Introduction
Breast cancer is the most common cancer in women in
western countries (following skin neoplasms). Advances in gene
profiling and molecular methods have brought about a shift from
traditional morphology-based diagnostics to more precise molecular
classification of breast cancers. Specific gene profiling and
protein expressions are reflected in a distinct disease behaviour,
prognosis and treatment response for each subgroup of breast
cancers. HER2-positive breast cancer is one of these subgroups and
is characterized by overexpression of HER2 protein and/or
amplification of HER2/neu gene. The HER2/neu gene (also known as
ErbB-2) is a proto-oncogene and encodes the trans-membrane receptor
protein HER2. HER2 is a member of the human epidermal growth factor
receptor family and is overexpressed/amplified in ∼15–25% of breast
cancers (1,2). To reflect the high importance of HER2
receptor signalling pathway for HER2-positive breast cancers,
molecular taxonomy refers to HER2-positive breast cancers as a
separate entity. HER2 receptor activation, either by
homodimerization when there is an abundance of HER2 receptors or
heterodimerization after stimulation of certain dimerization
partner receptors with a pertinent ligand, leads to subsequent
activation of PI3K/Akt and Ras/ERK/MAPK signalling pathways
responsible for the naturally high proliferation and aggressiveness
of these tumours. Without targeted anti-HER2 therapy, currently
available in the form of monoclonal antibodies and tyrosin kinase
inhibitors, HER2-positive breast cancer would continue to have one
of the poorest prognosis among breast cancers.
Despite the efficacy of targeted therapy, about half
of HER2-positive breast cancer patients have primary resistance to
trastuzumab or acquire resistance in the course of trastuzumab
therapy. Disease progression at the first radiological assessment,
or primary resistance, was observed in 50% of patient with
metastatic disease treated with single-agent trastuzumab (3–5).
Similar results were obtained for combined treatment with
trastuzumab and chemotherapy in metastatic disease (50% overall
response rate/ORR/) or in adjuvant settings (disease-free
survival/DFS/odds ratio/OR/ = 0.69 and locoregional recurrence OR =
0.53) (6,7).
Potential mechanisms of resistance to trastuzumab
include factors related to HER2 interactions with other members of
the HER family or trastuzumab, including the loss of, or increased,
HER2 expression; increased HER1 or HER3 expression; increased TGF-α
expres sion (a ligand for EGFR/HER1); steric hindrance of
HER2-antibody interaction by membrane-associated glycoproteins; and
inhibition of trastuzumab binding by HER2 ECD (extracellular
domain) fragments cleaved from the HER2 receptor. Incomplete HER
family blockade might be an important resistance mechanism, since
it could allow another HER receptor to compensate when one receptor
is blocked (8). Resistance to
trastuzumab might also arise through alternative signalling
pathways or through constitutive activation of the PI3K/Akt
signalling pathway, which is activated by HER2 signalling (and
therefore suppressed by HER2 inhibitors such as trastuzumab).
Constitutive activation might occur, for example, due to mutations
in the PIK3CA gene and/or loss of PTEN. Similar to HER2, the
IGF-1R, which can form heterodimers or heterotrimers with HER2,
activates the PI3K/Akt pathway and this mechanism is thought to be
an important source of trastuzumab resistance. Conversely, PTEN
suppresses the activation of the PI3K/Akt pathway and loss of PTEN
activity results in increased Akt activity and resistance to
trastuzumab (8). Hence, PI3K/Akt
pathway and Akt kinase seem to play a crucial role in oncogenic
potential of HER2 and the development of resistance to anti-HER2
targeted therapy (8–10).
Activation (phosphorylation) of Akt is a multistep
process (11). The signalling
cascade is initiated at cell membrane when a ligand binds to a
receptor (such as growth factor receptors, integrins, etc.) and
triggers conformational change of the receptor. Consequently, this
attracts PI3K (phosphatidylinositol 3-kinases) to the plasma
membrane and activates it. Phosphorylated PI3K generates the second
messenger PIP3 that recruits Akt to the inner cell membrane,
enabling phosphorylation of the Akt by its activating kinases, at
the threonine 308 residue (by PDK1) and at the serine 473 residue
[by rictor-mTOR complex in response to growth factor stimulation
(12) or by DNA-PK in response to
DNA damage (13)]. Once Akt is
activated, it dissociates from the plasma membrane and proceeds to
phosphorylate both cytoplasmic and nuclear downstream
effectors.
Akt family consists of three serine/threonine
kinases (Akt1, Akt2 and Akt3), with slightly different preferences
in their downstream effectors and varied presence in different
types of tissue. HER2-positive breast cancer cell lines and primary
tumours were found to have high expressions of Akt1, Akt2 and their
activated forms (14–18). Various Akt isoforms were also found
to have different biological functions in breast cancer. Akt1
accelerates tumourigenesis through increased cell proliferation and
is thus predominantly involved in the control of cell malignant
transformation, whereas, simultaneously, Akt1 suppresses tumour
invasion (19). Akt2
overexpression enhances invasive potential of breast cancer cells
and their ability to metastasize, Akt2 expression and activity
suppress anoikis and apoptosis caused by deprivation of nutrients
(19,20). The role of Akt3 in breast cancer
tumourigenesis is less clear. A number of studies examined the role
of Akt, especially the Akt1 and Akt2, in breast cancer and its
prognostic and predictive value (16–18,21–28).
However, no study so far have evaluated the correlation between
total Akt expression, subcellular localization of the activated
phosphorylated Akt (pAkt) and the results of anti-HER2 targeted
anticancer therapy that, through HER2 receptor, significantly
affects this signalling pathway. Therefore, we sought to explore
the relationship between activation and compartmentalization of
Akt1 and Akt2 and the outcome of targeted therapy in a sample of
patients with metastatic HER2-positive breast cancer treated with
trastuzumab.
Materials and methods
We enrolled 74 breast cancer patients treated
between 2001 and 2009 at the Masaryk Memorial Cancer Institute
(Brno, Czech Republic) for metastatic disease. The eligibility
criteria included: confirmed HER2 positivity, availability of
formalin-fixed and paraffin-embedded (FFPE) tissue samples of
primary tumours for immunohistochemistry evaluation, history of
trastuzumab based therapy for metastatic breast cancer and
availability of medical records for review. Informed consent was
obtained from each participating subject. Clinical data were
reviewed retrospectively from medical records. Immunohistochemistry
(IHC) evaluation was performed on tissue microarrays (TMAs). TMAs
were constructed from FFPE tissue sections using a technique
developed at our institution. The expression of HER2 protein was
determined by Dako Herceptest (Dako, Sweden) and scored on a
qualitative scale from 0 to 3+ according to Dako manual and
American Society of Clinical Oncology/College of American
Pathologists guideline recommendations for human epidermal growth
factor receptor 2 testing in breast cancer. HER2 gene status was
evaluated by FISH method using Abbott PathVysion HER2 kit (Abbott
Laboratories, USA). HER2 gene status was considered as positive
(FISH amplified) in case where a HER2 gene/centromer of chromosome
17 ratio was higher than 2.2 or if the number of HER2 gene copies
was higher than 6 per nucleus as measured by FISH. All tumours were
IHC 3+ and/or FISH-positive. The estrogen receptor (ER) and
progesterone receptor (PgR) status was examined by IHC, using
antibodies provided by Lab Vision (SP1 resp. SP2 monoclonal rabbit
antibody, Lab Vision Thermo Fisher Scientific, Fremont, CA, USA).
