Introduction
The morphological processes leading to cell death
include apoptosis, necrosis and autophagy (1–3).
Autophagy (or called self-eating) is a process maintaining cellular
homeostasis (4,5). When the cells undergo cellular
damage, autophagy is required for the promotion of cellular
survival (4,6). Autophagy involves the autophagosome
formation (a double-membrane structure), which fuses with a
lysosome to form an autophagolysosome, finally resulting in
degradation of the captured proteins or organelles by lysosomal
enzymes (7,8). Several reports have shown that
autophagy-related (Atg) proteins and microtubule-associated protein
1 light chain 3 (LC3) are major proteins involved in autophagy
processes (9–11). Induction of Atg and LC3 protein
levels has been linked with altering a variety of cellular
signaling pathways, such as adenosine monophosphate-activated
protein kinase (AMPK) (12–14),
mitogen-activated protein kinase (MAPK) (15,16)
and PI3K/Akt pathways (17,18).
Previous studies indicated that suppression of PI3K/Akt is involved
in regulating autophagy formation (19–21).
Chinese herbs are used for treatment of diseases in
Taiwan and in China for a long time (22,23).
Baicalin is one of the major flavonoids (molecular formula:
C21H18O11; Fig. 1) in the
traditional Chinese medicinal herb ‘Huang qin’ (Scutellaria
baicalensis Georgi) (24,25).
The baicalin exhibits many different pharmacological actions such
as anti-oxidant (26),
photo-protective (27), neural
protective (28,29), anti-depressant (30), anti-inflammatory (31,32),
anti-viral (33,34), anti-hepatotoxicity (35,36)
and anticancer effects (37–39).
Baicalin induces CA46 Burkitt lymphoma cell apoptosis through
inhibiting the PI3K/Akt kinase activity (40). Baicalin induces apoptosis in SW620
colorectal cancer cells in vitro and anticancer activity in
HCT-116 cells in vivo(41,42),
and Zheng et al demonstrated that baicalin induces apoptosis
in leukemia HL-60/ADR cells through inhibiting the PI3K/Akt kinase
(43). Our previous study
demonstrated that baicalin induced apoptosis in leukemia HL-60
cells through ER stress and mitochondrial-dependent pathways
(44). Recently, Zhang et
al pointed out that baicalin induces autophagy in human
hepatocellular carcinoma SMMC-7721 cells (45); however, there is no evidence to
show the effects of baicalin on the induction of autophagy in human
bladder cancer T24 cells. In the present study, we investigated the
pharmacological effects of the baicalin on inhibition of cell
growth and induction of cell autophagy in T24 cells. Our results
indicated that baicalin might contribute to cell autophagy via the
Akt pathway in T24 cells.
Materials and methods
Chemicals and reagents
Acridine orange (AO), Baicalin, 3- methyladenine
(3-MA), monodansylcadaverine (MDC) and tetrazolium
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA). Fetal
bovine serum (FBS), L-glutamine, penicillin/streptomycin and
trypsin-EDTA were obtained from Invitrogen Life Technologies
(Carlsbad, CA, USA). AKT kinase assay kit was obtained from Cell
Signaling Technology (Danvers, MA, USA). Tdt-mediated deoxyuridine
triphosphate nick end labeling (TUNEL) assay kit was purchased from
Roche Diagnostics (GmBH, Mannheim, Germany). Caspase-3 activity
assay kit was purchased from R&D Systems Inc. (Minneapolis, MN,
USA). The primary antibodies against Atg 5, Atg 7 and Atg 12,
Beclin, LC3-II, AKT and phospho-AKT (Ser473) were purchased from
Cell Signaling Technology. Antibody against β-actin was obtained
from Sigma Chemical Co. All peroxidase-conjugated secondary
antibodies were obtained from Santa Cruz Biotechnology Inc. (Santa
Cruz, CA, USA). The enhanced chemiluminescence (ECL) detection kit
was obtained from Pierce Chemical (Rockford, IL, USA).
Cell culture
The T24 human bladder cancer cell line was purchased
from the Food Industry Research and Development Institute (Hsinchu,
Taiwan). The cells were grown in McCoy’s 5a medium fortified with
10% FBS, 2 mM L-glutamine and penicillin/streptomycin and incubated
at 37°C under a humidified 5% CO2 atmosphere (46).
Cell viability and morphology
The cell viability was assessed by the MTT assay.
Briefly, the T24 cells were cultured in a 96-well plate at the
density of 1×104 cells/per well and were incubated with
0, 50, 100, 150 and 200 μM of baicalin for 24 h. For the
study of autophagy inhibition, cells were pre-treated with 3-MA (10
mM) for 1 h before the 24-h treatment of baicalin with indicated
concentrations. At the end of baicalin treatment, culture medium
containing MTT (0.5 mg/ml) was added to each well after washing the
cells. The cells were then incubated at 37°C for 4 h and the
supernatant was removed. The formed blue formazan crystals in
viable T24 cells were dissolved with isopropanol/0.04 N HCl,
followed by measurement of the absorbance of each well at 570 nm
with the ELISA reader with a reference wavelength of 620 nm. All
experiments were performed in triplicate. The cell viability of
each treatment was expressed as percentage of the control. The
morphological examination of autophagic vacuoles in
baicalin-treated cells was determined under a phase-contrast
microscope (18).
Trypan blue exclusion assay for cell
death
Trypan blue exclusion assay was used to evaluate
cell death induced by baicalin treatment. T24 cells in a 24-well
plate (2.5×105 cells/per well) were incubated with 0,
50, 100, 150 and 200 μM of baicalin. After 24 h, cells were
stained with 0.25% trypan blue solution and the numbers of dead
cells were determined by Countess Automated Cell Counter
(Invitrogen/Life Technologies) (18).
TUNEL staining
TUNEL staining was performed to detect apoptotic
cells according to the manufacturer’s protocol (in situ cell
death detection kit; Roche Diagnostics). T24 cells in a 24-well
plate (2.5×105 cells/per well) were exposed to 0, 50,
100, 150 and 200 μM of baicalin for 24 h. At the end of the
incubation, cells were collected, fixed with 70% ethanol and washed
twice with ice-cold PSB. After incubated in the dark for 30 min at
37°C in 100 μl of TdT-containing solution, the T24 samples
were washed once before flow cytometry analysis of the
TUNEL-positive cells using a FACSCalibur (Becton-Dickinson). The
median fluorescence intensity was quantified by CellQuest software
(18).
Caspase-3 activity assays
The caspase-3 activity assay was performed according
to the manufacturer’s instructions (Caspase Colorimetric Kit;
R&D Systems Inc.). Briefly, after a 24-h incubation with 0, 50,
100, 150 and 200 μM of baicalin, T24 cells
(∼1×107/75-T flask) were harvested. The collected cells
were then lysed in the lysis buffer [50 mM Tris-HCl (pH 7.4), 1 mM
EDTA, 10 mM EGTA, 10 mM digitonin and 2 mM DTT], followed by
centrifugation to collect total proteins in the supernatant. The
cell lysate containing 50 μg proteins were then incubated
for 1 h at 37°C with caspase-3 specific substrate (Ac-DEVD-pNA) in
the reaction buffer. The caspase-3 activity was determined by
measuring OD405 of the released pNA (18).
Detection of acidic vesicular organelles
(AVO) with acridine orange (AO) and acidic autophagic vacuoles with
monodan-sylcadaverine (MDC)
T24 cells were seeded on sterile coverslips in
tissue culture plates with a density of 5×104 cells/per
coverslip. After 0 or 200 μM of baicalin treatment for 24 h,
cells were stained with either acridine orange (AO) or 0.1 mM
monodansylcadaverine (MDC) at 37°C for 10 min. After three washes
with PBS, cells were immediately visualized by fluorescence
microscopy (Nikon, Melville, NY, USA) for the detection of acidic
vesicular organelles and MDC-positive autophagic vacuoles (18,47).
