Introduction
Malignant pleural mesothelioma (MPM) is a rare and
highly aggressive neoplasm, which arises from the pleural,
pericardial, or peritoneal lining. Most patients with MPM have a
history of exposure to carcinogenic asbestos fibers (1,2),
particularly those of the amphibole type, or to naturally occurring
erionite in some regions of Turkey (3,4).
Although surgery, chemotherapy, radiotherapy, and combinations
thereof play an important role in the treatment of MPM patients,
the median survival of patients treated for MPM is dismal, at only
6–18 months (5–7). Despite advances in modern systemic
chemotherapy using the combination of pemetrexed and cisplatin,
long-term survival in patients with MPM remains limited (8). Therefore, more specific, effective,
and less toxic therapies are needed. Research into the molecular
pathways of MPM has led to novel targeted strategies that inhibit
specific key molecules in tumor growth and progression.
Epidermal growth factor receptor (EGFR) is a
tyrosinekinase (TK) receptor involved in cell death and
proliferation, cell motility, angiogenesis, and extracellular
matrix composition (9). EGFR is
overexpressed in many human malignancies, including lung, head and
neck, colorectal, and breast cancers, where it is variably
associated with patient prognosis (10,11).
EGFR is reported to be overexpressed in 44–97% of MPM patients, as
determined by various immunohistochemical studies with variability
in outcomes (12–15). In a recent study, Destro et
al demonstrated that both the immunohistochemical expression
and corresponding mRNA levels of EGFR were higher in tumor
specimens than in normal pleural samples (12). These data confirmed those of a
previous study suggesting that EGFR could play an important role in
the oncogenic phenotype of MPM disease (9).
Two types of EGFR inhibitors have been developed:
small molecule EGFR tyrosine kinase inhibitors (TKIs) (16,17)
and monoclonal antibodies directed against the extracellular domain
of EGFR (18–20). Gefitinib, a quinazoline derivative,
is the first TKI developed that specifically inhibits the
activation of EGFR TK through competitive binding to the
ATP-binding domain of the receptor. Gefitinib has been shown to be
effective in preclinical studies and clinical trials, and it
received approval for use in Japan in patients with advanced
non-small cell lung cancer refractory to chemotherapy in July 2002.
Subsequently, it has gained approval in over 30 countries,
including the United States. Gefitinib reduced the proliferation of
MPM cells by inhibiting the EGFR signaling pathway in
vitro(9); however, the
clinical study revealed that gefitinib was not active in MPM
patients (21). The same is true
of erlotinib (14). These
disappointing results for EGFR TK inhibitors have led to increased
interest in monoclonal antibodies directed against EGFR, because
these 2 classes of agents may have substantially different
mechanisms of action.
Cetuximab is a chimeric mouse-human antibody
directed against the extracellular domain of EGFR (22), thereby inhibiting the binding of
activating ligands to the receptor. Consequently, cetuximab
inhibits ligand-dependent activation of the EGFR and inhibits the
downstream pathways that cause cell cycle progression, cell growth,
and angiogenesis. In addition, the binding of cetuximab initiates
EGFR internalization and degradation that leads to signal
termination (23–25). In addition to these direct
inhibitory effects to EGFR signaling, cetuximab potentially
provokes immunologic antitumor effects called antibody-dependent
cellular cytotoxicity (ADCC). This effect takes place in the
presence of the host effector system, such as natural killer (NK)
cells, because cetuximab has a human IgG1 backbone. Recently, we
and others showed that this ADCC activity is crucial for the
antitumor effects of cetuximab (26–28).
Because this immunological mechanism is not activated by TKIs,
cetuximab is expected to have more potent antitumor activities
against MPM than TKIs, especially in vivo. However, no
published in vitro or in vivo studies have focused on
the effect of cetuximab against MPM cells, particularly with
respect to ADCC activity.
In the present study, we investigated the biologic
activity of cetuximab against a panel of MPM cells with respect to
ADCC activity and the survival effects of intrathoracic treatment
using an orthotopic implantation mouse model that reproduces the
clinical behavior and therapeutic responsiveness of MPM in
humans.
Materials and methods
Cell lines and cell culture
Five MPM cell lines (EHMES-1, MSTO-211H, H2052,
EHMES-10 and H28) and an epidermoid carcinoma cell line (A431) were
used in this study. MSTO-211H, H2052, H28 and A431 were purchased
from American Type Culture Collection (ATCC, Manassas, VA, USA).
The other lines (EHMES-1, EHMES-10) were established from the
pleural effusion of a patient with MPM at Ehime University (Ehime,
Japan). All cell lines were maintained in RPMI-1640 supplemented
with 10% FCS, 50 U/ml penicillin, 50 U/ml streptomycin and 2.05
mmol/l glutamine. The cells were incubated at 37°C in 5%
CO2.
Monoclonal antibody
Cetuximab was obtained from Bristol-Myers Squibb
(New York, NY, USA). Rituximab, used as a control antibody, was
obtained from Chugai Pharmaceutical (Tokyo, Japan). Anti-EGF
receptor antibody (clone 528) for flow cytometry was obtained from
Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-EGF receptor
antibody (clone 31G7) for immunohistochemical analysis was obtained
from Zymed (South San Francisco, CA, USA).
Flow cytometric analysis
Cell surface EGFR expression of MPM cell lines was
examined by flow cytometry (Becton-Dickinson, Franklin Lakes, NJ,
USA) using a monoclonal antibody (clone 528). To determine the
absolute number of antibody-binding sites per cell, we carried out
a quantitative flow cytometric analysis using Dako QIFIKIT
(DakoCytomation, Copenhagen, Denmark). Briefly, 1×104
cells were incubated for 1 h at 4°C with 0.4 μg of the primary
antibody or the isotype-control IgG2a antibody (Sigma-Aldrich, St.
Louis, MO, USA) in phosphate-buffered saline (PBS) containing 1%
bovine serum albumin (BSA) and 0.01% sodium azide. After washing
thrice with PBS, cells were incubated for 1 h with FITC-conjugated
anti-mouse IgG (DakoCytomation) at 4°C. Similar to samples labeled
with FITC-conjugated anti-mouse IgG from this kit, standard beads
coated with a known amount of mouse IgG molecules were labeled with
this secondary antibody. The labeled samples were washed thrice
with PBS and analyzed using FACScan flow cytometer (Becton
Dickinson). The number of antibody binding sites per cell was
calculated by comparing the mean fluorescent intensity (MFI) value
of the labeled cells with a calibration curve obtained by
regression analysis of the MFI values of the standard beads.
Growth inhibition assay
Cell viability was assessed using the
2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulphophenyl)2H-tetrazolium
monosodium salt (WST-8) assay (Dojindo, Kumamoto, Japan). Cells
were plated at 3×104 cells/well in triplicate in 96-well
plates in complete medium. Following an overnight incubation,
cetuximab (0-1,000 μg/ml) was added in varying concentrations and
incubated. After 72 h, WST-8 solution (Dojindo) was added to each
well, followed by incubation for 4 h at 37°C, and absorbance was
measured using a Model 680 microplate reader (Bio-Rad Laboratories,
Hercules, CA, USA) at test and reference wavelengths of 450 and 655
nm, respectively. Cell viability was calculated by dividing the
mean absorbance of wells containing treated cells by those of
control wells with untreated cells. The concentration of cetuximab
resulting in 50% growth inhibition (IC50) was
calculated. All experiments were done at least in triplicate and
repeated at least 3 times.
