Introduction
Glioblastoma is one of the most intractable tumors
of the central nervous system and is often fatal. Complete
remission is rarely achieved. Compared to 30 years ago, the
survival time has been prolonged only by a few months (1). Although chemo-therapy, usually by
oral temozolomide, may prolong survival of glioblastoma patients,
it does not significantly affect survival. Bevacizumab, which is an
anti-VEGF antibody, also extends survival time by approximately 2
months (2); it is not, however,
curative. To date, the majority of glioblastoma patients succumb to
the disease within 1 year of initial diagnosis. The therapeutic
resistance of glioblastoma possibly results from the presence of
cancer stem cells. The cancer stem cell theory does not apply to
all types of cancer, but glioblastoma is considered a type of
cancer possessing cancer stem/initiating cells (3), which have been isolated from the U87
human glioma cell line (4). The
characteristics of glioma cancer stem cells include CD133
positivity, self-reproduction ability, neurosphere formation in
non-serum medium containing growth factors, tumorigenicity in SCID
mice, chemotherapy and radiotherapy resistance and cellular
hierarchy. Signal transducer and activator of transcription 3
(STAT3) was reported to play a critical role in the tumorigenicity
of glioma-initiating cells (5,6).
Inhibition of translation of STAT3 mRNA by STAT3 siRNA results in
inhibition of cell proliferation and self-renewal (7). In addition, the expression of
microRNA (miRNA)-21 in glioma-initiating cells is negatively
regulated by activated STAT3 (8).
It has been suggested that the JAK/STAT pathway is repressed by the
von Hippel-Lindau (VHL) protein (9) and that BC-box proteins, including
VHL, possibly inhibit the JAK/STAT pathway by binding to Elongin BC
(10). Upregulated expression of
PTEN coordinately inhibits the PI3K/Akt pathway (11) as well as VHL and PTEN function
(12). Glioma stem-like cells
(GSLCs) are suggested to be in a hypoxic environment (13). Furthermore, it was recently
reported that TRAIL and paclitaxel synergize to kill U87-derived
GSLCs in vitro(14) and
that miRNA-34a suppresses cell proliferation and induces apoptosis
in U87-derived GSLCs. In this study, we demonstrated that VHL
downregulated the tumorigenicity and self-renewal of U87-derived
GSLCs by inhibiting the JAK/STAT signaling pathway and upregulated
the expression of PTEN, which acted coordinately with VHL. We also
analyzed the role of STAT3 in GSLCs and the possibility of therapy
using VHL.
Materials and methods
Isolation of CD133+
U87-derived GSLCs
The human glioblastoma cell line U87MG was purchased
from ATCC. U87MG is derived from a human glioblastoma and is widely
used in biological studies on gliomas, particularly those examining
the pathway involved in glioma proliferation. We initially cultured
naive U87MG cells in Dulbecco’s modified Eagle’s medium (DMEM)
containing 10% fetal calf serum (FCS) (Fig. 1A) which was, according to a
previous study (4), subsequently
changed to non-serum DMEM/F12 containing B27 supplement
(Gibco-Invitrogen, Grand Island, NY, USA), basic FGF (PeproTech EC
Ltd., Rocky Hill, NJ, USA) and EGF (Upstate Biotechnology, Lake
Placid, NY, USA). CD133+ cells were then selected by
using an autoMACS™ Pro Separator (Miltenyi Biotec, Bergisch
Gladbach, Germany) and neurosphere-forming cells were cultured in
the same medium (Fig. 1B). Serial
passage of the cells was performed by a mechanical dissociation
method and passaged cells, i.e., GSLCs, were used for subsequent
experiments.
Characterization of U87-derived
GSLCs
Characterization of U87 GSLCs was carried out by
immunocytochemistry using the following antibodies: anti-CD133
(Santa Cruz Biotechnology, San Diego, CA, USA), anti-STAT3 (Cell
Signaling Technology, Danvers, MA, USA), anti-JAK2 (Santa Cruz
Biotechnology), anti-VHL (Santa Cruz Biotechnology), anti-Elongin A
(Abgent, San Diego, CA, USA), anti-PTEN (Santa Cruz Biotechnology),
anti-NeuroD (Santa Cruz Biotechnology), anti-MAP2 (Sigma-Aldrich,
St. Louis, MO, USA) and anti-GFAP antibodies (Dako, Glostrup,
Denmark), as described below. In addition, cell proliferation,
tumorigenicity in the subcutaneous tissue of SCID mice (Charles
River, Yokohama, Japan), as well as soft agar colony and
neurosphere formation were examined as described below.
Influence of VHL on U87-derived
GSLCs
We hypothesized that STAT3 was inhibited by VHL,
since most BC-box family proteins, including VHL, have the ability
to inhibit the JAK/STAT pathway. STAT3 plays a role in stem cell
maintenance. Therefore, we investigated whether VHL was able to
downregulate STAT3 in GSLCs. Initially, we constructed a
VHL-expressing adenovirus vector, as previously described (15).