Western blot analysis
T24 cells (1×107/75-T flask) were treated
with 0, 50, 100, 150 and 200 μM of baicalin for 24 h, then
harvested, lysed and the total proteins were collected by SDS
sample buffer. In brief, ∼30 μg of protein from each
treatment was resolved on 10% SDS-polyacrylamide gel
electrophoresis (SDS-PAGE) and electro-transferred to a
nitro-cellulose membrane. The transferred membranes were blocked in
5% non-fat dry milk in 20 mM Tris-buffered saline/0.05% Tween-20
for 1 h at room temperature followed by incubation with primary
antibodies against indicated autophagic-associated proteins or AKT
and autophagy pathway-related proteins at 4°C overnight. At the end
of incubation, membranes were washed with Tris-buffered
saline/Tween-20 and incubated with secondary antibodies conjugated
with horseradish peroxidase (HRP). The blots were developed by a
chemiluminescence kit (Millipore, Bedford, MA, USA), followed by
X-ray film exposure. Each membrane was stripped and reprobed with
anti-β-actin antibody to ensure equal protein loading during the
experiment (18).
In vitro AKT kinase assay
In brief, T24 cells (1×107/75-T flask)
were treated with 0, 50, 100, 150 and 200 μM of baicalin for
6 h. Cells were lysed in ice-cold lysis buffer provided by the kit.
The 200 μg of protein from each time-point of treatment was
immuno-precipitated with 2 μg of anti-AKT antibody
overnight. Immuno-precipitates were extensively washed and then
incubated with 1 μg of GSK-3 α/β fusion protein substrate in
50 μl of kinase buffer for 30 min at 30°C. Reactions were
stopped by SDS loading buffer and samples were separated on 12%
SDS-PAGE. The phospho-GSK-3 α/β (Ser219) was detected by
immunoblotting (18).
Statistical analysis
All the statistical results are presented as the
mean ± SEM for the indicated numbers of separate experiments.
Statistical analyses of data were done using one-way ANOVA followed
by Student’s t-test and *P<0.05,
**P<0.01, ***P<0.001 were considered
statistically significant (18).
Results
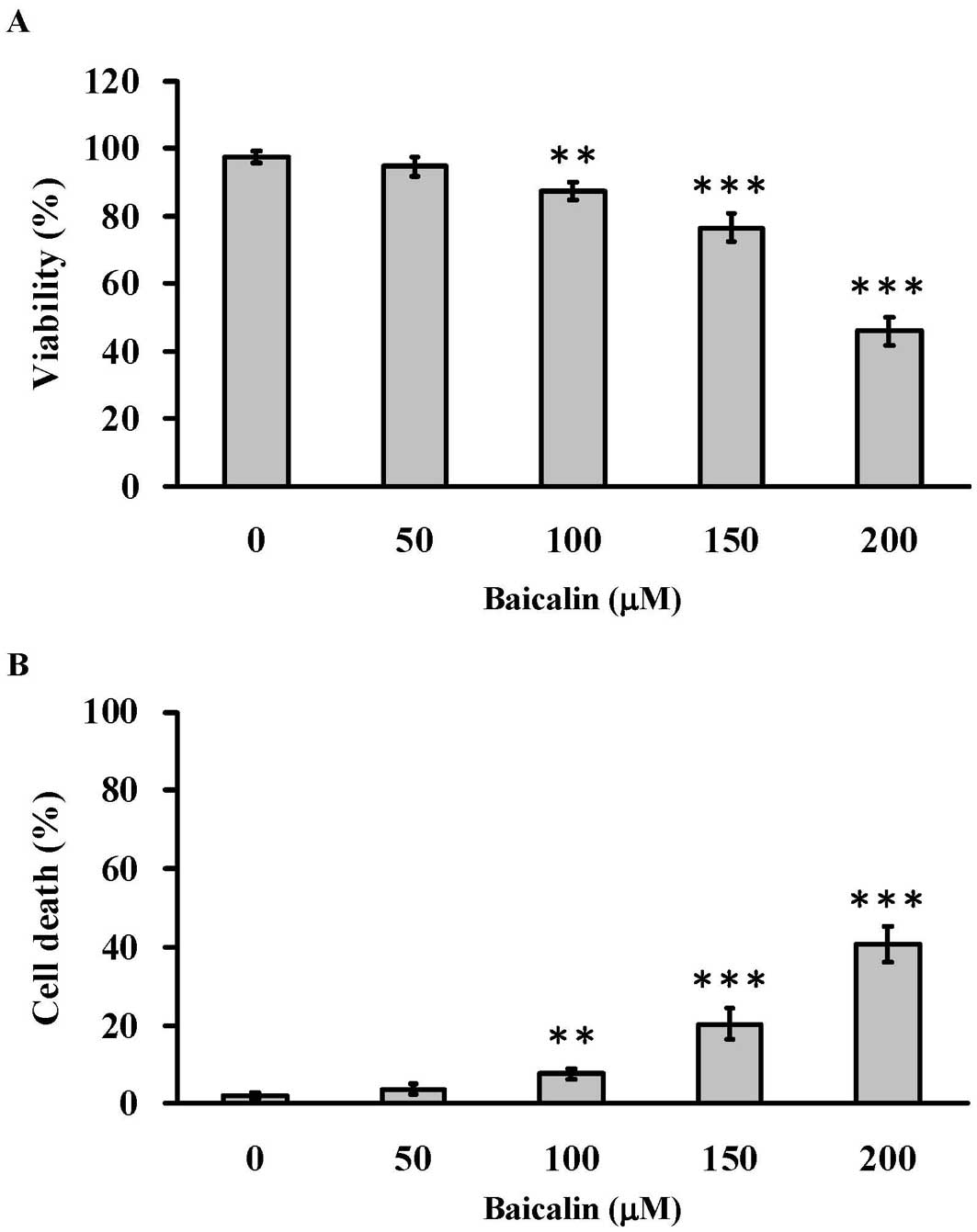
Baicalin decreased the viability of T24
human bladder cancer cells
The human bladder cancer cells T24 were treated with
baicalin (0, 50, 100, 150 and 200 μM) for 24 h. Results from
the MTT assay showed that even though 50 μM of baicalin did
not reduce cell viability, increased concentrations of baicalin
treatment (100, 150 and 200 μM) significantly led to
decrease of cell viability in T24 cells in a
concentration-dependent manner (Fig.
2A). Fig. 2B showed that
baicalin increased the number of cell death at 100, 150 and 200
μM in a concentration-dependent manner using the trypan blue
exclusion assay.
