Introduction
Human glioblastoma is one of the most lethal cancer
types and, in spite of the mass of in vitro and in
vivo studies accumulated in the literature, currently used
standard therapies still result in a median duration of patient
survival of 12–18 months after diagnosis. The key objective to
improve glioblastoma pharmacological therapy lies in the ability to
prevent the dissemination of single cancer cells that eventually
contributes to the reformation of new solid tumor masses.
The invasiveness of brain cancer cells is a complex
mechanism that involves several steps such as initial detachment of
tumorigenic cells from the tumor mass, migration through brain
parenchyma, resistance to apoptotic damage and finally adhesion to
distal cells in the tumoral niche. The endogenous extracellular
matrix (ECM) proteins, such as laminin, collagens, tenascin and
vitronectin, play a fundamental role in cancer cell invasiveness
since their binding to integrins modulates cell attachment and
other processes such as proliferation and migration.
Integrins are heterodimeric glycoprotein membrane
receptors formed by the combination of α and β subunits that give
rise to 24 distinct integrins whose subunit composition leads to
their ECM ligand specificity. The α5β1, αvβ3 and αvβ5 integrins,
recognizing the tripeptide sequence Arg-Gly-Asp (RGD) present in
many ECM proteins (1), are
actively exploited as potential targets in the development of
antitumorigenic and antiangiogenic compounds as they are
overexpressed in glioma and peritumoral endothelial cells (2).
The binding of ECM ligands to integrins activates
the cytosolic tyrosine kinase Src, constitutively bound to the
integrin β cytoplasmic tail and the focal adhesion kinase (FAK)
(3) that, in turn, leads to the
activation of downstream ERK-and AKT-dependent signaling
pathways.
FAK appears to play key roles in tumor growth and
metastatic spread. It is overexpressed in glioblastoma tumor biopsy
samples. It modulates proliferation, survival and migration of
glioblastoma cells in vitro and in animal model (4) and its activation, mediated by
integrin-ECM ligands, provides essential survival signals and
protects glioma cells from anoikis, a detachment-induced cell
death. For these reasons, inhibition of FAK activity is an
appealing target.
Resistance to anoikis confers a selective advantage
for tumor cell invasion and metastasis; therefore, reducing cancer
cell dissemination by enhancing anoikis via integrin antagonists
appears promising. However, although the validity of this
hypothesis has been confirmed in different cancer cell types and
endothelial cells with significant results (1), in glioma cells the complexity of the
mechanisms involved in the induction and resistance to anoikis is a
serious obstacle.
The first small molecule integrin antagonist
developed was cilengitide (EMD 121974), a cyclic peptide belonging
to the RGD-peptide family that, upon binding to the integrin β
chain, prevents the interaction of integrins with their endogenous
ECM ligands. Previous studies have demonstrated the promising
features of RGD-peptide molecules, as these compounds display
relative efficacy, good tolerability and low toxicity in clinical
trials. Although cilengitide blocks glioblastoma (GBM) growth in
nude mice (5), evidence in
patients with recurrent GBM has shown that cilengitide monotherapy
is well tolerated but displays modest antitumor activity (6). This finding has prompted efforts
aimed at the synthesis of new peptidic and non-peptidic integrin
antagonists with a different pattern of binding properties. These
molecules are currently under investigation for their
anti-angiogenic and anticancer activity, administered alone or in
combination with other therapeutic agents such as temozolomide
(7).
The new integrin antagonist 1a-RGD, unlike the
cyclic peptide cilengitide, is an RGD-like molecule containing a
bicyclic pseudopentapeptide that binds αvβ3, αvβ5 and α5β1
integrins with in vitro preferential affinity towards αvβ3.
However, it is still unknown whether and how the novel chemical
structure of 1a-RGD may interfere with the functional effects
elicited by the ECM-integrin interaction in glioma cells in
vitro.
In this study we evaluated several cellular effects
induced by 1a-RGD treatment in human U251 and U373 glioblastoma
cell lines that express αvβ3 and αvβ5 and α5β1 integrins. We showed
here that 1a-RGD decreased cell migration and attachment,
disassembled the actin cytoskeleton, reduced FAK phosphorylation,
decreased the expression of target integrins at transcriptional
level and induced anoikis. Our data highlight the importance of
small-molecule integrin antagonists as novel tools to reduce the
survival of glioma cells.
Materials and methods
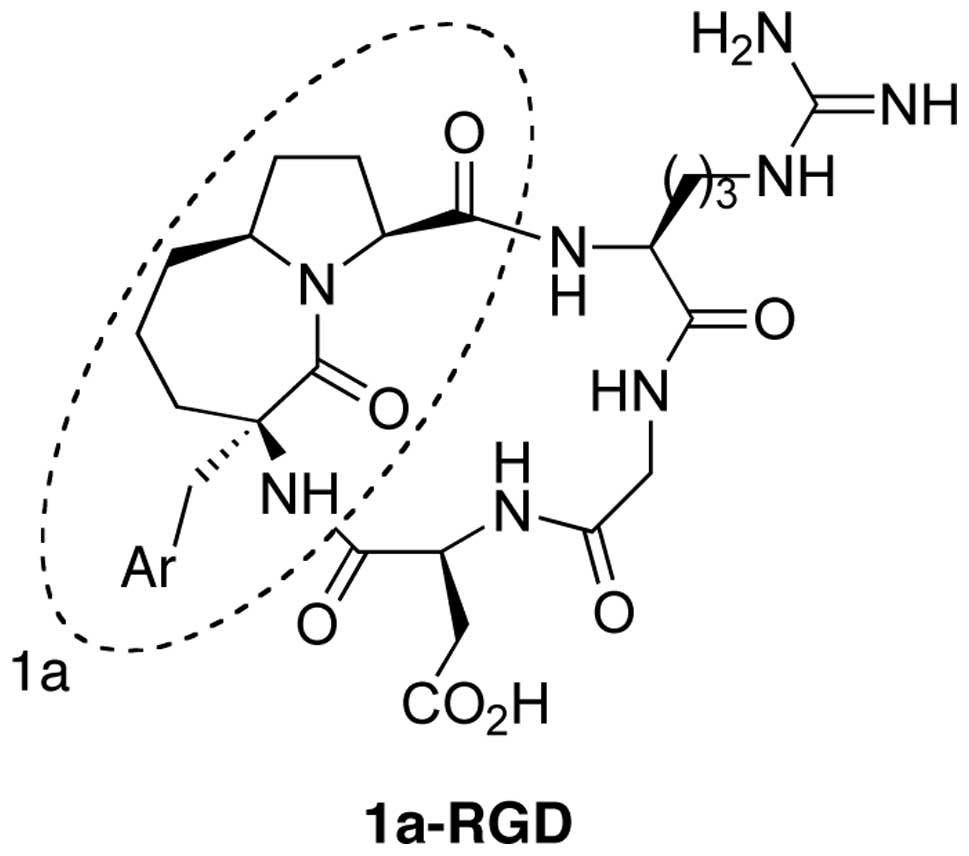
Synthesis of 1a-RGD
The synthesis of the cyclic-RGD derivative 1a-RGD
was carried out by exploiting a solution phase method. In the first
step the carboxy function of the azabicycloalkane scaffold 1a was
coupled with a suitably protected NH2-Arg-Gly-OH
dipeptide and the N-terminus of the scaffold was then coupled with
an Asp residue. The resulting linear peptide sequence was finally
cyclized to give the fully protected RGD-based cyclopenta peptide.
The final compound 1a-RGD (Fig. 1)
was purified by HPLC (8).
Cell culture
The U251 and U373 human glioblastoma cell lines were
purchased from Istituto Zootecnico Regione Lombardia (Brescia,
Italy). The cell lines were grown in DMEM supplemented with 5%
fetal bovine serum (FBS), 2 mM glutamine, penicillin-streptomycin
(10,000 U/ml) and cells were grown at 37°C in a controlled
atmosphere (5% CO2/95% air). Confluent cells were split
(1:5–1:10 ratio) by trypsinization and used at the third-fourth
passage after thawing. For all the experiments the cells were
plated at a density of 10,000 cells/cm2. The reagents
used for the cell cultures were from Euroclone, Italy.
Cell viability assay
Cells lines plated in 96-multiwells were treated
with 20 μM 1a-RGD56 for 24, 48 and 72 h. At the end, 20 ml
of CellTiter 96 reagent (Promega) was added to each well and after
3 h the colorimetric signal was detected by a multiwell plate
reader at 490 nm. Five wells for each experimental point were used.
Each experiment was repeated three times.
FACS analysis
Cell surface αvβ3, αvβ5 and α5β1 integrin receptors
were detected on fixed cells using αvβ3, αvβ5 and α5β1 Alexa
488-conjugated antibodies. Briefly, 10,000 cells for each sample
were mechanically collected, fixed in 10% formaldehyde in PBS for 5
min and washed three times in PBS. Cells were incubated for 2 h at
room temperature with antibodies. Samples were analyzed by a
FACSCalibur instrument (Becton-Dickinson, Franklin Lakes, NJ). Each
experiment was repeated three times.
