Introduction
COM1 (candidate of metastasis 1) was
initially found to be upregulated in the metastatic lesions of the
central nervous system upon injection of cancer cells originally
isolated from micrometastases in the bone marrow of a breast cancer
patient (1). Subsequently
COM1 was found to be the human counterpart of the rat
p8, which was first reported as a new gene and strongly
activated in pancreatic acinar cells during the acute phase of
pancreatitis in rat described by Mallo et al(2). COM1/P8 also known as nuclear
protein transcriptional regulator 1 (NUPR1), is located on
chromosome 16 at position p11.2. The COM1 gene encodes two
isoforms in protein products, one with 100 amino acids, the other
with 82 amino acids. The role of the 18 amino acids region absent
in the latter is presently unclear.
Overexpression of COM1 has been indicated in
the pancreatic cancer and thyroid cancer (3,4). Su
et al reported that COM1 was overexpressed in 59% of
the 44 pancreatic cancer cohort. In addition, its expression was
inversely correlated to apoptosis in pancreatic cancer. However,
COM1 did not have any significant correlation with the overall
survival of the patients with pancreatic cancer. Moreover, COM1 was
more prominent in lower age group patients, moderately or poorly
differentiated cancers and lymph node positive cases.
Overexpression of COM1 and loss of apoptosis were significantly
correlated with poor differentiation and lymph node metastasis.
This interesting expression pattern might reflect not only the
growth activity and differentiation of cancer cells but also
invasion and metastasis of human pancreatic cancer (3). Ito et al reported that COM1
was significantly overexpressed in thyroid papillary carcinomas and
that COM1 overexpression was more frequently observed in larger
papillary carcinoma with lymph node metastasis (4).
On the other hand, reduced expression of COM1 was
revealed in other cancer types including breast and prostate
cancer. Breast cancer cells have markedly reduced nuclear
distribution of the COM1 protein compared with normal mammary
epithelial cells. Low levels of COM1 were associated with nodal
status and poor clinical outcome (5). A follow-up study showed that the
nuclear presence of the COM1 protein was crucial to its inhibitory
role in cancer cells, and that loss of its nuclear presence of this
molecule had a detrimental effect on its overall negative control
of cancer growth (6). Compared
with normal prostate tissues, the expression of COM1 in prostate
cancer cells was substantially reduced. Knockdown of COM1 in
prostate cancer cells resulted in increased cellular motility and
growth. In contrast, overexpression of COM1 suppressed invasiveness
and growth of prostate cancer cells and tumour growth in
vivo. COM1 was proposed to act as a potential tumour suppressor
in prostate cancer (7).
Collectively these studies suggest that COM1 plays
contrasting roles in different malignancies. Although the mode of
action of COM1 in cancer cells remains unclear, it has been
recently indicated that Com1 may be connected to doxobubicin-indued
chemoresistence in cancer cells and that this may be linked to the
regulation of p53 and p21 (8).
Although COM1 has been implicated in the disease
progression of certain solid tumours including prostate cancer, its
role in bladder cancer remains unknown. In the present study we
first examined the expression of COM1 in normal bladder tissues and
bladder tissues with different invasion status and the effect of
COM1 on growth, adhesion, migration and invasion of bladder cancer
cells.
Materials and methods
Materials, cell lines and tissue
samples
Human bladder cancer cell lines RT112, EJ138 and T24
(ECACC, European Collection of Animal Cell Culture, Salisbury, UK)
were routinely maintained in DMEM-F12 medium supplemented with 10%
fetal bovine serum and antibiotics. Polyclonal goat anti-COM1
(SC-23283) and monoclonal mouse anti-GAPDH (SC-32233) were obtained
from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Other reagents
or kits were obtained from Sigma-Aldrich, Poole, UK.
Bladder tissues samples (n=37, including 14
non-invasive, 18 invasive and 5 normal bladder tissues) were
obtained from the Department of Urology, Chaoyang Hospital,
Beijing, China. All protocols were reviewed and approved by the
Ethics Committee and all patients gave written informed
consent.
Immunohistochemical staining procedure
for bladder tissues
Thin paraffin tissues sections were first dewaxed
using the gradient xylene/ethanol solutions. Antigen retrieval was
carried out using an EDTA antigen retrieving buffer in microwave
(25 min). After blocking the endogenous peroxidase, the sections
were incubated for 20 min in a horse serum blocking solution and
probed with the primary antibody (1 h). Following extensive
washing, sections were incubated for 30 min with a biotinylated
secondary antibody (multilink swine anti-goat/mouse/rabbit
immunoglobulin, Dako Inc. Carpinteria, CA, USA). Following washing,
an avidin-biotin complex (Vector Laboratories) was applied to the
sections followed by extensive washing. Diamino benzidine chromogen
(Vector Laboratories) was then added to the sections, which were
incubated in the dark for 5 min. Sections were then counterstained
in Gill’s haematoxylin and dehydrated in ascending grades of
methanol before clearing in xylene and mounting under a cover slip.
Staining of COM1 was assessed using a method modified from Allred
IHC scoring system (9). Score 0,
1, 2 and 3 was given to the staining being negative, faint,
moderate and strong, respectively.
