Introduction
Radiotherapy remains a foundational treatment for
thoracic malignancies (1,2);
however, its efficacy is often compromised by radiation-induced
lung injury (RILI) (3). This is
a debilitating complication affecting 14.2% of patients receiving
intensity-modulated radiotherapy (4) and 1.3% undergoing stereotactic
radiotherapy (5). Characterized
by progressive inflammation, fibrosis and respiratory failure
(6), RILI not only limits
radiation dose escalation, but also diminishes patient survival,
with mortality rates >30% in severe cases (7). Current management relies heavily on
glucocorticoids (8), which
transiently alleviate symptoms but paradoxically increase infection
risks and reduce tumor radiosensitivity (9,10). Given these limitations, it is
imperative to develop novel therapeutic strategies that address the
pathogenesis of RILI while maintaining anticancer
effectiveness.
Results of a previous study indicated that the
development of RILI is closely associated with
epithelial-mesenchymal transition (EMT), a process in which
alveolar epithelial cells lose their polarity, acquire
mesenchymal-like properties and thereby contribute to fibrotic
remodeling (11). TGF-β plays a
partial role in initiating EMT through triggering Smad-dependent
signaling pathways and enhancing the production of mesenchymal
proteins, including vimentin and α-smooth muscle actin (α-SMA)
(12-17). Moreover, the PI3K/Akt signaling
axis has been increasingly recognized for its role in intensifying
EMT and contributing to fibrosis, through repressing E-cadherin
expression via Snail and Twist regulation (18-20). Results of previous studies
revealed that PI3K inhibitors may inhibit the proliferation and
differentiation of lung fibroblasts into myofibroblasts (21). These results suggest that
targeting the PI3K/Akt signaling pathway, which is involved in
RILI, may serve as an effective strategy to attenuate
radiation-induced EMT (22).
Despite these advances, the precise mechanisms underlying the
association between EMT and the PI3K/Akt pathway in the context of
RILI remain to be fully elucidated.
Traditional Chinese Medicine includes a range of
bioactive compounds with multi-target potential (23). Results of a previous clinical
study demonstrate that patients treated with Huaxian Formula (HXF)
experienced a markedly lower incidence of radiotherapy interruption
due to severe RILI, compared with the control group (24). Moreover, results of previous
in vivo studies confirm that HXF may act as a potent
anti-RILI agent, reducing collagen deposition by 12.3% in murine
models (25-27). Through serum pharmaco-chemistry
and network pharmacology, HY-N7656 was isolated as the key active
component of HXF, demonstrating the high binding affinity to
PI3K/Akt targets (28). The
flavonoid compound, HY-N7656, is a naturally derived product that
has been investigated for multiple pharmacological activities,
including anti-inflammatory, antioxidative, radioprotective and
antifibrotic effects (23,29). Although HY-N7656 possesses the
aforementioned pharmacological properties, the specific efficacy
against RILI (30) and the
potential involvement in the EMT process and PI3K/Akt signaling
pathway remain unclear.
Thus, it was hypothesized that HY-N7656 may be among
the first natural flavonoid compounds to attenuate RILI via dual
inhibition of EMT and PI3K/Akt signaling. The establishment of a
RILI model using C57BL/6 mice with whole-lung irradiation at 17 Gy
is widely established, as it effectively replicates the key
pathological and inflammatory features observed in human lungs
(31,32). The present study aimed to
investigate the potential protective effects of HY-N7656 in RILI
both in vitro and in vivo, using TGF-β-stimulated
alveolar epithelial cells and a murine RILI model. Subsequently,
network pharmacology and molecular docking were used to explore the
potential underlying mechanisms. Collectively, results of the
present study indicated the potential role of HY-N7656 as a
radiosensitizer-adjuvant, addressing a crucial gap in therapeutics
targeting RILI.
Materials and methods
Animal experiments
C57BL/6 mice (SPF-grade; age, 8 weeks; weight, 20-25
g; n=80) were purchased from Beijing HFK Bio-Technology and used in
all animal experiments. All animals were acclimatized for 7 days
under standard conditions (22°C; 50±10% relative humidity; 12 h
light/dark cycle). To minimize sex-related variability in radiation
response (30), only male mice
were used. For experimental design, mice were randomized into four
cohorts (20 per group), including a control group without treatment
and an IR group receiving localized thoracic irradiation at 17 Gy.
Efficacy dose selection was carried out and 15 mg/kg HY-N7656 was
used for all experiments in order to limit toxicity and enhance
therapeutic efficacy. Mice in the HY-N7656 (15 mg/kg) group were
treated with irradiation and a daily intraperitoneal injection of
15 mg/kg HY-N7656. Mice in the drug-only group were treated with 15
mg/kg HY-N7656 without irradiation. On the second day following
treatment initiation, mice were subjected to localized 60Co
X-irradiation at a dose of 17 Gy (75 cGy/min), administered using a
Precision X-Ray X-RAD 320 irradiator. Notably, non-target tissues
of mice were protected using lead plates. HY-N7656 (purity,
>99.9%; cat. no. 23050-38-6; TargetMol Chemicals Inc.) was
administered intraperitoneally for 4 weeks post-irradiation and
samples were collected from all mice at week 16 post-irradiation.
In cases where severe distress or a weight loss >20% was
observed, the mice were humanely sacrificed by cervical
dislocation, in accordance with the guidelines set by the Animal
Care and Use Committee (IACUC) to minimize suffering. Mice were
anesthetized using isoflurane (3-4% for induction and 1.5-2% for
maintenance) using an animal anesthesia system. Following
anesthesia, 150-200 μl of orbital blood was collected from
each mouse. Following blood collection, all animals were euthanized
via cervical dislocation and death was confirmed by the absence of
heartbeat and respiration for at least 2 min. The Animal Ethics
Committee of Sichuan Cancer Hospital reviewed and approved all
procedures involving animals (approval no. SCC
HEC-04-2024-040).
Micro-computed tomography (CT) scan
At weeks 4, 8, 12 and 16 post-irradiation, mice were
anesthetized using 2% isoflurane and scanned using a Quantum GX
Micro-CT (Rigaku) with the following parameters: 80 kV, 100
μA, 360° rotation and 50 μm isotropic resolution.
Lung density was quantified in Hounsfield units (HU) using 3D
Slicer software (version 5.6.1; https://www.slicer.org), with healthy ventilation
defined as −900 to −500 HU and impaired ventilation defined as −500
to −100 HU (33). CT scans were
processed using 3D Slicer and lung HU values were quantified
separately for each group.
Enzyme-linked immunosorbent assay
(ELISA)
In Week 16, serum levels of TGF-β were determined
using a commercially available ELISA kit (cat. no. RK00057;
ABclonal Biotech Co., Ltd.). Notably, orbital blood was collected
and centrifuged at 1,000 × g for 20 min at 4°C and the supernatant
was extracted.
Histopathology and
immunohistochemistry
Pulmonary samples were fixed in 10% neutral buffered
formalin for 24 h at room temperature (20-25°C), dehydrated through
a graded ethanol series, cleared in xylene, paraffin-embedded, and
sectioned at a thickness of 5 μm. Hematoxylin and eosin
(H&E) staining was performed with hematoxylin for 5 min and
eosin for 2 min at room temperature, and scoring was performed
blindly according to the Szapiel criteria, which included the
evaluation of alveolar wall thickness and inflammation (34). Masson's trichrome staining was
performed at room temperature for a total duration of 60 min
according to standard protocols, and fibrosis severity was
subsequently assessed using Ashcroft scoring (35). Immunohistochemical (IHC) staining
for α-SMA was performed as follows: after antigen retrieval,
sections were permeabilized with 0.1% Triton X-100 in PBS and then
blocked with 5% non-fat milk at room temperature for 30 min. The
primary antibody against α-SMA (cat. no. H660016003; Huabio) was
diluted 1:5,000 and incubated at 4°C for 16 h. Sections were
subsequently incubated with an HRP-conjugated secondary antibody
(cat. no. SA00001-1; 1:5,000; ProteinTech Group, Inc.) at room
temperature for 30 min. After image acquisition using a light
microscope, quantitative analysis of the positive staining area was
performed using ImageJ 1.54p (National Institutes of Health).
Cell culture and HY-N7656 treatment
STR-verified A549 cells, derived from human lung
adenocarcinoma, were cultured in DMEM (cat. no. PYG0073; Boster
Biological Technology) containing 10% fetal bovine serum (cat. no.
40130ES; Shanghai Yeasen Biotechnology Co., Ltd.) at 37°C with 5%
CO2 in a humidified incubator. Following overnight
incubation in serum-free medium, cells were treated with or without
HY-N7656 or TGF-β. Subsequently, the culture medium was exchanged
for a serum-supplemented complete medium and cells underwent an
additional 48-h incubation period.
Assessment of cell viability
A549 cell viability was evaluated utilizing Cell
Counting Kit-8 (CCK-8; cat. no. 40203ES60; Shanghai Yeasen
Biotechnology Co., Ltd.). Initially, cells were distributed into
96-well plates with each well containing 3×103 cells.
Following a 24-h incubation period at 37°C, cells were treated with
different concentrations of HY-N765 and cultured for a further 48 h
at 37°C. All cells were incubated with CCK-8 reagent and cell
culture plates were incubated for 1 h at 37°C. Optical density was
measured at 450 nm and recorded with a Cytation 5 multimode cell
imaging plate reader (BioTek; Agilent Technologies, Inc.).
Cell migration assay
A uniform monolayer of A549 cells was established in
6-well plates and the surface was scratched using a sterile
200-μl pipette tip. Residual debris was eliminated via
washing with PBS. The cells were then treated with HY-N7656 (10
μM; cat. no. 23050-38-6; TargetMol Chemicals Inc.) and/or
TGF-β (5 ng/ml; cat. no. 91701ES10; Shanghai Yeasen Biotechnology
Co., Ltd.) in culture medium containing 0.5% FBS. Following 48 h of
incubation with the specified treatments, three random fields
corresponding to each scratch were selected and observed under a
light microscope to assess cell migration. ImageJ 1.54p (National
Institutes of Health) was used calculate wound healing, which was
expressed as the percentage of the initial wound area covered
during healing.
Immunofluorescence staining
A549 cells were seeded onto coverslips in 24-well
plates at a density of 1.2×104 cells per well. Following
washing with PBS, A549 cells distributed in 24-well plates were
fixed using 1 ml of 4% paraformaldehyde solution at room
temperature for 20 mins. Cells were fixed, treated with 0.5% Triton
X-100 to permeabilize membranes and rinsed using PBS. Subsequently,
membranes were blocked using 5% BSA (cat. no. BSAS 1.0; Bovostar;
Biovogen) for 1 h and incubated with the following primary
antibodies at 4°C overnight: Anti-E-cadherin (cat. no. 20874-1-AP;
1:500; ProteinTech Group, Inc.) and anti-vimentin (cat. no.
