Introduction
Hepatocellular carcinoma (HCC) is listed as the
fifth most common malignancy in the world. Although surgical
treatment and non-surgical therapeutic modalities such as
chemotherapy, radiotherapy and interventional therapy have been
employed, HCC is rarely curative (1,2). For
this reason more and more patients and oncologists are seeking
alternative medicines to improve the curative rate of HCC.
Traditional Chinese medicine (TCM) has been widely used in China to
treat HCC because it can improve immune function of patients and
alleviate chemoradiotherapy-related side effects (3).
In TCM, herbs commonly used for cancer treatment can
be divided into two categories. One is nutritious and tonic herbs
and the other is heat-clearing and detoxification herbs. Nutritious
and tonic herbs (Fuzheng herbs) act by improving the immune
function to fight cancer (such as Astragalus, Ganoderma
lucidum and ginseng) (4–6).
Heat clearing and detoxication herbs (Qingre Jiedu herbs) may
directively kill tumor cells [such as Hedyotis diffusa Willd
(HDW), Sophora flavescens (SF), Pseudobulbus
Cremastrae (PC), Prunella, Bidens, banzhilian] (7–9). In
TCM, cancer is considered to be caused by accumulation of foreign
toxins, and cancer itself is also considered to be a toxin that is
harmful to human body. The above herbs may contain ingredients that
can inhibit proliferation and promote apoptosis of tumor cells. For
example, we found that HDW water extract may inhibit proliferation
of cancer cells via regulating the cell cycle (10). Others reported HDW and
Prunella may induce cell apoptosis via the
mitochondrion-dependent pathway (11–14).
Cell proliferation is controlled by proteins
relating to cell cycle in the cytoplasm. Among them are cyclins and
cyclin-dependent kinases (CDKs). Protein phosphorylation by these
kinases is the basis of a cascade of signaling that pushes a cell
from one stage to the next. A set of checkpoints that monitor
completion of critical events is involved (15,16).
Kinase activation generally requires association with a second
subunit that is transiently expressed at the appropriate period of
the cell cycle. A cyclin may associate with a CDK to create an
active complex with unique substrate specificity. Regulating of
phosphorylation and dephosphorylation of CDK-cyclin complexes
ensure the normal transition of cell cycle stages (17,18).
Arresting cell cycle in G1 phase or G2/M phase is one of the
mechanisms used by anticancer medicines (19,20).
JXY is a polyherbal formula of TCM according to the
theory of Chinese medicine. It is composed of HDW (30 g),
Prunella (15 g), PC (15 g) and SF (15 g). These herbs are
capable of heat-clearing and detoxification. Our previous clinical
studies have shown that JXY can prolong the cancer patients’
overall survival time and improve the patients’ quality of life
(21). By using molecular docking
simulation, Chen et al showed that some components in HDW,
Prunella and PC can combine with CDK2 (22). In the present study, we evaluated
the effect of JXY on proliferation of hepatoma cells and its
effects on the cell cycle.
Materials and methods
Reagents
Dulbecco’s modified Eagle’s medium (DMEM), fetal
bovine serum (FBS), penicillin-streptomycin, western blot detection
stack/iblot dry blotting system, mouse antibodies against PCNA,
CDK1, CDK2, CDK4, cyclin B, cyclin D, cyclin E and cyclin A were
from Maixin Bio (Fuzhou, China). Cycle test™ plus DNA reagent kit
was purchased from Becton-Dickinson (San Jose, CA, USA). All other
chemicals, unless otherwise stated, were obtained from Sigma
Chemicals (St. Louis, MO, USA).
Preparation of ethyl acetate extract from
JXY
JXY is composed of HDW (30 g), Prunella (15
g), PC (15 g) and SF (15 g). Four herbs of JXY were purchased from
Guo Yi Tang Hospital of Fujian University of Traditional Chinese
Medicine (Fuzhou, China). JXY (7.5 kg) was refluxed with 75%
ethanol for 2×3 h to obtain total extract (TE-JXY). The alcohol was
removed under vacuum using a rotary evaporator. The residue was
suspended in water, which was partitioned sequentially with
petroleum ether, chloroform, ethyl acetate and n-BuOH to afford an
petroleum ether extract (PE-JXY), n-BuOH extract (BE-JXY), CE-JXY
and EE-JXY. Four extracts were evaporated in vacuum and stored at
4°C prior to use. PE-JXY, CE-JXY, EE-JXY and BE-JXY were diluted in
DMSO into 200 mg/ml for in vitro experiments. For in
vivo study EE-JXY was dissolved in NS to a final concentration
of 6 mg/ml.
Cell culture
Human hepatoma cells lines HepG2 was obtained from
American Type Culture Collection (ATCC, Manassas, VA, USA). The
cells were grown in DMEM containing 10% FBS, 100 U/ml penicillin
and 100 μg/ml streptomycin in 37°C humidified incubator with
5% CO2. The cells were subcultured at 80 to 90%
confluence. Cells used in this study were subjected to no more than
20 cell passages.
Tumor xenograft
Sixteen Male BALB/c nude mice with body weight from
18 to 22 g were injected with HepG2 cells suspension at the right
flank. After 7 days mice were randomly divided into two groups
(vehicle group and EE-JXY group). EE-JXY (0.06 g/kg) was
administered twice every day in the EE-JXY group while the same
volume of NS was administered for the vehicle group. During
treatment tumor size was measured and volume was calculated
according to the following formula: tumor volume (TV,
mm3) = d2×D/2, where d and D were the
shortest and longest diameter, respectively. On day 21 tumor was
excised and weighed. The animals were maintained in a pathogen-free
facility (23±2°C, 55±5% humidity). Food and water were provided
ad libitum. All procedures on treating mice were performed
according to Animal Care Guidelines issued by Ministry of Science
and Technology of the People’s Republic of China and the Animal
Care Committee of Fu Jian University of Traditional Chinese
Medicine approved our protocols.
Evaluation of cell viability by MTT
assay
Cell viability was evaluated by MTT colorimetric
assay; 1×104 cells/well were seeded into 96-well plates.
The cells were treated with different concentrations of PE-JXY,
EE-JXY, BE-JXY or CE-JXY for different times. Then 20 μl MTT
(5 mg/ml) was added to each well. After 4 h, MTT was discarded and
100 μl DMSO was added to each well. The absorbance was
measured at 490 nm with a microplate reader (Biotek, Winooski, VT,
USA). The cell viability was calculated according to the following
formula: cell viability(%) = average absorbance of JXY-containing
group/average absorbance of blank group × 100%.
