Introduction
Photodynamic therapy (PDT) is a treatment for the
selective destruction of cancerous and non-neoplastic cells that
involves the simultaneous presence of light, oxygen and a
light-activatable chemical known as a photosensitizer, with a
photosensitive molecule that can be localized in the target cells
(1). Photofrin, a representative
of the first generation photosensitizers, is transformed by
haematoporphyrin derivatives (HpD) and absorbs light up to 640 nm
(2). It is the most widely used
photosensitizer in clinical PDT, and has been approved by health
agencies to treat human solid malignancies (1). PDT has been indicated as a promising
treatment for a wide range of cancers, such as cervical cancer and
head and neck cancer (3–5). After PDT, the release of
mitochondrial cytochrome c into the cytosol leads to
apoptosis (6), which is
characterized by chromatin condensation, cleavage of chromosomal
DNA into internucleosomal fragments, cell shrinkage, membrane
blebbing, and the formation of apoptotic bodies without plasma
membrane breakdown (6). Lu et
al reported that methylene blue-mediated PDT induces
mitochondria-dependent apoptosis in HeLa cells (7).
The photosensitizer transfers energy from light to
molecular oxygen to generate reactive oxygen species (ROS),
particularly singlet oxygen, hydroxyl radicals and peroxides. This
photosensitizer can react with oxygen via energy transfer
processes, generating singlet oxygen (1O2) or
it can participate in electron transfer processes, leading to
radical formation (8). Normally,
direct and indirect evidence supports the prevalent role of
1O2 in the molecular processes initiated by
PDT (9). Both reactions can lead
to damage to the cellular components and cell death by either
apoptosis or necrosis. The apoptosis caused by ROS generated in
HeLa cells by Hematoporphyrin monomethyl ether (HMME)-induced PDT
was reported to be mediated by cytochrome c release and
caspase-3 activation (10).
Curcumin
(1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione), also
known as diferuloymethane, is the major yellow pigment in turmeric
(Curcuma longa) and is one of the most extensively studied
phytochemicals with chemo-preventive potential (11). Curcumin has been used in
traditional Asian, Indian medicine for thousands of years to treat
a range of diseases (12).
Curcumin has antitumor properties in cancers of the breast
(13), glioblastoma (14), head and neck (15), colon (16) and leukemia (17). The major mechanism by which
curcumin induces cytotoxicity in tumor cells is the induction of
apoptosis. Curcumin decreases the expression of anti-apoptotic
members of the Bcl-2 family and elevates the expression of Bax,
caspase-3, -8 and -9. In addition, it induces apoptosis through the
mitochondrial pathway involving the release of cytochrome c,
and then activates PARP cleavage (18). Karmakar et al reported that
curcumin activates caspases for apoptosis in human malignant
glioblastoma U87MG cells (14).
Conventional cancer therapies, including PDT and
chemotherapy as a single modality, have a limited but important
role in the overall treatment of most solid tumors. Therefore, the
strategies of cancer treatment using combined therapies are
considered more promising for higher efficacy, resulting in better
survival rates. Cancer therapies combining chemoprevention
including curcumin may have enhanced antitumor activity with a
decrease in the toxicity caused by PDT. Recent evidence suggests
that the photosensitizer effect of curcumin on UVB-irradiated HaCaT
cells occurs through activation of the caspase pathways (19). In addition, Dujic et al
reported that low concentrations of curcumin induce apoptosis in
skin keratinocytes only in combination with UVA or visible light
(20).
In this study, to confirm the interaction between
photofrin-induced PDT and curcumin during combined mortality, this
study examined effect and potential mechanism of a combination of
PDT and curcumin on apoptotic cell death in AMC-HN3 cells.
Materials and methods
Reagents
Photofrin, a derivative of the haematoporphyrin, was
purchased from LitePharm Tech, and was stable in PBS at −20°C in
the dark. Curcumin (Sigma, St. Louis, MO, USA) was stable in DMSO
(Sigma). A total of 100 mM of a stock solution of curcumin was
stored at −20°C in the dark. The following antibodies were used:
anti-caspase-8 (Calbiochem, La Jolla, CA, USA), anti-caspase-9
(Cell Signaling Technology, MA, USA), anti-capase-3 (Calbiochem),
anti-cytochrome c (BD Biosciences, Oxford, UK), anti-PARP
(Santa Cruz Biotechnology, Santa Cruz, CA, USA), horseradish
conjugated anti-mouse IgG (Santa Cruz Biotechnology) and
horseradish conjugated anti-rabbit IgG (Santa Cruz
Biotechnology).
Cell culture
The human head and neck cancer cell line (AMC-HN3)
was kindly provided by Asan Medical Center (Seoul, Korea). The
AMC-HN3 cells were cultured in RPMI-1640 medium (Hyclone, Logan,
UT, USA) supplemented with 10% fetal bovine serum (FBS) (Hyclone)
and 100 μg/ml streptomycin and 100 U/ml penicillin (Hyclone)
at 37°C in a 5% CO2 incubator.
Photodynamic therapy (PDT)
The cells were seeded in 6-well plates, 96-well
plates or plates, 100 mm in diameter. The cells were treated with a
series of 2-fold dilutions of photofrin, starting at 50
μg/ml and incubated for 6 h at 37°C in a 5% CO2
incubator. Subsequently, the photosensitized cells were irradiated
with 630 nm diode laser (0.83 mW/cm2) for 15 min at room
temperature. After irradiation, the cells were incubated in a
humidified atmosphere at 37°C and 5% CO2 for the
indicated times.