10366-1-AP; 1:1,000; ProteinTech Group, Inc.). Following washing
with PBS, membranes were incubated with secondary antibodies for 30
min at room temperature: Fluorescein (FITC)-conjugated Goat
Anti-Rabbit IgG(H+L) (cat. no. SA00003-2; 1:200; ProteinTech Group,
Inc.) for red fluorescence and Goat Anti-Rabbit IgG H&L (Alexa
Fluor® 488) (cat. no. ab150077; 1:200; Abcam) for green
fluorescence. Cells were washed twice with PBS for 5 min each time
and subsequently stained with DAPI for 10 min at room temperature
in the dark. Fluorescent images were obtained at a magnification of
×200 using a Nikon A1 confocal microscope with the addition of a
laser scanner.
Network pharmacology and molecular
docking analysis
To determine the potential targets of HY-N7656, data
was integrated from multiple open-access databases, such as TCMSP,
DrugBank, SwissTargetPrediction and PharmMapper.
Genes associated with RILI were determined using multiple
biomedical resources, including GeneCards, NCBI, OMIM and the
DisGeNET platform. Duplicate entries were removed and an
intersection analysis was conducted between the active compound
targets and RILI-associated targets, followed by protein-protein
interaction (PPI) analysis use the STRING database. To illustrate
the interaction results, Cytoscape 3.9.0 (Cytoscape Consortium;
https://cytoscape.org) was used for network
mapping, followed by degree centrality (DC)-based identification of
central targets (36). To gain
further insights into the biological roles of the overlapping
targets, functional enrichment analyses, including Gene Ontology
(GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment
analyses were conducted using the DAVID platform (https://david.ncifcrf.gov/). Following sorting
according to gene quantity and significance level, data were
visualized using an online tool provided by the Bioinformatics
platform. PI3K and Akt protein structures were obtained from the
Protein Data Bank (PDB) (https://www.rcsb.org/) and the mol2 structure of
HY-N7656 was obtained from the ChemSpider database (https://www.chemspider.com/). Structures were imported
into the CB-Dock2 online platform (https://cadd.labshare.cn/cb-dock2/) for molecular
docking visualization (37). The
relevant URLs for the databases are provided in Table SI.
Reverse transcription-quantitative (RT-q)
PCR analysis
Total RNA was extracted from A549 cells seeded at a
density of 8×104 cells per well using the SteadyPure
Quick Extraction kit (cat. no. AG21023; Accurate Biology) following
the manufacturer's protocol. RNA concentration and purity were
assessed using a NanoDrop 2000 spectrophotometer (Thermo Fisher
Scientific, Inc.). Total RNA was reverse-transcribed into cDNA
using the ExonScript RT SuperMix reverse transcription kit with
dsDNase (cat. no. 231106-A5; Exongen) according to the
manufacturer's protocol. qPCR analysis was conducted using
UltraStart SYBR Green qPCR Master Mix (cat. no. 231008-A4; Exongen)
on a CFX96 Real-Time system C1000™ thermal cycler (BioRad
Laboratories, Inc.). The PCR cycling conditions consisted of an
initial denaturation at 95°C for 30 sec, followed by 40 cycles of
denaturation at 95°C for 5 sec and annealing/extension at 60°C for
30 sec. Quantification of target gene mRNA was carried out using
the 2−ΔΔCq method based on comparative threshold cycles
(38). Sequences of primers are
listed in Table SII. All
experiments were performed in triplicate.
Western blot analysis
Lung tissues and A549 cells were washed in
pre-cooled PBS, lysed using RIPA lysis buffer (cat. no. PC101;
Epizyme) and heated to 95°C for denaturation. Protein concentration
was determined using a BCA protein assay kit. Proteins were
separated via electrophoresis on a 10% gel, with 30 μg of
protein loaded per lane and transferred to a PVDF membranes.
Subsequently, membranes were treated with 5% BSA for 1 h and
incubated with the following primary antibodies overnight at 4°C:
Anti-E-cadherin (cat. no. 20874-1-AP; 1:2,000; ProteinTech Group,
Inc.), anti-Vimentin (cat. no. 10366-1-AP; 1:2,000; ProteinTech
Group, Inc.), anti-AKT (cat. no. ET1609-51; 1:2,000; Huabio),
anti-phosphorylated (p)-AKT (cat. no. ab81283; 1:5,000; Abcam),
anti-PI3K (cat. no. 251221; 1:2,000; ZenBio), anti-p-PI3K (cat. no.
341468; 1:2,000; ZenBio), anti-Ncadherin (cat. no. sc-525409;
1:1,000; Santa Cruz Biotechnology, Inc.) and anti-GAPDH (cat. no.
AC002; 1:2,000; ABclonal Biotech Co., Ltd.). Following primary
incubation, membranes were incubated with the following secondary
antibodies for 1 h at room temperature: Goat anti-rabbit IgG (cat.
no. AS014; 1:5,000; ABclonal Biotech Co., Ltd.) and goat anti-mouse
IgG (cat. no. SA00001-1; 1:5,000; ProteinTech Group, Inc.).
Proteins bands were visualized using an ultrasensitive ECL-based
chemiluminescence assay (cat. no. RM02867; ABclonal Biotech Co.,
Ltd.) and expression was quantified using ImageJ software (version
1.54p; National Institutes of Health).
Statistical analysis
Statistical analysis was carried out using GraphPad
Prism 9 (Dotmatics). Data are presented as the mean ± standard
error of the mean (SEM). For statistical analysis, differences
between two groups were analyzed using unpaired Student's t-tests
following normality testing, while one-way ANOVA followed by
Tukey's post-hoc test was used for comparisons between multiple
groups. Investigators were blinded during histological scoring and
data analysis to eliminate any potential bias. The scoring results
were analyzed using the nonparametric Kruskal-Wallis test followed
by Dunn's post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
HY-N7656 dose optimization and survival
benefits in RILI mice
Mice in the RILI model received HY-N7656
intraperitoneally at doses of 7.5, 15 and 30 mg/kg, corresponding
to low, medium and high concentrations. The results revealed that
all three doses alleviated the radiation-induced increase in lung
density and reduced pulmonary pathological changes (Fig. 1A). As displayed in Fig. 1B, the rate of increase in lung
density gradually decreased from 47.7 to 34.7 and 30.9% with
increasing drug dosage, compared with the control group. Relative
to the IR group, treatment with HY-N7656 at a dose of 7.5 mg/kg led
to a 10.9% reduction in the lung density values of mice. For the 15
and 30 mg/kg dosages, the decreases in lung density values were
recorded as 23.9 and 27.7%, respectively. The pathological scoring
results displayed in Fig. 1C
revealed that, compared to the control group, the effects of the
low dose (P<0.001) were significantly lower than those of the
medium and high doses (P<0.0001). Compared with the 15 mg/kg
dose, the 30 mg/kg dose showed a trend toward improved efficacy;
however, statistical analysis revealed no significant difference
between the two (P>0.05). Accordingly, 15 mg/kg was selected as
the optimal dose for subsequent experiments. During the 16-week
period following irradiation, multiple parameters were monitored to
evaluate the therapeutic efficacy of HY-N7656 on RILI in mice. As
shown in Fig. S1A, compared
with the control group, the fur in the irradiated chest region
progressively changed from black to gray-white by Week 16.
Whole-chest irradiation resulted in high mortality and survival
status in each group was recorded at Week 16. The HY-N7656+IR group
exhibited a survival benefit at Week 4 and a prolonged survival
advantage was maintained over time, with a higher survival rate
(60%) compared with the irradiated group alone (20%), using the
control group as the baseline (Fig.
S1B). Of the 20 irradiated mice, 75% (15 mice) died, while 5
survived to 16 weeks, consistent with previous reports on thoracic
irradiation lethality (39,40). Based on our previous studies
(27,28), 5 mice per group were considered
sufficient to obtain statistically significant results. Body weight
was also monitored as an indicator of general health. As shown in
Fig. S1C, mice in the IR group
exhibited a weight loss of 29.2% compared with the control group
(P<0.0001), whereas HY-N7656 treatment led to a weight loss of
14.9% (P<0.001). Collectively, these findings indicated that
prophylactic administration of HY-N7656 markedly reduced
radiation-induced mortality in mice and improved their overall
health status.
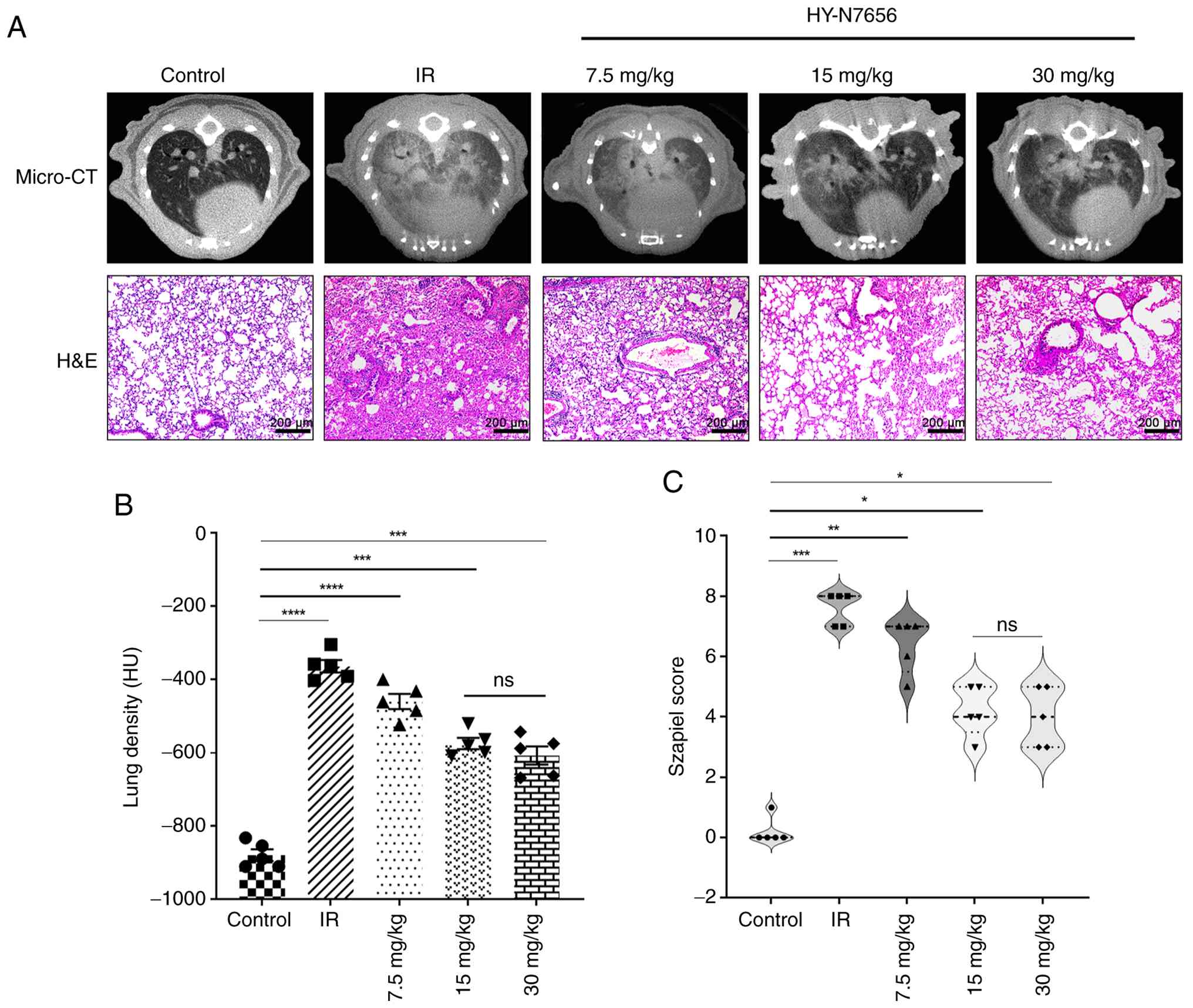 | Figure 1Effect of HY-N7656 on lung injury in
an IR-induced model. (A) Micro-CT images and H&E-stained lung
tissue sections from mice in the Control, IR and HY-N7656 treatment
groups (7.5, 15 and 30 mg/kg). Magnification, ×100. Scale bar, 200
μm. (B) Quantification of lung density (HU) based on
micro-CT imaging demonstrated a significant decrease in lung
density in the IR group relative to the control group, with
HY-N7656 producing a dose-dependent improvement. (C)
Histopathological scoring (Szapiel score) demonstrated a
significant reduction in tissue damage in HY-N7656-treated mice
compared with the IR group. Data are presented as mean ± SEM (n=5).
