML1. Regen Biomater. 11:rbae0182024. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Hossain MA, Adithan A, Alam MJ, Kopalli
SR, Kim B, Kang CW, Hwang KC and Kim JH: IGF-1 facilitates
cartilage reconstruction by regulating PI3K/AKT, MAPK, and NF-kB
signaling in rabbit osteoarthritis. J Inflamm Res. 14:3555–3568.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Fuentes EN, Björnsson BT, Valdés JA,
Einarsdottir IE, Lorca B, Alvarez M and Molina A: IGF-I/PI3K/Akt
and IGF-I/MAPK/ERK pathways in vivo in skeletal muscle are
regulated by nutrition and contribute to somatic growth in the fine
flounder. Am J Physiol Regul Integr Comp Physiol. 300:R1532–R1542.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Sudha S, Upmanyu A, Saraswat D and Singh
M: Pharmacological impacts of tanshinone on osteogenesis and
osteoclastogenesis: A review. Naunyn Schmiedebergs Arch Pharmacol.
398:135–146. 2025. View Article : Google Scholar
|
|
181
|
Luo L, Avery SJ and Waddington RJ:
Exploring a chemotactic role for EVs from progenitor cell
populations of human exfoliated deciduous teeth for promoting
migration of naïve BMSCs in bone repair process. Stem Cells Int.
2021:66817712021. View Article : Google Scholar
|
|
182
|
Olivieri F, Prattichizzo F, Giuliani A,
Matacchione G, Rippo MR, Sabbatinelli J and Bonafè M: miR-21 and
miR-146a: The microRNAs of inflammaging and age-related diseases.
Ageing Res Rev. 70:1013742021. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Jann J, Gascon S, Roux S and Faucheux N:
Influence of the TGF-β superfamily on osteoclasts/osteoblasts
balance in physiological and pathological bone conditions. Int J
Mol Sci. 21:75972020. View Article : Google Scholar
|
|
184
|
Yu Y, Cai W, Xu Y and Zuo W:
Down-regulation of miR-19b-3p enhances IGF-1 expression to induce
osteoblast differentiation and improve osteoporosis. Cell Mol Biol
(Noisy-le-grand). 68:160–168. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Sabbieti MG, Agas D, Marchetti L, Coffin
JD, Xiao L and Hurley MM: BMP-2 differentially modulates FGF-2
isoform effects in osteoblasts from newborn transgenic mice.
Endocrinology. 154:2723–2733. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Zhang C, Yang J, Zhu Z, Qin J, Yang L,
Zhao X, Su W, Cai Y, Yang J, Wang F, et al: Exosomal lncRNA HOTAIR
promotes osteoclast differentiation by targeting
TGF-beta/PTHrP/RANKL pathway. Basic Clin Pharmacol Toxicol.
132:242–252. 2023. View Article : Google Scholar
|
|
187
|
Wang J, Li X, Wang S, Cui J, Ren X and Su
J: Bone-targeted exosomes: Strategies and applications. Adv Healthc
Mater. 12:e22033612023. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Sayer AA and Cruz-Jentoft A: Sarcopenia
definition, diagnosis and treatment: consensus is growing. Age
Ageing. 51:afac2202022. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Jun L, Robinson M, Geetha T, Broderick TL
and Babu JR: Prevalence and mechanisms of skeletal muscle atrophy
in metabolic conditions. Int J Mol Sci. 24:29732023. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Dong Q, Li D, Zhang K, Shi H, Cai M, Li Y,
Zhao R and Qin D: Muscle-bone biochemical crosstalk in
osteosarcopenia: Focusing on mechanisms and potential therapeutic
strategies. J Endocrinol. 266:e2502342025. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Zhang H, Du Y, Tang W, Chen M, Yu W, Ke Z,
Dong S and Cheng Q: Eldecalcitol prevents muscle loss and
osteoporosis in disuse muscle atrophy via NF-ĸB signaling in mice.
