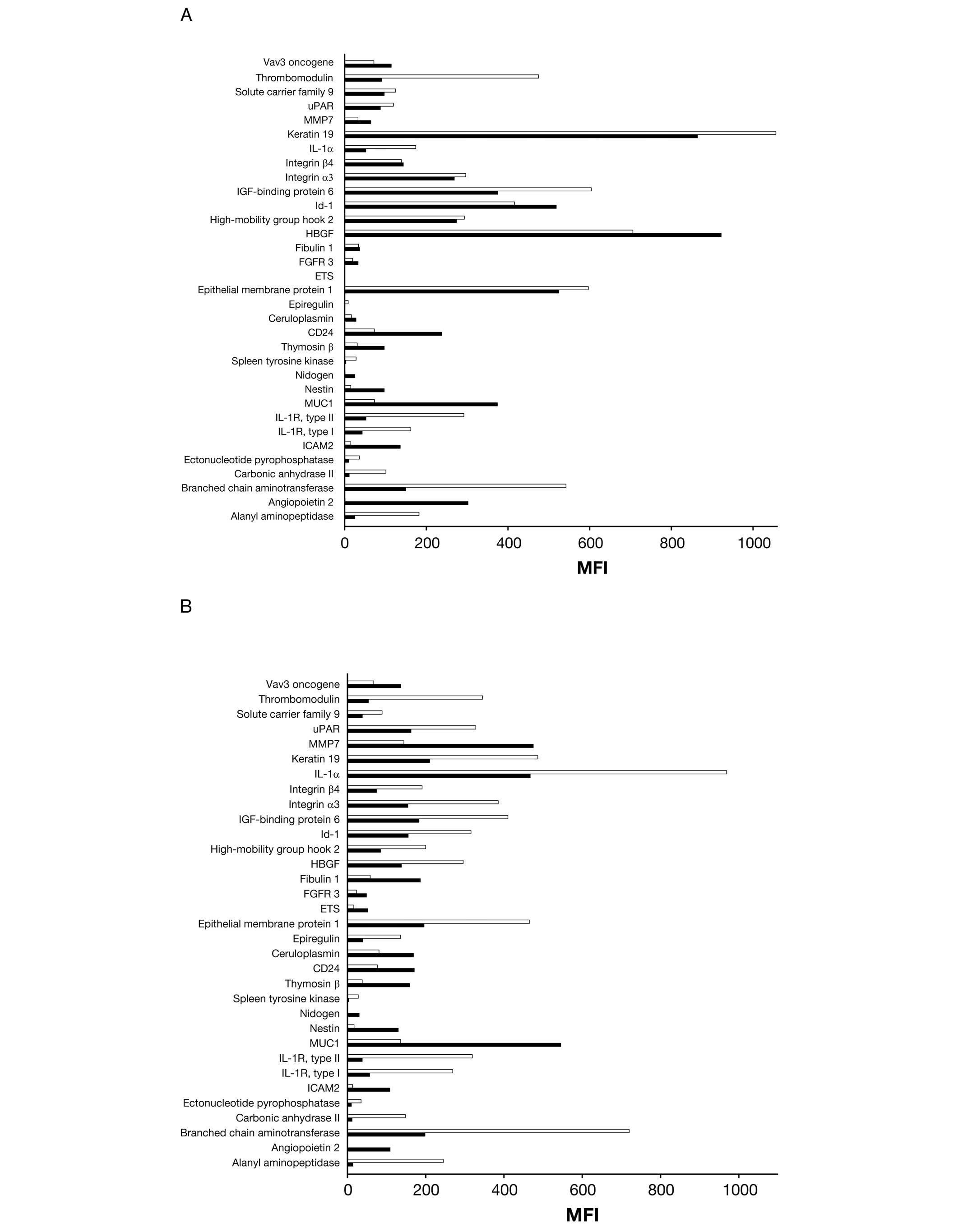
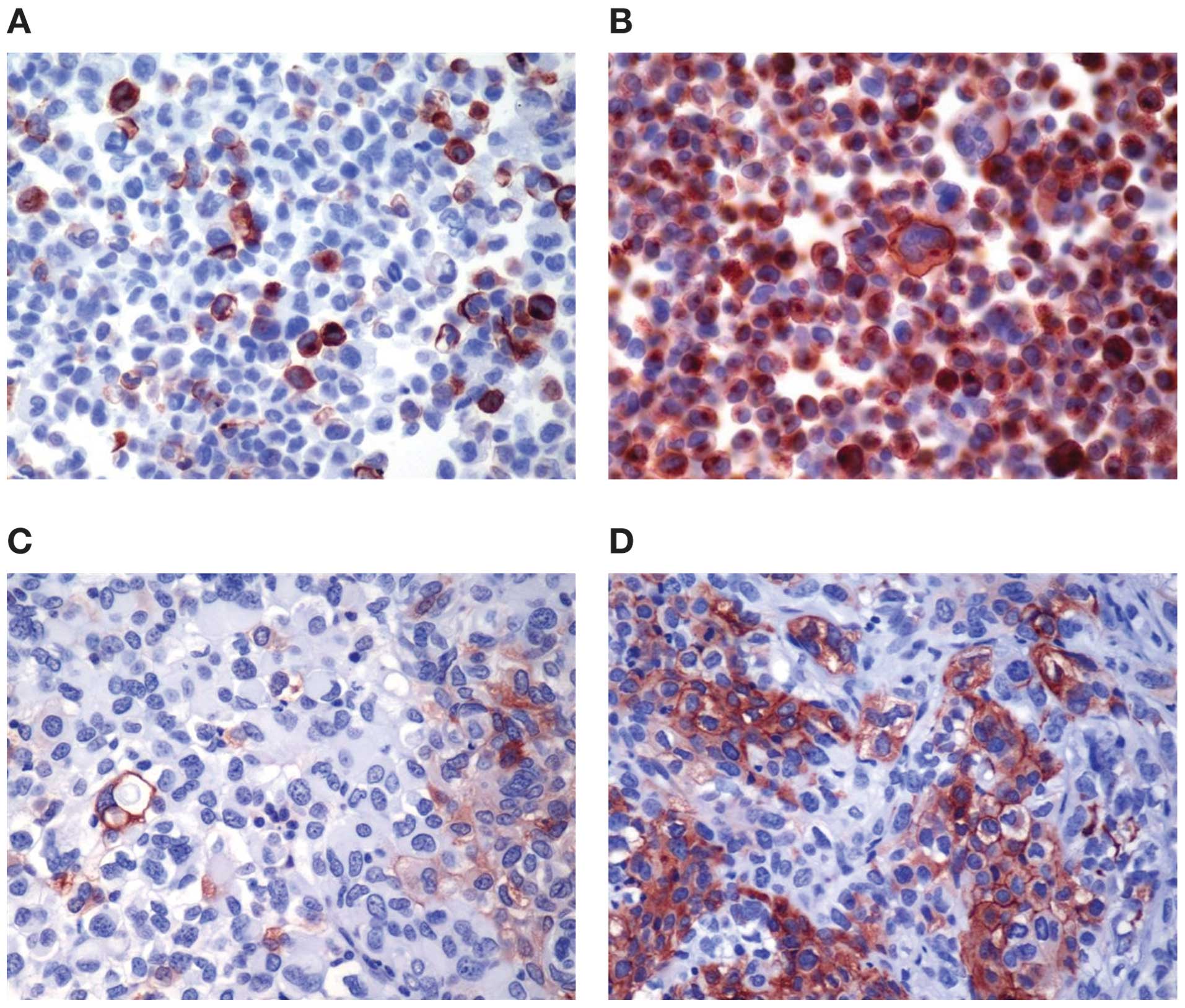
|
1
|
Alroy I and Yarden Y: The ErbB signaling
network in embryogenesis and oncogenesis: signal diversification
through combinatorial ligand-receptor interactions. FEBS Lett.
