Introduction
Pancreatic cancer is a devastating disease with a
poor 5-year survival rate of 6% (1). Local recurrences and systematic
metastasis are the major reasons for treatment failure, even after
curative operation for early stage tumors (2–4).
Lymphatic metastases are one of the common routes for tumor
recurrences and metastases in pancreatic cancer and also
significantly contribute to its poor prognosis (5–7).
However, the underlying mechanism of lymphatic metastases in
pancreatic cancer is not clear (8).
Cancer stem cells (CSCs) are a subpopulation of
tumor cells that has the capacity to self-renew (by symmetric and
asymmetric division), sustains the heterogeneous lineages of cancer
cells and continually maintains tumorigenesis (9). A growing body of evidence has
indicated the critical role of CSCs in many solid tumors (10–12).
In addition to contributing to primary tumor formation, CSCs are
also key players in the metastatic processes (12). However, the role of CSCs in
pancreatic cancer lymphatic metastasis has not been well
elucidated.
We have previously established a pancreatic cancer
cell line BxPC-3-LN with aggressive features and highly lymphatic
metastatic potential, which can serve as an ideal platform to study
the mechanism of lymphatic metastasis in pancreatic cancer
(8,13). Given the critical function of CSCs
in cancer metastasis, we investigated the stem cell-like properties
of BxPC-3-LN cells to confirm the involvement of CSCs in lymphatic
metastases of pancreatic cancer. We also examined the expression
profile of microRNAs (miRNAs) involved in CSCs and cancer
metastasis.
Materials and methods
Cell, tissue culture and animal
model
The human pancreatic cancer cell line BxPC-3 was
purchased from the American Type Culture Collection (Rockville, MD,
USA). Cells were cultured at 37°C in a humidified atmosphere of 95%
air and 5% CO2. Eight-week-old immunodeficient male mice
(BALB/c nu/nu and NOD/SCID) were obtained from Shanghai SLAC
Laboratory Animal Co. Ltd, Shanghai, China. All animal procedures
were approved by the Institutional of Animal Care Committee, Fudan
University, Shanghai, China.
Lymphatic metastatic potential
The BxPC-3-LN pancreatic cancer cells with highly
lymphatic metastatic capability were previously established by
consecutive in vivo selection processes (8). Eight-week-old BALB/c mice were
orthotopically injected with 1×106/ml cells into the
pancreas (n=3). Mice were sacrificed at 6-week end-points to
examine lymphatic involvement and metastatic lymph nodes were
confirmed by pathological examination. Histological features of
primary tumors were examined by hematoxylin and eosin staining.
Flow cytometry
Flow cytometry was used to detect the surface
markers of CD133 (Miltenyi Biotec, Bergisch Gladbach, Germany) and
CXCR4 (eBioscience, San Diego, CA, USA) by using Beckman Coulter
FC500 (Miami, FL, USA). Cells were collected, fixed by resuspensing
in 10 ml of 70% ethanol for 30 min and washed in PBS. They were
incubated in PBS solution containing CD133 and CXCR4 antibody at
4°C for 40 min. Quantitative values are means ± SEM from 3
independent experiments.
Self-renewal ability
Eight-week-old NOD/SCID mice were injected with
10,000 cells into the right flank (n=3). The formation of primary
tumors was examined every week. Mice were sacrificed at 4-week
end-points to check tumor formation.
Sphere formation of tumor cells was performed as
previously described with a slight modification (14). Cells were maintained in serum-free
DMEM/Ham’s F12 medium (Invitrogen, Carlsbad, CA) containing 1% N2
supplement (Invitrogen), 2% B27 supplement (Invitrogen), epidermal
growth factor (10 ng/ml; PeproTech), basic fibroblast growth factor
(20 ng/ml; PeproTech), heparin (2 μg/ml; Sigma-Aldrich, St.
Louis, MO) and plated at a density of 1,000 cells/ml in 6-well
ultra-low cluster plates (Corning Incorporated, Corning, NY).
Sphere formation was observed under light microscope.
Clone formation ability was examined as previously
described (15). In brief, 500
single cells were added to a 6-well culture plate. Culture medium
was changed every 3 days. At 2-week end-points, cells were stained
with hematoxylin solution. The number of colonies was counted under
a light microscope.
Immunofluorescence
Immunofluorescence was performed as previously
described (16). In brief, on
preincubation with normal blocking serum (diluted 1:20 in PBS) for
30 min, cells were incubated for 1 h with sonic hedgehog (Shh)
antibody (Epitomics, Burlingame, CA, USA) at 1:100 dilution. The
cells were labeled with an anti-rabbit IgG-PE secondary antibody
(Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 1:200 dilution.
DNA was counterstained with DAPI. Microscope was used to observe
the expression of specific markers.
RT-PCR
Shh gene expression was tested by
reverse-transcription polymerase chain reaction (RT-PCR). The total
RNA of cells was extracted using TRIzol isolation reagent
(Invitrogen). The primers used in the PCR reactions were designed
using information from the human genomic data base (forward primer
5′-CGGAGCGAGGAAGGGAAAG-3′ and reverse primer
5′-TTGGGGATAAACTGCTTGTAGGC-3′).