Cell viability
The MTT assay was used to assess the cell viability
of AMC-HN3 cells after the combination treatment. The cells
attached in a 96-well plate (1,000 cells/well) were treated with 25
μM of curcumin for 6 h. Subsequently, the cells were
incubated with 3 μg/ml photofrin for 6 h. The
photosensitized cells were then irradiated with a 630 nm diode
laser for 15 min. The cells were then incubated for 24 h at 37°C in
a 5% CO2 incubator and exposed to MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide] (2
mg/ml, Sigma) for 4 h. The solution was changed to 150 μl of
dimethylsulfoxide (DMSO, Kanto, Japan). After 5-min incubation and
shaking in microplate mixer (Amersham Pharmacia Biotech, Amersham,
UK), the optical density (OD) was measured using a microplate
reader (Bio-Rad, Hercules, CA, USA) at 540 nm wavelength. The cell
viability was calculated using the following formula: Cell
viability (%) = Mean optical density of treated wells/Mean optical
density of control wells × 100.
Detection of apoptosis and necrosis using
Hoechst 33342 and propidium iodine (PI)
Hoechst 33342 (Sigma) and PI (Sigma) double staining
was used to identify the cell death pattern. The nuclear morphology
was assessed with the cell membrane-permeant supravital DNA dye
Hoechst 33342 (excitation wavelength, 348 nm; emission, 479 nm).
Hoechst 33342, unlike PI, enters and stains the nucleus of both
viable cells and cells with apoptosis or necrosis. The plasma
membrane integrity was assessed using the cell membrane-impermeant
DNA dye PI (excitation wavelength 535 nm; emission 617 nm).
Necrosis was determined based on the positive PI staining in red
color, which is indicative of a loss of membrane integrity
(21). Briefly, 3 h after PDT, the
cells were stained with Hoechst 33342 (1 μg/ml) for 30 min.
The medium was then changed and the cells were incubated with PI (1
μg/ml) for 10 min before the observations by confocal laser
scanning microscopy (Carl Zeiss, Oberkochen, Germany).
Measurement of reactive oxygen species
(ROS)
The intracellular accumulation of ROS was determined
using H2DCFDA (2′,7′-Dichlorodihydro fluorescein
diacetate, Molecular Probes, Eugene, OR, USA), as previously
described (22). Briefly, 1 h
after PDT, the cells were incubated with 2 μM
H2DCFDA for 30 min and washed gently twice with DPBS.
Images of green H2DCFDA were collected by LSM-510-META
confocal microscopy (Carl Zeiss) with an excitation wavelength of
488 nm, a 560 nm dichroic mirror, and a 505 to 550 nm band pass
barrier filter.
Mitochondrial membrane potential
(Δψm)
Rhodamine 123 (Molecular Probes) was used to
evaluate the mitochondrial membrane potential (Δψm), as
predicted previously (22).
Briefly, 2 h after PDT, the cells were re-suspended and loaded with
1 μM rhodamine 123 for 30 min. The signal for rhodamine 123
was detected by the FL1-H (530 nm) channel and the data were
analyzed using the CELLQuest Program (Becton Dickinson, San Jose,
CA, USA). At least 20,000 events were counted.
Protein extraction and western blot
analysis
Six hours after PDT, the cells were washed twice
with cold DPBS, and the cytosolic fraction and total protein were
extracted in CE1 buffer (Qiagen, Valencia, CA, USA) and RIPA
buffer, respectively. A Bradford assay (Bio-Rad) was used to
determine the protein concentration by measuring the optical
density at 595 nm using a spectrophotometer (Biochrom, Cambridge,
UK). The protein samples were mixed with a 5X loading buffer (250
mM Tris, pH 6.8, 40% glycerol, 4% β-mercaptoethanol, 0.08%
bromophenol blue, 8% sodium dodecyl sulfate), heat-denatured at
95°C for 10 min, loaded onto the 10% sodium dodecyl sulfate
polyacrylamide gel and at 100 V for 90 min. After electrophoresis,
gels with the resolved proteins were transferred to PVDF membranes
(Bio-Rad) and blocked for 1 h in 10% skim milk. Each primary
antibody (caspase-3, -9, cyto-chrome c and PARP) was diluted
in 5% skim milk and added to the membrane for 90 min. The membranes
were washed five times with DPBS and detected with horseradish
peroxidaseconjugated secondary IgG for 1 h. The labeled protein
bands were detected using a Kodak in vivo image analyzer
(Eastern Kodak, Rochester, NY, USA).
Treatment with antioxidant
The attached cells were co-treated with 25 μM
of curcumin and either 40 mM D-mannitol or 5 mM glutathione for 6
h. The cells were then incubated with 3 μg/ml photofrin in
the presence of the individual antioxidant for 6 h. After washing
with fresh medium, the cells were subjected to irradiation under
the aforementioned conditions.
Statistical analysis
The significance of the differences was evaluated
using a Student’s t-test. A p-value <0.05 was considered
significant.
