Introduction
Ovarian cancer is the most lethal gynecological
malignancy in women because of occult metastases within the
peritoneal cavity and the advanced stage at detection when curative
therapy is ineffective. Approximately 80–90% ovarian cancer is
origin from ovarian surface epithelium. The etiology of ovarian
epithelial cancer (OEC) remains to be clarified, multiple factors
involved in OEC development, for example, hormonal, environmental
and genetic factors may play a role. Currently, the gonadotropin
theory of ovarian cancer proposes that elevated serum
gonadotropins, follicle-stimulating hormone (FSH) and luteinizing
hormone (LH), contribute significantly to the development of
ovarian cancer. In previous study, Choi et al have reported
that FSH enhanced ovarian cancer cells proliferation and invasion
by PI3K/AKT signal pathway (1,2).
Recent study in our laboratory demonstrated that FSH inhibits
ovarian cancer cell apoptosis by upregulating survivin and
downregulating PDCD6 and DR5 (3).
These studies indicate FSH plays an important role in OEC
occurrence, especially in postmenopausal women. In our previous
work, it was also reported that activation of the PI3K/AKT pathway
mediates FSH-stimulated VEGF expression in ovarian serous
cystadenocarcinoma (4). This study
also showed that survivin and HIF1α are involved in FSH-mediated
VEGF expression by PI3K/AKT signal pathway. Survivin is a member of
the inhibitor of apoptosis protein (IAP) family, in addition to the
anti-apoptosis function, the role of survivin to regulate VEGF
expression has been described in various tumor cell types (5,6).
These factors contribute to tumor angiogenesis. Inflammation also
facilitate tumor angiogenesis, Cox2 is an important effector
molecule of inflammation and was reported to be involve in VEGF
expression and tumor angiogenesis. In our recent study, it was
found FSH could significantly upregulate Cox2 expression in a
dose-dependent manner (unpublish data). Although these molecules
contributing to tumor angiogenesis have positive response to FSH
treatment, the detail mechanism and relative signal pathway is not
clear.
Mertens-Talcott et al showed evidence that
microRNA-27a: ZBTB10-specificity protein pathway contributed to
breast cancer angiogenesis, decreased microRNA-27a resulted in
attenuating expression of survivin and VEGF, whereas overexpression
of ZBTB10 reduced the protein levels of both molecules (7). MicroRNAs are endogenous 20–25 bp
small non-coding RNAs that interact with complementary bingding
sites in 3′-untranslated regions of target mRNA to inhibit their
expression by blocking translation or enhance mRNA cleavage
(8), and play essential roles in a
variety of cellular processes, including cell differentiation,
proliferation and fat metabolism (9–13).
MicroRNA-27a also possesses these oncogenic activities. In
addition, microRNA-27a was demonstrated to modulate the cardiac
β-myosin heavy chain gene via thyroid hormone signaling (14). Li et al confirmed that
antisense microRNA-27a and overexpression of ZBTB10 blocked
estrogen-induced transactivation in breast cancer (15). These studies suggest that
microRNA-27a: ZBTB10-specificity protein pathway mediates
hormone-induced bio-function. However, the role of this pathway in
expression of FSH-induced OEC angiogenesis related molecules has
not been addressed. Therefore, in this study, we focused on the
roles of microRNA-27a, ZBTB10 and Sp1 in mediating FSH-induced
VEGF, Cox2 and survivin expressions.
Materials and methods
Chemicals, antibodies, plasmids and
reagents
Lipofectamine 2000, DMEM/F12 medium and fetal bovine
serum were purchased from Invitrogen. Human follicle stimulating
hormone was obtained from Sigma-Aldrich. ZBTB10, SP1, Cox2,
survivin, VEGF, GAPDH and β-actin antibodies were purchased from
Abcam (Cambridge, UK). MicroRNA mirvaRNA extraction kits, the
reverse transcription and real-time PCR amplification kits were
obtained from Applied Biosciences. As-microRNA-27a (as-miR-27a) was
purchased from Applied Biosciences. The ZBTB10 expression plasmid
and empty plasmid (pCMV6-XL4) were get from Origene. Sp1 siRNA was
obtained from Dharmacon.
Cell lines and cell culture
Human ovarian cancer cell lines, A2780, OVCAR-3,
ES-2, HO8910PM, Hey and HO8910, were obtained from the American
Type Culture Collection (Manassas, VA) and cultured in 1:1
DMEM/F12. These cell lines were cultured in medium supplemented
with 100 U/ml penicillin, 100 μg/ml streptomycin and 10%
fetal bovine serum. Cultures were maintained at 37°C in a
humidified incubator containing 95% room air and 5% CO2
atmosphere. In addition, the Moody cell line was kindly provided by
Dr W. Zheng (Arizona University, Tucson, AZ, USA), it is a normal
ovarian epithelial cell line that was transfected with hTERT. The
cells were maintained in MCDB109/M199 medium supplemented with 15%
FBS.
Hormone treatment
Ovarian cancer Hey and HO8910 cells were plated in
6-well plates with a cell density of 1×105 cells/ml,
respectively. At 60% confluence, the media were changed to Opti-MEM
without serum, starvation for 24 h, the cells were treated with
different doses of FSH (0, 25, 50 and 100 mIU/ml) for 24 h, the
cells were harvested and used for determining the effect of FSH on
microRNA-27a expression by real-time PCR. Similarly, both cell
lines were treated with different doses of FSH for 48 h, following
the cells were collected for western blot analysis to examine the
expression patterns of Cox2, survivin and VEGF protein. To
investigate the effects of as-microRNA-27a on FSH-induced VEGF,
Cox2 and survivin, prior to 50 mIU/ml FSH treatment, Hey and HO8910
cells were treated with as-microRNA-27a, the cells were harvested
and the microRNA27a expression was examined by real-time PCR,
checked the proteins expression by western blot analysis. In
addition, in order to investigate the roles of ZBTB10 and Sp1 on
FSH-induced VEGF, Cox2 and survivin expressions, after
overexpression of ZBTB10 plasmid or knockdown Sp1, 50 mIU/ml FSH
was used to treat the cells for another 48 h, the different
expression profiles of VEGF, Cox2 and survivin were analyzed by
western blot analysis.