*P<0.05, **P<0.01,
***P<0.001, ****P<0.0001; ns, no
significance. HY-N7656, 5,7,8-trimethoxyflavone; IR, ionizing
radiation; HU, Hounsfield unit; micro-CT, micro-computed
tomography; H&E, haematoxylin and eosin; SEM, standard error of
the mean. |
HY-N7656 preserves lung architecture and
reduces fibrosis
The experimental procedure for animal experiments is
outlined in Fig. 2A. As
illustrated in Fig. 2B,
longitudinal micro-CT imaging of the irradiated group at 4, 8, 12
and 16 weeks revealed progressive damage in the lungs of the IR
group. Radiological examination at 16 weeks demonstrated classical
fibrotic indicators, including distorted septal architecture,
peripheral reticular densities and honeycomb-like lesions. Notably,
HY-N7656 intervention markedly alleviated these morphological signs
of RILI in treated mice. A computational assessment of HU values
was carried out to determine alterations in lung parenchymal
density following radiation exposure. In the IR group, high-density
foci were observed in the lungs after 8 weeks, with a significant
increase in lung density 16 weeks post-irradiation (Fig. 2D). Lung densitometric progression
was reduced by 27.6% in mice receiving HY-N7656 and the results
were statistically significant (P<0.001).
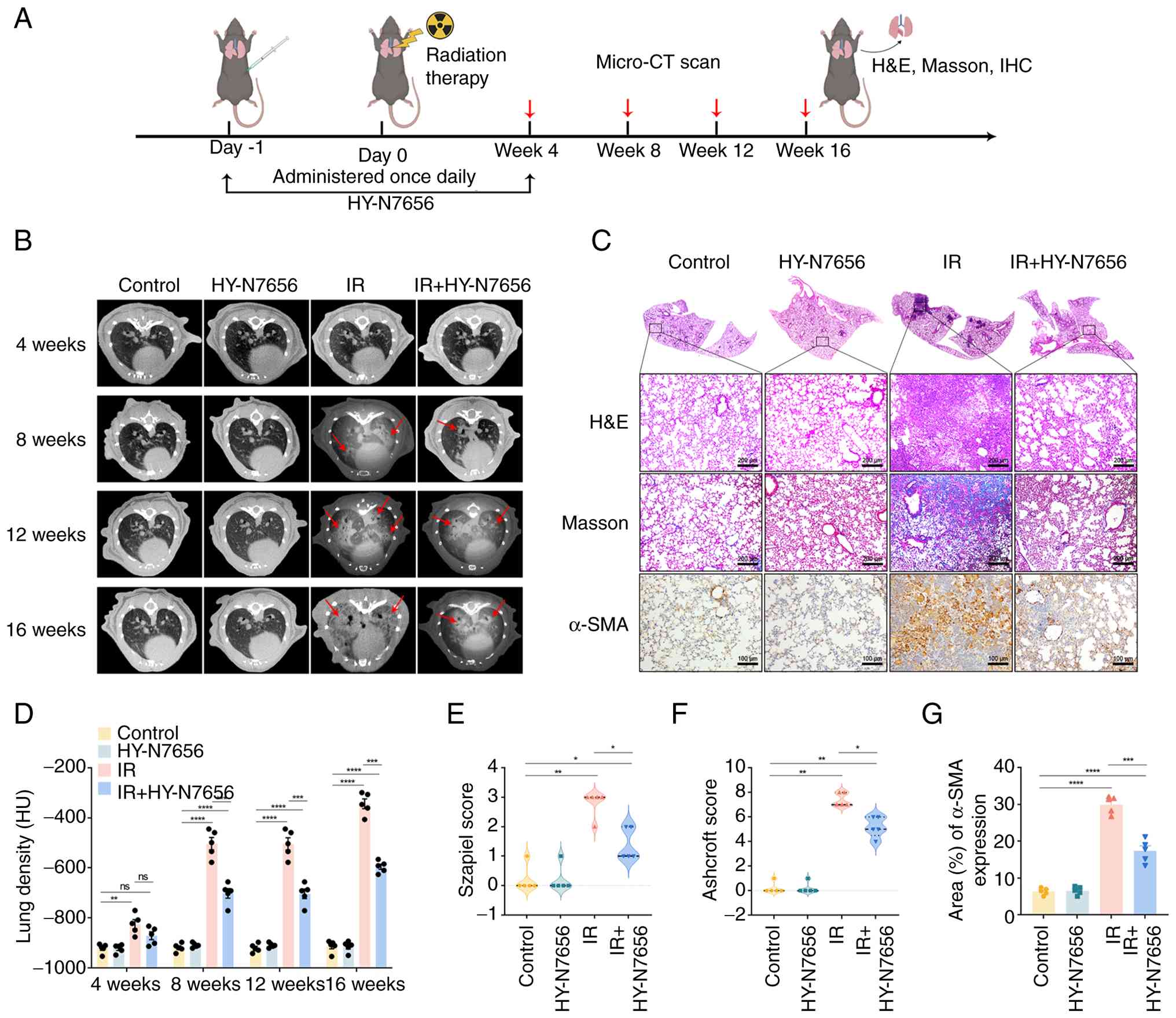 | Figure 2Effects of HY-N7656 on lung
morphology and histopathological changes in irradiated mice. (A)
Experimental design showing the timeline of radiation therapy,
HY-N7656 administration and evaluation at Weeks 4, 8, 12 and 16
through micro-CT scan, H&E staining, Masson staining and IHC
analysis. (B) Representative micro-CT images of lung morphology at
4, 8, 12 and 16 weeks post-treatment. Radiation-induced lung injury
is indicated by arrows. (C) Representative histological analysis of
lung tissue at 16 weeks. H&E, Masson (magnification, ×100;
scale bar, 200 μm) and α-SMA staining (magnification, ×200;
scale bar, 100 μm) revealed progressive fibrosis in the IR
group, while HY-N7656 treatment reduced fibrosis and α-SMA
expression, indicating a protective effect. (D) Quantification of
lung density (HU) based on micro-CT imaging at each time point
showed significant improvement in lung density in HY-N7656-treated
groups compared with the IR group. (E) Szapiel score for H&E
staining in lung tissue sections. (F) Ashcroft score for Masson
staining in lung tissue sections. (G) Quantification of
α-SMA-positive area (%) in lung tissue. Data are presented as mean
± SEM (n=5), *P<0.05, **P<0.01,
***P<0.001, ****P<0.0001, ns, no
significance. HY-N7656, 5,7,8-trimethoxyflavone; IR, ionizing
radiation; HU, Hounsfield unit; micro-CT, micro-computed
tomography; H&E, hematoxylin and eosin; IHC,
immunohistochemistry; α-SMA, α-smooth muscle actin; SEM, standard
error of the mean. |
At 16 weeks post-irradiation, tissues were collected
for morphological analysis and the results confirmed that all
organs, including the heart, liver, spleen and kidneys, remained
visibly healthy across all groups, excluding the lungs (Fig. S2). Lung tissue sections stained
with H&E exhibited a healthy pulmonary morphology in both the
HY-N7656 and control groups, with open alveolar spaces and no
evidence of inflammatory cell infiltration. Significant
histopathological alterations were detected in the IR group, with
features such as alveolar collapse, interstitial edema,
inflammatory cell accumulation and thickening of the alveolar
septa. Lung tissues obtained from the IR+HY-N7656 group exhibited
attenuated pathological changes, including alleviation of alveolar
septal thickening and suppression of immune cell infiltration
(Fig. 2C). According to the
Szapiel criteria, the IR group exhibited markedly elevated
inflammation scores relative to both the control and HY-N7656-only
groups (P<0.0001). Notably, the IR + HY-N7656 group exhibited a
reduction of >50% in lung inflammation score, compared with the
IR group (P<0.01; Fig. 2E).
Collagen accumulation in lung tissue was assessed using Masson's
trichrome staining. The findings indicated that lung structure
appeared healthy in both the HY-N7656 and control groups. Compared
with the control group, the IR group demonstrated pathological
evidence of fibrosis, characterized by substantial extracellular
matrix accumulation. Relative to the IR group, lungs obtained from
the IR + HY-N7656 group exhibited suppressed extracellular matrix
deposition and reduced fibrotic severity. The Ashcroft score
further indicated that HY-N7656 inhibited the radiation-induced
increase in score by 27% (Fig.
2F).
To further examine the role of HY-N7656 in
modulating fibrosis, a marker associated with RILI; namely α-SMA,
was analyzed via immunohistochemistry. Results of the present study
highlighted that the levels of α-SMA in lung tissue increased by
4.7-fold from baseline following irradiation, while HY-N7656
treatment effectively reduced α-SMA levels to 2.7 times the
expected level, thereby alleviating radiation-induced myofibroblast
differentiation (P<0.001; Fig.