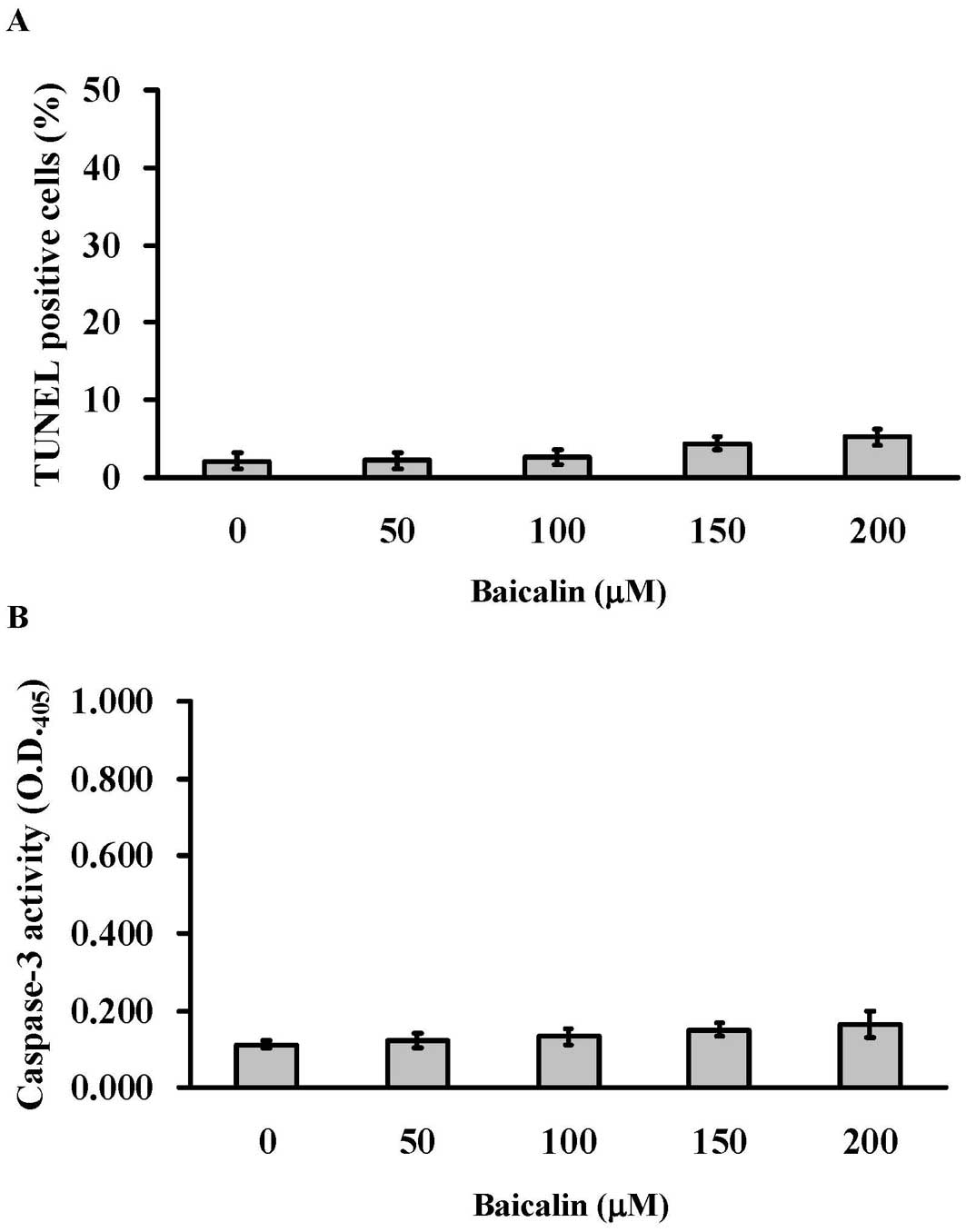
Baicalin induces caspase-independent cell
death in T24 cells
To verify whether baicalin induced apoptosis in T24
cells, cells were treated with baicalin (0, 50, 100, 150 and 200
μM) for 24 h before subjected to TUNEL staining. Fig. 3A indicated that the percentages of
TUNEL-positive cells in baicalin-treated groups were <5%. In
addition, to further examine whether the cell death caused by
baicalin treatment was mediated through caspase-3 activation,
protein samples collected from T24 cells after baicalin (0, 50,
100, 150 and 200 μM) treatment were analyzed for caspase-3
activity. The caspase-3 activity assay showed no changes in
baicalin-treated cells regardless of the baicalin concentrations
(Fig. 3B). Our results
demonstrated that apoptosis and the activation of caspase-3 were
not involved in baicalin-induced cell death.
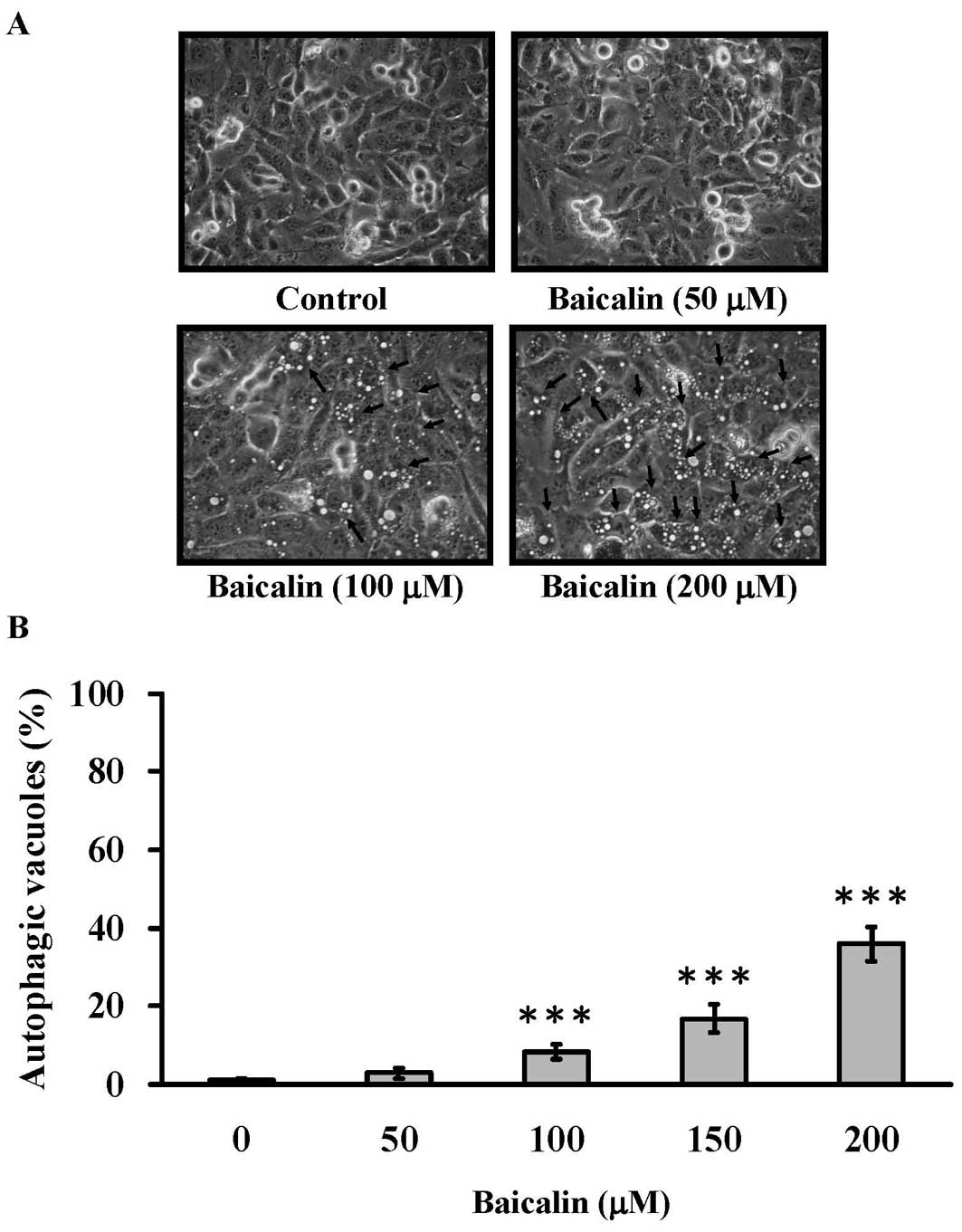
Baicalin induces cell autophagy in T24
cells
We further investigated whether the cell death
caused by baicalin treatment was mediated by autophagy. T24 cells
were treated with baicalin (0, 50, 100, 150 and 200 μM) for
24 h and the formation of autophagic vacuoles was examined under a
phase contrast microscope. As shown in Fig. 4A, 100 and 200 μM of baicalin
treatment induced the formation of autophagic vacuoles, while
baicalin at control and 50 μM induced hardly any formation
of autophagic vacuoles. In addition, the amount of autophagic
vacuole formation was significantly elevated in a
concentration-dependent manner in higher baicalin concentration
groups (≥100 μM) (Fig. 4B).
Especially, upon the challenge of 200 μM baicalin for 24 h,
∼40% of cells manifested autophagic vacuoles.
