Introduction
Sepsis, an infection-driven systemic inflammatory
response syndrome, poses a major global health challenge due to its
high incidence and mortality (1,2).
According to World Health Organization estimates, nearly 19 million
people develop sepsis annually and 20-30% succumb to severe
complications (2). In intensive
care units, mortality is up to 56%, making sepsis the third leading
cause of death worldwide (3,4).
Among complications, sepsis-induced myocardial dysfunction (SIMD)
is especially common and carries the worst prognosis. A total of
~70% of septic patients experience impaired myocardial
contractility, decreased ejection fraction and arrhythmia. These
cardiac impairments often coincide with hypotension and lactic
acidosis, hastening circulatory collapse and multi-organ failure
(5-7).
SIMD arises from a multifactorial network of
inflammatory and oxidative processes. Pathogen-associated molecular
patterns (PAMPs) engage toll-like receptor (TLR)4 signaling through
MyD88 and TIR-domain-containing adaptor inducing interferon-β,
thereby activating NF-κB and MAPKs (p38, JNK, ERK). This cascade
triggers the release of proinflammatory cytokines such as TNF-α and
IL-1β (8). The cytokine surge,
in turn, stimulates NADPH oxidase to generate reactive oxygen
species (ROS), which induce lipid peroxidation and protein damage
(9). Oxidative stress further
compromises mitochondrial integrity, leading to decreased ATP
synthesis and the activation of caspase-3-mediated apoptosis.
Collectively, these events depress ejection fraction, promote
ventricular dilation and drive circulatory failure (8).
Current clinical management of SIMD follows the
Surviving Sepsis Campaign Guidelines, focusing on fluid
resuscitation, broad-spectrum antibiotics and vasopressors.
Inotropes or β-blockers may be used as adjunctive therapies, but
they typically yield only transient hemodynamic benefits and can
increase myocardial oxygen demand or provoke arrhythmia (5). Given the intertwined
inflammation-oxidation-mitochondria-apoptosis network in SIMD,
single-target drugs are unlikely to provide comprehensive
cardioprotection. However, natural products offer promising
multi-target interventions with low toxicity profiles (10). Traditional compounds such as
flavonoids, glycosides, alkaloids and saponins exhibit
anti-inflammatory, antioxidant, antiapoptotic and
mitochondrial-restorative effects. Preclinical study demonstrates
their capacity to attenuate key aspects of SIMD pathology (10); for example, curcumin has been
found to have extensive cardiovascular protective effects by
regulating endothelial function (10).
To the best of our knowledge, a systematic synthesis
of how natural products counteract SIMD is lacking. The present
review therefore summarizes the clinical features and
pathophysiology of SIMD and the structure-activity-mechanism
relationships of major natural product classes. Furthermore, the
present study aimed to highlight the primary signaling pathways
involved and evaluate advanced delivery strategies that improve
bioavailability and representative compound binding to SIMD
targets. By offering an integrated framework, the present study
aimed to guide the development of novel multi-target therapeutic
agents, including dietary supplements and lead compounds, for SIMD
therapy.
Pathophysiology of SIMD
SIMD is a complex, dynamic process in which
overwhelming infection and systemic inflammation disrupt normal
cardiac function. Multiple interrelated mechanisms, including
dysregulated cytokine release, oxidative stress, mitochondrial
damage and impaired calcium handling, converge to depress
myocardial performance (5). A
comprehensive understanding of these pathways is key for accurate
diagnosis and designing targeted therapies (Fig. 1).
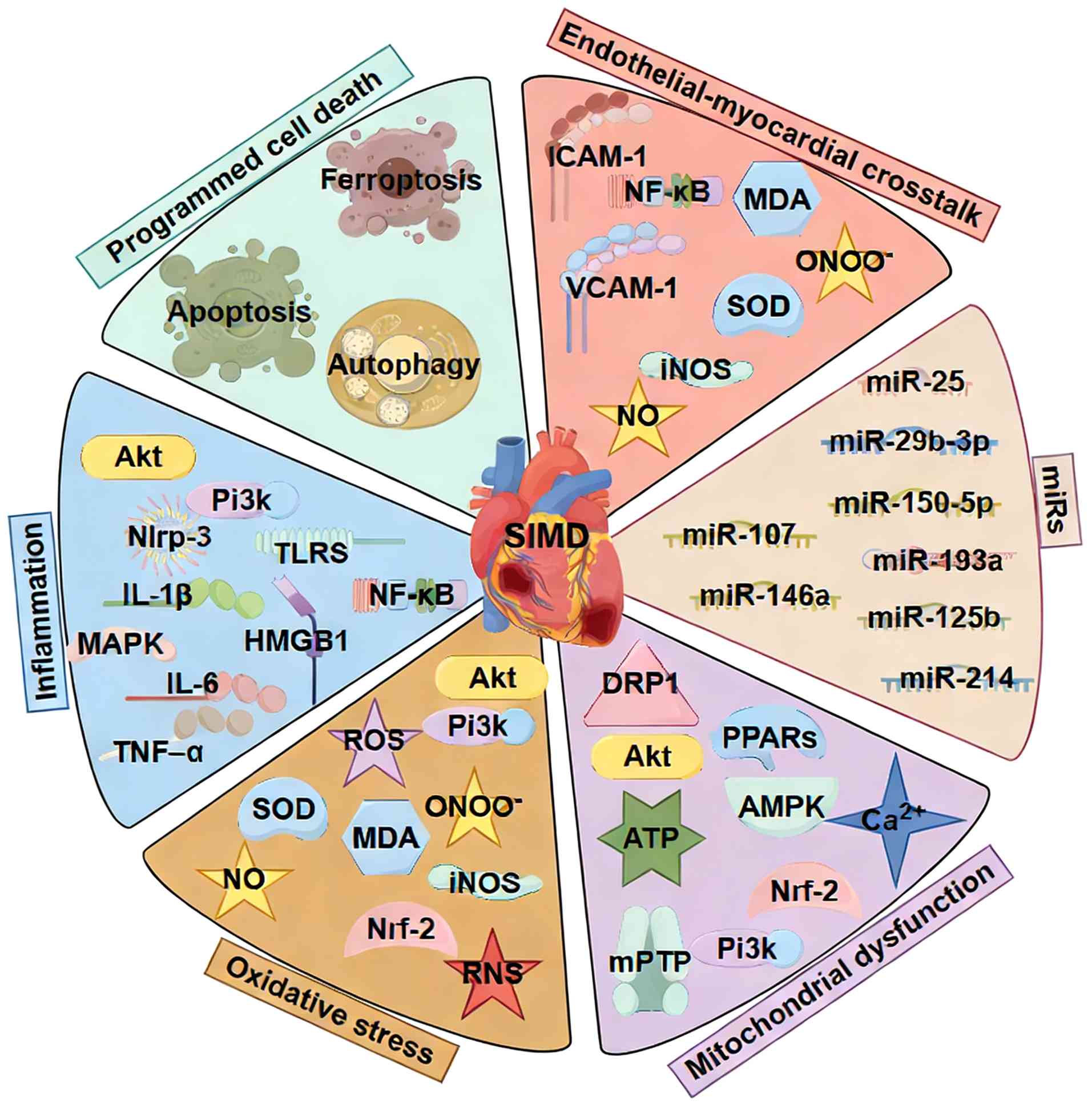 | Figure 1Pathophysiological mechanisms of
SIMD. This schematic illustrates the key interrelated pathways
(such as PI3K/Akt, NLRP3 and NF-κB) contributing to SIMD, including
dysregulated cytokine release, oxidative stress, mitochondrial
damage, impaired calcium handling and the regulatory roles of
specific. miRs. SIMD, sepsis-induced myocardial dysfunction; miR,
microRNA HMGB, high mobility group box; TLRS, Toll like receptors;
ROS, reactive oxygen species; SOD, superoxide dismutase; MDA,
malondialdehyde; iNOS, inducible nitric oxide synthase; RNS,
reactive nitrogen species; DRP, dynamin-related protein; PPAR,
peroxisome proliferator activated-receptors; mPTP, mitochondrial
permeability transition pore. |
Clinical characteristics of SIMD
SIMD describes a transient, sepsis-associated
decline in myocardial function that can involve both the left and
right ventricles and may affect systolic and/or diastolic
performance (11). Many studies
define SIMD by a reversible decrease in left ventricular ejection
fraction (LVEF) accompanied by ventricular dilation and poor
response to fluid resuscitation or catecholamines (3,5,7).
However, because LVEF is load-dependent, it may not reliably
reflect contractile reserve during sepsis (12). Consequently, SIMD is now
recognized as a spectrum of load-independent myocardial depression
manifesting as left ventricular systolic or diastolic dysfunction,
right ventricular dysfunction or a combination of both, often with
fluctuating hemodynamics (13).
Clinically, SIMD typically emerges within the first
hours to days of septic illness and presents with a heterogeneous
cardiac phenotype (14).
Systolic abnormalities range from decreased contractility to
ventricular dilation in certain patients (15), while diastolic dysfunction is
identified by altered filling parameters. Right ventricular
involvement is not uncommon and may exacerbate hemodynamic
instability (16). SIMD is often
reversible, although persistent dysfunction and worse outcomes
occur depending on sepsis severity, underlying comorbidities and
the level of hemodynamic support required (17). The clinical presentation may
evolve over time, with an initial high-output state potentially
progressing to a low-output phase, reflecting the interplay between
preload, afterload, myocardial depression and microcirculatory
redistribution (18). Thus, in
clinical practice, the diagnosis of SIMD relies on a comprehensive,
multi-faceted assessment. The typically involves confirming the
presence of sepsis, identifying otherwise unexplained myocardial
dysfunction, evidenced by new and often reversible
echocardiographic abnormality and associating these findings with
clinical signs of hemodynamic compromise, such as persistent
vasopressor dependency or objective evidence of tissue
hypoperfusion (14). While
elevated biomarkers such as cardiac troponins and natriuretic
peptides support the diagnosis by indicating myocardial injury or
stress, they are not standalone diagnostic criteria due to their
lack of specificity in the septic context (14).
Biomarkers of SIMD
Biomarkers of SIMD reflect diverse
pathophysiological domains, including direct myocardial injury,
ventricular wall stress, inflammatory remodeling and systemic
perfusion. It is crucial to note that most currently used
biomarkers are not specific to SIMD but indicate general myocardial
injury, stress, inflammation, or dysfunction. High-sensitivity
troponins I and T levels frequently rise in septic patients with
cardiac involvement, signaling myocardial injury rather than an
acute coronary syndrome. Troponin levels are associated with
illness severity and adverse outcomes (12,19), but their release in sepsis is
multifactorial, including demand ischemia, microvascular injury,
cytokine-mediated toxicity and catecholamine effects, underscoring
their lack of specificity for SIMD (19,20). B-type natriuretic peptides (BNP)
and N-terminal pro-B-type natriuretic peptide (NT-proBNP) increase
with myocardial wall stress and diastolic dysfunction and also
predict higher mortality (21,22). However, their variable
association with LVEF reduces their value as standalone markers of
intrinsic myocardial depression in SIMD (23).
Inflammatory and remodeling markers add prognostic
information beyond troponin and NPs. Soluble ST2 and galectin-3
rise in septic patients with myocardial involvement and are
associated with severity and adverse outcomes, reflecting ongoing
inflammation and fibrosis (24).
Similar to other markers, they are not specific to SIMD, and their
interpretation requires integration with imaging and clinical
context. Early injury markers such as heart-type fatty acid-binding
protein (H-FABP) may increase before troponin in certain cases,
offering potential for early risk stratification (25). Metabolic and perfusion markers,
including lactate, indicate global tissue hypoperfusion and shock
severity and thus complement cardiac-specific biomarkers in a
comprehensive hemodynamic assessment (26). Specific circulating microRNAs
(miRNAs or miRs) implicated in inflammatory and apoptotic pathways
(such as miR-21, miR-155 and miR-146a) represent a promising class
of biomarkers. They hold potential for more precise SIMD detection
and risk stratification but require prospective validation before
routine clinical use (27,28).
In summary, a multimodal strategy that integrates
biomarkers with load-independent echocardiographic measures and
detailed hemodynamic data is the most robust approach to defining
myocardial involvement, monitoring progression and guiding
management.
Pathology of SIMD
Programmed cell death
Cardiomyocyte loss in sepsis occurs through multiple
interrelated programmed cell death pathways (29). Apoptosis proceeds through
intrinsic mitochondrial pathways, where Bax and Bak mediate outer
mitochondrial membrane permeabilization and caspase activation, and
through endoplasmic reticulum stress pathways involving
glucose-regulated protein 78 kDa, CHOP and caspase-12 (30-32). Death receptor signaling via
TNF-R1 and FAS also contributes to caspase-8-mediated apoptosis.
Pharmacological modulation of these signals modestly improves
cardiac function in CLP (Cecum ligation and puncture (CLP)-induced
rats (33). Necroptosis, driven
by receptor interacting serine/threonine kinase RIPK1, RIPK3 and
MLKL (Mixed lineage kinase domain-like) MLKL, amplifies
inflammation and damages cardiomyocytes; inhibiting RIPK or MLKL
attenuates myocardial injury in CLP-induced septic mice (34). Pyroptosis, a lytic form of cell
death dependent on caspase-1-mediated gasdermin D (GSDMD) pore
formation, releases IL-1β and IL-18 and amplifies systemic
inflammation (35,36). Ferroptosis, an iron-dependent
form of lipid peroxidation, also contributes to cardiomyocyte
death; iron chelators and ferroptosis inhibitors lower ROS and
lipid peroxidation to preserve cardiac function in endotoxemia
(37,38). Autophagy plays a dual role in
SIMD. Moderate autophagic flux supports cell homeostasis and
cardiac resilience, but excessive or insufficient autophagy may
worsen injury (39).
Pharmacological induction of autophagy can restore homeostasis and
improve cardiac outcomes in septic rats (40).
Inflammation
Sepsis releases PAMPs and damage-associated
molecular patterns, which activate TLRs on cardiomyocytes,
fibroblasts and endothelial cells (ECs) (41). Except for TLR3, most TLR
signaling proceeds via the adaptor protein MyD88, activating
downstream effectors such as the MAPKs JNK, ERK1/2 and p38, as well
as the transcription factor NF-κB (42). This cascade drives production of
proinflammatory cytokines (such as IL-1β, IL-6, TNF-α, IL-12,
IL-17, IL-18) and chemokines such as CCL2/MCP-1 and CXCL8, which
recruit and activate immune cells (13,43-45). Activation of the NLRP3
inflammasome further amplifies IL-1β and IL-18 release, thereby
creating an inflammatory milieu that impairs cardiomyocyte
contractility by altering autophagy and lysosomal function
(46). High mobility group box
1) HMGB1 and extracellular histones released during sepsis impair
myocardial performance through TLR-dependent pathways, reinforcing
the inflammatory cascade (47).
Autonomic dysregulation also interacts with inflammation: Altered
adrenergic signaling can modulate cytokine production and
cardiomyocyte responsiveness, creating self-perpetuating feedback
loops that influence disease progression (48).
Oxidative stress
Alongside inflammation, sepsis triggers a surge of
ROS and nitrogen species (RNS) in cardiac tissue (49). ROS originate from electron leak
in mitochondrial complexes I-III and from NO-derived species such
as peroxynitrite (ONOO) (50).
Oxidative and nitrosative stress damage lipids, protein and DNA,
deplete antioxidants including superoxide dismutase, catalase and
glutathione, and disrupt calcium handling and
excitation-contraction coupling (51). NO produced by inducible nitric
oxide synthase (iNOS) contributes to myocardial depression, while
ONOO− nitrates tyrosine residues and further impairs
mitochondrial and contractile protein (52). This redox imbalance shifts
signaling toward proinflammatory pathways, creating a cycle that
worsens contractile dysfunction (53).
Mitochondrial dysfunction
Mitochondria are key for energy supply and cell
survival rate in SIMD (54).
Sepsis induces structural changes, swelling and cristae disruption,
alongside declines in oxidative phosphorylation and ATP synthesis
(55). Excessive mitochondrial
fission, driven by the GTPase dynamin-related protein 1 (DRP1),
fragments the organelle and worsens energy deficits, whereas
promoting fusion or mitophagy can maintain mitochondrial integrity
in LPS-induced mice (56). The
mitochondrial permeability transition pore (mPTP) opens under
calcium overload, oxidative stress and ATP depletion, collapsing
membrane potential and releasing cytochrome c (57). Agents that prevent mPTP opening
or stimulate mitochondrial biogenesis improve mitochondrial
function and cardiac performance in septic rats (58). In SIMD, energy metabolism shifts
from fatty acid oxidation to glycolysis, decreasing ATP yield;
downregulation of lipid-oxidation regulators such as PPARs and α
subunit of peroxisome proliferators activated receptor-γ
coactivator-1 (PGC-1α exacerbates energetic failure (58). Additional mitochondrial insults
include mitochondrial DNA oxidation and impaired activity of
electron transport chain complexes II and IV, limiting ATP
production and contractile capacity (59). Restoring mitochondrial integrity
with targeted antioxidants and NAD+-boosting strategies
offers a promising therapeutic approach in SIMD (60).
Endothelial-myocardial crosstalk
ECs coordinate vascular tone, perfusion and
inflammation in the cardiovascular system (61). In sepsis, EC dysfunction
transforms the vascular bed into a source of injurious signals that
affect adjacent cardiomyocytes, fibroblasts and the
microcirculation (62). Under
inflammatory and oxidative stress, ECs activate TLRs, triggering
NF-κB and MAPK pathways and increasing production of ROS and RNS
(63). This pro-oxidant
environment impairs myocardial relaxation and contraction and
worsens microvascular perfusion. Upregulation of intercellular cell
adhesion molecule-1 vascular cell adhesion molecule 1) promotes
leukocyte trafficking into the myocardium, while degradation of the
glycocalyx increases vascular permeability, edema and microvascular
leakage, collectively decreasing oxygen delivery and substrate
availability to the heart (64,65). Emerging evidence supports
bidirectional communication between ECs and cardiomyocytes:
Endothelial-derived signals modulate cardiomyocyte function and
energy metabolism, while cardiomyocytes influence endothelial
behavior via paracrine pathways and hemodynamic feedback (66). For example, endothelial-derived
reactive species and altered NO signaling contribute to calcium
handling abnormality and energetic stress in cardiomyocytes
(67). Conversely, interventions
that dampen endothelial inflammation or NO production yield
downstream cardioprotective effects (68,69).
Role of miRNAs
Dysregulated miRNAs serve as key epigenetic
regulators and downstream effectors in the pathogenesis of SIMD
(70). These small non-coding
(nc)RNAs fine-tune gene expression post-transcriptionally,
predominantly by modulating central inflammatory and apoptotic
pathways.
Numerous miRNAs critically regulate the inflammatory
cascade in SIMD, primarily through targeting key components of the
NF-κB signaling pathway. Certain miRNAs serve as endogenous
negative feedback regulators to attenuate excessive inflammation.
For example, miR-146a and miR-125b dampen NF-κB activation and
subsequent pro-inflammatory cytokine production by targeting key
adaptor proteins IL-1 receptor-associated kinase) and TNF receptor
associated factor 6) (71,72). Similarly, miR-25 inhibits the
TLR4/NF-κB pathway by directly targeting PTEN (73) and miR-29b-3p suppresses
MAPK/NF-κB signaling by targeting FOXO3A (74). miR-335, although upregulated in
SIMD, appears to exert a net beneficial effect on inflammation and
injury when overexpressed, suggesting a complex, context-dependent
regulatory role (75).
Conversely, specific miRNAs exacerbate myocardial inflammation.
miR-193a, expression of which is enhanced via METTL3-mediated m6A
modification, promotes inflammation by targeting the anti-apoptotic
gene BCL2L2 (76). The
circROCK1/miR-96-5p/oxidative stress responsive kinase 1) pathway
also promotes myocardial injury and NF-κB activation (77). Furthermore, hsa-miR-23a-3p,
hsa-miR-3175 and hsa-miR-23b-3p are implicated in regulating NF-κB
signaling via histone deacetylase 7)/ACTN4 (A-actinin-4),
contributing to inflammatory damage (78).
Beyond inflammation, miRNAs determine cardiomyocyte
fate by fine-tuning apoptotic and other cell death pathways.
Multiple miRNAs confer cardioprotection by inhibiting pro-apoptotic
signals or enhancing survival pathways. These include miR-214,
which improves cardiac function and suppresses apoptosis (79). miR-21 inhibits apoptosis by
targeting programmed cell death 4) to activate the PI3K/Akt
pathway, as well as by directly regulating Bcl-2 and CDK6 (80). miR-150-5p alleviates apoptosis by
modulating Akt2 expression (80). miR-107 promotes cardiomyocyte
proliferation and inhibits apoptosis via the PTEN/PI3K/AKT pathway
(81,82). Regulatory networks involving long
(l)ncRNAs further augment this layer of control. For example, the
lncRNA KCNQ1OT1 serves as a competitive endogenous RNA to sponge
miR-192-5p, thereby upregulating the anti-apoptotic protein
X-linked inhibitor of apoptosis (83), while the lncRNA ZFAS1 protects
against apoptosis by sequestering miR-34b-5p to upregulate sirtuin
(SIRT)1 (84). Conversely,
detrimental miRNAs such as miR-208a-5p and miR-21-3p promote
apoptosis (85).
Additionally, miRNAs participate in specialized cell
death modalities and mitochondrial dysfunction associated with
SIMD. miR-383-3p has been shown to alleviate SIMD by inhibiting
ferroptosis via the activating transcription factor 4
(ATF)-CHOP-ChaC glutathione specific γ-glutamylcyclotransferase 1)
(86), whereas miR-194-5p
aggravates oxidative stress and apoptosis by targeting DUSP9
(87). The Xist/miR-7a-5p
pathway serves a key role in sepsis-induced mitochondrial
dysfunction. Inhibition of either Xist or miR-7a-5p upregulates the
key mitochondrial biogenesis factor PGC-1α, increases ATP
production, and decreases cardiomyocyte apoptosis, highlighting
their key role in maintaining mitochondrial homeostasis (88,89).