Western blot analysis
The western blot analysis was performed as
previously reported. Briefly, after lysis, 60 μg proteins
were loaded on 10% SDS-PAGE ges, transferred to polyvinylidene
fluoride (PVDF) membranes, and incubated with specific primary
antibodies at 4°C overnight, followed by 1-h incubation with the
appropriate secondary antibody at room temperature, then the bands
were visualized with the ECL Plus system (Amersham, GE Healthcare).
GAPDH or β-actin served as a loading control.
Transfection with as-miR-27a and
real-time PCR
Transfecion with as-miR-27a was done using siPORT™
NeoFX™ Transfection Agent (Invitrogen) according the manufacturer’s
instruction. After transfection, the treated cells were harvested
and the microRNA was extracted using Taqman microRNA RT kit,
followed by amplification using Taqman universal PCR Master
mix.
Sp1 siRNA and ZBTB10 plasmid
transfections
siRNA transfections were performed as previously
reported, briefly, 1×106 cells were seeded in 6-cm
dishes and incubated for 24 h, the culture mediums were changed
with opti-MEM without serum prior to transfection with 50 nM Sp1
siRNA. After 12 h, the cells were treated with 50 mIU/ml FSH or PBS
for another 48 h.
ZBTB10 plasmid transfection was carried out using
Lipofectamine 2000 reagent following the manufacturer’s protocol.
Briefly, after serum starvation for 24 h, 4 μg plasmid or
empty plasmid were mixed with serum-free DMEM/F12 and Lipofectamine
2000 reagent, respectively, following incubated in room temperature
for 25 min, then added to the ovarian cancer cells.
Data analysis
Data are presented as the mean ± standard deviation
(SD). The statistical significance of the results was assessed by
the Student’s t-test or a one way ANOVA using SPSS 11.5 software
with p<0.05 being considered significant.
Results
The expression patterns of microRNA-27a
and Sp1 in normal ovarian epithelial cells and ovarian cancer
cells
To examine the possible contribution of
microRNA-27a: ZBTB10-specificity protein pathway on ovarian cancer
development, we evaluated the expression profiles of microRNA-27a
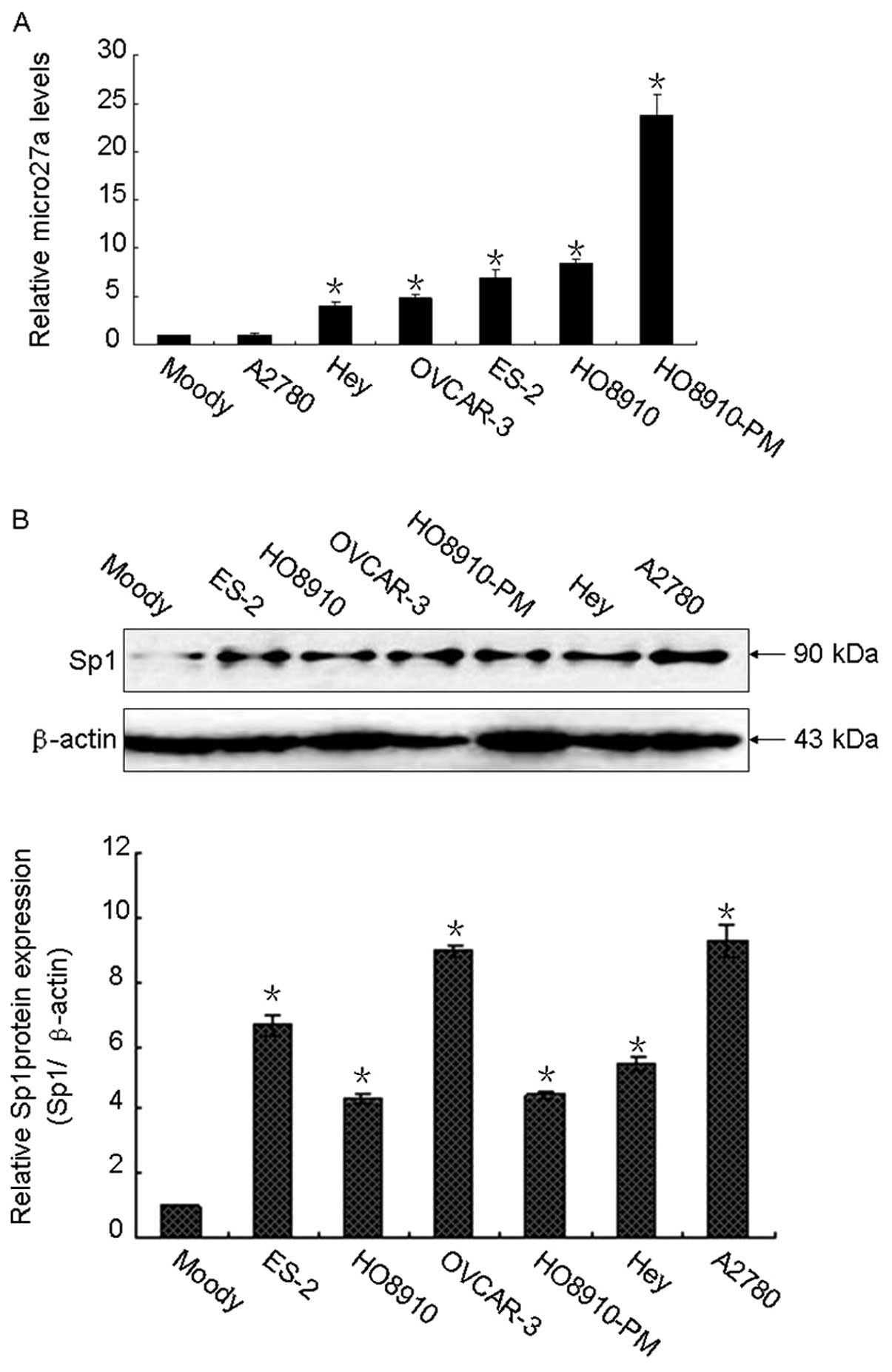
and Sp1 proteins. As illustrated in Fig. 1A, microRNA-27a was overexpressed in
ovarian cancer cells compared with that of Moody cells, which is a
normal ovarian epithelial cell line, transfected with hTERT,
suggesting that microRNA-27a may play an important role in ovarian
cancer development. Similar expression pattern was observed in Sp1,
the Sp1 protein levels were higher in ovarian cancer cells than
that in Moody cells (Fig. 1B).
