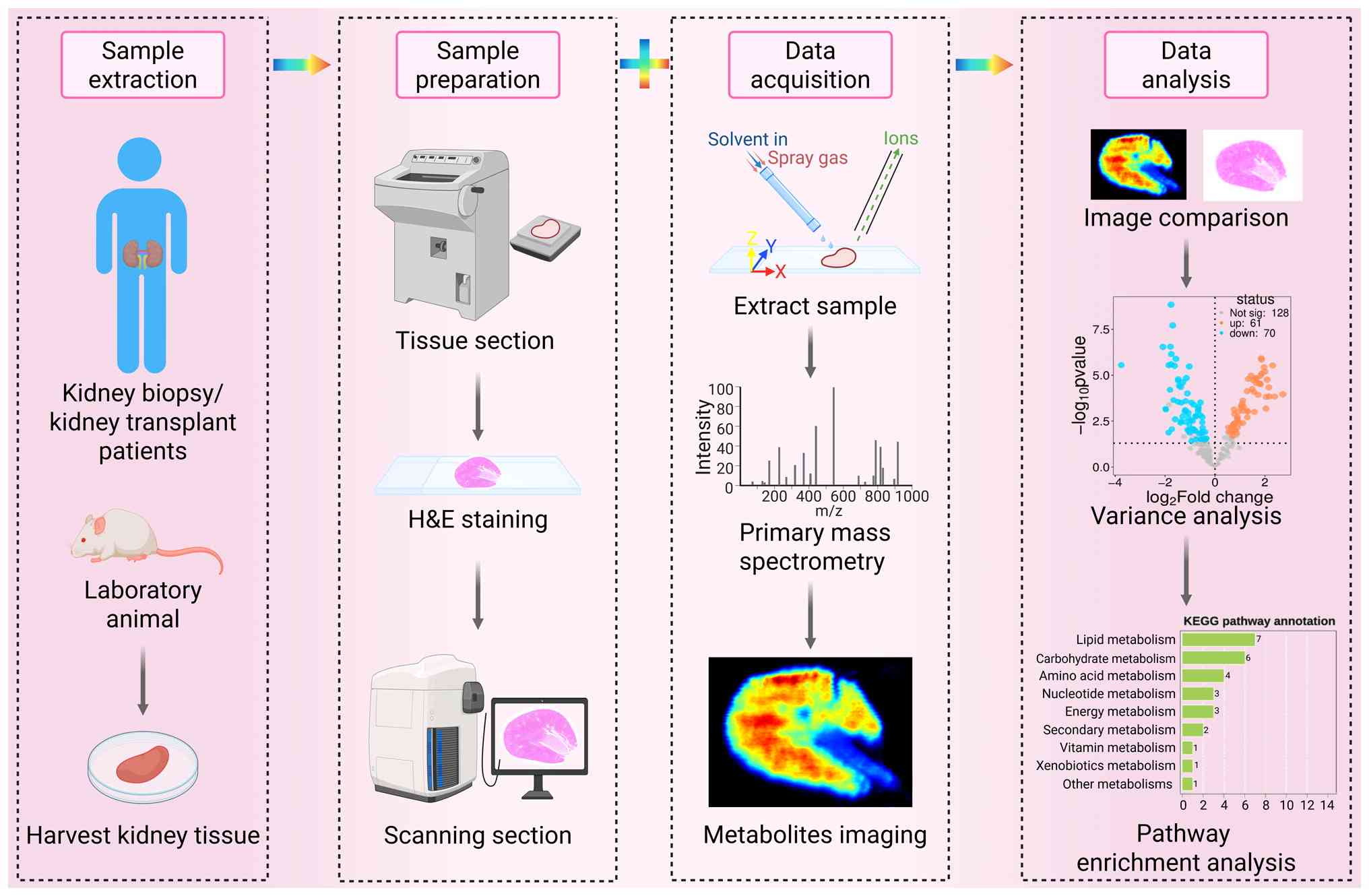
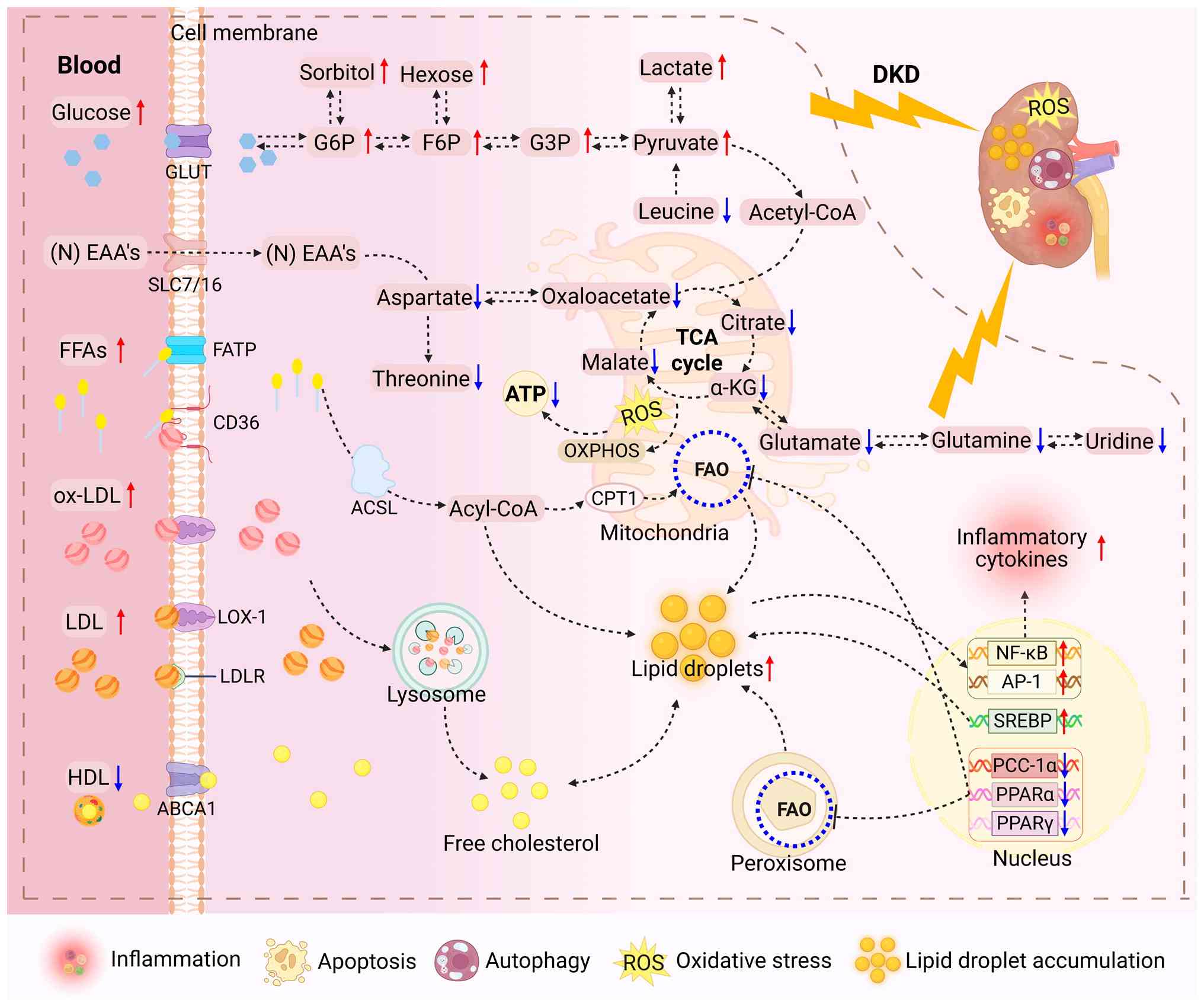
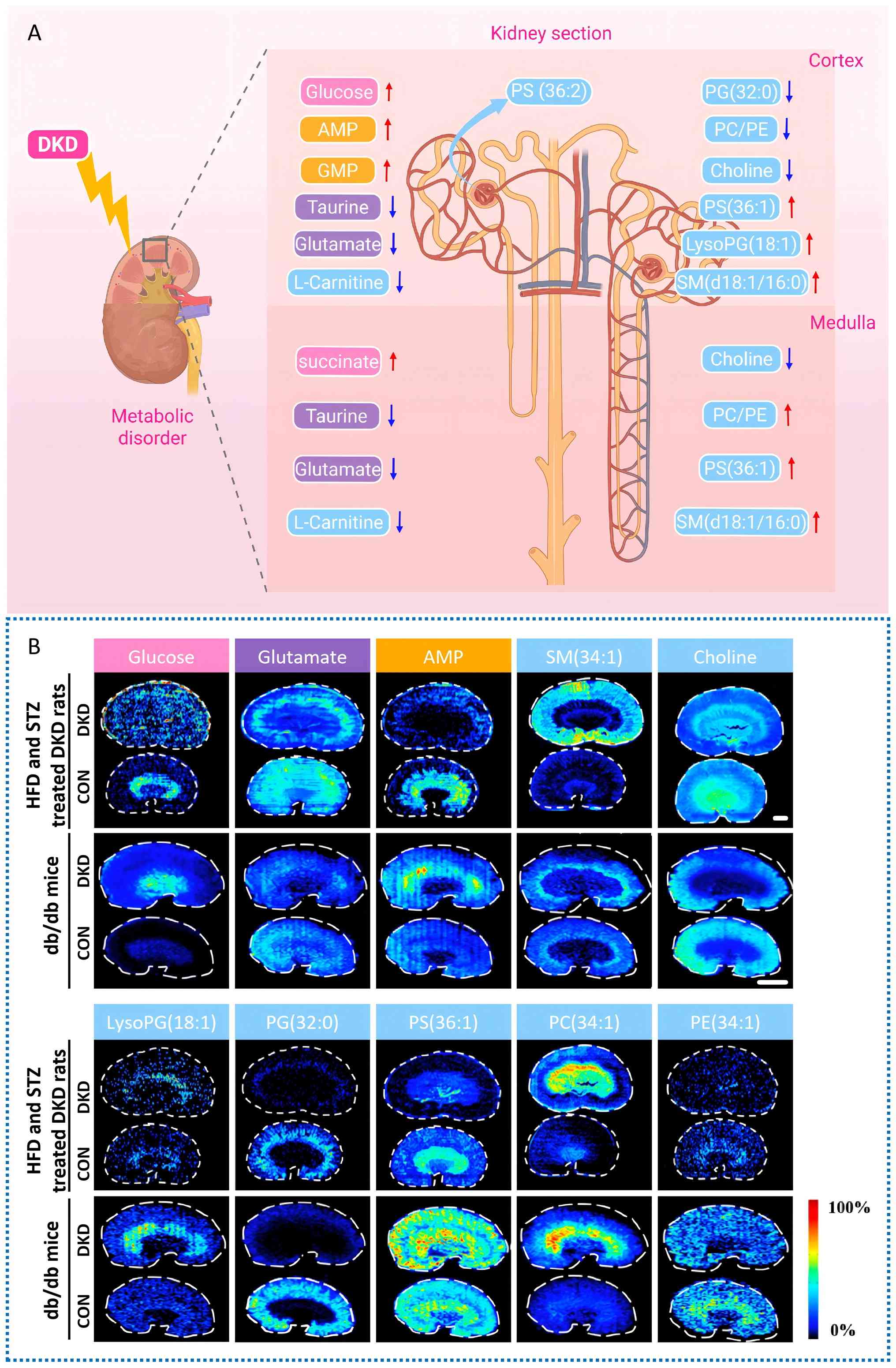
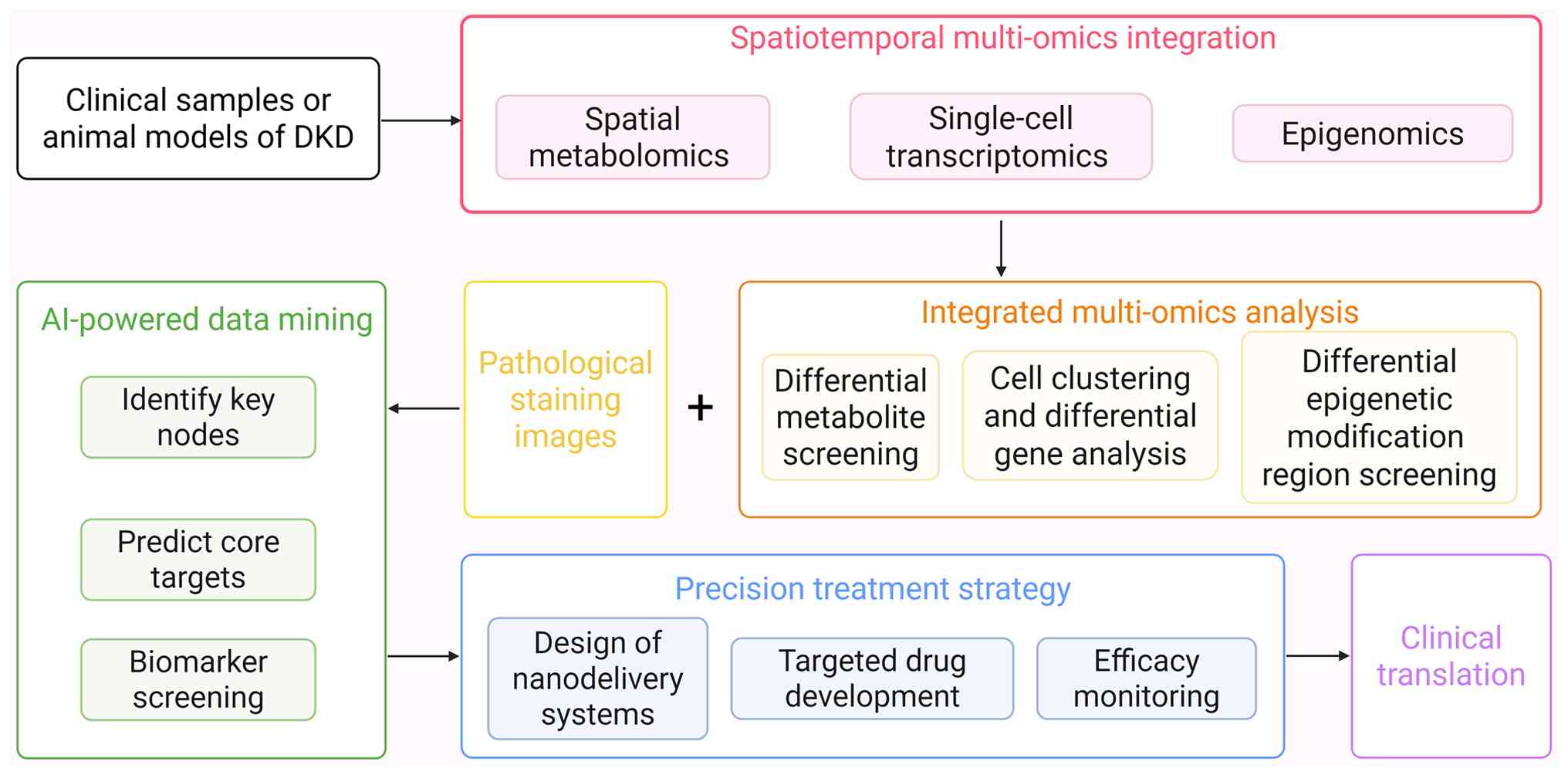
|
1
|
GBD 2021 Diabetes Collaborators: Global,
regional, and national burden of diabetes from 1990 to 2021, with
projections of prevalence to 2050: A systematic analysis for the
Global Burden of Disease Study 2021. Lancet. 402:203–234. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Joumaa JP, Raffoul A, Sarkis C, Chatrieh
E, Zaidan S, Attieh P, Harb F, Azar S and Ghadieh HE: Mechanisms,
biomarkers, and treatment approaches for diabetic kidney disease:
Current insights and future perspectives. J Clin Med. 14:7272025.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Johansen KL, Gilbertson DT, Li SL, Li S,