Isolation of peripheral blood mononuclear
cells (PBMCs) and interleukin-2 (IL-2) treatment
PBMCs were isolated from heparinized peripheral
blood by lymphocyte-separation-medium (MP Biomedicals, Irvine, CA,
USA) density gradient centrifugation. To investigate the effect of
IL-2 (Sigma-Aldrich) on ADCC activity, PBMCs (106
cells/ml) were pre-incubated at 37°C for up to 18 h before
cytotoxic assay in the presence of IL-2 (30 IU/ml) (29–31).
Blood samples were collected at Tottori University in accordance
with the Tottori University Review Board, and the healthy
individuals provided written informed consent.
Test for ADCC and NK activity
After the target MPM cells were labeled with 100 μCi
51Cr (PerkinElmer Life and Analytical Sciences, Boston,
MA, USA) for 60 min, target cells (104/well) and
effector cells at various effector:target (E/T) ratios were
co-incubated in 200 μl of DMEM or RPMI-1640 in a 96-well U-bottomed
plate in triplicate for 4 h at 37°C with 0.5 μg/ml of cetuximab
(Bristol-Myers Squibb) or control antibody, rituximab (Chugai
Pharmaceutical). Next, the amount of radioactivity in the
supernatant liquid was measured by a gamma counter. The percentage
of specific cytolysis was calculated as previously described
(27). ADCC activity was
calculated as the percentage of lysis in the presence of cetuximab
minus the percentage of lysis in the presence of control antibody
that is attributed to NK activity.
Immunohistochemical analysis
Paraffin-embedded cell blocks were prepared from
each MPM cell lines, which were fixed in 4% paraformaldehyde.
Tissue sections (3 μm) were de-waxed in xylene, rehydrated through
a graded series of ethanol solutions, rinsed in distilled water for
5 min, and then immersed in 0.6% hydrogen peroxide in methanol for
30 min to block endogenous peroxidase. For antigen retrieval, the
sections then were microwaved in 0.01 mol/l of sodium
citrate-buffered saline, pH 6.0, for 20 min at 92°C using a
Microwave Processor model MI-77 (Azumaya, Tokyo, Japan). After
rinsing in PBS for 5 min, the slides were pre-blocked with 10%
normal rabbit serum at room temperature for 20 min and incubated at
4°C overnight with the primary antibody, anti-EGF receptor antibody
(clone 31G7) (Zymed). The immunoreaction was visualized with
3.3′-diaminobenzidine and 100 μl of hydrogen peroxidase in 0.05 M
Tris-HCl buffer, pH 7.6. Finally, the slides were counterstained
with a 0.1% hematoxylin solution. The staining results were
measured semiquantitatively on a scale of 0, 1+, 2+ and 3+ as
follows: 0, no membranous staining in any of the cells; 1+, weak
intensity membranous and cytoplasmic staining of nearly equal
intensity; 2+, moderate to strong intensity staining predominantly
in the membranes; and 3+, strong intensity staining clearly
localized to the cell membranes. Representative examples of 0, 1+,
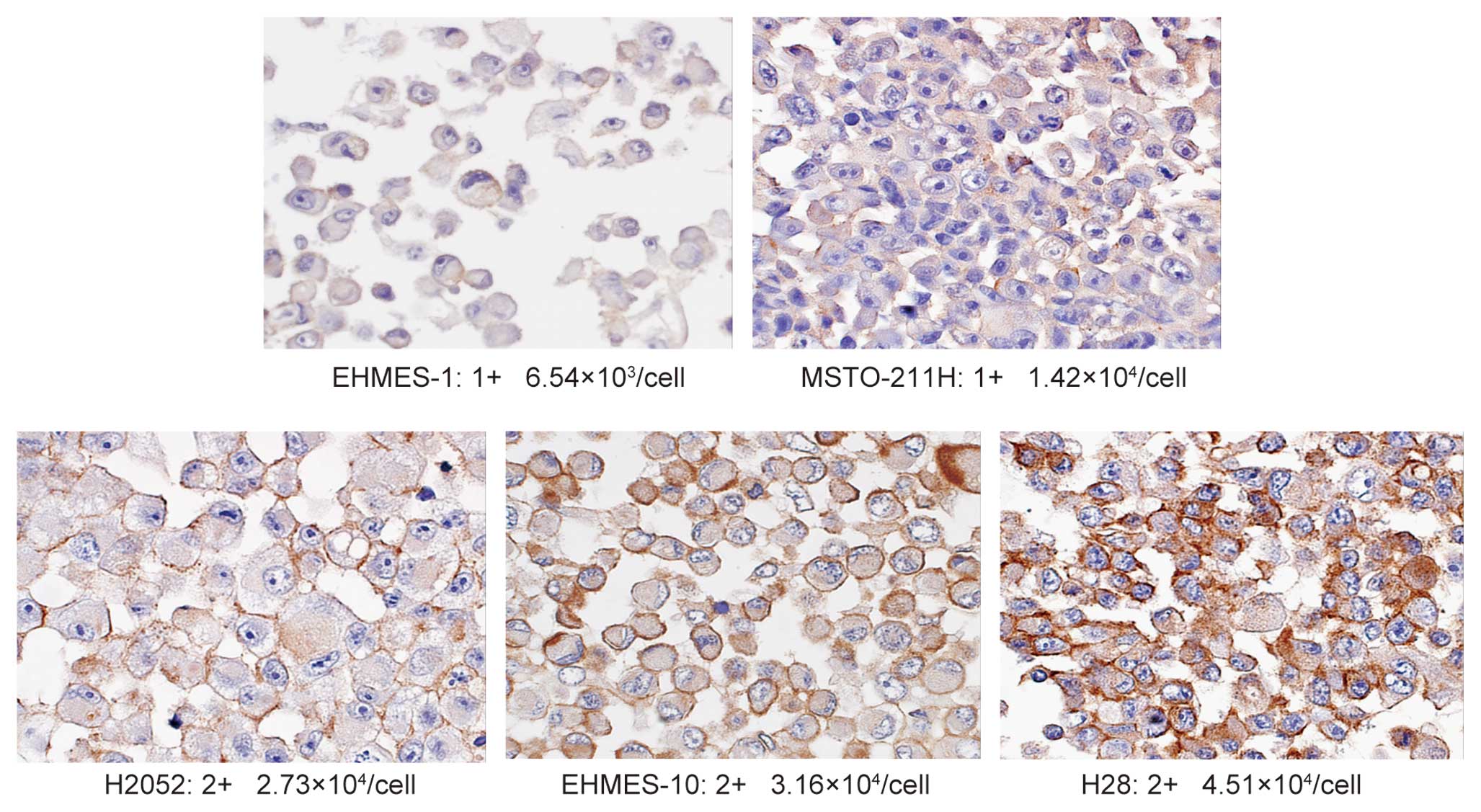
2+ and 3+ IHC staining for EGFR are demonstrated in Fig. 1. We performed the staining for the
each cell line 3 times, and the intensity was evaluated by 2
independent pathologists.
