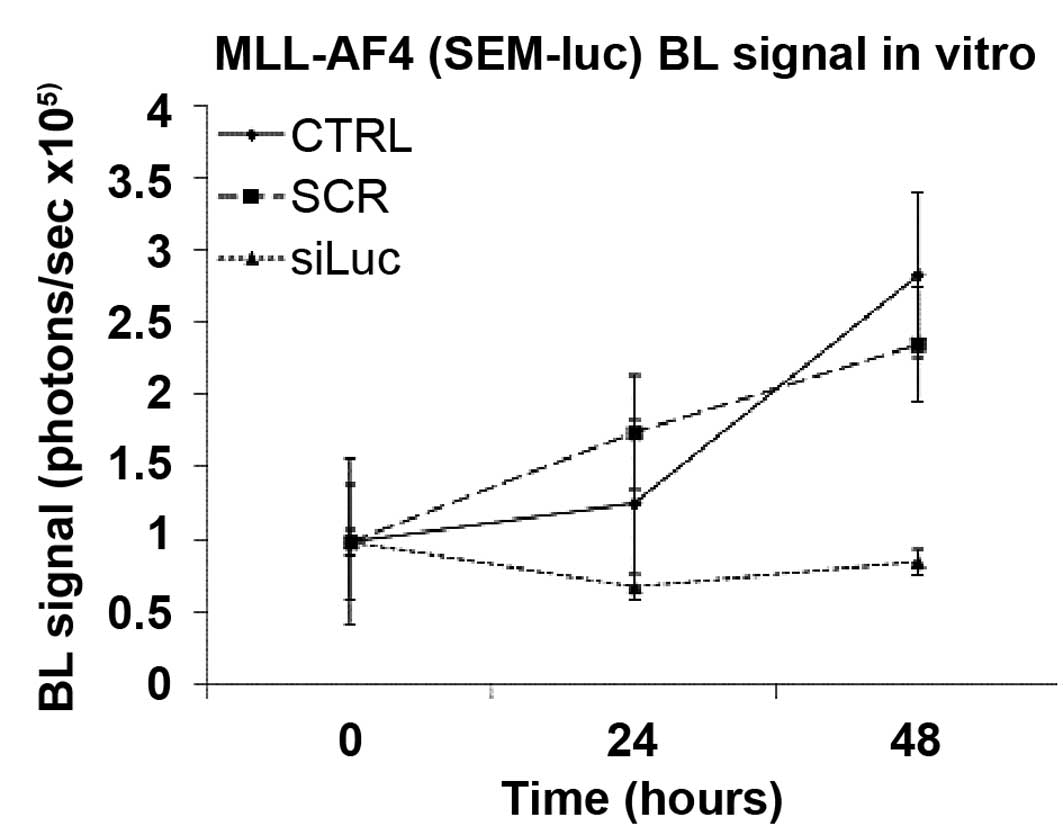
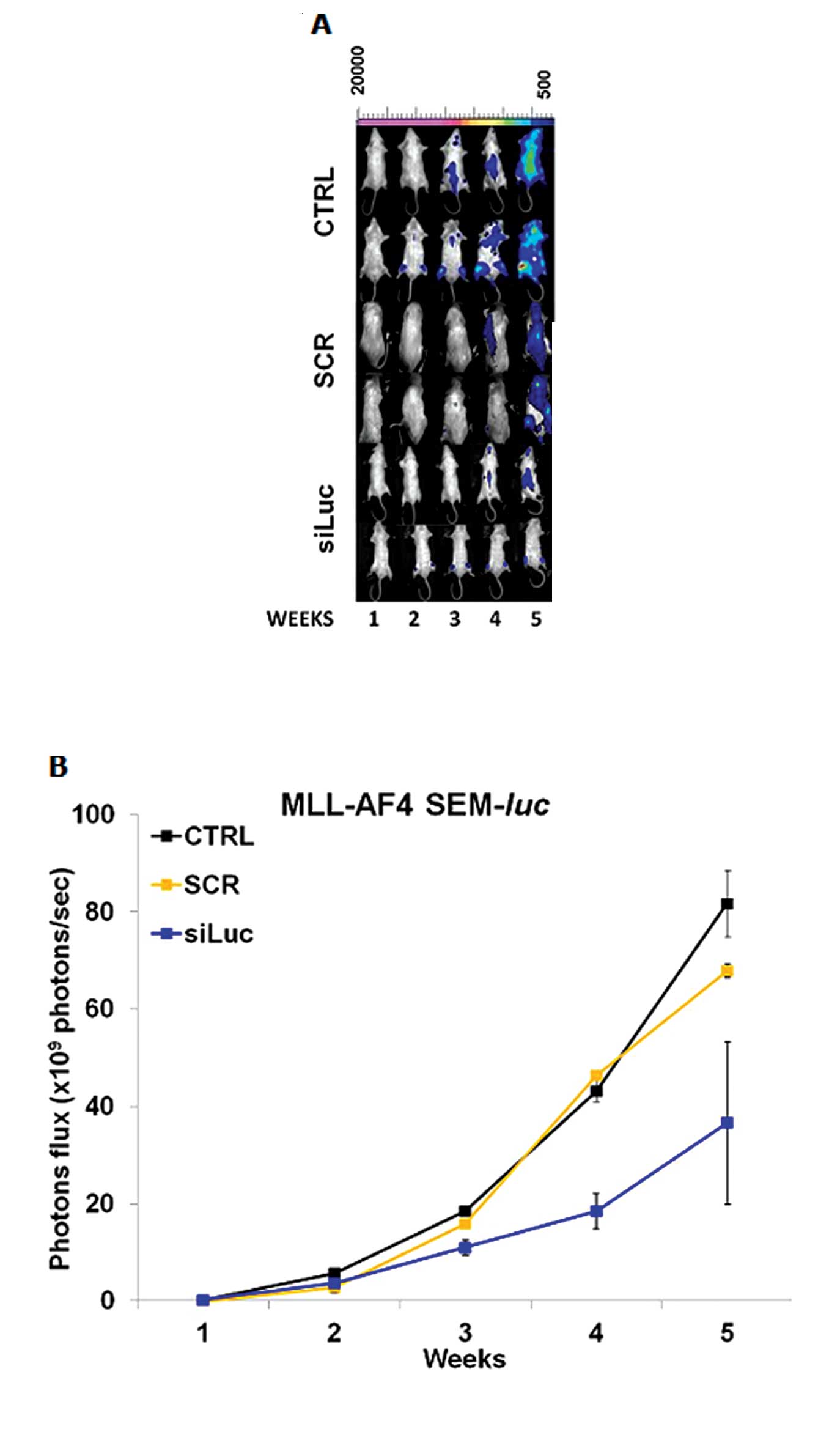
|
1
|
Krivtsov AV and Armstrong SA: MLL
translocations, histone modifications and leukaemia stem-cell
development. Nat Rev Cancer. 7:823–833. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bach C and Slany RK: Molecular pathology
of mixed-lineage leukemia. Future Oncol. 5:1271–1281. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thirman MJ, Gill HJ, Burnett RC, et al:
Rearrangement of the MLL gene in acute lymphoblastic and acute
myeloid leukemias with 11q23 chromosomal translocations. N Engl J
Med. 329:909–914. 1993. View Article : Google Scholar
|
|
4
|
Meyer C, Kowarz E, Hofmann J, et al: New
insights to the MLL recombinome of acute leukemias. Leukemia.
23:1490–1499. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pui CH, Chessells JM, Camitta B, et al:
Clinical heterogeneity in childhood acute lymphoblastic leukemia
with 11q23 rearrangements. Leukemia. 17:700–706. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pui CH, Schrappe M, Ribeiro RC and
Niemeyer CM: Childhood and adolescent lymphoid and myeloid
leukemia. Hematology Am Soc Hematol Educ Program. 118–145.
2004.PubMed/NCBI
|
|
7
|
Hess JL: MLL: a histone methyltransferase
disrupted in leukemia. Trends Mol Med. 10:500–507. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pizzo PA and Poplack DG: Principles and
Practice of Pediatric Oncology. 5th Edition. Lippincott Williams
& Wilkins; Philadelphia, PA: 2006
|
|
9
|
Greil J, Gramatzki M, Burger R, et al: The
acute lymphoblastic leukaemia cell line SEM with t(4;11)