VHL-expressing adenovirus vector
The VHL-expressing adenovirus vector was prepared as
previously described (15).
Adenovirus vector encoding human VHL (VHL54-213 amino acids) was
generated by the use of the cosmid vector pAxCAwt. As a control
vector, the vector for green fluorescent protein (GFP), generated
by the use of pAxCAwt, was obtained from the Riken Gene Bank
(Saitama, Japan). For adenovirus infection, U87 GSCs were seeded
into 6-cm dishes (1×106 cells/cm2) 1 day
prior to infection. The cells were then incubated for 1 h with 5 μl
of the virus solution diluted to 1×107 plaque-forming
units per milliliter in DMEM/F12 medium containing 5% FCS at a
multiplicity of infection of 10, which is a condition sufficient
for nearly 100% cell infection. VHL-expressing adenovirus vector
was transferred to U87 GSLCs following dissociation of the
neurospheres into single cells. Two days following the transfection
in dishes, some of the cultured cells were transferred onto a cover
glass and subsequently fixed for immunocytochemical study while the
remaining were used to extract protein for western blot
analysis.
Fluorescence-immunocytochemical study
using confocal laser microscopy
Naive U87MG cells attached to round cover slips were
fixed for 24 h with 10% formalin naturally deodorized buffer
solution. After irrigating twice with phosphate-buffered saline
(PBS), the fixed cells were blocked with 5% normal donkey serum for
30 min. Subsequently, primary antibodies (anti-NeuroD, anti-MAP2,
anti-STAT3, anti-VHL, anti-PTEN, anti-JAK2 and anti-GFAP) were
diluted with PBS (1:200) and incubated with the cells for 1 h at
room temperature. Following irrigation 3 times with PBS, the cells
were incubated in the dark for 45 min at room temperature with
FITC-linked anti-mouse IgG antibody (Sigma-Aldrich) or TRIC-linked
anti-rabbit IgG antibody (Sigma-Aldrich) as secondary antibodies.
Following a further 3 irrigations with PBS, the cells were
incubated for 5 min with DAPI (Molecular Probes, Eugene, OR, USA)
diluted 1:3,000 with PBS. Finally, the cover glasses bearing the
cells were placed on glass slides following application of ProLong
Gold Antifade Reagent (Life Technologies, Grand Island, NY, USA) to
the slides. The cells were then observed under an FV300 confocal
microscope (Olympus, Tokyo). Cell nuclei appeared as red
fluorescence and primary antibody-reactive antigens as green
fluorescence.
Western blot analysis
Protein was extracted from the cells with RIPA
buffer (Thermo Scientific, Rockford, IL, USA; 0.05 M Tris-HCl, pH
7.4, containing 0.15 M NaCl, 0.25% deoxycholic acid, 1% NP-40, 0.1%
SDS, 1 mM EDTA, 1 mM PMSF, 1 mM sodium orthovanadate, 1 mM sodium
fluoride, 1 μg/ml leupeptin and 1 μg/ml aprotinin). Extracted
proteins were electrophoresed on 8–15% SDS-PAGE gels and then
transferred to polyvinylidene difluoride (PVDF) membranes by using
an iBlot™ Gel Transfer Device (Life Technologies) for 7 min. The
blots were probed with primary and secondary antibodies by using a
SNAP id Protein Detection system (Merck Millipore, Darmstadt,
Germany). Immunoreactive bands were visualized by chemiluminescence
with ECL Western Blotting Detection Reagents (GE Healthcare,
Japan). Images were analyzed with LAS-1000 (Fujifilm, Tokyo, Japan)
and the density of the bands was determined by using Image Gauge
software (Fujifilm). The following primary antibodies were used:
anti-CD133 (Biorbyt, Cambridge, UK), anti-STAT3, anti-JAK2,
anti-VHL and anti-PTEN. Horseradish peroxidase (HRP)-linked
anti-rabbit IgG and HRP-linked anti-mouse IgG were used as the
secondary antibodies.
Neurosphere formation assay
The neurosphere formation assay was performed as
follows (16): neurosphere-forming
U87 GSLCs were dissociated into single cells by continuous
pipetting for 10 min. Following confirmation of their single-cell
status and dilution up to 5 cells/ml, 200 μl of the cell solution
was placed into each well of a 96-well plate (mean, one cell per
well). Subsequently, neurospheres ≥50 μm in diameter in a single
plate were counted under a phase-contrast microscope 1 week
following placement of the cells.
Soft agar colony formation assay
This assay was performed as previously described
(17). Briefly, following
dissociation of the U87 GSLC neurospheres, a single-cell suspension
(1,000 cells/ml) in 0.5 ml of 0.3% agar in a medium consisting of
10% FCS in DMEM/F12 was overlaid onto 0.5 ml of 0.6% agar medium in
the wells of 24-well plates. Following 3 weeks of cultivation in a
5% CO2 incubator, each well was examined under a
stereoscopic microscope for colonies consisting of >40
cells.