Cell cycle analysis
After incubation with different concentrations of
EE-JXY for 24 h, cells were digested with trypsinase and washed
twice with PBS. Single cell suspension with a final concentration
of 1×106/ml was prepared. Cell cycle analysis was
evaluated with flow cytometry according to the instructions of DNA
Plus kit and the percents of G0/G1-phase, S-phase and G2/M-phase
were calculated with the ModFit software (BD Biosciences, San Jose,
CA, USA).
Soft-agar colony formation assay
After HepG2 cells were treated with EE-JXY for 24 h,
cells were harvested and pipetted well to become single-cell
suspension in DMEM with 10% FBS at a concentration of
5×104 cells/ml. Normal melting point agar (0.4 ml of
0.4% agar in 1.6 ml DMEM containing 10% FBS) was placed into each
well of a 6-well plate. After solidification of the bottom agar, 1
ml of cell mixture consisting of 0.02 ml of cell suspension
(5×104 cells/ml) and 0.98 ml of 0.8% lower melting point
agar in DMEM containing 10% FBS were poured over the bottom agar.
After solidification of the upper agar wells were incubated at 37°C
in a humidified 5% CO2 atmosphere for 1 week. Colony
formation in the agar was then photographed and counted under a
phase-contrast microscope.
Western blot analysis
Tumor were lysed with lysis buffer (M-PER; Thermo
Scientific, Rockford, IL, USA) containing protease and phosphatase
inhibitor cocktails (EMD Biosciences La Jolla, CA, USA and Sigma
Chemical, respectively). The lysates were resolved in 12% SDS-PAGE
gels and electroblotted using the iblot western detection
stack/iblot dry blotting system. The PVDF membranes were blocked
with SuperBlock T20 (TBS) blocking buffer (Thermo Scientific) for
30 min and washed in TBS with 0.25% Tween-20 (TBST), followed by
incubation overnight at 4°C with primary antibody. After washing
with TBST, the membranes were incubated with secondary antibody for
1 h. The membranes were developed using Super Signal Pico Substrate
(Thermo Scientific), and images were taken using a Kodak Image
Station 400R (Kodak, Rochester, NY, USA).
Immunohistochemistry assay
After fixing in 10% formalin buffer for 24 h, tumor
samples were processed in a routine method for paraffin-embedded
tumor sections. Sections were subjected to antigen retrieval and
blocking of endogenous peroxidase activity. For immunostaining,
sections were incubated with the primary antibodies (mouse
monoclonal anti-PCNA (1:150; Maixin Bio), mouse monoclonal
anti-CDK1, anti-CDK2, anti-CDK4 (1:150; 1:100, 1:150, Maixin Bio),
mouse monoclonal anti-cyclin D, anti-cyclin E, anti-cyclin A and
anti-cyclin B (1:100; 1:150, 1:150, 1:100, Maixin Bio). Sections
were then incubated with biotinylated appropriate secondary
antibody followed by conjugated horseradish peroxidase
(HRP)-streptavidin (Maixin Bio). Then 3,3′-diaminobenzidine (DAB;
Sigma Chemicals) was added, incubated at room temperature and
counterstaining with diluted Harris hematoxylin (Sigma Chemicals).
Cells were quantified by counting positive cells and total number
of cells at five arbitrarily selected fields from each tumor at
magnifications, ×100. Data are presented as percentage of positive
cells.
Statistical analysis
Data are shown as mean values ± SD and analyzed
using SPSS package for Windows (Version 11.5). Statistical analysis
was performed with the Student’s t-test and one way analysis of
variance (ANOVA). Dunnett’s was used as post hoc test. Differences
at P<0.05 were considered statistically significant.
Results
Effects of JXY extracts on the growth of
HepG2 cells
The effects of PE-JXY, BE-JXY, CE-JXY and EE-JXY on
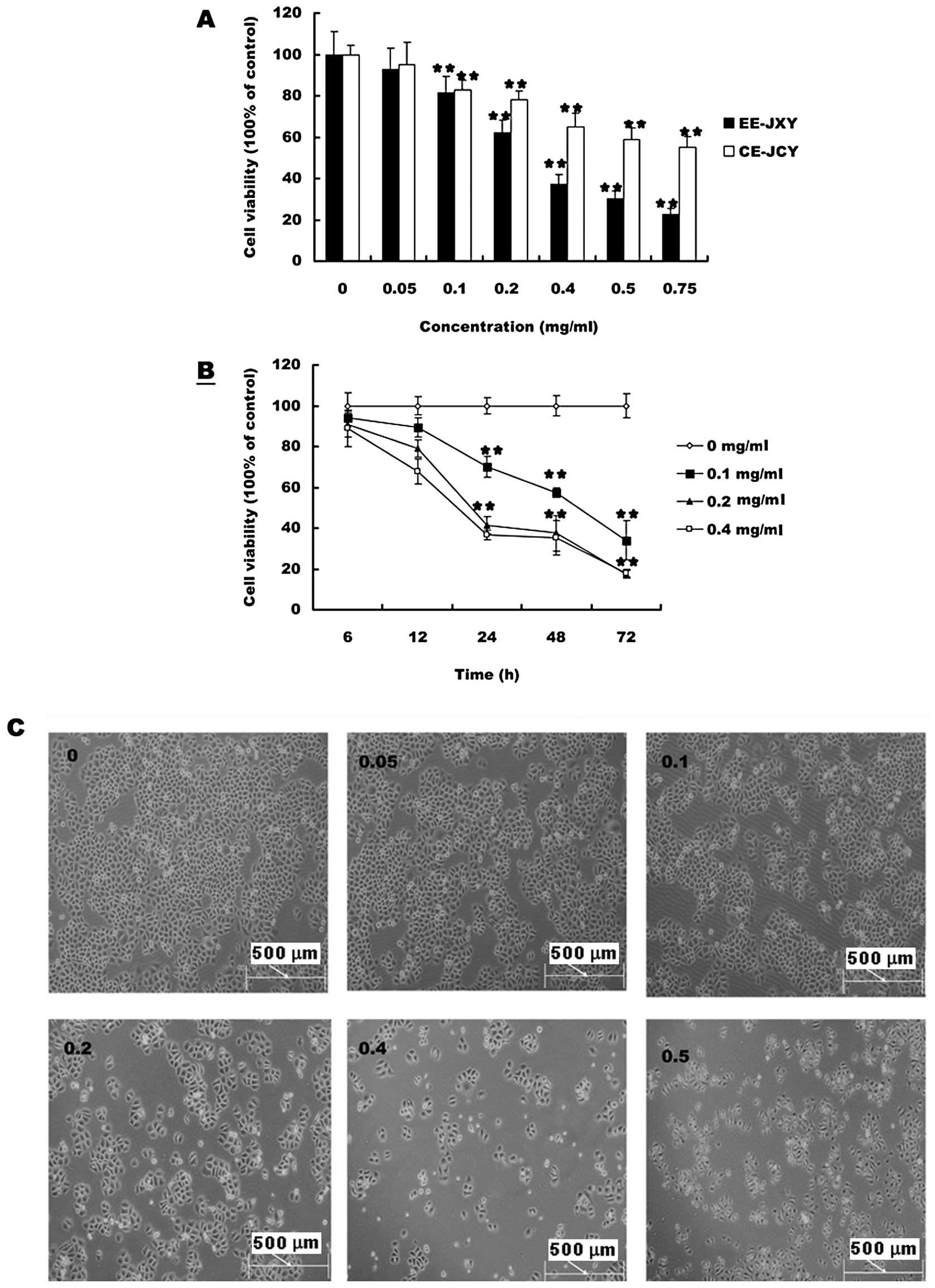
the growth of HepG2 cells was detected by MTT assay. As shown in
Fig. 1A cell viability was
inhibited by both CE-JXY and EE-JXY in a dose-dependent manner.