ER and PgR status was considered positive if >1% of cells were
stained in cell nuclei, and was considered negative in all other
cases. Similarly, Akt expression was assessed using IHC. Murine
monoclonal antibody was used for Akt1 IHC and rabbit monoclonal
antibody was used for Akt2 expression detection, both purchased
from Cell Signalling Technology (Beverly, MA, USA) and applied
according to the manual provided by the manufacturer. The tumours
with <5% of cells stained for protein were considered
Akt1/Akt2-negative. If ≥5% cells were stained with the respective
antibodies, the tumours were considered Akt1 or Akt2-positive. The
total Akt1 or Akt2 expression was considered to be strong if
>80% of cells were stained positive for the respective antibody.
Rabbit monoclonal antibodies, both from Cell Signalling Technology,
were used to detect phosphorylated (activated) Akt (pAkt) at Thr308
and phosphorylated Akt at Ser473. The expression was considered
positive if ≥5% tumour cells were stained with the antibody.
Cytoplasmic (c) and nuclear (n) fractions were assessed separately
for pAkt expression and tumours were divided into three groups:
group 1, pAkt expression negative; group 2, cytoplasmic-only pAkt
expression (pAkt-c); group 3, nuclear and cytoplasmic pAkt
expression (pAkt-n+c). The immunohistochemistry assessment was
performed by pathologists with an extensive experience in
evaluating tissue arrays and blinded to patient characteristic and
treatment outcomes.
Statistical analysis
Data are summarized using standard descriptive
statistics and frequency tabulations. Correlations between
expressions of various biomarkers (Akt1, Akt2, pAkt Thr308, pAkt
Ser473, ER, PgR) were analyzed using Spearman’s rank correlation
test. χ2 tests were used to determine correlation
between Akt expression or activation status and other clinical or
pathological characteristics. Response to therapy was evaluated
with RECIST criteria version 1.1. Time to progression (TTP) was
defined as the time from trastuzumab-based treatment initiation to
the first documented objective disease progression. Overall
survival was defined as the time from trastuzumab-based therapy
initiation to death from any cause (OSt), or time from diagnosis of
a metastatic breast cancer to death from any cause (OSm). Survival
data were plotted using the Kaplan-Meier method. The log-rank test
was used to analyze differences in TTP and OS. Univariate and
multivariate analyses of predictive factors were performed using
Cox’s proportional hazard regression. All tests were two-sided and
the significance level was set at α = 0.05. Statistical analysis
was performed with the support of MedCalc 9.1 software.
Results
Patient and tumour characteristics
We analyzed data from 74 patients. Patient and
tumour characteristics are summarized in Table I. All patients were female.
Sixty-five patients were diagnosed with early breast cancer and
progressed to metastatic cancer, 9 patients were diagnosed with
metastatic breast cancer. All patients were treated with
trastuzumab-based therapy only for metastatic breast cancer. The
use of trastuzumab corresponded to the knowledge then available.
Three patients were treated with trastuzumab monotherapy, all other
patients (96%) were treated with a combination of chemotherapy and
trastuzumab. Most frequently, trastuzumab was combined with taxanes
(57 patients, 77%) and was administered as the first line therapy
for metastatic breast cancer (44 patients, 59.5%). Initial
trastuzumab therapy led to complete remission in 9 patients (12.2%)
and partial remissions in 33 patients (44.6%). Overall response
rate was 56.8%. Stable disease as the best response was achieved in
19 patients (25.6%) and no response to therapy with disease
progression was seen in 8 patients (10.8%). Clinical benefit (CR or
PR or SD for at least 6 months) was achieved in 55 patients
(74.3%). Response could not be clearly identified in 5 patients, in
whom the disease metastasized predominantly to the skeleton. Median
follow-up time was 41 months. Disease progression was documented in
64 (86.5%) patients. Median TTP for the entire group was 9.2 months
(range from 1.3 to 56.2 months), median OSt was 20.1 months (range
1.3 to 68.3 months) and OSm was 29.8 months (range from 1.75 to 83
months).
 | Table IPatient and tumour
characteristics. |
Table I
Patient and tumour
characteristics.
| Characteristic | Patients no. | % |
|---|
| Age (years): median
54 (range 32–74) | | |
| <60 | 57 | 77.0 |
| ≥60 | 17 | 23.0 |
| Performance
status | | |
| 0 | 25 | 33.7 |
| 1 | 39 | 52.7 |
| 2 | 5 | 6.8 |
| NA | 5 | 6.8 |
| Histology | | |
| Ductal | 59 | 79.7 |
| Lobular | 7 | 9.5 |
| Mixed | 2 | 2.7 |
| Other | 6 | 8.1 |
| Tumour grade | | |
| G1 | 1 | 1.3 |
| G2 | 13 | 17.6 |
| G3 | 44 | 59.5 |
| UN | 16 | 21.6 |
| ER status | | |
| Positive | 32 | 43.2 |
| Negative | 40 | 54.1 |
| NA | 2 | 2.7 |
| PgR status | | |
| Positive | 20 | 27.0 |
| Negative | 49 | 66.2 |
| NA | 5 | 6.8 |
| Position of
trastuzumab in palliative therapy | | |
| 1. line | 44 | 59.5 |
| 2. line | 23 | 31.1 |
| ≥3. line | 7 | 9.4 |
| Combination of
trastuzumab with cytostatics | | |
| Paclitaxel | 39 | 52.7 |
| Docetaxel | 18 | 24.3 |
| Vinorelbine | 8 | 10.8 |
| CBDCA +
paclitaxel | 6 | 8.1 |
| No cytostatics
(trastuzumab monotherapy) | 3 | 4.1 |
| Best response to
therapy | | |
| CR | 9 | 12.2 |
| PR | 33 | 44.6 |
| SD | 19 | 25.6 |
| PD | 8 | 10.8 |
| NA | 5 | 6.8 |
The majority of tumours were invasive ductal
carcinomas (59 patients, 79.7%) and scored as grade 3 (59.5%), 32
patients had an ER-positive tumour (43.2%), 20 patients had a
PgR-positive tumour (27.0%) and 35 had an ER and/or PgR-positive
tumour (47.3%).
Akt expression and compartmentalization
in breast cancer
Seventy-four primary tumour samples were analyzed
for Akt1, Akt2, pAkt Thr308 and pAkt Ser408 expression and scored
as described above. Seventeen tumours (23.0%) were negative on Akt1
staining, 46 (62.1%) were Akt1 weak positive and 9 (12.2%) were
strong positive, results for two tumours were not interpretable.