Liu J, Roetker NS, Ku E, Schulman IH, Greer RC, Chan K, et al: US
renal data system 2023 annual data report: Epidemiology of kidney
disease in the United States. Am J Kidney Dis. 83:A8–A13. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vartak T, Godson C and Brennan E:
Therapeutic potential of pro-resolving mediators in diabetic kidney
disease. Adv Drug Deliv Rev. 178:1139652021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cleveland KH and Schnellmann RG:
Pharmacological targeting of mitochondria in diabetic kidney
disease. Pharmacol Rev. 75:250–262. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tuttle KR, Agarwal R, Alpers CE, Bakris
GL, Brosius FC, Kolkhof P and Uribarri J: Molecular mechanisms and
therapeutic targets for diabetic kidney disease. Kidney Int.
102:248–260. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sharma V, Khokhar M, Panigrahi P, Gadwal
A, Setia P and Purohit P: Advancements, Challenges, and clinical
implications of integration of metabolomics technologies in
diabetic nephropathy. Clin Chim Acta. 561:1198422024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rayego-Mateos S, Rodrigues-Diez RR,
Fernandez-Fernandez B, Mora-Fernández C, Marchant V, Donate-Correa
J, Navarro-González JF, Ortiz A and Ruiz-Ortega M: Targeting
inflammation to treat diabetic kidney disease: The road to 2030.
Kidney Int. 103:282–296. 2023. View Article : Google Scholar
|
|
9
|
Xu F, Jiang H, Li X, Pan J, Li H, Wang L,
Zhang P, Chen J, Qiu S, Xie Y, et al: Discovery of PRDM16-Mediated
TRPA1 induction as the mechanism for low Tubulo-interstitial
fibrosis in diabetic kidney disease. Adv Sci (Weinh).
