Introduction
The acetylation status of histones plays an
important role in the regulation of gene expression by altering
chromatin structure. Histone deacetylases (HDAC) facilitate a
closed chromatin structure and hence transcriptional repression.
HDAC function is commonly affected in human cancers. Therefore,
inhibition of HDAC represents a novel therapeutic approach for
cancer therapy. In our previous study, we compared the activity of
structurally similar bicyclic depsipeptide compounds using a panel
of 39 human cancer cell lines and found that spiruchostatin B
(SP-B) was the most potent inhibitor of HDAC1 and displayed the
most potent growth-inhibitory activity (1).
The anti-tumor effects of HDAC inhibitors (HDIs)
have been shown to be mediated by various signaling mechanisms. One
such mechanism involves release of HDAC1 from its binding to the
p21waf1/cip1 promoter, including to the Sp1 sites on
this promoter, which results in enhanced histone acetylation around
these promoter sites and strong activation of the expression of the
cyclin-dependent kinase inhibitor p21waf1/cip1(2). p21waf1/cip1 was initially
identified as a cell cycle regulatory protein that can cause cell
cycle arrest (3). Therefore,
p21waf1/cip1 is a key gene expression target of HDI
anti-tumor activity, and induction of p21waf1/cip1 by
inhibition of HDAC function is critical for HDI blockage of cell
cycle progression (4–7). Indeed, we have shown that NALM-6, a B
cell leukemia cell line that has higher expression of
p21waf1/cip1 mRNA compared with other typical
leukemia cell lines, was susceptible to SP-B-induced cytotoxicity
that depended on induction of apoptosis (8). This result suggests that the
introduction of exogenous p21waf1/cip1 into human
leukemia cells that have a low susceptibility to SP-B will enhance
their susceptibility to SP-B.
There are several major problems with the use of
anti-cancer drugs for the chemotherapy of cancer patients. One such
problem is the acquisition of drug resistance by tumor cells.
Development of resistance to chemotherapy is therefore a major
concern with any new therapy. It is well established that one
mechanism of resistance to chemotherapeutic agents involves the
expression of MDR1 (P-glycoprotein, P-gp), which can induce
increased efflux of anticancer agents from tumor cells (9). P-gp is a major contributor to the
resistance of cancer cells to FK228, a chemical with a similar
structure to that of SP-B (10–12).
It has also been shown that treatment with other HDIs such as
suberoylanilide hydroxamic acid (SAHA, vorinostat) can result in
the acquisition of irreversible and multidrug
resistance-independent HDI resistance in HCT colon tumor cells
(13,14). To date, there has been no report on
the establishment of an SP-B-resistant human leukemia cell line,
characterization of such an SP-B-resistant cell line or
determination of what molecule might reverse such resistance.
In the present study, we generated and characterized
a stable, SP-B-resistant NALM-6 cell (NALM-6/SP-B) line by
continuous exposure of the cells to SP-B, starting with a low
concentration. We also tested whether introduction of exogenous
p21waf1/cip1 would reverse the SP-B resistance of
NALM-6/SP-B cells, or enhance the susceptibility of the K562 human
erythroleukemia leukemia cell line, which is less susceptible to
SP-B than NALM-6 cells, to SP-B-induced apoptosis. The ultimate
aims of this study were to better understand the acquisition of
resistance or susceptibility to SP-B with a view of its clinical
application for the chemotherapy of leukemia.
Materials and methods
Materials and cell culture
SP-B was prepared as previously described (1,15).
Trichostatin A (TSA), sodium butyrate (NaB) and all other reagents,
unless stated, were of the highest grade available and were
supplied by either Sigma (St. Louis, MO, USA) or Wako Pure Chemical
Industries, Ltd (Osaka, Japan). All cells were supplied by the Cell
Resource Center for Biomedical Research, Tohoku University (Sendai,
Japan). Cells were routinely cultured using standard methods as
described in our previous report (16).
Cytotoxicity and apoptosis
Cytotoxicity was assessed using the MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide)
assay, and apoptosis was estimated based on nuclear morphological
observation using by our previously described method (17).
Establishment of a spiruchostatin
B-resistant cell line, NALM-6/SP-B
NALM-6/SP-B cells were obtained using a modification
of a method for obtaining Ara-C-resistant cells (17). NALM-6 parental cells were cultured
in increasing concentrations of SP-B, starting at 0.1 nM. Viable
cells were then passaged into a higher concentration of NALM-6 in
0.1 nM increments until a concentration of 6 nM SP-B was reached.
NALM-6/SP-B cells were then maintained in complete RPMI-1640 medium
containing 6 nM SP-B.
