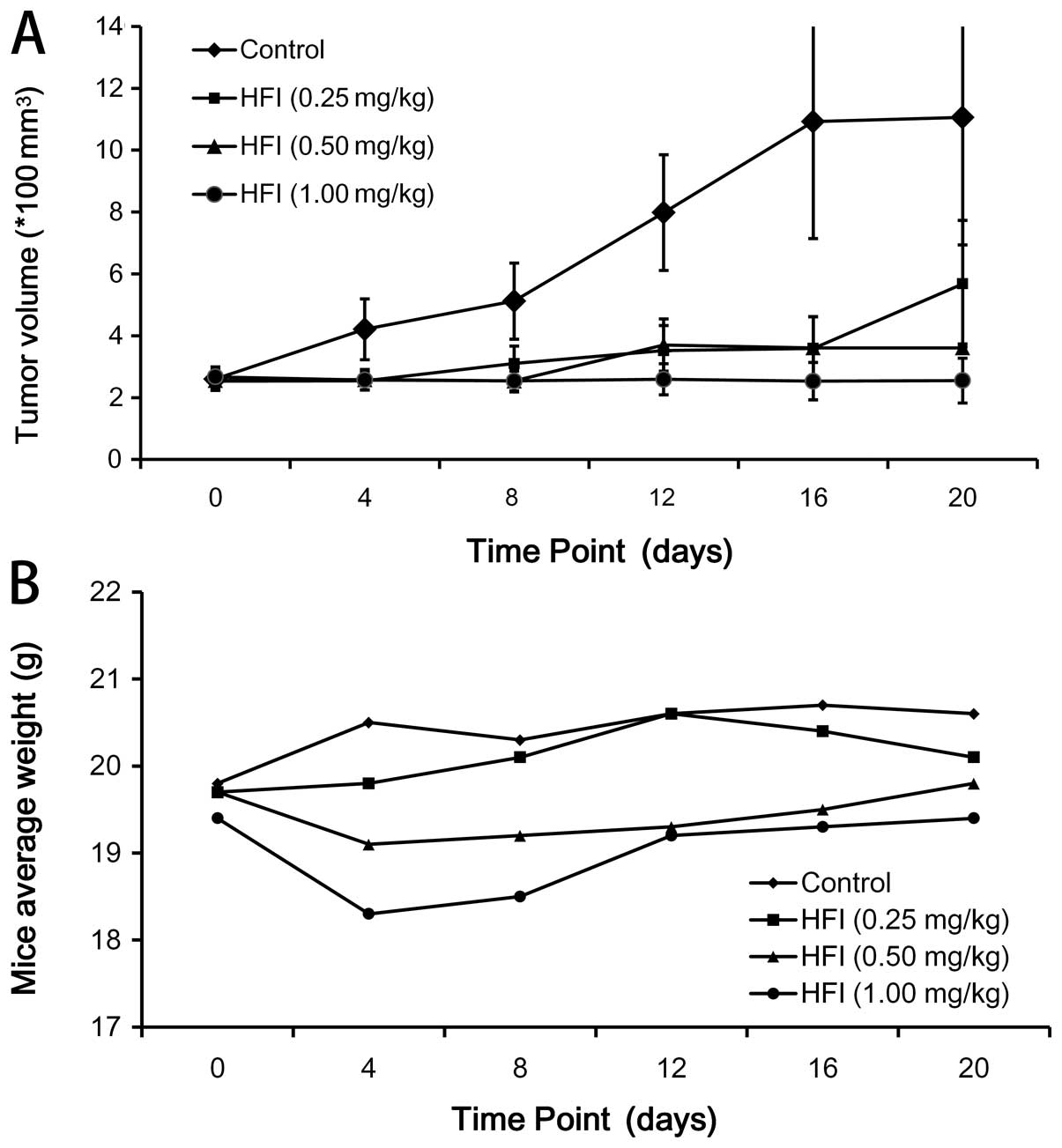
