Introduction
Ovarian cancer is the fifth leading cause of
cancer-related-death in the US. Approximately 22,000 women suffered
from ovarian cancer in 2010, about 14,000 of whom died of this
disease (1). Since most patients
with early-stage ovarian cancer seldom have any symptoms, at the
time of diagnosis, over 75% are already in advanced stages with
peritoneal dissemination and ascites, which are the typical
symptoms (2). The standard
treatment for ovarian cancer is cytoreductive surgery with
platinum/taxane combination chemotherapy. Ovarian cancer is mostly
sensitive to chemotherapy (3,4), but
becomes ineffective over time due to the development of
chemoresistance. The 5-year survival rate is only 40%, and has not
improved in the last decade (1).
Therefore, new strategies, such as immunotherapy and
molecular-targeted therapy, may prove useful in improving the
prognosis of ovarian cancer. The most common form of ovarian cancer
spread is peritoneal dissemination (2). Although the mechanism involved in
this process are largely unknown, studies indicate that,
immunotolerance induction plays an important role (5,6).
Indoleamine-2,3-dioxygenase (IDO) is an enzyme that
catalyzes the first and rate-limiting step in the kynurenine
pathway of tryptophan catabolism. IDO was originally discovered in
1967 (7,8) in the rabbit small intestine and was
first purified in 1978 (9).
Subsequently, it was reported that IDO could be induced in the
mouse lung with either influenza virus infection (10) or bacterial endotoxin shock
(11). Proinflammatory mediators,
such as interferon-γ or other cytokines can also stimulate IDO
induction (12). The first study
that described IDO as an immunosuppressant found that IDO in the
mouse placenta prevented rejection of the allogeneic fetus
(13). Recently, it was clarified
that IDO can induce immunotolerance in patients with autoimmune
diseases (14–17), chronic infections (18), and cancer (19). It was also reported that most human
tumors express IDO (19) and that
IDO can contribute to tumor-induced immunosuppression by starving T
cells, which are sensitive to tryptophan deficiency. In this
situation, tumor cells can escape immune surveillance via the
action of IDO (13). Natural
killer (NK) cells are important members of the innate immune
system, which plays a role in inhibiting the growth and
dissemination of several kinds of tumors (20). A series of receptors expressed by
NK cells are known to modulate the cytotoxicity of NK cells against
tumors (21). The
tryptophan-derived catabolic kynurenine can reduce NK cell number
and weaken NK cell cytotoxicity by inhibiting NK cell receptors,
thus contributing to tumor progression (22). IDO is frequently expressed in many
cancers such as gastric, pancreatic, colorectal, and prostate
cancers (19). In the
gynecological field, IDO expression has been observed in cervical,
endometrial, and ovarian cancer (19), and associations between its
expression and the prognosis of these cancers have been reported
(23–26).
RNA interference (RNAi) is a good technique for gene
silencing, that involves a post-transcriptional gene-silencing
mechanism (27). Among the
different types of RNAi techniques, the use of small interfering
RNAs (siRNAs) effectively suppresses gene expression, but the
suppression is transient (28),
which limits its therapeutic use. Short hairpin RNAs (shRNAs)
driven by polymerase III promoters have been developed as an
alternative strategy to attain long-term stable target gene
silencing and understand the consequence of stable silencing
(29,30).
In this study, we used an shRNA vector targeting IDO
to silence IDO expression in an IDO-expressing ovarian cancer cell
line to clarify the relationship between IDO expression and
peritoneal dissemination of ovarian cancer. Moreover, we
investigated the function of NK cells in ovarian cancer progression
in order to develop an IDO-targeted molecular therapy that inhibits
peritoneal dissemination.
Materials and methods
Cell culture
The human ovarian cancer cell line SKOV-3 (31) (American Type Culture Collection,
Manassas, VA) were cultured in RPMI-1640 medium (Gibco, Grand
Island, NY) containing 10% inactivated fetal calf serum (Sigma, St.
Louis, MO), 100 U/ml penicillin, and 100 μg/ml streptomycin (Gibco)
at 37°C in a 5% CO2 atmosphere for no longer than 8
weeks after recovery from frozen stocks.
The NK cell line KHYG-1 (32) was purchased from the Japanese
Collection of Research Bioresources (JCRB, Osaka, Japan). Cells
were cultured in RPMI-1640 medium supplemented with 100 nM of human
interleukin-2 (R&D Systems, Minneapolis, MN) and 10%
inactivated fetal calf serum (Sigma), at 37°C in a 5%
CO2 atmosphere for no longer than 8 weeks after recovery
from frozen stocks.
Antibodies
Anti-human IDO monoclonal antibody was prepared as
previously reported (33).
Anti-human actin antibody (Sigma) and anti-mouse CD49b antibody
(R&D) were used according to the manufacturer’s protocols.
shRNA stable cell line and control cell
line
The DNA oligonucleotides encoding shRNA targeting
the IDO gene (forward: 5′-CACCGGGGCAGATTATAAGAATTACGTGTGCTGTCC
GTAATTCTTGTAGTCTGCTCCTTTTT-3′, reverse: 5′-CCC
CGTCTAATATTCTTAATGCACACGACAGGCATTAAGA ACATCAGACGAGGAAAAATACG-3′)
were synthesized, annealed, and inserted into the BspMI site
of the vector piGENE PURhU6 (34),
which contained a human U6 promoter, and a puromycin resistance
gene. The shRNA expression plasmid (piGENE PURhU6/shIDO) and
control plasmid (piGENE PURhU6) were transfected into SKOV-3 using
Lipofectamine LTX and Plus Reagent (Invitrogen, Carlsbad, CA)
according to the manufacturer’s instructions. The cells were
selected using 0.5 μg/ml puromycin (Calbiochem, Darmstadt,
Germany). Resistant clones were obtained after 4 weeks as
SKOV-3/shIDO, SKOV-3/Mock. The cells were subsequently maintained
in the presence of 0.5 μg/ml puromycin.