RNA isolation and quantitative real-time
polymerase chain reaction (qPCR) assay
The mRNA expression level of
p21waf1/cip1 (GenBank Accession no. NM_000389.4),
HDAC1 (NM_004964.2), p53 (NM_000546.4), Bax
(NM_138761.3), Bcl-2 (NM_000633.2), Fas
(NM_000043.3), caspase-3 (Cas-3, NM_004346.3),
c-Myc (NM_002467.3), or multidrug resistance
(MDR1, NM_000927.3) in NALM-6 or NALM-6/SP-B cells was
quantified using the real-time polymerase chain reaction (qPCR)
with a Light Cycler (Roche, Basel, Switzerland). Briefly, total-RNA
was extracted from each cell line with the Isogen reagent (Nippon
Gene, Tokyo, Japan) and 0.1 μg of total-RNA was then reverse
transcribed to single-strand cDNA using the RverTra Ace®
qPCR RT Kit (Toyobo, Osaka, Japan). Aliquots of the cDNA
preparations were subjected to qPCR analysis using SYBR®
Premix Ex Taq™ (Takara Bio, Shiga, Japan) to quantify the
expression of each target gene and of the internal standard
β-actin (GenBank Accession no. NM_001101.3) using Light
Cycler. The primer pairs used were from the QuantiTect®
Primer Assay (Qiagen, Valencia, CA, USA) or were Takara Perfect
Real Time Primers (Takara Bio). The results of all assays were
checked with the melting curves to confirm the presence of single
PCR products.
Plasmid constructs and transfection
studies
The p21waf1/cip1 gene was isolated from
parental NALM-6 cells by PCR using KOD plus DNA polymerase (Toyobo)
and was cloned into pcDNA 3.1 Directional TOPO®
Expression vector (Invitrogen), according to the protocol supplied
by the manufacturer. Briefly, the sequences of the primers used
were 5′-CACCATGTCAGAACCGGCTGGGGATG-3′ for the upstream primer
(sense strand, residues 126–147), and
5′-TTAGGGCTTCCTCTTGGAGAAGATCAGC-3′ for the downstream primer
(antisense strand, residues 593–620), both of which were
synthesized by Operon (Tokyo, Japan), and which yielded a product
of about 0.5 kb. PCR amplification was performed for 1 cycle at
94°C for 2 min, which was immediately followed by 30 cycles of 15
sec at 94°C (dissociation), 30 sec at 61°C (primer annealing), and
2 min at 68°C (extension). The PCR products were resolved by
electrophoresis on a 1% agarose gel and the gel was stained with
ethidium bromide and imaged. The identity of the PCR product was
confirmed by direct sequence analysis. The open reading frame of
p21waf1/cip1 that was generated by PCR was then
inserted into the pcDNA 3.1 expression vector. The obtained cDNA
sequence was analyzed and confirmed to be identical with the
reported sequence. Empty vectors were used as controls in the
experiments.
NALM-6/SP-B or K562 cells were transfected with the
pcDNA 3.1 plasmid DNA alone (Empty) or with pcDNA 3.1 containing
p21waf1/cip1 cDNA, using the Neon™ Transfection
System (Invitrogen) according to the instructions provided by the
manufacturer. Briefly, 70–90% confluent cells were harvested and
then washed twice with PBS. Subsequently, 2×105 cells
were transfected with 1 μg of plasmid in a volume of 10
μl using a Neon tip and electroporation. The conditions of
electroporation were: 1,325 V for K562 cells or 1,410 V for
NALM-6/SP-B cells, a pulse length of 10 msec, and a total of 3
pulses. The cells were then cultured for 18 h without antibiotics
and used for the cytotoxicity assay.
Western blotting
Changes in the expression level of the
p21waf1/cip1 protein were detected using western
blotting as described in our previous report (18). All antibodies used were purchased
from Cell Signaling Technology Inc. (Beverly, MA, USA).
Statistical analysis
Statistical analysis of the results was performed
using a one-way analysis of variance (ANOVA) followed by the
Williams’ type multiple comparison test or a Bonferroni test among
multiple groups. A p-value of <0.05 was considered statistically
significant.
Results
Characteristics of SP-B-resistant NALM-6
cells
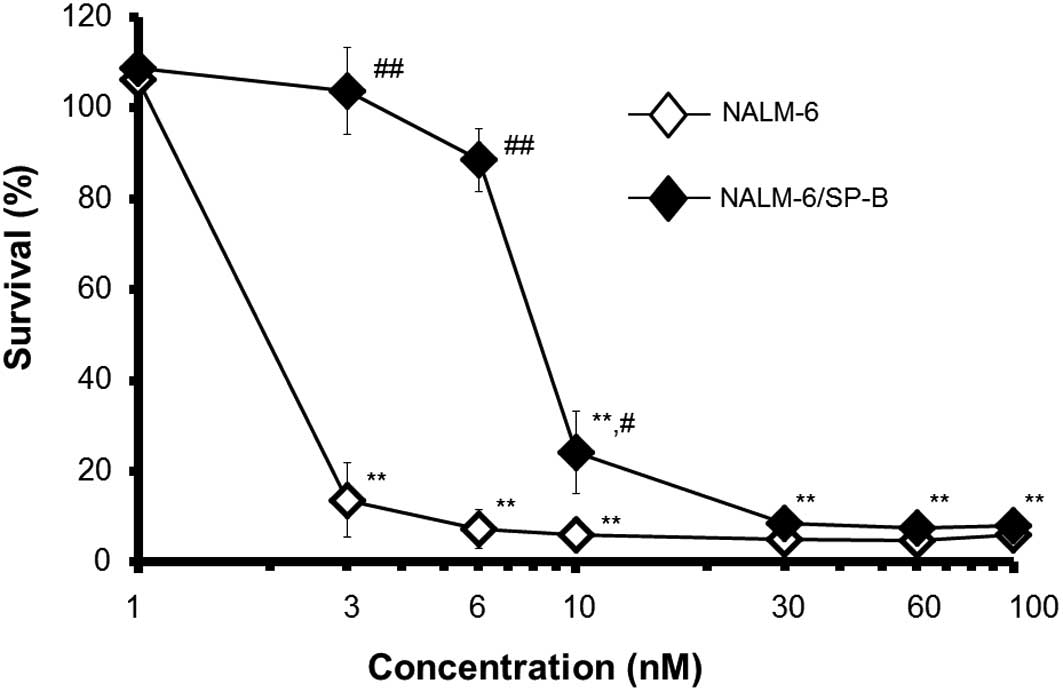
We first established an SP-B-resistant cell line
termed NALM-6/SP-B. We had previously calculated that 50% cell
growth inhibition (IC50) of parental NALM-6 cells by
SP-B after 24 h of culture occurred at an SP-B concentration of 5.2
nM (8). We therefore aimed to
develop NALM-6 cells that were resistant to SP-B concentrations of
up to 6 nM. Whereas SP-B was cytotoxic towards the parental cells
at concentrations of SP-B above 1 nM following incubation for 72 h,
SP-B did not induce any significant decrease in cell survival of
NALM-6/SP-B cells, even at SP-B concentrations of up to 6 nM
(Fig. 1). As indicated in Table I, the IC50 of SP-B for
NALM-6/SP-B cells was 5.9-fold higher than that for parental cells.
