Introduction
Bladder cancer is one of the most common malignant
tumors in the United States (1).
Approximately 70% of newly diagnosed bladder cancers are of the
superficial type (2). A mainstay
of therapy for superficial bladder cancer is complete transurethral
resection. However, bladder cancer locally relapses in 70% of
patients, and progresses to muscle invasive cancer after
transurethral resection in 30%. To reduce this recurrence and
progression, intravesical instillation of antitumor agents has been
used since the early seventies (3).
Bacillus Calmette-Guérin (BCG) was isolated from
Mycobacterium bovis which caused bovine tuberculosis in 1921
(4). Since the intravesical
instillation of BCG for bladder cancer therapy was first reported
in 1976 (5), it has become the
most successful immunotherapy for non-invasive bladder cancer
(6). However, the instillation of
BCG has serious side effects, such as disseminated infections,
sepsis and multiple organ failure (7,8).
Therefore, the discovery of other bacteria which have the same
anticancer effects with less side effects is required for bladder
cancer patients. Indeed, intravesical treatments with other
bacteria have been reported (9,10).
Although BCG intravesical therapy is one of the most
successful immunotherapies, its precise mechanism is still unknown.
Recently, it has been reported that the concentration of urinary
tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) was
higher in BCG-responsive patients than non-responders (11). Furthermore, polymorphonuclear
neutrophils (PMNs) migrating to the bladder after BCG instillation
release large amounts of TRAIL (12). TRAIL is a cytokine inducing
apoptosis in malignant tumor cells without affecting normal cells
(13–15) and is considered a promising
anticancer agent in clinical trials (16,17).
It has been reported that matrix metalloproteinases
(MMPs) stimulate the release of cytokines and chemokines via
proteolytic processing (18–20).
Other proteases, such as a disintegrin and metalloproteases (ADAMs)
or cathepsin E, have been also reported to be required for shedding
of cytokines from the cell surface (21,22).
However, little is known about the mechanism of TRAIL release in
PMNs (23).
In this study, we have found that Clostridium
butyricum MIYAIRI 588 (CBM588) induces the release of TRAIL
from PMNs, resulting in marked anticancer effects in vitro
and in vivo. We have additionally found that MMP-8 is one of
the key molecules for TRAIL release. We therefore hypothesize that
CBM588 might be useful for a novel intravesical therapy against
bladder cancer.
Materials and methods
Reagents
Clostridium butyricum MIYAIRI 588 (Miyarisan
Pharma., Tokyo, Japan), BCG (Nippon Kayaku, Tokyo, Japan and
Sanofi-Aventis, Tokyo, Japan), Lactobacillus casei (Yakult,
Tokyo, Japan), Krestin (Daiichi Sankyo, Tokyo, Japan), Lentinan
(Astellas Pharma, Tokyo, Japan) and IFN-α (Dainippon Sumitomo
Pharma, Osaka, Japan) were dissolved in PBS. 5Z-7-oxozeaenol
(Chugai Pharma., Tokyo, Japan), SB203580, SP600125 (Jena
Bioscience, Jena, Germany), cathepsin G inhibitor (Santa Cruz
Biotechnology, Santa Cruz, CA, USA), MMP-8 inhibitor and MMP-9
inhibitor (Merck, Darmstadt, Germany) were dissolved in dimethyl
sulfoxide (DMSO). Cycloheximide, leupeptin and EDTA were purchased
from Nacalai Tesque (Kyoto, Japan). Human recombinant DR5/Fc
chimera and zVAD-fmk were obtained from R&D Systems
(Minneapolis, MN, USA). Actinomycin D and 1,10-phenanthroline were
obtained from Sigma (St. Louis, MO, USA). Anti-human TLR2 and TLR4
were purchased from eBioscience (San Diego, CA, USA).
Cell culture
Human bladder cancer 253J-BV cells (24) provided by Dr K. Inoue (University
of Kochi), human lung cancer H460, human renal cancer 786-O, human
embryonic kidney HEK293 and murine bladder cancer MBT-2 cell lines
were maintained in RPMI-1640 medium with 10% fetal bovine serum
(FBS), 2 mM glutamine, 100 U/ml penicillin and 100 μg/ml
streptomycin. Normal PMNs were isolated using Polymorphprep
(Axis-Shield, Oslo, Norway) and normal PBMCs were isolated using
Lymphoprep (Axis-Shield), and maintained in RPMI-1640 medium with
10% FBS and 2 mM glutamine. All cells were incubated at 37°C with
humidity and 5% CO2. PMNs and PBMCs were acquired from
healthy volunteers after obtaining informed consent. This study was
approved by the Kyoto Prefectural University of Medicine Research
Ethics Committee (permission no. C-425 and C-919). All cell lines
except 253J-BV used in this study were obtained from American Type
Culture Collection (ATCC).
Enzyme-linked immunosorbent assay
(ELISA)
PMNs (2.5×106/ml) were incubated in the
presence or absence of CBM588, BCG, or other reagents for 6 h. PMNs
were centrifuged and the supernatant was collected. A human TRAIL
ELISA kit (Abcam, Cambridge, UK) was used according to the
manufacturer’s instructions.
RNA isolation and quantification by
real-time reverse transcription-PCR
RNA isolation and quantitative real-time RT-PCR were
carried out as described (25).
The real-time quantitative reverse transcription-PCR primer-probe
sets for TRAIL mRNA (Hs00234355_m1) and GAPDH mRNA (Hs99999905_m1)
were purchased from Applied Biosystems (Foster City, CA, USA).
Western blot analysis
Western blot analysis was carried out as described
previously (25). Rabbit
polyclonal MMP-8 (Chemicon, Temecula, CA, USA), MMP-9 (Abcam),
caspase-3, and mouse specific caspase-8 (Cell Signaling Technology,
Beverly, MA, USA) antibodies, and mouse monoclonal human TRAIL
(Santa Cruz Biotechnology) and β-actin (Sigma) antibodies were used
as the primary antibodies.
Stable transfection and siRNA
transfection
The human TRAIL expression plasmid pCAGGS NEO-TRAIL
was described previously (26).
786-O cells and HEK293 cells were transfected with pCAGGS NEO-TRAIL
or pCAGGS NEO, a vacant vector plasmid, using Lipofectamine 2000
(Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s
instructions. Transfected cells were selected in growth medium with
1 mg/ml G418 (Sigma) and kept stable for more than 1 month before
experiments.
siRNA transfections were carried out using
Lipofectamine RNAiMAX Reagent (Invitrogen) according to the
manufacturer’s instructions. Briefly, 1×105 cells were
seeded into 6-well tissue culture plates 1 day before transfection
with 50 nM MMP-8 siRNA (Hs00233972_m1), MMP-9 siRNA
(Hs00234579_m1), or a control siRNA (Silencer Select Negative
Control #2 siRNA) (Ambion, Austin, TX, USA). After 48 h, we
analyzed the culture medium of transfected cells by western
blotting to evaluate the release of soluble TRAIL.
