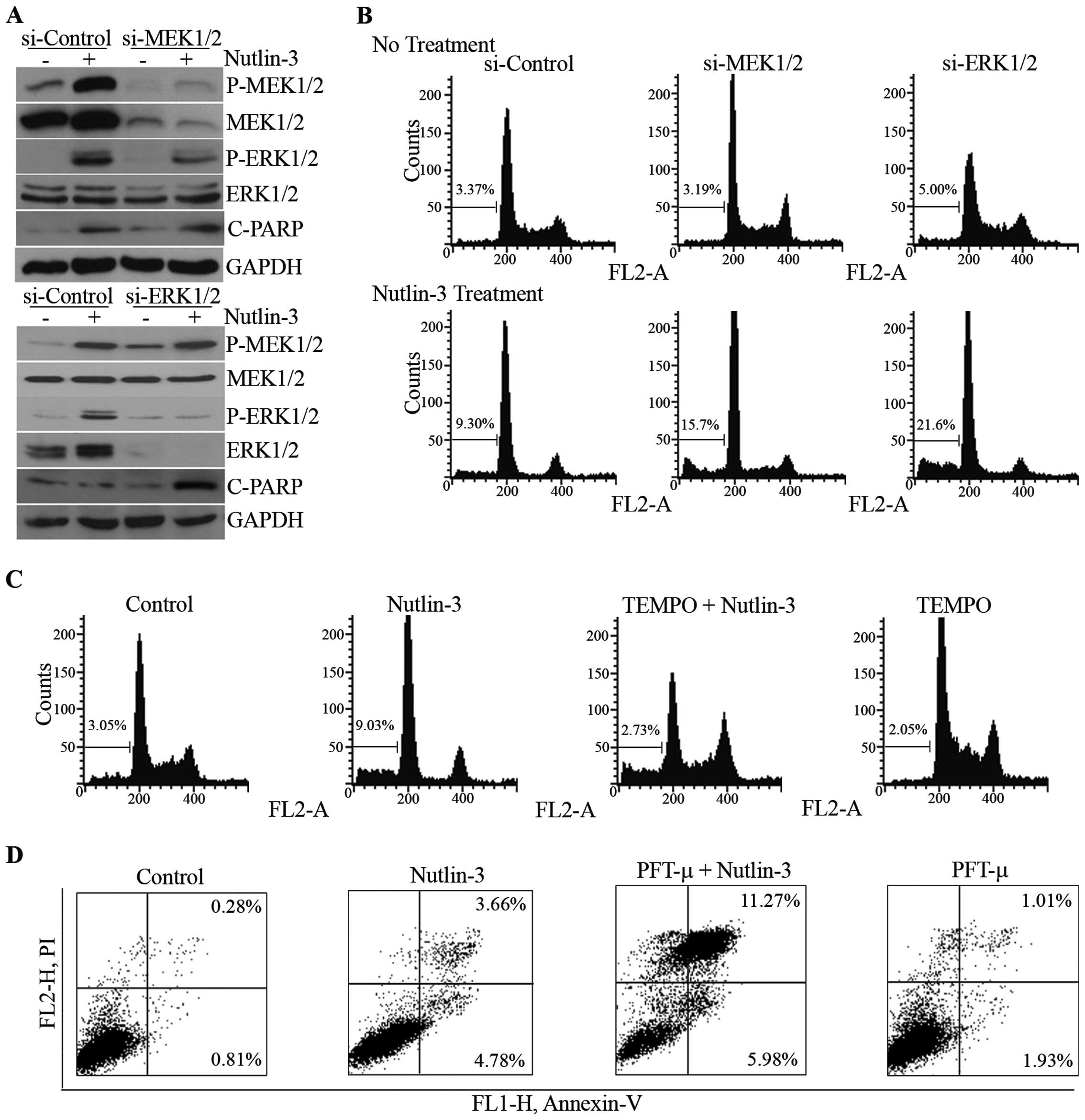
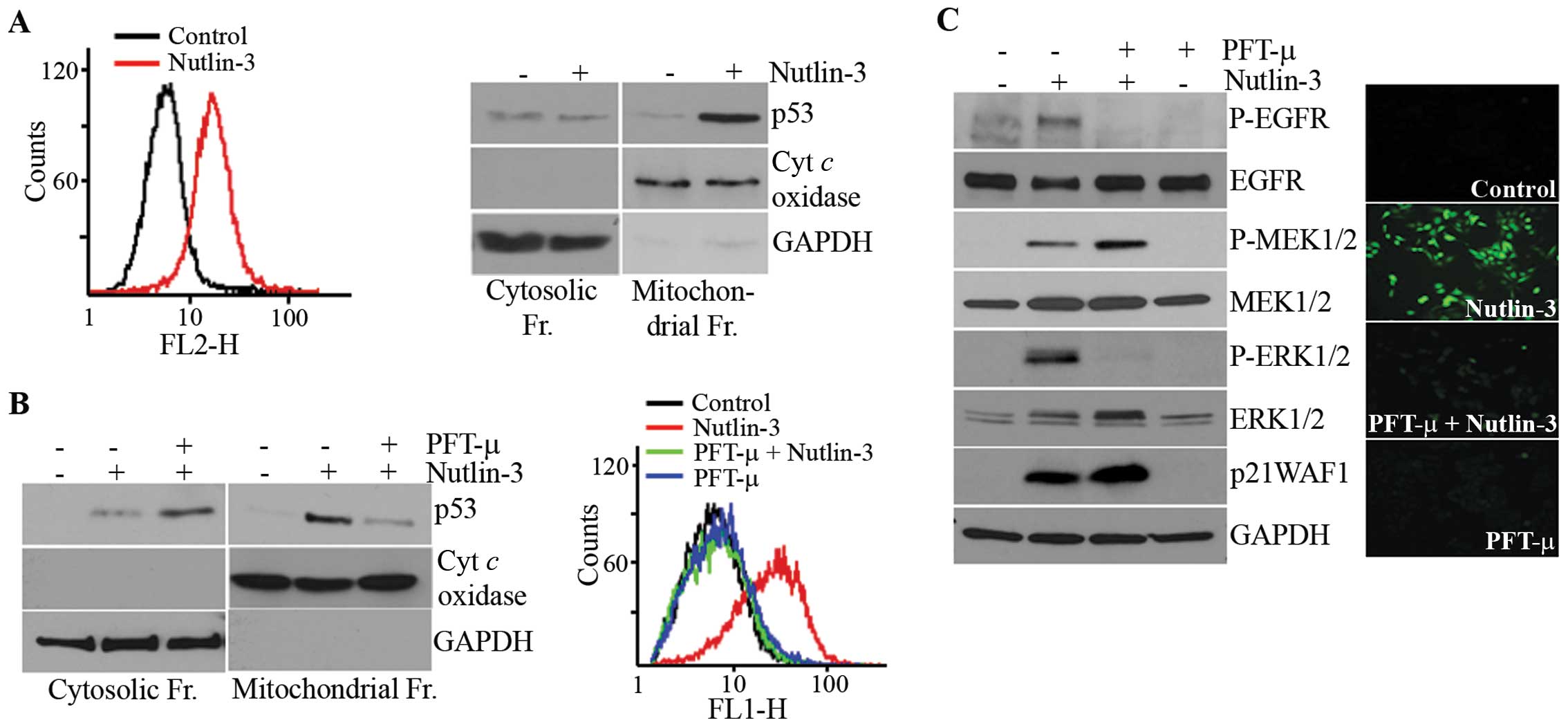
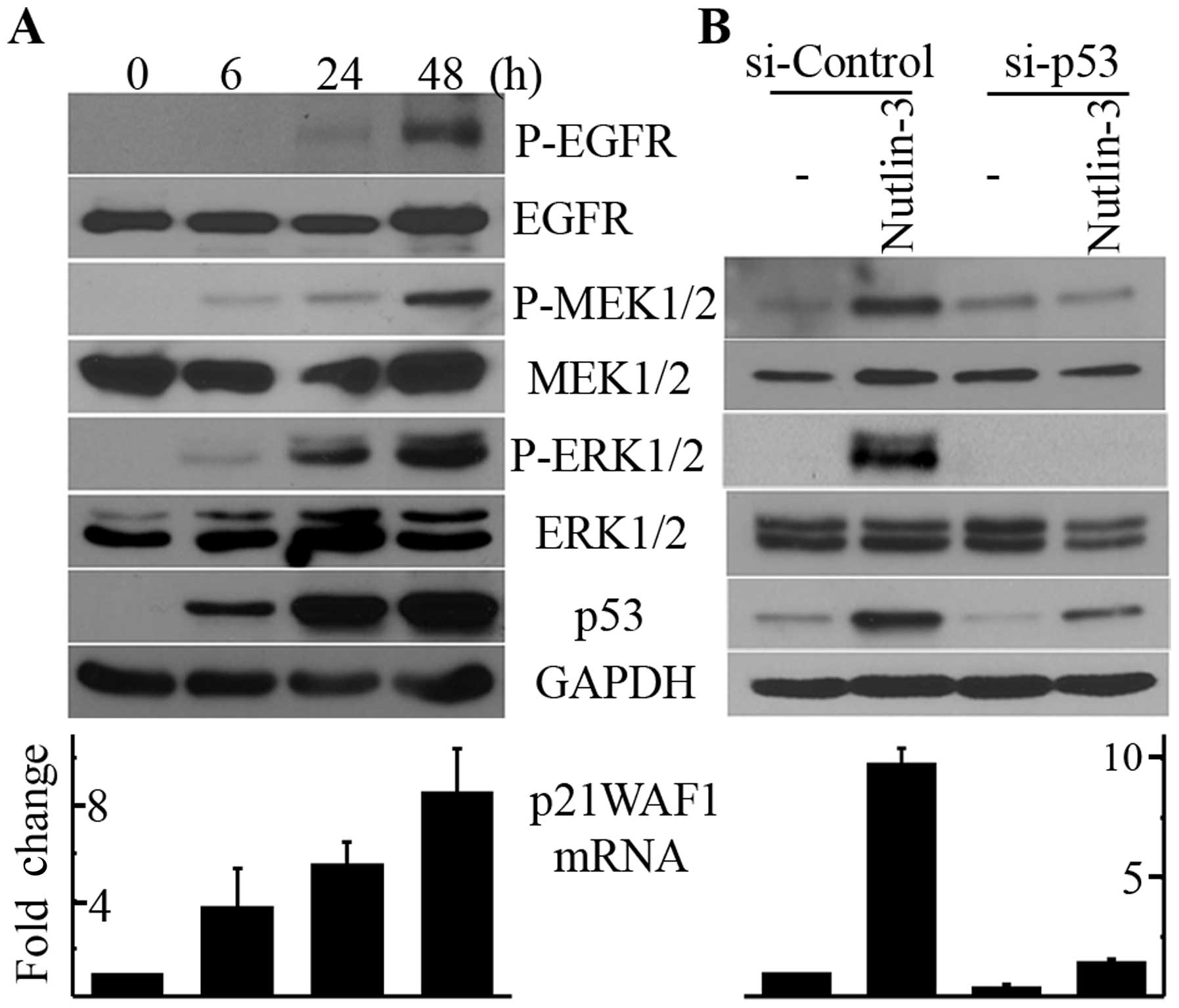