Western blotting
Protein (10 μg) extracted from a homogenate of
cultured cells was mixed with 2X SDS-PAGE sample buffer [120 mM
Tris-HCl (pH 6.8), 4% SDS, 20% glycerol, 0.004% bromophenol blue,
and 10% 2-mercaptoethanol]. The mixture was heated at 95°C for 2
min, and electrophoresed on a 0.1% SDS-10% polyacrylamide gel, and
then the proteins were blotted onto a polyfluorovinylidene
membrane. The menbranes were blocked with Non-Protein Blocking
Agent (ATTO Corporation, Tokyo, Japan) at room temperature for 1 h,
and incubated with anti-human IDO monoclonal antibody (1:1,000) and
anti-human actin polyclonal antibody (1:200) for 1 h at room
temperature. The membrane was washed with phosphate-buffered saline
(PBS)-Tween-20 three times, and then incubated with horseradish
peroxidase-conjugated secondary anti-mouse antibody (Thermo,
Rockford, IL) or anti-rabbit antibody (Thermo). Signals were
detected by chemiluminescence (ECL kit; Amersham Biosciences,
Piscataway, NJ) on X-ray film.
In vitro cell growth kinetics
SKOV-3/shIDO and SKOV-3/Mock cells (500 of each
line) were seeded into a 96-well plate, and cultured in RPMI-1640
medium containing 10% fetal calf serum. Every 24 h, cells were
counted using a colorimetric assay with the Cell Proliferation kit
II (XTT) (Boehringer Mannheim GmbH Biochemica, Mannheim, Germany),
and a growth curve was drawn from the results.
Sensitivity of transfectants to NK cells
in vitro
The sensitivity of SKOV-3/shIDO and SKOV-3/Mock
cells to NK cells was investigated by colorimetric assay using XTT.
SKOV-3/shIDO and SKOV-3/Mock cells (500 of each line) were seeded
into a 96-well plate and co-cultured with KHYG-1 cells (0, 500,
1000, or 2000 cells) in RPMI-1640 medium containing 10% fetal calf
serum for 72 h. After 3 washes with PBS to exclude KHYG-1 cells
completely, the viable cell count was determined by colorimetric
assay and calculated as the percent of control cells (cultured
without KHYG-1 cells).
Experimental animals
Four- to six-week-old female BALB/c nude mice (Japan
Clea Laboratories, Tokyo, Japan) were used. All animal experiments
were conducted according to the institutional and national
guidelines for animal experiments.
Subcutaneous tumor growth in vivo
SKOV-3/shIDO and SKOV-3/Mock cells (5×106
cells of each line) were inoculated subcutaneously into the back of
mice to induce tumor growth. The tumor volume [(long diameter) ×
(short diameter)2 × 1/2] was measured twice a week to
draw a tumor growth curve.
Peritoneal dissemination in vivo
SKOV-3/shIDO and SKOV-3/Mock cells (5×106
cells of each line) were injected intraperitoneally into nude mice,
and the mice were observed until death. A survival curve was
constructed using the Kaplan-Meier method. The mice were checked
for survival twice a day.
Immunohistochemical staining
At one week after subcutaneous tumor cell
inoculation, mice were sacrificed under isoflurane anesthesia, and
the tumor was removed. After formalin fixation, paraffin sections
were prepared, deparaffinized, and treated with hydrogen peroxide
for 30 min to block endogenous peroxidase. The sections were then
reacted with a 1:10 dilution (5 μg/ml) of anti-mouse CD49b primary
antibody for 16 h at room temperature, washed 3 times washes with
PBS, and then incubated with enzyme-conjugated streptavidin for 30
min. The sections were again washed with PBS 3 times, and color was
developed using the diaminobenzidin method. The number of stained
NK cells was counted under high-power magnification (x400).
Statistical analysis
Except for the comparison of survival curves, the
test of significance between the 2 groups was performed using
Student’s t-test. The generalized Wilcoxon test was used to compare
survival curves between the 2 groups. A P-value of <0.05 was
considered significant.
Results
Establishing an IDO-downregulated cell
line
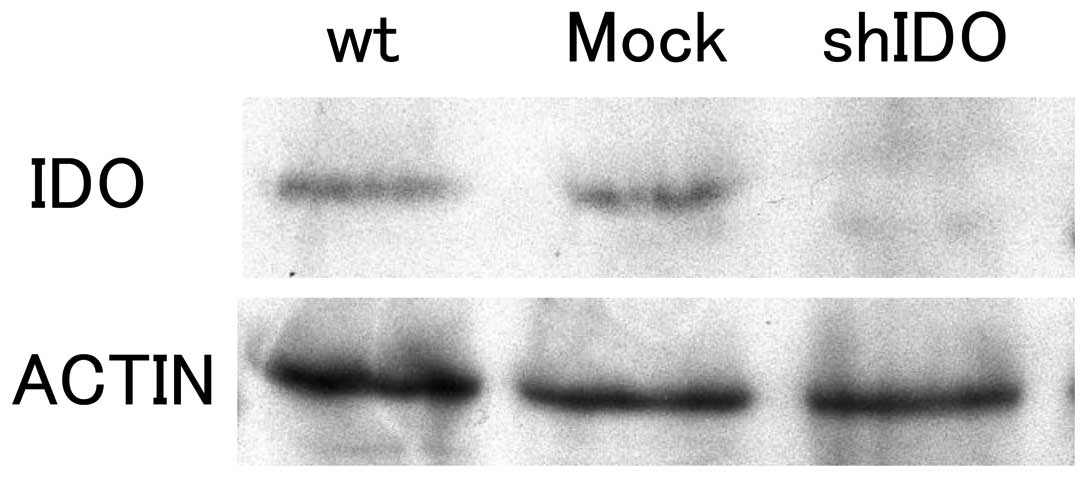
Fig. 1 shows the
results of Western blot analysis of the shIDO expression vector- or
control vector-transfected ovarian cancer cell line SKOV-3.
