Introduction
Pancreatic cancer is a very aggressive disease with
a median survival of 6 months and a dismal 5-year survival rate of
5% (1–3). Surgical resection remains the only
curative option for pancreatic cancer (4–6).
However, only 15–20% of patients undergo potentially curative
surgery, as the majority of cases are unresectable at the time of
diagnosis due to local major vessel invasion or remote metastases
(7). Moreover, the median survival
rate for those with clean microscopic surgical margins is
approximately 2 years, with a 5-year survival of 15–20% (8). The malignancy of pancreatic cancer is
strongly associated with its local recurrences and systemic
metastases, even after curative resection for early stage
pancreatic cancer (7,9,10).
As one of the most important routes for recurrence
and metastases in pancreatic cancer, lymphatic metastases
significantly correlate with poor prognosis (6,11–13).
The number of metastatic lymph nodes and the ratio of metastatic
lymph nodes to total number of examined lymph nodes have both been
reported to significantly correlate with survival after surgical
resection of pancreatic cancer (12,14).
Lymphatic metastases also play important roles in systemic
dissemination of cancer cells, as tumor cells in lymph nodes may
provide a reservoir of cells leading to distant, lethal metastases
(15–17). Moreover, the lymph node may also
promote metastasis formation in distant organs by lymphatic-venous
communications (18). However, the
mechanisms of lymphatic metastasis in pancreatic cancer have not
been fully studied for the lack of animal models that faithfully
replicate the process of lymphatic metastasis in humans.
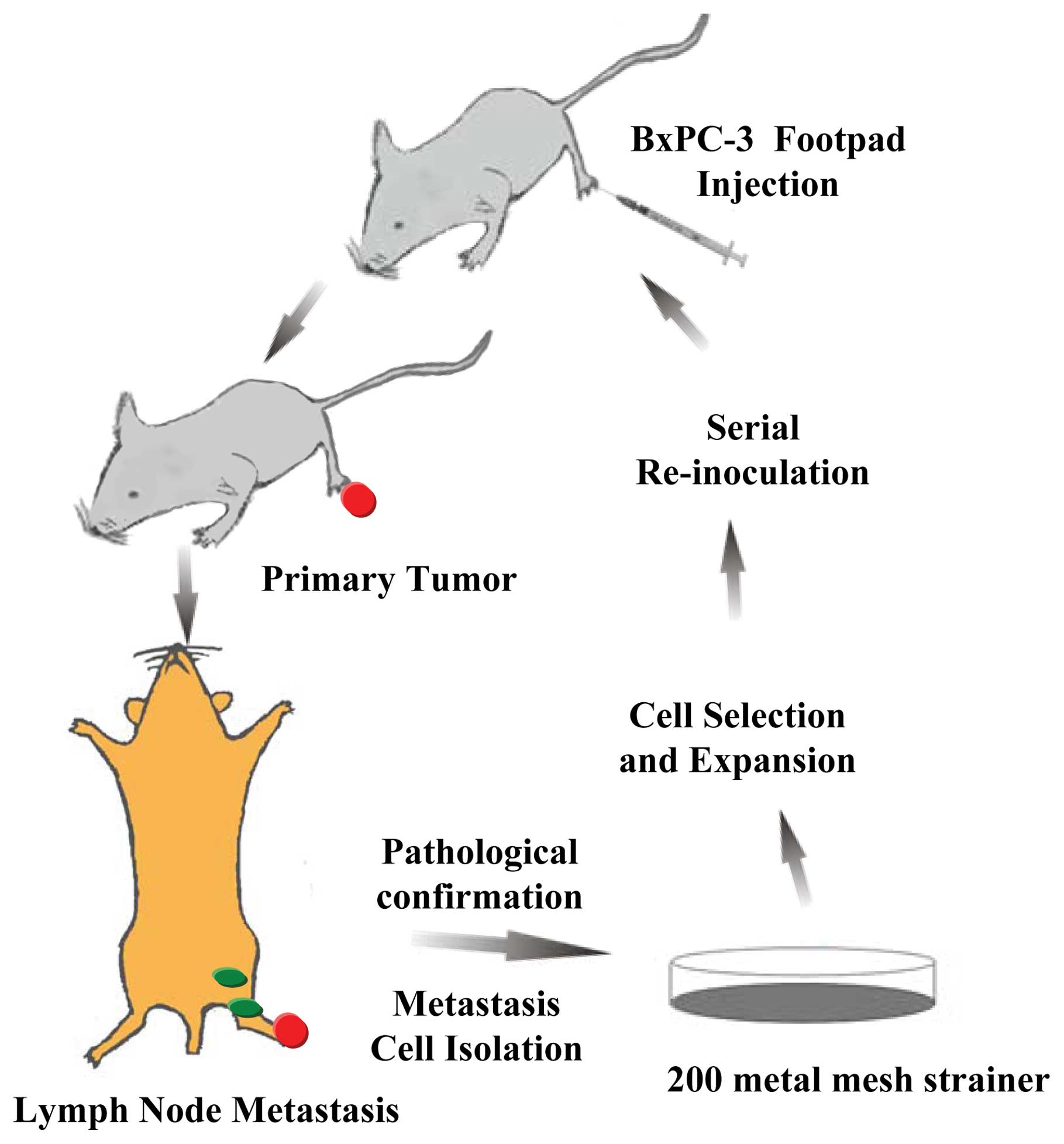
In the present study, we developed a BxPC-3-LN
subline from a BxPC-3 pancreatic cancer cell line by serial in
vivo selection. Subsequently, we characterized the phenotypes
of the BxPC-3-LN cells by comparing them to the parental cells.
Furthermore, to investigate the molecular mechanism in lymphatic
metastasis, a comparative analysis of differential gene expression
profiles in these two cell lines was performed by cDNA
microarray.