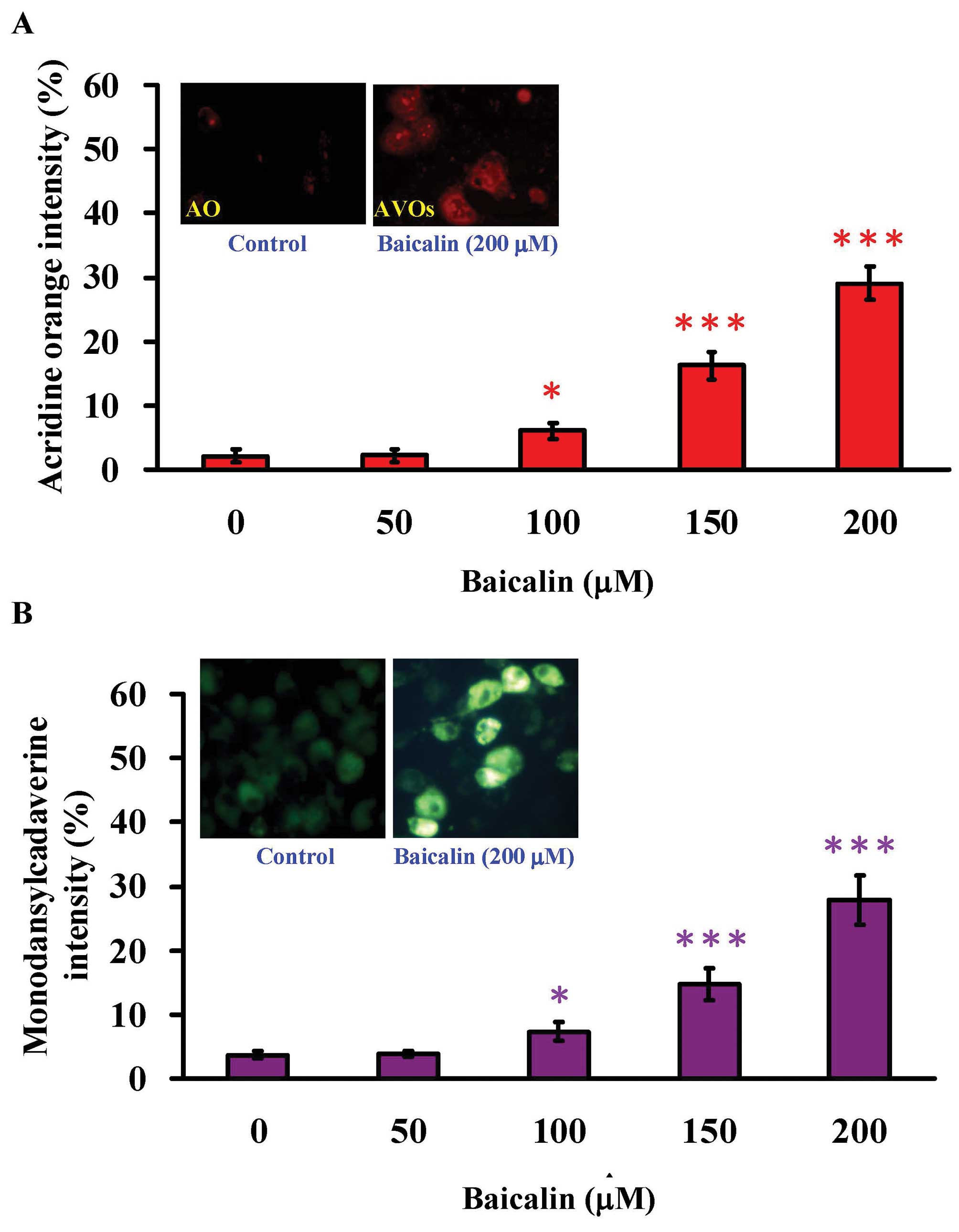
One of the hallmarks of autophagic cell death is the
cytosolic acidic vesicular organells (AVO) (48). Through the staining of acridine
orange (AO), a lysotropic dye that emits bright red fluorescence
inside the low pH acidic vesicles, AVO were noticeably observed in
the cytoplasm of baicalin-treated T24 cells (200 μM of
baicalin) when compared to the control group by the fluorescence
microscopy. In addition, as the concentration of baicalin
increased, the measured AO intensity became stronger (Fig. 5A). Furthermore, we confirmed the
autophagic cell death caused by baicalin treatment using
monodansylcadaverine (MDC) staining. MDC is another widely used
fluorescent marker that preferentially accumulates in autophagic
vacuoles. As shown in Fig. 5B, T24
cells treated 200 μM of baicalin for 24 h clearly showed
autophagic vacuoles, while very few autophagic vacuoles were
observed in the control group. Again, the MDC intensity increased
as the baicalin concentration increased. Our results indicated that
autophagy was the mechanism underlying baicalin-induced cell
death.
Baicalin regulates the
autophagy-associated protein levels in T24 cells
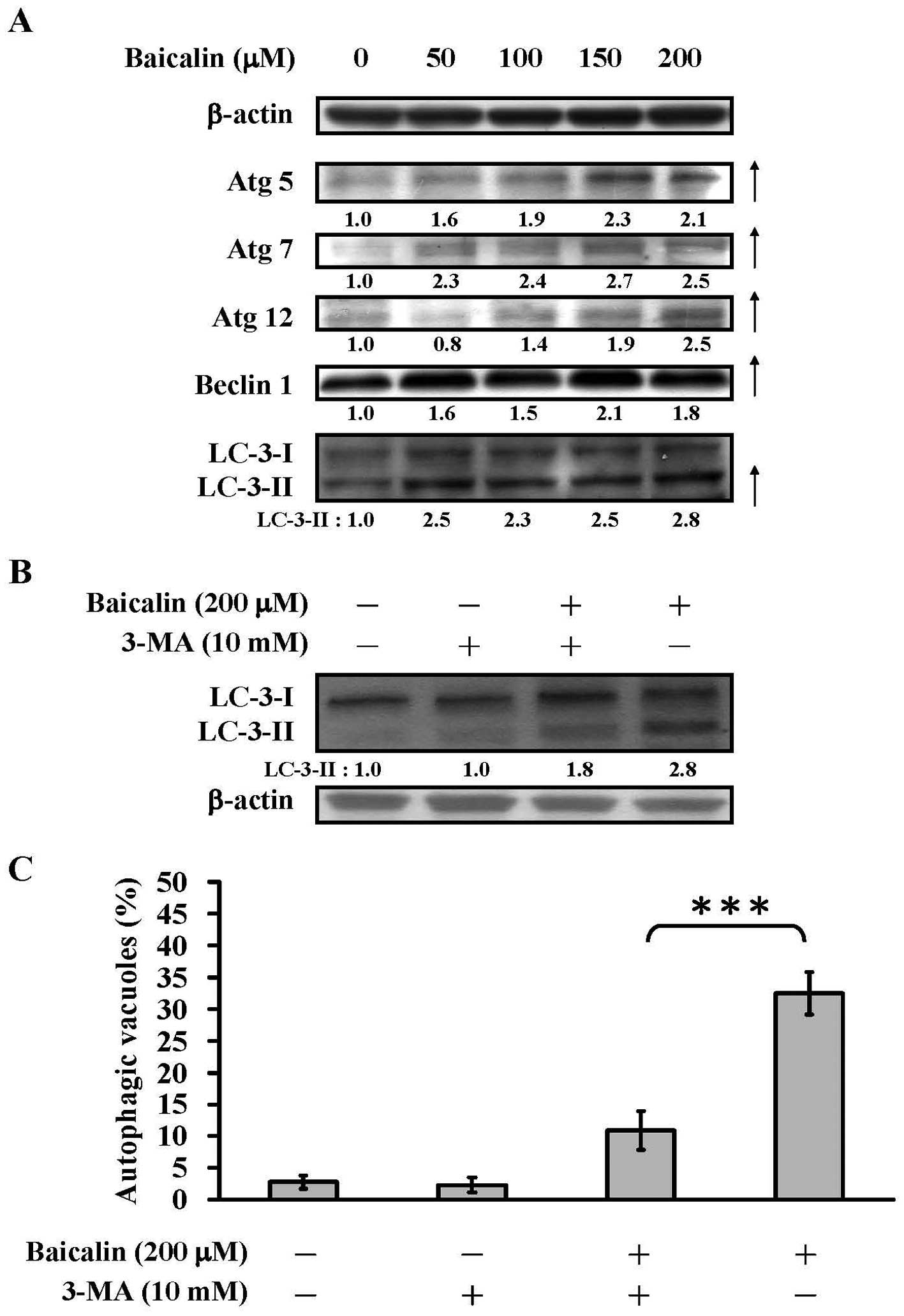
It has been shown that the autophagic cell death is
associated with the elevations of autophagosome formation protein
levels. Those proteins includes light-chain-3 (LC-3), Atg complex
(Atg 5, Atg 7 and Atg 12) and Beclin-1 (18,49).