Generation of mutations in the
transmembrane domain of TRAIL
Two constructs were generated, replacing the
trans-membrane domain of TRAIL using a site-directed mutagenesis
kit (Stratagene, La Jolla, CA, USA). The first mutant construct
(MT1) was obtained by substitution of the TRAIL trans-membrane
domain (residues 32–33: Ala-Val) with the peptide sequence
(Ala-Asp). The second mutant (MT2) was replaced with the peptide
sequence (Glu-Val).
Sequence analysis
The transmembrane domain of TRAIL was calculated
using version 2.0 of TMHMM (http://www.cbs.dtu.dk/services/TMHMM/). SignalP
(http://www.cbs.dtu.dk/services/SignalP/) was used for
the prediction of signal peptides.
Detection of apoptosis
PMNs (5×106/ml) were incubated in the
presence or absence of CBM for 24 h. PMNs were centrifuged and the
supernatant was collected. Cancer cells were pretreated with the
supernatant for 48 h. DNA fragmentation was quantified by the
percentage of hypodiploid DNA (sub-G1) as described previously
(25).
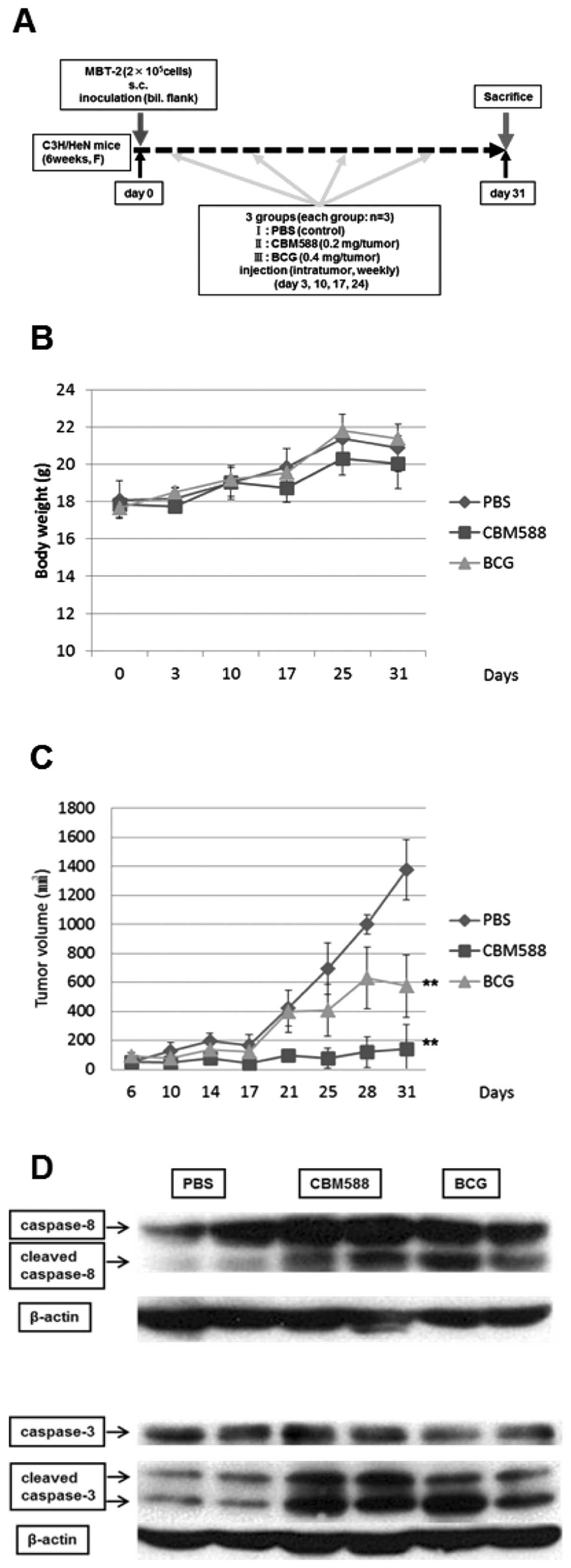
Implantation of MBT-2 cells into C3H/HeN
mice and the protocol in vivo
C3H/HeN female mice were obtained from Shimizu
Laboratory Supplies (Kyoto, Japan). The present study complied with
the principles and guidelines of the Japanese Council on Animal
Care and it was also approved by the Committee for Animal Research
of Kyoto Prefectural University of Medicine (permission no.
M23-180). MBT-2 cells were harvested and resuspended in 1:1
PBS/Matrigel mixture. Cells (2×105) in 100 μl of
mixture were injected s.c. into both flanks of each mouse. Mice
were randomized into the following treatment groups (n=3): group I,
untreated control (PBS, 100 μl/tumor); group II, CBM588 (0.2
mg/tumor); and group III, BCG (0.4 mg/tumor), once weekly,
intratumor injection. Therapy was continued for 31 days. Tumor
dimensions were measured with vernier calipers. Tumor volume was
calculated using the formula: (shortest diameter)2 ×
(longest diameter)/2. Tumor volume was compared among groups using
an unpaired Student’s t-test.
Results
Clostridium butyricum MIYAIRI 588
(CBM588) enhances the release of TRAIL from PMNs
It has been reported that BCG stimulates the release
of TRAIL from PMNs (12). We then
evaluated whether other bacteria have similar effects. We added
BCG, CBM588, Lactobacillus casei, Krestin (an extract from
Trametes Versicolor), Lentinan (an extract from an edible
mushroom), and IFN-α to PMNs for 6 h and measured concentrations of
soluble TRAIL by ELISA. CBM588, as well as BCG, increased soluble
TRAIL levels in the culture supernatant of PMNs, but the other
bacteria and IFN-α did not (Fig.
1A).