Natural products against SIMD
The pathophysiology of SIMD is complex and
multifactorial. Interplay of cytokine storm, excessive generation
of ROS and RNS, mitochondrial dysfunction and dysregulated
endothelial-myocardial crosstalk collectively drive cardiomyocyte
apoptosis, ferroptosis and microcirculatory impairment.
Regulation of programmed cell death
Programmed cell death pathways implicated in SIMD
include apoptosis, pyroptosis, autophagy and ferroptosis. Natural
products can confer cardioprotection by either enhancing cell
survival signals or inhibiting specific death pathways (Fig. 2).
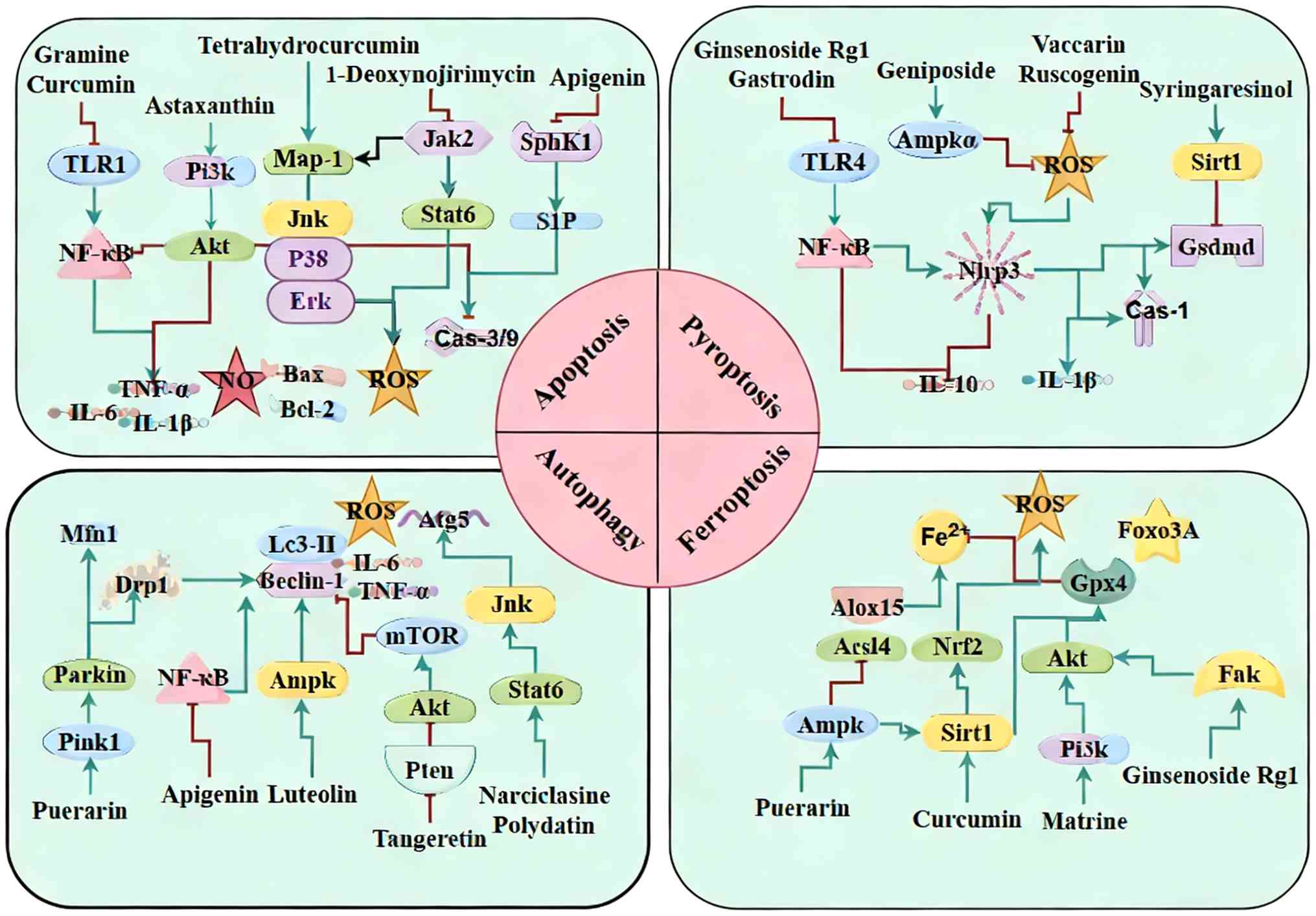 | Figure 2Regulation of programmed cell death
by natural products in SIMD. Natural products attenuate SIMD
through suppression of pro-oxidant NF-κB/TLR signaling, modulation
of PI3K/Akt, AMPK/autophagy, TFEB, NLRP3/pyroptosis and mitophagy
pathways and restoration of GPX4-associated antioxidant defenses.
SIMD, sepsis-induced myocardial dysfunction; TLR, Toll like
receptor; TFEB, T cell transcription factor EB; GPX, Glutathione
peroxidase; SphK, Sphingosine kinases; S1P, Sphingosine Kinase 1;
ROS, reactive oxygen species; MFN, mitofusin; SIRT, sirtuin 1;
GSDMD, gasdermin D; ALOX, arachidonate 5-lipoxygenase. |
Inhibition of apoptosis
Cardiomyocyte apoptosis is an early pathogenic event
in SIMD, contributing to amplified inflammation and progressive
cardiac dysfunction.
NF-κB, a master regulator of inflammation and
apoptosis, is a common target for numerous natural compounds that
also inhibit upstream TLR signaling. In CLP-induced septic mice,
compounds such as gramine, curcumin, andrographolide and berberine
suppress the TLR1/NF-κB axis, leading to lower TNF-α, IL-1β and NO
levels, decreased caspase-3 activation and apoptosis and restored
cardiac contractility, which translates into decreased mortality
(90-94). Notoginsenoside R1 activates the
PI3K/Akt pathway while concurrently inhibiting NF-κB, resulting in
suppressed caspase-3 activation and downregulated TNF-α and IL-1β
levels in both cardiomyocyte H9c2 and septic mice, thereby
attenuating inflammation and apoptosis (95,96).
The MAPK family also influences stress-induced
apoptosis. In LPS-challenged septic mice, tetrahydrocurcumin
upregulates mitogen activated protein kinase phosphatase 1),
suppresses ERK and JNK signaling and limits ROS production and
caspase-3-mediated apoptosis, preserving cardiac function (97). Astaxanthin similarly inhibits
ERK, JNK and p38 MAPK pathways while activating PI3K/Akt pathway,
resulting in lower serum levels of TNF-α, IL-6 and BNP, and reduced
myocardial injury and apoptosis in septic mice (98). Furthermore, 1-deoxynojirimycin
decreases ROS generation by inhibiting JAK2/STAT6 signaling,
thereby decreasing cardiomyocyte apoptosis and improving function
in septic mice (99).
Astragaloside IV protects against LPS-induced myocardial injury in
rats by inhibiting the JNK/Bax pathway, reducing levels of
caspase-3, CK-MB (Creatine kinase (CK-MB) and c-TnI (Cardiac
troponin I c-TnI) and increasing the Bcl-2/Bax ratio to mitigate
myocardial dysfunction (100).
In CLP-induced septic rats, apigenin inhibits the
Sphingosine kinase 1/sphingosine 1-phosphate) pathway, leading to
downregulation of cleaved caspase-3/-9 and Bax, upregulation of
Bcl-2, decreased serum CK-MB and lactate dehydrogenase (LDH)
levels, suppressed apoptosis and improved myocardial histology
(101). Gastrodin, tested in
CLP-induced septic mice and AC16 cardiomyocytes, modulates the
denticleless E3 ubiquitin protein ligase-homolog histone
acetyltransferase-driven ubiquitination-acetylation axis to promote
degradation of pro-caspase-3/-9 and Bax, thereby attenuating
apoptosis and alleviating myocardial damage (102).
Inhibition of pyroptosis
Pyroptosis is an inflammatory form of programmed
cell death triggered by inflammasome activation, characterized by
GSDMD-mediated pore formation and the release of IL-1β and IL-18
(103). In SIMD, pyroptosis not
only accelerates cardiomyocyte loss but also perpetuates a local
inflammatory cycle.
In CLP-induced septic mice, vaccarin and ruscogenin
attenuate myocardial injury by suppressing the NLRP3 inflammasome,
decreasing IL-1β and IL-18 release and limiting pyroptosis
(103,104). Artemisinin decreases NLRP3 and
caspase-1 expression in burned septic mice, lowers IL-1β and IL-18
mRNA in mouse monocytic macrophage leukemia RAW264.7 cells and
decreases neutrophil infiltration, thereby mitigating myocardial
damage (105). Similarly,
thymoquinone inhibits the NLRP3/caspase-1/IL-1β/IL-18 pathway,
downregulates p62 and c-TnT and upregulates IL-10, reducing
inflammation and pyroptosis in septic mice (106). Additionally, in LPS-challenged
septic mice, syringaresinol activates the endoplasmic reticulum
(ER)/SIRT1 pathway and suppresses NLRP3/GSDMD signaling to block
IL-1β and IL-18 release, significantly improving myocardial injury
(107). Both ginsenoside Rg1
and gastrodin inhibit the TLR4/NF-κB/NLRP3 signaling cascade,
downregulate Bax, upregulate Bcl-2 and restore cardiac function by
reducing pyroptosis (108,109). Nifuroxazide similarly targets
TLR4/NLRP3/IL-1β signaling to lower LDH and CK-MB levels, suppress
pyroptosis and protect myocardial function (110).
ROS also regulate NLRP3 activation. In LPS-induced
septic mice, carvacrol and emodin inhibit the ROS/NLRP3/GSDMD
pathway, decreasing IL-1β and IL-18 levels, which enhances survival
rate and restores cardiac function (111,112). Plumbagin also suppresses
ROS-mediated NLRP3/GSDMD signaling, decreases HMGB1, caspase-3 and
Bax expression and improves cardiac function through preventing
pyroptosis (113). Geniposide
downregulates neutrophil cytoplasmic factor 1 and suppresses the
AMPKα/ROS/NLRP3 pathway, leading to reduced ROS levels and improved
cardiac function and survival rate in septic mice (114). Finally, oxycodone markedly
improves EF and fractional shortening (FS), decreases levels of
ROS, c-TnI and CK-MB and activates the Nrf2/heme oxygenase (HO)-1
pathway to inhibit NLRP3-mediated pyroptosis, yielding notable
cardioprotective effects (115).
Autophagy
Autophagy is a key homeostatic process that removes
damaged organelles and proteins via lysosomal degradation. In
cardiomyocytes, it maintains mitochondrial quality, balances energy
metabolism and supports antioxidant defenses (39). In SIMD, autophagy exhibits a dual
role: Moderate activation clears dysfunctional mitochondria and
limits oxidative stress, whereas either excessive or insufficient
autophagic flux can exacerbate cell death and impair cardiac
function (116).
The AMP-activated protein kinase (AMPK) pathway
induces autophagy. In CLP-induced septic mice, luteolin activates
AMPK pathway signaling, which increases LC3-II and Beclin-1
expression, enhances autophagic flux, improves LVEF and FS,
decreases levels of TNF-α, IL-6 and ROS, restores mitochondrial
architecture and inhibits apoptosis (116). Conversely, in CLP-induced
septic rats, tangeretin suppresses excessive autophagy by
inhibiting PTEN and activating the Akt/mTOR pathway. This lowers
serum levels of cardiac myosin light chain-1 (cMLC1), c-TnI, LDH
and (CK), alleviates oxidative stress and inflammation and
preserves myocardial function (117).
The NF-κB/TFEB (T cell transcription factor EBTFEB
pathway also regulates autophagy. In LPS-challenged septic mice,
apigenin inhibits NF-κB to decrease inflammation and oxidative
stress while upregulating TFEB and its downstream target genes such
as ATG5, lysosome-associated membrane protein 1), p62,
microtubule-associated protein 1 light chain 3), vacuolar protein
sorting 11), thereby boosting autophagic flux. These combined
effects lower cardiac injury markers (CK, LDH, c-TnI, cMLC1),
improve survival rate and maintain cardiac function (118,119).
Mitochondrial autophagy (mitophagy) is key for
clearing damaged mitochondria. In LPS-stimulated H9c2
cardiomyocytes, puerarin upregulates Drp1 and mitofusin 1 to
preserve mitochondrial dynamics and activates the PINK1/Parkin
pathway, thereby reducing ROS, inhibiting apoptosis and restoring
cell viability (120).
Other natural compounds modulate autophagy through
alternative signaling nodes. In LPS-induced septic mice,
narciclasine and polydatin activate SIRT6 and JNK signaling, which
elevates LC3-II and Beclin-1 while lowering TNF-α and IL-6 levels,
thereby suppressing inflammation and attenuating myocardial injury
(121,122). Phlorizin modulates the
Hif-1α/BCL2 interacting protein 3) axis to promote Beclin-1
release, autophagosome formation and lysosomal degradation,
reducing oxidative stress and apoptosis, improving cardiac function
and enhancing survival rate (123).
Inhibition of ferroptosis
Ferroptosis is an iron-dependent form of programmed
cell death characterized by glutathione peroxidase 4 (GPX4)
inactivation, accumulation of lipid peroxides and iron overload
(37). In SIMD, inflammatory
disruption of iron homeostasis coupled with mitochondrial
dysfunction accelerates lipid peroxidation, triggering ferroptosis
and exacerbating cardiomyocyte injury (124). Consequently, targeting
antioxidant defenses, iron metabolism and lipid peroxidation
represents a promising therapeutic strategy.
The AMPK signaling axis serves a central role in
regulating ferroptosis. In LPS-induced septic mice, puerarin
activates AMPK to upregulate GPX4 and ferritin and downregulate
ACSL4 and the transferrin receptor (TFRC), reducing myocardial iron
content, lipid peroxidation and ferroptosis (124). Curcumin engages the AMPK/SIRT1
pathway to increase expression of GPX4, ferritin and translocase of
outer mitochondrial membrane 20 while decreasing levels of
Fe2+, MDA and prostaglandin-endoperoxide synthase 2,
thereby preventing ferroptotic cell death (125). Similarly, matrine activates
PI3K/Akt to enhance GPX4 and the Bcl-2/Bax ratio, reduce ACSL4 and
ROS and inhibit ferroptosis, improving cardiac performance
(126). Ginsenoside Rg1
stimulates FAK/Akt and upregulates FOXO3A, which decreases levels
of TNF-α, IL-1β and Fe2+ and increases Bcl-2,
collectively alleviating myocardial injury (127).
Direct inhibition of key lipid peroxidation enzymes
provides another mechanism to block ferroptosis. In LPS-stimulated
AC16 cardiomyocytes, resveratrol upregulates miR-149 and
downregulates HMGB1, decreasing Fe2+ accumulation and
lipid ROS to protect the myocardium (128). In CLP-induced septic mice,
narciclasine restores glutathione, downregulates TFRC and
upregulates GPX4 and HO-1, thereby inhibiting lipid peroxidation
and preserving cardiac function (129). Furthermore, resveratrol
activates the SIRT1/Nrf2 pathway to mitigate mitochondrial damage
and lipid peroxidation (130).
Arachidonic acid 15-lipoxygenase (ALOX15) is a key enzyme in lipid
peroxidation. Wogonin directly inhibits ALOX15, mitigating lipid
peroxidation and ferroptosis in the myocardium (131). Quercetin also activates
PI3K/Akt to inhibit ALOX5-mediated lipid oxidation, restores GPX4
and glutathione (GSH) levels, lowers Fe2+ and ROS, and
suppresses myocardial inflammation and ferroptosis (132).
Collectively, natural products, such as flavonoids,
alkaloids and saponins, attenuate SIMD through diverse mechanisms.
These include suppression of pro-oxidant NF-κB/TLR signaling,
modulation of PI3K/Akt, AMPK/autophagy, TFEB, NLRP3/pyroptosis and
mitophagy pathways and restoration of GPX4-related antioxidant
defenses. For example, curcumin, resveratrol, and quercetin
concurrently inhibit apoptosis and pyroptosis, promote adaptive
autophagy and restrain ferroptosis. Similarly, astaxanthin,
apigenin and puerarin protect the myocardium through combined
activation of autophagy and inhibition of ferroptosis. This
multimodal pharmacological profile highlights the potential of
natural products to target the interconnected cell death networks
that drive SIMD.
Regulation of inflammation
Sepsis provokes a systemic inflammatory response
that contributes directly to myocardial injury. Endotoxins and
other PAMPs activate key signaling cascades, most notably the
TLR4/NF-κB, MAPK and NLRP3 inflammasome pathways, leading to the
release of proinflammatory cytokines (TNF-α, IL-1β, IL-6, MCP-1)
and recruitment of neutrophils and macrophages into cardiac tissue.
The resulting inflammatory milieu impairs cardiomyocyte
contractility and survival (Fig.
3).
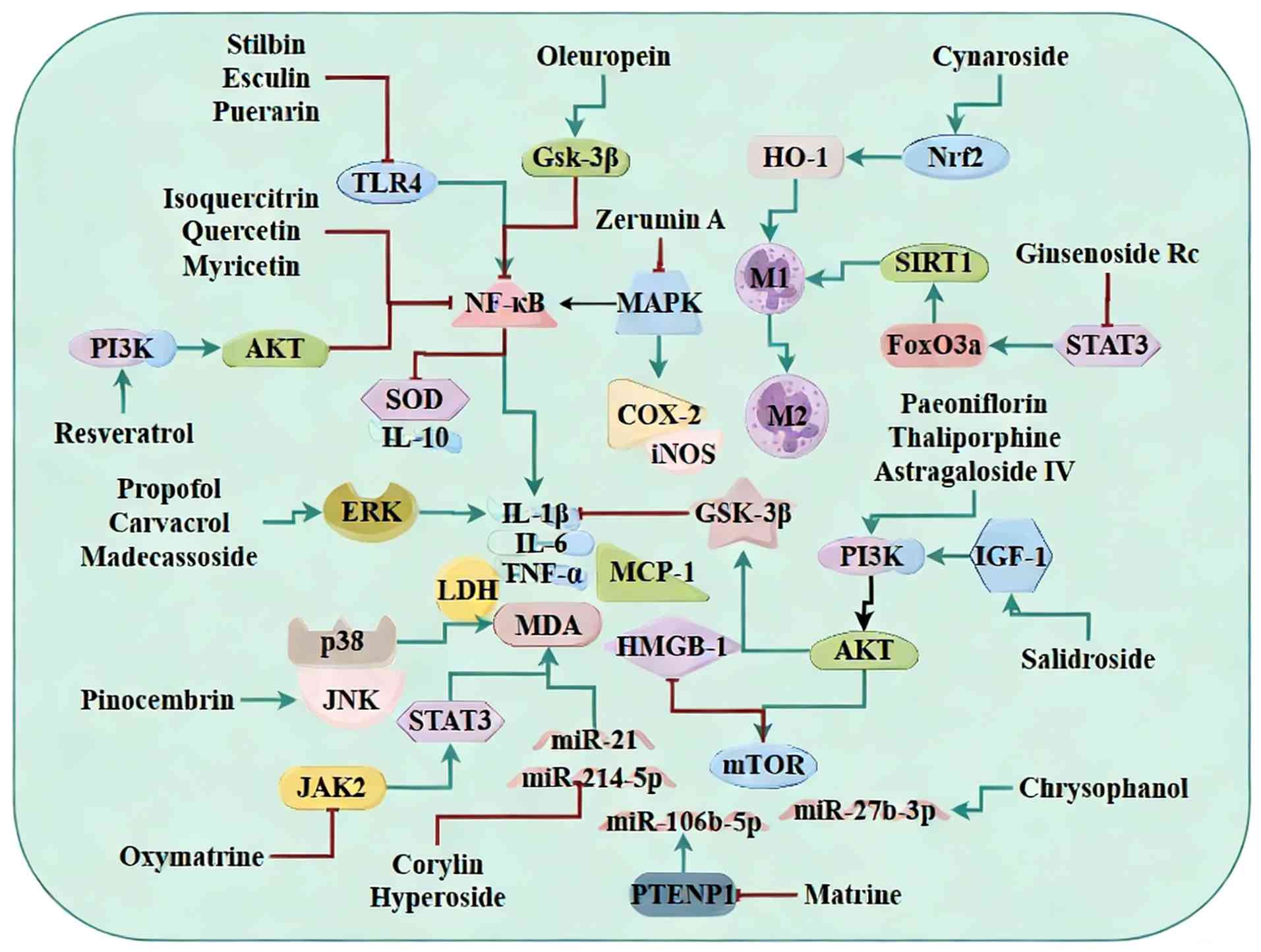 | Figure 3Anti-inflammatory mechanisms of
natural products in sepsis-induced myocardial dysfunction). Natural
products confer protection by inhibiting pro-inflammatory
TLR4/NF-κB and MAPK signaling cascades, activating the PI3K/Akt
survival pathway, modulating specific miR networks (miR-214-5p,
miR-21) and promoting the polarization of macrophages towards the
reparative M2 phenotype. TLR, toll-like receptor; miR, miRNA; GSK,
glycogen synthase kinase; HO, heme oxygenase; SIRT, sirtuin 1; COX,
cyclooxygenase; iNOS, inducible nitric oxide synthase; IGF, insulin
like growth factor; LDH, lactate dehydrogenase; MCP, monocyte
chemotactic protein; MDA, malondialdehyde; HMGB, high mobility
group box. |
Inhibition of the TLR4/NF-κB pathway
Numerous flavonoids and other phytochemicals
suppress TLR4-driven NF-κB activation to decrease cytokine release
and improve cardiac function. In LPS-challenged septic mice,
isoquercitrin and quercetin inhibit NF-κB p65 phosphorylation,
lower TNF-α, IL-6 and MCP-1 levels and significantly improve LVEF
and FS (133,134). Ciprofol similarly blocks NF-κB
activation in septic mice, decreasing levels of CK-MB, LDH, TNF-α
and IL-6 and restoring LVEF and FS (135). In CLP-induced septic rats,
astragaloside IV prevents NF-κB p65 phosphorylation, decreases
levels of LDH, CK-MB, IL-6, IL-1β and HMGB1 and enhances both
systolic and diastolic function as well as survival rate (136). Myricetin inhibits IκBα
degradation and NF-κB/p65 nuclear translocation in septic rats,
which lowers TNF-α, IL-6 and IL-1β and preserves cardiac function
(137,138). Similarly, naringin blocks NF-κB
nuclear translocation, decreases TNF-α, IL-1β and IL-6 levels and
enhanced superoxide dismutase activity while lowering MDA levels
and leads to improved cardiac performance in septic rats (139,140). Additionally, astilbin and
esculin both target the TLR4/NF-κB pathway to downregulate TNF-α,
IL-6 and MDA, correct QT prolongation and reduce the risk of
ventricular arrhythmia (141,142). Puerarin also inhibits
TLR4/NF-κB signaling in Langendorff-perfused hearts, decreasing
LDH, CK and TNF-α release and improving LV end-diastolic pressure
in septic rats (143). By
contrast, oleuropein activates the glycogen synthase kinase (GSK)-3
beta)/NF-κB pathway, decreases c-TnI, CK-MB, IL-6 and HMGB1 levels
and increases anti-inflammatory IL-10 levels, thereby protecting
cardiac function in septic rats (144).
