Introduction
The search for newer and more reliable biomarkers is
a continuing research endeavour. Biomarkers are crucial in
narrowing down on a precise diagnosis and are equally important
during the follow-up stage in disease management. Preliminary
experiments in our laboratory indicated that endothelial protein C
could be a possible biomarker candidate.
The endothelial protein C along with its receptor
displays pleiotropic functions. One such recognized function is its
role as an important regulator of homeostasis, besides being
implicated in the systemic response to acute inflammation. Protein
C zymogen binds to endothelial protein C receptor (EPCR) with high
affinity and stimulates its activation via the
thrombinthrombomodulin complex. Activated protein C (aPC), together
with its co-factor, protein S, degrade factors Va and VIIIa and
thereby interfere with thrombin generation (1–3).
EPCR is a type 1 transmembrane glycoprotein (CD201)
that shares considerable homology with the major histocompatibility
complex (4). EPCR is known to be
constitutively released in the plasma in a free soluble form as a
result of proteolytic cleavage. This soluble form of EPCR (sEPCR)
has the ability to trap free aPC, thereby depriving it of its
anti-coagulant function within the surrounding environment
(5,6). It has been shown that the shedding of
EPCR by human umbilical cord endothelial cells (HUVECs) is
effectively regulated by IL-1β and TNF-α, and downstream by MAP
kinase signaling pathways and metalloproteinases (7).
During acute inflammation, a significant increase in
circulating sEPCR is observed which in turn could provide an early
biological marker of sepsis outcome (8). It is known that cytokines, such as
IL1-β, and TNF-α, as well as endotoxins, can attenuate the
expression of EPCR which then leads to diminution in plasma aPC
(9). However, the nature of the
anti inflammatory action of aPC remains unclear.
The EPCR gene carries 13 single nucleotide
polymorphisms, which define 3 haplotypes. One of these haplotypes,
A3, encodes a protein, which is more sensitive than the other two
to the action of shedding enzymes, which can result in a marked
increase in the level of sEPCR. The presence of the A3 haplotype
therefore coincides with the presence of a high sEPCR level, the
latter being a candidate risk factor for venous thrombosis
(10–12). Interest in EPCR/aPC in relation to
tumor biology is gaining momentum with the appearance of an
increasing number of publications (13–16).
The appearance of high levels of sEPCR in malignant
tumors during disease and during relapse could have an implication
in venous thrombosis and if detected early enough could serve as a
useful indicator and therefore, preventive measures could be taken.
Taking this evidence into consideration, we undertook a detailed
study of aPC/EPCR not only in a large cohort of tumor biopsies,
patient ascitic fluid and plasma, but we also extended our study to
include in vitro cultured tumor cell lines.
Materials and methods
Reagents
Reagents were obtained from the following sources:
primary antibody AF2245 against EPCR (R&D Systems, Minneapolis,
MN, USA); primary antibody ATAP2 against PAR-1 (Invitrogen,
Carlsbad, CA, USA); biotinylated anti-rabbit, anti-mouse and
anti-goat IgG, streptavidin-fluorescein conjugate (Amersham,
Buckinghamshire, UK); rabbit anti-goat HRP (DakoCytomation,
Glostrup, Denmark); phycoerythrin-coupled anti-P-gp antibody
(Millipore, Billerica, MA, USA); human recombinant aPC (Lilly,
Suresnes, France); U0126 and wortmannin (Calbiochem, San Diego, CA,
USA). The recombinant form of human aPC marketed as Xigris, was
obtained from Eli Lilly (Indianapolis, IN, USA). The PCR primers
were designed with the Primer3 program, BLAST verified, and
synthesized by Eurobio (Les Ulis, France).
Cells
The human cancer cell lines used were: ovarian
(OVCAR, ATCC), breast (MDA-MB231 ATCC), lung (A549) and colorectal
(HT-29, HCT-8R ATCC). Cells were cultured in RPMI-1640 medium
containing 10% fetal calf serum, penicillin (50 U/ml), and
streptomycin (50 μg/ml) and incubated in a humidified
atmosphere containing 5% CO2 at 37°C, as recommended by
the supplier (PAA Laboratories Inc., Etobicoke, ON, USA).
Conditioned medium
Cells, seeded in two 25-cm2 flasks, were
grown to 80% confluency and then incubated in 1 ml serum-free
culture medium. aPC (10 μg/ml) was added to one flask, while
the second one, without aPC, served as the control. Cells were
pelleted by centrifugation after 18 h and the conditioned medium
was collected and aliquoted.
Plasma, ascites and mononuclear
cells
Plasma samples
Blood (n=79) samples from patients with ovarian
cancer were obtained from the Oncology Department of Hôtel-Dieu
Hospital (Paris, France) after informed consent, in accordance with
the rules of the revised Helsinki protocol. A total of 79 patients
with an age range of 39–90 years (mean ± SD, 62±14 years) were
selected. Patients received anti-coagulants.
Ascitic cells
Peritoneal fluid from 23 cancer patients of the
Hospital Hôtel-Dieu was collected. As ascite evacuation is part of
the routine management of patients, only oral consent was obtained
from them. Cells from ascitic fluid were pelleted by a short spin
at 1,000 rpm and the supernatant was collected.
EPCR-specific antibody production
Three EPCR-specific peptides were chosen based on
information provided by the crystallographic structure (21) and with the aid of the Cn3D 4.1
program for the tridimensional structure of EPCR. The EPCR-specific
peptides synthesized were as follows: peptide no. 1, 51-GGHLT HVLEG
PDTNT TIIQL-70; peptide no. 2, 68-IQLQP LQEPE SWART QSGLQ-87; and
peptide no. 3, 157-TSGVV TFTLQ QLNAY NRTRY-176. One of the peptides
(peptide no. 3) contained three essential residues required for aPC
binding (Q166, R173 and T174). The peptides were KLH-coupled and
injected in combination with Freund’s adjuvant every two weeks into
New Zealand white rabbits (150 to 300 μg per injection)
provided on contract by Agro-Bio (La Ferté St. Aubin, France),
followed by immunoglobulin purification. The fifth and last antigen
injection was on the 56th day and sera from rabbits were collected
on the 77th day. The specifity of polyclonal antibodies to peptides
was tested using a competition assay by the ELISA method using
purified peptides.