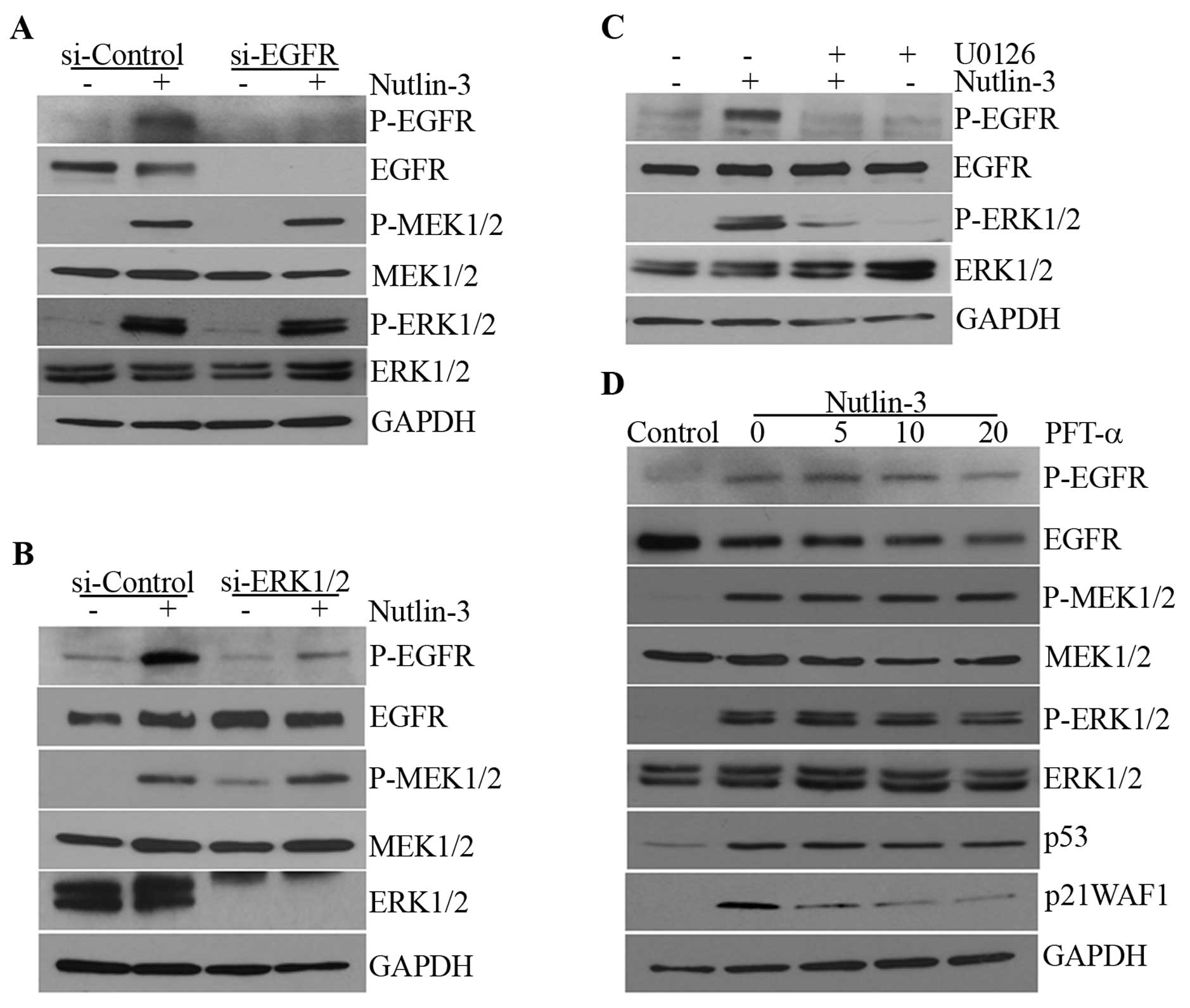
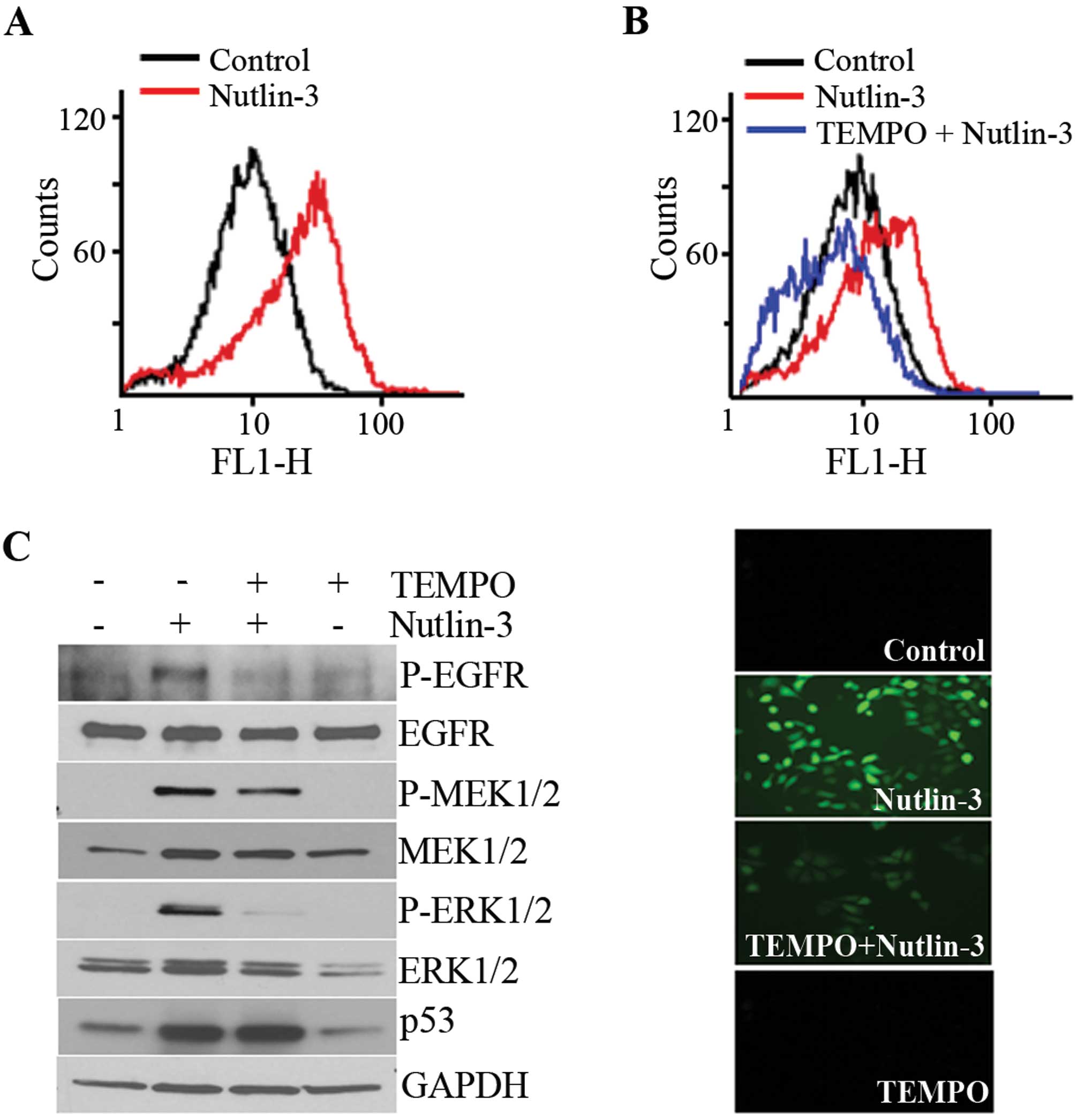