There were no Akt2-negative tumours, 45 (60.8%) samples were weak
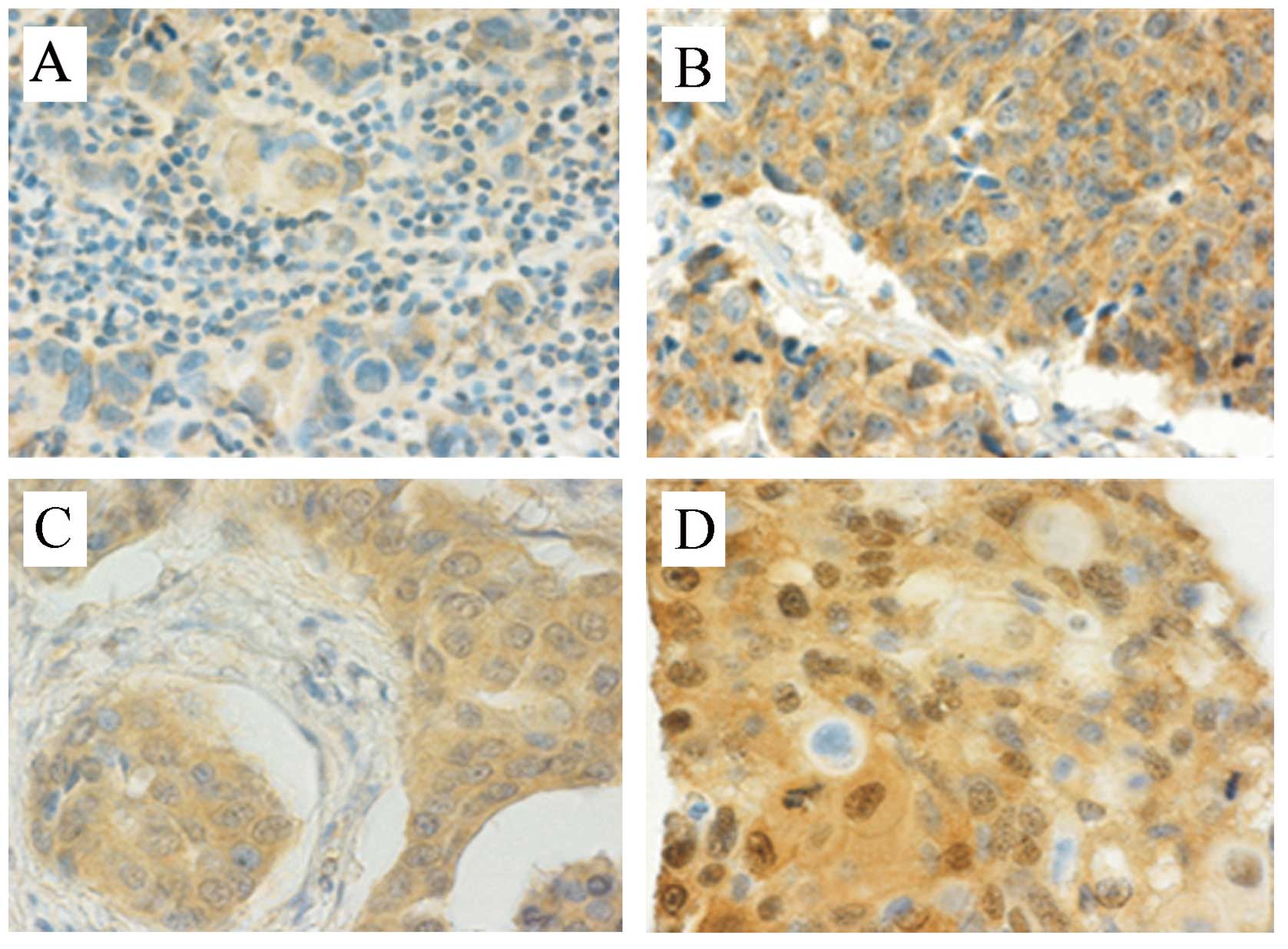
positive (Fig. 1A) and 26 (35.1%)
were strong positive on Akt2 staining (Fig. 1B), results for three tumours were
not interpretable. For phosphorylated Akt, cytoplasmic and nuclear
fractions were assessed separately. Ten tumours (13.5%) were
negative on any pAkt Thr308 staining, 22 tumours (29.7%) were
positive on cytoplasmic staining only (Fig. 1C) and 38 (51.4%) were positive on
both nuclear and cytoplasmic (n+c) staining (Fig. 1D), 4 cases (5.4%) could not be
assessed. For pAkt Ser473 staining, 5 cases were negative, 16 cases
(21.6%) were positive on cytoplasmic staining only and 49 (66.2%)
were positive on both nuclear and cytoplasmic staining, 4 cases
(5.4%) could not be interpreted. Akt expression results are
summarized in Table II.
 | Table IIAkt isoforms expression and
compartmentalization in a group of patients with HER2-positive
metastatic breast cancer. |
Table II
Akt isoforms expression and
compartmentalization in a group of patients with HER2-positive
metastatic breast cancer.
| Akt isoform | IHC staining | No. of
patients | % |
|---|
| Akt1 | Negative | 17 | 23.0 |
| Weak | 46 | 62.1 |
| Strong | 9 | 12.2 |
| NA | 2 | 2.7 |
| Akt2 | Negative | 0 | |
| Weak | 45 | 60.8 |
| Strong | 26 | 35.1 |
| NA | 3 | 4.1 |
| pAkt Thr308 | Negative | 10 | 13.5 |
| Cytoplasmic
only | 22 | 29.7 |
| Nuclear and
cytoplasmic | 38 | 51.4 |
| NA | 4 | 5.4 |
| pAkt Ser473 | Negative | 5 | 6.8 |
| Cytoplasmic
only | 16 | 21.6 |
| Nuclear and
cytoplasmic | 49 | 66.2 |
| NA | 4 | 5.4 |
We found no correlation between expression of Akt1,
Akt2 or activated forms of Akt and expression of estrogen or
progesterone receptors or tumour grading.
Correlation of Akt expression and
compartmentalization with time to progression
For patients with metastatic breast cancer treated
with trastuzumab, we tested the predictive value of expression of
distinct forms of Akt. There was no association between total Akt1
expression and TTP. We observed an unexpected trend towards
improved TTP in patients with tumours with strong Akt2 expression
(13.1 vs. 7.2 months) but the difference was not statistically
significant (P=0.133; HR 0.682; 95% CI 0.42–1.12). We evaluated
cytoplasmic and nuclear localization of pAkt separately. Even
though we did not observe any statistically significant difference
in TTP with respect to different pAkt Thr308 or pAkt Ser473
localization, there was, once again, an unexpected trend to longer
TTP in patients with activated Akt in both the cytoplasm and
nucleus (pAkt-n+c) compared to tumours with pAkt detected in the
cytoplasm only (pAkt-c): pAkt Thr308-n+c vs. pAkt Thr308-c, 10.2
vs. 8.3 months; pAkt Ser473-n+c vs. pAkt Ser473-c, 9.4 vs. 8.1
months, none of these results were, however, statistically
significant.
