Introduction
Increasing evidence shows that a small subset of
cells within a malignant neoplasm, named cancer stem cells (CSCs)
or tumor-initiating cells (TICs), are capable of initiating and
driving tumor growth (1,2). CSCs are so defined because they
possess many characteristics of normal stem cells, which have the
potential for self-renewal, in vitro sphere formation,
differentiation and tumorigenicity (3,4). In
addition they are relatively quiescent and resistant to many
chemotherapy drugs, and thus become sources of tumor recurrence
(5–7). CSCs are a new target of the next
generation of tumor therapeutic agents.
CSCs were first isolated from leukemia (8), and then from solid tumors including
glioblastoma (GBM, WHO grade IV) (9). In vitro culturing of CSCs is
widely used to test their characterization and functionality. In
the majority of studies CSCs are maintained and enriched in a
suspension culture of spheres in serum-free medium. This sphere
culture system attempts to decrease the secretion of
differentiation factors and to avoid differentiation stimulation by
adherence (10–12).
Although CSCs are defined by self-renewal,
differentiation, and tumorigenicity, they are usually identified
and isolated by stem cell markers. Markers such as Prominin-1
(CD133) and CD44 are frequently used in many types of tumors
(13–15). ATP-binding cassette (ABC)
transporter (breast cancer-resistance protein-1, ABCG2/BCRP1),
which is a marker of side-population cells, has also been used to
identify CSCs (16). Mostly,
multiple stem cell markers are used to identify CSCs and these
markers may differ among different tumor types. In breast cancer,
for example, the tumor-initiating cells have a characteristic
CD44(+)CD24(-/low) epithelial-specific antigen (ESA)(+) antigenic
pattern (17). For prostate
cancer, MDR1 and Oct-4 may be used as stem cell markers (18). In cells derived from glioblastoma,
neural stem/progenitor cell markers such as nestin, Sox2 and
Musashi-1 have been used to identify CSCs (19–22).
CSCs have been isolated not only from tumor tissues,
but also from established tumor cell lines including human breast
cancer (17), prostate cancer
(23), epithelial ovarian
carcinoma (24), melanoma
(25), colon cancer (26,27),
brain tumor (28,29) and others, and the list is expanding
rapidly. Traditionally, tumor cell lines were cultured in
serum-containing medium with monolayer morphology (excluding blood
system tumor cells). Generally, the vigorously dividing tumor cells
in serum-containing medium are considered differentiated cells.
Additionally, tumor cell lines have been used in studies for
decades, and cells have undergone various genomic and morphological
changes to adapt to the in vitro growth environment. Li
et al, observed several recurrent aberrations in established
glioma cell lines and these aberrations are not frequently observed
in primary tumors (30). Thus,
cultured tumor cell lines are unlikely to contain CSCs even if they
conserve some features of primary tumor biology. However, the
presence of CSCs in tumor cell lines suggests that some of the cell
lines may still possess stem cell potential. It is notable that the
reported CSCs in tumor cell lines were often obtained from floating
cells in the medium or cells which were cultured as spheres in
serum-free medium. These findings have led to interesting
questions. Can CSCs be re-induced from long-term cultured tumor
cells? Is the sphere culture system critical for the induction of
CSCs in tumor cell lines? Why do some cancer cell lines contain
CSCs and others do not? Answering these questions would be helpful
in understanding the development of CSCs.
In this study, we cultured the established
glioblastoma cell lines as spheres in a medium with or without
serum. We will examine the expression of generally accepted CSC
markers in these cells and compare CSC properties such as colony
formation, migration and chemotherapy resistance. Our main goal is
to determine whether those CSCs identified by stem cell markers
truly possess the ability to differentiate.
Materials and methods
Cell culture
LN229, T98G, U251n and U87 glioblastoma cell lines
were obtained from American Type Culture Collection (ATCC). Cells
were grown as monolayers when cultured in cell culture flasks with
DMEM containing 10% (v/v) fetal bovine serum (FBS), 2 mM
L-glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin
and 1% nonessential amino acid.
To culture tumor spheres, cells were seeded in 2%
poly (2-hydroxyethyl methacrylate) (poly-HEMA, Sigma)-coated cell
culture flasks to prevent cell adhesion. Tumor spheres were formed
either in serum-containing medium (i.e., serum sphere) or in
serum-free medium (i.e., serum-free sphere). Serum spheres were
cultured in the same medium as monolayer culture. Serum-free
spheres were cultured in DMEM/F12 supplemented with N2 supplement
and 0.5 mg/ml bovine serum albumin (BSA). Epidermal growth factor
(EFG) and basic fibroblast growth factor (FGFb) (20 ng/ml each)
were added to the medium before culturing.
Glioma cells exhibited variant growth speeds
especially when cultured as spheres. For subsequent sphere
formation 100–150 μm spheres were dissociated in 0.05%
trypsin-EDTA and seeded at a density of 10,000 cells/ml. Cell
culture medium was changed every 3 to 4 days. Spheres were cultured
at least 2 months in serum-containing medium or serum-free medium.
All cells were cultured at 37°C in a humidified atmosphere of 5%
CO2. Cell culture medium and additives were obtained
from Invitrogen.
Cell proliferation in soft agar
To observe the clonogenic ability of cells in
different culture conditions, monolayer cells and spheres were
dissociated with trypsin-EDTA. A total of 1x104 cells
were suspended in 0.3% low-melt agarose (Cambrex) and then seeded
onto the top of 2% poly-HEMA precoated 6-well plates (Corning). A
total volume of 4 ml of the serum-containing medium and serum-free
medium were added on top of the agarose layer with cells inside.
Medium was changed every 3 days. After 14 days in culture, the
plates were stained by 0.05% crystal violet for colony
quantification. Colonies with more than 20 cells were counted under
an inverted light microscope.
Sphere migration assay
Spheroids of approximately 200 μm diameter
were selected for the experiments. Six to ten spheroids were used
for each experimental condition in each experiment. Spheroids were
transferred individually to 24-well plates containing serum or
serum-free medium and allowed to migrate for 24 h. For
quantification, the mean diameter of glioma cells that had migrated
from the tumor spheroid was measured in a blinded manner and
expressed in relation to the mean radial distance at time 0 h.