To assess the onset of apoptosis the Annexin
technique was used. Cells (10,000) for each sample were
mechanically collected and, after washing in PBS, incubated for 5
min in the presence of Annexin and propidium iodide (PI,
Sigma-Aldrich). Each experiment was repeated three times.
BrdU-ELISA cell proliferation assay
Cells were plated in 96-multiwells in growth medium
and treated with 20 μM 1a-RGD for increasing time periods.
At the end, 10 μl of BrdU from the cell proliferation ELISA
BrdU kit (Roche Diagnostics) was added to each well. After a 5-h
incubation the assay was performed following the manufacturer’s
instructions. The samples were evaluated using a multiwell reader
at 450 nm. Eight wells were used for each experimental point and
each independent experiment was repeated three times.
Cell migration assay
Cells were plated in serum-free DMEM on a Matrigel
coated Transwell (Costar). In the bottom of the well 500 μl
DMEM containing 10% FBS was placed as a chemo attractant. The
migration assay was carried out for 12 h in the presence of 20
μM 1a-RGD in the cell culture incubator. After removing the
Matrigel, the cells present on the lower face of the membrane were
stained using DAPI (Sigma-Aldrich) and counted using a fluorescence
microscope. Cells were counted in 10 fields for each membrane.
Adhesion assay
Cells were harvested in PBS-EDTA 5 μM,
resuspended in DMEM containing 5% FBS, and plated for 1 h in
fibronectin-coated wells (10 μg/ml) in the presence of 20
μM 1a-RGD. Unattached cells were removed by two washes with
PBS and attached cells were subjected to cell viability MTS assay
(Promega) for quantification (9).
Immunocytochemical analysis
For actin cytoskeleton detection, cells were plated
onto polylysine-coated coverslips and incubated in the presence of
20 μM 1a-RGD for 4 h. At the end of the treatments the cells
were washed three times in PBS and fixed in 3.7% formaldehyde for
15 min at 37°C. The cells were then washed in PBS and incubated for
45 min in PBS containing 0.1% Triton X-100, 1% bovine serum albumin
(BSA) and 10% normal goat serum. The cells were then incubated for
90 min at 37°C with Alexafluor 633 phalloidin diluted 1:7000 in PBS
containing 0.01% Triton X-100 and 0.1% BSA (wash solution). At the
end of the incubation, cells were repeatedly washed with PBS and
counterstained with 1 μM Hoechst 33342 for 15 min at room
temperature. At the end of the incubation, cells were washed twice
with PBS and once with distilled water. Coverslips were mounted
with Mowiol. Images were acquired by laser scanning confocal
microscopy, x40 oil immersion objective (TCP-SP3, Leica) and
analyzed by dedicated Leica software. Each experiment was repeated
at least twice.
Western blot analysis
Cells grown in 60-mm dishes were treated for the
indicated time with 20 mM 1a-RGD. The cells were then rinsed twice
in ice-cold PBS and 200 ml of cell lysis buffer was added to the
dishes (composition: 50 mM Tris-HCl pH 7.4, 1% v/v NP-40, 0.25% w/v
sodium deoxycholate, 1 mM phenylmethylsulphonyl-fluoride (PMSF), 1
mM Na3VO4, 1 mM EDTA, 30 mM sodium
pyrophosphate, 1 mM NaF, 1 mg/ml leupeptin, 1 mg/ml pepstatin A, 1
mg/ml aprotinin and 1 mg/ml microcystin). After scraping, the cells
were sonicated for 10 sec, centrifuged at 12,000 × g for 5 min at
4°C and the amount of proteins in the supernatant was measured by
the BCA protein assay kit (Pierce). For western blot analysis, 30
μg of proteins was separated by 10% SDS-PAGE at 150 V for 2
h and blotted onto 0.22-mm nitrocellulose membranes at 50 mA for 16
h. The membranes were first blocked for 2 h in Tris-buffered saline
solution (TBST composition: Tris 10 mM, NaCl 150 mM, 0.1% Tween-20)
plus 5% low fat dry milk (TBSTM) and then incubated with the
appropriate antibody diluted 1:1000 in TBSTM, for 16 h at 4°C under
gentle agitation. The membranes were rinsed three times in TBST and
then incubated for 2 h at 21°C with a goat anti-rabbit IgG
horseradish peroxidase-conjugated secondary antibody (Upstate
Biotechnology), diluted 1:10,000 in TBSTM. The membranes were
rinsed three times in TTBS and the luminescent signal was detected
by the ECL Plus Western Blotting Detection system (Amersham). Each
experiment was repeated at least twice.
ELISA apoptosis assay
For the relative quantification of apoptosis a
sandwich immunoassay was performed to detect nucleosomes (Cell
Death Detection ELISA, Roche Diagnostics). Cells were plated in
12-multiwell plates in growth medium and treated with 20 μM
1a-RGD for increasing times. At the end of the incubation time, the
assay was performed following the manifacturer’s instructions.
Finally, the samples were read in a multiwell reader at 405 nm.
Eight wells were used for each experiment and each experiment was
repeated three times.
Real-time quantitative PCR
The primers were designed by using the ‘Primer3
Input’ software (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3.cgi/primer3_www.cgi)
and the specificity of each primer was controlled by the BLAST
software. Cells were treated with 20 μM 1a-RGD for 24 h and
total RNA was extracted. Quantitative real-time RT-PCR reactions
were performed as previously reported (9). At the end of the reaction, a melting
curve analysis was carried out to check for the presence of
primer-dimers. Comparison of the expression of each gene between
its control and stimulated states was determined with the ΔΔCt
method using RPL6 as housekeeping gene (10). Experiments were performed on three
different cell preparations and each run was analyzed in
duplicate.
Statistical analysis
Statistical analysis was performed by Instat3
software. Data are expressed as the means ± SD. The statistic
significance values (p) are referred to control values.
Results
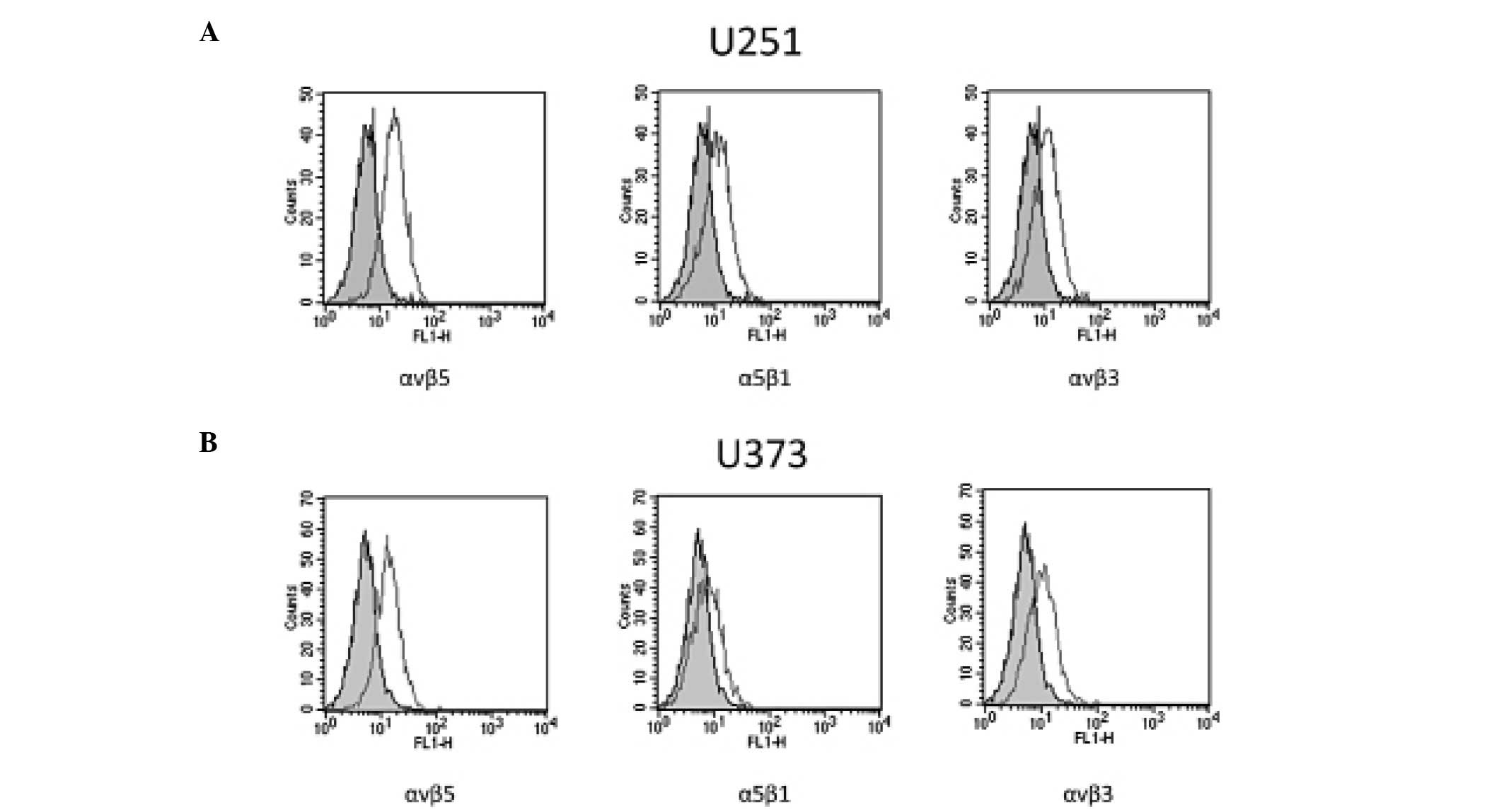
Expression of αvβ3, αvβ5 and α5β1
integrins in glioblastoma cell lines
To validate our model, preliminary experiments were
performed to assess whether U251 and U373 cell lines, grown in our
culture conditions, expressed the αvβ3, αvβ5 and α5β1 integrins
targeted by 1a-RGD. Classic RT-PCR experiments demonstrated that
U251 and U373 cells expressed mRNA for these integrin subunits
(data not shown).