Materials and methods
Cell culture and laboratory animals
The human pancreatic cancer cell line BxPC-3 was
obtained from the American Type Culture Collection (Rockville, MD).
Monolayer cultures of BxPC-3 cells were maintained in RPMI-1640
medium supplemented with 10% fetal bovine serum, 1%
penicillin-streptomycin (Invitrogen, Carlsbad, CA). Cultures were
maintained at 37°C in a humidified atmosphere of 95% air and 5%
CO2.
Seven to eight-week old immunodeficient male mice
(BALB/c nu/nu) were purchased from Shanghai SLAC Laboratory Animal
Co., Ltd., China, and housed in laminar-flow cabinets under
specific pathogen-free conditions. All animal procedures were
approved by the institutional animal care committee, Fudan
University, China.
Establishment of new cell subline
The BxPC-3 cell subline was established by a serial
in vivo selection process performed as previously described
with some modifications (19,20)
(Fig. 1). Each male nude mouse
(n=6) was injected subcutaneously in the left hind footpad with 0.1
ml of BxPC-3 cells (1×107/ml). The mice were sacrificed
at 8 weeks after tumor implantation. The possible metastatic lymph
nodes, including homolateral popliteal, inguinal, iliac,
para-aortic, para-renal, and mesenteric lymph nodes, were dissected
and dissociated sterilely. Metastatic lymph nodes were confirmed by
hematoxylin and eosin staining. The suspension was filtered using a
200-metal mesh strainer (Xinrui Biotechnology, Shanghai, China) and
centrifuged at 1000 rcf/ min for 5 min at 4°C. The cell pellet was
resuspended in culture medium and cultivated until stable
populations of human tumor cells were established under the same
conditions as detailed above. Cultured tumor cells were then
serially re-inoculated into new recipient mice. After five rounds
of in vivo selection, a variant of the BxPC-3 human
pancreatic cancer cell line (BxPC-3-LN) with high lymphatic
metastasis capacity was established.
Cell morphology and ultrastructure
Cell morphology was viewed and photographed under a
light microscope (Estativo Eclipse TE 2000S, Nikon, Spain). For
ultrastructural observation, cells were fixed with 2.5%
glutaraldehyde in 0.1 M PBS for 2 h at room temperature. For
observation under scanning electron microscope (SEM), the specimens
were immersed in isoamyl acetate, air-dried, and sputter-coated
with gold before examination under the Hitachi S-520 microscope
(Hitachi Ltd., Tokyo, Japan). For transmission electron microscopy
(TEM), the specimens were immersed twice in absolute propylene
oxide and embedded in Quetol 812. Following staining with uranyl
acetate and lead citrate, the specimens were observed with a Jeol
JEM-1230 electron microscope (Jeol, Tokyo, Japan) at 80 kV.
Cell cycle analysis
Cells were harvested, fixed by resuspension in 10 ml
of 70% ethanol for 30 min, and washed in ice-cold PBS. They were
incubated, washed and resuspended in DNA staining solution
containing 20 μg/ml propidium iodide (Sigma-Aldrich) and 100
μg/ml RNase (Invitrogen). DNA content was determined by flow
cytometry (BD FACS Aria, USA).
In vitro growth rate assay
To evaluate in vitro growth kinetics, growth
curves were prepared for each cell line. A total of
2×103 cells/well were plated in triplicate in a 96-well
plate and grown for 1–7 days. Cells were then incubated at 37°C for
4 h with 20 μl of MTS (Promega) per well, and absorbance was
read at 492 nm. The number of viable cells was counted at the
indicated time-points in triplicate.
Cell adhesion assay
The cell adhesion assay was performed as previously
described with a slight change (21). Twenty-four-well plates were coated
with 10 μg/ml of rat tail type IV collagen (BD Biosciences,
Bedford, MA). The wells were blocked with 1% BSA for 1 h at 37°C.
After the BSA solution was aspirated, 1×106 cells in
0.1% BSA were added to the wells in triplicate. At 15 and 30 min,
the wells were washed to remove unattached cells, incubated at 37°C
for 4 h in 100 μl MTS and measured for absorbance at 492 nm.
The assay was repeated at least 3 times.
Cell migration assay
The cell migration assay was performed using a
24-well transwell chamber (Corning Costar, Corning, NY) migration
assay as previously described with some change (22). Briefly, exponentially growing cells
were trypsinized and washed in RPMI-1640/0.1% BSA medium twice.
Cells (∼1×106) were seeded in the upper chamber of each
transwell unit, which contained a polycarbonate 8-μm pore
membrane pre-coated with 50 μl of 10 μg/ml
fibronectin (BD Biosciences) overnight. The cells were allowed to
migrate at 37°C and 5% CO2 for 24 h. The unattached
cells were rinsed off with PBS and the membranes containing
attached cells were fixed in 10% formalin for 30 min and washed
with PBS. The cells were stained with crystal violet for 20 min and
rinsed with water. Cells on the unmigrated side were gently wiped
off with a wet cotton tip applicator and the membrane was rinsed
with water. The membranes containing the migrated cells were dried
and mounted onto slides with permount. The number of migrated cells
per high power field (hpf) was determined by averaging 20 randomly
counted hpfs. The assays were performed in triplicate and were
reproducible in at least three batches of independent
experiments.
Cell invasion assay
Transwell chamber inserts (Corning Costar) with
filter membrane pore size of 8 μm were coated with 0.5 mg/
ml Matrigel (BD Biosciences) overnight. The membranes were
rehydrated and 1×105 cells in serum-free RPMI-1640
medium were placed onto the inner chamber of each insert unit.
Media with 10% FBS was placed into the outer chamber as a
chemoattractant. The cells were allowed to invade at 37°C and 5%
CO2 for 24 h. Cells were fixed in 10% formalin and
washed with PBS. The cells were stained with crystal violet and
rinsed with water. Cells on the unmigrated side were gently wiped
off with a wet cotton tip applicator and the membrane was rinsed
with water. The membranes containing the invaded cells were dried,
and mounted onto slides with permount. The number of migrated cells
per hpf was determined by averaging 20 randomly counted hpfs. The
assays were performed in triplicate and were reproducible in at
least three batches of independent experiments.
