Introduction
Bladder cancer (BCa) represents the ninth most
common malignancy worldwide (1).
For Europe, 139,500 new cases of BCa and 51,300 BCa-related deaths
were estimated for the year 2008 (2). At the time of diagnosis 20–30% of
patients present with muscle invasive disease and will be treated
by radical cystectomy. Depending on the tumour stage, the 5-year
survival rates range from 27 to 67% (3). For patients with metastatic BCa
systemic chemotherapy is recommended. However, median survival of
patients treated with gemcitabine and cisplatin is only 12.8 months
(4). Altogether, despite intensive
treatment with surgery and chemotherapy the prognosis for BCa
patients particularly at advanced stages is poor. Therefore, the
main goals of experimental BCa research are the improvement of
existing therapies as well as the development of alternative
therapeutic approaches.
One promising attempt is the knockdown of genes
which play important roles in the survival or progression of cancer
cells. Apoptosis (programmed cell death) is a highly conserved and
strictly regulated biological process that is essential for the
maintenance of normal tissue homeostasis as well as for the
selective and non-inflammatory removal of infected or damaged cells
(5). The ability to evade
apoptosis is one of the hallmarks that characterise tumour cells
(6). One mechanism by which cancer
cells escape apoptosis is the overexpression of antiapoptotic
genes, particularly of BCL2, Bcl-xL, XIAP and survivin.
BCL2 and Bcl-xL, two members of the BCL2 family,
inhibit cell death by preventing the release of cytochrome c
from the mitochondria and subsequent caspase activation (7). Activated caspases are the mediators
of apoptosis which cleave proteins that are essential for cell
function and stability (8). The
detection of BCL2 protein in BCa tissue samples was generally
associated with worse outcome (9–12).
Bcl-xL positivity was found in 81% of BCa samples and correlated
with high tumour stage and grade (13).
Survivin and XIAP are the two most important members
of the inhibitor of apoptosis protein (IAP) family. XIAP executes
its antiapoptotic function by direct inhibition of caspases
(14). Sixty-one percent of
nonmuscle-invasive BCa showed XIAP protein staining which was
associated with a high risk of recurrence (15). Survivin is the fourth most common
transcript found in human tumours (16). Survivin blocks cell death mainly by
interactions with other proteins. For example, the survivin-XIAP
complex enhances stability and activity of XIAP (17). Numerous studies documented the
extraordinary importance of survivin for BCa diagnosis and
prognosis as well as for the prediction of therapy response
(reviewed in ref. 18). While
survivin is absent in normal urothelium this IAP is found in
64–100% of BCa. Survivin expression is associated with high BCa
stage and grade as well as with an elevated risk of recurrence
(19–21).
The antiapoptotic factors BCL2, Bcl-xL, XIAP and
survivin can protect BCa cells from natural cell death. Hence, the
inhibition of these targets by small interfering RNAs (siRNAs)
could cause the reactivation of apoptotic signalling and
consequently a decrease in tumour growth. Small interfering RNAs
are synthetic double-stranded ribonucleic acids that mediate
specific gene silencing by inducing RNA interference (22). Thereby, siRNAs are bound into the
RNA-induced silencing complex (RISC) which mediates the cleavage of
the target mRNA by its intrinsic endonuclease function (23). Since cancer cells may bypass the
inhibition of one target, e.g. a reduction in BCL2 protein content
can be compensated by the induction of Bcl-xL (24), the combined knockdown of
antiapoptotic genes may be more suitable for BCa therapy.
Therefore, we analysed effects of single and combined
siRNA-mediated knockdown of BCL2, Bcl-xL, XIAP and survivin on
human BCa cell lines.
Materials and methods
Cell culture, siRNAs and
transfection
The human BCa cell lines EJ28 (University of
Frankfurt, Frankfurt, Germany), J82 and 5637 (ATCC, Manassas, VA,
USA) were cultured under standard conditions (37°C, humidified
atmosphere containing 5% CO2) without antibiotics. For
EJ28 and J82, DMEM (4.5 g/l glucose) containing 10% fetal calf
serum (FCS), 1% MEM non-essential amino acids and 1% HEPES (all
from Invitrogen, Karlsruhe, Germany) was used. 5637 cells were
cultured in RPMI-1640 (Invitrogen) including 10% FCS and 1% MEM
non-essential amino acids.
The target-directed siRNAs (abbreviations are shown
in Table I) and the negative
control siRNA ‘ns-si’ (reference: SR-CL000-005), which was used for
normalisation, were synthesised by Eurogentec (Seraing, Belgium).
After seeding and adherence for 24 or 72 h, cells were washed with
PBS and transfected with the siRNAs for 4 h in serum-free OptiMEM
(Invitrogen) using DOTAP liposomal transfection reagent (ratio
1:30, w/w) according to the manufacturer’s instructions (Roche,
Mannheim, Germany). Unless otherwise stated, the siRNAs were
transfected either separately with 40 nM of one construct (single
target treatments) or with combinations of four (M4-1 and M4-2, 10
nM per siRNA) or all eight target-directed siRNAs (M8, 5 nM per
siRNA). In the M4 combination treatments the siRNAs B2-1, BX-1, X-1
as well as S-1 (= M4-1) or B2-2, BX-2, X-2 as well as S-2 (= M4-2)
were incubated simultaneously. After 4 h, transfection medium was
replaced by fresh culture medium and cells were incubated for 24–96
h. For further analyses cells were harvested by trypsin treatment
(0.05% trypsin/0.02% EDTA, 5 min, 37°C). Detached and adherent
cells were pooled and analysed together.
 | Table IDesignations and target sequences of
the siRNAs. |
Table I
Designations and target sequences of
the siRNAs.
| Target gene | siRNA name | siRNA target
sequence |
|---|
| BCL2 | B2-1 |
CGGUGGUGGAGGAGCUCUU |
| BCL2 | B2-2 |
GCAUGCGGCCUCUGUUUGA |
| Bcl-xL | BX-1 |
GGGACAGCAUAUCAGAGCU |
| Bcl-xL | BX-2 |
CAGCUGGAGUCAGUUUAGU |
| XIAP | X-1 |
CGAGCAGGGUUUCUUUAUA |
| XIAP | X-2 |
CUGGGCAGGUUGUAGAUAU |
| Survivin | S-1 |
GAAGCAGUUUGAAGAAUUA |
| Survivin | S-2 |
CCAACAAUAAGAAGAAAGA |
Viability assay, apoptosis detection and
cell cycle analysis
Using the cell proliferation reagent WST-1 (Roche)
cellular viability was examined in quadruplicates 96 h after
transfection according to the manufacturer’s instructions.