FSH activates microRNA-27a:
ZBTB10-specificity protein pathway and induces tumor angiogenesis
factor expression
In a previous study, we demonstrated that FSH
enhanced VEGF expression and promoted angiogenesis (4), however, the detail signal pathway
involved in FSH regulation of VEGF and angiogenesis remains to be
clarified. Considering that microRNA-27a and Sp1 were overexpressed
in ovarian cancer, we further determined whether FSH activated the
microRNA-27a: ZBTB10-specificity protein pathway. As showed in
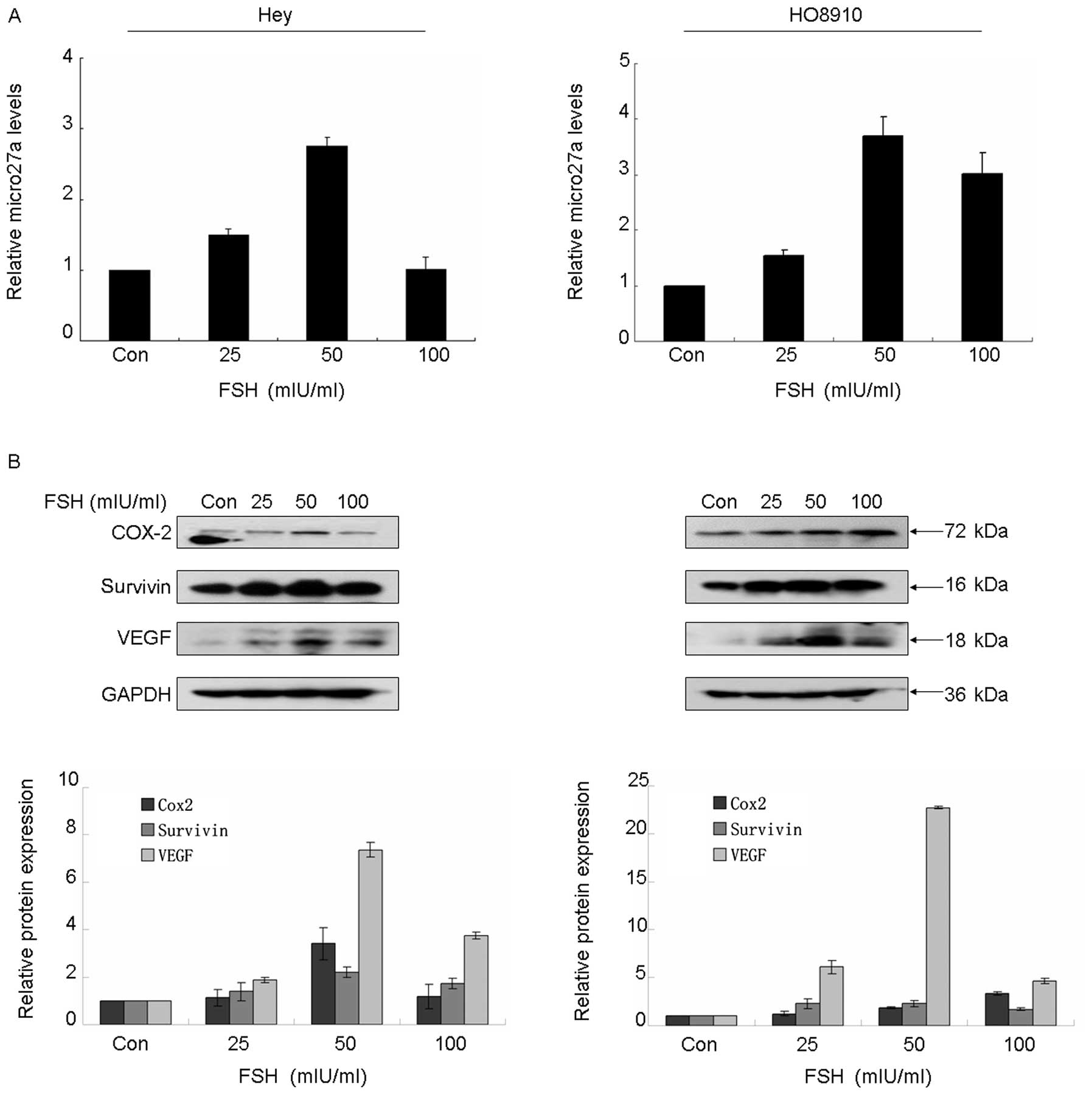
Fig. 2A, FSH potently enhanced
microRNA-27a expression in both Hey and HO8910 cell lines in a
dose-dependent manner, the maximal peak of increase of microRNA-27a
was observed when the cells were treated with 50 mIU/ml FSH for 24
h. Moreover, western blot analysis demonstrated that FSH treatment
resulted in significantly increased expression of survivin, Cox2,
VEGF and Sp1 (Fig. 2B and C), on
the contrary, it showed a reverse expression pattern in ZBTB10
protein, decreasing ZBTB10 protein was observed accompanying the
increasing dose of FSH (Fig.
2C).