11:e23067042024. View Article : Google Scholar
|
|
10
|
Liu D, Chen X, He W, Lu M, Li M, Zhang S,
Xie J, Zhang Y and Wang W: Update on the pathogenesis, diagnosis,
and treatment of diabetic tubulopathy. Integrat Med Nephrol Androl.
11:e23–00029. 2024.
|
|
11
|
Empitu MA, Rinastiti P and
Kadariswantiningsih IN: Targeting endothelin signaling in podocyte
injury and diabetic nephropathy-diabetic kidney disease. J Nephrol.
38:49–60. 2025. View Article : Google Scholar
|
|
12
|
Muthubharathi BC, Gowripriya T and
Balamurugan K: Metabolomics: Small molecules that matter more. Mol
Omics. 17:210–229. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou M, Sun W, Gao Y, Jiang B, Sun T, Xu
R, Zhang X, Wang Q, Xuan Q and Ma S: Metabolomic profiling reveals
interindividual metabolic variability and its association with
cardiovascular-kidney-metabolic syndrome risk. Cardiovasc Diabetol.
24:3152025. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barovic M, Hahn JJ, Heinrich A, Adhikari
T, Schwarz P, Mirtschink P, Funk A, Kabisch S, Pfeiffer AFH, Blüher
M, et al: Proteomic and metabolomic signatures in prediabetes
progressing to diabetes or reversing to normoglycemia within 1
year. Diabetes Care. 48:405–415. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pereira PR, Carrageta DF, Oliveira PF,
Rodrigues A, Alves MG and Monteiro MP: Metabolomics as a tool for
the early diagnosis and prognosis of diabetic kidney disease. Med
Res Rev. 42:1518–1544. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Md Dom ZI, Moon S, Satake E, Hirohama D,
Palmer ND, Lampert H, Ficociello LH, Abedini A, Fernandez K, Liang
X, et al: Urinary Complement proteome strongly linked to diabetic
kidney disease progression. Nat Commun. 16:72912025. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alexandrov T: Spatial metabolomics and
imaging mass spectrometry in the age of artificial intelligence.
Annu Rev Biomed Data Sci. 3:61–87. 2020. View Article : Google Scholar
|
|
18
|
Sharma K, Hansen J, Susztak K, Eberlin L,
Anderton CR, Alexandrov T and Iyengar R: Spatial metabolomics and
multiomics integration for breakthroughs in precision medicine for
kidney disease. Nat Rev Nephrol. Oct 9–2025. View Article : Google Scholar : Epub ahead of
print. PubMed/NCBI
|
|
19
|
Najumudeen AK and Vande voorde J: Spatial
metabolomics to unravel cellular metabolism. Nat Rev Genet.
26:2282025. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Allam M and Coskun AF: Combining spatial
metabolomics and proteomics profiling of single cells. Nat Rev
Immunol. 24:7012024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun N, Krauss T, Seeliger C, Kunzke T,
Stöckl B, Feuchtinger A, Zhang C, Voss A, Heisz S, Prokopchuk O, et
al: Inter-organ cross-talk in human cancer cachexia revealed by
spatial metabolomics. Metabolism. 161:1560342024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jia G, He P, Dai T, Goh D, Wang J, Sun M,
Wee F, Li F, Lim JCT, Hao S, et al: Spatial immune scoring system
predicts hepatocellular carcinoma recurrence. Nature.
640:1031–1041. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang X, Li T, Zhou Y, Wang X, Dan Z,
Huang J and He J: A new direction in metabolomics: Analysis of the
central nervous system based on spatially resolved metabolomics.
TrAC Trends Analytical Chemist. 165:1171032023. View Article : Google Scholar
|
|
24
|
Miller A, York EM, Stopka SA,
Martínez-François JR, Hossain MA, Baquer G, Regan MS, Agar NYR and
Yellen G: Spatially resolved metabolomics and isotope tracing
reveal dynamic metabolic responses of dentate granule neurons with
acute stimulation. Nat Metab. 5:1820–1835. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dean DA, Klechka L, Hossain E, Parab AR,
Eaton K, Hinsdale M and McCall LI: Spatial metabolomics reveals
localized impact of influenza virus infection on the lung tissue
metabolome. mSystems. 7:e00353222022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu X, Liu Z and Sun X: Single-cell and
spatial multi-omics in the plant sciences: Technical advances,
applications, and perspectives. Plant Commun. 4:1005082023.
View Article : Google Scholar :
|
|
27
|
Wang X, Zhang J, Zheng K, Du Q, Wang G,
Huang J, Zhou Y, Li Y, Jin H and He J: Discovering metabolic
vulnerability using spatially resolved metabolomics for antitumor
small molecule-drug conjugates development as a precise cancer
therapy strategy. J Pharm Anal. 13:776–787. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bag S, Oetjen J, Shaikh S, Chaudhary A,
Arun P and Mukherjee G: Impact of spatial metabolomics on
immune-microenvironment in oral cancer prognosis: A clinical
report. Mol Cell Biochem. 479:41–49. 2024. View Article : Google Scholar
|
|
29
|
He T, Lin K, Xiong L, Zhang W, Zhang H,
Duan C, Li X and Zhang J: Disorder of phospholipid metabolism in
the renal cortex and medulla contributes to acute tubular necrosis
in mice after cantharidin exposure using integrative lipidomics and
spatial metabolomics. J Pharm Anal. 15:1012102025. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qiu S, Wang Z, Wang X, Guo S, Cai Y, Xie
D, Hu Z, Wang S, Yang Q and Zhang A: Spatial metabolomics
identifies riboflavin metabolism as a therapeutic target of Huangqi
Guizhi Wuwu decoction in diabetic nephropathy. Biomed Chromatogr.
39:e702392025. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Z, Fu W, Huo M, He B, Liu Y, Tian L,
Li W, Zhou Z, Wang B, Xia J, et al: Spatial-resolved metabolomics
reveals tissue-specific metabolic reprogramming in diabetic
nephropathy by using mass spectrometry imaging. Acta Pharm Sin B.