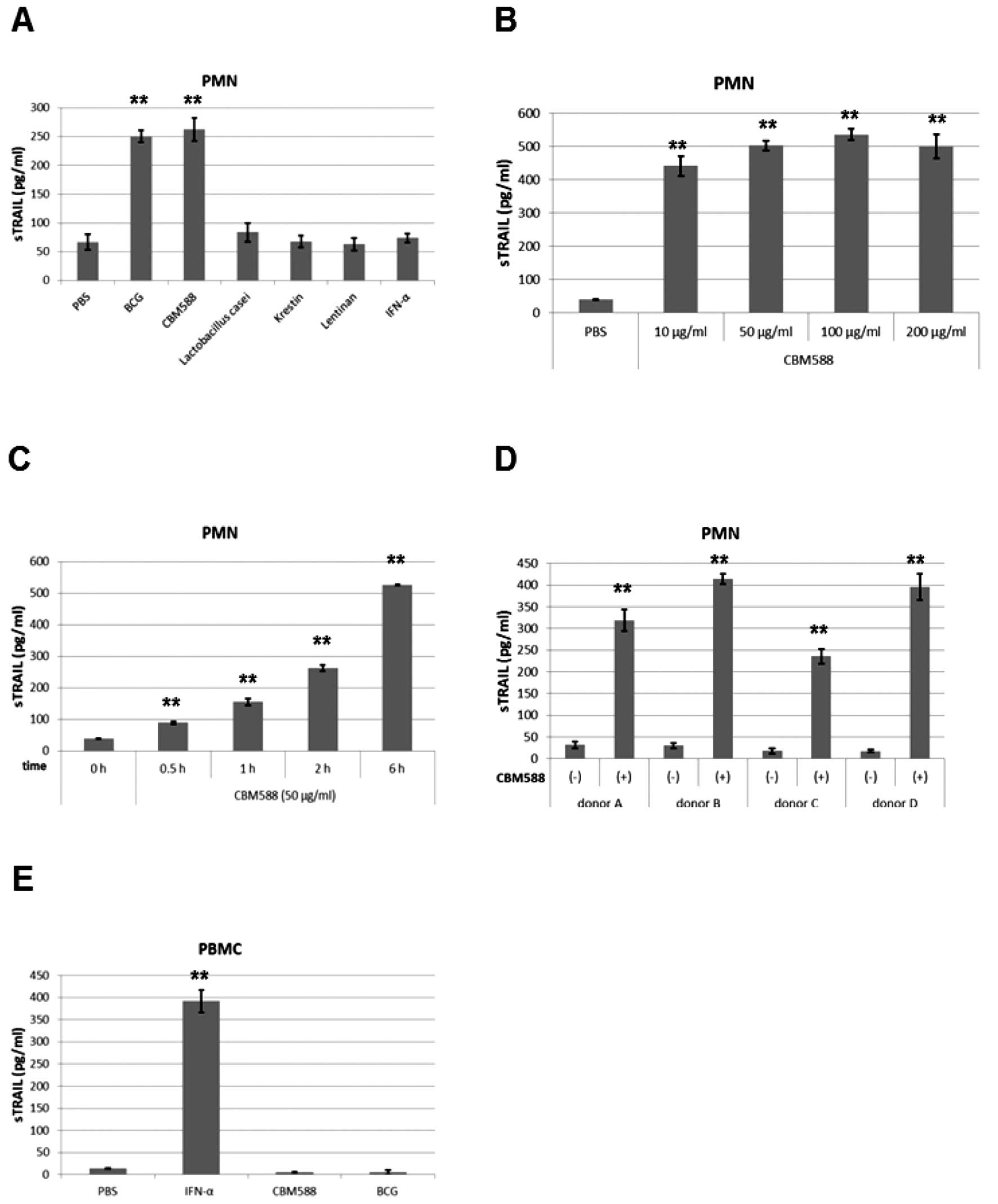 | Figure 1CBM588 enhances the release of TRAIL
from PMNs. (A and B) Human PMNs or PBMCs were isolated from blood
of healthy donors and seeded (2.5×106 cells/ml). After
incubation, the culture supernatant was assayed by ELISA for TRAIL.
(A) PMNs were stimulated with BCG, CBM588, Lactobacillus
casei, Krestin, Lentinan (each 10 μg/ml) or IFN-α (1,000
IU/ml) for 6 h. All bacteria were sterilized by heating.
**P<0.01 versus PBS. (B) PMNs were stimulated with
CBM588 at the doses indicated for 6 h. **P<0.01
versus PBS. (C) PMNs were stimulated with CBM588 (50 μg/ml)
for the period indicated. **P<0.01 versus PBS. (D)
PMNs were isolated from different plural donors. Each donor’s PMNs
were stimulated with PBS or CBM588 (50 μg/ml) for 6 h.
**P<0.01 versus each donor’s CBM588 (-). (E) PBMCs
were stimulated with IFN-α (1,000 IU/ml), CBM588 (50 μg/ml)
or BCG (100 μg/ml) for 24 h. **P<0.01 versus
PBS. Columns, mean; bars, mean ± SD. |
Clostridium butyricum is a gram-positive
anaerobe which produces butyric acid. It is found in soil or the
intestines of animals and humans. The MIYAIRI 588 strain of
Clostridium butyricum has been used as a probiotic for
treating and preventing diarrhea or constipation (27,28).
MIYA-BM® tablets containing CBM588 were approved by the
Japanese Ministry of Health and Welfare for clinical use in 1970.
Soluble TRAIL was drastically induced by CBM588 at 10 μg/ml
or more (Fig. 1B) and
time-dependently induced by 50 μg/ml of CBM588 (Fig. 1C). CBM588 similarly increased the