Apoptosis was assessed by Annexin V-FITC/propidium iodide (PI)
staining (Annexin V-FITC Apoptosis Detection Kit I, BD Biosciences,
Heidelberg, Germany) 48 h after transfection according to the
manufacturer’s instructions by flow cytometry (FACScan, BD
Biosciences). Percentage of early (Annexin V-FITC positive, PI
negative) and late (Annexin V-FITC positive, PI positive) apoptotic
cells was determined by quadrant analysis of Annexin V-FITC/PI
plots using WinMDI2.8 software (http://facs.scripps.edu/software.html). Cell cycle
distribution was assessed by propidium iodide staining (CycleTest
Plus DNA Reagent Kit, BD Biosciences) 48 h after transfection
according to the manufacturer’s instructions by flow cytometry
(FACScan, BD Biosciences).
RNA isolation, cDNA synthesis and
quantitative PCR
Total-RNA was isolated according to the
manufacturer’s instructions (InviTrap Spin Cell RNA Mini Kit;
Invitek, Berlin, Germany) and reverse transcribed into cDNA
(SuperScript II Reverse Transcriptase; Invitrogen). Transcript
amounts of the targets and the reference gene TBP (TATA box binding
protein) were determined by quantitative real-time PCR (qPCR) using
the primers, probes and kits listed in Table II.
 | Table IISequences of primers and probes and
the kits used for quantitative PCR. |
Table II
Sequences of primers and probes and
the kits used for quantitative PCR.
| Target | Sequence 5′→3′ |
|---|
| BCL2a | Target-specific
Real-Time Reagent Mix (AJ Roboscreen, Leipzig, Germany) containing
the appropriate primers and probes |
| Bcl-xLa |
| Survivinb | Primers: for:
GAACTGGCCCTTCTTGGAG, rev: AAGTCTGGCTCGTTCTCAGTG |
| Probe: Universal
ProbeLibrary Probe no. 86 (Roche, Germany, cat. no.
04689119001) |
| TBPa | Primers: for:
GAATATAATCCCAAGCGGTTTG, rev: ACTTCACATCACAGCTCCCC |
| Probes:
TTTCCCAGAACTGAAAATCAGTGCC-FL, LC-TGGTTCGTGGCTCTCTTATCCTCATG-PH |
| XIAPa | Primers: for:
GTGATAAAGTAAAGTGCTTTCACTGT, rev: GTAGTTCTTACCAGACACTCCTCAA |
| Probes:
GTGAAGACCCTTGGGAACAACAT-FL,
LC-CTAAATGGTATCCAGGGTGCAAATATCTG-PH |
Western blot analysis
Cells (5x104 per sample) were lysed in 20
µl loading buffer (20% glycerol, 2% SDS, 125 mM Tris pH 6.8, 5%
β-mercaptoethanol, bromophenol blue), incubated at 95°C for 5 min
and separated on 8–16% Precise Protein Gels (Fisher Scientific,
Schwerte, Germany). Proteins were transferred onto PVDF membranes
(GE Healthcare, Freiburg, Germany) which were incubated with
primary antibodies against BCL2 (1:200; clone 124; Dako, Glostrup,
Denmark), Bcl-xL (1:100; clone 2H12; QED Bioscience Inc., San
Diego, CA, USA), XIAP (1:250; clone 28; BD Biosciences) or survivin
(1:1,000; NB500-201; Novus Biologicals, Littleton, CO, USA).
β-actin detected by a monoclonal anti-β-actin antibody (1:20,000;
Sigma-Aldrich, St. Louis, MO, USA) served as a loading control. The
secondary polyclonal rabbit anti-mouse immunoglobulin HRP-linked
antibody (1:1,000; Dako; for β-actin, BCL2, Bcl-xL and XIAP) or the
polyclonal swine anti-rabbit immunoglobulin HRP-linked antibody
(1:1,000, Dako, for survivin), respectively, as well as the
Enhanced chemiluminescence Kit (GE Healthcare) were used for
visualization.
Results
BCL2, Bcl-xL, XIAP and survivin
expression in bladder cancer cell lines
Quantitative PCR analysis was used to determine
expression levels of the four selected antiapoptotic genes in EJ28,
J82 and 5637 BCa cells. As shown in Table III, BCL2, Bcl-xL, XIAP and survivin
are expressed simultaneously in all BCa cell lines. Since EJ28 and
J82 cells express all targets at high levels these cell lines were
chosen for target inhibition studies.
 | Table IIITarget mRNA expression levels in
EJ28, J82 and 5637 bladder cancer cell lines. |
Table III
Target mRNA expression levels in
EJ28, J82 and 5637 bladder cancer cell lines.
