Introduction
Gliomas are the most common primary brain tumors in
adults, representing more than 50% of all brain tumors. Among them,
glioblastoma (GBM) is the most biologically aggressive type,
accounting for approximately 50% of all glial tumors, and is
associated with the worst prognosis (1). Malignant gliomas are associated with
dismal prognoses because glioma cells can actively migrate within
the brain, often traveling relatively long distances, and making
them elusive targets for effective surgical management (1,2).
After the surgical resection and the adjuvant treatment of a
glioma, the residual tumor cells peripheral to the excised dense
cellular tumor core give rise to a recurrent tumor. In more than
90% of cases, this tumor develops immediately adjacent to the
resection margin or within 2 cm of the resection cavity (2). Furthermore, studies have demonstrated
that invasive glioma cells show a decrease in their rate of
proliferation and a relative resistance to apoptosis when compared
to the highly cellular tumor core, which may play roles in their
resistance to conventional pro-apoptotic chemotherapy and
radiotherapy (2). The multimodal
standard treatment protocol for GBM consists of surgery followed by
concurrent radiotherapy and chemotherapy and finally adjuvant
chemotherapy with the alkylating drug TMZ (1,3,4). It
has been demonstrated that a more extensive surgical resection is
associated with a longer life expectancy for both low- and
high-grade gliomas (5,6). The 5-year survival rate is 9.8% with
the combination of radio- and TMZ therapy compared to 1.9% with
radiotherapy alone (3).
This treatment regimen is often associated with
significant lymphopenia, thrombocytopenia, and progressive
blood-brain-barrier dysfunction that can result in clinical and
radiologic deterioration without true tumor progression (7). Recently, there has been increased
awareness of progressive and enhancing lesions and peritumoral
edema visible by magnetic resonance imaging (MRI) immediately after
RT/TMZ treatment (8–10). Although in some cases, these
changes reflect tumor growth due to the treatment resistant nature
of GBM, they can remain stable or diminish over time and may be a
treatment effect, referred to as pseudoprogression. Enlargement of
the lesion, even during the first follow-up MRI, is frequent,
occurring in close to 50% of the patients (10,11).
Therefore, TMZ treatment is not abandoned on the basis of seemingly
discouraging imaging results within the first months following
RT/TMZ. However, the percentage of true progression within the
early progression cohort is highly variable in the published
literature, ranging from 35% to >80% (9,11–16).
Therefore, there is a need for novel imaging
techniques or biochemical markers that can better distinguish
pseudoprogression from true progression to avoid unnecessary and
potentially harmful surgical interventions or time lost, as TMZ
treatment becomes ineffective in almost half of radiologically
progressive GBM patients.
The aim of this retrospective study was to
potentially correlate clinical, radiological and pathological data
from GBM patients with the existence of pseudoprogression.
Patients and methods
Patients
All patients receiving RT/TMZ for newly diagnosed
GBM between July 2005 and December 2009 were identified from the
retrospective database of the Department of Neurosurgery at Erasme
Hospital. TMZ was administered at a daily dose of 75 mg per
m2 concurrent with radiotherapy and followed by 150–200
mg per m2 for five days every 28 days. Local research
ethics board approval was obtained for this retrospective chart
review (ref Erasme P2010/073). The clinical data collected included
age, sex, extent of surgery, number of adjuvant TMZ cycles, and
date of death.