The expression and membrane localization of αvβ3,
αvβ5 and α5β1 receptors were further assessed by FACS analysis. The
results showed that the three receptors were detected on the cell
membrane surface in the U373 and U251 cells (Fig. 2).
1a-RGD decreases the cell viability of
glioblastoma cells
To ascertain whether 1a-RGD exerts any effect on
cell growth, we first studied the effect of 1a-RGD on U251 and U373
cell viability by treating the cells with increasing concentrations
of 1a-RGD. Concentration/response curves were constructed following
treatment of the cells with increasing concentrations of 1a-RGD for
24, 48 and 72 h. An IC50 value of 10.2±0.8 μM was
found when cells were exposed to the compound for 72 h and this
result prompted us to use 20 μM 1a-RGD in the following
experiments. When the U251 and U373 cells were treated with 20
μM 1a-RGD for 24, 48 and 72 h, a statistically significant
decrease in cell viability was observed after 72 h of treatment
(data not shown).
To rule out the possibility that the observed
decrease in cell viability was due to a toxic effect of 1a-RGD on
the cells, LDH release at different time-points was measured. As
expected, no changes in LDH release were observed even when
concentrations >20 μM were used, indicating that, under
our conditions, 1a-RGD did not decrease cell viability by an
aspecific toxic mechanism.
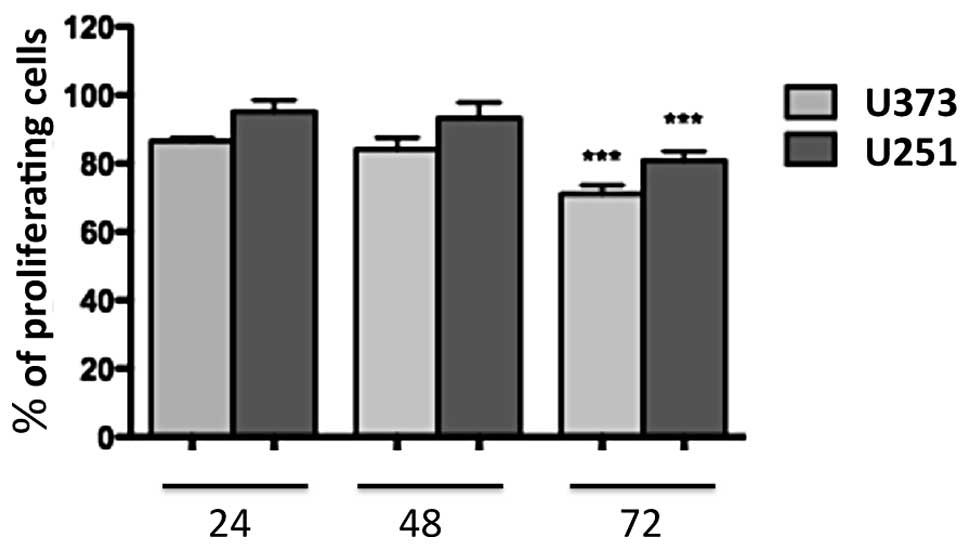
1a-RGD reduces the proliferation of
glioblastoma cells
U251 and U373 cells were treated for 24, 48 and 72 h
with 20 μM 1a-RGD, and BrdU assays were performed to
evaluate the effect of 1a-RGD on cell proliferation. A significant
decrease in the cell proliferation rate was observed after 72 h
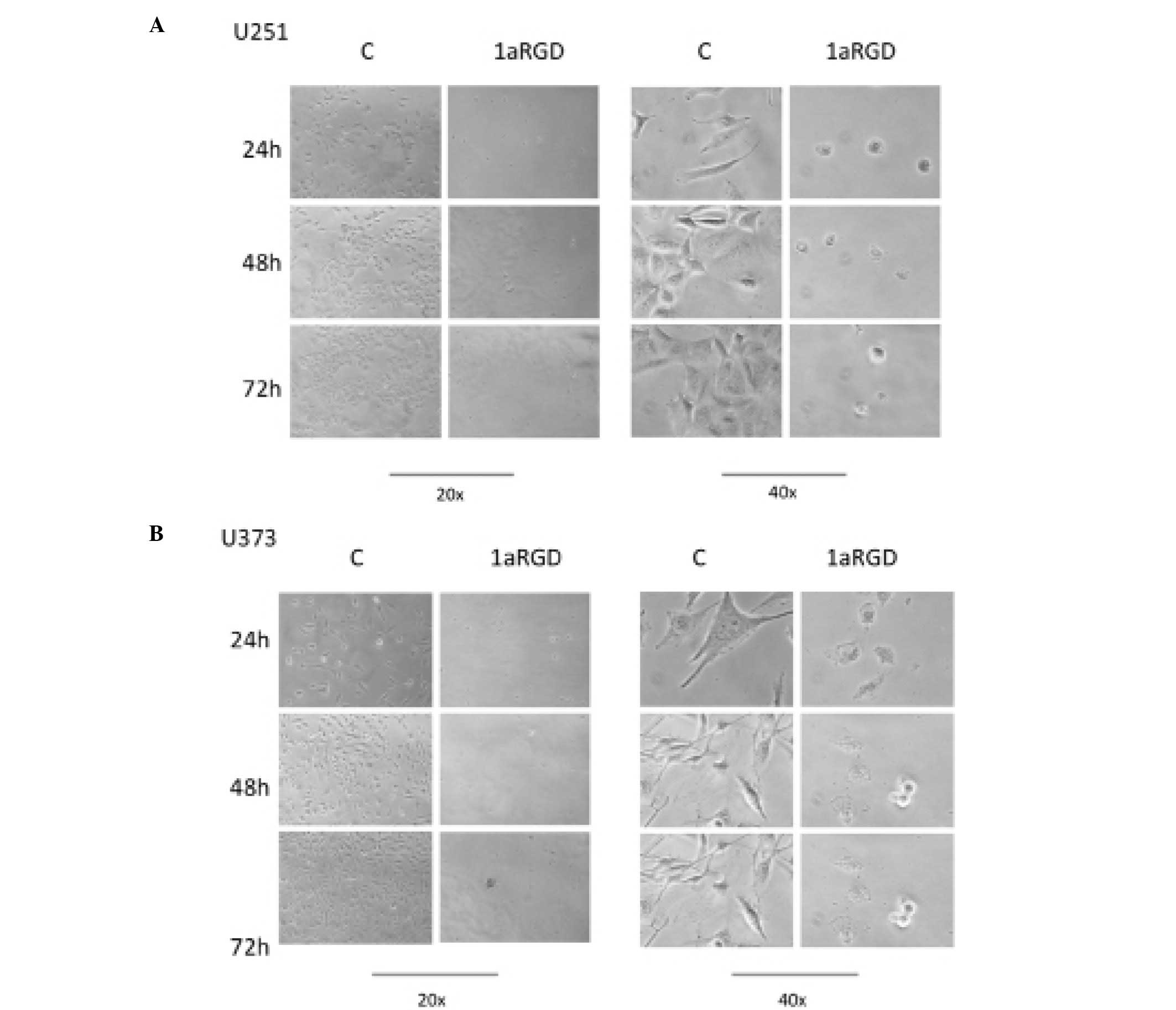
(Fig. 3). During these time-course
experiments, phase contrast images of the cells were captured. The
images clearly display detachment and changes in cell morphology
following 72 h of treatment (Fig.
4).