2G). Collectively, these observations demonstrated that
HY-N7656 may alleviate pulmonary damage following radiation
exposure by dampening inflammatory processes and inhibiting
extracellular matrix deposition.
HY-N7656 inhibits RILI progression via
suppression of EMT in vivo
As illustrated in Fig. 3A, circulating TGF-β levels in
irradiated mice increased by 110.8 pg/ml (2.8-fold) compared with
the control group (P<0.0001). By contrast, HY-N7656 treatment
attenuated this radiation-induced elevation by 1.2-fold relative to
the IR group, further supporting the compound anti-RILI effects of
HY-N7656. Blood parameters (Fig.
S3) and liver and kidney function indices (Fig. S4) remained within expected
ranges in all groups, confirming the biosafety of HY-N7656. The
lung index (lung-to-body-weight ratio), an indicator of pulmonary
edema, was markedly elevated in irradiated mice compared with
controls (Fig. 3B). HY-N7656
markedly mitigated this increase, reducing the lung coefficient by
73.4% and bringing it closer to physiological levels (P<0.001).
Consistent with these findings, hydroxyproline content in lung
tissue at 16 weeks post-irradiation was elevated 2.6-fold in the IR
group, whereas HY-N7656 limited this increase to 1-fold,
effectively suppressing radiation-induced collagen accumulation
(P<0.001; Fig. 3C). Results
of a previous study demonstrate that EMT plays a key role in the
progression of radiation-induced pulmonary fibrosis (41). To further validate results of the
in vivo studies, the expression levels of EMT-associated
proteins in mouse lung tissues were investigated. Western blot
analysis confirmed that HY-N7656 effectively reversed
radiation-induced EMT (Fig. 3D).
Specifically, E-cadherin, an epithelial marker that was markedly
downregulated following irradiation, was restored to twice its
irradiated level following HY-N7656 treatment (P<0.05; Fig. 3E). Moreover, HY-N7656 reduced the
irradiation-induced upregulation of the mesenchymal marker vimentin
by 33.9%, with the final expression level being comparable to that
of the control group (P>0.05; Fig. 3F). Collectively, these results
highlighted the protective effects of HY-N7656 against
radiation-induced pulmonary injury and further supported its
translational potential in preclinical models.
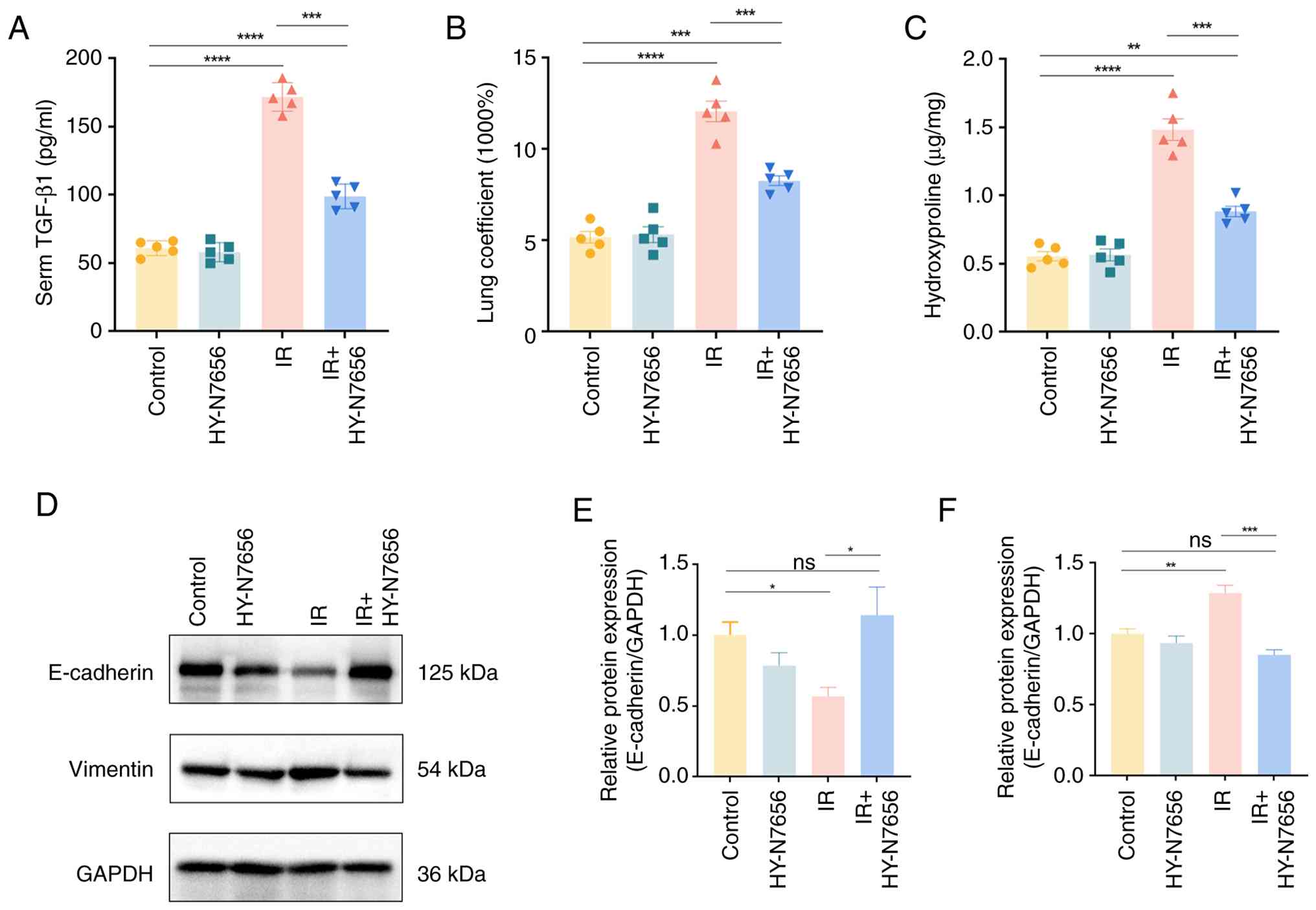 | Figure 3HY-N7656-mediated inhibition of RILI
and associated mechanisms in irradiated mice. (A) Detection of
serum TGF-β levels using ELISA at 16 weeks post-irradiation in each
group of mice (n=5). (B) Statistical analysis of relative lung
coefficient in each group of mice (n=5). (C) Quantification of
hydroxyproline content in lung tissues at 16 weeks post-radiation
(n=5). (D) Western blot analysis of EMT markers in RILI mice at 16
weeks post-irradiation. Quantification of (E) E-cadherin and (F)
vimentin protein expression levels. Data are presented as mean ±
SEM, *P<0.05, **P<0.01,
***P<0.001, ****P<0.0001, ns: no
significance. HY-N7656, 5,7,8-trimethoxyflavone; RILI,
radiation-induced lung injury; TGF-β, transforming growth factor-β;
ELISA, enzyme-linked immunosorbent assay; EMT,
epithelial-mesenchymal transition; SEM, standard error of the mean;
ns, not significant. |
HY-N7656 alleviates TGF-β-induced
cellular EMT
A safe working concentration range for HY-N7656 was
first determined using the CCK-8 assay. A549 cells, representing
human alveolar type II epithelial cells, were exposed to HY-N7656
at 5-30 μM and cell viability was assessed at 24 and 48 h
(Fig. S5A and B). Based on
these results, 5, 10 and 20 μM were selected as safe
intervention concentrations for subsequent experiments. In the
TGF-β-induced A549 fibrosis model, HY-N7656 was used at these
doses. Notably, 10 μM HY-N7656 (Fig. S6B) elicited the most robust
anti-fibrotic response, markedly downregulating EMT markers;
namely, N-cadherin and vimentin, while restoring E-cadherin
expression levels, compared with 5 μM (Fig. S6A) and 20 μM (Fig. S6C).
EMT is a key process through which epithelial cells
acquire mesenchymal characteristics. Upon TGF-β stimulation, A549
cells underwent a marked morphological transition, from orderly
cobblestone-like epithelial cells to elongated mesenchymal-like
cells with reduced intercellular adhesion. HY-N7656 effectively
prevented these morphological changes, helping to preserve the
epithelial phenotype (Fig. S7).
To further assess the effect of HY-N7656 on cell motility, a wound
healing assay was performed. TGF-β markedly enhanced A549 cell
migration over 48 h; however, compared to the IR group, HY-N7656
reduced this increase from 2.5- to 1.6-fold (P<0.001),
demonstrating a significant inhibitory effect on migration
(Fig. 4A and B).
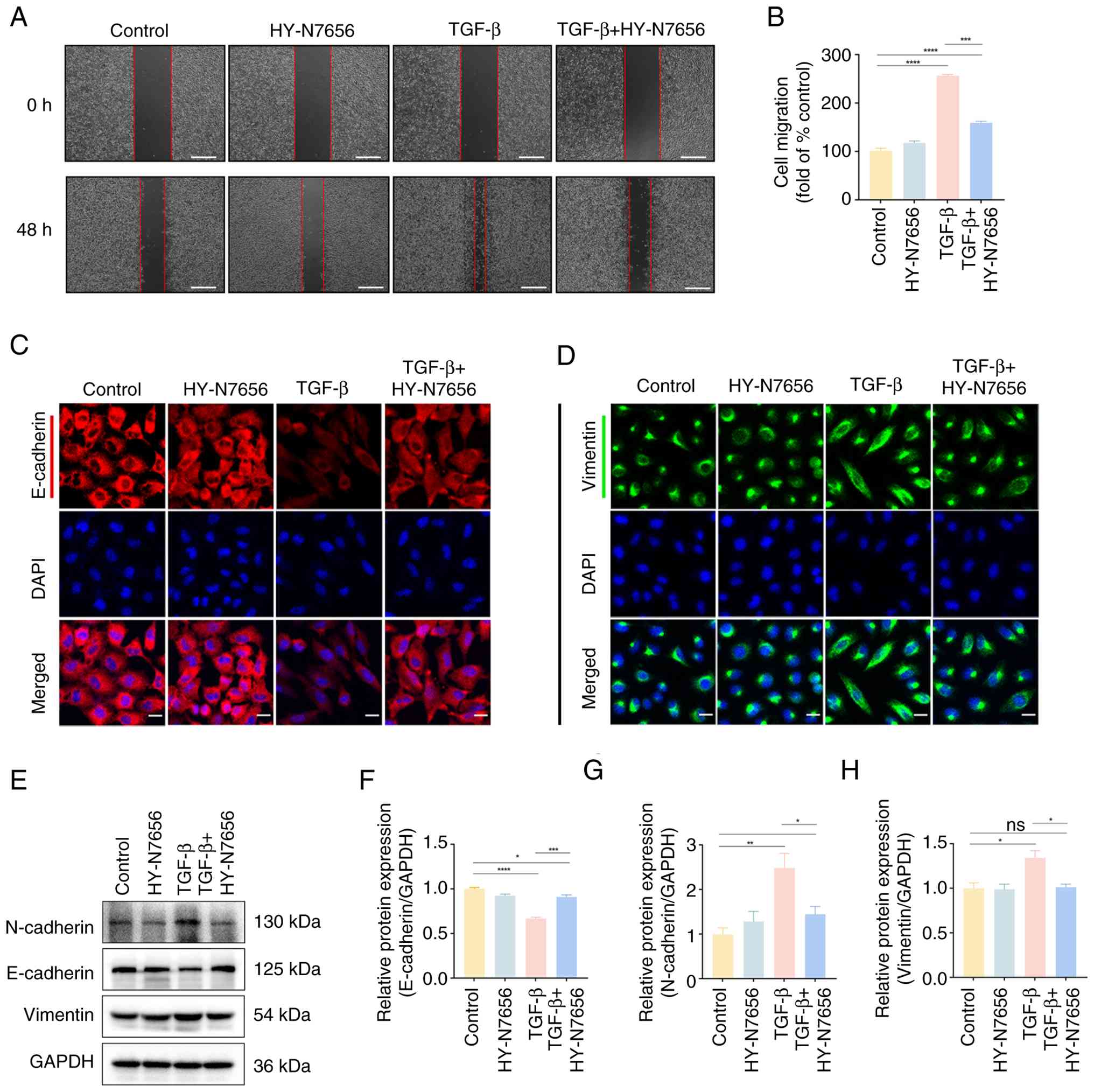 | Figure 4The effect of HY-N7656 on cell
migration and EMT markers. (A) Representative images of wound
healing assays highlighting cell migration at 0 and 48 h after
treatment in control, HY-N7656, TGF-β and TGF-β + HY-N7656 groups.
