Introduction
Lysophosphatidic acid (LPA;
acyl-glycerol-3-phosphate) is a phospholipid growth factor that
exerts diverse biological effects in cellular and organ systems
(1–3). LPA exhibits multiple physiological
activities through the activation of LPA receptors
(LPA1–6), which are G-protein coupled receptors (GPCRs)
that act through various downstream signal transduction pathways
(4). LPAs are produced from
lysophosphatidylcholine by autotaxin (ATX) (also known as
lysophospholipase D, lyso PLD) in the blood (5–7).
Of note, in LPA-related cancer pathophysiology,
melanoma cell lines secrete ATX and facilitate cell migration. In
previous studies, the ATX-LPA axis has been shown to function as a
mitogen and motility factor for various types of cancer, including
neuroblastoma, hepatoma, lung cancer, ovarian cancer, metastatic
breast cancer and melanoma, and these cancers produce high levels
of ATX (7). Thus, ATX was first
identified as an autocrine tumor cell motility factor (8). However, LPA is a strong negative
feedback ATX inhibitor with high affinity (9). Therefore, LPA-related compounds, such
as Brp-LPA and cyclic phosphatidic acids, which inhibit ATX
activity, have been investigated as putative anticancer or
antimetastatic agents (10–12).
Ginseng, a traditional herbal medicine, has been
used for cancer prevention (13,14).
However, little is known about the active components and molecular
mechanisms underlying the effects of ginseng. Recently, we isolated
a novel glycolipoprotein from Panax ginseng, gintonin, which
was identified as a LPA complex with ginseng proteins (15). Similar to LPA, gintonin activates
LPA receptors in cells expressing endogenous and heterologous LPA
receptors (16). Gintonin contains
approximately 9.5% LPA and the major LPA of gintonin is LPA
C18:2(16). A previous
study demonstrated that LPA C18:2 potently inhibits ATX
activity compared to other LPAs (17). However, the effects of gintonin on
ATX activity, on metastasis-related cellular effects in
vitro and on metastasis and tumor growth in vivo remain
unknown.
In the present study, we report that gintonin
potently inhibits ATX activity. In addition, gintonin inhibits
cellular metastasis-related cell migration. The oral administration
of gintonin inhibits lung metastasis and tumor growth in mice. We
discuss the mechanisms involved in the gintonin-mediated
antimetastatic effects and inhibition of tumor growth. Finally, we
propose that gintonin may be useful for targeted metastasis
prevention or therapy.
Materials and methods
Materials
Gintonin was isolated from Panax ginseng as
described in our previous study (15). The BrdU incorporation assay ELISA
kit was purchased from Roche Diagnostics (Mannheim, Germany).
Penicillin, streptomycin, DMEM and FBS were purchased from Life
Technologies (Grand Island, NY, USA). ATX Inhibitor Screening kits,
FS-3, Brp-LPA and LPA C18:2 were purchased from Echelon
Biosciences Inc. (Salt Lake City, UT, USA). Zoletil 50 was
purchased from Virbac (Carros, France). Rompun was purchased from
Bayer Korea (Seoul, Korea). All other reagents used were purchased
from Sigma-Aldrich Co. (St. Louis, MO, USA).
ATX inhibition assay
First, the inhibitory effects of gintonin on ATX
activity were examined using ATX Inhibitor Screening kits according
to the manufacturer’s instructions. The ATX- mediated hydrolysis of
the fluorogenic ATX substrate, FS-3, produces fluorescein
fluorescence by a fluorescence resonance energy transfer (18). Briefly, ATX was incubated with
either gintonin or Brp-LPA as a standard inhibitor at different
concentrations in a 96-well plate. FS-3 was then added and the
resulting fluorescence was measured over time [excitation (Ex), 485
nm; emission (Em), 528 nm]. The percent inhibition was calculated
from the slopes of the fluorescence versus time graphs of each
standard, sample and blank.
Cell culture
The B16/F10 murine melanoma cell line was purchased
from the Korean Cell Line Bank (KCLB; Seoul, Korea) (19) and the MDA-MB-435 human melanoma
cancer cell line was a kind gift from Professor Woong-Yang Park
(College of Medicine, Seoul National University, Seoul, Korea). The
cells were cultured in DMEM supplemented with 10% (v/v) FBS, 100
U/ml penicillin and 100 μg/ml streptomycin.
Measurement of ATX activity in the
conditioned medium from melanoma cells
The conditioned medium containing ATX was collected
and concentrated from melanoma cells, as previously described and
ATX activity in the medium was measured using fluorogenic ATX
substrate FS-3 (20–22). Briefly, the cells were grown in
100-mm cell culture dishes until 60–70% confluence. The growth
medium was removed and washed with serum-free DMEM. The medium was
replaced with 10 ml of DMEM containing 0.1% BSA and subsequently
incubated for 48 h at 37°C. The conditioned medium was collected
and centrifuged at 1,500 × g for 10 min at 4°C. The supernatant was
collected and concentrated 100-fold using an Amicon Ultra 4
centrifugal filter device (50K) (EMD Millipore, Billerica, MA,
USA). The conditioned media were washed with storage buffer (50 mM
Tris, 20% ethylene glycol, pH 7.5) 3 times in the same Centrifugal
Filter Device. The concentrated and conditioned medium was diluted
in Tris-buffered saline solution (TBS: 140 mM NaCl, 5 mM KCl, 1 mM
CaCl2, 1 mM MgCl2, 50 mM Tris-HCl, pH 8.0)
and incubated with different concentrations of gintonin or LPA
C18:2 for 10 min in a 96-well plate. FS-3 in TBS
containing 0.1% charcoal-treated BSA was then added to each well of
the 96-well plate (final concentration of FS-3, 2.5 μM) and
the resulting fluorescence was measured for 24 h (Ex, 485 nm; Em,
528 nm). The percentage inhibition was calculated from the slopes
of the fluorescence intensity versus the time graphs of each
well.
Scratch-wound healing assay
An in vitro migration (scratch-wound healing)
assay was subsequently performed as described previously (23). Briefly, cells were seeded in
24-well plates (1.5×105 cells/well for B16/F10 cells;
3×105 cells/well for MDA-MB-435 cells). The cells were
incubated in serum-reduced medium for 6 h and wounded in a line
across the well with a 200-μl pipette tip, which was
followed by washing twice with serum-reduced medium. The cells were
incubated with different concentrations of gintonin for 20–24 h.
The image of the wounded area was captured and the recovery of the
area was analyzed with an inverted fluorescence microscope
(AxioVert200; Carl Zeiss AG, Oberkochen, Germany) at ×100
magnification.