Inhibition of MAPK-associated
pathways
The MAPK family intersects with NF-κB signaling to
amplify inflammation. In LPS-stimulated H9C2 cells, zerumin A
suppresses MAPK-mediated NF-κB activation, resulting in lower
levels of iNOS, ROS, COX-2, NO, MCP-1, TNF-α, IFN-γ and IL-1β and
higher IL-10 expression, which improves cell viability (145). In LPS-challenged septic mice,
propofol and carvacrol both inhibit ERK1/2 phosphorylation,
decrease IL-6 and TNF-α levels, alleviate myocardial injury and
enhance survival rate (146,147). Pinocembrin blocks the p38/JNK
MAPK pathway, decreasing myocarditis and arrhythmia risk in
LPS-induced septic rats (148).
Similarly, madecassoside inhibits ERK1/2 and p38 activation in
septic mice, delays arterial pressure decline and lowers TNF-α
levels, thereby mitigating tachycardia (149). Additionally, alisol B
23-acetate suppresses TLR4/NOX2 and p38/MAPK/ERK signaling,
decreasing IL-6, IL-1β and TNF-α levels, which alleviates
myocardial injury and improves survival rate (150). In LPS-treated septic rats,
oxymatrine also inhibits the p38/MAPK/caspase-3 and JAK2/STAT3
pathways, decreases IL-1β and TNF-α levels and improves cardiac
function (151,152).
Activation of the PI3K/AKT pathway
The PI3K/Akt pathway exerts anti-inflammatory and
pro-survival effects in SIMD. In LPS-challenged septic mice,
astragaloside IV activates PI3K/Akt signaling to reduce LDH and
c-TnI levels, suppress myocardial inflammation and improve cardiac
performance (153).
Paeoniflorin and resveratrol also stimulate PI3K/Akt to decrease
levels of TNF-α, IL-1β, IL-6, IL-12, MCP-1, IFN-γ and iNOS, thereby
protecting against myocardial injury (154,155). In CLP-induced septic mice,
thaliporphine activates the PI3K/Akt/mTOR pathway to decrease
levels of TNF-α, c-TnI and LDH and enhance LV function (156). Salidroside modulates the
IGF-1/PI3K/Akt/GSK-3β pathway to decrease levels of CK, LDH, TNF-α,
IL-6 and IL-1β, thereby alleviating myocardial edema and
dysfunction (157).
miRNA-dependent anti-inflammatory
pathways
miRNAs have emerged as critical regulators of
myocardial inflammation in sepsis. For example, corylin improves
LPS-induced cardiac dysfunction in mice by downregulating
myocardial miR-214-5p, which attenuates inflammatory signaling
(158). In CLP-induced septic
mice, hyperoside exerts protective effects by suppressing the
sepsis-associated upregulation of miR-21, leading to decreased
myocardial inflammation (159).
Furthermore, matrine acts via the PTENP1/miR-106b-5p pathway to
inhibit proinflammatory pathways and enhance cardiomyocyte
viability, thereby improving cardiac function in CLP-induced mice
(160). Chrysophanol
downregulates miR-27b-3p and upregulates PPARγ, which suppresses
inflammatory cytokines and apoptotic proteins to repair CLP-induced
cardiac injury in mice (161).
Targeting macrophage polarization
Macrophage polarization strongly influences the
inflammatory milieu in SIMD. Pinostrobin inhibits TLR4/myeloid
differentiation protein-2 signaling in mouse monocytic macrophage
leukemia RAW264.7cells, decreasing expression of NO, prostaglandin
E2, TNF-α, IL-12, iNOS and COX-2, and improves heart rate and
survival rate in LPS-treated zebrafish (162). Cynaroside activates the
Nrf2/HO-1 pathway to lower IL-1β and TNF-α levels and promotes M2
macrophage polarization, thereby decreasing systemic inflammation
and protecting the myocardium in septic mice (163). Similarly, ginsenoside Rc
suppresses the STAT3/FoxO3a/Sirt1 pathway to limit M1
macrophage-mediated myocardial damage during sepsis (164).
Other anti-inflammatory agents
Several additional natural products exhibit potent
anti-inflammatory and cardioprotective effects in SIMD. In
LPS-induced septic mice, cyanidin lowers levels of LDH, CK, c-TnI,
TNF-α, IL-1β, MIP-2, MCP-1 and cMLC1, inhibits caspase-3 and PARP
cleavage and attenuates myocardial injury (165). Monotropein and baicalein
suppress TNF-α, IL-6, HMGB1 and iNOS/NO production while decreasing
MMP-2/9 and ROS levels to alleviate septic myocardial hypertrophy
and dysfunction (166,167). Paeoniflorin and honokiol
markedly decrease c-TnI, LDH, IL-6, TNF-α and IL-1β and raise IL-10
levels, improving cardiac contractility and enhancing survival rate
in CLP-induced septic mice (168,169). Similarly, dobutamine increases
serum levels of IL-10 and lowers c-TnI, NT-proBNP and H-FABP,
thereby enhancing survival rate in CLP-induced rats (170).
In LPS-challenged septic rats, magnolol corrects
hypotension and bradycardia by decreasing levels of iNOS, NO, TNF-α
and alanine aminotransferase/aspartate aminotransferase) (171). Sodium tanshinone IIA sulfonate
also improves LV function by inhibiting expression of TNF-α, IL-6,
HMGB1, C-reactive protein, PCT (Procalcitonin (PCT), c-TnI/c-TnT
and BNP (172). Additionally,
micromeria congesta downregulates IL-2, caspase-3 and heat shock
protein (HSP)-27 to attenuate septic myocardial pathology (173). Naringenin and tubeimoside I
activate the SIRT3 or HIF-1α pathways to suppress IL-1β, IL-6 and
TNF-α, improving myocardial injury (174,175). Yohimbine blocks α2A-adrenergic
receptors at cardiac sympathetic terminals, promotes norepinephrine
release and decreases NO and TNF-α levels to alleviate cardiac
dysfunction in septic rats (176).
In summary, these natural products protect against
SIMD by inhibiting TLR4/NF-κB and MAPK signaling, activating
PI3K/Akt, modulating miRNA networks and promoting macrophage M2
polarization. They collectively reduce proinflammatory cytokine
levels, limit inflammatory cell infiltration and improve cardiac
function and survival rate.
Regulation of oxidative stress
Oxidative stress is a key driver of cardiac injury
in SIMD. Inflammation, endotoxins and mitochondrial dysfunction
increase ROS/RNS levels, leading to lipid peroxidation, protein
carbonylation and DNA damage (49). These changes worsen contractile
dysfunction and promote cardiomyocyte death (Fig. 4).
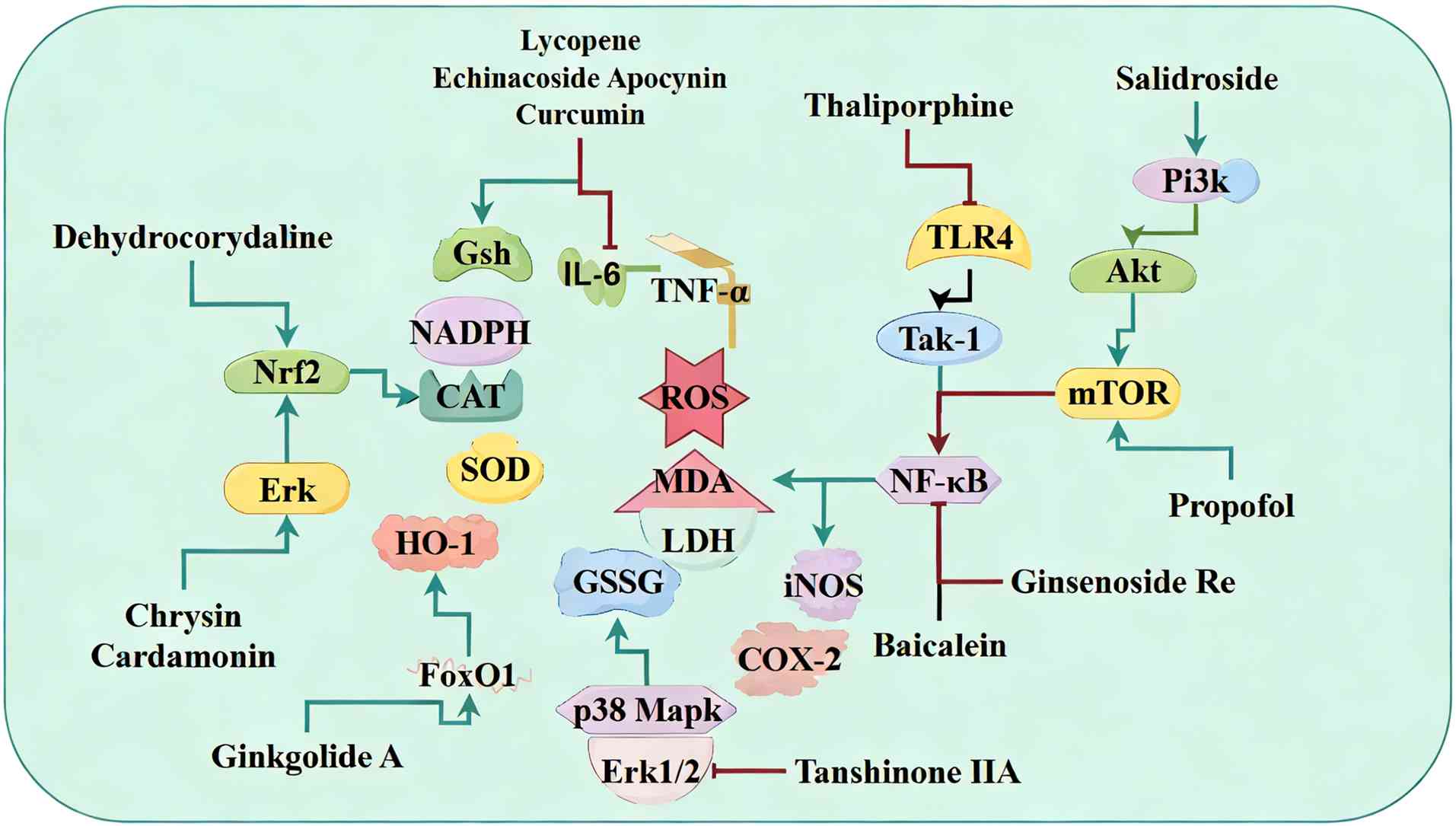 | Figure 4Modulation of oxidative stress by
natural products in sepsis-induced myocardial dysfunction). Natural
products primarily enhance antioxidant defenses by activating
enzymes such as SOD, CAT and GSH-Px or by stimulating the Nrf2/HO-1
pathway. They also dampen proinflammatory signaling via NF-κB, TLR4
and ERK1/2-p38 MAPK signaling to reduce NOX2/iNOS-mediated ROS/RNS
production. SOD, superoxide dismutase; CAT, catalase; GSH-Px,
glutathione peroxidase; HO, heme oxygenase; TLR, Toll like
receptor; NOX, nitrogen oxide; iNOS, inducible nitric oxide
synthase; ROS, reactive oxygen species; RNS, reactive nitrogen
species; GSSG, glutathione oxidized; MDA, malondialdehyde; LDH,
lactate dehydrogenase; TAK, transforming growth factor-β-activated
kinase 1. |
Activation of antioxidant enzymes
Natural products directly enhance the activity of
endogenous antioxidant systems. In LPS-induced septic mice,
lycopene, echinacoside and apocynin lower myocardial ROS and MDA
levels while increasing NADPH oxidase and SOD activity (177-179). Similarly, rutin reduces CK,
LDH, MDA, ROS, TNF-α and IL-6 and increases SOD and catalase (CAT)
activity, thereby protecting cardiac structure and function
(180). In CLP-induced rats,
curcumin decreases plasma levels of ROS, c-TnI and MDA, restores
the GSH/oxidized glutathione (GSSG) ratio and enhances SOD activity
to improve LVEF and FS (181,182). Quercetin similarly upregulates
endothelial (e)NOS and maintains the GSH/GSSG balance, alleviating
hypotension and tachycardia in septic mice (183).
Activation of the Nrf2 pathway
The transcription factor Nrf2 orchestrates the
cellular antioxidant response. In LPS-challenged septic mice,
resveratrol activates Nrf2 to decrease myocardial ROS and increase
the expression of antioxidant genes (184). Chrysin and cardamonin engage
the ERK-Nrf2-HO-1 axis, upregulating HO-1 and SOD while lowering
levels of ROS, MDA, TNF-α, IL-6 and IL-1β, which improves cardiac
function (185,186). Dehydrocorydaline also triggers
the Nrf2/HO-1 pathway, decreases levels of ROS, TNF-α and IL-1β and
restores SOD and glutathione peroxidase (GSH-Px) activity, leading
to improved survival rate (187).
Regulation of the PI3K/Akt/NF-κB
pathway
NF-κB links inflammation with oxidative stress. In
LPS-treated septic rats, baicalein inhibits the NF-κB pathway to
decrease levels of iNOS, ROS and NO, improving blood pressure,
heart rate and survival rate (188,189). Thaliporphine also suppresses
the TLR4/transforming growth factor-β-activated kinase 1
(TAK1)/NF-κB pathway to lower myocardial NO, ROS, TNF-α and iNOS
levels and improve LV pressure-volume indices (190). Ginsenoside Re corrects the
iNOS/eNOS imbalance by inhibiting NF-κB signaling, thereby
reversing myocardial injury (191). In LPS-treated rats, salidroside
activates the PI3K/Akt/mTOR pathway while inhibiting NF-κB, which
decreases levels of iNOS, COX-2 and ROS and improves cardiac
function (192). Propofol also
engages mTOR signaling to lower ROS and MDA levels and boosts SOD
activity, thereby reverse myocardial damage (193).
Other antioxidant agents
Additional natural products modulate oxidative
stress via unique pathways. In LPS-induced sepsis, ginkgolide A
activates FoxO1 and downstream effectors such as Kruppel-like
factor 15, thioredoxin 2), Notch1(Notch receptor 1), XBP1(X-box
binding protein 1), which upregulates antioxidant enzymes and
preserves mitochondrial function (194). Tanshinone IIA inhibits ERK1/2
and p38 MAPK phosphorylation, decreases NOX2 expression and lowers
MDA and ROS levels to alleviate cardiac dysfunction (195).
In summary, natural products primarily enhance
antioxidant defenses by activating enzymes such as SOD, CAT and
GSH-Px or by stimulating the Nrf2/HO-1 pathway. They also dampen
proinflammatory signaling via NF-κB, TLR4 and ERK1/2/p38 MAPK
pathways to reduce NOX2/iNOS-mediated ROS/RNS production. Through
simultaneous engagement of PI3K/Akt/mTOR and FoxO1 pathways, these
compounds preserve mitochondrial integrity and myocardial
contractility, thereby markedly improving SIMD outcomes and
survival rate.
Regulation of mitochondrial
dysfunction
Mitochondria serve not only as the energy source of
cardiomyocytes but also as a key hub for ROS production and the
regulation of programmed cell death (54). In SIMD, inflammatory mediators,
oxidative stress and impaired mitochondrial quality control cause
mitochondrial fragmentation, loss of membrane potential,
respiratory defects, diminished ATP synthesis and ultimately cell
death (55) (Fig. 5).
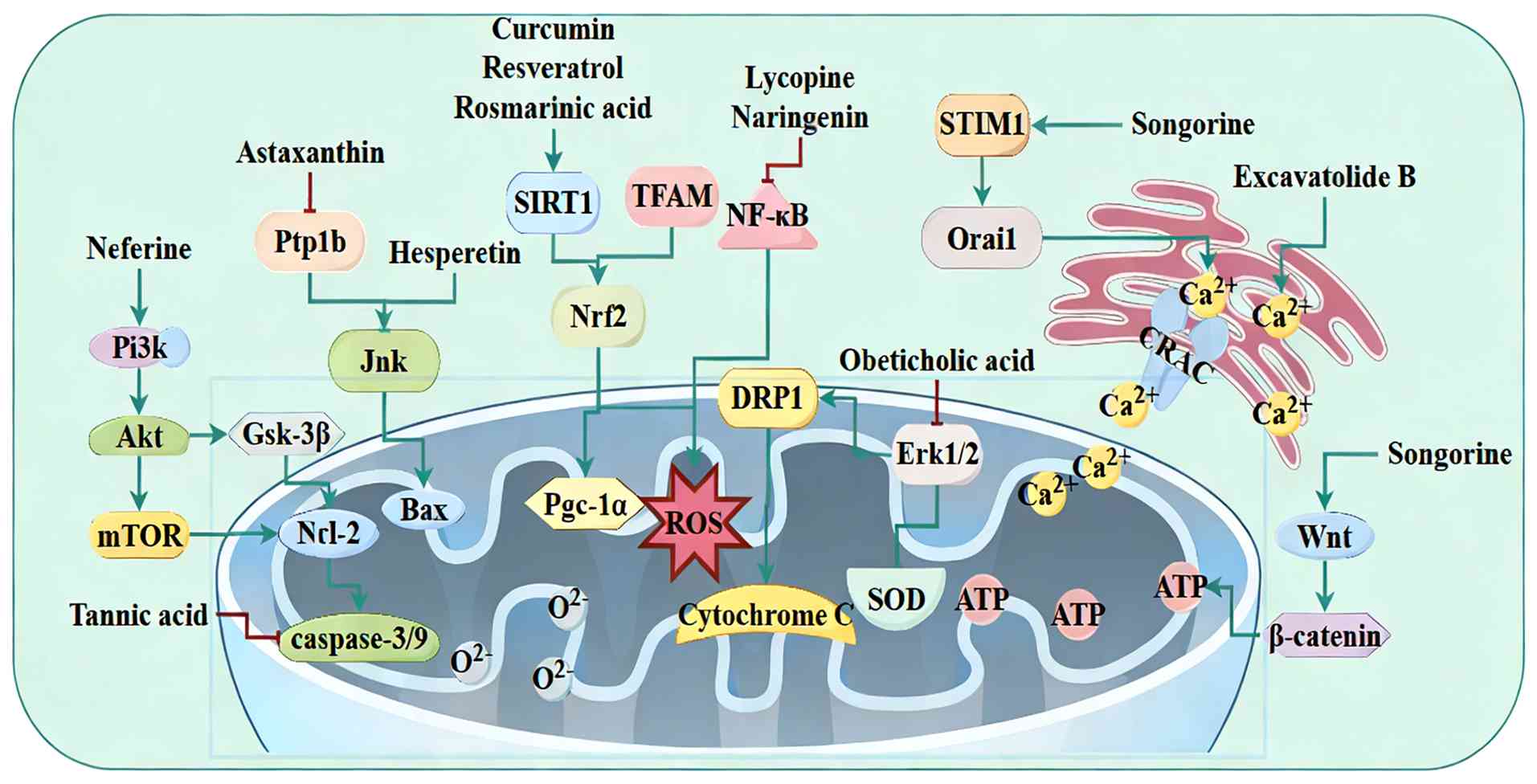 | Figure 5Targeting mitochondrial dysfunction
with natural products in SIMD. Natural products counteract SIMD by
engaging multiple mitochondrial-protective pathways, most notably
SIRT1/Nrf2, PI3K/Akt/mTOR and AMPK, and by promoting mitochondrial
biogenesis via PGC-1α/TFAM and UPRmt. SIMD, sepsis-induced
myocardial dysfunction; SIRT, sirtuin 1; PGC, peroxisome
proliferator-activated receptor γ coactivator; UPRmt, mitochondrial
unfolded protein response; GSK, glycogen synthase kinase; PTP1B,
protein tyrosine phosphatase 1B; ROS, reactive oxygen species; SOD,
superoxide dismutase; DRP, dynamin-related protein 1; STIM, stromal
interaction molecule 1; CRAC, calcium release-activated
channels. |
Activation of Nrf-associated
pathways
Nrf pathways are key for maintaining mitochondrial
redox homeostasis. In LPS-induced septic mice, curcumin and
resveratrol upregulate PGC-1α and mitochondrial transcription
factor A (TFAM), inhibit DRP1-mediated excessive mitochondrial
fission and activate the SIRT1/Nrf2 pathway to normalize
mitochondrial morphology and respiration, thereby markedly
improving cardiac function (196,197). Rosmarinic acid similarly
preserves mitochondrial membrane potential and ATP production by
triggering the SIRT1/Nrf2 pathway, resulting in decreased ROS
levels and enhanced survival rate (198). Songorine further boosts
Nrf1/TFAM signaling and promotes PGC-1α-Nrf2 interactions,
suppressing calcium release-activated channels) channel formation,
lowering mitochondrial ROS levels, restoring calcium homeostasis
and increasing contractile power (199).