| Cell line | BCL2/TBP | Bcl-xL/TBP | XIAP/TBP | Survivin/TBP |
|---|
| EJ28 | 0.283 | 27.8 | 3.25 | 4.35 |
| J82 | 0.084 | 24.0 | 2.00 | 2.77 |
| 5637 | 0.058 | 7.0 | 1.92 | 1.10 |
Optimising siRNA transfection for
combined knockdown of four target genes
To achieve strong target inhibition and to avoid
undesirable side effects, utilisation of highly effective siRNAs at
low concentrations is recommendable. Therefore, target knockdown
depending on the siRNA concentration was examined using the
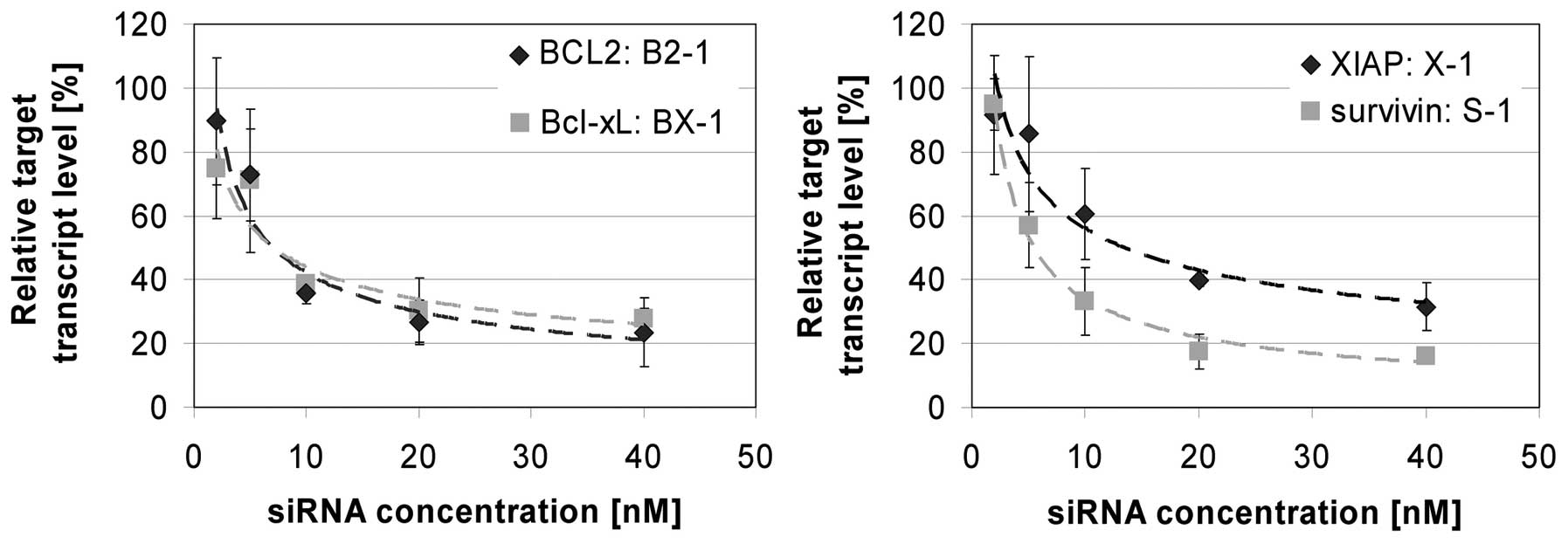
constructs B2-1, BX-1, X-1 and S-1 in EJ28 cells. Already by using
10 nM of the siRNAs a marked reduction of the target mRNA levels by
40–67% was achieved 24 h after treatment (Fig. 1). A quadruplication of the applied
siRNA concentration increased the target inhibition rate to 69–84%
(Fig. 1). To ensure comparability
among single target and target combination treatments, an equal
total amount of siRNAs as well as an equal amount of DOTAP
transfection reagent was used. Therefore, a final concentration of
40 nM siRNA was selected, whereby 40 nM siRNA were used in the
single target treatments and 10 nM (M4-1, M4-2) or 5 nM (M8) per
siRNA in the combinations. Thus, a marked target inhibition in all
treatments is ensured while side effects are maintained at the same
level by constant amounts of DOTAP.
Molecular effects of single and combined
siRNA-mediated target inhibition
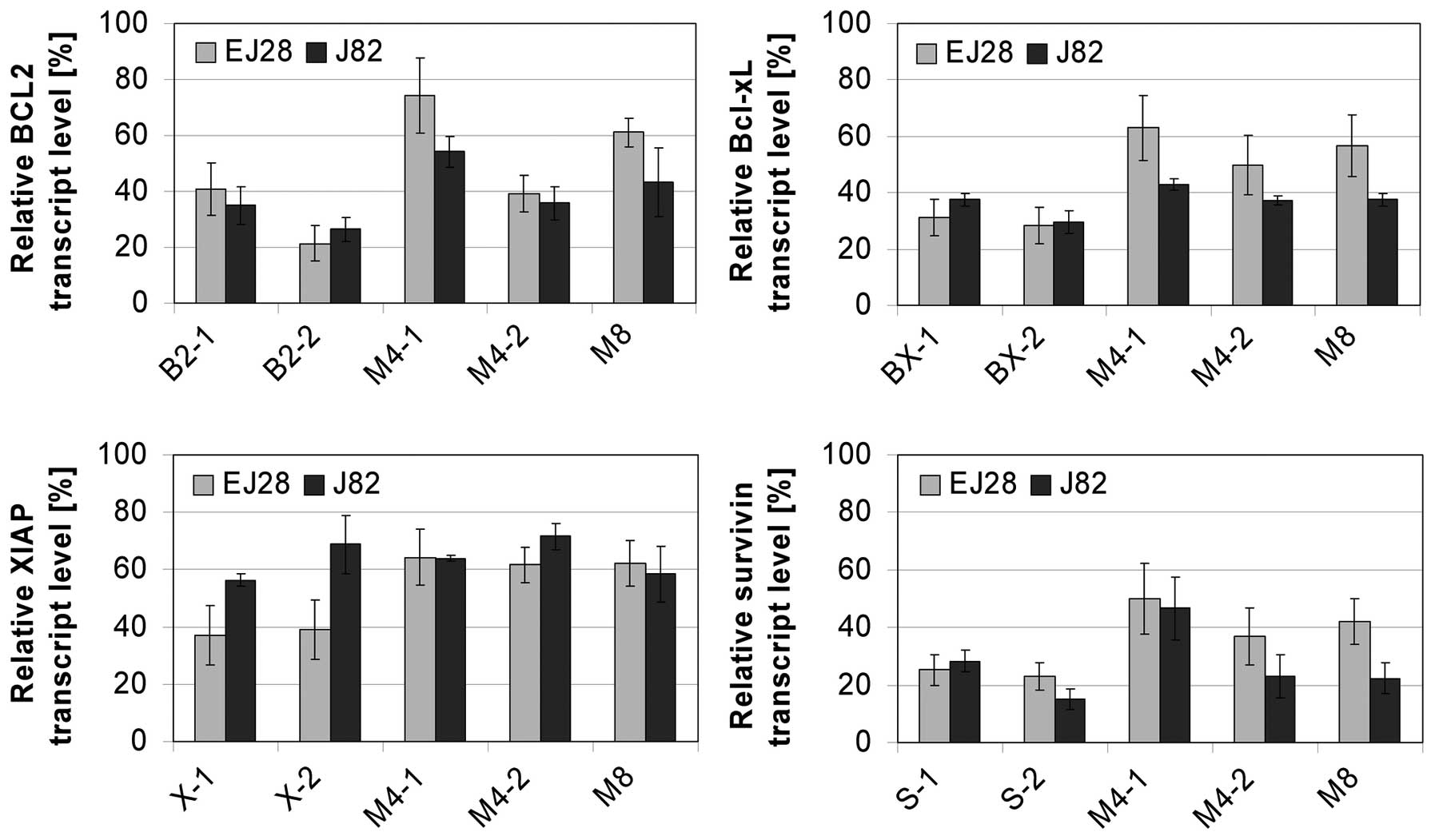
The selected target-specific siRNAs (Table I) potently decreased the mRNA
expression levels of their appropriate target in both BCa cell
lines 48 h after transfection (Fig.
