Introduction
Hepatocellular carcinoma (HCC) is the most frequent
primary liver cancer; it is the fifth most common malignancy
worldwide and the third most common cause of cancer-related
mortality (1). Current options for
the treatment of HCC include surgical resection, liver
transplantation, transcatheter arterial embolization (TAE) or
chemoembolization (TACE), transcatheter arterial infusion
chemotherapy (TAI), percutaneous ethanol injection therapy (PEIT),
percutaneous radiofrequency thermal ablation (RFA) therapy,
radioembolization and molecular-targeted drugs such as sorafenib
(2–5). However, HCC frequently recurs after
treatment, leading to high mortality rates. In 68 to 96% of
patients recurrence only occurs at intrahepatic sites (6). Therefore, prevention and effective
management of intrahepatic distant recurrence (IDR) are important
strategies for improving overall survival after curative treatment
of HCC (7).
Interferon treatment for chronic hepatitis C and
nucleoside analogue treatment for chronic hepatitis B have been
reported as being useful in preventing IDR after curative treatment
for HCC (8–10).
The objectives of adjuvant regional chemotherapy are
to prevent tumor recurrence from the primary tumor or to reduce the
incidence of a second HCC. Compared with systemic chemotherapy, TAI
has the advantages of increasing the local concentration of
chemotherapeutic agents for killing cancer cells, while causing
less damage to healthy liver tissue and reducing systemic
side-effects (11). However, to
our knowledge, there have been few reports on effective neoadjuvant
regional chemotherapy or adjuvant regional chemotherapy for
preventing IDR (7,12,13).
In our department, we have routinely performed
hepatic arterial infusion chemotherapy using an
epirubicin-mitomycin-lipiodol emulsion for HCC when carrying out
angiography, since the approval of these chemotherapeutic agents
for the treatment of HCC in Japan. However, convincing evidence
supporting the effectiveness of TAI with an
epirubicin-mitomycin-lipiodol emulsion before RFA in preventing IDR
is currently lacking.
The aim of the present study was to elucidate the
effectiveness of TAI using an epirubicin-mitomycin-lipiodol
emulsion, administered via the proper hepatic artery to the whole
liver (TAI-EML), prior to RFA, in preventing IDR for single
HCC.
Materials and methods
Study design
The present study was a single center and
retrospective study. Prior to angiography and RFA, written informed
consent was obtained from all the patients. The protocols for
angiography and RFA were approved by the ethics committee of our
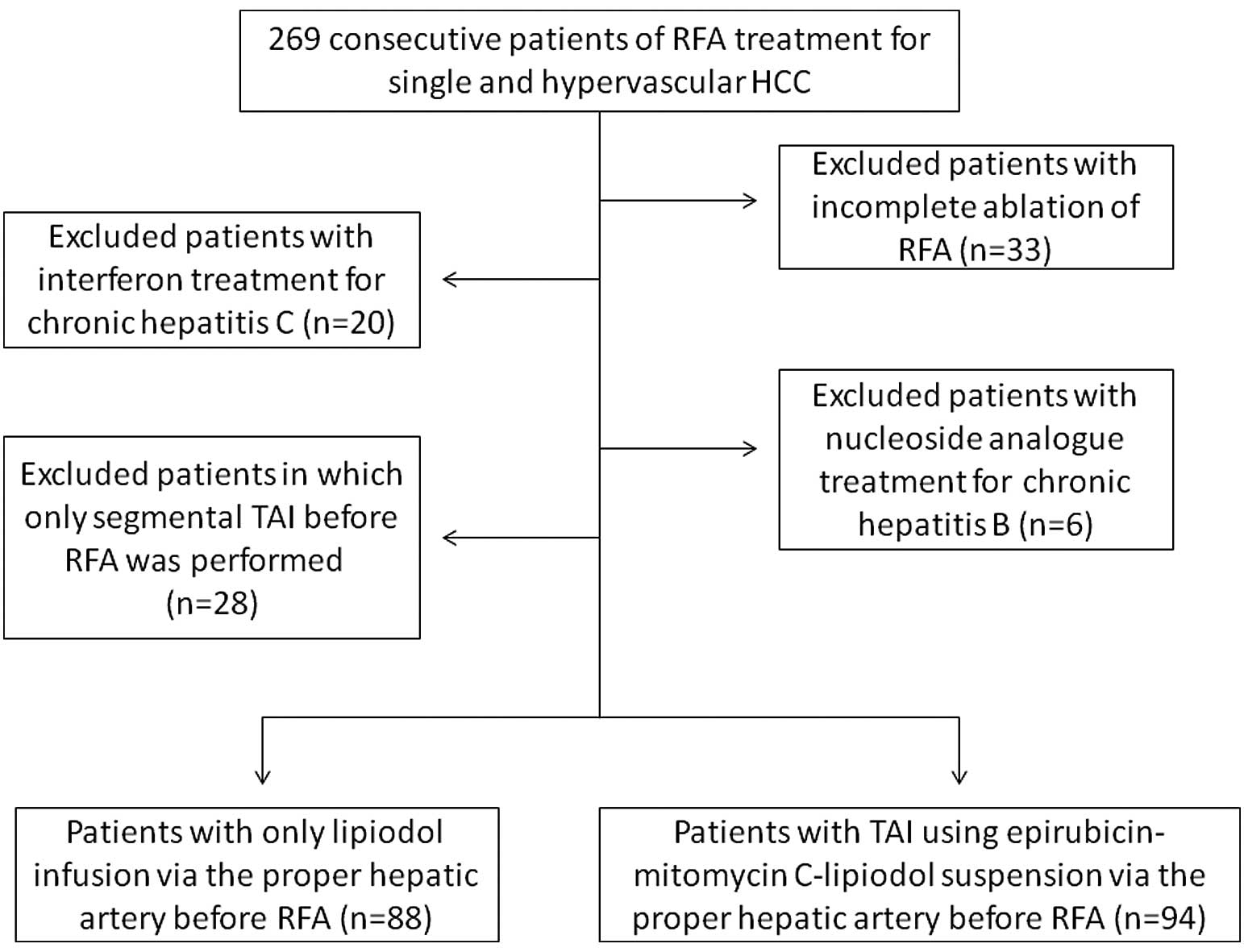
department (Fig. 1).