Results
Increase of cytotoxic and apoptotic
effect
A MTT assay was used to measure the cytotoxicity 24
h after PDT to assess the combination effect of curcumin and
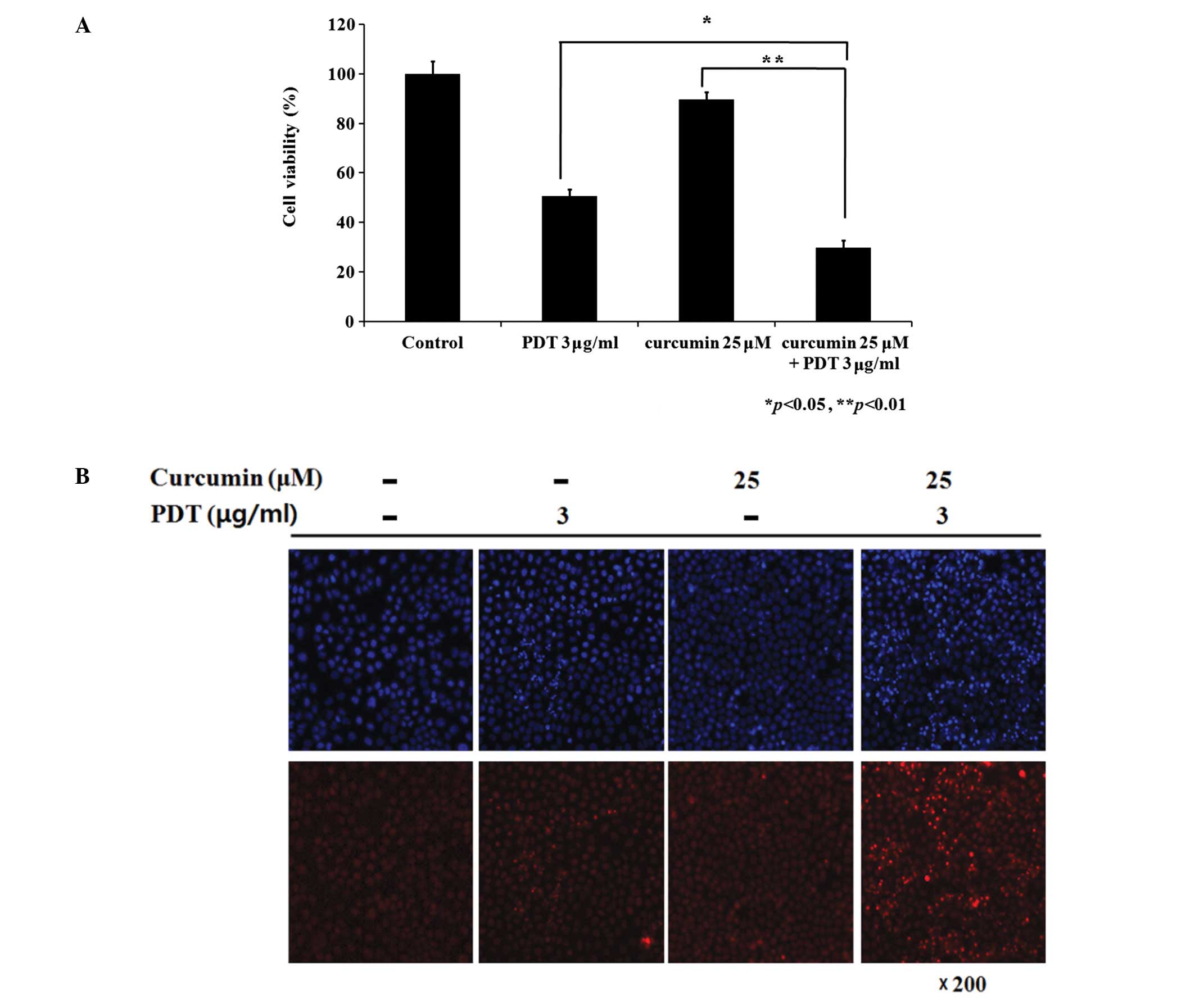
photofrin-induced PDT on AMC-HN3 cells. As shown in Fig. 1A, curcumin alone and PDT alone,
respectively, inhibited approximately 10 and 50% of the cell
viability, whereas a combination treatment with curcumin and
photofrin-induced PDT inhibited approximately 70% of the cell
viability. Dividing the effect from curcumin, there was an
approximately 10% extra cytotoxic effect from the combination group
comparing to the PDT only group.
Hoechst 33342 and PI double staining were performed
to determine if the combination treatment induced enhanced
apoptosis. There were only infrequent apoptotic bodies in curcumin
only and PDT only groups. In contrast, more condensed/fragmented
blue and pink nuclei as well as some pink intact nuclei were
observed in the combination group. (Fig. 1B) This suggests that the
combination treatment had a more intense apoptotic effect than each
single treatment.
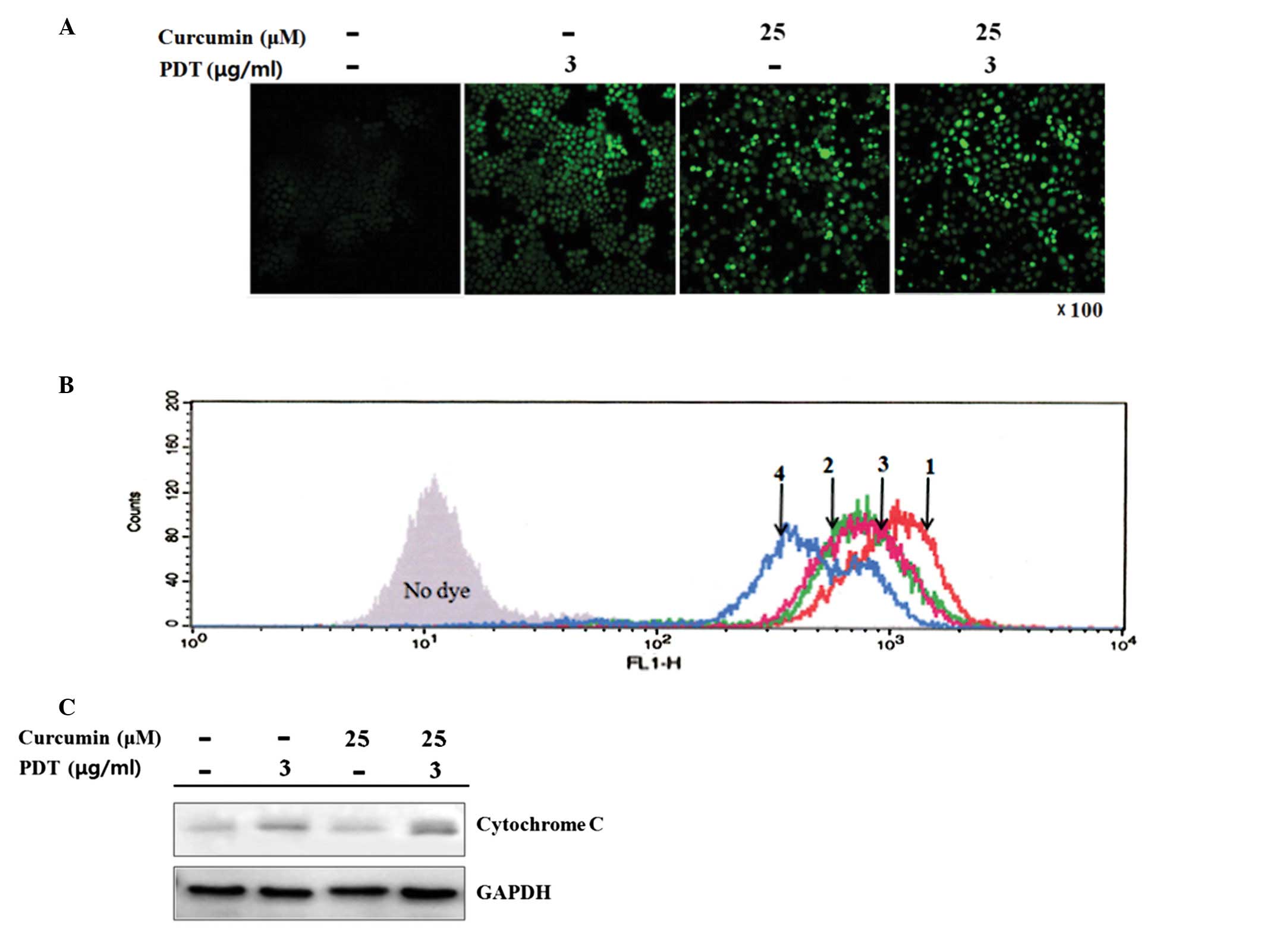
Changes of reactive oxygen species (ROS)
generation, mitochondrial membrane potential (Δψm), and
cytochrome c release
To determine if a pretreatment with curcumin affects
the generation of ROS by PDT, the intracellular ROS level was
detected using the fluorescent probe H2DCFDA, which is
readily oxidized to 2′,7′-dichlorofluorescein (DCF) in the presence
