Introduction
Prostate cancer is the most frequently diagnosed
malignancy and the second most common cause of cancer death in men.
It occurs predominantly in persons over 50 years of age (1), typically progressing at a slow rate
(2). Prostate cancer in elderly
males accounts for 33% of all newly diagnosed malignancies among
men in the United States (3).
Moreover, the number of patients with prostate cancer is increasing
in Asia (4,5). Therefore, the exploration and
development of novel and more effective antitumor agents for
patients with prostate cancer are urgently needed.
Apoptosis is a highly regulated process of
programmed cell death that plays an important role in the
maintenance of cellular homeostasis. Disruption of this process
represents a major contributing factor in the pathology of cancer.
Thus, apoptosis activation has been considered a good target in
cancer therapies (6,7). In general, apoptosis is regulated by
pro-apoptotic and anti-apoptotic gene products, such as the Bcl-2
and inhibitor of apoptosis protein (IAP) family members, and
executed through caspases and cysteine-aspartic proteases, chiefly
via two major and inter-related pathways (i.e., the
mitochondria-dependent ‘intrinsic’ cytochrome c/caspase-9
pathway and the death receptor-mediated ‘extrinsic’ caspase-8
pathway) (8,9). Caspase activation further leads to
protein cleavage resulting in DNA fragmentation, chromatin
condensation and cell shrinkage. Additionally, reactive oxygen
species (ROS) play a key role in mitochondria-mediated apoptosis.
Mitochondria are the prime source of ROS, which are byproducts of
aerobic respiration (10,11). High levels of ROS in mitochondria
can result in free radical attack of membrane phospholipids and
cause mitochondrial membrane depolarization. This is an
irreversible step, which is associated with the release of
mitochondrial factors including cytochrome c, triggering
caspase cascades (12,13,14).
Therefore, ROS plays an important role in mitochondria-mediated
apoptotic pathway.
Cordycepin, 3′-deoxyadenosine, is a major functional
component in the Cordyceps militaris fungus (Fig. 1) (15,16).
Due to the absence of oxygen in the 30-position of its ribose
moiety, the incorporation of cordycepin during RNA synthesis will
result in termination of chain elongation. This activity has been
well described in vitro with purified RNA polymerases and
poly(A) polymerases from a number of organisms, including yeast and
mammals (17,18). Cordycepin has also demonstrated
various properties, such as antitumor (18,19,20),
anti-fungal (21), anti-bacterial
(22) and anti-inflammatory
effects (23,24). Indeed, for centuries, Cordyceps
militaris has been a widely administered traditional Chinese
medicine, with cordycepin believed to be one of the bioactive
components mediating its beneficial effects (16,25).
While well known as a therapeutic agent due to its unique
properties, the molecular mechanisms underlying the anticancer
effects of cordycepin are not yet completely understood.
The purpose of this study was to evaluate the role
of mitochondria in apoptosis induced by cordycepin, using human
prostate carcinoma cells. We examined whether ROS were critical
mediators of cordycepin-induced PC-3 cell death, and we determined
the sequence of events leading to the activation of downstream
caspases and apoptosis. The study furnishes evidence that
cordycepin elicits ROS, which in turn triggers a decrease in
mitochondria membrane potential (MMP), consequently leading to
caspase activation.
Materials and methods
Reagents and antibodies
Cordycepin (MW, 251.2; product no. C3394),
4,6-diamidino-2-phenylindole (DAPI), dimethyl sulfoxide (DMSO),
N-acetyl-L-cysteine (NAC),
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT), 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine
iodide (JC-1) and propidium iodide (PI) were purchased from the
Sigma-Aldrich Chemical Co. (St. Louis, MO). Fetal bovine serum
(FBS) and caspase activity assay kits were obtained from Gibco-BRL
(Grand Island, NY) and R&D Systems (Minneapolis, MN),
respectively. The DNA staining kit (CycleTEST™ Plus Kit) and
enhanced chemiluminescence (ECL) kit were purchased from
Becton-Dickinson (San Jose, CA) and Amersham Co. (Arlington
Heights, IL), respectively. Antibodies specific for XIAP, cIAP-1,
cIAP-2, Bcl-2, Bax, Bcl-xL, caspase-3, -8 and -9, and
poly(ADP-ribose)polymerases (PARP) were obtained from Santa Cruz
Biotechnology (Santa Cruz, CA). Anti-cytochrome c and actin
antibodies were purchased from Cell Signaling (Beverly, MA) and
Sigma-Aldrich Chemical Co., respectively. The peroxidase-labeled
donkey anti-rabbit immunoglobulin and peroxidase-labeled sheep
anti-mouse immunoglobulin were purchased from Amersham Co.
Cell lines, cell culture and MTT
assay
Human prostate cancer cell lines (PC-3, DU145 and
LNCaP) were obtained from the American Type Culture Collection
(Rockville, MD). The culture medium used throughout the experiments
was RPMI-1640 medium (Gibco-BRL), containing 10% FBS, 2 mM
L-glutamine, and 100 U/ml penicillin and streptomycin. Cells were
cultured at 37°C in a humidified chamber containing 5%
CO2. For the cell viability assay, cells were seeded in
6-well plates and treated with various concentrations of cordycepin
for 24 h. After treatments, MTT working solution was added to
6-well culture plates and incubated continuously at 37°C for 2 h.
The culture supernatant was removed from the wells and DMSO was
added to dissolve the formazan crystals. The absorbance of each
well was measured at 540 nm with an ELISA reader (Molecular
Devices, Sunnyvale, CA).
Flow cytometry analysis
After treatment with cordycepin, the cells were
collected, washed with cold phosphate-buffered saline (PBS) and
fixed in 75% ethanol at 4°C for 30 min. Prior to analysis, the
cells were washed once again with PBS, suspended in a cold PI
solution containing 100 μg/ml RNase A, 50 μg/ml PI,
0.1% (w/v) sodium citrate and 0.1% (v/v) NP-40, and further
incubated on ice for 30 min in the dark. Flow cytometry analyses
were carried out using a flow cytometer (FACSCalibur;
Becton-Dickinson). Cell-Quest software was used to determine the
relative DNA content based on the presence of red fluorescence. The
sub-G1 population was calculated to estimate the apoptotic cell
population (26).
DNA fragmentation assay
Cells were lysed in 100 μl of 10 mM Tris-HCl
buffer (pH 7.4) containing 10 mM EDTA and 0.5% Triton X-100. After
centrifugation for 5 min at 15,000 rpm, supernatant samples were
treated with RNase A and proteinase K. Subsequently, 20 μl
of 5 M NaCl and 120 μl isopropanol were added to the
samples, which were then kept at −20°C for 6 h. Then, following
centrifugation for 15 min at 15,000 rpm, DNA pellets were dissolved
in 20 μl of TE buffer (10 mM Tris-HCl and 1 mM EDTA) as
loading samples. To assay the DNA fragmentation pattern, samples
were loaded onto 1.5% agarose gel and electrophoresis was carried
out.