There was an interesting trend to a longer time to
progression in patients whose tumours had strong expression of
total Akt2 or activated Akt (pAkt) detectable in both the cytoplasm
and nucleus. Consequently, we explored interrelationships between
these factors and found that patients with strong Akt2 expression
and activated Akt (pAkt) detectable in both the cytoplasm and
nucleus had statistically significantly longer time to progression
than other patients (any = any total Akt2 expression and
simultaneously pAkt limited to cytoplasm only). The results were as
follows: a) tumours with strong total Akt2 expression and
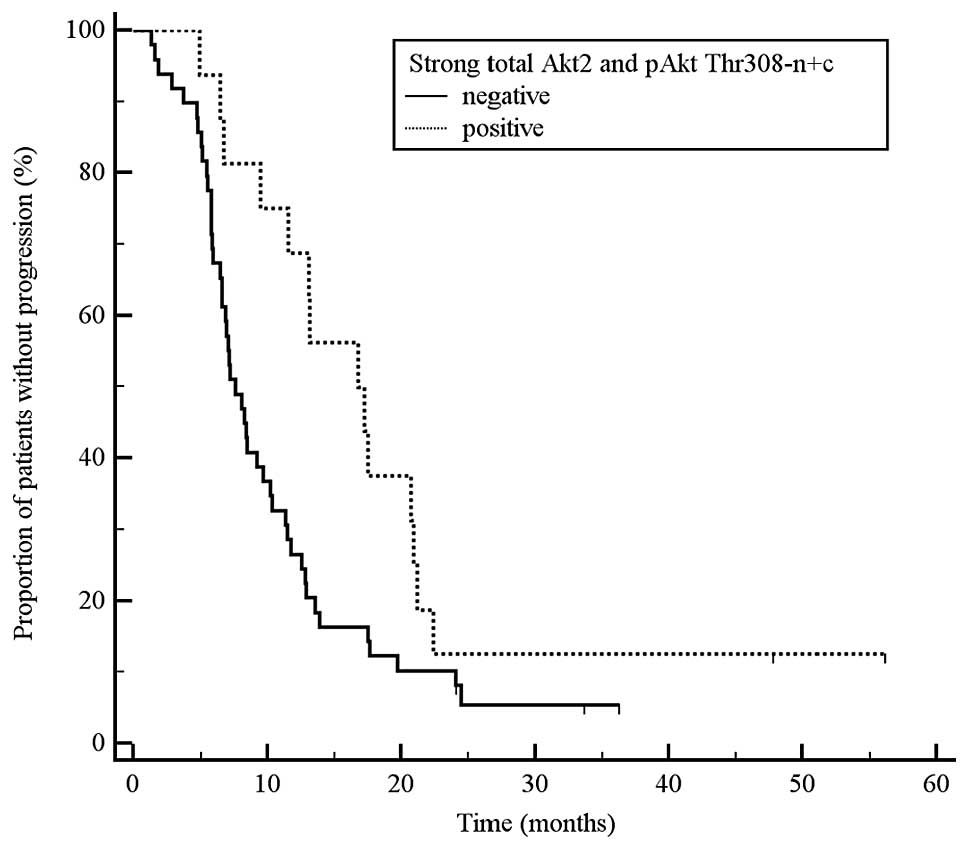
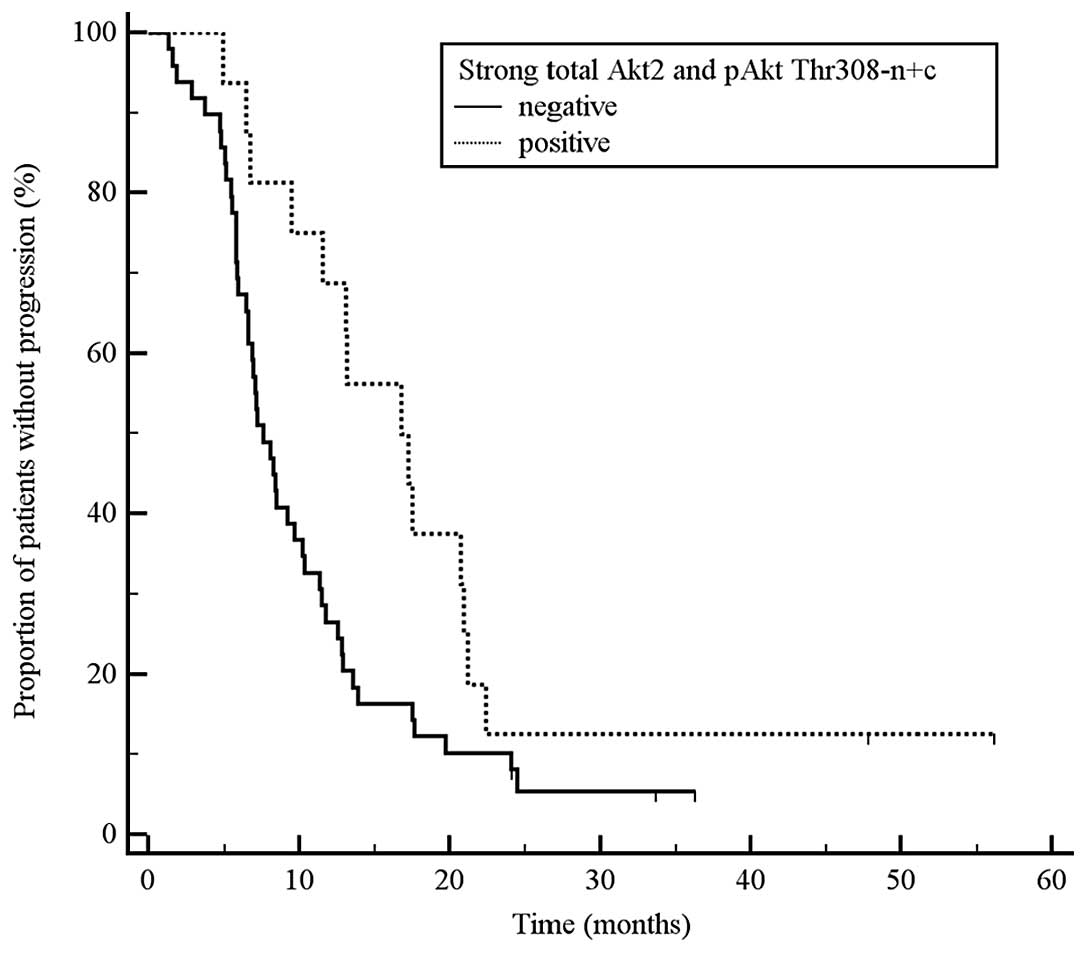
simultaneous pAkt Thr308 n+c vs. all other tumours: TTP 17.0 vs.
7.6 months, P=0.024, HR 0.52, 95% CI 0.31–0.87; Fig. 2; b) tumours with strong total Akt2
expression and simultaneous pAkt Ser473 n+c vs. all other tumours:
TTP 13.1 vs. 7.2 months, P=0.085, HR 0.62, 95% CI 0.37–1.03; c)
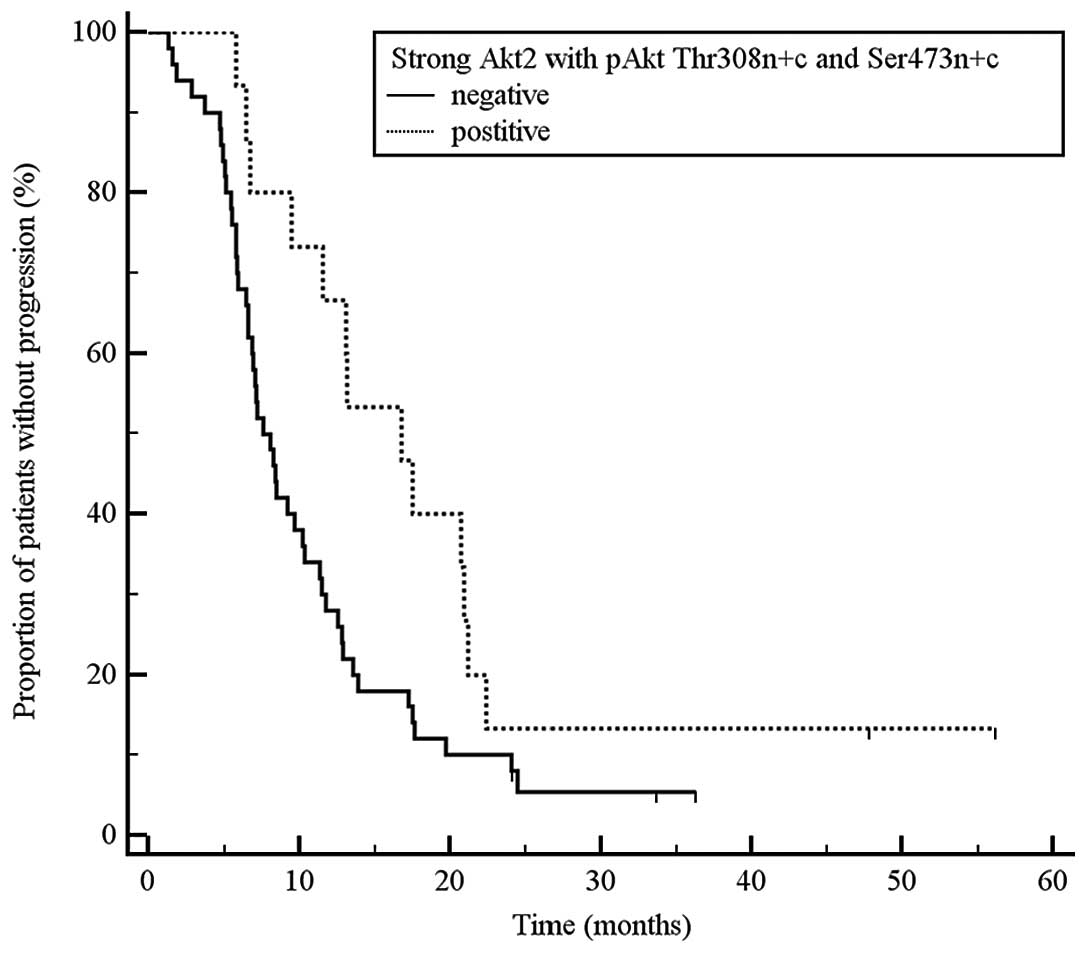
tumours with strong total Akt2 expression and simultaneous pAkt
Ser473 n+c and Thr308 n+c vs. all other tumours: 16.8 vs. 7.6
months, P=0.029, HR 0.52, 95% CI 0.30–0.88; Fig. 3.