Cell-proliferation assay
Cell proliferation was examined as previously
described (18). After U87 GSLCs
had been dissociated into single cells by 10-min continuous
pipetting using a long thin pipette, the cells were transfected
with the VHL-expressing adenovirus vector or GFP-expressing
adenovirus vector as a control. They were then cultured in the U87
GSLC maintenance medium. Starting 1 day after the cultures had been
prepared, the number of cells was counted as follows: control
vector-transfected and VHL-expressing vector-transfected U87 GSLCs
were washed once with PBS, then dissociated into single cells by
10-min continuous pipetting using a long thin pipette. The number
of viable cells was counted following staining with trypan
blue.
Implantation into SCID mice
Subcutaneous implantation of control- or
VHL-transfectant U87 GSLCs (1×104 U87 GSLCs) into 4 SCID
Hairless Outbred (SHO-Prkdc Hr) mice (Charles River) for each
vector was performed. The mice were housed in a clean,
temperature-controlled room on a 12-h day/night cycle with free
access to food and water. One month following implantation, the
mice were examined for subcutaneously-formed tumors. Formed tumors
were histologically examined with hematoxylin-eosin staining and
immunohistochemically with anti-CD133, anti-GFAP and anti-STAT3
antibodies. The animal experimental procedure was approved by the
Institutional Animal Use Committee of Yokohama City University and
was in accordance with the National Institutes of Health Guidelines
for the care and use of laboratory animals.
Statistical analysis