DAPI staining
Cells were washed with cold PBS and fixed with 4%
paraformaldehyde (Sigma-Aldrich Chemical Co.) in PBS for 10 min at
room temperature. The fixed cells were washed with PBS and stained
with DAPI solution for 10 min at room temperature. The cells were
then washed twice with PBS and analyzed with a fluorescence
microscope (Carl Zeiss, Germany).
Determination of caspase activity
The activities of caspases were determined by
colorimetric assay kits, which utilize synthetic tetrapeptides
[Asp-Glu-Val-Asp (DEAD) for caspase-3; Ile-Glu-Thr-Asp (IETD) for
caspase-8; and Leu-Glu-His-Asp (LEHD) for caspase-9] labeled with
p-nitroaniline (pNA), according to the manufacturer’s protocol. The
cells were briefly lysed in the supplied lysis buffer. The
supernatants were collected and incubated with the supplied
reaction buffer containing dithiothreitol (DTT) and substrates at
37°C for 2 h in the dark. The caspase activity was determined by
measuring changes in absorbance at 405 nm using the ELISA
reader.
Isolation of total-RNA and reverse
transcription-PCR
Total-RNA was isolated using TRIzol reagent
(Invitrogen, Carlsbad, CA). Total-RNA (1.0 μg) obtained from
cells was primed with random hexamers to synthesize complementary
DNA using M-MLV reverse transcriptase (Promega, Madison, WI)
according to the manufacturer’s instructions. Single stranded cDNA
was amplified by polymerase chain reaction (PCR) with the indicated
primers. Amplification products obtained by PCR were
electrophoretically separated on 1% agarose gel and visualized by
ethidium bromide (EtBr, Sigma-Aldrich Chemical Co.) staining.
Protein extraction and western blot
analysis
The cells were harvested and lysed with lysis buffer
(20 mM sucrose, 1 mM EDTA, 20 μM Tris-Cl, pH 7.2, 1 mM DTT,
10 mM KCl, 1.5 mM MgCl2 and 5 μg/ml aprotinin)
for 30 min. The protein concentration was measured using a Bio-Rad
protein assay (Bio-Rad Laboratories, Hercules, CA) according to the
manufacturer’s instructions. In a parallel experiment, cells were
washed with cold PBS and scraped; cytoplasmic and nuclear proteins
were then extracted using a mitochondrial fractionation kit
according to the manufacturer’s instructions (Activemotif,
Carlsbad, CA). For western blot analysis, an equal amount of
protein was subjected to electrophoresis on SDS-polyacrylamide gel
and transferred by electroblotting to a nitrocellulose membrane
(Schleicher & Schuell, Keene, NH). The blots were probed with
the desired antibodies for 1 h, incubated with the diluted
enzyme-linked secondary antibody and visualized by ECL kit
according to the recommended procedure.
Mitochondrial membrane potential (MMP,
ΔΨm) assay
The MMP of intact cells was measured by DNA flow
cytometry with the lipophilic cation JC-1. JC-1 is a ratiometric,
dual-emission fluorescent dye that is internalized and concentrated
by respiring mitochondria; therefore, it can reflect changes in MMP
in living cells. There are two excitation wavelengths: at low
values of MMP, it remains a monomer (FL-1, green fluorescence; 527
nm) while it forms aggregates at high MMP (FL-2, red fluorescence;
590 nm), according to the recommended procedure (Calbiochem). For
this study, the cells were trypsinized and the cell pellets were
resuspended in PBS and incubated with 10 μM JC-1 for 20 min
at 37°C. The cells were subsequently washed once with cold PBS,
suspended and analyzed using a flow cytometer.
Measurement of intracellular ROS
generation
The generation of ROS was determined in cells
treated with cordycepin in the presence and absence of NAC, and was
evaluated with 5-(and 6)-carboxy-2′7′-dichlorodihydrofluorescein
diacetate (DCF-DA; Molecular Probes, Leiden, The Netherlands) as
described previously (27). The
cells were incubated with 10 μM DCF-DA at 37°C for 30 min.
The cells were then washed with PBS and FL-1 fluorescence was
measured with a flow cytometer.
Statistical analysis
The data are expressed as a mean ± SD. A statistical
comparison was performed using one-way ANOVA followed by a Fisher’s
test. The significant (p<0.05) differences between the groups
were determined using an unpaired Student’s t-test.
Results
Induction of apoptosis by cordycepin in
PC-3 cells
To investigate the effect of cordycepin on cell
growth of human prostate carcinoma cell lines (PC-3, LNCaP and
DU145), the cells were exposed to various concentrations (0, 5, 10,
15, 20 and 25 μg/ml) of cordycepin for 24 h and then cell
viability was measured by the MTT assay. As shown in Fig. 2A, cordycepin elicited a decrease in
cell viability in a dose-dependent manner in prostate carcinoma
cells; notably, the cytotoxicity of cordycepin was more potent in
PC-3 cells than in DU145 and LNCaP cells. Morphological analysis of
DAPI-stained PC-3 cells treated with cordycepin indicated that they
had undergone gross morphological changes indicative of apoptosis,
including cell shrinkage, chromatin condensation and the loss of
nuclear construction (Fig. 2B).
Further experiments were performed to determine if this inhibitory
effect of cordycepin on cell viability was the result of apoptotic
cell death. As shown in Fig. 2C,
cordycepin treatment resulted in an increased accumulation of PC-3
cells at the apoptotic sub-G1 phase of the cell cycle and that this
response occurred in a concentration-dependent manner. We also
examined whether or not cordycepin induces DNA fragmentation,
another hallmark of apoptosis. Following agarose gel
electrophoresis of cells treated with cordycepin, a typical ladder
pattern of inter-nucleosomal DNA fragmentation was observed
(Fig. 2D). These results clearly
suggest that cordycepin-induced apoptosis took place in PC-3
cells.