We did not identify any correlation between survival
(TTP or OSt) and Akt1 expression and its combination with various
pAkt localizations.
Correlation of Akt expression and
compartmentalization with response to therapy
We found no statistically significant correlations
between expression of Akt isoforms or Akt compartmentalization and
trastuzumab therapy overall response rate or clinical benefit rate.
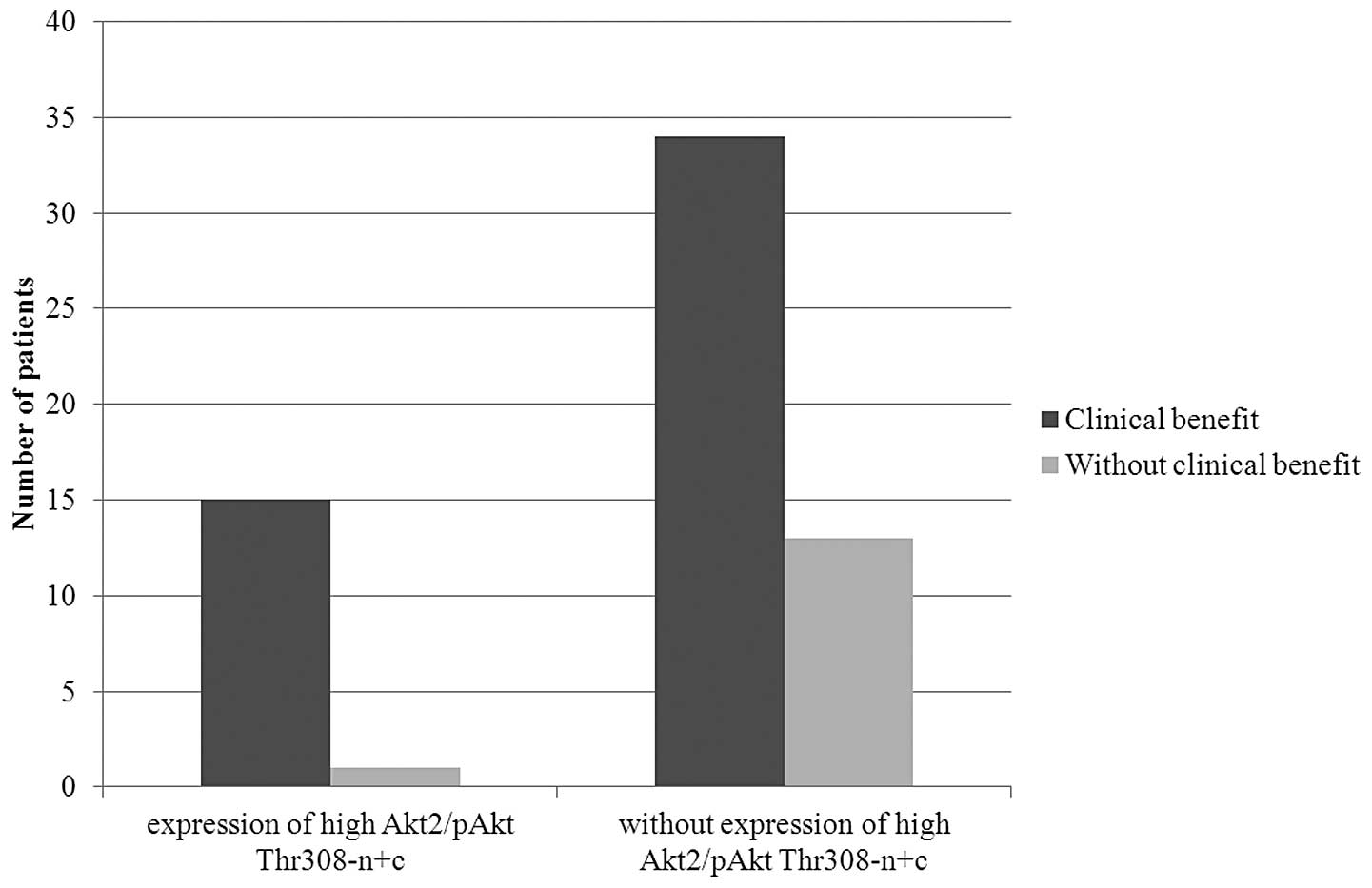
Patients with concurrent strong total Akt2 expression and pAkt
presence in both the cytoplasm and nucleus were more likely to
achieve clinical benefit than other patients. Of 16 patients with
strong total Akt2 expression and pAkt Thr308-n+c, 15 (94%) achieved
clinical benefit (P=0.077, Fig.
4). Similarly, 15 out of 17 (88%) patients with strong total
Akt2 expression and pAkt Ser473-n+c achieved clinical benefit. Even
though these results were not statistically significant, there was
a clear trend towards clinical benefit in this patient group. These
observations, once again, were not reproduced for the Akt1
expression and its combination with pAkt localization.
Correlation of Akt expression with
overall survival
For OSt (time from the initiation of trastuzumab
therapy for metastatic breast cancer to death from any cause),
strong expression of total Akt2 was associated with prolonged
survival (40.0 vs. 17.9 months, P=0.03, HR 0.55, 95% CI 0.32–0.93).
On the other hand, expression of Akt1, pAkt Thr308 or pAkt Ser473
alone was not associated with better OSt. OSt was longer in
patients whose tumours had strong total Akt2 expression and
concurrent cytoplasmic and nuclear pAkt activity (strong Akt2 and
pAkt Thr308-n+c vs. all others: 51.8 vs. 16.8 months, P=0.0009, HR
0.34, 95% CI 0.19–0.59; strong Akt2 and pAkt Ser473-n+c: 50.8 vs.
17.0 months, P=0.009, HR 0.45, 95% CI 0.26–0.78; Fig. 5).
Similar results were obtained for OSm (time from
diagnosis of metastatic breast cancer to death). Strong expression
of total Akt2 was associated with prolonged OSm (58.0 vs. 26.4
months, P=0.040, HR 0.57, 95% CI 0.33–0.97). When other markers
were analyzed separately, i.e., Akt1, pAkt Thr308 or pAkt Ser473,
no correlation was found between these markers and OSm. Analysis of
a combination of total Akt expression and compartmentalization of
pAkt provide similar results. Strong expression of total Akt2
together with cytoplasmic and nuclear activity of pAkt was
associated with longer median OSm. OSm for tumours with strong
expression of total Akt2 and pAkt Thr308-n+c vs. all other tumours
was 59.2 vs. 24.1 months, P=0.006, HR 0.39, 95% CI 0.22–0.69; and
it was 59.2 vs. 24.5 months, P=0.008, HR 0.45, 95% CI 0.26–0.79 for
strong expression of total Akt2 and pAkt Ser473-n+c.
Correlation of Akt expression with
disease-free interval
We analyzed the correlation between Akt status and
time to disease recurrence in patients who were first diagnosed
with early breast cancer and then progressed. We found no
association between Akt status (expression of Akt1 and Akt2, and
expression and compartmentalization of pAkt Thr308 and pAkt Ser473)
and disease free survival, including the analyses of combinations
of markers. Neither were there any correlations between Akt
expression pattern and the clinical stage of the primary breast
cancer at the time of breast cancer diagnosis.