Assays were repeated at least twice.
Western blot analysis
Glioma monolayer cells and spheres cultured in
different conditions were washed two times with ice cold
phosphate-buffered saline (PBS) and lysed in RIPA buffer (50 mM
Tris pH 7.4, 250 mM NaCl, 5 mM EDTA, 1% NP-40, 0.1% SDS, 0.5%
sodium deoxycholate, 1 mM phenylmethylsulphonyl fluoride)
containing 1% protease inhibitor cocktail (Calbiochem). Cell
lysates were centrifuged at 13,000 x g for 10 min to remove debris
and protein concentration was determined using the BCA protein
assay kit (Pierce). Total protein of 10–20 μg was subjected
to SDS-PAGE, transferred to polyvinylidene fluoride (PVDF)
membrane, and probed with antibodies, followed by HRP-conjugated
secondary antibodies. Specific proteins were detected by ECL
Western Blotting Detection Reagents (GE Healthcare Biosciences).
The experiments were repeated in triplicate. β-actin antibody was
used as the internal protein control. Antibodies against Nestin and
Sox2 were purchased from Millipore; antibodies against Musashi-1
and CD133 were the products of Cell Signaling Technology Inc.;
antibodies against CD44 and β-actin were obtained from Sigma; and
ABCG2 (anti-BCRP, clone BXP-21) was the product of Kamiya
Biomedical Company.
For quantification of relative protein levels, X-ray
film, and densitometric analysis was carried out using ImageJ
software (National Institutes of Health). Each immunoblot was
performed 3 to 6 times.
Cytotoxicity assay
Cytotoxicity of sphere cells responding to
temozolomide (TMZ, Sigma) was assessed by release of lactate
dehydrogenase (LDH) from damaged cells into the medium using a
cytotoxicity detection kit (Roche Applied Science). The method was
performed as specified by the manufacturer, with minor
modifications. Serum spheres and serum-free spheres were
dissociated with trypsin-EDTA and 2x105 single cells
were seeded in 2% poly-HEMA coated 24-well cell culture plates with
the same serum concentration as their sphere culture. Cells were
treated with TMZ for 48 h at concentrations of 800 and 1,000
μM. The same amount of DMSO was added into the medium as
control. Then supernatants were collected and centrifuged at 10,000
rpm for 5 min to remove floating cells. After sedimentation, 20
μl of supernatant from samples was transferred to a 96-well,
flat-bottomed plate (Costar) and mixed with 80 μl PBS, to
which 100 μl of substrate mix in assay buffer was then
added. Plates were kept protected from light for up to 30 min at
room temperature. The absorbance was monitored at 492 nm with the
reference wavelength 620 nm in a plate reader (Multiskan MCC/340,
Labsystems). The percentage of cytotoxicity was calculated relative
to absorbance values for blank medium controls and values resulting
from total lysis of cells by Triton X-100 (100% cell kill)
according to the following formula: Cytotoxicity (%) =
(ODtreatment−ODcontrol)/(ODtotal−ODcontrol)
x 100.
Immunocytochemistry staining
Lab-TeK tissue culture chambers (2-well) (Nunc) were
coated with poly-L-ornithine (Sigma)/fibronectin (Sigma).
Serum-containing and serum-free cultured spheres were dissociated
into single cells and 1x104 cells were seeded in each
well. Cells were cultured with 1% FBS containing DMEM medium for
7–10 days to undergo differentiation. Then cells were fixed with 4%
paraformaldehyde or methanol (O4 staining) and permeabilized with
PBS containing 0.5% Triton X-100 for 5 min. Cells were stained by
lineage-specific markers namely Glial Fibrillary Acidic Protein
(GFAP, astrocyte marker) (Millipore), Beta III tubulin (Tuj1,
neuron marker) (Covance) and O4 (Oligodendrocyte marker) (RnD
Systems). Signals were developed with diaminobenzidine (DAB) for
GFAP, Romulin AEC for Tuj1 and DAB/NiCl2 for O4 with 4
plus HRP detection system (Biocare). Cells were counter-stained
with hematoxylin (GFAP and Tuj1) and methyl-green (O4) (Sigma).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Each experiment was repeated three times with duplicates.
Statistical differences were evaluated using one-way ANOVA. A
probability value of p<0.05 was considered statistically
significant between two groups.
Results
Nestin, Sox2 and Musashi-1 were induced
in serum spheres of LN229 and U251n
Traditionally, glioblastoma cell lines were kept in
10% FBS containing medium and grown as monolayers. When cells were
cultured in serum-free medium, most of the tumor cells were
floating and form spheres. To make spheres of cells both in
serum-containing medium and serum-free medium, we coated cell
culture apparatus with 2% poly-HEMA to prevent cell adhesion.