Construction of COM1 expression vectors
and transfection
The first strand cDNA was synthesized from RNA
isolated from normal human mammary tissues using a DuraScript™
RT-PCR kit. PCR was then used to amplify the coding sequence of
human COM1 using the Extensor Hi-Fidelity PCR Master mix (ABgene
Ltd., Epsom, UK). The sequences of primers are shown in Table I. The verified COM1 insert was
cloned into a mammalian expression plasmid vector (pEF/His TOPO TA
plasmid vector, Invitrogen Inc., Paisley, UK). The recombinant
plasmid vectors were transformed into chemically competent TOP10
E. coli (Invitrogen), and the colonies were then analyzed.
Colonies carrying correct recombinant plasmids were amplified and
plasmids extracted. Purified COM1 transgenes and control plasmid
vectors were then transfected into RT112 and EJ138 cells
individually using an Easjet Plus electroporator (EquiBio Ltd,
Kent, UK). After up to 3 weeks of selection with blasticidin the
transfectants were verified for their expression of COM1 and
successful clones were used in subsequent studies.
 | Table IPrimer sequences for PCR. |
Table I
Primer sequences for PCR.
| Primer | Forward | Reverse |
|---|
| COM1 |
CCTGGATGAATCTGACCTC |
AGCAGCTTCTCTCTTGGTG |
| COM1 expression | ATGGCCACCTTCCCAC |
ACTGCGCCGTGCCCCTCG |
| COM1 ribozyme |
CTGCAGCTTCTCTCTTGGTGCCTGATGAGTCCGTGAGGA |
ACTAGTGGAGGCCGGAAAGGTTTCGTCCTCACGGACT |
| uPA |
GGTTGTGTGTGGGTTAGACT |
GTGGCTACCAGACATTGATT |
| p21 |
CGGGATGAGTTGGGAGGAG |
ACAGGTCCACATGGTCTTCC |
| C-myc |
TGCTCCATGAGGAGACAC |
TTTCATTGTTTTCCAACTCC |
| GAPDH |
GGCTGCTTTTAACTCTGGTA |
GACTGCGGTCATGAGGCCGT |
Generation of COM1 ribozyme
transgenes
Anti-COM1 hammerhead ribozyme transgenes were
synthesised and cloned into pEF6/V5-His-TOPO plasmid vector as
described in previous studies (5–7).
Empty plasmid vectors and the ribozyme transgenes were then
transfected into RT112 and EJ138 cells and the cells underwent
selection with 5 μg/ml blasticidin for approximately 2 weeks
before verification.
RNA isolation and reverse transcription
PCR
RNA was isolated using total-RNA Isolation Reagent
(ABgene Ltd). Reverse transcription was performed using the
DuraScript™ RT-PCR kit, followed by PCR using a REDTaq™ ReadyMix
PCR reaction mix (primer sequences shown in Table I). Cycling conditions were 94°C for
5 min, followed by 30 cycles of 94°C for 30 sec, 55°C for 30 sec
and 72°C for 40 sec. This was followed by a final 10-min extension
period at 72°C. The products were visualized on 2% agarose gel
stained with ethidium bromide.
Immunoprecipitation and western blot
analysis
The protein concentration in cell lysates were
determined using the DC Protein Assay kit (Bio-Rad) and an ELx800
spectrophotometer (Bio-Tek™). Equal amount of proteins were
separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and blotted onto nitrocellulose sheets.
Proteins were then respectively probed with anti-COM1 antibody and
peroxidase-conjugated secondary antibody, with stringent washings
between each step. Protein bands were visualized using the
Supersignal™ West Dura system (Pierce Biotechnology, Inc.,
Rockford, IL, USA), and photographed using a UVITech imager
(UVITech Inc., Cambridge, UK).
In vitro cell growth assay
Cells were plated into a 96-well plate (3,000
cells/well). Cell growth was assessed after a period of incubation
(up to 5 days). Crystal violet was used to stain cells. Following
washing, stained crystal was extracted with 10% acetic acid and
absorbance was determined at a wavelength of 540 nm using a
spectrophotometer (Elx800, Bio-Tek, Potton, UK).
In vitro invasion assay
Transwell inserts with 8 μm pore size were
coated with 50 μg Matrigel (BD Matrigel™ Basement Membrane
Matrix) and air dried. The Matrigel was rehydrated before use.
Cells (30,000) were added to each insert, same number cells were
loaded into another well as control. After 96 h cells that had
migrated through the matrix to the other side of the insert were
fixed in 4% formalin, stained with 0.5% (w/v) crystal violet. The
stained crystal was extracted with 10% acetic acid and absorbance
was determined at a wavelength of 540 nm using a spectrophotometer
(Bio-Tek, Elx800). Percentage of invaded cells was calculated as
absorbance of invaded cells divided by absorbance of control
cells.
Flow cytometric analysis of
apoptosis
All cells including those floating in the culture
medium were harvested after a period of incubation. Cells were
washed in ice cold PBS and resuspended in 1X Annexin-binding buffer
at a density of 1×106 cells/ml after centrifugation.
FITC Annexin V (5 μl) and PI working solution (1 μl)
(100 μg/ml) (Molecular Probes, Eugene, OR, USA) were added
to 100 μl of the cell suspension. After a 30-min incubation
at room temperature, 400 μl of 1X Annexin-binding buffer was
added, mixed gently and the samples were kept on ice. The stained
cells were immediately analyzed using the flow cytometer and
FlowMax software package.
Statistical analysis
Statistical analysis was performed using the Minitab
statistical software package (version 14). Non-normally distributed
data were assessed using the Mann-Whitney test, while the two
sample t-test was used for normally distributed data. χ2
test was used to analyse the IHC staining of COM1 in bladder
specimens. Differences were considered to be statistically
significant at p<0.05.