Drug cytotoxicity assay
Drug cytotoxicity assay was performed as previously
described with a slight modification (8). In brief, the BxPC-3-LN cells were
plated at a density of 104 cells per well into 96-multiwell plates
and were allowed to grow for 24 h. Cyclopamine (6 μmol/l,
Sigma-Aldrich), gemcitabine (100 nM, Eli Lilly) and cyclopamine (6
μmol/l) combined with gemcitabine (100 nM) were added to the
cells and then incubated for 48 h at 37°C. After initial
incubation, cells were incubated at 37°C for 4 h with 20 μl
of MTS (Promega) and absorbance was read at 492 nm. Viability
levels are presented as a percentage of the level obtained from the
blank control (untreated cells) (mean ± SE, n=6).
MicroRNA PCR array
Isolation of miRNAs from cells was carried out using
the mirVana miRNA Isolation Kit (Applied Biosystems). miRNAs
expression was quantified by using TaqMan miRNA qRT-PCR assays as
previously described (TaqMan Array Human MicroRNA A+B Cards Set
v3.0, Applied Biosystems) (17).
The PCR reaction was performed on 7900HT Real-Time PCR System
(Applied Biosystems) with the following conditions: 16°C 2 min,
42°C 1 min, 50°C 1 sec for 40 cycles. The fold change of miRNAs
expression (BxPC-3 vs. BxPC-3-LN) was calculated.
Statistical analysis
Fisher’s exact test and Student’s t-test were
applied to compare enumeration data and measure mean t data. Stata
10.0 was used for the tests and p<0.05 was considered
statistically significant.
Results
Lymphatic metastasis potential
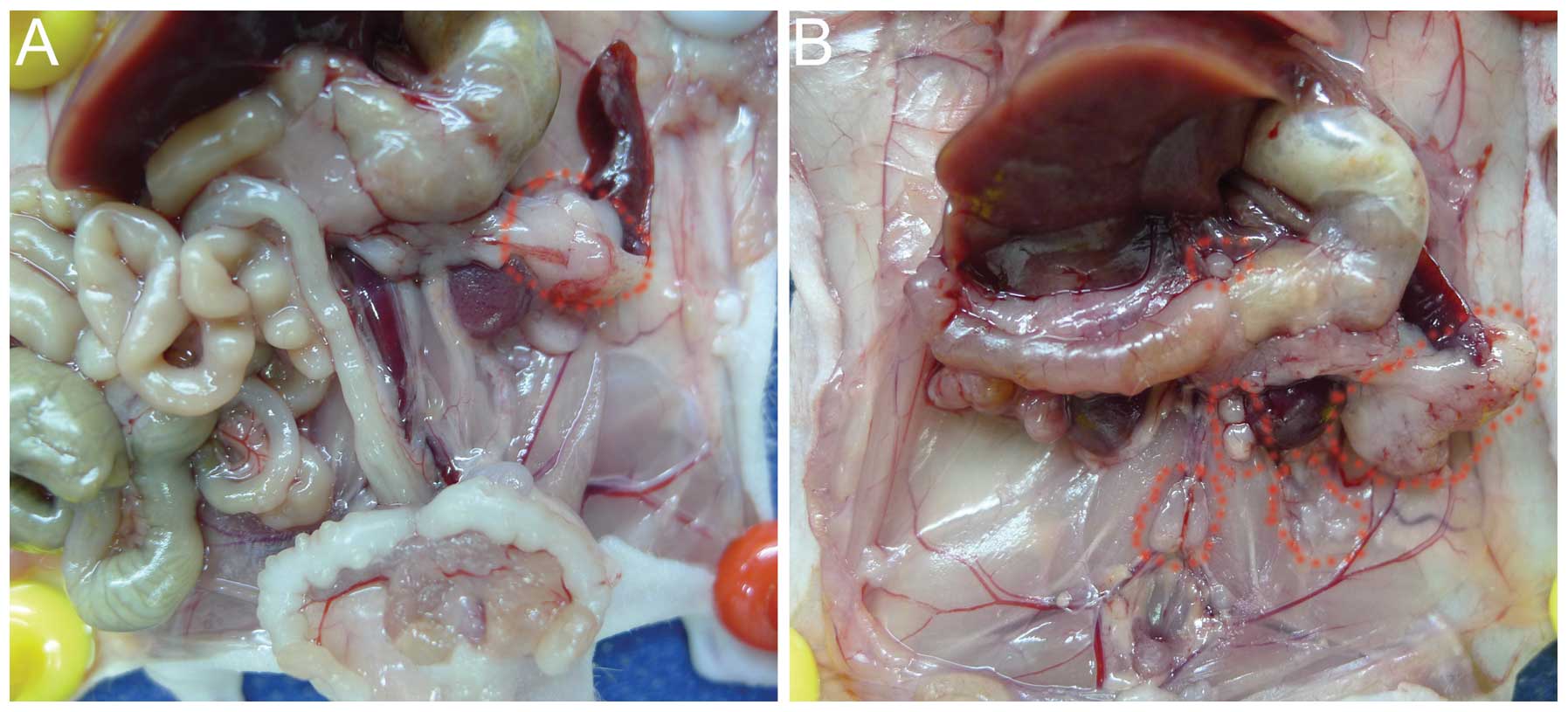
After 6 weeks of tumor cell implantation
orthotopically, the BxPC-3-LN cells generated celiac axis,
para-aortic and para-renal lymph node metastases confirmed by
pathological examination, with larger primary tumors compared with
the parental BxPC-3 cells (Fig. 1A and
B). The distribution of metastatic lymph nodes by BxPC-3-LN
cells was similar to human disease. No obvious lymphatic metastasis
was observed for the parental BxPC-3 cells (Fig. 1A).