2). In EJ28 cells, BCL2 was reduced down to 21%, Bcl-xL down to
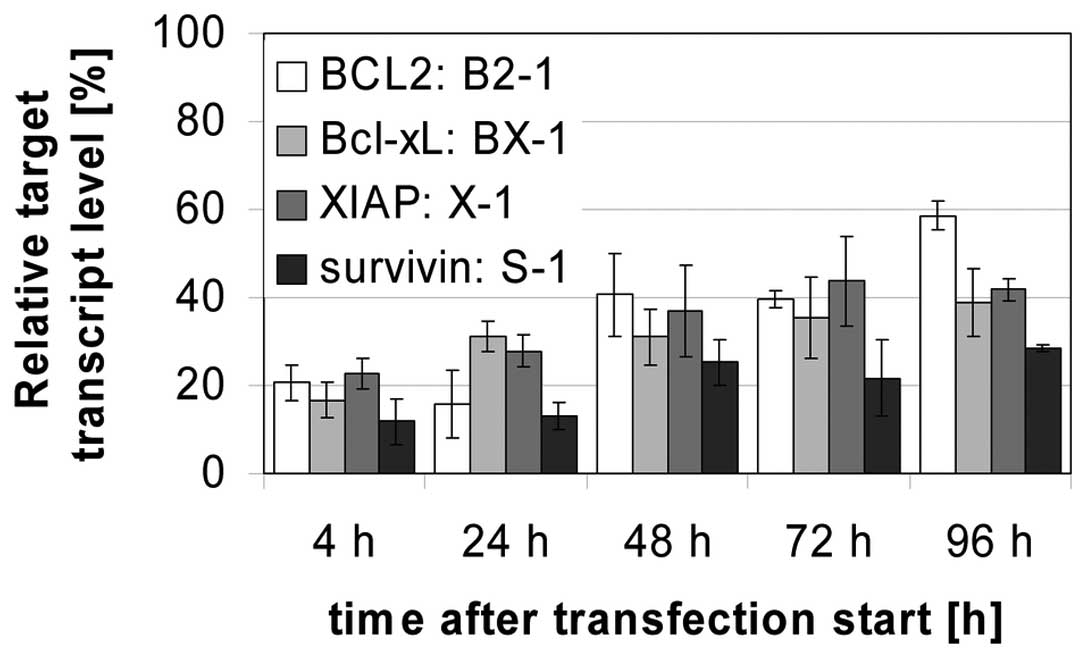
28%, XIAP down to 37% and survivin down to 23% at best. Even 96 h
after transfection a target inhibition down to 28% (survivin) and
to 59% (BCL2) was measured (Fig.
3). The simultaneous inhibition of all four antiapoptotic genes
resulted in mRNA downregulation of all targets. Using a combination
of all eight siRNAs, BCL2 was reduced down to 61 and 43%, Bcl-xL
down to 57 and 38%, XIAP down to 62 and 59%, and survivin down to
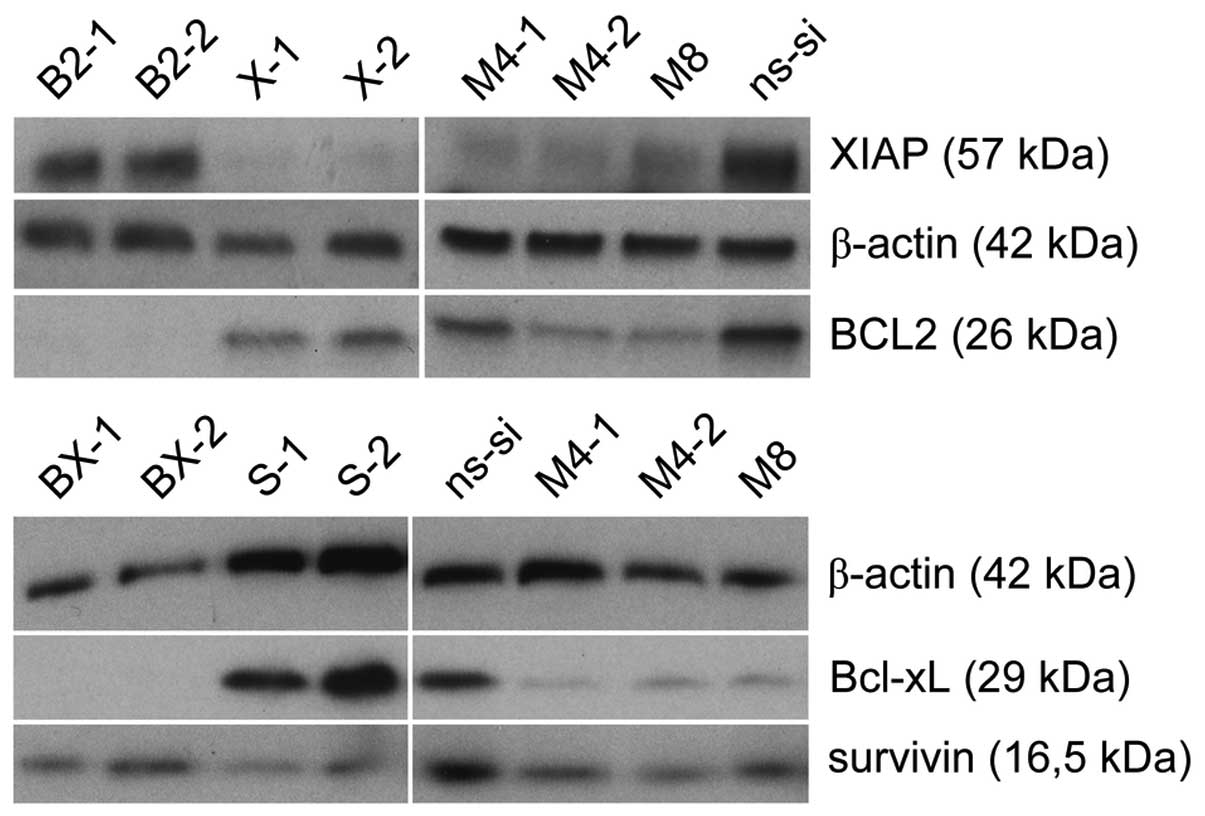
42 and 22% in EJ28 and J82 BCa cells, respectively (Fig. 2). Western blot analysis showed
specific protein reduction 48 h after siRNA transfection in single
target and target combination treatments in both BCa cell lines
(Fig. 4, representative western
blots are shown for EJ28 cells, similar results were obtained with
J82 cells).