Results
Expression of COM1 in human bladder
tissues
We first examined the protein expression of COM1 in
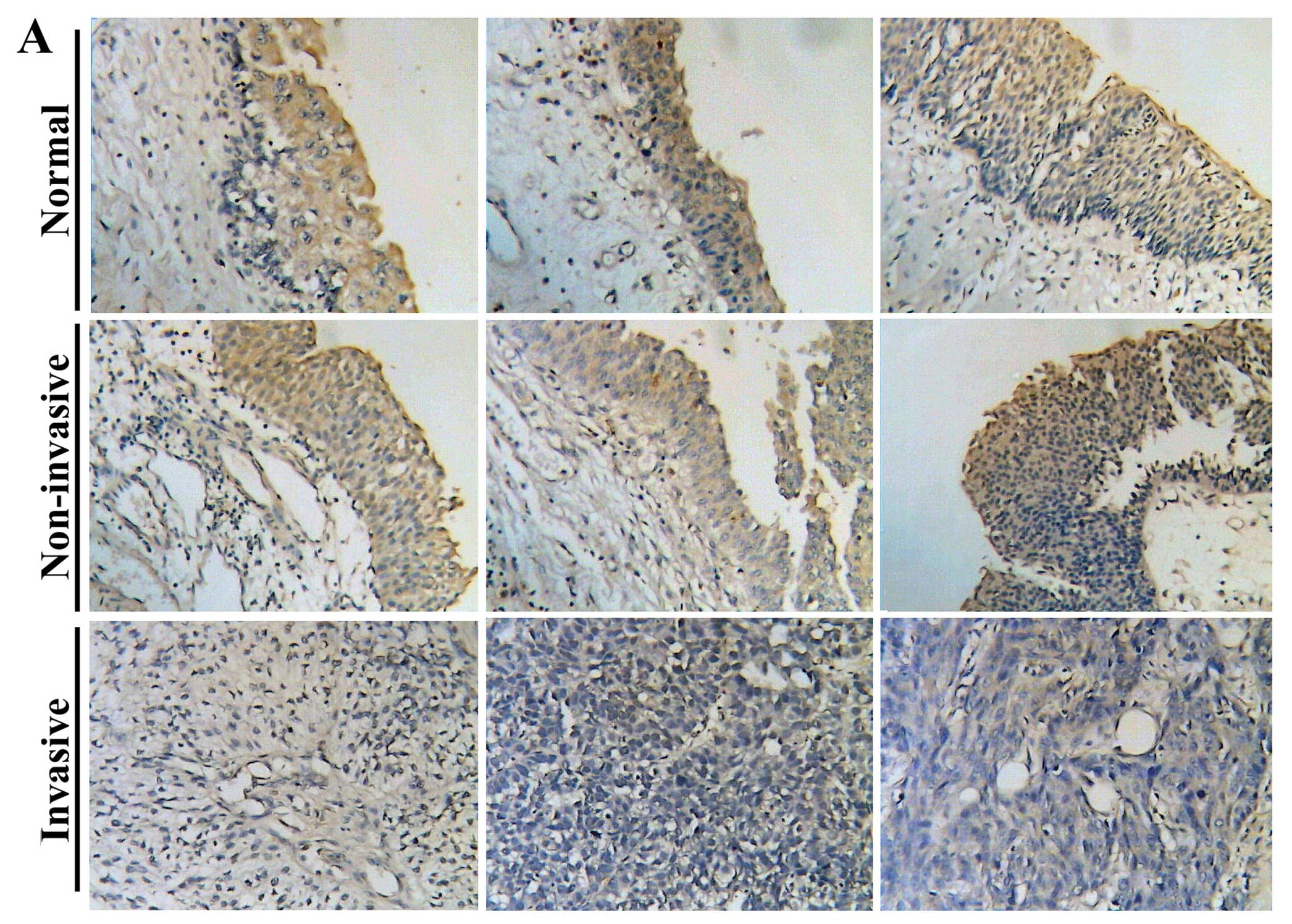
human bladder tissues using immunohistochemical method. As shown in
Fig. 1A, staining of COM1 was
mainly confined in cytoplasm of bladder urothelial cells, with no
obvious staining seen in nuclei of these cells or stromal tissue.
Normal bladder urothelial cells and cancer cells of non-invasive
tumours exhibited a stronger staining of COM1 in the cytoplasm. In
contrast, the staining was weaker or absent from the cancer cells
of invasive tumour tissues (Fig.
1A). Invasive tumours had significantly low levels of staining
in comparison with non-invasive tumours (p=0.012, Table II). In both normal and tumour
tissues, nucleic staining of COM1 was not demonstrated. The
expression of COM1 was also examined in three bladder cancer cell
lines using RT-PCR. COM1 transcript was detected in all three human
bladder cancer cells (Fig.
1B).
 | Table IIIHC staining of COM1 in bladder cancer
specimens. |
Table II
IHC staining of COM1 in bladder cancer
specimens.
| | Score 0 (%) | Score 1 (%) | Score 2 (%) | Score 3 (%) | Total |
|---|
| Normal | | 0 | 2 (40) | 3 (60) | 0 | 5 |
| Cancer | Non-invasive | 0 | 2 (14.3) | 9 (64.3) | 3 (21.4) | 14 |
| Invasive | 3 (16.7) | 10 (55.6) | 4 (22.2) | 1 (5.6) | 18 |
Knockdown and overexpression of COM1 in
bladder cancer cells
To investigate the impact of COM1 on functions of
bladder cancer cells, the constructed COM1 expression vectors and
anti-COM1 ribozyme transgenes were utilised to over-express or
knockdown COM1 in bladder cancer cells, which were weakly positive
for COM1. After the selection using blasticidin, the expression of
COM1 in the transfected cells was verified using both RT-PCR and
western blot analysis (Fig. 2).