Immunolabeling
The presence of EPCR proteins in cancer cells was
revealed by immunocytochemistry as previously described (17). As the controls, isotypic antibodies
were used in parallel and the nuclei were DAPI-labeled. FACS
analysis was performed on cells detached by accutase (PAA)
treatment and immunolabeled as previously described (17) with EPCR (20 μg/ml) antibody
and compared with the isotypic control. Ascitic cells were examined
as previously described (17).
Cells were observed after staining with methylene blue and
eosin.
Tissue microarray (TMA)
EPCR expression was examined on 4 mm-thick routinely
processed paraffin sections of tumor biopsies as described by Rafii
et al(18). Four different
TMAs were prepared. Ovarian cancer TMA contained 146 samples
(before treatment, n=84 and after treatment, n=62), breast tumor
TMA 120 samples, while colon cancer TMA had 30 samples and the lung
cancer TMA had 24. EPCR proteins were revealed by fluorescent
immunolabeling and compared to the controls using isotypic
antibodies.
sEPCR-ELISA assay
sEPCR in cultured cell supernatants and in ascitic
fluid was measured using Asserachrom sEPCR immunoassay as
recommended by the commercial supplier (Diagnostica Stago,
Parsippany, NJ, USA).
Rerverse transcription-polymerase
chain reaction (RT-PCR) analysis
The cancer cell RNA extracts were prepared using the
Nucleospin RNA-II kit (Macherey-Nagel EURL, Hoerdt, France).
Following reverse transcription [Mu-MLV reverse transcriptase and
oligo(dT) primers], PCR was performed with TaqDNA polymerase
(Gibco-BRL, Paisley, UK). Specific primers for EPCR synthesis were
as follows: sense, 5′-CAA CTT CAG GAT GTT GAC AA-3′; antisense,
5′-CTA CAG CCA CAC CAG CAA T-3′ to yield a product size of 692 bp
(18). The PCR products, along
with a 100-bp DNA ladder, were analyzed by electrophoresis on
agarose gels containing ethidium bromide.
Gene sequencing
The cancer cell RNA extracts were prepared and
subjected to reverse transcription as described above. Cancer cell
DNA extracts were prepared using the Nucleospin Tissue kit
(Macherey-Nagel EURL). Genomic DNA and cDNA samples were shipped on
dry ice to the Qiagen Sequencing Service. Samples were amplified
with HotStarTaq Plus DNA Polymerase using custom-made primers,
designed by Qiagen. These primers were selected according to the
EPCR sequence available under GenBank accession nos. AF106202 or
BC01445. Reference AF106202 includes the entire gene and promoter