We additionally compared the effect of other HDIs such as FK228,
TSA and NaB, as well as the effect of several typical
chemotherapeutic agents, on the growth of NALM-6 and NALM-6/SP-B
cells. Although NALM-6/SP-B cells were more resistant to FK228 than
the parental cells (4.8-fold higher resistance), the parental and
resistant cells showed similar susceptibility to the effects of the
other HDIs and of paclitaxel (Taxol; TAX). NALM-6/SP-B cells were
slightly more resistant to the P-gp substrates doxorubicin (DOX)
and vincristine (VCR) than the parental cells, but this increased
resistance was not statistically significant. To further ascertain
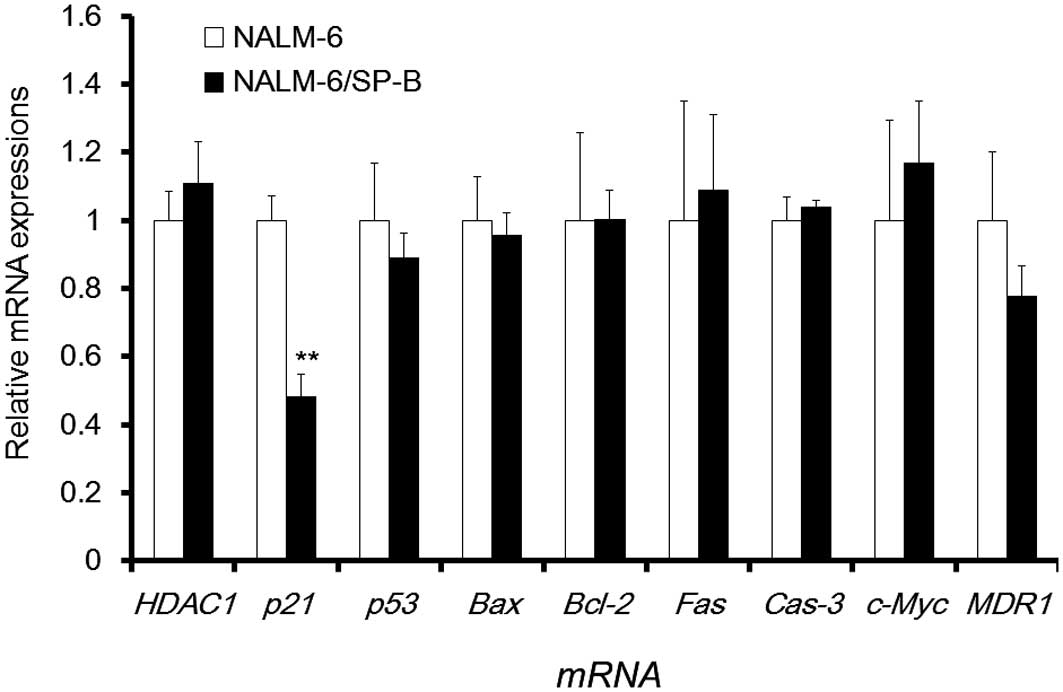
the characteristics of NALM-6/SP-B cells, we compared the mRNA
expression of 9 genes related to cellular growth or apoptosis,
including HDAC1, p21waf1/cip1, p53,
Bax, Bcl-2, Fas, Cas-3, c-Myc,
and MDR1 in NALM-6/SP-B and control NALM-6 cells using qPCR
(Fig. 2). The mRNA expression of
p21waf1/cip1 was significantly decreased in
NALM-6/SP-B cells to less than half the level of parental cells,
but the mRNA expression level of the other genes showed little
difference between the two cell types. Thus, downregulation of
p21waf1/cip1 appeared to be a characteristic of
NALM-6/SP-B cells that is important for their resistance to
SP-B.
 | Table IThe cytotoxicity of anti-cancer drugs
towards NALM-6 and NALM-6/SP-B. |
Table I
The cytotoxicity of anti-cancer drugs
towards NALM-6 and NALM-6/SP-B.