410:83–86. 1997. View Article : Google Scholar
|
|
2
|
Olayioye MA, Neve RM, Lane HA and Hynes
NE: The ErbB signaling network: receptor heterodimerization in
development and cancer. EMBO J. 19:3159–3167. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Slamon DJ, Godolphin W, Jones LA, Holt JA,
Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al:
Studies of the HER-2/neu proto-oncogene in human breast and ovarian
cancers. Science. 244:707–712. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ross JS, Slodkowska EA, Symmans WF,
Pusztai L, Ravdin PM and Hortobagyi GN: The HER-2 receptor and
breast cancer: ten years of targeted anti-HER-2 therapy and
personalized medicine. Oncologist. 14:320–368. 2009.PubMed/NCBI
|
|
5
|
Dawood S, Broglio K, Buzdar AU, Hortobagyi
GN and Giordano SH: Prognosis of women with metastatic breast
cancer by HER2 status and trastuzumab treatment: an
institutional-based review. J Clin Oncol. 28:92–98. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bookman MA, Darcy KM, Clarke-Pearson D,
Boothby RA and Horowitz IR: Evaluation of monoclonal anti-HER2
antibody, trastuzumab, in patients with recurrent or refractory
ovarian or primary peritoneal carcinoma with overexpression of
HER2: a phase II trial of the Gynecologic Oncology Group. J Clin
Oncol. 21:283–290. 2003. View Article : Google Scholar
|
|
7
|
McAlpine JN, Wiegand KC, Vang R, Ronnett
BM, Adamiak A, Köbel M, Kalloger SE, Swenerton KD, Huntsman DG,
Gilks CB and Miller DM: HER2 overexpression and amplification is
present in a subset of ovarian mucinous carcinomas and can be
targeted with trastuzumab therapy. BMC Cancer. 9:4332009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Slamon DJ, Leyland-Jones B, Shak S, Fuchs
H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M,
Baselga J and Norton L: Use of chemotherapy plus a monoclonal
antibody against HER2 for metastatic breast cancer that
overexpresses HER2. N Engl J Med. 344:783–792. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cobleigh MA, Vogel CL, Tripathy D, Robert
NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman
G and Slamon DJ: Multinational study of the efficacy and safety of
humanized anti-HER2 monoclonal antibody in women who have
HER2-overexpressing metastatic breast cancer that has progressed
after chemotherapy for metastatic disease. J Clin Oncol.
17:2639–2648. 1999.
|
|
10
|
Romond EH, Perez EA, Bryant J, Suman VJ,
Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman
PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG,
Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas
EP, Lingle WL, Klein PM, Ingle JN and Wolmark N: Trastuzumab plus
adjuvant chemotherapy for operable HER2-positive breast cancer. N
Engl J Med. 353:1673–1684. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Perez EA, Romond EH, Suman VJ, Jeong JH,
Davidson NE, Geyer CE Jr, Martino S, Mamounas EP, Kaufman PA and
Wolmark N: Four-year follow-up of trastuzumab plus adjuvant
chemotherapy for operable human epidermal growth factor receptor
2-positive breast cancer: joint analysis of data from NCCTG N9831
and NSABP B-31. J Clin Oncol. 29:3366–3373. 2011.PubMed/NCBI
|
|
12
|
Smith I, Procter M, Gelber RD, Guillaume
S, Feyereislova A, Dowsett M, Goldhirsch A, Untch M, Mariani G,
Baselga J, Kaufmann M, Cameron D, Bell R, Bergh J, Coleman R,
Wardley A, Harbeck N, Lopez RI, Mallmann P, Gelmon K, Wilcken N,
Wist E, Sánchez Rovira P and Piccart-Gebhart MJ: 2-year follow-up
of trastuzumab after adjuvant chemotherapy in HER2-positive breast
cancer: a randomised controlled trial. Lancet. 369:29–36.