region (8,167 bp) whereas BC01445 represents the mRNA (1,381 bp).
Both strands of DNA were sequenced with BigDye 3.1 Terminator
Chemistry (Applied Biosystems, Carlsbad, CA, USA), using the ABI
Sequence Analyzer 3730XL. The genomic DNA sequence was aligned with
the GenBank reference sequence AF106202 whereas the cDNA sequenced
was aligned with BC01445.
Results
Detection of protein C receptor in
situ in ovarian cancer
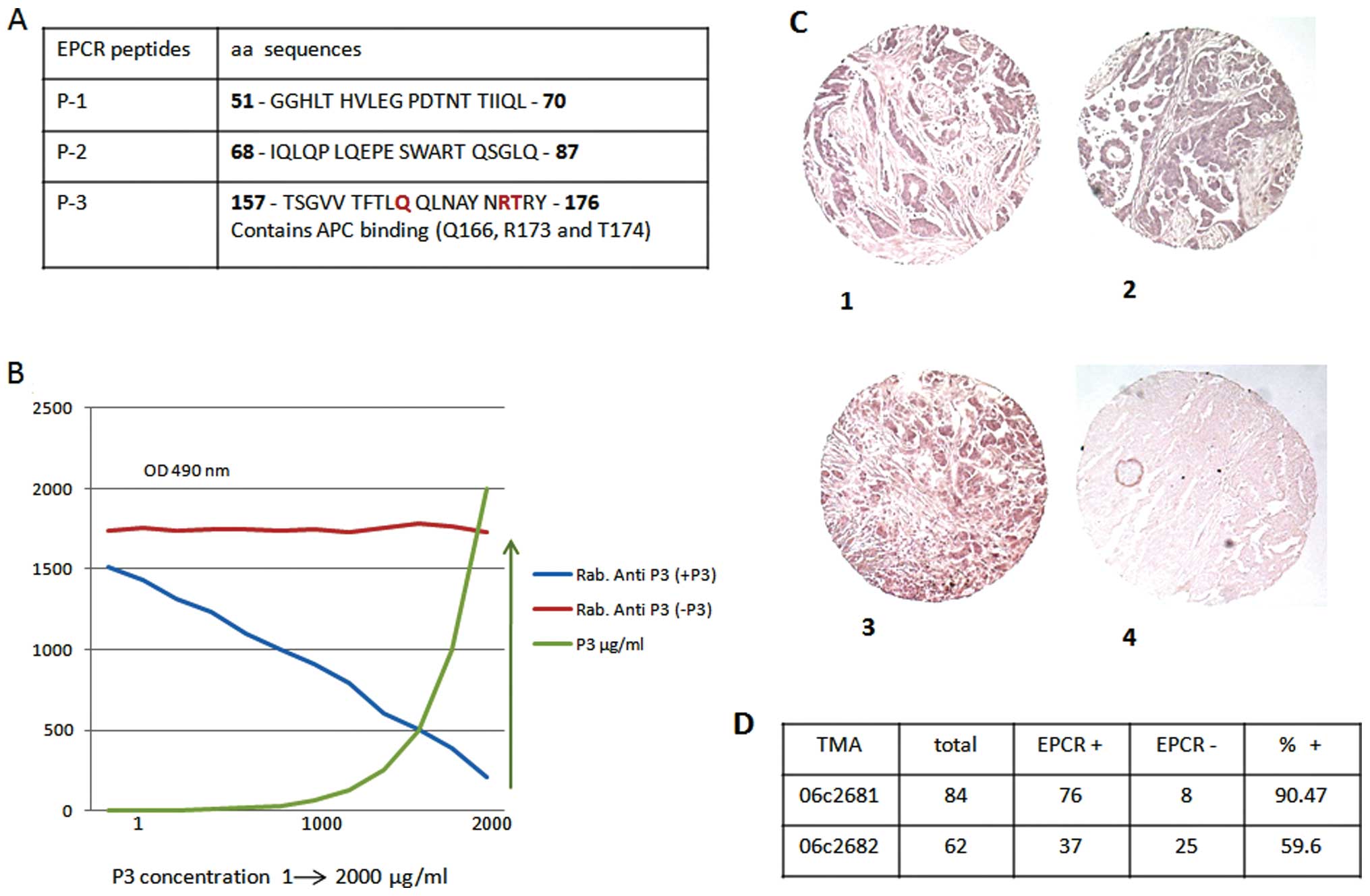
Three rabbit anti-EPCR-specific peptides were
produced. One of these peptides (peptide 3) contained an aPC
binding (Q166, R173 and T174) domain (Fig. 1A). The specificity of these
antibodies was tested using a competitive immune assay based on the
binding competition between rabbit anti-peptides and the
corresponding peptide. Results are presented only for peptide 3
(Fig. 1B). These antibodies were
used for the immune analysis of EPCR in the present study. EPCR
expression in situ was evaluated in various cancer biopsies
by TMA using anti-peptide 3 antibody. As presented in Fig. 1C and D, of the 146 biopsies from
ovarian cancer tested before (n=84, Fig. 1C–1 and C–2) and after (n=62,
Fig. 1C–3) treatment, 90.47 and
56.6% of biopsies were positive for the protein C receptor,
respectively. The control is presented in Fig. 1C–4.
Similarly, EPCR was detected in 20 out of 24 lung
cancer biopsies, representing approximately 80% of positive samples
in this case. Also, of the 30 colon cancer biopsies tested, 20
(65%) were positive for EPCR expression (data not shown).
Therefore, the presence of EPCR in tumors, whatever their origin,
seems to be, more or less, common.
Evaluation of sEPCR in ascitic cell
clusters, fluid samples and plasma from ovarian cancer
patients
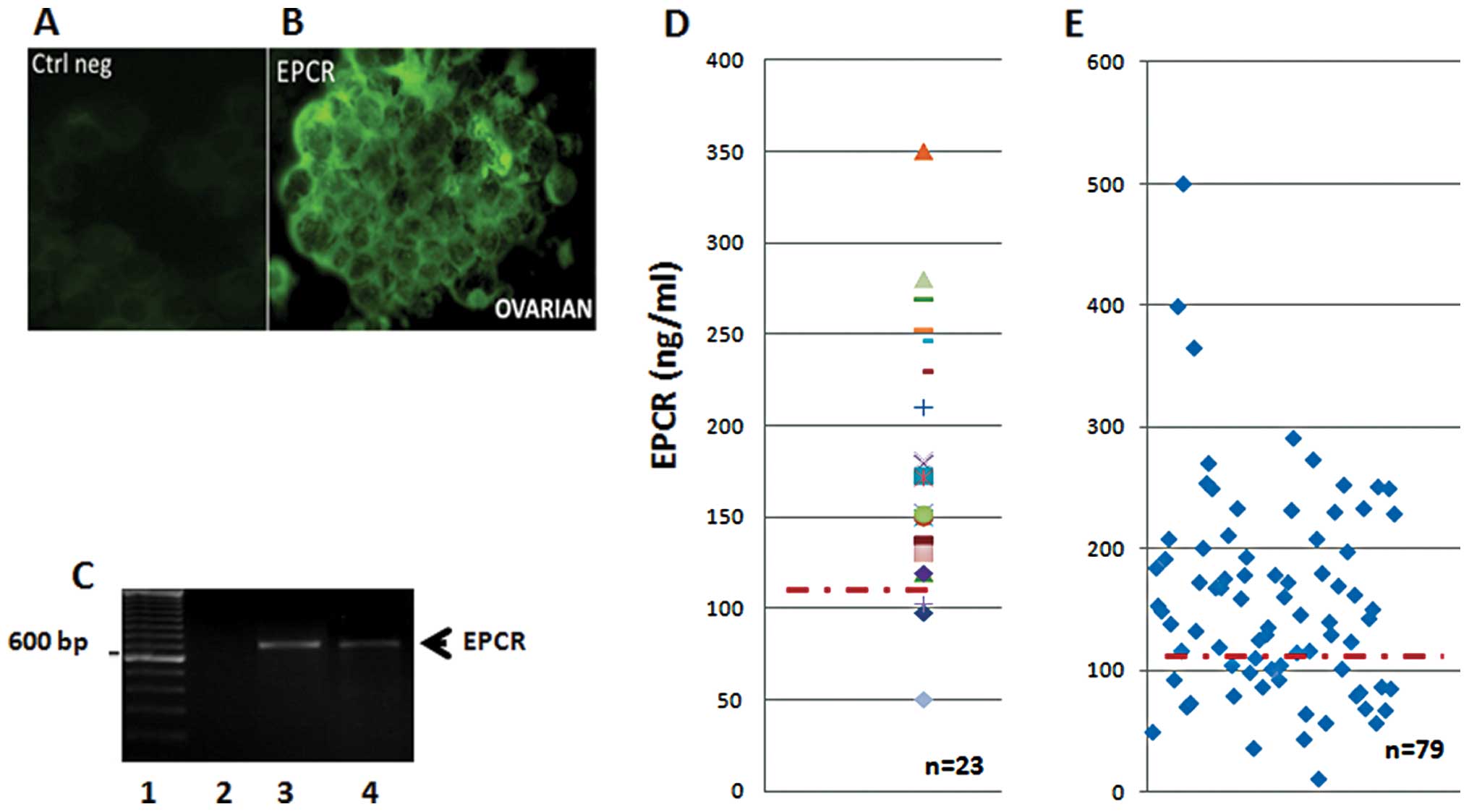
Twenty-three ascitic fluid samples and their
floating cell clusters were also screened for the presence of EPCR
(Fig. 2). The data in Fig. 2 indicate the presence of
membrane-bound EPCR detected by immunohistochemistry (Fig. 2B), while the isotype control
remained negative (Fig. 2A). All
ascitic cell clusters were found to be positive for EPCR protein
expression. Similar results were also obtained when we used rabbit
anti-EPCR peptides as the probe (data not shown). These results
were then confirmed using RT-PCR analysis. Cells in the floating
aggregates (clusters) were found to transcribe the EPCR gene as an
amplified band consistent with the predicted size (Fig. 2C).