Cellular effects of single and combined
siRNA-mediated target inhibition
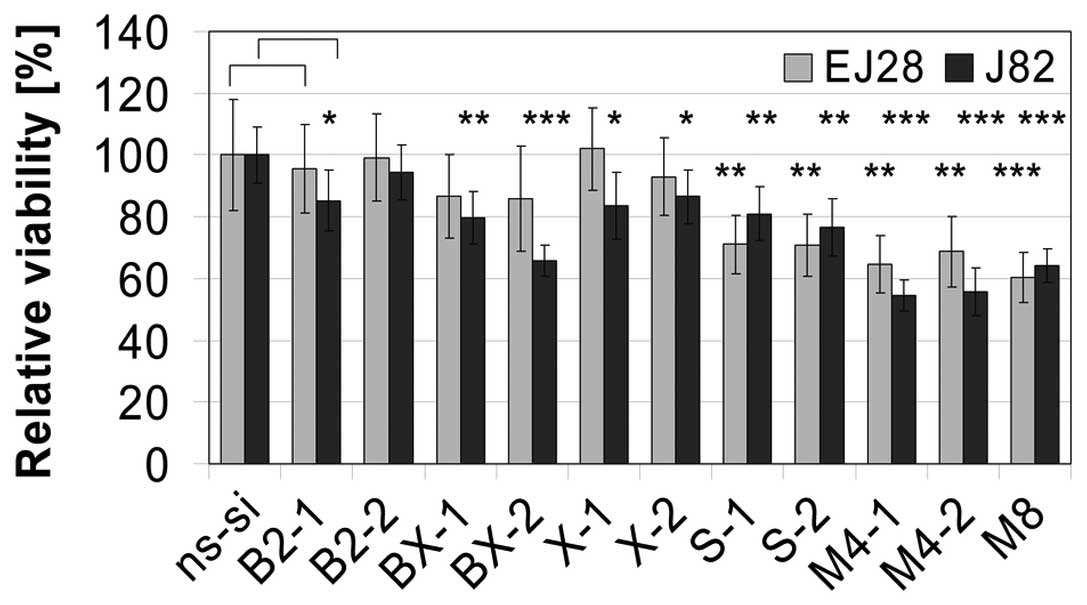
Inhibition of BCL2 and XIAP did not or only
marginally affect the growth of the BCa cell lines EJ28 and J82
(Figs. 5 and 6). Knockdown of Bcl-xL reduced BCa cell
viability by 13–34% (Fig. 5).
Strongest decrease in cell viability as well as a profound
reduction in cell counts were observed after siRNA-mediated
inhibition of survivin (Figs. 5
and 6). Antiproliferative effects
of the siRNA combination treatments M4-1, M4-2 and M8 with one or
two siRNAs per target were comparable (Figs. 5 and 6). On average, simultaneous knockdown of
BCL2, Bcl-xL, XIAP and survivin inhibited BCa cell viability by 39%
and BCa cell counts by 46%.
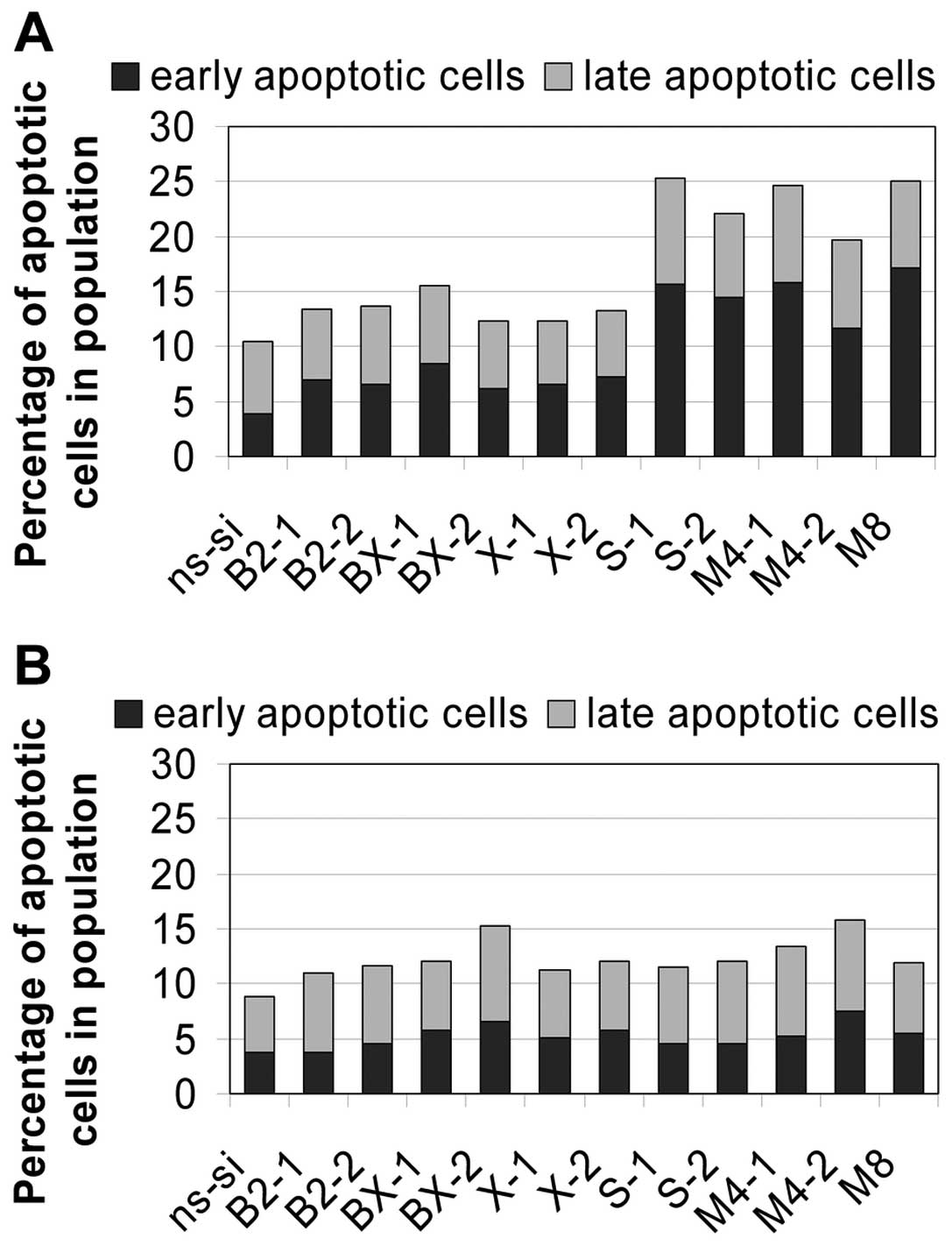
In EJ28 cells single knockdown of survivin as well
as simultaneous inhibition of all four antiapoptotic genes caused a
1.9 to 2.5-fold enhancement in apoptosis rate (Fig. 7A). For example, percentage of
apoptotic cells in population increased from 10% in the ns-si
control to 25% after treatment with the anti-survivin siRNA S-1. In
J82 cells only a marginal enhancement of apoptosis by factor 1.4 on
average was seen (Fig. 7B). No
changes in cell cycle distribution were found in EJ28 and J82 cells
after inhibition of BCL2, Bcl-xL or XIAP whereas knockdown of
survivin caused polyploidy in both BCa cell lines (data not shown).
For example, 10% of the EJ28 cells showed DNA content of 8N after
treatment with S-1 or S-2 compared to 1% in the ns-si control. In
the combination treatments M4-1, M4-2 and M8 similar changes were
found but effects were less prominent, e.g. 48 h after transfection
with M4-2, 2 and 4% of EJ28 and J82 cells, respectively, showed DNA
content of 8N.
Discussion
Deregulation of apoptosis is a key factor in
tumourigenesis (6). The members of
the BCL2 and IAP families are of particular importance for the
regulation of apoptotic signalling (25). BCL2, Bcl-xL, XIAP and survivin are
the most important antiapoptotic members of these two families and
are frequently upregulated in human tumours including BCa (15,26,27).
Therefore, these genes represent interesting candidates for a
target-directed molecular-based antitumour therapy. Since the
knockdown of a single antiapoptotic target might be bypassed by the
upregulation of other prosurvival genes the simultaneous inhibition
of BCL2, Bcl-xL, XIAP and survivin could be more potent in
decreasing BCa cell proliferation.
Using 40 nM of new siRNAs with optimised design,
comparable mRNA inhibition rates were obtained in the single target
treatments as with much higher concentrations of different siRNAs
targeted at BCL2, Bcl-xL, XIAP or survivin in previous studies.
Only 40 nM of B2-1, BX-1 and X-1 reduced mRNA levels of their
appropriate target down to 59, 39 and 42% in EJ28 cells 96 h after
transfection (Fig. 3) whereas 200
nM of the previously used siRNAs (28) decreased mRNA levels down to 59, 37