Inhibition of microRNA-27a blocks
FSH-activating microRNA-27a: ZBTB10-specificity protein pathway and
abolishes FSH-induced tumor angiogenesis factor expression
To investigate the role of microRNA-27a on
FSH-induced tumor angiogenesis factor expression, inhibition of
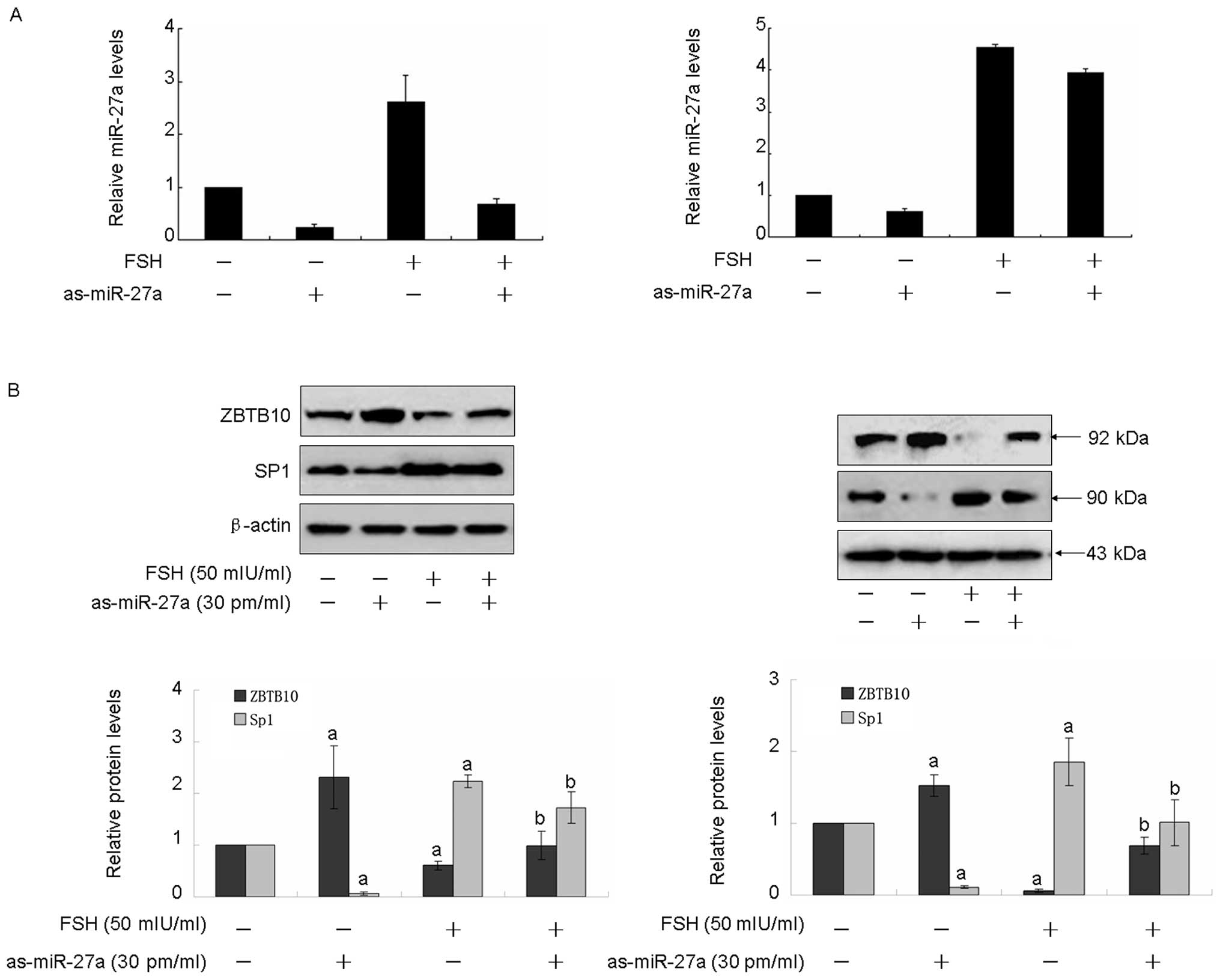
microRNA-27a was performed using as-miR-27a. As showed in Fig. 3A, transfection of as-miR-27a
obviously decreased micro-27a expression in both Hey and HO8910
cell lines. As illustrated in Fig.
3B, transfection of as-miR-27a induced approximately a 2-fold
(Hey) or 1.5-fold (HO8910) increase of ZBTB10, the effect was
abolished by 50 mIU/ml FSH treatment for 48 h. However,
transfection of as-miR-27a resulted in a 10–16-fold decrease of
Sp1, 7–9-fold decrease of Cox2, 2–3-fold decrease of survivin,
2–3-fold decrease of VEGF. Moreover, the FSH-induced Sp1, Cox2,
survivin and VEGF expression were blocked by transfection of
as-miR-27a (Fig. 3B and C).
Overexpression of ZBTB10 blocks
microRNA-27a: ZBTB10-specificity protein pathway and FSH induces
tumor angiogenesis factor expression