Data were analyzed by ANOVA (SPSS II; SPSS, IBM,
Tokyo, Japan) and a P-value of <0.05 was considered to indicate
a statistically significant difference.
Results
Immunocytochemical study on naive U87MG
cells
Naive cells of the U87MG cell line were cultured as
substrate-attached cells in DMEM containing 10% FCS and the cells
were passaged every 4–5 days. The cells displayed 2 or 3 cellular
processes and were Elongin A positive, rarely CD133 positive, VHL
negative and MAP2 negative. Also, the majority of the cells were
STAT3 positive, partially JAK1 positive and PTEN negative.
U87MG-derived GSLCs
To obtain GSLCs from the U87MG cell line, we changed
the medium to non-serum DMEM containing basic FGF, EGF and B27
supplement. One month later, the cultured cells tended to float. By
using the MACS method with anti-CD133 antibody, we collected
CD133-positive fractioned cells, according to the manufacturer’s
instructions, and then cultured them in the above medium for 2–3
weeks. The cultured cells formed numerous floating neurospheres.
The neurospheres were dissociated and some of the single cells were
stained by fluorescence immunocytochemistry for characterization,
whereas the remaining were used for soft agar colony and
neurosphere formation, as well as for cell proliferation assays. In
addition, protein was extracted from neurospheres for western blot
analysis.
Fluorescence-immunocytochemical study on
U87 GSLCs
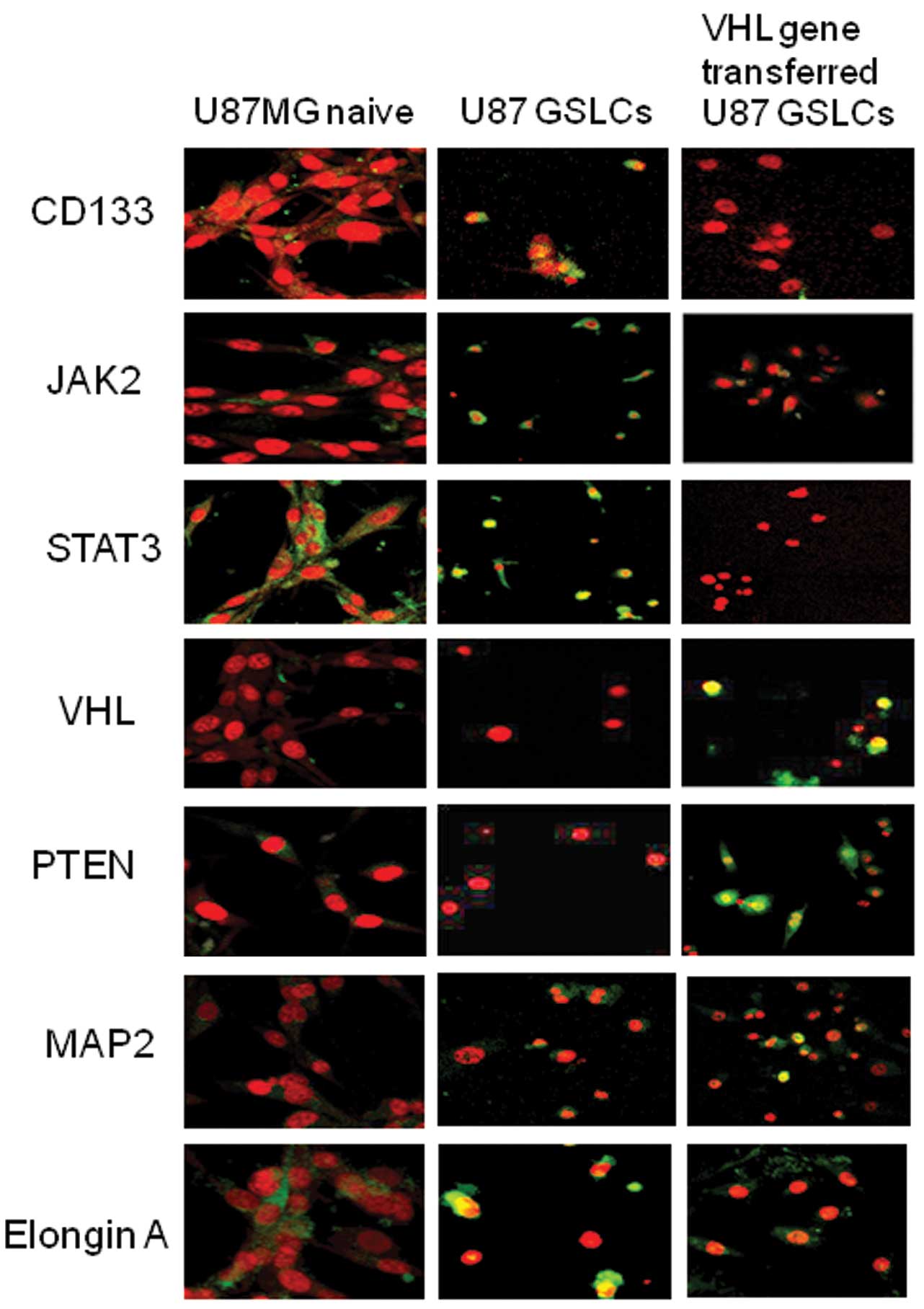
Results of the immunocytochemical analysis of U87
GSLCs are depicted in Figs. 2 and
3A. The percentage of