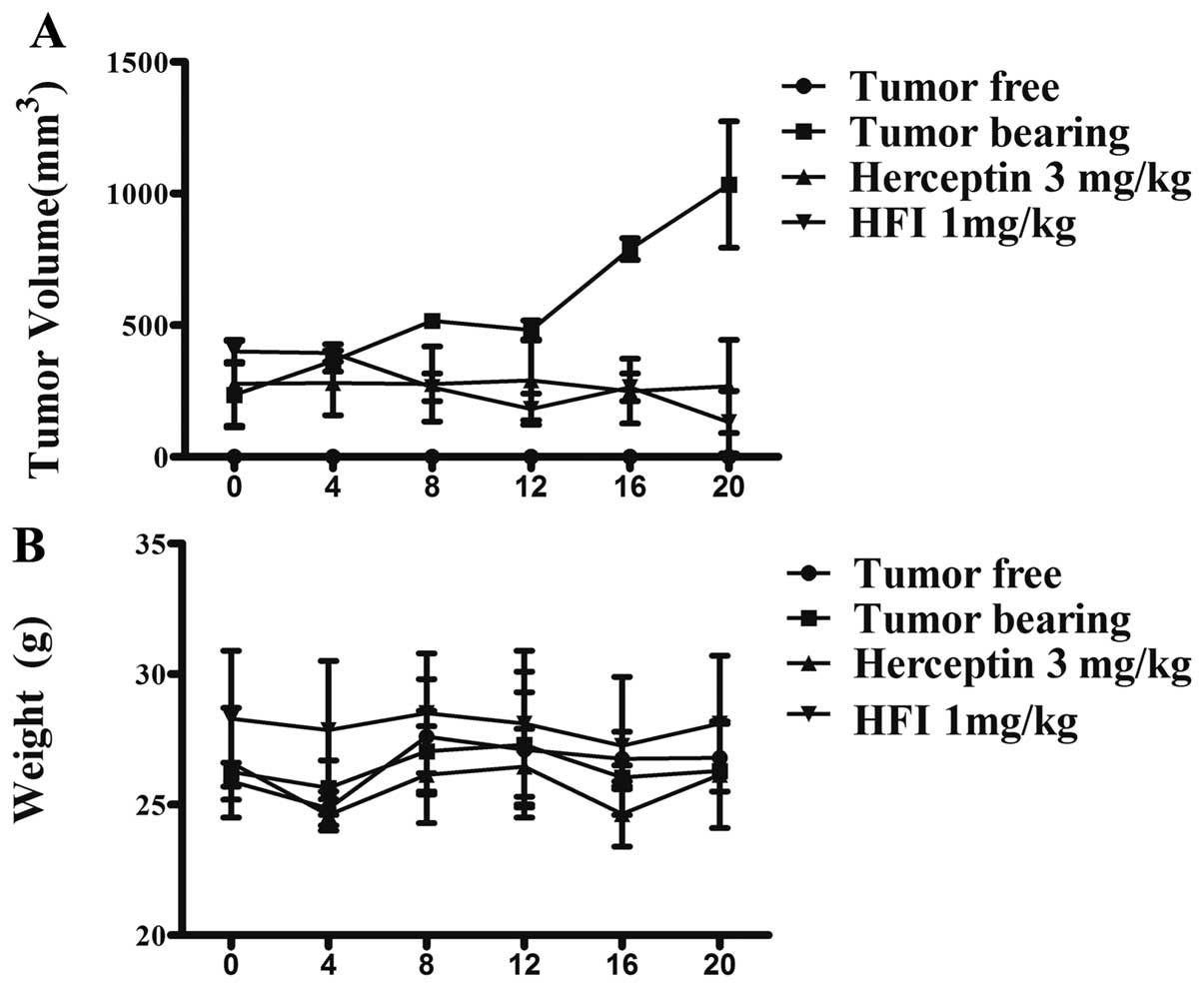
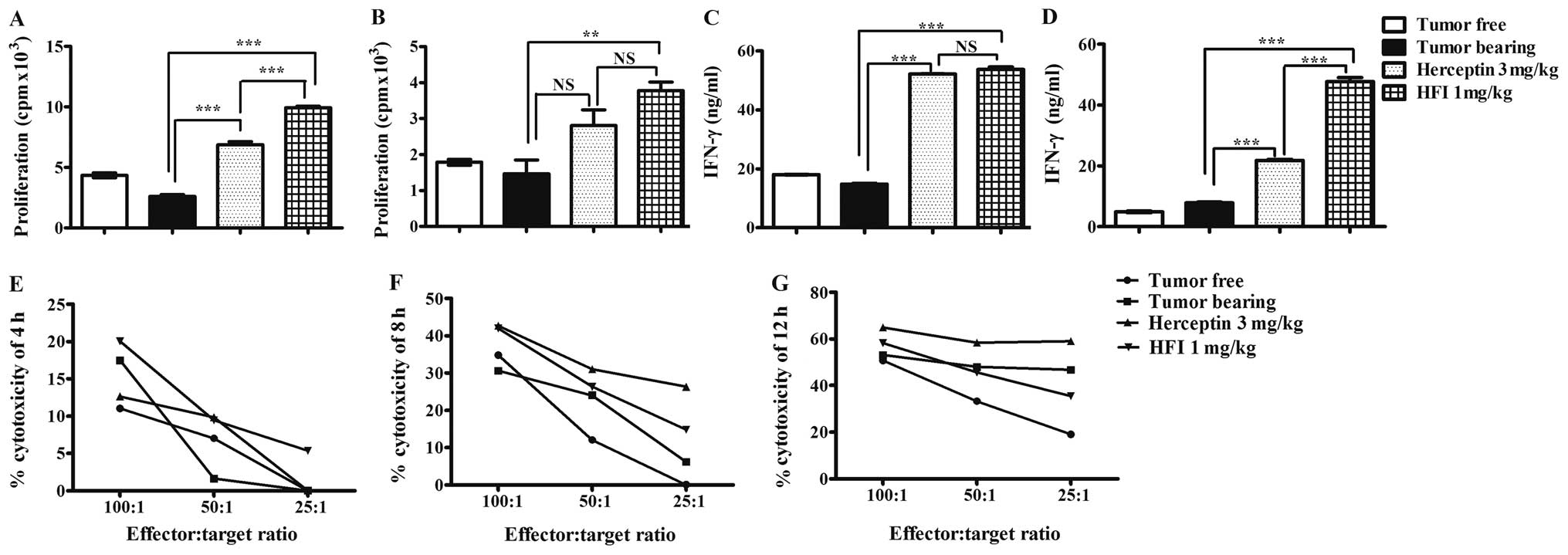
