Introduction
Pancreatic cancer (PC) is one of the most malignant
tumors. It is the fourth leading cause of cancer death in the
United States (1) and has an
increasing trend of mortality rate in China (2). Due to the lack of obvious symptoms or
specific manifestation in the early stage, the disease is often
diagnosed too late to have available therapeutic intervention
options.
Cancer stem cells (CSCs) are a small subpopulation
of multipotent cells exhibiting self-renewal capacity, multilineage
differentiation and high carcinogenesis (3,4), and
have been identified in several types of human cancers including
pancreatic cancer (5–8). Tumorsphere under suspension culture
is one of the important techniques to isolate cancer stem cell.
Dysregulation of CSC self-renewal may lead to expansion of the
stem-like cells, which might result in early-stage carcinogenesis
(9,10). Chemoresistance of CSCs is one of
the most critical reasons leading to failure of chemotherapy, which
might be followed by tumor recurrence.
Hedgehog (Hh) family members are key regulators of
carcinogenesis (4,11). Hh signal transduction is initiated
by binding Hh ligand, including Shh, Dhh and Ihh, to its receptor
Patched (PTCH). In the absence of Hh protein, PTCH represses signal
transduction by inhibiting Smoothened (Smo). When Hh ligand binds
to PTCH receptor, inhibitory effects of PTCH on Smo diminish, Hh
pathway is activated and the ultimate step is mediated by the zinc
finger transcription factors-Gli family which turns on genes
regulating cell cycle, and determinating cell-fate. Hh pathway is
active in many tumors, such as medulloblastoma (12), glioma (13), gastric cancer (14) and pancreatic cancer (15). Increasing evidence supports that
hedgehog pathway has a role in the maintenance and progression of
pancreatic cancer (16–18). It also has a crucial role in
reverse chemoresistance of some CSCs, such as CD34+
leukaemic cells (19) and
glioblastoma CSCs (20), but the
mechanism is still unclear.
The breast cancer resistance protein (BCRP/ABCG2), a
member of the G-subfamiliy of the ATP-binding cassette
(ABC)-transporter superfamily, is implied to be associated with
multidrug resistance in some cancer stem cells, such as side
population in lung cancer (21),
and prostate tumorshpere (22). In
some cancers, there is relationship between Hh pathway and ABCG2.
In diffuse large B-cell lymphoma (23), ABCG2 is the target of Hh
pathway.
In the present study, we isolated pancreatic
tumorspheres under floating-culture system, and identified the
stemness potential of self-renewal, differentiation, high
carcinogenesis and chemoresistance. We elucidated the important
role of Hedgehog pathway in regulation of self-renewal and
chemoresistance of pancreatic CSCs.
Materials and methods
Cell lines and animals
The human pancreatic adenocarcinoma cell line PANC-1
(Chinese Academy of Sciences, Shanghai, China) was cultured in
RPMI-1640 (Gibco, Grand Island, NY, USA) supplemented with 10%
fetal bovine serum (FBS, HyClone, Logan, UT, USA), 100 U/ml
penicillin G, and 100 μg/ml streptomycin. In all experiments, cells
were maintained at 37°C in a humidified 5% CO2 air
atmosphere. Male non-obese diabetic/severe combined
immunodeficiency (NOD/SCID) mice (6-8 weeks) were purchased from
Chinese Academy of Sciences (Beijing, China). Animal care and
experimental protocols were performed in accordance with procedures
and guidelines established by Chinese Academy of Sciences
Experimental Animal Care Commission.
Expansion of PANC-1 tumorspheres
Viable, floating single cells were collected from
the supernatant of PANC-1 cells by centrifugation at 1,000 rpm for
5 min and plated at 1,000 cells/ml in serum-free DMEM-F12 (Gibco)
supplemented with 20 ng/ml recombinant human epidermal growth
factor (rhEGF, Invitrogen, NY, USA), 0.4% bovine serum albumin
(BSA, Sigma, St. Louis, MO, USA), 5 μg/ml insulin, 1:50 B27
supplement (Gibco, Invitrogen), 100 U/ml penicillin, and 100 mg/ml
streptomycin. Cells grown in this condition as non-adherent spheres
were enzymatically dissociated after 12 days by incubation in a
trypsin-EDTA solution and then cultured to generate tumorspheres of
the next generation. Differentiation was induced by culturing
tumorsphere-derived cells in RPMI-1640 supplemented with 10% fetal
bovine serum without rhEGF.
Sphere formation assay
PANC-1 tumorspheres and its parental cell line
PANC-1 were dissociated, and 1,000 cells per well were plated in
6-well culture dishes in 5 ml DMEM-F12 medium with rhEGF. The
number of spheres for each well was evaluated after 15 days of
culture.
Immunofluorescence analysis
To detect the differential potential of PANC-1
tumorspheres, the spheres were cultured in DMEMF12 supplemented
with 10% FBS. Observation of morphology was performed by
microscope. The expression of CK18, a differentiation marker, was
detected by immunofluorescence on 1st, 5th and 10th day. The cells
were washed with PBS and fixed in 4% paraform for 15 min on ice.
After two more phosphate-buffered solution (PBS) washes, the cells
were covered with 0.5% Triton-100 for 15 min on ice, then washed
with PBS and incubated with 5% non-fat milk for 1 h at room
temperature to block non-specific binding of IgG. The cells were
incubated with primary antibody anti-human CK18 (Cell Signaling
Technology, Boston, MA, USA) for 2 h at room temperature, then
washed with PBS and incubated with fluorochrome-conjugated
secondary antibody at room temperature for 30 min in a dark
chamber. The cells were washed with PBS and covered with DAPI to
stain the nuclei. Random photographs were taken at ×200
magnification.
Tumorsphere xenografts
To explore the tumorigenic capacity, PANC-1
tumorsphere cells in concentrations ranging from 200 to
2×104 were injected into the subcutaneous space of the
pad of NOD/SCID mice. Tumor growth was monitored every 2 days after
the second week of inoculation. When the xenograft tumors had
reached the desired size, mice were sacrificed and a portion of the
tumor tissue was collected, fixed in 4% paraform, and embedded in
paraffin for hematoxylin and eosin (H&E) staining to assess
tumor pathology. Tumors of three animals were harvested per
experiment.
Quantitative real-time RT-PCR
(qRT-PCR)
To analyse the hedgehog pathway components, qRT-PCR
was performed to examine the expression level of Smo, Gli 1 and Gli
2 mRNA in PANC-1 tumorspheres and PANC-1. Briefly, total RNA was
extracted from the cells using TRIzol (Invitrogen, CA, USA)
according to the manufacturer’s instructions. For qRT-PCR, 1 μl of
gene primers with SYBR Green in 20 μl of reaction volume was
applied. 18S ribosomal RNA was used as an endogenous control.