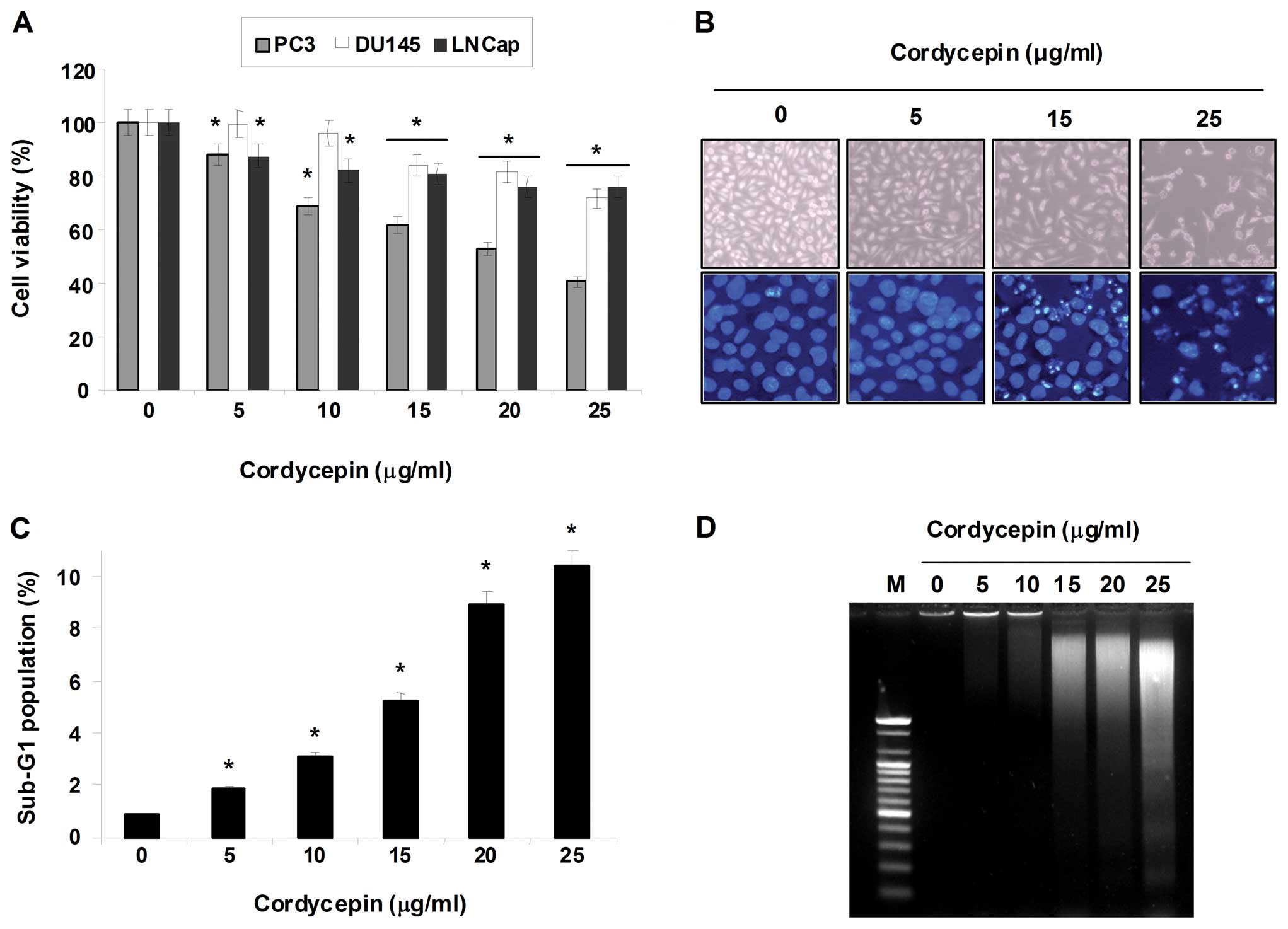 | Figure 2Inhibition of cell viability and
induction of apoptosis by cordycepin in human prostate cancer
cells. (A) PC-3, DU145 and LNCaP cells (2×105
cells/well) were plated in 6-well tissue culture plates; next, the
cells were treated with variable concentrations of cordycepin for
24 h. Following treatment, the cell viability was determined by MTT
assays. Data are the mean ± SD of three different experiments. The
significance was determined by a Student’s t-test
(*p<0.05, compared with control). (B) The cellular
(upper panels) and nuclear (lower panels) morphological changes of
PC-3 cells incubated with or without cordycepin for 24 h were
examined under an inverted microscope (magnification, ×200) and a
fluorescence microscope (×400), respectively. For DAPI staining
(lower panels), the cells were fixed and stained with DAPI solution
for 10 min at room temperature. (C) To quantify the degree of
apoptosis induced by cordycepin, cells grown under the same
conditions as (A) were evaluated by a flow cytometer for sub-G1 DNA
content, which represents the cells undergoing apoptotic DNA
degradation. Data are the mean ± SD of three different experiments.
The significance was determined by the Student’s t-test
(*p<0.05, compared with control). (D) To analyze the
DNA fragmentation, the cells were treated with the indicated
concentrations of cordycepin for 24 h; DNA was extracted, resolved
in 1.5% agarose gel and then visualized using EtBr. The results
presented here are from one representative experiment of three
performed that showed similar patterns. |
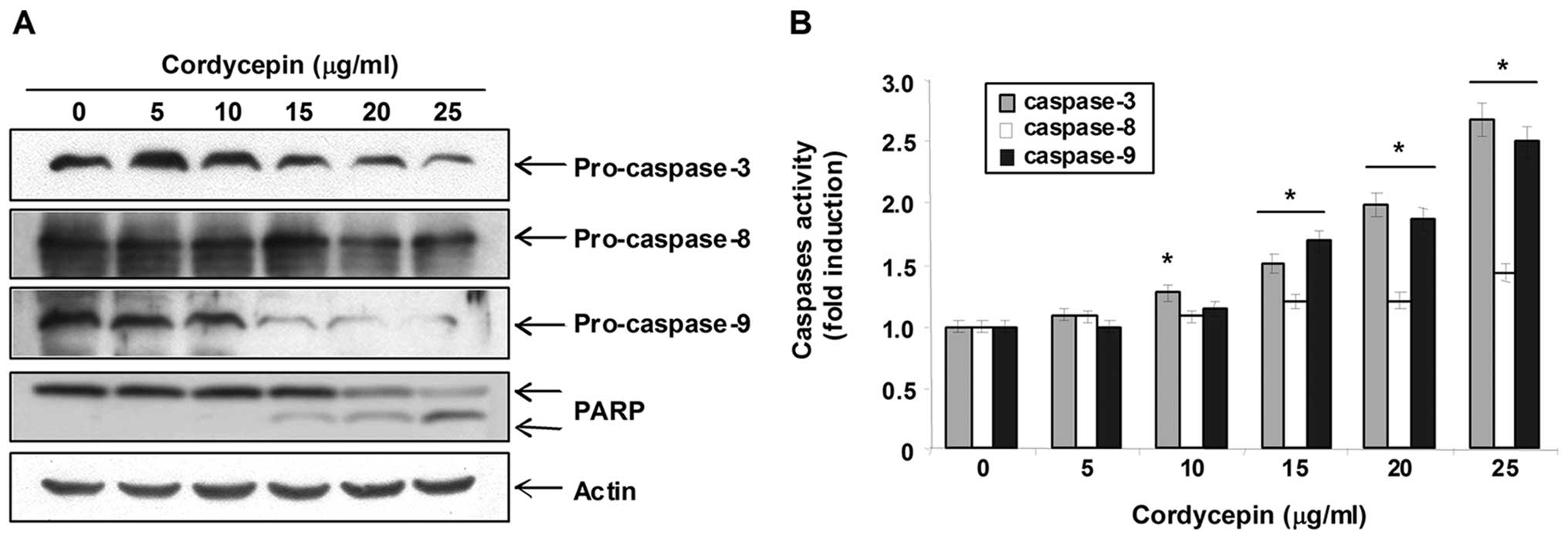
Activation of caspase-3 and -9 by
cordycepin in PC-3 cells
Caspases are a family of cysteine proteases that
play essential roles as important mediators in apoptosis and as
determinants of the general apoptotic morphology through the
cleavage of various cellular substrates including PARP, an
endogenous substrate of activated caspase-3 (28). Caspase-3, a pivotal mediator of
apoptosis in mammalian cells, can be activated by upstream
initiator caspases such as caspase-8 or -9 through two distinct
pathways (i.e., the death receptor-mediated extrinsic caspase-8
pathway or the mitochondria dependent-cytochrome c/caspase-9
intrinsic pathway, respectively) (8,9).