Inhibition of AMPK-associated
pathways
Although AMPK often supports mitochondrial
homeostasis, certain stress contexts benefit from modulating its
downstream effectors. In LPS-stimulated H9C2 cells, hesperetin
upregulates Bcl-2, downregulates Bax and caspase-3/9 expression
through inhibiting JNK phosphorylation, thus preserving
mitochondrial membrane potential and decreasing apoptosis (200). In CLP-induced septic mice,
tannic acid attenuates ER stress and decreases expression of ROS,
Bax, cytochrome c and caspase-3/-9/-12, thereby safeguarding
mitochondrial function in cardiomyocytes (201). Additionally, astaxanthin
inhibits the PTP1B/JNK pathway to maintain membrane potential and
limit ROS generation, which decreases cardiomyocyte apoptosis
(202). In LPS-challenged
septic mice, lycorine and naringenin activate AMPK and suppress
NF-κB signaling, together protecting cardiac function (203,204). Obeticholic acid blocks the
ERK1/2-DRP1 axis to prevent cytochrome c release and upregulates
GPX1 and SOD1/2, thereby restoring mitochondrial integrity
(205).
Activation of PI3K/Akt signaling
The PI3K/Akt pathway also contributes to
mitochondrial preservation. In LPS-induced septic rats, neferine
activates PI3K/Akt/mTOR signaling to elevate the Bcl-2/caspase-3
ratio, decreases ROS levels and restores mitochondrial structure
and function, leading to improved myocardial performance (206). Ginsenoside Rg1 also engages the
recombinant purinergic receptor P2X to stimulate the Akt/GSK-3β
pathway, suppressing mitochondrial superoxide generation and
cytochrome c release, stabilizing calcium handling and enhancing
survival rate (207).
Other pathways
In LPS-treated septic mice, verbascoside promotes
mitochondrial biogenesis and morphological repair, thereby
improving cardiac function (208). Silibinin also decreases CCR2
levels and activates LXRα (Liver X receptors) signaling to dampen
inflammation and oxidative damage while improving mitochondrial
function (209,210). Additionally, chicoric acid
inhibits α-tubulin acetylation and NLRP3 inflammasome assembly,
indirectly restoring mitochondrial architecture and alleviating
myocardial injury (211).
Songorine also activates Wnt/β-catenin signaling to boost
mitochondrial biogenesis and suppress inflammation and apoptosis,
thereby improving LVEF and FS (212). Furthermore, excavatolide B
modulates intracellular and extracellular Ca2+ channels
to reverse calcium dysregulation and repair mitochondrial structure
in septic hearts (213).
Salvianolic acid B induces an ATF5-mediated mitochondrial unfolded
protein response (UPRmt), prevents excessive mitochondrial fission,
preserves membrane potential and significantly enhances contractile
function and mitochondrial ultrastructure (214).
In summary, natural products counteract SIMD by
engaging multiple mitochondrial-protective pathways, most notably
SIRT1/Nrf2, PI3K/Akt/mTOR and AMPK pathways, and by promoting
mitochondrial biogenesis via PGC-1α/TFAM and UPRmt. These
interventions preserve mitochondrial membrane potential, augment
ATP production, decrease ROS accumulation and suppress apoptosis,
collectively leading to substantial improvements in cardiac
function and survival rate in SIMD.
Regulation of endothelial-myocardial
crosstalk
ECs constitute the first line of defense in
maintaining microcirculatory stability and myocardial function
(61). In sepsis, endothelial
barrier dysfunction increases vascular permeability, tissue edema
and inflammatory cell infiltration, while endothelial-derived
cytokines and exosomes propagate injurious signals to
cardiomyocytes, exacerbating SIMD (63).
In LPS-induced septic mice, neohesperidin
dihydrochalcone markedly suppresses vascular hyperpermeability and
myocardial tissue damage. It decreases ROS production, preserves
mitochondrial membrane potential and enhances antioxidant defenses
(CAT, SOD, GSH) while lowering MDA levels (215). Mechanistically, it inhibits
phosphorylation of TAK1, ERK1/2 and NF-κB, stabilizes endothelial
junction protein and diminishes THP-1 monocyte adhesion and
infiltration (215). These
combined effects reinforce endothelial barrier function and
mitigate myocardial injury (215). In LPS-induced septic shock
rats, anisodamine restores hemodynamics and repairs the endothelial
glycocalyx, thereby decreasing myocardial damage (8). It lowers levels of lactate, IL-1β,
IL-6, TNF-α, CK, c-TnT, NT-proBNP, syndecan-1 and hyaluronic acid
via inhibition of NF-κB and NLRP3 signaling and activation of the
PI3K/Akt pathway (8). Moreover,
exosomes released by LPS-stimulated human umbilical vein ECs
transfer inflammatory and oxidative signals to the myocardium;
anisodamine attenuates these exosome-mediated effects, indicating
that it disrupts harmful endothelial-myocardial crosstalk to confer
cardioprotection in SIMD (216).
Classification and structure-activity
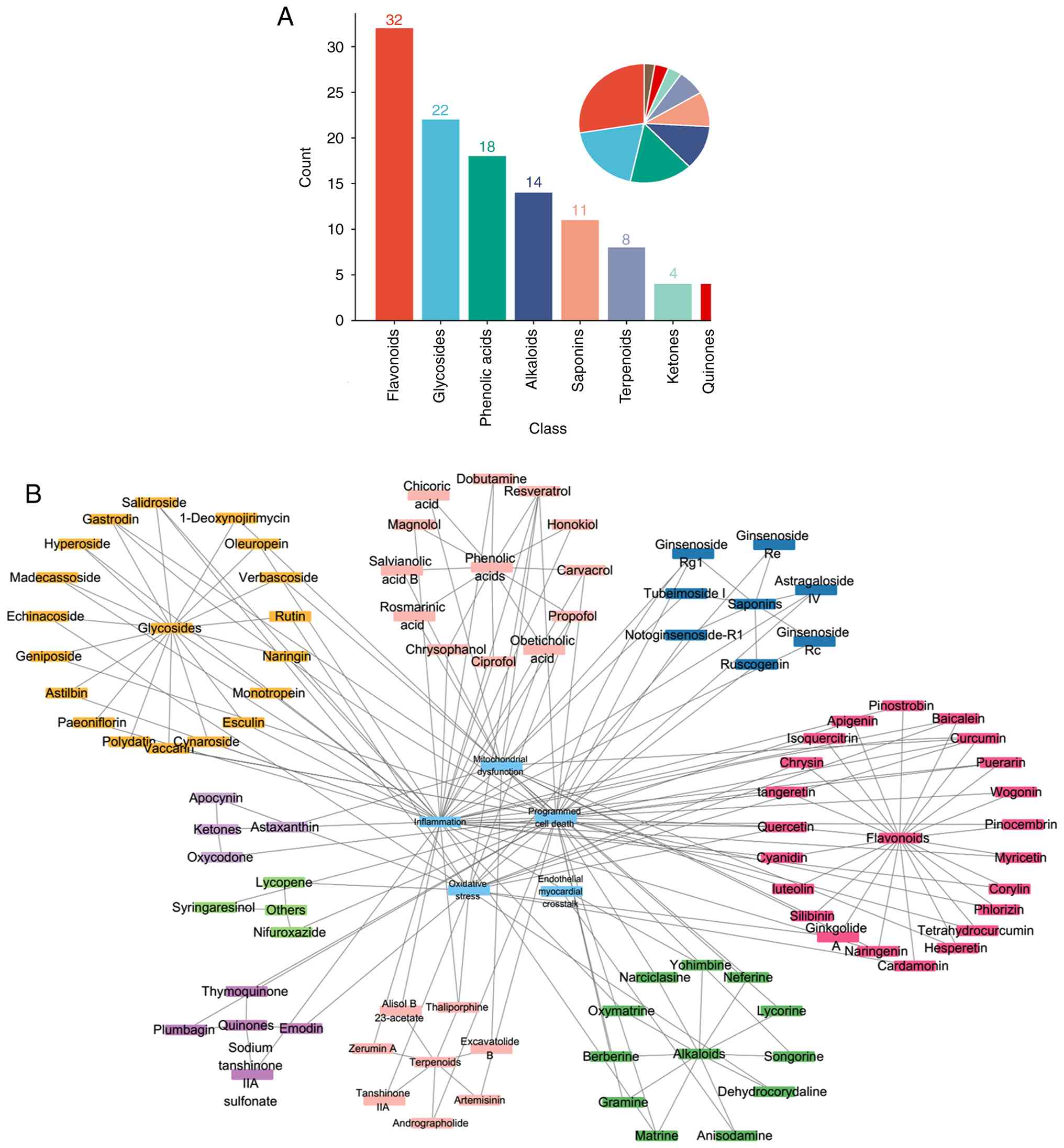
relationships
Natural products with efficacy against SIMD can be
divided into five major chemical classes: Flavonoids, glycosides,
phenolic acids, alkaloids and saponins (Fig. 6A). Notably, flavonoids and
glycosides account for approximately half of the identified
compounds. The prominent bioactivity of these classes is associated
with their characteristic chemical scaffolds. Specifically, the
presence of multiple hydroxyl (-OH) groups confers potent
antioxidant capacity by serving as hydrogen donors to directly
neutralize free radicals (217). Concurrently, conjugated double
bond systems (extended π-electron networks) enable electron
delocalization, which stabilizes antioxidant reaction intermediates
and facilitates key interactions with cellular signaling proteins
(kinases, transcription factors) (217). These combined physicochemical
properties underpin their broad-spectrum antioxidant,
anti-inflammatory and cytoprotective activities observed in SIMD
models (Fig. 6B).
Flavonoids
Flavonoids share a characteristic C6-C3-C6
three-ring scaffold. Critical structural features include a C2-C3
double bond, hydroxyl groups at C3, C5 and/or C7 and a
catechol-type dihydroxy arrangement on the B-ring (positions 3',4')
(217). This configuration
maximizes electron delocalization and hydrogen-donating capacity,
explaining their strong radical scavenging activity and their
direct, Structure-activity relationship (SAR)-driven inhibition of
central pro-inflammatory signaling hubs such as NF-κB and MAPK
(218). Mechanistically,
flavonoids thereby modulate the TLR4/NF-κB pathway, NLRP3
inflammasome activation and MAPK pathways to lower proinflammatory
cytokines (IL-1β, TNF-α) and attenuate cardiomyocyte apoptosis
(219).
Glycosides
Glycosides consist of aglycone cores (flavonoid,
sesquiterpene or other scaffolds) linked to sugar moieties via O-
or C-glycosidic bonds. Glycosylation is a key SAR-modifying factor:
It markedly enhances aqueous solubility and pharmacokinetic
properties, improving tissue distribution and bioavailability
(220). Notable glycosides in
SIMD include isoquercitrin, paeoniflorin and salidroside. The sugar
units promote interactions with cell-surface receptors or
transporters, facilitate macrophage M2 polarization and improve
cardiomyocyte survival rate (220). Glycosides often retain and
enhance the anti-inflammatory and anti-apoptotic properties of
their parent aglycones, while also demonstrating distinct benefits
such as stabilizing mitochondrial membrane potential (220).
Phenolic acids
Phenolic acids feature an aromatic ring substituted
with ≥1 hydroxyl groups and a carboxyl group. The phenolic
hydroxyls are primary sites for antioxidant activity via hydrogen
atom transfer. The carboxyl group introduces an additional SAR
dimension, allowing coordination with metal ions or formation of
hydrogen bonds with target proteins (such as those involved in
redox signaling), thereby stabilizing mitochondrial function and
inhibiting NF-κB (221). In
SIMD rats, phenolic acids efficiently scavenge ROS, preserve
mitochondrial membrane potential, decrease intracellular
Ca2+ overload and mitigate cardiomyocyte necrosis and
apoptosis (128).
Alkaloids
Alkaloids contain ≥1 basic nitrogen atom within
heterocyclic or polycyclic scaffolds. Examples relevant to SIMD
include yohimbine and anisodamine. The basic nitrogen forms ionic
interactions with acidic residues in receptors or enzymes, thereby
modulating neurotransmitter release and inflammatory mediator
levels (222). In sepsis,
alkaloids often exhibit anti-inflammatory and anti-apoptotic
effects by blocking α2-adrenergic receptors or inhibiting the NLRP3
inflammasome, helping to preserve cardiomyocyte function (199).
Saponins
Saponins are composed of triterpene or steroid
aglycones linked to ≥1 sugar chains. This amphipathic structure
defines their unique SAR: Aglycone enables interactions with and
modulation of cell membrane properties and associated signaling
pathways, while the sugar chains confer water solubility and
influence pharmacokinetics (223). This structure allows saponins
to modulate membrane fluidity and receptor clustering, leading to
inhibition of caspase activation and decreased inflammatory
mediator release, thereby mitigating apoptosis in cardiomyocytes
(207).
Certain natural products display multi-target
actions across SIMD-relevant pathways, combining anti-inflammatory
and antioxidant effects with mitochondrial protection and
anti-apoptotic signaling. Notable multi-target compounds include
astragaloside IV, baicalein, carvacrol, curcumin, emodin,
ginsenoside Re, matrine, propofol, quercetin and resveratrol
(91,95,100,128,131). These agents exemplify the
potential of pleiotropic natural products to regulate inflammation,
oxidative stress, mitochondrial dysfunction and programmed cell
death in SIMD (Table SI).
Notable therapeutic targets in SIMD
Key signaling pathways in SIMD
Natural products exert protective effects in SIMD
by modulating multiple disease-associated pathways and molecular
targets. Their primary actions are divided into four categories:
Anti-inflammatory, antioxidant, anti-cell death and mitochondrial
protection and repair (Fig. 7A).
Notable signaling cascades include NF-κB, the MAPK family, the
TLR4/MyD88 pathway, PI3K/Akt/mTOR, Nrf2/HO-1 and the NLRP3
inflammasome.
 | Figure 7Notable therapeutic targets and
network pharmacology analysis in SIMD. (A) Primary actions of
natural products are categorized into anti-inflammatory,
antioxidant, anti-cell death and mitochondrial protection. Major
targeted signaling cascades include NF-κB, MAPK family, TLR4/MyD88,
PI3K/Akt/mTOR, Nrf2/HO-1 and the NLRP3 inflammasome. (B) Network
pharmacology comparison highlights high-frequency SIMD-associated
targets, such as iNOS (NOS2), NF-κB, MAPK members (JNK, ERK1/2),
PI3K/Akt/mTOR, NLRP3, SIRT1 and STAT3. SIMD, sepsis-induced
myocardial dysfunction; TLR, Toll like receptor; HO, heme
oxygenase; iNOS, inducible nitric oxide synthase ; SIRT, sirtuin
1. |
NF-κB serves as a key regulator of inflammation in
SIMD (224). In cardiomyocytes
and cardiac macrophages, LPS or endotoxin binding to TLR4/MyD88
activates the IKK complex, which phosphorylates and degrades IκBα.
This frees NF-κB (p65/p50) to enter the nucleus and upregulate
levels of IL-1β, IL-6, TNF-α and iNOS, thereby amplifying local
inflammation and disturbing calcium homeostasis (225). As an upstream switch in early
SIMD, TLR4 recruits TIR domain containing adaptor protein/MyD88
adaptors following LPS engagement, propagating both NF-κB and MAPK
signaling and exacerbating myocardial injury (226,227).
The MAPK family, including p38, JNK, and ERK, also
governs myocardial stress responses, inflammation and death in SIMD
(228). p38 and JNK generally
promote inflammation and apoptosis, while ERK supports survival or
death depending on context (228). Overactivation of p38/JNK
triggers c-Jun and ATF-2, leading to caspase-3-mediated apoptosis
in SIMD (229). Nrf2/HO-1
provides the chief antioxidant defense. Under oxidative stress,
Nrf2 dissociates from Keap1, translocates to the nucleus and binds
antioxidant response elements to induce HO-1, NQO1 and other
protective genes (230). Excess
ROS in SIMD further damages membranes and amplifies NF-κB-driven
inflammation (231).
PI3K/Akt/mTOR and SIRT1/AMPK form core networks
that support cardiomyocyte survival and energy balance (232). PI3K activation leads to Akt
phosphorylation and mTOR signaling, which promotes protein
synthesis and restrains autophagy (233). SIRT1 deacetylates PGC-1α, p53
and NF-κB, coordinating mitochondrial biogenesis with
anti-inflammatory effects (234). AMPK senses low ATP levels and
works in concert with SIRT1 to maintain energy homeostasis
(235). In SIMD, these pathways
are typically downregulated, contributing to mitochondrial
dysfunction and cell death (124).
Finally, the NLRP3 inflammasome and ferroptosis
pathways drive inflammatory cell death in SIMD. NLRP3 assembly
activates caspase-1, leading to IL-1β maturation and pyroptosis
(236). Ferroptosis depends on
iron-catalyzed lipid peroxidation, with key regulators including
GPX4, acyl-CoA synthetase long chain family member 4 (ACSL4) and
nuclear receptor coactivator 4) (237).
Rather than acting on a single target, natural
products coordinate the aforementioned hubs to deliver combined
anti-inflammatory, antioxidant, anti-death and
mitochondrial-protective effects. Future studies should map the
spatiotemporal dynamics and crosstalk between these pathways to
guide the development of multitarget natural derivatives with
improved precision and synergy.
Network pharmacology comparison of
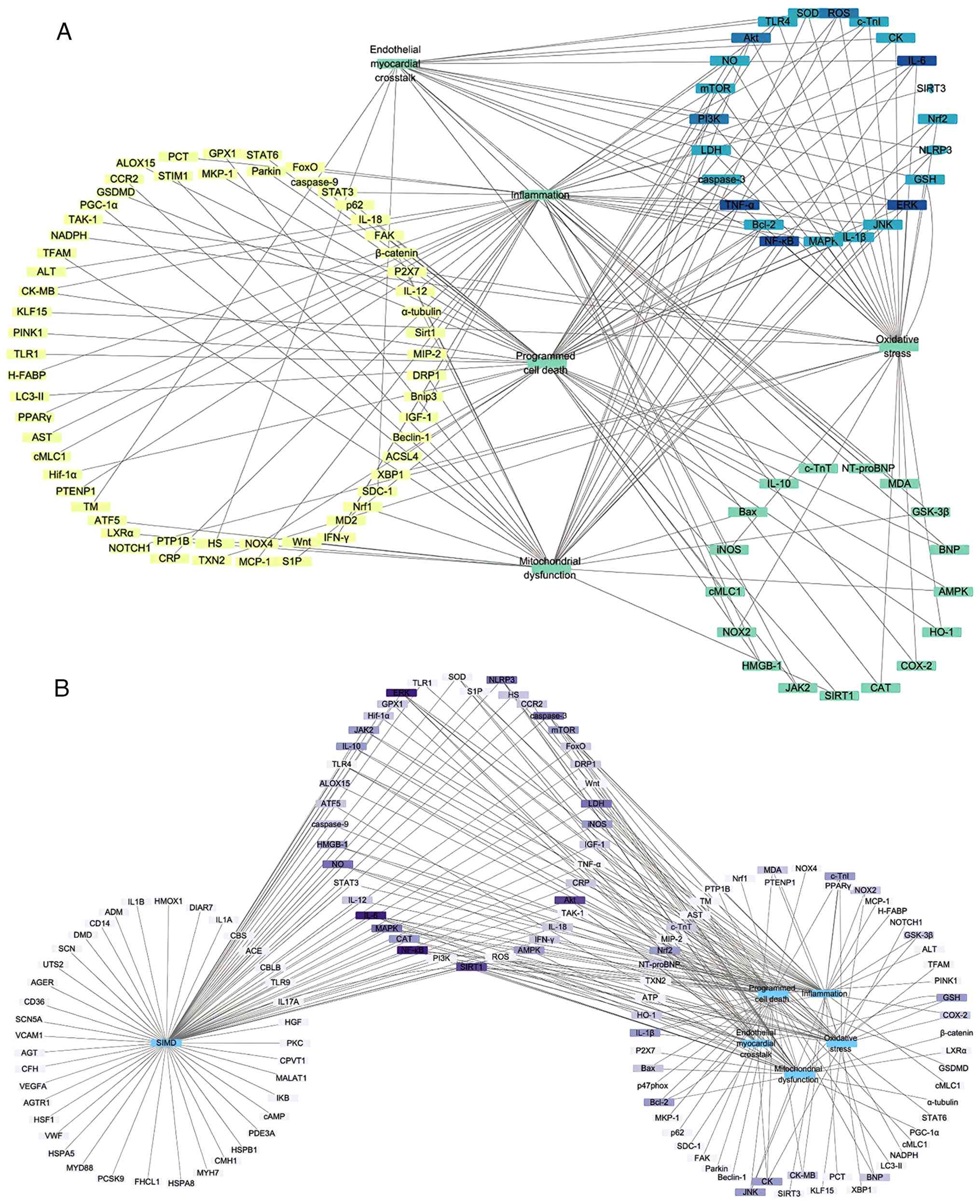
SIMD-associated targets
To evaluate how natural product targets align with
known SIMD genes, 'sepsis', 'myocardial injury', and 'myocardial
dysfunction' were in the Online Mendelian inheritance in Man and
GeneCards databases (8,10). This search yielded over 350
unique disease-associated genes, with 41 common targets (Fig. 7B). High-frequency targets
included iNOS (NOS2), NF-κB, MAPK family members (JNK, ERK1/2),
PI3K/Akt/mTOR, NLRP3, SIRT1 and STAT3.
Notably, certain predicted targets, such as VEGF,
cAMP, HSPs, Von Willebrand factor), MMP9 and PKC (Protein kinase
C), lack thorough experimental validation in SIMD. Conversely,
high-frequency targets such as NOX2, HO-1, GSK-3β, COX-2 and Nrf2
were not annotated as core SIMD targets in database queries. This
discrepancy may reflect annotation lags, limited natural
product-target interaction data and the inherent constraints of
network pharmacology analyses. Integrating network pharmacology
with systematic literature mining, multi-omics datasets and
experimental validation is essential to build a comprehensive
disease-drug-target network and inform the rational development of
new SIMD therapies.