| IC50
| |
|---|
| Drugs
(concentration) | NALM-6 | NALM-6/SP-B | Index |
|---|
| SP-B (nM) | 1.6
(1.03–2.73) | 9.9
(5.98–16.55) | ×5.9 |
| FK228 (nM) | 2.1
(1.31–3.54) | 10.3
(6.48–16.36) | ×4.8 |
| TSA (nM) | 33.3
(23.13–48.08) | 36.5
(24.08–55.45) | ×1.1 |
| NaB (mM) | 0.86
(0.668–1.120) | 1.3
(0.88–2.10) | ×1.6 |
| DOX (nM) | 15.8
(9.99–25.23) | 42.6
(24.84–73.06) | ×2.7 |
| VCR (nM) | 3.4
(2.10–5.53) | 8.9
(5.53–14.43) | ×2.6 |
| TAX (nM) | 4.5
(2.26–9.13) | 5.4
(2.85–10.45) | ×1.2 |
The introduction of exogenous
p21waf1/cip1 leads to the reversal of SP-B
resistance
Fig. 2 shows that,
of the mRNAs expression tested, only p21waf1/cip1
mRNA expression was decreased in NALM-6/SP-B cells. We therefore
next constructed a p21waf1/cip1 expression vector, and
attempted to reverse the SP-B resistance of NALM-6/SP-B cells by
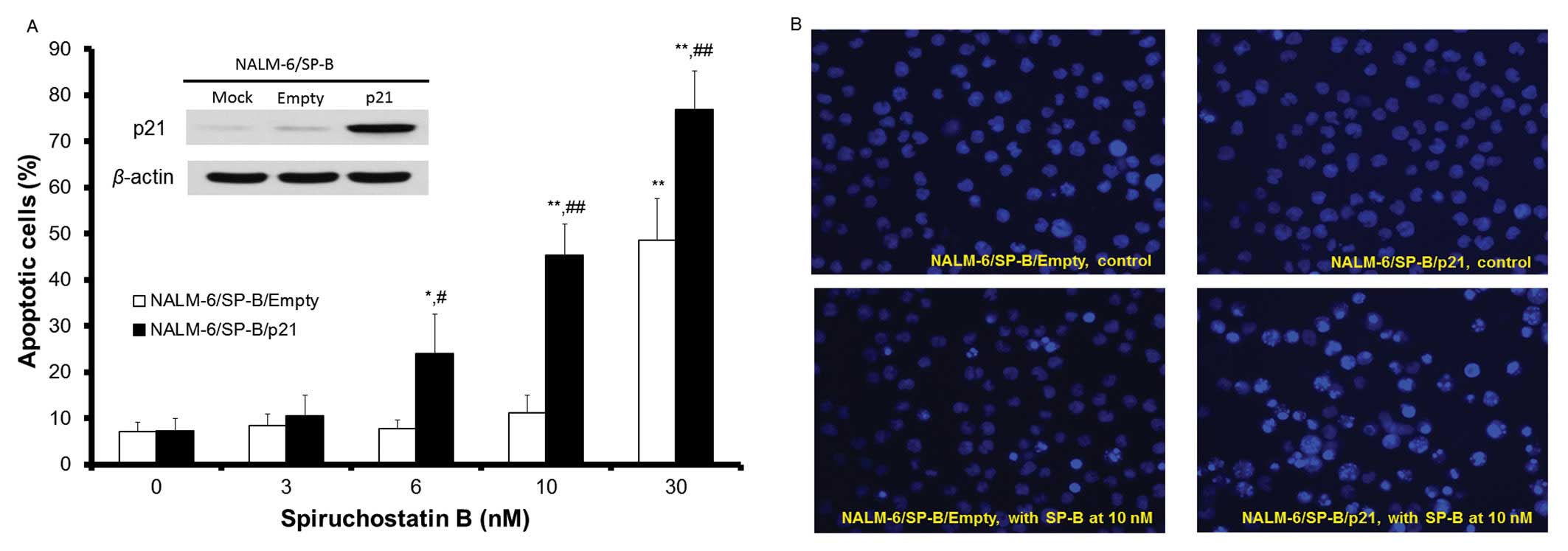
transfection of this vector. As shown in the inset to Fig. 3A, the p21waf1/cip1
protein was strongly expressed in NALM-6/SP-B cells 24 h after
transfection with the p21waf1/cip1 expression vector.
Overexpression of p21waf1/ cip1 was maintained following
incubation for 48 h, there was little change of in cell viability,
cell cycle arrest or induction of apoptosis of
p21waf1/cip1 overexpressing NALM-6/SP-B
(NALM-6/SP-B/p21) cells compared to NALM-6/SP-B cells (data not
shown). These results suggest that introduction of exogenous
p21waf1/cip1 alone is not sufficient to critically
affect cell growth or the induction of apoptosis in NALM-6/SP-B
cells. Although apoptosis of NALM-6/SP-B/Empty (transfected with
empty control vector) cells was not induced by SP-B at SP-B
concentrations of 10 nM or less over a 24-h incubation, apoptosis
of NALM-6/SP-B/p21 cells was induced by SP-B at concentrations
starting from 6 nM and there was a concentration-dependent increase
in NALM-6/SP-B/p21 cell apoptosis between 6 and 30 nM of SP-B.