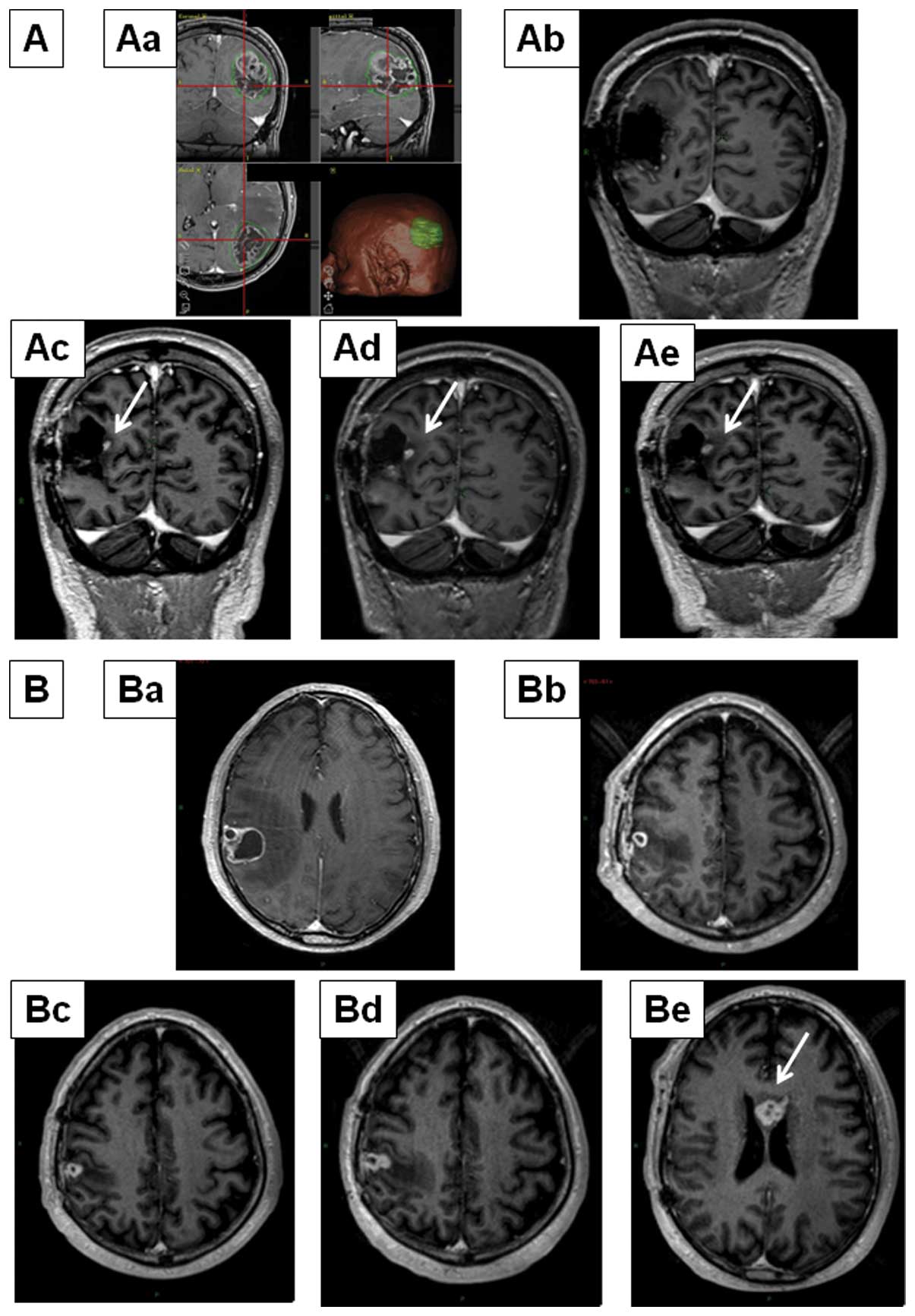
Patients were first radiologically categorized as
early progression, which was defined as progression within 8 weeks
of completing RT/TMZ. Patients with early radiological progression
were further subdivided into pseudoprogression and true progression
groups (Fig. 1). We defined
radiological pseudoprogression as progressive enhancing lesions
with peritumoral edema at MRI within eight weeks of completing
RT/TMZ treatment, without clinical signs of deterioration, that
stabilizes or even resolves after additional cycles of adjuvant
TMZ.
Anatomopathological data and immunohistochemical
markers relating to the expression of p53 protein and to the level
of cellular proliferation (measured by Ki-67 index by means of the
MIB-1 antibody) were analyzed.