CD133-positive cells as well as STAT3- and JAK2-positive cells was
higher in the control-vector than in the VHL-vector transfectants.
The majority of the naive U87 cells were Elongin A positive, as
were the U87 GCLSs. U87 GSLCs were also VHL and PTEN negative,
similar to U87 naive cells. Approximately half of those cells were
NeuroD positive, of which some were GFAP positive.
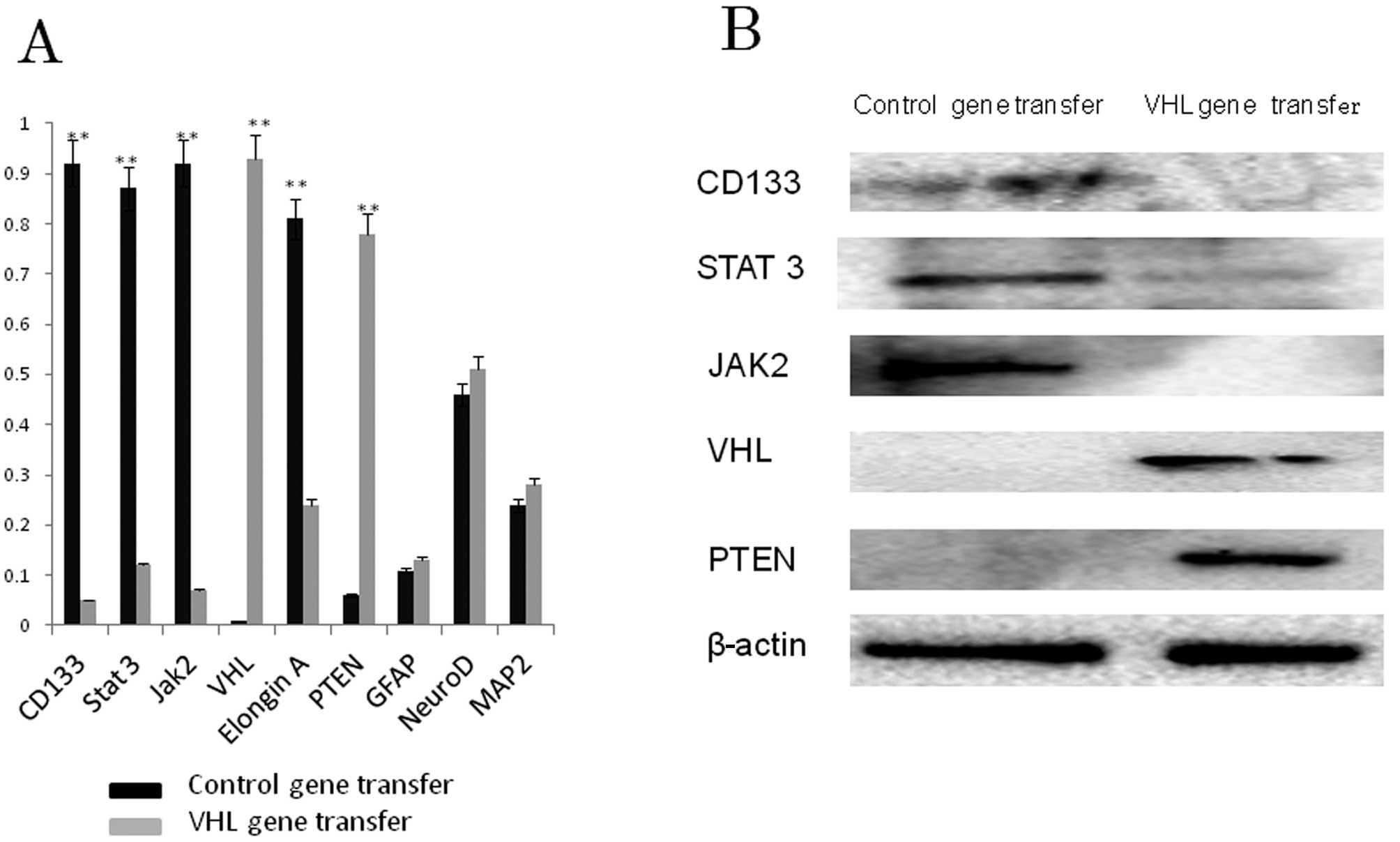
Western blot analysis
The results of western blot analysis (Fig. 3B) supported the immunocytochemical
data. U87 GSLCs expressed CD133, STAT3 and JAK2, but not VHL or
PTEN. Expression of CD133, STAT3 and JAK1 was not detected
following VHL gene transfer, contrary to VHL and PTEN expression,
which was detected. Following VHL gene transfer to U87 GSLCs, the
percentage of VHL and PTEN positive cells was increased, whereas
that of CD133, STAT3 and JAK2 positive cells was decreased.
Soft agar colony formation, neurosphere
formation and cell proliferation
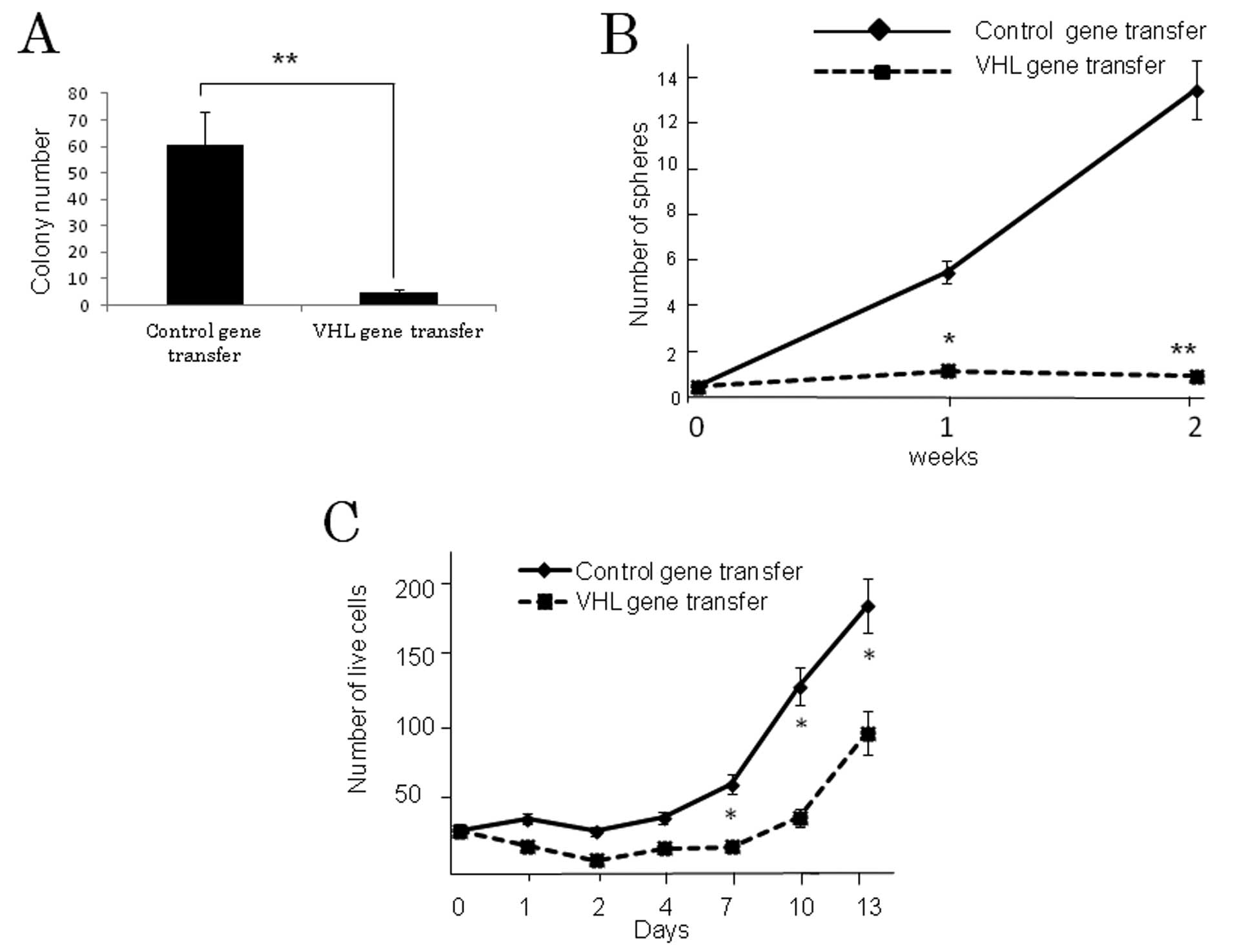
The results of the colony-formation assay in soft
agar medium demonstrated significantly greater colony formation in
the control vector-transfected compared to the VHL-expressing
vector-transfected U87 GSLCs (P<0.001) (Fig. 4A). Neurosphere formation, which is
a reflection of the self-renewal ability, was also significantly
greater in the former than in the latter (P<0.001) (Fig. 4B). Proliferation of control
vector-transfected U87 GSLCs was significantly more pronounced
compared to the VHL gene-transfected GSLCs 7 days following
transfection (P<0.01) (Fig.