Therefore, we investigated whether cordycepin induces the
activation of caspases and cleavage of PARP in PC-3 cells. As shown
in Fig. 3A, western blot analyses
showed that cordycepin concentration-dependently induced a marked
decrease of pro-caspase-3 and -9, and cleavage of PARP. In
addition, to quantify the proteolytic activation of the caspases,
we evaluated in vitro caspase activities using fluorogenic
substrates. As shown in Fig. 3B,
treatment with cordycepin significantly increased the activities of
caspase-3 and -9 compared with control cells, though it did not
affect that of caspase-8, suggesting a likely involvement of
mitochondria-dependent cascade for caspase activation.
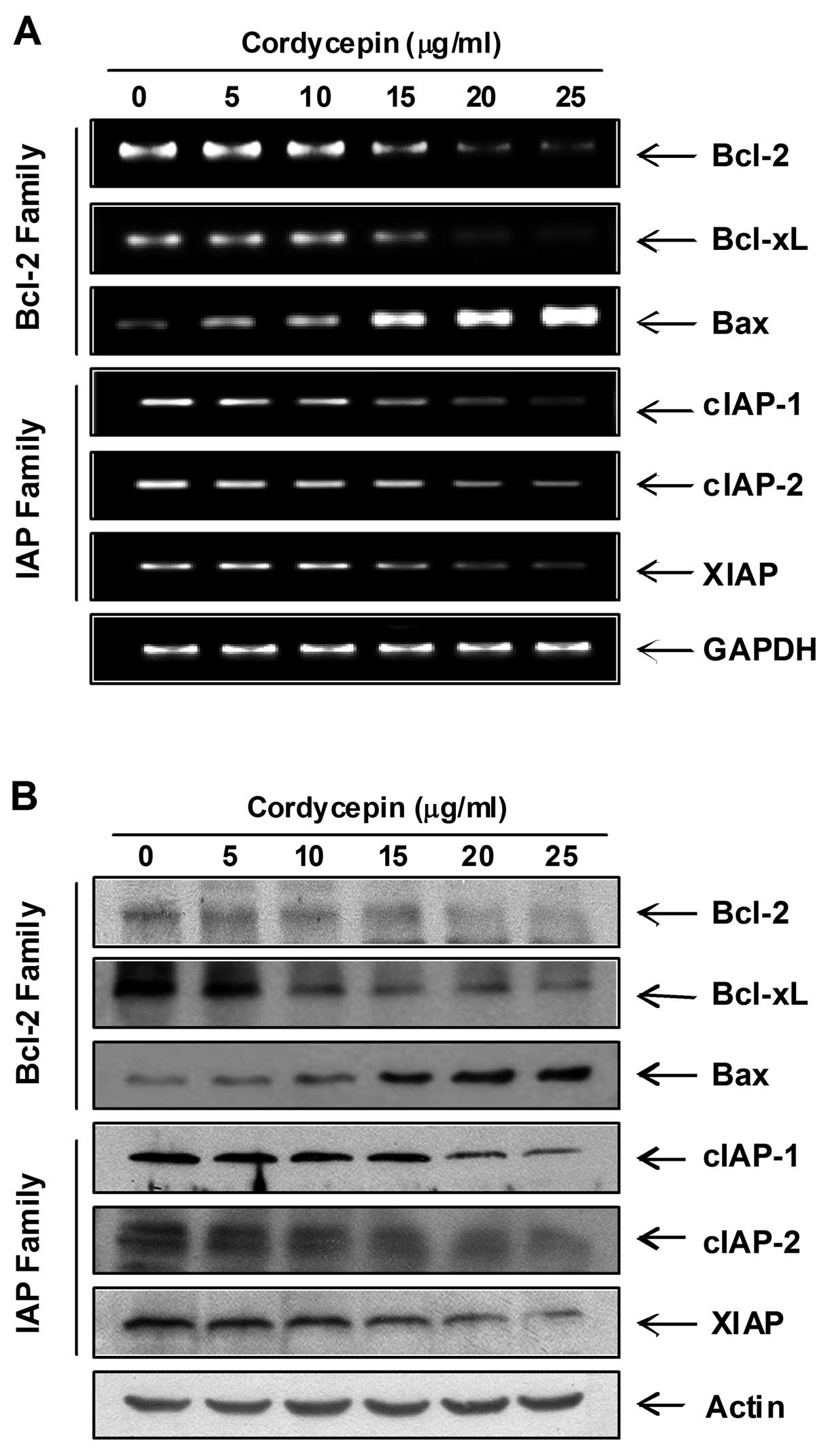
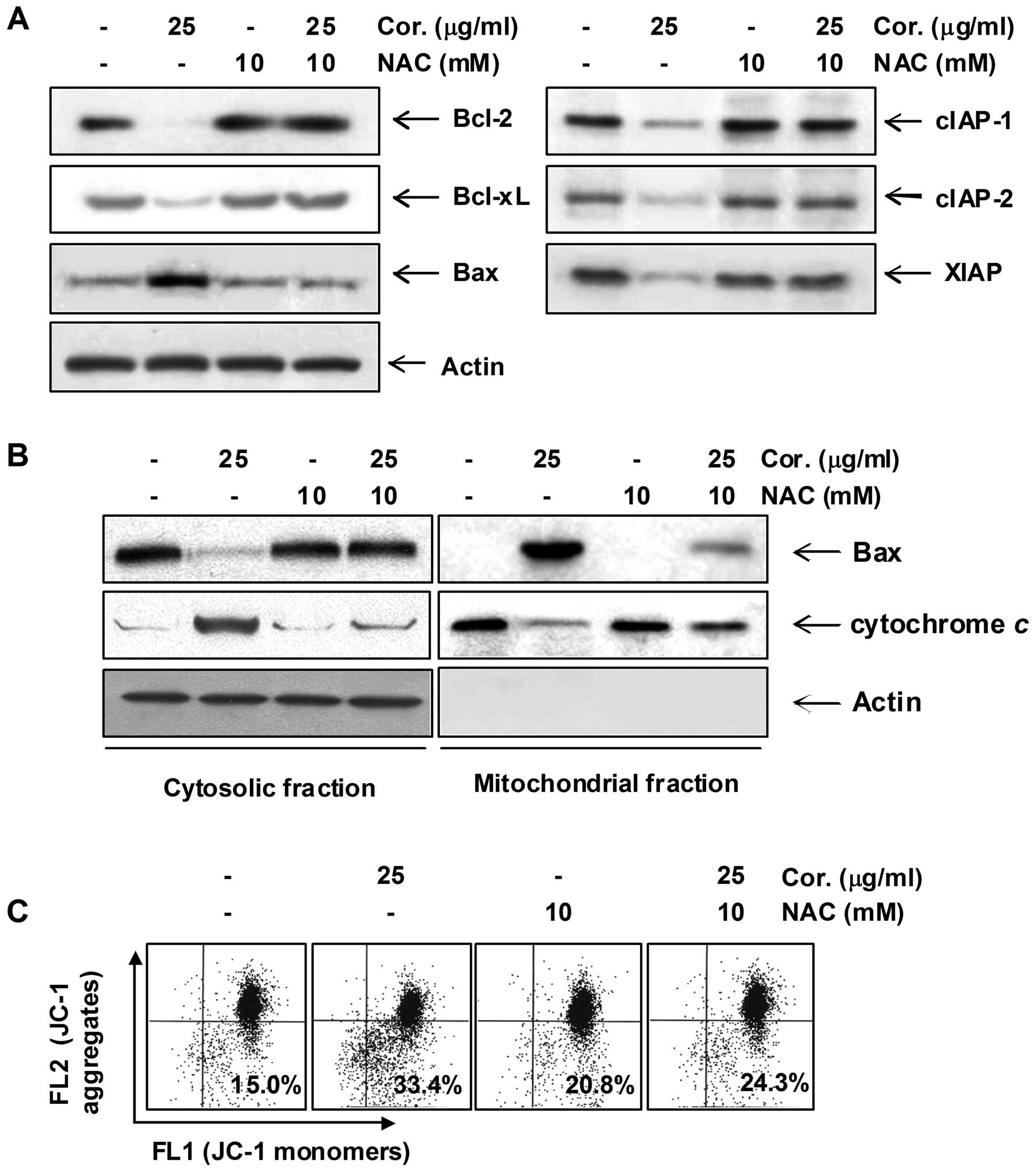
Modulation of Bcl-2 and IAP family and
dysfunction of mitochondria by cordycepin in PC-3 cells
Next, we examined the effect of cordycepin on the