11:3665–3677. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Min X, Zhao Y, Yu M, Zhang W, Jiang X, Guo
K, Wang X, Huang J, Li T, Sun L and He J: Spatially resolved
metabolomics: From metabolite mapping to function visualizing. Clin
Transl Med. 14:e700312024. View Article : Google Scholar
|
|
33
|
Tuck M, Grélard F, Blanc L and Desbenoit
N: MALDI-MSI towards multimodal imaging: Challenges and
perspectives. Front Chem. 10:9046882022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kumar BS: Desorption electrospray
ionization mass spectrometry imaging (DESI-MSI) in disease
diagnosis: An overview. Anal Methods. 15:3768–3784. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang S, Wang Z, Liu Y, Zhang X, Zhang H,
Wang Z, Zhou Z and Abliz Z: Dual mass spectrometry imaging and
spatial metabolomics to investigate the metabolism and
nephrotoxicity of nitidine chloride. J Pharm Anal. 14:1009442024.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Song X, Zang Q, Li C, Zhou T and Zare RN:
Immuno-desorption electrospray ionization mass spectrometry imaging
identifies functional macromolecules by using
Microdroplet-cleavable mass tags. Angew Chem Int Ed Engl.
62:e2022169692023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lockyer NP, Aoyagi S, Fletcher JS, Gilmore
I, van der heide P, Moore KL, Tyler BJ and Weng LT: Secondary ion
mass spectrometry. Nat Rev Methods Primers. 4:322024. View Article : Google Scholar
|
|
38
|
Coello Y, Jones AD, Gunaratne TC and
Dantus M: Atmospheric pressure femtosecond laser imaging mass
spectrometry. Anal Chem. 82:2753–2758. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen H, Durand S, Bawa O, Bourgin M,
Montégut L, Lambertucci F, Motiño O, Li S, Nogueira-Recalde U,
Anagnostopoulos G, et al: Biomarker identification in liver cancers
using desorption electrospray ionization mass spectrometry
(DESI-MS) imaging: An approach for spatially resolved metabolomics.
Methods Mol Biol. 2769:199–209. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
He MJ, Pu W, Wang X, Zhong X, Zhao D, Zeng
Z, Cai W, Liu J, Huang J, Tang D and Dai Y: Spatial metabolomics on
liver cirrhosis to hepatocellular carcinoma progression. Cancer
Cell Int. 22:3662022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lakkimsetty SS, Weber A, Bemis KA, Stehl
V, Bronsert P, Föll MC and Vitek O: MSIreg: An R package for
unsupervised coregistration of mass spectrometry and H&E
images. Bioinformatics. 40:btae6242024. View Article : Google Scholar
|
|
42
|
Zickuhr GM, Um IH, Laird A, Harrison DJ
and Dickson AL: DESI-MSI-guided exploration of metabolic-phenotypic
relationships reveals a correlation between PI 38:3 and
proliferating cells in clear cell renal cell carcinoma via
single-section co-registration of multimodal imaging. Anal Bioanal
Chem. 416:4015–4028. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang G, Heijs B, Kostidis S, Rietjens RGJ,
Koning M, Yuan L, Tiemeier GL, Mahfouz A, Dumas SJ, Giera M, et al:
Spatial dynamic metabolomics identifies metabolic cell fate
trajectories in human kidney differentiation. Cell Stem Cell.
29:1580–1593.e7. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang G, Heijs B, Kostidis S, Mahfouz A,
Rietjens RGJ, Bijkerk R, Koudijs A, van der Pluijm LAK, van den
Berg CW, Dumas SJ, et al: Analyzing cell-type-specific dynamics of
metabolism in kidney repair. Nat Metab. 4:1109–1118. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lin J, Lin H, Li C, Liao N, Zheng Y, Yu X,
Sun Y and Wu L: Unveiling characteristic metabolic accumulation
over enzymatic-catalyzed process of Tieguanyin oolong tea
manufacturing by DESI-MSI and multiple-omics. Food Res Int.
181:1141362024. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Banerjee S, Wong AC, Yan X, Wu B, Zhao H,
Tibshirani RJ, Zare RN and Brooks JD: Early detection of unilateral
ureteral obstruction by desorption electrospray ionization mass
spectrometry. Sci Rep. 9:110072019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Qi K, Wu L, Liu C and Pan Y: Recent
advances of ambient mass spectrometry imaging and its applications
in lipid and metabolite analysis. Metabolites. 11:7802021.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Blanc T, Goudin N, Zaidan M, Traore MG,
Bienaime F, Turinsky L, Garbay S, Nguyen C, Burtin M, Friedlander
G, et al: Three-dimensional architecture of nephrons in the normal
and cystic kidney. Kidney Int. 99:632–645. 2021. View Article : Google Scholar
|
|
49
|
Li H, Li D and Humphreys BD: Chromatin
conformation and histone modification profiling across human kidney
anatomic regions. Sci Data. 11:7972024. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang SY and Mahler GJ: A glomerulus and
proximal tubule microphysiological system simulating renal
filtration, reabsorption, secretion, and toxicity. Lab Chip.
23:272–284. 2023. View Article : Google Scholar
|
|
51
|
Fan G, Jiang C, Huang Z, Tian M, Pan H,
Cao Y, Lei T, Luo Q and Yuan J: 3D autofluorescence imaging of
hydronephrosis and renal anatomical structure using
cryo-micro-optical sectioning tomography. Theranostics.
13:4885–4904. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hinze C, Karaiskos N, Boltengagen A,
Walentin K, Redo K, Himmerkus N, Bleich M, Potter SS, Potter AS,
Eckardt KU, et al: Kidney Single-cell transcriptomes predict
spatial corticomedullary gene expression and tissue osmolality
gradients. J Am Soc Nephrol. 32:291–306. 2021. View Article : Google Scholar :
|
|
53
|
Gao G, Sumrall ES, Pitchiaya S, Bitzer M,
Alberti S and Walter NG: Biomolecular condensates in kidney
physiology and disease. Nat Rev Nephrol. 19:756–770. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ding Y, Zhao F, Hu J, Zhao Z, Shi B and Li
S: A conjoint analysis of renal structure and omics characteristics
reveal new insight to yak high-altitude hypoxia adaptation.