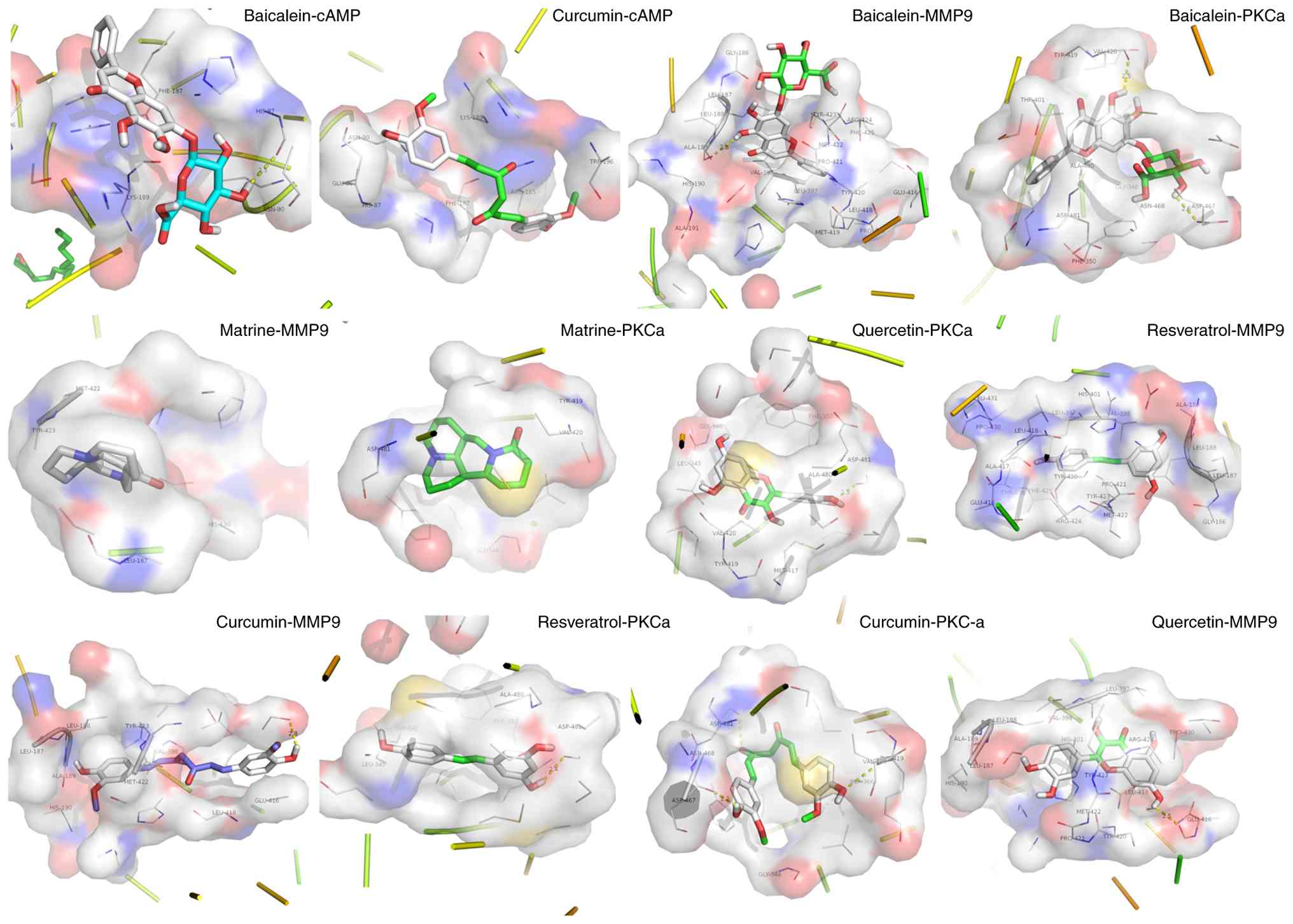
Docking of natural products to novel SIMD
targets
To identify additional molecular targets for
natural products in SIMD, molecular docking was performed for three
proteins not yet validated in vitro or in vivo:
cAMP-dependent protein kinase (cAMP), MMP9 and PKC). A total of
five representative ligands (baicalein, quercetin, curcumin,
matrine and resveratrol) were drawn in ChemDraw (14.0,
revvitysignals. com/products/research/chemdraw), energy-minimized
using the Molecular mechanics 2 force field, and docked to each
target with AutoDock Vina (1.1.2,
github.com/ccsb-scripps/AutoDock-Vina). The lowest-energy poses
exhibiting the most favorable orientations were selected for
analysis (238). All five
compounds bound tightly to both MMP9 and PKC, with calculated
binding energies <-8.0 kcal/mol (Fig. 8). Baicalein and curcumin also
demonstrated notable affinity for PKA, with binding energies
<-5.0 kcal/mol, suggesting potential direct modulation of this
kinase. Key interacting residues and hydrogen-bond contacts are
detailed in Table SII. These
findings indicate that multitarget natural products may exert
cardioprotective effects not only through established
anti-inflammatory and antioxidant pathways but also by directly
engaging cAMP-dependent protein kinase, MMP9 and PKC. This in
silico evidence lays the groundwork for experimental validation
of these novel interactions.
Novel formulations for natural product
therapeutics in SIMD
Although natural products offer multitarget synergy
against SIMD, their clinical translation is hampered by poor
solubility, low bioavailability and rapid clearance (239). Recent advances in formulation
science, including nanotechnology, supramolecular chemistry and
metal-organic coordination, provide new routes to improve delivery,
targeting and efficacy (240).
Metal-organic nanozymes
Self-assembled nanozymes that incorporate
metal-organic coordination combine drug delivery with enzyme-like
catalysis (239,240). For example, ceria
(CeO2) or iron oxide (Fe3O4)
nanoparticles mimic superoxide dismutase and catalase through
reversible Ce3+/Ce4+ or
Fe2+/Fe3+ redox cycling, efficiently
scavenging O2•- and H2O2 (241,242). By coordinating curcumin onto
ceria, researchers have created ceria-curcumin hybrids (CeCHs) that
exhibit dual SOD- and CAT-like activity in vitro (242). CeCHs neutralizes ROS, prevent
glutathione peroxidase 4-induced ferroptosis in cardiomyocytes and
shift macrophages toward an M2 phenotype (243). In LPS- and CLP-induced septic
mice, CeCH decreases myocarditis and restores cardiac function
(243). Similarly, Brazilin-Ce
(IV) metal-organic nanoparticles inhibit IKKβ phosphorylation,
suppress NF-κB signaling and deliver strong anti-inflammatory and
cardioprotective effects in mice with myocardial infarction and
sepsis (244).
Polymeric and lipid-based
nanocarriers
Polymeric and lipid nanoparticles enhance the
stability, circulation time and tissue distribution of natural
products. Common polymers include poly(lactic-co-glycolic acid),
PVA (Polyvinyl alcohol) and chitosan. Liposomal systems, solid
lipid nanoparticles (SLNs) and mesoporous silica nanoparticles also
serve as versatile carriers (245,246). In LPS-induced septic mice,
curcumin-loaded SLNs decrease IL-6, TNF-α and IL-1β more
effectively than free curcumin, while boosting IL-10 levels. This
improvement is associated with stronger suppression of the
TLR2/TLR4/NF-κB pathway and decreased multi-organ injury (247). Nano-curcumin further enhances
mTOR pathway regulation and offers superior protection against
myocardial ultrastructural damage in SIMD mice compared with the
unformulated compound (248).
Cyclodextrin (CD)-based inclusion
complexes
CD hosts form non-covalent inclusion complexes with
hydrophobic drugs, improving their solubility and stability
(249,250). Hydroxypropyl-β-CD (HPβCD) and
methyl-β-CD (MβCD) are used (249). In neonatal mice with
LPS-induced sepsis, naringenin/HPβCD complexes more effective than
naringin in decreasing inflammatory cell infiltration in the lung,
heart, kidney and brain. They also lower TNF-α, IL-1β and IL-6,
increase IL-10, catalase and SOD activity and decrease lipid
peroxidation and protein carbonylation, leading to improved
survival rate (251).
Quercetin/β-CD complexes extend plasma half-life of quercetin and
enhance myocardial accumulation in CLP-induced septic rats,
strengthening TLR4/NF-κB inhibition and improving cardiac function
(252). Chemical
derivatization, such as converting steviol glycosides into
isosteviol sodium salt, further boosts water solubility and, in
LPS-induced septic mice, enhances survival rate, multi-organ
function and reduces inflammation and macrophage infiltration
(253).
However, systematic pharmacokinetics and
pharmacodynamics analyses, long-term toxicity studies and
validation in large-animal models remain outstanding needs.
Standardization of manufacturing methods, drug-loading efficiency
and in vivo kinetics is also required. Future efforts should
integrate multi-omics, high-resolution imaging and large-animal
models to optimize formulations, clarify dosing regimens, define
safety margins and accelerate clinical translation of these
advanced delivery systems.
Discussion
Sepsis ranks as the third leading cause of death
worldwide, affecting nearly 20 million people each year. A total of
40-60% of these patients develop SIMD, and their 28-day mortality
is ~3 times higher than that of patients without cardiac
involvement (11). This
underscores the urgent need for effective, low-toxicity treatments.
Natural products modulate key signaling networks, including
TLR4/MyD88/NF-κB, MAPKs (p38/JNK/ERK), the NLRP3 inflammasome,
PI3K/Akt/mTOR, SIRT1/AMPK and Nrf2/HO-1, to restore mitochondrial
membrane potential, maintain ATP production and decrease
inflammation, oxidative stress and cell death (207,217,220-222).
Multi-center randomized controlled trials indicate
that formulations such as Xuebijing injection can decrease 28-day
mortality and lower cardiac injury biomarkers (cTnI, NT-proBNP) and
inflammatory markers (PCT), indicating systemic and possible
myocardial protective effects (254,255). Similarly, the JinHong Formula
decreases mortality and improves organ function scores, potentially
through inhibition of inflammatory pathways such as IL-17 and TNF
(256). Other formulations,
including Dachaihu Tang and Shenfu injection, improve organ
dysfunction scores, microcirculation and inflammatory or
coagulation parameters (257,258). The alkaloid anisodamine has
decreases serum lactate and improves mortality in septic shock
(259,260). However, challenges remain. Most
trials did not specifically enroll patients with confirmed SIMD,
limiting direct conclusions about cardiac-specific efficacy.
Furthermore, notable heterogeneity exists in study designs, sample
size and the compositions of natural product formulations.
Additionally, pharmacokinetic challenges constrain the use of
natural products. Poor water solubility, low oral bioavailability
and rapid systemic clearance all decrease therapeutic impact. To
overcome these issues, researchers are developing novel
formulations, such as polymeric or lipid nanoparticles,
metal-organic frameworks, CD inclusion complexes, supramolecular
assemblies and chemical derivatives, to improve solubility,
stability, half-life and tissue targeting (261). Well-designed clinical trials
are essential to assess safety, pharmacokinetics, pharmacodynamics
and real-world efficacy in patients with SIMD.
Furthermore, a deeper understanding of the
pathogenic network of SIMD is also needed. Extracellular vesicles
and their miRNA cargo mediate crosstalk between immune cells and
cardiomyocytes, regulating inflammation and apoptosis (262). Specific miRNAs fine-tune gene
networks that control survival, fibrosis and autophagy (263). Endothelial-myocardial
interactions, governed by adhesion molecules, inflammatory
mediators and the glycocalyx, critically influence microcirculatory
perfusion and cardiac stress responses (63,264). Mitochondria-endoplasmic
reticulum contacts sites serve as hubs for calcium homeostasis,
lipid metabolism and cell fate decisions (265,266). Additionally, emerging evidence
highlights the role of the gut-heart axis in SIMD (267). Sepsis-induced disruption of the
intestinal barrier leads to microbial translocation, systemic
dissemination of PAMPs and elevated levels of gut-derived
metabolites (267). This fuels
a persistent systemic inflammatory state and may contribute
directly to remote organ injury, including myocardial dysfunction
(267). Conversely, beneficial
microbial metabolites, such as short-chain fatty acids, modulate
host immune responses and exert anti-inflammatory effects.
Interventions targeting the gut microbiota or its metabolites hold
promise for attenuating systemic inflammation and improving cardiac
outcomes in sepsis, presenting a novel therapeutic avenue for SIMD
(268). Thus, future efforts
should leverage single-cell sequencing, organoid models and
multi-omics approaches to build a comprehensive molecular map of
SIMD and natural product interventions. Such integrative studies
may identify new precision targets and accelerate the development
of optimized, multitarget natural therapies for clinical use.
In conclusion, the present study provides a
systematic overview of the structure-activity-mechanism
relationships between natural products and SIMD. The mechanisms
involve inhibition of the TLR4/MyD88-NF-κB/MAPK pathway and the
NLRP3 inflammasome and activation of antioxidant and anti-apoptotic
pathways, including PI3K/Akt/mTOR, SIRT1/AMPK and Nrf2/HO-1. The
present review also maps the association between natural product
classes and pharmacological activities and validated potential
compounds against new SIMD targets via network pharmacology and
molecular docking. Collectively, the present study clarified how
natural product structures are associated with SIMD pathology and
outlined a clear path from molecular design to clinical
translation. The present study provides a systematic theoretical
basis and practical avenues for multitarget natural product
interventions in SIMD, with implications for drug discovery and
clinical strategy advancement.
Supplementary Data
Availability of data and materials
The data generated in the present study are
included in the figures and/or tables of this article.
Authors' contributions
FT contributed to study design, performed the
literature review and drafted the manuscript. DL, SCZ and HMZ
performed the literature review. XWQ supervised the study. All
authors contributed to manuscript revision. All authors have read
and approved the final manuscript. FT and XWQ confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
HO-1
|
heme oxygenase-1
|
|
PAMP
|
pathogen-associated molecular
pattern
|
|
PI3K
|
phosphoinositide 3-kinase
|
|
PKC
|
protein kinase C
|
|
SIMD
|
sepsis-induced myocardial
dysfunction
|
|
SIRT1
|
sirtuin 1
|
Acknowledgements
Not applicable.
Funding
The present study was supported by Natural Science Foundation of
Chongqing (grant no. CSTB2024NSCQ-KJFZZDX0023) and Chongqing
Medical Scientific Research Project (Joint Project of Chongqing
Health Commission and Science and Technology Bureau; grant no.
2022MSXM114).
References
|
1
|
Singer M, Deutschman CS, Seymour CW,
Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche
JD, Coopersmith CM, et al: The third international consensus
definitions for sepsis and septic shock (Sepsis-3). JAMA.
315:801–810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Angus DC and van der Poll T: Severe sepsis
and septic shock. N Engl J Med. 369:840–851. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yan J, Li Z, Li Y and Zhang Y: Sepsis
induced cardiotoxicity by promoting cardiomyocyte cuproptosis.
Biochem Biophys Res Commun. 690:1492452024. View Article : Google Scholar
|
|
4
|
Cecconi M, Evans L, Levy M and Rhodes A:
Sepsis and septic shock. Lancet. 392:75–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Iyer S, Kennedy JN, Jentzer JC, Senussi MH
and Seymour CW: Cardiac function before sepsis and clinical
outcomes. JAMA. 331:1496–1499. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van der Poll T and van Deventer SJ:
Cytokines and anticytokines in the pathogenesis of sepsis. Infect
Dis Clin North Am. 13:413–426. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu J, Li J, Tian P, Guli B, Weng G, Li L
and Cheng Q: H2S attenuates sepsis-induced cardiac dysfunction via
a PI3K/Akt-dependent mechanism. Exp Ther Med. 17:4064–4072.
2019.PubMed/NCBI
|
|
8
|
Tang F, Zhang JN, Xu LY, Zhao XL, Wan F,
Ao H and Peng C: Endothelial-derived exosomes: A novel therapeutic
strategy for LPS-induced myocardial damage with anisodamine. Int J
Biol Macromol. 282:1369932024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang X, Zhang C, Tian T, Dai X, Xing Y,
Wang Y, Yang D, Li H, Wang Y, Lv X and Wang H: Posttreatment with
dexmedetomidine aggravates LPS-induced myocardial dysfunction
partly via activating cardiac endothelial α2A-AR in mice. Int
Immunopharmacol. 116:1097242023. View Article : Google Scholar
|
|
10
|
Tang F, Liu D, Zhang L, Xu LY, Zhang JN,
Zhao XL, Ao H and Peng C: Targeting endothelial cells with golden
spice curcumin: A promising therapy for cardiometabolic
multimorbidity. Pharmacol Res. 197:1069532023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen Y, Weng D, Shi W, Wei S, Ji W, Wang
X, Xu Y, Wang X, Mei X and Guo S: Integrative network pharmacology
and multi-omics reveal anisodamine hydrobromide's multi-target
mechanisms in sepsis. Sci Rep. 15:279962025. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Palmieri V, Innocenti F, Guzzo A, Guerrini
E, Vignaroli D and Pini R: Left ventricular systolic longitudinal
function as predictor of outcome in patients with sepsis. Circ
Cardiovasc Imaging. 8:e0038652015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fan R, Liu H and Liang Q: Roles and
therapeutic targeting of exosomes in Sepsis-induced cardiomyopathy.
J Cell Mol Med. 29:e705592025. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Antonucci E, Fiaccadori E, Donadello K,
Taccone FS, Franchi F and Scolletta S: Myocardial depression in
sepsis: From pathogenesis to clinical manifestations and treatment.
J Crit Care. 29:500–511. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Repessé X, Charron C and Vieillard-Baron
A: Evaluation of left ventricular systolic function revisited in
septic shock. Crit Care. 17:1642013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aissaoui N, Boissier F, Chew M, Singer M
and Vignon P: Sepsis-induced cardiomyopathy. Eur Heart J.
46:3339–3353. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Parker MM, Shelhamer JH, Bacharach SL,
Green MV, Natanson C, Frederick TM, Damske BA and Parrillo JE:
Profound but reversible myocardial depression in patients with
septic shock. Ann Intern Med. 100:483–490. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zaky A, Deem S, Bendjelid K and Treggiari
MM: Characterization of cardiac dysfunction in sepsis: An ongoing
challenge. Shock. 41:12–24. 2014. View Article : Google Scholar
|
|
19
|
ver Elst KM, Spapen HD, Nguyen DN, Garbar
C, Huyghens LP and Gorus FK: Cardiac troponins I and T are
biological markers of left ventricular dysfunction in septic shock.
Clin Chem. 46:650–657. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sheyin O, Davies O, Duan W and Perez X:
The prognostic significance of troponin elevation in patients with
sepsis: A meta-analysis. Heart Lung. 44:75–81. 2015. View Article : Google Scholar
|
|
21
|
Clerico A, Iervasi G and Mariani G:
Pathophysiologic relevance of measuring the plasma levels of
cardiac natriuretic peptide hormones in humans. Horm Metab Res.
31:487–498. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chua G and Kang-Hoe L: Marked elevations
in N-terminal brain natriuretic peptide levels in septic shock.
Crit Care. 8:R248–R250. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Roch A, Allardet-Servent J, Michelet P,
Oddoze C, Forel JM, Barrau K, Loundou A, Perrin G, Auffray JP,
Portugal H and Papazian L: NH2 terminal pro-brain natriuretic
peptide plasma level as an early marker of prognosis and cardiac
dysfunction in septic shock patients. Crit Care Med. 33:1001–1007.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alam ML, Katz R, Bellovich KA, Bhat ZY,
Brosius FC, de Boer IH, Gadegbeku CA, Gipson DS, Hawkins JJ,
Himmelfarb J, et al: Soluble ST2 and Galectin-3 and Progression of
CKD. Kidney Int Rep. 4:103–111. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chang X, Guo Y, Wang J, Liu J, Ma Y, Lu Q
and Han Y: Heart-type fatty acid binding protein (H-FABP) as an
early biomarker in sepsis-induced cardiomyopathy: A prospective
observational study. Lipids Health Dis. 23:2832024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weinberger J, Klompas M and Rhee C: What
is the utility of measuring lactate levels in patients with sepsis
and septic shock? Semin Respir Crit Care Med. 42:650–661. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Benz F, Roy S, Trautwein C, Roderburg C
and Luedde T: Circulating MicroRNAs as biomarkers for sepsis. Int J
Mol Sci. 17:782016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Manetti AC, Maiese A, Paolo MD, De Matteis
A, La Russa R, Turillazzi E, Frati P and Fineschi V: MicroRNAs and
Sepsis-induced cardiac dysfunction: A systematic review. Int J Mol
Sci. 22:3212020. View Article : Google Scholar
|
|
29
|
Ketelut-Carneiro N and Fitzgerald KA:
Apoptosis, pyroptosis, and Necroptosis-Oh My! The many ways a cell
can die. J Mol Biol. 434:1673782022. View Article : Google Scholar
|
|
30
|
Communal C, Sumandea M, de Tombe P, Narula
J, Solaro RJ and Hajjar RJ: Functional consequences of caspase
activation in cardiac myocytes. Proc Natl Acad Sci USA.
99:6252–6256. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nevière R, Fauvel H, Chopin C, Formstecher
P and Marchetti P: Caspase inhibition prevents cardiac dysfunction
and heart apoptosis in a rat model of sepsis. Am J Respir Crit Care
Med. 163:218–225. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hu H, Tian M, Ding C and Yu S: The C/EBP
Homologous protein (CHOP) Transcription factor functions in
endoplasmic reticulum Stress-induced apoptosis and microbial
infection. Front Immunol. 9:30832018. View Article : Google Scholar
|
|
33
|
Li L, Peng X, Guo L, Zhao Y and Cheng Q:
Sepsis causes heart injury through endoplasmic reticulum
stress-mediated apoptosis signaling pathway. Int J Clin Exp Pathol.