Primers were designed as: Smo, forward, 5′-cat ccc tga ctg tga gat
ca-3′; reverse, 5′-cac cat ctt ggt gac atg ct-3′. Gli 1, forward,
5′-cca tac atg tgt gag cac ga-3′; reverse, 5′-ggc aca gtc agt ctg
ctt t-3′. Gli 2, forward, CAA CGC CTA CTC TCC CAG AC; reverse, GAG
CCT TGA TGT ACT GTA CCA C. QRT-PCR was performed with SYBR Green
PCR System (Toyobo). Amplification data were analyzed with Applied
Biosystems Prism Sequence Detection Software version 2.1 (Applied
Biosystems).
Cell proliferation assay by WST-8
method
PANC-1 tumor-spheres were harvested and dissociated
into single cell suspension, 3×104 PANC-1 sphere cells
were seeded in 96-well plate per well. PANC-1 was also dissociated
into single cell suspension, 3×103 cells were seeded in 96-well
plate per well. The cells were treated in different concentration
of cyclopamine (Sigma-Aldrich, St. Louis, MO) (0, 0.5, 1, 2, 5 and
10 μmol/l, respectively) in triplicate for 24, 48 and 72 h,
respectively. WST-8 reagent (10 μl per well) from Cell Counting
kit-8 (Dojindo, Kumamoto, Japan) was added, incubated for 4 h, and
absorbance was determined with a multi-well spectrophotometer
(BioTek, VT, USA) at 450 and 630 nm. Inhibition rate = (1 -
absorbance of treated cells/control cells) ×100%.
Western blot analysis
The concentration of total protein extracted from
PANC-1 and PANC-1 tumorsphere cells with and without cyclopamine
was determined with a BCA Protein Assay kit (Pierce, USA). Equal
amounts of protein were separated by 10% SDS-PAGE and
electrophoretically transferred to PVDF membranes (Millipore,
Bedford, MA, USA) using a mini trans-blot. Rat anti-human CK18
(Cell Signaling Technology), Bmi-1 (Abcam, MA, USA), ABCG2 (Abcam),
Smo (Abcam), rabbit anti-human Gli 1 (Santa Cruz Biotechnology,
Santa Cruz, CA, USA) and Gli 2 (Santa Cruz Biotechnology) were used
to detect the expression of homologous proteins. β-actin (Santa
Cruz Biotechnology) was used as an internal control.
Electrochemiluminescence was performed with a Chemilmager 5500
imaging system (San Leandro, CA, USA), according to the
manufacturer’s instructions.
Flow cytometry analysis
To detect the influence of tumorspheres on cell
cycle after treatment of cyclopamine, flow cytometry analysis was
performed. Briefly, tumorsphere cells and PANC-1 were treated with
10 μmol/l cyclopamine for 72 h. Then the cells were trypsinized and
fixed with ice-cold 70% ethanol for 18 h at 4°C. The fixed cells
were stained with 50 mg/ml propidium iodide (BD Pharmingen, San
Diego, CA) and 50 mg/ml RNase and then analysed using a flow
cytometer (BD Pharmingen). PANC-1 tumorspheres and PANC-1 without
cyclopamine incubation were performed as control.
CD133 is implied as a stem cell marker in many
tumors, to detect phenotypic difference of CD133 in PANC-1 and
PANC-1 spheres, cells were harvested with trypsin-EDTA to produce a
single cell suspension. The cells were pelleted by centrifugation,
washed twice with PBS and incubated with FITC conjugated CD133
(Ancell, Bayport, MN, USA) for 30 min in the dark. The cells were
washed twice with PBS after incubation and analyzed using a flow
cytometer.
Statistical analysis
Data are presented as mean ± standard deviation
(SD), using the SPSS software, version 11.0. The means were then
compared using a one-way ANOVA with LSD among groups or Student’s
t-test between groups. P<0.05 was considered statistically
significant.
Results
PANC-1 tumorspheres cultured under
floating-culture system possess self-renewal potential
One of the probable method to identify the CSCs is
serum-free floating-culture system. It was sucessfully used to
culture neural stem cell and other cancer stem cells, such as
breast CSCs and brain CSCs. We applied this method to isolate
PANC-1 tumorspheres from human pancreatic cancer cell line PANC-1.
The adherent PANC-1 was trypsinized into single-cell suspension,
and was seeded in serum-free media supplemented with growth factors
at clonal density (1,000 cells/ml). Five days later, PANC-1
tumorspheres began to form in culture and they propagated after
10-15 days (Fig. 1). The 1st
spheres could be enzymatically dissociated into single cells, which
in turn generated the 2nd spheres. This procedure could be repeated
and the PANC-1 tumorspheres have been passaged over 20 times in
vitro.
We subsequently examined whether PANC-1 tumorsphere
cells possess self-renewal capacity. Sphere formation assay was
performed to calculate the number of stem cell spheres and measure
the self-renewal capacity of each sphere generation. Each
generation of tumorsphere cells (103) were plated in
6-well dish, and cultured for 15 days in triplicate. The 1st PANC-1
tumorsphere cells form 5.5±1.3 spheres; The 4th PANC-1 tumorsphere
cells form 4.5±1.3 spheres (P>0.05). The capacity of
serial-passage in vitro and the number of tumorspheres that
remained equivalent show the self-renewal potential of PANC-1
tumorspheres.