Incubation with 10 nM SP-B for 24 h induced morphological changes
typical of apoptosis such as chromatin condensation and apoptotic
bodies in NALM-6/SP-B/p21 cells, but induced little change in the
morphology of NALM-6/SP-B/Empty cells (Fig. 3B). The percentage cell survival of
NALM-6/SP-B/Empty and NALM-6/SP-B/p21 cells following incubation
for 48 h with SP-B at concentrations of 3, 6, 10 and 30 nM was
99.8, 91.1, 88.4 and 10.2%, and 73.3, 62.7, 47.5 and 3.4%,
respectively. These data indicate that induction of exogenous
p21waf1/cip1 expression reversed the SP-B resistance of
the NALM-6/SP-B cells.
The introduction of exogenous
p21waf1/cip1 enhances the susceptibility of another
leukemia cell line to SP-B-induced apoptosis
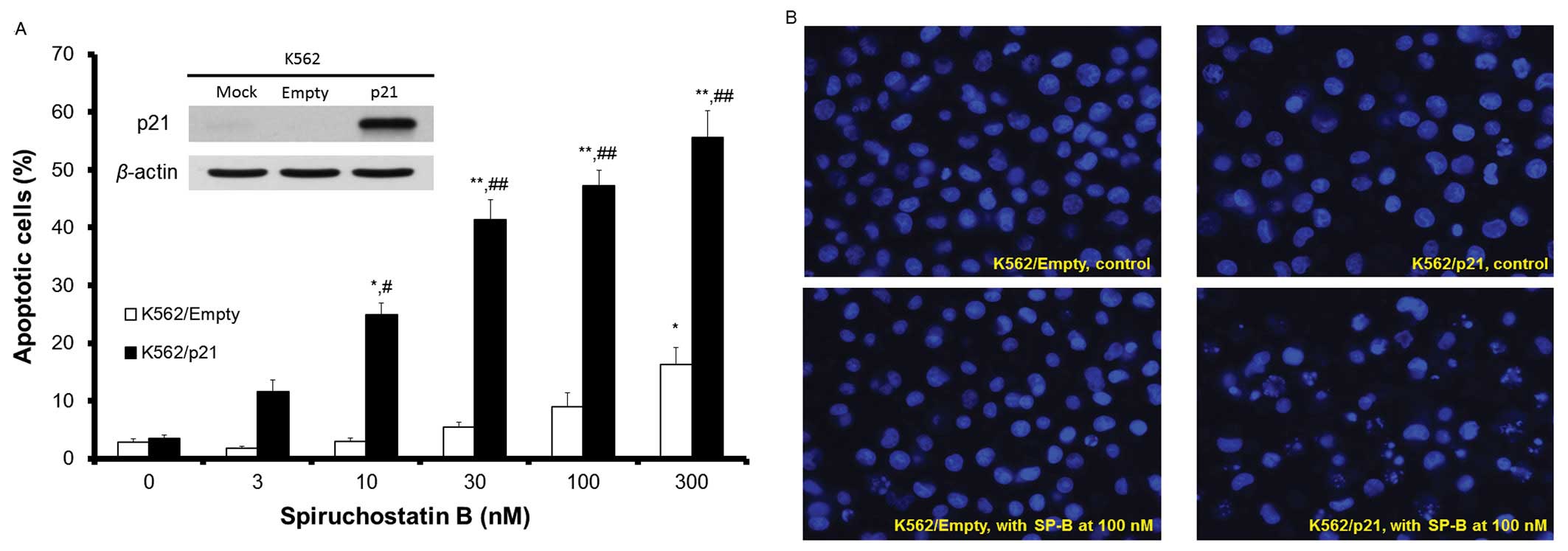
Our previous results indicated that the human
erythroleukemia cell line K562 was considerably less susceptible to
SP-B than typical leukemia cell lines (8). Thus, the IC50 of SP-B for
K562 cells was more than 100-fold higher than that for the parental
NALM-6 cells. To determine whether introduction of exogenous
p21waf1/cip1 expression improves the susceptibility of
leukemia cells to SP-B, we transfected K562 cells with
p21waf1/cip1 and analyzed its effect on the
susceptibility of these cells to apoptosis induced by SP-B
(Fig. 4A and B). As shown in the
inset to Fig. 4A, the
p21waf1/cip1 protein was expressed in K562 cells 24 h
after p21waf1/cip1 transfection (K562/p21). Following
SP-B treatment of K562 control cells (transfected with empty
vector, K562/Empty), there was no significant induction of
apoptosis by SP-B compared to non-treated cells at SP-B
concentrations of up to 100 nM, although apoptosis of these cells
was increased at an SP-B concentration of 300 nM. Apoptosis was
induced by SP-B in K562/p21 cells in an SP-B
concentration-dependent manner and apoptosis of these cells was
significantly increased compared to K562/Empty cells at all SP-B
concentrations tested between 10 and 300 nM (Fig. 4A). The percentage cell survival of
K562/Empty or K562/p21 cells following incubation for 48 h with
SP-B at concentrations of 3, 10, 30, 100 and 300 nM was 102.8,
98.4, 87.4, 72.8 and 28.4%, and 85.2, 79.9, 38.2, 19.1 and 13.8%,
respectively. Thus, expression of exogenous p21waf1/cip1
enhanced the susceptibility of K562 cells to SP-B.
Discussion
Our previous report showed that SP-B is the most
potent HDI for a typical human leukemia cell line and further
demonstrated that the expression of p21waf1/cip1 plays a
pivotal role in SP-B induction of apoptosis and in the resulting
anti-tumor activity of SP-B (8).