We assessed the electronic medical records of
patients registered in our database. The primary endpoint was
post-RFA IDR-free survival, and the secondary endpoints were local
tumor progression (LTP) and overall survival (OS).
Patients
We performed RFA in 269 consecutive treatment-naive
patients diagnosed with solitary and hypervascular HCC in the
Department of Gastroenterology and Hepatology, Osaka Red Cross
Hospital, Japan, between January 2004 and October 2010. During the
period from January 2004 to April 2005 and from December 2008 to
October 2010, we routinely performed TAI-EML prior to RFA. Between
May 2005 and October 2008, only lipiodol infusion was performed
prior to RFA, as we were using the protocols of several clinical
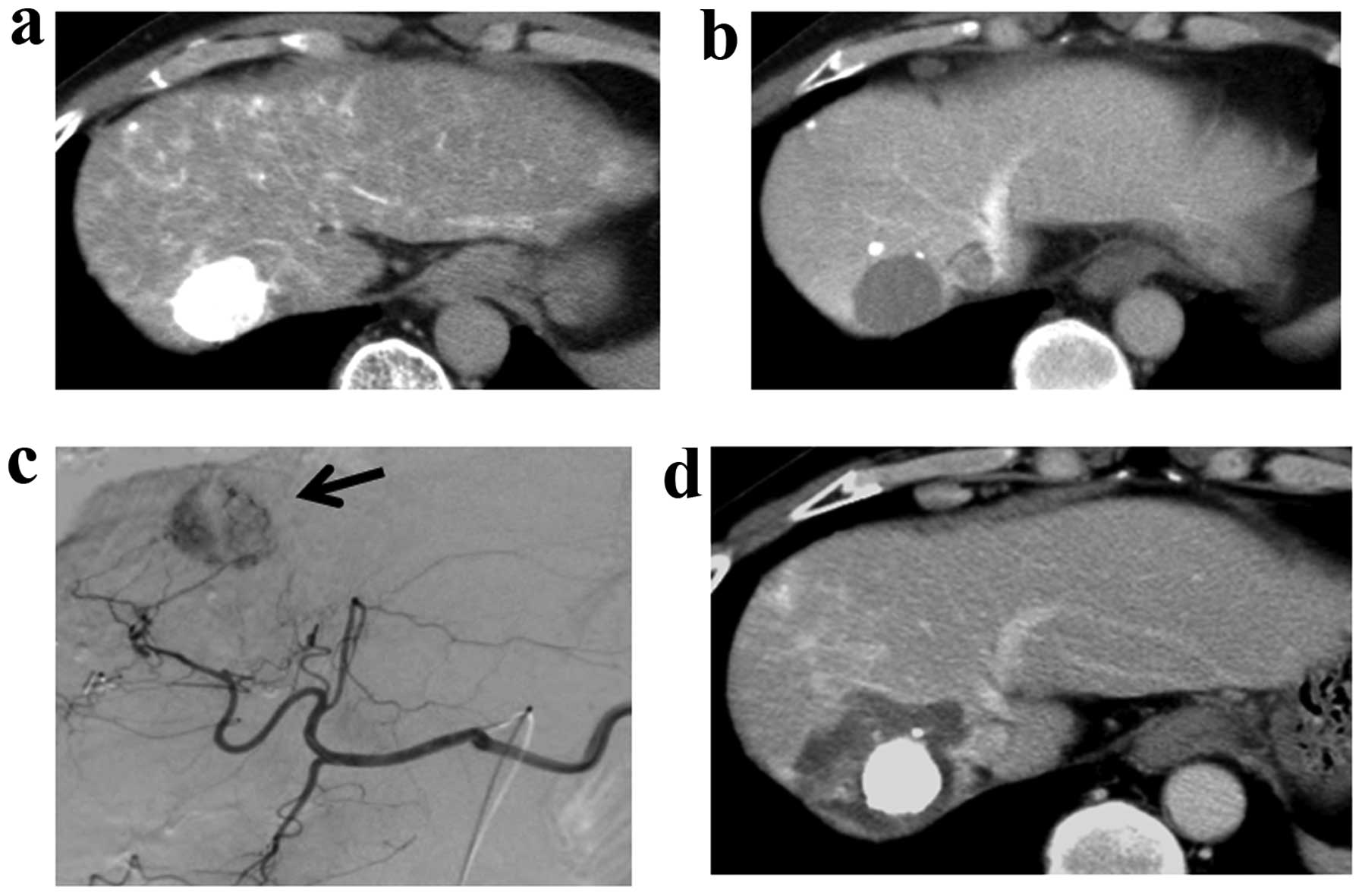
studies that we had participated in. Lipiodol (Lipiodol
Ultra-Fluid, Schering Japan, Osaka, Japan) accumulates selectively
in tumors when infused intra-arterially (14). Therefore, lipiodol infusion was
performed to improve tumor visibility when assessing the
effectiveness of RFA using dynamic computed tomography (CT). A
representative case is shown in Fig.
2.
Of the 269 patients mentioned above, patients who
met the following inclusion criteria were analyzed: a) complete
ablation of RFA; and b) no evidence of other malignancies. We
excluded patients who had: a) incomplete ablation of RFA; b)
interferon treatment for chronic hepatitis C; c) nucleoside
analogue treatment for chronic hepatitis B; and d) only segmental
TAI before RFA due to the decision of attending physicians
(Fig. 1). Complete ablation was
defined as no apparent residual tumors on dynamic CT performed
within 7 days after RFA and was determined by three radiologists
(not blinded) experienced in liver imaging. In patients with
incomplete RFA, TACE was performed after RFA.