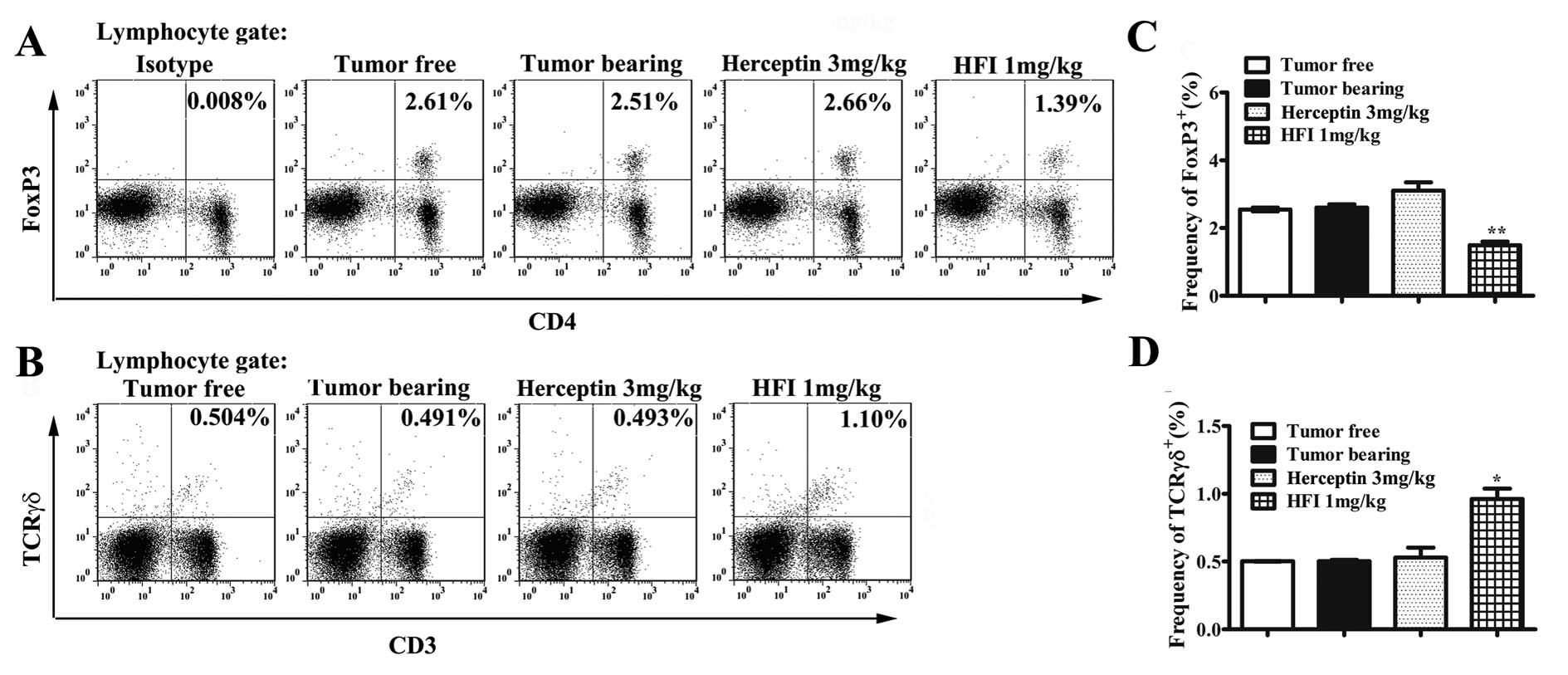
|
1.