In the present study, we developed and characterized SP-B-resistant
NALM-6 cells, and, by comparing SP-B-resistant and -susceptible
leukemia cells, showed that the introduction of exogenous
p21waf1/cip1 into cells reversed their SP-B resistance
and enhanced their susceptibility to SP-B.
The SP-B-resistant NALM-6 cells we established were
also resistant to FK228 (Table I).
All previously established FK228-resistant cells are highly
resistant to FK228 compared with parental cells and this resistance
depends on increased expression of the P-gp protein that is encoded
by the MDR1 gene (10–12).
These previously established FK228-resistant cells acquired drug
resistance within a short period (continuous culture for 1 to 3
months) compared with the SP-B-resistant cells that we established
(continuous culture for almost 6 months). The SP-B concentration
and the culture period necessary for acquisition of resistance to
SP-B in this study suggest that it was more difficult to achieve
SP-B resistance than FK228 resistance. Furthermore, SP-B resistance
had little effect on MDR1 mRNA expression in NALM-6 cells.
However, despite the fact that MDR1 mRNA expression of
NALM-6/SP-B was nearly the same as that of parental cells (Fig. 2), the NALM-6/SP-B cells displayed
lower susceptibility to DOX and VCR, which are better substrates
for P-gp, than the parental cells (Table I). A previous DNA microarray
analysis study indicated that the level of
p21waf1/cip1 is significantly decreased in
DOX-resistant acute myelocytic leukemia cells (19). This result suggests that a decline
in p21waf1/ cip1 contributes to lower sensitivity
to DOX. Our data further showed that NALM-6/SP-B cells are slightly
more resistant to VCR than parental cells, but show similar
resistance to TAX. Both VCR and TAX are M-phase-specific drugs that
stabilize microtubules. A previous study indicated that
cytotoxicity and apoptosis induced by these agents were potentiated
by overexpression of p21waf1/cip1 in sarcoma cells
(20). In contrast, enforced
expression of p21waf1/cip1 in leukemia cells attenuated
TAX-mediated apoptosis (21).
Thus, the involvement of p21waf1/cip1 in the action of
M-phase-specific drugs may be cell specific. Yamada et
al(11), using cDNA microarray
analysis, showed that gene expression of
p21waf1/cip1 increased in FK228-resistant KU812
cells established from a patient with myeloid leukemia in blastic
crisis. Unexpectedly, in our study, NALM-6/SP-B showed decreased
p21waf1/cip1 mRNA expression compared with
parental cells (Fig. 2). We
propose that the underlying mechanism is as follows: SP-B has a
stronger effect on p21waf1/cip1 expression than other
HDIs (8); therefore, continuous
exposure of NALM-6 parental cells to SP-B might lead to
counteraction of HDI effects on p21waf1/cip1 expression,
resulting in downregulation of p21waf1/cip1 mRNA
expression. Such a decrease may not occur following continuous
exposure of cells to other HDIs, which have a lower effect on
histone deacetylases, and therefore on p21waf1/cip1
expression.
As shown in Fig. 2,
although endogenous p21waf1/cip1 mRNA expression
was decreased in NALM-6 cells that had acquired SP-B resistance,
neither the mRNA expression of HDAC1 nor that of other
transcription factors such as p53 or c-Myc, or of
apoptosis-related genes showed any change. This HDAC1 result
is in contrast to HL-60 human promyelocytic leukemia cells that are
resistant to HDIs, which have been shown to possess significantly
higher levels of HDAC1 than parental cells (22). Several other studies in which p53
was assayed as a representative apoptosis-related transcription
factor indicated that HDI-induced apoptosis involves the p53
pathway (23,24). In contrast, it has also been shown
that HDI induces apoptosis through a p53-independent pathway in
leukemia cells (25,26). Our result suggests that the SP-B
resistance of NALM-6 cells does not involve p53 expression. Indeed,
SP-B did not change p53 expression and pifithrin-α, an inhibitor of
p53, and did not affect SP-B-induced apoptosis in either parental
NALM-6 or NALM-6/SP-B cells (data not shown). The mRNA expression
of the proto-oncogene c-Myc, in addition to that of
p21waf1/cip1, has been shown to be a useful
indicator of the anti-tumor activity of FK228 (27). Moreover, cDNA microarray analysis
of a human acute T cell leukemia cell line (CEM) has shown that
HDIs regulate not only c-Myc, but also other genes such as
Bax, Bcl-2 and Cas-3 that are involved in the
intrinsic apoptotic pathway (28).
Another study has proposed that FR901228 (also called FK228) can
upregulate the Fas system in osteosarcoma cells, resulting in
caspase activation (29). However,
as mentioned above, no significant change in the mRNA levels of
these previously reported signals was observed in the
SP-B-resistant NALM-6 cells. Here, we demonstrated that the
decrease in p21waf1/cip1 mRNA expression was a
unique characteristic of the SP-B-resistant NALM-6 cells. This is
how induction of apoptosis by SP-B in NALM-6/SP-B was reversed, by
the introduction of exogenous p21waf1/cip1 expression,
which compensated for the decrease in endogenous
p21waf1/cip1 in the resistant cells (Fig. 3).