Diagnosis of HCC
HCC was diagnosed using abdominal ultrasound and
dynamic CT scans (hyperattenuation during the arterial phase in all
or some part of the tumor and hypoattenuation in the portal-venous
phase) and/or magnetic resonance imaging (MRI), mainly based on the
recommendations of the American Association for the Study of Liver
Diseases (15). Arterial and
portal phase dynamic CT images were obtained at approximately 30
and 120 sec, respectively, after the injection of the contrast
material. In our department, abdominal angiography combined with CT
(angio-CT) assistance was performed on all patients before RFA; CT
during hepatic arteriography (CTHA) was performed with the catheter
tip in the proper hepatic artery and CT during arterial-portography
(CTAP) was performed with the catheter tip in the superior
mesenteric artery, as Yamasaki et al reported that this
technique was useful for detecting small satellite nodules
(16). Then, we confirmed the
presence of single and hypervascular HCC with no vascular invasion
using CTHA and CTAP. Immediately after evaluation using angio-CT,
TAI-EML or lipiodol infusion-alone via the proper hepatic artery
was performed in the same session.
Treatment procedure
TAI
A catheter was introduced into the proper hepatic
artery using the Seldinger technique. This was followed by an
intra-arterial infusion via the proper hepatic artery according to
tumor size and liver function, of an emulsion containing epirubicin
(mean dose, 28.2 mg; range, 10–40 mg), mitomycin (mean dose, 6.2
mg; range, 4–10 mg) and lipiodol (mean dose, 2.1 ml; range, 1–3 ml)
in the TAI group and of lipiodol alone (mean dose, 1.9 ml; range,
1–3 ml) in the lipiodol-alone group, respectively. When patients
had poor liver function, the dosage of the anticancer agents and
lipiodol were reduced.
Epirubicin-mitomycin-lipiodol
emulsion
Epirubicin (Farmorubicin; Kyowa Hakko Kirin Company,
Ltd., Tokyo, Japan) is an anthracycline-based anticancer drug and
is the 4′-epimer of doxorubicin. Epirubicin, as well as
doxorubicin, binds to the deoxyribonucleic acid (DNA) in tumor
cells, leading to suppression of DNA synthesis (17). Mitomycin (Mitomycin C; Kyowa Hakko
Kirin Company, Ltd., Tokyo, Japan) is changed into activated
metabolites by various enzymes, and these block DNA replication in
tumor cells leading to antitumor effects (18). Due to the fact that epirubicin
easily undergoes glucuronidation, its toxicity is milder than that
of doxorubicin. In 1986, the Japan Epirubicin Study Group for
Hepatocellular Carcinoma reported that in 53 HCC patients who had
received TAI with epirubicin, eight of the patients had an
objective response. A retrospective comparison of this result with
that of patients that were treated using TAI with doxorubicin
demonstrated that epirubicin was more effective than doxorubicin in
terms of survival rate (17).
Since that time, epirubicin has been conventionally used in TAI for
HCC in Japan, and additional clinical trials have been performed in
several faculties. Epirubicin alone or in combination with other
chemotherapeutic agents such as mitomycin C has been used in TAI
for HCC in Asian countries, including Japan (17). Considering this background, we have
conventionally performed TAI for HCC in our department with an
epirubicin-mitomycin-lipiodol emulsion.
RFA
In all patients, the first session of RFA was
performed within 7 days after angiography. We routinely used a
cool-tip needle (Radionics Corp., Burlington, MA, USA) while
performing RFA. Under local anesthesia, using the intercostal or
subcostal approach, an electrode was inserted under real-time
ultrasound guidance. The initial treatment was planned with one
ablation for tumors of <2 cm in diameter, and two or more
ablations with the overlapping technique for tumors of ≥2 cm in
diameter. When tumor ablation was complete, thermal ablation was
performed along the needle track. We did not use the coaxial
technique. All patients were carefully observed for
treatment-related complications. The procedures were all conducted
under ultrasound guidance by one of five operators who had at least
3 years of experience in performing RFA.
Follow-up
Follow-up after RFA consisted of monthly blood tests
and monitoring of tumor markers, including α-fetoprotein (AFP) and
des-γ-carboxy prothrombin (DCP), which were measured using a
chemiluminescent enzyme immunoassay (Lumipulse PIVKAII Eisai,
Tokyo, Japan). Abdominal ultrasonography and dynamic CT scans were
obtained every 3–4 months following RFA.
Definition of IDR and LTP
We defined IDR as the recurrence of a new lesion
(excluding extrahepatic metastasis) at a distant site from the
ablation zone detected using a dynamic CT scan. When a new lesion
appeared in the same segment as the original tumor, we defined it
as IDR if it was not adjacent to the ablation zone. We defined
local tumor progression (LTP) as the presence of a hypervascular
nodule adjacent to the ablation zone after RFA revealed using a
dynamic CT scan (19). Recurrence
that was distant from the ablation zone in the same segment was not
included in the definition of LTP. IDR and LTP were determined by
the same three radiologists (not blinded) as described above.
Statistical analysis
Intercomparison of the lipiodol-alone group and the
TAI group was performed using the unpaired t-test or Fisher’s exact
test. Data were expressed as the mean ± standard deviation and were
analyzed using univariate and multivariate analyses. The cumulative
post-RFA IDR-free survival rate, the cumulative LTP rate and the
cumulative OS rate were calculated using the Kaplan-Meier method,
and tested using the log-rank test. Regarding factors contributing
to IDR, the Cox proportional hazard model was used for multivariate
analyses of factors that were considered significant in univariate
analysis. These statistical methods were used to estimate the
interval from RFA treatment. Data were analyzed using SPSS
software, version 9.0 (SPSS Inc., Chicago, IL, USA) for Microsoft
Windows. P-values <0.05 were considered to indicate
statistically significant differences.
Results
Patients and recurrence
In 94 of the 182 patients analyzed in the present
study, between January 2004 and April 2005 and between December
2008 and October 2010, TAI-EML was performed prior to RFA. Over the
period from May 2005 to October 2008, infusion involving
lipiodol-alone via the proper hepatic artery was performed prior to
RFA in 88 patients.