release of TRAIL from the PMNs of all other donors’ blood (Fig. 1D), while neither CBM588 nor BCG did
so in peripheral blood mononuclear cells (PBMCs) (Fig. 1E). These results suggest that
CBM588, as well as BCG, induces the release of TRAIL from PMNs, but
not from PBMCs.
CBM588, similarly to BCG, does not induce
TRAIL synthesis but stimulates the release of TRAIL from
intracellular stores in PMNs
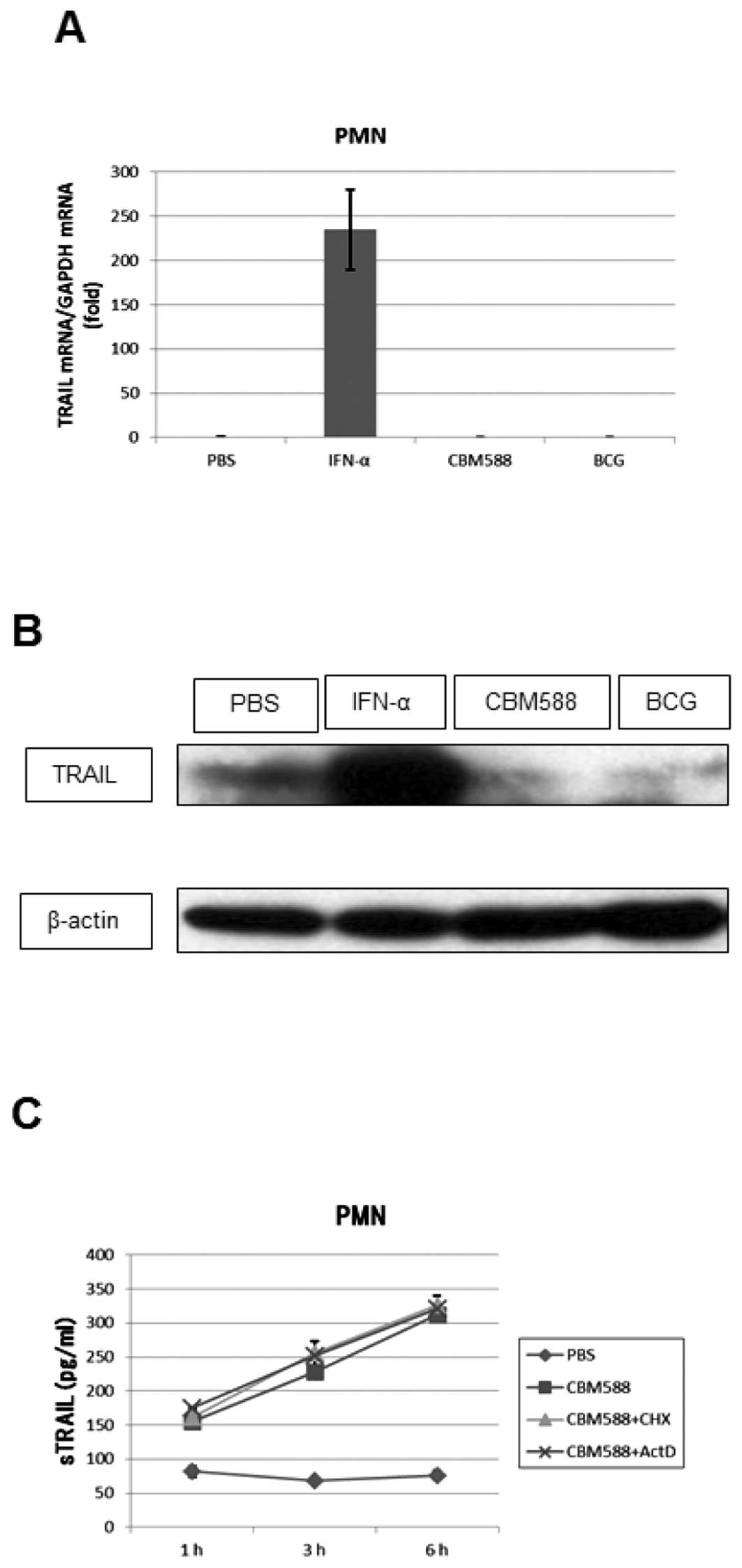
Kemp (12) reported
that BCG did not increase TRAIL mRNA levels in PMNs and we have
found that BCG or CBM588 did not induce TRAIL mRNA expression,
whereas IFN-α did (Fig. 2A). We
additionally evaluated the expression of the TRAIL protein in PMNs
by western blotting. The amount of the protein was increased by
IFN-α, whereas it was decreased by CBM588 or BCG (Fig. 2B). Furthermore, to determine
whether CBM588 or BCG induces de novo synthesis of TRAIL in
PMNs, we treated PMNs with a protein or RNA synthesis inhibitor,
cycloheximide (10 μg/ml) or actinomycin D (1 μg/ml),
respectively, for 1 h before adding CBM588 or BCG. The inhibitors
did not suppress the release of TRAIL from PMNs (Fig. 2C). These results suggest that PMNs
possess intracellular stores of TRAIL and that CBM588 or BCG
induces the release of TRAIL from these stores without inducing
TRAIL synthesis.
CBM588 induces the release of TRAIL from
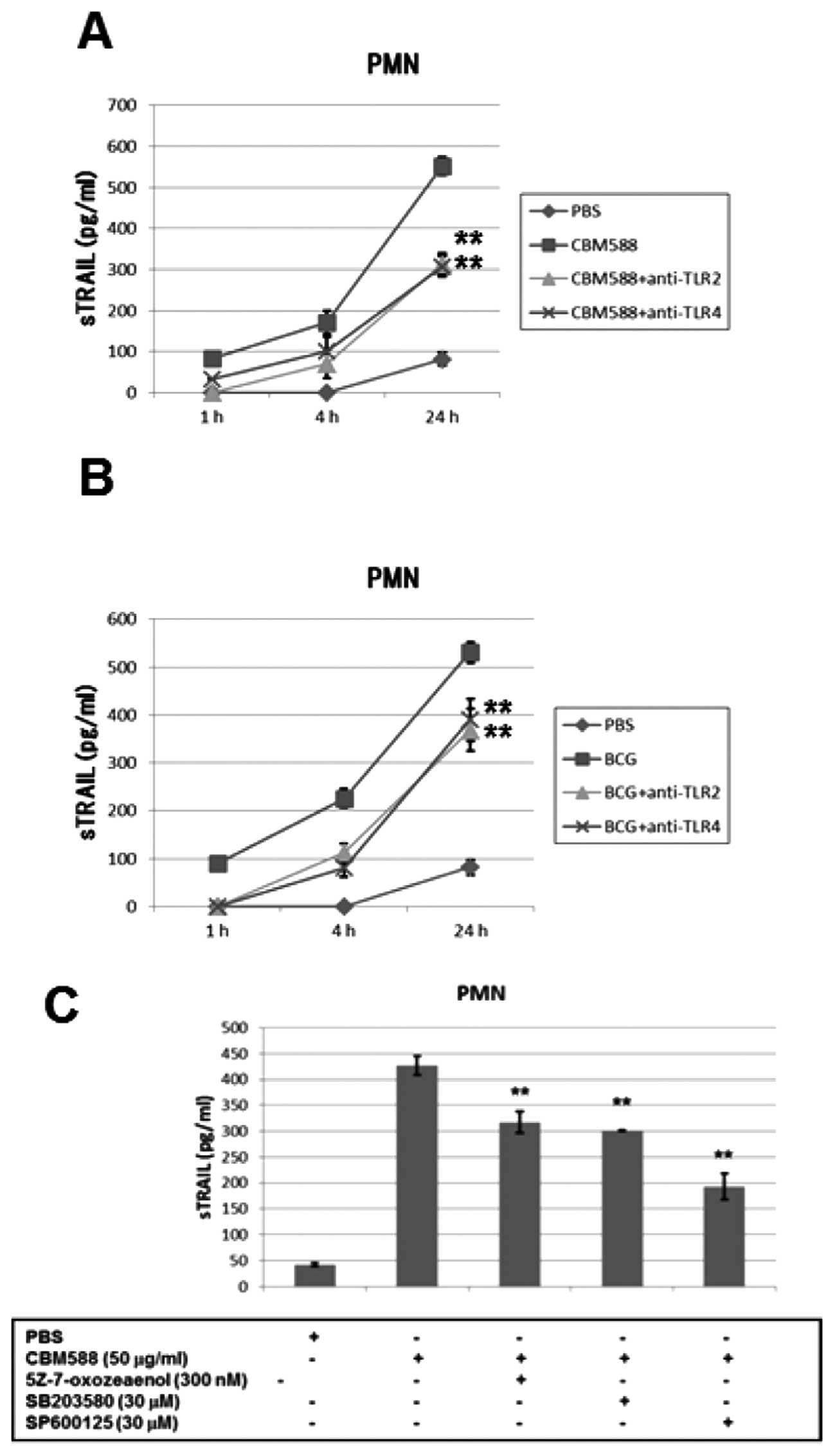
PMNs through the TLR2/4 signaling pathway
Toll-like receptors (TLRs) play a role in mediating
innate immune reactions (29,30)
and TLR2 and TLR4 have been reported to be involved in the host
immune response to BCG (31,32).