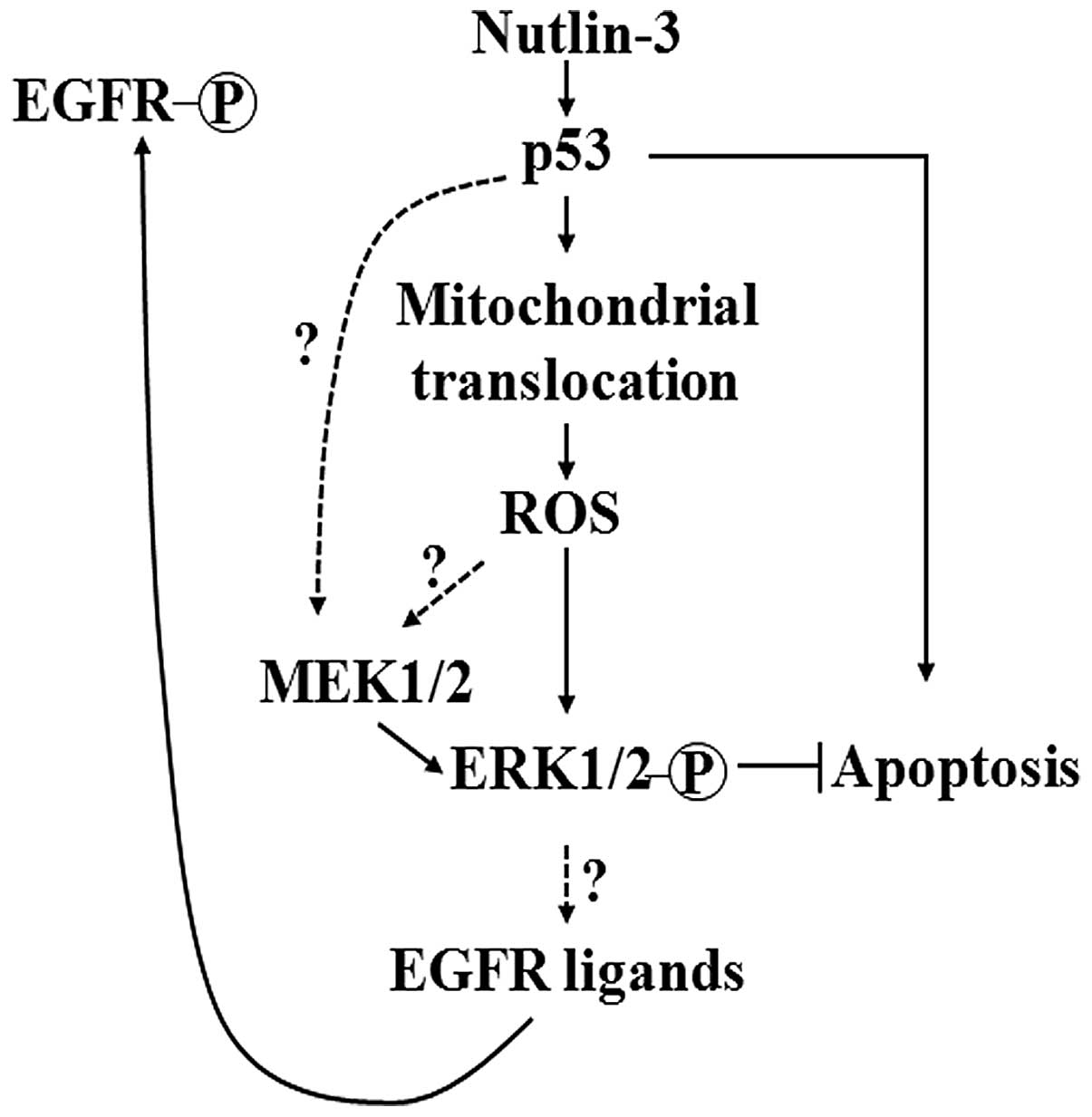
|
1
|
Menendez D, Inga A and Resnick MA: The
expanding universe of p53 targets. Nat Rev Cancer. 9:724–737. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Riley T, Sontag E, Chen P and Levine A:
Transcriptional control of human p53-regulated genes. Nat Rev Mol
Cell Biol. 9:402–412. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Honda R, Tanaka H and Yasuda H:
Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53.