Drug cytotoxicity assay
Cells were plated at a density of 104
cells per well into 96-multiwell plates and were allowed to grow
for 24 h before the experiment. The different concentrations (from
0 to 104 nM) of gemcitabine resuspended in 100 μl
RPMI-1640 medium were added to the cells, which were then incubated
for 24 h at 37°C. After initial incubation, cells were incubated at
37°C for 4 h with 20 μl of MTS (Promega), and absorbance was
read at 492 nm. Chemosensitivity was expressed as the drug
concentration that inhibited cell proliferation by 50%
(IC50 values). The number of viable cells was counted at
the indicated time-points in triplicate. Expression of
chemoresistance genes related to gemcitabine, including
ribonucleotide reductase M1 (RRM-1), ribonucleotide reductase M2
(RRM-2), human equilibrative nucleoside transporter-1 (hENT-1), and
deoxycytidine kinase (dCK), were examined by reverse-transcription
polymerase chain reaction (RT-PCR) as previously described
(23).
In vivo tumor growth and metastatic
potential
Eight-week old mice were injected with
1×106/ml BxPC-3 and BxPC-3-LN cells into the footpad
(n=6). The primary tumors were allowed to form and the weight was
measured every week. Tumor volume was calculated according to the
formula: (length in centimeters x (width)2)/2. Six mice
in each group were sacrificed separately at 8-week end-points to
examine lymphatic involvement. The possible metastatic lymph nodes
were divided into three groups: N1 (popliteal, inguinal), N2
(iliac, para-aortic), N3 (para-renal). At end-point, animals were
euthanized and autopsies were performed. Organs (including lymph
nodes, lungs, liver, spleen, pancreas, kidneys, ovaries, and any
other tissues of abnormal appearance) were examined superficially
for evidence of metastases using a dissecting microscope. Upon
sacrifice, the tumors, metastatic lymph nodes, and other organs
were fixed for hematoxylin and eosin staining.
cDNA microarray
Total RNA was extracted from BxPC-3 and BxPC-3-LN
cells, respectively, with TRIzol (Life Technologies, USA) and
dissolved in Milli-Q H2O. The RNA from BxPC-3 was