|
Jallal B, Schlessinger J and Ullrich A:
Tyrosine phosphatase inhibition permits analysis of signal
transduction complexes in p185HER2/neu-overexpressing human tumor
cells. J Biol Chem. 267:4357–4363. 1992.
|
|
2.
|
Rusnak DW, Lackey K, Affleck K, et al: The
effects of the novel, reversible epidermal growth factor
receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of
human normal and tumor-derived cell lines in vitro and in vivo. Mol
Cancer Ther. 1:85–94. 2001.
|
|
3.
|
Agus DB, Akita RW, Fox WD, et al:
Targeting ligand-activated ErbB2 signaling inhibits breast and
prostate tumor growth. Cancer Cell. 2:127–137. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Konopleva M, Zhang W, Shi YX, et al:
Synthetic triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic
acid induces growth arrest in HER2-overexpressing breast cancer
cells. Mol Cancer Ther. 5:317–328. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Bergman I, Barmada MA, Griffin JA and
Slamon DJ: Treatment of meningeal breast cancer xenografts in the
rat using an anti-p185/HER2 antibody. Clin Cancer Res. 7:2050–2056.
2001.PubMed/NCBI
|
|
6.
|
Spiridon CI, Ghetie MA, Uhr J, et al:
Targeting multiple Her-2 epitopes with monoclonal antibodies
results in improved anti-growth activity of a human breast cancer
cell line in vitro and in vivo. Clin Cancer Res. 8:1720–1730.
2002.PubMed/NCBI
|
|
7.
|
Meng S, Tripathy D, Shete S, et al: HER-2
gene amplification can be acquired as breast cancer progresses.
Proc Natl Acad Sci USA. 101:9393–9398. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Jerome L, Alami N, Belanger S, et al:
Recombinant human insulin-like growth factor binding protein 3
inhibits growth of human epidermal growth factor
receptor-2-overexpressing breast tumors and potentiates herceptin
activity in vivo. Cancer Res. 66:7245–7252. 2006. View Article : Google Scholar
|
|
9.
|
Merlin JL, Barberi-Heyob M and Bachmann N:
In vitro comparative evaluation of trastuzumab (Herceptin) combined
with paclitaxel (Taxol) or docetaxel (Taxotere) in HER2-expressing
human breast cancer cell lines. Ann Oncol. 13:1743–1748. 2002.
View Article : Google Scholar
|
|
10.
|
Nahta R, Hung MC and Esteva FJ: The
HER-2-targeting antibodies trastuzumab and pertuzumab
synergistically inhibit the survival of breast cancer cells. Cancer
Res. 64:2343–2346. 2004. View Article : Google Scholar
|
|
11.
|
Moulder SL, Yakes FM, Muthuswamy SK,
Bianco R, Simpson JF and Arteaga CL: Epidermal growth factor
receptor (HER1) tyrosine kinase inhibitor ZD1839 (Iressa) inhibits
HER2/neu (erbB2)-overexpressing breast cancer cells in vitro and in
vivo. Cancer Res. 61:8887–8895. 2001.PubMed/NCBI
|
|
12.
|
Liang K, Lu Y, Jin W, Ang KK, Milas L and
Fan Z: Sensitization of breast cancer cells to radiation by
trastuzumab. Mol Cancer Ther. 2:1113–1120. 2003.PubMed/NCBI
|
|
13.
|
Tseng PH, Wang YC, Weng SC, et al:
Overcoming trastuzumab resistance in HER2-overexpressing breast
cancer cells by using a novel celecoxib-derived
phosphoinositide-dependent kinase-1 inhibitor. Mol Pharmacol.