Reduced COM1 expression of both mRNA (Fig. 2A) and protein (Fig. 2B) was seen in RT112 COM1rib, in
comparison with RT112 WT and RT112 pEF, while an increased
expression of COM1 was seen in RT112 COMexp compared to the
controls. Similarly, knockdown and overexpression of COM1 were
confirmed in EJ138 COMrib and EJ138 COMexp cells respectively, in
comparison with EJ138 WT and EJ138 pEF control cells.
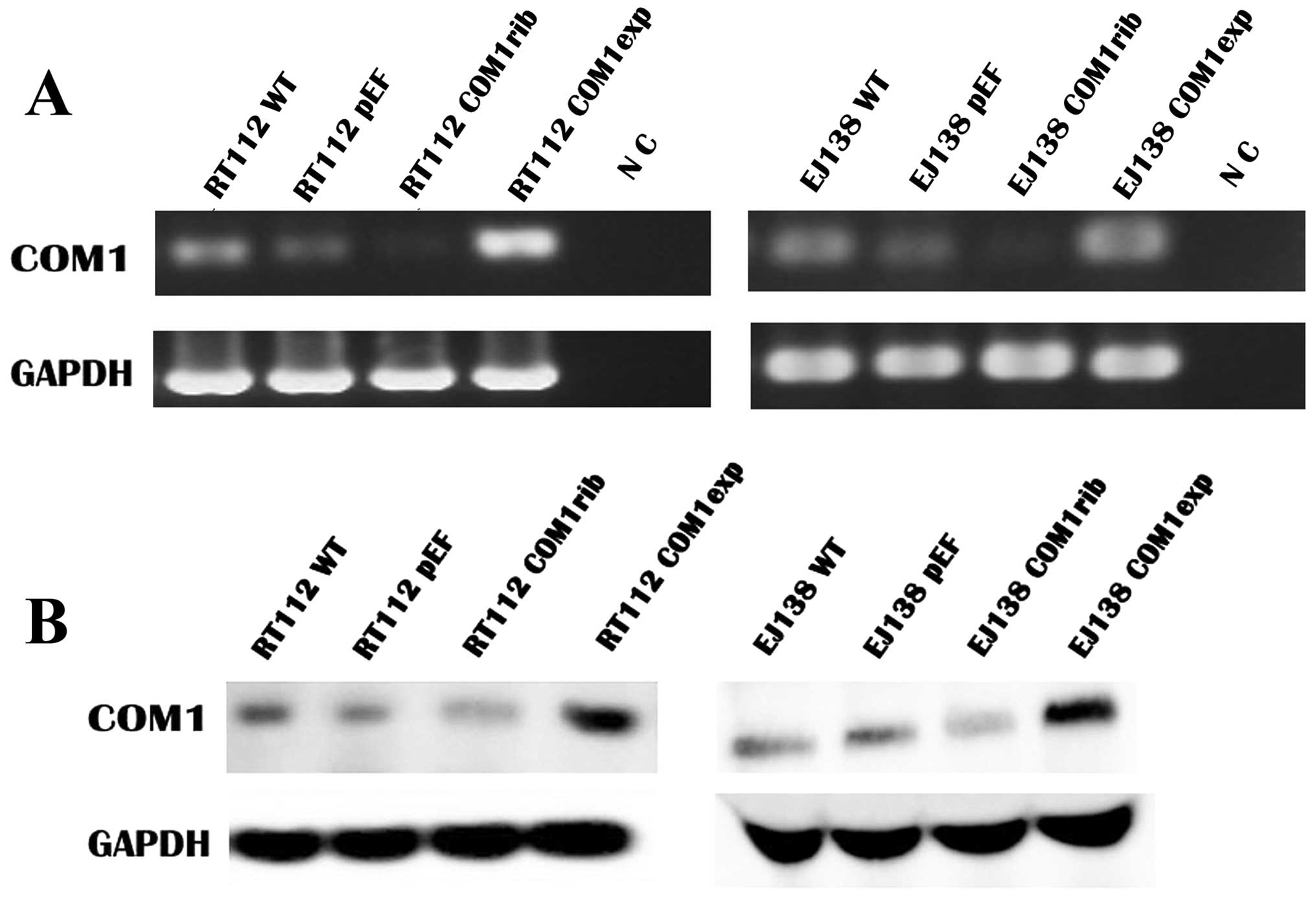 | Figure 2Knockdown and overexpression of COM1
in bladder cancer cells. (A) The mRNA expression of COM1 in the
transfected cells by RT-PCR. mRNA of COM1 was knocked down in RT112
COM1rib and EJ138 COM1rib, and overexpressed in RT112 COM1exp,
compared with the wild-type (RT112WT and EJ138WT) and empty plasmid
(RT112pEF and EJ138pEF) control cells. Reduced COM1 expression of
mRNA was seen in RT112 COM1rib, in comparison with RT112 WT and
RT112 pEF, while an increased expression of COM1 was seen in RT112
COMexp compared to the controls. Similarly, knockdown and
overexpression of COM1 were confirmed in EJ138 COMrib and EJ138
COMexp cells respectively, in comparison with EJ138 WT and EJ138
