Introduction
Natural products comprise a vast diversity of
complex structures and new chemical entities, whereas synthetic
libraries typically show considerably less diversity (1). Similarly to vincristine, more than
60% of currently applied anticancer drugs are derived from natural
sources, i.e., plants, microorganisms and marine organisms
(2). Vincristine is an alkaloid in
the leaves of the Madagaskar rosy periwinkle C. roseus, a
plant that was traditionally used to treat conditions like
hemorrhage, toothache, wounds, hyperglycemia and diabetic ulcers
(3). In less-developed countries,
a majority of the population still relies on traditional healing
plants in treating various conditions. Traditional healing plants
have the advantage that they have been successfully tested over the
centuries, exhibit specificity and are therefore tolerable with
little side effects. This makes them a prime source for the study
of new therapeutic perspectives. Therefore, natural products from
high biodiversity areas such as the lowland rain forests of El
Peten, Guatemala, are in this respect of particular interest. In
the present work, the Maya ethno-pharmacological plant
Neurolaena lobata was selected for investigations based on
its long history as anti-protozoan medicine (4). N. lobata is still widely used
in Guatemala and Belize to cure a variety of diseases, particularly
malaria and amoebiasis, fungus, ringworm and intestinal parasites
(5). More recently it is used also
as oncolytic home remedy. Hypoglycemic activity of the ethanol
extract was demonstrated in vivo(6) and a recent study reported an
inhibitory effect of N. lobata extracts on the transfer of
HIV from dendritic cells to lymphocytes in vitro(7). A few characteristic sesquiterpene
lactones which were isolated of N. lobata(8) exhibit cytotoxic effects in GLC4 and
COLO 320 tumor cell lines (9), but
show only weak toxicity on brine shrimp larvae Artemia
salina(10).
Anaplastic large cell lymphoma (ALCL) represents
appoximately 10–15% of childhood lymphoma, whereby the majority of
them carry chromosomal aberrations involving the anaplastic
lymphoma kinase (ALK) of which the t(2;5)(p23;q35) translocation,
which fuses the ALK gene to the nucleophosmin (NPM)
gene (11), represents the most
frequent one (12). The resulting
NPM/ALK fusion protein with constitutive tyrosine kinase activity
is considered to play an essential role in the pathogenesis of ALCL
through its impact on proliferation, differentiation and apoptosis
(13) and malignant transformation
in co-operation with other oncogenes (14). Therefore, ALK is suggested as
target for therapeutic intervention. Most ALK+ ALCL
patients respond with complete remission upon first-line treatment.
However, high relapse rates as well as long-term effects of
chemotherapy and radiation therapy have to be considered,
particularly in pediatric and adolescent patients, as both
treatments potentially damage normal cells which might turn into
secondary malignancies within decades of life time (15–17).
Combinatorial chemotherapy (CHOP: cyclophoshamide,
hydroxydaunorubicin (doxorubicin), oncovin (vincristine),
prednisone) is applied in the first treatment approach of ALCL
patients, sometimes combined with radiotherapy. Ongoing research
tries to discover a treatment directly targeting ALK (18) and an ALK inhibitor, critozinib, is
currently tested in a phase III trial (19). Here, we describe the property of
the dichloromethane extract of the ethno-pharmacological plant
N. lobata, which inhibits NPM/ALK expression and its
downstream effectors and its apoptosis inducing effects on leukemia
and lymphoma cells at low concentrations.
Materials and methods
Plant material
Neurolaena lobata was collected in Guatemala,
Departamento Petén, near the north-western shore of Lago Petén
Itzá, 0.5 km NNW of San José in the area of the Chakmamantok-rock
formation (16 59′16″ N, 89 53′45″ W). Voucher specimens (leg. R.O.
Frisch, det. R.O. Frisch Nr. 7-2009 28. 04. 2009, Herbarium W) were
archived at the Museum of Natural History, Vienna, Austria. The
fresh plant material (the aerial plant parts, leaves, caulis and
florescence) of N. lobata was stored deep-frozen until
lyophilization and subsequent extraction.
Plant extraction
Lyophilized aerial plant parts (leaves, florescence
and stipes) of N. lobata were powdered and consecutively
extracted with petroleum ether (PE), dichloromethane
(CH2Cl2), ethyl acetate (EA), methanol (MeOH)
and water (H2O), leading to five extracts of distinct
polarity. For this, plant powder was mixed at a concentration of
1:10 (w/v) with solvent and treated in an ultra sonic bath for 10
min to break the plant cells. The extraction was performed under
reflux on a waterbath for 1 h. Afterwards the content of the flask
was filtered. The solid plant residue was air-dried overnight and
reused for the following extraction step with the next, more polar
solvent, whereas the dissolved material of the liquid phase was
dried by rotary evaporation. Dried extracts were stored in a vacuum
desiccator protected from light at 4°C. Extract weights obtained
from serial extraction of 20.0 g lyophilized plant material with
five solvents of different polarity are shown in Table I. For further use in cell culture
experiments only small amounts of the gained extracts were
dissolved in DMSO. To account for detrimental effects of DMSO on
cell proliferation, apoptosis and cell cycle, controls were treated
with the respective concentrations of DMSO used for sample
treatment. Maximum concentration of DMSO was limited to 0.5% to
avoid cell damage due to toxicity of DMSO.
 | Table I.N. lobata extract weights used
for HL-60 treatment. |
Table I.
N. lobata extract weights used
for HL-60 treatment.
| Extract type | Used extract
concentrations (μg/ml medium) | Corresponding dried
plant weight (μg) |
|---|
| Petroleum
ether | 5 | 113.1 |
| 15 | 339.2 |
| 30 | 678.3 |
| 60 | 1356.7 |
|
Dichloromethane | 5 | 168.9 |
| 15 | 506.8 |
| 30 | 1013.7 |
| 60 | 2027.3 |
| Ethyl acetate | 5 | 341.4 |
| 15 | 1024.2 |
| 30 | 2048.4 |
| 60 | 4096.8 |
| Methanol | 5 | 92.4 |
| 15 | 277.3 |
| 30 | 554.6 |
| 60 | 1109.2 |
| Water | 5 | 41.6 |
| 15 | 124.8 |
| 30 | 249.5 |
| 60 | 499.0 |
Cell culture
HL-60, human promyelocytic leukemia cells, were
purchased from ATCC (American Type Culture Collection). HL-60,
SR-786, NPM-ALK positive human ALCL (anaplastic large cell
lymphoma) cells, and CD4-417, NPM-ALK positive mouse ALCL cells
were grown in RPMI-1640 medium supplemented with 10% heat
inactivated fetal calf serum (FCS), 1% L-glutamine and 1%
penicillin/streptomycin. Primary human lung fibroblasts (HLFs),
were cultivated in DMEM medium supplemented with 10% FCS and 1%
penicillin/streptomycin. Media and supplements were purchased from
Life Technologies. All cells were maintained in a humidified
atmosphere containing 5% CO2 at 37°C.
Proliferation assay
HL-60 cells were treated with the plant extracts to
discover the most anti-proliferative ones. For this, HL-60 cells
were seeded in 24-well plates at a concentration of
1×105 cells/ml allowing logarithmic growth within 72 h.
Afterwards cells were incubated with increasing concentrations of
plant extracts. The cell number was determined twice within 24 h
using a KX-21 N microcell counter (Sysmex Corp., Kobe, Japan).
Proliferation rates were calculated as described (20,21).
Only the dichloromethane extract, which exhibited the strongest
anti-neoplastic activity, was tested in other cell systems
including SR-786, CD4-417 and HLF. ALCL SR-786 (human) and CD-417
(mouse) cell lines were counted using an electronic cell counter
(Casy Roche). SR-786 cells were seeded in a 48-well plate at a
concentration of 2×105 cells/ml. Then, the cells were
incubated with increasing extract concentrations for 48 h. CD4-417
cells were seeded in 48-well plates at a concentration of
1×106 cells/ml and incubated with extract for 72 h.
Alamar-blue cytotoxicity assay
The alamar-blue assay (Invitrogen, Life
Technologies) was applied to measure cytotoxicity. The active
component of alamar-blue is resazurin, which is a non-toxic and
cell permeable compound. Upon entering an active cell resazurin is
converted to bright red fluorescent resorufin via reduction
reactions of cell metabolism. The amount of fluorescence produced
is proportional to the number of living cells and corresponds to
the cells metabolic activity. HLF cells were seeded into 500
μl medium of a 48-well plate at a concentration providing
confluence within the wells after 48 h. CD4-417 cells were seeded
into 48-well plates at a concentration of 1×106
cells/ml. Each well was filled with 500 μl cell suspension.