Boyden chamber assay
The chemotactic motility of the cells through the
membrane was measured using a modified Boyden chamber (Neuro Probe
Inc., Gaithersburg, MD, USA) (23). A polycarbonate membrane with
8-μm pores was coated with fibronectin. Either gintonin,
Brp-LPA or LPA C18:2 in DMEM (0.1% BSA and 0.2% FBS) was
added to the lower chambers. The Boyden chamber was assembled by
first laying down the membrane coated with fibronectin and
subsequently, the top chamber on the lower chambers. Cells
(5×104 cells/well) were added to the top chambers and
incubated for the indicated time-periods at 37°C. The cells on the
membrane were fixed and stained with Diff-Quik (Sysmex Corporation,
Kobe, Japan) and mounted on slide glass. Non-migrated cells were
removed with Kimwipes. The migrated cells in 4 fields were counted
under a microscope (light microscopy) at ×200 magnification. The
images were photographed with a dark-field microscope (Eclipse 80i;
Nikon Corporation, Tokyo, Japan).
Cell proliferation assay
Cell proliferation was determined using a BrdU
incorporation assay, which measures DNA synthesis (16) and a XTT-based assay, which measures
cell viability based on the activity of mitochondrial enzymes
(24). Briefly, the cells were
seeded at 3×103 cells per well in 96-well plates and
incubated for 24 h. The cells were then washed with DMEM and
incubated for 6 h with DMEM containing 0.2% FBS. The cells were
washed with fresh DMEM (0.2% FBS) again and incubated with either
gintonin or Brp-LPA at the indicated concentrations. After 48 h,
cell proliferation was assessed by a BrdU ELISA assay (16) and XTT assay as previously described
(24).
Evaluation of in vivo antitumor
activity
Male C57BL/6 mice (Koatech Technology Corporation,
Seoul, Korea) 6 weeks of age were housed under specific
pathogen-free conditions. In the metastatic model, B16/F10 melanoma
cells (1×105 cells/0.1 ml PBS per mouse) were injected
into the tail vein. Mice were orally administered saline solution
(control), gintonin (50 or 100 mg/kg, 1 or 2 mg/0.1 ml saline per
dose) or LPA C18:2 (2 mg/kg in 0.1% BSA) daily for 3
weeks, commencing 3 days before or 1, 4 or 7 days after the cell
injection. Two or three weeks after the cell injection, the mice
were anesthetized with Zoletil 50 and Rompun, sacrificed, and the
lungs were excised. The number of nodules was counted. In the
subcutaneous model, tumors were produced by injecting B16/F10
melanoma cells (5×104 cells/0.2 ml PBS per mouse) into
the left flank of each mouse (n=5 per group). Mice were orally
administered saline solution (control) or gintonin (100 mg/kg, 0.1
ml each dose) daily for 3 weeks, commencing 3 days before and
continuing after the cell injection. Tumor growth was recorded with
a caliper and calculated as V = 0.52 × d2 × D (D, long
diameter; d, short diameter). Body weight was also determined 3
times a week. After 3 weeks of treatment, the mice were sacrificed
and the tumors were excised and examined histologically.
Histology and histochemistry
Tumor tissues were fixed with 4% formaldehyde and
embedded in paraffin. The paraffin tissue sections were fixed and
stained with hematoxylin and eosin in order to examine tumor
progression. In order to observe the microvessels of the tumor
tissues, tissue sections were fixed and stained with a von
Willebrand factor (vWF) antibody according to the staining protocol
for immunohistochemistry. The vessels were observed under a
microscope (×200 magnification) and the number in each field (0.322
mm2) was counted. Ten fields were randomly chosen for
each group.
Ethics
Animal experiments were conducted in strict
accordance with the recommendations in the Guide for the Care and
Use of Laboratory Animals of the National Institutes of Health. The
experimental protocol was approved by the Institutional Animal Care
and Use Committee of Konkuk University, Seoul, Korea (no.
KU08095).
Statistical analysis
The data are expressed as the means ± standard error
of the mean (SEM). Statistical comparisons between the controls and
the treated experimental groups were performed using Student’s
t-tests. A value of p<0.05 was considered to indicate a
statistically significant difference.
Results
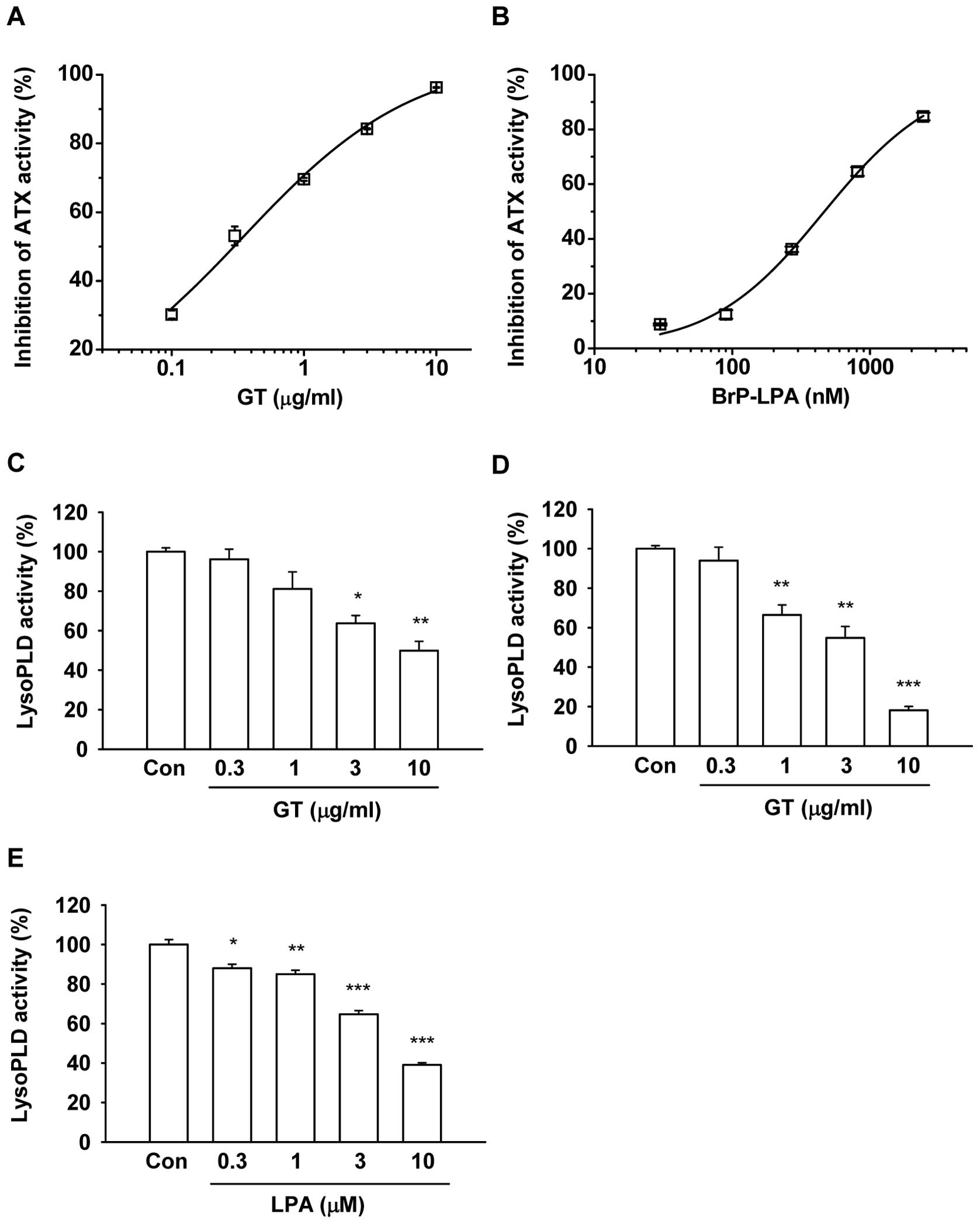
Gintonin effects on ATX activity
The inhibitory effects of gintonin on ATX activity
were determined using an ATX assay kit with the fluorogenic
substrate, FS-3, at the range of 0.1 to 10 μg/ml (Fig. 1A) (18). Gintonin (10 μg/ml) potently
inhibited ATX activity in a concentration-dependent manner. The
IC50 was 0.35±0.09 μg/ml. As a positive ATX
activity inhibitor, Brp-LPA also inhibited ATX activity at
concentrations of 30 to 2,430 nM (Fig.