As examined by western blot analysis, baicalin increased the
protein expression of Atg 5, Atg 7 and Atg 12, Beclin-1 and LC-3 II
(Fig. 6A). For example, when
compared with the control group, the respective protein levels of
Atg 5, Atg 7 and Atg 12, Beclin-1 and LC-3 II were 2.1-, 2.5-,
2.5-, 1.8- and 2.8-fold higher after treated with 200 μM of
baicalin for 24 h.
Among the afore-mentioned proteins, the
microtubule-associated protein light-chain 3 (LC-3) is a reliable
autophagic membrane marker for the detection of early autophagosome
formation (50,51). The conversion of LC-3I to LC-3II is
indicative of autophagic activity. We next examined whether
3-methyladenine (3-MA), a commonly used reagent that inhibits
autophagy by blocking autophagosome formation via the inhibition of
type III phosphatidylinositol 3-kinases (PI-3K), could attenuate
the elevated LC-3 II expression induced by baicalin. As shown in
Fig. 6B, 200 μM of baicalin
treatment upregulated the LC-3 II protein levels to 2.8-fold when
compared to the control group. Nevertheless, 3-MA pretreatment (10
mM) decreased the LC-3II expression level to 1.8-fold in the
presence of 200 μM of baicalin. The quantitative data from
the numbers of autophagic vacuoles also indicated that the
baicalin-induced autophagic vacuoles formation was sharply
diminished upon 3-MA pretreatment (Fig. 6C). The experimental results
(Fig. 6) indicated that baicalin
induced autophagic cell death through upregulation of proteins
associated with autophagosome formation and it could be attenuated
by 3-MA.
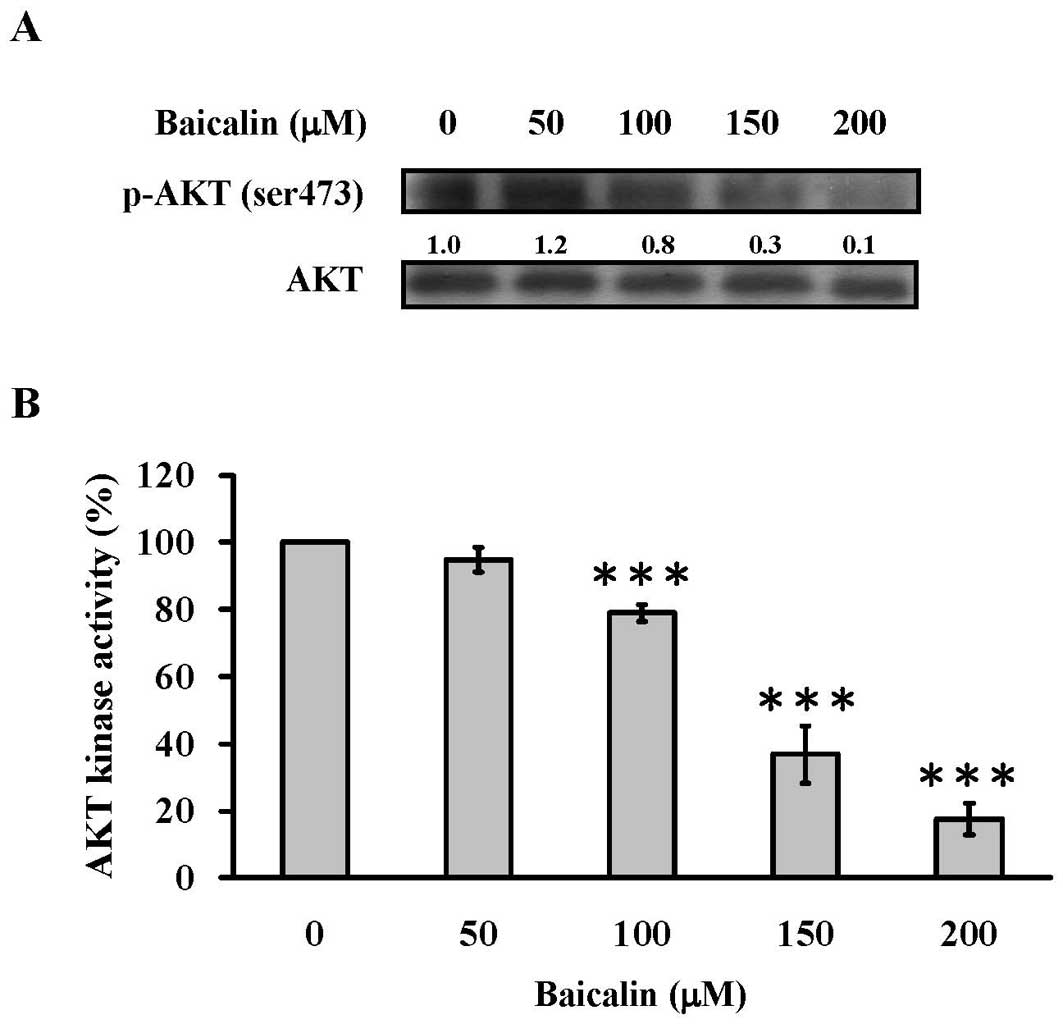
Baicalin blocks the AKT signaling in T24
cells
The AKT activity has been demonstrated to contribute
to autophagic cell death (6,40).
We next performed western blot analysis and AKT kinase activity
assay to investigate whether the AKT signaling was involved in the
baicalin-induced autophagic cell death in T24 cells. The present
study showed that baicalin decreased the phosphor-AKT (Ser473)
protein levels in T24 cells in a concentration-dependent manner
(Fig. 7A). In addition, baicalin
inhibited AKT kinase activity and the inhibition was
concentration-dependent (Fig. 7B).
Our data implied that baicalin induced cell autophagy in T24 cells
through blocking the AKT signaling.
Discussion
Previous studies have showed that Scutellaria
baicalensis Gerogi containing over 30 different kinds of
flavonoids (24,52,53),
including baicalin, baicalein, oroxylin A and wogonin (53–55).
It was reported that ethanol extracts of Scutellaria
baicalensis Gerogi prevent oxidative damage (56,57)
and has anti-inflammation (58,59)
and anti-angiogenesis effects (60). In addition, Scutellaria
baicalensis Gerogi extract triggers G2/M arrest and
caspase-dependent apoptosis by modulating ERK pathway in HSC-T6
cells (58). In the present study,
we focused on the baicalin from Scutellaria baicalensis
Gerogi for their anticancer effect on human bladder cancer T24
cells. Baicalin is a natural flavonoids compound with anticancer
activity and low toxicity against normal cells (61,62).
Previous reports showed that baicalin exerted anti-proliferative
ability and induced apoptotic effects in many cancer cell lines
(CA46, SW620, HCT-116, HL-60/ADR and HL-60) (40,41,43,44,63,64).
In this study, we investigated the anticancer effects of baicalin
on T24 human bladder cancer cells in vitro. Our results
showed that baicalin exerted a significant anti-proliferative
effect on T24 cells (Fig. 2A).
Baicalin is a new anticancer agent and has apoptotic effect on T24
cells. However, the apoptotic TUNEL-positive cells and caspase-3
activity did not change in baicalin-treated T24 cells (Fig. 3). Data suggested that there may be
another mechanism involved in baicalin-induced cell death in T24
cells.
Many studies have suggested the autophagy has a
cancer suppressor role (65).
Several traditional Chinese medicines such as arsenic trioxide
(AS2O3) (66), berberine (67), bufalin (18) and kaempferol (68) have been demonstrated to induce
autophagy and to exert anticancer activity in cancer cells.