Skelet Muscle. 13:222023. View Article : Google Scholar
|
|
192
|
Bettis T, Kim BJ and Hamrick MW: Impact of
muscle atrophy on bone metabolism and bone strength: Implications
for muscle-bone crosstalk with aging and disuse. Osteoporos Int.
29:1713–1720. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Rong S, Wang L, Peng Z, Liao Y, Li D, Yang
X, Nuessler AK, Liu L, Bao W and Yang W: The mechanisms and
treatments for sarcopenia: Could exosomes be a perspective research
strategy in the future? J Cachexia Sarcopenia Muscle. 11:348–365.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Chu R, Li M, Xie Y, Du Y and Ni T:
Exercise interventions and serum IGF-1 levels in older adults with
frailty and/or sarcopenia: A systematic review and meta analysis.
Front Public Health. 13:16606942025. View Article : Google Scholar :
|
|
195
|
Zhu J, Fan J, Xia Y, Wang H, Li Y, Feng Z
and Fu C: Potential therapeutic targets of macrophages in
inhibiting immune damage and fibrotic processes in musculoskeletal
diseases. Front Immunol. 14:12194872023. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Hu S, Wang S, Yang X, Li P, Li Z, Luo B,
Liang Y and Pan X: Exosomes promise better bone regeneration. Regen
Ther. 30:389–402. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Liu W, Li L, Rong Y, Qian D, Chen J, Zhou
Z, Luo Y, Jiang D, Cheng L, Zhao S, et al: Hypoxic mesenchymal stem
cell-derived exosomes promote bone fracture healing by the transfer
of miR-126. Acta Biomater. 103:196–212. 2020. View Article : Google Scholar
|
|
198
|
Holliday LS, McHugh KP, Zuo J, Aguirre JI,
Neubert JK and Rody WJ Jr: Exosomes: Novel regulators of bone
remodelling and potential therapeutic agents for orthodontics.
Orthod Craniofac Res. 20(Suppl 1): S95–S99. 2017. View Article : Google Scholar
|
|
199
|
Hatakeyama J, Inoue S, Jiang H, Yokoi R
and Moriyama H: Exercise-induced interactions between skeletal
muscle and bone via myokines and osteokine in mice: Role of
FNDC5/irisin, IGF-1, and osteocalcin. Bone. 190:1173142025.
View Article : Google Scholar
|
|
200
|
Krylova SV and Feng D: The machinery of
exosomes: Biogenesis, release, and uptake. Int J Mol Sci.
24:13372023. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Fridman E, Ginini L, Gil Z and Milman N:
The purification and characterization of exosomes from macrophages.
Methods Mol Biol. 2184:77–90. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Xu WM, Li A, Chen JJ and Sun EJ: Research
development on exosome separation technology. J Membr Biol.
256:25–34. 2023. View Article : Google Scholar
|
|
203
|
Auquière M, Muccioli GG and des Rieux A:
methods and challenges in purifying drug-loaded extracellular
vesicles. J Extracell Vesicles. 14:e700972025. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Yousaf I, Kegler U, Hofner M and Noehammer
C: Evaluation of commercially available kits for parallel DNA and
microRNA isolation suitable for epigenetic analyses from cell-free
saliva and salivary extracellular vesicles. Int J Mol Sci.