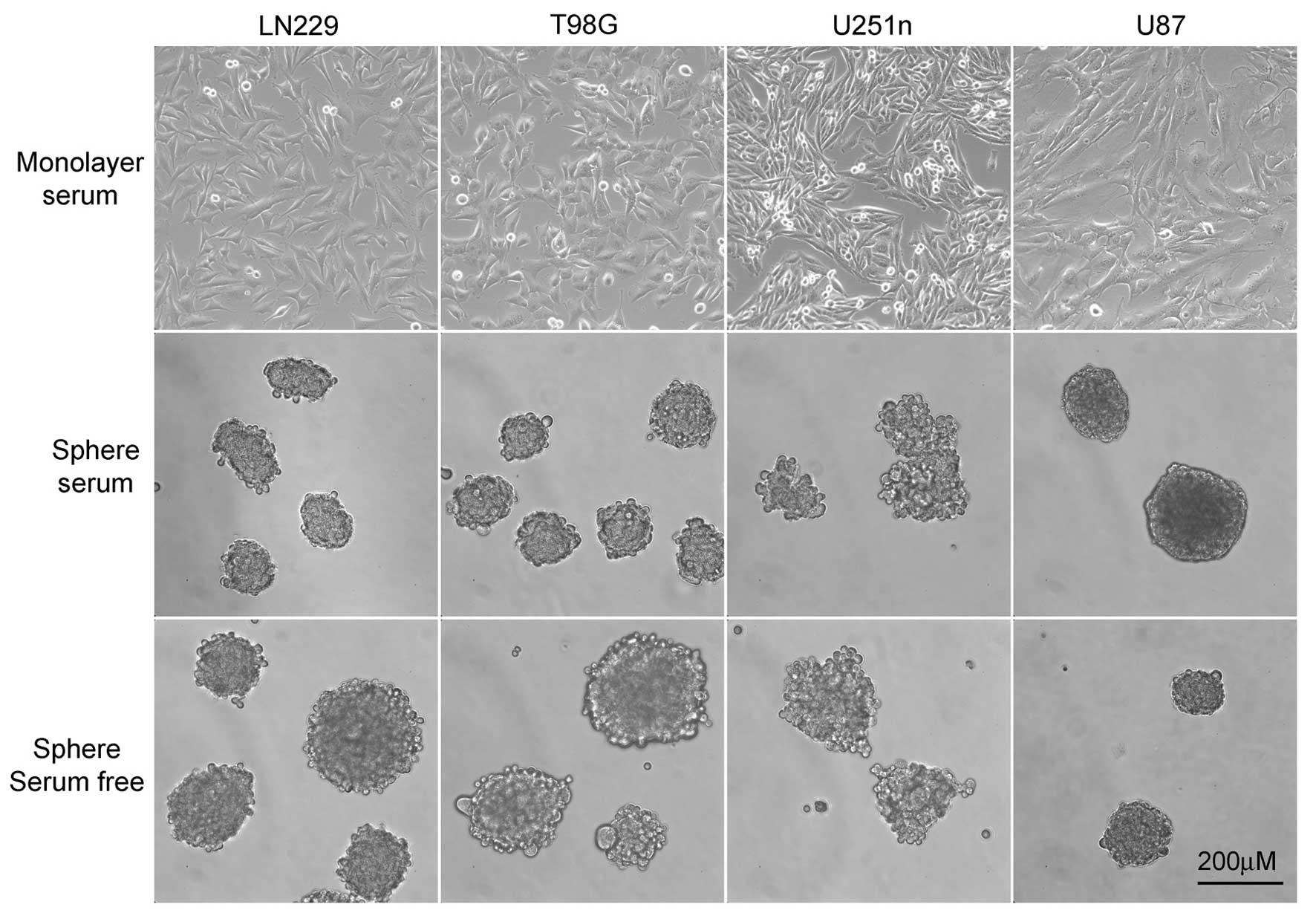
LN229, T98G and U87 formed spheres that were round with tightly
connected cells. U251n formed spheres that were not exactly round
from both serum-containing medium and serum-free medium. The cells
in U251n spheres were loosely connected and easy to expel from the
spheres. U87 cells grow extremely slowly when cultured as spheres.
The number and size of U87 spheres exhibited little change during
the process, even though these spheres were suspended in culture
for at least 8 weeks before further testing took place (Fig. 1).
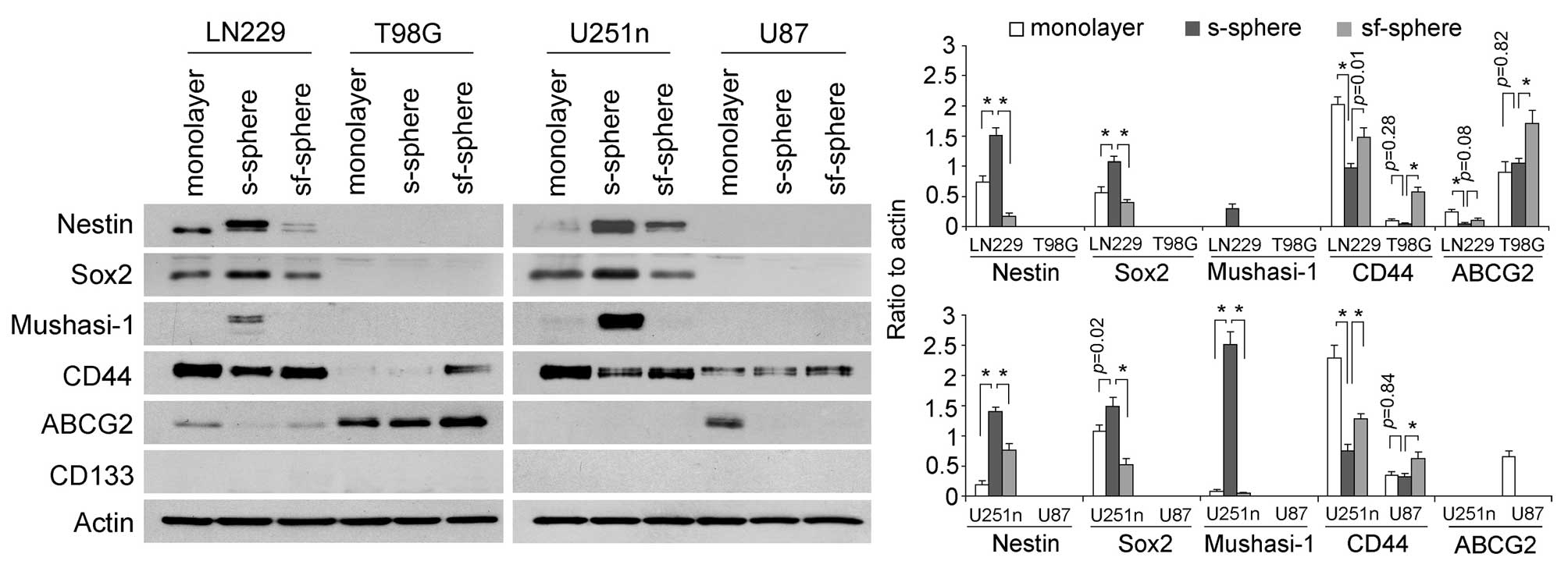
Expressions of stem cell markers were determined in
cells from different culture systems by western blot assay. We
found glioblastoma cells exhibited different expression patterns of
stem cell markers. Nestin, Sox2 and Mushashi-1 were expressed only
by LN229 and U251n cells but not by T98G and U87 cells. Serum
spheres expressed higher levels of these markers than monolayer
cells and spheres from serum-free medium. Specifically, Mushasi-1
was greatly induced only in serum spheres. CD44 expressed in an
opposite way to Nestin, Sox2 and Mushasi-1 in LN229 and U251n cells
and serum spheres expressed lower levels of CD44 than monolayers
and serum-free spheres. CD44 was also detected by T98G and U87
cells and the highest level of CD44 was expressed by serum-free
spheres. ABCG2 was detected in Ln229, T98G and U87 cells. However,
ABCG2 expression was not found to be correlated with other stem
cell markers. CD133 was not detected in any of the cell lines.