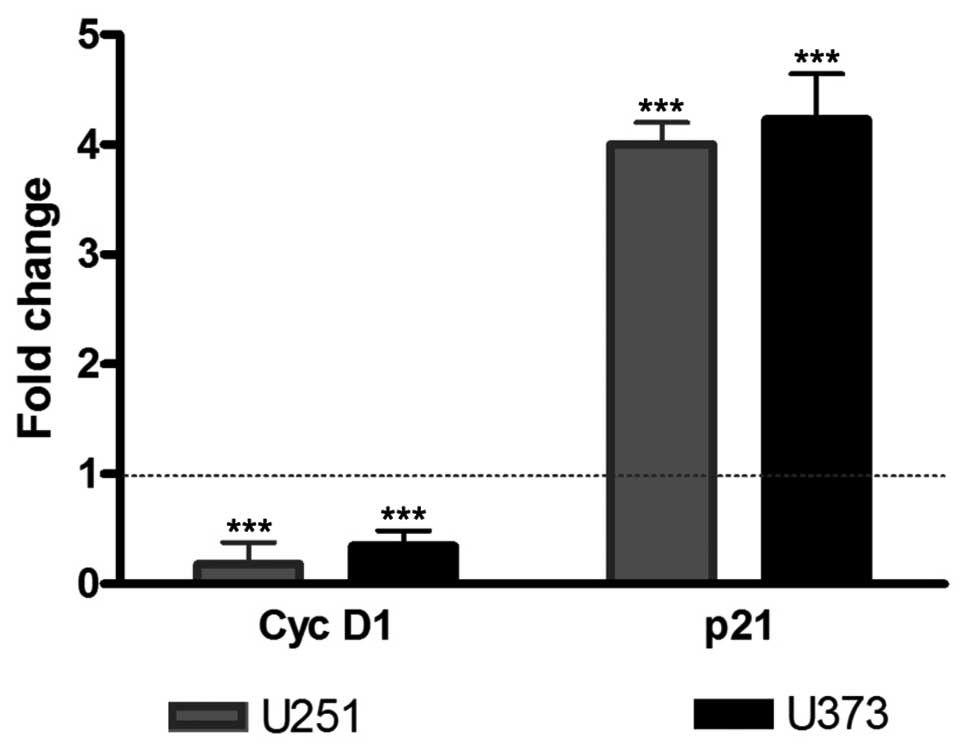
To confirm the reduction in the cell proliferation
rate observed in BrdU experiments, cyclin D1 and p21 mRNA
expression levels were measured by real-time quantitative PCR. U251
and U373 cells were treated with 1a-RGD for 72 h, and total RNA was
extracted and retrotranscribed to cDNA. The treatment of U251 and
U373 cell lines with 20 μM 1a-RGD elicited a significant
decrease in cyclin D1 mRNA and a significant increase in p21
mRNA.
Single transcript variations were calculated by the
ΔΔCt method using RPL6 as a reference gene as reported in Materials
and methods. Data are expressed as fold change and the results are
summarized in Fig. 5.
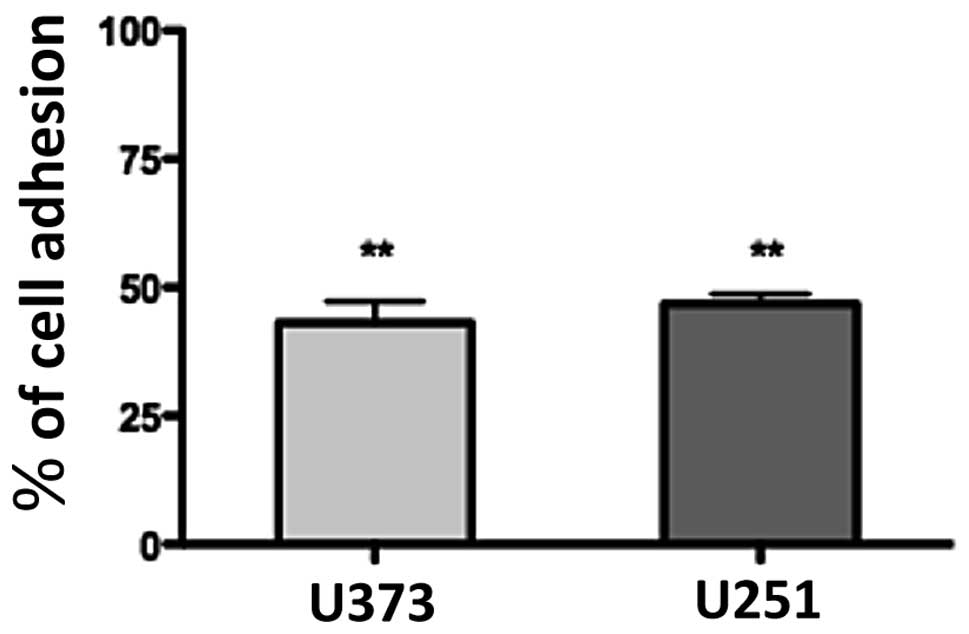
1a-RGD reduces the adhesive capability of
glioma cell lines
To ascertain whether 1a-RGD affects cell attachment
and interferes with integrin binding to cytoskeleton ECM,
attachment tests were performed as described. When cells were
plated on fibronectin-coated wells in the presence of 20 μM
1a-RGD, 1 h after plating only ∼50% of the cells were attached
compared to the controls; the quantification of attached cells was
performed by an MTS assay, as described in Materials and methods
(Fig. 6). This result indicates
that the compound exerts a strong inhibitory effect on the cell
attachment process. Similar results were obtained when the wells
were coated with Matrigel.
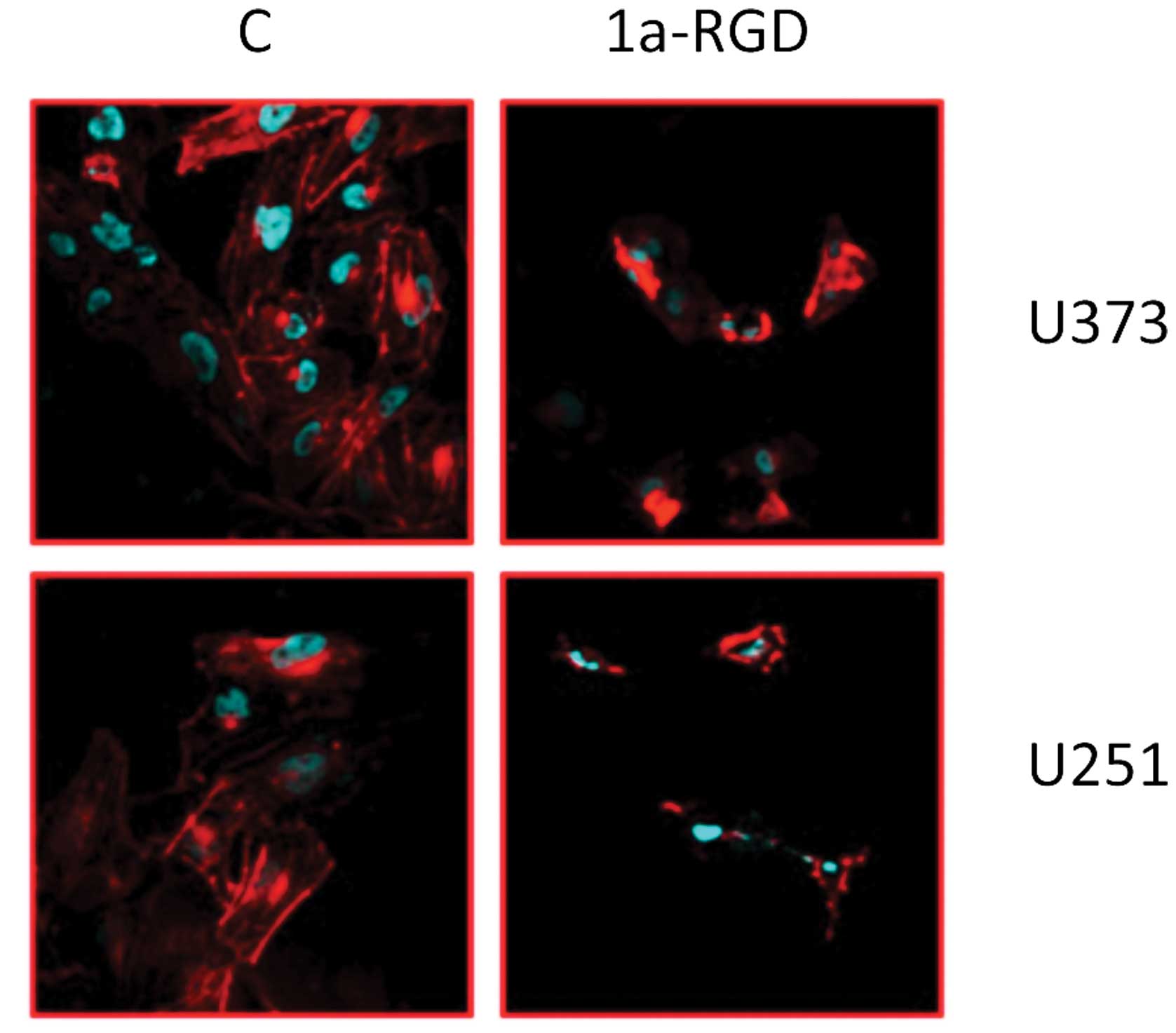
1a-RGD induces cytoskeleton disassembly
in glioma cell lines
To evaluate whether the observed effects on cell
adhesion are related to cytoskeleton alterations, we investigated
the effect of short-term (4 h) 1a-RGD treatment on the cytoskeleton
by the use of confocal microscopy. The U373 and U251 cells were
plated on polylysine-coated slides and treated with 20 μM
1a-RGD for 4 h. After fixing, they were marked with fluorescent
phalloidin and DAPI, as described in Materials and methods. The
results showed that the compound induced structural cytoskeletal
disassembly. Actin fibers appeared ruffled and the cell shape was
deeply affected (Fig. 7). These
data indicate that 1a-RGD, by antagonizing integrins binding to the
well, caused marked changes in the cytoskeletal structure.