Treatment with 0.05 to 0.75 mg/ml of CE-JXY and EE-JXY for 24 h
reduced cell viability by 5 to 40% and 9 to 75%, respectively,
compared to untreated control cells (P<0.01 for both). Greater
inhibition was observed for EE-JXY than for CE-JXY at the same
concentration. Inhibition of cell viability by PE-JXY or BE-JXY was
weak (data not shown). We also evaluated the effect of 0.1, 0.2 and
0.4 mg/ml of EE-JXY on cell viability with different culture time.
As shown in Fig. 1B and C, cell
viability was inhibited by EE-JXY in a time-dependent manner.
EE-JXY inhibits colony formation ability
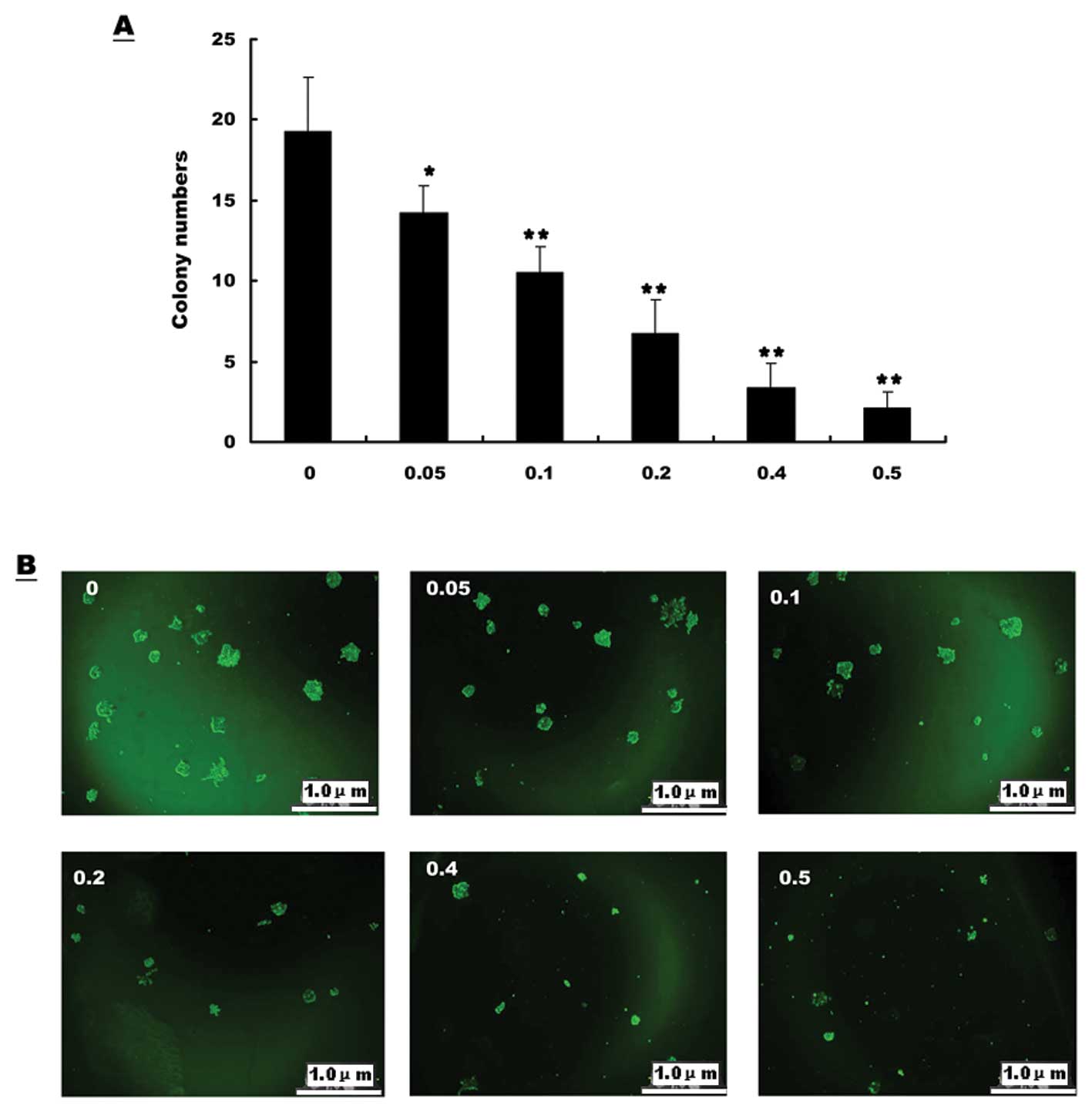
of tumor cells
In order to determine the effect of EE-JXY on colony
formation of HepG2, cells treated with different concentrations of
EE-JXY were cultured in semisolid culture media for 1 week followed
by manual counting of colonies. The results showed that as the
concentration of EE-JXY increased, the colony size and the colony
number decreased (Fig. 2A). Thus
the colony formation ability of HepG2 cells was significantly
inhibited by EE-JXY (Fig. 2B).