labeled with Cy3-dUTP and that from BxPC-3-LN with Cy5-dUTP.
Fluorescent probe mixtures were denatured at 95°C for 5 min, and
applied to the prehybridized chip (Affymetrix, Inc., USA) under a
cover glass, which was hybridized at 42°C for 15–20 h. After
washing, the microarray was scanned on a scanner (Agilent, G2565BA)
and the image was analyzed using Feature Extraction software
(Agilent). The signal intensity of each spot was calibrated by
subtraction from the intensity of the negative control. The assay
was performed twice and only genes showing the same tendency of
differential expression were eligible for the final-result
analysis.
Statistical analysis
Fisher’s exact test and Student’s t-test were used
for comparisons of enumeration data and to measure mean t data,
respectively. The statistical analysis software package Stata 10.0
was used for the tests, and P<0.05 was considered statistically
significant.
Results
Morphology and ultrastructure
Under a light microscope, the morphology of the
BxPC-3-LN cell line was similar to the parental BxPC-3 cell line
observed in culture. Both of them demonstrated polygonal epithelial
morphology with large nuclei and 3–6 conspicuous nucleoli (Fig. 2A1 and A2). In addition, under SEM,
in comparison to the parental cells, the BxPC-3-LN cells exhibited
discernible aggressive morphological alterations such as filopodia
and lamellipodia, protrusions emerging from the cellular membrane
(Fig. 2B1 and B2). Under TEM,
compared to the parental cells, the BxPC-3-LN cells contained more
secretory granules (∼50–400 nm in diameter) (Fig. 2C1 and C2).
In vitro cell growth
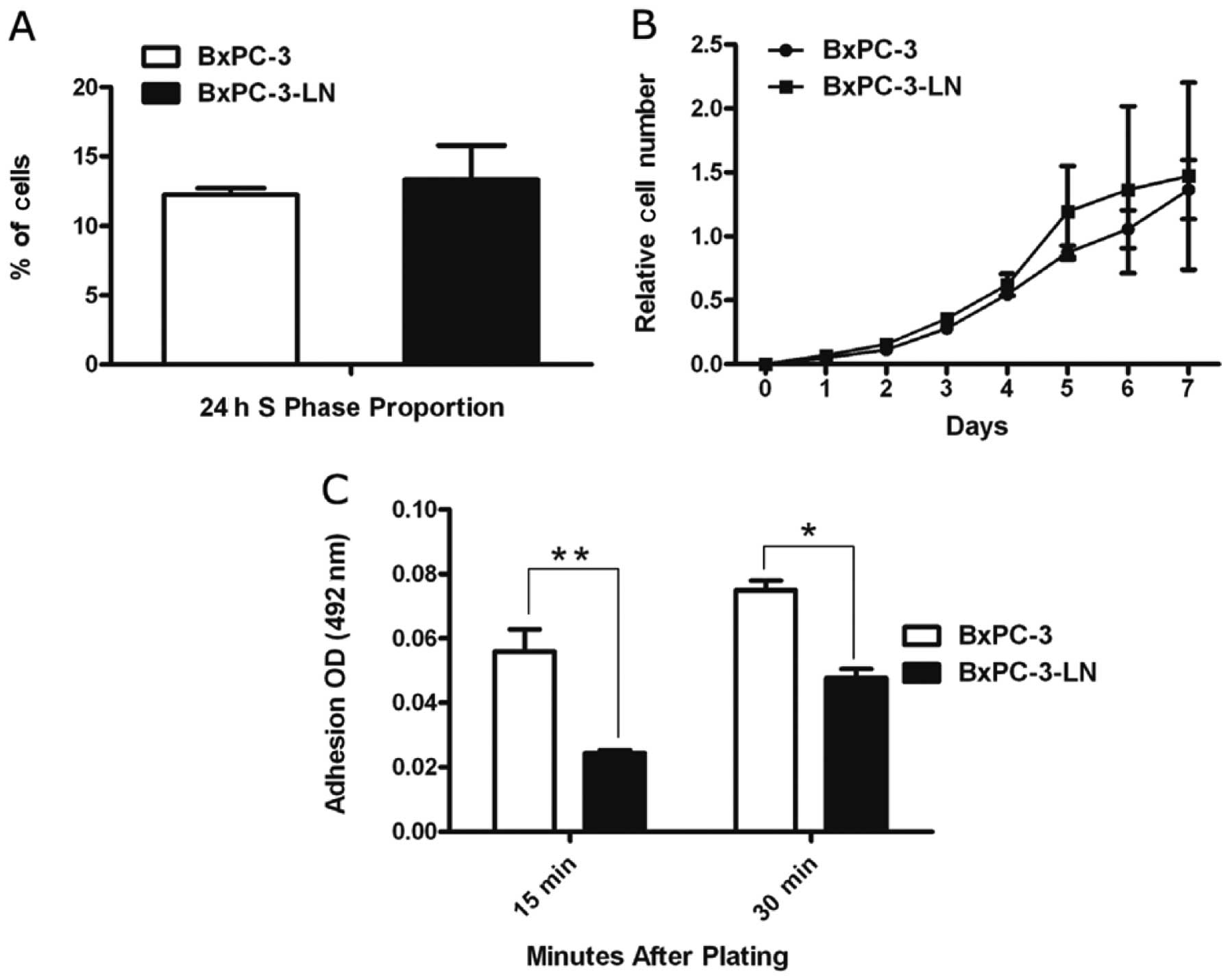
Cell cycle analysis by flow cytometry revealed that
the percentage of BxPC-3-LN cells in S phase (13.3%) was similar to
that of the parental cells (12.2%) (Fig. 3A). Growth curves were employed to
evaluate the in vitro growth kinetics through MTS assay.
BxPC-3-LN cells exhibited similar in vitro proliferation to
BxPC-3 cells (Fig. 3B).
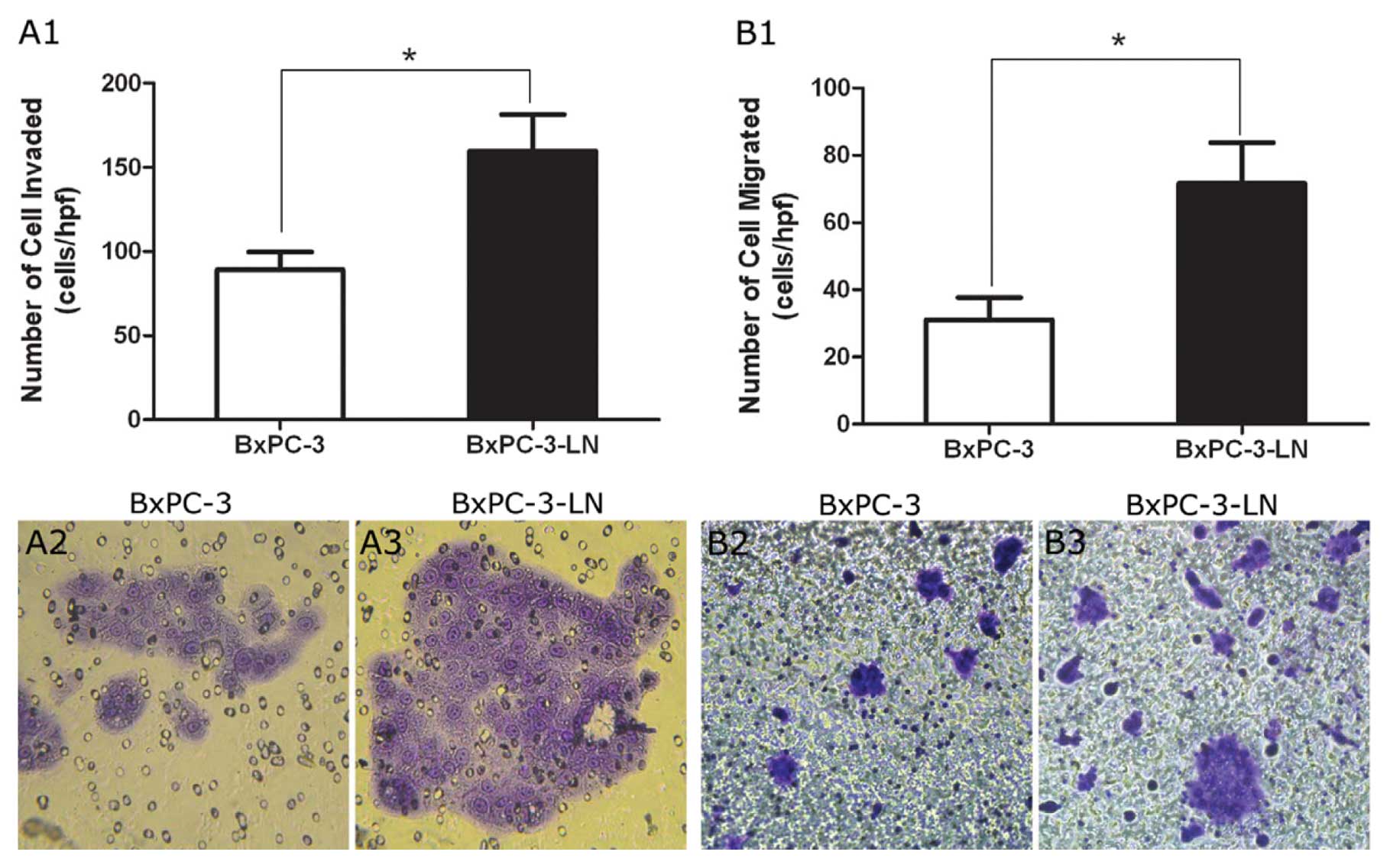
Cell adhesion, migration, and invasion
assay
As shown in Fig.