The red lines indicate the wound area (scale bar, 500 μm).
(B) Quantification of cell migration represented as fold change
relative to control. (C) Immunofluorescence staining of E-cadherin
(red) and DAPI (blue) in A549 cells (scale bar, 20 μm). (D)
Immunofluorescence staining of vimentin (green) and DAPI (blue) in
A549 cells (scale bar, 20 μm). (E) Western blot analysis of
N-cadherin, E-cadherin and vimentin protein expression levels in
cells. GAPDH was used as a loading control. Quantification of (F)
E-cadherin, (G) N-cadherin and (H) vimentin protein expression.
Data are presented as mean ± SEM (n=3), *P<0.05,
**P<0.01, ***P<0.001,
****P<0.0001, ns, not significant. HY-N7656,
5,7,8-trimethoxyflavone; EMT, epithelial-mesenchymal transition;
TGF-β, transforming growth factor-β; DAPI,
4',6-diamidino-2-phenylindole; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase; SEM, standard error of the mean. |
Notably, E-cadherin is essential for maintaining
epithelial integrity and its loss is a hallmark of EMT and vimentin
is strongly upregulated during the mesenchymal transition. Thus,
expression levels were examined using immunofluorescence staining
in the TGF-β-induced model (Fig. 4C
and D). Results of the present analysis revealed that, compared
to the IR group, TGF-β reduced intracellular E-cadherin to 40% of
control levels, whereas HY-N7656 increased expression to 77% of
expected levels. Conversely, HY-N7656 decreased aberrant vimentin
expression by 16.3% (P<0.001; Fig. S8A and B).
Radiation-induced EMT disrupts epithelial polarity,
cell-cell adhesion and cytoskeletal structure. To further clarify
the mechanism by which HY-N7656 mitigates EMT, western blot
analysis was performed (Fig.
4E). Results of the present study revealed that HY-N7656
suppressed TGF-β-induced N-cadherin upregulation, limiting its
increase to 40% (P<0.001), reduced vimentin expression by 24.6%
(P<0.05) and attenuated E-cadherin downregulation by 36.8%
(P<0.05; Fig. 4F-H). Overall,
these findings demonstrated that HY-N7656 effectively inhibited
TGF-β-induced EMT in vitro, reduced cell migration and
preserved epithelial phenotype, highlighting its therapeutic
potential in preventing fibrosis-associated cellular
reprogramming.
Network pharmacology and molecular
docking analysis
As displayed in Fig.
5A, 58 overlapping target genes were identified between 118
HY-N7656- and 1,712 RILI-associated targets. The PPI network
constructed via STRING and visualized in Cytoscape 3.9.0 revealed
six hub genes; namely, ALB, EGFR, MMP9, PTGS2, ESR1 and SRC,
through CytoNCA centrality analysis (Fig. 5B). Previous studies demonstrated
that targeting the aforementioned pathways may improve DNA repair,
inflammatory responses and apoptosis in irradiated cells, thereby
protecting lung tissue from radiation toxicity and potentially
enhancing the efficacy of radiotherapy (42-46). As displayed in Fig. 5C, GO enrichment analysis
indicated that these targets mainly regulate biological processes
associated with oxidative stress, inflammation and cell
proliferation (Table SIII).
KEGG analysis further revealed significant enrichment of multiple
signaling pathways, including the PI3K/Akt pathway (Fig. 5D; Table SIV), suggesting that this may be
a potential core mechanism underlying the therapeutic effects of
HY-N7656 in the treatment of RILI. Fig. 5E and F illustrate the docking
visualization between the small molecule compound, HY-N7656 and
PI3K and Akt proteins, with binding energies of −7.3 and −9.4
kcal/mol, respectively. These results highlighted a strong binding
affinity between HY-N7656 and PI3K/Akt, meaning HY-N7656 may exert
its therapeutic effects through targeting the PI3K/Akt signaling
pathway. These findings may provide a validation basis for further
mechanistic studies of HY-N7656.
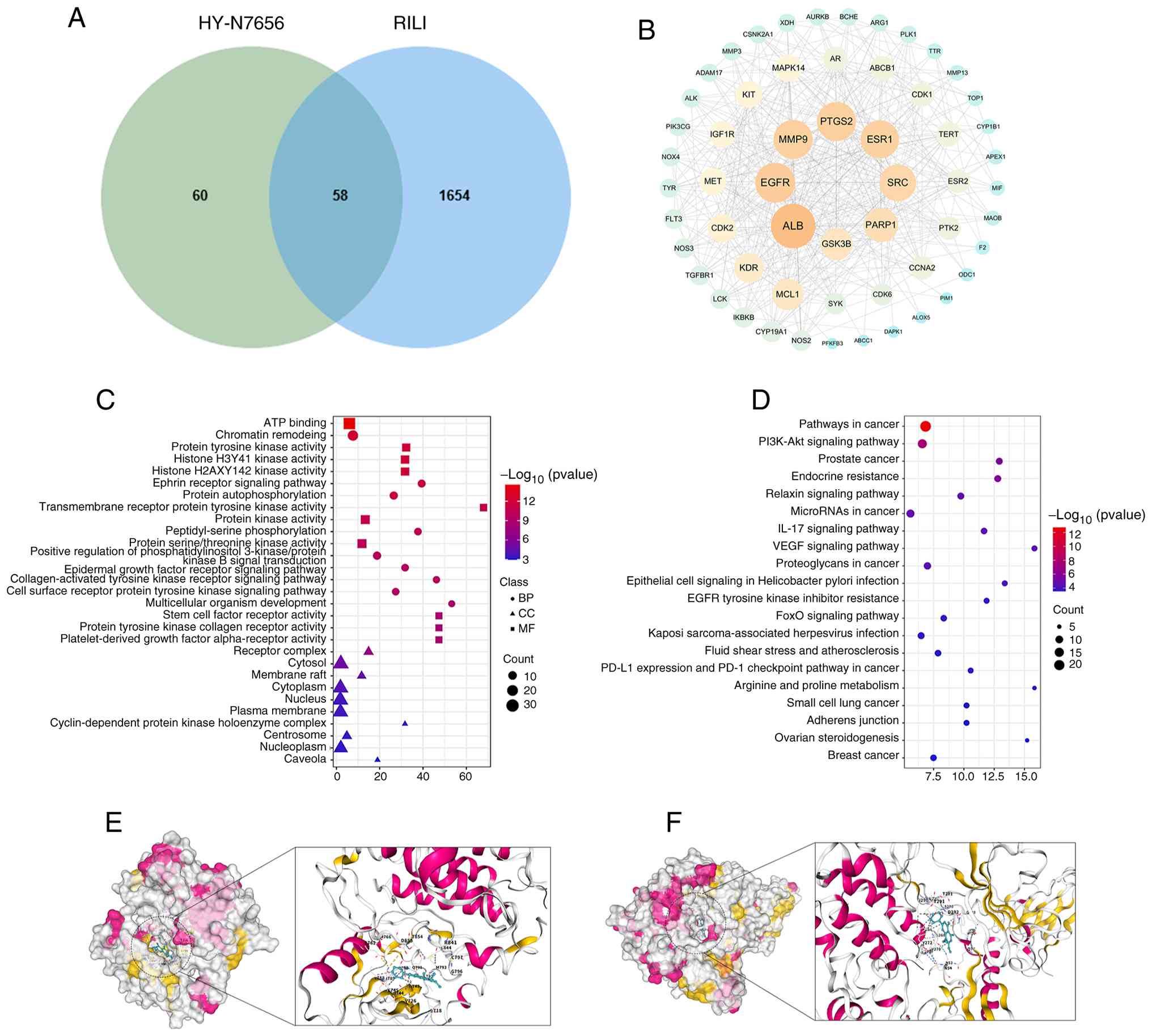 | Figure 5Network pharmacology analysis. (A)
After intersecting 118 targets of HY-N7656 with 1,712 RILI-related
targets, 58 common targets of HY-N7656 acting on RILI were
identified. (B) PPI network diagram of the 58 intersecting targets,
with node importance decreasing from large to small and color
transitioning from yellow to green. (C) GO enrichment of HY-N7656
targets for the treatment of RILI, highlighting the top 10 enriched
BPs, CCs and MFs. (D) Top 20 KEGG pathways enriched for HY-N7656 in
the treatment of RILI. (E) Visualization of molecular docking
between HY-N7656 and PI3K. (F) Visualization of molecular docking
between HY-N7656 and Akt. HY-N7656, 5,7,8-trimethoxyflavone; RILI,
radiation-induced lung injury; PPI, protein-protein interaction;
GO, Gene Ontology; BP, biological process; CC, cellular component;
MF, molecular function; KEGG, Kyoto Encyclopedia of Genes and
Genomes; PI3K, phosphoinositide 3-kinase. |
HY-N7656 inhibits the PI3K/Akt
pathway
Based on the previous KEGG pathway enrichment
analysis using network pharmacology, the PI3K/Akt axis may serve as
a critical mechanism by which HY-N7656 exerts its inhibitory effect
on RILI. Results of the western blot analysis revealed that Akt and
PI3K phosphorylation levels were upregulated by ~1.6- and 1.8-fold,
respectively, following treatment with TGF-β, compared with the
control group (P<0.05 and P<0.001; Fig. 6A-C). Notably, a significant
decline in phosphorylated (p)-Akt and p-PI3K levels was observed
following treatment with HY-N7656, with reductions of 32.8 and
45.6%, respectively. Thus, HY-N7656 inhibited the PI3K/Akt
signaling triggered by TGF-β and this inhibitory effect was
associated with the suppression of EMT progression. A schematic
diagram of the effect of HY-N7656 on RILI is displayed in Fig. 6D.