The baseline characteristics of the two groups are
shown in Table I. The mean tumor
size was 2.06 cm (range, 0.9–3.2 cm) in the TAI group and 1.97 cm
(range, 0.9–3.3 cm) in the lipiodol-alone group, respectively.
There were no significant differences between the two groups in
terms of baseline characteristics. At a median follow-up interval
of 36.4 months (range, 6.2–83.6 months), 55 (62.5%) of the 88
patients in the lipiodol-alone group and 31 (33.0%) of the 94
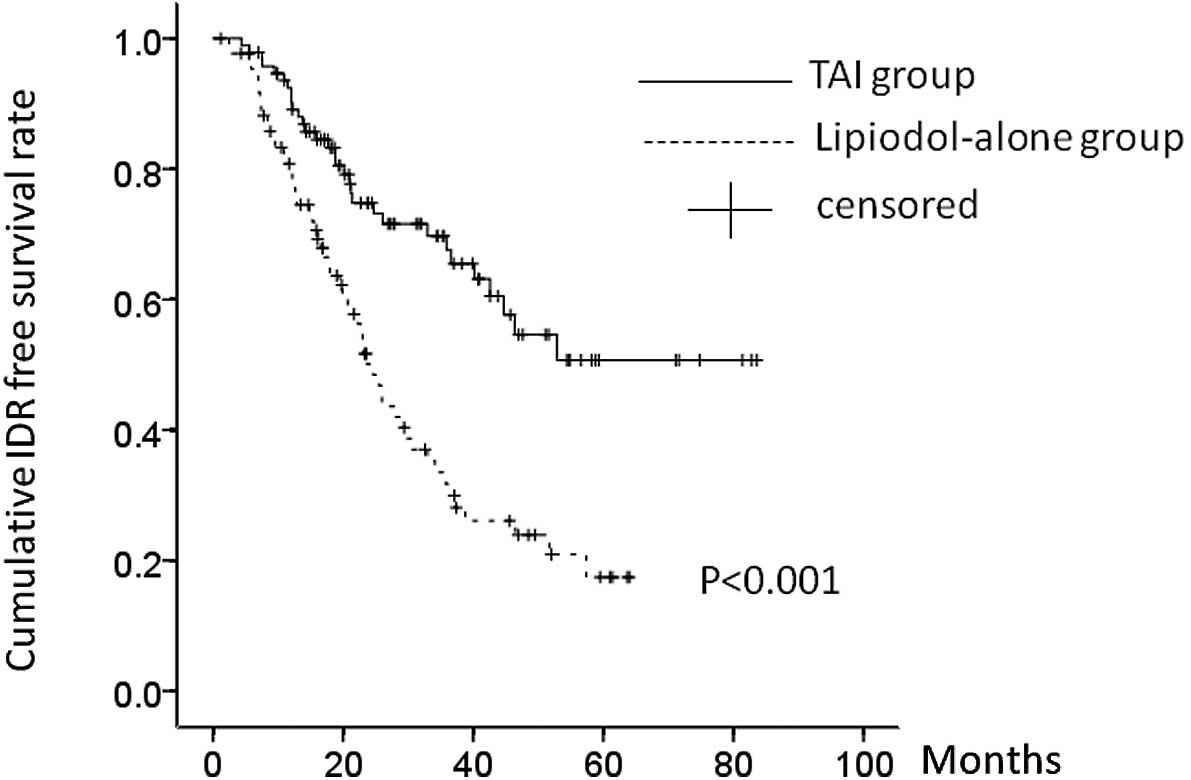
patients in the TAI group had IDR. The cumulative post-RFA IDR-free
survival rates at 1, 2 and 3 years were 74.0, 50.8 and 34.9%,
respectively, in the lipiodol-alone group, and 90.8, 74.8 and
70.0%, respectively, in the TAI group (P<0.001). There was a
significant difference in the post-RFA IDR-free survival rate
between the two groups (P<0.001) (Fig. 3).
 | Table IBaseline characteristics of patients
in the TAI and lipiodol-alone groups. |
Table I
Baseline characteristics of patients
in the TAI and lipiodol-alone groups.
| Lipiodol-alone group
(n=88) | TAI group (n=94) | P-value |
|---|
| Gender (male/female),
n | 58/30 | 56/38 | 0.444a |
| Age (mean ± SD) | 69.6±9.8 | 69.3±8.1 | 0.819b |
| Etiology of liver
disease, n | | | |
| B/C/non B, non
C | 2/70/16 | 4/80/10 | 0.229a |
| Child-Pugh
classification, n | | | |
|
Child-A/Child-B/Child-C | 77/8/3 | 80/13/1 | 0.358a |
| Diabetes Mellitus
(yes/no), n | 28/60 | 32/62 | 0.755a |
| Body mass index
(kg/m2) (mean ± SD) | 23.3±3.6 | 23.1±3.5 | 0.764b |
| Total bilirubin
(mg/dl) (mean ± SD) | 0.94±0.56 | 0.95±0.53 | 0.859b |
| Albumin (g/dl) (mean
± SD) | 3.83±0.52 | 3.83±0.53 | 0.988b |
| Platelet
(×104/μl) (mean ± SD) | 10.6±4.7 | 11.4±4.9 | 0.247b |
| Prothrombin time
(%) (mean ± SD) | 87.2±15.6 | 87.4±16.3 | 0.922b |
| AST (IU/l) (mean ±
SD) | 61.0±37.2 | 53.9±31.2 | 0.164b |
| ALT (IU/l) (mean ±
SD) | 58.4±64.7 | 54.9±28.9 | 0.157b |
| AFP (ng/ml) (mean ±
SD) | 120.7±530.4 | 111.2±335.9 | 0.885b |
| DCP (mAU/ml) (mean
± SD) | 215.0±1114.5 | 288.0±1559.4 | 0.718b |
| Tumor size (cm)
(mean ± SD) | 1.97±0.63 | 2.06±0.66 | 0.397b |
Univariate and multivariate
analysis
Using univariate analysis, age ≥70 years (P=0.046),
platelet count ≥10×104/μl (P<0.001),
des-γ-carboxy prothrombin level (DCP ≥100 mAU/ml) (P=0.030) and
TAI-EML before RFA (P<0.001) were found to be significant
factors contributing to IDR (Table
II). In the multivariate analyses involving the four factors
that were found to be significant in the univariate analysis,
hazard ratios (HRs), 95% confidence interval and P-values are
detailed in Table III. A platelet
count of ≥10×104/μl and TAI-EML were found to be
significant independent factors linked to IDR.