Previously, the CD133 antibody had been successfully used in
detecting primary cultured neurospheres from glioblastoma tissues
(31) (Fig. 2).
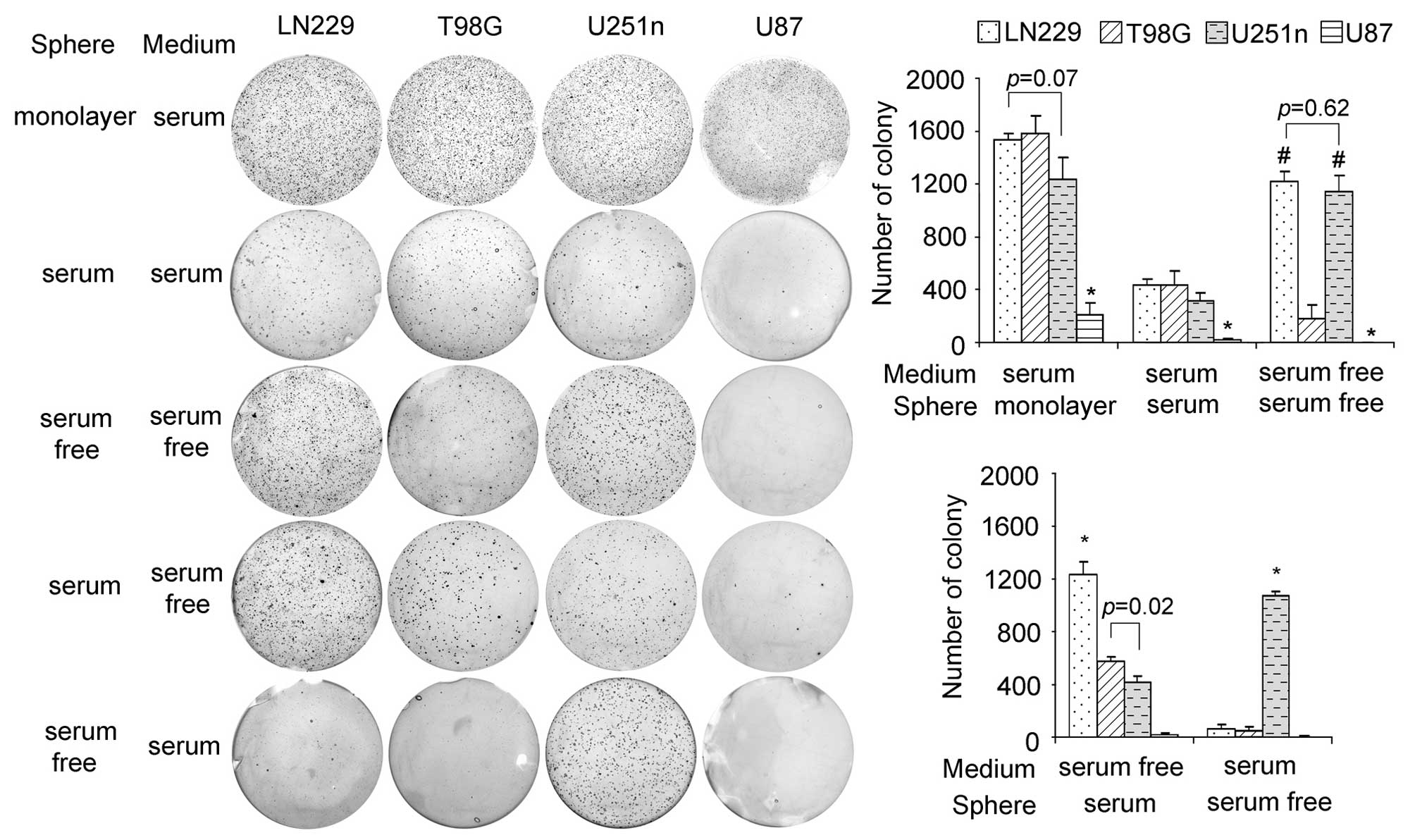
Colony formation potentials in Ln229,
U251n, T98G and U87 spheres
When grown as monolayers, LN229, T98G and U251n
cells showed similar colony formation and U87 cells grew slower
than the other three types. In the same medium as monolayer cells,
the sphere cells grew more slowly than the monolayer cells but with
the same growth pattern. Interestingly, serum-free sphere cells
from LN229 and U251n showed a much higher rate of colonization than
those from T98G and U87. LN229 and U251n sphere cells also
exhibited special colony formation potential when their growth
media were changed. When changed to serum-free medium, serum
spheres from LN229 presented a higher colony formation rate than
the other 3 cell lines. However, when changed to serum-containing
medium, serum-free spheres of U251n exhibited a much higher rate of
colonization than the other 3 cell lines, which suggests that LN229
and U251n have stronger survival ability than T98G and U87 in
response to environmental changes (Fig. 3).