Increasing concentrations of dichloromethane extract or solvent
were added and after 24 and 48 h, respectively, 50 μl
alamar-blue reagent was added to each well and incubated for ∼90
min at 37°C until colour changed from blue to red. Afterwards the
48-well plate was placed into a multi-detection reader for
fluorescence and absorbance (Bio-Tek Instrument, Inc., Vermont,
USA). Plate reader software KC-4 (Bio-Tek) was used to determine
absorption at 570 nm. To calculate the differences in cell
viability, mean blank value (only medium) was subtracted from all
other measurement values to take fluorescence of the medium into
account. Mean value of the control samples was considered as 100%
cell viability. The mean values of treated samples are described as
percentage of control sample viability.
Apoptosis assay
Hoechst 33258 (HO) and propidium iodide (PI) double
staining (both Sigma, St. Louis, MO) allows the determination of
the type of death the cell is undergoing, i.e., apoptosis (early or
late) or necrosis (22–24). HL-60 cells were seeded in a 24-well
plate at a concentration of 1×105 cells/ml and treated
with increasing concentrations of the specified extracts. After 24
h of incubation, 100 μl cell suspension of each well were
transferred into separate wells of a 96-well plate and Hoechst
33285 and propidium iodide were added at final concentrations of 5
and 2 μg/ml, respectively. After 1 h of incubation at 37°C,
stained cells were examined and photographed on a fluorescence
microscope (Axiovert, Zeiss) equipped with a DAPI filter. Type and
number of cell deaths was evaluated by visual examination of the
images according to the morphological characteristics revealed by
HOPI staining.
Cell cycle distribution (FACS)
analysis
CD-417 cells were seeded in a 6-well plate at a
concentration of 1×106 cells/ml. Then dichloromethane
extract of N. lobata was added to a final concentration of
5, 10 and 15 μg/ml. After 24 h of incubation at 37°C, cells
were harvested, transferred into 15-ml tubes and centrifuged (4°C,
800 rpm, 5 min). The supernatant was discarded and the cell pellet
was washed with cold PBS, centrifuged (4°C, 800 rpm, 5 min),
resuspended in 1 ml cold ethanol (70%), and either fixed for 30 min
at 4°C or stored at −20°C prior further handling. After two washing
steps with cold PBS, the cell pellet was resuspended in 500
μl cold PBS and transferred into a 5 ml polystyrene round
bottom tube. RNAse A and propidium iodide were added to a final
concentration of 50 μg/ml each and incubated for 1 h at 4°C.
The final cell number was adjusted between 0.5 and 1×106
cells in 500 μl. Cells were analyzed by FACSCalibur flow
cytometer (BD Bioscience, San Jose, CA, USA). Cell cycle
distribution was calculated with ModFid LT software (Verity
Software House, Topsham, ME, USA).
Western blotting
CD4-417 cells were seeded in a 6-well plate at a
concentration of 106 cells/ml and treated with 10
μg/ml of the dichloromethane extract of N. lobata.
SR-786 cells were seeded in a T-75 tissue culture flask at a
concentration of 2.5×105 cells/ml and incubated with 15
μg/ml dichloromethane extract. Cells (106) were harvested
after 4, 8 and 24 h. Additionally, Proteasome Inhibitor IV (cat.
no. 539175, Merck) was added to a final concentration of 50
μM in a single experiment. In another experiment lysosome
inhibitor ammonium chloride (NH4Cl) was added at a
concentration of 20 mM. HL-60 cells were seeded in T-75 tissue
culture flasks at a concentration of 1.8×105 cells/ml
and incubated with 15 μg/ml extract. HLFs were grown in a
6-well plate and incubated with 10 and 15 μg/ml
dichloromethane extract of N. lobata. Cells were washed
twice with cold PBS and centrifuged at 1000 rpm for 5 min at 4°C.
The cell pellet was lysed in a buffer containing 150 mM NaCl, 50 mM
Tris pH 8.0, 1% Triton-X-100, 1 mM phenylmethylsulfonyl fluoride
(PMSF) and 1 mM Protease Inhibitor Cocktail (PIC), (Sigma,
Schnelldorf, Germany). The lysate was centrifuged at 12000 rpm for
20 min at 4°C. Supernatant was transferred into a 1.5-ml tube and
stored at −20°C until further analysis. Equal amounts of protein
lysate were mixed with SDS (sodium dodecyl sulphate) sample buffer
and loaded onto a 10% polyacrylamide gel. Proteins were separated
by polyacrylamide gel electrophoresis (PAGE) at 120 V and
electro-transferred onto a PVDF (polyvinylidene difluoride)
membrane (Hybond, Amersham, Buckinghamshire, UK) at 95 V for 80
min. Membranes were allowed to dry for ≥30 min up to 2 h to provide
fixing of the proteins to the membrane. Methanol was used to
remoisten the membranes. Equal sample loading was checked by
staining the membrane with Ponceau S (Sigma). After removing
Ponceau S with PBS or TBS (Tris-buffered saline, pH 7.6), membranes
were blocked in PBS- or TBS-milk (5% non-fat dry milk in PBS
containing 0.5% Tween-20 or TBS containing 0.1% Tween-20) for 1 h.
Then, membranes were washed with PBS/T (PBS containing 0.5%
Tween-20) or TBS/T (TBS containing 0.1% Tween-20), changing the
washing solution 4–5 times, for ≥20 min. Next, membranes were
incubated with the primary antibody in blocking solution (according
to the data sheet TBS-, PBS-milk or TBS-, PBS-BSA) diluted
1:500–1:1000, gently shaking at 4°C, overnight. Thereafter,
membranes were washed again with PBS/T or TBS/T and incubated with
the secondary antibody (peroxidase conjugated anti-rabbit IgG or
anti-mouse IgG) diluted 1:2000 in PBS- or TBS-milk at room
temperature for 1 h. Chemiluminescence was developed by ECL
detection kit (Amersham) and membranes were exposed to Amersham
Hyperfilm.
The used antibodies were: CD246, ALK protein,
monoclonal mouse, clone ALK1, code M7195 and Nucleophosmin,
monoclonal mouse, clone 376, code M7305 (DakoCytomation); PDGF
Receptor β (28E1) Rabbit mAB, no. 3169; Chk1 (2G1D5) Mouse mAb, no.
2360; Phospho-Chk1 (Ser345) Antibody, no. 2341; Chk2 Antibody, no.
2662; Phospho-Chk2 (Thr68) Antibody, no. 2661; Cleaved PARP
(Asp214) Antibody (Mouse Specific), no. 9544; Cleaved Caspase 3
(Asp175) Antibody, no. 9661; Caspase 3 Antibody, no. 9662;
Phospho-cdc2 (Tyr15)(10A11) Rabbit mAb, no. 4539; Phospho-p53
(Ser20) Antibody, no. 9287; Phospho-Akt (Ser473)(587F11) Mouse mAb,
no. 4051; Akt Antibody, no. 9272, all from (Cell Signaling);
PhosphoDetect Anti-H2AX (pSer139), DR 1017 (EMD4Biosciences); p53,
mouse monoclonal, cat. no. 1767 (Immunotech, Coulter Co.); PARP-1
(F-2): sc-8007, mouse monoclonal; Cdc25A (F-6): sc-7389, mouse
monoclonal; Cdc25B (C-20): sc-326, rabbit polyclonal; Cdc25C
(C-20): sc-327, rabbit polyclonal; Cdc2 p34 (17): sc-54, mouse monoclonal; JunB (120):
sc-73, rabbit polyclonal; c-Jun (H-79): sc-1694, rabbit polyclonal;
p21 (C-19): sc-397, rabbit polyclonal, all from (Santa Cruz
Biotechnology, Inc.); c-Myc Ab-2 (9E10.3), no. MS-139-P1, mouse
monoclonal (Thermo Fisher Scientific, Inc.); β-actin, monoclonal
mouse ascites fluid, clone AC-15, cat. no. A5441 (Sigma).
Quantitative RT-PCR
SR-786 cells were seeded in T-75 tissue culture
flasks at a concentration of 2.5×105 cells/ml and
incubated with 15 μg/ml dichloromethane extract of N.
lobata. After 4 and 8 h cells were harvested and homogenized
using Qia-shredder (Qiagen) and RNA isolated according to the
instructions of RNeasy Mini Kit (Qiagen). RNA concentration was
measured using a NanoDrop Fluorospectrometer (Thermo Fisher
Scientific, Inc.) and RNA was stored at −80°C until further
processing. First-strand cDNA synthesis from 1 μg RNA was
performed using Superscript first-strand synthesis systems for
RT-PCR (Invitrogen). cDNA synthesis reaction was primed using
random hexamers. Desired mRNA was obtained by choosing specific
primers in the PCR. NPM/ALK transcript levels in SR-786 cells were
investigated by RT-PCR using TaqMan detection system. The
housekeeping gene glyceralaldehyde 3-phosphate dehydrogenase
(GAPDH) served as reference gene. For each sample, 7 μl
H2O, 10 μl TaqMan universal PCR master mix
(Applied Biosystems), 1 μl primer and probe and 2 μl
cDNA, or 2 μl H2O for negative controls, were
filled into a well of a 96-well optical reaction plate. In case of
GAPDH amplification, the primer and probe mixture from TaqMan gene
expression kit (Applied Biosystems) was used. To detect NPM/ALK
transcripts, forward primer (GTG GTC TTA AGG TTG AAG TGT GGT T) and
reverse primer (GCT TCC GGC GGT ACA CTA CTA A) were mixed with the
probe (TGC TGT CCA CTA ATA TGC ACT GGC CCT) prior adding to the
reaction mix. Final concentration of primers and probe in the
sample mixtures were 0.5 and 0.25 μM, respectively. Cycle
program (95°C for 10 min to activate polymerase followed by 40
cycles of 95°C for 15 sec and 60°C for 1 min) was started on an ABI
PRISM 7000 Sequence Detection System (Applied Biosystems). RT-PCR
was performed in duplicates for each cDNA template and gene
investigated. Negative controls, containing water instead of cDNA,
confirmed the absence of RNA/DNA in all reagents applied in the
assay. Comparative CT (ΔΔCT) method (25) was used to quantify NPM/ALK
expression relative to GAPDH expression using the formula below.