Parental cells (wt) and control vector-transfected cells (Mock)
showed evident IDO expression. In contrast, the shIDO expression
vector-transfected cells (shIDO) did not show IDO expression,
confirming IDO downregulation in the SKOV-3/shIDO cell line.
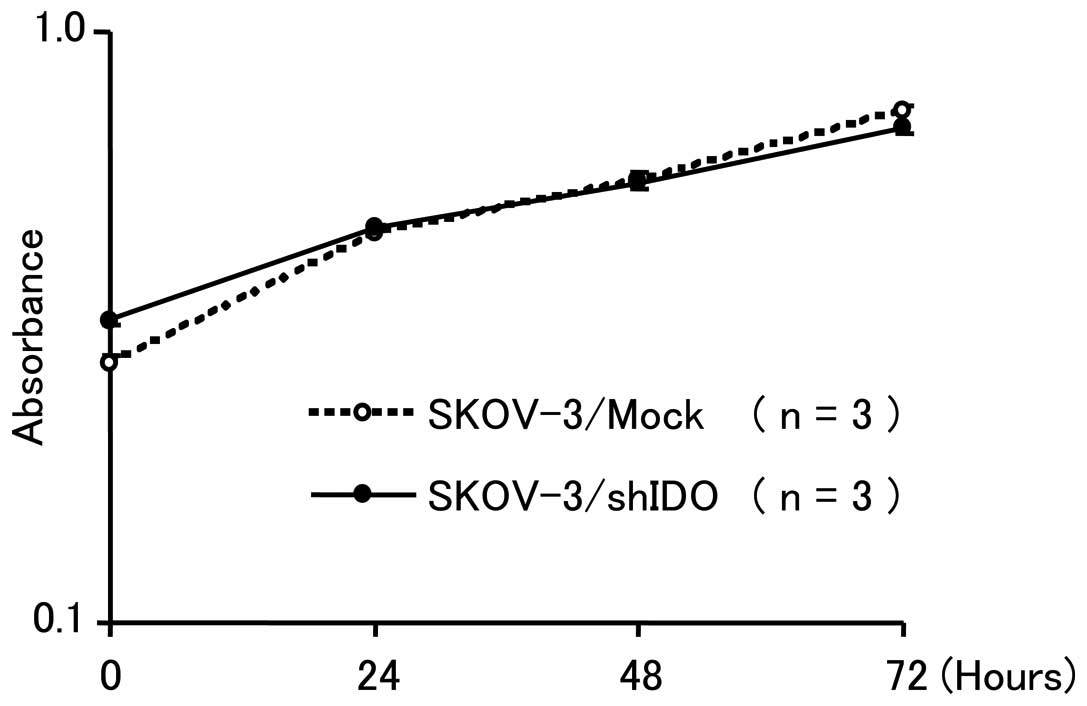
In vitro cell growth kinetics
Growth curve analyses of SKOV-3/shIDO and
SKOV-3/Mock cells showed no significant difference between the two
groups, suggestiong that the downregulation of IDO did not affect
cell growth in vitro (Fig.
2).
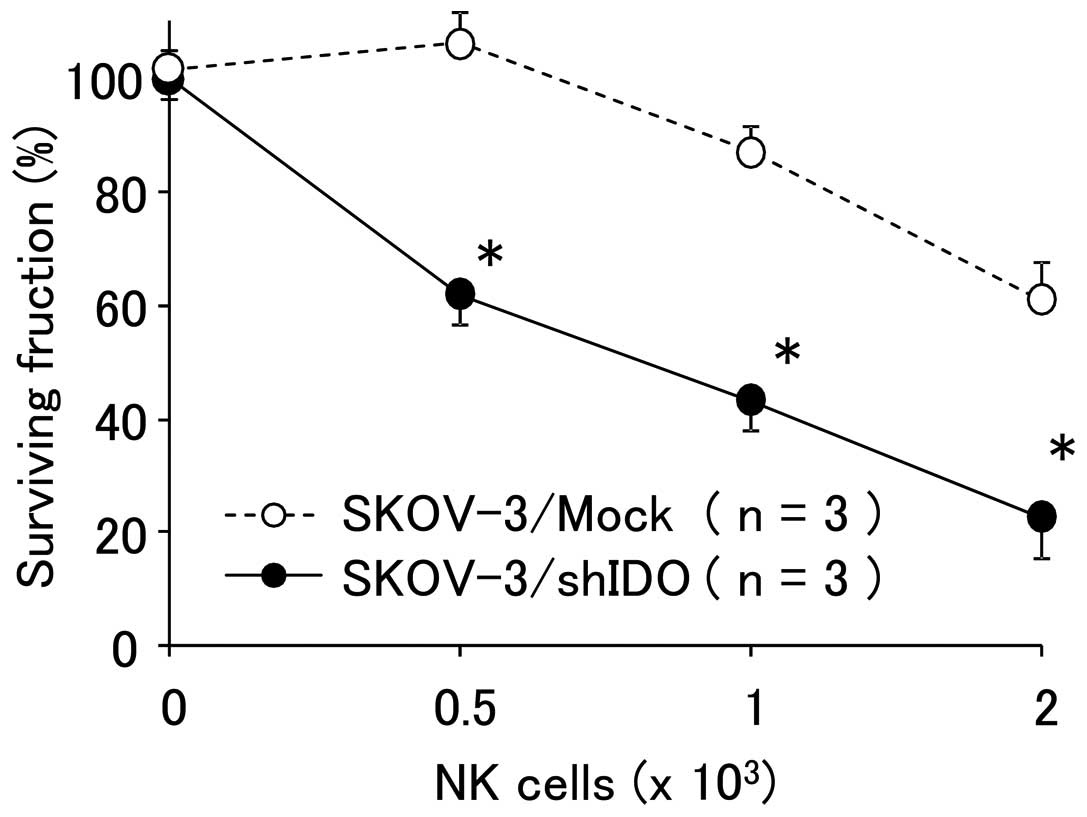
Sensitivity of transfectants against NK
cells in vitro
The proportion of viable tumor cells co-cultured
with NK cells is shown in Fig. 3.