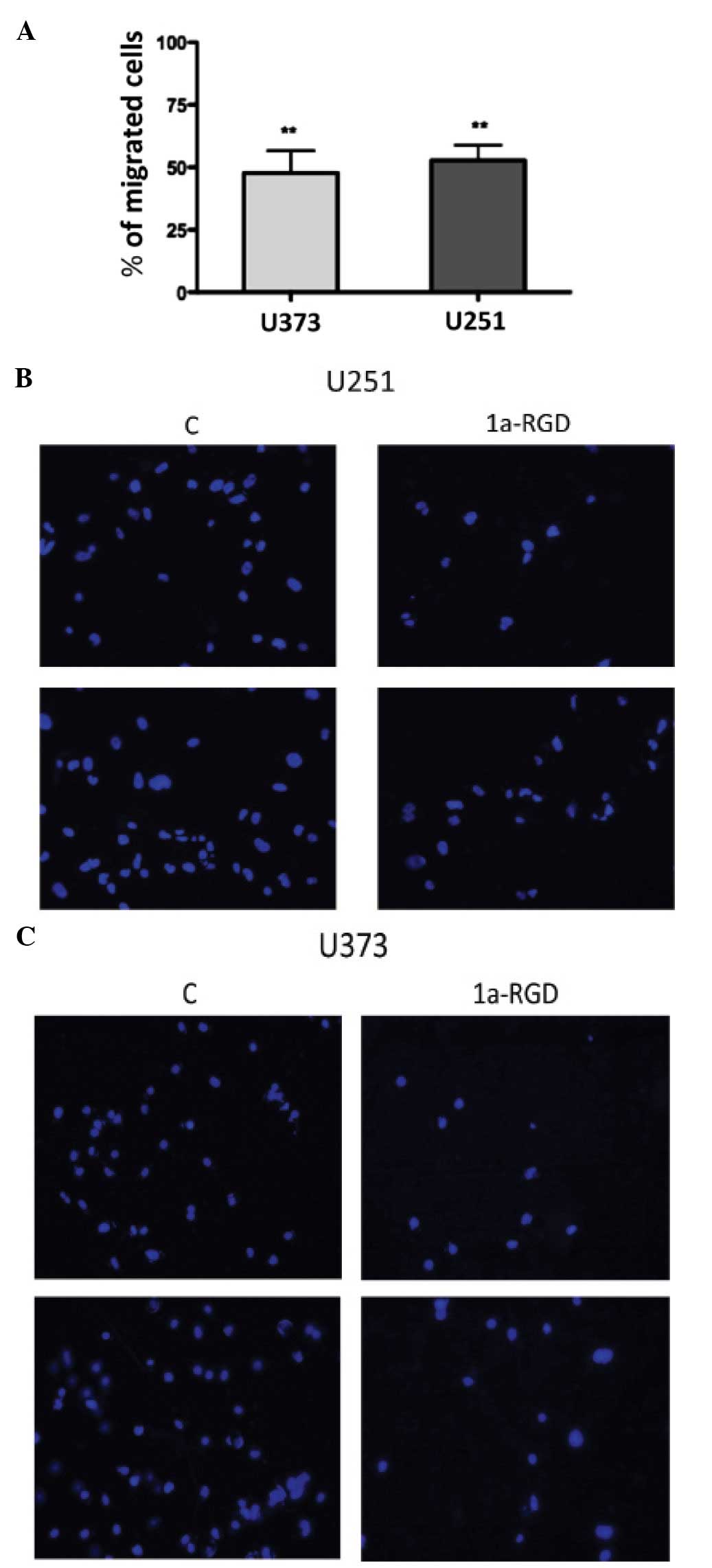
1a-RGD inhibits the cell migration of
glioma cell lines
The cellular machinery that is activated during cell
motility and cell migration processes requires a functional
cytoskeleton (11). The
observation that 1a-RGD treatment induces changes in actin fibers,
led us to investigate the effect of this compound on cell motility
by migration assays as described in Materials and methods. The U373
and U251 cells were plated on a Matrigel- coated membrane in
serum-free medium in the presence of 20 μM 1a-RGD for 12 h.
At the end of the treatment, the cells on the lower side of the
membrane were stained with DAPI and counted (see Materials and
methods). The results, expressed as a percentage compared to the
control, showed a marked decrease in cell number on the lower side
of the membranes in the presence of 1a-RGD (Fig. 8A); representative fields of the
lower membrane sides are shown in Fig.
8B and C.
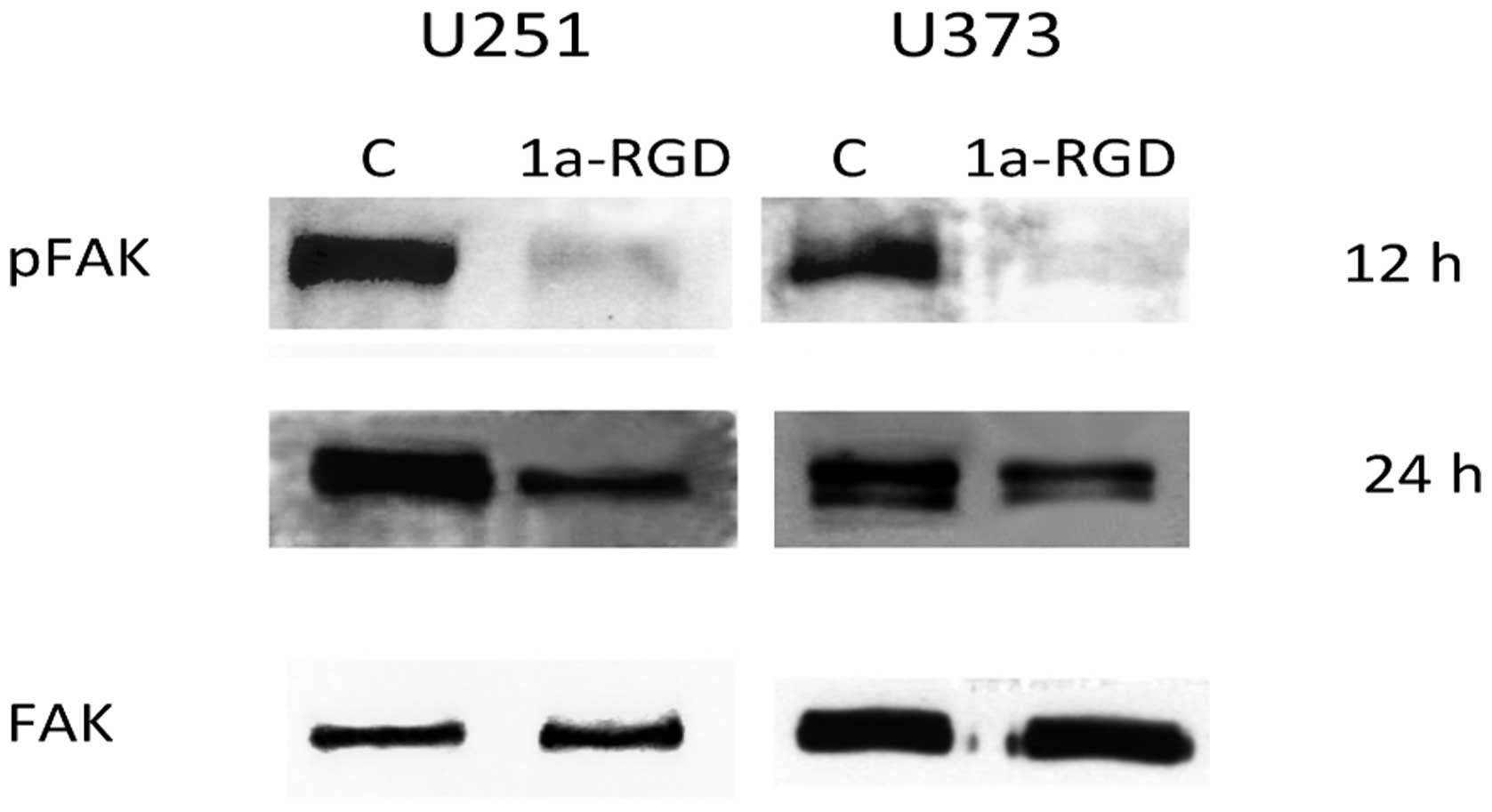
1a-RGD inhibits FAK phosphorylation but
not AKT and ERK phosphorylation
The tyrosine kinase FAK resides in focal adhesions
formed by integrin clustering and is functionally linked to
integrin activation. As 1a-RGD appears to interfere with cell
attachment, cell migration and cytoskeleton assembly, we evaluated
the effect of this antagonist on FAK phosphorylation. Western blot
experiments were performed as indicated above (Fig. 9). In both cell lines, a marked
decrease in pFAK signal is detected after 12 h of treatment and the
signal was downregulated even after 24 h. These data confirm that
1a-RGD binding to integrin receptors inhibits cellular processes
required for cell attachment and cell migration.