The CT value is determined as the number of PCR cycles
that is needed to reach a defined level of fluorescence and
therefore newly synthesized DNA. Ratio = 2−ΔΔCT.
ΔCT = CT target gene (NPM-ALK) -
CT control gene (GAPDH). ΔΔCT =
ΔCT drug treatment - ΔCT control sample.
Statistical analysis
The apoptosis, proliferation, toxicity, FACS and
Q-PCR experiments were analyzed by t-test using GraphPad Prism
version 4 (GraphPad Prim Sofware, Inc., San Diego, CA, USA). Data
were considered statistically significant at p≤0.05.
Results
Anti-neoplastic activities of N. lobata
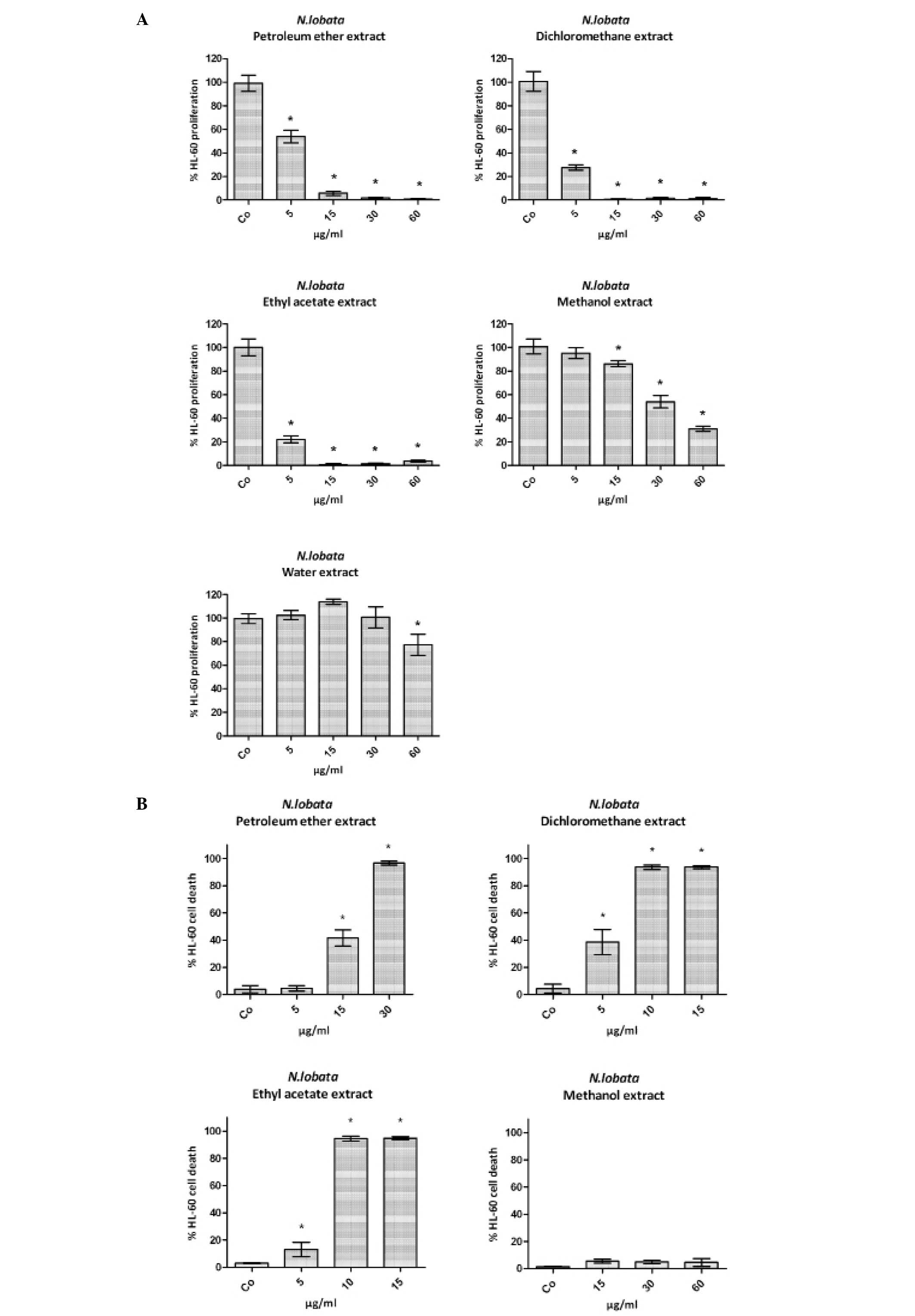
extracts in HL-60 cells
To explore anti-neoplastic effects logarithmically
growing HL-60 cells were treated with increasing extract
concentrations (5–60 μg/ml) of the aerial parts of N.
lobata and the effects on cell proliferation and apoptosis
were monitored. All extracts dose-dependently inhibited
proliferation whereby the dichloromethane and ethyl acetate extract
exhibited the highest and the methanol and water extract the lowest
activities (Fig. 1A). Therefore,
the water extract was excluded from further analyses. From the
remaining four extracts the dichloromethane extract induced the
strongest pro-apoptotic effect (Fig.
1B) and subsequent investigations were hence continued with
this extract type.
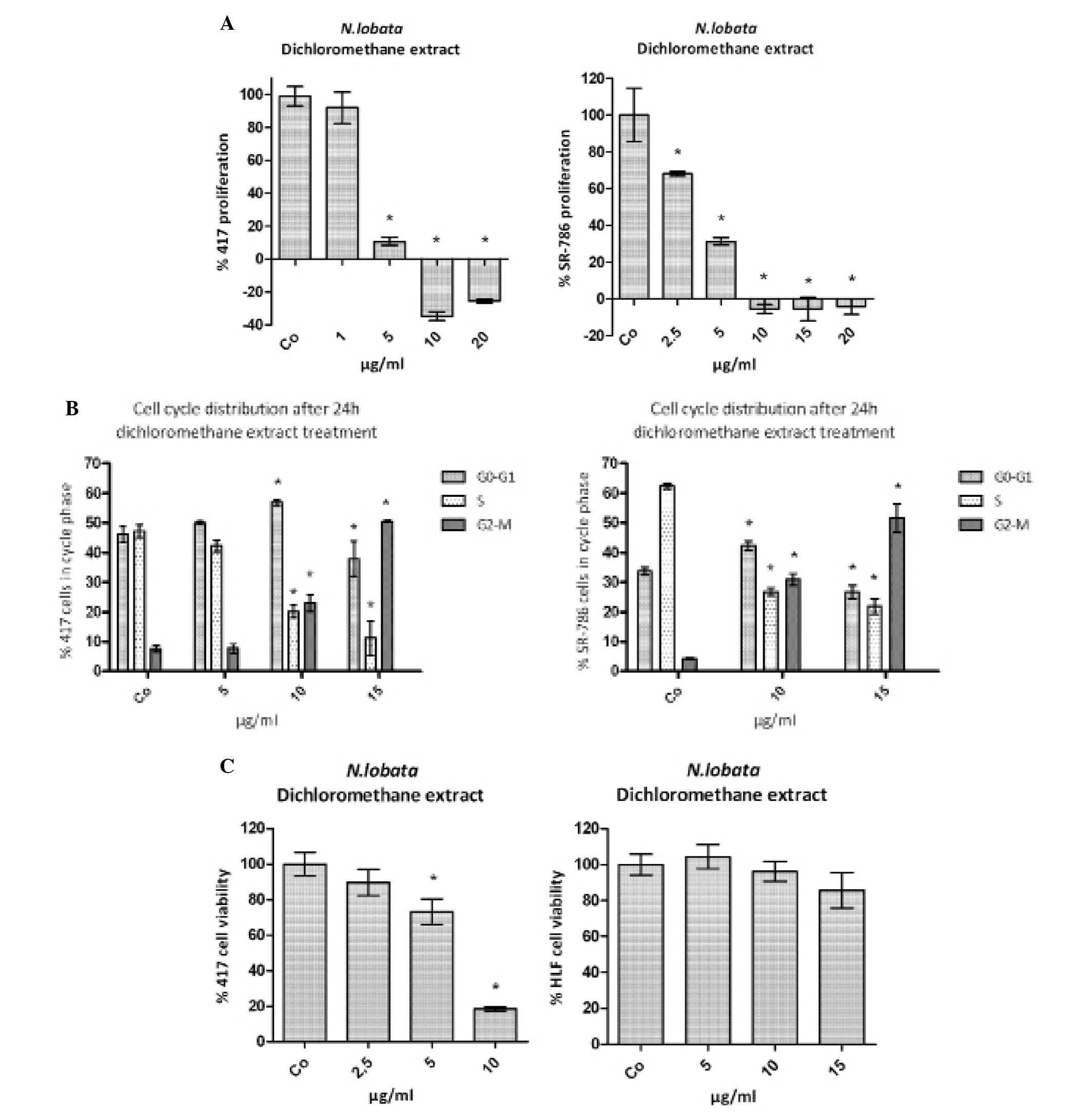
The dichloromethane extract inhibits proliferation
of human and murine ALCL cells. We decided to test the N.