Statistical analyses
The descriptive statistics consisted in the use of
box-and-whisker plot. In the basic box-and-whisker plot, the
central box represents the values from the lower to upper quartile
(25–75 percentile, interquartile range, IQR). The middle line
represents the median. The whiskers represent the lowest datum
still within 1.5 IQR of the lower quartile, and the highest datum
still within 1.5 IQR of the upper quartile. The categorical data
were compared using a χ2 test and continuous data using
a Mann-Whitney non-parametric test. A p<0.05 was considered
significant. The analyses were performed using Statistix
9® (Analytical Software, Tallahassee FL, USA).
Results
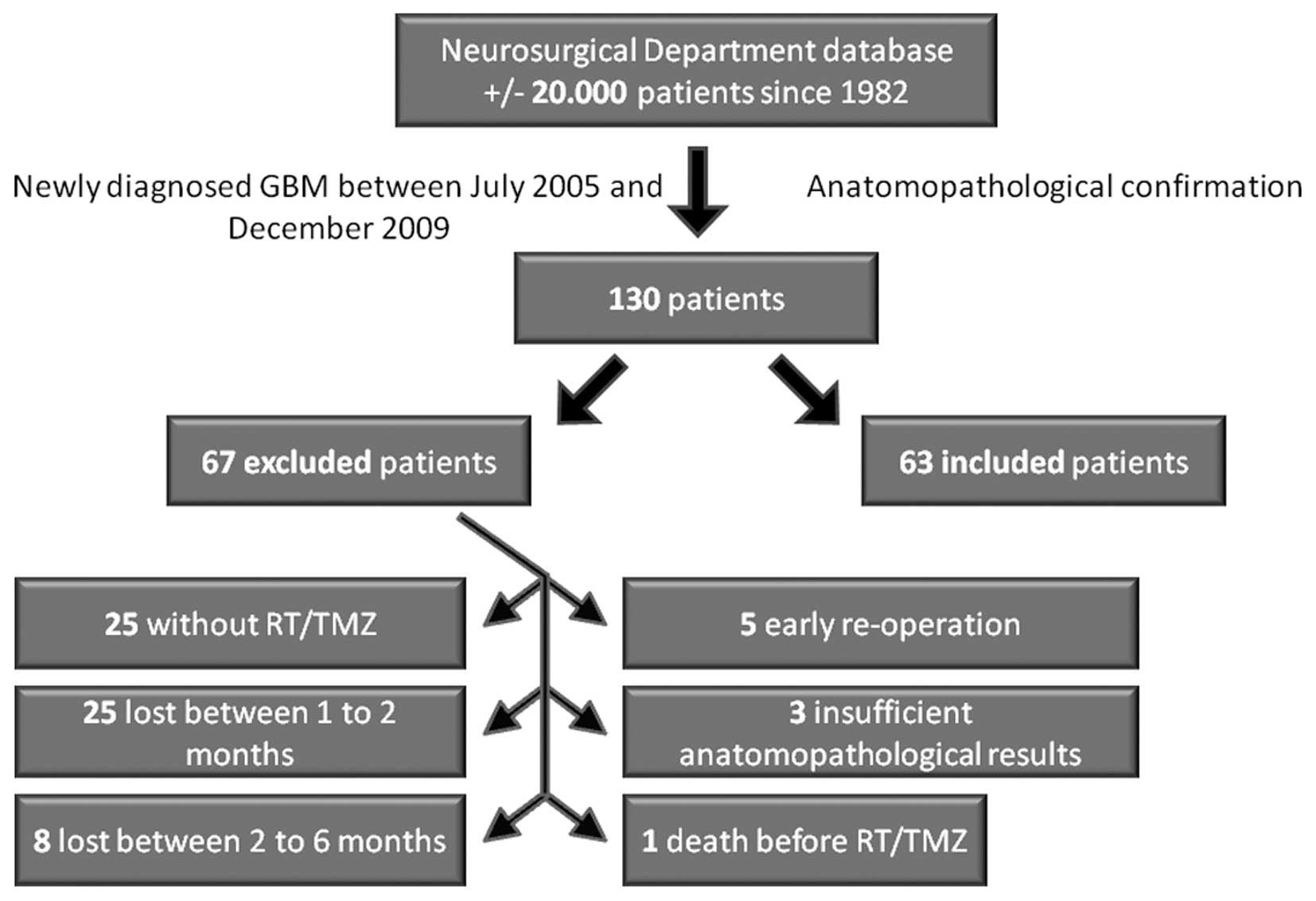
A total of 130 patients with newly
histopathologically confirmed GBM were identified
Sixty-seven patients were excluded: 25 did not
receive RT/TMZ, 25 were lost to follow-up at 1–2 months and 8 at
2–6 months following combined RT/TMZ, 5 were re-operated early on,
3 showed insufficient anatomopathological markers and 1 died before
RT/TMZ (Fig. 2). Sixty-three
patients between the ages of 27 and 78 were therefore selected for
the study. Demographic data are shown in Table I. Thirty-three of 63 (52%) patients
showed early progression (progression within eight weeks after
completing RT/TMZ), of which 7 (21%) were identified as showing
pseudoprogression, and 26 (79%) were identified as true progression
(Fig. 3). In the group with no
early progression (30 patients), 14 (47%) followed a pejorative
evolution between two to six months following the end of the
concomitant RT/TMZ treatment (Fig.