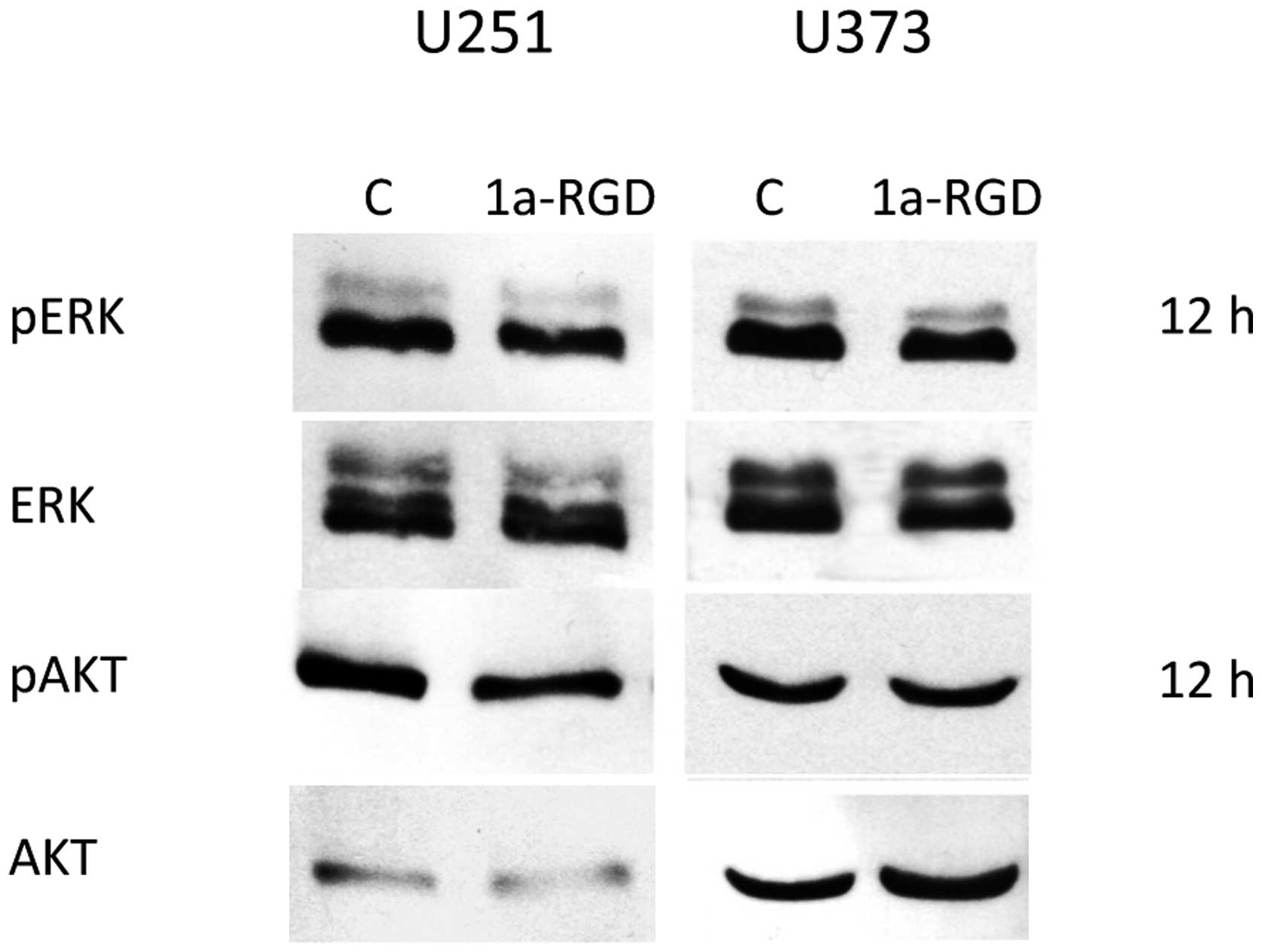
Western blot experiments were performed to further
investigate the effect of 1a-RGD treatment on integrin-linked
signal transduction. Cells were treated with the compound at
different time points. After 72 h of treatments, no effect on Akt
or ERK phosphorylation was observed in both cell lines (Fig. 10).
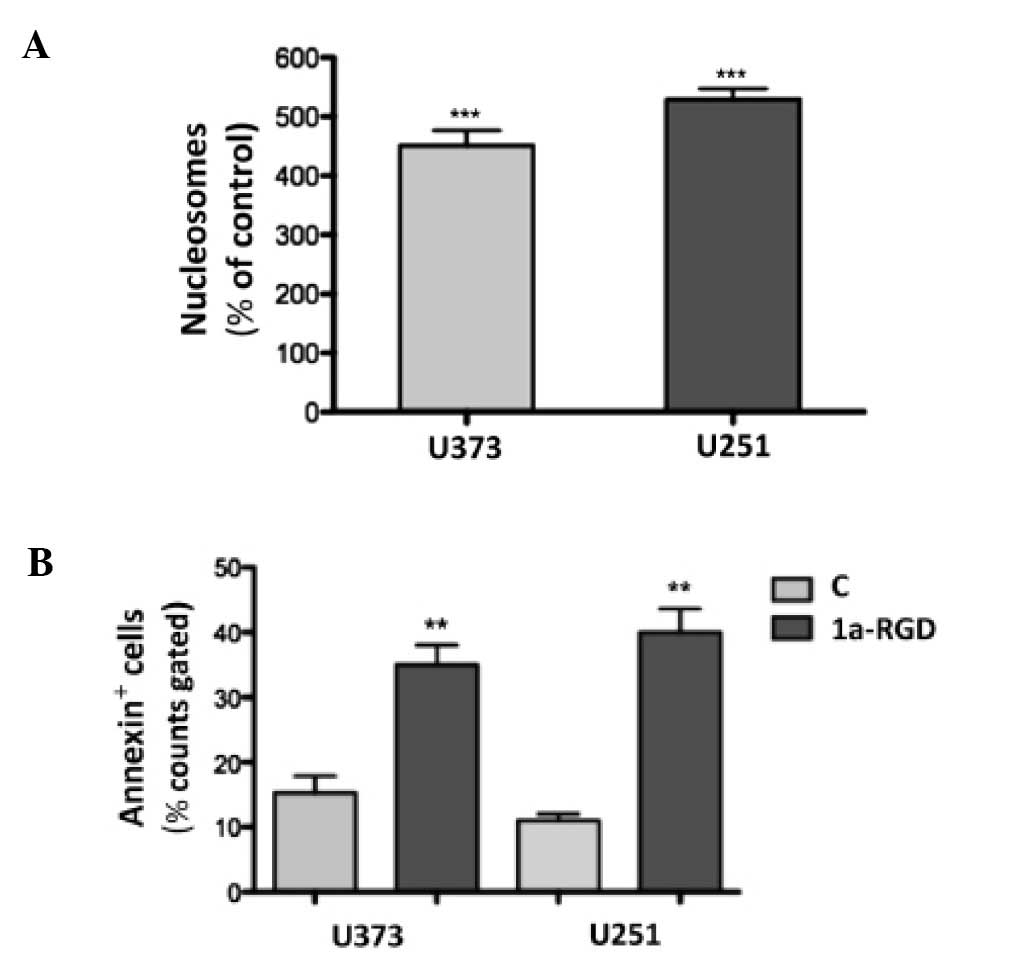
1a-RGD induces anoikis in glioma cell
lines
In order to assess whether the 1a-RGD treatment
induces apoptosis, cells were treated with 20 μM 1a-RGD for
increasing times and then cell death was assessed by nucleosome
assay. After 72 h, 1a-RGD induced a significant increase in
fragmented DNA (Fig. 11A).
Similar results were obtained when cell death was measured by
Annexin V staining (Fig.
11B).
FACS experiments were also separately performed on
attached and detached cells from the same wells treated with 20
μM 1a-RGD for 72 h. Notably, Annexin-positive cells were
almost exclusively found in the detached fraction.