pEF control cells. NC, negative control. (B) The potein expression
of COM1 (∼10 kDa) in the transfected cells by western blot
analysis. Protein of COM1 was knocked down in RT112 COM1rib and
EJ138 COM1rib and overexpression in RT112 COM1exp, compared with
the wild-type (RT112WT and EJ138WT) and empty plasmid (RT112pEF and
EJ138pEF) control cells. Reduced COM1 expression of protein was
seen in RT112 COM1rib, in comparison with RT112 WT and RT112 pEF,
while an increased expression of COM1 was seen in RT112 COMexp
compared to the controls. Similarly, knockdown and overexpression
of COM1 were confirmed in EJ138 COMrib and EJ138 COMexp cells
respectively, in comparison with EJ138 WT and EJ138 pEF control
cells. GAPDH (38 kDa) was used as the housekeeping control. |
COM1 is associated with the growth rate
and invasion of bladder cancer cells
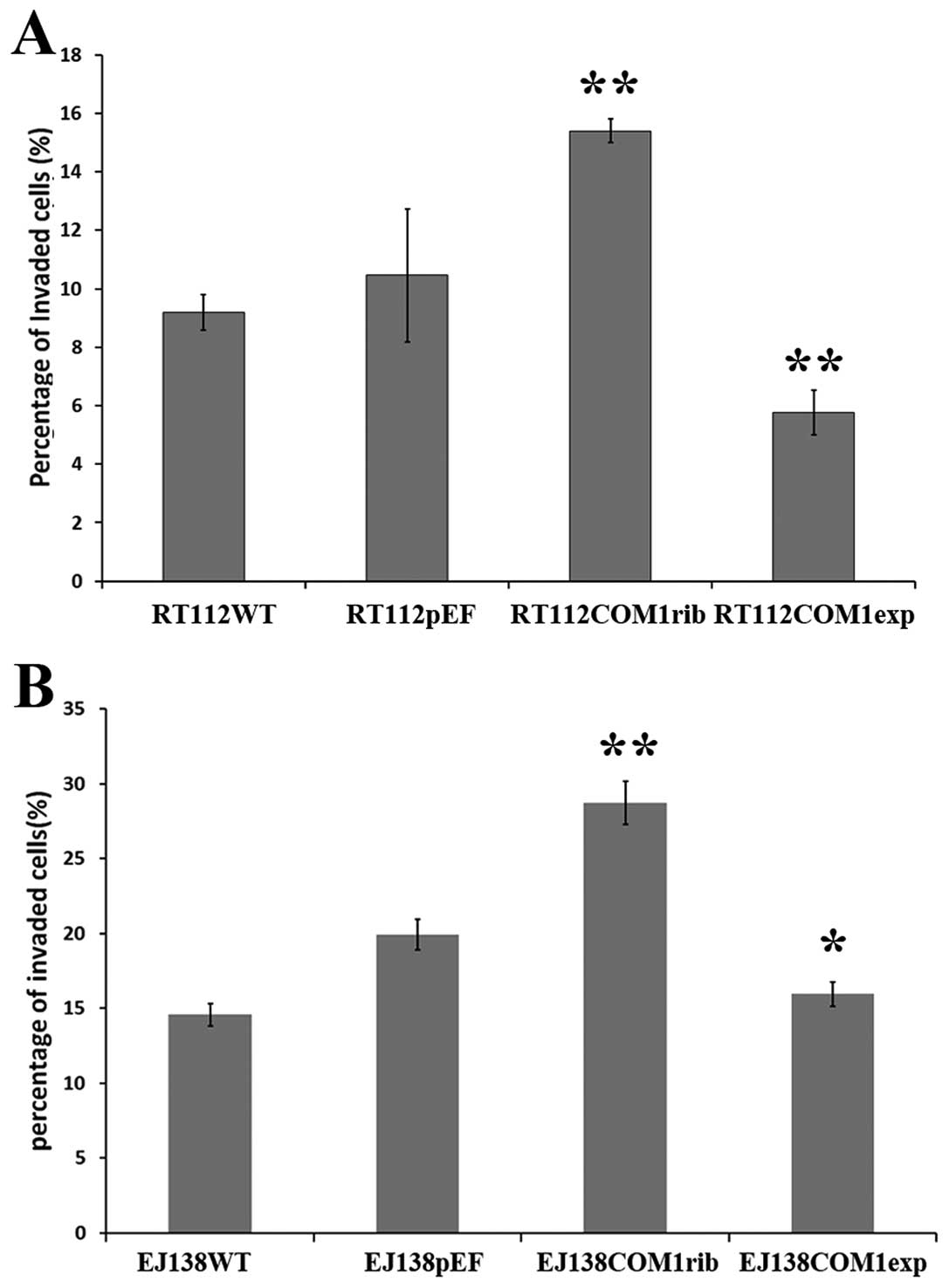
Following verification, influence of COM1 on
invasiveness was examined in the genetically modified cells.
Knockdown of COM1 in the bladder cancer cells rendered the cells
more invasive (Fig. 3).