 | Table IIUnivariate analysis of parameters
contributing to post-RFA IDR. |
Table II
Univariate analysis of parameters
contributing to post-RFA IDR.
| N | P-valuea |
|---|
| Gender (male)
(yes/no) | 114/68 | 0.763 |
| Age >70 years (
yes/no) | 102/80 | 0.046 |
| Child-Pugh
classification (Child-Pugh A) (yes/no) | 157/25 | 0.382 |
| Diabetes Mellitus
(yes/no) | 60/122 | 0.243 |
| Body mass index
(>25 kg/m2) (yes/no) | 51/131 | 0.560 |
| Total bilirubin
(>1.0 mg/dl) (yes/no) | 64/118 | 0.190 |
| Albumin (>3.5
g/dl) (yes/no) | 142/40 | 0.710 |
| Platelet
(>10×104/μl) (yes/no) | 101/81 | <0.001 |
| Prothrombin time
(>80%) (yes/no) | 124/58 | 0.211 |
| AST (>60 IU/l)
(yes/no) | 65/117 | 0.277 |
| ALT (>60 IU/l)
(yes/no) | 51/131 | 0.399 |
| AFP (>100 ng/ml)
(yes/no) | 30/152 | 0.201 |
| DCP (>100
mAU/ml) (yes/no) | 36/146 | 0.030 |
| Tumor size (>2.0
cm) (yes/no) | 92/90 | 0.284 |
| TAI to the whole
liver (yes/no) | 94/88 | <0.001 |
 | Table IIIMultivariate analysis contributing to
post-RFA IDR. |
Table III
Multivariate analysis contributing to
post-RFA IDR.
| Hazard ratio | 95% CI | P-valuea |
|---|
| Age >70 | | | |
| Yes | 1.000 | | |
| No | 0.673 | 0.435–1.042 | 0.076 |
| Platelet
>10×104/μl | | | |
| Yes | 1.000 | | |
| No | 2.279 | 1.469–3.536 | <0.001 |
| DCP >100
mAU/ml | | | |
| Yes | 1.578 | 0.830–3.001 | 0.164 |
| No | 1.000 | | |
| TAI before RFA | | | |
| Yes | 1.000 | | |
| No | 2.260 | 1.448–3.528 | <0.001 |
LTP
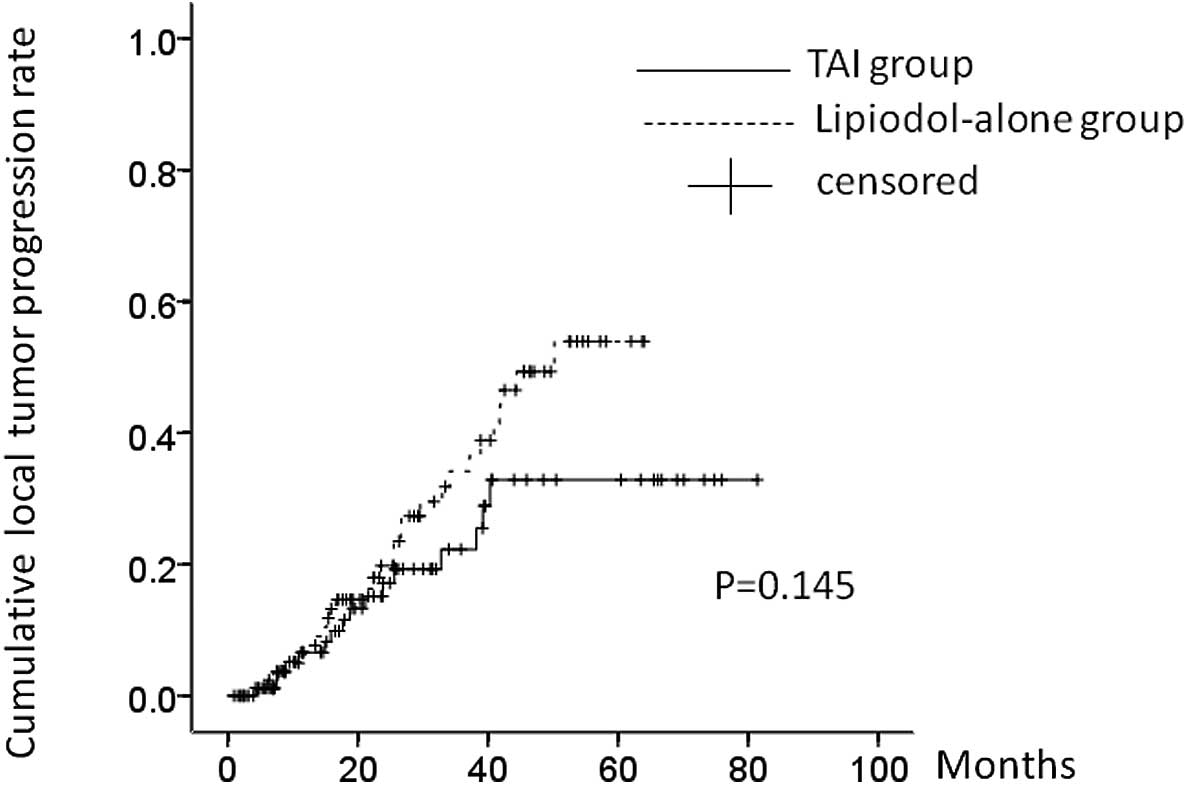
During the observation period, 16 patients (17.0%)
in the TAI group and 28 patients (31.8%) in the lipiodol-alone
group, respectively, had LTP. The 1-, 2- and 3-year LTP rates were:
8.4, 17.4 and 22.2%, respectively, in the TAI group and 8.6, 19.6
and 34.9%, respectively, in the lipiodol-alone group. In terms of
the LTP rate, there was no significant difference between these two
groups (P=0.145) (Fig. 4).