Animals
Male C.B-17 SCID mice (5 weeks) were obtained from
CLEA Japan (Osaka, Japan) and maintained under specific
pathogen-free conditions throughout the study. Experiments were
carried out in accordance with the guidelines established by the
Tottori University Committee on Animal Care and Use.
Orthotopic implantation model
The cultured MSTO-211H cells were harvested by
pipetting. The cells were washed 3 times and resuspended in
Ca2+- and Mg2+-free PBS. For orthotopic
implantation, SCID mice were anesthetized with ether and had their
right chest wall shaved. After sterilization of the chest wall with
70% ethanol, the right chest skin and subcutaneous tissue was cut,
and the parietal pleura was exposed. Thereafter, the tumor cells
(106/100 μl PBS) were injected into the thoracic cavity
of SCID mice using a 27G needle as described previously (32). Finally, the incisions were sutured
to close the wound. The mice were treated with cetuximab (0.05
mg/mouse i.t.) or in combination with IL-2 (30 IU/ml i.t.) using
the same methods on day 7, and sacrificed on day 21 to evaluate
tumor development. The pleura-disseminated tumors were inspected
macroscopically.
Area measurements
The intrathoracic tumor area was manually defined on
intrathoracic pictures, and was measured with the image analysis
software program Scion Image for Windows (PC version of NIH
Image).
Statistics
The statistical comparison between the 2 groups was
analyzed using Student’s t-test. The survival times of SCID mice
bearing MSTO-211H cells was determined using the Kaplan-Meier
estimation (PRISM for Windows; GraphPad Software, La Jolla, CA,
USA).
Results
Analysis of EGFR expression in MPM cell
lines using flow cytometry and IHC
We first examined the expression of EGFR in 5 MPM
cell lines. A431, an epidermoid carcinoma cell line, was used as a
positive control for EGFR expression in most studies, since it has
been reported to express high levels of EGFR (33,34).
We measured the number of EGFRs on each MPM cell line by
quantitative flow cytometric analysis (Dako QIFIKIT) (35) and compared them to the evaluation
by immunohistochemistry (IHC) (scored from 0 to 3+). As shown in
Table I, the level of EGFR
expression in each MPM cell line, in ascending order, is as
follows: EHMES-1, MSTO-211H, H2052, EHMES-10 and H28. As assessed
using IHC, 2 cell lines (EHMES-1 and MSTO-211H), which express a
low number of EGFRs (ranging from 6.54×103 to
1.42×104/cell), were stained and scored as 1+. The other
3 cell lines of MPM, which expressed moderate numbers of EGFR
(ranging from 2.73×104 to 4.51×104/cell),
were scored as 2+. The positive control cell line A431, expressing
a large number of EGFRs (3.51×106/cell) scored 3+ (data
not shown) (Fig. 1). These results
indicated a good correlation between the number of EGFR molecules
on the cells and their EGFR status as estimated by IHC.
 | Table I.EGFR expression analysis by
quantitative flow cytometry and IHC in malignant pleural
mesothelioma cell lines. |
Table I.
EGFR expression analysis by
quantitative flow cytometry and IHC in malignant pleural
mesothelioma cell lines.
| Cell lines | EGFR expression
(nos. of EGFR/cells) | Immunohistochemical
score |
|---|
| EHMES-1 |
6.54×103 | 1+ |
| MSTO-211H |
1.42×104 | 1+ |
| H2052 |
2.73×104 | 2+ |
| EHMES-10 |
3.16×104 | 2+ |
| H28 |
4.51×104 | 2+ |
Direct effects of cetuximab on growth
inhibition in MPM cells
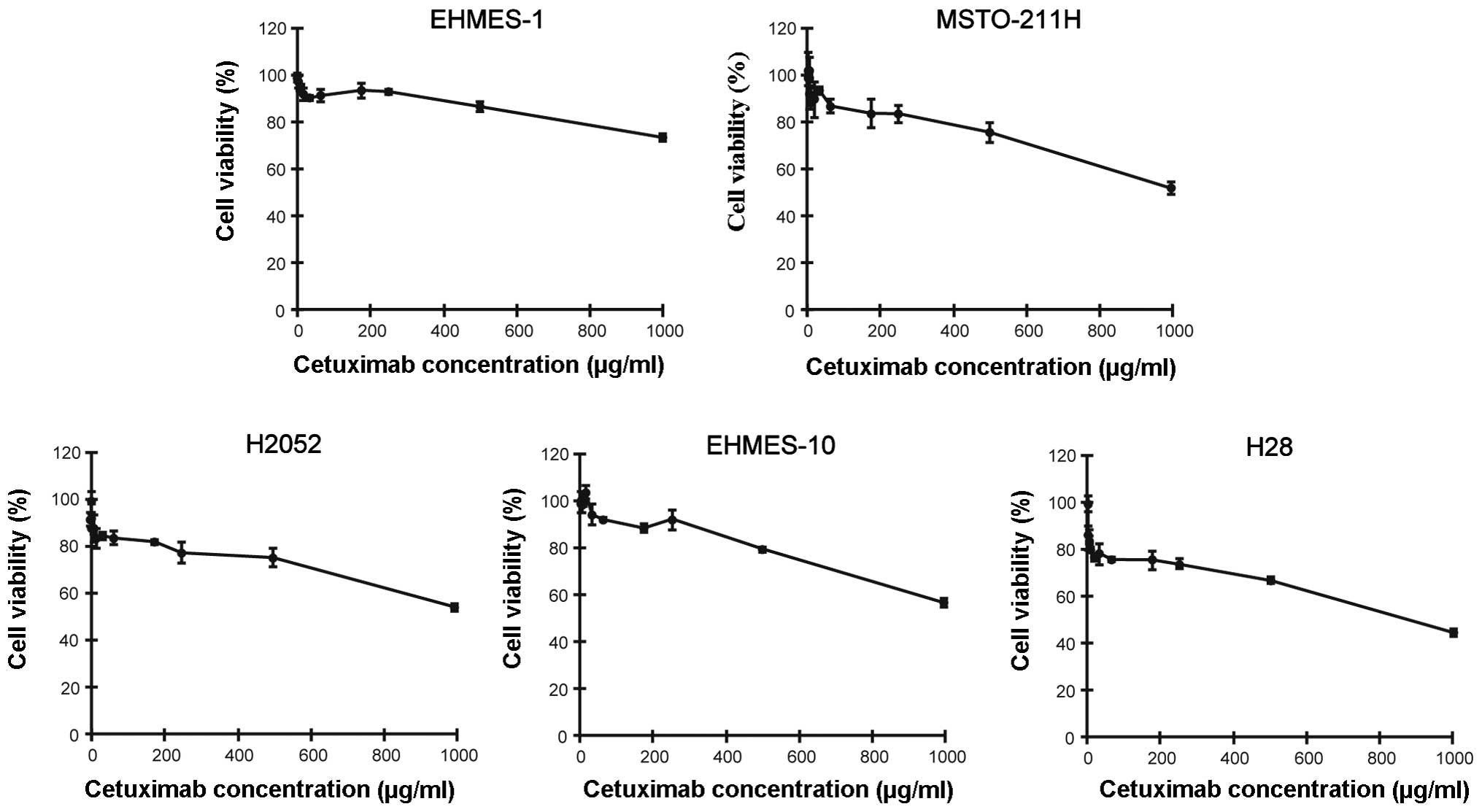
We next examined the effect of cetuximab against the
proliferation of MPM cells using the WST-8 assay, which is a
modified MTT assay. We found that all MPM cell lines were
completely resistant to cetuximab treatment irrespective of the
surface amount of EGFR (Fig. 2).