1B). The IC50 was 492.5±118.7 nM, which was
consistent with that presented in previous reports (Fig. 1B) (10). These results demonstrate that
gintonin is effective as an ATX inhibitor, similar to Brp-LPA.
Gintonin inhibits the ATX secretory
activity of melanoma cells
The ATX secretory activity was determined using the
concentrated medium obtained from the melanoma cells in the absence
or presence of gintonin. Gintonin significantly inhibited the ATX
secretory activity of mouse B16/F10 and human MDA-MB-435 cells
(Fig. 1C and D). Gintonin (10
μg/ml) inhibited the ATX secretory activity of the B16/F10
and MDA-MB-435 cells by 50.1±4.7 and 81.8±1.9%, respectively. As a
positive control, we also used LPA C18:2. LPA
C18:2 also inhibited ATX secretory activity in a
concentration-dependent manner (Fig.
1E). These results indicate that gintonin inhibits the ATX
secretory activity of melanoma cells.
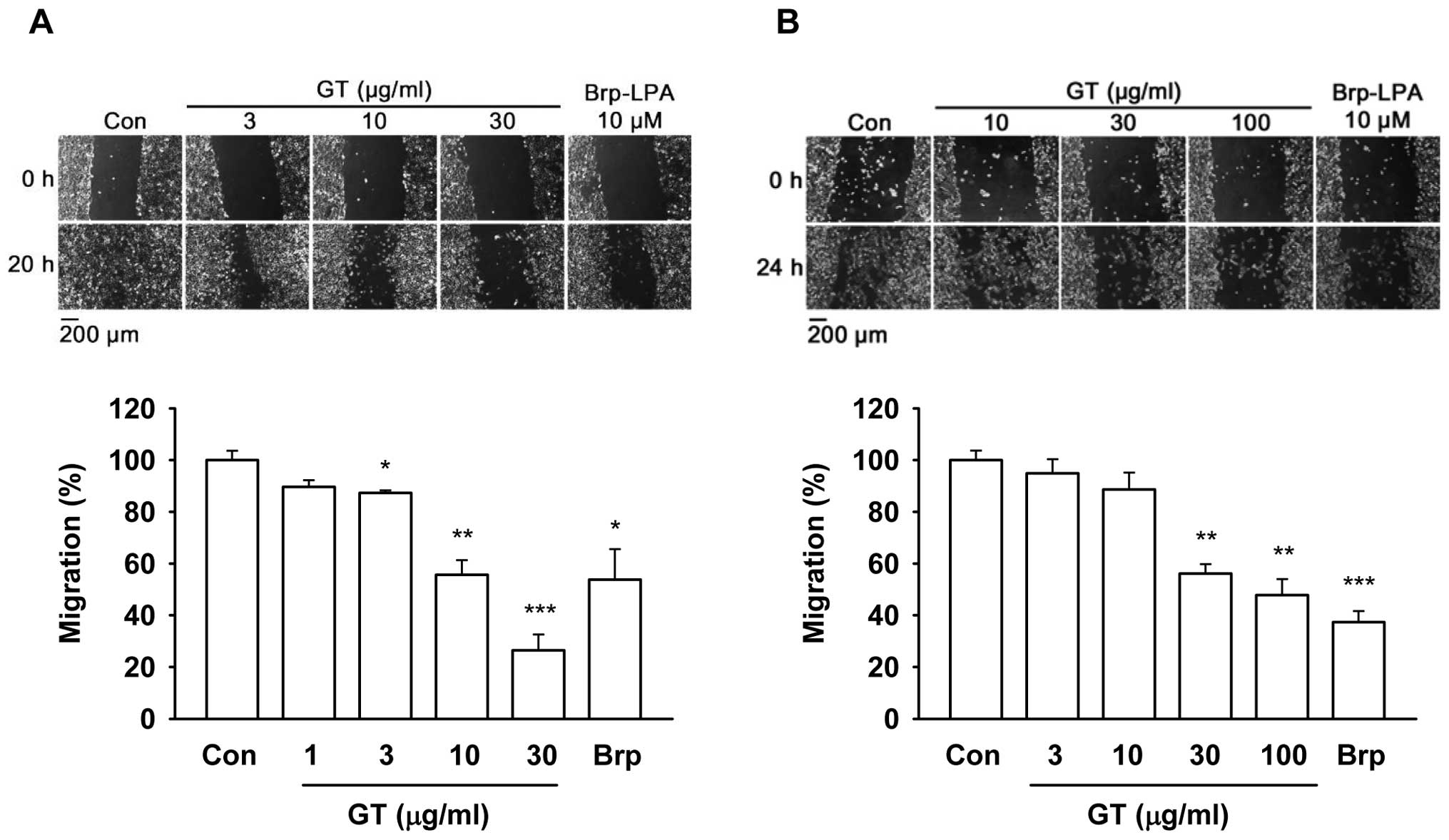
Gintonin inhibits melanoma cell
migration
Since the secretion of ATX stimulates cell migration
and gintonin inhibits ATX secretory activity, we examined whether
the gintonin-induced inhibition of ATX activity could be coupled
with the cell migration inhibition. The gintonin effects on
melanoma cell migration were determined by a scratch-wound healing
assay (Fig. 2) and the modified
Boyden chamber assay (Fig. 3). In
the scratch-wound healing assay, gintonin dose-dependently reduced
the migration and motility of the B16/F10 cells (Fig. 2A). Gintonin (30 μg/ml)
reduced the migration of B16/F10 cells by 73.5±6.1%. Similarly,
gintonin inhibited MDA-MB-435 cell migrations by 40–60% at
concentrations of 30–100 μg/ml (Fig. 2B). The IC50 values for
cell migration were 14.9±17.2 and 69.4±31.2 μg/ml in the
mouse and human melanoma cells, respectively. Brp-LPA (10
μM), which was the positive control, inhibited the migration
of B16/F10 and MDA-MB-435 cells by 46.2±11.7 or 62.6±4.2%,
respectively.