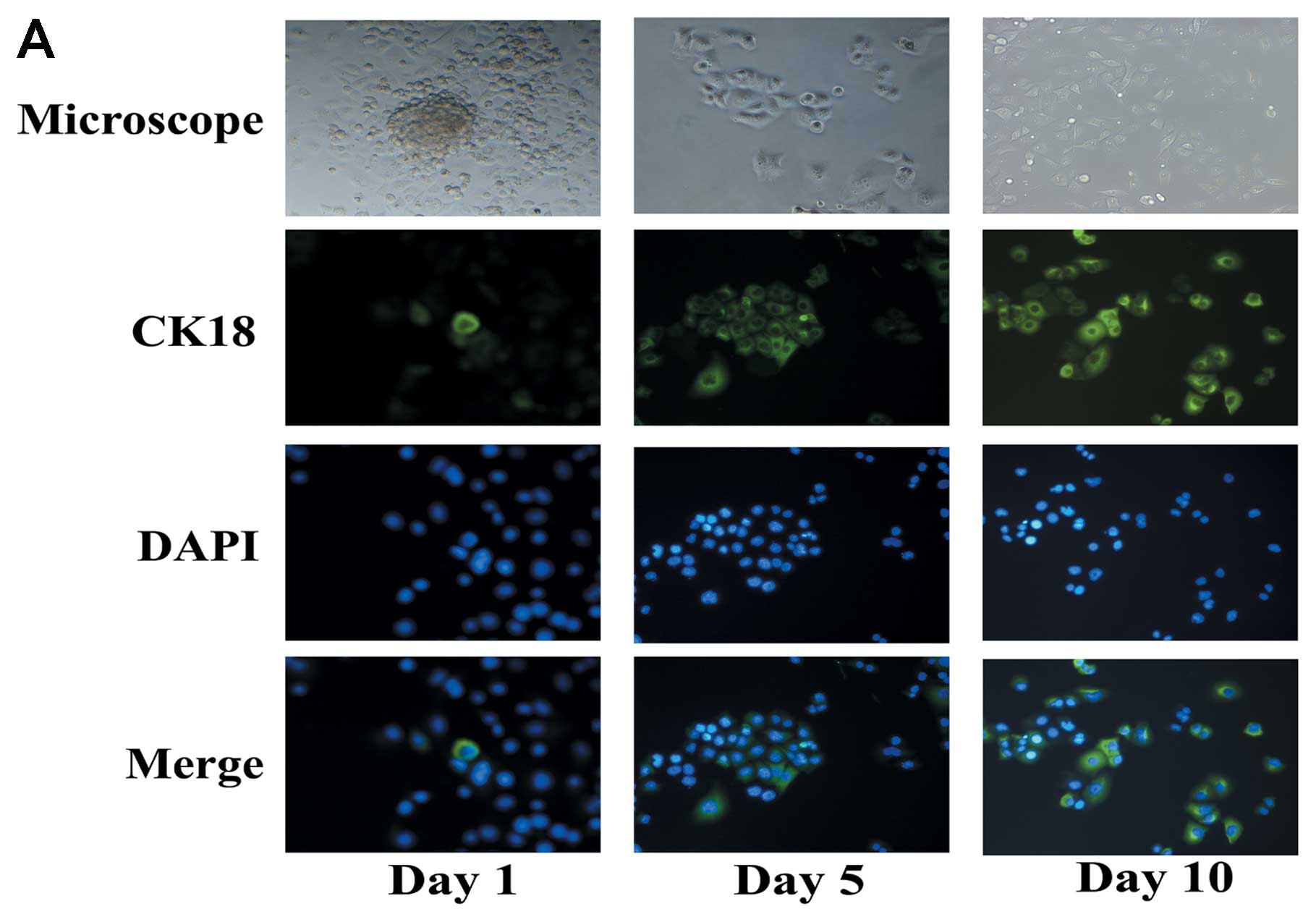
PANC-1 tumorspheres have differentiation
potential
Differentiation potential is one of the capacites of
CSCs. CK18 is a mature marker associated to luminal/ductal
epithelial cells, and acts as a differentiation marker in this
study. Both immunofluorescence analysis and western blot analyses
were performed to detect the expression of CK18. To induce
differentiation, floating PANC-1 tumorspheres were cultured in the
medium without growth factors and with 10% fetal bovine serum.
Under differentiating conditions, floating PANC-1 tumorspheres
began to adhere and acquired epithelium-like morphology. Expression
of CK18 was increased after induction of differentiation (Fig. 2).
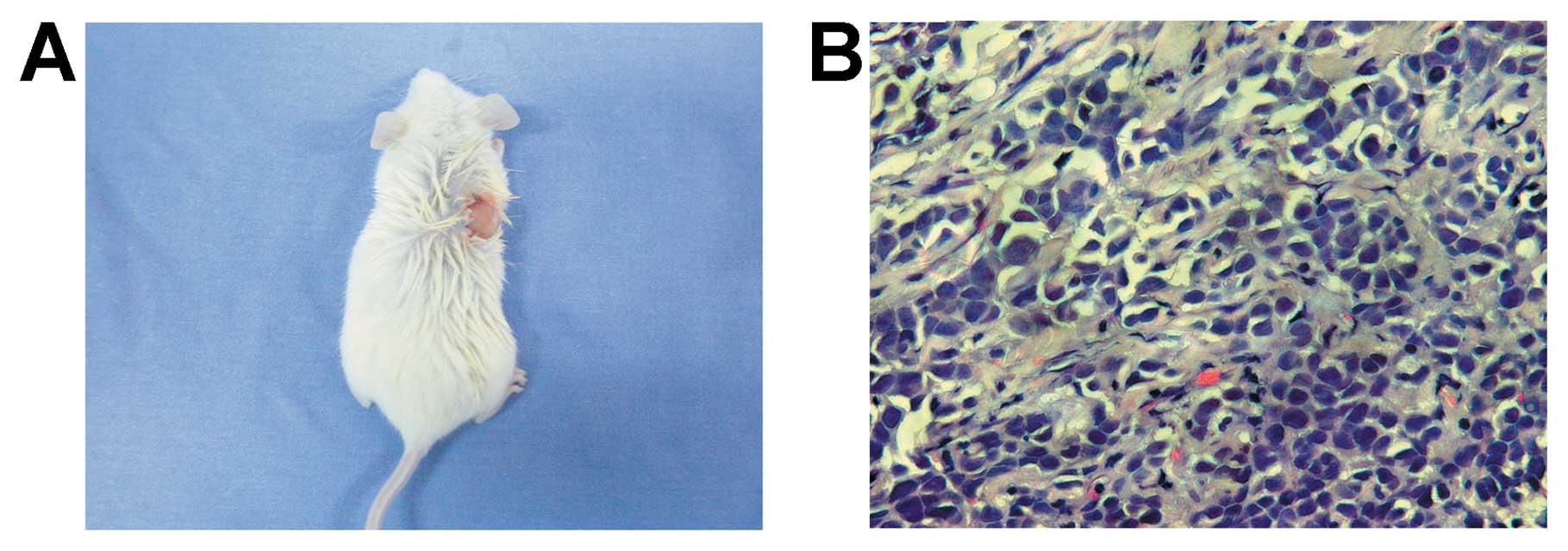
PANC-1 tumorspheres have higher
tumorigenic potential
High tumorigenic potential is one of the
characteristics of CSCs. To detect the tumorigenicity of PANC-1
tumorspheres, a concentration ranging from 200 to 2×104
tumorsphere cells were injected into the subcutaneous space of the
pad of NOD/SCID mice. After 11 weeks, 2×103 PANC-1
tumorsphere cells initiated a tumor in 1 of 3 NOD/SCID mice
(Fig. 3). Whereas, ≥105
PANC-1 cells initiated a tumor in NOD/SCID mice (Table I). The data imply that PANC-1
tumorsphere cells are more tumorigenic than its parental cell line.
Collectively, floating PANC-1 tumorspheres have potential of
self-renewal, differentiation and high tumori-genesis, which are
the characteritics of CSCs.
 | Table I.Tumor formation initiated by PANC-1
tumorsphere cells in NOD/SCID mice. |
Table I.