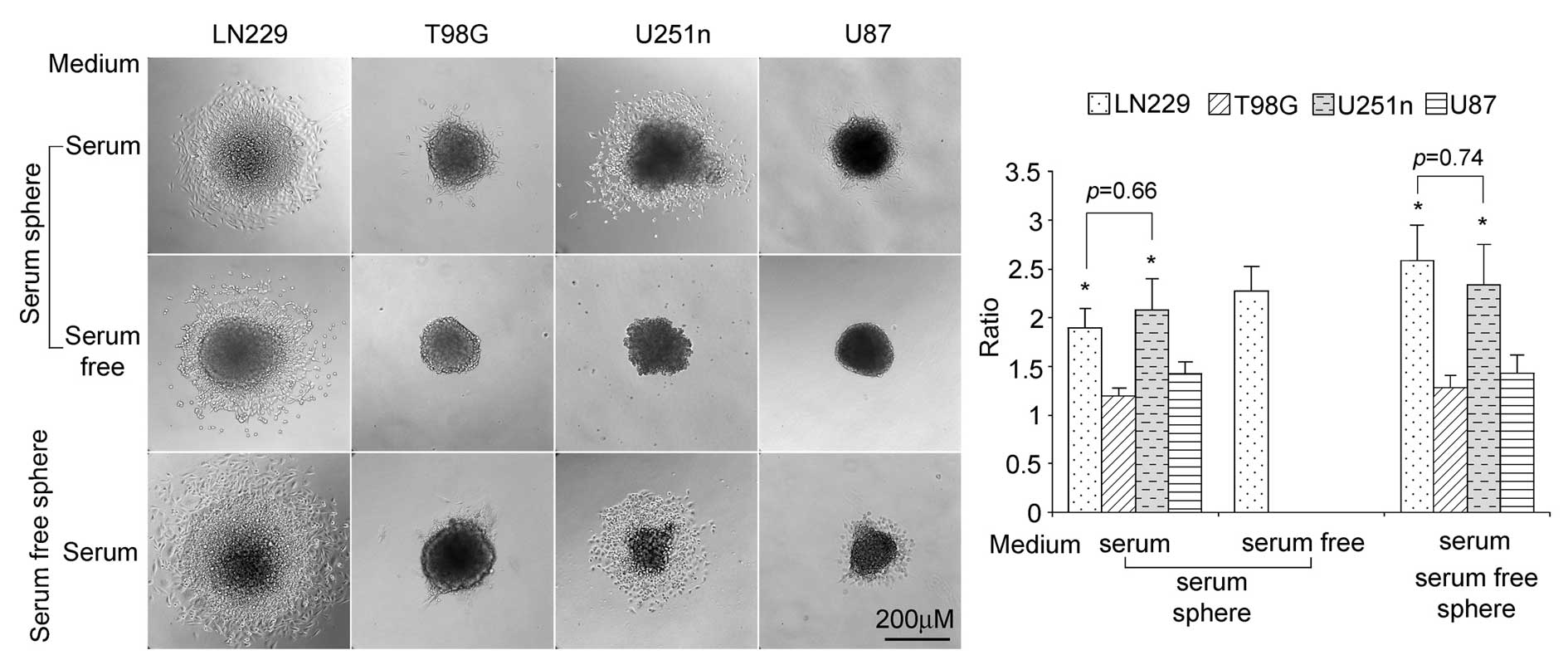
Migration ability of LN229, U251n, T98G
and U87 spheres
We seeded spheres in plated cell culture (with no
poly-HEMA coating) to test cell migration ability in
serum-containing medium or serum-free medium. When seeded in
serum-containing medium, both serum spheres and serum-free spheres
from LN229 and U251n cells exhibited higher rates of distant
migration than those from T98G and U87. Compared with LN229 and
U251 spheres, only small numbers of the T98G and U87 cells migrated
outside their spheres. In serum-free medium, only serum spheres of
LN229 showed migration (Fig.
4).
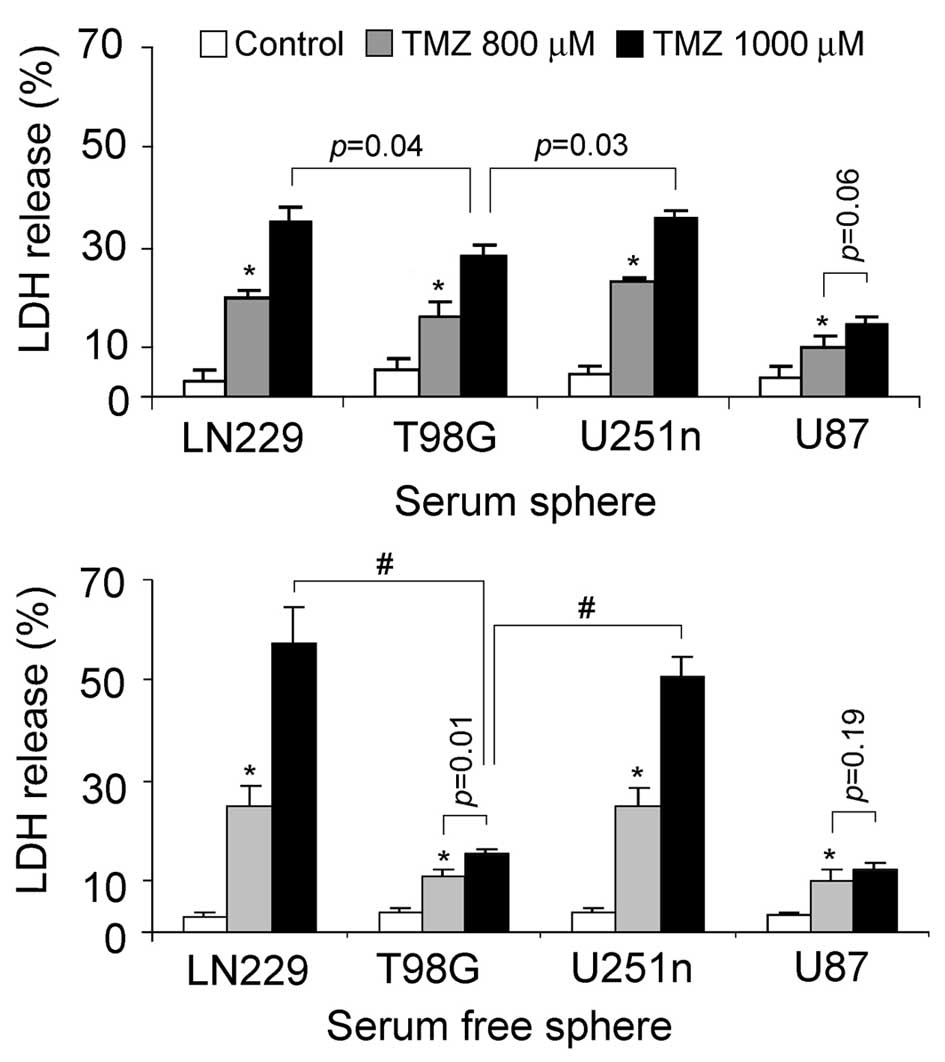
TMZ-induced cytotoxicity
The sensitivity of glioblastoma sphere cells to
chemotherapies was evaluated by testing LDH released from damaged
cells. TMZ, which is currently used in fighting glioblastoma, was
chosen as the cytotoxic reagent. LN229 and U251n sphere cells from
the same culture system exhibited similar cytotoxic effects
following TMZ treatment. LN229 and U251n serum spheres treated
after 48 h with 1,000 μM TMZ showed cell death rates of 34
and 35%, respectively. LN229 and U251n serum-free spheres treated
after 48 h with 1,000 μM TMZ showed cell death rates of 57
and 51%, respectively. U87 sphere cells showed relatively lower
cytotoxicity than the other 3 cells. Noticeably, serum spheres of
T98G exhibited lower toxic effect than those of LN229 and U251n,
while they had similar colonization rates in serum-containing
medium (Fig. 5).