of ROS. Compared to the control group, curcumin and PDT alone
induced remarkable generation of ROS. The ROS signal induced by the
combination group was higher than that of each single treatment
(Fig. 2A).
To further examine the activation of mitochondria,
the collapse of Δψm was quantified by flow cytometry.
Compared to the control group, a decrease in Δψm, a
leftward shift in the fluorescence curve, was clearly observed in
the curcumin or PDT treatment alone group. The combination group
showed a more intense loss of Δψm than that each single
treatment group (Fig. 2B).
The collapse of Δψm by PDT has been
suggested to be a key factor in the release of cytochrome c
from the mitochondria to the cytosol. As shown in Fig. 2C, the release of cytochrome
c was increased markedly 6 h after PDT. Furthermore, a
pretreatment with curcumin clearly enhanced the release of
cytochrome c from the mitochondria by PDT.
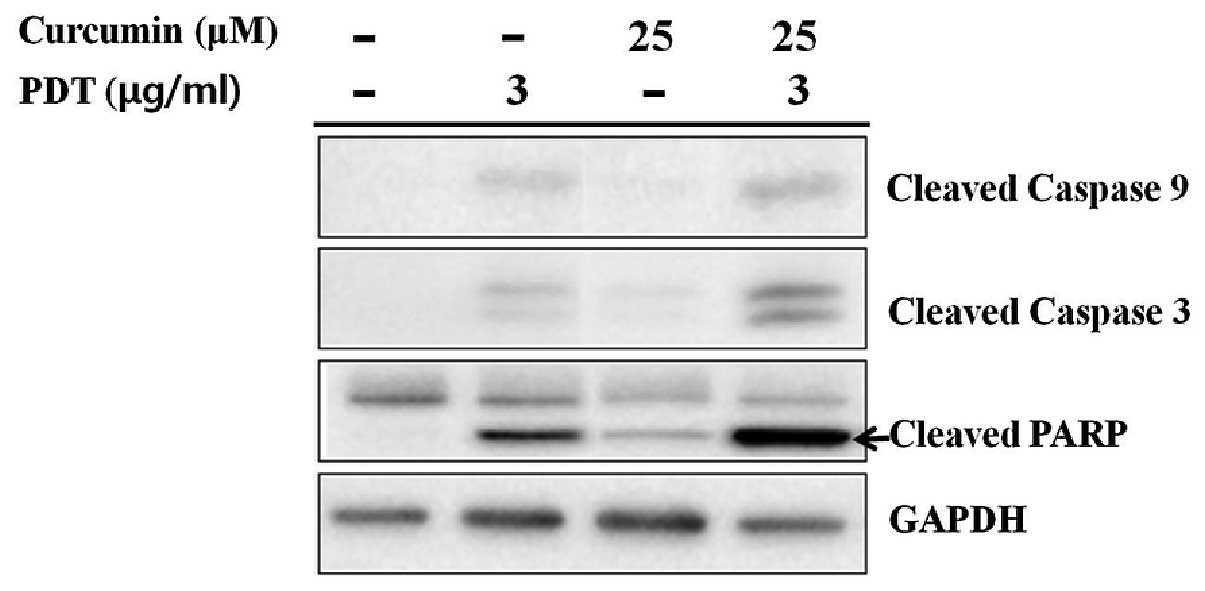
Increase of caspase-9, -3 and PARP
activities by combination treatment
Mitochondrial release of cytochrome c into
the cytosol may lead to the activation of caspase-9 and -3 for
apoptosis. The level of caspase-9 and caspase-3 activation was
determined to confirm the induction of mitochondrial-mediated
apoptosis. There was stronger expression of the cleaved form of
caspase-9 as well as its downstream executioner caspase-3 in the
combination group than that in PDT or curcumin only groups
(Fig. 3). PARP, as a native
substrate of caspase-3, showed a similar expression pattern to
cleaved caspase-3.