13:964–971. 2020.PubMed/NCBI
|
|
34
|
Xu X, Liu Q, He S, Zhao J, Wang N, Han X
and Guo Y: Qiang-Xin 1 formula prevents Sepsis-induced apoptosis in
murine cardiomyocytes by suppressing endoplasmic Reticulum- and
Mitochondria-associated pathways. Front Pharmacol. 9:8182018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zheng X, Chen W, Gong F, Chen Y and Chen
E: The role and mechanism of pyroptosis and potential therapeutic
targets in sepsis: A review. Front Immunol. 12:7119392021.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xue Z, Xi Q, Liu H, Guo X, Zhang J, Zhang
Z, Li Y, Yang G, Zhou D, Yang H, et al: miR-21 promotes NLRP3
inflammasome activation to mediate pyroptosis and endotoxic shock.
Cell Death Dis. 10:4612019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fang X, Wang H, Han D, Xie E, Yang X, Wei
J, Gu S, Gao F, Zhu N, Yin X, et al: Ferroptosis as a target for
protection against cardiomyopathy. Proc Natl Acad Sci USA.
116:2672–2680. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li W, Li W, Leng Y, Xiong Y and Xia Z:
Ferroptosis is involved in diabetes myocardial Ischemia/reperfusion
injury through endoplasmic reticulum stress. DNA Cell Biol.
39:210–225. 2020. View Article : Google Scholar
|
|
39
|
Parzych KR and Klionsky DJ: An overview of
autophagy: Morphology, mechanism, and regulation. Antioxid Redox
Signal. 20:460–473. 2014. View Article : Google Scholar :
|
|
40
|
Liu AB, Li SJ, Yu YY, Zhang JF and Ma L:
Current insight on the mechanisms of programmed cell death in
sepsis-induced myocardial dysfunction. Front Cell Dev Biol.
11:13097192023. View Article : Google Scholar :
|
|
41
|
Denk S, Perl M and Huber-Lang M: Damage-
and pathogen-associated molecular patterns and alarmins: Keys to
sepsis? Eur Surg Res. 48:171–179. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Vénéreau E, Ceriotti C and Bianchi ME:
DAMPs from cell death to new life. Front Immunol. 6:4222015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hobai IA, Morse JC, Siwik DA and Colucci
WS: Lipopolysaccharide and cytokines inhibit rat cardiomyocyte
contractility in vitro. J Surg Res. 193:888–901. 2015. View Article : Google Scholar
|
|
44
|
Zhang YY and Ning BT: Signaling pathways
and intervention therapies in sepsis. Signal Transduct Target Ther.
6:4072021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fujimura K, Karasawa T, Komada T, Yamada
N, Mizushina Y, Baatarjav C, Matsumura T, Otsu K, Takeda N,
Mizukami H, et al: NLRP3 inflammasome-driven IL-1β and IL-18
contribute to lipopolysaccharide-induced septic cardiomyopathy. J
Mol Cell Cardiol. 180:58–68. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Busch K, Kny M, Huang N, Klassert TE,
Stock M, Hahn A, Graeger S, Todiras M, Schmidt S, Chamling B, et
al: Inhibition of the NLRP3/IL-1β axis protects against
sepsis-induced cardiomyopathy. J Cachexia Sarcopenia Muscle.
12:1653–1668. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kumar V: Toll-like receptors in
sepsis-associated cytokine storm and their endogenous negative
regulators as future immunomodulatory targets. Int Immunopharmacol.
89:1070872020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hoover DB, Ozment TR, Wondergem R, Li C
and Williams DL: Impaired heart rate regulation and depression of
cardiac chronotropic and dromotropic function in polymicrobial
sepsis. Shock. 43:185–191. 2015. View Article : Google Scholar :
|
|
49
|
Nolfi-Donegan D, Braganza A and Shiva S:
Mitochondrial electron transport chain: Oxidative phosphorylation,
oxidant production, and methods of measurement. Redox Biol.
37:1016742020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen YR and Zweier JL: Cardiac
mitochondria and reactive oxygen species generation. Circ Res.
114:524–537. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sun F, Huo X, Zhai Y, Wang A, Xu J, Su D,
Bartlam M and Rao Z: Crystal structure of mitochondrial respiratory
membrane protein complex II. Cell. 121:1043–1057. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tsolaki V, Makris D, Mantzarlis K and
Zakynthinos E: Sepsis-induced cardiomyopathy: Oxidative
implications in the initiation and resolution of the damage. Oxid
Med Cell Longev. 2017:73935252017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chen YR, Chen CL, Yeh A, Liu X and Zweier
JL: Direct and indirect roles of cytochrome b in the mediation of
superoxide generation and NO catabolism by mitochondrial
succinate-cytochrome c reductase. J Biol Chem. 281:13159–13168.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Takasu O, Gaut JP, Watanabe E, To K,
Fagley RE, Sato B, Jarman S, Efimov IR, Janks DL, Srivastava A, et
al: Mechanisms of cardiac and renal dysfunction in patients dying
of sepsis. Am J Respir Crit Care Med. 187:509–517. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Vanasco V, Saez T, Magnani ND, Pereyra L,
Marchini T, Corach A, Vaccaro MI, Corach D, Evelson P and Alvarez
S: Cardiac mitochondrial biogenesis in endotoxemia is not
accompanied by mitochondrial function recovery. Free Radic Biol
Med. 77:1–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Haileselassie B, Mukherjee R, Joshi AU,
Napier BA, Massis LM, Ostberg NP, Queliconi BB, Monack D, Bernstein
D and Mochly-Rosen D: Drp1/Fis1 interaction mediates mitochondrial
dysfunction in septic cardiomyopathy. J Mol Cell Cardiol.
130:160–169. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wagner S, Schürmann S, Hein S, Schüttler J
and Friedrich O: Septic cardiomyopathy in rat LPS-induced
endotoxemia: Relative contribution of cellular diastolic Ca(2+)
removal pathways, myofibrillar biomechanics properties and action
of the cardiotonic drug levosimendan. Basic Res Cardiol.
110:5072015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lin Y, Xu Y and Zhang Z: Sepsis-induced
myocardial dysfunction (SIMD): The pathophysiological mechanisms
and therapeutic strategies targeting mitochondria. Inflammation.
43:1184–1200. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ravikumar N, Sayed MA, Poonsuph CJ, Sehgal
R, Shirke MM and Harky A: Septic cardiomyopathy: From basics to
management choices. Curr Probl Cardiol. 46:1007672021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cao T, Ni R, Ding W, Ji X, Fan GC, Zhang Z
and Peng T: Nicotinamide mononucleotide as a therapeutic agent to
alleviate multi-organ failure in sepsis. J Transl Med. 21:8832023.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Furian T, Aguiar C, Prado K, Ribeiro RV,
Becker L, Martinelli N, Clausell N, Rohde LE and Biolo A:
Ventricular dysfunction and dilation in severe sepsis and septic
shock: Relation to endothelial function and mortality. J Crit Care.
27:319.e9–e15. 2012. View Article : Google Scholar
|
|
62
|
Kacimi R, Karliner JS, Koudssi F and Long
CS: Expression and regulation of adhesion molecules in cardiac
cells by cytokines: Response to acute hypoxia. Circ Res.
82:576–586. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Tang F, Zhao XL, Xu LY, Zhang JN, Ao H and
Peng C: Endothelial dysfunction: Pathophysiology and therapeutic
targets for sepsis-induced multiple organ dysfunction syndrome.
Biomed Pharmacother. 178:1171802024. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhan JH, Wei J, Liu YJ, Wang PX and Zhu
XY: Sepsis-associated endothelial glycocalyx damage: A review of
animal models, clinical evidence, and molecular mechanisms. Int J
Biol Macromol. 295:1395482025. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Burg N, Malpass R, Alex L, Tran M,
Englebrecht E, Kuo A, Pannelini T, Minett M, Athukorala K and
Worgall T: Endothelial cell sphingosine 1-phosphate receptor 1
restrains VE-cadherin cleavage and attenuates experimental
inflammatory arthritis. JCI Insight. 9:e1714672024. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
de Oliveira J and Miranda CH: Doxycycline
protects against sepsis-induced endothelial glycocalyx shedding.
Sci Rep. 14:104772024. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
van de Sandt AM, Windler R, Gödecke A,
Ohlig J, Zander S, Reinartz M, Graf J, van Faassen EE, Rassaf T,
Schrader J, et al: Endothelial NOS (NOS3) impairs myocardial
function in developing sepsis. Basic Res Cardiol. 108:3302013.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zeng N, Xu J, Yao W, Li S, Ruan W and Xiao
F: Brain-derived neurotrophic factor attenuates septic myocardial
dysfunction via eNOS/NO pathway in rats. Oxid Med Cell Longev.
2017:17214342017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hong G, Zheng D, Zhang L, Ni R, Wang G,
Fan GC, Lu Z and Peng T: Administration of nicotinamide riboside
prevents oxidative stress and organ injury in sepsis. Free Radic
Biol Med. 123:125–137. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Mirna M, Paar V, Rezar R, Topf A, Eber M,
Hoppe UC, Lichtenauer M and Jung C: MicroRNAs in inflammatory heart
diseases and Sepsis-induced cardiac dysfunction: A potential scope
for the future? Cells. 8:13522019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Gao M, Wang X, Zhang X, Ha T, Ma H, Liu L,
Kalbfleisch JH, Gao X, Kao RL, Williams DL and Li C: Attenuation of
cardiac dysfunction in polymicrobial sepsis by MicroRNA-146a is
mediated via targeting of IRAK1 and TRAF6 expression. J Immunol.
195:672–682. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ma H, Wang X, Ha T, Gao M, Liu L, Wang R,
Yu K, Kalbfleisch JH, Kao RL, Williams DL and Li C: MicroRNA-125b
prevents cardiac dysfunction in polymicrobial sepsis by targeting
TRAF6-Mediated nuclear factor κB activation and p53-Mediated
apoptotic signaling. J Infect Dis. 214:1773–1783. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Yao Y, Sun F and Lei M: miR-25 inhibits
sepsis-induced cardiomyocyte apoptosis by targetting PTEN. Biosci
Rep. 38:BSR201715112018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Li Z, Yi N, Chen R, Meng Y, Wang Y, Liu H,
Cao W, Hu Y, Gu Y, Tong C, et al: miR-29b-3p protects
cardiomyocytes against endotoxin-induced apoptosis and inflammatory
response through targeting FOXO3A. Cell Signal. 74:1097162020.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Long X, Huang Y, He J, Zhang X, Zhou Y,
Wei Y, Tang Y and Liu L: Upregulation of miR-335 exerts protective
effects against sepsis-induced myocardial injury. Mol Med Rep.
24:8062021. View Article : Google Scholar :
|
|
76
|
Liang L, Liu S, Wu Q, Chen R, Jiang S and
Yang Z: m6A-mediated upregulation of miRNA-193a aggravates
cardiomyocyte apoptosis and inflammatory response in sepsis-induced
cardiomyopathy via the METTL3/miRNA-193a/BCL2L2 pathway. Exp Cell
Res. 430:1137122023. View Article : Google Scholar
|
|
77
|
He Z, Xu L, Zeng X, Yang B, Liu P, Han D,
Xue H and Luo B: circROCK1 Promotes septic myocardial injury
through regulating miR-96-5p/OXSR1 axis. Acta Biochim Pol.
70:567–574. 2023.PubMed/NCBI
|
|
78
|
Wang H, Bei Y, Shen S, Huang P, Shi J,
Zhang J, Sun Q, Chen Y, Yang Y, Xu T, et al: miR-21-3p controls
sepsis-associated cardiac dysfunction via regulating SORBS2. J Mol
Cell Cardiol. 94:43–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Ge C, Liu J and Dong S: miRNA-214 protects
Sepsis-induced myocardial injury. Shock. 50:112–118. 2018.
View Article : Google Scholar
|
|
80
|
Li Y, Sun G and Wang L: MiR-21
participates in LPS-induced myocardial injury by targeting Bcl-2
and CDK6. Inflamm Res. 71:205–214. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhu XG, Zhang TN, Wen R and Liu CF:
Overexpression of miR-150-5p alleviates apoptosis in Sepsis-induced
myocardial depression. Biomed Res Int. 2020:30231862020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zhang L, Li B, Li W, Jiang J, Chen W, Yang
H and Pan D: miR-107 attenuates Sepsis-induced myocardial injury by
targeting PTEN and activating the PI3K/AKT signaling pathway. Cells
Tissues Organs. 212:523–534. 2023. View Article : Google Scholar
|
|
83
|
Sun F, Yuan W, Wu H, Chen G, Sun Y, Yuan
L, Zhang W and Lei M: LncRNA KCNQ1OT1 attenuates sepsis-induced
myocardial injury via regulating miR-192-5p/XIAP axis. Exp Biol Med
(Maywood). 245:620–630. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Chen DD, Wang HW and Cai XJ: Long
non-coding RNA ZFAS1 alleviates sepsis-induced myocardial injury
via target miR-34b-5p/SIRT1. Innate Immun. 27:377–387. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Xu LJ, Yang Y, Yuan LF, Liu H, Xu NP, Yang
Y and Huang L: SP1-stimulated miR-208a-5p aggravates sepsis-induced
myocardial injury via targeting XIAP. Exp Cell Res. 435:1139052024.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Li Y, Shao Y, Su J and Dong S: MiR-383-3p
attenuates sepsis-induced myocardial ferroptosis by targeting ATF4
and inhibiting the ATF4-CHOP-CHAC1 signaling axis. Cell Signal.
136:1121692025. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wang J, Wei T, Zhang W, Chu Y, Zhang D,
Zhang M, Hu J, Ji Z and Hao Q: Inhibition of miR-194-5p avoids
DUSP9 downregulation thus limiting sepsis-induced cardiomyopathy.
Sci Rep. 14:203132024. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Liang D, Jin Y, Lin M, Xia X, Chen X and
Huang A: Down-regulation of Xist and Mir-7a-5p improves LPS-induced
myocardial injury. Int J Med Sci. 17:2570–2577. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Gong M, Tao L and Li X:
MicroRNA-21-3p/Rcan1 signaling axis affects apoptosis of
cardiomyocytes of sepsis rats. Gen Physiol Biophys. 42:217–227.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Dao L, Liu H, Xiu R, Yao T, Tong R and Xu
L: Gramine improves sepsis-induced myocardial dysfunction by
binding to NF-κB p105 and inhibiting its ubiquitination.
Phytomedicine. 125:1553252024. View Article : Google Scholar
|
|
91
|
Chen D, Wang H and Cai X: Curcumin
interferes with sepsis-induced cardiomyocyte apoptosis via TLR1
inhibition. Rev Port Cardiol. 42:209–221. 2023.In English,
Portuguese. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zhang J, Zhu D, Wang Y and Ju Y:
Andrographolide attenuates LPS-induced cardiac malfunctions through
Inhibition of IκB phosphorylation and apoptosis in mice. Cell
Physiol Biochem. 37:1619–1628. 2015. View Article : Google Scholar
|
|
93
|
Wang YY, Li HM, Wang HD, Peng XM, Wang YP,
Lu DX, Qi RB, Hu CF and Jiang JW: Pretreatment with berberine and
yohimbine protects against LPS-induced myocardial dysfunction via
inhibition of cardiac I-[kappa]B[alpha] phosphorylation and
apoptosis in mice. Shock. 35:322–328. 2011. View Article : Google Scholar
|
|
94
|
Meng YY, Liu Y, Hu ZF, Zhang Y, Ni J, Ma
ZG, Liao HH, Wu QQ and Tang QZ: Sanguinarine attenuates
lipopolysaccharide-induced inflammation and apoptosis by inhibiting
the TLR4/NF-κB pathway in H9c2 Cardiomyocytes. Curr Med Sci.
38:204–211. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhong L, Zhou XL, Liu YS, Wang YM, Ma F,
Guo BL, Yan ZQ and Zhang QY: Estrogen receptor α mediates the
effects of notoginsenoside R1 on endotoxin-induced inflammatory and
apoptotic responses in H9c2 cardiomyocytes. Mol Med Rep.
12:119–126. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Sun B, Xiao J, Sun XB and Wu Y:
Notoginsenoside R1 attenuates cardiac dysfunction in endotoxemic
mice: An insight into oestrogen receptor activation and PI3K/Akt
signalling. Br J Pharmacol. 168:1758–1770. 2013. View Article : Google Scholar :
|
|
97
|
Zhu H, Zhang L, Jia H, Xu L, Cao Y, Zhai
M, Li K, Xia L, Jiang L, Li X, et al: Tetrahydrocurcumin improves
lipopolysaccharide-induced myocardial dysfunction by inhibiting
oxidative stress and inflammation via JNK/ERK signaling pathway
regulation. Phytomedicine. 104:1542832022. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Xie WJ, Hou G, Wang L, Wang SS and Xiong
XX: Astaxanthin suppresses lipopolysaccharide-induced myocardial
injury by regulating MAPK and PI3K/AKT/mTOR/GSK3β signaling. Mol
Med Rep. 22:3338–3346. 2020.PubMed/NCBI
|
|
99
|
Jiang L, Zhang L, Yang J, Shi H, Zhu H,
Zhai M, Lu L, Wang X, Li XY, Yu S, et al: 1-Deoxynojirimycin
attenuates septic cardiomyopathy by regulating oxidative stress,
apoptosis, and inflammation via the JAK2/STAT6 signaling pathway.
Biomed Pharmacother. 155:1136482022. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Su Y, Yin X, Huang X, Guo Q, Ma M and Guo
L: Astragaloside IV ameliorates sepsis-induced myocardial
dysfunction by regulating NOX4/JNK/BAX pathway. Life Sci.
310:1211232022. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zhang T, Yan T, Du J, Wang S and Yang H:
Apigenin attenuates heart injury in lipopolysaccharide-induced
endotoxemic model by suppressing sphingosine kinase 1/sphingosine
1-phosphate signaling pathway. Chem Biol Interact. 233:46–55. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Yu H, Du Q, Wu J, Feng F, Hou S, Liu M,
Wang S, Liu X, Wang C and Xu K: Gastrodin regulates H3K14la through
the CDT2-KAT2A axis to treat sepsis-induced myocardial dysfunction.
Int Immunopharmacol. 161:1150652025. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Wang RY, Wang MG, Tang HZ, Du H, Luo Y, Li
Q, Zhang XH, Fu J and Lv CZ: The protective effects of ruscogenin
against Lipopolysaccharide-induced myocardial injury in septic
mice. J Cardiovasc Pharmacol. 84:175–187. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zhu XX, Meng XY, Zhang AY, Zhao CY, Chang
C, Chen TX, Huang YB, Xu JP, Fu X, Cai WW, et al: Vaccarin
alleviates septic cardiomyopathy by potentiating NLRP3
palmitoylation and inactivation. Phytomedicine. 131:1557712024.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Long H, Xu B, Luo Y and Luo K: Artemisinin
protects mice against burn sepsis through inhibiting NLRP3
inflammasome activation. Am J Emerg Med. 34:772–777. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Liu H, Sun Y, Zhang Y, Yang G, Guo L, Zhao
Y and Pei Z: Role of thymoquinone in cardiac damage caused by
sepsis from BALB/c mice. Inflammation. 42:516–525. 2019. View Article : Google Scholar
|
|
107
|
Wei A, Liu J, Li D, Lu Y, Yang L, Zhuo Y,
Tian W and Cong H: Syringaresinol attenuates sepsis-induced cardiac
dysfunction by inhibiting inflammation and pyroptosis in mice. Eur
J Pharmacol. 913:1746442021. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Luo M, Yan D, Sun Q, Tao J, Xu L, Sun H
and Zhao H: Ginsenoside Rg1 attenuates cardiomyocyte apoptosis and
inflammation via the TLR4/NF-κB/NLRP3 pathway. J Cell Biochem.
121:2994–3004. 2020. View Article : Google Scholar
|
|
109
|
Shao F, Zhou L, Zhang Y, Chen H, Zhang Y
and Guan Z: Gastrodin alleviates inflammatory injury of
cardiomyocytes in septic shock mice via inhibiting NLRP3
expression. In Vitro Cell Dev Biol Anim. 57:571–581. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Khodir AE, Samra YA and Said E: A novel
role of nifuroxazide in attenuation of sepsis-associated acute lung
and myocardial injuries; role of TLR4/NLPR3/IL-1β signaling
interruption. Life Sci. 256:1179072020. View Article : Google Scholar
|
|
111
|
Song P, Shen DF, Meng YY, Kong CY, Zhang
X, Yuan YP, Yan L, Tang QZ and Ma ZG: Geniposide protects against
sepsis-induced myocardial dysfunction through AMPKα-dependent
pathway. Free Radic Biol Med. 152:186–196. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Dai S, Ye B, Chen L, Hong G, Zhao G and Lu
Z: Emodin alleviates LPS-induced myocardial injury through
inhibition of NLRP3 inflammasome activation. Phytother Res.
35:5203–5213. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Du R, Yun Q, Wang Y, Dou X, Ye H, Wang J
and Gao Q: Plumbagin protect against sepsis-induced myocardial
injury in mice by inhibiting the JAK2/STAT3 signaling pathway to
reduce cardiomyocyte pyroptosis. Nan Fang Yi Ke Da Xue Xue Bao.
44:2209–2219. 2024.In Chinese. PubMed/NCBI
|
|
114
|
Joshi S, Kundu S, Priya VV, Kulhari U,
Mugale MN and Sahu BD: Anti-inflammatory activity of carvacrol
protects the heart from lipopolysaccharide-induced cardiac
dysfunction by inhibiting pyroptosis via NLRP3/Caspase1/Gasdermin D
signaling axis. Life Sci. 324:1217432023. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Wang Y, Feng W, Li S, Liu C, Jia L, Wang
P, Li L, Du H and Yu W: Oxycodone attenuates
lipopolysaccharide-induced myocardial injury by inhibiting
inflammation, oxidation and pyroptosis via Nrf2/HO-1 signalling
pathway. Clin Exp Pharmacol Physiol. 51:e139102024. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Wu B, Song H, Fan M, You F, Zhang L, Luo
J, Li J, Wang L, Li C and Yuan M: Luteolin attenuates
sepsis-induced myocardial injury by enhancing autophagy in mice.
Int J Mol Med. 45:1477–1487. 2020.PubMed/NCBI
|
|
117
|
Shiroorkar PN, Afzal O, Kazmi I, Al-Abbasi
FA, Altamimi ASA, Gubbiyappa KS and Sreeharsha N: Cardioprotective
effect of tangeretin by inhibiting PTEN/AKT/mTOR axis in
experimental Sepsis-induced myocardial dysfunction. Molecules.