The quantity of sEPCR in ascitic supernatants and
plasma from ovarian cancer was assessed by ELISA. All samples
tested positive, and exhibited a concentration well above the
baseline plasma value of 100 ng/ml (Fig. 2D). Ascite samples (91%) tested
positive, revealing a concentration noticeably higher than the
plasma baseline value estimated at 100 ng/ml. The above cited
results correlate with fibrin deposits generally observed in
ascites (data not shown). Finally, in plasma samples from ovarian
cancers, once again, 70% of the samples revealed a concentration
well above the baseline plasma value (Fig. 2E).
sEPCR expressed by ovarian cancer
cells may be a biomarker of cancer onset
We assessed by ELISA the quantity of sEPCR and
detectable CA125 in a relatively small number (n=29) of patients
with ovarian cancer before treatment. This allowed us to establish,
using the Spearman’s test, a positive correlation between plasma
sEPCR and CA125 in the patient population (Spearman’s coefficient,
Rho = 0.36).
We divided all patients into two subgroups: group 1
(n=12) with the amount of plasmatic sEPCR below the baseline (68 to
117 ng/ml) and group 2 (n=17) where the concentration of sEPCR was
noticeably higher than the plasma baseline value (140 to 250 ng/ml,
n=17). The results are presented in Table I.
 | Table IQuantification of EPCR and CA125 in
all the patients. |
Table I
Quantification of EPCR and CA125 in
all the patients.
| A, group 1 | | |
|
| Patients | EPCR, ng/ml | CA125 J0 |
| 1 | 68 | 54 |
| 2 | 86 | 83 |
| 3 | 64 | 84 |
| 4 | 69 | 114 |
| 5 | 57 | 127 |
| 6 | 117 | 195 |
| 7 | 85 | 199 |
| 8 | 57 | 273 |
| 9 | 82 | 279 |
| 10 | 43 | 369.5 |
| 11 | 11 | 553 |
| 12 | 80 | 637 |
| Median | 68.5 | 197 |
| B, group 2 | | |
|
| Patients | EPCR, ng/ml | CA125 J0 |
|
| 1 | 146 | 30.6 |
| 2 | 229 | 99.4 |
| 3 | 250 | 101.3 |
| 4 | 170 | 122 |
| 5 | 150 | 140 |
| 6 | 143 | 148 |
| 7 | 162 | 265 |
| 8 | 130 | 296.1 |
| 9 | 180 | 339 |
| 10 | 291 | 370 |
| 11 | 198 | 387 |
| 12 | 251 | 668 |
| 13 | 274 | 798 |
| 14 | 140 | 928 |
| 15 | 253 | 946 |
| 16 | 230 | 1130 |
| 17 | 234 | 3620 |
| Median | 198 | 339 |
The median value obtained was 68.5 ng/ml for sEPCR
and 197 ng/ml for CA125 in the first group and 198 ng/ml for sEPCR
and 339 ng/ml for CA125 in the second group. The results obtained
show that in the second group, when CA125 increased by 1.72-fold,
the amount of sEPCR also increased by 2.9-fold. Thus, in the second
group, there was a positive correlation between sEPCR and CA125,
indicating that sEPCR could be measured as an indicator of ovarian
cancer along with the currently used measure of CA125.