EE-JXY induce cell cycle arrest of HepG2
cells
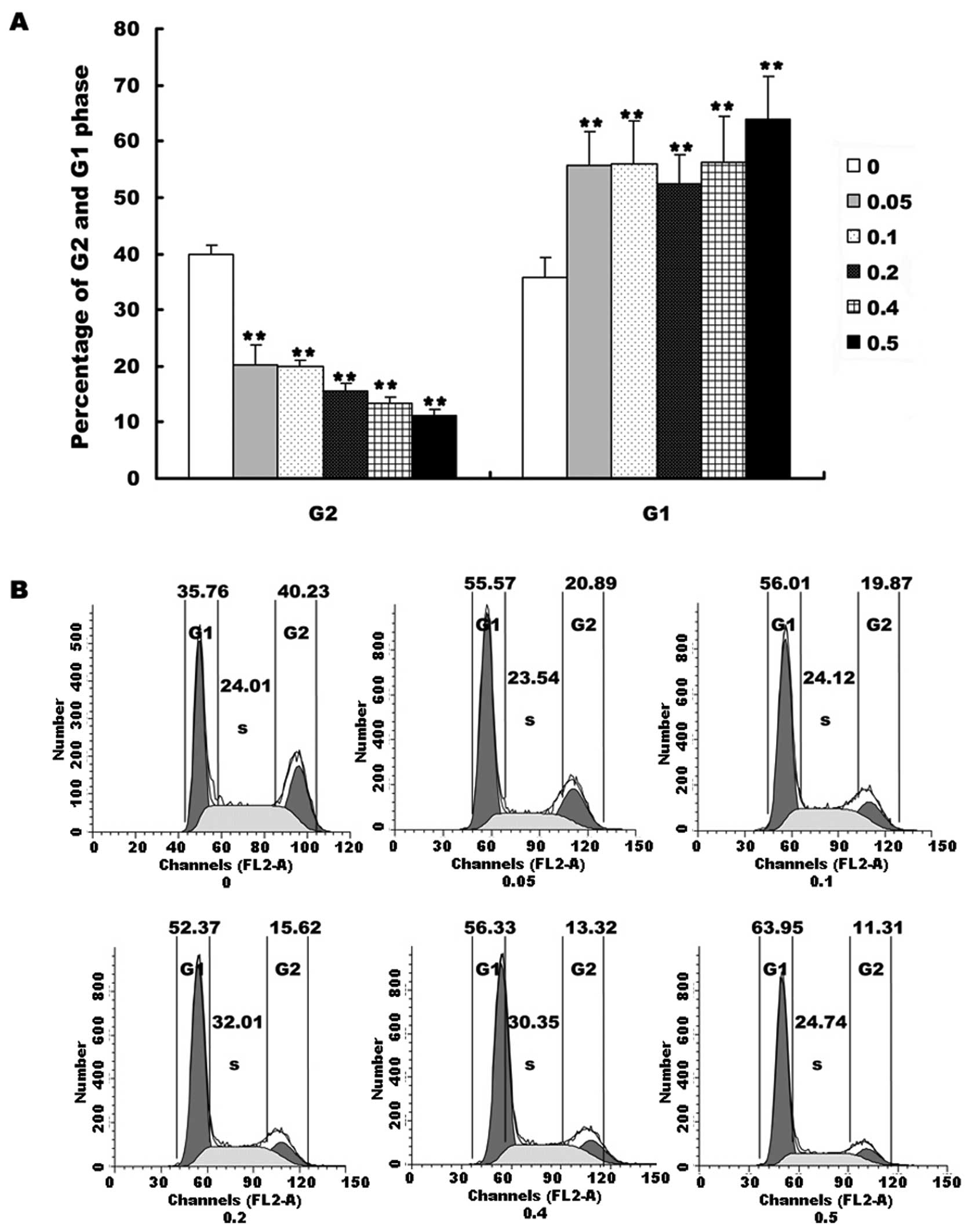
We investigated the cell cycle after HepG2 cells
were treated with different concentrations of EE-JXY for 24 h. The
results showed that EE-JXY markedly increased the percentage of
cells in G1 phase (Fig. 3A,
P<0.01) and decreased the percentage of cells in G2 phase in a
dose-dependent manner (Fig. 3A,
P<0.01). These results demonstrated that EE-JXY induced cell
cycle arrest of HepG2 cells. The representative results of cell
cycle are shown in Fig. 3B.
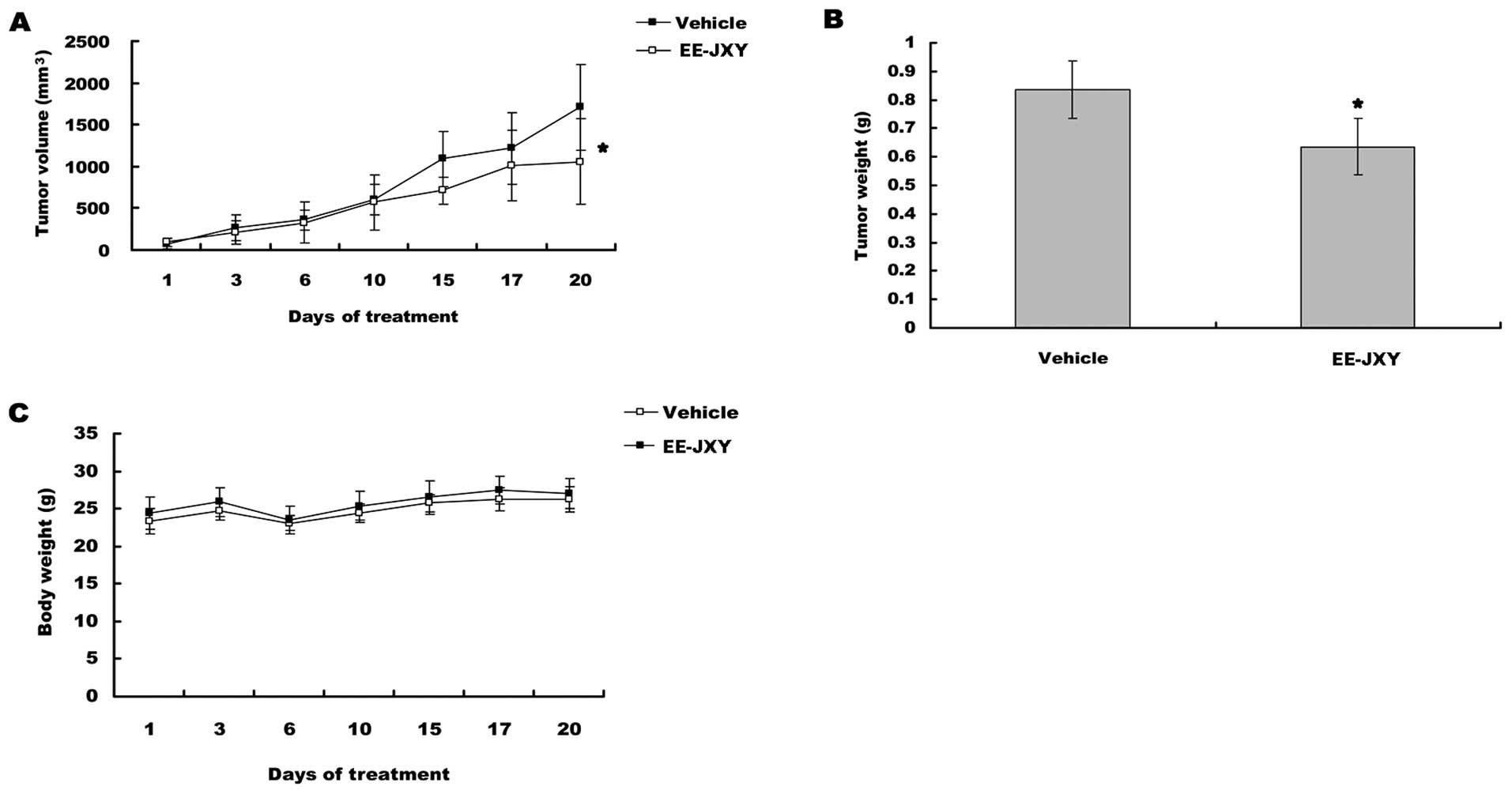
EE-JXY inhibits tumor growth in vivo
After mice were treated for 20 days, tumor volume
was reduced by 39% (P<0.05) in EE-JXY group compared with