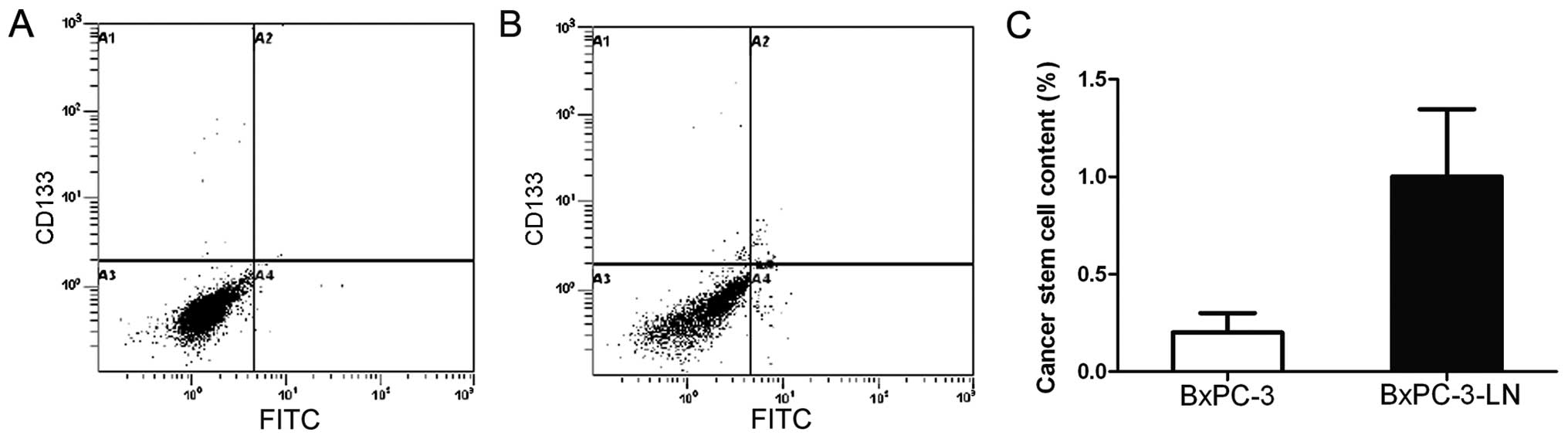
BxPC-3-LN cells possess CSC markers
In order to determine whether the lymphatic
metastatic BxPC-3-LN cells have CSC-like properties, we examined
the expression of the CSC markers CD133+ and
CXCR4+ by flow cytometry. As shown in Fig. 2, the BxPC-3-LN cells contained an
average of 1.0% of CD133+/CXCR4+ cells, which
was 5 times of that in the BxPC-3 cells (0.2%), indicating
BxPC-3-LN cells possess CSC-like properties, which may explain the
matastatic potential of this cell line.
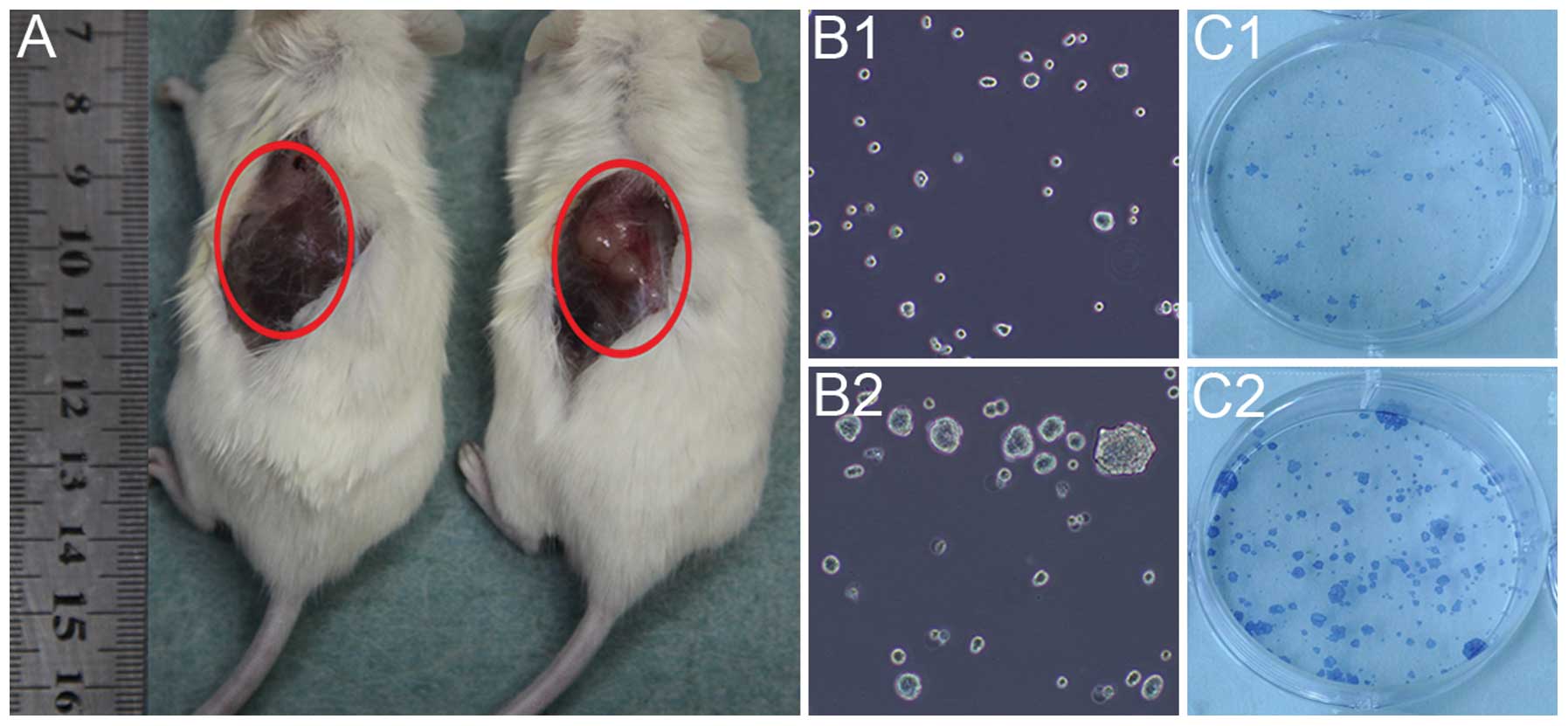
Self-renewal ability
The self-renewal ability of the BxPC-3-LN and BxPC-3
cells was detected by in vivo tumorigenicity and in