Genomics. 116:1108572024. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gurung RL, Zheng H, Tan JLI, Liu S, Chan
C, Ang K, Tan C, Liu JJ, Subramaniam T, Sum CF and Lim SC:
Integrative metabolomic and proteomic analysis of diabetic kidney
disease progression with younger-onset type 2 diabetes. Diabetes
Obes Metab. 27:7454–7463. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jiang X, Liu X, Qu X, Zhu P, Wo F, Xu X,
Jin J, He Q and Wu J: Integration of metabolomics and peptidomics
reveals distinct molecular landscape of human diabetic kidney
disease. Theranostics. 13:3188–3203. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Fan X, Yang M, Lang Y, Lu S, Kong Z, Gao
Y, Shen N, Zhang D and Lv Z: Mitochondrial metabolic reprogramming
in diabetic kidney disease. Cell Death Dis. 15:4422024. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li S and Susztak K: Mitochondrial
dysfunction has a central role in diabetic kidney disease. Nat Rev
Nephrol. 21:77–78. 2025. View Article : Google Scholar
|
|
59
|
Poorna R, Chen WW, Qiu P and Cicerone MT:
Toward Gene-correlated spatially resolved metabolomics with
fingerprint coherent Raman imaging. J Phys Chem B. 127:5576–5587.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Alexandrov T: Spatial metabolomics: From a
niche field towards a driver of innovation. Nat Metabolism.
5:1443–1445. 2023. View Article : Google Scholar
|
|
61
|
Zhang J, Wu T, Li C and Du J: A
glycopolymersome strategy for 'drug-free' treatment of diabetic
nephropathy. J Control Release. 372:347–361. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Luo A, Wang R, Gong J, Wang S, Yun C, Chen
Z, Jiang Y, Liu X, Dai H, Liu H and Zheng Y: Syntaxin 17
translocation mediated mitophagy switching drives
hyperglycemia-induced vascular injury. Adv Sci (Weinh).
12:e24149602025. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Rebelos E, Mari A, Oikonen V, Iida H,
Nuutila P and Ferrannini E: Evaluation of renal glucose uptake with
[18F] FDG-PET: Methodological advancements and metabolic
outcomes. Metabolism. 141:1553822023. View Article : Google Scholar
|
|
64
|
Liu X, Du H, Sun Y and Shao L: Role of
abnormal energy metabolism in the progression of chronic kidney
disease and drug intervention. Ren Fail. 44:790–805. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhang G, Zhang J, DeHoog RJ, Pennathur S,
Anderton CR, Venkatachalam MA, Alexandrov T, Eberlin LS and Sharma
K: DESI-MSI and METASPACE indicates lipid abnormalities and altered
mitochondrial membrane components in diabetic renal proximal
tubules. Metabolomics. 16:112020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhang X, Liu Y, Yang S, Gao X, Wang S,
Wang Z, Zhang C, Zhou Z, Chen Y, Wang Z and Abliz Z: Comparison of
local metabolic changes in diabetic rodent kidneys using mass
spectrometry imaging. Metabolites. 13:3242023. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhang G, Liu L, Tamayo IM, De Leon NGP,
Vigers TB, Tommerdahl KL, Nelson RG, Ladd PE, Alexandrov T,
Birznieks C, et al: 406-P: Spatial metabolomics of human kidney
tissues reveal impaired tricarboxylic acid (TCA) cycle turnover in
type 1 Diabetes (T1D). Diabetes. 72:4062023. View Article : Google Scholar
|
|
68
|
Qi W, Keenan HA, Li Q, Ishikado A, Kannt
A, Sadowski T, Yorek MA, Wu IH, Lockhart S, Coppey LJ, et al:
Pyruvate kinase M2 activation may protect against the progression
of diabetic glomerular pathology and mitochondrial dysfunction. Nat
Med. 23:753–762. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hasegawa S and Inagi R: Harnessing
metabolomics to describe the pathophysiology underlying progression
in diabetic kidney disease. Curr Diab Rep. 21:212021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Murphy DP, Wolfson J, Reule S, Johansen
KL, Ishani A and Drawz PE: A cohort study of sodium-glucose
cotransporter-2 inhibitors after acute kidney injury among Veterans
with diabetic kidney disease. Kidney Int. 106:126–135. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wang Y, Liu T, Wu Y, Wang L, Ding S, Hou
B, Zhao H, Liu W and Li P: Lipid homeostasis in diabetic kidney
disease. Int J Biol Sci. 20:3710–3724. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Mitrofanova A, Merscher S and Fornoni A:
Kidney lipid dysmetabolism and lipid droplet accumulation in
chronic kidney disease. Nat Rev Nephrol. 19:629–645. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Iizuka K: Commentary: Comprehensive
lipidome profiling of the kidney in early-stage diabetic
nephropathy. Front Endocrinol (Lausanne). 13:10153052022.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhang YR, Piao HL and Chen D:
Identification of spatial specific lipid metabolic signatures in
Long-standing diabetic kidney disease. Metabolites. 14:6412024.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hao Y, Fan Y, Feng J, Zhu Z, Luo Z, Hu H,
Li W, Yang H and Ding G: ALCAT1-mediated abnormal cardiolipin
remodelling promotes mitochondrial injury in podocytes in diabetic
kidney disease. Cell Commun Signal. 22:262024. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Grove KJ, Voziyan PA, Spraggins JM, Wang
S, Paueksakon P, Harris RC, Hudson BG and Caprioli RM: Diabetic
nephropathy induces alterations in the glomerular and tubule lipid
profiles. J Lipid Res. 55:1375–1385. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
McCrimmon A, Corbin S, Shrestha B, Roman
G, Dhungana S and Stadler K: Redox phospholipidomics analysis
reveals specific oxidized phospholipids and regions in the diabetic
mouse kidney. Redox Biol. 58:1025202022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Vianello E, Ambrogi F, Kalousová M,
Badalyan J, Dozio E, Tacchini L, Schmitz G, Zima T, Tsongalis GJ
and Corsi-Romanelli MM: Circulating perturbation of
phosphatidylcholine (PC) and phosphatidylethanolamine (PE) is
associated to cardiac remodeling and NLRP3 inflammasome in
cardiovascular patients with insulin resistance risk. Exp Mol
Pathol. 137:1048952024. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wunderling K, Zurkovic J, Zink F,
Kuerschner L and Thiele C: Triglyceride cycling enables
modification of stored fatty acids. Nat Metab. 5:699–709. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Darshi M, Tumova J, Saliba A, Kim J, Baek
J, Pennathur S and Sharma K: Crabtree effect in kidney proximal
tubule cells via late-stage glycolytic intermediates. Iscience.