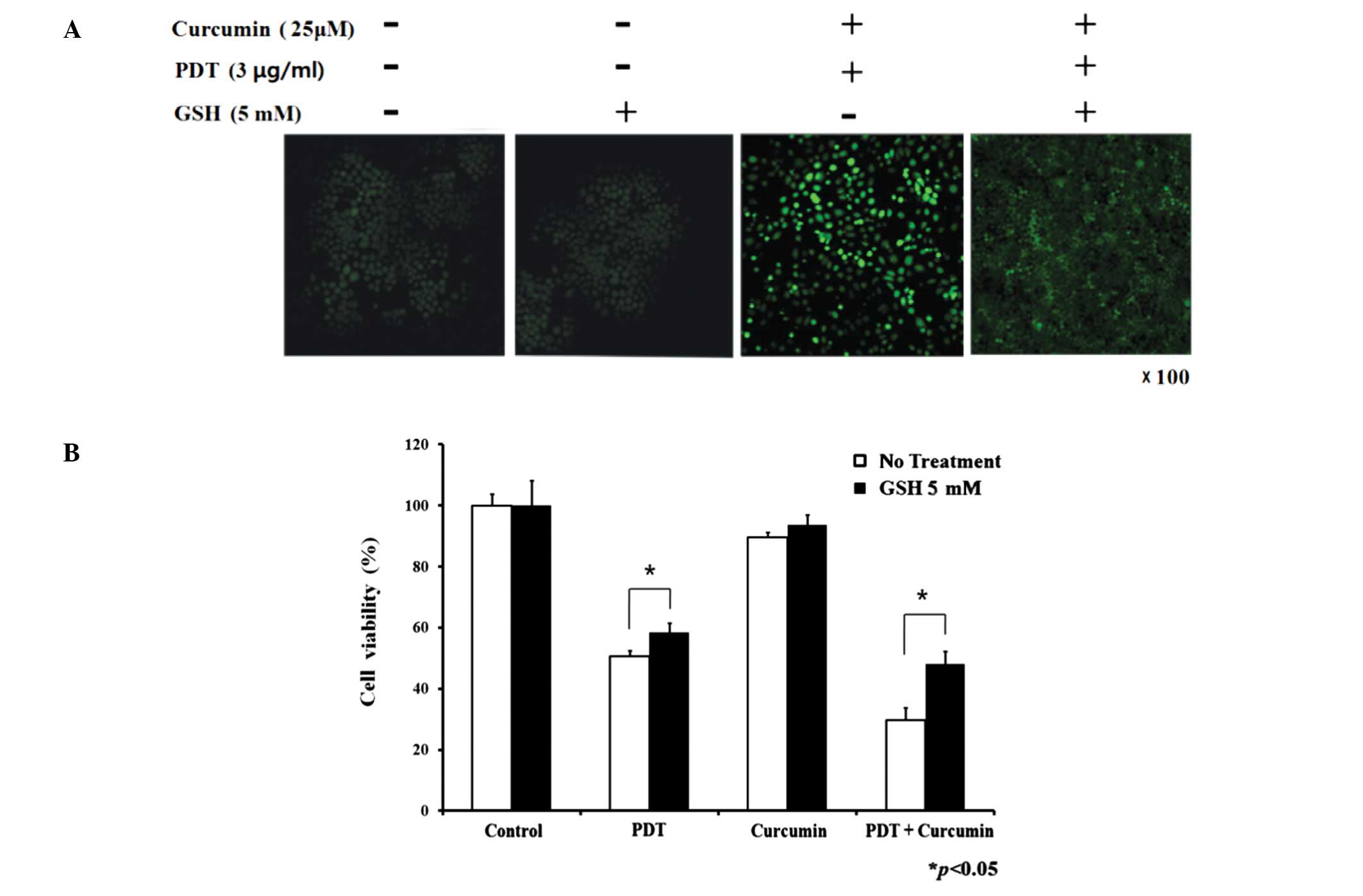
Decrease of combination treatment induced
ROS and cytotoxicity by glutathione
In Fig. 4A, the
enhanced intracellular ROS levels of the combination treatment
group were attenuated by glutathione (singlet oxygen quencher). As
described above, the combination group exhibited a more intense
cytotoxic effect than the single treatment groups. The decrease in
cell viability induced by PDT and the combination group was
prevented by glutathione (Fig.
4B), but not by D-mannitol (hydroxyl radical scavenger),
indicating that singlet oxygen plays a key role in PDT and
combination treatment. In the presence of glutathione, the cell
survival rate was elevated by approximately 17 and 23% in the PDT
only group and combination group, respectively.
Inhibition of apoptosis by
glutathione
The mitochondria-related apoptotic signals were
investigated to further confirm the molecular mechanisms by which
glutathione prevents apoptosis induced by the combination
treatment. The collapse of Δψm in the combination
therapy group was protected by a concomitant treatment with
glutathione (Fig. 5A). Similarly
to the change in Δψm, the release of cytochrome c
also decreased in the combination therapy group with glutathione
(Fig. 5B). Moreover, the
expression of caspase-3 and PARP protein were inhibited by
glutathione (Fig. 5C).