1a-RGD reduces mRNA expression of the
α5 and β1 integrin subunits
It is known that, in some cases, long term
occupation of cell surface receptors by specific antagonists may
induce compensatory and adaptive changes of their expression at the
transcriptional level by complex positive or negative feedback
mechanisms. Since in the literature no data concerning the
long-term effects of RGD-antagonist treatment on integrin
expression are available, we decided to evaluate whether 1a-RGD
modifies mRNA levels of α5 and β1 integrin
subunits in long-term experiments. U251 and U373 cells were treated
with 20 μM 1a-RGD for 72 h, total RNA was extracted,
retrotranscribed to cDNA and real-time PCR was performed. Data were
obtained by the ΔΔCt method using RPL6 as reference gene (Table I). In both cell lines, while the
amount of αv, β3 and β5 mRNA was
not changed, a statistically significant decrease in α5
and β1 mRNA compared to the control (=1) was found: U373
α5 0.51±0.03 (p<0.01); U373 β1 0.66±0.04
(p<0.05); U251 α5 0.60±0.03 (p<0.05); U251 β1 0.41±0.02
(p<0.01).
 | Table I.Primer sequences and PCR product size
(bp). |
Table I.
Primer sequences and PCR product size
(bp).
| αv | NM_002210 | F:
actggcttaagagagggctgtg | 110 |
| | R:
tgccttacaaaaatcgctga | |
| β3 | NM_000212 | F:
agacactcccacttggcatc | 123 |
| | R:
tcctcaggaaaggtccaatg | |
| β5 | NM_002213 | F:
agcctatctccacgcacact | |
| | R:
cctcggagaaggaaacatca | 91 |
| α5 | NM_002205 | F:
cctgctgtccaccatgtcta | |
| | R:
ttaatggggtgattggtggt | 138 |
| β1 | NM_133376 | F:
tccaatggcttaatttgtgg | |
| | R:
cgttgctggcttcacaagta | 190 |
| CycD1 | NM_053056 | F:
ctcacgcttacctcaaccatc | |
| | R:
ctttggcctctcgatacacac | 130 |
| p21 | NM_000389 | F:
atatggggctgggagtagttg | |
| | R:
ccaggccagtatgttacagga | 132 |
| RPL6 | NM_001024662.1 | F:
agattacggagcagcagcgcaagattg | |
| | R:
gcaaacacagatcgcaggtagccc | 105 |
Discussion
The metastatic cascade of tumor cells involves
several sequential steps: detaching from the original tumor mass,
migration to distant sites and attachment to the tumoral niche
(12). During the steps of
metastasis formation, cancer cell survival depends on the ability
of cells to interact with ECM proteins and integrins regulate both
migration and adhesion processes. For this reason, preventing tumor
cells binding to the ECM is an important strategy to inhibit cancer
cell spreading and induction of anoikis. Small-molecule integrin
antagonists bearing the RGD sequence, such as the prototype
compound cilengitide, have been shown to exert antiangiogenic and
anti-proliferative activity in glioma therapy (13).
However, the mechanisms underlying the
antiangiogenic and antiproliferative effects of small-molecule
integrin antagonists are still unclear.
Preclinical studies in patients with recurrent GBM
have shown that cilengitide monotherapy inhibits glioblastoma
growth with modest antitumor activity (6) and, in combination with radiotherapy,
decreases tumor proliferation (7).
However, a recent study has raised concern about the use of
cilengitide monotherapy in glioma demonstrating that, under certain
experimental conditions and at nanomolar concentrations, this
molecule promotes rather than inhibits angiogenesis (14).
Considerable efforts have been made to synthesize
new RGD compounds with anticancer activity and 1-aRGD belongs to a
family of new RGD compounds synthesized in our laboratory (15).
In a main attempt to characterize the functional
effects elicited by 1a-RGD in human glioma cell lines grown in
vitro, we found that 1a-RGD, although it induces modifications
in cell functionality already described for other similar
compounds, nevertheless it displays certain novel features not
previously reported for other RGD integrin antagonists.
The ability of 1a-RGD to decrease adhesion and
proliferation in glioma cell lines are in excellent agreement with
the effects elicited by the small α5β1 integrin antagonist SJ749
that dose-dependently inhibits adhesion to fibronectin and
proliferation in the glioma cell lines A172 and U87 (16).
In a similar study, an RGD peptide that potently
binds to the αvβ3, αvβ5 and α5β1 integrins was found to decrease
the proliferation rate and adhesion in a panel of rat and human
glioma cells (17).
Another study reported that in glioma cell lines
cilengitide induces detachment and cell death with only a modest
effect on cell viability and without appreciable perturbation on
cell migration and invasivenesss (18).
Our data indicate that the main triggering event
underlying the observed cellular effects elicited by 1a-RGD was the
inhibition of cell attachment via direct antagonism with endogenous
ECM ligands towards target integrins as demonstrated by
cytoskeleton disassembly and inhibition of focal adhesion kinase
FAK. However, under our conditions, 1a-RGD markedly affected not
only cell attachment but also cell migration, and FAK inhibition
appears to be an important step in this process.
FAK is the link between integrins and downstream
signaling transduction pathways and activates ERK and PI3K/AKT
pathways involved in cell proliferation and migration (19); short term cilengitide treatment
inhibited the FAK/Src/AKT-dependent pathway in endothelial and
glioma cells but did not affect ERK activation in HUVEC cells
(20). In partial contrast with
this observation, we found a significant FAK phosphorylation
decrease in cells treated up to 24 h with 1a-RGD but no appreciable
effect on AKT and ERK phosphorylation was observed. The most likely
explanation for this discrepancy is that other receptor systems,
merging on the AKT- and ERK-dependent pathways, may likely overcome
the weak inhibitory effect elicited by 1a-RGD.
The concept that the pattern of integrin expression
affects cell migration has been demonstrated in some cancer cell
types. In melanomas, expression of αvβ3 integrin correlates with
tumor invasion (21) and
overexpression of αvβ3 is reported to mediate the migratory and
invasive phenotype of imatinib-resistant adherent chronic
myelogenous leukemia cells (22).
In addition integrin β5 expression plays a critical role in breast
carcinoma cell migration and enhances their proliferative
capacities (23).
We found that prolonged treatment with 1a-RGD
induced, at the transcriptional level, a downregulation of α5 and
β1 subunits in glioma cell lines and this event is likely to
contribute to the reduction in cell migration. The capability of
decreasing the expression of selected target integrins by
pharmacological treatment is quite intriguing as α5β1 integrin,
together with concomitant EGFR activation, is required for mutated
p53 driven enhancement of cancer cell motility (24).
The combined reduction in cell migration and
attachment are critical factors in reducing cancer cell
dissemination together with their ability to form new peritumoral
niches in the brain. These effects elicited by 1a-RGD have crucial
implications for the management of glioma recurrence since the grim
prognosis of glioma can be mainly ascribed to the ability of a
subpopulation of cancer cells to disseminate throughout the brain
giving rise to new local solid mass reformation. Migrating
non-adherent cells undergo anoikis but metastatic cells acquire
resistance to anoikis and therefore the search for new proapoptotic
drugs is the most important unsolved problem in oncology.
Although widely investigated in several cancer cell
lines, few studies have reported a proapoptotic effect of
small-molecule RGD integrin antagonists in glioblastoma cells.
However, due to their limited efficacy in monotherapy in recent
years, other approches have been devised to enhance the induction
of cell death elicited by small-molecule integrin antagonists with
the purpose to improve their clinical relevance. For example, these
molecules could amplify the proapoptotic effect of other anticancer
drugs, since in glioma cells the co-administration of two selective
α5β1 integrin inhibitors was found to facilitate cell apoptosis
induced by two currently used chemotherapeutic agents, ellipticine
and temozolomide, in brain tumor therapy (25). Furthermore, in endothelial cells,
the administration of the selective αvβ3 and αvβ5 antagonist RGDfV
was found to potentiate the apoptotic cell death induced by the
c-Abl kinase inhibitor STI-571 (26).
In addition, novel RGD-like compounds could be
screened as shuttle molecules when chemically linked to antitumoral
drugs to improve local bioavailability and to reduce the dose
sufficient for cell killing. In MCF-7 breast cancer cells
expressing αvβ3 integrin, the integrin antagonist
cyclic-RGD-bioshuttle functionalized with the antitumoral drug
temozolomide (TMZ) displayed a 10-fold decrease in the
IC50 when compared to the underivatized TMZ (27). The co-administration or chemical
coupling of these inhibitors with clinically relevant drugs
deserves further testing first in different in vitro models
and warrants additional in vivo studies in animal
experimental models.