Univariate and multivariate analyses of
TTP, OSt and OSm
The results of univariate Cox regression analyses
for TTP, OSt and OSm are summarized in Table III. These results provided the
basis for multivariate Cox regression analyses that confirmed
independence of some of these parameters in predicting TTP, OSt and
OSm (Table III). Strong total
expression of Akt2 and concurrent presence of activated pAkt Thr308
in the cytoplasm and nucleus (strong Akt2 and pAkt Thr308-n+c) was
an independent positive predictive factor in multivariate analyses
for TTP as well as OSt. We consider this as very important, as both
these intervals (TTP and OSt) are directly associated with targeted
anti-HER2 therapy with trastuzumab. Multivariate analyses also
confirmed that the number of metastases is an important negative
predictor for all analyzed survival intervals.
 | Table IIIRelationship between studied clinical
and molecular factors and survival intervals. |
Table III
Relationship between studied clinical
and molecular factors and survival intervals.
| TTP
| OSt
| OSm
|
|---|
| Factors | P-valuea | HR-95% CIa | P-valuea | HR-95% CIa | P-valuea | HR-95% CIa |
|---|
| Akt1 | 0.263 | 0.411–1.272 | 0.131 | 0.334–1.150 | 0.408 | 0.417–1.425 |
| Akt2 | 0.136 | 0.406–1.128 | 0.033 | 0.288–0.946 | 0.044 | 0.292–0.980 |
| pAkt Thr308 | 0.391 | 0.797–1.222 | 0.223 | 0.541–1.152 | 0.179 | 0.536–1.122 |
| pAkt Ser473 | 0.229 | 0.488–1.186 | 0.162 | 0.459–1.137 | 0.155 | 0.464–1.127 |
| Strong Akt2 + pAkt
Thr308-n+c | 0.027b | 0.276–0.921 | 0.002b | 0.131–0.620 | 0.008 | 0.179–0.772 |
| Strong Akt2 + pAkt
Ser473-n+c | 0.088 | 0.350–1.072 | 0.011 | 0.205–0.808 | 0.011 | 0.196–0.803 |
| ER | 0.637 | 0.685–1.858 | 0.121 | 0.365–1.122 | 0.014b | 0.270–0.861 |
| PgR | 0.308 | 0.769–2.308 | 0.301 | 0.757–2.477 | 0.387 | 0.718–2.364 |
| Age (<60 vs. ≥60
years) | 0.162 | 0.359–1.184 | 0.026 | 0.197–0.899 | 0.056 | 0.324–1.011 |
| Position of
trastuzumab therapy | 0.194 | 0.860–1.840 | 0.385 | 0.793–1.832 | 0.293 | 0.553–1.194 |
| Number of
metastatic sites | 0.004b |
1.114–1.751 | 0.002b |
1.164–1.947 | 0.014b |
1.070–1.797 |
Discussion
In this retrospective study, we investigated
associations between Akt expression, activation and
compartmentalization and the efficacy of anti-HER2 targeted therapy
on a model of metastatic HER2-positive breast cancer patients
treated with monoclonal antibody against HER2 receptor,
trastuzumab. In this molecular subtype of breast cancers, PI3K/Akt
pathway seems to have the highest impact on oncogenic potential of
HER2 and the development of resistance to anti-HER2 targeted
therapy (8–10).
We confirmed that Akt1 and Akt2 are widely expressed
in HER2-positive breast tumours and, simultaneously, tumours
contain their activated form. Surprisingly, pAkt did not cross into
the nucleus and was found in the cytoplasm only in >20% of
tumours (29.7% for pAkt Thr308 and 21.6% for pAkt Ser473). The
reasons for this are not known. Nevertheless, biological impact of
different compartmentalization of pAkt has been previously shown
(29–34). We found that patients with
HER2-positive breast cancer treated with anti-HER2 targeted therapy
with trastuzumab, whose tumours strongly expressed Akt2, had
significantly longer overall survival from targeted treatment
initiation (OSt). Furthermore, we found that this anti-HER2
targeted treatment-associated positive effect was even more
powerful in patients whose tumours also had strong expression of
Akt2 as well as cytoplasmic and nuclear localization of pAkt (pAkt
Thr308 and/or pAkt Ser473). These patients had prolonged time to
progression, overall survival and more likely achieved clinical
benefit (complete or partial remission or disease stabilization).
Multivariate analysis confirmed that strong Akt2 expression and
pAkt Thr308 (n+c) is a positive and independent predictor of TTP
and OSt.
Positive predictive value of Akt2 expression for the
outcome of targeted antitumour therapy has been previously
described. On a sample of 402 ER-α positive breast cancer patients,
Kirkergaard et al showed significantly longer survival in a
group with tumours presenting strong cytoplasmic expression of Akt2
(HR 1.8, CI 95% 1.14–2.97, P=0.0115) (23). There are two features common to our
and Kirgergaard et al patient samples. First, all patients
were treated with targeted therapy, i.e., a therapy that affects
Akt signalling pathway (35). We
used anti-HER2 therapy with trastuzumab, Kirgergaard et al
used anti-ER endocrine therapy with tamoxifen. Second, Akt
expression was determined on primary, i.e., treatment naïve
tumours. Therefore, we may hypothesize that the reduction of Akt
signalling pathway activity by targeted treatment may be associated
with better treatment outcome. Similarly to our results,
Kirgergaard et al did not confirm an association between
Akt1 and survival parameters. This may be due to different
biological effects of Akt1 and Akt2 in HER2-positive breast cancer
(19,20,36,37).
For many years, the oncogenic potential of Akt was
considered to originate from its cytoplasmic localization, possibly
through regulation of apoptosis, proliferation, energy metabolism
and motility, by phosphorylating downstream effectors such as Bad,
RAF, CREB, NF-κB, caspases, GSK-3 α/β, mTOR, p21WAF1 and
others (11,38). However, several studies have
identified a pool of activated Akt inside the nucleus (nuclear
pAkt). The presence of pAkt in the nucleus mostly results from
translocation of pAkt from the cytoplasm (39), although phosphorylation of Akt
directly in the nucleus has also been shown, achieved through
activated PDK1 that also has the capacity to pass into the nucleus
(40). Nuclear Akt has an impact
on different biological functions. It has been shown that nuclear
Akt regulates cell cycle and apoptosis through phosphorylation of
the Forkhead transcription factor (29), GSK-3 (30) and Ebp1 (31). Moreover, nuclear pAkt
phosphorylates both cyclin-dependent kinase inhibitors
p21WAF1 and p27KIP1, resulting in their
expulsion from the nucleus and subsequent cytoplasmic degradation,
thus preventing cell cycle arrest (32–34).
The research studies discussed below provide
explanations for the observed impact of pAkt compartmentalization
(through direct effect on PI3K/Akt signalling pathway mediated, in
our case, by HER2 receptor) on treatment outcome.
Yoo et al showed that stimulation of HER3
receptor with its natural ligand heregulin leads to activation and
dissociation of Ebp1, a ubiquitously expressed protein, from HER3
and its translocation from the cytoplasm into the nucleus (41). Ahn et al confirmed that
phosphorylated Ebp1 binds to phosphorylated nuclear Akt and the
resulting complex interacts with CAD and inhibits its DNA
fragmentation activity; this leads to suppression of apoptosis in
the final stage. Moreover, Ebp1 also suppresses caspase-3 substrate
ICAD apoptotic degradation (31).
These findings suggest that Ebp1 is involved in inhibiting
apoptosis on multiple levels. HER3 receptor is then the most
frequent heterodimerization partner for HER2 receptor in
HER2-positive breast cancer and this dimerization pair forms the
most active kinase domain and the strongest PI3K/Akt signalling
pathway stimulator (9).