OVCAR-3 ovarian cancer cell line
expresses PAR-1 antigens, CD133 and CD117
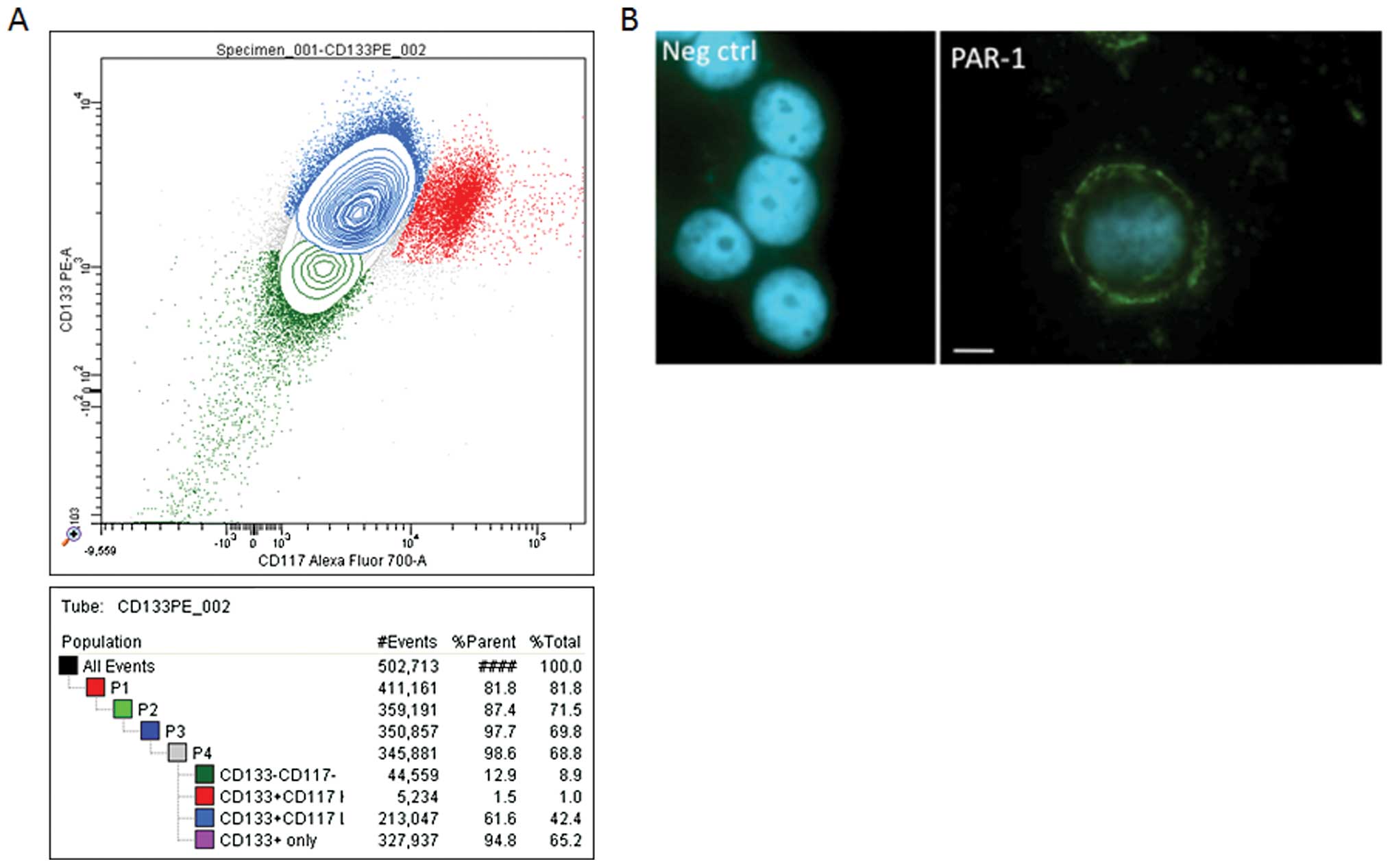
Analysis by cytometry of OVCAR-3 cells using CD133
and CD117 antibodies revealed a positive immune reactivity with
these. The representative experiments presented in Fig. 3A show that 65.2% of OVCAR-3
wild-type cells expressed CD133, while 42% of these cells expressed
both CD133 and CD117. The same results (70–75%) were obtained using
CD133 microbeads. As CD133 and CD117 are recognized markers of
pro-stem cells, their presence tends to favour the idea that
OVCAR-3 cells carry pro-stem cell characteristics.
Due to the crucial role of PAR-1 in EPCR function,
we tested the presence of this protein in OVCAR cells. As shown in
Fig. 3B, these cells express the
PAR-1 protein. This finding was also confirmed by flow cytometry
(data not shown).
Expression of EPCR by the OVCAR-3
cancer cell line
EPCR expression in OVCAR-3 cells was evaluated.
RT-PCR (Fig. 4A) and FACS analysis
(Fig. 4B) indicated that both EPCR
mRNAs and proteins were detectable in these cells. Other cell
lines, derived from lung (A549), colorectal (HT-29) and ileocecal
(HCT-8R) cancers, were also screened for EPCR transcription by
RT-PCR amplification. Analysis of the product of RT-PCR by gel
electrophoresis exhibited a prominent amplification band for all
three cell lines. The band size was consistent with the predicted
size for EPCR (Fig. 4A).