Tumor formation initiated by PANC-1
tumorsphere cells in NOD/SCID mice.
| Dilutions
(cells/ml)
|
|---|
| Cell type of
inoculation |
2×102 |
2×103 |
2×104 |
2×105 |
2×106 |
|---|
| Tumorsphere
cells | 0/3 | 1/3 | 3/3 | - | - |
| PANC-1 | - | - | 0/3 | 2/3 | 3/3 |
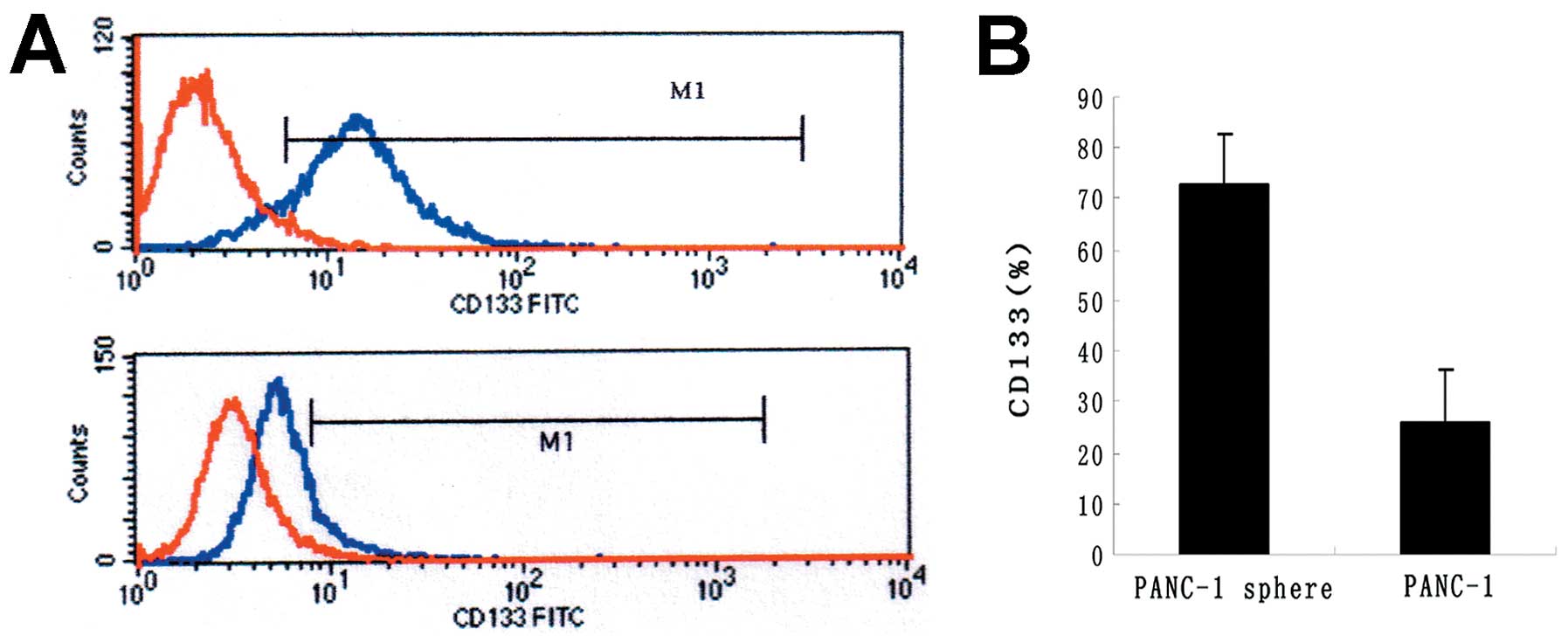
CD133 is highly expressed in PANC-1
tumorspheres
CD133 is well-known as stem cell marker in many
tumors. To detect the phenotypic difference between PANC-1
tumorspheres and PANC-1, FACS was carried out. The
CD133+ subfraction was dramatically increased in PANC-1
tumorspheres compared with PANC-1 (72.73±4.38 vs 26.16±2.13%)
(P<0.05) (Fig. 4).
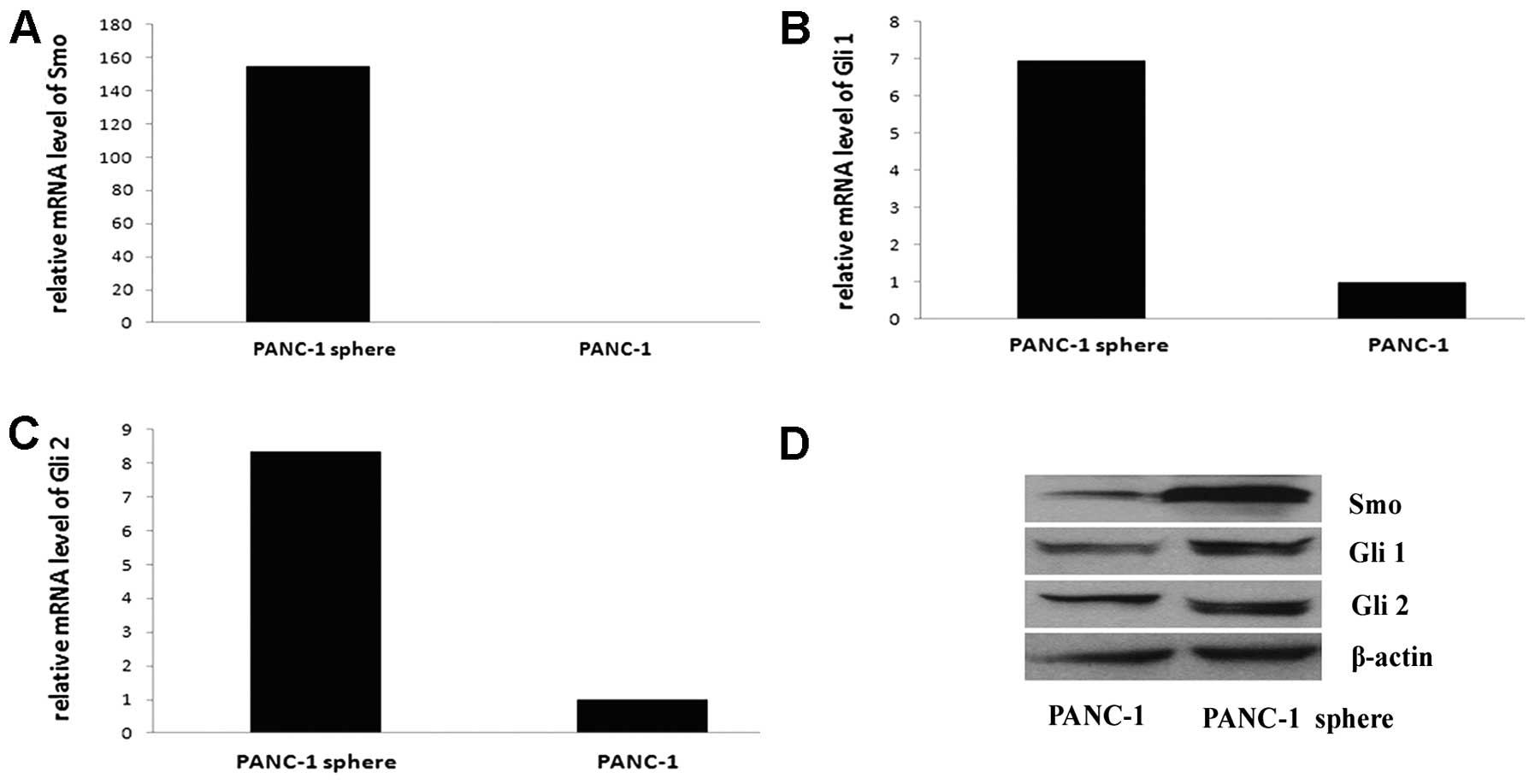
The Hh pathway is active in PANC-1
tumorspheres
Hh pathway is active in some CSCs, such as glioma
CSCs and breast CSCs. Also, Hh pathway is active in pancreatic
ductal adenocarcinoma. To evaluate the possibility that Hh
signaling is active in pancreatic CSCs, we examined the expression
of Smo, Gli 1 and Gli 2 mRNA and protein in PANC-1 tumorspheres and
PANC-1 cells by qRT-PCR and western blotting. Hh pathway is active
in both PANC-1 tumorspheres and PANC-1 cells, Smo, Gli 1 and Gli 2
mRNA are high expressed, especially in PANC-1 tumorspheres, the
expression of Smo mRNA is 154.76-fold as that of PANC-1 cells, the
expression of Gli 1 mRNA is 6.94-fold that of PANC-1 cells and Gli
2 is 8.36-fold. The results of western blot analysis are consistent