26:63652025. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Coughlan C, Bruce KD, Burgy O, Boyd TD,
Michel CR, Garcia-Perez JE, Adame V, Anton P, Bettcher BM, Chial
HJ, et al: Exosome isolation by ultracentrifugation and
precipitation and techniques for downstream analyses. Curr Protoc
Cell Biol. 88:e1102020. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Sidhom K, Obi PO and Saleem A: Review of
exosomal isolation methods: Is size exclusion chromatography the
best option? Int J Mol Sci. 21:64662020. View Article : Google Scholar
|
|
207
|
Gao M, Cai J, Zitkovsky HS, Chen B and Guo
L: comparison of yield, purity, and functional properties of
large-volume exosome isolation using ultrafiltration and
polymer-based precipitation. Plast Reconstr Surg. 149:638–649.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Greening DW, Xu R, Ji H, Tauro BJ and
Simpson RJ: A protocol for exosome isolation and characterization:
Evaluation of ultracentrifugation, density-gradient separation, and
immunoaffinity capture methods. Methods Mol Biol. 1295:179–209.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Contreras-Naranjo JC, Wu HJ and Ugaz VM:
Microfluidics for exosome isolation and analysis: Enabling liquid
biopsy for personalized medicine. Lab Chip. 17:3558–3577. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Tang YT, Huang YY, Zheng L, Qin SH, Xu XP,
An TX, Xu Y, Wu YS, Hu XM, Ping BH and Wang Q: Comparison of
isolationmethods of exosomes and exosomal RNA from cell culture
medium and serum. Int J Mol Med. 40:834–844. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Huang Y, Liu Y, Huang Q, Sun S, Ji Z,
Huang L, Li Z, Huang X, Deng W and Li T: TMT-Based quantitative
proteomics analysis of synovial fluid-derived exosomes in
inflammatory arthritis. Front Immunol. 13:8009022022. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Huang LH, Rau CS, Wu SC, Wu YC, Wu CJ,
Tsai CW, Lin CW, Lu TH and Hsieh CH: Identification and
characterization of hADSC-derived exosome proteins from different
isolation methods. J Cell Mol Med. 25:7436–7450. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Lai JJ, Chau ZL, Chen SY, Hill JJ, Korpany
KV, Liang NW, Lin LH, Lin YH, Liu JK, Liu YC, et al: Exosome
processing and characterization approaches for research and
technology development. Adv Sci (Weinh). 9:e21032222022. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
D'Acunzo P, Kim Y, Ungania JM,
Pérez-González R, Goulbourne CN and Levy E: Isolation of
mitochondria-derived mitovesicles and subpopulations of
microvesicles and exosomes from brain tissues. Nat Protoc.
17:2517–2549. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Doyle LM and Wang MZ: Overview of
extracellular vesicles, their origin, composition, purpose, and
methods for exosome isolation and analysis. Cells. 8:7272019.
View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Veerman RE, Teeuwen L, Czarnewski P,
Güclüler Akpinar G, Sandberg A, Cao X, Pernemalm M, Orre LM,
Gabrielsson S and Eldh M: Molecular evaluation of five different
isolation methods for extracellular vesicles reveals different
clinical applicability and subcellular origin. J Extracell
Vesicles. 10:e121282021. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Zhao R, Zhao T, He Z, Cai R and Pang W:
Composition, isolation, identification and function of adipose
tissue-derived exosomes. Adipocyte. 10:587–604. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Corona ML, Hurbain I, Raposo G and van
Niel G: Characterization of extracellular vesicles by transmission
electron microscopy and immunolabeling electron microscopy. Methods
Mol Biol. 2668:33–43. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Wu X, Showiheen SAA, Sun AR, Crawford R,
Xiao Y, Mao X and Prasadam I: Exosomes extraction and
identification. Methods Mol Biol. 2054:81–91. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Chen Z, Luo L, Ye T, Zhou J, Niu X, Yuan
J, Yuan T, Fu D, Li H, Li Q and Wang Y: Identification of specific
markers for human pluripotent stem cell-derived small extracellular
vesicles. J Extracell Vesicles. 13:e124092024. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Rahmatinejad F, Kharat Z, Jalili H, Renani
MK and Mobasheri H: Comparison of morphology, protein
concentration, and size distribution of bone marrow and Wharton's
jelly-derived mesenchymal stem cells exosomes isolated by
ultracentrifugation and polymer-based precipitation techniques.