The percent survival of SKOV-3/shIDO cells was significantly lower
than that of the control cells, indicating that the downregulation
of IDO reinforced the sensitivity of tumor cells against NK
cells.
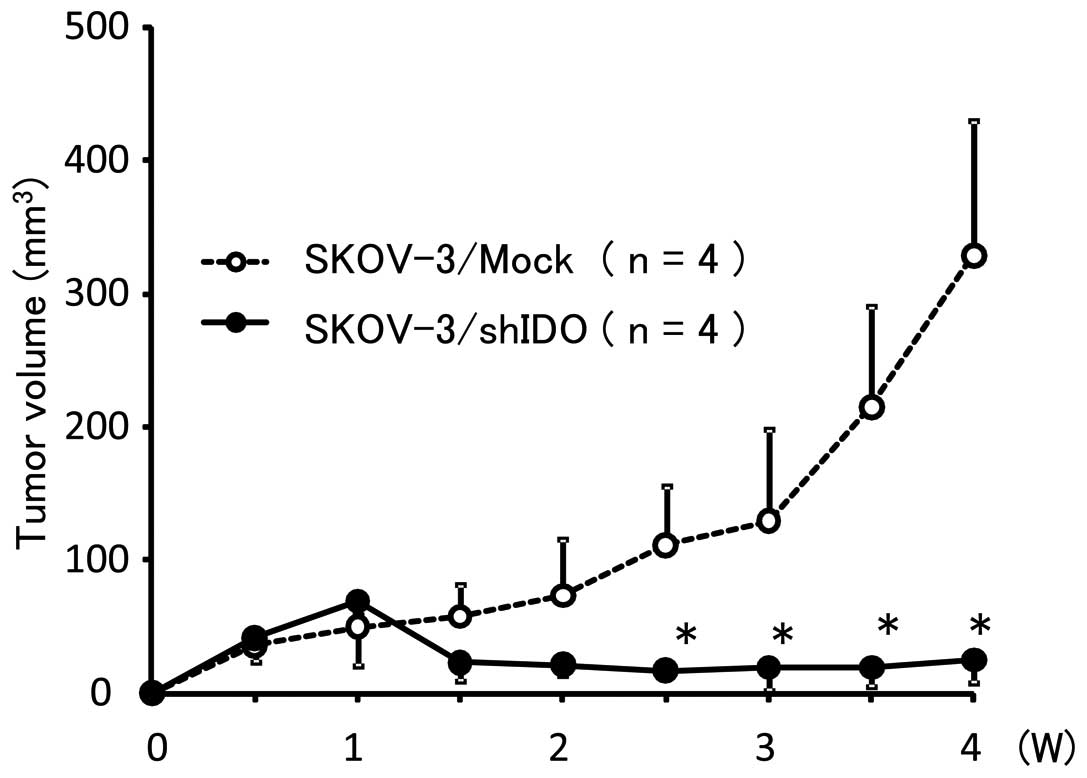
Tumor growth in vivo
Both SKOV-3/shIDO and control cells formed small
nodules one week after inoculation (Fig. 4). Subsequently, the tumors in the
control group were enlarged, whereas those in the SKOV-3/shIDO
group were reduced, suggesting that the downregulation of IDO
inhibited tumor growth in vivo.
Number of NK cells in the tumor
stroma
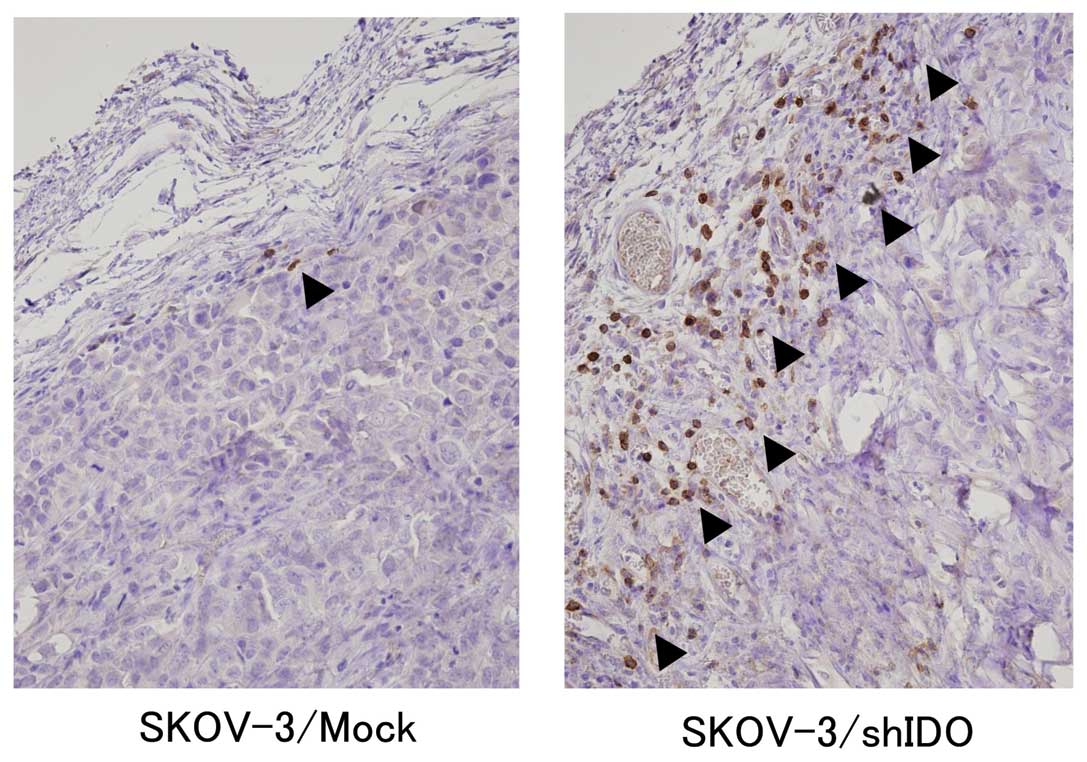
Immunostaining of NK cells (black arrowhead) shows
accumulation of NK cells in the stroma of SKOV-3/shIDO and control
subcutaneous tumors (Fig. 5). The
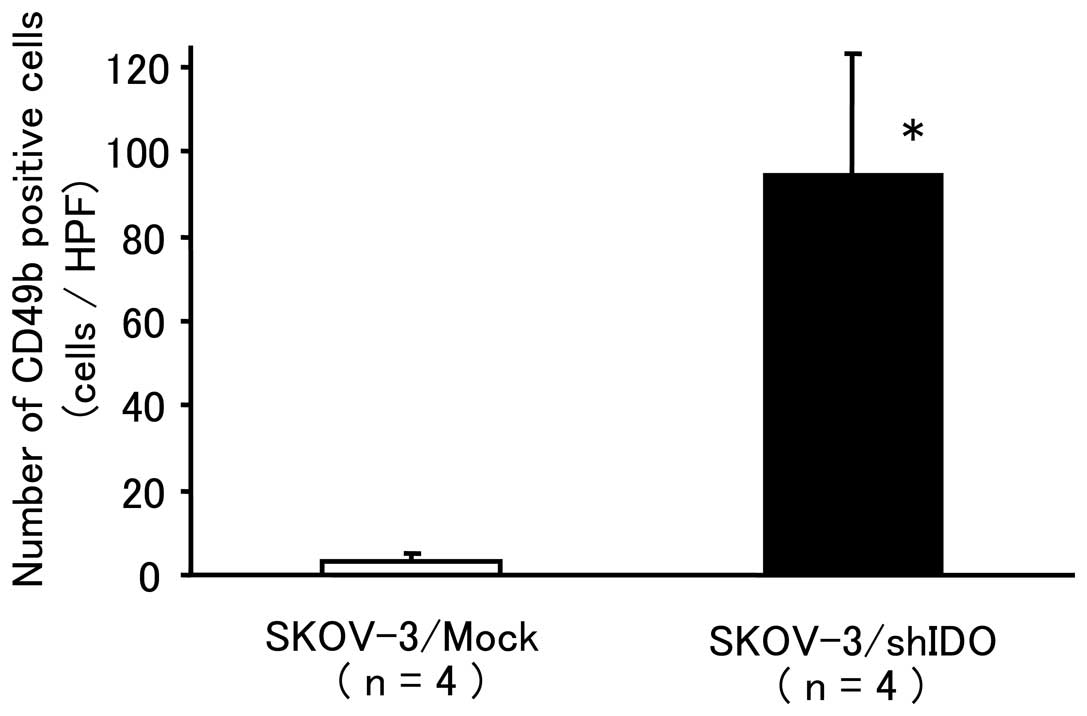
number of NK cells (94±29) that accumulated in the SKOV-3/shIDO
tumors was significantly higher than that (3±2) in the control
tumors (P<0.01) (Fig. 6). These
results suggest that the downregulation of IDO promoted NK cell
accumulation around the tumor.
Peritoneal dissemination in vivo
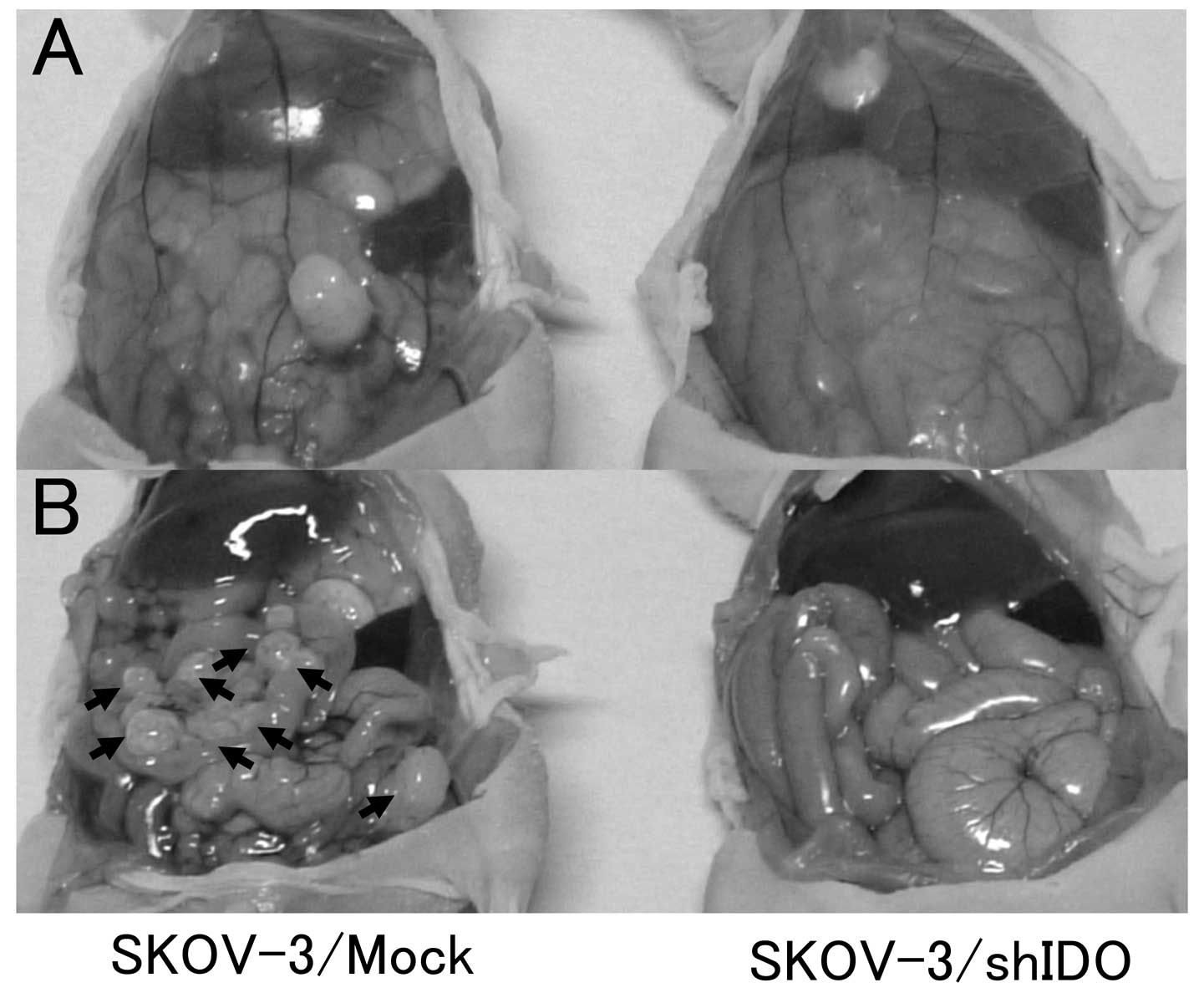
Four weeks after intraperitoneal tumor cell
inoculation, mice with intraperitoneally-injected control cells
demonstrated bloody ascites and marked peritoneal dissemination,
whereas those receiving the intraperitoneal injection of