FEBS Lett. 420:25–27. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oliner JD, Pietenpol JA, Thiagalingam S,
Gyuris J, Kinzler KW and Vogelstein B: Oncoprotein MDM2 conceals
the activation domain of tumor suppressor p53. Nature. 362:857–860.
1993. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reinhardt HC and Schumacher B: The p53
network: cellular and systemic DNA damage responses in aging and
cancer. Trends Genet. 28:128–136. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sill H, Olipitz W, Zebisch A, Schulz E and
Wölfler A: Therapy-related myeloid neoplasms: pathobiology and
clinical characteristics. Br J Pharmacol. 162:792–805. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Travis LB, Ng AK, Allan JM, et al: Second
malignant neoplasms and cardiovascular disease following
radiotherapy. J Natl Cancer Inst. 104:357–370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shangary S and Wang S: Targeting the
MDM2-p53 interaction for cancer therapy. Clin Cancer Res.
14:5318–5324. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dickens MP, Fitzgerald R and Fischer PM:
Small-molecule inhibitors of MDM2 as new anticancer therapeutics.
Semin Cancer Biol. 20:10–18. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vassilev LT, Vu BT, Graves B, et al: In
vivo activation of the p53 pathway by small-molecule antagonists of
MDM2. Science. 303:844–848. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Secchiero P, Bosco R, Celeghini C and
Zauli G: Recent advances in the therapeutic perspectives of
nutlin-3. Curr Pharm Des. 17:569–577. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tovar C, Rosinski J, Filipovic Z, et al:
Small-molecule MDM2 antagonists reveal aberrant p53 signaling in
cancer: implications for therapy. Proc Natl Acad Sci USA.
103:1888–1893. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mendrysa SM, O’Leary KA, McElwee MK, et
al: Tumor suppression and normal aging in mice with constitutively
high p53 activity. Genes Dev. 20:16–21. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mebratu Y and Tesfaigzi Y: How ERK1/2
activation controls cell proliferation and cell death: Is
subcellular localization the answer? Cell Cycle. 8:1168–1175. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McCubrey JA, Steelman LS, Chappell WH, et
al: Roles of the Raf/MEK/ERK pathway in cell growth, malignant
transformation and drug resistance. Biochim Biophys Acta.
1773:1263–1284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Persons DL, Yazlovitskaya EM and Pelling
JC: Effect of extracellular signal-regulated kinase on p53
accumulation in response to cisplatin. J Biol Chem.
275:35778–35785. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sablina AA, Chumakov PM, Levine AJ and
Kopnin BP: p53 activation in response to microtubule disruption is
mediated by integrin-Erk signaling. Oncogene. 20:899–909. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
She QB, Bode AM, Ma WY, Chen NY and Dong
Z: Resveratrol-induced activation of p53 and apoptosis is mediated
by extracellular-signal-regulated protein kinases and p38 kinase.
Cancer Res. 61:1604–1610. 2001.PubMed/NCBI
|
|
19
|
Lin T, Mak NK and Yang MS: MAPK regulate
p53-dependent cell death induced by benzo[a]pyrene: involvement of
p53 phosphorylation and acetylation. Toxicology. 247:145–153.
2008.PubMed/NCBI
|
|
20
|
Lee SW, Fang L, Igarashi M, Ouchi T, Lu KP
and Aaronson SA: Sustained activation of Ras/Raf/mitogen-activated
protein kinase cascade by the tumor suppressor p53. Proc Natl Acad
Sci USA. 97:8302–8305. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fang L, Li G, Liu G, Lee SW and Aaronson
SA: p53 induction of heparin-binding EGF-like growth factor
counteracts p53 growth suppression through activation of MAPK and
PI3K/Akt signaling cascades. EMBO J. 20:1931–1939. 2001. View Article : Google Scholar
|
|
22
|
Ongusaha PP, Kim JI, Fang L, et al: p53
induction and activation of DDR1 kinase counteracts p53-mediated
apoptosis and influence p53 regulation through a positive feedback
loop. EMBO J. 22:1289–1301. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yin Y, Liu YX, Jin YJ, Hall EJ and Barrett
JC: PAC1 phosphatase is a transcription target of p53 in signaling
apoptosis and growth suppression. Nature. 422:527–531. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ueda K, Arakawa H and Nakamura Y:
Dual-specificity phosphatase 5 (DUSP5) as a direct transcriptional
target of tumor suppressor p53. Oncogene. 22:5586–5591. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li M, Zhou JY, Ge Y, Matherly LH and Wu
GS: The phosphatase MKP1 is a transcriptional target of p53
involved in cell cycle regulation. J Biol Chem. 278:41059–41068.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kojima K, Konopleva M, Samudio IJ, Ruvolo
V and Andreeff M: Mitogen-activated protein kinase kinase
inhibition enhances nuclear proapoptotic function of p53 in acute
myelogenous leukemia cells. Cancer Res. 67:3210–3219. 2007.