vitro sphere and clone formation. The BxPC-3-LN cells generated
big tumors with 10,000 cells injected after 4 week of implantation
(Fig. 3A right), while no tumor
was observed for BxPC-3 cells with the same amount of cells
injected (Fig. 3A left). In
addition, the BxPC-3-LN cells showed more spheres (Fig. 3B2) and clones formation (Fig. 3C2) than the BxPC-3 cells (Fig. 3B1 and C1).
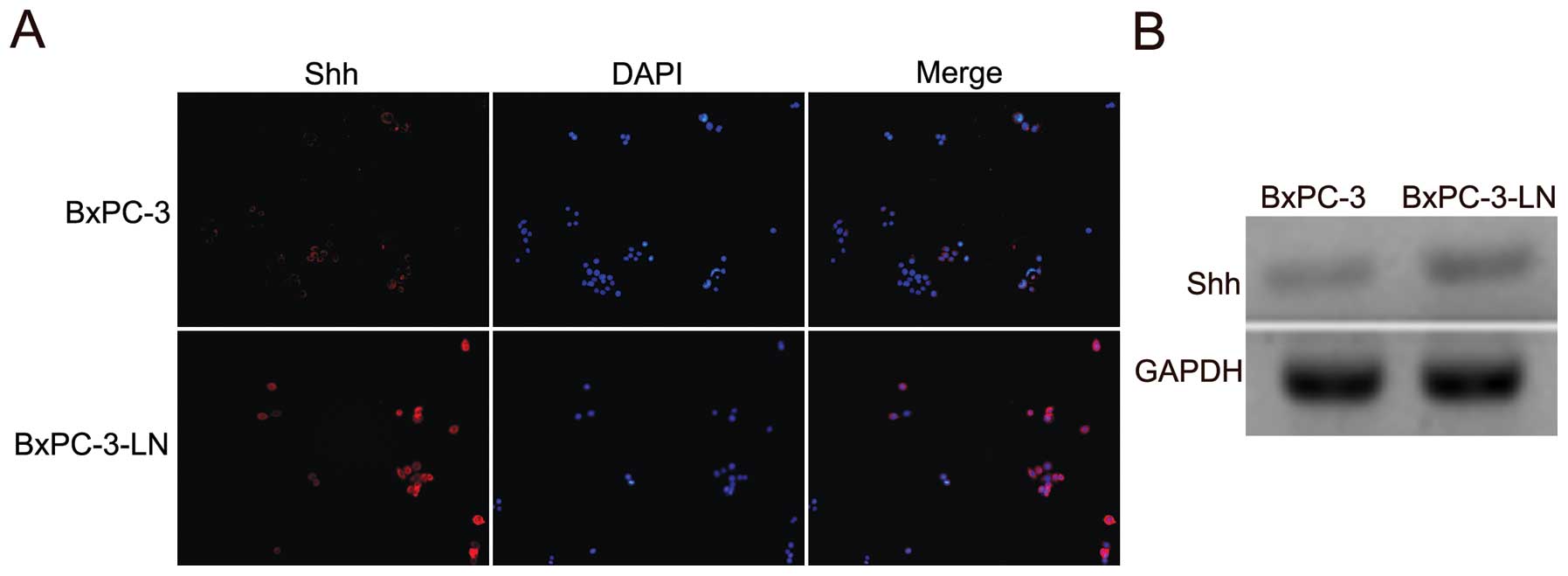
Shh expression is increased in BxPC-3-LN
cells
The expression of Shh in BxPC-3 and the lymphatic
metastatic BxPC-3-LN cells was examined by immunofluorescence. The
BxPC-3-LN cells showed higher expression of Shh compared with the
parental BxPC-3 cells (Fig. 4A).