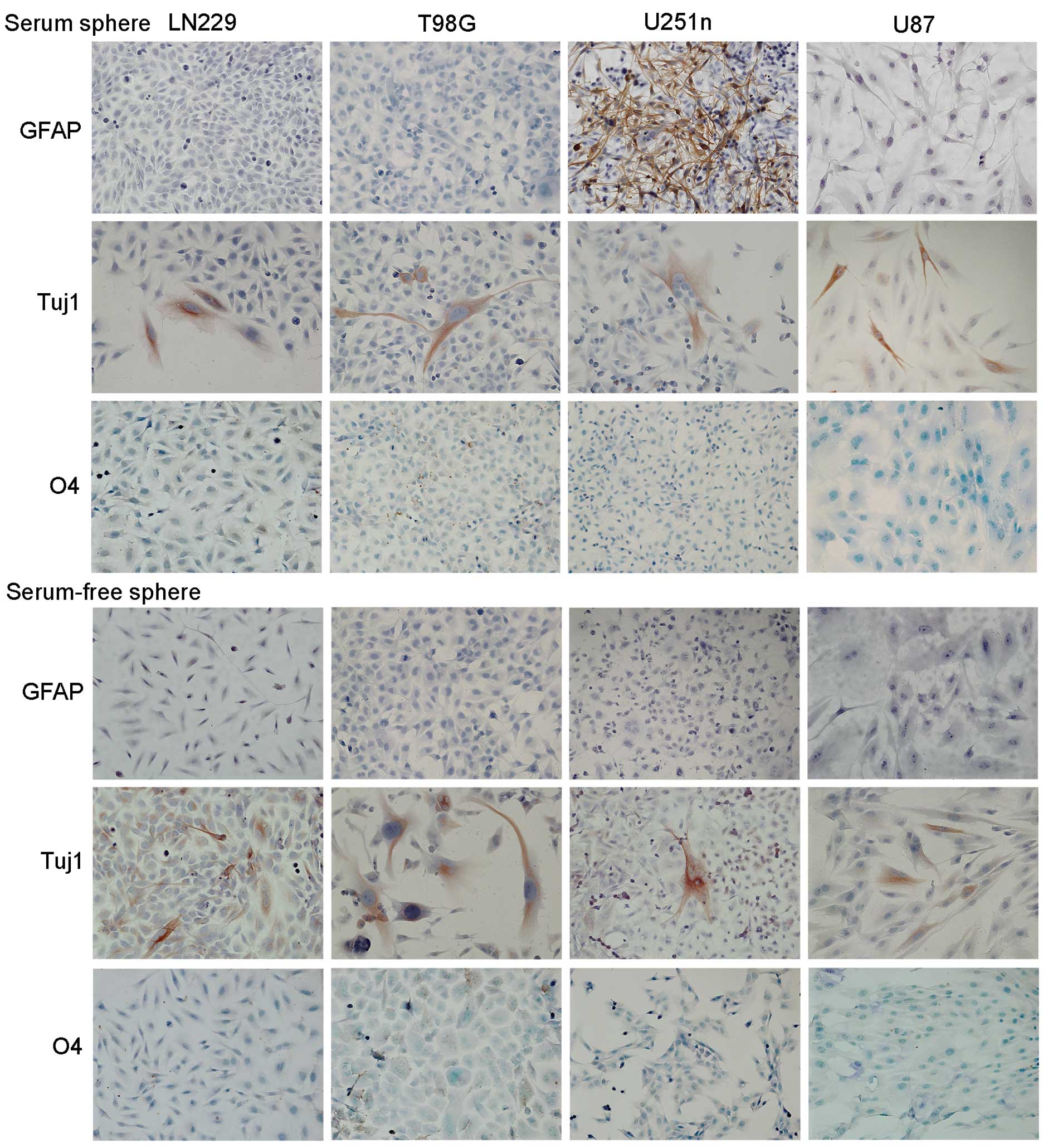
In vitro differentiation
Cell differentiation of sphere cells was evaluated
after 7–10 days adhesive growth in medium containing 1% FBS. Both
serum spheres and serum-free spheres showed neuronal
differentiation. Tuj1 positive neuronal cells were observed among
cell populations. Only cells from serum spheres of U251n exhibited
astrocytic differentiation, which was demonstrated as GFAP-positive
cells. We did not observe oligodendrocytic differentiation in any
of the four cell lines in either culture system as detected by O4
staining (Fig. 6).