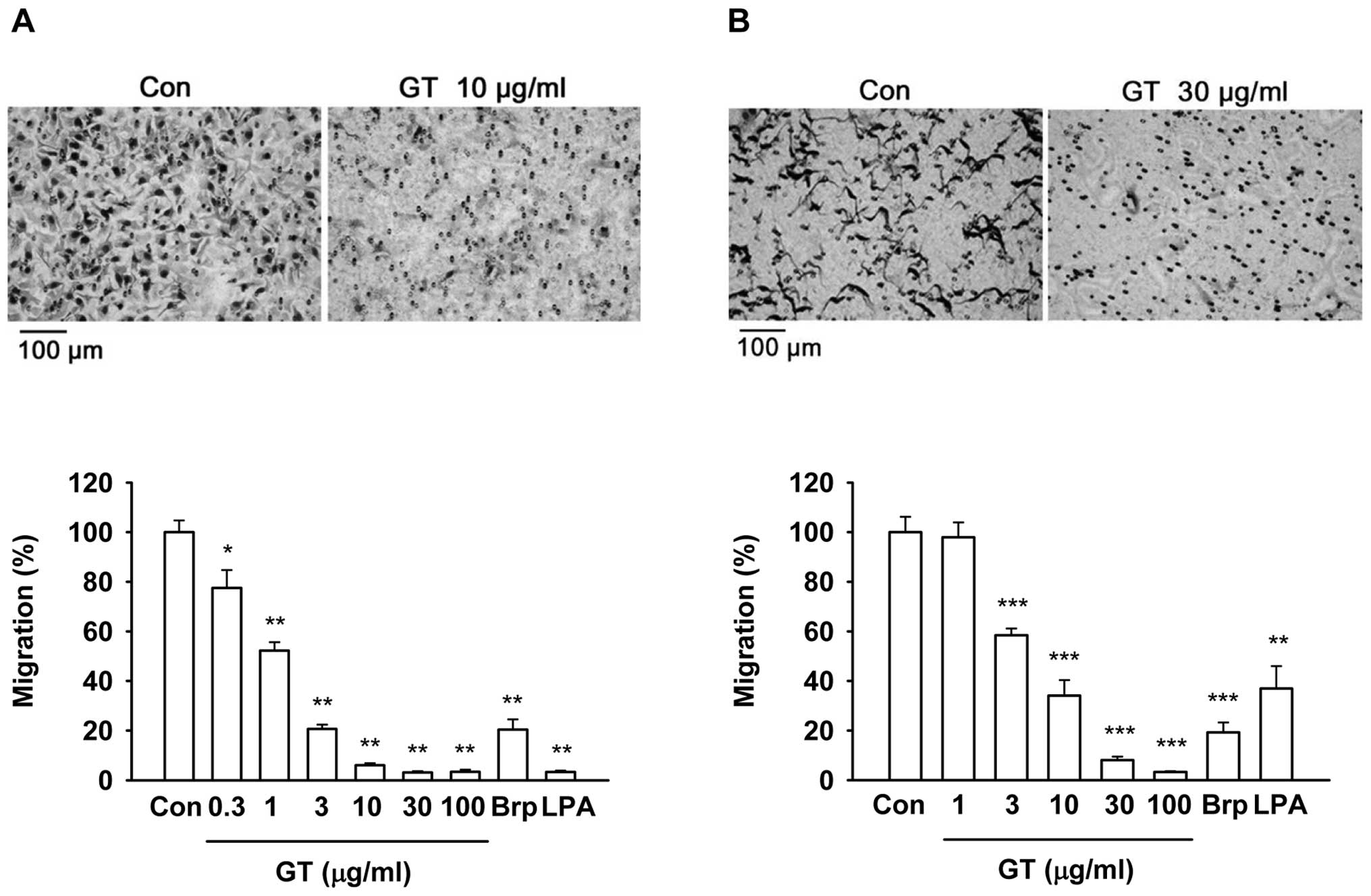
In a modified Boyden chamber assay, gintonin also
reduced the chemotactic motility (transmigration) of B16/F10 and
MDA-MB-435 cells in a dose-dependent manner (Fig. 3A and B). Gintonin at 10 and 30
μg/ml induced approximately 90% inhibition of migration in
both cells. The IC50 values for migration were 0.96±0.08
and 4.82±1.48 μg/ml in the mouse and human melanoma cells,
respectively. Brp-LPA (10 μM) and LPA C18:2 (10
μM), which were the positive controls, inhibited the
migration of B16/F10 cells by 79.6±4.2 and 96.7±0.6%, respectively.
Similarly, Brp-LPA (10 μM) and LPA C18:2 (10
μM) inhibited the migration of MDA-MB-435 cells by 80.8±4.0
and 63.1±9.0%, respectively. These results demonstrate that
gintonin exerts inhibitory effects on cancer cell migration.
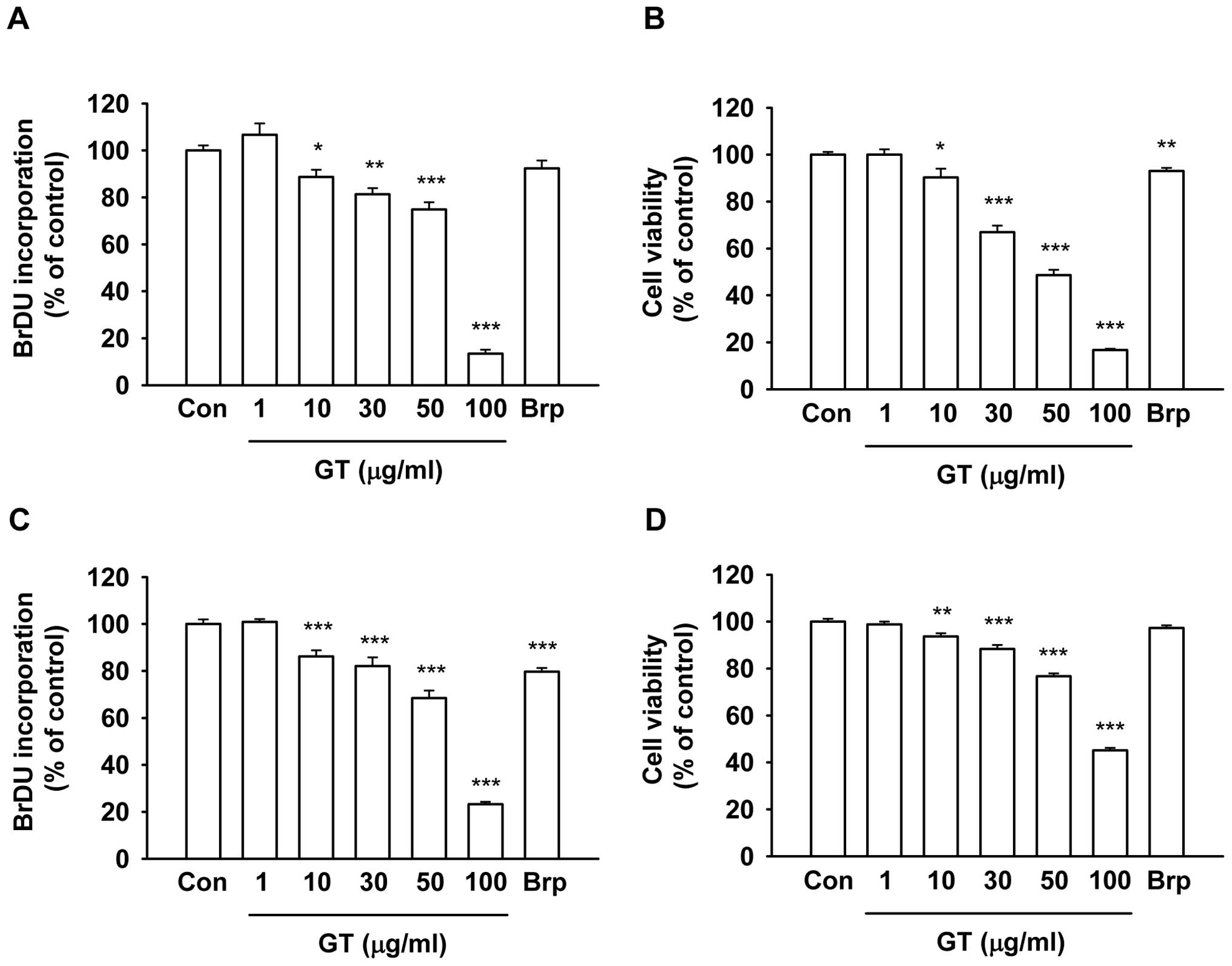
Gintonin effects on melanoma cell
growth
To determine whether the gintonin-induced inhibition
of cancer cell migration was due to cell growth inhibition, the
gintonin effects on cell growth were examined by BrdU incorporation
(25) and XTT-based assays
(26) (Fig. 4). Gintonin slightly reduced cell
proliferation at 10 μg/ml, which is a concentration that
exerts a strong inhibitory effect on cancer cell migration
(Figs. 2 and 3). However, gintonin at concentrations
>50 μg/ml inhibited BrdU incorporation and mitochondrial
respiratory enzyme activity by 80–90% in B16/F10 (Fig. 4A and B) and MDA-MB-435 cells
(Fig. 4C and D). Brp-LPA at 10
μM inhibited <20% of the proliferation of B16/F10
(Fig. 4A and B) and MDA-MB-435
cells (Fig. 4C and D). These