2007.PubMed/NCBI
|
|
13
|
Slamon D, Eiermann W, Robert N, Pienkowski
T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M,
Pinter T, Valero V, Liu MC, Sauter G, von Minckwitz G, Visco F, Bee
V, Buyse M, Bendahmane B, Tabah-Fisch I, Lindsay MA, Riva A and
Crown J: Adjuvant trastuzumab in HER2-positive breast cancer. N
Engl J Med. 365:1273–1283. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J and Kang YK:
Trastuzumab in combination with chemotherapy versus chemotherapy
alone for treatment of HER2-positive advanced gastric or
gastro-oesophageal junction cancer (ToGA): a phase 3, open-label,
randomised controlled trial. Lancet. 376:687–697. 2010. View Article : Google Scholar
|
|
15
|
Spector NL and Blackwell KL: Understanding
the mechanisms behind trastuzumab therapy for human epidermal
growth factor receptor 2-positive breast cancer. J Clin Oncol.
27:5838–5847. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Albanell J and Baselga J: Unraveling
resistance to trastuzumab (Herceptin): insulin-like growth factor-I
receptor, a new suspect. J Natl Cancer Inst. 93:1830–1832. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nahta R, Takahashi T, Ueno NT, Hung MC and
Esteva FJ: p27Kip1 down-regulation is associated with
trastuzumab resistance in breast cancer cells. Cancer Res.
64:3981–3986. 2004.
|
|
18
|
Nagata Y, Lan KH, Zhou X, Tan M, Esteva
FJ, Sahin AA, Klos KS, Li P, Monia BP, Nguyen NT, Hortobagyi GN,
Hung MC and Yu D: PTEN activation contributes to tumor inhibition
by trastuzumab, and loss of PTEN predicts trastuzumab resistance in
patients. Cancer Cell. 6:117–127. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nagy P, Friedländer E, Tanner M, Kapanen
AI, Carraway KL, Isola J and Jovin TM: Decreased accessibility and
lack of activation of ErbB2 in JIMT-1, a Herceptin-resistant,
MUC4-expressing breast cancer cell line. Cancer Res. 65:473–482.
2005.PubMed/NCBI
|
|
20
|
Dhillon J, Astanehe A, Lee C, Fotovati A,
Hu K and Dunn SE: The expression of activated Y-box binding
protein-1 serine 102 mediates trastuzumab resistance in breast
cancer cells by increasing CD44+ cells. Oncogene.
29:6294–6300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang S, Huang WC, Li P, Guo H, Poh SB,
Brady SW, Xiong Y, Tseng LM, Li SH, Ding Z, Sahin AA, Esteva FJ,
Hortobagyi GN and Yu D: Combating trastuzumab resistance by
targeting SRC, a common node downstream of multiple resistance
pathways. Nat Med. 17:461–469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fernandes H, Cohen S and Bishayee S:
Glycosylation-induced conformational modification positively
regulates receptor-receptor association: a study with an aberrant
epidermal growth factor receptor (EGFRvIII/DeltaEGFR) expressed in
cancer cells. J Biol Chem. 276:5375–5383. 2001. View Article : Google Scholar
|
|
23
|
Meric F, Lee WP, Sahin A, Zhang H, Kung HJ
and Hung MC: Expression profile of tyrosine kinases in breast
cancer. Clin Cancer Res. 8:361–367. 2002.PubMed/NCBI
|
|
24
|
Unger MA, Rishi M, Clemmer VB, Hartman JL,
Keiper EA, Greshock JD, Chodosh LA, Liebman MN and Weber BL:
Characterization of adjacent breast tumors using oligonucleotide
microarrays. Breast Cancer Res. 3:336–341. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Raponi M, Belly RT, Karp JE, Lancet JE,
Atkins D and Wang Y: Microarray analysis reveals genetic pathways
modulated by tipifarnib in acute myeloid leukemia. BMC Cancer.