To analyse the role of ZBTB10 on FSH-induced tumor
angiogenesis factor expression, over-expression of ZBTB10 was
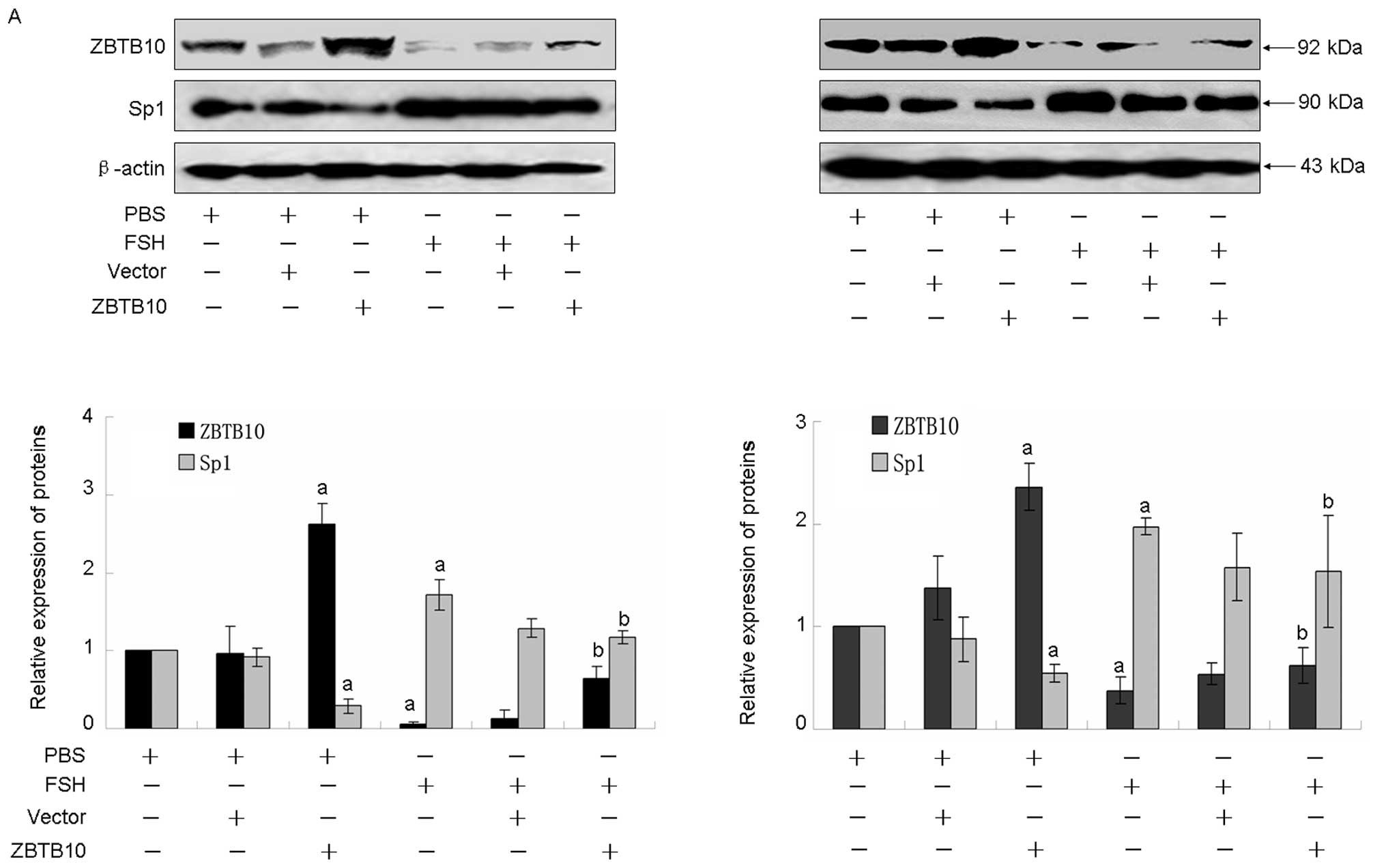
performed. As showed in Fig. 4A,
transfection with ZBTB10 significantly enhanced ZBTB10 protein
expression, whereas FSH treatment attenuated this effect. As
expected, overexpression of ZBTB10 potently inhibited Sp1,
survivin, Cox2 and VEGF expression in both Hey and HO8910 cell
lines, moreover, the increased expression induced by FSH also was
attenuated (Fig. 4A and B).
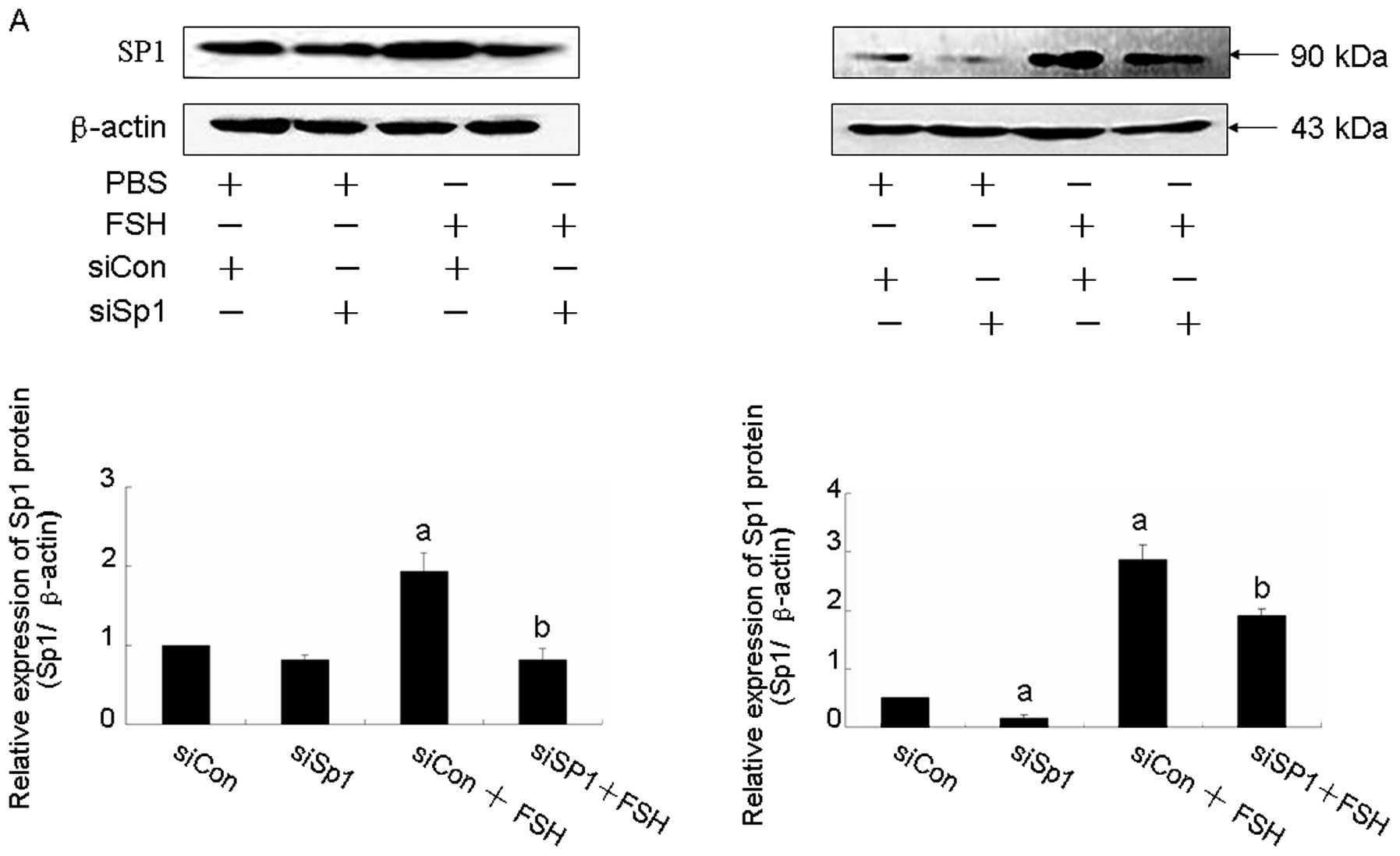
Knockdown of Sp1 abolishes microRNA-27a:
ZBTB10-specificity protein pathway and FSH induces tumor
angiogenesis factor expression
To test whether Sp1 is involved in FSH-induced tumor
angiogenesis factor expression, knockdown of Sp1 was performed by
siRNA. Transient transfection of Sp1 siRNA obviously blocked Sp1
protein expression in both Hey and HO8910 cell lines (Fig. 5A). In addition, it resulted in
decreasing of Cox2, survivin and VEGF levels. Moreover, FSH-induced
Cox2, survivin and VEGF expressions were abolished by knocking down
siSp1 (Fig. 5B).