with the mRNA expression (Fig.
5).
Cyclopamine-mediated blockade of Hh
pathway inhibits proliferation of PANC-1 CSCs via Bmi-1
To detect the effects on inhibition of Hh pathway,
we used cyclopamine, a special inhibitor of Smo, to block the Hh
pathway. PANC-1 tumorsphere cells were treated in different
concentration of cyclopamine (0, 0.5, 1, 2, 5 and 10 μmol/l,
respectively) in triplicate for different times (24, 48 and 72 h).
Cyclopamine-mediated Hh pathway blockade inhibited the overall
growth rate of the culture in a dose-and time-dependent fashion.
After treatment with 10 μmol/l cyclopamine for 72 h, the inhibition
rate of PANC-1 tumorsphere cells was (37.85±13.69)% (P<0.05).
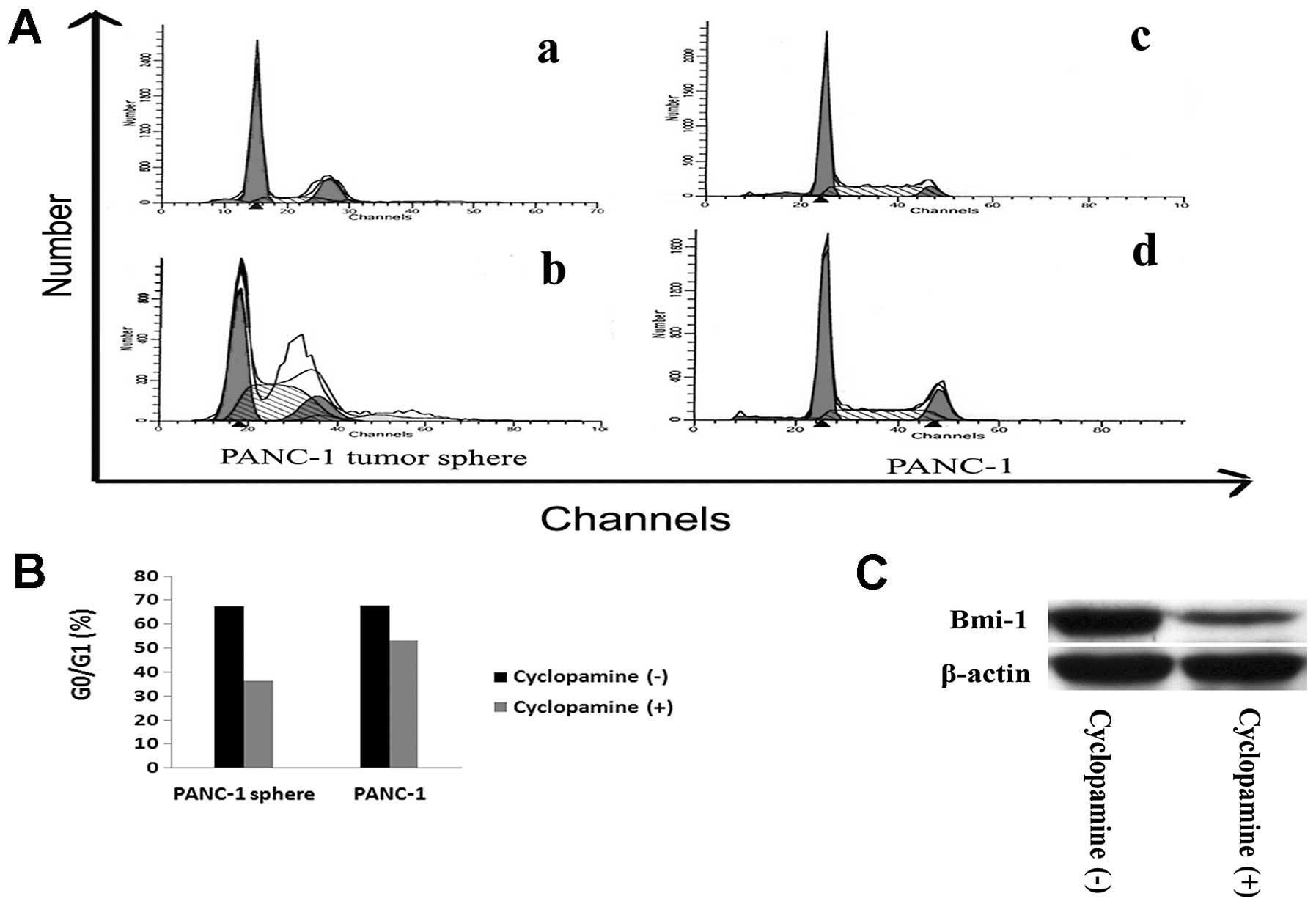
Furthermore, to investigate whether cell cycle of PANC-1
tumorsphere cells change after cyclopamine treatment, flow
cytometry was performed to detect the cell cycle of tumorspheres
with and without cyclopamine. After incubation with 10 μmol/l
cyclopamine for 72 h, percentage of G0/G1 of
PANC-1 tumorspheres was decreased (36.53±6.03)%, compared to
(67.41±6.35)% before incubation (P<0.05) (Fig. 6A and B). We hypothesized that Bmi-1
may function as a downstream target of the Hh pathway. To test the
hyposis, we examine the expression of Bmi-1 protein of PANC-1
tumor-spheres with and without cyclopamine incubation by western
blotting. After incubation with 10 μmol/l cyclopamine for 72 h,
expression of Bmi-1 protein was reduced (Fig. 6C).