vehicle group (Fig. 4A). A
comparison of tumor weight between EE-JXY group and vehicle group
showed a similar tendency (Fig.
4B, P<0.05) with tumor volume. More importantly, the body
weights between vehicle group and EE-JXY group was similar at the
end of treatment, indicating EE-JXY is safe (Fig. 4C).
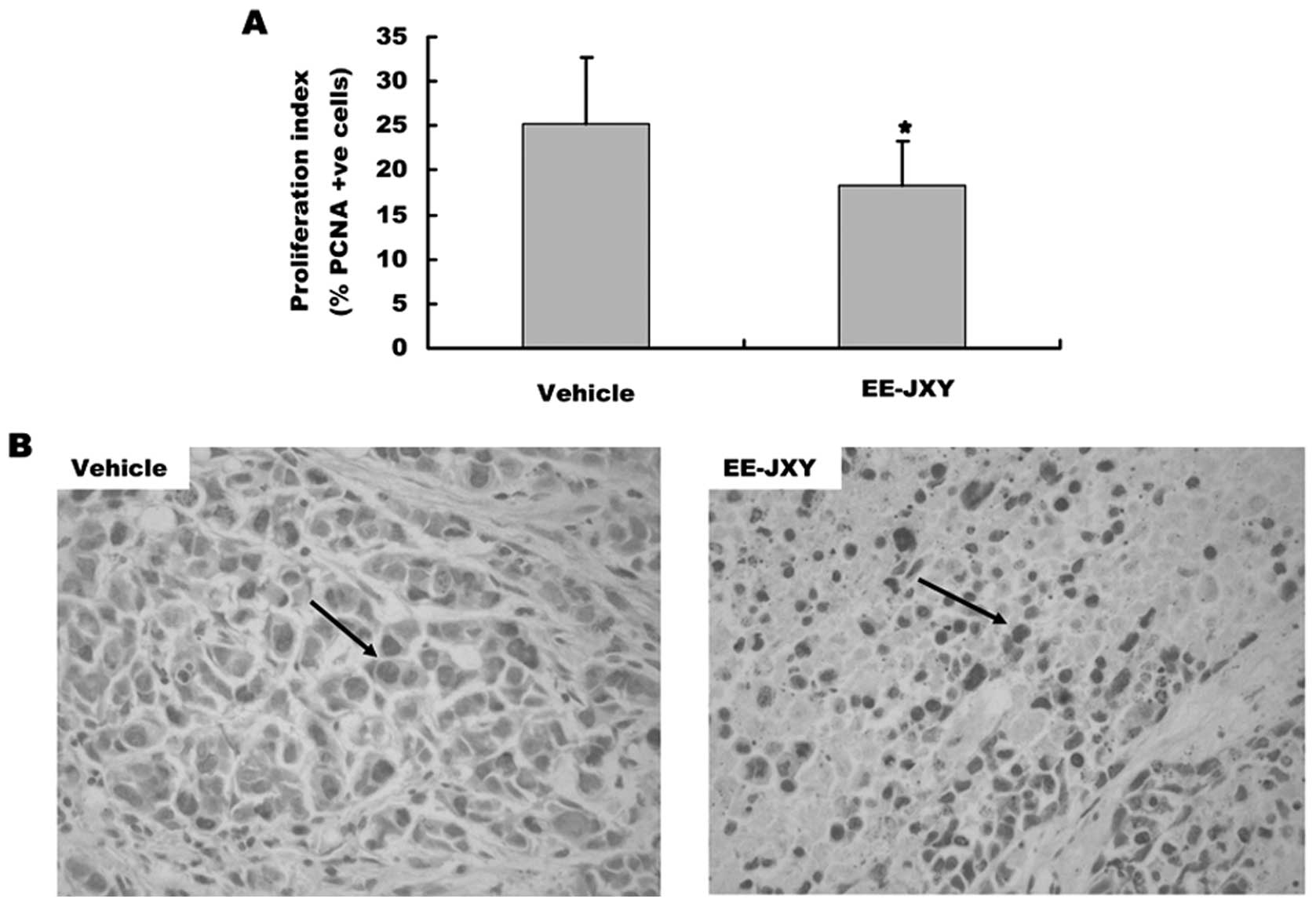
The effect of EE-JXY on proliferation of
tumor
We also detected the proliferation index of tumor
after mice treated with EE-JXY for 20 days by immunohistochemical
staining assay. The results showed that the proliferation index of
EE-JXY group was significantly lower than the vehicle group
(Fig. 5A, P<0.01). The
representative images of tumor cell proliferation are shown in
Fig. 5B.