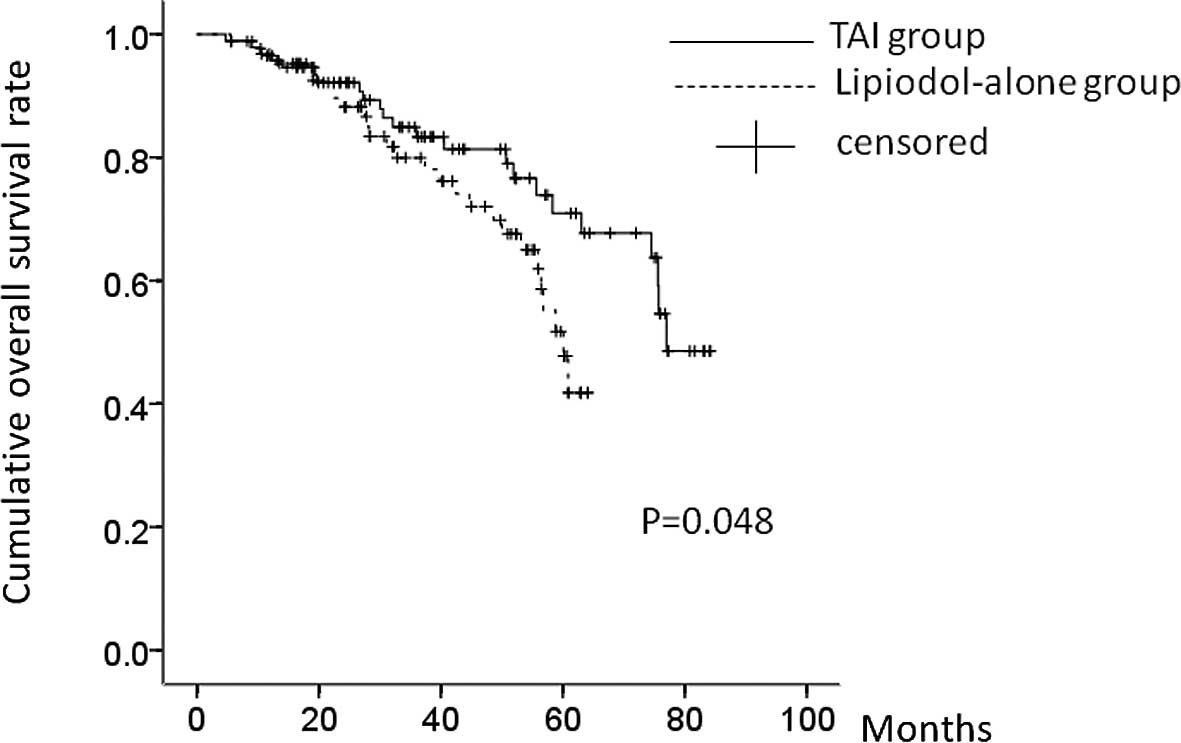
OS
During the observation period, 23 patients (24.5%)
in the TAI group and 30 patients (34.1%) in the lipiodol-alone
group, respectively, succumbed to the disease. Four patients (4.3%)
in the TAI group and six patients (6.8%) in the lipiodol-alone
group, respectively, were lost to follow-up. The causes of
mortality were HCC recurrence (17 patients), liver failure (four
patients) and miscellaneous causes (two patients) in the TAI group,
and HCC recurrence (23 patients), liver failure (four patients) and
miscellaneous causes (three patients) in the lipiodol-alone group,
respectively. The 1-, 3- and 5-year OS rates were: 96.0, 83.7 and
71.1%, respectively, in the TAI group and 96.0, 79.6 and 51.0%,
respectively, in the lipiodol-alone group. In terms of the OS rate,
there was a significant difference between these two groups
(P=0.048) (Fig. 5).
TAI adverse events
Patients in the TAI group experienced transient
fever, abdominal pain, appetite loss, nausea and elevation of liver
enzymes after TAI. However, these side-effects were all grade 1 (as
determined using the National Cancer Institute Common Terminology
Criteria for Adverse Events, version 3.0) (20). Side-effects improved within 3 or 4
days folowing TAI. In the lipiodol-alone group, no side-effects
were observed.
Complications associated with RFA
In terms of complications associated with RFA
itself, pneumothorax in one patient, biloma in one patient and
intra-abdominal bleeding in one patient in the TAI group, and
biloma in one patient, refractory ascites in one patient and
retroperitoneal bleeding in one patient in the lipiodol-alone group
were observed. All patients with complications related to RFA
improved during the same hospitalization period. However, one
patient in the TAI group required re-admission due to biloma and
improved after percutaneous drainage. There were no needle tract
implantations and there were no mortalities due to RFA related
complications.
Discussion
HCC is suitable for treatment with regional
chemotherapy as it has a tendency to stay in the liver until it is
at an advanced stage, with extrahepatic metastasis generally
occurring late. This suggests that an effective regional
chemotherapy would have a great impact on the course of HCC
(21). The rationale for regional
chemotherapy stems from the difference in the dual blood inflow
supply via the portal vein and the hepatic artery between HCC and
nontumorous liver. In hypervascular HCC, the hepatic artery
generally becomes the only vessel supplying blood to the tumor.
Therefore, the hepatic artery is used as a roadway to treat the
tumor, whereas the non-tumorous liver is least affected since the
portal vein is responsible for supplying most of its blood
(6,22). Several clinical trials related to
regional chemotherapy have been performed with the aim of reducing
the incidence of IDR (6,7,12,13,23,24).