Tissue Cell. 88:1024272024. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Kim J, Lyu HZ, Jung C, Lee KM, Han SH, Lee
JH and Cha M: Osteogenic response of MC3T3-E1 and Raw264.7 in the
3D-encapsulated co-culture environment. Tissue Eng Regen Med.
18:387–397. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Wragg NM, Mosqueira D, Blokpeol-Ferreras
L, Capel A, Player DJ, Martin NRW, Liu Y and Lewis MP: Development
of a 3D tissue-engineered skeletal muscle and bone co-culture
system. Biotechnol J. 15:e19001062020. View Article : Google Scholar
|
|
224
|
Ostrovidov S, Ahadian S, Ramon-Azcon J,
Hosseini V, Fujie T, Parthiban SP, Shiku H, Matsue T, Kaji H,
Ramalingam M, et al: Three-dimensional co-culture of C2C12/PC12
cells improves skeletal muscle tissue formation and function. J
Tissue Eng Regen Med. 11:582–595. 2017. View Article : Google Scholar
|
|
225
|
Liu J, Yang D, Shi S, Lin L, Xiao M, Yuan
Z and Yu M: Overexpression of vasostatin-1 protects
hypoxia/reoxygenation injuries in cardiomyocytes-endothelial cells
transwell co-culture system. Cell Biol Int. 38:26–31. 2014.
View Article : Google Scholar
|
|
226
|
Liu M, Han Y, Wang J, Zhu Y, Zhang Y, Chu
Q, Yang C, Chen B and Sun G: Skeletal muscle-derived IL-33 mediates
muscle-to-bone crosstalk and regulates bone metabolism via CD8(+) T
cell-secreted CCL5. EBioMedicine. 122:1060242025. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Le VL, Chang CY, Chuang CW, Syu SH, Shih
HJ, Nguyen Vo HP, Van MN and Huang CJ: Therapeutic effects of
engineered exosomes from RAW264.7 Cells Overexpressing
hsa-let-7i-5p against Sepsis in Mice-A comparative study with human
placenta-derived mesenchymal stem cell exosomes. J Pers Med.
14:6192024. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Bier A, Berenstein P, Kronfeld N,
Morgoulis D, Ziv-Av A, Goldstein H, Kazimirsky G, Cazacu S, Meir R,
Popovtzer R, et al: Placenta-derived mesenchymal stromal cells and
their exosomes exert therapeutic effects in Duchenne muscular
dystrophy. Biomaterials. 174:67–78. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Cai H and Guo H: Mesenchymal stem cells
and their exocytotic vesicles. Int J Mol Sci. 24:20852023.
View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Zha Y, Li Y, Lin T, Chen J, Zhang S and
Wang J: Progenitor cell-derived exosomes endowed with VEGF plasmids
enhance osteogenic induction and vascular remodeling in large
segmental bone defects. Theranostics. 11:397–409. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Tenkumo T, Aobulikasimu A, Asou Y, Shirato
M, Shishido S, Kanno T, Niwano Y, Sasaki K and Nakamura K:
Proanthocyanidin-rich grape seed extract improves bone loss, bone
healing, and implant osseointegration in ovariectomized animals.
Sci Rep. 10:88122020. View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Halloran D, Pandit V, Chukwuocha K and
Nohe A: Methyl-beta-cyclodextrin restores aberrant bone
morphogenetic protein 2-signaling in bone marrow stromal cells
obtained from aged C57BL/6 Mice. J Dev Biol. 12:302024. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Yashima N, Minamizono W, Matsunaga H, Lyu
J, Fujikawa K, Suito H, Okunuki T, Nakai S and Ohsako M:
Non-contact electrical stimulation via a Vector-potential
transformer promotes bone healing in drill-hole injury model. J
Bone Miner Metab. 43:348–359. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Muwanga GPB, Siliezar-Doyle J, Ortiz AA,
Kaslow J, Haight ES and Tawfik VL: The tibial fracture-pin model: A
clinically relevant mouse model of orthopedic injury. J Vis Exp.