Cyclopamine reverses chemoresistance of
PANC-1 tumor-spheres via ABCG2
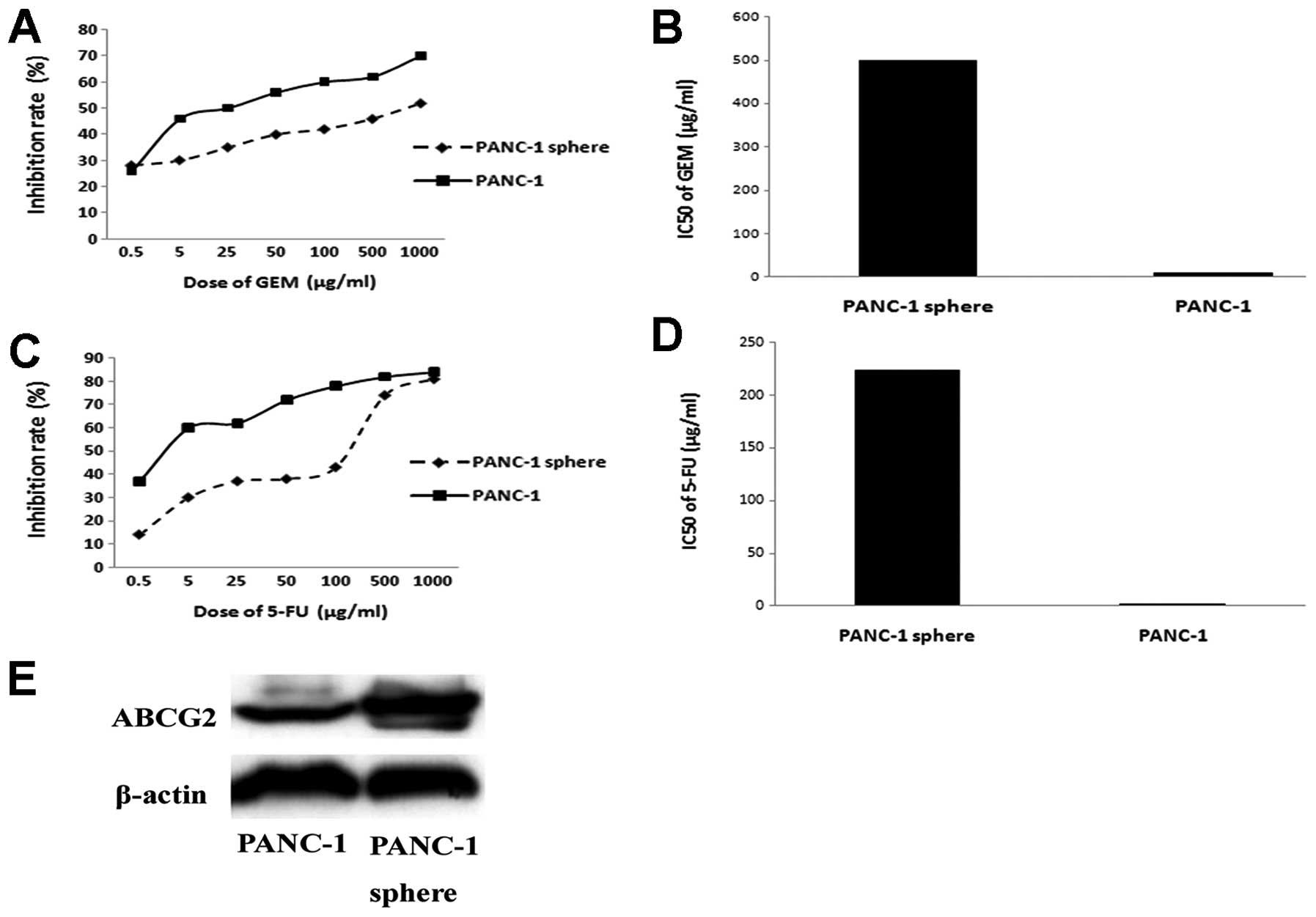
Drug sensitivity assay was carried out to detect the
chemotherapy resistance of PANC-1 tumorspheres and its parental
cell line PANC-1. PANC-1 tumorspheres are more resistant to
gemcitabine and 5-FU than PANC-1. IC50 of gemcitabine in
PANC-1 tumorspheres is 500.75±10.51 μg/ml, while PANC-1 is
11.43±2.10 μg/ml (P<0.05). IC50 of 5-FU in PANC-1
tumorspheres and PANC-1 is 224.37±5.71 μg/ml vs 2.19±0.32 μg/ml
(P<0.05). Expression of ABCG2 is detected by western blotting.
As shown, expression of ABCG2 in PANC-1 tumorspheres is much higher
than that of PANC-1 (Fig. 7).