Discussion
It has been revealed that almost all tissues contain
tissue-specific stem cells, which continuously generate the
residential differentiated cells responsible for tissue function
and homeostasis (32). The uneven
cell growth and heterogeneity of tumor phenotype led to the
postulation that tumors may be derived from tissue stem cells. The
CSCs may proliferate to generate differentiated tumor mass and may
also generate new CSCs through self-renewal. In glioblastoma, CSCs
are thought to be responsible for the invasive growth and tumor
recurrence due to their resistance to chemotherapy drugs.
Sphere formation is a typical characteristic of CSCs
when cultured in serum-free medium. The sphere culture of CSCs
minimizes stimulation from the environment, thus keeping CSCs in an
undifferentiated state. Interestingly, we found that all the tested
tumor cell lines exhibited similar effects such as floating growth
and sphere formation in the serum-free condition. Cell lines like
U251n may not entirely float in the medium and some cells still
remain adhered to the regular cell culture flasks. Additionally, we
found that part of the dissociated spheres may re-attach to a
non-poly-HEMA coated flask when subcultured to secondary spheres,
indicating the uneven growth of the cells within the spheres.
LN229 and U251n cells are distinguished from T98G
and U87 cells by expression of Nestin, Sox2, Musashi-1 and CD44.
With the expression of multiple stem cell markers, LN229 and U251n
cells exhibited higher migration and colonization abilities than
T98G and U87 cells. Note that higher colonization was observed only
in spheres cultured in serum-free medium. In serum-containing
medium, the external stimulation of growth factors from serum may
overwhelm the internal cell signaling. However, even with the
similar expression of stem cell markers, LN229 and U251n cells
showed different colonization ability when they underwent immediate
medium changes. When serum spheres were placed in serum-free
medium, LN229 cells showed a higher colonization rate than the
other cell lines, while serum-free spheres of U251n cells placed in
serum-containing medium showed significantly higher colonization
than the other cell lines, indicating that LN229 and U251n cells
have special abilities in adapting to environmental change. Whether
the unique colonization abilities of U251n serum-free spheres in
serum-containing medium predicts its multilineage differentiation
potential has not been determined and needs further testing. CD133,
which was used extensively as a stem cell marker, is not expressed
by these cells. Recent studies indicate that both CD133-negative
and CD133-positive cancer cells can initiate tumors (33,34).
Serum spheres of LN229 and U251n cells exhibited
more resistance to TMZ than their serum-free spheres. This result
is consistent with our finding that serum spheres of LN229 and
U251n express higher levels of stem cell markers. Cytotoxicity to
TMZ is closely correlated with cell proliferation rate. Stem cell
markers may be involved in the modulation of cell cycle progression
and thus change cell response to chemotherapy agents. Expression
levels of the drug-resistance-related gene ABCG2 is not correlated
with TMZ-induced cell damage. ABCG2 also is not correlated with
stem cell properties, suggesting the ABCG2 may not be crucial for
stem cell function. The lower cytotoxic effects of T98G serum
sphere may be due to its expression of O6-Methylguanine-DNA
Methyltransferase (MGMT), which is a response to repair TMZ-induced
DNA damage (35).
When checking differentiation abilities, we found
that most glioblastoma cells are maintained in an undifferentiated
state. Through differentiation stimulation, few tumor cells
differentiated to neuronal cells. Only cells from serum spheres of
U251n exhibited both astrocytic and neuronal differentiation. The
limited neuronal differentiation of glioblastoma cell lines
predicts that these tumor cells may originally be derived from
adult neural stem or progenitor cells (36).
It is unclear why serum spheres are more stem-like
than serum-free spheres. Cells cultured as spheres may change
cell-to-cell interaction compared to monolayer cells. In spheres,
cells in the inner part of the spheres may receive less nutrients
compared to the outer layer. The sphere structure formed in
serum-containing medium may be more suitable for inner layer cells
to transform to quiescent or dormant cells which are more
stem-like. Considering the similar levels of stem cell markers
expressed by LN229 and U251n, differentiation ability cannot be
predicted only by the presence of stem cell markers. We assume that
some cancer cells such as U251n cells have the potential to
dedifferentiate and this ability is decided by the intrinsic
changes within cells and may not depend only on the expression of
stem cell markers. Astrocytes from p53-deficient mice were reported
to dedifferentiate to stem-like cells (36,37).
It is possible that U251n cells acquired dedifferentiation ability
through genomic changes.
Our study provides evidence that some cancer cell
lines may retain stem cell properties and their multi-potential
ability can be restored if they are kept in the proper conditions.
Serum-containing medium is a favorable environment for stem cell
enrichment. Stem cell markers may predict some stem cell potential
but not the ability to differentiate. U251n cells possess the most
stem cell properties compared with other glioblastoma cell lines.
The tumorigenic ability and invasive behavior of U251n serum
spheres in vivo needs to be tested in animal models. Future
studies are necessary to determine the differences between cells
located at the core and the outer surface of the spheres.
Understanding the molecular mechanisms of tumor dedifferentiation
and formation will help provide future clinical therapies for
patients with glioblastoma.
Acknowledgements
This study was supported by the
Hermelin Brain Tumor Center. We thank Sue MacPhee-Gray for
editorial assistance in preparing the manuscript.
References
|
1.
|
Qiao B, Johnson NW, Chen X, Li R, Tao Q
and Gao J: Disclosure of a stem cell phenotype in an oral squamous
cell carcinoma cell line induced by BMP-4 via an
epithelial-mesenchymal transition. Oncol Rep. 26:455–461.
2011.PubMed/NCBI
|
|
2.
|
Dalerba P, Cho RW and Clarke MF: Cancer
stem cells: models and concepts. Annu Rev Med. 58:267–284. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Venere M, Fine HA, Dirks PB and Rich JN:
Cancer stem cells in gliomas: identifying and understanding the
apex cell in cancer’s hierarchy. Glia. 59:1148–1154.
2011.PubMed/NCBI
|
|
4.