However, to our knowledge, there have been few reports on
neoadjuvant regional chemotherapy or adjuvant regional chemotherapy
that was effective in preventing IDR, either prior to or following
curative treatment of HCC (7,12,13).
In contrast to TAI with platinum agents such as
cisplatin, TAI with an epirubicin-mitomycin-lipiodol emulsion
generally does not cause renal toxicity or a hypersensitivity
reaction (17,25). In fact, in the present study, there
were no serious complications due to TAI-EML. In all patients, RFA
was performed safely within 7 days following TAI. These results
suggest that TAI-EML is a safe procedure in terms of adverse
effects.
Using univariate analysis, age ≥70 years, platelet
count ≥10×104/μl, DCP ≥100 mAU/ml and TAI-EML
were found to be significant factors contributing to IDR. Asahina
et al (26) reported that
aging has become one of the most important risk factors for HCC,
and Kubo et al (27)
reported that the platelet count was well correlated with hepatic
fibrosis and liver carcinogenesis. Findings in these two studies
were similar to our findings using univariate analysis. In the
present study, 36 cases (19.8%) had a DCP value of ≥100 mAU/ml. It
has also been reported (28) that
high DCP levels reflect the aggressiveness and progression of HCC
tumors and that the DCP level is a predictor of microvascular
invasion. These findings seem to correlate with our results.
However, using multivariate analysis, the platelet count
≥10×104/μl and TAI-EML before RFA were found to
be significant factors linked to IDR. Moreover, in terms of the OS
rate, there was a significant difference between these two groups
in the present study. These results suggest that suppression of the
progression of hepatic fibrosis as well as TAI-EML before RFA
appears useful in preventing post-RFA recurrence and might
contribute to improved survival rates.
It is generally believed that recurrences after
curative treatment for HCC in the early post-treatment period
arise, not because of incomplete treatment of the primary tumor but
because of pre-existing microscopic tumor foci that are not
detected by imaging modalities; in addition, recurrence can be
caused by malignant cells that have been disseminated during
treatment (29). TAI-EML before
RFA may prevent an increase in size of pre-existing microscopic
tumor foci.
In terms of LTP, there was no significant difference
in the two groups. In reducing LPT after RFA, obtaining sufficient
ablative margin is considered to be essential (30).
The present study had several limitations: i) it was
a retrospective study carried out over a long period of 6 years;
ii) histological examination of each target HCC was not performed,
although it has been reported that pathological and biological
factors have been found to be useful and have helped guide
clinicians in the management of HCC patients (31); iii) the median observation period
in the present study was relatively short compared with previous
studies; iv) patients with incomplete ablation of RFA were excluded
in the present study, leading to a bias; v) there were some
patients lost to follow-up, leading to the possibility of
under-estimating the true recurrence and survival rates. Therefore,
a prospective and randomized study of whole liver treatment with an
epirubicin-mitomycin-lipiodol emulsion is required in the future.
However, our findings demonstrate a significant IDR preventive
effect in the TAI group, suggesting the usefulness of whole liver
treatment with TAI using an epirubicin-mitomycin-lipiodol
emulsion.
In conclusion, RFA and sequential TAI-EML may
contribute to a longer recurrence-free period and overall
survival.
References
|
1.
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar
|
|
2.
|
Kudo M, Izumi N, Kokudo N, Matsui O,
Sakamoto M, Nakashima O, Kojiro M and Makuuchi M: HCC Expert Panel
of Japan Society of Hepatology. Management of hepatocellular
carcinoma in Japan: Consensus-Based Clinical Practice Guidelines
proposed by the Japan Society of Hepatology (JSH) 2010 updated
version. Dig Dis. 29:339–364. 2011. View Article : Google Scholar
|
|
3.
|
Lencioni R: Loco-regional treatment of
hepatocellular carcinoma. Hepatology. 52:762–773. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: SHARP Investigators Study Group: Sorafenib in advanced
hepatocellular carcinoma. New Eng J Med. 359:378–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Guy J, Kelley RK, Roberts J, Kerlan R, Yao
F and Terrault N: Multidisciplinary management of hepatocellular
carcinoma. Clin Gastroenterol Hepatol. 10:354–362. 2012. View Article : Google Scholar
|
|
6.
|
Zhou WP, Lai EC, Li AJ, Fu SY, Zhou JP,
Pan ZY, Lau WY and Wu MC: A prospective, randomized, controlled
trial of preoperative transarterial chemoembolization for
resectable large hepatocellular carcinoma. Ann Surg. 249:195–202.
2009. View Article : Google Scholar
|
|
7.
|
Izumi R, Shimizu K, Iyobe T, Ii T, Yagi M,
Matsui O, Nonomura A and Miyazaki I: Postoperative adjuvant hepatic
arterial infusion of lipiodol containing anticancer drugs in
patients with hepatocellular carcinoma. Hepatology. 20:295–301.
1994. View Article : Google Scholar
|
|
8.
|
Nishiguchi S, Shiomi S, Nakatani S, Takeda
T, Fukuda K, Tamori A, Habu D and Tanaka T: Prevention of
hepatocellular carcinoma in patients with chronic active hepatitis
C and cirrhosis. Lancet. 357:196–197. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Shiratori Y, Shiina S, Teratani T, Imamura
M, Obi S, Sato S, Koike Y, Yoshida H and Omata M: Interferon
therapy after tumor ablation improves prognosis in patients with
hepatocellular carcinoma associated with hepatitis C virus. Ann
Intern Med. 138:299–306. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Papatheodoridis GV, Lampertico P,
Manolakopoulos S and Lok A: Incidence of hepatocellular carcinoma
in chronic hepatitis B patients receiving nucleos(t)ide therapy: a
systematic review. J Hepatol. 53:348–356. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Schwartz JD, Schwartz M, Mandeli J and
Sung M: Neoadjuvant and adjuvant therapy for resectable
hepatocellular carcinoma: review of the randomized clinical trials.