SKOV-3/shIDO cells showed no abnormal changes (Fig. 7A and B). All control
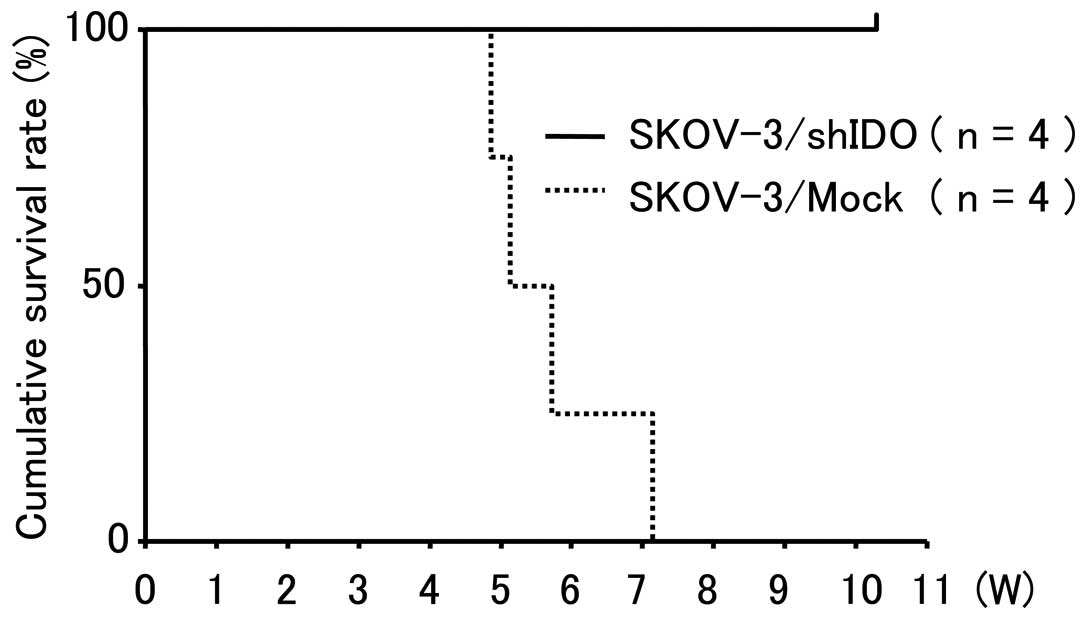
cell-inoculated mice died of peritoneal dissemination with ascites
within 50 days after inoculation, whereas all SKOV-3/shIDO
cell-inoculated mice survived longer than 70 days after inoculation
(P<0.01) (Fig. 8). Thus,
downregulating IDO inhibited peritoneal dissemination formation and
ascites accumulation in tumor-inoculated mice.
Discussion
The experiments described herein aimed to clarify
the relationship between the immunosuppressive enzyme IDO and
ovarian cancer progression, as well as to develop a molecular
therapy-targeting IDO. Previously, we transfected an IDO expression
vector into a non-IDO-expressing human ovarian cancer cell line and
established an IDO-expressing cell line to examine the relationship
between IDO expression and ovarian cancer progression, especially
in term of peritoneal dissemination in vivo (35). In the present study, we utilized an
shRNA expression vector targeting the IDO gene to examine whether
inhibition of IDO can control peritoneal dissemination of ovarian
cancer. We found that the downregulation of IDO expression did not
influence cancer cell growth in vitro, but controlled tumor
growth and peritoneal dissemination in vivo. In addition,
the downregulation of IDO reinforced the sensitivity of cancer
cells to NK cells in vitro and promoted NK cell accumulation