70:1534–1541. 2006. View Article : Google Scholar
|
|
14.
|
Arpino G, Gutierrez C, Weiss H, et al:
Treatment of human epidermal growth factor receptor
2-overexpressing breast cancer xenografts with multiagent
HER-targeted therapy. J Natl Cancer Inst. 99:694–705. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Talmadge JE, Phillips H, Schindler J,
Tribble H and Pennington R: Systematic preclinical study on the
therapeutic properties of recombinant human interleukin 2 for the
treatment of metastatic disease. Cancer Res. 47:5725–5732.
1987.PubMed/NCBI
|
|
16.
|
Kovacs JA, Vogel S, Albert JM, et al:
Controlled trial of interleukin-2 infusions in patients infected
with the human immunodeficiency virus. N Engl J Med. 335:1350–1356.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Kozlowski T, Sablinski T, Basker M, et al:
Decreased graft-versus-host disease after haplotype mismatched bone
marrow allografts in miniature swine following interleukin-2
treatment. Bone Marrow Transplant. 25:47–52. 2000. View Article : Google Scholar
|
|
18.
|
Gaffen SL and Liu KD: Overview of
interleukin-2 function, production and clinical applications.
Cytokine. 28:109–123. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Malek TR and Bayer AL: Tolerance, not
immunity, crucially depends on IL-2. Nat Rev Immunol. 4:665–674.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Cesana GC, DeRaffele G, Cohen S, et al:
Characterization of CD4+CD25+ regulatory T
cells in patients treated with high-dose interleukin-2 for
metastatic melanoma or renal cell carcinoma. J Clin Oncol.
24:1169–1177. 2006.PubMed/NCBI
|
|
21.
|
Goldsmith MA, Lai SY, Xu W, et al: Growth
signal transduction by the human interleukin-2 receptor requires
cytoplasmic tyrosines of the beta chain and non-tyrosine residues
of the gamma c chain. J Biol Chem. 270:21729–21737. 1995.
View Article : Google Scholar
|
|
22.
|
Hughes-Fulford M, Sugano E, Schopper T, Li
CF, Boonyaratanakornkit JB and Cogoli A: Early immune response and
regulation of IL-2 receptor subunits. Cell Signal. 17:1111–1124.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Dybkaer K, Iqbal J, Zhou G, et al: Genome
wide transcriptional analysis of resting and IL2 activated human
natural killer cells: gene expression signatures indicative of
novel molecular signaling pathways. BMC Genomics. 8:2302007.
View Article : Google Scholar
|
|
24.
|
Harvill ET and Morrison SL: An IgG3-IL2
fusion protein activates complement, binds Fc gamma RI, generates
LAK activity and shows enhanced binding to the high affinity IL-2R.
Immunotechnology. 1:95–105. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Budagian V, Nanni P, Lollini PL, et al:
Enhanced inhibition of tumour growth and metastasis, and induction
of antitumour immunity by IL-2-IgG2b fusion protein. Scand J
Immunol. 55:484–492. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Heuser C, Ganser M, Hombach A, et al: An
anti-MUC1-antibody-interleukin-2 fusion protein that activates
resting NK cells to lysis of MUC1-positive tumour cells. Br J
Cancer. 89:1130–1139. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Neal ZC, Yang JC, Rakhmilevich AL, et al:
Enhanced activity of hu14.18-IL2 immunocytokine against murine NXS2
neuroblastoma when combined with interleukin 2 therapy. Clin Cancer
Res. 10:4839–4847. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Gillies SD, Lan Y, Williams S, et al: An
anti-CD20-IL-2 immunocytokine is highly efficacious in a SCID mouse
model of established human B lymphoma. Blood. 105:3972–3978. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Wagner K, Schulz P, Scholz A, Wiedenmann B
and Menrad A: The targeted immunocytokine L19-IL2 efficiently
inhibits the growth of orthotopic pancreatic cancer. Clin Cancer
Res. 14:4951–4960. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Marlind J, Kaspar M, Trachsel E, et al:
Antibody-mediated delivery of interleukin-2 to the stroma of breast
cancer strongly enhances the potency of chemotherapy. Clin Cancer
Res. 14:6515–6524. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Shi M, Xie Z, Feng J, et al: A recombinant
anti-erbB2, scFv-Fc-IL-2 fusion protein retains antigen specificity
and cytokine function. Biotechnol Lett. 25:815–819. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Shi M, Xie Z, Yu M, Shen B and Guo N:
Controlled growth of Chinese hamster ovary cells and high
expression of antibody-IL-2 fusion proteins by temperature
manipulation. Biotechnol Lett. 27:1879–1884. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Shi M, Zhang L, Gu HT, et al: Efficient
growth inhibition of ErbB2-overexpressing tumor cells by anti-ErbB2
ScFv-Fc-IL-2 fusion protein in vitro and in vivo. Acta Pharmacol
Sin. 28:1611–1620. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Arakawa T, Prestrelski SJ, Kenney WC and
Carpenter JF: Factors affecting short-term and long-term
stabilities of proteins. Adv Drug Deliv Rev. 46:307–326. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Chen L, Xie Z, Teng Y, et al: Highly
efficient selection of the stable clones expressing antibody-IL-2
fusion protein by a dicistronic expression vector containing a
mutant neo gene. J Immunol Methods. 295:49–56. 2004. View Article : Google Scholar
|
|
36.