28: View Article : Google Scholar : 2022.
|
|
235
|
Wang BW, Jiang Y, Yao ZL, Chen PS, Yu B
and Wang SN: Aucubin protects chondrocytes against IL-1β-Induced
apoptosis in vitro and inhibits osteoarthritis in mice model. Drug
Des Devel Ther. 13:3529–3538. 2019. View Article : Google Scholar :
|
|
236
|
Suh HR, Cho HY and Han HC: Development of
a novel model of intervertebral disc degeneration by the
intradiscal application of monosodium iodoacetate (MIA) in rat.
Spine J. 22:183–192. 2022. View Article : Google Scholar
|
|
237
|
Li Y, Qiao X, Feng Y, Zhou R, Zhang K, Pan
Y, Yan T, Yan L, Yang S, Wei X, et al: Characterization of the gut
microbiota and fecal metabolome in the osteosarcoma mouse model.
Aging (Albany NY). 16:10841–10859. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Ferrena A, Wang J, Zhang R,
Karadal-Ferrena B, Al-Hardan W, Singh S, Borjihan H, Schwartz EL,
Zhao H, Oktay MH, et al: SKP2 knockout in Rb1/p53-Deficient mouse
models of osteosarcoma induces immune infiltration and drives a
transcriptional program with a favorable prognosis. Mol Cancer
Ther. 23:223–234. 2024. View Article : Google Scholar :
|
|
239
|
Wang C, Luo D, Zheng L and Zhao M:
Anti-diabetic mechanism and potential bioactive peptides of casein
hydrolysates in STZ/HFD-induced diabetic rats. J Sci Food Agric.
104:2947–2958. 2024. View Article : Google Scholar
|
|
240
|
Yao Y, Cai X, Chen Y, Zhang M and Zheng C:
Estrogen deficiency-mediated osteoimmunity in postmenopausal
osteoporosis. Med Res Rev. 45:561–575. 2025. View Article : Google Scholar
|
|
241
|
Rangel LBA, de Siqueira D, Soares ODR,
Santana HS, Miguel EC, da Cunha M, Oliveira ALA, Pedrosa DF,
Resgala LCR, Neto HAR, et al: Vitamin K supplementation modulates
bone metabolism and ultra-structure of ovariectomized mice. Cell
Physiol Biochem. 51:356–374. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Chen MY, Zhao FL, Chu WL, Bai MR and Zhang
DM: A review of tamoxifen administration regimen optimization for
Cre/loxp system in mouse bone study. Biomed Pharmacother.
165:1150452023. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Gelles K, Butylina M and Pietschmann P:
Animal models for age-related osteoporosis. Gerontology. 71:1–29.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Rong K, Chen P, Lang Y, Zhang Y, Wang Z,
Wen F and Lu L: Morinda officinalis polysaccharide attenuates
osteoporosis in rats underwent bilateral ovariectomy by suppressing
the PGC-1α/PPARү pathway. J Orthop Surg (Hong Kong).
30:102255362211308242022. View Article : Google Scholar
|
|
245
|
Liu Y, Dimango E, Bucovsky M, Agarwal S,
Nishiyama K, Guo XE, Shane E and Stein EM: Abnormal
microarchitecture and stiffness in postmenopausal women using
chronic inhaled glucocorticoids. Osteoporos Int. 29:2121–2127.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Abdi S, Javanmehr N, Ghasemi-Kasman M,
Bali HY and Pirzadeh M: Stem cell-based therapeutic and diagnostic
approaches in Alzheimer's disease. Curr Neuropharmacol.