in the tumor stroma in vivo. These findings indicate that
the downregulation of IDO controls peritoneal dissemination of
ovarian cancer by promoting NK cell accumulation in tumors,
suggesting that IDO is a useful therapeutic target for patients
with ovarian cancer.
Lack of the essential amino acid tryptophan and
accumulation of its metabolite, kynurenine, inhibit cell growth and
induce apoptosis. T cells are particularly sensitive to this type
of stress (13). Regarding the
mechanism of cancer cell immunotolerance, IDO has been shown to
promote local tryptophan depletion, resulting in T-cell function
suppression around IDO-expressing cancer cells and local
immunotolerance (19). The
possibility cannot be excluded that IDO expression is involved in
the immunotolerance of ovarian cancer through such a T
cell-mediated mechanism. We initially obtained a murine ovarian
tumor cell line (OV2944-HM-1) with the ability to develop into
subcutaneous tumor and disseminate peritoneally in immunocompetent
mice. However, IDO was hardly detected in this cell line, according
to the results of Western blot analysis using an anti-mouse IDO
antibody (data not shown). Therefore, we chose the human ovarian
cancer cell line (SKOV-3) that constitutively expresses IDO and
implanted them in nude mice. Nude mice congenitally lack T cells;
therefore, in this experimental system, we could not examine the
effect of IDO on T-cell function.