The upregulation of Shh mRNA in the BxPC-3-LN cells was also
confirmed by RT-PCR (Fig. 4B).
Those data suggest that Shh pathway might be involved in the
lymphatic metastasis of pancreatic cancer.
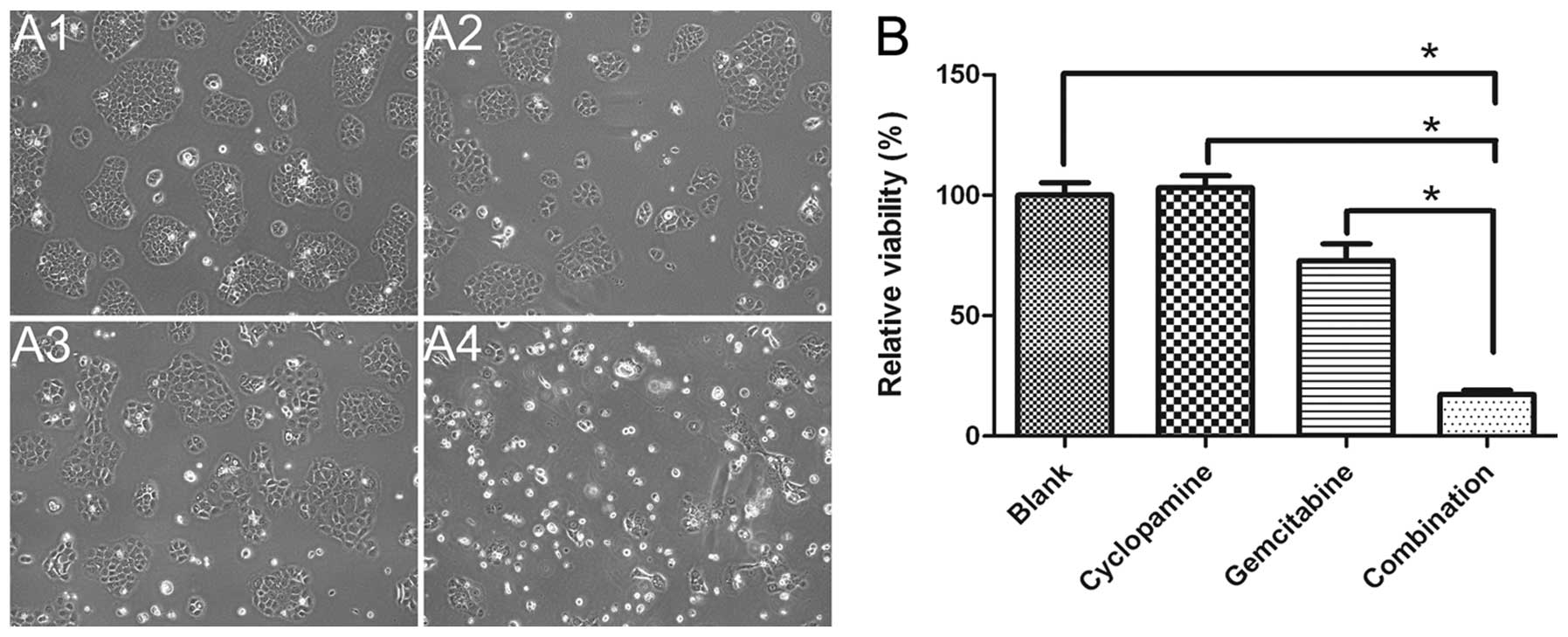
Drug cytotoxicity
MTS assay was used to examine the growth inhibition
of cyclopamine, gemcitabine and cyclopamine combined with
gemcitabine in BxPC-3 cells. As shown in Fig. 5, cyclopamine combined with
gemcitabine (17.3%) caused significant inhibition of cell growth
compared with gemcitabine alone (p<0.0001) and cyclopamine alone
(p<0.0001) (Fig. 5), indicating
that blocking Shh pathway is needed in controling the lymphatic
metastasis of pancreatic cancer.