Interestingly, overexpression of COM1 in these cells made the cells
less invasive compared with the control cells. Using the same
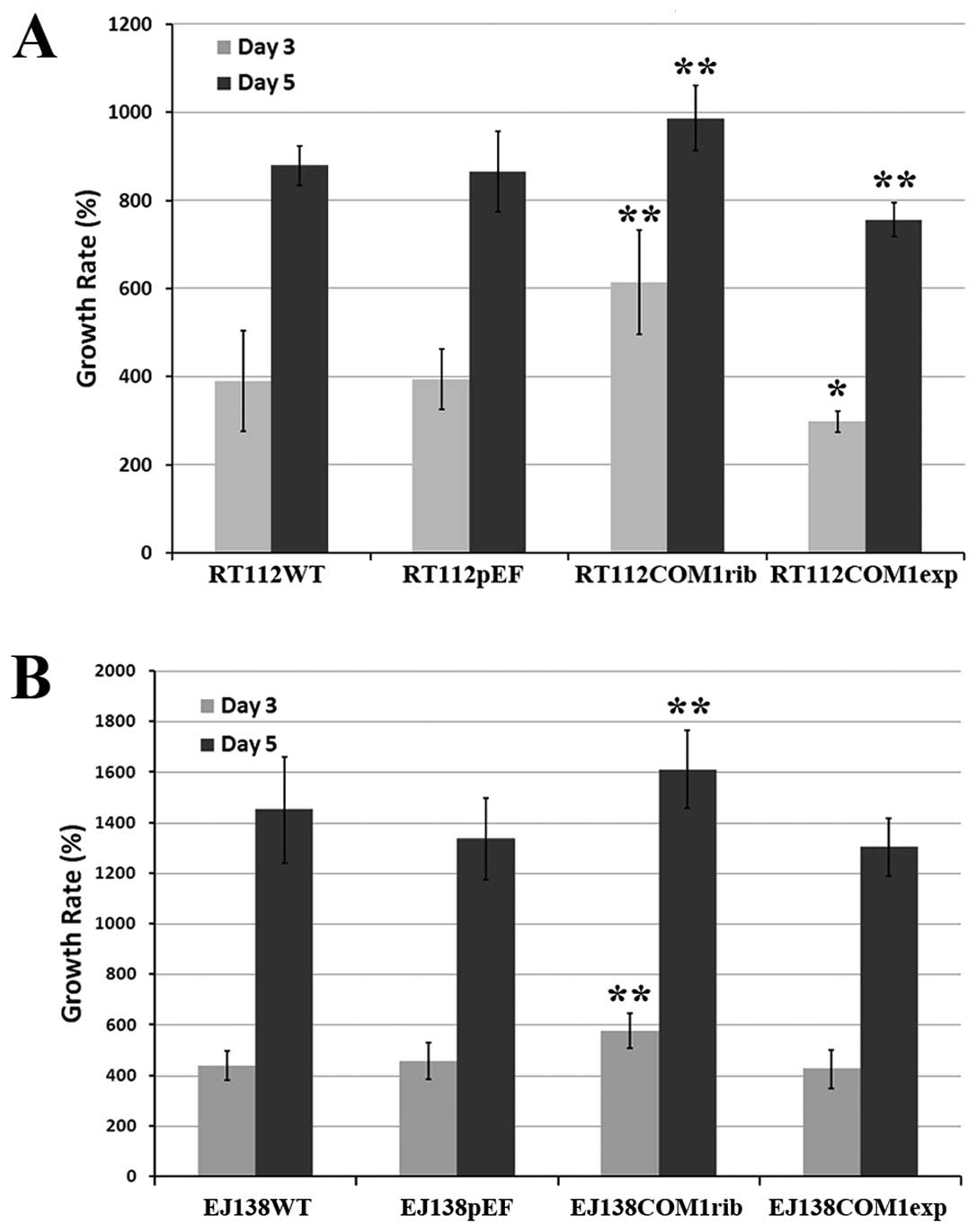
cells, we further tested the rate of cell growth over a 5-day
period. RT112 COM1rib and EJ138 COM1rib both displayed a faster
rate of growth, compared with the controls (Fig. 4). Furthermore, cells with forced
overexpression of COM1 displayed a slower growth rate.
Effects of COM1 on apoptosis of bladder
cancer cells
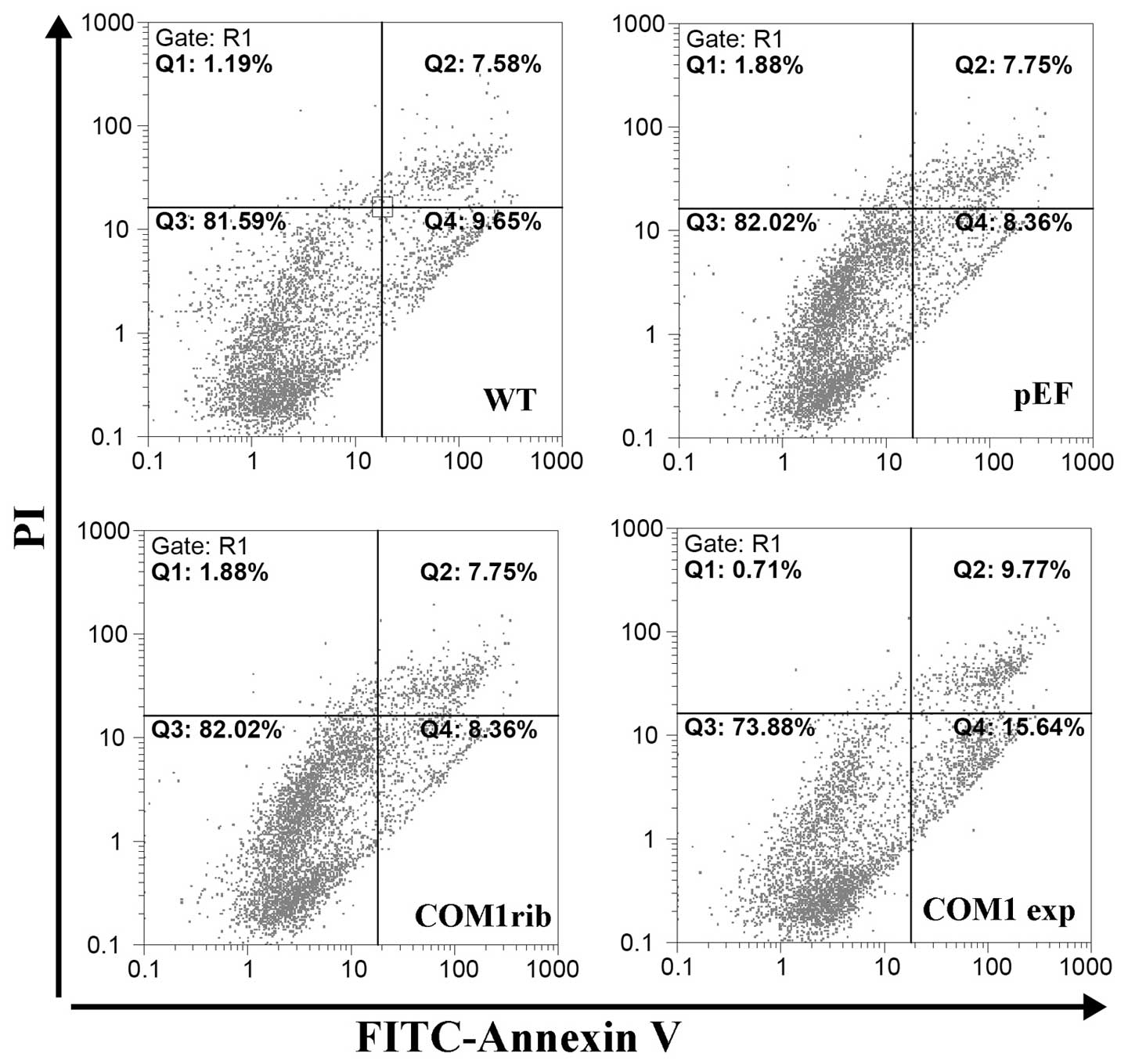
In order to determine the mechanism by which COM1
inhibits bladder cancer cell growth, the proportion of apoptotic