3C, the highly metastatic BxPC-3-LN cells had decreased
relative adherence compared to the parental BxPC-3 cells. At the
15- and 30-min time-points, the decrease in relative adherence
ranged from 36.0 to 57.0% (P<0.05). Conversely, BxPC-3-LN cells
demonstrated increased cell migration and invasion compared to the
parental BxPC-3 cells (Fig. 4). In
fact, when the migrated cells were counted, there was more than
twice the number of cells migrated per high power field in
BxPC-3-LN samples than in BxPC-3 samples (Fig. 4B1, B2 and B3).
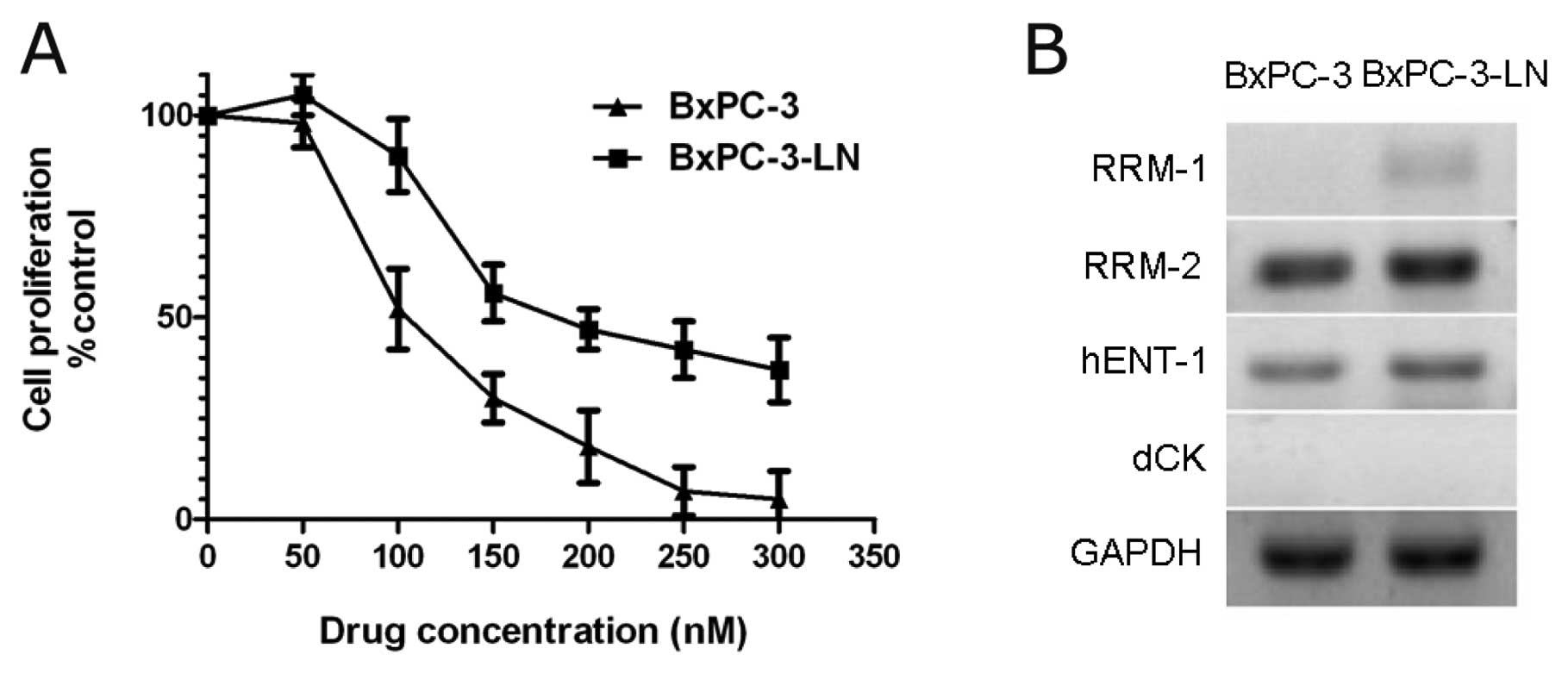
Drug cytotoxicity assay
We examined the cellular sensitivity of BxPC-3 cells
and BxPC-3-LN cells to gemcitabine in vitro using the
proliferation assay. Cells were treated with increasing
concentrations of gemcitabine (50–300 nM) (Fig. 5A). BxPC-3-LN cells exhibited native
resistance to gemcitabine and the IC50 value of
BxPC-3-LN cells (210.5 nM) was higher than that of BxPC-3 cells
(125.3 nM) (P<0.05). In addition, expression of chemoresistance
genes related to gemcitabine was examined by RT-PCR. RRM-1, RRM-2
and hENT-1 expression was higher in BxPC-3-LN cells compared to
BxPC-3 cells (Fig. 5B).