These data suggest that direct growth inhibitory effects would not
be expected in the anti-MPM action of cetuximab.
Cetuximab-mediated cytotoxicity against
MSTO-211H cells by healthy human PBMCs
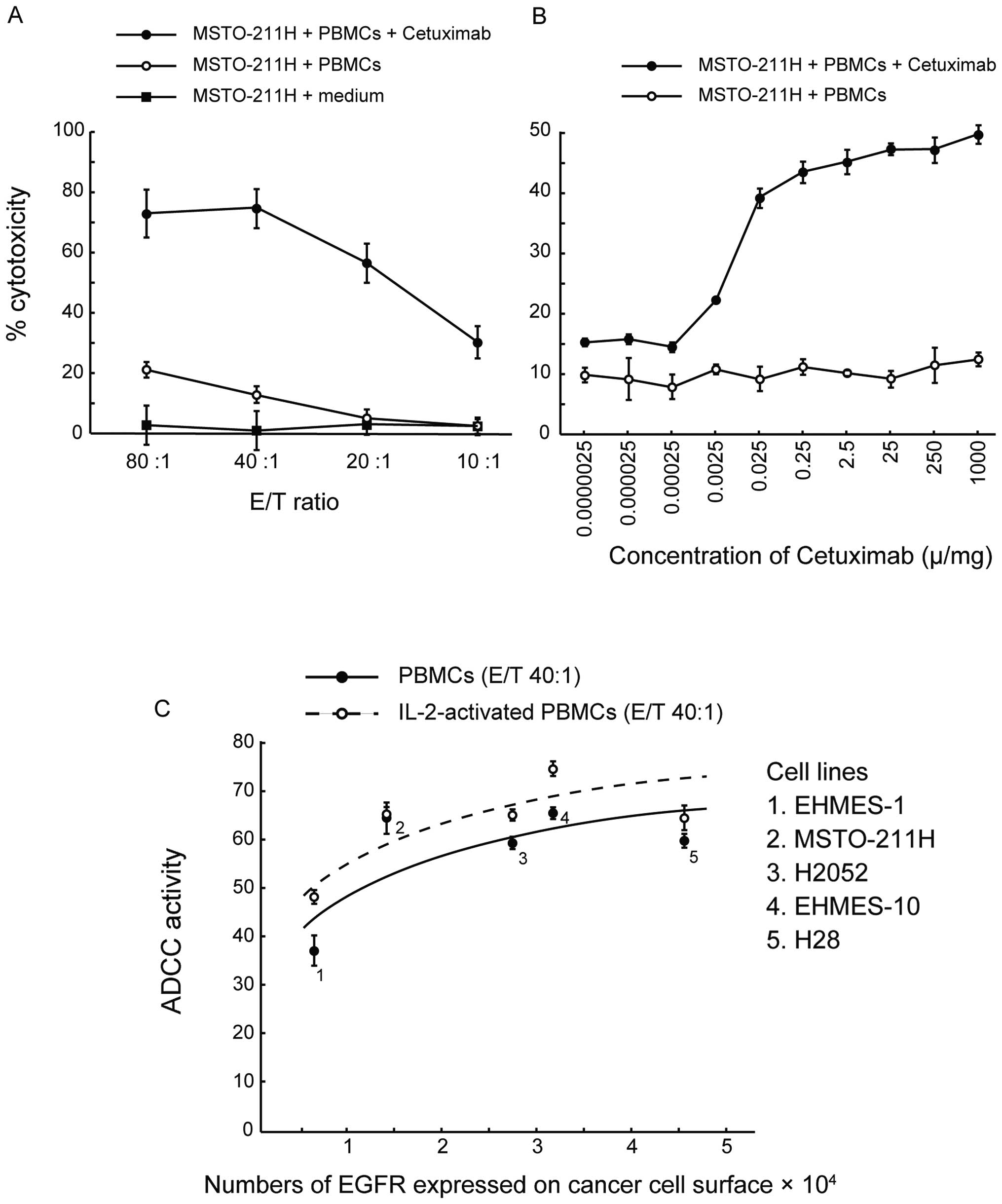
To test whether cetuximab induces ADCC activity
against MPM cell lines, we performed a 4-h 51Cr release
assay of MSTO-211H cells that weakly express EGFR using human PBMCs
at various E/T ratios (Fig. 3A).
While low levels of cytolysis of MSTO-211H cells were induced by
PBMCs at the higher E/T ratios of 80:1 and 40:1 in the absence of
cetuximab (known as NK activity), the lytic activity of PBMCs
increased significantly in the presence of cetuximab at both E/T
ratios. There was no significant increase in lytic activity in the
presence of the control antibody, rituximab (data not shown). These
data suggest that cetuximab was capable of inducing ADCC activity
efficiently, even against MPM cells that weakly express EGFR.
Next, to identify the optimal cetuximab
concentration for ADCC activity, we determined the ADCC activity
with increasing concentrations of cetuximab, ranging from
2.5×10−6 to 1,000 mg/ml at an E/T ratio of 20:1. As
shown in Fig. 3B,
cetuximab-mediated ADCC activity against MSTO-211H cells was
already detectable at a concentration of 2.5×10−3 mg/ml
and was saturated at 0.25 mg/ml. These data indicate that a
cetuximab concentration in excess of 0.25 mg/ml was sufficient for
maximum ADCC activity. We used this concentration of cetuximab for
the subsequent assays.
Cetuximab-mediated ADCC activity against
MPM cell lines with various EGFR expression levels
To evaluate the correlation between the ADCC
activity induced by cetuximab and EGFR expression levels on target
MPM cells, we determined the ADCC activity in MPM cell lines with
various EGFR expression levels at an E/T ratio of 40:1 in the
presence of the optimal dose of cetuximab (0.25 mg/ml). As shown in
Fig. 3C, the ADCC activity
correlated logarithmically with the number of EGFR molecules
expressed on the MPM cell surface. Near-maximum ADCC activity was
observed in MSTO-211H cells, which have small numbers of EGFRs and
scored 1+ by IHC. ADCC activity did not increase in cells with
higher EGFR expression.
In addition, as IL-2 is known to activate PBMCs, we
tested the effects of overnight treatment of PBMCs with IL-2 on
cetuximab-mediated ADCC activity. Low doses of IL-2 increased ADCC
activity in all cell lines, regardless of EGFR expression level
(Fig. 3C). These data suggest that
the very weak EGFR expression in MPM cells is enough to mediate
ADCC activity and that IL-2 is capable of enhancing this
activity.