3).
 | Table IDemographics of population. |
Table I
Demographics of population.
| Age |
| Median (years) | 59.5 |
| <50 | 15 (23.8%) |
| 50–59 | 16 (25.4%) |
| 60–69 | 19 (30.2%) |
| ≥70 | 13 (20.6%) |
| Gender |
| Female/Male | 24/39
(38.1%/61.9%) |
| Surgery |
| Gross total | 58 (92%) |
| Partial
resection | 2 (3.2%) |
| Open biopsy | 1 (1.6%) |
| Stereotactic
biopsy | 2 (3.2%) |
The evaluation of p53 overexpression in GBM tissue
was categorized into four classes: no, low, intermediate and high
overexpression. While levels of p53 expression were of no
predictive value in the identification of pseudoprogression (data
not shown), the Ki67 index, related to the level of cellular
proliferation, was predictive (Fig.
4). Indeed, the median level of cellular proliferation within
the group of pseudoprogression GBP patients was significantly
(p=0.0016) higher [20% (IQR 20–50%)] compared to the group of GBM
patients associated with true progression [10% (IQR 3.5–20%]
(Fig. 4).
Discussion
The diagnosis of pseudoprogression is highly
relevant to the practice of neuro-oncology. In a phase III
prospective study, De Wit and collaborators (17) showed that radiotherapy alone could
bring about pseudoprogression in three out of 32 GBM patients (9%).
This finding could be one possible explanation for the worsening of
imaging studies and clinical deterioration observed following the
completion of RT/TMZ. The incidence of pseudoprogression in the
early progression patient population reported in the literature
varies from 12 to 64% of pseudoprogression cases (8–16).
In our study, seven of the 63 patients studied developed
pseudoprogression, representing 11% of the patients receiving
RT/TMZ treatment and 21% (7/33) of the patients with signs of early
progression.
Suspicion of pseudoprogression may influence a
clinician’s recommendation to continue with standard adjuvant TMZ
chemotherapy rather than beginning a second line therapy for
recurrence. Imaging changes consistent with pseudoprogression
commonly persist for up to three months following the completion of
RT/TMZ and occasionally are persistent for longer periods.
In the pivotal EORTC-NCIC-CTG trial, up to 20% of
patients did not receive maintenance TMZ therapy, usually due to
deterioration in post-treatment imaging (4). However, TMZ, given as maintenance
therapy for at least six months in appropriate patients, is one of
the most active agents currently approved for GBM. The therapeutic
benefits of TMZ are due to the fact that it induces double strand
DNA breaks via the generation of methyl-guanosine (18) concomitantly with sustained
autophagy-related processes (19,20),
with both of these effects resulting in the apoptosis of GBM cells
(21). TMZ also displays
anti-angiogenic effects (22). In
contrast, TMZ treatment of GBMs can lead to the emergence of
TMZ-resistant tumors, at least at the experimental level (23,24).
Therefore, the occurrence of pseudoprogression
following standard therapy for GBM raises important issues related
to the determination of disease progression, the optimal timing and
methods to judge treatment efficacy, when to recommend second line
or experimental therapy, and how to evaluate new agents
administered ‘on the back of’ RT/TMZ.