lobata dichloromethane extract (further on simply called
‘extract’) in ALCL cells, because there is still no specific cure
available for this malignancy. Human ALCL SR-786 and murine ALCL
CD4-417 cells were treated with increasing concentrations of
extract to analyze the inhibition of cell proliferation. The
concentration inhibiting 50% of the proliferation of SR-786 cells
was ∼3.8 μg/ml and of CD4-417 cells ∼2.5 μg/ml
(Fig. 2A). In both cell lines the
extract inhibited the cell cycle dose-dependently in the G2/M
phase, which resulted in an accumulation of the cells in this cycle
phase paralleled by a decreasing number of cells particularly in
S-phase (Fig. 2B). The
anti-proliferative effect of the extract was specific for the
tested leukemia and lymphoma cell lines, because there was no
significant toxicity observed in normal human lung fibroblasts
(HLF) compared to CD4-417 cells (Fig.
2C).
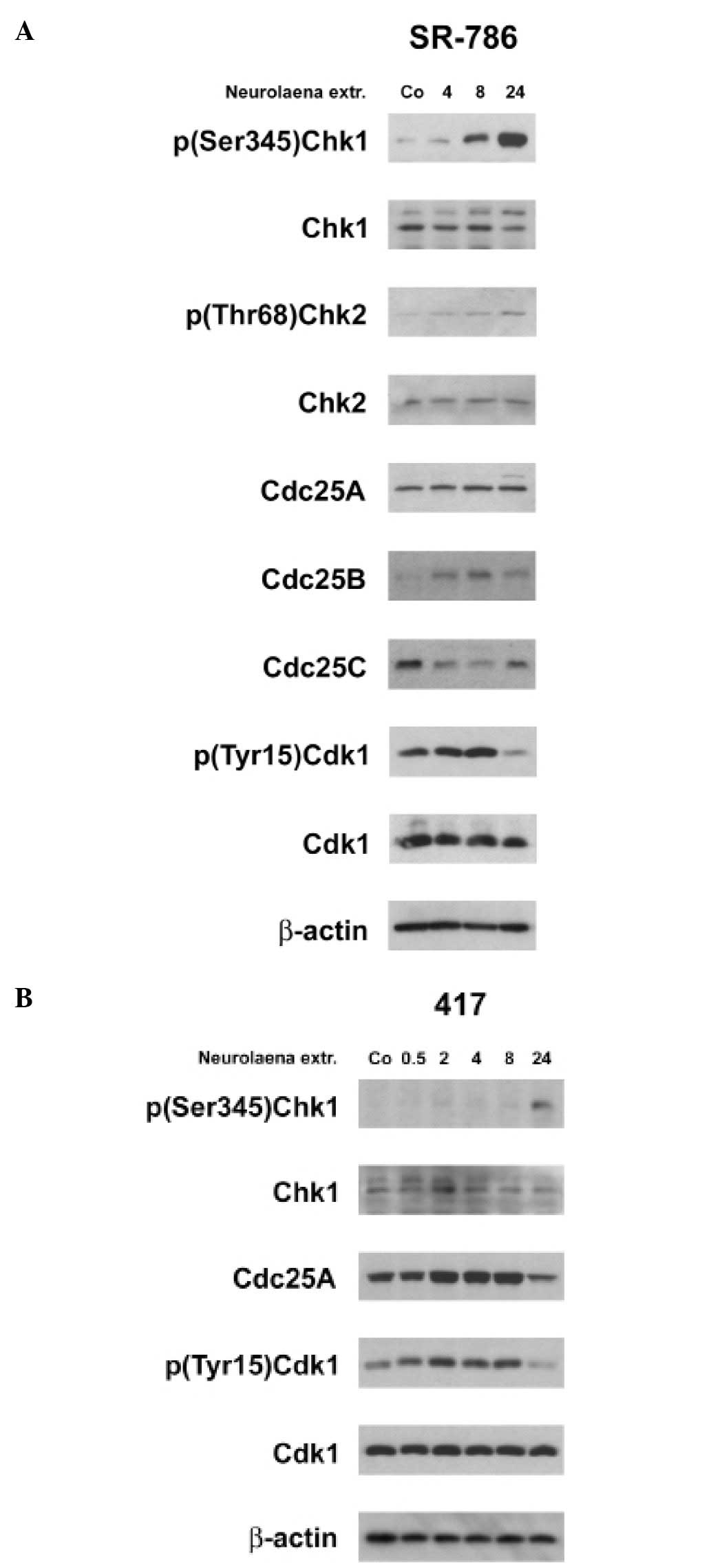
N. lobata extract activates DNA damage
checkpoints
In both ALCL cell lines the extract induced the
phosphorylation (activation) of Chk1 and in human SR-786 cells also
Chk2 activation was evident (Fig.
3A). This was consistent with the downregulation of Cdc25C, the
concomitant increase of the inhibitory Cdk1 phosphorylation and
with cell cycle arrest in G2/M. In contrast, the expression of
Cdc25B was upregulated and Cdc25A unaffected in SR-786 cells. In
murine CD4-417 cells Cdc25A became downregulated after 24 h
(Fig. 3B). Conflicting signaling
of Cdc25 family proteins is an alternative explanation for the
observed G2/M cell cycle arrest.
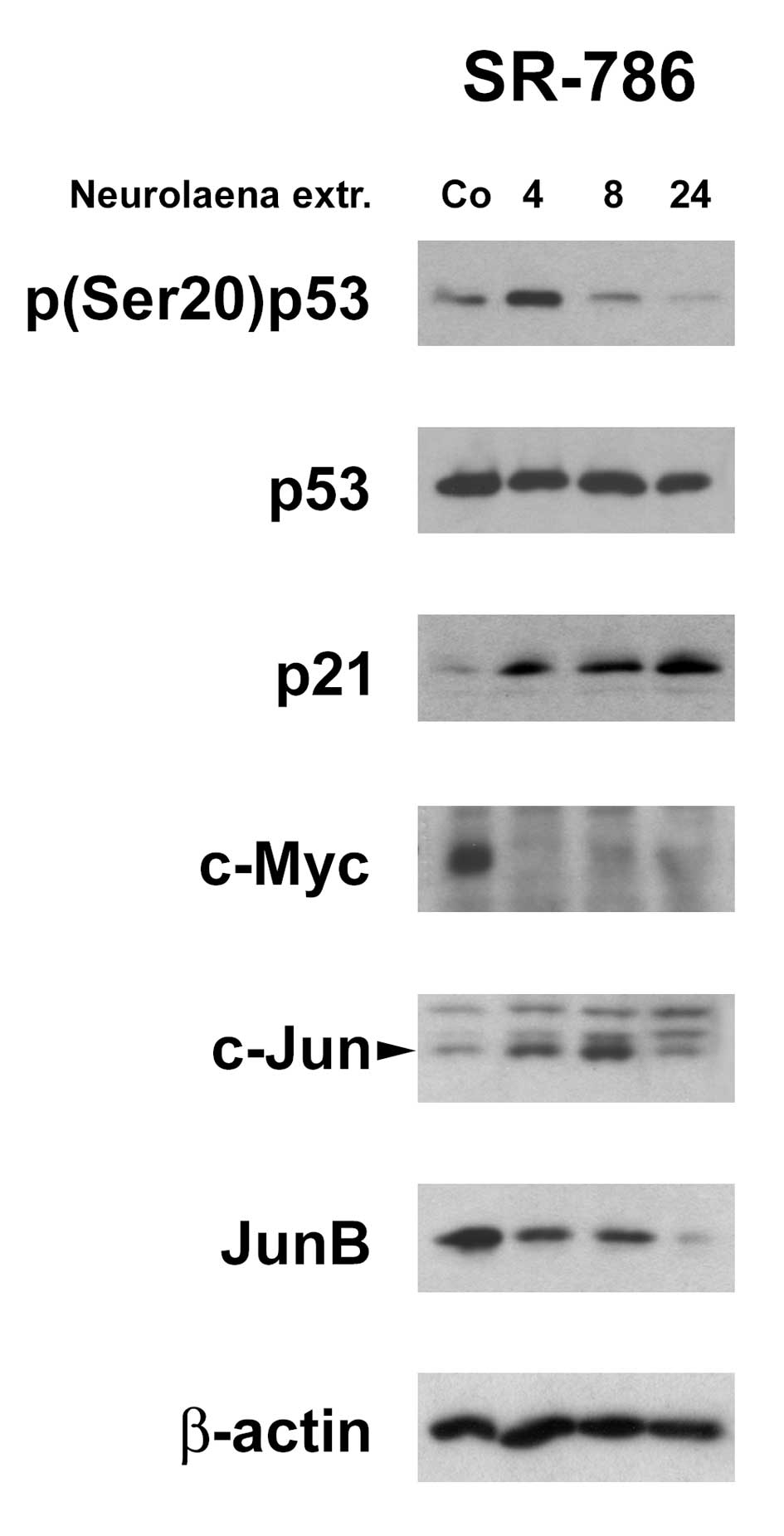
N. lobata extract induces tumor
suppressors and inhibits oncogenes
The induction of DNA checkpoints was paralleled by
the activation of p53 indicated by its phosphorylation at serine 20
and concomitant increase of its direct transcriptional target p21.