Trastuzumab blocks this stimulation. Boehme et al showed,
that downregulation of Akt2, but not Akt1, by siRNA prevented
phosphorylation of GSK-3 and strongly reduced the accumulation of
p53 after ionizing irradiation (IR). IR activated predominantly
nuclear Akt in a DNA-PK-dependent manner. Nuclear pAkt
phosphorylates and thus inactivates GSK-3 very efficiently.
Subsequently to inactivation of GSK-3, MDM2 was hypophosphorylated
and it was incapable of mediating p53 degradation. In consequence,
p53 was accumulated in the nucleus and ready to exert its
biological function (30).
Our results and the studies discussed above allow us
to hypothesise why patients with HER2-positive breast cancers
treated with targeted anti-HER2 therapy achieve better treatment
results if their primary tumours have high Akt2 expression and,
simultaneously, nuclear pAkt. Constitutive activation of HER2 prior
to targeted treatment initiation leads to increased activation of
Akt and, through its dimerization partners, HER3 and HER4, also to
activation of Ebp1. Activated Akt2 exerts its antiapoptotic and
proliferative effects in the cytoplasm. In addition, both
phosphorylated molecules, pAkt a pEbp1, cross into the nucleus
where they further potentiate these effects. Nuclear pAkt also
facilitates stabilization of p53 and its accumulation in the
nucleus. Inhibition of PI3K/Akt signalling pathway, with targeted
anti-HER2 receptor anticancer treatment in our case, reduces
antiapoptotic and proproliferative activity of Akt kinase. On the
other hand, in the nuclei of cells with accumulated pAkt and
protein p53 leads to cell cycle arrest and subsequent apoptosis.
Moreover, lack of pAkt in the nucleus leads to nucleic accumulation
of cyclin-dependent kinase inhibitors p21WAF1 and
p27KIP1, resulting in cell cycle arrest (32–34).
This hypothesis is supported by the fact that our observations were
valid for the survival intervals associated with trastuzumab
anti-HER2 therapy (TTP, OSt and OSm) only, not the disease-free
survival (DFS). In patients with HER2-positive cancer, DFS depends
on adjuvant treatment that, in our sample, did not contain
trastuzumab. We found only one study that correlated specifically
to nuclear location of Akt with clinical outcome and involved
ER-positive breast tumours. Badve et al showed that in
ER-positive tumours treated with targeted hormonal therapy
(circumstances analogous to our study), nuclear location of pAkt
was associated with better prognosis (26).
To provide the full picture in this discussion, it
should be mentioned that several studies described reverse
relationship between Akt and response to different therapy
modalities and clinical outcome in breast cancer patients.
Activation of Akt was associated with shortened disease-free
survival (16,17,21,23,24,28)
or overall survival in breast cancer (22). However, cell compartmentalization
of pAkt was either not reflected at all in these studies or
evidence of pAkt in the cytoplasm was considered as a positive
result. In addition, these studies analysed the relationship
between Akt and DFS and, with respect to these particular findings,
our results do not contravene those of other authors; we did not
confirm positive predictive value of strong total Akt2 expression
and concurrent pAkt (n+c) on DFS.
No study has been published so far evaluating a
relationship between total Akt expression and concurrent
subcellular localization of pAkt in primary tumours and the outcome
of anti-HER2 targeted therapy. Considering certain inconsistencies
in studies on the significance of Akt as well as potential
limitations of our study (relatively small numbers of patients,
different chemotherapy regimens administered with trastuzumab,
different order in which trastuzumab is added to treatment) we
suggest that further studies evaluating relationships between Akt,
its expression and compartmentalization and the outcome of
anticancer treatment affecting the Akt signalling pathway,
including in vitro studies on cell lines, are conducted
before firm conclusions can be made.
In conclusion, even though important advances have
been made in the treatment of HER2-positive breast cancer, there is
an important group of patients that does not benefit from anti-HER2
targeted therapy as expected. We focused on PI3K/Akt pathway that
appears to have the most pronounced impact on oncogenic potential
of HER2 and development of resistance to anti-HER2 targeted
therapy. We are the first to show the significance of Akt kinase
isoform, activity and compartmentalization for prediction of
response to trastuzumab-based anti HER2 targeted therapy in
patients with HER2-positive metastatic breast cancer; we found that
strong Akt2 expression and concurrent presence of activated pAkt in
the cytoplasm and nucleus was linked to better outcome. From the
confirmed differences in biological function of the various Akt
kinase isoforms and the importance of nuclear presence of activated
pAkt, we hypothesised why these patients in particular benefited
from anticancer treatment that targets the Akt signalling
pathway.
Acknowledgements
This study was supported by the IGA MZ
CR, project no. NR/8335-3, and the Czech Ministry of Health,
project no. MZ0MOU2005 and by Biomedreg CZ.1.05/2.1.00/01.0030.
References
|
1
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Owens MA, Horten BC and Da Silva MM: HER2
amplification ratios by fluorescence in situ hybridization and
correlation with immunohistochemistry in a cohort of 6556 breast
cancer tissues. Clin Breast Cancer. 5:63–69. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cobleigh MA, Vogel CL, Tripathy D, Robert
NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman
G and Slamon DJ: Multinational study of the efficacy and safety of
humanized anti-HER2 monoclonal antibody in women who have
HER2-overexpressing metastatic breast cancer that has progressed
after chemotherapy for metastatic disease. J Clin Oncol.
17:2639–2648. 1999.PubMed/NCBI
|
|
4
|
Baselga J, Tripathy D, Mendelsohn J,
Baughman S, Benz CC, Dantis L, Sklarin NT, Seidman AD, Hudis CA,
Moore J, et al: Phase II study of weekly intravenous recombinant
humanized anti-p185HER2 monoclonal antibody in patients
with HER2/neu-overexpressing metastatic breast cancer. J Clin
Oncol. 14:737–744. 1996.PubMed/NCBI
|
|
5
|
Vogel CL, Cobleigh MA, Tripathy D, Gutheil
JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF,
Burchmore M, et al: Efficacy and safety of trastuzumab as a single
agent in first-line treatment of HER2-overexpressing metastatic
breast cancer. J Clin Oncol. 20:719–726. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Slamon DJ, Leyland-Jones B, Shak S, Fuchs
H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M,
et al: Use of chemotherapy plus a monoclonal antibody against HER2
for metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yin W, Jiang Y, Shen Z, Shao Z and Lu J:
Trastuzumab in the adjuvant treatment of HER2-positive early breast
cancer patients: a meta-analysis of published randomized controlled
trials. PLoS One. 6:e210302011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arteaga CL, Sliwkowski MX, Osborne CK,
Perez EA, Puglisi F and Gianni L: Treatment of HER2-positive breast
cancer: current status and future perspectives. Nat Rev Clin Oncol.
9:16–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hsieh AC and Moasser MM: Targeting HER
proteins in cancer therapy and the role of the non-target HER3. Br
J Cancer. 97:453–457. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
She QB, Chandarlapaty S, Ye Q, Lobo J,
Haskell KM, Leander KR, DeFeo-Jones D, Huber HE and Rosen N: Breast
tumor cells with PI3K mutation or HER2 amplification are
selectively addicted to Akt signaling. PLoS One. 3:e30652008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sarbassov DD, Guertin DA, Ali SM and
Sabatini DM: Phosphorylation and regulation of Akt/PKB by the
rictor-mTOR complex. Science. 307:1098–1101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bozulic L, Surucu B, Hynx D and Hemmings
BA: PKBalpha/Akt1 acts downstream of DNA-PK in the DNA
double-strand break response and promotes survival. Mol Cell.