20:1093–1115. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Henderson S, Ibe I, Cahill S, Chung YH and
Lee FY: Bone quality and fracture-healing in type-1 and type-2
diabetes mellitus. J Bone Joint Surg Am. 101:1399–1410. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Castillo ÍMP, Argilés JM, Rueda R, Ramírez
M and Pedrosa JML: Skeletal muscle atrophy and dysfunction in
obesity and type-2 diabetes mellitus: Myocellular mechanisms
involved. Rev Endocr Metab Disord. 26:815–836. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
249
|
Edgar L, Akbar N, Braithwaite AT,
Krausgruber T, Gallart-Ayala H, Bailey J, Corbin AL, Khoyratty TE,
Chai JT, Alkhalil M, et al: Hyperglycemia induces trained immunity
in macrophages and their precursors and promotes atherosclerosis.
Circulation. 144:961–982. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Kishore A and Petrek M: roles of
macrophage polarization and macrophage-derived miRNAs in pulmonary
fibrosis. Front Immunol. 12:6784572021. View Article : Google Scholar : PubMed/NCBI
|
|
251
|
Cazzanelli P, Lamoca M, Hasler J, Hausmann
ON, Mesfin A, Puvanesarajah V, Hitzl W and Wuertz-Kozak K: The role
of miR-155-5p in inflammation and mechanical loading during
intervertebral disc degeneration. Cell Commun Signal. 22:4192024.
View Article : Google Scholar : PubMed/NCBI
|
|
252
|
Adamopoulos IE: Inflammation in bone
physiology and pathology. Curr Opin Rheumatol. 30:59–64. 2018.
View Article : Google Scholar :
|
|
253
|
Sun Y, Kuek V, Liu Y, Tickner J, Yuan Y,
Chen L, Zeng Z, Shao M, He W and Xu J: MiR-214 is an important
regulator of the musculoskeletal metabolism and disease. J Cell
Physiol. 234:231–245. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Ma-On C, Sanpavat A, Whongsiri P,
Suwannasin S, Hirankarn N, Tangkijvanich P and Boonla C: Oxidative
stress indicated by elevated expression of Nrf2 and 8-OHdG promotes
hepatocellular carcinoma progression. Med Oncol. 34:572017.
View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Palma FR, Gantner BN, Sakiyama MJ, Kayzuka
C, Shukla S, Lacchini R, Cunniff B and Bonini MG: ROS production by
mitochondria: Function or dysfunction? Oncogene. 43:295–303. 2024.
View Article : Google Scholar
|
|
256
|
Song Y and Chung J: Aging aggravates
periodontal inflammatory responses and alveolar bone resorption by
porphyromonas gingivalis infection. Curr Issues Mol Biol.
45:6593–6604. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
257
|
Liu J, Zhao Y, Zhang Y, Yao X and Hang R:
Exosomes derived from macrophages upon Zn ion stimulation promote
osteoblast and endothelial cell functions. J Mater Chem B.
9:3800–3807. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
258
|
Zhu Y, Zhao S, Cheng L, Lin Z, Zeng M,
Ruan Z, Sun B, Luo Z, Tang Y and Long H: Mg2+-mediated
autophagy-dependent polarization of macrophages mediates the
osteogenesis of bone marrow stromal stem cells by interfering with
macrophage-derived exosomes containing miR-381. J Orthop Res.
40:1563–1576. 2022. View Article : Google Scholar
|
|
259
|
Wei F, Li M, Crawford R, Zhou Y and Xiao
Y: Exosome-integrated titanium oxide nanotubes for targeted bone
regeneration. Acta Biomater. 86:480–492. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
260
|
Bai X, Gao M, Syed S, Zhuang J, Xu X and
Zhang XQ: Bioactive hydrogels for bone regeneration. Bioact Mater.
3:401–417. 2018.PubMed/NCBI
|
|
261
|
Moura MLA, Fugimoto M, Kawachi APM, de
Oliveira ML, Lazaretti-Castro M and Reginato RD: Estrogen therapy
associated with mechanical vibration improves bone
microarchitecture and density in osteopenic female mice. J Anat.