Also c-Myc was shown to negatively regulate the transcription of
p21 (26) and hence, c-Myc
downregulation may have caused p21 induction. It is of note that
p21 upregulation (after 4 h) precisely correlated with the
downregulation of c-Myc in SR-786 cells. Also the expression of
JunB was dramatically inhibited whereas c-Jun levels increased and
an additional protein band with reduced electrophoretic mobility
appeared on the western blotting indicating a post-translational
modified c-Jun protein (Fig. 4).
JunB and c-Jun are overexpressed in ALCL favoring progression
(27). Taking together, the
activation of p53, the induction of p21, and the inhibition of
c-Myc and JunB expression certainly contributed to proliferation
arrest.
N. lobata extract inhibits expression of
NPM/ALK
As mentioned above, high JunB levels are common in
ALCL (27) and caused by NPM/ALK
(28). Hence, the decrease of JunB
protein levels tempted us to analyze the effect of the extract on
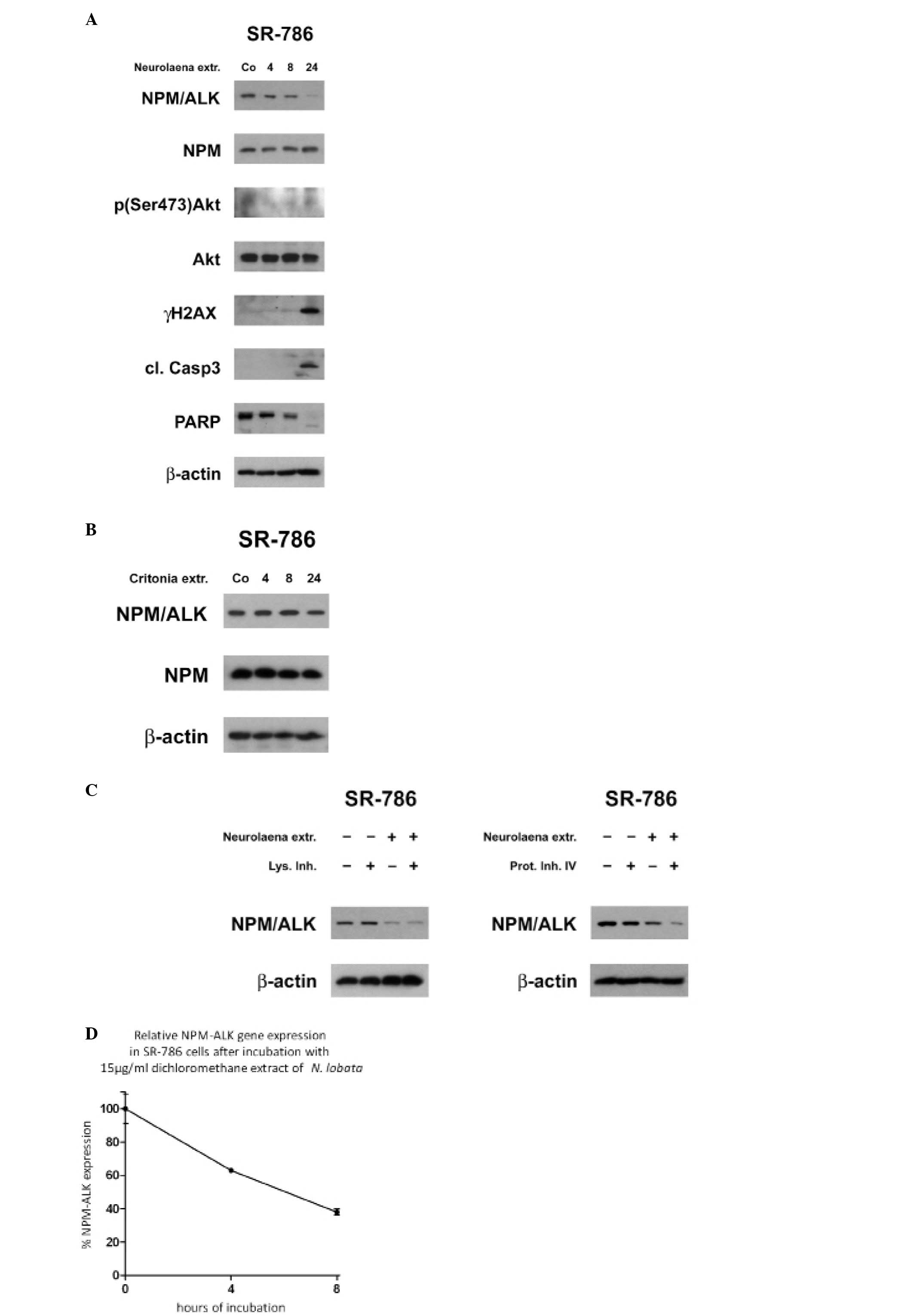
the expression of NPM/ALK. Treatment of SR-786 cells with the
extract decreased the level of NPM/ALK with similar kinetics as
JunB which is consistent with the role of NPM/ALK in the induction
of JunB (Fig. 5A). Therefore, we
challenged the specificity of the N. lobata extract and
treated SR-786 cells with the petroleum ether extract of
Critonia morifolia, which is another plant with strong
anti-neoplastic properties (29).
C. morifolia extract (20 μg/ml) exhibits a similar
cytotoxic activity against HL-60 cells as 10 μg/ml N.
lobata extract (data not shown). However, 20 μg/ml of
C. morifolia extract had no effect on NPM/ALK expression in
SR-786 cells (Fig. 5B). Therefore,
we considered the effect of the N. lobata extract on NPM/ALK
suppression as being specific. NPM/ALK was reported to permanently
activate Akt via PI3K (30,31).
The western blot analysis of untreated control cells showed a low
constitutive Akt serine 473 phosphorylation level, which further
and transiently decreased upon treatment with N. lobata
extract. The downregulation of NPM/ALK correlated also with the
activation of caspase 3 and the occurrence of the signature type
cleavage product of PARP and an increase of γH2AX. In contrast, the
expression of NPM, the 5-prime fusion partner of the
t(2;5)(p23;q35) translocation was not inhibited. Next, we tested
whether NPM/ALK downregulation was due to accelerated protein
degradation. However, neither 20 mM ammonium chloride, which
inhibits the lysosomal pathway (32) nor 50 μM Proteasome inhibitor
IV reversed the reduction of NPM/ALK expression levels (Fig. 5C). Instead, the extract decreased
the NPM/ALK transcript levels (Fig.
5D).