4C), although the difference between them was smaller than in
the case of the soft agar colony or neurosphere formation
assay.
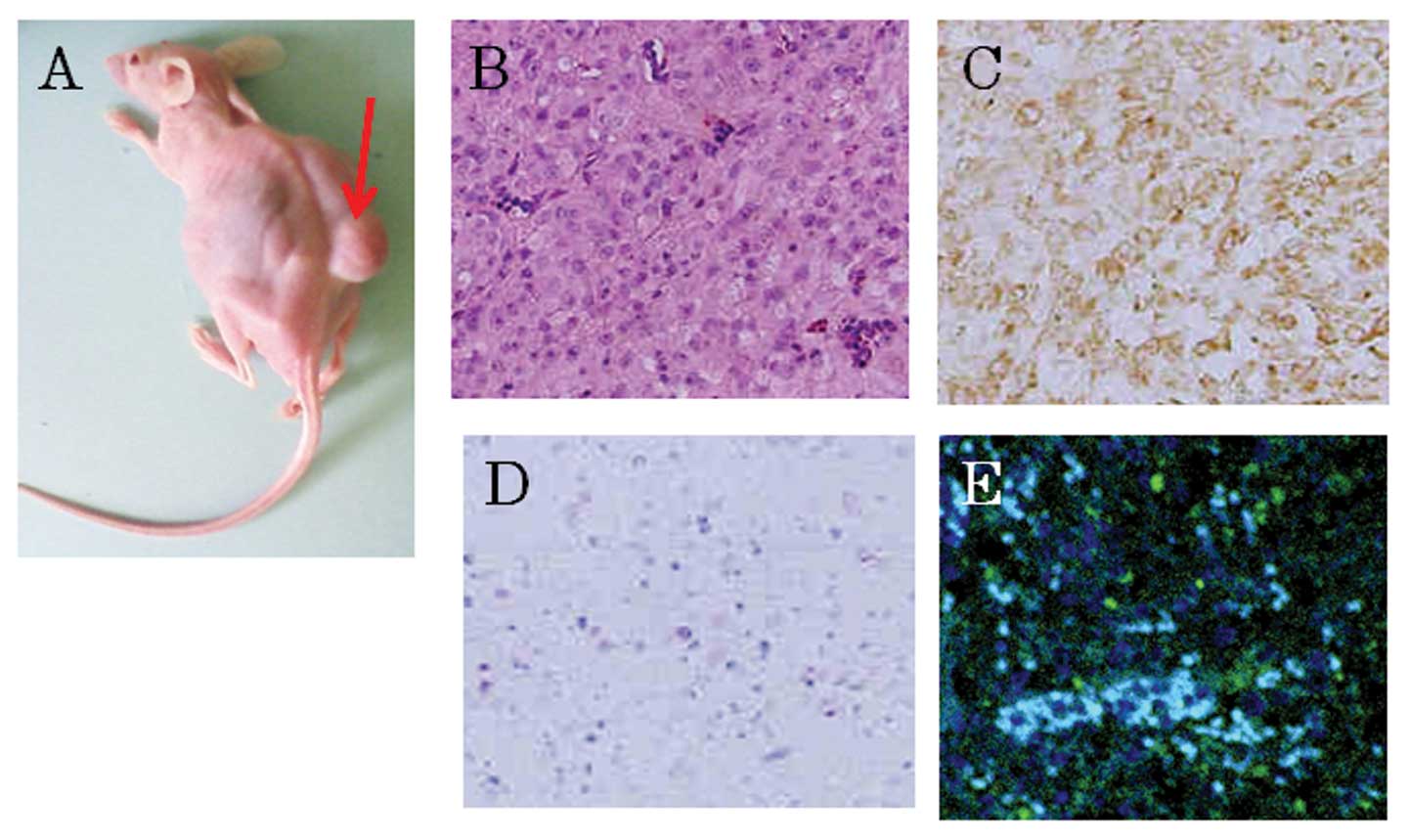
Implantation of U87 GSLCs into SCID
mice
Although subcutaneous transplantation of U87 GSLCs
into SCID mice always resulted in tumor formation, transplantation
of VHL gene-transfected U87 GSLCs resulted in markedly reduced or
no tumor formation. The tumors arising from the U87 GSLCs
transplanted into the SCID mice exhibited the pathological features
of glioblastoma and most of the cells in the tumor tissue were
CD133- and STAT3- positive but showed no immunoreactivity
indicative of GFAP (Fig. 5).