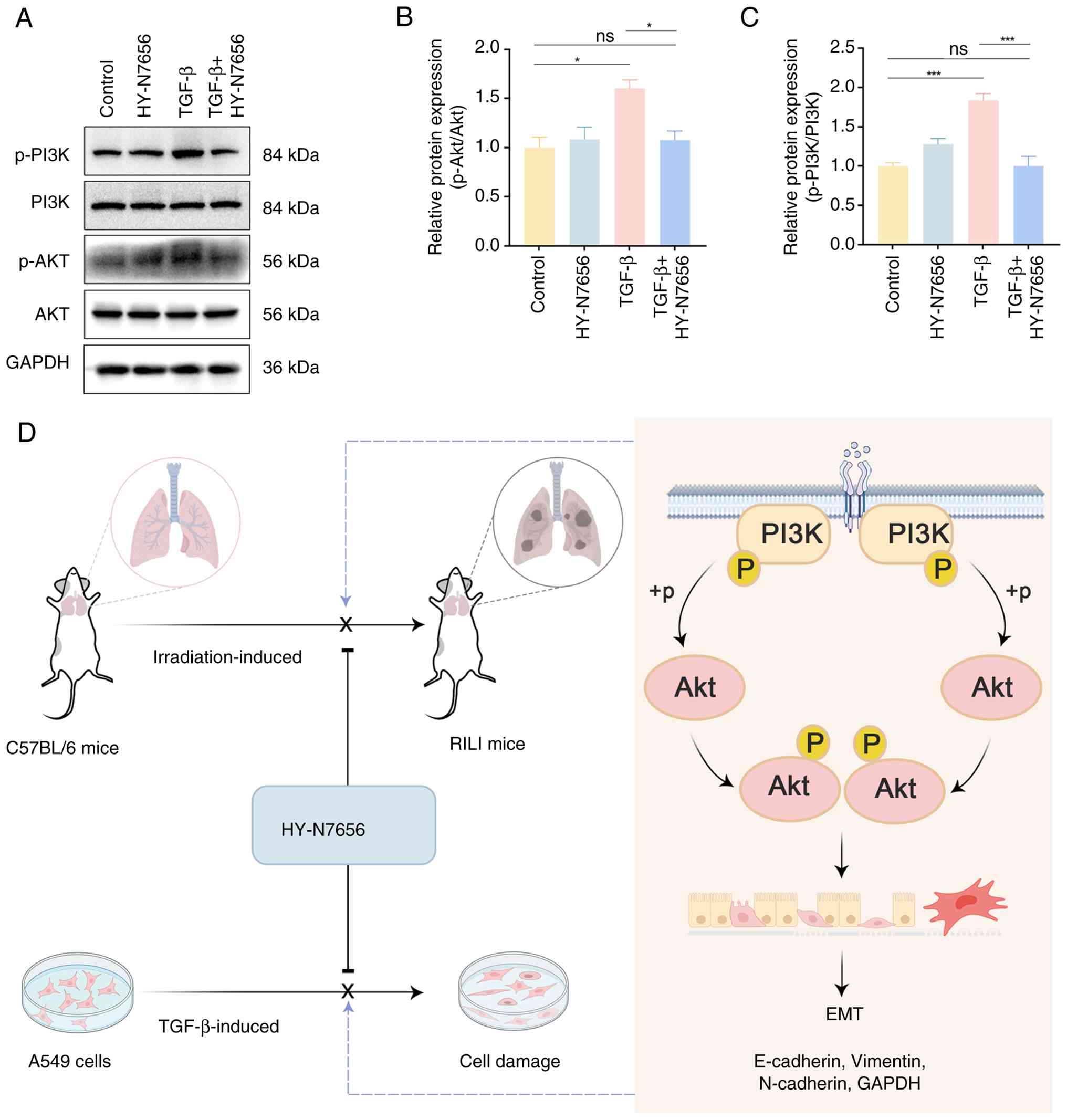 | Figure 6HY-N7656 inhibits activation of the
PI3K/Akt signaling pathway in A549 cells. (A) Western blot analysis
of PI3K, AKT, PI3K phosphorylation and AKT phosphorylation in A549
cells. Quantitative analysis of (B) p-Akt and (C) p-PI3K protein
levels. GAPDH was used as the loading control. Data are presented
as mean ± SEM. *P<0.05, ***P<0.001. (D)
Mechanism of action for HY-N7656 in alleviating RILI via inhibition
of PI3K/Akt mediated partial EMT. HY-N7656,
5,7,8-trimethoxyflavone; PI3K, phosphoinositide 3-kinase; AKT,
protein kinase B; RILI, radiation-induced lung injury; EMT,
epithelial-mesenchymal transition; GAPDH, glyceraldehyde
3-phosphate dehydrogenase; SEM, standard error of the mean; p-,
phosphorylated. |
Discussion
RILI is a well-recognized complication of
radiotherapy for thoracic tumors (47-49), ultimately leading to impaired
lung function, respiratory failure and, in some cases, mortality
(50). Despite its clinical
severity, effective strategies for preventing and managing RILI
remain limited, highlighting the requirement for novel therapeutic
interventions. A previous study demonstrated that the natural
flavonoid, HY-N7656, possesses anti-inflammatory activity (29). In the present study, HY-N7656 was
identified as a first-in-class dual inhibitor of EMT and PI3K/Akt
signaling, offering a mechanistically novel approach for the
treatment of RILI. The findings demonstrated that early
administration of HY-N7656 effectively inhibited the progression of
RILI.
The progression of RILI is strongly associated with
EMT, which functions as a fundamental mechanistic pathway (51). Results of a previous study
demonstrated that oral administration of the heat shock protein 70
inducer geranylgeranylacetone in irradiated mice sustains
E-cadherin expression and suppresses EMT markers, thereby exerting
protective effects against RILI (52). Emetine dihydrochloride has
similarly been reported to reduce EMT-associated markers and
decrease Smad3 phosphorylation, mitigating RILI through EMT
inhibition (53). Across diverse
contexts, EMT is characterized by shared regulatory features,
including alterations in cell morphology, increased cell motility,
loss of epithelial traits and acquisition of mesenchymal properties
(54). Consistent with previous
findings (55,56), thoracic irradiation in mice was
confirmed to induce pulmonary EMT, as indicated by reduced
epithelial polarity, reduced E-cadherin expression, impaired
intercellular junctions and increased vimentin levels. TGF-β is
widely recognized as a key driver of EMT (57) and acts as a major upstream
regulator in the initiation of acute lung inflammation, as well as
the progression of chronic fibrosis (13). Given its central role in
RILI-associated pulmonary fibrosis, inhibition of TGF-β activation
and expression is considered an effective strategy for slowing RILI
progression. For example, Jiawei Maxing Shigan Tang, a traditional
Chinese herbal formula, has been reported to improve RILI outcomes,
potentially via the modulation of Tregs, inhibiting the TGF-β/Smad
axis and attenuating EMT-associated processes (58). In the present study, HY-N7656
treatment restored E-cadherin levels in lung tissue, reduced
vimentin expression and effectively inhibited radiation-induced
EMT. Further experiments indicated that this effect was
attributable to suppression of TGF-β elevation during RILI. These
findings demonstrated that HY-N7656 improves RILI through the
inhibition of TGF-β-induced EMT progression.
Network pharmacology analysis revealed that the
PI3K/Akt signaling pathway was markedly enriched. As one of the
most frequently activated pathways in RILI, this finding suggested
that HY-N7656 may exert its therapeutic effects through this axis,
warranting further investigation. Subsequent molecular docking
validation indicated that HY-N7656 possesses a strong binding
association with key components of the PI3K/Akt signaling pathway.
In addition to canonical TGF-β signaling, aberrant activation of
the PI3K-Akt pathway plays a critical role in driving EMT
initiation and promoting tissue structural alterations (59,60). At elevated levels, TGF-β
activates the PI3K/Akt signaling pathway, which subsequently
downregulates FOXO3a expression; thus, inducing EMT in alveolar
epithelial cells (61). This
pathway is frequently emphasized in studies of cancer, inflammation
and fibrosis (62). AKT, the
principal downstream effector of PI3K, exhibits markedly increased
phosphorylation following radiation exposure. Results of a previous
study demonstrated that targeting the AKT/GSK3β signaling pathway
effectively attenuated radiation-induced EMT in alveolar epithelial
cells, offering a potential therapeutic strategy for RILI (63).
Akt signaling facilitates EMT progression through
the suppression of Snail and Twist, transcription factors that
regulate E-cadherin (64). For
example, Myrtol reduces phosphorylated AKT expression and enhances
E-cadherin levels, thereby inhibiting EMT and providing protective
effects against RILI (65).
Results of a previous study demonstrate that Akt inhibitors
decreased E-cadherin and elevated α-SMA expression, further
highlighting the role of the PI3K/Akt pathway in promoting EMT
progression (22). Moreover,
Re-Du-Ning, a formulation derived from Artemisia and its
bioactive constituents, has been reported to mitigate RILI through
inhibition of PI3K/Akt signaling and suppression of EMT progression
(66). This highlights the
PI3K/Akt pathway as a potential target for EMT inhibition (67). Results of a previous study also
demonstrates that low molecular weight fucose may attenuate EMT and
fibrotic remodeling through modulation of PI3K/Akt pathway activity
in both in vivo and in vitro models (68). Collectively, this evidence
supports the hypothesis that PI3K phosphorylation-induced Akt
signaling plays a central role in activating EMT in AT2 cells,
establishing the PI3K/Akt pathway as an effective and strategic
target for RILI intervention. In vitro experimental data
further confirmed that HY-N7656 effectively blocks transmission
through the PI3K/Akt signaling pathway, thereby suppressing EMT
progression in the context of RILI. Compared with compounds, such
as Myrtol and emetine, which mitigate EMT in RILI, HY-N7656 more
precisely delineates the PI3K/Akt pathway as a critical therapeutic
target, demonstrating distinct mechanistic advantages.