N. lobata extract inhibits expression of
PDGF-Rβ
It was shown that NPM/ALK induces JunB and c-Jun
(27,28). Recently JunB and c-Jun were
identified as transcriptional activators of PDGF-Rβ which
synergizes with ALK in ALCL development as confirmed in an ALCL
mouse model. In human ALCL PDGF-Rα takes this part. It was recently
shown that imatinib and nilotinib are potent treatment options for
ALCL because both inhibit PDGF-R (33). Interestingly, in established human
ALCL cell lines harboring the t(2;5)(p23;q35) translocation PDGF-Rα
expression disappears, whereas in murine ALCL cell lines PDGF-Rβ
remains expressed in certain cell lines (33). Therefore, we tested whether extract
treatment affects the expression of PDGF-Rβ in the murine CD4-417
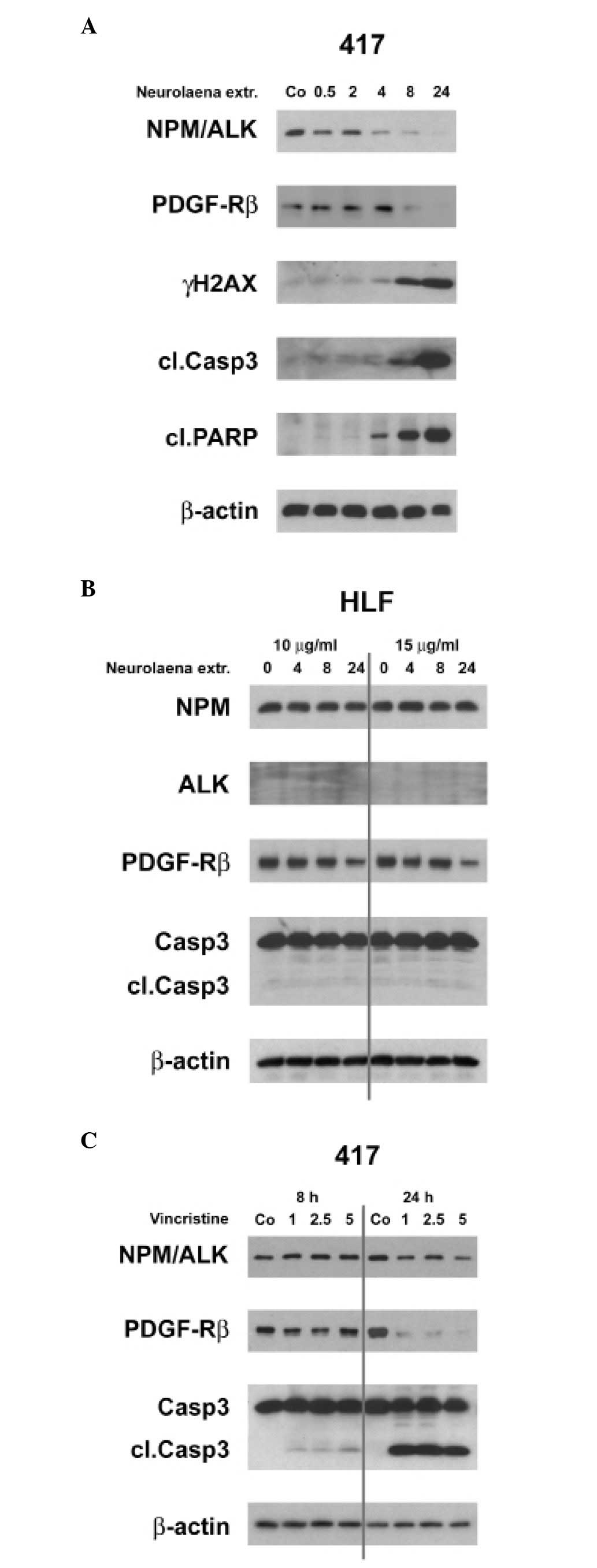
ALCL cell line. The extract inhibited first the expression of
NPM/ALK and its mediator JunB (4 h) and then the PDGF-Rβ (8 h)
(Fig. 6A). While it was shown that
PDGF-Rβ promotes tumor growth and progression (34,35)
we demonstrate that reduced NPM/ALK expression (after 4 h)
correlated with caspase 3 activation, signature type cleavage of
PARP and occurrence of γH2AX, rather than whith downregulation of
PDGF-Rβ expression (after 8 h) (Fig.
6A). This was consistent with the observation that tyrosine
kinase inhibition induced caspase-dependent apoptosis of
ALK+ALCL (36). In
normal HLFs PDGF-Rβ was only slightly reduced after 24 h of extract
treatment and caspase 3 was not activated (Fig. 6B) indicating specificity of the
extract towards malignant cells.
We wanted to know whether an ALCL standard therapy,
vincristine, also downregulates NPM/ALK and PDGF-Rβ. Although
vincristine dose- and time-dependently inhibited PDGF-Rβ protein
expression it only marginally inhibited NPM/ALK expression
(Fig. 6C). Therefore, vincristine
seems to exert its anti-ALCL activity through downregulation of
PDGF-Rβ and thus, targets the same protagonist as imatinib, yet by
a different mechanism. In contrast, N. lobata extract
targets mainly NPM/ALK, which further interferes with signaling
downstream to JunB and to PDGF-Rβ. This points towards a specific
‘active principle’ which is contained in the extract and could be
used to combat ALCL. Inhibition of ALK with critozinib and of
PDGF-R with imatinib increased the survival rates in an ALCL mouse
model dramatically (33).
Therefore, it can be expected that the extract of N. lobata
may have a strong effect in the repression of ALCL. Furthermore,
the extract must contain also other anti-neoplastic components,
because HL-60 cells, which neither harbor the t(2;5)(p23;q35)
translocation nor express p53 were killed by low extract
concentrations.
Discussion
In search of novel cancer and lymphoma therapies we
tested traditional healing plants and purified plant compounds
regarding their anti-neoplastic properties (37–46).
The rationale for plant selection was their ethno-pharmaceutical
use against chronic inflammation or severe skin defects (4). In the present study we analysed N.
lobata, a plant which is widely used in Maya folk medicine to
treat malaria (4). A panel of
characteristic sesquiterpene lactones were isolated of N.
lobata such as neurolenin A- F and lobatin A–C (8) and also pyrrolizidine alkaloids
(47). The plant extract and pure
sesquiterpene lactones (i.e., neurolenin B), which were isolated
from the dichloromethane extract, were most active against
Plasmodium falciparum and P. berghei, whereas lobatin
B was most cytotoxic in GLC4 and COLO320 tumor cell lines (9). This strongly suggests that the active
principles of the dichloromethane extract used in our research were
also sesquiterpene derivatives. Other drugs of this substance
class, i.e., artemisinin, thapsigargin, and parthenolide, have
already reached clinical trials. These compounds exhibit higher
selectivity toward cancer cells then other commonly used
chemotherapeutic drugs (48,49).
In recent years also Maya shamans of Guatemala treat cancer
patients with N. lobata preparations. In the present study
we demonstrate that the dichloromethane extract inhibits oncogenes
such as c-Myc and JunB and induces the tumor suppressors p53 and
p21. It blocks NPM/ALK expression at the transcriptional level and
to the best of our knowledge this is the first description of a
remedy with this specific property. It was shown that NPM/ALK
activates PI3K and the survival promoting kinase Akt (30,31).
Here, the downregulation of NPM/ALK by the N. lobata extract
correlated with the activation of caspase 3 and apoptosis. In
addition, NPM/ALK induces the expression of the oncogene JunB
(28) that is associated with
NPM/ALK carcinogenic transformation (50). JunB overexpression is a hallmark of
non-Hodgkin lymphomas such as ALCL (27) and drives the proliferation and
progression of these malignancies. The kinetics of extract-mediated
NPM/ALK- and JunB downregulation were similar implicating that
NPM/ALK inhibition and JunB suppression were connected. Most
recently it was shown that JunB and c-Jun are transcription factors
of PDGF-Rβ (33). PDGF-Rβ, a
receptor tyrosine kinase, which plays an important role in
proliferation and differentiation (51) is overexpressed in ALCL causing the
vascularization and progression of this lymphoma (33) and therefore, correlates with bad
prognosis. The treatment of a terminal ALCL patient with a
t(2;5)(p23;q35) translocation with imatinib, which inhibits the
tyrosine kinase activity of PDGF-Rβ, caused complete remission
within 14 days and this further confirms that the downstream
effectors of NPM/ALK are JunB and PDGF-Rβ (33). Thus, the inhibition of NPM/ALK by
the N. lobata extract most likely caused the downregulation
of JunB and with some delay the decrease of PDGF-Rβ expression. The
inhibition of NPM/ALK expression by N. lobata was specific,
because the extract of C. morifolia or vincristine (which is
used for ALCL treatment) did not affect NPM/ALK levels. N.
lobata was toxic for malignant cells, but not for normal HLFs.
In mice, oral and intra-peritoneal administration of 500 mg/kg of
the water, ethanol and dichloromethane extract every 48 h for three
weeks did not exhibit sub-acute toxicity and oral dosages up to 5
g/kg did not exhibit acute toxicity (52). This highlights the specificity of
this N. lobata extract against malignant cells.
Acknowledgements
We wish to thank Toni Jäger for
preparing the images. This study was supported by the Funds for
Innovative and Interdisciplinary Cancer Research to M.F.-S. and G.K
and the Hochschuljubiläumsstiftung der Stadt Wien to G.K. H.D. is
supported by the Herzfelder Family Foundation and the NÖ
Forschungs- und Bildungsges.m.b.H. (NFB).
References
|
1.
|
Koehn FE and Carter GT: The evolving role
of natural products in drug discovery. Nat Rev Drug Discov.