Intriguingly, baicalin-induced autophagy in T24 cells was
demonstrated by autophagic vesicle formation (Fig. 4). Baicalin induced autophagy
generation shown by larger bright-red AO-stained vacuoles (Fig. 5A) and induction of the LC3 cleavage
(Fig. 6A). In contrast, protein
levels of the LC3-II, Beclin-1, Atg 5, Atg 7 and Atg 12 were
upregulated in T24 cells after baicalin treatment (Fig. 6A). When T24 cells were pre-treated
with 3-MA followed by treatment with baicalin, LC3 protein cleavage
(Fig. 6B) and autophagic vesicle
formation (Fig. 6C) were
significantly decreased compared with the baicalin alone treatment
group. Our results demonstrated that baicalin-induced cell death
possibly involved autophagy, and is the first detailed evidence
that baicalin induced autophagy in T24 cells. Our findings are in
agreement with previous studies that baicalin induced autophagy in
SMMC-7721 cells (45).
Akt serine/threonine kinase [also called protein
kinase B (PKB)] is one of the most regularly activated protein
kinases in human bladder cancer (69–71).
Activation of Akt is associated with anti-apoptosis, cell
proliferation and cellular energy metabolism (72). The AKT pathway is frequently
activated in human bladder cancer cells. Askham et al
demonstrated that the AKT1 G49A (E17K) mutation led to constitutive
AKT1 activation and was found in 4.8% bladder cancer cell lines and
2.7% bladder tumors (73).
Regulating the Akt pathway is potentially essential for developing
therapeutic inhibitors in human bladder cancer. Dickstein et
al demonstrated that the AKT inhibitor AZ7328 has synergistic
effect on inducing apoptosis with autophagy inhibitors in human
bladder cancer cells (74). Wu
et al demonstrated that PI-3 kinase inhibitor LY294002
inhibits cell proliferation and sensitizes doxorubicin in human
bladder cancer cells (75). Our
study demonstrated baicalin induced autophagy accompanied with
downregulation of phospho-AKT (Ser473) protein level (Fig. 7A) and Akt kinase activity (Fig. 7B). Previous studies demonstrated
that baicalin induced apoptotic cell death through inhibiting the
AKT signaling pathway in CA46 Burkitt lymphoma and leukemia
HL-60/ADR cells (40,76). In the present study, the result
showed that the AKT pathway is associated with the induction of
autophagy in baicalin-treated T24 cells.
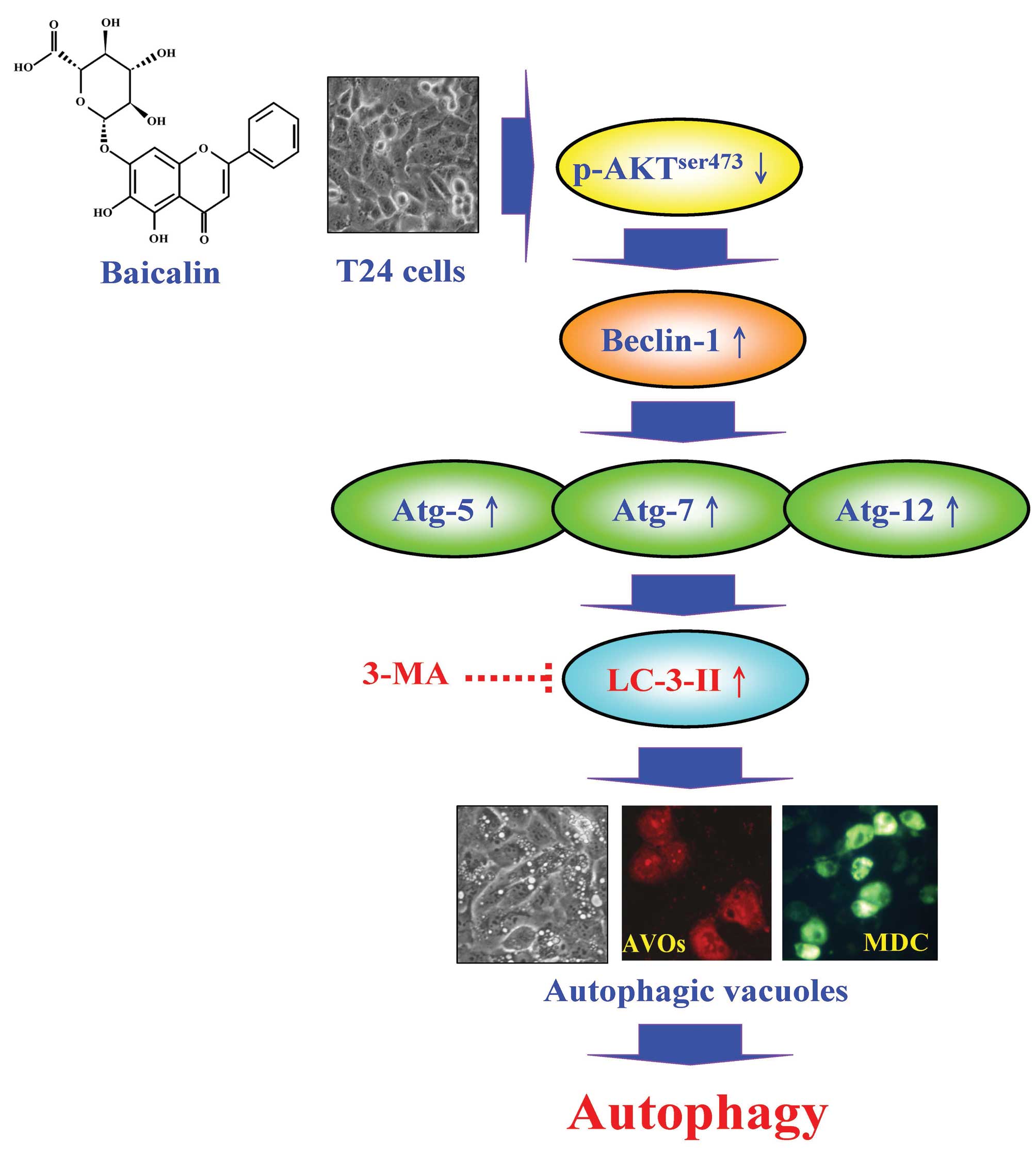
The molecular mechanisms underlying the inhibitory
effect of baicalin on T24 cell proliferation are summarized in
Fig. 8. In conclusion, baicalin
induces autophagy through the Akt signaling pathway in T24 human
bladder cancer cells. Our findings imply that baicalin may be used
as a novel anticancer drug candidate for the treatment of human
bladder cancer.
Acknowledgements
We thank the grant-in-aid
DOH101-TD-C-111-005 from Taiwan Department of Health, China Medical
University Hospital Cancer Research Center of Excellence. This
study was supported by the grant from the National Science Council,
Republic of China (Taiwan). This study was also supported in part
by grant from China Medical University (CMU101-S-27) awarded to
J.-S. Yang.
References
|
1
|
Golstein P and Kroemer G: Cell death by
necrosis: towards a molecular definition. Trends Biochem Sci.
32:37–43. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bustamante-Marin X, Quiroga C, Lavandero
S, Reyes JG and Moreno RD: Apoptosis, necrosis and autophagy are
influenced by metabolic energy sources in cultured rat
spermatocytes. Apoptosis. 17:539–550. 2012.PubMed/NCBI
|
|
3
|
Edinger AL and Thompson CB: Death by
design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol.
16:663–669. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mah LY and Ryan KM: Autophagy and cancer.