Therefore, we evaluated whether TLR2 or TLR4 was associated with
TRAIL release from PMNs by CBM588 or BCG. We stimulated PMNs with
CBM588 or BCG for 6 h after incubating with TLR2 or TLR4
neutralization antibody for 0.5 h and then measured concentrations
of soluble TRAIL using ELISA. TLR2 or TLR4 neutralization antibody
inhibited the release of TRAIL by CBM588 or BCG (Fig. 3A and B). Inhibition of the
downstream pathway of TLR2 or TLR4 by a TAK1 inhibitor
5Z-7-oxozeaenol (33), a p38 MAPK
inhibitor SB203580 and a JNK inhibitor SP600125 suppressed the
release of TRAIL by CBM588 (Fig.
3C). CBM588 or BCG upregulated the phosphorylation of p38 and
JNK in PMNs (data not shown). These results strongly suggest that
CBM588 or BCG releases TRAIL from PMNs at least partially through a
TLR2 or TLR4 signaling pathway.
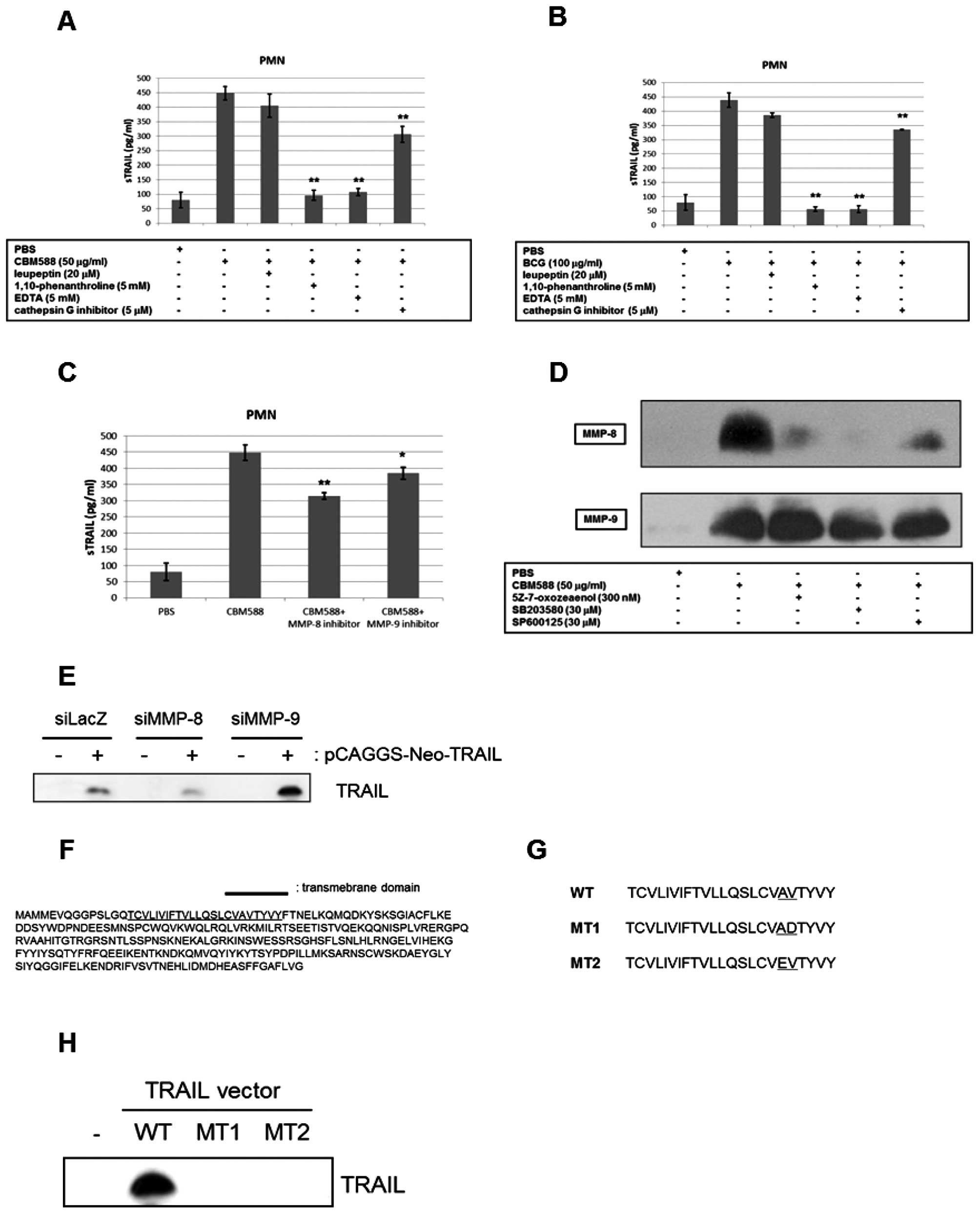
MMP-8 is one of the key molecules for the
release of TRAIL from PMNs
To determine what protease was associated with the
release of TRAIL from PMNs, we examined whether protease inhibitors
suppressed the release. Among the inhibitors examined, general MMP
inhibitors, such as 1,10-phenanthroline and EDTA, markedly
decreased the concentration of soluble TRAIL in the culture
supernatant of PMNs stimulated by CBM588 or BCG (Fig. 4A and B), while the cathepsin G
inhibitor had only a slight effect. PMNs are known to have abundant
MMP-8 and -9, and therefore we examined the effect of an MMP-8 or
-9 specific inhibitor on the release of TRAIL by CBM588. As shown
in Fig. 4C, the MMP-8 specific
inhibitor suppressed the accelerated release of TRAIL by CBM588,
whereas the MMP-9 specific inhibitor only weakly inhibited the
release. When PMNs are activated, MMPs are released from their
granules to the extracellular area (34–36).
We therefore detected MMP-8 and -9 in culture supernatant by
western blotting and found that CBM588 drastically increased MMP-8
and -9 protein levels. Whereas the MMP-8 level was decreased by
5Z-7-oxozeaenol, SB203580 or SP600125, the level of MMP-9 was not
changed (Fig. 4D). These results
suggest that MMP-8 at least partially releases TRAIL and is
regulated by TLR2/4 downstream signaling.
We next examined the effects of MMP-8/9 siRNA on the
release of TRAIL. We transfected the TRAIL expression vector into
TRAIL-resistant human renal cancer 786-O cells, and established a
stable TRAIL-expressing cell line. Subsequently, we transiently
transfected MMP-8 siRNA or MMP-9 siRNA into this cell line. As
shown in Fig. 4E, knockdown of
MMP-8, but not MMP-9, decreased TRAIL levels in the culture
supernatant. Therefore, this result strongly suggests that MMP-8 is
crucial to the release of TRAIL. Fig.