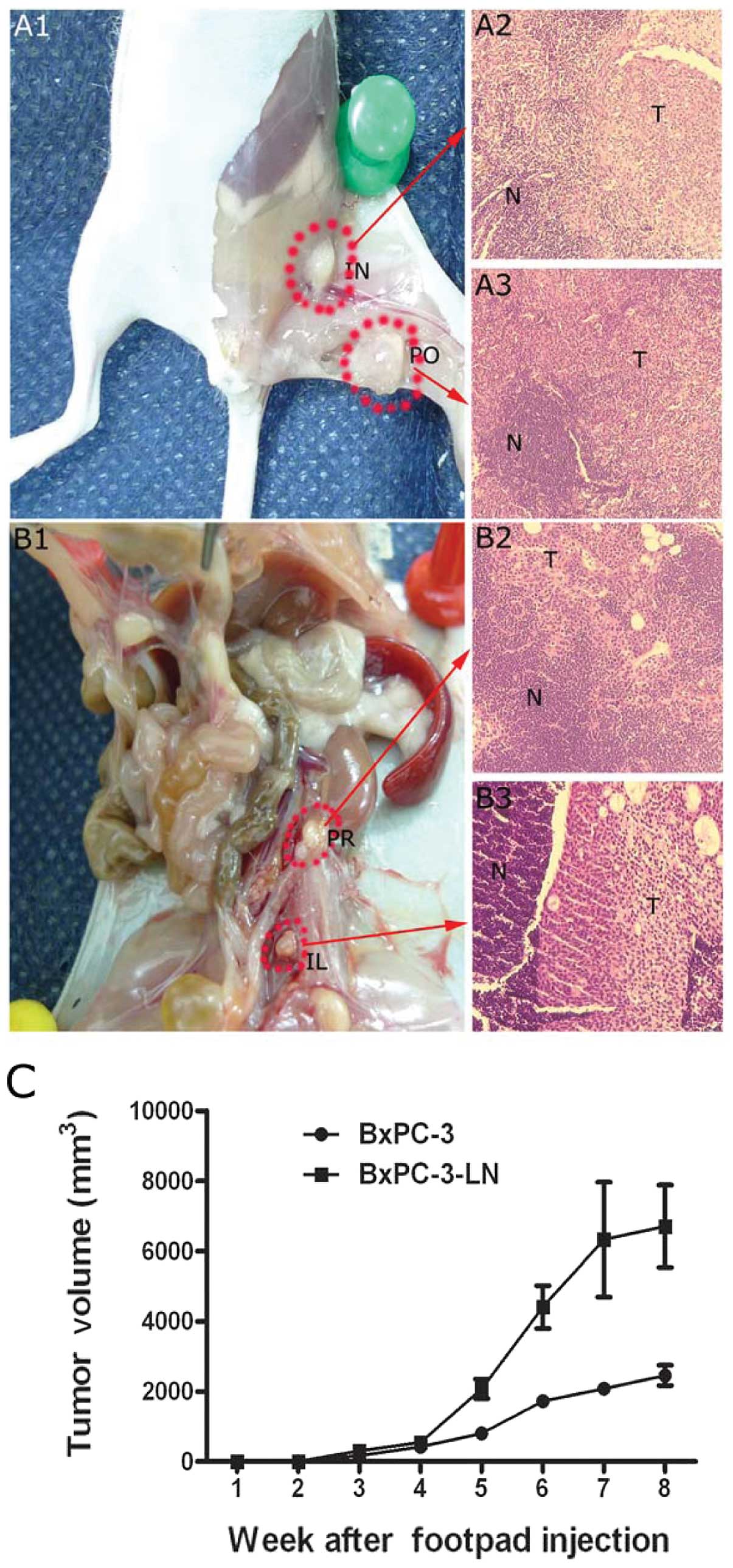
In vivo spontaneous lymphatic metastasis
via footpad injection
After five rounds of consecutive in vivo
selection, the lymphatic metastases of BxPC-3-LN cells were
obvious. When injected into nude mice, BxPC-3-LN cells had a
>12-fold increase in presence of lymphatic metastases compared
to the parental BxPC-3 cells (Fig.
6 and Table I). BxPC-3-LN
cells also formed larger primary tumors when compared to the
parental BxPC-3 cells (Fig. 6C).
Total lymph node and total animal metastatic ratio of the BxPC-3-LN
cells was 71.1 and 100.0%, which was higher than that of the
parental BxPC-3 cells (5.6 and 16.7%, respectively) (Table I). No metastases were found in the
liver, other abdominal viscera or the lungs.
 | Table I.Lymphatic metastasis of each lymph
node group. |
Table I.
Lymphatic metastasis of each lymph
node group.
| Cell lines | N1 (M-LNs/T-LNs)
(%) | N2 (M-LNs/T-LNs)
(%) | N3 (M-LNs/T-LNs)
(%) | All lymph nodes
(M-LNs/T-LNs) (%) | Total animals (M
/T) (%) |
|---|
| BxPC-3 | 1/12 (8.3) | 1/12 (8.3) | 0/12 (0.0) | 2/36 (5.6) | 1/6 (16.7) |
| BxPC-3-LN | 12/12 (100.0) | 10/14 (71.4) | 5/12 (41.7) | 27/38 (71.1) | 6/6 (100.0) |
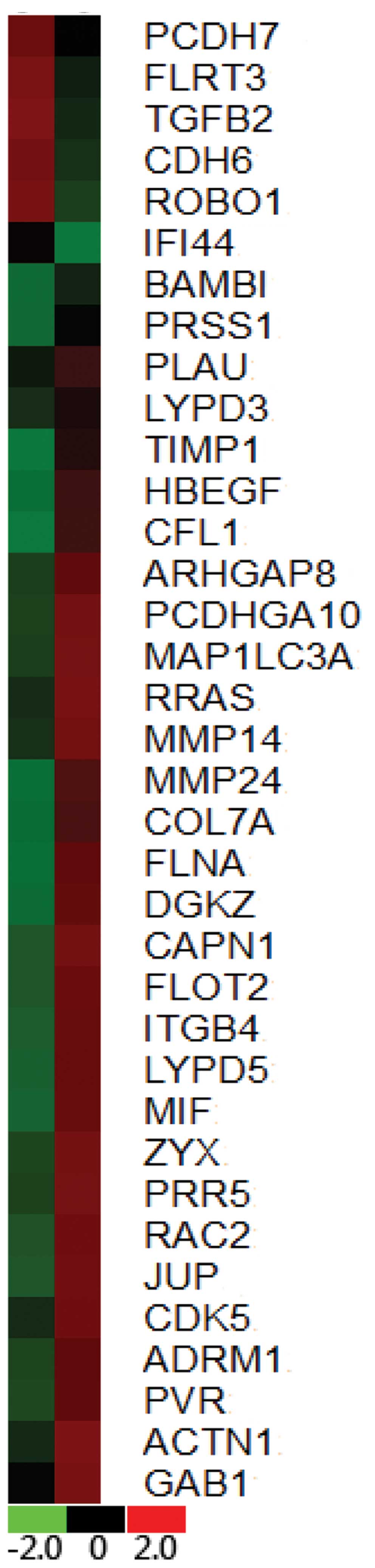
Comparison of gene expression between
BxPC-3 and BxPC-3-LN cell lines
The differences in the gene expression levels
between the two cell lines were assessed by measuring the ratios of
their expression. Genes were identified as differentially expressed
if the absolute value of the natural logarithm of the ratios was
<0.3 or >2.0. Thirty-six genes were identified as being
differentially expressed, including 6 with decreased expression and
30 with increased expression in BxPC-3 vs BxPC-3-LN (Fig. 7).