The biology of pseudoprogression is not clear, and
several hypotheses are found in the literature (8,9,11,17,25).
While Chamberlain et al (26) have demonstrated that some patients
develop early radionecrosis following RT/TMZ, the issue of
pseudoprogression is different. Pseudoprogression likely involves
early changes to the vascular endothelium and the
blood-brain-barrier associated with vasogenic edema; however, the
precise mechanism remains complex and poorly understood (8). Combined with the radiotherapy effect,
TMZ, which induces cellular replication arrest in the G2/M cell
cycle phase (the phase most sensitive to radiotherapy) and
increases the number of DNA breaks in GBM cells (18), could have a role in the
pseudoprogression phenomenon. The increase in contrast enhancement
during pseudoprogression could also be due to cellular hypoxia
secondary to the combined treatment (25). Cellular hypoxia leads to
dysregulation of the expression of several molecules including
hypoxia-inducible factor-1α (HIF-1α) (25). In the absence of HIF-1α regulation,
DNA promoter regions are activated, leading to an increase in the
transcription of hypoxia response elements (HREs) (25). These HREs conduct the transcription
of more than one hundred genes, leading to an increase in the
synthesis of vascular endothelial growth factor (25). With the goal being to help hypoxic
cells, these processes increase vascular permeability and therefore
lead to an increase in contrast enhancement and angiogenesis
(25).
Future studies will likely take advantage of
developments in modern MRI-based vascular permeability, flow
imaging, spectroscopy and PET scanning (27,28)
and in alternative end-points and response criteria developed by an
international working group (29)
to elucidate the nature and timing of these changes. A recent study
suggests that relative cerebral blood volume measured by dynamic
susceptibility-weighted contrast enhanced perfusion MRI has an
impact on the predictability of pseudoprogression in patients with
GBM (30). Perhaps an imaging tool
can be developed to assist the clinician to determine the
difference between a patient with a robust treatment response
(conferring a survival advantage) versus a patient with disease
resistance. Until then, we must be cautious with the interpretation
of imaging following the treatment of GBM.
Our study suggests a statistically significant
difference in the levels of cellular proliferation, observed by
means of the percentage of Ki67 antigen expression, between
pseudoprogression and true progression. All patients with
pseudoprogression showed a GBM tumor associated with a level of
Ki67 expression ≥20%. To our knowledge, this is the first time that
a link between the level of cellular proliferation in GBMs and the
development of a pseudoprogression phenomenon during or just after
RT/TMZ has been reported. This phenomenon could be explained, at
least in part, by the fact that RT/TMZ induces cell death during
the replication phase of the cell cycle. Thus, this phase would
show the highest level of cellular replication and the highest
observed initial effects of the treatment (8,9,11,25).
The study by Brandes et al (11) argues this point because that group
has demonstrated that the level of pseudoprogression is
significantly higher in the presence of MGMT
(O6-methylguanine methyltranferase) promoter methylation
compared to its absence (66 vs. 34%). The inactivation of the
repairing enzyme MGMT increases the efficiency of TMZ (3,18,31).
A recent study revealed that methylation-specific multiplex
ligation probe amplification, an assay that permits the
semi-quantitative evaluation of promoter methylation, is a useful
method for predicting radiological progression versus
pseudoprogression in GBM patients, and the interpretation of the
results, in combination with methylation-specific polymerase chain
reaction results, will provide good practical guidelines for
clinical decision making regarding GBM treatment (32).
Our observation, suggesting that GBM associated with
high levels of cellular proliferation may differentiate tumor
progression from pseudoprogression, warrants further investigation
in a prospective study with a larger number of patients. We plan
also to analyze the individual versus combined prognostic
information contributed by MGMT status and Ki67-related cell
proliferation levels.
Acknowledgements
F.L. is a Clinical Research Fellow with the Fonds
National de la Recherche Scientifique (FNRS, Belgium).