It has been reported that IDO induces the
accumulation of the tryptophan metabolite kynurenine, which
suppresses NK cell receptor expression, and thereby inhibits NK
cell function (22). Similarly, in
our previous experiments, IDO expression inhibited the cytotoxic
activity of NK cells in vitro and suppressed NK cell
accumulation in the tumor stroma in vivo (35). Herein, we demonstrated that IDO
downregulation enhanced the sensitivity of cancer cells to NK cells
in vitro and promoted NK cell accumulation in the tumor
stroma in vivo. Thus, the downregulation of IDO reinforced
the sensitivity of cancer cells to NK cells, mediating peritoneal
dissemination and growth of ovarian cancer.
Typical methods of inhibiting IDO function include
the use of 1-methly-tryptophan (1-MT) and gene silencing by RNAi.
In IDO-catalyzed tryptophan metabolism, 1-MT competes with
tryptophan for IDO, acting as an IDO inhibitor (36). Inaba et al reported that the
oral administration of 1-MT to the host suppressed the tumor growth
of IDO-overexpressing ovarian cancer cells with enhanced
proliferative activity (26).
Similarly, in our previous study, we showed that oral
administration of 1-MT inhibited the tumor growth potential of
IDO-transfected ovarian cancer cells with enhanced proliferative
activity (35). In our study, mice
given 1-MT orally showed no fatal side effects (35). These findings suggest the
possibility of IDO-targeted molecular therapy for ovarian cancer
using the oral administration of 1-MT or its analogues. Muller
et al reported that the combination of 1-MT with paclitaxel
synergistically regressed an autochthonous breast cancer (37). In addition, Inaba et al
demonstrated that treatment with 1-MT plus paclitaxel
synergistically prolonged mouse survival compared to treatment with
paclitaxel alone in an IDO-overexpressing ovarian cancer peritoneal
carcinomatosis model (26). Since
paclitaxel is a key drug in the chemotherapy of ovarian cancer, the
combined use of such an anticancer drug and targeted therapy
against IDO may be advantageous in treating ovarian cancer.
Compared to 1-MT treatment, RNAi demonstrates higher
potency and efficiency (38). To
date, chemically synthesized siRNA and vector-mediated expression
of shRNA are the more commonly used RNAi techniques for gene
silencing in mammalian cells (30,39).
Although siRNA can be more easily transfected into cancer cells,
and its silencing function is more effective, its function is
transient. The remarkable advantages of shRNA is that the
inhibition of target genes can last for weeks or even months,
making it possible to elucidate the consequences of long-term
stable silencing of a gene (30).
In actual clinical settings, nanoparticle-based vectors (40) or viral-based expression vectors
could be used to deliver the IDO shRNA to the cancer cells.
The results of this study demonstrate that the
downregulation of IDO in human ovarian cancer cells constitutively
expressing IDO inhibits ovarian cancer progression, suggesting that
the use of IDO-targeted shRNA as a potentially effective
molecular-targeted therapy for ovarian cancer.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2
|
Heintz AP: Surgery in advanced ovarian
carcinoma: is there proof to show the benefit? Eur J Surg Oncol.
14:91–99. 1988.PubMed/NCBI
|
|
3
|
McGuire WP, Hoskins WJ, Brady MF, et al:
Cyclophosphamide and cisplatin compared with paclitaxel and
cisplatin in patients with stage III and stage IV ovarian cancer. N
Engl J Med. 334:1–6. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takei Y, Suzuki M, Ohwada M, et al: A
feasibility study of paclitaxel and carboplatin therapy in Japanese
patients with epithelial ovarian cancer. Oncol Rep. 10:951–955.
2003.PubMed/NCBI
|
|
5
|
Dunn GP, Bruce AT, Ikeda H, Old LJ and
Schreiber RD: Cancer immunoediting: from immunosurveillance to
tumor escape. Nat Immunol. 3:991–998. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schreiber RD, Old LJ and Smyth MJ: Cancer
immunoediting: integrating immunity’s roles in cancer suppression
and promotion. Science. 331:1565–1570. 2011.PubMed/NCBI
|
|
7
|
Higuchi K and Hayaishi O: Enzymic
formation of D-kynurenine from D-tryptophan. Arch Biochem Biophys.
120:397–403. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamamoto S and Hayaishi O: Tryptophan
pyrrolase of rabbit intestine. D- and L-tryptophan-cleaving enzyme
or enzymes. J Biol Chem. 242:5260–5266. 1967.PubMed/NCBI
|
|
9
|
Shimizu T, Nomiyama S, Hirata F and
Hayaishi O: Indoleamine 2,3-dioxygenase. Purification and some
properties. J Biol Chem. 253:4700–4706. 1978.PubMed/NCBI
|
|
10
|
Yoshida R, Urade Y, Tokuda M and Hayaishi
O: Induction of indoleamine 2,3-dioxygenase in mouse lung during
virus infection. Proc Natl Acad Sci USA. 76:4084–4086. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoshida R and Hayaishi O: Induction of
pulmonary indoleamine 2,3-dioxygenase by intraperitoneal injection
of bacterial lipopolysaccharide. Proc Natl Acad Sci USA.
75:3998–4000. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fujigaki S, Saito K, Sekikawa K, et al:
Lipopolysaccharide induction of indoleamine 2,3-dioxygenase is
mediated dominantly by an IFN-gamma-independent mechanism. Eur J
Immunol. 31:2313–2318. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Munn DH, Zhou M, Attwood JT, et al:
Prevention of allogeneic fetal rejection by tryptophan catabolism.