expression of Bcl-2 and IAP family members, which have been
reported to play an important role in regulating apoptosis. RT-PCR
and western blot analysis data showed that cordycepin
concentration-dependently induced the expression of pro-apoptotic
Bax mRNA and protein, whereas those levels of anti-apoptotic Bcl-2
and Bcl-xL were decreased in response to cordycepin treatment. The
levels of IAP family members such as XIAP, cIAP-1 and cIAP-2 were
markedly inhibited by cordycepin treatment in a dose-dependent
manner (Fig. 4). In order to
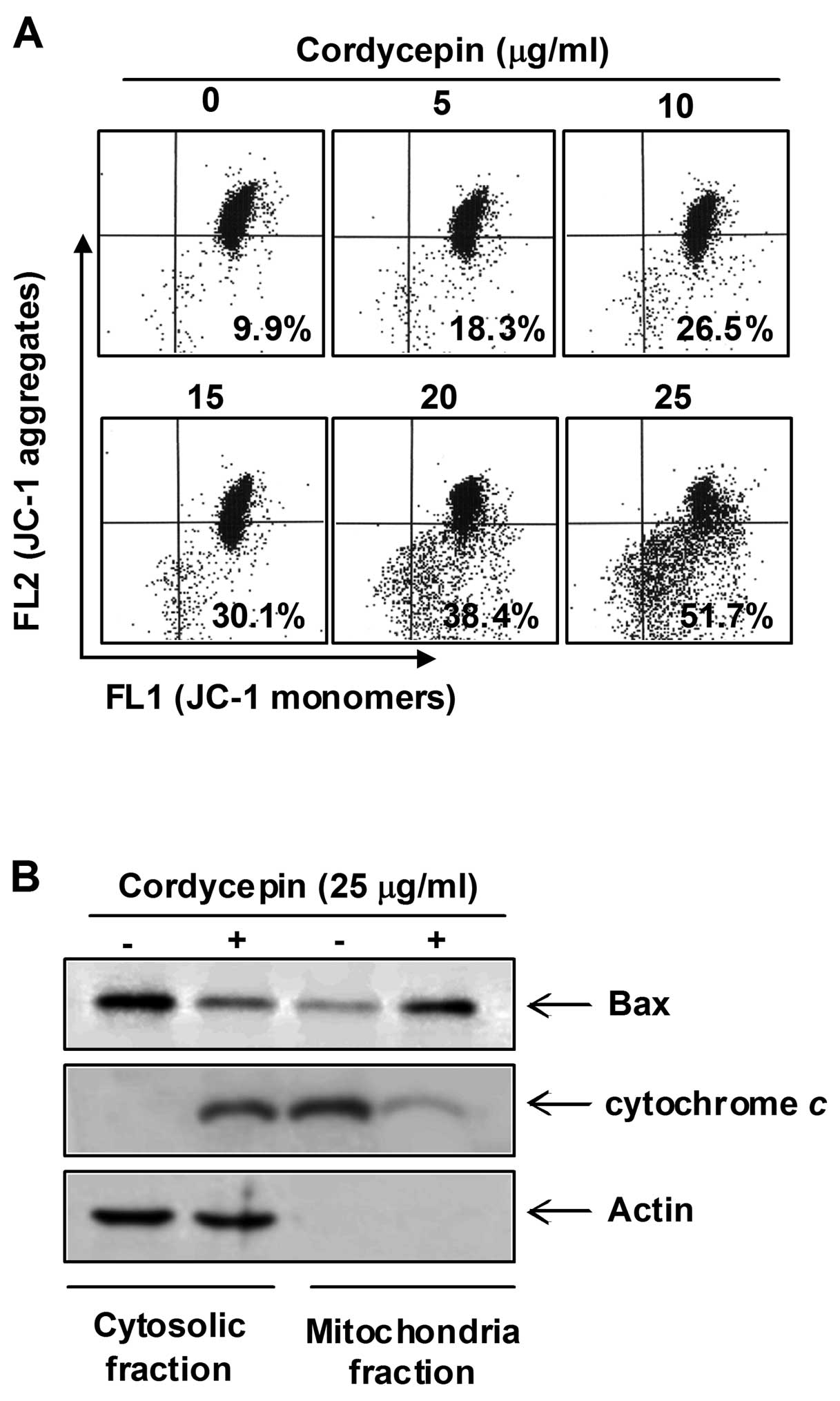
assess the role of the mitochondria in cordycepin-induced apoptosis
of PC-3 cells, we investigated the effects of cordycepin on the
levels of cytosolic and mitochondrial Bax and cytochrome c
as well as the MMP values.
As shown in Fig.
5A, treatment with cordycepin concentration-dependently caused
a significant reduction in the MMP value in PC-3 cells.