4:562004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sliwkowski MX, Lofgren JA, Lewis GD,
Hotaling TE, Fendly BM and Fox JA: Nonclinical studies addressing
the mechanism of action of trastuzumab (Herceptin). Semin Oncol.
26(Suppl): 60–70. 1999.PubMed/NCBI
|
|
27
|
Schroeder JA, Thompson MC, Gardner MM and
Gendler SJ: Transgenic MUC1 interacts with epidermal growth factor
receptor and correlates with mitogen-activated protein kinase
activation in the mouse mammary gland. J Biol Chem.
276:13057–13064. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nielsen DL, Andersson M and Kamby C:
HER2-targeted therapy in breast cancer. Monoclonal antibodies and
tyrosine kinase inhibitors. Cancer Treat Rev. 35:121–136. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
du Manoir JM, Francia G, Man S, Mossoba M,
Medin JA, Viloria-Petit A, Hicklin DJ, Emmenegger U and Kerbel RS:
Strategies for delaying or treating in vivo acquired resistance to
trastuzumab in human breast cancer xenografts. Clin Cancer Res.
12:904–916. 2006.
|
|
30
|
Fessler SP, Wotkowicz MT, Mahanta SK and
Bamdad C: MUC1* is a determinant of trastuzumab
(Herceptin) resistance in breast cancer cells. Breast Cancer Res
Treat. 118:113–124. 2009.
|
|
31
|
Valabrega G, Capellero S, Cavalloni G,
Zaccarello G, Petrelli A, Migliardi G, Milani A, Peraldo-Neia C,
Gammaitoni L, Sapino A, Pecchioni C, Moggio A, Giordano S, Aglietta
M and Montemurro F: HER2-positive breast cancer cells resistant to
trastuzumab and lapatinib lose reliance upon HER2 and are sensitive
to the multitargeted kinase inhibitor sorafenib. Breast Cancer Res
Treat. 130:29–40. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ramanathan RK, Hwang JJ, Zamboni WC,
Sinicrope FA, Safran H, Wong MK, Earle M, Brufsky A, Evans T,
Troetschel M, Walko C, Day R, Chen HX and Finkelstein S: Low
overexpression of HER-2/neu in advanced colorectal cancer limits
the usefulness of trastuzumab (Herceptin) and irinotecan as
therapy. A phase II trial Cancer Invest. 22:858–865. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pietras RJ, Fendly BM, Chazin VR, Pegram
MD, Howell SB and Slamon DJ: Antibody to HER-2/neu receptor blocks
DNA repair after cisplatin in human breast and ovarian cells.
Oncogene. 9:1829–1838. 1994.PubMed/NCBI
|
|
34
|
Ritter CA, Bianco R, Dugger T, Forbes J,
Qu S, Rinehart C, King W and Arteaga CL: Mechanisms of resistance
development against trastuzumab (Herceptin) in an in vivo breast
cancer model. Int J Clin Pharmacol Ther. 42:642–643. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
O’Brien NA, Browne BC, Chow L, Wang Y,
Ginther C, Arboleda J, Duffy MJ, Crown J, O’Donovan N and Slamon
DJ: Activated phosphoinositide 3-kinase/AKT signaling confers
resistance to trastuzumab but not lapatinib. Mol Cancer Ther.
9:1489–1502. 2010.PubMed/NCBI
|
|
36
|
Scheuer W, Freiss T, Burtscher H,
Bossenmaier B, Endl J and Hasmann M: Strongly enhanced antitumor
activity of trastuzumab and pertuzumab combination treatment on
HER2-positive human xenograft tumor models. Cancer Res.
69:9330–9336. 2009. View Article : Google Scholar
|
|
37
|
Vogel CL, Cobleigh MA, Tripathy D, Gutheil
JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF,
Burchmore M, Shak S, Stewart SJ and Press M: Efficacy and safety of
trastuzumab as a single agent in first-line treatment of
HER2-overexpressing metastatic breast cancer. J Clin Oncol.