4:206–220. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Cragg GM and Newman DJ: Natural Product
Sources of Drugs: Plants, Microbes, Marine Organisms, and Animals.
Comprehensive Medicinal Chemistry II. Chapter 1.08. 355–403.
2007.
|
|
3.
|
Gidding CE, Kellie SJ, Kamps WA and de
Graaf SS: Vincristine revisited. Crit Rev Oncol Hematol.
29:267–287. 1999. View Article : Google Scholar
|
|
4.
|
Arvigo R and Balick M: Rainforest
Remedies. 2nd edition. Lotus Press; Twin Lakes, WI: 1998
|
|
5.
|
Berger I, Passreiter CM, Cáceres A and
Kubelka W: Antiprotozoal activity of Neurolaena lobata.
Phytother Res. 15:327–330. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Gupta MP, Solis NG, Avella ME and Sanchez
C: Hypoglycemic activity of Neurolaena lobata (L.) R. Br J
Ethnopharmacol. 10:323–327. 1984.
|
|
7.
|
Bedoya LM, Alvarez A, Bermejo M, González
N, Beltrán M, Sánchez-Palomino S, Cruz SM, Gaitán I, del Olmo E,
Escarcena R, García PA, Cáceres A, San Feliciano A and Alcamí J:
Guatemalan plants extracts as virucides against HIV-1 infection.
Phytomedicine. 15:520–524. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Passreiter CM, Wendisch D and Gondol D:
Sesquiterpene lactones from Neurolaena lobata.
Phytochemistry. 39:133–137. 1995. View Article : Google Scholar
|
|
9.
|
François G, Passreiter C, Woerdenbag H and
van Looveren M: Antiplasmodial activities and cytotoxic effects of
aqueous extracts and sesquiterpene lactones from Neurolaena
lobata. Planta Med. 62:126–129. 1996.PubMed/NCBI
|
|
10.
|
Berger I, Barrientos AC, Cáceres A,
Hernández M, Rastrelli L, Passreiter CM and Kubelka W: Plants used
in Guatemala for the treatment of protozoal infections: II.
Activity of extracts and fractions of five Guatemalan plants
against Trypanosoma cruzi. J Ethnopharmacol. 62:107–115. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Morris SW, Kirstein MN, Valentine MB,
Dittmer KG, Shapiro DN, Saltman DL and Look AT: Fusion of a kinase
gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s
lymphoma. Science. 263:1281–1284. 1994.
|
|
12.
|
Stein H, Foss HD, Dürkop H, Marafioti T,
Delsol G, Pulford K, Pileri S and Falini B: CD30(+) anaplastic
large cell lymphoma: a review of its histopathologic, genetic, and
clinical features. Blood. 96:3681–3695. 2000.
|
|
13.
|
Piva R, Chiarle R, Manazza AD, Taulli R,
Simmons W, Ambrogio C, D’Escamard V, Pellegrino E, Ponzetto C,
Palestro G and Inghirami G: Ablation of oncogenic ALK is a viable
therapeutic approach for anaplastic large-cell lymphomas. Blood.
107:689–697. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Simonitsch I, Polgar D, Hajek M, Duchek P,
Skrzypek B, Fassl S, Lamprecht A, Schmidt G, Krupitza G and Cerni
C: The cytoplasmic truncated receptor tyrosine kinase ALK homodimer
immortalizes and cooperates with ras in cellular transformation.
FASEB J. 15:1416–1428. 2001.PubMed/NCBI
|
|
15.
|
Meadows AT, Friedman DL, Neglia JP,
Mertens AC, Donaldson SS, Stovall M, Hammond S, Yasui Y and Inskip
PD: Second neoplasms in survivors of childhood cancer: findings
from the Childhood Cancer Survivor Study cohort. J Clin Oncol.
27:2356–2362. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Reiter A: Diagnosis and treatment of
childhood non-Hodgkin lymphoma. Hematology Am Soc Hematol Educ
Program. 285–296. 2007. View Article : Google Scholar
|
|
17.
|
Freed J and Kelly KM: Current approaches
to the management of pediatric Hodgkin lymphoma. Paediatr Drugs.
12:85–98. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Palmer RH, Vernersson E, Grabbe C and
Hallberg B: Anaplastic lymphoma kinase: signalling in development
and disease. Biochem J. 420:345–361. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Hallberg B and Palmer RH: Crizotinib -
latest champion in the cancer wars? N Engl J Med. 363:1760–1762.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Maier S, Strasser S, Saiko P, Leisser C,
Sasgary S, Grusch M, Madlener S, Bader Y, Hartmann J, Schott H,
Mader RM, Szekeres T, Fritzer-Szekeres M and Krupitza G: Analysis
of mechanisms contributing to AraC-mediated chemoresistance and
re-establishment of drug sensitivity by the novel
heterodinucleoside phosphate 5-FdUrd-araC. Apoptosis. 11:427–440.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Strasser S, Maier S, Leisser C, Saiko P,
Madlener S, Bader Y, Bernhaus A, Gueorguieva M, Richter S, Mader
RM, Wesierska-Gadek J, Schott H, Szekeres T, Fritzer-Szekeres M and
Krupitza G: 5-FdUrd-araC heterodinucleoside re-establishes
sensitivity in 5-FdUrd- and AraC-resistant MCF-7 breast cancer
cells overexpressing ErbB2. Differentiation. 74:488–498. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Fassl S, Leisser C, Huettenbrenner S,
Maier S, Rosenberger G, Strasser S, Grusch M, Fuhrmann G, Leuhuber
K, Polgar D, Stani J, Tichy B, Nowotny C and Krupitza G:
Transferrin ensures survival of ovarian carcinoma cells when
apoptosis is induced by TNFalpha, FasL, TRAIL, or Myc. Oncogene.
22:8343–8355. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Leisser C, Rosenberger G, Maier S,
Fuhrmann G, Grusch M, Strasser S, Huettenbrenner S, Fassl S, Polgar
D, Krieger S, Cerni C, Hofer-Warbinek R, deMartin R and Krupitza G:
Subcellular localisation of Cdc25A determines cell fate. Cell Death
Differ. 11:80–89. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Grusch M, Polgar D, Gfatter S, Leuhuber K,
Huettenbrenner S, Leisser C, Fuhrmann G, Kassie F, Steinkellner H,
Smid K, Peters GJ, Jayaram HN, Klepal W, Szekeres T, Knasmüller S
and Krupitza G: Maintenance of ATP favours apoptosis over necrosis
triggered by benzamide riboside. Cell Death Differ. 9:169–178.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2[-Delta Delta C(T)] method. Methods. 25:402–408. 2001.
|
|
26.
|
Coller HA, Grandori C, Tamayo P, Colbert
T, Lander ES, Eisenman RN and Golub TR: Expression analysis with
oligonucleotide microarrays reveals that MYC regulates genes
involved in growth, cell cycle, signaling, and adhesion. Proc Natl
Acad Sci USA. 97:3260–3265. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Mathas S, Hinz M, Anagnostopoulos I,
Krappmann D, Lietz A, Jundt F, Bommert K, Mechta-Grigoriou F, Stein
H, Dörken B and Scheidereit C: Aberrantly expressed c-Jun and JunB
are a hallmark of Hodgkin lymphoma cells, stimulate proliferation
and synergize with NF-kappa B. EMBO J. 21:4104–4113. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Staber PB, Vesely P, Haq N, Ott RG, Funato
K, Bambach I, Fuchs C, Schauer S, Linkesch W, Hrzenjak A, Dirks WG,
Sexl V, Bergler H, Kadin ME, Sternberg DW, Kenner L and Hoefler G:
The oncoprotein NPM-ALK of anaplastic large-cell lymphoma induces
JUNB transcription via ERK1/2 and JunB translation via mTOR
signaling. Blood. 110:3374–3383. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Unger C, Popescu R, Giessrigl B, Rarova L,
Herbacek I, Seelinger M, Diaz R, Wallnöfer B, Fritzer-Szekeres M,
Szekeres T, Frisch R, Doležal K, Strnad M, De Martin R, Grusch M,
Kopp B and Krupitza G: An apolar extract of Critonia
morifolia inhibits c-Myc, cyclin D1, Cdc25A, Cdc25B, Cdc25C and
Akt and induces apoptosis. Int J Oncol. 40:2131–2139.
2012.PubMed/NCBI
|
|
30.
|
Slupianek A, Nieborowska-Skorska M, Hoser
G, Morrione A, Majewski M, Xue L, Morris SW, Wasik MA and Skorski
T: Role of phosphatidylinositol 3-kinase-Akt pathway in
nucleophosmin/anaplastic lymphoma kinase-mediated lymphomagenesis.
Cancer Res. 61:2194–2199. 2001.PubMed/NCBI
|
|
31.
|
Polgar D, Leisser C, Maier S, Strasser S,
Rüger B, Dettke M, Khorchide M, Simonitsch I, Cerni C and Krupitza
G: Truncated ALK derived from chromosomal translocation
t(2;5)(p23;q35) binds to the SH3 domain of p85-PI3K. Mutat Res.