and 46%, respectively. Reduction in survivin expression of about 70
and 50% was shown in EJ28 and J82 BCa cells, respectively, 48 h
after transfection with 250 nM siRNA (29). In the present study, survivin mRNA
was decreased with 40 nM of the new siRNAs in EJ28 and J82 cells by
76 and 79% on average, respectively (Fig. 2). Even 96 h after treatment target
mRNA levels were downregulated by up to 72% (Fig. 3). These facts verify that the novel
siRNAs induced an effective and long-lasting inhibition of their
target expression. In addition, the risk of undesired side effects
is minimised due to the significantly lower siRNA concentrations
applied.
The simultaneous transfection of various siRNAs
might induce a competition between the constructs as to their
incorporation into RISC (30).
Therefore, the effectiveness of individual siRNAs might be limited
in combination treatments. With the siRNAs used in this study no
competition between the constructs regarding their incorporation
into RISC is assumed. The slightly decreased mRNA inhibition rate
in the combination treatments (M4-1, M4-2, M-8) compared to the
single target treatments seemed to be mediated basically by the
different siRNA concentrations with 40 nM per siRNA in the single
target treatments and 10 nM siRNA per target in the combinations
treatments (Fig. 2). Comparably,
Yang et al demonstrated effective protein knockdown of the
three IAPs livin, XIAP and survivin with a siRNA combination
comprising of 10 nM siRNA per target. The protein reduction in the
combination treatment was only slightly decreased in comparison to
the single target treatments with 30 nM siRNA (31).
All siRNAs used in the present study, either
separately or combined, sufficiently decreased the mRNA and protein
levels of their targets (Figs. 2
and 4). Of the single target
treatments, the inhibition of survivin caused strongest
antiproliferative effects on EJ28 and J82 BCa cells, namely
considerable reductions in cell viability and cell counts (Figs. 5 and 6). This is mediated by apoptosis
induction and the formation of polyploid cells. Survivin knockdown
induces polyploidy because survivin is, besides its function as
inhibitor of apoptosis, an integral part of the chromosomal
passenger complex, thereby regulating chromosome segregation and
cytokinesis (32). In agreement
with the results of this study, Ning et al and Takizawa
et al showed apoptosis induction and an arrest in G2/M cell
cycle phase after treatment of BCa cells with siRNAs targeted at
survivin (29,33).
Despite marked decreases in target protein contents
(Fig. 4), BCL2 and XIAP single
knockdown had no or only marginal impact on BCa cell growth
(Figs. 5 and 6). Using other siRNA sequences previous
studies showed a reduction in EJ28 cell counts of about 43% after
transfection of 200 nM siRNA targeting BCL2 or XIAP (28). Since target mRNA inhibition rates
were comparable to the present study, differences might be due to
off-target effects mediated by higher siRNA concentrations.