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Marques DS, Sandrini JZ, Boyle RT, Marins
LF and Trindade GS: Relationships between multidrug resistance
(MDR) and stem cell markers in human chronic myeloid leukemia cell
lines. Leuk Res. 34:757–762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Storci G, Sansone P, Mari S, et al:
TNFalpha up-regulates SLUG via the NF-kappaB/HIF1alpha axis, which
imparts breast cancer cells with a stem cell-like phenotype. J Cell
Physiol. 225:682–691. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Yip NC, Fombon IS, Liu P, et al:
Disulfiram modulated ROS-MAPK and NFkappaB pathways and targeted
breast cancer cells with cancer stem cell-like properties. Br J
Cancer. 104:1564–1574. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Lapidot T, Sirard C, Vormoor J, et al: A
cell initiating human acute myeloid leukaemia after transplantation
into SCID mice. Nature. 367:645–648. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Galli R, Binda E, Orfanelli U, et al:
Isolation and characterization of tumorigenic, stem-like neural
precursors from human glioblastoma. Cancer Res. 64:7011–7021. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Singh SK, Clarke ID, Hide T and Dirks PB:
Cancer stem cells in nervous system tumors. Oncogene. 23:7267–7273.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Leis O, Eguiara A, Lopez-Arribillaga E, et
al: Sox2 expression in breast tumours and activation in breast
cancer stem cells. Oncogene. 31:1354–1365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Miki J and Rhim JS: Prostate cell cultures
as in vitro models for the study of normal stem cells and cancer
stem cells. Prostate Cancer Prostatic Dis. 11:32–39. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Zhu Z, Hao X, Yan M, et al: Cancer
stem/progenitor cells are highly enriched in
CD133+CD44+ population in hepatocellular
carcinoma. Int J Cancer. 126:2067–2078. 2010.PubMed/NCBI
|
|
14.
|
Wang T, Ong CW, Shi J, et al: Sequential
expression of putative stem cell markers in gastric carcinogenesis.
Br J Cancer. 105:658–665. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Hwang-Verslues WW, Kuo WH, Chang PH, et
al: Multiple lineages of human breast cancer stem/progenitor cells
identified by profiling with stem cell markers. PLoS One.
4:e83772009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Salcido CD, Larochelle A, Taylor BJ,
Dunbar CE and Varticovski L: Molecular characterisation of side
population cells with cancer stem cell-like characteristics in
small-cell lung cancer. Br J Cancer. 102:1636–1644. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Fillmore CM and Kuperwasser C: Human
breast cancer cell lines contain stem-like cells that self-renew,
give rise to phenotypically diverse progeny and survive
chemotherapy. Breast Cancer Res. 10:R252008. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Rentala S and Mangamoori LN: Isolation,
characterization and mobilization of prostate cancer tissue derived
CD133+ MDR1+ cells. J Stem Cells. 5:75–81.
2010.PubMed/NCBI
|
|
19.
|
Strojnik T, Rosland GV, Sakariassen PO,
Kavalar R and Lah T: Neural stem cell markers, nestin and musashi
proteins, in the progression of human glioma: correlation of nestin
with prognosis of patient survival. Surg Neurol. 68:133–144. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Uchida N, Buck DW, He D, et al: Direct
isolation of human central nervous system stem cells. Proc Natl
Acad Sci USA. 97:14720–14725. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Singh SK, Hawkins C, Clarke ID, et al:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Ying M, Wang S, Sang Y, et al: Regulation
of glioblastoma stem cells by retinoic acid: role for Notch pathway
inhibition. Oncogene. 30:3454–3467. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Luk SU, Lee TK, Liu J, et al:
Chemopreventive effect of PSP through targeting of prostate cancer
stem cell-like population. PLoS One. 6:e198042011. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Shi MF, Jiao J, Lu WG, et al:
Identification of cancer stem cell-like cells from human epithelial
ovarian carcinoma cell line. Cell Mol Life Sci. 67:3915–3925. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Chandrasekaran S and DeLouise LA:
Enriching and characterizing cancer stem cell sub-populations in
the WM115 melanoma cell line. Biomaterials. 32:9316–9327. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Fan X, Ouyang N, Teng H and Yao H:
Isolation and characterization of spheroid cells from the HT29
colon cancer cell line. Int J Colorectal Dis. 26:1279–1285. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Zou J, Yu XF, Bao ZJ and Dong J: Proteome
of human colon cancer stem cells: a comparative analysis. World J
Gastroenterol. 17:1276–1285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Zheng X, Shen G, Yang X and Liu W: Most C6
cells are cancer stem cells: evidence from clonal and population
analyses. Cancer Res. 67:3691–3697. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Qiang L, Yang Y, Ma YJ, et al: Isolation
and characterization of cancer stem like cells in human
glioblastoma cell lines. Cancer Lett. 279:13–21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Li A, Walling J, Kotliarov Y, et al:
Genomic changes and gene expression profiles reveal that
established glioma cell lines are poorly representative of primary
human gliomas. Mol Cancer Res. 6:21–30. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
deCarvalho AC, Nelson K, Lemke N, et al:
Gliosarcoma stem cells undergo glial and mesenchymal
differentiation in vivo. Stem Cells. 28:181–190. 2010.PubMed/NCBI
|
|
32.
|
Weissman IL, Anderson DJ and Gage F: Stem
and progenitor cells: origins, phenotypes, lineage commitments, and
transdifferentiations. Annu Rev Cell Dev Biol. 17:387–403. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Wu Y and Wu PY: CD133 as a marker for
cancer stem cells: progresses and concerns. Stem Cells Dev.
18:1127–1134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Clevers H: The cancer stem cell: premises,
promises and challenges. Nat Med. 17:313–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Okamoto R, Takano H, Okamura T, et al:
O(6)-methylgua-nine-DNA methyltransferase (MGMT) as a determinant
of resistance to camptothecin derivatives. Jpn J Cancer Res.
93:93–102. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Lee JS, Gil JE, Kim JH, et al: Brain
cancer stem-like cell genesis from p53-deficient mouse astrocytes
by oncogenic Ras. Biochem Biophys Res Commun. 365:496–502. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Moon JH, Kwon S, Jun EK, et al:
Nanog-induced dedifferentiation of p53-deficient mouse astrocytes
into brain cancer stem-like cells. Biochem Biophys Res Commun.
412:175–181. 2011.PubMed/NCBI
|