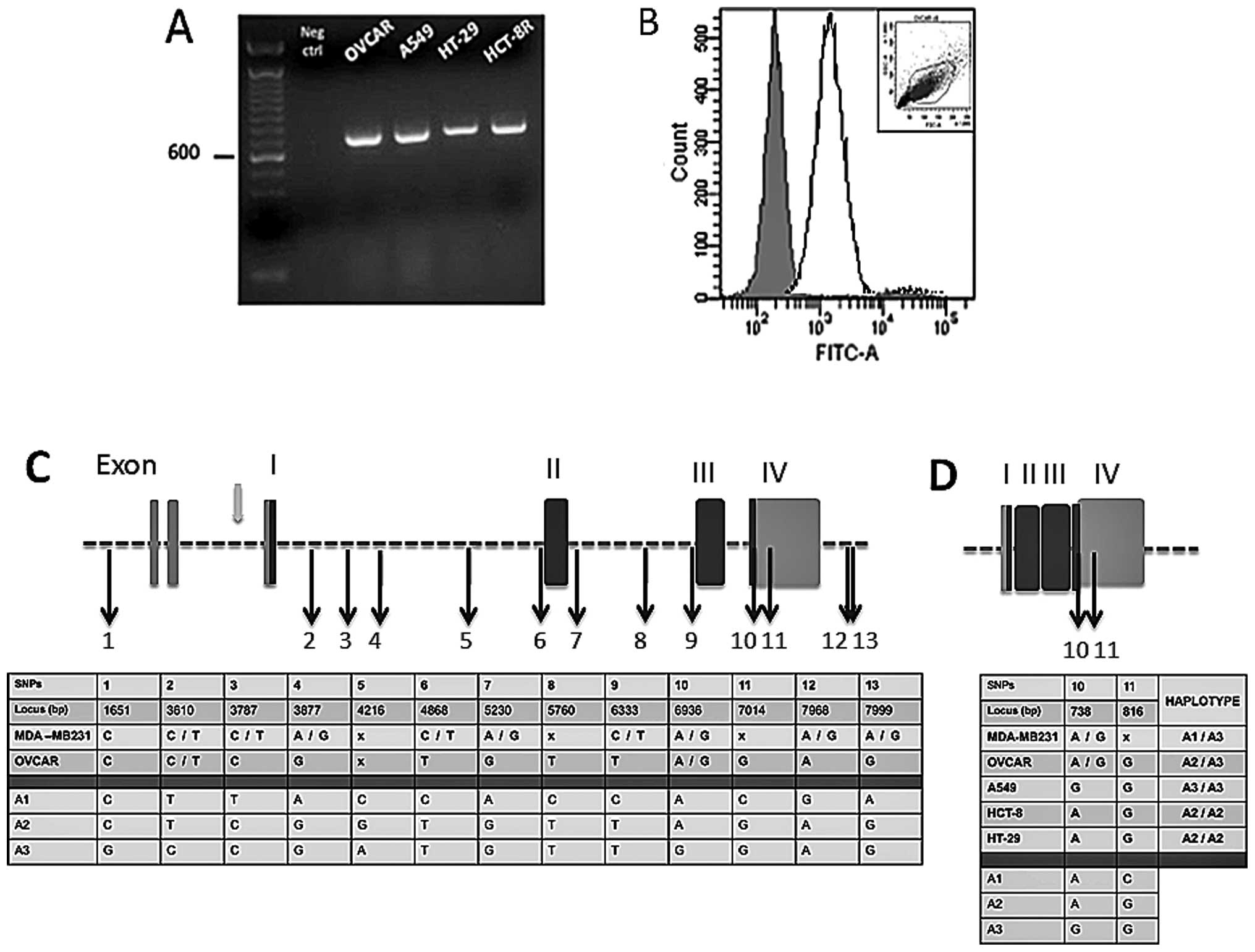 | Figure 4EPCR gene sequence analysis from
ovarian, breast, lung and colorectal cancer cell lines. (A) Gel
electrophoresis of EPCR (RT-PCR products) of ovarian (OVCAR), lung
(A549), colorectal (HT-29) and ileocecal (HCT-8R) cancer cell
lines. Expected bands of 692 bp were observed as shown and the
control without nucleic acid (Neg ctrl) remained negative. (B) Flow
cytometry analysis of EPCR in OVCAR. The solid line shows EPCR
staining and the isotype control as represented by the shaded area.
The cytogram gate is displayed in the upper corner. (C) Breast and
ovarian cancer cell EPCR DNA alignment with GenBank data (reference
no. AF106202). (D) Ovarian, breast, lung and colon cancer cell EPCR
cDNA alignment with GenBank data (reference no. BC01445). DNA and
cDNA extracts were shipped to the Qiagen Sequencing Service. Both
DNA strands were sequenced and nucleotides were numbered according
to the appropriate GenBank reference no. The 13 single nucleotide
polymorphisms (SNPs) initially described in endothelial cells were
also found here allowing us to designate 3 haplotypes (A1, A2, A3).
Exons are presented as vertical rectangular blocks; darkly shaded
regions are the ones that are transcribed. Black arrows indicate
the SNPs. Gray arrow shows a promoter region. The ‘x’ indicates
positions of bases that the commercial firm, Qiagen, was uncertain
of; it can represent any one of the four bases. |
The integrity of the coding sequences and the EPCR
gene locus was further scrutinized through sequencing of the EPCR
cDNA and the EPCR gene. The sequence obtained was compared to a
consensus sequence corresponding to the endothelial gene. It was
observed that the cancer cell and endothelial cell EPCR haplotypes
share similarity with respect to single nucleotide polymorphisms
(SNPs). For this purpose, both genomic and cDNA were sequenced for
OVCAR and MDA-MB231 cell lines, whereas only cDNA was sequenced for
A549, HCT-8R and HT-29 cells. The cDNA and genomic DNA sequences
were compared with BC01445 (1,381 bp) and AF106202 (8,167 bp)
GenBank loci, respectively (Fig.
4). We detected the 13 SNPs already described in endothelial
cell genes (Saposnik et al) (11). In addition, within the 5′UTR
region, a thymidine insertion (locus 260) and an adenosine deletion
(locus 840) were found in both MDA-MB231 and OVCAR cells. Moreover,
a SNP was observed between the second and the third exon for both
cell lines (C5727T). Two other SNPs (G7965C and T8153C) were
detected only in the OVCAR cells within the 3′UTR region.
OVCAR, a human ovarian adenocarcinoma cell line
carries both C and T at the nucleotide position 3787 and both A and
G at nucleotide position 6936 (Fig.
4C). This reflects a heterozygosity for A1 and A3 haplotypes.
In the same manner, the breast cancer cell line, MDA-MB231, is
heterozygous for A2 and A3 since only C is detected at position
3787, which is located in intron 1, while both A and G are found at
position 6936.
The data in Fig. 4D
show that the lung cancer cell line, A549, is homozygous for the A3
haplotype, since exon 4 contains G nucleotides at loci 738 and 817.
Similar analysis revealed that both colorectal and ileocecal cancer
cell lines (HT-29 and HCT-8R) carry the A2 haplotype in the
homozygous form.