233:715–723. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
262
|
Vilaca T, Eastell R and Schini M:
Osteoporosis in men. Lancet Diabetes Endocrinol. 10:273–283. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
263
|
Cauley JA: Estrogen and bone health in men
and women. Steroids. 99(Pt A): 11–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
264
|
Pellegrino A, Tiidus PM and Vandenboom R:
Mechanisms of estrogen influence on skeletal muscle: Mass,
regeneration, and mitochondrial function. Sports Med. 52:2853–2869.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
265
|
Alexander SE, Pollock AC and Lamon S: The
effect of sex hormones on skeletal muscle adaptation in females.
Eur J Sport Sci. 22:1035–1045. 2022. View Article : Google Scholar
|
|
266
|
Jardí F, Laurent MR, Dubois V, Kim N,
Khalil R, Decallonne B, Vanderschueren D and Claessens F: Androgen
and estrogen actions on male physical activity: A story beyond
muscle. J Endocrinol. 238:R31–R52. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
267
|
Unger CA, Aladhami AK, Hope MC, Cotham WE,
Nettles KW, Clegg DJ, Velázquez KT and Enos RT: skeletal muscle
endogenous estrogen production ameliorates the metabolic
consequences of a high-fat diet in male mice. Endocrinology.
164:bqad1052023. View Article : Google Scholar : PubMed/NCBI
|
|
268
|
Collins BC, Mader TL, Cabelka CA, Iñigo
MR, Spangenburg EE and Lowe DA: Deletion of estrogen receptor α in
skeletal muscle results in impaired contractility in female mice. J
Appl Physiol (1985). 124:980–992. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
269
|
Tramunt B, Smati S, Grandgeorge N, Lenfant
F, Arnal JF, Montagner A and Gourdy P: Sex differences in metabolic
regulation and diabetes susceptibility. Diabetologia. 63:453–461.
2020. View Article : Google Scholar :
|
|
270
|
O'Reilly J, Ono-Moore KD, Chintapalli SV,
Rutkowsky JM, Tolentino T, Lloyd KCK, Olfert IM and Adams SH: Sex
differences in skeletal muscle revealed through fiber type,
capillarity, and transcriptomics profiling in mice. Physiol Rep.
9:e150312021.PubMed/NCBI
|
|
271
|
Farhat F, Amérand A, Simon B, Guegueniat N
and Moisan C: Gender-dependent differences of mitochondrial
function and oxidative stress in rat skeletal muscle at rest and
after exercise training. Redox Rep. 22:508–514. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
272
|
Clynes MA, Gregson CL, Bruyère O, Cooper C
and Dennison EM: Osteosarcopenia: Where osteoporosis and sarcopenia
collide. Rheumatology (Oxford). 60:529–537. 2021. View Article : Google Scholar
|
|
273
|
Gálvez I, Navarro MC, Martín-Cordero L,
Otero E, Hinchado MD and Ortega E: The influence of obesity and
weight loss on the bioregulation of innate/inflammatory responses:
Macrophages and immunometabolism. Nutrients. 14:6122022. View Article : Google Scholar : PubMed/NCBI
|
|
274
|
Jin Z, Huang Q, Peng J, Liu Z, Hu R, Wu J
and Wang F: MiR-125a-3p alleviates hyperproliferation of
keratinocytes and psoriasis-like inflammation by targeting
TLR4/NF-ĸB pathway. Postepy Dermatol Alergol. 40:447–461. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
275
|
Paoli A, Cenci L, Pompei P, Sahin N,
Bianco A, Neri M, Caprio M and Moro T: Effects of two months of
very low carbohydrate ketogenic diet on body composition, muscle
strength, muscle area, and blood parameters in competitive natural
body builders. Nutrients. 13:3742021. View Article : Google Scholar : PubMed/NCBI
|
|
276
|
Ying W, Riopel M, Bandyopadhyay G, Dong Y,
Birmingham A, Seo JB, Ofrecio JM, Wollam J, Hernandez-Carretero A,
Fu W, et al: Adipose tissue macrophage-derived exosomal miRNAs can
modulate in vivo and in vitro insulin sensitivity. Cell.