Lancet Oncol. 3:593–603. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Ishikawa T, Higuchi K, Kubota T, Seki K,
Honma T, Yoshida T and Kamimura T: Prevention of intrahepatic
distant recurrence by transcatheter arterial infusion chemotherapy
with platinum agents for stage I/II hepatocelluar carcinoma.
Cancer. 117:4018–4025. 2011. View Article : Google Scholar
|
|
13.
|
Chua TC, Liauw W, Saxena A, Chu F, Glenn
D, Chai A and Morris DL: Systematic review of neoadjuvant
transarterial chemoembolization for resectable hepatocellular
carcinoma. Liver Int. 30:166–174. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kanematsu T, Inokuchi K, Sugimachi K,
Furuta T, Sonoda T, Tamura S and Hasuo K: Selective effects of
lipiodolized antitumor agents. J Surg Oncol. 25:218–226. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Bruix J and Sherman M: Practice Guidelines
Committee, American Association for the Study of Liver Diseases:
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar
|
|
16.
|
Yamasaki T, Kurokawa F, Shirahashi H,
Kusano N, Hironaka K and Okita K: Percutaneous radiofrequency
ablation therapy with combined angiography and computed tomography
assistance for patients with hepatocellular carcinoma. Cancer.
91:1342–1348. 2001. View Article : Google Scholar
|
|
17.
|
[No authors listed]. Intra-arterial
administration of epirubicin in the treatment of nonresectable
hepatocellular carcinoma. Epirubicin Study Group for Hepatocellular
Carcinoma. Cancer Chemother Pharmacol. 19:183–189. 1987.
|
|
18.
|
Kumari R, Sharma A, Ajay AK and Bhat MK:
Mitomycin C induces bystander killing in homogenous and
heterogeneous hepatoma. Molecular Cancer. 8:872009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Goldberg SN, Grassi CJ, Cardella JF,
Charboneau JW, Dodd GD 3rd, Dupuy DE, Gervais D, Gillams AR, Kane
RA, Lee FT Jr, et al: Society of Interventional Radiology
Technology Assessment Committee; International Working Group on
Image-Guided Tumor Ablation. Image-guided tumor ablation:
Standardization of terminology and reporting criteria. Radiology.
235:728–739. 2005. View Article : Google Scholar
|
|
20.
|
National Cancer Institute (USA): Common
terminology criteria for adverse events, version 3.0. http://ctep.cancer.gov/reporting/ctc.html.
Published August 9, 2006.
|
|
21.
|
Lau WY, Yu SC, Lai EC and Leung TW:
Transarterial chemoembolization for hepatocellular carcinoma. J Am
Coll Surg. 202:155–168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Lau WY, Lai EC, Leung TW and Yu SC:
Adjuvant intra-arterial iodine-131-labeled lipiodol for resectable
hepatocellular carcinoma: a prospective randomized trial-update on
5-year and 10-year survival. Ann Surg. 247:43–48. 2008.PubMed/NCBI
|
|
23.
|
Wu CC, Ho YZ, Ho WL, Wu TC, Liu TJ and
P’eng FK: Preoperative transcatheter arterial chemoembolization for
resectable large hepatocellular carcinoma: a reappraisal. Br J
Surg. 82:122–126. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Zhang Z, Liu Q, He J, Yang J, Yang G and
Wu M: The effect of preoperative transcatheter hepatic arterial
chemoembolization on disease-free survival after hepatectomy for
hepatocellular carcinoma. Cancer. 89:2606–2612. 2000. View Article : Google Scholar
|
|
25.
|
Uyama N, Hatano E, Maetani Y, Isoda H,
Shibata T, Taura K, Oe S, Naito M, Yasuchika K, Fujii H, Ikai I and
Uemoto S: Efficacy and toxicity of transcatheter arterial
chemoembolization with cisplatin suspended in lipiodol for
unresectable hepatocellular carcinoma. Gan To Kagaku Ryoho.
35:775–780. 2008.(In Japanese).
|
|
26.
|
Asahina Y, Tsuchiya K, Tamaki N, Hirayama
I, Tanaka T, Sato M, Yasui Y, Hosokawa T, Ueda K, Kuzuya T,
Nakanishi H, et al: Effect of aging on risk for hepatocellular
carcinoma in chronic hepatitis C virus infection. Hepatology.
52:518–527. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Kubo S, Tanaka H, Shuto T, Takemura S,
Yamamoto T, Uenishi T, Tanaka S, Ogawa M, Sakabe K, Yamazaki K and
Hirohashi K: Correlation between low platelet count and
multicentricity of hepatocellular carcinoma in patients with
chronic hepatitis C. Hepatol Res. 30:221–225. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Kobayashi M, Ikeda K, Kawamura Y, Yatsuji
H, Hosaka T, Sezaki H, Akuta N, Suzuki F, Suzuki Y, Saitoh S, Arase
Y and Kumada H: High serum des-gamma-carboxy prothrombin level
predicts poor prognosis after radiofrequency ablation of
hepatocellular carcinoma. Cancer. 115:571–580. 2009. View Article : Google Scholar
|
|
29.
|
Mazzaferro V, Romito R, Schiavo M, Mariani
L, Camerini T, Bhoori S, Capussotti L, Calise F, Pellicci R, Belli
G, et al: HCC Italian Task Force: Prevention of hepatocellular
carcinoma recurrence with alpha-interferon after liver resection in
HCV cirrhosis. Hepatology. 44:1543–1554. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Kim YS, Lee WJ, Rhim H, Lim HK, Choi D and
Lee JY: The minimal ablative margin of radiofrequency ablation of
hepatocellular carcinoma (>2 and <5 cm) needed to prevent
local tumor progression: 3D quantitative assessment using CT image
fusion. AJR Am J Roentgenol. 195:758–765. 2010.
|
|
31.
|
Ng IO: Prognostic significance of
pathological and biological factors in hepatocellular carcinoma. J
Gastroenterol Hepatol. 13:666–670. 1998. View Article : Google Scholar : PubMed/NCBI
|