Furthermore, exposure of cells to cordycepin led to a significant
increase in the release of the mitochondrial pro-apoptotic protein
cytochrome c to the cytosol and a decreased Bax level of
cytosol (Fig. 5B). In contrast,
treatment with cordycepin induced a significant decrease in
mitochondrial cytochrome c and an increase of Bax protein
into the mitochondria, indicating a direct role of the mitochondria
in cordycepin-induced apoptosis of PC-3 cells.
Involvement ROS generation in
cordycepin-induced apoptosis in PC-3 cells
Many reports have suggested that the mitochondrial
apoptotic pathway is an important downstream signal of ROS in
apoptotic cell death. High levels of ROS can induce apoptosis by
triggering mitochondrial permeability transition pore opening,
release of pro-apoptotic factors and activation of caspase-9 and -3
(12,13). Thus, to examine whether the ROS
accumulation is involved in cordycepin-induced apoptosis,
intracellular ROS levels were examined using DCFH-DA and flow
cytometry. As shown in Fig. 6A,
treatment of PC-3 cells with cordycepin resulted in a significant
elevation of intracellular ROS, compared with the vehicle control.
In a parallel experiment, pre-treatment of the ROS scavenger NAC,
along with cordycepin, significantly reduced ROS generation as
compared to the cordycepin-treated group, whereas treatment with
NAC alone did not alter ROS level in comparison to control.
Furthermore, blocking of the generation of ROS by pre-treatment of
the cells with NAC prevented the cordycepin-induced increased
accumulation of sub-G1 population, activation of caspases (-3 and
-9), and proteolytic cleavage of PARP (Fig. 6B–D). In addition, NAC blocked
modulation of Bcl-2 and IAP family proteins, loss of MMP, and
translocation of cytochrome c and Bax (Fig. 7). Taken together, the above
findings suggest that cordycepin induces apoptosis via ROS
generation-dependent mechanisms in PC-3 cells.
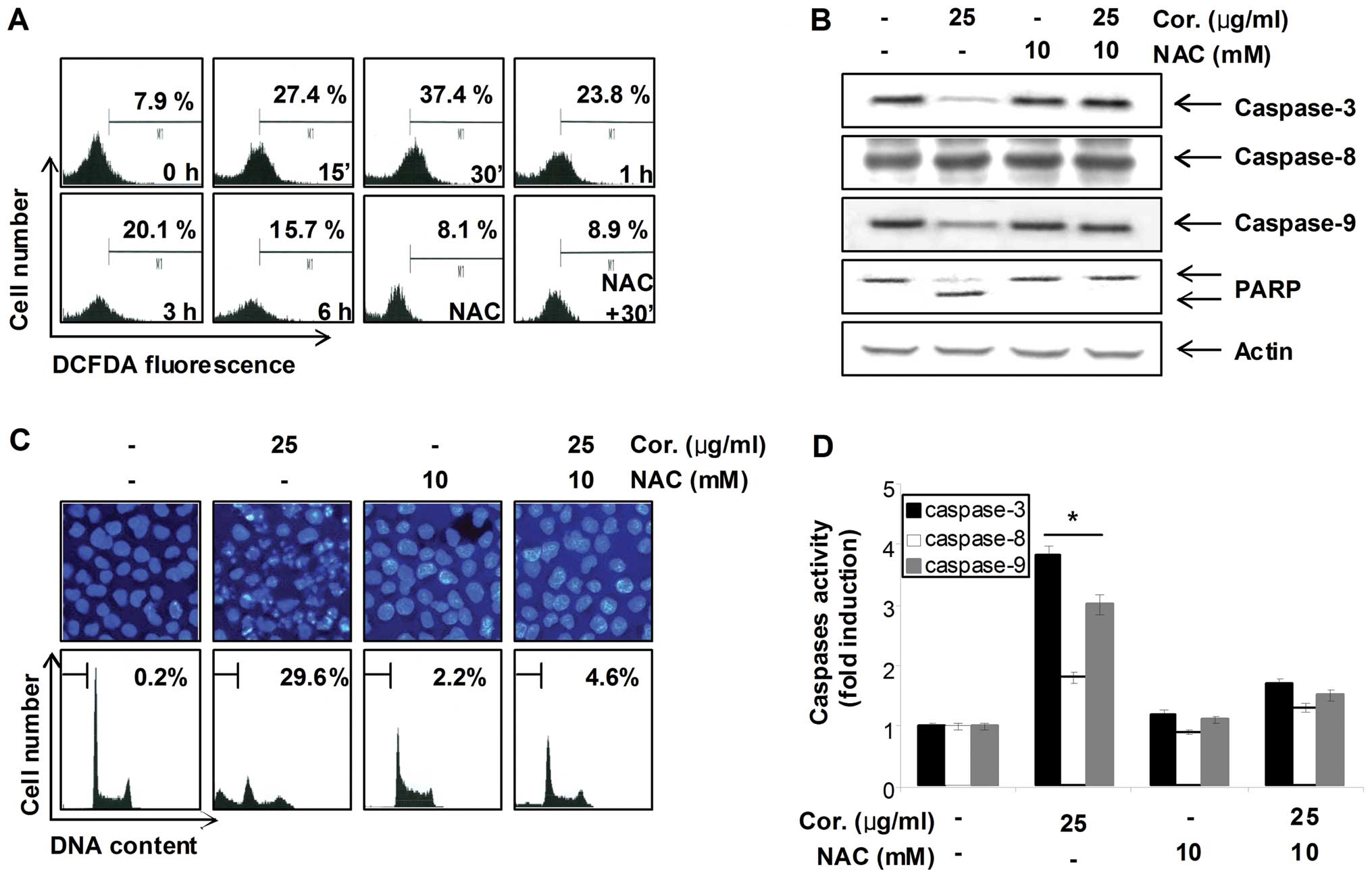 | Figure 6Cordycepin-induced apoptosis is
associated with ROS generation in PC-3 cells. (A) Cells were
treated with or without NAC (10 mM) for 1 h before being challenged
with 30 μg/ml of cordycepin for the indicated times. ROS
generation was measured by a flow cytometer. (B) Cells were treated
with or without NAC for 1 h before being challenged with 30
μg/ml of cordycepin for 30 min. Equal amounts of cell
lysates (40 μg) were then resolved by SDS-polyacrylamide
gels, transferred to nitrocellulose membranes, and probed with
antibodies against caspase-3, -8, -9 and PARP. The proteins were
then visualized using ECL detection. Actin was used as an internal
control. (C) The nuclear morphological changes in PC-3 cells
incubated under the same conditions as (B) were analyzed via
fluorescence microscope (magnification, ×400). For DAPI staining,
the cells were fixed and stained with DAPI solution for 10 min at
room temperature (upper panels). To quantify the degree of
apoptosis induced by cordycepin, cells grown under the same
conditions as (B) were evaluated by a flow cytometer for sub-G1 DNA
content. Each point represents the average of two independent
experiments (lower panels). (D) The cell lysates obtained from
cells grown under the same conditions as (B) were assayed for in
vitro caspase-3, -8 and -9 activity using DEVD-pNA, IETD-pNA
and LEHD-pNA, respectively, as substrates. The relative fluorescent
products were measured. Data are means ± SD from representative
experiments performed at least three times. The significance was
determined by a Student’s t-test (*p<0.05, compared
with control). |
Discussion
Targeting apoptosis pathways is considered an
effective strategy for cancer chemoprevention as well as therapy.