View Article : Google Scholar
|
|
27
|
Zhang W, Konopleva M, Burks JK, et al:
Blockade of mitogen-activated protein kinase/extracellular
signal-regulated kinase kinase and murine double minute
synergistically induces apoptosis in acute myeloid leukemia via
BH3-only proteins Puma and Bim. Cancer Res. 70:2424–2434. 2010.
View Article : Google Scholar
|
|
28
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jang JY, Kim MK, Jeon YK, et al:
Adenovirus adenine nucleotide translocator-2 shRNA effectively
induces apoptosis and enhances chemosensitivity by the
down-regulation of ABCG2 in breast cancer stem-like cells. Exp Mol
Med. 44:251–259. 2012. View Article : Google Scholar
|
|
30
|
Sauer L, Gitenay D, Vo C and Baron VT:
Mutant p53 initiates a feedback loop that involves Egr-1/EGF
receptor/ERK in prostate cancer cells. Oncogene. 29:2628–2637.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McCubrey JA, Lahair MM and Franklin RA:
Reactive oxygen species-induced activation of the MAP kinase
signaling pathways. Antioxid Redox Signal. 8:1775–1789. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu CM, Sun YZ, Sun JM, Ma JQ and Cheng C:
Protective role of quercetin against lead-induced inflammatory
response in rat kidney through the ROS-mediated MAPKs and NF-κB
pathway. Biochim Biophys Acta. 1820:1693–1703. 2012.PubMed/NCBI
|
|
33
|
Liu B, Chen Y and St Clair DK: ROS and
p53: a versatile partnership. Free Radic Biol Med. 44:1529–1535.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sansome C, Zaika A, Marchenko ND and Moll
UM: Hypoxia death stimulus induces translocation of p53 protein to
mitochondria. Detection by immunofluorescence on whole cells. FEBS
Lett. 488:110–115. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mihara M, Erster S, Zaika A, et al: p53
has a direct apoptogenic role at the mitochondria. Mol Cell.
11:577–590. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Palacios G and Moll UM: Mitochondrially
targeted wild-type p53 suppresses growth of mutant p53 lymphomas in
vivo. Oncogene. 25:6133–6139. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Talos F, Petrenko O, Mena P and Moll UM:
Mitochondrially targeted p53 has tumor suppressor activities in
vivo. Cancer Res. 65:9971–9981. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vaseva AV, Marchenko ND and Moll UM: The
transcription-independent mitochondrial p53 program is a major
contributor to nutlin-induced apoptosis in tumor cells. Cell Cycle.
8:1711–1719. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao Y, Chaiswing L, Velez JM, et al: p53
translocation to mitochondria precedes its nuclear translocation
and targets mitochondrial oxidative defense protein-manganese
superoxide dismutase. Cancer Res. 65:3745–3750. 2005. View Article : Google Scholar
|
|
40
|
Strom E, Sathe S, Komarov PG, et al:
Small-molecule inhibitor of p53 binding to mitochondria protects
mice from gamma radiation. Nat Chem Biol. 2:474–479. 2006.
View Article : Google Scholar : PubMed/NCBI
|