However, the present study exhibits limitations. For
example, results from the initial experiments of the present study
demonstrated that a 15 mg/kg dose of HY-N7656 effectively prevents
and treats RILI. Although associated pathological and biochemical
experiments have confirmed the safety of HY-N7656, pharmacokinetic
evaluation remains indispensable for future clinical translation.
In addition, inclusion of female mice alongside male mice will be
required in future work to address the limitation of using only
male mice in the present study. Molecular docking and associated
experimental evidence demonstrated that HY-N7656 inhibited RILI
through the PI3K/Akt signaling pathway. However, it has not yet
been determined whether HY-N7656 also exerts effects on
radiation-activated Wnt/β-catenin and NF-κB signaling pathways,
which promote lung fibrosis and inflammation (69,70). Based on this, future
comprehensive analyses should incorporate additional mechanistic
experiments, such as immunoprecipitation and kinase activation
assays, to further elucidate the crosstalk between HY-N7656 and
upstream and downstream signaling involved in RILI (PI3K/Akt,
Wnt/β-catenin and NF-κB pathways) (22).
To the best of the authors' knowledge, the present
study is the first to demonstrate that the natural flavonoid,
HY-N7656, may serve as a novel therapeutic agent for mitigating
RILI. Results of the present study indicated that HY-N7656 targets
the PI3K/Akt signaling pathway to suppress EMT progression,
markedly reduced pulmonary inflammation and fibrosis, preserved
epithelial integrity and ultimately ameliorated RILI. Future
research should prioritize preclinical validation in humanized
models and Phase I evaluation of its combinatorial potential with
radiotherapy, offering a novel strategy for RILI treatment.
Supplementary Data
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CCG designed and conducted animal and cell
experiments, processed the data and wrote the manuscript. HKL
collated the experimental results, plotted charts and revised the
manuscript. YZM, JYC, ZYF, SLF and LQ were involved in the
experimental process and data collection. BL, KX and MHC provided
financial support. JYL and QC provided ideas for the design of the
project. CCG and MHC confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the Animal
Ethics Committee of Sichuan Cancer Hospital (approval no.
SCCHEC-04-2024-040).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
The present study was supported by Sichuan Medical Association
(grant no. 2024HR15), The Chengdu Technology Bureau Program (grant
nos. 2024-YF05-02230-SN and 2024-YF05-01283-SN) and the Sichuan
Provincial Administration of Traditional Chinese Medicine Project
(grant no. 2023MS433).
References
|
1
|
Xu T, Zhang Y, Chang P, Gong S, Shao L and
Dong L: Mesenchymal stem cell-based therapy for radiation-induced
lung injury. Stem Cell Res Ther. 9:182018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bradley JD, Paulus R, Komaki R, Masters G,
Blumenschein G, Schild S, Bogart J, Hu C, Forster K, Magliocco A,
et al: Standard-dose versus high-dose conformal radiotherapy with
concurrent and consolidation carboplatin plus paclitaxel with or
without cetuximab for patients with stage IIIA or IIIB
non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two
factorial phase 3 study. Lancet Oncol. 16:187–199. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sharma GP, Fish BL, Frei AC, Narayanan J,
Gasperetti T, Scholler D, Pierce L, Szalewski N, Blue N, Medhora M
and Himburg HA: Pharmacologic ACE-inhibition mitigates
radiation-induced pneumonitis by suppressing ACE-expressing lung
myeloid cells. Int J Radiat Oncol Biol Phys. 113:177–191. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Onishi H, Marino K, Yamashita H, Terahara
A, Onimaru R, Kokubo M, Shioyama Y, Kozuka T, Matsuo Y, Aruga T and
Hiraoka M: Case series of 23 patients who developed fatal radiation
pneumonitis after stereotactic body radiotherapy for lung cancer.
Technol Cancer Res Treat. 17:15330338188013232018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meng Y, Yang H, Wang W, Tang X, Jiang C,
Shen Y and Luo W: Excluding PTV from lung volume may better predict
radiation pneumonitis for intensity modulated radiation therapy in
lung cancer patients. Radiat Oncol. 14:72019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu S, Liu C and Ji HL: Concise review:
Therapeutic potential of the mesenchymal stem cell derived
secretome and extracellular vesicles for radiation-induced lung
injury: Progress and hypotheses. Stem Cells Transl Med. 8:344–354.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Giuranno L, Ient J, De Ruysscher D and
Vooijs MA: Radiation-induced lung injury (RILI). Front Oncol.
9:8772019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bledsoe TJ, Nath SK and Decker RH:
Radiation pneumonitis. Clin Chest Med. 38:201–208. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deng Y, Xia X, Zhao Y, Zhao Z, Martinez C,
Yin W, Yao J, Hang Q, Wu W, Zhang J, et al: Glucocorticoid receptor
regulates PD-L1 and MHC-I in pancreatic cancer cells to promote
immune evasion and immunotherapy resistance. Nat Commun.
12:70412021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li L, Wu D, Deng S, Li J, Zhang F, Zou Y,
Zhang T and Xu Y: NVP-AUY922 alleviates radiation-induced lung
injury via inhibition of autophagy-dependent ferroptosis. Cell
Death Discov. 8:862022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Simone CB II: Thoracic radiation normal
tissue injury. Semin Radiat Oncol. 27:370–377. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Froese AR, Shimbori C, Bellaye PS, Inman
M, Obex S, Fatima S, Jenkins G, Gauldie J, Ask K and Kodlb M:
Stretch-induced activation of transforming growth factor-β1 in
pulmonary fibrosis. Am J Respir Crit Care Med. 194:84–96. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Singh V, Torricelli AA, Nayeb-Hashemi N,
Agrawal V and Wilson SE: Mouse strain variation in SMA(+)
myofibroblast development after corneal injury. Exp Eye Res.
115:27–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tatler AL and Jenkins G: TGF-β activation
and lung fibrosis. Proc Am Thorac Soc. 9:130–136. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim KK, Kugler MC, Wolters PJ, Robillard
L, Galvez MG, Brumwell AN, Sheppard D and Chapman HA: Alveolar
epithelial cell mesenchymal transition develops in vivo during
pulmonary fibrosis and is regulated by the extracellular matrix.
Proc Natl Acad Sci USA. 103:13180–13185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park HR, Jo SK and Jung U: Ionizing
radiation promotes epithelial-to-mesenchymal transition in lung
epithelial cells by TGF-β-producing M2 macrophages. In Vivo.
33:1773–1784. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sohn SH, Lee JM, Park S, Yoo H, Kang JW,
Shin D, Jung KH, Lee YS, Cho J and Bae H: The inflammasome
accelerates radiation-induced lung inflammation and fibrosis in
mice. Environ Toxicol Pharmacol. 39:917–926. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moghbeli M: PI3K/AKT pathway as a pivotal
regulator of epithelial-mesenchymal transition in lung tumor cells.
Cancer Cell Int. 24:1652024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bustamante A, Baritaki S, Zaravinos A and
Bonavida B: Relationship of signaling pathways between RKIP
expression and the inhibition of EMT-inducing transcription factors
SNAIL1/2, TWIST1/2 and ZEB1/2. Cancers (Basel). 16:31802024.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yan Z, Zhu J, Liu Y, Li Z, Liang X, Zhou
S, Hou Y, Chen H, Zhou L, Wang P, et al: DNA-PKcs/AKT1 inhibits
epithelial-mesenchymal transition during radiation-induced
pulmonary fibrosis by inducing ubiquitination and degradation of
Twist1. Clin Transl Med. 14:e16902024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Conte E, Fruciano M, Fagone E, Gili E,
Caraci F, Iemmolo M, Crimi N and Vancheri C: Inhibition of PI3K
prevents the proliferation and differentiation of human lung
fibroblasts into myofibroblasts: the role of class I P110 isoforms.
PLoS One. 6:e246632011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang XL, Xing RG, Chen L, Liu CR and Miao
ZG: PI3K/Akt signaling is involved in the pathogenesis of
bleomycin-induced pulmonary fibrosis via regulation of
epithelial-mesenchymal transition. Mol Med Rep. 14:5699–5706. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang B, Wei J, Meng L, Wang H, Qu C, Chen
X, Xin Y and Jiang X: Advances in pathogenic mechanisms and
management of radiation-induced fibrosis. Biomed Pharmacother.
121:1095602020. View Article : Google Scholar
|
|
24
|
Lin B, Zhang P and Lang J: Clinical
observation of Huaxian decociton on preventing 70 cases of
radio-pulmonarylesion. J Sichuan Tradit Chin Med. 30:76–78. 2012.In
Chinese.
|
|
25
|
Lin B, Zhang P and Lang J: Experimental
research of using Huaxian decociton to prevent and treating
radiation fibrosis of lung. J Sichuan Tradit Chin Med. 33:54–57.
2015.In Chinese.
|
|
26
|
Chen J, Zou P, Fang Z, Gong C, Yin J, Chen
M, Lin B and Lang J: Hua Xian Fang alleviates radiation-induced
pulmonary fibrosis by upregulating the level of IFN-γ in blood and
tissues. Chin J Radiat Oncol. 33:554–561. 2024.In Chinese.
|
|
27
|
Chen J, Zou P, Quan L, Gong C, Fang Z, Lin
B, Lang J and Chen M: Huaxian formula prevents the progression of
radiation-induced pulmonary fibrosis by inhibiting the pro-fibrotic
effects of macrophages. J Ethnopharmacol. 338:1190262025.
View Article : Google Scholar
|
|
28
|
Gong C, Chen J, Zou P, Fang Z, Quan L,
Wang J, Yin J, Lin B, Lang J and Chen M: Serum pharmacochemistry
and network pharmacology reveal active compounds and mechanisms of
the huaxian formula in alleviating radiation-induced pulmonary
fibrosis. Drug Des Devel Ther. 19:627–644. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shen DY, Juang SH, Kuo PC, Huang GJ, Chan
YY, Damu AG and Wu TS: Chemical constituents from andrographis
echioides and their anti-inflammatory activity. Int J Mol Sci.
14:496–514. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Travis EL, Rachakonda G, Zhou X, Korhonen
K, Sekhar KR, Biswas S and Freeman ML: NRF2 deficiency reduces life
span of mice administered thoracic irradiation. Free Radic Biol
Med. 51:1175–1183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Niu S and Zhang Y, Cong C, Wu Z, Wang Z,
Sun M, Yao C and Zhang Y: Comparative study of radiation-induced
lung injury model in two strains of mice. Health Phys. 122:579–585.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lierova A, Kasparova J, Pejchal J,
Kubelkova K, Jelicova M, Palarcik J, Korecka L, Bilkova Z and
Sinkorova Z: Attenuation of radiation-induced lung injury by
hyaluronic acid nanoparticles. Front Pharmacol. 11:11992020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gattinoni L, Caironi P, Pelosi P and
Goodman LR: What has computed tomography taught us about the acute
respiratory distress syndrome? Am J Respir Crit Care Med.