chromosomal rearrangement is biphenotypic and responsive to
interleukin-7. Br J Haematol. 86:275–283. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Balgobind BV, Raimondi SC, Harbott J, et
al: Novel prognostic subgroups in childhood 11q23/MLL-rearranged
acute myeloid leukemia: results of an international retrospective
study. Blood. 114:2489–2496. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Frascella E, Rondelli R, Pigazzi M, et al:
Clinical features of childhood acute myeloid leukaemia with
specific gene rearrangements. Leukemia. 18:1427–1429. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dothager RS, Flentie K, Moss B, Pan MH,
Kesarwala A and Piwnica-Worms D: Advances in bioluminescence
imaging of live animal models. Curr Opin Biotechnol. 20:45–53.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luker GD and Luker KE: Optical imaging:
current applications and future directions. J Nucl Med. 49:1–4.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Klerk CP, Overmeer RM, Niers TM, et al:
Validity of bioluminescence measurements for noninvasive in vivo
imaging of tumor load in small animals. Biotechniques. 43:7–13.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Greer LF and Szalay AA: Imaging of light
emission from the expression of luciferases in living cells and
organisms: a review. Luminescence. 17:43–74. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Söling A and Rainov NG: Bioluminescence
imaging in vivo-application to cancer research. Expert Opin Biol
Ther. 3:1163–1172. 2003.Erratum in: Expert Opin Biol Ther 3: 1315,
2003.