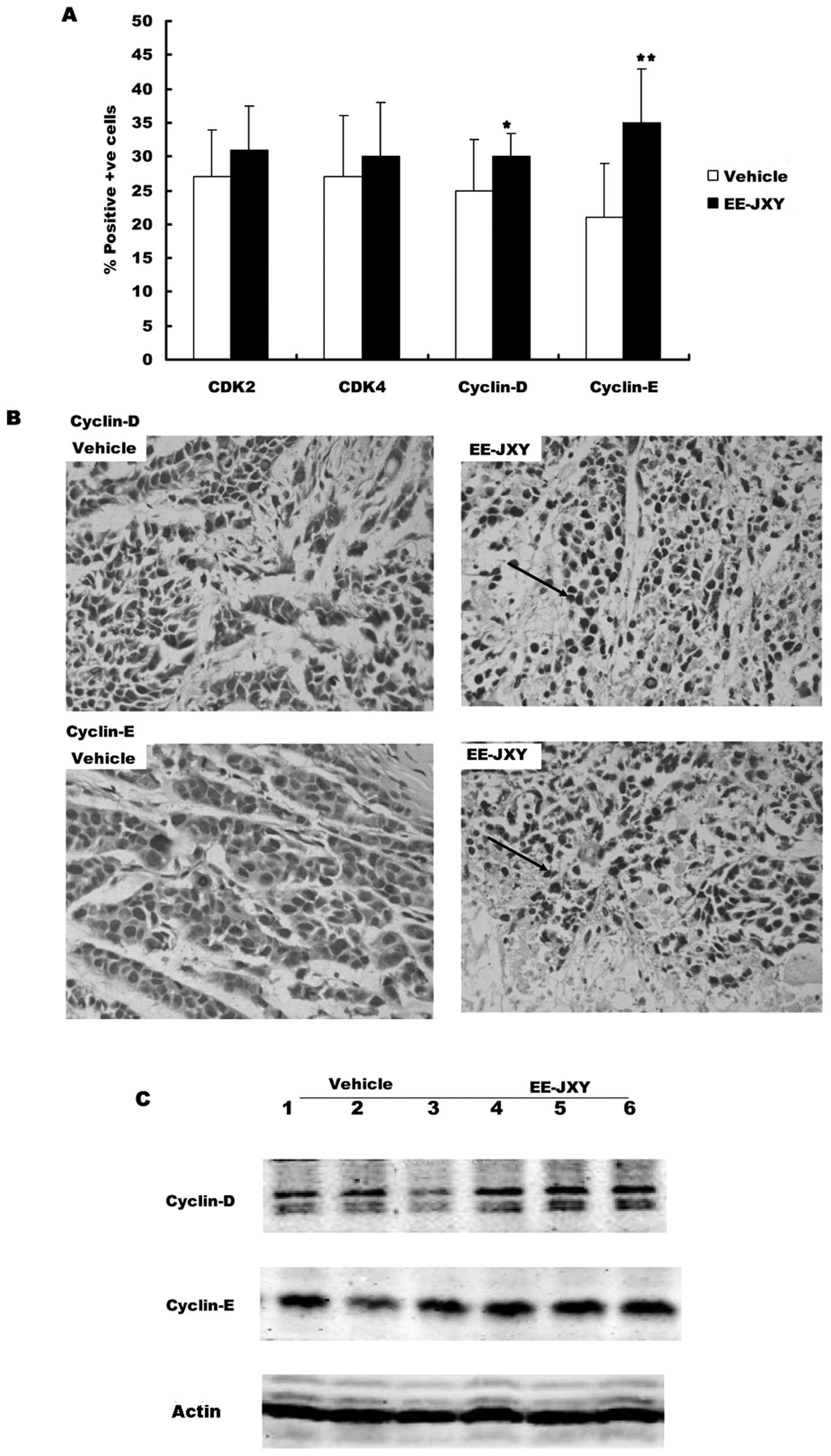
Effects of EE-JXY on cell cycle-related
proteins
First we detected the expression of G1 phase related
proteins (CDK2, CDK4, cyclin D and cyclin E) in the tumor tissue
with immunohistochemical staining and western blot analysis.
Immunochemistry showed there were more cells positive for CDK2 and
CDK4 in the EE-JXY group than in the vehicle group, but the
difference was not significant (Fig.
6B). There were significantly more cells positive for cyclin D
and cyclin E in the EE-JXY group than in the vehicle group
(Fig. 6A, P<0.05 and
P<0.01). The results were further confirmed by western blot
analysis (Fig. 6C).
Next we detected the expression of G2 phase related
proteins (CDK1, cyclin A and cyclin B). Immunohistochemical
staining showed that the percentages of cells positive for CDK1,
cyclin A or cyclin B were similar between the EE-JXY group and
vehicle group (data not shown). These results were confirmed by
western blot analysis (data not shown).
Discussion
According to TCM theory, toxins are one of common
causes of cancer. Toxins are referred to as ‘fire toxins’ and
‘heat’ which, when accumulating in liver, may lead to liver qi
stagnation. In Chinese medicine, a variety of symptoms associated
with menopause, depression, headaches, insomnia and fibromyalgia
are attributed to liver qi stagnation (23). Toxins can also cause blood stasis.
Toxins and heat accumulating in organs will cause cancer. Several
herbs are commonly used in China to treat cancer by clearing heat
and expelling toxin. These herbs include HDW, Selaginellae
doederleinii, Sophora Tonkinensis, Rhizoma Curcumae
Ezhu, Prunella, SF and PC (4,24).
Pharmacological studies have shown that these herbal medicines have
antitumor effect via inducing cell apoptosis, inhibiting cell
proliferation and angiogenesis (25–27).
JXY is composed of HWD, Prunella, SF and PC. It is used to
treat abscesses, ulcerations, swellings, sore throat, bronchitis,
tonsillitis, damp heat jaundice (infectious hepatitis) and
malignant tumors of liver, lung and stomach, which are all
considered to be caused by excess of heat, fire and toxin in the
tissues or organs. Our results showed that JXY extracts, especially
EE-JXY, can decrease cell viability through arresting cells in
G0/G1 phase in vitro and inhibit tumor growth by regulating
the level of G1 phase related proteins in vivo.