4F shows the amino acid sequence of human TRAIL, including a
putative transmembrane domain identified using TMHMM. Using SignalP
3.0, we found a proteolytic cleavage site of TRAIL, which could be
digested by MMP-8 and -9. We constructed plasmids with a mutation
at this site, and named them MT1 and MT2 (Fig. 4G). As shown in Fig. 4H, the introduction of a mutation at
the putative proteolytic cleavage site remarkably inhibited the
release of TRAIL from TRAIL-expressing HEK293 cells. Therefore,
these results clearly show that the site is absolutely necessary
for the release of TRAIL and that MMP-8 is a candidate for the
proteolytic enzyme of TRAIL.
Release of TRAIL from PMNs stimulated by
CBM588 results in the apoptosis of human cancer cells in vitro
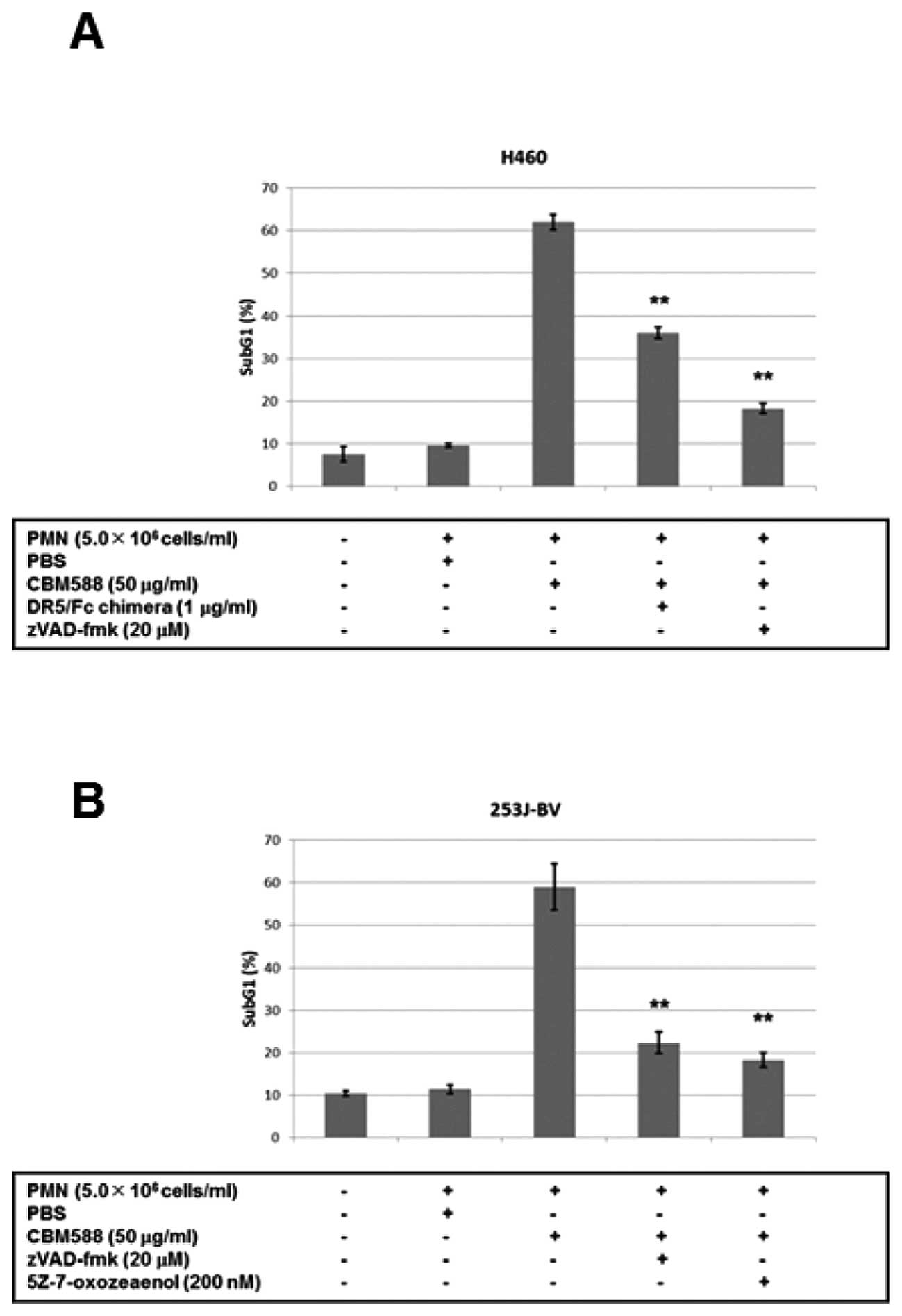
A recent study demonstrated that BCG enhanced the
cytotoxicity of PMNs against bladder cancer cells (37). We then added CBM588 to PMNs, and
found that the supernatant drastically enhanced the apoptosis of
human lung cancer H460 cells (Fig.
5A). The pan-caspase inhibitor zVAD-fmk inhibited apoptosis,
while a dominant negative protein against DR5, the DR5/Fc chimera,
partially inhibited it (Fig. 5A).
We also confirmed the results by crystal violet staining, and found
that CBM588 alone had little effect on cancer cell death (data not
shown). We performed the next experiment using bladder cancer
253J-BV cells and found that apoptosis was similarly induced by
PMNs with CBM588 and inhibited by zVAD-fmk or 5Z-7-oxozeaenol, a
TAK1 inhibitor (Fig. 5B). These
results suggest that TRAIL released from PMNs by CBM588 induces
caspase-dependent apoptosis of cancer cells.
CBM588 suppresses the growth of murine
bladder cancer cells in C3H/HeN mice
Previous studies showed that BCG induced antitumor
effects against bladder cancer cells in vivo(37,38).
We then examined the effects of CBM588 and BCG against murine
bladder cancer MBT-2 cells inoculated in C3H/HeN mice. As shown in
Fig. 6A, on day 0, MBT-2 cells
were subcutaneously inoculated into both flanks of mice. On days 3,
10, 17 and 24, CBM588, BCG or PBS as a control was injected into
each tumor. The tumor volume was measured by vernier calipers on
each day indicated. A significant difference in weight was not
observed among the three groups (Fig.
6B). Intratumor injection of BCG partially suppressed tumor
growth, while CBM588 almost completely inhibited it (Fig. 6C). We additionally evaluated the
activation of caspase-8 and -3 in tumor cell lysate by western
blotting. We detected the cleaved form of caspase-8 and -3 from
lysate of tumors treated with CBM588 or BCG (Fig. 6D). These results suggest that
CBM588 is also useful in vivo to suppress the growth of
bladder cancer cells.
Discussion
BCG is recognized as one of the most effective
agents against superficial bladder cancer. Despite great effort,
however, the serious side effects of BCG, such as disseminated
infections, sepsis, and multiple organ failure (7,8) have
yet to be eliminated. In addition, the best use of BCG has not been
clearly determined, because of a poor understanding of its
mechanisms of action. It is therefore necessary to clarify the
antitumor mechanisms of BCG for safer and more effective use.