It is interesting to note that A3 carriers were
found to be associated with a large amount of sEPCR shed into their
culture medium. It was observed that one million A3 homozygous A549
cells shed approximately 4,250 ng/ml of sEPCR, whereas A3
heterozygous MDA-MB231 and OVCAR cells shed only 450 and 145 ng/ml
per million cells, respectively (data not shown).
Discussion
Data generated recently in our laboratory encouraged
us to investigate the role of aPC/sEPCR in ovarian cancers. In the
present study, we adressed the question of sEPCR and its levels of
expression in ovarian and other cancers, as well as in established
cancer cell lines. Our aim was to find out whether sEPCR could be a
candidate biomarker.
Our data provide evidence of the presence of EPCR in
ovarian cancer cells and also in a large cohort of tumor biopsies.
The release of sEPCR is significant since its secretion in the
plasma of patients with ovarian cancer is related to enhanced cell
survival, invasion and immune down regulation. However, the
mechanisms behind this remain to be elucidated. It is of vital
importance, in routine clinical practice, to avail of reliable
markers of tumor behaviour. CA125 is the currently used marker for
ovarian tumors. An additional biomarker of tumor cells, if
revealed, would contribute towards making a sounder diagnosis.
EPCR proteins were detected in situ, by
antibodies against the synthetic peptides of EPCR. In ovarian
cancer (90.47%), in the breast (60%), lung (80%) and colon (65%)
cancer biopsies. The relatively low percentages of positive samples
observed could be an underestimate as TMA samples may contain,
besides tumor cells, necrosed tissue or the biopsy may simply miss
the tumor nodule and aspirate adipose and/or tumor adjacent healthy
tissue. However, TMA analysis of ovarian cancers after treatment
showed a decrease in EPCR immunostaining.
aPC is a key inhibitor of fibrin formation. Other
than hemostastic function, aPC is also known to exert pleiotropic
effects, depending on the cell types expressing EPCR. Among others,
aPC interferes with the endothelial cell p53 pathway (19,20).
It also promotes endothelial cell proliferation through the MAPK
and PI3K signaling pathways (20–22).
Cancer cells expressing EPCR may therefore benefit from the
cytoprotective effect imparted by aPC (23). Scheffer et al found a high
expression of EPCR in a large panel of tumor cell lines and
interpreted this in the light of the role of EPCR in coagulation
(15). Beaulieu and Church
(16) claimed that aPC increases
breast cancer cell invasion and chemotaxis through EPCR and PAR-1.
These findings are demonstrative of the importance of EPCR
expression in tumor cell behavior (17). Of note, the OVCAR-3 cell line used
in the present study was found to express high levels of CD133 and
CD117, which are markers characteristic of pre-stem cells. We also
noticed an increased expression of PAR-1, which is a known cofactor
for the EPCR signaling pathway in cancer cells.
In addition, we found that ovarian cell-associated
EPCR is functional. Treatment of cancer cells with aPC induced cell
survival that could be inhibited by the neutralizing antibody,
anti-peptide 3. The study of ascites from patients showed that EPCR
in the soluble form (sEPCR) could be detected on the cancer cell
membranes as well as in the ascitic fluid. In the ascitic fluid,
membrane-associated EPCR, via aPC, can inhibit fibrin formation and
participate in fluid expansion in ovarian cancer, whereas under
intravascular conditions, sEPCR, due to its trapping action, may be
a leading cause of hypercoagulable state associated with
cancer.
RT-PCR analysis detected the presence of EPCR mRNA
in ascitic cell clusters of ovarian cancers, OVCAR-3 cells and
several other tumor cell lines, such as breast (MDA-MB231), lung
(A549) and colorectal (HT-29, HCT-8R). The presence of
EPCR-specific mRNA is in line with and confirms our observation by
TMA analysis.
The increase in expression levels of EPCR/sEPCR
observed by immune antibodies and TMA was further corroborated by
examining the sequence integrity of the EPCR gene and its mRNA
transcript. This allowed us to characterize the cancer cell
haplotypes which reflects one of the originalities of the current
study. EPCR gene sequencing provided evidence for A3 haplotype
expression in heterozygous (OVCAR and MDA-MB231) and homozygous
(A549) forms. Of note, among the 10 alleles sequenced, 4
represented the A3 haplotype, which is a non-negligible figure.
Knowing the frequency in the healthy population (7%), it is
questionable whether the values we obtained indeed reflect the
malignant nature of the cells studied (11). It might be pertinent to compare the
haplotypes of tumor and healthy cells of a patient to see whether
the genotype changes with malignancy. The A3 haplotype favors the
proteolytic cleavage of EPCR and is thus associated with increased
plasma levels of sEPCR (11). It
is interesting to note that the cultured cells carrying the A3
haplotype shed large amounts of sEPCR into the medium. Elevated
sEPCR plasmatic level increases the risk of venous thrombosis
(11,24). Thus, cancer cells that express the
A3 haplotype may expose patients to a higher risk level. The A3
haplotype and elevated sEPCR, both of which are inter-related,
contribute synergistically to the thrombophilic state in ovarian
cancer patients (24).
The results from our study present strong evidence
in favour of the role of EPCR/sEPCR in tumor cells, whether in
tumor biopsies or in established cancer cell lines. There is a
clear increase in sEPCR concomitant with the existence of malignant
cells.
CA125 is the currently used marker for ovarian
tumors. Nevertheless, on the basis of our observations, we propose
that the measurement of plasma sEPCR at regular intervals during
the remission period in cancer patients, particularly those with
cancer of the ovaries, may provide clinically relevant information
and perhaps an early signal warranting attention. The measurement
of sEPCR and CA125 simultaneously in ovarian cancer patients could
go a step further towards providing a sounder diagnosis.