25:56222020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Cardenas H, Arango D, Nicholas C, Duarte
S, Nuovo GJ, He W, Voss OH, Gonzalez-Mejia ME, Guttridge DC,
Grotewold E and Doseff AI: Dietary apigenin exerts
Immune-regulatory activity in vivo by reducing NF-κB activity,
halting leukocyte infiltration and restoring normal metabolic
function. Int J Mol Sci. 17:3232016. View Article : Google Scholar
|
|
119
|
Li F, Lang F, Zhang H, Xu L, Wang Y, Zhai
C and Hao E: Apigenin alleviates Endotoxin-induced myocardial
toxicity by modulating inflammation, oxidative stress, and
autophagy. Oxid Med Cell Longev. 2017:23028962017. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Chang X, He Y, Wang L, Luo C, Liu Y and Li
R: Puerarin alleviates LPS-induced H9C2 cell injury by inducing
mitochondrial autophagy. J Cardiovasc Pharmacol. 80:600–608. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Tang R, Jia L, Li Y, Zheng J and Qi P:
Narciclasine attenuates sepsis-induced myocardial injury by
modulating autophagy. Aging (Albany NY). 13:15151–15163. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Yuan X, Chen G, Guo D, Xu L and Gu Y:
Polydatin alleviates septic myocardial injury by promoting
SIRT6-mediated autophagy. Inflammation. 43:785–795. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Yu YW, Chen X, Yan JY, Hu J, Huang KY, Ji
KT and Cai HL: Phlorizin, a novel caloric restriction mimetic,
stimulates hypoxia and protects cardiomyocytes through activating
autophagy via modulating the Hif-1α/Bnip3 axis in sepsis-induced
myocardial dysfunction. Int Immunopharmacol. 126:1112412024.
View Article : Google Scholar
|
|
124
|
Zhou B, Zhang J, Chen Y, Liu Y, Tang X,
Xia P, Yu P and Yu S: Puerarin protects against sepsis-induced
myocardial injury through AMPK-mediated ferroptosis signaling.
Aging (Albany NY). 14:3617–3632. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Lin X, Zhao X, Chen Q, Wang X, Wu Y and
Zhao H: Quercetin ameliorates ferroptosis of rat cardiomyocytes via
activation of the SIRT1/p53/SLC7A11 signaling pathway to alleviate
sepsis-induced cardiomyopathy. Int J Mol Med. 52:1162023.
View Article : Google Scholar :
|
|
126
|
Xiao Y, Yu Y, Hu L, Yang Y, Yuan Y, Zhang
W, Luo J and Yu L: Matrine alleviates Sepsis-induced myocardial
injury by inhibiting ferroptosis and apoptosis. Inflammation.
46:1684–1696. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Lin LQ, Mao FK, Lin J, Guo L, Yuan WR and
Wang BY: Ginsenoside Rg1 induces ferroptosis by regulating the
focal adhesion kinase/protein kinase B-forkhead box O3A signaling
pathway and alleviates sepsis-induced myocardial damage. J Physiol
Pharmacol. 75:2024. View Article : Google Scholar
|
|
128
|
Wang X, Simayi A, Fu J, Zhao X and Xu G:
Resveratrol mediates the miR-149/HMGB1 axis and regulates the
ferroptosis pathway to protect myocardium in endotoxemia mice. Am J
Physiol Endocrinol Metab. 323:e21–e32. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Tang R, Jiang M, Tang X, Chen S, Xu H, Pan
Y, Lin B, Wei X, Ye Q, Wu M and Qi P: Narciclasine mitigates
sepsis-induced cardiac dysfunction by enhancing BNIP3-mediated
mitophagy and suppressing ferroptosis. Free Radic Biol Med.
238:220–234. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Zeng Y, Cao G, Lin L, Zhang Y, Luo X, Ma
X, Aiyisake A and Cheng Q: Resveratrol attenuates Sepsis-induced
cardiomyopathy in rats through Anti-Ferroptosis via the Sirt1/Nrf2
pathway. J Invest Surg. 36:21575212023. View Article : Google Scholar
|
|
131
|
Ye H, Wu L, Liu YM, Zhang JX, Hu HT, Dong
ML and Ren J: Wogonin attenuates septic cardiomyopathy by
suppressing ALOX15-mediated ferroptosis. Acta Pharmacol Sin.
46:2407–2422. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Guan F, Du H, Li J, Ren H and Dong A:
Quercetin alleviates LPS-stimulated myocardial injury through
regulating ALOX5/PI3K/AKT pathway in sepsis. Cardiovasc Toxicol.
24:1116–1124. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Huang SH, Xu M, Wu HM, Wan CX, Wang HB, Wu
QQ, Liao HH, Deng W and Tang QZ: Isoquercitrin attenuated cardiac
dysfunction via AMPKα-Dependent pathways in LPS-Treated mice. Mol
Nutr Food Res. 62:e18009552018. View Article : Google Scholar
|
|
134
|
Wei X, Meng X, Yuan Y, Shen F, Li C and
Yang J: Quercetin exerts cardiovascular protective effects in
LPS-induced dysfunction in vivo by regulating inflammatory cytokine
expression, NF-κB phosphorylation, and caspase activity. Mol Cell
Biochem. 446:43–52. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Zhou Q, Zeng X, Kang W, Pan X, Wang L and
Xia Z: Ciprofol attenuates sepsis-induced cardiomyopathy via α7
nicotinic acetylcholine receptor-dependent modulation of myocardial
inflammation and NF-κB/STAT3 signaling. Eur J Pharmacol.
1003:1779832025. View Article : Google Scholar
|
|
136
|
Huang X, Zhang MZ, Liu B, Ma SY, Yin X and
Guo LH: Astragaloside IV Attenuates polymicrobial Sepsis-induced
cardiac dysfunction in rats via IKK/NF-κB pathway. Chin J Integr
Med. 27:825–831. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Chen S and Fan B: Myricetin protects
cardiomyocytes from LPS-induced injury. Herz. 43:265–274. 2018.
View Article : Google Scholar
|
|
138
|
Zhang N, Feng H, Liao HH, Chen S, Yang Z,
Deng W and Tang QZ: Myricetin attenuated LPS induced cardiac injury
in vivo and in vitro. Phytother Res. 32:459–470. 2018. View Article : Google Scholar
|
|
139
|
Xianchu L, Lan PZ, Qiufang L, Yi L,
Xiangcheng R, Wenqi H and Yang D: Naringin protects against
lipopolysaccharide-induced cardiac injury in mice. Environ Toxicol
Pharmacol. 48:1–6. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Sun LJ, Qiao W, Xiao YJ, Cui L, Wang X and
Ren WD: Naringin mitigates myocardial strain and the inflammatory
response in sepsis-induced myocardial dysfunction through
regulation of PI3K/AKT/NF-κB pathway. Int Immunopharmacol.
75:1057822019. View Article : Google Scholar
|
|
141
|
Fang Z, Wang G, Huang R, Liu C,
Yushanjiang F, Mao T and Li J: Astilbin protects from
sepsis-induced cardiac injury through the NRF2/HO-1 and TLR4/NF-κB
pathway. Phytother Res. 38:1044–1058. 2024. View Article : Google Scholar
|
|
142
|
Su Z, Gao M, Weng L and Xu T: Esculin
targets TLR4 to protect against LPS-induced septic cardiomyopathy.
Int Immunopharmacol. 131:1118972024. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Shaojun Z, Yanyan X, Jian C, Xia Z, Qiang
F and Saiping J: Effects of puerarin on lipopolysaccharide-induced
myocardial dysfunction in isolated rat hearts. Pak J Pharm Sci.
30:1195–1202. 2017.PubMed/NCBI
|
|
144
|
Xing C, Xu L and Yao Y: Beneficial role of
oleuropein in sepsis-induced myocardial injury. Possible
Involvement of GSK-3β/NF-κB pathway. Acta Cir Bras. 36:e3601072021.
View Article : Google Scholar
|
|
145
|
Shyni GL, Renjitha J, B Somappa S and
Raghu KG: Zerumin A attenuates the inflammatory responses in
LPS-stimulated H9c2 cardiomyoblasts. J Biochem Mol Toxicol.
35:1–11. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Yan C, Kuang W, Jin L, Wang R, Niu L, Xie
C, Ding J, Liao Y, Wang L, Wan H and Ma G: Carvacrol protects mice
against LPS-induced sepsis and attenuates inflammatory response in
macrophages by modulating the ERK1/2 pathway. Sci Rep.
13:128092023. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Tang J, Hu JJ, Lu CH, Liang JN, Xiao JF,
Liu YT, Lin CS and Qin ZS: Propofol inhibits
lipopolysaccharide-induced tumor necrosis factor-alpha expression
and myocardial depression through decreasing the generation of
superoxide anion in cardiomyocytes. Oxid Med Cell Longev.
2014:1573762014. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Li C, Wan W, Ye T, Sun Y, Chen X, Liu X,
Shi S, Zhang Y, Qu C, Yang B, et al: Pinocembrin alleviates
lipopolysaccharide-induced myocardial injury and cardiac
dysfunction in rats by inhibiting p38/JNK MAPK pathway. Life Sci.
277:1194182021. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Cao W, Li XQ, Zhang XN, Hou Y, Zeng AG,
Xie YH and Wang SW: Madecassoside suppresses LPS-induced TNF-alpha
production in cardiomyocytes through inhibition of ERK, p38, and
NF-kappaB activity. Int Immunopharmacol. 10:723–729. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Wang B, Chen L, Dai L, Fang W and Wang H:
Alisol B 23-Acetate ameliorates Lipopolysaccharide-induced cardiac
dysfunction by suppressing Toll-Like Receptor 4 (TLR4)/NADPH
Oxidase 2 (NOX2) signaling pathway. Med Sci Monit. 25:8472–8481.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Zhang M, Wang X, Wang X, Hou X, Teng P,
Jiang Y, Zhang L, Yang X, Tian J, Li G, et al: Oxymatrine protects
against myocardial injury via inhibition of JAK2/STAT3 signaling in
rat septic shock. Mol Med Rep. 7:1293–1299. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Zhang M, Wang X, Bai B, Zhang R, Li Y and
Wang Y: Oxymatrine protects against sepsis-induced myocardial
injury via inhibition of the TNF-α/p38-MAPK/caspase-3 signaling
pathway. Mol Med Rep. 14:551–559. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Zhao P, Wang Y, Zeng S, Lu J, Jiang TM and
Li YM: Protective effect of astragaloside IV on
lipopolysaccharide-induced cardiac dysfunction via downregulation
of inflammatory signaling in mice. Immunopharmacol Immunotoxicol.
37:428–433. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Zhai J and Guo Y: Paeoniflorin attenuates
cardiac dysfunction in endotoxemic mice via the inhibition of
nuclear factor-κB. Biomed Pharmacother. 80:200–206. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Shang X, Lin K, Yu R, Zhu P, Zhang Y, Wang
L, Xu J and Chen K: Resveratrol protects the myocardium in sepsis
by activating the phosphatidylinositol 3-Kinases
(PI3K)/AKT/Mammalian target of rapamycin (mTOR) pathway and
inhibiting the nuclear Factor-κB (NF-κB) signaling pathway. Med Sci
Monit. 25:9290–9298. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Lee AS, Chen WP, Kuo YL, Ho YJ, Lee SS and
Su MJ: Thaliporphine preserves cardiac function of endotoxemic
rabbits by both directly and indirectly attenuating NFκB signaling
pathway. PLoS One. 7:e391742012. View Article : Google Scholar
|
|
157
|
He H, Chang X, Gao J, Zhu L, Miao M and
Yan T: Salidroside mitigates Sepsis-Induced myocarditis in rats by
regulating IGF-1/PI3K/Akt/GSK-3β signaling. Inflammation.
38:2178–2184. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Li C, Hou D, Huang Y, Liu Y, Li Y and Wang
C: Corylin alleviated sepsis-associated cardiac dysfunction via
attenuating inflammation through downregulation of microRNA-214-5p.
Toxicol Res (Camb). 13:tfae0812024. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Zhang J, Liu Y and Liu L: Hyperoside
prevents sepsis-associated cardiac dysfunction through regulating
cardiomyocyte viability and inflammation via inhibiting miR-21.
Biomed Pharmacother. 138:1115242021. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Liu Y, Liu L and Zhang J: Protective role
of matrine in sepsis-associated cardiac dysfunction through
regulating the lncRNA PTENP1/miR-106b-5p axis. Biomed Pharmacother.
134:1111122021. View Article : Google Scholar
|
|
161
|
Zhao H, Wang Y and Zhu X: Chrysophanol
exerts a protective effect against sepsis-induced acute myocardial
injury through modulating the microRNA-27b-3p/Peroxisomal
proliferating-activated receptor gamma axis. Bioengineered.
13:12673–12690. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Athapaththu A, Lee KT, Kavinda MHD, Lee S,
Kang S, Lee MH, Kang CH, Choi YH and Kim GY: Pinostrobin
ameliorates lipopolysaccharide (LPS)-induced inflammation and
endotoxemia by inhibiting LPS binding to the TLR4/MD2 complex.
Biomed Pharmacother. 156:1138742022. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Feng J, Liu Z, Chen H, Zhang M, Ma X, Han
Q, Lu D and Wang C: Protective effect of cynaroside on
sepsis-induced multiple organ injury through Nrf2/HO-1-dependent
macrophage polarization. Eur J Pharmacol. 911:1745222021.
View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Jinzhong Wang MS and Jian Fu MS:
STAT3/FoxO3a/Sirt1 pathway inhibition by ginsenoside Rc ameliorates
cardiomyocyte damage in septic cardiomyopathy by altering
macrophage polarization. J Mol Histol. 56:1482025. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Li F, Lang F, Wang Y, Zhai C, Zhang C,
Zhang L and Hao E: Cyanidin ameliorates endotoxin-induced
myocardial toxicity by modulating inflammation and oxidative stress
through mitochondria and other factors. Food Chem Toxicol.
120:104–111. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Chen HM, Liou SF, Hsu JH, Chen TJ, Cheng
TL, Chiu CC and Yeh JL: Baicalein inhibits HMGB1 release and
MMP-2/-9 expression in lipopolysaccharide-induced cardiac
hypertrophy. Am J Chin Med. 42:785–797. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Wu W, Wang J, Wang G, Wang F, Yang Y, Liu
Z, Song Q, Chen S and Chen H: Monotropein inhibits MMP9-mediated
cardiac oxidative stress, inflammation, matrix degradation and
apoptosis in a mouse and cell line models of septic cardiac injury.
Mol Biol Rep. 52:3292025. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Cao W, Zhang W, Liu J, Wang Y, Peng X, Lu
D, Qi R, Wang Y and Wang H: Paeoniflorin improves survival in
LPS-challenged mice through the suppression of TNF-α and IL-1β
release and augmentation of IL-10 production. Int Immunopharmacol.
11:172–178. 2011. View Article : Google Scholar
|
|
169
|
Liu A, Xun S, Zhou G, Zhang Y and Lin L:
Honokiol alleviates sepsis-associated cardiac dysfunction via
attenuating inflammation, apoptosis and oxidative stress. J Pharm
Pharmacol. 75:397–406. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Tang X, Xu Y, Dai X, Xing Y, Yang D, Huang
Q, Li H, Lv X, Wang Y, Lu D and Wang H: The Long-term effect of
dobutamine on intrinsic myocardial function and myocardial injury
in septic rats with myocardial dysfunction. Shock. 56:582–592.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Tsai YC, Cheng PY, Kung CW, Peng YJ, Ke
TH, Wang JJ and Yen MH: Beneficial effects of magnolol in a rodent
model of endotoxin shock. Eur J Pharmacol. 641:67–73. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Meng ZJ, Wang C, Meng LT, Bao BH, Wu JH
and Hu YQ: Sodium tanshinone IIA sulfonate attenuates cardiac
dysfunction and improves survival of rats with cecal ligation and
puncture-induced sepsis. Chin J Nat Med. 16:846–855.
2018.PubMed/NCBI
|
|
173
|
Dörtbudak MB, Demircioğlu M and Kapucuk
FS: Micromeria congesta alleviates LPS-Induced inflammation,
apoptosis, oxidative stress and DNA damage in rat heart and
kidneys. Vet Med Sci. 11:e702642025. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Pan J, Meng L, Li R, Wang Z, Yuan W, Li Y,
Chen L, Shen Q, Liu W and Zhu L: Naringenin protects against septic
cardiomyopathy in mice by targeting HIF-1α. Biochem Biophys Res
Commun. 704:1496132024. View Article : Google Scholar
|
|
175
|
Cheng Z, Lv D, Luo M, Wang R, Guo Y, Yang
X, Huang L, Li X, Li C, Shang FF, et al: Tubeimoside I protects
against sepsis-induced cardiac dysfunction via SIRT3. Eur J
Pharmacol. 905:1741862021. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Wang Y, Yu X, Wang F, Wang Y, Wang Y, Li
H, Lv X, Lu D and Wang H: Yohimbine promotes cardiac NE release and
prevents LPS-induced cardiac dysfunction via blockade of
presynaptic α2A-adrenergic receptor. PLoS One. 8:e636222013.
View Article : Google Scholar
|
|
177
|
Duzen IV, Oguz E, Yilmaz R, Taskin A,
Vuruskan E, Cekici Y, Bilgel ZG, Goksuluk H, Candemir B and Sucu M:
Lycopene has a protective effect on septic shock-induced cardiac
injury in rats. Bratisl Lek Listy. 120:919–923. 2019.
|
|
178
|
Ben-Shaul V, Lomnitski L, Nyska A,
Zurovsky Y, Bergman M and Grossman S: The effect of natural
antioxidants, NAO and apocynin, on oxidative stress in the rat
heart following LPS challenge. Toxicol Lett. 123:1–10. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Li X, Zhang Z, Zhang X, Yin Y, Yuan X, You
X and Wu J: Echinacoside prevents Sepsis-induced myocardial damage
via targeting SOD2. J Med Food. 27:123–133. 2024. View Article : Google Scholar
|
|
180
|
Xianchu L, Lan Z, Ming L and Yanzhi M:
Protective effects of rutin on lipopolysaccharide-induced heart
injury in mice. J Toxicol Sci. 43:329–337. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Yang C, Wu K, Li SH and You Q: Protective
effect of curcumin against cardiac dysfunction in sepsis rats.
Pharm Biol. 51:482–487. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Sompamit K, Kukongviriyapan U, Nakmareong
S, Pannangpetch P and Kukongviriyapan V: Curcumin improves vascular
function and alleviates oxidative stress in non-lethal
lipopolysaccharide-induced endotoxaemia in mice. Eur J Pharmacol.
616:192–199. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Kukongviriyapan U, Sompamit K,
Pannangpetch P, Kukongviriyapan V and Donpunha W: Preventive and
therapeutic effects of quercetin on lipopolysaccharide-induced
oxidative stress and vascular dysfunction in mice. Can J Physiol
Pharmacol. 90:1345–1353. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Hao E, Lang F, Chen Y, Zhang H, Cong X,
Shen X and Su G: Resveratrol alleviates endotoxin-induced
myocardial toxicity via the Nrf2 transcription factor. PLoS One.
8:e694522013. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Xingyue L, Shuang L, Qiang W, Jinjuan F
and Yongjian Y: Chrysin ameliorates Sepsis-induced cardiac
dysfunction through upregulating Nfr2/Heme oxygenase 1 pathway. J
Cardiovasc Pharmacol. 77:491–500. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Tan Y, Wan HH, Sun MM, Zhang WJ, Dong M,
Ge W, Ren J and Peng H: Cardamonin protects against
lipopolysaccharide-induced myocardial contractile dysfunction in
mice through Nrf2-regulated mechanism. Acta Pharmacol Sin.
42:404–413. 2021. View Article : Google Scholar :
|
|
187
|
Li Y, Zhang L, Zhang P and Hao Z:
Dehydrocorydaline protects against Sepsis-induced myocardial injury
through modulating the TRAF6/NF-κB pathway. Front Pharmacol.
12:7096042021. View Article : Google Scholar
|
|
188
|
Lee YM, Cheng PY, Chim LS, Kung CW, Ka SM,
Chung MT and Sheu JR: Baicalein, an active component of Scutellaria
baicalensis Georgi, improves cardiac contractile function in
endotoxaemic rats via induction of heme oxygenase-1 and suppression
of inflammatory responses. J Ethnopharmacol. 135:179–185. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Cheng PY, Lee YM, Wu YS, Chang TW, Jin JS
and Yen MH: Protective effect of baicalein against endotoxic shock
in rats in vivo and in vitro. Biochem Pharmacol. 73:793–804. 2007.
View Article : Google Scholar
|
|
190
|
Chen WP, Tzeng HJ, Ku HC, Ho YJ, Lee SS
and Su MJ: Thaliporphine ameliorates cardiac depression in
endotoxemic rats through attenuating TLR4 signaling in the
downstream of TAK-1 phosphorylation and NF-κB signaling. Naunyn
Schmiedebergs Arch Pharmacol. 382:441–453. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Chen RC, Wang J, Yang L, Sun GB and Sun
XB: Protective effects of ginsenoside Re on
lipopolysaccharide-induced cardiac dysfunction in mice. Food Funct.
7:2278–2287. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Chen L, Liu P, Feng X and Ma C:
Salidroside suppressing LPS-induced myocardial injury by inhibiting
ROS-mediated PI3K/Akt/mTOR pathway in vitro and in vivo. J Cell Mol
Med. 21:3178–3189. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Xie L, Zhao M, Zong L and Yue Y: Propofol
ameliorates Sepsis-induced myocardial dysfunction via
Anti-Apoptotic, Anti-Oxidative properties, and mTOR signaling.
Discov Med. 36:2088–2097. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Wang L, Zhao Y, Su Z, Zhao K, Li P and Xu
T: Ginkgolide A targets forkhead box O1 to protect against
lipopolysaccharide-induced septic cardiomyopathy. Phytother Res.