Cold Spring Harb Perspect Biol. 4:a0088212012.PubMed/NCBI
|
|
5
|
Lockshin RA and Zakeri Z: Cell death in
health and disease. J Cell Mol Med. 11:1214–1224. 2007. View Article : Google Scholar
|
|
6
|
Glick D, Barth S and Macleod KF:
Autophagy: cellular and molecular mechanisms. J Pathol. 221:3–12.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaminskyy V and Zhivotovsky B: Proteases
in autophagy. Biochim Biophys Acta. 1824:44–50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen Y and Yu L: Autophagic lysosome
reformation. Exp Cell Res. 19:142–146. 2013. View Article : Google Scholar
|
|
9
|
Mizushima N: The role of the Atg1/ULK1
complex in autophagy regulation. Curr Opin Cell Biol. 22:132–139.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Reggiori F: 1. Membrane origin for
autophagy. Curr Top Dev Biol. 74:1–30. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tanida I, Ueno T and Kominami E: LC3
conjugation system in mammalian autophagy. Int J Biochem Cell Biol.
36:2503–2518. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang WL, Perillo W, Liou D, Marambaud P
and Wang P: AMPK inhibitor compound C suppresses cell proliferation
by induction of apoptosis and autophagy in human colorectal cancer
cells. J Surg Oncol. 106:680–688. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ge W, Guo R and Ren J: AMP-dependent
kinase and autophagic flux are involved in aldehyde
dehydrogenase-2-induced protection against cardiac toxicity of
ethanol. Free Radic Biol Med. 51:1736–1748. 2011. View Article : Google Scholar
|
|
14
|
Wu Y, Li X, Zhu JX, et al:
Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of
Parkinson’s disease. Neurosignals. 19:163–174. 2011.PubMed/NCBI
|
|
15
|
Li ZY, Yang Y, Ming M and Liu B:
Mitochondrial ROS generation for regulation of autophagic pathways
in cancer. Biochem Biophys Res Commun. 414:5–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dagda RK, Zhu J, Kulich SM and Chu CT:
Mitochondrially localized ERK2 regulates mitophagy and autophagic
cell stress: implications for Parkinson’s disease. Autophagy.
4:770–782. 2008.PubMed/NCBI
|
|
17
|
Nishiyama Y, Shimada Y, Yokoi T, et al:
Akt inactivation induces endoplasmic reticulum stress-independent
autophagy in fibroblasts from patients with Pompe disease. Mol
Genet Metab. 107:490–495. 2012. View Article : Google Scholar
|
|
18
|
Tsai SC, Yang JS, Peng SF, et al: Bufalin
increases sensitivity to AKT/mTOR-induced autophagic cell death in
SK-HEP-1 human hepatocellular carcinoma cells. Int J Oncol.
41:1431–1442. 2012.
|
|
19
|
Chen J, Crawford R and Xiao Y: Vertical
inhibition of the PI3K/Akt/mTOR pathway for the treatment of
osteoarthritis. J Cell Biochem. Aug 28–2012.(Epub ahead of print).
View Article : Google Scholar
|
|
20
|
Zeng T, Zhang CL, Song FY, et al: PI3K/Akt
pathway activation was involved in acute ethanol-induced fatty
liver in mice. Toxicology. 296:56–66. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martelli AM, Evangelisti C, Follo MY, et
al: Targeting the phosphatidylinositol 3-kinase/Akt/mammalian
target of rapamycin signaling network in cancer stem cells. Curr
Med Chem. 18:2715–2726. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hugel HM, Jackson N, May BH and Xue CC:
Chinese herbs for dementia diseases. Mini Rev Med Chem. 12:371–379.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen S, Wu T, Kong X and Yuan H: Chinese
medicinal herbs for measles. Cochrane Database Syst Rev. Nov
9–2011.CD005531 View Article : Google Scholar
|
|
24
|
Yuan Y, Shuai L, Chen S, Huang L, Qin S
and Yang Z: Flavonoids and antioxidative enzymes in
temperature-challenged roots of Scutellaria baicalensis Georgi. Z
Naturforsch C. 67:77–85. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma AT, Zhong XH, Liu ZM, et al: Protective
effects of baicalin against bromocriptine induced abortion in mice.
Am J Chin Med. 37:85–95. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Waisundara VY, Siu SY, Hsu A, Huang D and
Tan BK: Baicalin upregulates the genetic expression of antioxidant
enzymes in Type-2 diabetic Goto-Kakizaki rats. Life Sci.
88:1016–1025. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bing-Rong Z, Song-Liang J, Xiao EC, et al:
Protective effect of the Baicalin against DNA damage induced by
ultraviolet B irradiation to mouse epidermis. Photodermatol
Photoimmunol Photomed. 24:175–182. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li HY, Hu J, Zhao S, et al: Comparative
study of the effect of baicalin and its natural analogs on neurons
with oxygen and glucose deprivation involving innate immune
reaction of TLR2/TNFalpha. J Biomed Biotechnol. Mar 21–2012.(Epub).
267890 View Article : Google Scholar
|
|
29
|
Cao Y, Mao X, Sun C, et al: Baicalin
attenuates global cerebral ischemia/reperfusion injury in gerbils
via anti-oxidative and anti-apoptotic pathways. Brain Res Bull.
85:396–402. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
de Carvalho RS, Duarte FS and de Lima TC:
Involvement of GABAergic non-benzodiazepine sites in the
anxiolytic-like and sedative effects of the flavonoid baicalein in
mice. Behav Brain Res. 221:75–82. 2011.PubMed/NCBI
|
|
31
|
Zhu J, Wang J, Sheng Y, et al: Baicalin
improves survival in a murine model of polymicrobial sepsis via
suppressing inflammatory response and lymphocyte apoptosis. PLoS
One. 7:e355232012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fu S, Sun C, Tao X and Ren Y:
Anti-inflammatory effects of active constituents extracted from
Chinese medicinal herbs against Propionibacterium acnes. Nat Prod
Res. 26:1746–1749. 2012. View Article : Google Scholar
|
|
33
|
Chu ZY, Chu M and Teng Y: Effect of
baicalin on in vivo anti-virus. Zhongguo Zhong Yao Za Zhi.
32:2413–2415. 2007.(In Chinese).
|
|
34
|
Kitamura K, Honda M, Yoshizaki H, et al:
Baicalin, an inhibitor of HIV-1 production in vitro. Antiviral Res.
37:131–140. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qiao H, Han H, Hong D, Ren Z, Chen Y and
Zhou C: Protective effects of baicalin on carbon tetrachloride
induced liver injury by activating PPARgamma and inhibiting
TGFbeta1. Pharm Biol. 49:38–45. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hwang JM, Wang CJ, Chou FP, et al:
Protective effect of baicalin on tert-butyl hydroperoxide-induced
rat hepatotoxicity. Arch Toxicol. 79:102–109. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chiu YW, Lin TH, Huang WS, et al:
Baicalein inhibits the migration and invasive properties of human
hepatoma cells. Toxicol Appl Pharmacol. 255:316–326. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shieh DE, Cheng HY, Yen MH, Chiang LC and
Lin CC: Baicalin-induced apoptosis is mediated by Bcl-2-dependent,
but not p53-dependent, pathway in human leukemia cell lines. Am J
Chin Med. 34:245–261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Motoo Y and Sawabu N: Antitumor effects of
saikosaponins, baicalin and baicalein on human hepatoma cell lines.
Cancer Lett. 86:91–95. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang Y, Hu J, Zheng J, et al:
Down-regulation of the PI3K/Akt signaling pathway and induction of
apoptosis in CA46 Burkitt lymphoma cells by baicalin. J Exp Clin
Cancer Res. 31:482012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen WC, Kuo TH, Tzeng YS and Tsai YC:
Baicalin induces apoptosis in SW620 human colorectal carcinoma
cells in vitro and suppresses tumor growth in vivo. Molecules.
17:3844–3857. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee DH, Kim C, Zhang L and Lee YJ: Role of
p53, PUMA, and Bax in wogonin-induced apoptosis in human cancer
cells. Biochem Pharmacol. 75:2020–2033. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zheng J, Hu JD, Chen YY, et al: Baicalin
induces apoptosis in leukemia HL-60/ADR cells via possible
down-regulation of the PI3K/Akt signaling pathway. Asian Pac J
Cancer Prev. 13:1119–1124. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lu HF, Hsueh SC, Ho YT, et al: ROS
mediates baicalin-induced apoptosis in human promyelocytic leukemia
HL-60 cells through the expression of the Gadd153 and
mitochondrial-dependent pathway. Anticancer Res. 27:117–125.