References
|
1
|
Lefranc F, Sadeghi N, Camby I, Metens T,
De Witte O and Kiss R: Present and potential future issues in
glioblastoma treatment. Expert Rev Anticancer Ther. 6:719–732.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lefranc F, Brotchi J and Kiss R: Possible
future issues in the treatment of glioblastomas: special emphasis
on cell migration and the resistance of migrating glioblastoma
cells to apoptosis. J Clin Oncol. 23:2411–2422. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stupp R, Hegi M, Mason W, van den Bent MJ,
Taphoorn MJ, Janzer RC, et al: Effects of radiotherapy with
concomitant and adjuvant temozolomide versus radiotherapy alone on
survival in glioblastoma in a randomised phase III study: 5-year
analysis of the EORTC-NCIC trial. Lancet Oncol. 10:459–466.
2009.
|
|
4
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, et al: Radiotherapy plus concomitant and
adjuvant temozolomide for glioblastoma. N Engl J Med. 352:987–996.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sanai N and Berger MS: Glioma extent of
resection and its impact on patient outcome. Neurosurgery.
62:753–764. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stummer W, van den Bent MJ and Westphal M:
Cytoreductive surgery of glioblastoma as the key to successful
adjuvant therapies: new arguments in an old discussion. Acta
Neurochir (Wien). 153:1211–1218. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Holdhoff M and Grossman SA: Controversies
in the adjuvant therapy of high-grade gliomas. Oncologist.
16:351–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brandsma D, Stalpers L, Taal W, Sminia P
and van den Bent MJ: Clinical features, mechanisms, and management
of pseudo-progression in malignant gliomas. Lancet Oncol.
9:453–461. 2008. View Article : Google Scholar
|
|
9
|
Taal W, Brandsma D, de Bruin HG, Bromberg
JE, Swaak-Kragten AT, Smitt PA, et al: Incidence of early
pseudo-progression in a cohort of malignant glioma patients treated
with chemoirradiation with temozolomide. Cancer. 113:405–410. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Topkan E, Topuk S, Oymak E, Parlak C and
Pehlivan B: Pseudo-progression in patients with glioblastoma
multiforme after concurrent radiotherapy and temozolomide. Am J
Clin Oncol. March 10–2011.(Epub ahead of print).
|
|
11
|
Brandes A, Franceschi E, Tosoni A, Blatt
V, Pession A, Tallini G, et al: MGMT promoter methylation statu
scan predict the incidence and outcome of pseudoprogression after
concomitant radiochemotherapy in newly diagnosed glioblastoma
patients. J Clin Oncol. 26:2192–2197. 2008. View Article : Google Scholar
|
|
12
|
Chaskis C, Neyns B, Michotte A, De Ridder
M and Everaert H: Pseudoprogression after radiotherapy with
concurrent temozolomide for high-grade glioma: clinical
observations and working recommendations. Surg Neurol. 72:423–428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Clarke JL, Abrey LE, Karimi S and Lassman
AB: Pseudo-progression (PsPr) after concurrent radiotherapy (RT)
and temozolomide (TMZ) for newly diagnosed glioblastoma multiforme
(GBM). J Clin Oncol. 26(Suppl): abs. 20252008.
|
|
14
|
Gerstner ER, McNamara MB, Norden AD,
Lafrankie D and Wen PY: Effect of adding temozolomide to radiation
therapy on the incidence of pseudo-progression. J Neurooncol.
94:97–101. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jefferies S, Burton K, Jones P and Burnet
N: Interpretation of early imaging after concurrent radiotherapy
and temozolomide for glioblastoma. Clin Oncol. 19(Suppl): abs.
332007.
|
|
16
|
Sanghera P, Perry J, Sahgal A, Symons S,
Aviv R, Morrison M, et al: Pseudoprogression following
chemoradiotherapy for glioblastoma multiforme. Can J Neurol Sci.
37:36–42. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
De Wit M, De Bruin H, Eijkenboom W,
Sillevis Smitt PA and van den Bent MJ: Immediate post-radiotherapy
changes in malignant glioma can mimic tumor progression. Neurology.