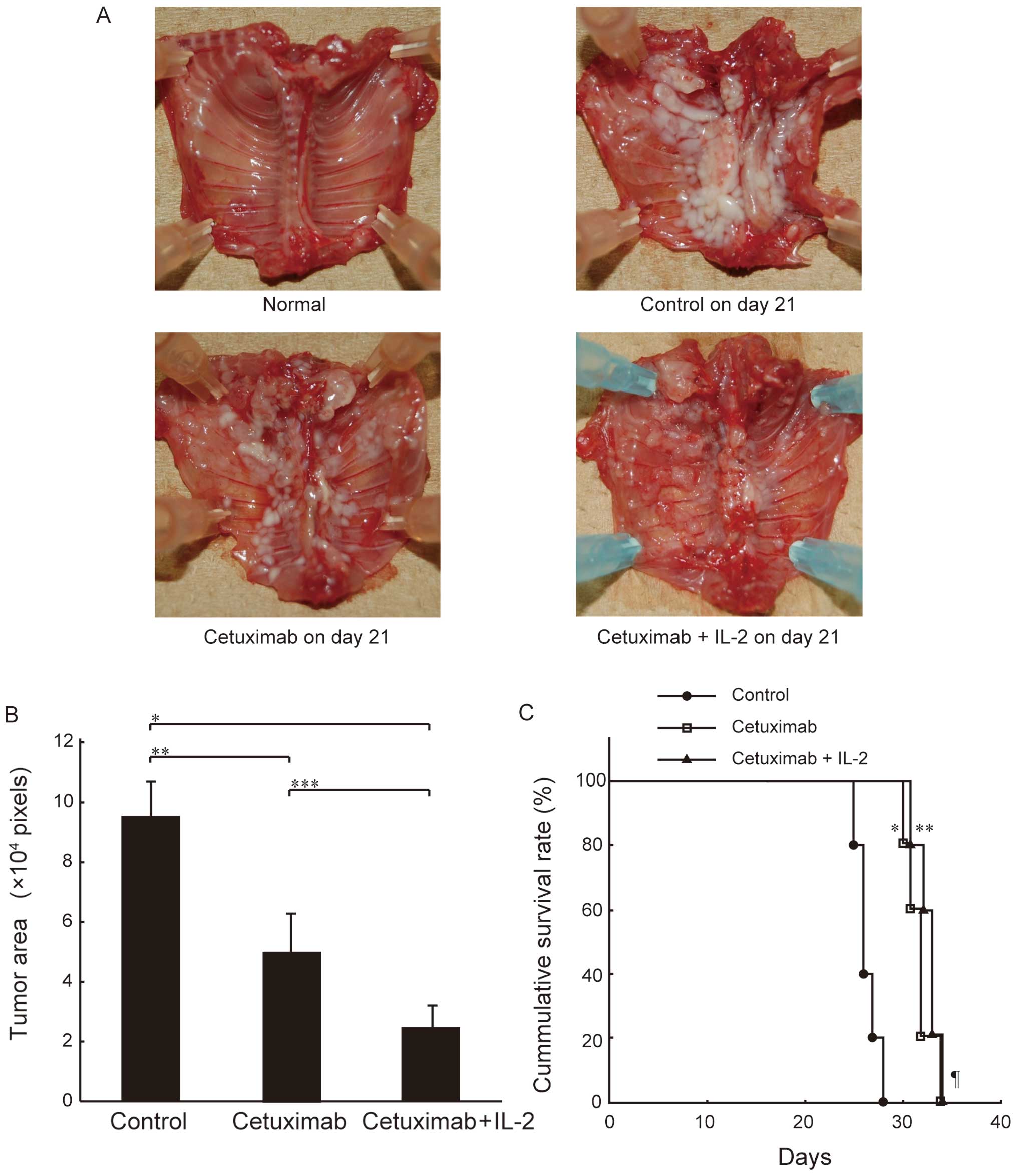
Effect of cetuximab and IL-2 on SCID mice
bearing MSTO-211H cells
To test the antitumor effects of cetuximab in
vivo, we used an orthotopic implantation mouse model as a
clinically relevant animal model. In this model, cells from the
mesothelioma cell line MSTO-211H were implanted into the thoracic
cavity of SCID mice, which possess robust NK cell activity
(36). The implanted mice were
treated with cetuximab by direct administration into the thoracic
cavity with and without IL-2 on day 7, and then sacrificed on day
21 as described in Materials and methods. To determine the optimal
dose of cetuximab for the treatment of SCID mice bearing MSTO-211H,
we first determined the survival times of the mice using various
amounts of cetuximab (0.5 mg/mouse, 0.05 mg/mouse and 0.005
mg/mouse i.t. on day 7). This preliminary experiment showed that
there was no statistically significant difference in survival time
between the mice (data not shown). In addition, in a separate study
that evaluated the pharmacokinetics of cetuximab in nude mice
bearing human colon carcinoma xenografts, the efficacious range for
antitumor activity was demonstrated to be 0.04-1 mg/mouse (37). We therefore administered cetuximab
at a dose of 0.05 mg/mouse in our subsequent in vivo
experiments to ensure biological activity yet minimize side
effects. As shown in Fig. 4A,
cetuximab inhibited intrathoracic mesothelioma growth in the mice,
and this inhibition was markedly enhanced by IL-2
co-administration. This inhibitory effect of cetuximab alone and
its enhancement by the addition of IL-2 was confirmed by
quantitative measurements of the tumor area using Scion Image
Software (Fig. 4B). Furthermore,
intrathoracic administration of cetuximab significantly prolonged
the survival of the mice, and the combination of cetuximab with
IL-2 tended to improve survival (Fig.
4C). These results suggest that cetuximab exerts antitumor
effects against MPM cells in the presence of the mouse effector
system, and that ADCC activity is highly involved in this
effect.
Discussion
In the present study, we evaluated cetuximab as a
novel molecular targeting agent for MPM. We found that cetuximab
induces potent ADCC activity but not growth inhibition against MPM
cell lines. Cetuximab-induced ADCC activity has several
characteristics that are relevant to clinical therapeutic
applications. First, low concentrations of cetuximab are sufficient
to induce maximum ADCC activity. Second, the low EGFR expression
levels on MPM cells, which are scored as 1+ by IHC, could be
sufficient for maximum ADCC activity mediated by cetuximab. Third,
ex vivo IL-2 treatment of PBMCs can enhance
cetuximab-mediated ADCC activity against MPM cell lines. Finally,
intrathoracic administration of cetuximab in an orthotopic
implantation mouse model significantly inhibited tumor growth and
prolonged the survival time in the presence of the mouse effector
system. These data indicate the important role of ADCC activity in
the mechanism of action of cetuximab against cancer cells and
underscore the promising potential of cetuximab as a new class of
therapeutic agent for use against MPM.
In this study, we examined the correlation between
the number of EGFRs on the cell surface and cetuximab-induced ADCC
activity in MPM cells and found that there is a logarithmic
relationship between them. This finding is in agreement with our
previous observations and those reported by others in relation to
other cancers. The ADCC activities of trastuzumab (38), anti-Ep-CAM antibody (39) and cetuximab (27) have been reported to weakly
correlate with the logarithm of the number of target cell surface
antigens in breast or lung cancer cells. The correlation observed
in this study indicates that low EGFR expression levels could be
sufficient for maximum ADCC activity of cetuximab against MPM cells
and that an increase in the expression level of EGFR has no obvious
effect on ADCC activity.