2007.
|
|
45
|
Zhang X, Tang X, Liu H, Li L, Hou Q and
Gao J: Autophagy induced by baicalin involves downregulation of
CD147 in SMMC-7721 cells in vitro. Oncol Rep. 27:1128–1134.
2012.PubMed/NCBI
|
|
46
|
Huang WW, Yang JS, Pai SJ, et al: Bufalin
induces G(0)/G(1) phase arrest through inhibiting the levels of
cyclin D, cyclin E, CDK2 and CDK4, and triggers apoptosis via
mitochondrial signaling pathway in T24 human bladder cancer cells.
Mutat Res. 732:26–33. 2012. View Article : Google Scholar
|
|
47
|
Kim JY, Cho TJ, Woo BH, et al:
Curcumin-induced autophagy contributes to the decreased survival of
oral cancer cells. Arch Oral Biol. 57:1018–1025. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Munafo DB and Colombo MI: A novel assay to
study autophagy: regulation of autophagosome vacuole size by amino
acid deprivation. J Cell Sci. 114:3619–3629. 2001.PubMed/NCBI
|
|
49
|
McCoy F, Hurwitz J, McTavish N, et al:
Obatoclax induces Atg7-dependent autophagy independent of beclin-1
and BAX/BAK. Cell Death Dis. 1:e1082010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Barth S, Glick D and Macleod KF:
Autophagy: assays and artifacts. J Pathol. 221:117–124. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mizushima N and Yoshimori T: How to
interpret LC3 immuno-blotting. Autophagy. 3:542–545. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yu K, Gong Y, Lin Z and Cheng Y:
Quantitative analysis and chromatographic fingerprinting for the
quality evaluation of Scutellaria baicalensis Georgi using
capillary electrophoresis. J Pharm Biomed Anal. 43:540–548. 2007.
View Article : Google Scholar
|
|
53
|
Zhou XQ, Liang H, Lu XH, Cai SQ, Wang B
and Zhao YY: Flavonoids from Scutellaria baicalensis and their
bioactivities. Beijing Da Xue Xue Bao. 41:578–584. 2009.(In
Chinese).
|
|
54
|
Liu B, Shi RB and Zhu LJ: HPLC fingerprint
of flavonoids of Kushen Tang and its correlation to Scutellaria
baicalensis and Sophora flavescens. Zhongguo Zhong Yao Za Zhi.
32:1631–1634. 2007.(In Chinese).
|
|
55
|
Kim YH, Jeong DW, Kim YC, Sohn DH, Park ES
and Lee HS: Pharmacokinetics of baicalein, baicalin and wogonin
after oral administration of a standardized extract of Scutellaria
baicalensis, PF-2405 in rats. Arch Pharm Res. 30:260–265. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hirunuma M, Shoyama Y, Sasaki K, et al:
Flavone-catalyzed apoptosis in Scutellaria baicalensis.
Phytochemistry. 72:752–760. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Choi J, Conrad CC, Malakowsky CA, Talent
JM, Yuan CS and Gracy RW: Flavones from Scutellaria baicalensis
Georgi attenuate apoptosis and protein oxidation in neuronal cell
lines. Biochim Biophys Acta. 1571:201–210. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Pan TL, Wang PW, Leu YL, Wu TH and Wu TS:
Inhibitory effects of Scutellaria baicalensis extract on hepatic
stellate cells through inducing G2/M cell cycle arrest and
activating ERK-dependent apoptosis via Bax and caspase pathway. J
Ethnopharmacol. 139:829–837. 2012. View Article : Google Scholar
|
|
59
|
Li HB, Jiang Y and Chen F: Separation
methods used for Scutellaria baicalensis active components. J
Chromatogr B Analyt Technol Biomed Life Sci. 812:277–290. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wang S, Zheng Z, Weng Y, et al:
Angiogenesis and anti-angiogenesis activity of Chinese medicinal
herbal extracts. Life Sci. 74:2467–2478. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhu H, Wang Z, Xing Y, et al: Baicalin
reduces the permeability of the blood-brain barrier during hypoxia
in vitro by increasing the expression of tight junction proteins in
brain microvascular endothelial cells. J Ethnopharmacol.
141:714–720. 2012. View Article : Google Scholar
|
|
62
|
Hu Q, Noor M, Wong YF, et al: In vitro
anti-fibrotic activities of herbal compounds and herbs. Nephrol
Dial Transplant. 24:3033–3041. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yang BL, Chen HJ, Chen YG, et al:
Inhibitory effects of baicalin on orthotopic xenografts of
colorectal cancer cells that are deficient in a mismatch repair
gene in nude mice. Int J Colorectal Dis. Aug 23–2012.(Epub ahead of
print).
|
|
64
|
Ren X, Li CL, Wang HX, et al: Molecular
mechanism of HL-60 cell apoptosis induced by baicalin. Zhongguo Shi
Yan Xue Ye Xue Za Zhi. 20:847–851. 2012.(In Chinese).
|
|
65
|
Carew JS, Kelly KR and Nawrocki ST:
Autophagy as a target for cancer therapy: new developments. Cancer
Manag Res. 4:357–365. 2012.
|
|
66
|
Goussetis DJ, Altman JK, Glaser H, McNeer
JL, Tallman MS and Platanias LC: Autophagy is a critical mechanism
for the induction of the antileukemic effects of arsenic trioxide.
J Biol Chem. 285:29989–29997. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wang N, Feng Y, Zhu M, et al: Berberine
induces autophagic cell death and mitochondrial apoptosis in liver
cancer cells: the cellular mechanism. J Cell Biochem.
111:1426–1436. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Filomeni G, Desideri E, Cardaci S, et al:
Carcinoma cells activate AMP-activated protein kinase-dependent
autophagy as survival response to kaempferol-mediated energetic
impairment. Autophagy. 6:202–216. 2010. View Article : Google Scholar
|
|
69
|
Shahjee HM, Koch KR, Guo L, Zhang CO and
Keay SK: Antiproliferative factor decreases Akt phosphorylation and
alters gene expression via CKAP4 in T24 bladder carcinoma cells. J
Exp Clin Cancer Res. 29:1602010. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Chen M, Cassidy A, Gu J, et al: Genetic
variations in PI3K-AKT-mTOR pathway and bladder cancer risk.
Carcinogenesis. 30:2047–2052. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Champelovier P, El Atifi M, Mantel F, et
al: In vitro tumoral progression of human bladder carcinoma: role
for TGFbeta. Eur Urol. 48:846–851. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Stueckle TA, Lu Y, Davis ME, et al:
Chronic occupational exposure to arsenic induces carcinogenic gene
signaling networks and neoplastic transformation in human lung
epithelial cells. Toxicol Appl Pharmacol. 261:204–216. 2012.
View Article : Google Scholar
|
|
73
|
Askham JM, Platt F, Chambers PA, Snowden
H, Taylor CF and Knowles MA: AKT1 mutations in bladder cancer:
identification of a novel oncogenic mutation that can co-operate
with E17K. Oncogene. 29:150–155. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Dickstein RJ, Nitti G, Dinney CP, Davies
BR, Kamat AM and McConkey DJ: Autophagy limits the cytotoxic
effects of the AKT inhibitor AZ7328 in human bladder cancer cells.
Cancer Biol Ther. 13:1325–1338. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wu D, Tao J, Xu B, et al:
Phosphatidylinositol 3-kinase inhibitor LY294002 suppresses
proliferation and sensitizes doxorubicin chemotherapy in bladder
cancer cells. Urol Int. 86:346–354. 2011. View Article : Google Scholar
|
|
76
|
Zheng J, Hu JD, Huang Y and Chen BY:
Effects of baicalin on proliferation and apoptosis of
adriamycin-resistant human leukemia HL-60/ADR cells. Zhongguo Shi
Yan Xue Ye Xue Za Zhi. 17:1198–1202. 2009.(In Chinese).
|