Many chemopreventive agents have been found to modulate key
molecules or events in apoptosis-inducing signal transduction
pathways. In the present study, we evaluated the mechanisms by
which cordycepin induced apoptotic cell death in PC-3 human
prostate cancer cells through the generation of ROS. Our study
demonstrated that treatment of cordycepin mediated mitochondria
membrane dysfunction, resulting in the release of apoptotic genes,
such as cytochrome c, from mitochondria into the cytosol.
Ultimately, these results activate a caspase cascade and apoptotic
cell death. Suppression of ROS generation attenuated this
cordycepin-induced activation of caspase and subsequent
apoptosis.
The process of apoptosis is controlled by a wide
range of cellular signals, which can be divided into both extrinsic
and intrinsic pathways. Apoptosis requires caspases activity, and
caspases become active when cleaved (29,30).
Adaptor proteins facilitate the auto-cleavage of initiator caspases
(e.g., caspase-8 and -9), initiator caspases cleave effector
caspases (e.g., caspase-3), and effector caspases disrupt cell
function to elicit cell death. Two events signal adaptor-mediated
caspase cleavage: the binding of ligand to death receptors (the
death receptor pathway) and the release of cytochrome c from
mitochondria (the mitochondrial pathway) (8,9).
Death receptors activate caspase-8, whereas cytochrome c
activates caspase-9. Caspase-3 is common to both pathways. When
apoptosis occurs, many proteins modulate apoptotic signaling,
including the IAPs and the Bcl-2 proteins. IAPs inactivate cleaved
caspases; thus, they impede the apoptotic process once it has
begun. Caspases targeted by IAPs include caspase-9 and -3 but not
caspase-8 (31). The Bcl-2
proteins damage or protect mitochondria; types of Bcl-2 proteins
are: multidomain apoptotic (Bax and Bak), single domain apoptotic
(termed BH3-only), and anti-apoptotic (Bcl-2 and Bcl-xL) (32). When receiving an apoptosis signal,
Bax and Bak perforate mitochondrial membranes to release cytochrome
c. BH3-only proteins facilitate activation of Bax and Bak,
whereas anti-apoptotic Bcl-2 proteins oppose activation. Bak is
constitutively mitochondrial, whereas Bax translocates from the
cytosol to the mitochondria in response to stress (33,34).
Our results showed that cordycepin induced apoptosis in PC-3 cells,
and this apoptosis was associated with increased activity of
intrinsic caspase cascades, such as caspase-9 (Fig. 3). Treatment of cordycepin also
reduced the expression of IAP family proteins, such as XIAP, cIAP-1
and cIAP-2, and the anti-apoptotic Bcl-2 and Bcl-xL, whereas the
expression of pro-apoptotic Bax was markedly raised in PC-3 cells
(Fig. 4). Additionally, cordycepin
mediated the loss of MMP and release of cytochrome c to
cytosol, consistent with mitochondria-dependent apoptosis (Fig. 5), which was connected with the
activation of caspase-9 and -3, and the concomitant degradation of
PARP (Fig. 2). Therefore, the
apoptotic effects of cordycepin on PC-3 cells appeared to involve
activation of the mitochondrial pathways.
Additionally, ROS are known to mediate other
intracellular signaling cascades, such as mitochondrial apoptosis
(35). Oxidative stress is
generally considered an important regulator of apoptosis (36). Many studies have suggested that a
disproportionate production of ROS in mitochondria leads to
oxidative stress and dysfunction of cell organelles like the
mitochondria. This is associated with the release of mitochondrial
factors, triggering caspase cascade and eventually apoptosis or
necrosis (12,13). We found a significant
overproduction of ROS in cordycepin-treated PC-3 cells; however,
treatment of cordycepin with NAC, a commonly used ROS scavenger,
effectively blocked this ROS generation and almost completely
suppressed the cordycepin-induced activation of caspases,
degradation of PARP, decrease of sub-G1 population, modulation of
Bcl-2 as well as IAP family proteins, loss of MMP and release of
cytochrome c from mitochondria to cytosol (Figs. 6 and 7). Because ROS have the potential to
induce the collapse of the MMP, and consequently trigger the series
of events leading to the mitochondria-associated apoptotic pathway
(36,37), our findings suggest the involvement
of ROS and mitochondrial dysfunction in cordycepin-induced
caspase-mediated apoptosis in PC-3 cells.
In conclusion, the present study demonstrated that
cordycepin could significantly induce apoptosis in human prostate
PC-3 cells through a mitochondria-mediated caspase-dependent
pathway. The positive correlation between the overproduction of ROS
and mitochondrial dysfunction, together with the protective effect
of NAC on the cordycepin-induced apoptosis, suggest that ROS
generation may play a key role in the apoptotic process induced by
cordycepin. Taken together, these results suggest that cordycepin
may be a potential chemotherapeutic agent for the treatment of
prostate cancer patients.
Acknowledgements
This research was supported by
Technology Development Program for Agriculture and Forestry
(610003-03-1-SU000), Ministry for Food, Agriculture, Forestry and
Fisheries, and Basic Science Research Program through the National
Research Foundation of Korea (NRF) grant funded by the Korea
government (no. 2012046358).
References
|
1
|
Jemal A, Ward E and Thun M: Declining
death rates reflect progress against cancer. PLoS One.
5:e9584–e9591. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sciarra A, Salciccia S and Panebianco V:
Proton spectroscopic and dynamic contrast-enhanced magnetic
resonance: a modern approach in prostate cancer imaging. Eur Urol.
54:485–488. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Crawford ED: Epidemiology of prostate
cancer. Urology. 62:3–12. 2003. View Article : Google Scholar
|
|
4
|
Zhang L, Yang BX, Zhang HT, Wang JG, Wang
HL and Zhao XJ: Prostate cancer: an emerging threat to the health
of aging men in Asia. Asian J Androl. 13:574–578. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xia SJ, Cui D and Jiang Q: An overview of
prostate diseases and their characteristics specific to Asian men.
Asian J Androl. 14:458–464. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Neto CC, Amoroso JW and Liberty AM:
Anticancer activities of cranberry phytochemicals: an update. Mol
Nutr Food Res. 52:S18–S27. 2008.PubMed/NCBI
|
|
7
|
Kaur M, Pop M, Shi D, Brignone C and
Grossman SR: hHR23B is required for genotoxic-specific activation
of p53 and apoptosis. Oncogene. 26:1231–1237. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Igney FH and Krammer PH: Immune escape of
tumors: apoptosis resistance and tumor counterattack. J Leukoc
Biol. 71:907–920. 2002.PubMed/NCBI
|
|
9
|
Hu W and Kavanagh JJ: Anticancer therapy
targeting the apoptotic pathway. Lancet Oncol. 4:721–729. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pathak N and Khandelwal S: Role of
oxidative stress and apoptosis in cadmium induced thymic atrophy
and splenomegaly in mice. Toxicol Lett. 169:95–108. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chatterjee S, Kundu S, Bhattacharyya A,
Hartinger CG and Dyson PJ: The ruthenium(II)-arene compound RAPTA-C
induces apoptosis in EAC cells through mitochondrial and p53-JNK
pathways. J Biol Inorg Chem. 13:1149–1155. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huppertz B, Kadyrov M and Kingdom JC:
Apoptosis and its role in the trophoblast. Am J Obstet Gynecol.