Cyclins and CDKs are two key classes of cell cycle
regulatory proteins that determine a cell’s progress through cell
cycle (28). Cyclin D is the first
cyclin produced in response to extracellular signals (e.g. growth
factors). Cyclin D binds to existing CDK4 to form the active cyclin
D-CDK4 complex and then phosphorylates the retinoblastoma
susceptibility protein (Rb) (29)
to activate E2F. Activation of E2F results in transcription of
various genes such as cyclin E, cyclin A, DNA polymerase, and
thymidine kinase. Cyclin E binds to CDK2, forming the cyclin E-CDK2
complex, which pushes the cell from G1 to S phase (G1/S
transition). Our results showed that EE-JXY increased the level of
cyclin D and cyclin E, which may be responsible on its effects on
cell cycle arrest in G1 phase.
Studies have shown that the HDW and Prunella
may inhibit the proliferation of tumor cells by regulating
expression of cell cycle-related proteins (14,30).
Similarly we found water extract of HWD can arrest the HepG2 cells
in G0/G1 phase via downregulating the mRNA and protein levels of
CDK2, CDK4 and E2F (10). Matrine,
a component rich in SF, inhibited cell proliferation through
arresting cells in G0/G1 phase in gallbladder cancer cells
(31), prostate cancer cells
(32) and hepatoma cells (33). Qin et al and Zhao et
al reported that matrine downregulated the expression of cyclin
D1 to inhibit the growth of retinoblastoma cells (34,35).
The results of the present study with EE-JXY are consistent with
these reports, although the underlying mechanism is unknown.
In p53-dependent G1-arrest signal transduction
pathway, DNA damage signalling stabilizes the p53 and then induces
expression of many target genes. The gene encoding p21 constitutes
a crucial target which can bind the cyclin E/CDK2 complex and
inhibits the complex activation, as its upregulation leads to
G1-arrest following DNA damage (36). In the p53-independent G1-arrest
signal transduction pathway, chemokines can ubiquitinate and
degradate cell division cycle 25 homolog A that is required for
activation of cyclin E/CDK2 complex to inhibit cell proliferation
(37,38). Whether EE-JXY actives the
p53-dependent or -independent G1-arrest signal transduction pathway
to inhibit tumor growth requires further study.
In summary, EE-JXY significantly inhibited the
growth of hepatocellular carcinoma in vivo without any toxic
effects. The anti-proliferation effect of EE-JXY regulates the
expression of cyclin D and cyclin E to arrest cells in G0/G1 phase.
The mechanism by which JXY regulates expression of cyclin D and
cyclin E need further investigation.
Abbreviations:
|
JXY
|
Jiedu Xiaozheng Yin
|
|
TCM
|
traditional Chinese medicine
|
|
EE-JXY
|
ethyl acetate fraction
|
|
CE-JXY
|
chloroform fraction
|
|
PCNA
|
proliferating cell nuclear antigen
|
|
CDK2
|
cyclin-dependent kinase2
|
|
CDK4
|
cyclin-dependent kinase4
|
|
CDK1
|
cyclin-dependent kinase1
|
|
HCC
|
hepatocellular carcinoma
|
|
HDW
|
Hedyotis Diffusa Willd
|
|
SF
|
Sophora flavescens
|
|
PC
|
Pseudobulbus Cremastrae
|
|
CDKs
|
cyclin-dependent kinases
|
|
DMEM
|
Dulbecco’s modified Eagle’s medium
|
|
FBS
|
fetal bovine serum
|
|
PE-JXY
|
ether extract
|
|
BE-JXY
|
n-BuOH extract
|
|
TV
|
tumor volume
|
|
TBS
|
SuperBlock T20
|
|
TBST
|
Tween-20
|
|
ANOVA
|
one-way analysis of variance
|
|
Rb
|
retinoblastoma susceptibility
protein
|
Acknowledgements
This study was supported by CHEN Ke-ji
Integrative Medicine Development Fund (CKJ2010020); International
Science Joint Project of the Ministry of Science and Technology of
China (2008DFA32200); Key Project of Fujian Province Department of
Science and Technology (2008KJB-01); Fujian Province Natural
Science Foundation (2010J01197, 2012J01393). National Natural
Science Foundation of China (81102582).
References
|
1.
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar
|
|
2.
|
Gish RG and Baron A: Hepatocellular
carcinoma (HCC): current and evolving therapies. IDrugs.
11:198–203. 2008.PubMed/NCBI
|
|
3.
|
Konkimalla VB and Efferth T:
Evidence-based Chinese medicine for cancer therapy. J
Ethnopharmacol. 116:207–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Beinfield H and Korngold E: Chinese
medicine and cancer care. Altern Ther Health Med. 9:38–52.
2003.
|
|
5.
|
Huang CF, Lin SS, Liao PH, Young SC and
Yang CC: The immunopharmaceutical effects and mechanisms of herb
medicine. Cell Mol Immunol. 5:23–31. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Pan B, Cheng T, Nan KJ, Qiu GQ and Sun XC:
Effect of Fuzheng Yiliu decoction combined with chemotherapy on
patients with intermediate and late stage gastrointestinal cancer.
World J Gastroenterol. 11:439–442. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Yan Y, Cook J, McQuillan J, Zhang G,
Hitzman CJ, Wang Y, Wiedmann TS and You M: Chemopreventive effect
of aerosolized polyphenon E on lung tumorigenesis in A/J mice.
Neoplasia. 9:401–405. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Fang Y, Zhang Y, Chen M, Zheng H and Zhang
K: The active component of Hedyotis diffusa Willd. Chinese
Trad Plant Med. 26:577–579. 2004.
|
|
9.
|
Wu YG and Song LR: Shanghai Science and
Technology Press. Zhong hua ben cao (Chin). 25:530–533. 1998.
|
|
10.
|
Chen XZ, Cao ZY, Chen TS, Zhang YQ, Liu
ZZ, Su YT, Liao LM and Du J: Water extract of Hedyotis
Diffusa Willd suppresses proliferation of human HepG2 cells and
potentiates the anticancer efficacy of low-dose 5-fluorouracil by
inhibiting the CDK2-E2F1 pathway. Oncol Rep. 28:742–748. 2012.
|
|
11.
|
Lin J, Chen Y, Wei L, Chen X, Xu W, Hong
Z, Sferra TJ and Peng J: Hedyotis Diffusa Willd extract
induces apoptosis via activation of the mitochondrion-dependent
pathway in human colon carcinoma cells. Int J Oncol. 37:1331–1338.