26:1064622023. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Hall AM: Protein handling in kidney
tubules. Nat Rev Nephrol. 21:241–252. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Panov AV, Mayorov VI, Dikalova AE and
Dikalov SI: Long-Chain and Medium-Chain fatty acids in energy
metabolism of murine kidney mitochondria. Int J Mol Sci.
24:3792023. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Li H, Li D, Ledru N, Xuanyuan Q, Wu H,
Asthana A, Byers LN, Tullius SG, Orlando G, Waikar SS and Humphreys
BD: Transcriptomic, epigenomic, and spatial metabolomic cell
profiling redefines regional human kidney anatomy. Cell Metab.
36:1105–1125.e10. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Kang HM, Ahn SH, Choi P, Ko YA, Han SH,
Chinga F, Park AS, Tao J, Sharma K, Pullman J, et al: Defective
fatty acid oxidation in renal tubular epithelial cells has a key
role in kidney fibrosis development. Nat Med. 21:37–46. 2015.
View Article : Google Scholar
|
|
85
|
Li C, Gao L, Lv C, Li Z, Fan S, Liu X,
Rong X, Huang Y and Liu J: Active role of amino acid metabolism in
early diagnosis and treatment of diabetic kidney disease. Front
Nutr. 10:12398382023. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Liu L, Xu J, Zhang Z, Ren D, Wu Y, Wang D,
Zhang Y, Zhao S, Chen Q and Wang T: Metabolic homeostasis of amino
acids and diabetic kidney disease. Nutrients. 15:1842023.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Linnan B, Yanzhe W, Ling Z, Yuyuan L,
Sijia C, Xinmiao X, Fengqin L and Xiaoxia W: In situ metabolomics
of metabolic reprogramming involved in a mouse model of type 2
diabetic kidney disease. Front Physiol. 12:7796832021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Han J, Li P, Sun H, Zheng Y, Liu C, Chen
X, Guan S, Yin F and Wang X: Integrated metabolomics and mass
spectrometry imaging analysis reveal the efficacy and mechanism of
Huangkui capsule on type 2 diabetic nephropathy. Phytomedicine.
138:1563972025. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Castoldi F, Kroemer G and Pietrocola F:
Spermidine rejuvenates T lymphocytes and restores anticancer
immunosurveillance in aged mice. Oncoimmunology. 11:21468552022.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zou D, Zhao Z, Li L, Min Y, Zhang D, Ji A,
Jiang C, Wei X and Wu X: A comprehensive review of spermidine:
Safety, health effects, absorption and metabolism, food materials
evaluation, physical and chemical processing, and bioprocessing.
Compr Rev Food Sci Food Saf. 21:2820–2842. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Aihara S, Torisu K, Uchida Y, Imazu N,
Nakano T and Kitazono T: Spermidine from arginine metabolism
activates Nrf2 and inhibits kidney fibrosis. Commun Biol.
6:6762023. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Li X, Zhou X, Liu X, Li X, Jiang X, Shi B
and Wang S: Spermidine protects against acute kidney injury by
modulating macrophage NLRP3 inflammasome activation and
mitochondrial respiration in an eIF5A hypusination-related pathway.
Mol Med. 28:1032022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Jia M, Lin L, Xun K, Li D, Wu W, Sun S,
Qiu H and Jin D: Indoxyl sulfate aggravates podocyte damage through
the TGF-β1/Smad/ROS signalling pathway. Kidney Blood Press Res.
49:385–396. 2024.
|
|
94
|
Zhao T, Zhang H, Yin X, Zhao H, Ma L, Yan
M, Peng L, Wang Q, Dong X and Li P: Tangshen formula modulates gut
Microbiota and reduces gut-derived toxins in diabetic nephropathy
rats. Biomed Pharmacother. 129:1103252020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Hejazi L, Sharma S, Ruiz A, Zhang G, Tucci
FC and Sharma K: Spatial metabolomics analysis by MSI-DeepPath
identifies key pathways in ZDF diabetic kidney disease model.
Diabetes. 72(Suppl 1): 400–P. 2023. View Article : Google Scholar
|
|
96
|
Varadaiah YGC, Sivanesan S, Nayak SB and
Thirumalarao KR: Purine metabolites can indicate diabetes
progression. Arch Physiol Biochem. 128:87–91. 2022. View Article : Google Scholar
|
|
97
|
Zubaidi SN, Wong PL, Qadi WSM, Dawoud EAD,
Hamezah HS, Baharum SN, Jam FA, Abas F, Moreno A and Mediani A:
Deciphering the mechanism of Annona muricata leaf extract in
alloxan-nicotinamide-induced diabetic rat model with 1H-NMR-based
metabolomics approach. J Pharm Biomed Anal. 260:1168062025.