Discussion
Based on the gonadotrophin theory, the hormone
environment is an important factor for ovarian cancer occurrence,
especially in postmenopausal women due to increased FSH and LH
levels resulting from loss of feedback of estrogen. Currently,
aberrant FSH level is considered as a high risk factor for OEC
development. Except for enhancing OEC cell proliferation, migration
and invasion, and blocking of apoptosis, increasing evidence
indicates that FSH induces angiogenesis which is a pivotal step in
ovarian cancer development, growth, and invasion beyond the
regional border (4,16,17).
VEGF is a glycoprotein which is associated with tumor angiogenesis,
higher levels of VEGF are consistent with ovarian cancer poor
prognosis and tumor progression (18). In our previous study, it was found
that FSH enhanced VEGF expression mediated by survivin and HIF1α
via PI3K/AKT signal pathway (4).
In this study, we showed the evidence that FSH upregulated not only
VEGF, but also Cox2 and survivin in a dose-dependent manner. Cox2
plays an important role in regulating VEGF expression, several
studies demonstrated that Cox2-mediated VEGF expression might
contribute to tumor metastasis via lymphangiogenesis or
angiogenesis pathways (19–23).
These results imply that overexpression of Cox2, survivin and VEGF
facilitates tumor angiogenesis. Although there are positive
response between these three molecule expressions and FSH
treatment, the detail mechanism and signal pathway are not
clear.
In the current study, it was found that microRNA-27a
is required for FSH-induced VEGF, Cox2 and survivin expression.
MicroRNA-27a possesses oncogenic activity, it is involved in cancer
development in various tumor types, such as breast and ovarian
cancer (7,24). It was found that microRNA-27a
overexpressed in several ovarian cancer cell lines, compared with
the Moody cell line, a normal ovarian epithelial cell line, which
suggests that microRNA-27a is functional in OEC occurrence. In
addition, obvious increase in microRNA-27a expression pattern was
identified in the present study together with increased dose of
FSH. We found that transfection of as-microRNA-27a reduced Cox2,
survivin and VEGF expressions, moreover, the induced expressions of
the three molecules by FSH were decreased by microRNA-27a, which
indicates microRNA-27a contributes to FSH-induced ovarian cancer
angiogenesis.
Under rich FSH conditions, reduced ZBTB10 expression
was observed. This putative zinc finger protein suppresses
specificity protein (Sp) transcription factors and Sp-dependent
gene expression (7,25). It was reported that microRNA-27a
targets ZBTB10 gene in breast cancer and regulates cell
proliferation (7). Our data
clearly showed that transfection of as-microRNA-27a induced ZBTB10
expression, the enhanced effect attenuated inhibition of
FSH-induced ZBTB10 expression. This is consistent with the results
of Mertens-Talcott et al(7). To investigate the role of ZBTB10 on
FSH-induced angiogenesis, transfection of ZBTB10 plasmid was
performed. Our present result showed that overexpression of ZBTB10
protein inhibited the expressions of VEGF, Cox2 and survivin, and
it abolished FSH-induced expression. These data imply that ZBTB10
plays an opposite role in mediating FSH-induced angiogenesis.
Sp1 is a critical molecule in microRNA-27a:
ZBTB10-specificity protein pathway and is a target gene of ZBTB10,
which belongs to the Sp/krüppel-like factor family of transcription
factors (26–28). Previous reports stated that Sp1 was
overexpressed in several type of cancer tissues (29–31),
moreover, it is a significant predictor of survival in human
gastric cancer (30–32). In the current study, overexpression
of Sp1 was observed in ovarian cancer cell lines, which indicates
that Sp1 may be involved in OEC occurrence. Moreover, the Sp1
protein level was elevated with increased dose of FSH, whereas,
transfection of as-microRNA-27a or overexpression of ZBTB10
inhibited Sp1 expression, the inhibition effect abolished
FSH-induced Sp1 expression. Present data also clearly showed that
RNA interference directed against Sp1 blocked the induction of
VEGF, Cox2 and survivin by FSH, suggesting that Sp1 is a critical
molecule in mediating FSH-induced angiogenesis. These results are
consistent with previous studies of Sp1 involved in regulation of
VEGF expression (33–38).