Our results indicate the possible usefulness of EGFR
IHC as a predictive marker of the effectiveness of
cetuximab-mediated ADCC activity against MPM cells. We demonstrated
that the demarcation point of the EGFR expression level to achieve
maximum ADCC activity is between EHMES-1 (6.54×103 EGFR
molecules/cell) and MSTO-211H (1.42×104 EGFR
molecules/cell), both of which are scored as 1+ by IHC. Therefore,
near-maximum ADCC activity could be expected as long as the cells
are stained by IHC, independent of the strength of the staining.
This feature could circumvent a common weak point of IHC, as the
semi-quantitative (40) nature of
IHC makes it prone to inter-observer scoring error. In addition,
IHC is superior to other methods for measuring EGFR levels in
clinical specimens, such as a ligand binding assay (41) and quantitative flow cytometry
(42), because it does not require
isolation of cells from fresh tissue or special equipment.
Therefore, IHC might be useful if it is scored simply as negative
or positive when assessing a tumor sample for predicting the
effectiveness of the ADCC activity of cetuximab.
We have demonstrated that cetuximab-mediated ADCC
activity against MPM cell lines is enhanced in response to IL-2.
This lymphokine is normally produced by T-lymphocytes and augments
the function of effector cells, such as B cells, NK cells, T cells
and monocytes (43). The
combination of IL-2 and a therapeutic monoclonal antibody has been
explored extensively in the case of rituximab (44,45)
and trastuzumab (46–49) and has been reported to enhance ADCC
activity in vitro(50) or
in vivo using mouse xenograft models (51). Based on these fundamental studies,
several preclinical trials of these combination therapies have been
conducted, including rituximab for B cell non-Hodgkin’s lymphoma
(52) and trastuzumab for
HER2-overexpressing cancer (53,54).
Therefore, our observation that IL-2 enhances cetuximab-meditated
ADCC activity against MPM cell lines might lend support to the
future concurrent use of cetuximab and IL-2 in patients with
MPM.
In our study, we showed that intrathoracic
administration of cetuximab significantly inhibited tumor growth
and prolonged the survival of mice. Several lines of evidences
suggest that the anti-MPM effects of cetuximab in vivo are
dependent on ADCC activity. First, murine spleen cells derived from
SCID mice have been reported to induce ADCC activity against
melanoma cells treated with cetuximab, though the effect is not as
potent as that by parental mouse monoclonal antibodies against EGFR
(26). In addition, several
reports have described that the mouse effector system can induce
the ADCC activity of human IgG1 antibody (55,56).
Taken together, it can be concluded that mouse effector cells can
bind to the Fc portion of human IgG to some extent, exerting some
level of ADCC activity, if not its full activity. Second, our
preliminary observation that there was no difference in survival
times between mice treated with different amounts of cetuximab is
strikingly similar to the dose-effector relationship of
cetuximab-induced ADCC activity shown in vitro; that is, a
cetuximab concentration in excess of 0.25 mg/ml is sufficient for
maximum ADCC activity in vitro, and higher concentrations
have no effect on the activity. Third, we used C.B-17 SCID mice,
which lack mature T- and B-lymphocytes but possess robust NK cell
activity (36). We have shown in a
previous report that only NK cells are major effectors of
cetuximab-mediated ADCC activity that is augmented by IL-2
(27). In parallel, IL-2
co-administration with cetuximab significantly inhibited MPM tumor
growth in our model. Considering these data, we believe that our
successful treatment of MPM in the SCID mouse model reflects
cetuximab-induced ADCC activity and that future efforts to enhance
this ADCC activity with effective adjuvants, such as cytokines
(57), would be of vital
importance.
This is the first study to investigate intrathoracic
treatment by cetuximab for MPM. Because mesothelioma tends to
remain localized in the pleural cavity for a long time, the
development of local treatments would be promising. For this
purpose, the orthotopic mouse model of MPM would be ideal for the
evaluation of cetuximab, because MPM cells mimic the clinical
behavior and progression of human MPM in this model (32). Local treatment with antitumor drugs
offers a theoretical advantage, because the tumor is exposed
directly to higher drug concentrations, while a lower incidence of
toxic side effect can be expected. To date, several local
treatments have been reported as successful in combination with
other therapeutic modalities, such as surgery. These local
treatments include intrathoracic chemotherapy (58), chemohyperthermia (59), and intraoperative photodynamic
therapy (IPDT) (60). Our proposed
combination therapy using cetuximab with IL-2 is preferable for the
local treatment of MPM, because IL-2 causes serious side effects
such as vascular leakage syndrome and systemic immuno-suppression
if administered systemically (61). Local treatment may not be expected
to cure MPM patients, but the improved local control of MPM in the
thoracic cavity in combination with systemic treatment could offer
potential benefits for MPM patients.
In this study, cetuximab treatment significantly
inhibited intrathoracic MPM tumor growth, and the addition of IL-2
enhanced this activity. However, contrary to our expectations, the
combined use of cetuximab and IL-2 did not improve survival of the
mice compared to cetuximab alone. There are 2 possible explanations
for this result. First, the number of mice we used in each group
(N=5) was not enough to detect the difference. Second, the effects
on tumor growth might not directly affect the survival of the mice
due to distant metastasis or the side effects of IL-2, such as
cardiac failure (62). Therefore,
further study is warranted to determine the cause of death in these
mice and to explore more effective and less toxic combination-use
of IL-2.
In conclusion, cetuximab induces ADCC activity
against EGFR-expressing MPM cell lines. Near-maximum ADCC activity
was observed in cells with very weak EGFR expression levels, which
was detectable as faint IHC staining. ADCC activity is enhanced at
any EGFR expression level in the presence of low doses of IL-2.
Intrathoracic administration of cetuximab in SCID mice bearing
MSTO-211H cells significantly inhibited tumor growth and prolonged
survival of the mice. These observations suggest the possible use
of cetuximab as a novel and effective therapeutic agent that could
be used in combination therapies for patients with MPM.
Acknowledgements
This study was supported by
Grant-in-Aid for Scientific Research (C) 21590994 (to H.C. and
E.S.) and 22590863 (to E.S. and H.C.) from the Ministry of
Education, Science and Culture, Sports, Science and Technology,
Japan.