View Article : Google Scholar
|
|
98
|
Shen XL, Liu H, Xiang H, Qin XM, Du GH and
Tian JS: Combining biochemical with (1)H NMR-based metabolomics
approach unravels the antidiabetic activity of genipin and its
possible mechanism. J Pharm Biomed Anal. 129:80–89. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Yuan Y, Huang L, Yu L, Yan X, Chen S, Bi
C, He J, Zhao Y, Yang L, Ning L, et al: Clinical metabolomics
characteristics of diabetic kidney disease: A meta-analysis of 1875
cases with diabetic kidney disease and 4503 controls. Diabetes
Metab Res Rev. 40:e37892024. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Jung I, Nam S, Lee DY, Park SY, Yu JH, Seo
JA, Lee DH and Kim NH: Association of succinate and adenosine
nucleotide metabolic pathways with diabetic kidney disease in
patients with type 2 diabetes mellitus. Diabetes Metab J.
48:1126–1134. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Mohandes S, Doke T, Hu H, Mukhi D, Dhillon
P and Susztak K: Molecular pathways that drive diabetic kidney
disease. J Clin Invest. 133:e1656542023. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Sharma K, Zhang GS, Hansen J, Bjornstad P,
Lee HJ, Menon R, Hejazi L, Liu JJ, Franzone A, Looker HC, et al:
Endogenous adenine mediates kidney injury in diabetic models and
predicts diabetic kidney disease in patients. J Clin Invest.
133:e1703412023. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Ragi N and Sharma K: Deliverables from
metabolomics in kidney disease: Adenine, new insights, and
implication for clinical Decision-making. Am J Nephrol. 55:421–438.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Drexler Y and Fornoni A: Adenine crosses
the biomarker bridge: From 'omics to treatment in diabetic kidney
disease. J Clin Invest. 133:e1740152023. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Hocher B and Adamski J: Metabolomics for
clinical use and research in chronic kidney disease. Nat Rev
Nephrol. 13:269–284. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Efiong EE, Maedler K, Effa E, Osuagwu UL,
Peters E, Ikebiuro JO, Soremekun C, Ihediwa U, Niu J, Fuchs M, et
al: Decoding diabetic kidney disease: A comprehensive review of
interconnected pathways, molecular mediators, and therapeutic
insights. Diabetol Metab Syndr. 17:1922025. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Sinha SK and Nicholas SB: Pathomechanisms
of diabetic kidney disease. J Clin Med. 12:73492023. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Huynh C, Ryu J, Lee J, Inoki A and Inoki
K: Nutrient-sensing mTORC1 and AMPK pathways in chronic kidney
diseases. Nat Rev Nephrol. 19:102–122. 2023. View Article : Google Scholar
|
|
109
|
Zhang J, Fuhrer T, Ye H, Kwan B,
Montemayor D, Tumova J, Darshi M, Afshinnia F, Scialla JJ, Anderson
A, et al: High-throughput metabolomics and diabetic kidney disease
progression: Evidence from the chronic renal insufficiency (CRIC)
study. Am J Nephrol. 53:215–225. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Hong YA and Nangaku M: Endogenous adenine
as a key player in diabetic kidney disease progression: An
integrated multiomics approach. Kidney Int. 105:918–920. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Demko J, Saha B, Takagi E, Manis A,
Richman P and Pearce D: Renal tubule mTORC2 deletion increases
gluconeogenesis and urinary glucose excretion. Physiology.
38:57350392023. View Article : Google Scholar
|
|
112
|
Qiu S, Xie D, Guo S, Wang Z, Wang X, Cai
Y, Lin C, Yao H, Guan Y, Zhao Q, et al: Spatially segregated
multiomics decodes metformin-mediated function-specific metabolic
characteristics in diabetic kidney disease. Life Metabolism.
4:loaf0192025. View Article : Google Scholar
|
|
113
|
Wu Q, Chu JL, Rubakhin SS, Gillette MU and
Sweedler JV: Dopamine-modified TiO2 monolith-assisted LDI MS
imaging for simultaneous localization of small metabolites and
lipids in mouse brain tissue with enhanced detection selectivity
and sensitivity. Chem Sci. 8:3926–3938. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Spraggins JM, Rizzo DG, Moore JL, Noto MJ,
Skaar EP and Caprioli RM: Next-generation technologies for spatial
proteomics: Integrating ultra-high speed MALDI-TOF and high mass
resolution MALDI FTICR imaging mass spectrometry for protein
analysis. Proteomics. 16:1678–1689. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Neumann EK, Migas LG, Allen JL, Caprioli
RM, Van de Plas R and Spraggins JM: Spatial metabolomics of the
human kidney using MALDI trapped ion mobility imaging mass
spectrometry. Anal Chem. 92:13084–13091. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Fu T, Oetjen J, Chapelle M, Verdu A,
Szesny M, Chaumot A, Degli-Esposti D, Geffard O, Clément Y,
Salvador A and Ayciriex S: In situ isobaric lipid mapping by
MALDI-ion mobility separation-mass spectrometry imaging. J Mass
Spectrom. 55:e45312020. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Spatial Omics DataBase (SODB): Increasing
accessibility to spatial omics data. Nat Methods. 20:359–360. 2023.
View Article : Google Scholar
|
|
118
|
Vandergrift GW, Veličković M, Day LZ,
Gorman BL, Williams SM, Shrestha B and Anderton CR: Untargeted
spatial metabolomics and spatial proteomics on the same tissue
section. Anal Chem. 97:392–400. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Abedini A, Levinsohn J, Klötzer KA,
Dumoulin B, Ma Z, Frederick J, Dhillon P, Balzer MS, Shrestha R,
Liu H, et al: Single-cell multi-omic and spatial profiling of human
kidneys implicates the fibrotic microenvironment in kidney disease
progression. Nat Genet. 56:1712–1724. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Chuang AE, Chen YL, Chiu HJ, Nguyen HT and
Liu CH: Nasal administration of polysaccharides-based nanocarrier
combining hemoglobin and diferuloylmethane for managing diabetic
kidney disease. Int J Biol Macromol. 282:1365342024. View Article : Google Scholar : PubMed/NCBI
|