171:372–384.e12. 2017. View Article : Google Scholar
|
|
277
|
Gao X, Chen Y, Wang J, Xu J, Wan H, Li X
and Shi Y: Mitochondria-Rich extracellular vesicles from bone
marrow stem cells mitigate muscle degeneration in rotator cuff
tears in a rat model through macrophage M2 phenotype conversion.
Arthroscopy. 41:3487–3499. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
278
|
Trentini M, D'Amora U, Ronca A, Lovatti L,
Calvo-Guirado JL, Licastro D, Monego SD, Delogu LG, Wieckowski MR,
Barak S, et al: Bone regeneration revolution: Pulsed
electromagnetic field modulates macrophage-derived exosomes to
attenuate osteoclastogenesis. Int J Nanomedicine. 19:8695–8707.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
279
|
Hu XM, Wang CC, Xiao Y, Liu Y, Huang HR,
Jiang P, Wang YK, Lin YJ, Li LC and Qi ZQ: Non-clinical safety
evaluation of exosomes derived from human umbilical cord
mesenchymal stem cells in cynomolgus monkeys. Int J Nanomedicine.
19:4923–4939. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
280
|
Li JJ, Wang B, Kodali MC, Chen C, Kim E,
Patters BJ, Lan L, Kumar S, Wang X, Yue J and Liao FF: In vivo
evidence for the contribution of peripheral circulating
inflammatory exosomes to neuroinflammation. J Neuroinflammation.
15:82018. View Article : Google Scholar : PubMed/NCBI
|
|
281
|
Wang Y, Kong Y, Du J, Qi L, Liu M, Xie S,
Hao J, Li M, Cao S, Cui H, et al: Injection of human umbilical cord
mesenchymal stem cells exosomes for the treatment of knee
osteoarthritis: From preclinical to clinical research. J Transl
Med. 23:6412025. View Article : Google Scholar : PubMed/NCBI
|
|
282
|
Muraca M and Cappariello A: The role of
extracellular vesicles (EVs) in the epigenetic regulation of bone
metabolism and osteoporosis. Int J Mol Sci. 21:86822020. View Article : Google Scholar : PubMed/NCBI
|
|
283
|
Rohm TV, Castellani Gomes Dos Reis F,
Isaac R, Murphy C, Rocha KCE, Bandyopadhyay G, Gao H, Libster AM,
Zapata RC, Lee YS, et al: Adipose tissue macrophages secrete small
extracellular vesicles that mediate rosiglitazone-induced insulin
sensitization. Nat Metab. 6:880–898. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
284
|
Ying W, Gao H, Dos Reis FCG, Bandyopadhyay
G, Ofrecio JM, Luo Z, Ji Y, Jin Z, Ly C and Olefsky JM: MiR-690, an
exosomal-derived miRNA from M2-polarized macrophages, improves
insulin sensitivity in obese mice. Cell Metab. 33:781–790.e5. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
285
|
Wang Y, Zhang X, Wang J, Zhang Y, Ye Q,
Wang Y, Fei D and Wang Q: Inflammatory periodontal ligament stem
cells drive m1 macrophage polarization via exosomal
miR-143-3p-Mediated regulation of PI3K/AKT/NF-κB signaling. Stem
Cells. 41:184–199. 2023. View Article : Google Scholar
|
|
286
|
Chen Y, Dong J, Li J, Li J, Lu Y, Dong W,
Zhang D and Dang X: Engineered macrophage-derived exosomes via
click chemistry for the treatment of osteomyelitis. J Mater Chem B.
12:10593–10604. 2024. View Article : Google Scholar : PubMed/NCBI
|