Similarly to the present results, Sensintaffar et al showed
that siRNA-induced XIAP knockdown did not affect T24 BCa cell
viability 72 and 96 h after treatment (34), neither did BCL2 inhibition in
prostate cancer cell lines induce phenotypic changes (35). Possibly, BCL2 and XIAP are of minor
importance in several monolayer cell cultures due to their
continuous supply with oxygen and nutrients. In contrast, cancer
cell aggregates like tumour spheroids that represent better models
for tumour structure contain nutrient-deficient and hypoxic
microenvironments which might show differing gene expression
profiles. Indeed, increased BCL2 and XIAP protein contents were
found in tumour spheroids of lung and breast cancer cells,
respectively, in comparison to the corresponding monolayer cell
cultures (36,37). Moreover, inhibition of BCL2 and
XIAP might not induce direct antiproliferative effects in cancer
cells but rather a sensitisation to exogenous apoptosis stimuli
similar to chemotherapy or radiation.
Bcl-xL reduction in BCa cells induced moderate
antiproliferative effects. For example in EJ28 cells, viability
decreased by 14% and apoptosis rate increased on average by 34%
relative to negative control siRNA treated cells (Figs. 5 and 7). In the same cell line 200 nM of
another Bcl-xL targeting siRNA induced apoptosis and sensitised
cells towards a subsequent chemotherapy with mitomycin C (28). Similarly, in prostate and ovarian
cancer cells siRNA-mediated Bcl-xL knockdown inhibited tumour cell
proliferation and sensitised cells towards cisplatin and tumour
necrosis factor-related apoptosis-inducing ligand (TRAIL),
respectively (38,39).
Combined inhibition of BCL2, Bcl-xL, XIAP and
survivin was carried out by simultaneous transfection of one (M4-1,
M4-2) or two (M8) siRNAs per target. All three combination
treatments induced comparable cellular effects (Figs. 5–7). After simultaneous inhibition of all
four targets, effective reductions in cell viability and cell
counts as well as induction of apoptosis were seen. These effects
were as strong as after survivin knockdown, which was the most
efficient single target treatment. Percentage of polyploid BCa
cells increased in the combination treatments but was lower than
after survivin single knockdown. Because survivin is the only
target which additionally functions in cytokinesis, the formation
of polyploid cells should represent the consequence of survivin
knockdown. Since downregulation of survivin in the single target
treatments with 40 nM siRNA is slightly stronger than after
simultaneous inhibition of BCL2, Bcl-xL, XIAP and survivin with 10
nM siRNA per target (Fig. 2), the
proportion of polyploid cells in the combination treatments might
be lower because of the higher amount survivin remaining. That the
degree of BCa growth reductions in the M4 and M8 treatments is not
different from the value after single survivin inhibition should be
the consequence of a synergistic action of the simultaneous
knockdown of multiple antiapoptotic genes, presumably survivin and
Bcl-xL.
Further studies showed the potential of the
simultaneous knockdown of multiple antiapoptotic genes. The
combined targeting of the three IAPs c-IAP1, c-IAP2 and XIAP in
prostate cancer cells decreased proliferation and sensitised cells
to TRAIL treatment (40). In
pancreatic cancer cells, the simultaneous inhibition of BCL2, XIAP
and survivin mediated induction of apoptosis (41). Moreover, Yang et al
demonstrated that the combined knockdown of livin, XIAP and
survivin in T24 BCa cells reduced cell proliferation and induced
apoptosis (31). These studies as
well as the present report prove that the simultaneous inhibition
of multiple antiapoptotic genes might be a promising treatment
option for cancer.
Acknowledgements
The authors would like to thank Dr
Matthias Kotzsch and Antje Zobjack (Institute of Pathology,
Technical University of Dresden) for their help with the
fluorometric analyses. This study was supported by a grant of the
Else Kröner-Fresenius-Stiftung.
References
|
1
|
Ploeg M, Aben KK and Kiemeney LA: The
present and future burden of urinary bladder cancer in the world.
World J Urol. 27:289–293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Parkin DM and Steliarova-Foucher
E: Estimates of cancer incidence and mortality in Europe in 2008.
Eur J Cancer. 46:765–781. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Herr HW, Dotan Z, Donat SM and Bajorin DF:
Defining optimal therapy for muscle invasive bladder cancer. J
Urol. 177:437–443. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dogliotti L, Carteni G, Siena S, et al:
Gemcitabine plus cisplatin versus gemcitabine plus carboplatin as
first-line chemotherapy in advanced transitional cell carcinoma of
the urothelium: results of a randomized phase 2 trial. Eur Urol.
52:134–141. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Burz C, Berindan-Neagoe I, Balacescu O and
Irimie A: Apoptosis in cancer: key molecular signaling pathways and
therapy targets. Acta Oncol. 48:811–821. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brunelle JK and Letai A: Control of
mitochondrial apoptosis by the Bcl-2 family. J Cell Sci.
122:437–441. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Taylor RC, Cullen SP and Martin SJ:
Apoptosis: controlled demolition at the cellular level. Nat Rev Mol
Cell Biol. 9:231–241. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Glick SH, Howell LP and White RW:
Relationship of p53 and bcl-2 to prognosis in muscle-invasive
transitional cell carcinoma of the bladder. J Urol. 155:1754–1757.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hussain SA, Ganesan R, Hiller L, et al:
BCL2 expression predicts survival in patients receiving synchronous
chemoradiotherapy in advanced transitional cell carcinoma of the
bladder. Oncol Rep. 10:571–576. 2003.PubMed/NCBI
|
|
11
|
Ong F, Moonen LM, Gallee MP, et al:
Prognostic factors in transitional cell cancer of the bladder: an
emerging role for Bcl-2 and p53. Radiother Oncol. 61:169–175. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pollack A, Wu CS, Czerniak B, Zagars GK,
Benedict WF and McDonnell TJ: Abnormal bcl-2 and pRb expression are
independent correlates of radiation response in muscle-invasive
bladder cancer. Clin Cancer Res. 3:1823–1829. 1997.PubMed/NCBI
|
|
13
|
Korkolopoulou P, Lazaris A, Konstantinidou
AE, et al: Differential expression of bcl-2 family proteins in
bladder carcinomas. Relationship with apoptotic rate and survival.