63:535–537. 2004.PubMed/NCBI
|
|
18
|
Hegi ME, Liu L, Herman JG, Stupp R, Wick
W, Weller M, et al: Correlation of O6-methylguanine
methyltransferase (MGMT) promoter methylation with clinical
outcomes in glioblastoma and clinical strategies to modulate MGMT
activity. J Clin Oncol. 26:4189–4199. 2008.PubMed/NCBI
|
|
19
|
Kanzawa T, Germano IM, Komata T, Ito H,
Kondo Y and Kondo S: Role of autophagy in temozolomide-induced
cytotoxicity for malignant glioma cells. Cell Death Differ.
11:448–457. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Katayama M, Kawaguchi T, Berger MS and
Pieper RO: DNA damaging agent-induced autophagy produces a
cytoprotective adenosine triphosphate surge in malignant glioma
cells. Cell Death Differ. 14:548–558. 2007. View Article : Google Scholar
|
|
21
|
Roos WP, Batista LF, Naumann SC, Wick W,
Weller M, Menck CF, et al: Apoptosis in malignant glioma cells
triggered by the temozolomide-induced DNA lesion
O6-methylguanine. Oncogene. 26:186–197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mathieu V, De Neve N, Le Mercier M,
Dewelle J, Gaussin JF, Dehoux M, et al: Combining bevacizumab with
temozolomide increases the antitumor efficacy of temozolomide in a
human glioblastoma orthotopic xenograft model. Neoplasia.
10:1383–1392. 2008.
|
|
23
|
Le Calvé B, Rynkowski M, Le Mercier M,
Bruyère C, Lonez C, Gras T, et al: Long-term in vitro treatment of
human glioblastoma cells with temozolomide increases resistance in
vivo through up-regulation of GLUT transporter and aldo-keto
reductase enzyme AKR1C expression. Neoplasia. 12:727–739.
2010.PubMed/NCBI
|
|
24
|
Le Mercier M, Lefranc F, Mijatovic T,
Debeir O, Haibe-Kains B, Bontempi G, et al: Evidence of galectin-1
involvement in glioma chemoresistance. Toxicol Appl Pharmacol.
229:172–183. 2008.PubMed/NCBI
|
|
25
|
Jensen R: Brain tumor hypoxia:
tumorigenesis, angiogenesis, imaging, pseudoprogression, and as a
therapeutic target. J Neurooncol. 92:317–335. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chamberlain MC, Glantz MJ, Chalmers L, van
Horn A and Sloan AE: Early necrosis following concurrent Temodar
and radiotherapy in patients with glioblastoma. J Neurooncol.
82:81–83. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu X, Wong KK, Young GS, Guo L and Wong
ST: Support vector machine multiparametric MRI identification of
pseudoprogression from tumor recurrence in patients with resected
glioblastoma. J Magn Reson Imaging. 33:296–305. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang I and Aghi MK: New advances that
enable identification of glioblastoma recurrence. Nat Rev Clin
Oncol. 6:648–657. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brandsma D and van den Bent MJ:
Pseudoprogression and pseudoresponse in the treatment of gliomas.
Curr Opin Neurol. 22:633–638. 2009. View Article : Google Scholar
|
|
30
|
Kong DS, Kim ST, Kim EH, Lim DH, Kim WS,
Suh YL, et al: Diagnostic dilemma of pseudoprogression in the
treatment of newly diagnosed glioblastomas: The role of assessing
relative cerebral blood flow volume and oxygen-6-methylguanine-DNA
methyltransferase promoter methylation status. AJNR Am J
Neuroradiol. 32:382–387. 2011.
|
|
31
|
Fabi A, Russillo M, Metro G, Vidiri A, Di
Giovanni S and Cognetti F: Pseudoprogression and MGMT status in
glioblastoma patients: implications in clinical practice.
Anticancer Res. 29:2607–2610. 2009.PubMed/NCBI
|
|
32
|
Park CK, Kim J, Yim SY, Lee AR, Han JH,
Kim CY, et al: Usefull of MS-MLPA for detection of MGMT promoter
methylation in the evaluation of pseudoprogression in glioblastoma
patients. Neurooncology. 13:195–202. 2011.PubMed/NCBI
|