570:9–15. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Cockle SM and Dean RT: The regulation of
proteolysis in normal fibroblasts as they approach confluence.
Evidence for the participation of the lysosomal system. Biochem J.
208:795–800. 1982.PubMed/NCBI
|
|
33.
|
Laimer D, Dolznig H, Kollmann K, Vesely
PW, Schlederer M, Merkel O, Schiefer AI, Hassler MR, Heider S,
Amenitsch L, Thallinger C, Staber PB, Simonitsch-Klupp I, Artaker
M, Lagger S, Turner SD, Pileri S, Piccaluga PP, Valent P, Messana
K, Landra I, Weichhart T, Knapp S, Shehata M, Todaro M, Sexl V,
Höfler G, Piva R, Medico E, Ruggeri BA, Cheng M, Eferl R, Egger G,
Penninger JM, Jaeger U, Moriggl R, Inghirami G and Kenner L: PDGFR
blockade is a rational and effective therapy for NPM-ALK driven
lymphomas. Nat Med. (In press).
|
|
34.
|
Andrae J, Gallini R and Betsholtz C: Role
of platelet-derived growth factors in physiology and medicine.
Genes Dev. 22:1276–1312. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Jechlinger M, Sommer A, Moriggl R, Seither
P, Kraut N, Capodiecci P, Donovan M, Cordon-Cardo C, Beug H and
Grünert S: Autocrine PDGFR signaling promotes mammary cancer
metastasis. J Clin Invest. 116:1561–1570. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Ergin M, Denning MF, Izban KF, Amin HM,
Martinez RL, Saeed S and Alkan S: Inhibition of tyrosine kinase
activity induces caspase-dependent apoptosis in anaplastic large
cell lymphoma with NPM-ALK (p80) fusion protein. Exp Hematol.
29:1082–1090. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Gridling M, Stark N, Madlener S, Lackner
A, Popescu R, Benedek B, Diaz R, Tut FM, Nha Vo TP, Huber D,
Gollinger M, Saiko P, Ozmen A, Mosgoeller W, De Martin R, Eytner R,
Wagner KH, Grusch M, Fritzer-Szekeres M, Szekeres T, Kopp B, Frisch
R and Krupitza G: In vitro anti-cancer activity of two
ethno-pharmacological healing plants from Guatemala Pluchea
odorata and Phlebodium decumanum. Int J Oncol.
34:1117–1128. 2009.
|
|
38.
|
Stark N, Gridling M, Madlener S, Bauer S,
Lackner A, Popescu R, Diaz R, Tut FM, Vo TP, Vonach C, Giessrigl B,
Saiko P, Grusch M, Fritzer-Szekeres M, Szekeres T, Kopp B, Frisch R
and Krupitza G: A polar extract of the Maya healing plant Anthurium
schlechtendalii (Aracea) exhibits strong in vitro anticancer
activity. Int J Mol Med. 24:513–521. 2009.PubMed/NCBI
|
|
39.
|
Madlener S, Svacinová J, Kitner M, Kopecky
J, Eytner R, Lackner A, Vo TP, Frisch R, Grusch M, De Martin R,
Dolezal K, Strnad M and Krupitza G: In vitro
anti-inflammatory and anticancer activities of extracts of
Acalypha alopecuroidea (Euphorbiaceae). Int J Oncol.
35:881–891. 2009.
|
|
40.
|
Ozmen A, Bauer S, Gridling M, Singhuber J,
Krasteva S, Madlener S, Vo TP, Stark N, Saiko P, Fritzer-Szekeres
M, Szekeres T, Askin-Celik T, Krenn L and Krupitza G: In
vitro anti-neoplastic activity of the ethno-pharmaceutical
plant Hypericum adenotrichum Spach endemic to Western Turkey. Oncol
Rep. 22:845–852. 2009.
|
|
41.
|
Ozmen A, Madlener S, Bauer S, Krasteva S,
Vonach C, Giessrigl B, Gridling M, Viola K, Stark N, Saiko P,
Michel B, Fritzer-Szekeres M, Szekeres T, Askin-Celik T, Krenn L
and Krupitza G: In vitro anti-leukemic activity of the
ethno-pharmacological plant Scutellaria orientalis ssp carica
endemic to western Turkey. Phytomedicine. 17:55–62. 2010.
View Article : Google Scholar
|
|
42.
|
Vo NT, Madlener S, Bago-Horvath Z,
Herbacek I, Stark N, Gridling M, Probst P, Giessrigl B, Bauer S,
Vonach C, Saiko P, Grusch M, Szekeres T, Fritzer-Szekeres M, Jäger
W, Krupitza G and Soleiman A: Pro- and anticarcinogenic mechanisms
of piceatannol are activated dose dependently in MCF-7 breast
cancer cells. Carcinogenesis. 31:2074–2081. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Khan M, Giessrigl B, Vonach C, Madlener S,
Prinz S, Herbaceck I, Hölzl C, Bauer S, Viola K, Mikulits W,
Quereshi RA, Knasmüller S, Grusch M, Kopp B and Krupitza G:
Berberine and a Berberis lycium extract inactivate Cdc25A and
induce alpha-tubulin acetylation that correlate with HL-60 cell
cycle inhibition and apoptosis. Mutat Res. 683:123–130. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Bauer S, Singhuber J, Seelinger M, Unger
C, Viola K, Vonach C, Giessrigl B, Madlener S, Stark N, Wallnofer
B, Wagner KH, Fritzer-Szekeres M, Szekeres T, Diaz R, Tut F, Frisch
R, Feistel B, Kopp B, Krupitza G and Popescu R: Separation of
anti-neoplastic activities by fractionation of a Pluchea odorata
extract. Front Biosci (Elite Ed). 3:1326–1336. 2011.PubMed/NCBI
|
|
45.
|
Madlener S, Saiko P, Vonach C, Viola K,
Huttary N, Stark N, Popescu R, Gridling M, Vo NT, Herbacek I,
Davidovits A, Giessrigl B, Venkateswarlu S, Geleff S, Jäger W,
Grusch M, Kerjaschki D, Mikulits W, Golakoti T, Fritzer-Szekeres M,
Szekeres T and Krupitza G: Multifactorial anticancer effects of
digalloyl-resveratrol encompass apoptosis, cell-cycle arrest, and
inhibition of lymphendothelial gap formation in vitro. Br J Cancer.
102:1361–1370. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Kerjaschki D, Bago-Horvath Z, Rudas M,
Sexl V, Schneckenleithner C, Wolbank S, Bartel G, Krieger S, Kalt
R, Hantusch B, Keller T, Nagy-Bojarszky K, Huttary N, Raab I,
Lackner K, Krautgasser K, Schachner H, Kaserer K, Rezar S, Madlener
S, Vonach C, Davidovits A, Nosaka H, Hämmerle M, Viola K, Dolznig
H, Schreiber M, Nader A, Mikulits W, Gnant M, Hirakawa S, Detmar M,
Alitalo K, Nijman S, Offner F, Maier TJ, Steinhilber D and Krupitza
G: Lipoxygenase mediates invasion of intrametastatic lymphatic
vessels and propagates lymph node metastasis of human mammary
carcinoma xenografts in mouse. J Clin Invest. 121:2000–2012. 2011.
View Article : Google Scholar
|
|
47.
|
Passreiter CM: Pyrrolizidine alkaloids
from Neurolaena lobata. Biochem Sys Ecol. 26:839–843. 1998.
View Article : Google Scholar
|
|
48.
|
Ghantous A, Gali-Muhtasib H, Vuorela H,
Saliba NA and Darwiche N: What made sesquiterpene lactones reach
cancer clinical trials? Drug Discov Today. 15:668–678. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Zhang S, Won YK, Ong CN and Shen HM:
Anti-cancer potential of sesquiterpene lactones: bioactivity and
molecular mechanisms. Curr Med Chem Anticancer Agents. 5:239–249.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Hsu FY, Johnston PB, Burke KA and Zhao Y:
The expression of CD30 in anaplastic large cell lymphoma is
regulated by nucleophosmin-anaplastic lymphoma kinase-mediated JunB
level in a cell type-specific manner. Cancer Res. 66:9002–9008.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Gotzmann J, Fischer AN, Zojer M, Mikula M,
Proell V, Huber H, Jechlinger M, Waerner T, Weith A, Beug H and
Mikulits W: A crucial function of PDGF in TGF-beta-mediated cancer
progression of hepatocytes. Oncogene. 25:3170–3185. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Cáceres A, López B, González S, Berger I,
Tada I and Maki J: Plants used in Guatemala for the treatment of
protozoal infections. I Screening of activity to bacteria, fungi
and American trypanosomes of 13 native plants. J Ethnopharmacol.
62:195–202. 1998.PubMed/NCBI
|