References
|
1.
|
L GreillierP AstoulMesothelioma and
asbestos-related pleural
diseasesRespiration76115200810.1159/00012757718583923
|
|
2.
|
JC WagnerCA SleggsP MarchandDiffuse
pleural mesothelioma and asbestos exposure in the North Western
Cape ProvinceBr J Ind Med17260271196013782506
|
|
3.
|
P DumortierL CopluV de MaertelaerS EmriI
BarisP De VuystAssessment of environmental asbestos exposure in
Turkey by bronchoalveolar lavageAm J Respir Crit Care
Med15818151824199810.1164/ajrccm.158.6.97121199847273
|
|
4.
|
B BarisAU DemirV ShehuY KarakocaG
KisacikYI BarisEnvironmental fibrous zeolite (erionite) exposure
and malignant tumors other than mesotheliomaJ Environ Pathol
Toxicol Oncol1518318919969216804
|
|
5.
|
P RuffieR FeldS MinkinDiffuse malignant
mesothelioma of the pleura in Ontario and Quebec: a retrospective
study of 332 patientsJ Clin Oncol71157116819892666592
|
|
6.
|
HI PassK KrandaBK TemeckI FeuersteinSM
SteinbergSurgically debulked malignant pleural mesothelioma:
results and prognostic factorsAnn Surg
Oncol4215222199710.1007/BF023066139142382
|
|
7.
|
VW RuschS PiantadosiEC HolmesThe role of
extra-pleural pneumonectomy in malignant pleural mesothelioma. A
Lung Cancer Study Group trialJ Thorac Cardiovasc
Surg1021919912072706
|
|
8.
|
NJ VogelzangJJ RusthovenJ SymanowskiPhase
III study of pemetrexed in combination with cisplatin versus
cisplatin alone in patients with malignant pleural mesotheliomaJ
Clin Oncol2126362644200310.1200/JCO.2003.11.13612860938
|
|
9.
|
PA JanneML TaffaroR SalgiaBE
JohnsonInhibition of epidermal growth factor receptor signaling in
malignant pleural mesotheliomaCancer Res6252425247200212234991
|
|
10.
|
CL ArteagaEpidermal growth factor receptor
dependence in human tumors: more than just
expression?Oncologist7Suppl
43139200210.1634/theoncologist.7-suppl_4-3112202786
|
|
11.
|
J BrabenderKD DanenbergR MetzgerEpidermal
growth factor receptor and HER2-neu mRNA expression in non-small
cell lung cancer is correlated with survivalClin Cancer
Res718501855200111448895
|
|
12.
|
A DestroGL CeresoliM FalleniEGFR
overexpression in malignant pleural mesothelioma. An
immunohistochemical and molecular study with clinico-pathological
correlationsLung
Cancer51207215200610.1016/j.lungcan.2005.10.01616384623
|
|
13.
|
K OkudaH SasakiO KawanoEpidermal growth
factor receptor gene mutation, amplification and protein expression
in malignant pleural mesotheliomaJ Cancer Res Clin
Oncol13411051111200810.1007/s00432-008-0384-418392851
|
|
14.
|
LL GarlandC RankinDR GandaraPhase II study
of erlotinib in patients with malignant pleural mesothelioma: a
Southwest Oncology Group StudyJ Clin
Oncol2524062413200710.1200/JCO.2006.09.763417557954
|
|
15.
|
V AgarwalMJ LindL CawkwellTargeted
epidermal growth factor receptor therapy in malignant pleural
mesothelioma: where do we stand?Cancer Treat
Rev37533542201010.1016/j.ctrv.2010.11.004
|
|
16.
|
ES KimV HirshT MokGefitinib versus
docetaxel in previously treated non-small-cell lung cancer
(INTEREST): a randomised phase III
trialLancet37218091818200810.1016/S0140-6736(08)61758-419027483
|
|
17.
|
FA ShepherdJ Rodrigues PereiraT
CiuleanuErlotinib in previously treated non-small-cell lung cancerN
Engl J Med353123132200510.1056/NEJMoa05075316014882
|
|
18.
|
J AlbanellJ Codony-ServatF RojoActivated
extracellular signal-regulated kinases: association with epidermal
growth factor receptor/transforming growth factor alpha expression
in head and neck squamous carcinoma and inhibition by
anti-epidermal growth factor receptor treatmentsCancer
Res61650065102001
|
|
19.
|
NI GoldsteinM PrewettK ZuklysP RockwellJ
MendelsohnBiological efficacy of a chimeric antibody to the
epidermal growth factor receptor in a human tumor xenograft
modelClin Cancer Res11311131819959815926
|
|
20.
|
DJ JonkerCJ O’CallaghanCS
KarapetisCetuximab for the treatment of colorectal cancerN Engl J
Med35720402048200710.1056/NEJMoa07183418003960
|
|
21.
|
R GovindanRA KratzkeJE Herndon IIGefitinib
in patients with malignant mesothelioma: a phase II study by the
Cancer and Leukemia Group BClin Cancer
Res1123002304200510.1158/1078-0432.CCR-04-194015788680
|
|
22.
|
S LiKR SchmitzPD JeffreyJJ WiltziusP
KussieKM FergusonStructural basis for inhibition of the epidermal
growth factor receptor by cetuximabCancer
Cell7301311200510.1016/j.ccr.2005.03.00315837620
|
|
23.
|
JD SatoT KawamotoAD LeJ MendelsohnJ
PolikoffGH SatoBiological effects in vitro of monoclonal antibodies
to human epidermal growth factor receptorsMol Biol
Med151152919836094961
|
|
24.
|
GN GillT KawamotoC CochetMonoclonal
anti-epidermal growth factor receptor antibodies which are
inhibitors of epidermal growth factor binding and antagonists of
epidermal growth factor binding and antagonists of epidermal growth
factor-stimulated tyrosine protein kinase activityJ Biol
Chem259775577601984
|
|
25.
|
T KawamotoJD SatoA LeJ PolikoffGH SatoJ
MendelsohnGrowth stimulation of A431 cells by epidermal growth
factor: identification of high-affinity receptors for epidermal
growth factor by an anti-receptor monoclonal antibodyProc Natl Acad
Sci USA8013371341198310.1073/pnas.80.5.1337
|
|
26.
|
M NaramuraSD GilliesJ MendelsohnRA
ReisfeldBM MuellerTherapeutic potential of chimeric and murine
anti-(epidermal growth factor receptor) antibodies in a metastasis
model for human melanomaCancer Immunol
Immunother37343349199310.1007/BF015184588402738
|
|
27.
|
J KuraiH ChikumiK
HashimotoAntibody-dependent cellular cytotoxicity mediated by
cetuximab against lung cancer cell linesClin Cancer
Res1315521561200710.1158/1078-0432.CCR-06-172617332301
|
|
28.
|
H KimuraK SakaiT AraoT ShimoyamaT TamuraK
NishioAntibody-dependent cellular cytotoxicity of cetuximab against
tumor cells with wild-type or mutant epidermal growth factor
receptorCancer
Sci9812751280200710.1111/j.1349-7006.2007.00510.x17498200
|
|
29.
|
CS HenneyK KuribayashiDE KernS
GillisInterleukin-2 augments natural killer cell
activityNature291335338198110.1038/291335a06164929
|
|
30.
|
Z LiuFT LeeN HanaiCytokine enhancement of
in vitro antibody-dependent cellular cytotoxicity mediated by
chimeric anti-GD3 monoclonal antibody KM871Cancer
Immun213200212747758
|
|
31.
|
QH NguyenRL RobertsBJ AnkSJ LinCK LauER
StiehmEnhancement of antibody-dependent cellular cytotoxicity of
neonatal cells by interleukin-2 (IL-2) and IL-12Clin Diagn Lab
Immunol59810419989455889
|
|
32.
|
E NakatakiS YanoY MatsumoriNovel
orthotopic implantation model of human malignant pleural
mesothelioma (EHMES-10 cells) highly expressing vascular
endothelial growth factor and its receptorCancer
Sci97183191200610.1111/j.1349-7006.2006.00163.x
|
|
33.