In the present study, we found that the non-toxic
and harmless Clostridium, CBM588, drastically suppressed the growth
of bladder cancer cells in vitro and in vivo. CBM588
is known to be a component of intestinal bacterial flora. This
strain has been shown to be non-toxic and harmless unlike other
Clostridium and is commonly used as a probiotic for diarrhea
(39). Therefore, CBM588 is
promising for the novel and safer treatment of superficial bladder
cancer.
We found that CBM588 as well as BCG induced the
release of TRAIL from intracellular stores in PMNs, while they did
not enhance TRAIL synthesis. On the other hand, it has been
reported that a combination of intravesical BCG with IFN-α was
effective (40,41). IFN-α upregulates the transcription
of TRAIL in PMNs (12) and
therefore it might be more effective for CBM588 intravesical
therapy to use IFN-α. Furthermore, CBM588 shows promise in
combination with various agents enhancing sensitivity to TRAIL,
such as inducers of a TRAIL receptor, death receptor (DR) 5. We
previously reported a variety of agents upregulating DR5 expression
(25,42,43).
Among them, sulforaphane in broccoli sprouts might be useful in
combination with the intravesical CBM588 therapy, because of its
own antitumor effects (44) and
effective transition to urine (45).
The proteolytic activities of various proteases,
such as lysosomal cathepsins and MMPs, have been associated with
many malignant tumors (46,47).
However, several strategies designed to broadly block MMPs have not
been successful as cancer therapy, perhaps due to their functional
diversity in vivo(48,49).
For example, several MMPs, such as MMP-3, -8 and -12, were found to
have antitumorigenic effects through the suppression of tumor
angiogenesis and degradation of chemokines that mediate
organ-specific metastasis (47).
In the present study, we demonstrated that MMP-8 induced the
release of TRAIL, consistent with the hypothesis described
above.
In conclusion, we have found that CBM588 is safer
than BCG and effective against human bladder cancer cells in
vitro and in vivo. We additionally clarified that TRAIL
release by CBM588 is important for the antitumor effect and that
MMP-8 is one of the key molecules responsible for the release. We
believe that this discovery will lead to a novel intravesical
therapy for bladder cancer.
Abbreviations:
|
CBM588
|
Clostridium butyricum MIYAIRI
588;
|
|
BCG
|
Bacillus Calmette-Guérin;
|
|
PMN
|
polymorphonuclear neutrophil;
|
|
PBMC
|
peripheral blood mononuclear cell;
|
|
MMP
|
matrix metalloproteinase;
|
|
TRAIL
|
tumor necrosis factor-related
apoptosis-inducing ligand;
|
|
TLR
|
toll-like receptor
|
Acknowledgements
The authors thank Drs K. Inoue and
C.P. Dinney for generously providing the human bladder cancer cell
line 253J-BV and Dr T. Yoshida for helpful discussions. The gifts
of Clostridium butyricum MIYAIRI 588 from Miyarisan
Pharmaceutical, BCG from Nippon Kayaku and Sanofi-Aventis
Pharmaceutical, and 5Z-7-oxozeaenol from Chugai Pharmaceutical are
gratefully acknowledged. This study was partly supported by the
Japanese Ministry of Education, Culture, Sports, Science and
Technology (22790547 to M. Horinaka).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2
|
Devesa SS, Blot WJ, Stone BJ, Miller BA,
Tarone RE and Fraumeni JF Jr: Recent cancer trends in the United
States. J Natl Cancer Inst. 87:175–182. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hasui Y, Osada Y, Kitada S and Nishi S:
Significance of invasion to the muscularis mucosae on the
progression of superficial bladder cancer. Urology. 43:782–786.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Crispen R: History of BCG and its
substrains. Prog Clin Biol Res. 310:35–50. 1989.PubMed/NCBI
|
|
5
|
Morales A, Eidinger D and Bruce AW:
Intracavitary Bacillus Calmette-Guerin in the treatment of
superficial bladder tumors. J Urol. 116:180–183. 1976.PubMed/NCBI
|
|
6
|
Herr HW and Morales A: History of bacillus
Calmette-Guerin and bladder cancer: an immunotherapy success story.
J Urol. 179:53–56. 2008. View Article : Google Scholar
|
|
7
|
Borre S, Brustia D, Rosa F, Brondolo R,
Rizzo G and Garavelli PL: Calmette-Guerin bacillus disseminated
infection after intra vesical instillation. Recenti Prog Med.
93:247–248. 2002.(In Italian).
|
|
8
|
Elmer A, Bermes U, Drath L, Buscher E and
Viertel A: Sepsis and multiple organ failure after BCG-instillation
for bladder cancer. Internist. 45:935–939. 2004.(In German).
|
|
9
|
Takahashi T, Kushiro A, Nomoto K, et al:
Antitumor effects of the intravesical instillation of heat killed
cells of the Lactobacillus casei strain Shirota on the murine
orthotopic bladder tumor MBT-2. J Urol. 166:2506–2511. 2001.
View Article : Google Scholar
|
|
10
|
Seow SW, Cai S, Rahmat JN, et al:
Lactobacillus rhamnosus GG induces tumor regression in mice bearing
orthotopic bladder tumors. Cancer Sci. 101:751–758. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ludwig AT, Moore JM, Luo Y, et al: Tumor
necrosis factor-related apoptosis-inducing ligand: a novel
mechanism for Bacillus Calmette-Guerin-induced antitumor activity.
Cancer Res. 64:3386–3390. 2004. View Article : Google Scholar
|
|
12
|
Kemp TJ: Neutrophil stimulation with
Mycobacterium bovis bacillus Calmette-Guerin (BCG) results in the
release of functional soluble TRAIL/Apo-2L. Blood. 106:3474–3482.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wiley SR, Schooley K, Smolak PJ, et al:
Identification and characterization of a new member of the TNF
family that induces apoptosis. Immunity. 3:673–682. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ashkenazi A, Pai RC, Fong S, et al: Safety
and antitumor activity of recombinant soluble Apo2 ligand. J Clin
Invest. 104:155–162. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Walczak H, Miller RE, Ariail K, et al:
Tumoricidal activity of tumor necrosis factor-related
apoptosis-inducing ligand in vivo. Nat Med. 5:157–163. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fesik SW: Promoting apoptosis as a
strategy for cancer drug discovery. Nat Rev Cancer. 5:876–885.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Soria JC, Smit E, Khayat D, et al: Phase
1b study of dulanermin (recombinant human Apo2L/TRAIL) in
combination with paclitaxel, carboplatin, and bevacizumab in
patients with advanced non-squamous non-small-cell lung cancer. J
Clin Oncol. 28:1527–1533. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schonbeck U, Mach F and Libby P:
Generation of biologically active IL-1 beta by matrix
metalloproteinases: a novel caspase-1-independent pathway of IL-1
beta processing. J Immunol. 161:3340–3346. 1998.PubMed/NCBI
|
|
19
|
Van den Steen PE, Proost P, Wuyts A, Van
Damme J and Opdenakker G: Neutrophil gelatinase B potentiates
interleukin-8 tenfold by aminoterminal processing, whereas it
degrades CTAP-III, PF-4, and GRO-alpha and leaves RANTES and MCP-2
intact. Blood. 96:2673–2681. 2000.
|
|
20
|
McQuibban GA, Gong JH, Tam EM, McCulloch
CA, Clark-Lewis I and Overall CM: Inflammation dampened by
gelatinase A cleavage of monocyte chemoattractant protein-3.