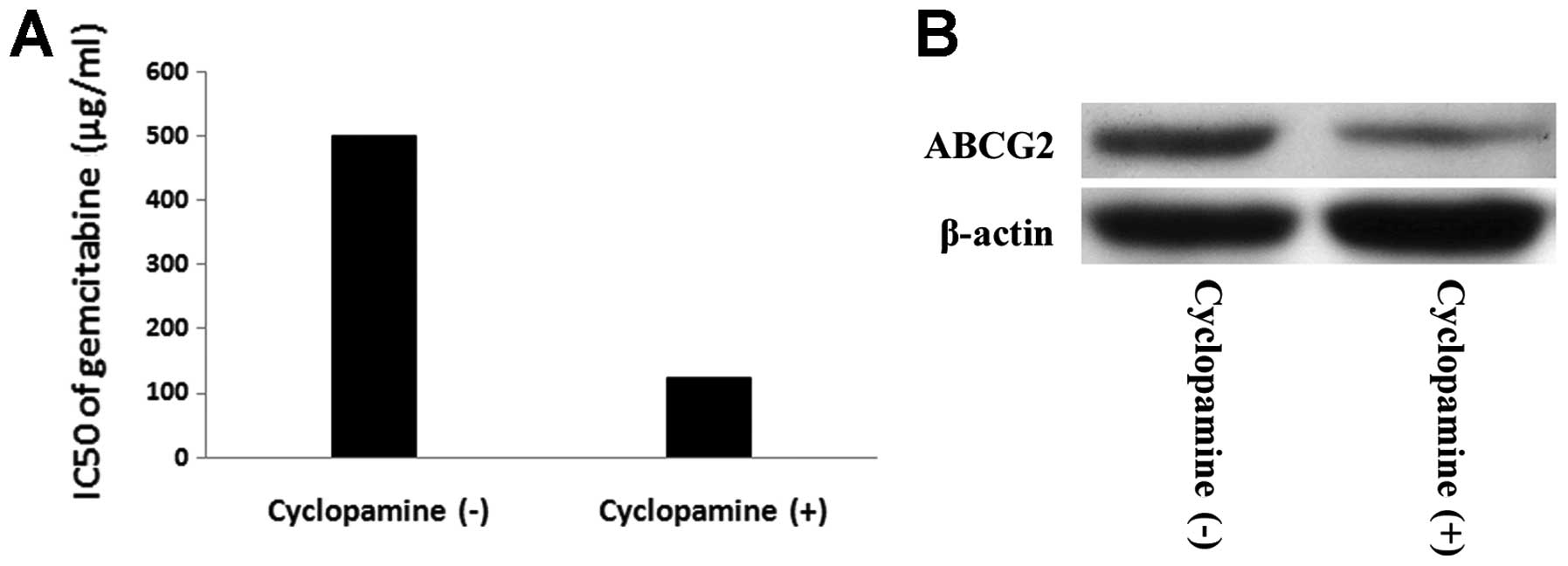
Moreover, we investigated whether inhibition of Hh pathway could
reverse chemoresistance of PANC-1 tumorspheres, and we used
cyclopamine to block the Hh pathway. PANC-1 tumor-sphere cells
preincubated with 10 μmol/l cyclopamine for 72 h were treated with
different concentration of gemcitabine (0, 0.5, 5, 25, 50, 100, 500
and 1000 μg/ml) for 72 h. IC50 of gemcitabine in PANC-1
tumorspheres with and without cyclopamine is 124.55±12.37 μg/ml vs
500.75±10.51 μg/ml (P<0.05). We hypothesized the expression of
ABCG2 in PANC-1 tumorsphere cells changed after cyclopamine
treatment. To consolidate this notion, western blot analysis was
performed to detect expression of ABCG2 protein of tumorspheres
with and withdrawl cyclopa-mine. PANC-1 tumorsphere after
incubation with cyclopamine displayed significantly reduced levels
of ABCG2 compared to tumorspheres without cyclopamine incubation
(P<0.05) (Fig. 8).
Discussion
Accumulating evidence has demonstrated that a tumor
is in essence heterogeneous and contains cancer stem cells which
possess the potential of self-renewal, multilineage
differentiation, high proliferation, and chemoresistance. More and
more studies support existance of tumor-initiating cells in
pancreatic adenocarcinoma (7,8).
Serum-free floating culture system has been used to identify CSCs
in several cancer types such as brain cancer, breast cancer and
colon cancer (24–26). The cells isolated using this system
were proved to have the stem cell features and represent the small
population in tumors which was the reason of tumor formation,
metastasis and resistance to chemotherapy. The advantage of this
strategy to enrich CSCs is its independence of specific cell
surface markers. In our study, we isolated PANC-1 tumor-spheres
under floating-culture system, and it could be serially propagated
in vitro over 20 passages. Sphere formation assay showed
that the number of spheres kept equivalent when being propagated
in vitro. Under differentiating conditions, PANC-1
tumorspheres were adherent and differentiated. They acquired
epithelial morphology and expressed mature marker CK18 associated
to luminal/ductal cells. More CK18 was expressed when the time of
differentiation was extended. Furthermore, as few as
2×103 PANC-1 sphere cells initiated a tumor in NOD/SCID
mice, a 100-fold enhanced tumorigenic potential compared to its
parental cell line, which is in accordance with Li et al
(7). Taken together, PANC-1
tumorspheres displayed potential of self-renewal, differentiation
and high tumorigenicity, which are the characteristics of cancer
stem cells.
Hedgehog signal pathway is active in pancreatic
cancer (27), also it is active in
many CSCs, such as prostate CSCs (28), and glioblastoma CSCs (20). Hedgehog has been associated with
the self-renewal process of CSCs (29). B lymphoma Mo-MLV insertion region 1
(Bmi-1), a transcriptional repressor belonging to the polycomb
group (PCG) of transcription factors, has been reported to play a
key role in regulating self-renewal of leukemic stem cell (30) and breast CSCs (23). It is reported that the Hh pathway
regulates self-renewal of human medulloblastoma brain
tumor-initiating cells via Bmi-1 (31). In our study, inhibition of Hh
pathway depressed self-renewal of the PANC-1 tumorsphere. To
illustrate whether Hh regulates PANC-1 tumorsphere via Bmi-1, we
examined the expression of Bmi-1 with and without
cyclopamine-mediated blockade of Hh. Expression of Bmi-1 was
reduced after blockade of the Hh pathway. Taken together, these
data suggested that Hedgehog pathway and Bmi-1 might be involved in
pancreatic tumorigenesis.
It is reported that CSCs are naturally
chemoresistant (32). ABCG2 with
drug capability are preferentially expressed in CSCs. Also, the
chemoresistant phenotype of CSCs can be mediated by ABCG2 protein.
Hh pathway was illustrated to have a relationship with
chemoresistance of pancreatic cancer stem cells (33), and ABCG2 is reported to be the
direct transcriptional target of Hh pathway in drug tolerance in
diffuse large B-cell lymphoma (34). In our study, we found that PANC-1
tumorspheres were more resistant to gemcitabine and 5-FU compared
to PANC-1. Expression of ABCG2 was significantly elevated in PANC-1
tumorspheres. Blockade of Hh pathway by cyclopamine reversed
chemoresistance of gemcitabine in PANC-1 tumorspheres. Moreover,
expression of ABCG2 was decreased after cyclopamine incubation.
Hence, it was implied in our study that inhibition of Hh pathway
reversed chemoresistance in PANC-1 tumorspheres via ABCG2.
In conclusion, our study illustrated that
tumorspheres derived from pancreatic cancer cell line PANC-1
possessed self-renewal, chemoresistance and other stemness
properties. Hh pathway was active in PANC-1 tumorspheres. More
importantly, our data suggested that inhibition of Hh pathway
depressed self-renewal of pancreatic CSCs via Bmi-1, which might be
involved in pancreatic tumorigenesis. Our study also demonstrated
the crucial role of Hh pathway of pancreatic CSCs in mediating
chemotherapy resistance associated with ABCG2.