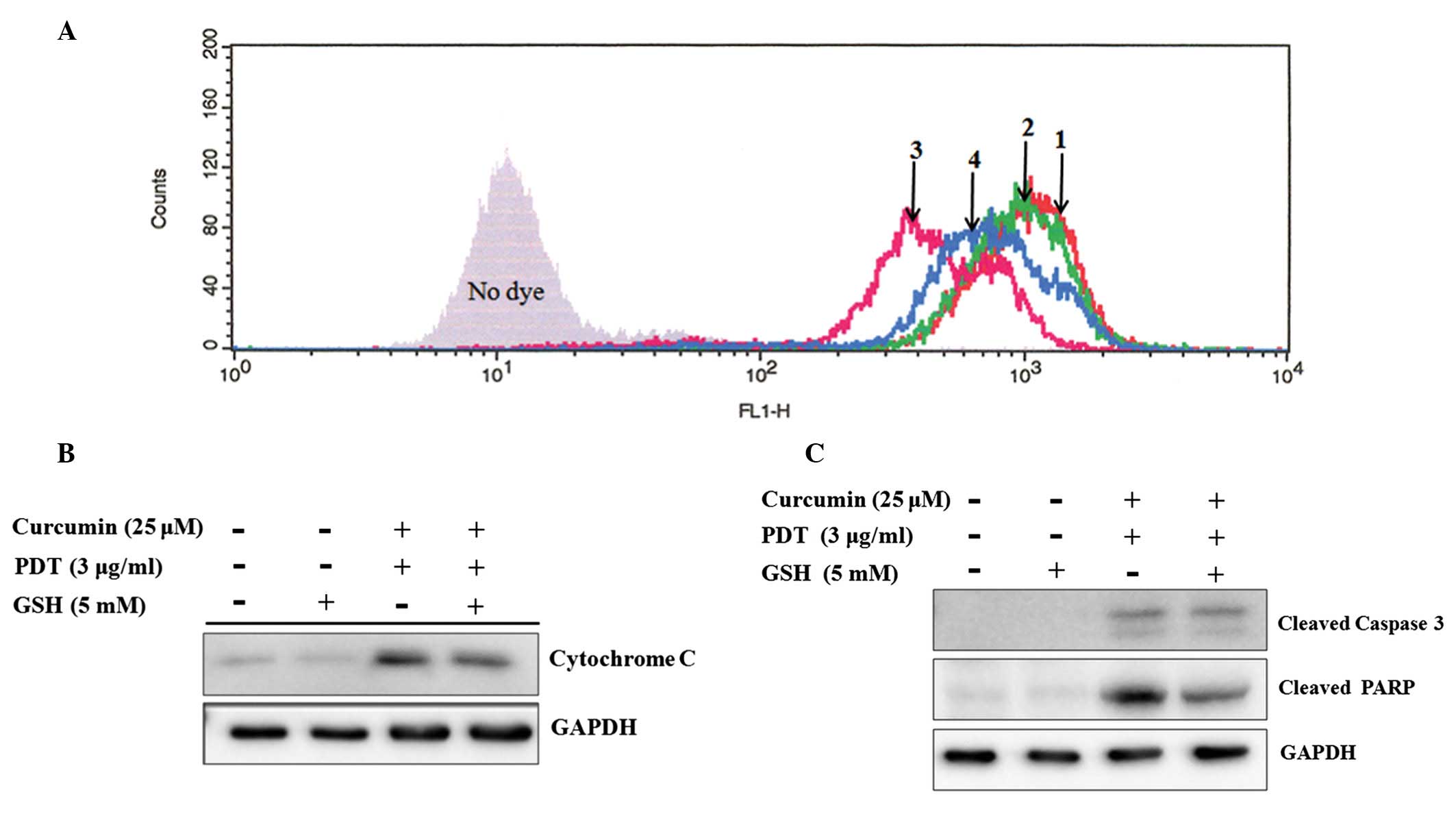 | Figure 5Effect of the singlet oxygen-specific
inhibitor glutathione on the combination treatment-induced MMP
collapse, cytochrome c release, as well as the activation of
caspase-3 and PARP. (A) The cells were incubated with curcumin and
glutathione for 6 h, treated with 3 μg/ml of photofrin and
glutathione for 6 h, then irradiated by laser without glutathione.
The treated cells were incubated at 2 h. The cells were stained
with Rhodamine 123 for 30 min at 37°C. MMP was analyzed by flow
cytometry. 1, control; 2, 5 mM of glutathione; 3, 25 μM
curcumin and 3 μg/ml of PDT, 4, 25 μM curcumin, 3
μg/ml of PDT and 5 mM of glutathione. (B) The cells were
treated with combination therapy or glutathione. Cytosolic fraction
(80 μg) was separated by 15% SDS-PAGE and then immunoblotted
with the anti-cytochrome c antibody. (C) The cells were
treated with combination therapy or glutathione. The total proteins
(80 μM) were extracted and separated by 10% SDS-PAGE. The
proteins were subjected to immunoblotting using the anti-caspase-3
and anti-PARP antibody, respectively. |
Discussion
PDT must provide an enhanced therapeutic response to
be used as a first-line curative modality (23). Consequently, combination regimens
consisting of PDT and a secondary treatment can be designed to
increase the effectiveness of PDT. The systemic toxicity might be
reduced due to the use of a lower dose of photosensitizer during
combination PDT.
The strategies of cancer treatment using combined
therapies or combined agents with distinct molecular mechanisms are
considered to be more promising for higher efficacy in the
induction of apoptosis. The number of publications regarding the
potentiated antitumor effects of cancer therapies using the
chemopreventive agent, curcumin, has increased dramatically. In
addition, common cancer therapies combined with these dietary
compounds may exert enhanced antitumor activity through synergistic
action (24).