results demonstrate that gintonin does not affect cell growth at
concentrations that inhibit migration and that the gintonin-induced
inhibition of cancer cell migration does not occur through cell
growth inhibition.
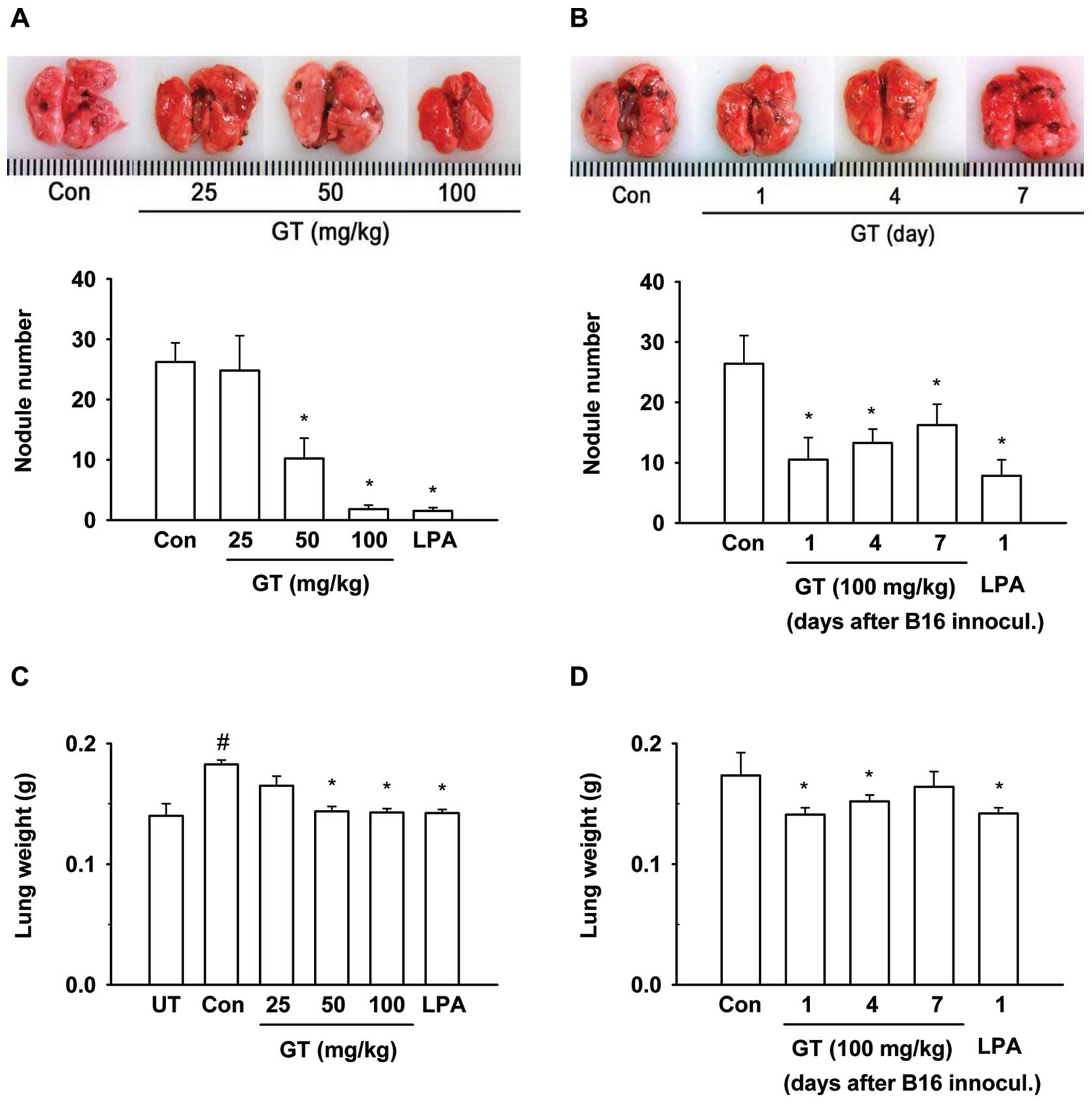
Gintonin inhibits lung metastasis of
melanoma cells in mice
Since the above results showed the possibility that
gintonin may affect metastasis in vivo through the
inhibition of ATX activity and cell migration, we examined the
antimetastatic effects of gintonin. For this purpose, we used
B16/F10 mouse melanoma cells for the in vivo lung metastasis
experiments (19,27). We examined the gintonin effects on
lung metastasis after the intravenous administration of B16/F10
mouse melanoma cells. Gintonin treatment at a daily oral dose of
25, 50 and 100 mg/kg for 3 weeks commencing 3 days before B16/F10
inoculation resulted in 24.8±5.8, 10.2±3.4 and 1.8±0.7 nodules,
respectively, compared to 26.2±3.2 nodules in the saline control
group (Fig. 5A). Thus, gintonin
suppressed the lung metastasis of melanoma cells in a
dose-dependent manner. The weights of the lungs also correlated
with metastasis (Fig. 5C). LPA
C18:2 (2 mg/kg), which was used as the positive control,
with treatment commencing 3 days before B16/F10 inoculation, also
inhibited lung metastasis. We then examined the effects of the
gintonin post-treatment in lung metastasis. To determine at which
time-point gintonin inhibits metastasis, a 2-week treatment of
gintonin [daily dose, 100 mg/kg, per os (p.o.)] was
initiated after 1, 4, or 7 days after B16/F10 inoculation into the
tail veins of the mice (Fig. 5B).