Acknowledgements
We would like to thank Dr Jing Wei for
assistance with flow cytometry. This research was supported in part
by a grant from the Natural Science Foundation of Guangdong
Province (no. 815100890100013, 04009381) and Medical Scientific
Research Foundation of Guangdong Province (no. B2009066).
References
|
1.
|
Greenlee RT, Murray T, Bolden S and Wingo
PA: Cancer statistics. CA Cancer J Clin. 50:7–33. 2000.
|
|
2.
|
Wang L, Yang GH, Lu XH, Huang ZJ and Li H:
Pancreatic cancer mortality in China (1991-2000). World J
Gastroenterol. 9:1819–1823. 2003.
|
|
3.
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Beachy PA, Karhadkar SS and Berman DM:
Tissue repair and stem cell renewal in carcinogenesis. Nature.
432:324–331. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Singh SK, Hawkins C, Clarke ID, et al:
Identification of human brain tumour initiating cells. Nature.
432:281–282. 2004. View Article : Google Scholar
|
|
7.
|
Li CW, Heidt DG, Dalerba P, et al:
Identification of pancreatic cancer stem cells. Cancer Res.
67:1030–1037. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Hermann PC, Huber SL, Herrler T, et al:
Distinct populations of cancer stem cells determine tumor growth
and metastatic activity in human pancreatic cancer. Cell Stem Cell.
1:241–242. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Dontu G, Al-Hajj M, Abdallah WM, Clarke MF
and Wicha MS: Stem cells in normal breast development and breast
cancer. Cell Prolif. 36:59–72. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Wicha MS, Liu S and Dontu G: Cancer stem
cells: an old idea - a paradigm shift. Cancer Res. 66:1883–1890.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Berman DM, Karhadkar SS, Maitra A, et al:
Widespread requirement for hedgehog ligand stimulation in growth of
digestive tract tumors. Nature. 425:846–851. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Berman DM, Karhadkar SS, Hallahan AR, et
al: Medulloblastoma growth inhibition by hedgehog pathway blockade.
Science. 297:1559–1561. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Clement V, Sanchez P, de Tribolet N,
Radovanovic I and Ruiz i Altaba A: HEDGEHOG-GLI1 signaling
regulates human glioma growth, cancer stem cell self-renewal, and
tumorigenicity. Curr Biol. 17:165–172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Han ME, Lee YS, Baek SY, Kim BS, Kim JB
and Oh SO: Hedgehog signaling regulates the survival of gastric
cancer cells by regulating the expression of Bcl-2. Int J Mol Sci.
10:3033–3043. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Thayer SP, di Magliano MP, Heiser PW, et
al: Hedgehog is an early and late mediator of pancreatic cancer
tumorigenesis. Nature. 425:851–856. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Jones S, Zhang X, Parsons DW, et al: Core
signaling pathways in human pancreatic cancers revealed by global
genomic analyses. Science. 321:1801–1806. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Feldmann G, Habbe N, Dhara S, et al:
Hedgehog inhibition prolongs survival in a genetically engineered
mouse model of pancreatic cancer. Gut. 57:1420–1430. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Jimeno A, Feldmann G, Suárez-Gauthier A,
et al: A direct pancreatic cancer xenograft model as a platform for
cancer stem cell therapeutic development. Mol Cancer Ther.
8:310–314. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Liu S, Dontu G, Ilia D, Mantle ID, et al:
Hedgehog signaling and Bmi-1 regulate self-renewal of normal and
malignant human mammary stem cells. Cancer Res. 66:6063–6071. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Bar EE, Chaudhry A, Lin A, et al:
Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like
cancer cells in glioblastoma. Stem Cells. 25:2524–2533. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Shi Y, Fu X, Hua Y, Han Y, Lu Y and Wang
J: The side population in human lung cancer cell line NCI-H460 is
enriched in stem-like cancer cells. PLoS One. 7:e333582012.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Zhang L, Jiao M, Li L, et al: Tumorspheres
derived from prostate cancer cells possess chemoresistant and
cancer stem cell properties. J Cancer Res Clin Oncol. 138:675–686.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Park IK, Qian D, Kiel M, et al: Bmi-1 is
required for maintenance of adult self-renewing haematopoietic stem
cells. Nature. 423:302–305. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Inagaki A, Soeda A, Oka N, et al:
Long-term maintenance of brain tumor stem cell properties under at
non-adherent and adherent culture conditions. Biochem Biophys Res
Commun. 361:586–592. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Ponti D, Costa A, Zaffaroni N, et al:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Ricci-Vitiani L, Lombardi DG and Pilozzi
E: Identification and expansion of human colon-cancer-initiating
cells. Nature. 445:111–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Inaguma S, Kasai K and Ikeda H: GLI1
facilitates the migration and invasion of pancreatic cancer cells
through MUC5AC-mediated attenuation of E-cadherin. Oncogene.
30:714–723. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Chang HH, Chen BY, Wu CY, et al: Hedgehog
overexpression leads to the formation of prostate cancer stem cells
with meta-static property irrespective of androgen receptor
expression in the mouse model. J Biomed Sci. 18:62011. View Article : Google Scholar
|
|
29.
|
Takebe N, Harris PJ, Warren RQ and Ivy SP:
Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog
pathways. Nat Rev Clin Oncol. 8:97–106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Dimri GP, Martinez JL, Jacobs JJ, et al:
The Bmi-1 oncogene induces telomerase activity and immortalizes
human mammary epithelial cells. Cancer Res. 62:4736–4745.
2002.PubMed/NCBI
|
|
31.
|
Wang X, Venugopal C, Manoranjan B, et al:
Sonic hedgehog regulates Bmi1 in human medulloblastoma brain
tumor-initiating cells. Oncogene. 31:187–199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Rich JN and Bao S: Chemotherapy and cancer
stem cells. Cell Stem Cell. 1:353–355. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Yao J, An Y, Wie JS, et al: Cyclopamine
reverts acquired chemoresistance and down-regulates cancer stem
cell markers in pancreatic cancer cell lines. Swiss Med Wkly.
141:w132082011.PubMed/NCBI
|
|
34.
|
Singh RR, Kunkalla K, Qu C, Schlette E,
Neelapu SS, Samaniego F and Vega F: ABCG2 is a direct
transcriptional target of hedgehog signaling and involved in
stroma-induced drug tolerance in diffuse large B-cell lymphoma.
Oncogene. 30:4874–4886. 2011. View Article : Google Scholar : PubMed/NCBI
|