Curcumin can both stimulate and inhibit apoptotic
signaling. For example, curcumin induces apoptosis in human
melanoma cells (30–60 μM for 24 h) (25), whereas recent studies reported that
curcumin attenuates UV irradiation-induced ROS formation and
apoptosis in epidermoid carcinoma A431 cells (25–50 μM for
2–3 h) (26), and prevents
PDT-induced cell death (27). In
this study, curcumin alone decreased the cell viability of AMC-HN-3
cells in a dose- and time-dependent manner (data not shown). The
curcumin plus PDT group showed an increased cytotoxic effect
compared to each single group (Fig.
1A).
Fig. 1B shows
infrequent apoptotic bodies in both the low concentration of PDT (3
μg/ml) and curcumin alone groups. On the other hand, the
combination group induced more apoptotic cells with typical
chromatin condensation, nuclear fragmentation, and the formation of
apoptotic bodies (Fig. 1B). This
suggests that the combination treatment initiated a more intense
apoptotic effect than that of each single treatment group.
The mitochondria are a major source of ROS in cells.
As reported, ROS play an important role in controlling a range of
cell functions, such as proliferation and apoptosis (28). In the apoptosis-inducing
concentrations, confocal microscopy analysis using the cell
permeable dye H2DCFDA as an indicator of ROS generation
showed that the intracellular oxidative stress caused by single
treatments were further enhanced by the combination therapy
(Fig. 2A). The generation of ROS
peaked at 1 h after PDT; the signal of which subsequently decreased
later, indicating that ROS formation is transient.
Loss of the mitochondrial membrane potential
(Δψm) is associated with a dysfunction of the
mitochondria, which can be detected in apoptotic cell death
(29). Previous studies reported
that the mitochondria are the targets of photodamage triggered by
photofrin-PDT (30). Biochemical
analysis indicated that HpD/photofrin PDT caused mitochondrial
damage and inactivation of the mitochondrial enzymes (31). Lam et al suggested that PDT
with Pc 4 triggers mitochondrial ROS production resulting in inner
membrane permeablization, mitochondrial depolarization and
swelling, which in turn leads to cytochrome c release and
apoptotic death (32). In this
study, the induction of ROS by curcumin or PDT alone was consistent
with the disruption of Δψm after treatment (Fig. 2B). Compared with the single
treatments, the combination group caused a more intense decrease in
Δψm and a much larger release of cytochrome c
from the mitochondria to the cytosol (Fig. 2C).
Caspases play critical roles in the initiation and
execution of apoptosis and are activated by cytochrome c
(33). In the present study, the
combination group showed stronger caspase-9, -3 and PARP activation
than those in the single treatment groups (Fig. 3).
Hydroxyl radical and singlet oxygen have been
reported to be important cellular mediators for PDT-induced
responses (34). The generation of
intracellular ROS initiated by the combination group was suppressed
by glutathione (Fig. 4A).
D-mannitol, hydroxyl radical scavenger, had no protective effect on
cell death induced by the single treatment groups or the
combination group. However, the cell viability induced by the
single or combination groups was protected by the co-treatment of
glutathione (singlet oxygen quencher) (Fig. 4B). This suggests that singlet
oxygen plays an important role in curcumin/photofrin-PDT-induced
cytotoxicity. Glutathione also inhibited the combination
therapy-induced Δψm collapse (Fig. 5A) and cytochrome c release
by suppressing the generation of ROS (Fig. 5B). The upregulation of caspase-3
and PARP by the combination group was prevented by glutathione
(Fig. 5C).
In summary, these results suggest that the combined
PDT with curcumin has enhanced cytotoxic and apoptotic effects on
AMC-HN3 cells via a mitochondria-dependent apoptosis pathway. In
addition, the generation of ROS plays an important role in this
combination therapy-induced apoptosis.
Acknowledgements
We would like to thank the Medical
Laser Research Center, Dankook University for the financial
support.
References
|
1.
|
Dougherty TJ, Gomer CJ, Henderson BW, et
al: Photodynamic therapy. J Natl Cancer Inst. 90:889–905. 1998.
View Article : Google Scholar
|
|
2.
|
Saczko J, Mazurkiewicz M, Chwilkowska A,
et al: Intracellular distribution of Photofrin in malignant and
normal endothelial cell lines. Folia Biol (Praha). 53:7–12.
2007.PubMed/NCBI
|
|
3.
|
Hopper C: Photodynamic therapy: a clinical
reality in the treatment of cancer. Lancet Oncol. 1:212–219. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Yamaguchi S, Tsuda H, Takemori M, et al:
Photodynamic therapy for cervical intraepithelial neoplasia.
Oncology. 69:110–116. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Copper MP, Triesscheijn M, Tan IB,
Ruevekamp MC and Stewart FA: Photodynamic therapy in the treatment
of multiple primary tumours in the head and neck, located to the
oral cavity and oropharynx. Clin Otolaryngol. 32:185–189. 2007.
View Article : Google Scholar
|
|
6.
|
Buytaert E, Dewaele M and Agostinis P:
Molecular effectors of multiple cell death pathways initiated by
photodynamic therapy. Biochim Biophys Acta. 1776:86–107.
2007.PubMed/NCBI
|
|
7.
|
Lu Y, Jiao R, Chen X, Zhong J, Ji J and
Shen P: Methylene blue-mediated photodynamic therapy induces
mitochondria-dependent apoptosis in HeLa cell. J Cell Biochem.
105:1451–1460. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Moor AC: Signaling pathways in cell death
and survival after photodynamic therapy. J Photochem Photobiol B.
57:1–13. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Niedre M, Patterson MS and Wilson BC:
Direct near-infrared luminescence detection of singlet oxygen
generated by photodynamic therapy in cells in vitro and tissues in
vivo. Photochem Photobiol. 75:382–391. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Ding X, Xu Q, Liu F, et al:
Hematoporphyrin monomethyl ether photodynamic damage on HeLa cells
by means of reactive oxygen species production and cytosolic free
calcium concentration elevation. Cancer Lett. 216:43–54. 2004.