Discussion
Glioma cancer stem cells are defined by their
self-renewal ability, CD133-positivity, and transplantation ability
in SCID mice (3). U87 GSLCs
constructed by us exhibited these properties and may thus be
considered a subpopulation of naive U87 cells. U87 GSLCs may be
cultured in non-serum medium containing growth factors such as
basic FGF and EGF. Glioma cancer stem cells are also known as
glioma-initiating cells. GSLCs possess various mechanisms related
to treatment tolerability, epigenetics and PTEN/PI3K/Akt signaling
(19), and they reside in a
hypoxic niche (14). Our results
suggest that U87 GSLCs had a high capacity for colony and
neurosphere formation. VHL inhibited STAT3, JAK2 and Elongin A. In
addition, VHL upregulated PTEN expression. However, GFAP, NeuroD
and MAP2 levels were not significantly affected by the VHL gene
transfer. These results suggest that VHL affected the JAK/STAT
pathway as well as the PTEN/PI3K/Akt pathway; they also suggest
that upregulation of PTEN by VHL gene transfer may affect the
PI3K/Akt pathway, since PTEN is a PI3K/Akt pathway inhibitor
(19). STAT3 has been reported to
play an important role in the self-renewal ability of cancer stem
cells. VHL inhibited the implantation ability, as well as soft agar
colony and neurosphere formation, all of which are key
characteristics of cancer stem cells. Our results suggest that
these characteristics may be related to the JAK/STAT or
PTEN/PI3K/Akt pathways in GSLCs. VHL inhibits HIF-1α under
normoxic, but not under hypoxic, conditions by acting through the
ubiquitin/proteasome system (20).
Although VHL gene mutations are not frequently found in
gliomas (21), function of the
VHL gene is maintained under normoxic, but not hypoxic,
conditions (20). In addition,
overexpression of VHL inhibits tumorigenesis and reduces the
proliferation of glioma cells (22). The core of glioblastomas is
reported to be in the hypoxic state and glioblastomas are
characterized by their necrotic regions due to poor
vascularization, which leads to inadequate blood supply and,
consequently, to hypoxic and necrotic areas (23). It is suggested that GSLCs are
maintained in vivo in a niche characterized by reduced
oxygen tension, i.e., in a hypoxic niche (13). U87-derived GCLSs exhibited negative
expression of the VHL protein and, thus, the use of U87 GCLSs may
be considered adequate for characterization of GCLSs.
Overexpression of VHL upregulated PTEN and downregulated the
JAK/STAT pathway, although it did not significantly affect the
expression of neuronal differentiation markers such as NeuroD, GFAP
and MAP2. In addition, overexpression of VHL significantly
downregulated the proliferation of U87 GSLCs. However, the
overexpression of VHL inhibited cell proliferation of U87 GSLCs to
a lesser extent than it did soft agar colony and neurosphere
formation, or implantation capacity into SCID mice. These results
suggest that VHL inhibited the tumorigenicity and self-renewal
ability via the JAK/STAT pathway and also affected the
PTEN/PI3K/Akt pathway, both of which are critical in GSLCs, but it
did not affect the differentiation of the GSLCs.
In conclusion, VHL overexpression downregulated the
tumorigenicity and self-renewal ability in U87 GCLSs via the
JAK/STAT pathway and upregulated PTEN. VHL over-expression therapy
may be promising for the regulation of GSLCs under hypoxic
conditions.
Acknowledgements
This study was supported by a grant
from the Education and Science Ministry of Japan [Basic Research
(B) 23390353]. The authors thank Ms. Akemi Miura for her technical
assistance.
References
|
1
|
Stupp R, Mason WP, van den Bent MJ, et al:
Radiotherapy plus concomitant and adjuvant temozolomide for
glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Friedman HS, Prados MD, Wen PY, et al:
Bevacizumab alone and in combination with irinotecan in recurrent
glioblastoma. J Clin Oncol. 27:4733–4740. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu SC, Ping YF, Yi L, Zhou ZH, Chen JH,
Yao XH, Gao L, Wang JM and Bian XW: Isolation and characterization
of cancer stem cells from a human glioblastoma cell line U87.