|
GJ TodaroJE De LarcoGrowth factors
produced by sarcoma virus-transformed cellsCancer
Res38414741541978212188
|
|
34.
|
CJ WikstrandRE McLendonAH FriedmanDD
BignerCell surface localization and density of the tumor-associated
variant of the epidermal growth factor receptor, EGFRvIIICancer
Res574130414019979307304
|
|
35.
|
G BrockhoffF HofstaedterR KnuechelFlow
cytometric detection and quantitation of the epidermal growth
factor receptor in comparison to Scatchard analysis in human
bladder carcinoma cell
linesCytometry177583199410.1002/cyto.9901701108001460
|
|
36.
|
K DorshkindSB PollackMJ BosmaRA
PhillipsNatural killer (NK) cells are present in mice with severe
combined immunodeficiency (scid)J Immunol1343798380119853989296
|
|
37.
|
FR LuoZ YangH DongCorrelation of
pharmacokinetics with the antitumor activity of Cetuximab in nude
mice bearing the GEO human colon carcinoma xenograftCancer
Chemother
Pharmacol56455464200510.1007/s00280-005-1022-315947929
|
|
38.
|
R NiwaM SakuradaY KobayashiEnhanced
natural killer cell binding and activation by low-fucose IgG1
antibody results in potent antibody-dependent cellular cytotoxicity
induction at lower antigen densityClin Cancer
Res1123272336200510.1158/1078-0432.CCR-04-2263
|
|
39.
|
N PrangS PreithnerK BrischweinCellular and
complement-dependent cytotoxicity of Ep-CAM-specific monoclonal
antibody MT201 against breast cancer cell linesBr J
Cancer92342349200515655555
|
|
40.
|
A RalletMJ FarouxS TheobaldEpidermal
growth factor receptors in breast cancer: comparison of radioligand
and immunocytochemical assaysAnticancer
Res141417142119948067716
|
|
41.
|
JG KlijnPM BernsPI SchmitzJA FoekensThe
clinical significance of epidermal growth factor receptor (EGF-R)
in human breast cancer: a review on 5232 patientsEndocr
Rev1331719921313356
|
|
42.
|
R KimmigD PfeifferH LandsmannH
HeppQuantitative determination of the epidermal growth factor
receptor in cervical cancer and normal cervical epithelium by
2-color flow cytometry: evidence for down-regulation in cervical
cancerInt J
Cancer74365373199710.1002/(SICI)1097-0215(19970822)74:4%3C365::AID-IJC1%3E3.0.CO;2-T
|
|
43.
|
KA SmithInterleukin-2: inception, impact,
and
implicationsScience24011691176198810.1126/science.31318763131876
|
|
44.
|
E HooijbergJJ SeinPC van den
BerkEradication of large human B cell tumors in nude mice with
unconjugated CD20 monoclonal antibodies and interleukin 2Cancer
Res552627263419957540106
|
|
45.
|
J GolayM ManganiniV
FacchinettiRituximab-mediated antibody-dependent cellular
cytotoxicity against neoplastic B cells is stimulated strongly by
interleukin-2Haematologica8810021012200312969808
|
|
46.
|
M KuboT MorisakiH KurokiCombination of
adoptive immunotherapy with Herceptin for patients with
HER2-expressing breast cancerAnticancer
Res2344434449200314666732
|
|
47.
|
WE CarsonR PariharMJ
LindemannInterleukin-2 enhances the natural killer cell response to
Herceptin-coated Her2/neu-positive breast cancer cellsEur J
Immunol3130163025200110.1002/1521-4141(2001010)31:10%3C3016::AID-IMMU3016%3E3.0.CO;2-J11592078
|
|
48.
|
K KonoA TakahashiF IchiharaH SugaiH FujiiY
MatsumotoImpaired antibody-dependent cellular cytotoxicity mediated
by herceptin in patients with gastric cancerCancer
Res6258135817200212384543
|
|
49.
|
AD SantinS BelloneM GokdenOverexpression
of HER-2/neu in uterine serous papillary cancerClin Cancer
Res812711279200212006548
|
|
50.
|
N BerinsteinR LevyTreatment of a murine B
cell lymphoma with monoclonal antibodies and IL-2J
Immunol13997197619873496394
|
|
51.
|
WM VuistF v BuitenenMA de RieA HekmanP
RumkeCJ MeliefPotentiation by interleukin 2 of Burkitt’s lymphoma
therapy with anti-pan B (anti-CD19) monoclonal antibodies in a
mouse xenotransplantation modelCancer Res49378337881989
|
|
52.
|
WL GluckD HurstA YuenPhase I studies of
interleukin (IL)-2 and rituximab in B-cell non-Hodgkin’s lymphoma:
IL-2 mediated natural killer cell expansion correlations with
clinical responseClin Cancer Res10225322642004
|
|
53.
|
GF FlemingNJ MeropolGL RosnerA phase I
trial of escalating doses of trastuzumab combined with daily
subcutaneous interleukin 2: report of cancer and leukemia group B
9661Clin Cancer Res837183727200212473581
|
|
54.
|
T RepkaEG ChioreanJ GayTrastuzumab and
inter-leukin-2 in HER2-positive metastatic breast cancer: a pilot
studyClin Cancer Res924402446200312855616
|
|
55.
|
M SuzukiM Kato-NakanoS KawamotoTherapeutic
antitumor efficacy of monoclonal antibody against Claudin-4 for
pancreatic and ovarian cancersCancer
Sci10016231630200910.1111/j.1349-7006.2009.01239.x19555390
|
|
56.
|
DE Lopes de MenezesK Denis-MizeY
TangRecombinant interleukin-2 significantly augments activity of
rituximab in human tumor xenograft models of B-cell non-Hodgkin
lymphomaJ Immunother306474200717198084
|
|
57.
|
JM RodaT JoshiJP ButcharThe activation of
natural killer cell effector functions by cetuximab-coated,
epidermal growth factor receptor positive tumor cells is enhanced
by cytokinesClin Cancer
Res1364196428200710.1158/1078-0432.CCR-07-086517962339
|
|
58.
|
T AzizA JilaihawiD PrakashThe management
of malignant pleural mesothelioma; single centre experience in 10
yearsEur J Cardiothorac Surg22298305200212142203
|
|
59.
|
O MonneuseAC BeaujardB GuibertLong-term
results of intrathoracic chemohyperthermia (ITCH) for the treatment
of pleural malignanciesBr J
Cancer8818391843200310.1038/sj.bjc.660100012799624
|
|
60.
|
H SchouwinkET RutgersJ van der
SijpIntraoperative photodynamic therapy after pleuropneumonectomy
in patients with malignant pleural mesothelioma: dose finding and
toxicity resultsChest12011671174200110.1378/chest.120.4.1167
|
|
61.
|
W Den OtterJJ JacobsJJ BattermannLocal
therapy of cancer with free IL-2Cancer Immunol
Immunother57931950200818256831
|
|
62.
|
B CastagnetoS ZaiL MuttiPalliative and
therapeutic activity of IL-2 immunotherapy in unresectable
malignant pleural mesothelioma with pleural effusion: results of a
phase II study on 31 consecutive patientsLung
Cancer31303310200110.1016/S0169-5002(00)00192-6
|