cells was analyzed by flow cytometry. It was demonstrated that
there was a significantly higher proportion of apoptotic cells
including both early apoptotic (Q4), late apoptotic and dead cells
(Q2) in RT112 COM1exp cells (Q2 9.77% + Q4 15.64%), compared with
the RT112 WT (Q2 7.58% + Q4 9.65%) and the RT112pEF (Q2 7.75% + Q4
8.36%) control (Fig. 5). There was
no difference of the apoptotic population was seen in RT112COM1rib
cells compared to both controls.
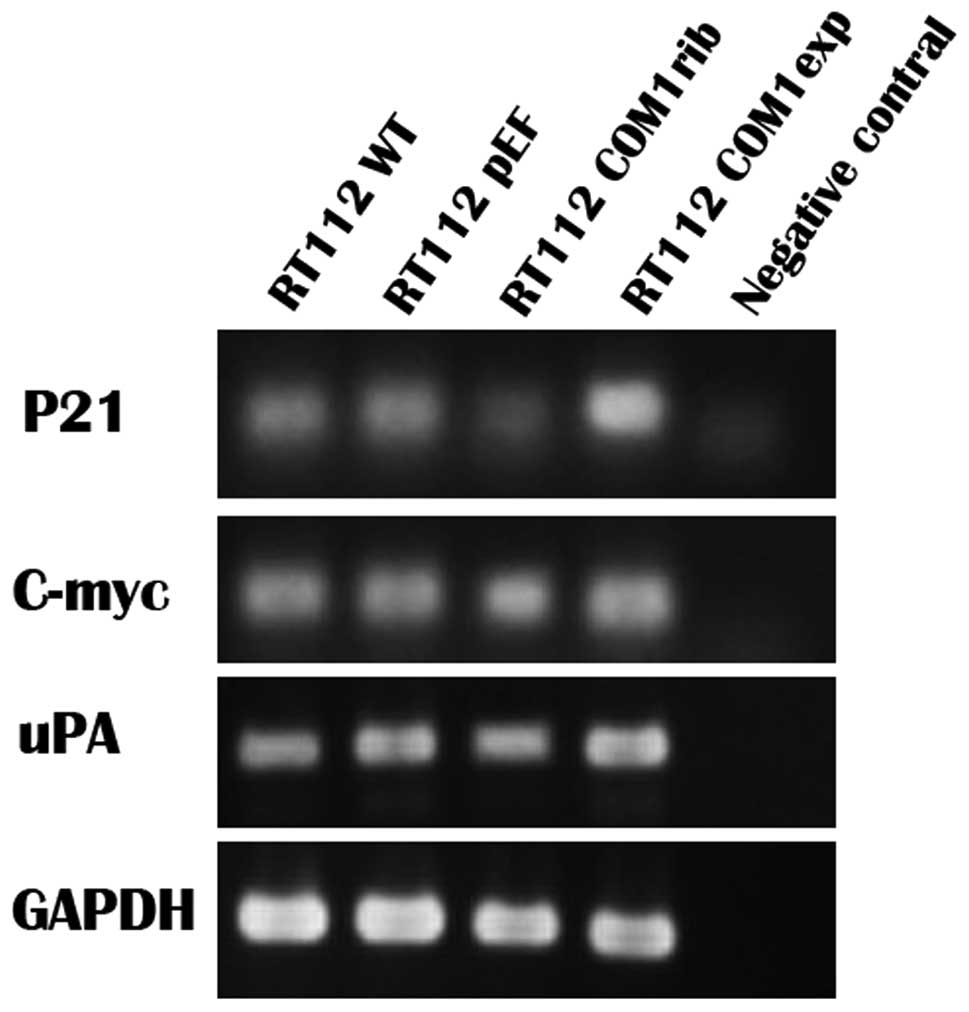
Expression of uPA, C-myc and P21 in
bladder cancer cell line RT112
We further examined a couple of COM1 responsive
genes which might be involved in the impact on growth and invasion.