References
|
1
|
Dahlbäck B and Villoutreix BO: Molecular
recognition in the protein C anticoagulant pathway. J Thromb
Haemost. 1:1525–1534. 2003.
|
|
2
|
Li W, Zheng X, Gu J, Hunter J, Ferrell GL,
Lupu F, Esmon NL and Esmon CT: Overexpressing endothelial cell
protein C receptor alters the hemostatic balance and protects mice
from endotoxin. J Thromb Haemost. 3:1351–1359. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Taylor FB Jr, Peer GT, Lockhart MS,
Ferrell G and Esmon CT: Endothelial cell protein C receptor plays
an important role in protein C activation in vivo. Blood.
97:1685–1688. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fukudome K and Esmon CT: Identification,
cloning, and regulation of a novel endothelial cell protein
C/activated protein C receptor. J Biol Chem. 269:26486–26491.
1994.PubMed/NCBI
|
|
5
|
Xu J, Qu D, Esmon NL and Esmon CT:
Metalloproteolytic release of endothelial cell protein C receptor.
J Biol Chem. 275:6038–6044. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fukudome K, Kurosawa S, Stearns-Kurosawa
DJ, He X, Rezaie AR and Esmon CT: The endothelial cell protein C
receptor. Cell surface expression and direct ligand binding by the
soluble receptor. J Biol Chem. 271:17491–17498. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Menschikowski M, Hagelgans A, Eisenhofer G
and Siegert G: Regulation of endothelial protein C receptor
shedding by cytokines is mediated through differential activation
of MAP kinase signaling pathways. Exp Cell Res. 315:2673–2682.
2009. View Article : Google Scholar
|
|
8
|
Guitton C, Gérard N, Sébille V,
Bretonnière C, Zambon O, Villers D and Charreau B: Early rise in
circulating endothelial protein C receptor correlates with poor
outcome in severe sepsis. Intensive Care Med. 37:950–956. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Esmon CT: Possible involvement of
cytokines in diffuse intravascular coagulation and thrombosis.
Baillieres Best Pract Res Clin Haematol. 12:343–359. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pintao MC, Roshani S, de Visser MC, Tieken
C, Tanck MW, Wichers IM, Meijers JC, Rosendaal FR, Middeldorp S and
Reitsma PH: High levels of protein C are determined by PROCR
haplotype 3. J Thromb Haemost. 9:969–976. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saposnik B, Reny JL, Gaussem P, Emmerich
J, Aiach M and Gandrille S: A haplotype of the EPCR gene is
associated with increased plasma levels of sEPCR and is a candidate
risk factor for thrombosis. Blood. 103:1311–1318. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qu D, Wang Y, Song Y, Esmon NL and Esmon
CT: The Ser219 −>Gly dimorphism of the endothelial protein C
receptor contributes to the higher soluble protein levels observed
in individuals with the A3 haplotype. J Thromb Haemost. 4:229–235.
2006.
|
|
13
|
Tsuneyoshi N, Fukudome K, Horiguchi S, Ye
X, Matsuzaki M, Toi M, Suzuki K and Kimoto M: Expression and
anticoagulant function of the endothelial cell protein C receptor
(EPCR) in cancer cell lines. Thromb Haemost. 85:356–361.
2001.PubMed/NCBI
|
|
14
|
Wang X, Wang E, Kavanagh JJ and Freedman
RS: Ovarian cancer, the coagulation pathway, and inflammation. J
Transl Med. 3:252005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Scheffer GL, Flens MJ, Hageman S,
Izquierdo MA, Shoemaker RH and Scheper RJ: Expression of the
vascular endothelial cell protein C receptor in epithelial
tumour cells. Eur J Cancer. 38:1535–1542. 2002.PubMed/NCBI
|
|
16
|
Beaulieu LM and Church FC: Activated
protein C promotes breast cancer cell migration through
interactions with EPCR and PAR-1. Exp Cell Res. 313:677–687. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ducros E, Berthaut A, Mirshahi SS, Faussat
AM, Soria J, Agarwal MK and Mirshahi M: Aldosterone modifies
hemostasis via upregulation of the protein-C receptor in human
vascular endothelium. Biochem Biophys Res Commun. 373:192–196.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rafii A, Mirshahi P, Poupot M, Faussat AM,
Simon A, Ducros E, Mery E, Couderc B, Lis R, Capdet J, Bergalet J,
Querleu D, Dagonnet F, Fournié JJ, Marie JP, Pujade-Lauraine E,
Favre G, Soria J and Mirshahi M: Oncologic trogocytosis of original
stromal cells induces chemoresistance of ovarian tumours. PLoS One.
3:e38942008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheng T, Liu D, Griffin JH, Fernández JA,
Castellino F, Rosen ED, Fukudome K and Zlokovic BV: Activated
protein C blocks p53-mediated apoptosis in ischemic human brain
endothelium and is neuroprotective. Nat Med. 9:338–342. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mosnier LO, Zlokovic BV and Griffin JH:
The cytoprotective protein C pathway. Blood. 109:3161–3172. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Suzuki K and Hayashi T: Protein C and its
inhibitor in malignancy. Semin Thromb Hemost. 33:667–672. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Uchiba M, Okajima K, Oike Y, Ito Y,
Fukudome K, Isobe H and Suda T: Activated protein C induces
endothelial cell proliferation by mitogen-activated protein kinase
activation in vitro and angiogenesis in vivo. Circ Res. 95:34–41.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kobayashi H, Moniwa N, Gotoh J, Sugimura M
and Terao T: Role of activated protein C in facilitating basement
membrane invasion by tumor cells. Cancer Res. 54:261–267.
1994.PubMed/NCBI
|
|
24
|
Uitte de Willige S, Van Marion V,
Rosendaal FR, Vos HL, de Visser MC and Bertina RM: Haplotypes of
the EPCR gene, plasma sEPCR levels and the risk of deep venous
thrombosis. J Thromb Haemost. 2:1305–1310. 2004.PubMed/NCBI
|