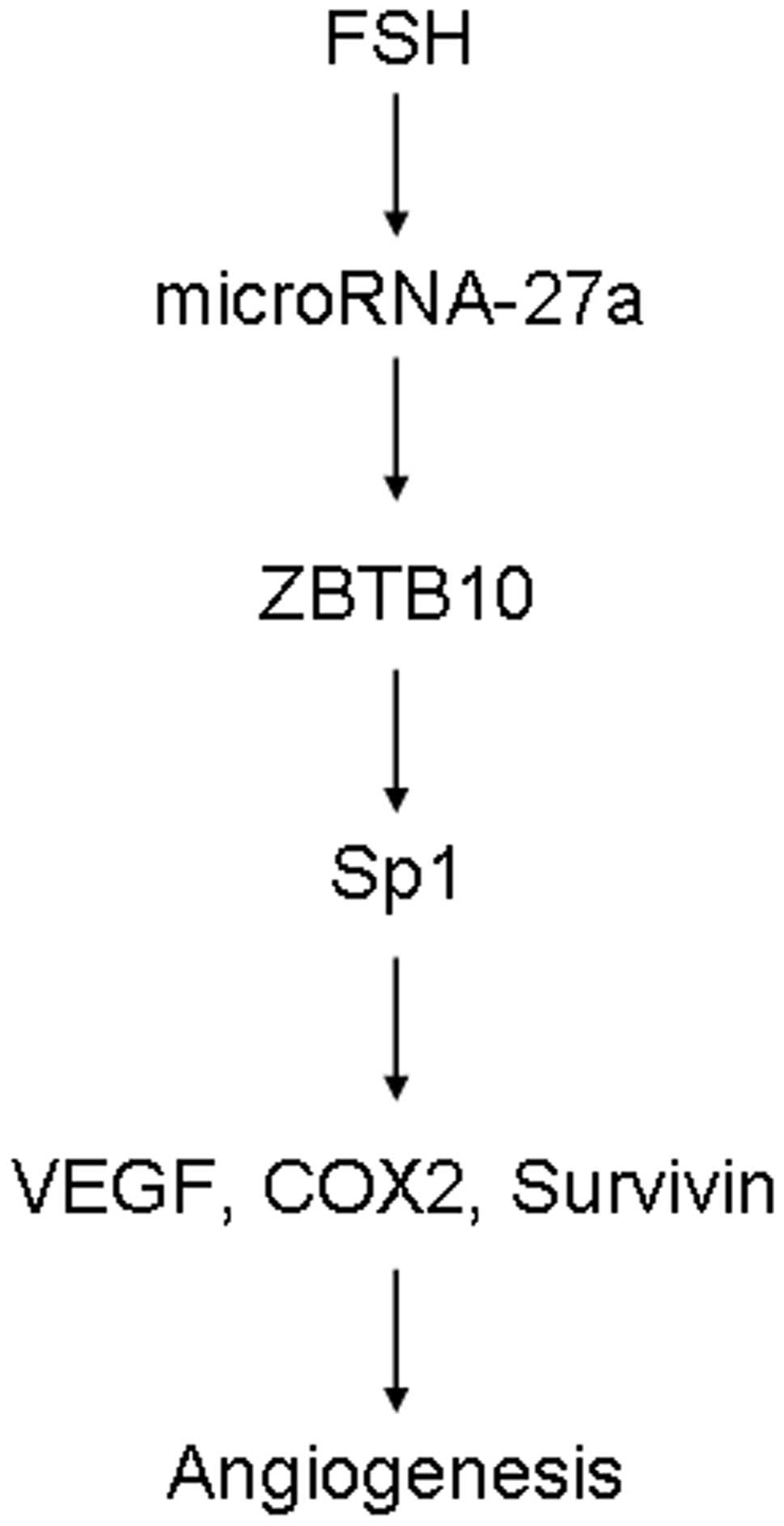
In conclusion, this study shows that FSH stimulates
the micoRNA-27a: ZBTB10-specificity protein pathway to induce VEGF,
Cox2 and survivin expression (Fig.
6), which is the first evidence of direct linkage of FSH to
microRNA activation and VEGF, Cox2 and survivin expression. Our
research may help to understand the molecular mechanism of
FSH-induced angiogenesis.
Acknowledgements
The study was supported by grants from
the National Natural Science Foundation of China (NSFC no.
81020108027, no. 30872755, no. 81172478), supported in part by the
grant (no. 10JC1413100) from Shanghai Science and Technology
Committee.
References
|
1
|
Choi JH, Wong AS, Huang HF and Leung PC:
Gonadotropins and ovarian cancer. Endocr Rev. 28:440–461. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Choi KC, Kang SK, Tai CJ, Auersperg N and
Leung PC: Follicle-stimulating hormone activates mitogen-activated
protein kinase in preneoplastic and neoplastic ovarian surface
epithelial cells. J Clin Endocrinol Metab. 87:2245–2253. 2002.
View Article : Google Scholar
|
|
3
|
Huang Y, Jin H, Liu Y, et al: FSH inhibits
ovarian cancer cell apoptosis by up-regulating survivin and
down-regulating PDCD6 and DR5. Endocr Relat Cancer. 18:13–26. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang Y, Hua K, Zhou X, et al: Activation
of the PI3K/AKT pathway mediates FSH-stimulated VEGF expression in
ovarian serous cystadenocarcinoma. Cell Res. 18:780–791. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ryan BM, Konecny GE, Kahlert S, et al:
Survivin expression in breast cancer predicts clinical outcome and
is associated with HER2, VEGF, urokinase plasminogen activator and
PAI-1. Ann Oncol. 17:597–604. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cai X, Ma S, Gu M, Zu C, Qu W and Zheng X:
Survivin regulates the expression of VEGF-C in lymphatic metastasis
of breast cancer. Diagn Pathol. 7:522012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mertens-Talcott SU, Chintharlapalli S, Li
X and Safe S: The oncogenic microRNA-27a targets genes that
regulate specificity protein transcription factors and the G2-M
checkpoint in MDA-MB-231 breast cancer cells. Cancer Res.
67:11001–11011. 2007. View Article : Google Scholar
|
|
8
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gagan J, Dey BK, Layer R, Yan Z and Dutta
A: MicroRNA-378 targets the myogenic repressor MyoR during myoblast
differentiation. J Biol Chem. 286:19431–19438. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kloosterman WP and Plasterk RH: The
diverse functions of microRNAs in animal development and disease.
Dev Cell. 11:441–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Naguibneva I, Ameyar-Zazoua M, Polesskaya
A, et al: The microRNA miR-181 targets the homeobox protein Hox-A11
during mammalian myoblast differentiation. Nat Cell Biol.
8:278–284. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sugatani T and Hruska KA: MicroRNA-223 is
a key factor in osteoclast differentiation. J Cell Biochem.