37:3309–3322. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Huang L, Zheng M, Zhou Y, Zhu J, Zhu M,
Zhao F and Cui S: Tanshinone IIA attenuates cardiac dysfunction in
endotoxin-induced septic mice via inhibition of NADPH oxidase
2-related signaling pathway. Int Immunopharmacol. 28:444–449. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Hou D, Liao H, Hao S, Liu R, Huang H and
Duan C: Curcumin simultaneously improves mitochondrial dynamics and
myocardial cell bioenergy after sepsis via the SIRT1-DRP1/PGC-1α
pathway. Heliyon. 10:e285012024. View Article : Google Scholar
|
|
197
|
Smeding L, Leong-Poi H, Hu P, Shan Y,
Haitsma JJ, Horvath E, Furmli S, Masoom H, Kuiper JW, Slutsky AS,
et al: Salutary effect of resveratrol on sepsis-induced myocardial
depression. Crit Care Med. 40:1896–1907. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Peng K, Yang F, Qiu C, Yang Y and Lan C:
Rosmarinic acid protects against lipopolysaccharide-induced cardiac
dysfunction via activating Sirt1/PGC-1α pathway to alleviate
mitochondrial impairment. Clin Exp Pharmacol Physiol. 50:218–227.
2023. View Article : Google Scholar
|
|
199
|
Li Y, Feng YF, Liu XT, Li YC, Zhu HM, Sun
MR, Li P, Liu B and Yang H: Songorine promotes cardiac
mitochondrial biogenesis via Nrf2 induction during sepsis. Redox
Biol. 38:1017712021. View Article : Google Scholar
|
|
200
|
Yang Z, Liu Y, Deng W, Dai J, Li F, Yuan
Y, Wu Q, Zhou H, Bian Z and Tang Q: Hesperetin attenuates
mitochondria-dependent apoptosis in lipopolysaccharide-induced H9C2
cardiomyocytes. Mol Med Rep. 9:1941–1946. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Yang YP, Zhao JQ, Gao HB, Li JJ, Li XL,
Niu XL, Lei YH and Li X: Tannic acid alleviates
lipopolysaccharide-induced H9C2 cell apoptosis by suppressing
reactive oxygen species-mediated endoplasmic reticulum stress. Mol
Med Rep. 24:5352021. View Article : Google Scholar :
|
|
202
|
Xie WJ, Liu M, Zhang X, Zhang YG, Jian ZH
and Xiong XX: Astaxanthin suppresses LPS-induced myocardial
apoptosis by regulating PTP1B/JNK pathway in vitro. Int
Immunopharmacol. 127:1113952024. View Article : Google Scholar
|
|
203
|
Ye G, Wang M, Liu D, Cheng L, Yin X, Zhang
Q and Liu W: Mechanism of naringenin blocking the protection of
LTB4/BLT1 receptor against septic cardiac dysfunction. Ann Clin Lab
Sci. 50:769–774. 2020.PubMed/NCBI
|
|
204
|
Zhao H, Chen Y, Qian L, Du L, Wu X, Tian
Y, Deng C, Liu S, Yang W, Lu C, et al: Lycorine protects against
septic myocardial injury by activating AMPK-related pathways. Free
Radic Biol Med. 197:1–14. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Miao H, Tang X, Cui Y, Shi J, Xiong X,
Wang C and Zhang Y: Obeticholic acid inhibit mitochondria
dysfunction via regulating ERK1/2-DRP pathway to exert protective
effect on lipopolysaccharide-induced myocardial injury. Adv Biol
(Weinh). 8:e23005762024. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Qi Z, Wang R, Liao R, Xue S and Wang Y:
Neferine ameliorates Sepsis-induced myocardial dysfunction through
Anti-apoptotic and antioxidative effects by regulating the
PI3K/AKT/mTOR signaling pathway. Front Pharmacol. 12:7062512021.
View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Liu Z, Pan H, Zhang Y, Zheng Z, Xiao W,
Hong X, Chen F, Peng X, Pei Y, Rong J, et al: Ginsenoside-Rg1
attenuates sepsis-induced cardiac dysfunction by modulating
mitochondrial damage via the P2X7 receptor-mediated Akt/GSK-3β
signaling pathway. J Biochem Mol Toxicol. 36:e228852022. View Article : Google Scholar
|
|
208
|
Zhu X, Sun M, Guo H, Lu G, Gu J, Zhang L,
Shi L, Gao J, Zhang D, Wang W, et al: Verbascoside protects from
LPS-induced septic cardiomyopathy via alleviating cardiac
inflammation, oxidative stress and regulating mitochondrial
dynamics. Ecotoxicol Environ Saf. 233:1133272022. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Lu C, Lei W, Sun M, Wu X, Liu Q, Liu J,
Yang Y, Yang W, Zhang Z, Li X, et al: Identification of CCR2 as a
hub in septic myocardial injury and cardioprotection of silibinin.
Free Radic Biol Med. 197:46–57. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Sun M, Zhao H, Jin Z, Lei W, Deng C, Yang
W, Lu C, Hou Y, Zhang Y, Tang R, et al: Silibinin protects against
sepsis and septic myocardial injury in an NR1H3-dependent pathway.
Free Radic Biol Med. 187:141–157. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Sun HJ, Zheng GL, Wang ZC, Liu Y, Bao N,
Xiao PX, Lu QB and Zhang JR: Chicoric acid ameliorates
sepsis-induced cardiomyopathy via regulating macrophage metabolism
reprogramming. Phytomedicine. 123:1551752024. View Article : Google Scholar
|
|
212
|
Chen M, Huang S, Weng S, Weng J, Guo R,
Shi B and Liu D: Songorine ameliorates LPS-induced sepsis
cardiomyopathy by Wnt/β-catenin signaling pathway-mediated
mitochondrial biosynthesis. Naunyn Schmiedebergs Arch Pharmacol.
397:4713–4725. 2024. View Article : Google Scholar
|
|
213
|
Hwang HR, Tai BY, Cheng PY, Chen PN, Sung
PJ, Wen ZH and Hsu CH: Excavatolide B modulates the
electrophysiological characteristics and calcium homeostasis of
atrial myocytes. Mar Drugs. 15:252017. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Chen R, Zheng A, Wang Y, Guo L, Dou H, Lu
L, Rafiq M, Li P, Chen X and Xiao Q: Salvianolic acid B improves
mitochondrial dysfunction of septic cardiomyopathy via enhancing
ATF5-mediated mitochondrial unfolded protein response. Toxicol Appl
Pharmacol. 491:1170722024. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Nong Y, Lu J, Yu D and Wei X:
Neohesperidin dihydrochalcone alleviates Lipopolysaccharide-induced
vascular endothelium dysfunction by regulating antioxidant
capacity. Immun Inflamm Dis. 12:e701072024. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Tang F, Liu D, Wan F, Zhang L, Xu LY,
Zhang JN, Zhao XL, Ao H and Peng C: Ameliorative effect of
anisodamine (654-1/654-2) against myocardial dysfunction induced by
septic shock via the NF-κB/NLRP-3 or the PI3K-AKT/NF-κB pathway.
Phytomedicine. 123:1552772024. View Article : Google Scholar
|
|
217
|
Chiorcea-Paquim AM: Electrochemistry of
flavonoids: A comprehensive review. Int J Mol Sci. 24:156672023.
View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Billowria K, Ali R, Rangra NK, Kumar R and
Chawla PA: Bioactive flavonoids: A comprehensive review on
pharmacokinetics and analytical aspects. Crit Rev Anal Chem.
54:1002–1016. 2024. View Article : Google Scholar
|
|
219
|
Jomova K, Alomar SY, Valko R, Liska J,
Nepovimova E, Kuca K and Valko M: Flavonoids and their role in
oxidative stress, inflammation, and human diseases. Chem Biol
Interact. 413:1114892025. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Patel S: Plant-derived cardiac glycosides:
Role in heart ailments and cancer management. Biomed Pharmacother.
84:1036–1041. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
de Araújo FF, de Paulo Farias D, Neri-Numa
IA and Pastore GM: Polyphenols and their applications: An approach
in food chemistry and innovation potential. Food Chem.
338:1275352021. View Article : Google Scholar
|
|
222
|
Cinelli MA and Jones AD: Alkaloids of the
genus datura: Review of a rich resource for natural product
discovery. Molecules. 26:26292021. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Jolly A, Hour Y and Lee YC: An outlook on
the versatility of plant saponins: A review. Fitoterapia.
174:1058582024. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Huang S, Liu D, Sun J, Zhang H, Zhang J,
Wang Q, Gan L, Qu G, Qiu J, Deng J, et al: Tim-3 regulates
sepsis-induced immunosuppression by inhibiting the NF-κB signaling
pathway in CD4 T cells. Mol Ther. 30:1227–1238. 2022. View Article : Google Scholar
|
|
225
|
Chen XS, Wang SH, Liu CY, Gao YL, Meng XL,
Wei W, Shou ST, Liu YC and Chai YF: Losartan attenuates
sepsis-induced cardiomyopathy by regulating macrophage polarization
via TLR4-mediated NF-κB and MAPK signaling. Pharmacol Res.
185:1064732022. View Article : Google Scholar
|
|
226
|
Yang Q, Wang Y, Cao G, Li X and Zhao T:
Anti-sepsis effect of Xiaochaihu decoction based on the
TLR4/MyD88/NF-κB signalling pathway. Heliyon. 10:e267122024.
View Article : Google Scholar
|
|
227
|
Huang L, Li Y, Cheng Z, Lv Z, Luo S and
Xia Y: PCSK9 promotes endothelial dysfunction during sepsis via the
TLR4/MyD88/NF-κB and NLRP3 Pathways. Inflammation. 46:115–128.
2023. View Article : Google Scholar
|
|
228
|
Wu Y, Wang Q, Li M, Lao J, Tang H, Ming S,
Wu M, Gong S, Li L, Liu L and Huang X: SLAMF7 regulates the
inflammatory response in macrophages during polymicrobial sepsis. J
Clin Invest. 133:e1502242023. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Al-Kadi A, Anter AF, Rofaeil RR,
Sayed-Ahmed MM, Hafez S and Ahmed AF: Endothelin system blockade
extenuates Sepsis-induced acute heart and kidney injuries via
modulating ET-1/Klotho/p38-MAPK. Clin Exp Pharmacol Physiol.
52:e700422025. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Wang Y, Yu W, Shi C and Hu P: Crocetin
attenuates Sepsis-induced cardiac dysfunction via regulation of
inflammatory response and mitochondrial function. Front Physiol.
11:5142020. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Li J, Wang L, Wang B, Zhang Z, Jiang L,
Qin Z, Zhao Y and Su B: NOX4 is a potential therapeutic target in
septic acute kidney injury by inhibiting mitochondrial dysfunction
and inflammation. Theranostics. 13:2863–2878. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Pan T, Sun S, Chen Y, Tian R, Chen E, Tan
R, Wang X, Liu Z, Liu J and Qu H: Immune effects of
PI3K/Akt/HIF-1α-regulated glycolysis in polymorphonuclear
neutrophils during sepsis. Crit Care. 26:292022. View Article : Google Scholar
|
|
233
|
Ma L, Zhang R, Li D, Qiao T and Guo X:
Fluoride regulates chondrocyte proliferation and autophagy via
PI3K/AKT/mTOR signaling pathway. Chem Biol Interact.
349:1096592021. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Lee J, Kim J, Lee JH, Choi YM, Choi H, Cho
HD, Cha GH, Lee YH, Jo EK, Park BH and Yuk JM: SIRT1 promotes host
protective immunity against toxoplasma gondii by controlling the
FoxO-autophagy axis via the AMPK and PI3K/AKT signalling pathways.
Int J Mol Sci. 23:135782022. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Yin Z, Tian L, Kou W, Cao G, Wang L, Xia
Y, Lin Y, Tang S, Zhang J and Yang H: Xiyangshen Sanqi Danshen
granules attenuated D-gal-induced C57BL/6J mouse aging through the
AMPK/SIRT1 signaling pathway. Phytomedicine. 136:1562132025.
View Article : Google Scholar
|
|
236
|
Chen Y, Chen J, Xing Z, Peng C and Li D:
Autophagy in neuroinflammation: A focus on epigenetic regulation.
Aging Dis. 15:739–754. 2024. View Article : Google Scholar :
|
|
237
|
Lu SM, Yang B, Tan ZB, Wang HJ, Xie JD,
Xie MT, Jiang WH, Huang JZ, Li J, Zhang L, et al: TaoHe ChengQi
decoction ameliorates sepsis-induced cardiac dysfunction through
anti-ferroptosis via the Nrf2 pathway. Phytomedicine.
129:1555972024. View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Tang F, Yan YM, Yan HL, Wang LX, Hu CJ,
Wang HL, Ao H, Peng C and Tan YZ: Chuanxiongdiolides R4 and R5,
phthalide dimers with a complex polycyclic skeleton from the aerial
parts of Ligusticum chuanxiong and their vasodilator activity.
Bioorg Chem. 107:1045232021. View Article : Google Scholar
|
|
239
|
Zhang T, Lu M, Yang Y, Ji X, Gu H, Sun Y,
Chen C and Sun T: Cold-adapted nanozymes. Adv Healthc Mater.
14:e25012112025. View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Li S, Wang F, Hao L, Zhang P, Song G,
Zhang Y, Wang C, Wang Z and Wu Q: Enhancing peroxidase activity of
NiCo2O4 nanoenzyme by Mn doping for catalysis of
CRISPR/Cas13a-mediated non-coding RNA detection. Int J Biol
Macromol. 283:1375942024. View Article : Google Scholar
|
|
241
|
Meng X, Fan K and Yan X: Nanozymes: An
emerging field bridging nanotechnology and enzymology. Sci China
Life Sci. 62:1543–1546. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Cao X, Jiang H, Huang X, Sun D and Qi G:
Hydrogel patch doped with nanoenzyme for SERS detection of hydrogen
peroxide in complex body fluids. Talanta. 285:1273282025.
View Article : Google Scholar
|
|
243
|
Jiang C, Shi Q, Yang J, Ren H, Zhang L,
Chen S, Si J, Liu Y, Sha D, Xu B and Ni J: Ceria nanozyme
coordination with curcumin for treatment of sepsis-induced cardiac
injury by inhibiting ferroptosis and inflammation. J Adv Res.
63:159–170. 2024. View Article : Google Scholar :
|
|
244
|
Li S, Wang K, Jiang K, Xing D, Deng R, Xu
Y, Ding Y, Guan H, Chen LL, Wang D, et al: Brazilin-Ce
nanoparticles attenuate inflammation by de/anti-phosphorylation of
IKKβ. Biomaterials. 305:1224662024. View Article : Google Scholar
|
|
245
|
Mai BT, Fernandes S, Balakrishnan PB and
Pellegrino T: Nanosystems based on magnetic nanoparticles and
thermo\or pH-Responsive polymers: An update and future
perspectives. Acc Chem Res. 51:999–1013. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
246
|
De Jong WH and Borm PJ: Drug delivery and
nanoparticles: Applications and hazards. Int J Nanomedicine.
3:133–149. 2008. View Article : Google Scholar :
|
|
247
|
Wang J, Wang H, Zhu R, Liu Q, Fei J and
Wang S: Anti-inflammatory activity of Curcumin-loaded solid lipid
nanoparticles in IL-1β transgenic mice subjected to the
lipopolysaccharide-induced sepsis. Biomaterials. 53:475–483. 2015.
View Article : Google Scholar
|
|
248
|
Rattis BAC, Piva HL, Duarte A, Gomes
FGFLR, Lellis JR, Soave DF, Ramos SG, Tedesco AC and Celes MRN:
Modulation of the mTOR pathway by curcumin in the heart of septic
mice. Pharmaceutics. 14:22772022. View Article : Google Scholar : PubMed/NCBI
|
|
249
|
Tian B, Hua S and Liu J:
Cyclodextrin-based delivery systems for chemotherapeutic anticancer
drugs: A review. Carbohydr Polym. 232:1158052020. View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Sahu KM, Patra S and Swain SK: Host-guest
drug delivery by β-cyclodextrin assisted polysaccharide vehicles: A
review. Int J Biol Macromol. 240:1243382023. View Article : Google Scholar
|
|
251
|
Heimfarth L, Dos Santos KS, Monteiro BS,
de Souza Oliveira AK, Coutinho HDM, Menezes IRA, Dos Santos MRV, de
Souza Araújo AA, Picot L, de Oliveira Júnior RG, et al: The
protective effects of naringenin, a citrus flavonoid, non-complexed
or complexed with hydroxypropyl-β-cyclodextrin against multiorgan
damage caused by neonatal endotoxemia. Int J Biol Macromol.
264:1305002024. View Article : Google Scholar
|
|
252
|
Penalva R, González-Navarro CJ, Gamazo C,
Esparza I and Irache JM: Zein nanoparticles for oral delivery of
quercetin: Pharmacokinetic studies and preventive anti-inflammatory
effects in a mouse model of endotoxemia. Nanomedicine. 13:103–110.
2017. View Article : Google Scholar
|
|
253
|
Wang S, Tan KS, Beng H, Liu F, Huang J,
Kuai Y, Zhang R and Tan W: Protective effect of isosteviol sodium
against LPS-induced multiple organ injury by regulating of
glycerophospholipid metabolism and reducing macrophage-driven
inflammation. Pharmacol Res. 172:1057812021. View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Liu S, Yao C, Xie J, Liu H, Wang H, Lin Z,
Qin B, Wang D, Lu W, Ma X, et al: Effect of an Herbal-based
injection on 28-Day mortality in patients with sepsis: The EXIT-SEP
randomized clinical trial. JAMA Intern Med. 183:647–655. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Zhang H, Wei L, Zhao G, Liu S, Zhang Z,
Zhang J and Yang Y: Protective effect of Xuebijing injection on
myocardial injury in patients with sepsis: A randomized clinical
trial. J Tradit Chin Med. 36:706–710. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
256
|
Wu X, He C, Liu C, Xu X, Chen C, Yang H,
Shi H, Fei Y, Sun Y, Zhou S and Fang B: Mechanisms of JinHong
Formula on treating sepsis explored by randomized controlled trial
combined with network pharmacology. J Ethnopharmacol.
305:1160402023. View Article : Google Scholar
|
|
257
|
Huang N, Tam YH, Zhang Z, Kao X, Yang Z,
Xu W, Yuan K, He M and Chen J: Efficacy and safety of Dachaihu
decoction for sepsis: A randomized controlled trial. Phytomedicine.
136:1563112025. View Article : Google Scholar
|
|
258
|
Liao J, Qin C, Wang Z, Gao L, Zhang S,
Feng Y, Liu J and Tao L: Effect of shenfu injection in patients
with septic shock: A systemic review and meta-analysis for
randomized clinical trials. J Ethnopharmacol. 320:1174312024.
View Article : Google Scholar
|
|
259
|
Yu Y, Zhu C, Hong Y, Chen L, Huang Z, Zhou
J, Tian X, Liu D, Ren B, Zhang C, et al: Effectiveness of
anisodamine for the treatment of critically ill patients with
septic shock: A multicentre randomized controlled trial. Crit Care.
25:3492021. View Article : Google Scholar : PubMed/NCBI
|
|
260
|
Zhang F, Mei X, Zhou P, Tian YP, Liu JX,
Dong X, Yuan DS, Lin ZF, Zhang L, Lin JH, et al: Anisodamine
hydrobromide in the treatment of critically ill patients with
septic shock: A multi-center randomized controlled trial. Ann Med.
55:22643182023. View Article : Google Scholar
|
|
261
|
Guo X, Luo W, Wu L, Zhang L, Chen Y, Li T,
Li H, Zhang W, Liu Y, Zheng J and Wang Y: Natural products from
herbal medicine Self-Assemble into advanced bioactive materials.
Adv Sci (Weinh). 11:e24033882024. View Article : Google Scholar : PubMed/NCBI
|
|
262
|
Li J, Sun S, Zhu D, Mei X, Lyu Y, Huang K,
Li Y, Liu S, Wang Z, Hu S, et al: Inhalable stem cell exosomes
promote heart repair after myocardial infarction. Circulation.
150:710–723. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
263
|
Yuan Y, Mei Z, Qu Z, Li G, Yu S, Liu Y,
Liu K, Shen Z, Pu J, Wang Y, et al: Exosomes secreted from
cardiomyocytes suppress the sensitivity of tumor ferroptosis in
ischemic heart failure. Signal Transduct Target Ther. 8:1212023.
View Article : Google Scholar : PubMed/NCBI
|
|
264
|
Tirziu D, Giordano FJ and Simons M: Cell
communications in the heart. Circulation. 122:928–937. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
265
|
Hu Y, Chen H, Zhang L, Lin X, Li X, Zhuang
H, Fan H, Meng T, He Z, Huang H, et al: The AMPK-MFN2 axis
regulates MAM dynamics and autophagy induced by energy stresses.
Autophagy. 17:1142–1156. 2021. View Article : Google Scholar :
|
|
266
|
Wu S, Lu Q, Ding Y, Wu Y, Qiu Y, Wang P,
Mao X, Huang K, Xie Z and Zou MH: Hyperglycemia-driven inhibition
of AMP-activated protein kinase α2 induces diabetic cardiomyopathy
by promoting mitochondria-associated endoplasmic reticulum
membranes in vivo. Circulation. 139:1913–1936. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
267
|
Zhou ZK, Yu MM, Shou ST, Chai YF and Liu
YC: Interaction between gut-heart axis in sepsis-induced
cardiomyopathy. Pharmacol Res. 217:1078062025. View Article : Google Scholar : PubMed/NCBI
|
|
268
|
Yuzefpolskaya M, Bohn B, Nasiri M, Zuver
AM, Onat DD, Royzman EA, Nwokocha J, Mabasa M, Pinsino A, Brunjes
D, et al: Gut microbiota, endotoxemia, inflammation, and oxidative
stress in patients with heart failure, left ventricular assist
device, and transplant. J Heart Lung Transplant. 39:880–890. 2020.
View Article : Google Scholar : PubMed/NCBI
|