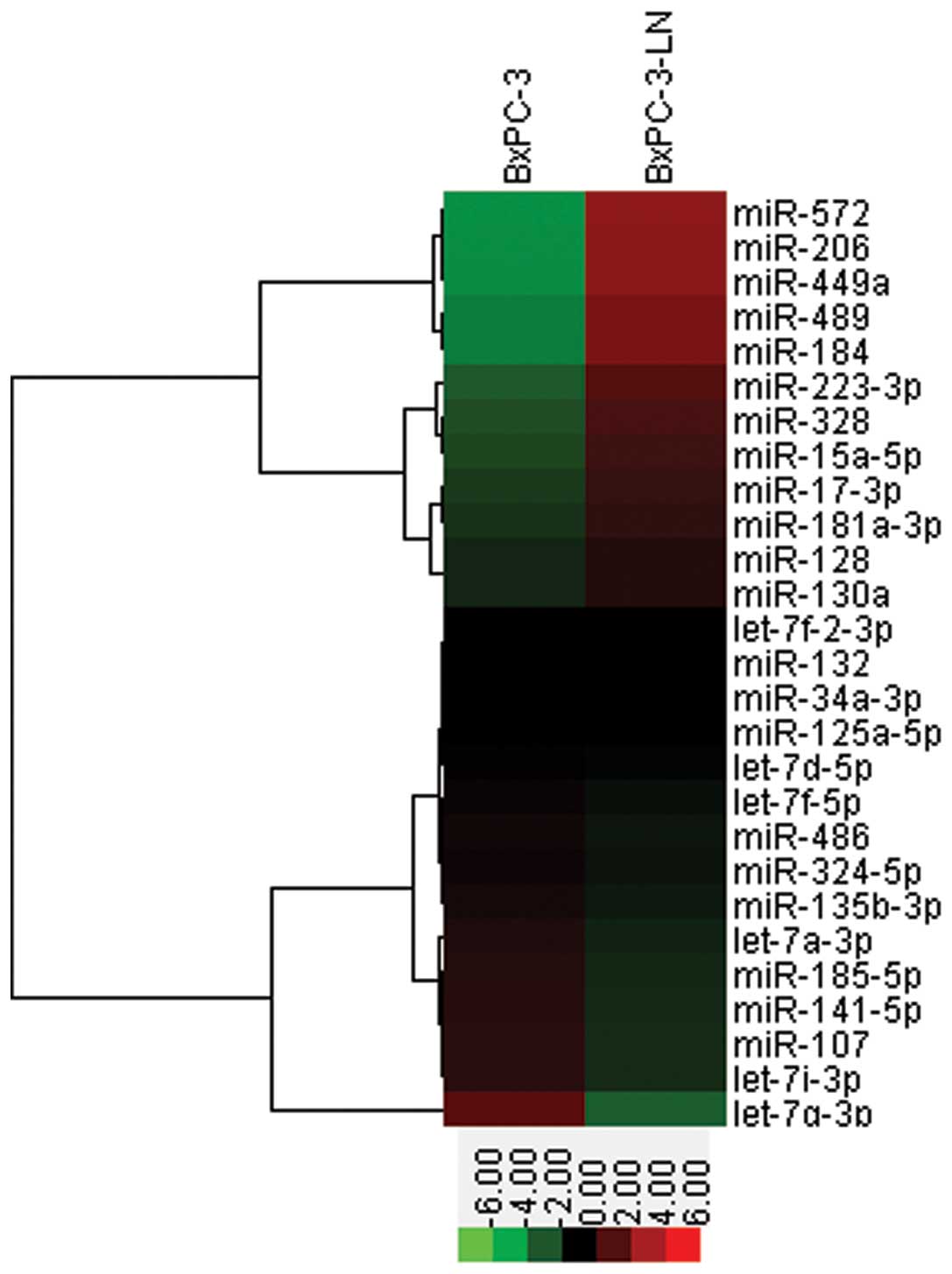
MicroRNA PCR array
Differential expression of miRNAs in the BxPC-3 and
BxPC-3-LN cells was quantified by using TaqMan miRNA qRT-PCR assay.
Compared with the BxPC-3 cells, several aberrantly expressed miRNAs
related to CSCs were found in the BxPC-3-LN cells, including
upregulation of miR-572, miR-206, miR-449a, miR-489, miR184 and
downregulation of let-7g-3p, let-7i-3p, let-7a-3p, miR-107, miR-128
and miR-141-5p (Fig. 6),
suggesting a possible regulatory role of microRNA in lymphatic
metastasis of pancreatic cancer.
Discussion
CSC is thought as the major source of tumorigenesis
and metastasis (9). CD133 is a
stem-like cell surface marker expressed in diverse solid tumors,
including pancreatic, brain and colon tumors (12,18,19).
In pancreatic cancer, CD133+ expressing cells were
associated with tumor initiation, chemoresistance, increased cell
migration and invasion (12,20).
More importantly, it was demonstrated that a
CD133+/CXCR4+ subpopulation was responsible
for pancreatic cancer metastasis (12). Previously, we have confirmed the
migration, invasive and chemoresistant capability of a lymphatic
metastatic pancreatic cancer cell line BxPC-3-LN established by
serial in vivo selection (8). In this study, we further demonstrated
that BxPC-3-LN cells presented stem cell-like properties, including
highly lymphatic metastastic potential, self-renewal ability and
chemoresistance. In addition, the BxPC-3-LN cells also expressed
higher levels of Shh, which was considered as one of the most
important stem cell-related signal pathways (11,12).
CD133+/CXCR4+ cells, which were identified as
the migrating CSCs in a previous study (12), were found to be enriched in the
BxPC-3-LN cells compared with the parental BxPC-3 cells.