|
|
17
|
Negrin RS and Contag CH: In vivo imaging
using bioluminescence: a tool for probing graft-versus-host
disease. Nat Rev Immunol. 6:484–490. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Inoue Y, Tojo A, Sekine R, et al: In vitro
validation of bioluminescent monitoring of disease progression and
therapeutic response in leukaemia model animals. Eur J Nucl Med Mol
Imaging. 33:557–565. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Inoue Y, Izawa K, Tojo A, et al:
Monitoring of disease progression by bioluminescence imaging and
magnetic resonance imaging in an animal model of hematologic
malignancy. Exp Hematol. 35:407–415. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Inoue Y, Izawa K, Kiryu S, Kobayashi S,
Tojo A and Ohtomo K: Bioluminescent evaluation of the therapeutic
effects of total body irradiation in a murine hematological
malignancy model. Exp Hematol. 36:1634–1641. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Armstrong SA, Kung AL, Mabon ME, et al:
Inhibition of FLT3 in MLL: Validation of a therapeutic target
identified by gene expression based classification. Cancer Cell.
3:173–183. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Edinger M, Cao YA, Verneris MR, Bachmann
MH, Contag CH and Negrin RS: Revealing lymphoma growth and the
efficacy of immune cell therapies using in vivo bioluminescence
imaging. Blood. 15:640–648. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maes W, Deroose C, Reumers V, et al: In
vivo bioluminescence imaging in an experimental mouse model for
dendritic cell based immunotherapy against malignant glioma. J
Neurooncol. 91:127–139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Inoue Y, Kiryu S, Izawa K, Watanabe M,
Tojo A and Ohtomo K: Comparison of subcutaneous and intraperitoneal
injection of D-luciferin for in vivo bioluminescence
imaging. Eur J Nucl Med Mol Imaging. 36:771–779. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wetterwald A, van der Pluijm G, Que I, et
al: Optical imaging of cancer metastasis to bone marrow: a mouse
model of minimal residual disease. Am J Pathol. 160:1143–1153.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aronin N: Target selectivity in mRNA
silencing. Gene Ther. 13:509–516. 2006. View Article : Google Scholar
|
|
27
|
Meister G and Tuschl T: Mechanisms of gene
silencing by double-stranded RNA. Nature. 431:343–349. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tuschl T: RNA interference and small
interfering RNAs. Chembiochem. 2:239–245. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sledz CA and Williams BR: RNA interference
in biology and disease. Blood. 106:787–794. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cejka D, Losert D and Wacheck V: Short
interfering RNA (siRNA): tool or therapeutic? Clin Sci. 110:47–58.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Behlke MA: Progress towards in vivo use of
siRNAs. Mol Ther. 13:644–670. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tsuchiya S, Yamabe M, Yamaguchi Y,
Kobayashi Y, Konno T and Tada K: Establishment and characterization
of a human acute monocytic leukemia cell line (THP-1). Int J
Cancer. 26:171–176. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Matsuo Y, MacLeod RA, Uphoff CC, et al:
Two acute monocytic leukemia (AML-M5a) cell lines (MOLM-13 and
MOLM-14) with interclonal phenotypic heterogeneity showing MLL-AF9
fusion resulting from an occult chromosome insertion, ins(11;9)
(q23;p22p23). Leukemia. 11:1469–1477. 1997. View Article : Google Scholar
|
|
34
|
Matsuo Y and Drexler HG: Establishment and
characterization of human B cell precursor-leukemia cell lines.
Leuk Res. 22:567–579. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mezzanotte L, Fazzina R, Michelini E, et
al: In vivo bioluminescence imaging of murine xenograft cancer
models with a red-shifted thermostable luciferase. Mol Imaging
Biol. 12:406–414. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Montemurro L, Tonelli R, Fazzina R,
Martino V, Marino F and Pession A: Identification of two MLL-MLLT3
(alias MLL-AF9) chimeric transcripts in the MOLM-13 cell line.
Cancer Genet Cytogenet. 154:96–97. 2004. View Article : Google Scholar : PubMed/NCBI
|