As shown in Fig. 5, the early
administration of gintonin or LPA C18:2 after B16/F10
cell inoculation was more effective for the inhibition of lung
metastasis of melanoma cells. These results indicated that the
pre-treatment with gintonin and LPA C18:2 prior to
B16/F10 cell inoculation was more effective in inhibiting lung
metastasis than gintonin post-treatment.
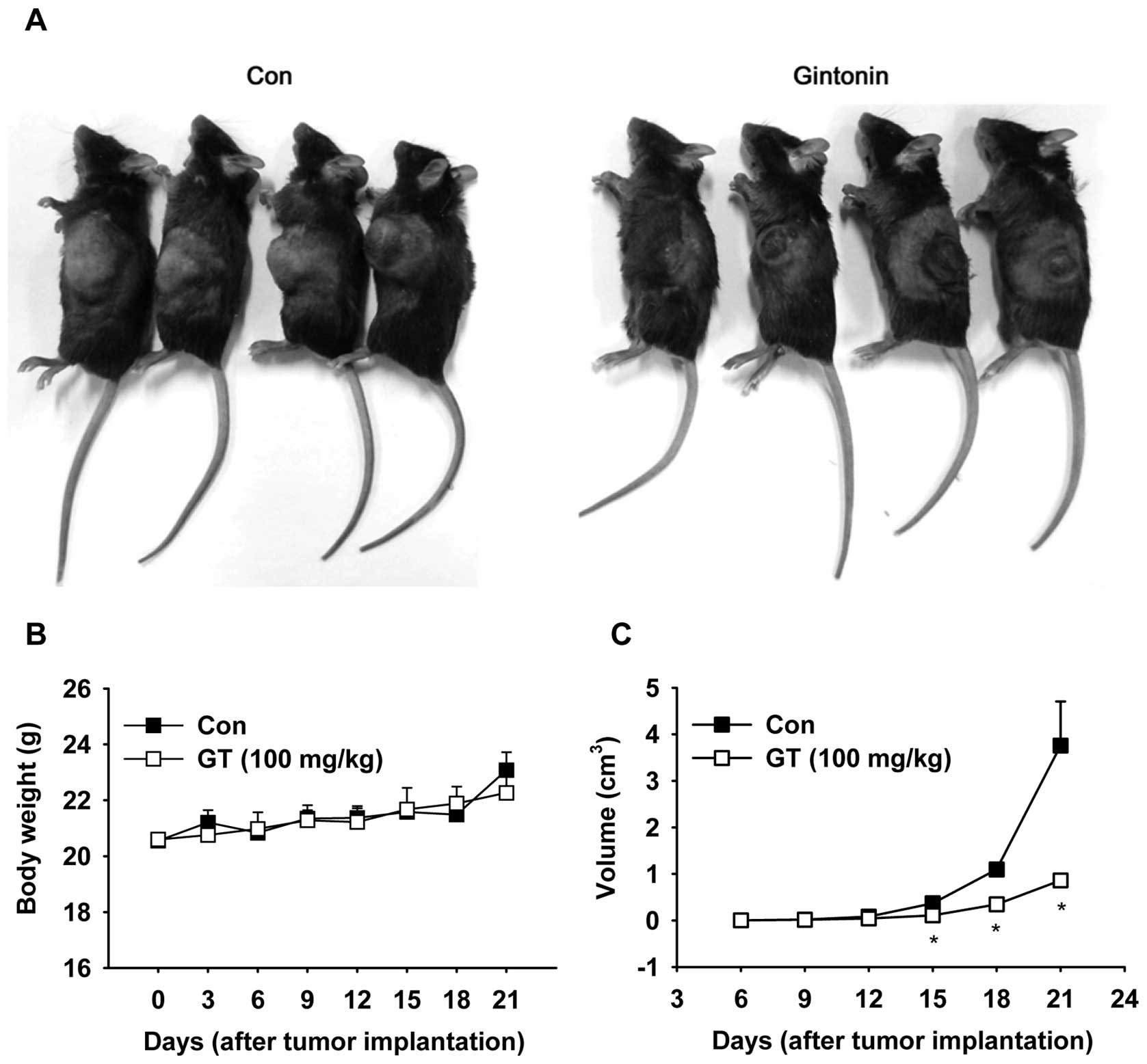
Gintonin inhibits tumor growth in
mice
We then examined the gintonin effects on tumor
growth. B16/F10 cells were injected subcutaneously into the left
flanks of mice. Tumor volume exponentially increased in the
saline-treated control mice after the cell injections, reaching
approximately 3,000 mm3 3 weeks after the cell injection
(Fig. 6A and C). The oral
administration of gintonin (100 mg/kg, p.o.) markedly suppressed
tumor growth, showing limited tumor volumes that were <1,000
mm3 3 weeks after the cell injection (Fig. 6A and C). Increased tumor volume
tended to correlate with increased body weight without significant
difference in body weights between the groups (Fig. 6B). Hematoxylin and eosin staining
of the tumor tissues revealed that the tumor tissues from the
control group were more vulnerable to necrosis compared to those
from the gintonin-treated group (Fig.
7). Moreover, the tumor tissues in the mice treated with
gintonin showed less mitosis and pleiomorphisms compared to those
from the control group (Fig. 7).
In addition, immunohistochemical analyses were performed using the
tumor tissues with antibodies for vWF in order to determine the
development of vessels and the numbers of vessels in the tumor
tissues. Gintonin treatment reduced vWF-stained blood vessel
numbers compared to the saline control mice (Fig. 7A and B). These results demonstrate
that gintonin exerts antimetastatic activities, as well as
antitumor activities in vivo.
Discussion
Metastasis is the process by which a certain cancer
spreads from the location at which a tumor first arises to distant
locations in the body (28).
Metastasis usually depends on cancer cells having 2 separate
abilities, increased motility and invasion. Cancer recurrence by
metastasis is one of the main causes of mortality in cancer
patients and is currently a main target for cancer therapy
(27).
In a previous study, we demonstrated that gintonin
activates LPA receptors and exhibits LPA receptor-mediated cellular
effects (16). However, since
ginseng has cancer preventive effects and anticancer activities
(13,14) and LPA C18:2 acts as a
strong negative regulator of ATX activity (17), we investigated whether gintonin,
which is rich in LPA C18:2, exerts in vitro and
in vivo antimetastatic activities.