Eur Urol. 41:274–283. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eckelman BP, Salvesen GS and Scott FL:
Human inhibitor of apoptosis proteins: why XIAP is the black sheep
of the family. EMBO Rep. 7:988–994. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li M, Song T, Yin ZF and Na YQ: XIAP as a
prognostic marker of early recurrence of nonmuscular invasive
bladder cancer. Chin Med J (Engl). 120:469–473. 2007.PubMed/NCBI
|
|
16
|
Velculescu VE, Madden SL, Zhang L, et al:
Analysis of human transcriptomes. Nat Genet. 23:387–388. 1999.
View Article : Google Scholar
|
|
17
|
Dohi T, Okada K, Xia F, et al: An IAP-IAP
complex inhibits apoptosis. J Biol Chem. 279:34087–34090. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Margulis V, Lotan Y and Shariat SF:
Survivin: a promising biomarker for detection and prognosis of
bladder cancer. World J Urol. 26:59–65. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schultz IJ, Kiemeney LA, Witjes JA, et al:
Survivin mRNA expression is elevated in malignant urothelial cell
carcinomas and predicts time to recurrence. Anticancer Res.
23:3327–3331. 2003.PubMed/NCBI
|
|
20
|
Shariat SF, Ashfaq R, Karakiewicz PI,
Saeedi O, Sagalowsky AI and Lotan Y: Survivin expression is
associated with bladder cancer presence, stage, progression, and
mortality. Cancer. 109:1106–1113. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Weikert S, Christoph F, Schrader M, Krause
H, Miller K and Muller M: Quantitative analysis of survivin mRNA
expression in urine and tumor tissue of bladder cancer patients and
its potential relevance for disease detection and prognosis. Int J
Cancer. 116:100–104. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dallas A and Vlassov AV: RNAi: a novel
antisense technology and its therapeutic potential. Med Sci Monit.
12:RA67–74. 2006.PubMed/NCBI
|
|
23
|
Kim DH and Rossi JJ: Strategies for
silencing human disease using RNA interference. Nat Rev Genet.
8:173–184. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han Z, Chatterjee D, Early J, Pantazis P,
Hendrickson EA and Wyche JH: Isolation and characterization of an
apoptosis-resistant variant of human leukemia HL-60 cells that has
switched expression from Bcl-2 to Bcl-xL. Cancer Res. 56:1621–1628.
1996.PubMed/NCBI
|
|
25
|
Tan ML, Ooi JP, Ismail N, Moad AI and
Muhammad TS: Programmed cell death pathways and current antitumor
targets. Pharm Res. 26:1547–1560. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hunter AM, LaCasse EC and Korneluk RG: The
inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis.
12:1543–1568. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shangary S and Johnson DE: Recent advances
in the development of anticancer agents targeting cell death
inhibitors in the Bcl-2 protein family. Leukemia. 17:1470–1481.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kunze D, Wuttig D, Fuessel S, et al:
Multitarget siRNA inhibition of antiapoptotic genes (XIAP, BCL2,
BCL-X(L)) in bladder cancer cells. Anticancer Res. 28:2259–2263.
2008.PubMed/NCBI
|
|
29
|
Ning S, Fuessel S, Kotzsch M, et al:
siRNA-mediated down-regulation of survivin inhibits bladder cancer
cell growth. Int J Oncol. 25:1065–1071. 2004.PubMed/NCBI
|
|
30
|
Castanotto D, Sakurai K, Lingeman R, et
al: Combinatorial delivery of small interfering RNAs reduces RNAi
efficacy by selective incorporation into RISC. Nucleic Acids Res.
35:5154–5164. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang D, Song X, Zhang J, et al:
Therapeutic potential of siRNA-mediated combined knockdown of the
IAP genes (Livin, XIAP, and Survivin) on human bladder cancer T24
cells. Acta Biochim Biophys Sin (Shanghai). 42:137–144. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jeyaprakash AA, Klein UR, Lindner D, Ebert
J, Nigg EA and Conti E: Structure of a Survivin-Borealin-INCENP
core complex reveals how chromosomal passengers travel together.
Cell. 131:271–285. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takizawa BT, Uchio EM, Cohen JJ, Wheeler
MA and Weiss RM: Downregulation of survivin is associated with
reductions in TNF receptors’ mRNA and protein and alterations in
nuclear factor kappa B signaling in urothelial cancer cells. Cancer
Invest. 25:678–684. 2007.PubMed/NCBI
|
|
34
|
Sensintaffar J, Scott FL, Peach R and
Hager JH: XIAP is not required for human tumor cell survival in the
absence of an exogenous death signal. BMC Cancer. 10:112010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Anderson EM, Miller P, Ilsley D, et al:
Gene profiling study of G3139- and Bcl-2-targeting siRNAs
identifies a unique G3139 molecular signature. Cancer Gene Ther.
13:406–414. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gallardo-Perez JC, Espinosa M,
Ceballos-Cancino G, et al: NF-kappa B is required for the
development of tumor spheroids. J Cell Biochem. 108:169–180. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang TM, Barbone D, Fennell DA and
Broaddus VC: Bcl-2 family proteins contribute to apoptotic
resistance in lung cancer multicellular spheroids. Am J Respir Cell
Mol Biol. 41:14–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mu P, Nagahara S, Makita N, Tarumi Y,
Kadomatsu K and Takei Y: Systemic delivery of siRNA specific to
tumor mediated by atelocollagen: combined therapy using siRNA
targeting Bcl-xL and cisplatin against prostate cancer. Int J
Cancer. 125:2978–2990. 2009. View Article : Google Scholar
|
|
39
|
Zhu H, Guo W, Zhang L, et al: Enhancing
TRAIL-induced apoptosis by Bcl-X(L) siRNA. Cancer Biol Ther.
4:393–397. 2005.PubMed/NCBI
|
|
40
|
Gill C, Dowling C, O’Neill AJ and Watson
RW: Effects of cIAP-1, cIAP-2 and XIAP triple knockdown on prostate
cancer cell susceptibility to apoptosis, cell survival and
proliferation. Mol Cancer. 8:392009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ruckert F, Samm N, Lehner AK, Saeger HD,
Grutzmann R and Pilarsky C: Simultaneous gene silencing of Bcl-2,
XIAP and Survivin re-sensitizes pancreatic cancer cells towards
apoptosis. BMC Cancer. 10:3792010. View Article : Google Scholar : PubMed/NCBI
|