101:996–999. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nishi H, Ono K, Horie T, et al:
MicroRNA-27a regulates beta cardiac myosin heavy chain gene
expression by targeting thyroid hormone receptor beta1 in neonatal
rat ventricular myocytes. Mol Cell Biol. 31:744–755. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li X, Mertens-Talcott SU, Zhang S, Kim K,
Ball J and Safe S: MicroRNA-27a indirectly regulates estrogen
receptor {alpha} expression and hormone responsiveness in MCF-7
breast cancer cells. Endocrinology. 151:2462–2473. 2010.PubMed/NCBI
|
|
16
|
Wang J, Luo F, Lu JJ, Chen PK, Liu P and
Zheng W: VEGF expression and enhanced production by gonadotropins
in ovarian epithelial tumors. Int J Cancer. 97:163–167. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liao H, Zhou Q, Gu Y, Duan T and Feng Y:
Luteinizing hormone facilitates angiogenesis in ovarian epithelial
tumor cells and metformin inhibits the effect through the mTOR
signaling pathway. Oncol Rep. 27:1873–1878. 2012.PubMed/NCBI
|
|
18
|
Wong C, Wellman TL and Lounsbury KM: VEGF
and HIF-1alpha expression are increased in advanced stages of
epithelial ovarian cancer. Gynecol Oncol. 91:513–517. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gallo O, Franchi A, Magnelli L, et al:
Cyclooxygenase-2 pathway correlates with VEGF expression in head
and neck cancer. Implications for tumor angiogenesis and
metastasis. Neoplasia. 3:53–61. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim MH, Seo SS, Song YS, et al: Expression
of cyclooxygenase-1 and -2 associated with expression of VEGF in
primary cervical cancer and at metastatic lymph nodes. Gynecol
Oncol. 90:83–90. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marrogi AJ, Travis WD, Welsh JA, et al:
Nitric oxide synthase, cyclooxygenase 2, and vascular endothelial
growth factor in the angiogenesis of non-small cell lung carcinoma.
Clin Cancer Res. 6:4739–4744. 2000.PubMed/NCBI
|
|
22
|
Shtivelband MI, Juneja HS, Lee S and Wu
KK: Aspirin and salicylate inhibit colon cancer medium- and
VEGF-induced endothelial tube formation: correlation with
suppression of cyclooxygenase-2 expression. J Thromb Haemost.
1:2225–2233. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang J, Ji J, Yuan F, et al:
Cyclooxygenase-2 expression is associated with VEGF-C and lymph
node metastases in gastric cancer patients. Biomed Pharmacother.
59(Suppl 2): S285–S288. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li ZM, Hu S, Xiao L, et al: Expression of
microRNA 27a and its correlation with drug resistance in human
ovarian cancer A2780/Taxol cells. Zhonghua Fu Chan Ke Za Zhi.
45:372–375. 2010.(In Chinese).
|
|
25
|
Chintharlapalli S, Papineni S, Abdelrahim
M, et al: Oncogenic microRNA-27a is a target for anticancer agent
methyl 2-cyano-3,11-dioxo-18beta-olean-1,12-dien-30-oate in colon
cancer cells. Int J Cancer. 125:1965–1974. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Philipsen S and Suske G: A tale of three
fingers: the family of mammalian Sp/XKLF transcription factors.
Nucleic Acids Res. 27:2991–3000. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bouwman P and Philipsen S: Regulation of
the activity of Sp1-related transcription factors. Mol Cell
Endocrinol. 195:27–38. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Safe S and Abdelrahim M: Sp transcription
factor family and its role in cancer. Eur J Cancer. 41:2438–2448.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lou Z, O’Reilly S, Liang H, Maher VM,
Sleight SD and McCormick JJ: Down-regulation of overexpressed sp1
protein in human fibrosarcoma cell lines inhibits tumor formation.
Cancer Res. 65:1007–1017. 2005.PubMed/NCBI
|
|
30
|
Wang L, Wei D, Huang S, et al:
Transcription factor Sp1 expression is a significant predictor of
survival in human gastric cancer. Clin Cancer Res. 9:6371–6380.
2003.PubMed/NCBI
|
|
31
|
Zhang J, Zhu ZG, Ji J, et al:
Transcription factor Sp1 expression in gastric cancer and its
relationship to long-term prognosis. World J Gastroenterol.
11:2213–2217. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yao JC, Wang L, Wei D, et al: Association
between expression of transcription factor Sp1 and increased
vascular endothelial growth factor expression, advanced stage, and
poor survival in patients with resected gastric cancer. Clin Cancer
Res. 10:4109–4117. 2004. View Article : Google Scholar
|
|
33
|
Akiyama H, Tanaka T, Maeno T, et al:
Induction of VEGF gene expression by retinoic acid through
Sp1-binding sites in retinoblastoma Y79 cells. Invest Ophthalmol
Vis Sci. 43:1367–1374. 2002.PubMed/NCBI
|
|
34
|
Bermudez Y, Yang H, Saunders BO, Cheng JQ,
Nicosia SV and Kruk PA: VEGF- and LPA-induced telomerase in human
ovarian cancer cells is Sp1-dependent. Gynecol Oncol. 106:526–537.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cho SG, Yi Z, Pang X, et al:
Kisspeptin-10, a KISS1-derived decapeptide, inhibits tumor
angiogenesis by suppressing Sp1-mediated VEGF expression and
FAK/Rho GTPase activation. Cancer Res. 69:7062–7070. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li ZY, Zhu F, Hu JL, et al: Sp1
inhibition-mediated upregulation of VEGF 165 b induced by
rh-endostatin enhances antiangiogenic and anticancer effect of
rh-endostatin in A549. Tumour Biol. 32:677–687. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Loeffler S, Fayard B, Weis J and
Weissenberger J: Interleukin-6 induces transcriptional activation
of vascular endothelial growth factor (VEGF) in astrocytes in vivo
and regulates VEGF promoter activity in glioblastoma cells via
direct interaction between STAT3 and Sp1. Int J Cancer.
115:202–213. 2005. View Article : Google Scholar
|
|
38
|
Novak EM, Metzger M, Chammas R, et al:
Downregulation of TNF-alpha and VEGF expression by Sp1 decoy
oligodeoxynucleotides in mouse melanoma tumor. Gene Ther.
10:1992–1997. 2003. View Article : Google Scholar : PubMed/NCBI
|