In the present study, we made 3 key observations
that indicate that gintonin has antimetastatic and antitumor
activities. First, we observed that gintonin and LPA
C18:2 potently inhibited the activity of ATX that was
secreted from melanoma cells. Second, gintonin strongly blocked
cell migration at low concentrations with a slight inhibition of
tumor cell proliferation. Third, the oral administration of
gintonin and LPA decreased the number of metastatic lung nodule
formations induced by the tail-vein inoculation of melanoma cells.
Gintonin also reduced the size of tumors induced by the
subcutaneous inoculation of melanoma cells in mice. Thus, the
results showed that the gintonin-induced inhibition of ATX activity
and cell migration may be coupled with the attenuation of
metastasis and tumor growth.
In previous reports, Deng et al demonstrated
that orally-administered LPA restored intestinal injuries induced
by radiation exposure (29).
Adachi et al also showed that orally-administered LPA
attenuated gastric ulcer formation induced by stress (30). In the present study, we further
demonstrate that orally-administered gintonin, as well as LPA
C18:2 exert metastasis-related anticancer activity. We
previously demonstrated that gintonin activated LPA receptor
subtypes with high affinity and increased the migration and
proliferation of human umbilical vein endothelial cells and induced
morphological changes in neuronal cells via LPA receptors (16). In the present study, gintonin
inhibited the migration of melanoma cells in vitro. Thus,
gintonin and LPA may have differential effects that are cell
type-dependent or may have dual actions. One action is as an ATX
inhibitor and the other is as a LPA receptor ligand. It is likely
that, in pathophysiological conditions such as cancer,
gintonin-induced antimetastatic and antitumor activities are
achieved through the inhibition of ATX activity. A line of evidence
supports this notion. Gintonin as well as LPA C18:2
inhibited the in vitro activities of purified ATX and
secreted ATX in cancer cell medium (Fig. 1). A number of studies have shown
that the inhibition of ATX activity results in a decrease in LPA
production and antimetastasis in different types of tumor (10–12,31–34).
Thus, we speculated that gintonin may negatively affect ATX
activity, resulting in an attenuation of endogenous LPA
production.
Of note, we observed that the inhibition of
metastasis of melanoma cells by gintonin was much more effective
when mice were orally pre-treated with gintonin prior to melanoma
cell inoculation (Fig. 5). Thus,
the oral gintonin pre-treatment may be also coupled with the
preventive effects against metastasis. In addition, although
pre-treatment with gintonin (100 mg/kg, p.o.) did not suppress
tumor formation in the mice subcutaneously injected with melanoma
cells as much as it did lung metastasis, it also significantly
reduced tumor size and angiogenesis compared to the saline control
group. Taken together, these results suggest that gintonin inhibits
ATX activity to suppress the subsequent ATX-mediated metastasis and
tumor growth. Currently, we are investigating how gintonin binds to
or interacts with ATX for the inhibition of ATX activity.
In conclusion, the results from the present study
demonstrate that the gintonin-induced inhibition of ATX activity
may be the molecular basis of the gintonin-induced antimetastatic
and antitumor activities. Finally, we propose that gintonin may be
a useful agent for the prevention of metastasis, particularly when
used in combination with cancer-related anti-proliferative
drugs.
Acknowledgements
The present study was supported by
grants from the Basic Science Research Program (2011-0021144) and
the Priority Research Centers Program through the National Research
Foundation of Korea (NRF), which is funded by the Ministry of
Education, Science and Technology (2012-0006686) and BK21 to S.-Y.
Nah.
References
|
1.
|
Moolenaar WH: LPA: a novel lipid mediator
with diverse biological actions. Trends Cell Biol. 4:213–219. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Mills GB and Moolenaar WH: The emerging
role of lysophosphatidic acid in cancer. Nat Rev Cancer. 3:582–591.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Chun J, Hla T, Lynch KR, Spiegel S and
Moolenaar WH: International Union of Basic and Clinical
Pharmacology. LXXVIII. Lysophospholipid receptor nomenclature.
Pharmacol Rev. 62:579–587. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Kranenburg O and Moolenaar WH: Ras-MAP
kinase signaling by lysophosphatidic acid and other G
protein-coupled receptor agonists. Oncogene. 20:1540–1546. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Tokumura A, Majima E, Kariya Y, Tominaga
K, Kogure K, Yasuda K and Fukuzawa K: Identification of human
plasma lysophospholipase D, a lysophosphatidic acid-producing
enzyme, as autotaxin, a multifunctional phosphodiesterase. J Biol
Chem. 277:39436–39442. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Umezu-Goto M, Kishi Y, Taira A, Hama K,
Dohmae N, Takio K, Yamori T, Mills GB, Inoue K, Aoki J and Arai H:
Autotaxin has lysophospholipase D activity leading to tumor cell
growth and motility by lysophosphatidic acid production. J Cell
Biol. 158:227–233. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Houben AJ and Moolenaar WH: Autotaxin and
LPA receptor signaling in cancer. Cancer Metastasis Rev.
30:557–565. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Stracke ML, Krutzsch HC, Unsworth EJ,
Arestad A, Cioce V, Schiffmann E and Liotta LA: Identification,
purification and partial sequence analysis of autotaxin, a novel
motility-stimulating protein. J Biol Chem. 267:2524–2529.
1992.PubMed/NCBI
|
|
9.
|
van Meeteren LA, Ruurs P, Christodoulou E,
Goding JW, Takakusa H, Kikuchi K, Perrakis A, Nagano T and
Moolenaar WH: Inhibition of autotaxin by lysophosphatidic acid and
sphingosine 1-phosphate. J Biol Chem. 280:21155–21161.
2005.PubMed/NCBI
|
|
10.
|
Zhang H, Xu X, Gajewiak J, Tsukahara R,
Fujiwara Y, Liu J, Fells JI, Perygin D, Parrill AL, Tigyi G and
Prestwich GD: Dual activity lysophosphatidic acid receptor
pan-antagonist/ autotaxin inhibitor reduces breast cancer cell
migration in vitro and causes tumor regression in vivo. Cancer Res.
69:5441–5449. 2009. View Article : Google Scholar
|
|
11.
|
Uchiyama A, Mukai M, Fujiwara Y, Kobayashi
S, Kawai N, Murofushi H, Inoue M, Enoki S, Tanaka Y, Niki T,
Kobayashi T, Tigyi G and Murakami-Murofushi K: Inhibition of
transcellular tumor cell migration and metastasis by novel
carba-derivatives of cyclic phosphatidic acid. Biochim Biophys
Acta. 1771:103–112. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Gupte R, Siddam A, Lu Y, Li W, Fujiwara Y,
Panupinthu N, Pham TC, Baker DL, Parrill AL, Gotoh M, Murakami-
Murofushi K, Kobayashi S, Mills GB, Tigyi G and Miller DD:
Synthesis and pharmacological evaluation of the stereoisomers of
3-carba cyclic-phosphatidic acid. Bioorg Med Chem Lett.