30:203–213. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bacus SS, Altomare DA, Lyass L, Chin DM,
Farrell MP, Gurova K, Gudkov A and Testa JR: AKT2 is frequently
upregulated in HER-2/neu-positive breast cancers and may contribute
to tumor aggressiveness by enhancing cell survival. Oncogene.
21:3532–3540. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park SS and Kim SW: Activated Akt
signaling pathway in invasive ductal carcinoma of the breast:
correlation with HER2 overexpression. Oncol Rep. 18:139–143.
2007.PubMed/NCBI
|
|
16
|
Zhou X, Tan M, Stone Hawthorne V, Klos KS,
Lan KH, Yang Y, Yang W, Smith TL, Shi D and Yu D: Activation of the
Akt/mammalian target of rapamycin/4E-BP1 pathway by ErbB2
overexpression predicts tumor progression in breast cancers. Clin
Cancer Res. 10:6779–6788. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tokunaga E, Kimura Y, Oki E, Ueda N,
Futatsugi M, Mashino K, Yamamoto M, Ikebe M, Kakeji Y, Baba H and
Maehara Y: Akt is frequently activated in HER2/neu-positive breast
cancers and associated with poor prognosis among hormone-treated
patients. Int J Cancer. 118:284–289. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stål O, Pérez-Tenorio G, Akerberg L,
Olsson B, Nordenskjöld B, Skoog L and Rutqvist LE: Akt kinases in
breast cancer and the results of adjuvant therapy. Breast Cancer
Res. 5:R37–R44. 2003.PubMed/NCBI
|
|
19
|
Hutchinson JN, Jin J, Cardiff RD, Woodgett
JR and Muller WJ: Activation of Akt-1 (PKB-alpha) can accelerate
ErbB-2-mediated mammary tumorigenesis but suppresses tumor
invasion. Cancer Res. 64:3171–3178. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arboleda MJ, Lyons JF, Kabbinavar FF, Bray
MR, Snow BE, Ayala R, Danino M, Karlan BY and Slamon DJ:
Overexpression of AKT2/protein kinase Bbeta leads to up-regulation
of beta1 integrins, increased invasion, and metastasis of human
breast and ovarian cancer cells. Cancer Res. 63:196–206.
2003.PubMed/NCBI
|
|
21
|
Pérez-Tenorio G and Stål O: Activation of
AKT/PKB in breast cancer predicts a worse outcome among endocrine
treated patients. Br J Cancer. 86:540–545. 2002.PubMed/NCBI
|
|
22
|
Schmitz KJ, Otterbach F, Callies R, Levkau
B, Hölscher M, Hoffmann O, Grabellus F, Kimmig R, Schmid KW and
Baba HA: Prognostic relevance of activated Akt kinase in
node-negative breast cancer: a clinicopathological study of 99
cases. Mod Pathol. 17:15–21. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kirkegaard T, Witton CJ, McGlynn LM, Tovey
SM, Dunne B, Lyon A and Bartlett JM: AKT activation predicts
outcome in breast cancer patients treated with tamoxifen. J Pathol.
207:139–146. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vestey SB, Sen C, Calder CJ, Perks CM,
Pignatelli M and Winters ZE: Activated Akt expression in breast
cancer: correlation with p53, Hdm2 and patient outcome. Eur J
Cancer. 41:1017–1025. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Andre F, Nahta R, Conforti R, Boulet T,
Aziz M, Yuan LX, Meslin F, Spielmann M, Tomasic G, Pusztai L, et
al: Expression patterns and predictive value of phosphorylated AKT
in early-stage breast cancer. Ann Oncol. 19:315–320. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Badve S, Collins NR, Bhat-Nakshatri P,
Turbin D, Leung S, Thorat M, Dunn SE, Geistlinger TR, Carroll JS,
Brown M, et al: Subcellular localization of activated AKT in
estrogen receptor-and progesterone receptor-expressing breast
cancers: potential clinical implications. Am J Pathol.
176:2139–2149. 2010. View Article : Google Scholar
|
|
27
|
Esteva FJ, Guo H, Zhang S, Santa-Maria C,
Stone S, Lanchbury JS, Sahin AA, Hortobagyi GN and Yu D: PTEN,
PIK3CA, p-AKT, and p-p70S6K status: association with trastuzumab
response and survival in patients with HER2-positive metastatic
breast cancer. Am J Pathol. 177:1647–1656. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu Y, Mohamed H, Chillar R, Ali I, Clayton
S, Slamon D and Vadgama JV: Clinical significance of Akt and
HER2/neu overexpression in African-American and Latina women with
breast cancer. Breast Cancer Res. 10:R32008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo
P, Hu LS, Anderson MJ, Arden KC, Blenis J and Greenberg ME: Akt
promotes cell survival by phosphorylating and inhibiting a Forkhead
transcription factor. Cell. 96:857–868. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Boehme KA, Kulikov R and Blattner C: p53
stabilization in response to DNA damage requires Akt/PKB and
DNA-PK. Proc Natl Acad Sci USA. 105:7785–7790. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ahn JY, Liu X, Liu Z, Pereira L, Cheng D,
Peng J, Wade PA, Hamburger AW and Ye K: Nuclear Akt associates with
PKC-phosphorylated Ebp1, preventing DNA fragmentation by inhibition
of caspase-activated DNase. EMBO J. 25:2083–2095. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou BP, Liao Y, Xia W, Spohn B, Lee MH
and Hung MC: Cytoplasmic localization of p21Cip1/WAF1 by
Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat
Cell Biol. 3:245–252. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Viglietto G, Motti ML, Bruni P, Melillo
RM, D’Alessio A, Califano D, Vinci F, Chiappetta G, Tsichlis P,
Bellacosa A, et al: Cytoplasmic relocalization and inhibition of
the cyclin-dependent kinase inhibitor p27Kip1 by
PKB/Akt-mediated phosphorylation in breast cancer. Nat Med.
8:1136–1144. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Le XF, Pruefer F and Bast RC Jr:
HER2-targeting antibodies modulate the cyclin-dependent kinase
inhibitor p27Kip1 via multiple signaling pathways. Cell
Cycle. 4:87–95. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Simoncini T, Hafezi-Moghadam A, Brazil DP,
Ley K, Chin WW and Liao JK: Interaction of oestrogen receptor with
the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature.
407:538–541. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Irie HY, Pearline RV, Grueneberg D, Hsia
M, Ravichandran P, Kothari N, Natesan S and Brugge JS: Distinct
roles of Akt1 and Akt2 in regulating cell migration and
epithelial-mesenchymal transition. J Cell Biol. 171:1023–1034.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yoeli-Lerner M, Yiu GK, Rabinovitz I,
Erhardt P, Jauliac S and Toker A: Akt blocks breast cancer cell
motility and invasion through the transcription factor NFAT. Mol
Cell. 20:539–550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Datta SR, Brunet A and Greenberg ME:
Cellular survival: a play in three Akts. Genes Dev. 13:2905–2927.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xuan Nguyen TL, Choi JW, Lee SB, Ye K, Woo
SD, Lee KH and Ahn JY: Akt phosphorylation is essential for nuclear
translocation and retention in NGF-stimulated PC12 cells. Biochem
Biophys Res Commun. 349:789–798. 2006.PubMed/NCBI
|
|
40
|
Kikani CK, Dong LQ and Liu F: ‘New’-clear
functions of PDK1: beyond a master kinase? J Cell Biochem.
96:1157–1162. 2005.
|
|
41
|
Yoo JY, Wang XW, Rishi AK, Lessor T, Xia
XM, Gustafson TA and Hamburger AW: Interaction of the PA2G4 (EBP1)
protein with ErbB-3 and regulation of this binding by heregulin. Br
J Cancer. 82:683–690. 2000.PubMed/NCBI
|