Recent studies have shown that
p21waf1/cip1 can mediate both pro- and anti-apoptotic
functions in response to anti-tumor agents depending on the cell
type and the cellular context (30). As shown in Fig. 4A and B, overexpressed
p21waf1/cip1 enhanced SP-B-induced apoptosis in K562
cells. This result suggests that p21waf1/cip1 has
pro-apoptotic functions in K562 cells. Furthermore, the enhancing
effect of exogenous p21waf1/cip1 on the susceptibility
of K562 cells to SP-B-induced apoptosis was stronger than its
effect on the reversal of the resistance of NALM-6/SP-B cells to
SP-B (Fig. 3). In other words,
overexpressed p21waf1/cip1 could not completely reverse
the resistance of NALM-6/SP-B so that it had the same level of SP-B
susceptibility as the parental cells. Most studies on induction of
apoptosis by p21waf1/cip1 were reported using cells that
expressed a non-functional p53 mutant (30). It has already been reported that
K562 cells harbor mutated p53 (31,32),
and that NALM-6 cells express wild-type p53 (18,32).
The ability of overexpressed p21waf1/cip1 to enhance
SP-B-induced apoptosis is assumed to depend on p53 status.
Elucidation of the relationship between p53 status and
p21waf1/cip1 expression may lead to the establishment of
effective SP-B chemotherapy for leukemia patients.
In conclusion, NALM-6 cells that were resistant to
SP-B showed a unique decrease in the mRNA expression of
p21waf1/cip1. The introduction of exogenous
p21waf1/cip1 not only reversed the SP-B resistance of
these NALM-6 cells, but also enhanced the susceptibility of K562
cells to apoptosis induced by SP-B. Based on these results,
introduction of exogenous p21waf1/cip1 into tumor cells
in a clinical setting should improve tumor resistance to SP-B or
may lead to successful chemotherapy using SP-B. Our findings may be
useful when establishing a therapeutic strategy using SP-B.
References
|
1
|
Narita K, Kikuchi T, Watanabe K, et al:
Total synthesis of the bicyclic depsipeptide HDAC inhibitors
spiruchostatins A and B, 5″-epi-spiruchostatin B, FK228 (FR901228)
and preliminary evaluation of their biological activity. Chemistry.
15:11174–11186. 2009.PubMed/NCBI
|
|
2
|
Ocker M and Schneider-Stock R: Histone
deacetylase inhibitors: signalling towards p21cip1/waf1. Int J
Biochem Cell Biol. 39:1367–1374. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xiong Y, Zhang H and Beach D: D type
cyclins associate with multiple protein kinases and the DNA
replication and repair factor PCNA. Cell. 71:505–514. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Han JW, Ahn SH, Kim YK, et al: Activation
of p21(WAF1/Cip1) transcription through Sp1 sites by histone
deacetylase inhibitor apicidin: involvement of protein kinase C. J
Biol Chem. 276:42084–42090. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fournel M, Trachy-Bourget MC, Yan PT, et
al: Sulfonamide anilides, a novel class of histone deacetylase
inhibitors, are anti-proliferative against human tumors. Cancer
Res. 62:4325–4330. 2002.
|
|
6
|
Archer SY, Meng S, Shei A and Hodin RA:
p21(WAF1) is required for butyrate-mediated growth inhibition of
human colon cancer cells. Proc Natl Acad Sci USA. 95:6791–6796.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sambucetti LC, Fischer DD, Zabludoff S, et
al: Histone deacetylase inhibition selectively alters the activity
and expression of cell cycle proteins leading to specific chromatin
acetylation and antiproliferative effects. J Biol Chem.
274:34940–34947. 1999. View Article : Google Scholar
|
|
8
|
Kanno SI, Maeda N, Tomizawa A, Yomogida S,
Katoh T and Ishikawa M: Involvement of p21waf1/cip1
expression in the cytotoxicity of the potent histone deacetylase
inhibitor spiruchostatin B towards susceptible NALM-6 human B cell
leukemia cells. Int J Oncol. 40:1391–1396. 2012.
|
|
9
|
Szakacs G, Annereau JP, Lababidi S, et al:
Predicting drug sensitivity and resistance: profiling ABC
transporter genes in cancer cells. Cancer Cell. 6:129–137. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiao JJ, Huang Y, Dai Z, et al:
Chemoresistance to depsipeptide FK228
[(E)-(1S,4S,10S,21R)-7-[(Z)-ethylidene]-4,21-diisopropyl-2-oxa-12,13-dithia-5,8,20,23-tetraazabicyclo[8,7,6]-tricos-16-ene-3,6,9,22-pentanone]
is mediated by reversible MDR1 induction in human cancer cell
lines. J Pharmacol Exp Ther. 314:467–475. 2005.PubMed/NCBI
|
|
11
|
Yamada H, Arakawa Y, Saito S, Agawa M,
Kano Y and Horiguchi-Yamada J: Depsipeptide-resistant KU812 cells
show reversible P-glycoprotein expression, hyper-acetylated
histones, and modulated gene expression profile. Leuk Res.
30:723–734. 2006. View Article : Google Scholar
|
|
12
|
Matsubara H, Watanabe M, Imai T, et al:
Involvement of extracellular signal-regulated kinase activation in
human osteosarcoma cell resistance to the histone deacetylase
inhibitor FK228
[(1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-bis(propan-2-yl)-2-oxa-12,13-dit
hia-5,8,20,23-tetraazabicyclo[8.7.6]
tricos-16-ene-3,6,9,19,22-pentone]. J Pharmacol Exp Ther.