20:7525–7528. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Mochizuki M, Yoo YC, Matsuzawa K, Sato K,
Saiki I, Tono-oka S, Samukawa K and Azuma I: Inhibitory effect of
tumor metastasis in mice by saponins, ginsenoside-Rb2, 20(R)-and
20(S)-ginsenoside-Rg3, of red ginseng. Biol Pharm Bull.
18:1197–1202. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Pan XY, Guo H, Han J, Hao F, An Y, Xu Y,
Xiaokaiti Y, Pan Y and Li XJ: Ginsenoside Rg3 attenuates cell
migration via inhibition of aquaporin 1 expression in PC-3M
prostate cancer cells. Eur J Pharmacol. 683:27–34. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Pyo MK, Choi SH, Hwang SH, Shin TJ, Lee
BH, Lee SM, Lim YH, Kim DH and Nah SY: Novel glycolipoproteins from
ginseng. J Ginseng Res. 35:92–103. 2011. View Article : Google Scholar
|
|
16.
|
Hwang SH, Shin TJ, Choi SH, Cho HJ, Lee
BH, Pyo MK, Lee JH, Kang J, Kim HJ, Park CW, Shin HC and Nah SY:
Gintonin, newly identified compounds from ginseng, is novel
lysophosphatidic acids-protein complexes and activates G
protein-coupled lysophosphatidic acid receptors with high affinity.
Mol Cells. 33:151–162. 2012. View Article : Google Scholar
|
|
17.
|
Liu XW, Sok DE, Yook HS, Sohn CB, Chung YJ
and Kim MR: Inhibition of lysophospholipase D activity by
unsaturated lysophosphatidic acids or seed extracts containing
1-linoleoyl and 1-oleoyl lysophosphatidic acid. J Agric Food Chem.
55:8717–8722. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Ferguson CG, Bigman CS, Richardson RD, van
Meeteren LA, Moolenaar WH and Prestwich GD: Fluorogenic
phospholipid substrate to detect lysophospholipase D/autotaxin
activity. Org Lett. 8:2023–2026. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Poste G, Doll J, Brown AE, Tzeng J and
Zeidman I: Comparison of the metastatic properties of B16 melanoma
clones isolated from cultured cell lines, subcutaneous tumors and
individual lung metastases. Cancer Res. 42:2770–2778. 1982.
|
|
20.
|
Gaetano CG, Samadi N, Tomsig JL, Macdonald
TL, Lynch KR and Brindley DN: Inhibition of autotaxin production or
activity blocks lysophosphatidylcholine-induced migration of human
breast cancer and melanoma cells. Mol Carcinog. 48:801–809. 2009.
View Article : Google Scholar
|
|
21.
|
Clair T, Lee HY, Liotta LA and Stracke ML:
Autotaxin is an exoenzyme possessing 5′-nucleotide
phosphodiesterase/ATP pyrophosphatase and ATPase activities. J Biol
Chem. 272:996–1001. 1997.PubMed/NCBI
|
|
22.
|
Nam SW, Clair T, Kim YS, McMarlin A,
Schiffmann E, Liotta LA and Stracke ML: Autotaxin (NPP-2), a
metastasis-enhancing motogen, is an angiogenic factor. Cancer Res.
61:6938–6944. 2001.PubMed/NCBI
|
|
23.
|
Moore LD, Isayeva T, Siegal GP and
Ponnazhagan S: Silencing of transforming growth factor-beta1 in
situ by RNA interference for breast cancer: implications for
proliferation and migration in vitro and metastasis in vivo. Clin
Cancer Res. 14:4961–4970. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Hwang SH, Rait A, Pirollo KF, Zhou Q,
Yenugonda VM, Chinigo GM, Brown ML and Chang EH: Tumor-targeting
nanodelivery enhances the anticancer activity of a novel
quinazolinone analogue. Mol Cancer Ther. 7:559–568. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Porstmann T, Ternynck T and Avrameas S:
Quantitation of 5-bromo-2-deoxyuridine incorporation into DNA: an
enzyme immunoassay for the assessment of the lymphoid cell
proliferative response. J Immunol Methods. 82:169–179. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Scudiero DA, Shoemaker RH, Paull KD, Monks
A, Tierney S, Nofziger TH, Currens MJ, Seniff D and Boyd MR:
Evaluation of a soluble tetrazolium/formazan assay for cell growth
and drug sensitivity in culture using human and other tumor cell
lines. Cancer Res. 48:4827–4833. 1988.PubMed/NCBI
|
|
27.
|
Scutti JA, Matsuo AL, Pereira FV, Massaoka
MH, Figueiredo CR, Moreira DF, Belizário JE and Travassos LR: Role
of SOCS-1 gene on melanoma cell growth and tumor development.
Transl Oncol. 4:101–109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Leberi MF and Efferth T: Molecular
principles of cancer invasion and metastasis (Review). Int J Oncol.
34:881–895. 2009.PubMed/NCBI
|
|
29.
|
Deng W, Shuyu E, Tsukahara R, Valentine
WJ, Durgam G, Gududuru V, Balazs L, Manickam V, Arsura M,
VanMiddlesworth L, Johnson LR, Parrill AL, Miller DD and Tigyi G:
The lysophosphatidic acid type 2 receptor is required for
protection against radiation-induced intestinal injury.
Gastroenterology. 132:1834–1851. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Adachi M, Horiuchi G, Ikematsu N, Tanaka
T, Terao J, Satouchi K and Tokumura A: Intragastrically
administered lysophosphatidic acids protect against gastric ulcer
in rats under water-immersion restraint stress. Dig Dis Sci.
56:2252–2261. 2011. View Article : Google Scholar
|
|
31.
|
Xu X and Prestwich GD: Inhibition of tumor
growth and angiogenesis by a lysophosphatidic acid antagonist in an
engineered three-dimensional lung cancer xenograft model. Cancer.
116:1739–1750. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Gierse J, Thorarensen A, Beltey K,
Bradshaw-Pierce E, Cortes-Burgos L, Hall T, Johnston A, Murphy M,
Nemirovskiy O, Ogawa S, Pegg L, Pelc M, Prinsen M, Schnute M,
Wendling J, Wene S, Weinberg R, Wittwer A, Zweifel B and Masferrer
J: A novel autotaxin inhibitor reduces lysophosphatidic acid levels
in plasma and the site of inflammation. J Pharmacol Exp Ther.
334:310–317. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Albers HM, Dong A, van Meeteren LA, Egan
DA, Sunkara M, van Tilburg EW, Schuurman K, van Tellingen O, Morris
AJ, Smyth SS, Moolenaar WH and Ovaa H: Boronic acid-based inhibitor
of autotaxin reveals rapid turnover of LPA in the circulation. Proc
Natl Acad Sci USA. 107:7257–7262. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
van Meeteren LA, Brinkmann V,
Saulnier-Blache JS, Lynch KR and Moolenaar WH: Anticancer activity
of FTY720: phosphorylated FTY720 inhibits autotaxin, a
metastasis-enhancing and angiogenic lysophospholipase D. Cancer
Lett. 266:203–208. 2008.PubMed/NCBI
|