|
Lin JX and Leonard WJ: Signaling from the
IL-2 receptor to the nucleus. Cytokine Growth Factor Rev.
8:313–332. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Lacosta S, Merali Z and Anisman H: Central
monoamine activity following acute and repeated systemic
interleukin-2 administration. Neuroimmunomodulation. 8:83–90. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Sereti I, Anthony KB, Martinez-Wilson H,
et al: IL-2-induced CD4+ T-cell expansion in
HIV-infected patients is associated with long-term decreases in
T-cell proliferation. Blood. 104:775–780. 2004.PubMed/NCBI
|
|
39.
|
Jo D, Liu D, Yao S, Collins RD and Hawiger
J: Intracellular protein therapy with SOCS3 inhibits inflammation
and apoptosis. Nat Med. 11:892–898. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Lewis GD, Figari I, Fendly B, et al:
Differential responses of human tumor cell lines to anti-p185HER2
monoclonal antibodies. Cancer Immunol Immunother. 37:255–263. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Smith KA and Griffin JD: Following the
cytokine signaling pathway to leukemogenesis: a chronology. J Clin
Invest. 118:3564–3573. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Friess T, Scheuer W and Hasmann M:
Combination treatment with erlotinib and pertuzumab against human
tumor xenografts is superior to monotherapy. Clin Cancer Res.
11:5300–5309. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Johnson BE and Janne PA: Rationale for a
phase II trial of pertuzumab, a HER-2 dimerization inhibitor, in
patients with non-small cell lung cancer. Clin Cancer Res.
12:4436s–4440s. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Konecny GE, Pegram MD, Venkatesan N, et
al: Activity of the dual kinase inhibitor lapatinib (GW572016)
against HER-2-overexpressing and trastuzumab-treated breast cancer
cells. Cancer Res. 66:1630–1639. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
King DM, Albertini MR, Schalch H, et al:
Phase I clinical trial of the immunocytokine EMD 273063 in melanoma
patients. J Clin Oncol. 22:4463–4473. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Zhang X, Feng J, Ye X, Yao Y, Zhou P and
Chen X: Development of an immunocytokine, IL-2-183B2scFv, for
targeted immunotherapy of ovarian cancer. Gynecol Oncol.
103:848–852. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Johnson EE, Lum HD, Rakhmilevich AL, et
al: Intratumoral immunocytokine treatment results in enhanced
antitumor effects. Cancer Immunol Immunother. 57:1891–1902. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Singh H, Serrano LM, Pfeiffer T, et al:
Combining adoptive cellular and immunocytokine therapies to improve
treatment of B-lineage malignancy. Cancer Res. 67:2872–2880. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Spiridon CI, Guinn S and Vitetta ES: A
comparison of the in vitro and in vivo activities of IgG and
F(ab’)2 fragments of a mixture of three monoclonal anti-Her-2
antibodies. Clin Cancer Res. 10:3542–3551. 2004.PubMed/NCBI
|
|
50.
|
Xie H, Lin L, Tong L, et al: AST1306, a
novel irreversible inhibitor of the epidermal growth factor
receptor 1 and 2, exhibits antitumor activity both in vitro and in
vivo. PLoS One. 6:e214872011. View Article : Google Scholar
|
|
51.
|
Zou W: Regulatory T cells, tumour immunity
and immunotherapy. Nat Rev Immunol. 6:295–307. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Koreth J, Matsuoka K, Kim HT, et al:
Interleukin-2 and regulatory T cells in graft-versus-host disease.
N Engl J Med. 365:2055–2066. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Vesely MD, Kershaw MH, Schreiber RD and
Smyth MJ: Natural innate and adaptive immunity to cancer. Annu Rev
Immunol. 29:235–271. 2011. View Article : Google Scholar : PubMed/NCBI
|