Discussion
Several human pancreatic cancer cell lines have been
employed in studying the molecular mechanisms of pancreatic cancer
lymphatic metastasis (24,25). Although these cell lines show
different phenotypes and genotypes that are representative of
pancreatic cancer, few of them have the capability for spontaneous
lymphatic metastasis in immunodeficient mice (26,27).
It is critical to establish appropriate cell lines and animal
models to interpret the molecular mechanisms of lymphatic
metastasis for pancreatic cancer.
The parental BxPC-3 cell line was first established
from the body of the pancreatic adenocarcinoma of a 61-year-old
female patient reported in 1986 (27). It is one of the most commonly
referenced cell lines of pancreatic cancer (28). Compared to other pancreatic cancer
cell lines, BxPC-3 shows moderate propensities of adhesion,
migration, invasion and tumorigenicity (27). Evidence of lymphatic metastasis was
rarely found to be associated with BxPC-3 (27). Therefore, it could serve as an
ideal parental cell line from which to select highly lymphatic
metastasis cell lines.
We used an in vivo selection process by
injecting tumor cells into the footpad of nude mice and then
examining and culturing the draining metastatic lymph nodes to
establish the subline. This method was first developed by Carr
et al in 1976 (19), and
later confirmed as a useful and efficient method to develop
lymphatic metastatic animal models (20,29).
The basic model is to inject tumor cells into the footpad and then
to examine the draining popliteal lymph node histologically at
varying times thereafter (29). We
have made some modifications from the previous methods. First, a
repetitive in vivo selection process was used to establish
the high lymphatic metastasis subline. Second, lymph nodes in the
lymph draining route, including popliteal, inguinal, iliac,
para-aortic, or para-renal lymph nodes, were removed and
pathologically confirmed. Last, but most importantly, we improved
this technique by using a metal mesh strainer to get rid of major
non-tumor cells.
After five rounds of in vivo selection, a
variant of the BxPC-3 human pancreatic cancer cell line with high
lymphatic metastatic potential, BxPC-3-LN, was established.
Although the morphology of BxPC-3-LN cells was similar to the
parental cells by light microscopy, the ultrastructure of BxPC-3-LN
cells observed under SEM and TEM showed a more aggressive change,
with the presence of filopodia, lamellipodia and a greater number
of secretory granules. In addition, our results demonstrated that
the BxPC-3-LN cells exhibited decreased cell adhesion, and
increased migration, invasion, drug resistance, and spontaneous
lymphatic metastases in vivo, indicating the aggressive
phenotype of the BxPC-3-LN cells.
BxPC-3-LN cells can be used as a valuable tool to
study the molecular mechanisms of lymphatic metastasis for
pancreatic cancer. For example, lymphatic metastatic related genes
and proteins can be identified by comparing BxPC-3 and BxPC-3-LN
cells. In the present study, we compared the gene expression
between BxPC-3 and BxPC-3-LN by cDNA array assay. As expected,
altered expressions of numerous genes related to invasion, motility
and tumorgenicity in these cells were revealed. Metastasis-related
gene alteration including upregulation of MMP14, MMP24, MIF, and
ADRM1, and downregulation of TGFB2 and ROBO1 were observed. Our
study demonstrated that gene expression patterns undergo complex
alterations during the course of lymphatic metastasis of pancreatic
cancer. In addition, this model may serve as an ideal platform to
study potential therapeutic approaches for lymphatic metastasis of
pancreatic cancer. For example, it can be used as a model to test
drugs that may potentially inhibit lymphatic metastasis and
eradicate tumor cells.
In conclusion, we have developed and characterized a
highly lymphatic metastatic human pancreatic cancer cell line that
could serve as a useful platform for the study of pancreatic cancer
lymphatic metastasis and potential therapeutics.
Acknowledgements
This study was supported in part by
the National Science Foundation of China (grant nos. 81101807,
81001058, 30901435 and 30972905), the Shanghai Science and
Technology Commission (10JC1418900), the MacDonald Research Fund,
and the William and Ella Owens Medical Research Foundation.
References
|
1.
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar
|
|
2.
|
Kim JW, Shin SS, Heo SH, et al: Diagnostic
performance of 64-section CT using CT gastrography in preoperative
T staging of gastric cancer according to 7th edition of AJCC cancer
staging manual. Eur Radiol. 22:654–662. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Braat H, Bruno M, Kuipers EJ and
Peppelenbosch MP: Pancreatic cancer: promise for personalised
medicine? Cancer Lett. 318:1–8. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Bouvet M, Bold RJ, Lee J, et al:
Adenovirus-mediated wild-type p53 tumor suppressor gene therapy
induces apoptosis and suppresses growth of human pancreatic cancer.