One serious limitation of this study is that the
effects exerted by 1a-RGD have been detected in glioma cell
cultures propagated for a long period in cultures that may not
mirror the real genotype of the original tumor. To overcome this
pitfall, the functional cellular effects elicited by 1a-RGD
reported here must be tested in a more reliable in vitro
cell model that more closely resembles the phenotype of glioma
cells in situ. The recent characterization of glioma cancer
stem cells, that can be grown either attached to a laminin
substrate or in suspension as neurospheres in the absence of serum
and that appear like true precursors of circulating metastatic
cells (28), could represent a
suitable alternative experimental in vitro model to shed new
light on this promising avenue of research.
In conclusion, we provide new insights into the
functional cellular effects induced by a novel small-molecule RGD
integrin antagonist in human glioblastoma cell lines that can
potentially improve the pharmacological approach and clinical
management of glioblastoma chemotherapy.
Acknowledgements
This study was supported by a PRIN
grant of the Italian Ministry of University and Research
(MIUR).
References
|
1.
|
Paolillo M, Russo MA, Serra M, Colombo L
and Schinelli S: Small molecule integrin antagonists in cancer
therapy. Mini Rev Med Chem. 9:1439–1446. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Schnell O, Krebs B, Wagner E, Romagna A,
Beer AJ, Grau SJ, Thon N, Goetz C, Kretzschmar HA, Tonn JC and
Goldbrunner RH: Expression of integrin αvβ3 in gliomas correlates
with tumor grade and is not restricted to tumor vasculature. Brain
Pathol. 18:378–386. 2008.
|
|
3.
|
Zhao J and Guan JL: Signal transduction by
focal adhesion kinase in cancer. Cancer Metastasis Rev. 28:35–49.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Assoian RK and Klein EA: Growth control by
intracellular tension and extracellular stiffness. Trends Cell
Biol. 18:347–352. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Yamada S, Bu XY, Khankaldyyan V,
Gonzales-Gomez I, McComb JG and Laug WE: Effect of the angiogenesis
inhibitor cilengitide (EMD 121974) on glioblastoma growth in nude
mice. Neurosurgery. 59:1304–1312. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Reardon DA, Fink KL, Mikkelsen T,
Cloughesy TF, O’Neill A, Plotkin S, Glantz M, Ravin P, Raizer JJ,
Rich KM, Schiff D, Shapiro WR, Burdette-Radoux S, Dropcho EJ,
Wittemer SM, Nippgen J, Picard M and Nabors LB: Randomized phase II
study of cilengitide, an integrin-targeting
arginine-glycine-aspartic acid peptide, in recurrent glioblastoma
multiforme. J Clin Oncol. 26:5610–5617. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Stupp R, Hegi ME, Neyns B, Goldbrunner R,
Schlegel U, Clement PM, Grabenbauer GG, Ochsenbein AF, Simon M,
Dietrich PY, Pietsch T, Hicking C, Tonn JC, Diserens AC, Pica A,
Hermisson M, Krueger S, Picard M and Weller M: Phase I/IIa study of
cilengitide and temozolomide with concomitant radiotherapy followed
by cilengitide and temozolomide maintenance therapy in patients
with newly diagnosed glioblastoma. J Clin Oncol. 28:2712–2718.
2010. View Article : Google Scholar
|
|
8.
|
Arosio D, Belvisi L, Colombo L, Colombo M,
Invernizzi D, Manzoni L, Potenza D, Serra M, Castorina M, Pisano C
and Scolastico C: A potent integrin antagonist from a small library
of cyclic RGD pentapeptide mimics including benzyl-substituted
azabicycloalkane amino acids. Chem Med Chem. 3:1589–1603. 2008.
View Article : Google Scholar
|
|
9.
|
Paolillo M, Russo MA, Curti D, Lanni C and
Schinelli S: Endothelin B receptor antagonists block proliferation
and induce apoptosis in glioma cells. Pharmacol Res. 61:306–315.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Dussault AA and Pouliot M: Rapid and
simple comparison of messenger RNA levels using real-time PCR. Biol
Proced Online. 8:1–10. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Wehrle-Haller B: Assembly and disassembly
of cell matrix adhesions. Curr Opin Cell Biol. July 19–2012.(Epub
ahead of print).
|
|
12.
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Desgrosellier JS and Cheresh DA: Integrins
in cancer: biological implications and therapeutic opportunities.
Nat Rev Cancer. 10:9–22. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Reynolds AR, Hart IR, Watson AR, Welti JC,
Silva RG, Robinson SD, Da Violante G, Gourlaouen M, Salih M, Jones
MC, Jones DT, Saunders G, Kostourou V, Perron-Sierra F, Norman JC,
Tucker GC and Hodivala-Dilke KM: Stimulation of tumor growth and
angiogenesis by low concentrations of RGD-mimetic integrin
inhibitors. Nat Med. 15:392–400. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Belvisi L, Bernardi A, Colombo M, Manzoni
L, Potenza D, Scolastico C, Giannini G, Marcellini M, Riccioni T,
Castorina M, LoGiudice P and Pisano C: Targeting integrins:
insights into structure and activity of cyclic RGD pentapeptide
mimics containing azabicycloalkane amino acids. Bioorg Med Chem.
14:169–180. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Maglott A, Bartik P, Cosgun S, Klotz P,
Ronde P, Fuhrmann G, Takeda K, Martin S and Dontenwill M: The small
alpha5beta1 integrin antagonist, SJ749, reduces proliferation and
clonogenicity of human astrocytoma cells. Cancer Res. 66:6002–6007.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Mattern RH, Read SB, Pierschbacher MD, Sze
CI, Eliceiri BP and Kruse CA: Glioma cell integrin expression and
their interactions with integrin antagonists. Cancer Ther.
3A:325–340. 2005.PubMed/NCBI
|
|
18.
|
Maurer GD, Tritschler I, Adams B,
Tabatabai G, Wick W, Stupp R and Weller M: Cilengitide modulates
attachment and viability of human glioma cells, but not sensitivity
to irradiation or temozolomide in vitro. Neurooncology. 11:747–756.
2009.PubMed/NCBI
|
|
19.
|
Schaller MD: Cellular functions of FAK
kinases: insight into molecular mechanisms and novel functions. J
Cell Sci. 123:1007–1013. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Oliveira-Ferrer L, Hauschild J, Fiedler W,
Bokemeyer C, Nippgen J, Celik I and Schuch G: Cilengitide induces
cellular detachment and apoptosis in endothelial and glioma cells
mediated by inhibition of FAK/src/AKT pathway. J Exp Clin Cancer
Res. 27:862008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Kuphal S, Bauer R and Bosserhoff AK:
Integrin signaling in malignant melanoma. Cancer Metastasis Rev.
24:195–222. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Puissant A, Dufies M, Fenouille N, Ben
Sahra I, Jacquel A, Robert G, Cluzeau T, Deckert M, Tichet M, Chéli
Y, Cassuto JP, Raynaud S, Legros L, Pasquet JM, Mahon FX, Luciano F
and Auberger P: Imatinib triggers mesenchymal-like conversion of
CML cells associated with increased aggressiveness. J Mol Cell
Biol. 4:207–220. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Bianchi-Smiraglia A, Paesante S and Bakin
AV: Integrin β5 contributes to the tumorigenic potential of breast
cancer cells through the Src-FAK and MEK-ERK signaling pathways.
Oncogene. July 23–2012.(Epub ahead of print). View Article : Google Scholar
|
|
24.
|
Muller PA, Caswell PT, Doyle B, Iwanicki
MP, Tan EH, Karim S, Lukashchuk N, Gillespie DA, Ludwig RL,
Gosselin P, Cromer A, Brugge JS, Sansom OJ, Norman JC and Vousden
KH: Mutant p53 drives invasion by promoting integrin recycling.
Cell. 139:1327–1341. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Martinkova E, Maglott A, Leger DY, Bonnet
D, Stiborova M, Takeda K, Martin S and Dontenwill M: alpha5beta1
integrin antagonists reduce chemotherapy-induced premature
senescence and facilitate apoptosis in human glioblastoma cells.
Int J Cancer. 127:1240–1248. 2010. View Article : Google Scholar
|
|
26.
|
Xu J, Millard M, Ren X, Cox OT and
Erdreich-Epstein A: c-Abl mediates endothelial apoptosis induced by
inhibition of integrins alphavbeta3 and alphavbeta5 and by
disruption of actin. Blood. 115:2709–2718. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Braun K, Wiessler M, Pipkorn R, Ehemann V,
Bauerle T, Fleischhacker H, Muller G, Lorenz P and Waldeck W: A
cyclic-RGD-BioShuttle functionalized with TMZ by DARinv
‘Click Chemistry’ targeted to αvβ3 integrin for therapy. Int J Med
Sci. 7:326–339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Pollard SM, Yoshikawa K, Clarke ID, Danovi
D, Stricker S, Russell R, Bayani J, Head R, Lee M, Bernstein M,
Squire JA, Smith A and Dirks P: Glioma stem cell lines expanded in
adherent culture have tumor-specific phenotypes and are suitable
for chemical and genetic screens. Cell Stem Cell. 4:568–580. 2009.
View Article : Google Scholar : PubMed/NCBI
|