328:839–848. 2009.PubMed/NCBI
|
|
13
|
Imesch P, Dedes KJ, Furlato M, Fink D and
Fedier A: MLH1 protects from resistance acquisition by the histone
deacetylase inhibitor trichostatin A in colon tumor cells. Int J
Oncol. 35:631–640. 2009.PubMed/NCBI
|
|
14
|
Dedes KJ, Dedes I, Imesch P, von Bueren
AO, Fink D and Fedier A: Acquired vorinostat resistance shows
partial cross-resistance to ‘second-generation’ HDAC inhibitors and
correlates with loss of histone acetylation and apoptosis but not
with altered HDAC and HAT activities. Anticancer Drugs. 20:321–333.
2009.PubMed/NCBI
|
|
15
|
Takizawa T, Watanabe K, Narita K, Oguchi
T, Abe H and Katoh T: Total synthesis of spiruchostatin B, a potent
histone deacetylase inhibitor, from a microorganism. Chem Commun
(Camb). 1677–1679. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kanno S, Kakuta M, Kitajima Y, et al:
Preventive effect of trimidox on oxidative stress in U937 cell
line. Biol Pharm Bull. 30:994–998. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kanno S, Hiura T, Ohtake T, et al:
Characterization of resistance to cytosine arabinoside (Ara-C) in
NALM-6 human B leukemia cells. Clin Chim Acta. 377:144–149. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kanno S, Higurashi A, Watanabe Y, Shouji
A, Asou K and Ishikawa M: Susceptibility to cytosine arabinoside
(Ara-C)-induced cytotoxicity in human leukemia cell lines. Toxicol
Lett. 152:149–158. 2004.PubMed/NCBI
|
|
19
|
Song JH, Choi CH, Yeom HJ, Hwang SY and
Kim TS: Monitoring the gene expression profiles of
doxorubicin-resistant acute myelocytic leukemia cells by DNA
microarray analysis. Life Sci. 79:193–202. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li W, Fan J, Banerjee D and Bertino JR:
Overexpression of p21(waf1) decreases G2-M arrest and apoptosis
induced by paclitaxel in human sarcoma cells lacking both p53 and
functional Rb protein. Mol Pharmacol. 55:1088–1093. 1999.PubMed/NCBI
|
|
21
|
Ahmed W, Rahmani M, Dent P and Grant S:
The cyclin-dependent kinase inhibitor p21(CIP1/WAF1) blocks
paclitaxel-induced G2M arrest and attenuates mitochondrial injury
and apoptosis in p53-null human leukemia cells. Cell Cycle.
3:1305–1311. 2004. View Article : Google Scholar
|
|
22
|
Fiskus W, Rao R, Fernandez P, et al:
Molecular and biologic characterization and drug sensitivity of
pan-histone deacetylase inhibitor-resistant acute myeloid leukemia
cells. Blood. 112:2896–2905. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bandyopadhyay D, Mishra A and Medrano EE:
Overexpression of histone deacetylase 1 confers resistance to
sodium butyrate-mediated apoptosis in melanoma cells through a
p53-mediated pathway. Cancer Res. 64:7706–7710. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Condorelli F, Gnemmi I, Vallario A,
Genazzani AA and Canonico PL: Inhibitors of histone deacetylase
(HDAC) restore the p53 pathway in neuroblastoma cells. Br J
Pharmacol. 153:657–668. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vrana JA, Decker RH, Johnson CR, et al:
Induction of apoptosis in U937 human leukemia cells by
suberoylanilide hydroxamic acid (SAHA) proceeds through pathways
that are regulated by Bcl-2/Bcl-XL, c-Jun, and p21CIP1, but
independent of p53. Oncogene. 18:7016–7025. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Insinga A, Monestiroli S, Ronzoni S, et
al: Inhibitors of histone deacetylases induce tumor-selective
apoptosis through activation of the death receptor pathway. Nat
Med. 11:71–76. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sasakawa Y, Naoe Y, Inoue T, et al:
Effects of FK228, a novel histone deacetylase inhibitor, on tumor
growth and expression of p21 and c-myc genes in vivo. Cancer Lett.
195:161–168. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Peart MJ, Smyth GK, van Laar RK, et al:
Identification and functional significance of genes regulated by
structurally different histone deacetylase inhibitors. Proc Natl
Acad Sci USA. 102:3697–3702. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Imai T, Adachi S, Nishijo K, et al:
FR901228 induces tumor regression associated with induction of Fas
ligand and activation of Fas signaling in human osteosarcoma cells.
Oncogene. 22:9231–9242. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu S, Bishop WR and Liu M: Differential
effects of cell cycle regulatory protein p21(WAF1/Cip1) on
apoptosis and sensitivity to cancer chemotherapy. Drug Resist
Updat. 6:183–195. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Law JC, Ritke MK, Yalowich JC, Leder GH
and Ferrell RE: Mutational inactivation of the p53 gene in the
human erythroid leukemic K562 cell line. Leuk Res. 17:1045–1050.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Filippini G, Griffin S, Uhr M, et al: A
novel flow cytometric method for the quantification of p53 gene
expression. Cytometry. 31:180–186. 1998. View Article : Google Scholar : PubMed/NCBI
|