Ann Surg Oncol. 5:681–688. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Luo G, Long J, Zhang B, et al: Stroma and
pancreatic ductal adenocarcinoma: an interaction loop. Biochim
Biophys Acta. 1826:170–178. 2012.PubMed/NCBI
|
|
6.
|
Ni X, Yang J and Li M: Imaging-guided
curative surgical resection of pancreatic cancer in a xenograft
mouse model. Cancer Lett. 324:179–185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Wray CJ, Ahmad SA, Matthews JB and Lowy
AM: Surgery for pancreatic cancer: recent controversies and current
practice. Gastroenterology. 128:1626–1641. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Geer RJ and Brennan MF: Prognostic
indicators for survival after resection of pancreatic
adenocarcinoma. Am J Surg. 165:63–72. 1993.PubMed/NCBI
|
|
9.
|
Warshaw AL and Fernandez-del Castillo C:
Pancreatic carcinoma. N Engl J Med. 326:455–465. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Shi WD, Meng ZQ, Chen Z, Lin JH, Zhou ZH
and Liu LM: Identification of liver metastasis-related genes in a
novel human pancreatic carcinoma cell model by microarray analysis.
Cancer Lett. 283:84–91. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Hirono S, Yamaue H, Hoshikawa Y, et al:
Molecular markers associated with lymph node metastasis in
pancreatic ductal adenocarcinoma by genome-wide expression
profiling. Cancer Sci. 101:259–266. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Isaji S, Kawarada Y and Uemoto S:
Classification of pancreatic cancer: comparison of Japanese and
UICC classifications. Pancreas. 28:231–234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Li Y, Kong D, Ahmad A, Bao B and Sarkar
FH: Pancreatic cancer stem cells: emerging target for designing
novel therapy. Cancer Lett. May 20–2012.(Epub ahead of print).
|
|
14.
|
Pedrazzoli S, DiCarlo V, Dionigi R, et al:
Standard versus extended lymphadenectomy associated with
pancreatoduodenectomy in the surgical treatment of adenocarcinoma
of the head of the pancreas: a multicenter, prospective, randomized
study. Lymphadenectomy Study Group. Ann Surg. 228:508–517. 1998.
View Article : Google Scholar
|
|
15.
|
Alitalo K, Tammela T and Petrova TV:
Lymphangiogenesis in development and human disease. Nature.
438:946–953. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Oliver G and Detmar M: The rediscovery of
the lymphatic system: old and new insights into the development and
biological function of the lymphatic vasculature. Genes Dev.
16:773–783. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Zhang Y, Tang H, Cai J, et al: Ovarian
cancer-associated fibroblasts contribute to epithelial ovarian
carcinoma metastasis by promoting angiogenesis, lymphangiogenesis
and tumor cell invasion. Cancer Lett. 303:47–55. 2011. View Article : Google Scholar
|
|
18.
|
Yin T, Ji XL and Shen MS: Relationship
between lymph node sinuses with blood and lymphatic metastasis of
gastric cancer. World J Gastroenterol. 9:40–43. 2003.PubMed/NCBI
|
|
19.
|
Carr I, McGinty F and Norris P: The fine
structure of neoplastic invasion: invasion of liver, skeletal
muscle and lymphatic vessels by the Rd/3 tumour. J Pathol.
118:91–99. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Carr J, Carr I, Dreher B and Betts K:
Lymphatic metastasis: invasion of lymphatic vessels and efflux of
tumour cells in the afferent popliteal lymph as seen in the Walker
rat carcinoma. J Pathol. 132:287–305. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Luu HH, Kang Q, Park JK, et al: An
orthotopic model of human osteosarcoma growth and spontaneous
pulmonary metastasis. Clin Exp Metastasis. 22:319–329. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Varney ML, Singh S, Li A, Mayer-Ezell R,
Bond R and Singh RK: Small molecule antagonists for CXCR2 and CXCR1
inhibit human colon cancer liver metastases. Cancer Lett.
300:180–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Luo G, Jin C, Long J, et al: RNA
interference of MBD1 in BxPC-3 human pancreatic cancer cells
delivered by PLGA-poloxamer nanoparticles. Cancer Biol Ther.
8:594–598. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Ochi N, Matsuo Y, Sawai H, et al: Vascular
endothelial growth factor-C secreted by pancreatic cancer cell line
promotes lymphatic endothelial cell migration in an in vitro model
of tumor lymphangiogenesis. Pancreas. 34:444–451. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Taniguchi S, Iwamura T and Katsuki T:
Correlation between spontaneous metastatic potential and type I
collagenolytic activity in a human pancreatic cancer cell line
(SUIT-2) and sublines. Clin Exp Metastasis. 10:259–266. 1992.
View Article : Google Scholar
|
|
26.
|
Lieber M, Mazzetta J, Nelson-Rees W,
Kaplan M and Todaro G: Establishment of a continuous tumor-cell
line (panc-1) from a human carcinoma of the exocrine pancreas. Int
J Cancer. 15:741–747. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Tan MH, Nowak NJ, Loor R, et al:
Characterization of a new primary human pancreatic tumor line.
Cancer Invest. 4:15–23. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Blanquicett C, Saif MW, Buchsbaum DJ, et
al: Antitumor efficacy of capecitabine and celecoxib in irradiated
and lead-shielded, contralateral human BxPC-3 pancreatic cancer
xenografts: clinical implications of abscopal effects. Clin Cancer
Res. 11:8773–8781. 2005. View Article : Google Scholar
|
|
29.
|
Carr I: Experimental lymphatic metastasis.
J Microsc. 131:211–220. 1983. View Article : Google Scholar : PubMed/NCBI
|