Cancer Lett. 265:124–134. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sherry MM, Reeves A, Wu JK and Cochran BH:
STAT3 is required for proliferation and maintenance of multipotency
in glioblastoma stem cells. Stem Cells. 27:2383–2392. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Villalva C, Martin-Lannerée S, Cortes U,
Dkhissi F, Wager M, Le Corf A, Tourani JM, Dusanter-Fourt I, Turhan
AG and Karayan-Tapon L: STAT3 is essential for the maintenance of
neurosphere-initiating tumor cells in patients with glioblastomas:
a potential for targeted therapy? Int J Cancer. 128:826–838. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang YP, Chang YL, Huang PI, et al:
Resveratrol suppresses tumorigenicity and enhances radiosensitivity
in primary glioblastoma tumor initiating cells by inhibiting the
STAT3 axis. J Cell Physiol. 227:976–993. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohno M, Natsume A, Kondo Y, Iwamizu H,
Motomura K, Toda H, Ito M, Kato T and Wakabayashi T: The modulation
of microRNAs by type I IFN through the activation of signal
transducers and activators of transcription 3 in human glioma. Mol
Cancer Res. 7:2022–2030. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ivanov SV, Salnikow K, Ivanova AV, Bai L
and Lerman MI: Hypoxic repression of STAT1 and its downstream genes
by a pVHL/HIF-1 target DEC1/STRA13. Oncogene. 26:802–812. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kamura T, Sato S, Haque D, Liu L, Kaelin
WG Jr, Conaway RC and Conaway JW: The Elongin BC complex interacts
with the conserved SOCS-box motif present in members of the SOCS,
ras, WD-40 repeat, and ankyrin repeat families. Genes Dev.
12:3872–3881. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dasari VR, Kaur K, Velpula KK, Gujrati M,
Fassett D, Klopfenstein JD, Dinh DH and Rao JS: Upregulation of
PTEN in glioma cells by cord blood mesenchymal stem cells inhibits
migration via downregulation of the PI3K/Akt pathway. PLoS One.
5:e103502010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Frew IJ, Thoma CR, Georgiev S, Minola A,
Hitz M, Montani M, Moch H and Krek W: pVHL and PTEN tumour
suppressor proteins cooperatively suppress kidney cyst formation.
EMBO J. 27:1747–1757. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bar EE: Glioblastoma, cancer stem cells
and hypoxia. Brain Pathol. 21:119–129. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qiu B, Sun X, Zhang D, Wang Y, Tao J and
Ou S: TRAIL and paclitaxel synergize to kill U87 cells and
U87-derived stem-like cells in vitro. Int J Mol Sci. 13:9142–9156.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamada H, Dezawa M, Shimazu S, Baba M,
Sawada H, Kuroiwa Y, Yamamoto I and Kanno H: Transfer of the von
Hippel-Lindau tumor suppressor gene to neuronal progenitor cells in
treatment for Parkinson’s disease. Ann Neurol. 54:352–359.
2003.PubMed/NCBI
|
|
16
|
Hägerstrand D, He X, Bradic Lindh M, Hoefs
S, Hesselager G, Ostman A and Nistér M: Identification of a
SOX2-dependent subset of tumor- and sphere-forming glioblastoma
cells with a distinct tyrosine kinase inhibitor sensitivity
profile. Neuro Oncol. 13:1178–1191. 2011.
|
|
17
|
Kanno H, Kuwabara T, Shinonaga M, Chang
CC, Tanaka Y, Sugio Y, Morita H, Yasumitsu H, Umeda M and Nagashima
Y: Establishiment of a human glioma cell line bearing a
homogeneously staining chromosomal region and releasing α- and
β-type transforming growth factors. Acta Neuropathol. 79:30–36.
1989.PubMed/NCBI
|
|
18
|
Yamada S, Kanno H and Kawahara N:
Trans-membrane peptide therapy for malignant glioma by use of a
peptide derived from the MDM2 binding site of p53. J Neurooncol.
109:7–14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bleau AM, Hambardzumyan D, Ozawa T,
Fomchenko EI, Huse JT, Brennan CW and Holland EC: PTEN/PI3K/Akt
pathway regulates the side population phenotype and ABCG2 activity
in glioma tumor stem-like cells. Cell Stem Cell. 4:226–235. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maxwell PH, Wiesener MS, Chang GW,
Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER and
Ratcliffe PJ: The tumour suppressor protein VHL targets
hypoxia-inducible factors for oxygen-dependent proteolysis. Nature.
399:271–275. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kanno H, Shuin T, Kondo K, Yamamoto I, Ito
S, Shinonaga M, Yoshida M and Yao M: Somatic mutations of the von
Hippel-Lindau tumor suppressor gene and loss of heterozygosity on
chromosome 3p in human glial tumors. Cancer Res. 57:1035–1038.
1997.PubMed/NCBI
|
|
22
|
Sun X, Liu M, Wei Y, Liu F, Zhi X, Xu R
and Krissansen GW: Overexpression of von Hippel-Lindau tumor
suppressor protein and antisense HIF-1alpha eradicates gliomas.
Cancer Gene Ther. 13:428–435. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Valk PE, Mathis CA, Prados MD, Gilbert JC
and Budinger TF: Hypoxia in human gliomas: demonstration by PET
with fluorine-18-fluoromisonidazole. J Nucl Med. 33:2133–2137.
1992.PubMed/NCBI
|