195:29–39. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou H, Liu X, Liu L, Yang Z, Zhang S,
Tang M, Tang Y, Dong Q and Hu R: Oxidative stress and apoptosis of
human brain microvascular endothelial cells induced by free fatty
acids. J Int Med Res. 37:1897–1903. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang MF, Liao YF, Hung YC, Lin CL, Hour
TC, Lue KH, Hung HC and Liu GY: Hydroxydibenzoylmethane induces
apoptosis through repressing ornithine decarboxylase in human
promyelocytic leukemia HL-60 cells. Exp Mol Med. 43:189–196. 2011.
View Article : Google Scholar
|
|
15
|
Cunningham KG, Manson W, Spring FS and
Hutchinson SA: Cordycepin, a metabolic product isolated from
cultures of Cordyceps militaris. Nature. 166:9491950.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Paterson RR: Cordyceps: a traditional
Chinese medicine and another fungal therapeutic biofactory?
Phytochemistry. 69:1469–1495. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Horowitz B, Goldfinger BA and Marmur J:
Effect of cordycepin triphosphate on the nuclear DNA-dependent RNA
polymerases and poly(A) polymerase from the yeast, Saccharomyces
cerevisiae. Arch Biochem Biophys. 172:143–148. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Müller WE, Seibert G, Beyer R, Breter HJ,
Maidhof A and Zahn RK: Effect of cordycepin on nucleic acid
metabolism in L5178Y cells and on nucleic acid-synthesizing enzyme
systems. Cancer Res. 37:3824–3833. 1977.
|
|
19
|
Foss FM: Combination therapy with purine
nucleoside analogs. Oncology. 14:31–35. 2000.PubMed/NCBI
|
|
20
|
Nakamura K, Yoshikawa N, Yamaguchi Y,
Kagota S, Shinozuka K and Kunitomo M: Antitumor effect of
cordycepin (3′-deoxyadenosine) on mouse melanoma and lung carcinoma
cells involves adenosine A3 receptor stimulation. Anticancer Res.
26:43–47. 2006.
|
|
21
|
Sugar AM and McCaffrey RP: Antifungal
activity of 3′-deoxyadenosine (cordycepin). Antimicrob Agents
Chemother. 42:1424–1427. 1998.
|
|
22
|
Ahn YJ, Park SJ, Lee SG, Shin SC and Choi
DH: Cordycepin: selective growth inhibitor derived from liquid
culture of Cordyceps militaris against Clostridium
spp. J Agric Food Chem. 48:2744–2748. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim HG, Shrestha B, Lim SY, Yoon DH, Chang
WC, Shin DJ, Han SK, Park SM, Park JH, Park HI, Sung JM, Jang Y,
Chung N, Hwang KC and Kim TW: Cordycepin inhibits
lipopolysaccharide-induced inflammation by the suppression of
NF-kappaB through Akt and p38 inhibition in RAW 264.7 macrophage
cells. Eur J Pharmacol. 545:192–199. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jeong JW, Jin CY, Kim GY, Lee JD, Park C,
Kim GD, Kim WJ, Jung WK, Seo SK, Choi IW and Choi YH:
Anti-inflammatory effects of cordycepin via suppression of
inflammatory mediators in BV2 microglial cells. Int
Immunopharmacol. 10:1580–1586. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou X, Gong Z, Su Y, Lin J and Tang K:
Cordyceps fungi: natural products, pharmacological functions
and developmental products. J Pharm Pharmacol. 61:279–291. 2009.
View Article : Google Scholar
|
|
26
|
Lee K, Lee MH, Kang YW, Rhee KJ, Kim TU
and Kim YS: Parkin induces apoptotic cell death in TNF-α-treated
cervical cancer cells. BMB Rep. 45:526–531. 2012.PubMed/NCBI
|
|
27
|
Kim IH, Kim SW, Kim SH, Lee SO, Lee ST,
Kim DG, Lee MJ and Park WH: Parthenolide-induced apoptosis of
hepatic stellate cells and anti-fibrotic effects in an in
vivo rat model. Exp Mol Med. 44:448–456. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang X, Chen S, Ma G, Ye M and Lu G:
Involvement of proinflammatory factors, apoptosis, caspase-3
activation and Ca2+ disturbance in microglia
activation-mediated dopaminergic cell degeneration. Mech Ageing
Dev. 126:1241–1254. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chang HY and Yang X: Proteases for cell
suicide: functions and regulation of caspases. Microbiol Mol Biol
Rev. 64:821–846. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jin Z and El-Deiry WS: Overview of cell
death signaling pathways. Cancer Biol Ther. 4:139–163.
2005.PubMed/NCBI
|
|
31
|
Deveraux QL, Roy N, Stennicke HR, Van
Arsdale T, Zhou Q, Srinivasula SM, Alnemri ES, Salvesen GS and Reed
JC: IAPs block apoptotic events induced by caspase-8 and cytochrome
c by direct inhibition of distinct caspases. EMBO J. 17:2215–2223.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Borner C: The Bcl-2 protein family:
sensors and checkpoints for life-or-death decisions. Mol Immunol.
39:615–647. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Griffiths GJ, Dubrez L, Morgan CP, Jones
NA, Whitehouse J, Corfe BM, Dive C and Hickman JA: Cell
damage-induced conformational changes of the pro-apoptotic protein
Bak in vivo precede the onset of apoptosis. J Cell Biol.
144:903–914. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wolter KG, Hsu YT, Smith CL, Nechushtan A,
Xi XG and Youle RJ: Movement of Bax from the cytosol to
mitochondria during apoptosis. J Cell Biol. 139:1281–1292. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bruce-Keller AJ, Begley JG, Fu W,
Butterfield DA, Bredesen DE, Hutchins JB, Hensley K and Mattson MP:
Bcl-2 protects isolated plasma and mitochondrial membranes against
lipid peroxidation induced by hydrogen peroxide and amyloid
beta-peptide. J Neurochem. 70:31–39. 1998. View Article : Google Scholar
|
|
36
|
Fiers W, Beyaert R, Declercq W and
Vandenabeele P: More than one way to die: apoptosis, necrosis and
reactive oxygen damage. Oncogene. 18:7719–7730. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fleury C, Mignotte B and Vayssière JL:
Mitochondrial reactive oxygen species in cell death signaling.
Biochimie. 84:131–141. 2002. View Article : Google Scholar : PubMed/NCBI
|