164:1701–1711. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Szapiel SV, Elson NA, Fulmer JD,
Hunninghake GW and Crystal RG: Bleomycin-induced interstitial
pulmonary disease in the nude, athymic mouse. Am Rev Respir Dis.
120:893–899. 1979.PubMed/NCBI
|
|
35
|
Hübner RH, Gitter W, El Mokhtari NE,
Mathiak M, Both M, Bolte H, Freitag-Wolf S and Bewig B:
Standardized quantification of pulmonary fibrosis in histological
samples. Biotechniques. 44:507–511. 514–517. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Missiuro PV, Liu K, Zou L, Ross BC, Zhao
G, Liu JS and Ge H: Information flow analysis of interactome
networks. PLoS Comput Biol. 5:e10003502009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu Y, Yang X, Gan J, Chen S, Xiao ZX and
Cao Y: CB-Dock2: Improved protein-ligand blind docking by
integrating cavity detection, docking and homologous template
fitting. Nucleic Acids Res. 50:W159–W164. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−delta delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
39
|
Kang SK, Rabbani ZN, Folz RJ, Golson ML,
Huang H, Yu D, Samulski TS, Dewhirst MW, Anscher MS and Vujaskovic
Z: Overexpression of extracellular superoxide dismutase protects
mice from radiation-induced lung injury. Int J Radiat Oncol Biol
Phys. 57:1056–1066. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Travis EL, Down JD, Holmes SJ and Hobson
B: Radiation pneumonitis and fibrosis in mouse lung assayed by
respiratory frequency and histology. Radiat Res. 84:133–143. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li X, Ma L, Huang K, Wei Y, Long S, Liu Q,
Zhang D, Wu S, Wang W, Yang G, et al: Regorafenib-attenuated,
bleomycin-induced pulmonary fibrosis by inhibiting the TGF-β1
signaling pathway. Int J Mol Sci. 22:19852021. View Article : Google Scholar
|
|
42
|
Yang K, Palm J, König J, Seeland U,
Rosenkranz S, Feiden W, Rübe C and Rübe CE:
Matrix-metallo-proteinases and their tissue inhibitors in
radiation-induced lung injury. Int J Radiat Biol. 83:665–676. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim SB, Ly P, Kaisani A, Zhang L, Wright
WE and Shay JW: Mitigation of radiation-induced damage by targeting
EGFR in noncancerous human epithelial cells. Radiat Res.
180:259–267. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen ZY, Xiao HW, Dong JL, Li Y, Wang B,
Fan SJ and Cui M: Gut microbiota-derived PGF2α fights against
radiation-induced lung toxicity through the MAPK/NF-κB pathway.
Antioxidants (Basel). 11:652021. View Article : Google Scholar
|
|
45
|
He G, Tang A, Xie M, Xia W, Zhao P, Wei J,
Lai Y, Tang X, Zou YM and Liu H: Blood gene expression profile
study revealed the activation of apoptosis and p53 signaling
pathway may be the potential molecular mechanisms of ionizing
radiation damage and radiation-induced bystander effects. Dose
Response. 18:15593258209141842020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lieverse RIY, Van Limbergen EJ, Oberije
CJG, Troost EGC, Hadrup SR, Dingemans AC, Hendriks LEL, Eckert F,
Hiley C, Dooms C, et al: Stereotactic ablative body radiotherapy
(SABR) combined with immunotherapy (L19-IL2) versus standard of
care in stage IV NSCLC patients, ImmunoSABR: A multicentre,
randomised controlled open-label phase II trial. BMC Cancer.
20:5572020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hou G, Li J, Liu W, Wei J, Xin Y and Jiang
X: Mesenchymal stem cells in radiation-induced lung injury: From
mechanisms to therapeutic potential. Front Cell Dev Biol.
10:11003052022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Konkol M, Śniatała P and Milecki P:
Radiation-induced lung injury - what do we know in the era of
modern radiotherapy? Rep Pract Oncol Radiother. 27:552–565.
2022.PubMed/NCBI
|
|
49
|
Drishya S, Dhanisha SS, Raghukumar P and
Guruvayoorappan C: Amomum subulatum mitigates experimental thoracic
radiation-induced lung injury by regulating antioxidant status and
inflammatory responses. Food Funct. 14:1545–1559. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fox MS, Ouriadov A, Thind K, Hegarty E,
Wong E, Hope A and Santyr GE: Detection of radiation induced lung
injury in rats using dynamic hyperpolarized (129)Xe magnetic
resonance spectroscopy. Med Phys. 41:0723022014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kun C, Tao L, Leiyuan H, Yunhao F, Ning W,
Zhe L, Yuanyuan C, Xiao L, Hongran Q, Jianming C, et al:
Heat-killed Salmonella typhimurium mitigated radiation-induced lung
injury. Clin Exp Pharmacol Physiol. 46:1084–1091. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kim JS, Son Y, Jung MG, Jeong YJ, Kim SH,
Lee SJ, Lee YJ and Lee HJ: Geranylgeranylacetone alleviates
radiation-induced lung injury by inhibiting
epithelial-to-mesenchymal transition signaling. Mol Med Rep.
13:4666–4670. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang X, Li M, Yin J, Fang J, Ying Y, Ye T,
Zhang F, Ma S, Qin H and Liu X: Emetine dihydrochloride alleviated
radiation-induced lung injury through inhibiting EMT. J Cell Mol
Med. 27:3839–3850. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nagaraja SS and Nagarajan D:
Radiation-induced pulmonary epithelial-mesenchymal transition: A
review on targeting molecular pathways and mediators. Curr Drug
Targets. 19:1191–1204. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Qu H, Liu L, Liu Z, Qin H, Liao Z, Xia P,
Yang Y, Li B, Gao F and Cai J: Blocking TBK1 alleviated
radiation-induced pulmonary fibrosis and epithelial-mesenchymal
transition through Akt-Erk inactivation. Exp Mol Med. 51:1–17.
2019. View Article : Google Scholar
|
|
57
|
Wang J, Bao L, Yu B, Liu Z, Han W, Deng C
and Guo C: Interleukin-1β promotes epithelial-derived alveolar
elastogenesis via αvβ6 integrin-dependent TGF-β activation. Cell
Physiol Biochem. 36:2198–2216. 2015. View Article : Google Scholar
|
|
58
|
Wang M, Feng Y, Zhang P, Shen K, Su J,
Zhong Y, Yang X, Lin S and Lu J: Jiawei Maxing Shigan Tang
alleviates radiation-induced lung injury via TGF-β1/Smad signaling
pathway mediated by regulatory T cells. J Ethnopharmacol.
320:1173892024. View Article : Google Scholar
|
|
59
|
Chen H, Chen N, Li F, Sun L, Du J, Chen Y,
Cheng F, Li Y, Tian S, Jiang Q, et al: Repeated radon exposure
induced lung injury and epithelial-mesenchymal transition through
the PI3K/AKT/mTOR pathway in human bronchial epithelial cells and
mice. Toxicol Lett. 334:4–13. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Polimeni M, Gulino GR, Gazzano E, Kopecka
J, Marucco A, Fenoglio I, Cesano F, Campagnolo L, Magrini A,
Pietroiusti A, et al: Multi-walled carbon nanotubes directly induce
epithelial-mesenchymal transition in human bronchial epithelial
cells via the TGF-β-mediated Akt/GSK-3β/SNAIL-1 signalling pathway.
Part Fibre Toxicol. 13:272016. View Article : Google Scholar
|
|
61
|
Qian W, Cai X, Qian Q, Zhang W and Wang D:
Astragaloside IV modulates TGF-β1-dependent epithelial-mesenchymal
transition in bleomycin-induced pulmonary fibrosis. J Cell Mol Med.
22:4354–4365. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Bakin AV, Tomlinson AK, Bhowmick NA, Moses
HL and Arteaga CL: Phosphatidylinositol 3-kinase function is
required for transforming growth factor beta-mediated epithelial to
mesenchymal transition and cell migration. J Biol Chem.
275:36803–38610. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Li Y, Shen Z, Jiang X, Wang Y, Yang Z, Mao
Y, Wu Z, Li G and Chen H: Mouse mesenchymal stem cell-derived
exosomal miR-466f-3p reverses EMT process through inhibiting
AKT/GSK3β pathway via c-MET in radiation-induced lung injury. J Exp
Clin Cancer Res. 41:1282022. View Article : Google Scholar
|
|
64
|
Karimi Roshan M, Soltani A, Soleimani A,
Rezaie Kahkhaie K, Afshari AR and Soukhtanloo M: Role of AKT and
mTOR signaling pathways in the induction of epithelial-mesenchymal
transition (EMT) process. Biochimie. 165:229–234. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhao DY, Qu HJ, Guo JM, Zhao HN, Yang YY,
Zhang P, Cao K, Lei X, Cui JG, Liu C, et al: Protective effects of
myrtol standardized against radiation-induced lung injury. Cell
Physiol Biochem. 38:619–634. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yang C, Song C, Wang Y, Zhou W, Zheng W,
Zhou H, Deng G, Li H, Xiao W, Yang Z, et al: Re-Du-Ning injection
ameliorates radiation-induced pneumonitis and fibrosis by
inhibiting AIM2 inflammasome and epithelial-mesenchymal transition.
Phytomedicine. 102:1541842022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Liu X, Shao C and Fu J: Promising
biomarkers of radiation-induced lung injury: A review.
Biomedicines. 9:11812021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wu N, Li Z, Wang J, Geng L, Yue Y, Deng Z,
Wang Q and Zhang Q: Low molecular weight fucoidan attenuating
pulmonary fibrosis by relieving inflammatory reaction and
progression of epithelial-mesenchymal transition. Carbohydr Polym.
273:1185672021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yang M, Che Y, Li K, Fang Z, Li S, Wang M,
Zhang Y, Xu Z, Luo L, Wu C, et al: [Detection and quantitative
analysis of tumor-associated tertiary lymphoid structures]. J
Zhejiang Univ Sci B. 24:779–795. 2023.In English, Chinese.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhou X, Bao WA, Zhu X, Lin J, Fan JF, Yang
Y, Du XH and Wang YZ: 3,3'-Diindolylmethane attenuates inflammation
and fibrosis in radiation-induced lung injury by regulating
NF-κB/TGF-β/Smad signaling pathways. Exp Lung Res. 48:103–113.
2022. View Article : Google Scholar : PubMed/NCBI
|




