20:719–726. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Reim F, Dombrowski Y, Ritter C, Buttmann
M, Häusler S, Ossadnik M, Krockenberger M, Beier D, Beier CP, Dietl
J, Becker JC, Hönig A and Wischhusen J: Immunoselection of breast
and ovarian cancer cells with trastuzumab and natural killer cells:
selective escape of
CD44high/CD24low/HER2low breast
cancer stem cells. Cancer Res. 69:8058–8066. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Packer LM, Williams SJ, Callaghan S,
Gotley DC and McGuckin MA: Expression of the cell surface mucin
gene family in adenocarcinomas. Int J Oncol. 25:1119–1126.
2004.PubMed/NCBI
|
|
40
|
Scibetta AG, Albanese I, Morris J, Cooper
L, Downward J, Rowe P and Taylor-Papadimitriou J: Regulation of
MUC1 expression in human mammary cell lines by the c-ErbB2 and ras
signaling pathways. DNA Cell Biol. 20:265–274. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hollingsworth MA and Swanson BJ: Mucins in
cancer: protection and control of the cell surface. Nat Rev Cancer.
4:45–60. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li Y, Kuwahara H, Ren J, Wen G and Kufe D:
The c-Src tyrosine kinase regulates signaling of the human DF3/MUC1
carcinoma-associated antigen with GSK3 beta and beta-catenin. J
Biol Chem. 276:6061–6064. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Baselga J, Gelmon KA, Verma S, Wardley A,
Conte P, Miles D, Bianchi G, Cortes J, McNally VA, Ross GA,
Fumoleau P and Gianni L: Phase II trial of pertuzumab and
trastuzumab in patients with human epidermal growth factor receptor
2-positive metastatic breast cancer that progressed during prior
trastuzumab therapy. J Clin Oncol. 28:1138–1144. 2010. View Article : Google Scholar
|
|
44
|
Krop IE, LoRusso O, Miller KD, Modi S,
Yardley D, Rodriguez G, Lu M, Burrington B, Agresta S and Rugo H: A
phase 2 study of the HER2 antibody-drug conjugate trastuzumab-DM1
(TDM-1) in patients (PTS) with HER2-positive metastatic breast
cancer (MBC) previously treated with trastuzumab, lapatinib, and
chemotherapy [abstract 2770]. Ann Oncol. 21(Suppl 8):
viii972010.
|
|
45
|
Burris HA III, Rugo HS, Vukelja SJ, Vogel
CL, Borson RA, Limentani S, Tan-Chiu E, Krop IE, Michaelson RA,
Girish S, Amler L, Zheng M, Chu YW, Klencke B and O’Shaughnessy JA:
Phase II study of the antibody drug conjugate trastuzumab-DM1 for
the treatment of human epidermal growth factor receptor 2
(HER2)-positive breast cancer after prior HER2-directed therapy. J
Clin Oncol. 29:398–405. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Konecny GE, Pegram MD, Venkatesan N, Finn
R, Yang G, Rahmeh M, Untch M, Rusnak DW, Spehar G, Mullin RJ, Keith
BR, Gilmer TM, Berger M, Podratz KC and Slamon DJ: Activity of the
dual kinase inhibitor lapatinib (GW572016) against
HER-2-overexpressing and trastuzumab-treated breast cancer cells.
Cancer Res. 66:1630–1639. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Geyer CE, Forster J, Lindquist D, Chan S,
Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A,
Kaufman B, Skarlos D, Campone M, Davidson N, Berger M, Oliva C,
Rubin SD, Stein S and Cameron D: Lapatinib plus capecitabine for
HER2-positive advanced breast cancer. N Engl J Med. 355:2733–2743.
2006. View Article : Google Scholar : PubMed/NCBI
|