2010.
|
|
12.
|
Lin J, Wei L, Xu W, Hong Z, Liu X and Peng
J: Effect of Hedyotis Diffusa Willd extract on tumor
angiogenesis. Mol Med Rep. 4:1283–1288. 2011.
|
|
13.
|
Jitka P, Milan K and Jaromír S: Biological
activities of Prunella vulgaris extract. Phytother Res.
17:1082–1087. 2003.
|
|
14.
|
Psotova J, Svobodova A, Kolarova H and
Walterova D: Photoprotective properties of Prunella vulgaris
and rosmarinic acid on human keratinocytes. J Photochem Photobiol
B. 84:167–174. 2006.
|
|
15.
|
Hunter T and Pines J: Cyclins and cancer.
II: Cyclin D and CDK inhibitors come of age. Cell. 79:573–582.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Morgan DO: Principles of CDK regulation.
Nature. 374:131–134. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Cobrinik D: Pocket proteins and cell cycle
control. Oncogene. 24:2796–2809. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Glotzer M: The molecular requirements for
cytokinesis. Science. 307:1735–1739. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Tin AS, Sundar SN, Tran KQ, Park AH,
Poindexter KM and Firestone GL: Antiproliferative effects of
artemisinin on human breast cancer cells requires the downregulated
expression of the E2F1 transcription factor and loss of E2F1-target
cell cycle genes. Anticancer Drugs. 23:370–379. 2012. View Article : Google Scholar
|
|
20.
|
Fang XM, Liu B, Liu YB, Wang JJ, Wen JK,
Li BH and Han M: Acetylbritannilactone suppresses growth via
upregulation of krüppel-like transcription factor 4 expression in
HT-29 colorectal cancer cells. Oncol Rep. 26:1181–1187.
2011.PubMed/NCBI
|
|
21.
|
Chen LW, Lin J, Chen W and Zhang W: Effect
of Chinese herbal medicine on patients with primary hepatic
carcinoma in III stage during perioperational period: a report of
42 cases. Zhongguo Zhong Xi Yi Jie He Za Zhi. 25:832–834. 2005.(In
Chinese).
|
|
22.
|
Chen L, Zheng C and Du J: Study on
antitumor mechanism of Qingre Xiaozheng drink by molecular docking
method. Clin Pharmacol Ther (Chin). 12:324–328. 2007.
|
|
23.
|
Li ZQ: Traditional Chinese medicine for
primary liver cancer. World J Gastroenterol. 4:360–364. 1998.
|
|
24.
|
Qui JX and Yang JK: Clinical observation
and experimental research in treatment of liver cancer at advanced
stage by traditional medication of strengthening the Spleen,
replenishing Qi, clearing away heat and toxic material. J
Integrated Trad Chin West Med. 7:275–277. 1987.
|
|
25.
|
Yadav SK and Lee SC: Evidence for
Oldenlandia diffusa-evoked cancer cell apoptosis through
superoxide burst and caspase activation. Zhong Xi Yi Jie He Xue
Bao. 4:485–489. 2006.
|
|
26.
|
Rasul A, Yu B, Yang LF, Ali M, Khan M, Ma
T and Yang H: Induction of mitochondria-mediated apoptosis in human
gastric adenocarcinoma SGC-7901 cells by kuraridin and
Nor-kurarinone isolated from Sophora flavescens. Asian Pac J
Cancer Prev. 12:2499–2504. 2011.PubMed/NCBI
|
|
27.
|
Lin CC and Namba T: Historical and
herbological studies on the traditional Japanese and Chinese crude
drugs. On the ‘Shān-cí-gū’. Yakushigaku Zasshi. 20:88–98. 1985.(In
Japanese).
|
|
28.
|
Nigg EA: Cyclin-dependent protein kinases:
key regulators of the eukaryotic cell cycle. Bioessays. 17:471–480.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Orlando DA, Lin CY, Bernard A, Wang JY,
Socolar JES, Iversen ES, Hartemink AJ and Haase SB: Global control
of cell-cycle transcription by coupled CDK and network oscillators.
Nature. 453:944–947. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Feng L, Jia XB, Shi F and Chen Y:
Identification of two polysaccharides from Prunella vulgaris
L and evaluation on their anti-lung adenocarcinoma activity.
Molecules. 15:5093–5103. 2010.
|
|
31.
|
Kang SC, Lee CM, Choi H, Lee JH, Oh JS,
Kwak JH, Lee JH, Oh JS, Kwak JH and Zee OP: Evaluation of oriental
medicinal herbs for estrogenic and antiproliferative activities.
Phytother Res. 20:1017–1019. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Zhang Z, Wang X, Wu W, Wang J, Wang Y, Wu
X, Fei X, Li S, Zhang J, Dong P, Gu J and Liu Y: Effects of matrine
on proliferation and apoptosis in gallbladder carcinoma cells
(GBC-SD). Phytother Res. 26:932–937. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Zhang P, Wang Z, Chong T and Ji Z: Matrine
inhibits proliferation and induces apoptosis of the
androgen-independent prostate cancer cell line PC-3. Mol Med Rep.
5:783–787. 2012.PubMed/NCBI
|
|
34.
|
Qin XG, Hua Z, Shuang W, Wang YH and Cui
YD: Effects of matrine on HepG2 cell proliferation and expression
of tumor relevant proteins in vitro. Pharm Biol. 48:275–281. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Zhao B, Li B, Bai S, Shen L, Ren R, Jonas
JB, Xu X, Lu Q and Liu Q: Effects of matrine on proliferation and
apoptosis of cultured retinoblastoma cells. Graefes Arch Clin Exp
Ophthalmol. 250:897–905. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Agami R and Bernards R: Convergence of
mitogenic and DNA damage signaling in the G1 phase of the cell
cycle. Cancer Lett. 177:111–118. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Brew CT, Aronchik I, Hsu JC, Sheen JH,
Dickson RB, Bjeldanes LF and Firestone GL: Indole-3-carbinol
activates the ATM signaling pathway independent of DNA damage to
stabilize p53 and induce G1 arrest of human mammary epithelial
cells. Int J Cancer. 118:857–868. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Alpan RS and Pardee AB: p21WAF1/CIP1/SDI1
is elevated through a p53-independent pathway by mimosine. Cell
Growth Differ. 7:893–901. 1996.PubMed/NCBI
|