View Article : Google Scholar
|
|
11.
|
Surh YJ: Cancer chemoprevention with
dietary phytochemicals. Nat Rev Cancer. 3:768–780. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Rahman I, Biswas SK and Kirkham PA:
Regulation of inflammation and redox signaling by dietary
polyphenols. Biochem Pharmacol. 72:1439–1452. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Choudhuri T, Pal S, Agwarwal ML, Das T and
Sa G: Curcumin induces apoptosis in human breast cancer cells
through p53-dependent Bax induction. FEBS Lett. 512:334–340. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Karmakar S, Banik NL and Ray SK: Curcumin
suppressed anti-apoptotic signals and activated cysteine proteases
for apoptosis in human malignant glioblastoma U87MG cells.
Neurochem Res. 32:2103–2113. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Aggarwal S, Takada Y, Singh S, Myers JN
and Aggarwal BB: Inhibition of growth and survival of human head
and neck squamous cell carcinoma cells by curcumin via modulation
of nuclear factor-kappaB signaling. Int J Cancer. 111:679–692.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Rashmi R, Kumar S and Karunagaran D: Human
colon cancer cells lacking Bax resist curcumin-induced apoptosis
and Bax requirement is dispensable with ectopic expression of Smac
or downregulation of Bcl-XL. Carcinogenesis. 26:713–723. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Liu HL, Chen Y, Cui GH and Zhou JF:
Curcumin, a potent anti-tumor reagent, is a novel histone
deacetylase inhibitor regulating B-NHL cell line Raji
proliferation. Acta Pharmacol Sin. 26:603–609. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Shishodia S, Chaturvedi MM and Aggarwal
BB: Role of curcumin in cancer therapy. Curr Probl Cancer.
31:243–305. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Park K and Lee JH: Photosensitizer effect
of curcumin on UVB-irradiated HaCaT cells through activation of
caspase pathways. Oncol Rep. 17:537–540. 2007.PubMed/NCBI
|
|
20.
|
Dujic J, Kippenberger S, Hoffmann S, et
al: Low concentrations of curcumin induce growth arrest and
apoptosis in skin keratinocytes only in combination with UVA or
visible light. J Invest Dermatol. 127:1992–2000. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Dursun B, He Z, Somerset H, Oh DJ, Faubel
S and Edelstein CL: Caspases and calpain are independent mediators
of cisplatin-induced endothelial cell necrosis. Am J Physiol Renal
Physiol. 291:F578–F587. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Chung PS, He P, Shin JI, Hwang HJ, Lee SJ
and Ahn JC: Photodynamic therapy with 9-hydroxypheophorbide alpha
on AMC-HN-3 human head and neck cancer cells: induction of
apoptosis via photoactivation of mitochondria and endoplasmic
reticulum. Cancer Biol Ther. 8:1343–1351. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Gomer CJ, Ferrario A, Luna M, Rucker N and
Wong S: Photodynamic therapy: combined modality approaches
targeting the tumor microenvironment. Lasers Surg Med. 38:516–521.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Sarkar FH and Li YW: Targeting multiple
signal pathways by chemopreventive agents for cancer prevention and
therapy. Acta Pharmacol Sin. 28:1305–1315. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Bush JA, Cheung KJ Jr and Li G: Curcumin
induces apoptosis in human melanoma cells through a Fas
receptor/caspase-8 pathway independent of p53. Exp Cell Res.
271:305–314. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Chan WH, Wu CC and Yu JS: Curcumin
inhibits UV irradiation-induced oxidative stress and apoptotic
biochemical changes in human epidermoid carcinoma A431 cells. J
Cell Biochem. 90:327–338. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Chan WH and Wu HJ: Anti-apoptotic effects
of curcumin on photosensitized human epidermal carcinoma A431
cells. J Cell Biochem. 92:200–212. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Thannickal VJ and Fanburg BL: Reactive
oxygen species in cell signaling. Am J Physiol Lung Cell Mol
Physiol. 279:L1005–L1028. 2000.PubMed/NCBI
|
|
29.
|
Hirsch T, Marchetti P, Susin SA, et al:
The apoptosis-necrosis paradox. Apoptogenic proteases activated
after mitochondrial permeability transition determine the mode of
cell death. Oncogene. 15:1573–1581. 1997. View Article : Google Scholar
|
|
30.
|
Kinzler I, Haseroth E, Hauser C and Ruck
A: Role of mitochondria in cell death induced by Photofrin-PDT and
ursodeoxycholic acid by means of SLIM. Photochem Photobiol Sci.
6:1332–1340. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Roberts WG, Liaw LH and Berns MW: In vitro
photosensitization II. An electron microscopy study of cellular
destruction with mono-L-aspartyl chlorin e6 and photofrin II.
Lasers Surg Med. 9:102–108. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Lam M, Oleinick NL and Nieminen AL:
Photodynamic therapy-induced apoptosis in epidermoid carcinoma
cells. Reactive oxygen species and mitochondrial inner membrane
permeabilization. J Biol Chem. 276:47379–47386. 2001. View Article : Google Scholar
|
|
33.
|
Budihardjo I, Oliver H, Lutter M, Luo X
and Wang X: Biochemical pathways of caspase activation during
apoptosis. Annu Rev Cell Dev Biol. 15:269–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Price M, Terlecky SR and Kessel D: A role
for hydrogen peroxide in the pro-apoptotic effects of photodynamic
therapy. Photochem Photobiol. 85:1491–1496. 2009. View Article : Google Scholar : PubMed/NCBI
|