Science. 281:1191–1193. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schroecksnadel K, Winkler C, Duftner C,
Wirleitner B, Schirmer M and Fuchs D: Tryptophan degradation
increases with stage in patients with rheumatoid arthritis. Clin
Rheumatol. 25:334–337. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brown RR, Ozaki Y, Datta SP, Borden EC,
Sondel PM and Malone DG: Implications of interferon-induced
tryptophan catabolism in cancer, auto-immune diseases and AIDS. Adv
Exp Med Biol. 294:425–435. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Labadarios D, McKenzie DY, Dickerson JW
and Parke DV: Metabolic abnormalities of tryptophan and nicotinic
acid in patients with rheumatoid arthritis. Rheumatol Rehabil.
17:227–232. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Varga J, Yufit T and Brown RR: Inhibition
of collagenase and stromelysin gene expression by interferon-gamma
in human dermal fibroblasts is mediated in part via induction of
tryptophan degradation. J Clin Invest. 96:475–481. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mellor AL and Munn DH: IDO expression by
dendritic cells: tolerance and tryptophan catabolism. Nat Rev
Immunol. 4:762–774. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Uyttenhove C, Pilotte L, Theate I, et al:
Evidence for a tumoral immune resistance mechanism based on
tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med.
9:1269–1274. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vivier E, Tomasello E, Baratin M, Walzer T
and Ugolini S: Functions of natural killer cells. Nat Immunol.
9:503–510. 2008. View
Article : Google Scholar
|
|
21
|
Lanier LL: Up on the tightrope: natural
killer cell activation and inhibition. Nat Immunol. 9:495–502.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Della Chiesa D, Carlomagno S, Frumento G,
et al: The tryptophan catabolite L-kynurenine inhibits the surface
expression of NKp46- and NKG2D-activating receptors and regulates
NK-cell function. Blood. 108:4118–4125. 2006.PubMed/NCBI
|
|
23
|
Inaba T, Ino K, Kajiyama H, et al:
Indoleamine 2,3-dioxygenase expression predicts impaired survival
of invasive cervical cancer patients treated with radical
hysterectomy. Gynecol Oncol. 117:423–428. 2010. View Article : Google Scholar
|
|
24
|
Ino K, Yoshida N, Kajiyama H, et al:
Indoleamine 2,3-dioxygenase is a novel prognostic indicator for
endometrial cancer. Br J Cancer. 95:1555–1561. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takao M, Okamoto A, Nikaido T, et al:
Increased synthesis of indoleamine-2,3-dioxygenase protein is
positively associated with impaired survival in patients with
serous-type, but not with other types of, ovarian cancer. Oncol
Rep. 17:1333–1339. 2007.
|
|
26
|
Inaba T, Ino K, Kajiyama H, et al: Role of
the immunosuppressive enzyme indoleamine 2,3-dioxygenase in the
progression of ovarian carcinoma. Gynecol Oncol. 115:185–192. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gartel AL and Kandel ES: RNA interference
in cancer. Biomol Eng. 23:17–34. 2006. View Article : Google Scholar
|
|
28
|
Scherr M and Eder M: Gene silencing by
small regulatory RNAs in mammalian cells. Cell Cycle. 6:444–449.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hannon GJ, Chubb A, Maroney PA, Hannon G,
Altman S and Nilsen TW: Multiple cis-acting elements are required
for RNA polymerase III transcription of the gene encoding H1 RNA,
the RNA component of human RNase P. J Biol Chem. 266:22796–22799.
1991.PubMed/NCBI
|
|
30
|
Walchli S and Sioud M: Vector-based
delivery of siRNAs: in vitro and in vivo challenges. Front Biosci.
13:3488–3493. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fogh J, Wright WC and Loveless JD: Absence
of HeLa cell contamination in 169 cell lines derived from human
tumors. J Natl Cancer Inst. 58:209–214. 1977.PubMed/NCBI
|
|
32
|
Yagita M, Huang CL, Umehara H, et al: A
novel natural killer cell line (KHYG-1) from a patient with
aggressive natural killer cell leukemia carrying a p53 point
mutation. Leukemia. 14:922–930. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takikawa O, Kuroiwa T, Yamazaki F and Kido
R: Mechanism of interferon-gamma action. Characterization of
indoleamine 2,3-dioxygenase in cultured human cells induced by
interferon-gamma and evaluation of the enzyme-mediated tryptophan
degradation in its anticellular activity. J Biol Chem.
263:2041–2048. 1988.
|
|
34
|
Miyagishi M and Taira K: Strategies for
generation of an siRNA expression library directed against the
human genome. Oligonucleotides. 13:325–333. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nonaka H, Saga Y, Fujiwara H, et al:
Indoleamine 2,3-dioxygenase promotes peritoneal dissemination of
ovarian cancer through inhibition of natural killercell function
and angiogenesis promotion. Int J Oncol. 38:113–120. 2011.
|
|
36
|
Cady SG and Sono M:
1-Methyl-DL-tryptophan, beta-(3-benzofuranyl)-DL-alanine (the
oxygen analog of tryptophan), and
beta-[3-benzo(b)thienyl]-DL-alanine (the sulfur analog of
tryptophan) are competitive inhibitors for indoleamine
2,3-dioxygenase. Arch Biochem Biophys. 291:326–333. 1991.PubMed/NCBI
|
|
37
|
Muller AJ, DuHadaway JB, Donover PS,
Sutanto-Ward E and Prendergast GC: Inhibition of indoleamine
2,3-dioxygenase, an immunoregulatory target of the cancer
suppression gene Bin1, potentiates cancer chemotherapy. Nat Med.
11:312–319. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yen MC, Lin CC, Chen YL, et al: A novel
cancer therapy by skin delivery of indoleamine 2,3-dioxygenase
siRNA. Clin Cancer Res. 15:641–649. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shiota M, Ikeda Y and Wadhwa R: The
factors that contribute to the long-term expression of siRNA.
Nucleic Acids Symp Ser (Oxf). 2006:243–244. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Serda RE, Godin B, Blanco E, Chiappini C
and Ferrari M: Multi-stage delivery nano-particle systems for
therapeutic applications. Biochim Biophys Acta. 1810:317–329. 2011.
View Article : Google Scholar : PubMed/NCBI
|