Science. 289:1202–1206. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Black RA, Rauch CT, Kozlosky CJ, et al: A
metalloproteinase disintegrin that releases tumour-necrosis
factor-alpha from cells. Nature. 385:729–733. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kawakubo T, Okamoto K, Iwata J, et al:
Cathepsin E prevents tumor growth and metastasis by catalyzing the
proteolytic release of soluble TRAIL from tumor cell surface.
Cancer Res. 67:10869–10878. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Weinlich R, Brunner T and Amarante-Mendes
GP: Control of death receptor ligand activity by posttranslational
modifications. Cell Mol Life Sci. 67:1631–1642. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dinney CP, Fishbeck R, Singh RK, et al:
Isolation and characterization of metastatic variants from human
transitional cell carcinoma passaged by orthotopic implantation in
athymic nude mice. J Urol. 154:1532–1538. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Taniguchi H, Yoshida T, Horinaka M, et al:
Baicalein overcomes tumor necrosis factor-related
apoptosis-inducing ligand resistance via two different
cell-specific pathways in cancer cells but not in normal cells.
Cancer Res. 68:8918–8927. 2008. View Article : Google Scholar
|
|
26
|
Matsubara H, Mizutani Y, Hongo F, et al:
Gene therapy with TRAIL against renal cell carcinoma. Mol Cancer
Ther. 5:2165–2171. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Okamoto T, Sasaki M, Tsujikawa T, Fujiyama
Y, Bamba T and Kusunoki M: Preventive efficacy of butyrate enemas
and oral administration of Clostridium butyricum M588 in dextran
sodium sulfate-induced colitis in rats. J Gastroenterol.
35:341–346. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Seki H, Shiohara M, Matsumura T, et al:
Prevention of antibiotic-associated diarrhea in children by
Clostridium butyricum MIYAIRI. Pediatr Int. 45:86–90. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Akira S, Takeda K and Kaisho T: Toll-like
receptors: critical proteins linking innate and acquired immunity.
Nat Immunol. 2:675–680. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takeda K and Akira S: Roles of Toll-like
receptors in innate immune responses. Genes Cells. 6:733–742. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Heldwein KA, Liang MD, Andresen TK, et al:
TLR2 and TLR4 serve distinct roles in the host immune response
against Mycobacterium bovis BCG. J Leukoc Biol. 74:277–286. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Godaly G and Young DB: Mycobacterium bovis
bacille Calmette Guerin infection of human neutrophils induces
CXCL8 secretion by MyD88-dependent TLR2 and TLR4 activation. Cell
Microbiol. 7:591–601. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ninomiya-Tsuji J, Kajino T, Ono K, et al:
A resorcylic acid lactone, 5Z-7-oxozeaenol, prevents inflammation
by inhibiting the catalytic activity of TAK1 MAPK kinase kinase. J
Biol Chem. 278:18485–18490. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Owen CA, Hu Z, Lopez-Otin C and Shapiro
SD: Membrane-bound matrix metalloproteinase-8 on activated
polymorphonuclear cells is a potent, tissue inhibitor of
metalloproteinase-resistant collagenase and serpinase. J Immunol.
172:7791–7803. 2004. View Article : Google Scholar
|
|
35
|
Pei D: Leukolysin/MMP25/MT6-MMP: a novel
matrix metal-loproteinase specifically expressed in the leukocyte
lineage. Cell Res. 9:291–303. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Faurschou M and Borregaard N: Neutrophil
granules and secretory vesicles in inflammation. Microbes Infect.
5:1317–1327. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kamat AM, Tharakan ST, Sung B and Aggarwal
BB: Curcumin potentiates the antitumor effects of Bacillus
Calmette-Guerin against bladder cancer through the downregulation
of NF-κB and upregulation of TRAIL receptors. Cancer Res.
69:8958–8966. 2009.PubMed/NCBI
|
|
38
|
Fujii H, Saimoto A, Nishikawa K, Yamazaki
S and Kameyama S: Effect of BCG Connaught strain on mouse bladder
tumor models. Biotherapy. 17:167–173. 2003.
|
|
39
|
Shimbo I, Yamaguchi T, Odaka T, et al:
Effect of Clostridium butyricum on fecal flora in Helicobacter
pylori eradication therapy. World J Gastroenterol. 11:7520–7524.
2005.PubMed/NCBI
|
|
40
|
Stricker P, Pryor K, Nicholson T, et al:
Bacillus Calmette-Guerin plus intravesical interferon alpha-2b in
patients with superficial bladder cancer. Urology. 48:952–961.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Esuvaranathan K, Chiong E, Thamboo TP, et
al: Predictive value of p53 and pRb expression in superficial
bladder cancer patients treated with BCG and interferon-alpha.
Cancer. 109:1097–1105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yoshida T, Horinaka M and Sakai T:
‘Combination-oriented molecular-targeting prevention’ of cancer: a
model involving the combination of TRAIL and a DR5 inducer. Environ
Health Prev Med. 15:203–210. 2010.
|
|
43
|
Matsui TA, Sowa Y, Yoshida T, et al:
Sulforaphane enhances TRAIL-induced apoptosis through the induction
of DR5 expression in human osteosarcoma cells. Carcinogenesis.
27:1768–1777. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shan Y, Sun C, Zhao X, Wu K, Cassidy A and
Bao Y: Effect of sulforaphane on cell growth, G(0)/G(1) phase cell
progression and apoptosis in human bladder cancer T24 cells. Int J
Oncol. 29:883–888. 2006.PubMed/NCBI
|
|
45
|
Munday R, Mhawech-Fauceglia P, Munday CM,
et al: Inhibition of urinary bladder carcinogenesis by broccoli
sprouts. Cancer Res. 68:1593–1600. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nomura T and Katunuma N: Involvement of
cathepsins in the invasion, metastasis and proliferation of cancer
cells. J Med Invest. 52:1–9. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Overall CM and Kleifeld O: Tumour
microenvironment -opinion: validating matrix metalloproteinases as
drug targets and anti-targets for cancer therapy. Nat Rev Cancer.
6:227–239. 2006. View Article : Google Scholar
|
|
48
|
Coussens LM, Fingleton B and Matrisian LM:
Matrix metalloproteinase inhibitors and cancer: trials and
tribulations. Science. 295:2387–2392. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Overall CM and Lopez-Otin C: Strategies
for MMP inhibition in cancer: innovations for the post-trial era.
Nat Rev Cancer. 2:657–672. 2002. View
Article : Google Scholar : PubMed/NCBI
|