Cyclopamine (a Shh signal pathway inhibitor) combined with
gemcitabine showed greater inhibitory effect on the BxPC-3-LN cells
than cyclopamine and gemcitabine alone. Our findings suggest that
CD133+/CXCR4+ cells might be the major
migrating CSCs and are responsible for the lymphatic metastases of
pancreatic cancer.
Previous studies reported that gemcitabine-resistant
cells showed cancer stem-like cell phenotype, which underwent
epithelial-to-mesenchymal transition (EMT) and showed increased
expression of the stem cell markers CD24, CD44 and epithelial
specific antigen (ESA) (21,22).
These findings indicate that cancer stem-like cells, which are
known for their chemoresistant ability, can be enriched during the
acquisition of chemoresistance and in therapeutic treatments
(23,24). The in vivo serial selection
process that was used shares common features to
gemcitabine-resistant cells established by exposure to serially
escalated doses of gemcitabine, which was also accompanied by an
enrichment of CSCs.
miRNAs are a group of short non-coding RNAs that
modulate gene expression at the post-transcriptional level, usually
leading to translational suppression or gene silencing (25). miRNAs are involved in tumor
maintenance, progression, and treatment resistance (26–28).
There are also evidence showing that miRNAs regulate CSCs by
suppressing the expression of ‘stem cell factors’ such as Sox2,
Kif4, CD44 and Notch (24,26–32).
Yu et al(24) found that
the CSC-enriched cells expressed low levels of let-7, miR-107,
miR-125, miR-128, miR-130, miR-132 and miR-141 compared with the
parental cells. Another study showed that restoration of miR-34
strongly suppressed the growth and invasion of p53-mutant
pancreatic cancer cells, and sensitized the cells to chemo- and
radiotherapy. Moreover, restoration of miR-34 resulted in an 87%
decrease in the CD133+/CXCR4+ CSCs by direct
regulating its downstream targets Notch1/2 and Bcl-2, suggesting
the potential role of miR-34 in CSCs (29). In our study, we also showed that
the CSC-enriched BxPC-3-LN cells expressed lower levels of let-7,
miR-34, miR-107, miR-125, miR-128, miR-130, miR-132 and miR-141
than the parental BxPC-3 cells, indicating miRNAs regulates the
lymphatic metastasis of pancreatic cancer by regulating CSCs. In
addition, we found upregulation of miR-184 in BxPC-3-LN cells,
which is epigenetically regulated by Methyl-CpG binding protein 1
(MBD1) for modulating stem cell growth and differentiation
(33). Our previous studies have
demonstrated that MBD1 plays an important role in pancreatic cancer
growth and lymphatic metastasis (34,35).
These findings suggest that MBD1 regulate pancreatic cancer
progression by epigenetic regulation of miR-184, which can affect
stem cell properties.
In conclusion, the BxPC-3-LN cells possess stem
cell-like properties. The in vivo serial selection processes
were similar to gemcitabine-resistant cells established by exposure
to serially escalated doses of gemcitabine, which was also
accompanied by an enrichment of CSCs. Our results suggest the
function of CSCs in pancreatic cancer lymphatic metastasis and
miRNAs may regulate pancreatic cancer metastasis through modulating
the properties of CSCs.
Acknowledgements
This study was supported in part by
the National Science Foundation of China (grant nos. 81172276,
81101807, 81001058, 30901435 and 30972905) (X.Y.), the Shanghai
Science and Technology Commission (grant no. 12QH1400600), ‘985
project’ Third Stage Oncology Project (grant no. 985III-YFX0102)
(J.L.), the MacDonald Research Fund, and the William and Ella Owens
Medical Research Foundation (M.L.). We thank Dr Bo Zhang and Dr
Huanyu Xia for their technical assistance.
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar
|
|
2
|
Luo G, Long J, Zhang B, et al: Stroma and
pancreatic ductal adenocarcinoma: An interaction loop. Biochim
Biophys Acta. 1826:170–178. 2012.PubMed/NCBI
|
|
3
|
Warshaw AL and Fernandez-del Castillo C:
Pancreatic carcinoma. N Engl J Med. 326:455–465. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li X, Ma Q, Xu Q, et al: SDF-1/CXCR4
signaling induces pancreatic cancer cell invasion and
epithelial-mesenchymal transition in vitro through non-canonical
activation of Hedgehog pathway. Cancer Lett. 322:169–176. 2012.
View Article : Google Scholar
|
|
5
|
Pedrazzoli S, DiCarlo V, Dionigi R, et al:
Standard versus extended lymphadenectomy associated with
pancreatoduodenectomy in the surgical treatment of adenocarcinoma
of the head of the pancreas: a multicenter, prospective, randomized
study. Lymphadenectomy Study Group. Ann Surg. 228:508–517. 1998.
View Article : Google Scholar
|
|
6
|
Li Y, Kong D, Ahmad A, Bao B and Sarkar
FH: Pancreatic cancer stem cells: Emerging target for designing
novel therapy. Cancer Lett. May 20–2012.(Epub ahead of print).
|
|
7
|
Ni X, Yang J and Li M: Imaging-guided
curative surgical resection of pancreatic cancer in a xenograft
mouse model. Cancer Lett. 324:179–185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Long J, Luo G, Liu C, et al: Development
of a unique mouse model for pancreatic cancer lymphatic metastasis.
Int J Oncol. 41:1662–1668. 2012.PubMed/NCBI
|
|
9
|
Clarke MF, Dick JE, Dirks PB, et al:
Cancer stem cells - perspectives on current status and future
directions: AACR Workshop on cancer stem cells. Cancer Res.
66:9339–9344. 2006. View Article : Google Scholar
|
|
10
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li C, Heidt DG, Dalerba P, et al:
Identification of pancreatic cancer stem cells. Cancer Res.
67:1030–1037. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hermann PC, Huber SL, Herrler T, et al:
Distinct populations of cancer stem cells determine tumor growth
and metastatic activity in human pancreatic cancer. Cell Stem Cell.
1:313–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo G, Yu X, Jin C, et al:
LyP-1-conjugated nanoparticles for targeting drug delivery to
lymphatic metastatic tumors. Int J Pharm. 385:150–156. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singh SK, Clarke ID, Terasaki M, et al:
Identification of a cancer stem cell in human brain tumors. Cancer
Res. 63:5821–5828. 2003.PubMed/NCBI
|
|
15
|
Shi WD, Meng ZQ, Chen Z, Lin JH, Zhou ZH
and Liu LM: Identification of liver metastasis-related genes in a
novel human pancreatic carcinoma cell model by microarray analysis.
Cancer Lett. 283:84–91. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Giodini A, Kallio MJ, Wall NR, et al:
Regulation of microtubule stability and mitotic progression by
survivin. Cancer Res. 62:2462–2467. 2002.PubMed/NCBI
|
|
17
|
Mitchell PS, Parkin RK, Kroh EM, et al:
Circulating microRNAs as stable blood-based markers for cancer
detection. Proc Natl Acad Sci USA. 105:10513–10518. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
O’Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110.
2007.PubMed/NCBI
|
|
19
|
Singh SK, Hawkins C, Clarke ID, et al:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moriyama T, Ohuchida K, Mizumoto K, et al:
Enhanced cell migration and invasion of CD133+
pancreatic cancer cells cocultured with pancreatic stromal cells.
Cancer. 116:3357–3368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shah AN, Summy JM, Zhang J, Park SI,
Parikh NU and Gallick GE: Development and characterization of
gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol.
14:3629–3637. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Z, Li Y, Kong D, et al: Acquisition
of epithelialmesenchymal transition phenotype of
gemcitabine-resistant pancreatic cancer cells is linked with
activation of the notch signaling pathway. Cancer Res.
69:2400–2407. 2009. View Article : Google Scholar
|
|
23
|
Hong SP, Wen J, Bang S, Park S and Song
SY: CD44-positive cells are responsible for gemcitabine resistance
in pancreatic cancer cells. Int J Cancer. 125:2323–2331. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu F, Yao H, Zhu P, et al: let-7 regulates
self renewal and tumorigenicity of breast cancer cells. Cell.
131:1109–1123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hatfield SD, Shcherbata HR, Fischer KA,
Nakahara K, Carthew RW and Ruohola-Baker H: Stem cell division is
regulated by the microRNA pathway. Nature. 435:974–978. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wellner U, Schubert J, Burk UC, et al: The
EMT-activator ZEB1 promotes tumorigenicity by repressing
stemness-inhibiting microRNAs. Nat Cell Biol. 11:1487–1495. 2009.
View Article : Google Scholar
|
|
27
|
Liu C, Kelnar K, Liu B, et al: The
microRNA miR-34a inhibits prostate cancer stem cells and metastasis
by directly repressing CD44. Nat Med. 17:211–215. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheng H, Shi S, Cai X, et al: microRNA
signature for human pancreatic cancer invasion and metastasis. Exp
Ther Med. 4:181–187. 2012.PubMed/NCBI
|
|
29
|
Ji Q, Hao X, Zhang M, et al: MicroRNA
miR-34 inhibits human pancreatic cancer tumor-initiating cells.
PLoS One. 4:e68162009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Leal JA and Lleonart ME: MicroRNAs and
cancer stem cells: Therapeutic approaches and future perspectives.
Cancer Lett. Apr 30–2012.(Epub ahead of print).
|
|
31
|
Ni X, Long J, Cen P, Chen L, Yang J and Li
M: Pancreatic cancer tumour initiating cells: the molecular
regulation and therapeutic values. J Cell Mol Med. 16:988–994.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu C and Tang DG: MicroRNA regulation of
cancer stem cells. Cancer Res. 71:5950–5954. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu C, Teng ZQ, Santistevan NJ, et al:
Epigenetic regulation of miR-184 by MBD1 governs neural stem cell
proliferation and differentiation. Cell Stem Cell. 6:433–444. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Luo G, Jin C, Long J, et al: RNA
interference of MBD1 in BxPC-3 human pancreatic cancer cells
delivered by PLGA-poloxamer nanoparticles. Cancer Biol Ther.
8:594–598. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu C, Chen Y, Yu X, et al: Proteomic
analysis of differential proteins in pancreatic carcinomas: Effects
of MBD1 knock-down by stable RNA interference. BMC Cancer.
8:1212008. View Article : Google Scholar : PubMed/NCBI
|