The mRNA expression of uPA, C-myc and P21 in RT112 cells were
detected using RT-PCR. The expression of P21 was significantly
higher in which bladder cancer cells that overexpressed COM1, and
was much lower in COM1 knockdown cells. But no significant
difference was found in mRNA levels of uPA and C-myc (Fig. 6).
Discussion
Bladder cancer is one of the commonly seen tumours
in males and females and the incidence is estimated to be the 7th
in the UK and 9th worldwide, based on the 2008 statistics (Cancer
Research UK, 2012) Approximately 382,660 new bladder cancer cases
occurred and 150,280 patients died of bladder cancer worldwide in
2008. At the same time, bladder cancer is the most common
malignancy of the urinary tract, accounting for 90–95% of
urothelial carcinomas (11). The
feature of bladder cancer is apt to recurrence. At initial
diagnosis, 75% patients present with non-muscle-invasive bladder
cancer (NMIBC), the remaining 25% present with muscle-invasive
bladder cancer (MIBC). The main problems of NMIBC are recurrence
and progression, while MIBC is frequently associated with
metastatic diseases and is the major cause of mortality. In NMIBC,
approximately 50–80% of patients will recur after managed with
transurethral resection (TUR) and intraversical therapy (12). Despite the improvements in both
early detection and treatment, there remains a significant
challenge to manage this disease.
The role of COM1, in tumour progression remains
unclear and in somewhat controversial. However, its effect on cell
growth, i.e. whether it is stimulating or inhibiting may vary in
different malignancies. COM1 has been found to aid the
establishment of metastasis and to play a key role in the
progression of malignancies of breast (1,5–7,13),
thyroid (4,13,14),
central nervous system (15) and
pancreas (3,16,17).
COM1 has been implicated in inducing chemoresistance in pancreatic
and breast cancer cells, protecting them from apoptosis and making
tumour cells genetically unstable (17). In prostate cancer, however, COM1
appears to have tumour suppressive activity (7). The current study has examined the
staining pattern of COM1 in human bladder tissues and tested the
impact on the growth, invasion and apoptosis of bladder cancer
cells by genetically manipulating the expression of COM1.
Similar to human breast cancer, thyroid and prostate
cancer, COM1 is seen at a lower level in bladder tumour cells,
compared with normal bladder urothelial cells and the COM1 staining
is linked to the muscle invasion of bladder cancer. This is in
contrast to that observed with pancreatic cancer cells, in which
cancer cells had higher staining than normal cells. This would
indicate that in solid human tumours, COM1 exists at lower levels,
although the opposite may also operate. It is interesting to note
that both normal urothelial cells and bladder cancer cells had
little COM1 in the nucleus. The nuclear existence of COM1 is
particularly interesting as it has been suggested that the
cytoplasmic/nuclear distribution pattern of the COM1 protein may be
a key feature in cancer and an important reason in deciding the
contrasting role of COM1 in different cancer types. While COM1
nucleic staining in breast cancer cells has been shown to be
significantly reduced compared with normal epithelial cells
(5), bladder cancer cells have a
marked reduction in the cytoplasmic region while no nuclear
staining was seen in normal and tumour cells of the bladder
tissues. The current study further indicates the possibility of a
nuclear connection in the function of COM1. Thus, changes in the
overall level of staining of COM1 in bladder cancer cells and in
intracellular distribution appear to be a feature in human bladder
tumour tissues.
In the present study, we employed methods to
genetically alter the expression of COM1 in bladder cancer cells,
namely the ribozyme approach and overexpression approach,
respectively. The loss of COM1 by way of hammerhead ribozyme
transgenes resulted in more invasiveness compared with control
cells. In clear contrast, forced overexpression of COM1 in two
bladder cancer cell lines, RT112 and EJ138, resulted in a reduction
of invasion. This indicates that COM1 plays a key role in the
control of the aggressiveness of bladder cancer cells. The current
study has also demonstrated that loss of COM1 resulted in an
accelerated cell growth in vitro. This growth regulatory
role of COM1 has been tentatively indicated in other tumour cells
including breast cancer cells and thyroid cancer cells, in which
Vit D3 inhibition of cell growth was associated with a rise of COM1
(18,19). In pancreatic cancer cells,
overexpression of COM1 is associated with inhibitory effects on
cell growth and/or with accelerated apoptosis (15). This together with the effects
observed in other solid tumours (breast, prostate and thyroid)
(4,6,14),
strongly suggest that COM1 is a potential tumour suppressor in
these solid tumours. This suggestion is further supported by our
in vitro results, in which COM1 exhibited inhibitory effect
on growth of bladder cancer cells.
In order to determine the mechanism by which COM1
inhibits bladder cancer cell growth, the proportion of apoptotic
cells was analyzed by flow cytometry in our study. There was an
increased proportion of apoptotic cells (both early and late) in
bladder cancer cells overexpressing COM1. A cyclin/cyclin dependent
kinase (cdk) inhibitor, p21 has been shown as a target gene
regulated by COM1 during doxobubicin induced chemoresistence
(17). In line with this finding,
an upregulation of p21 was seen in the bladder cancer cells of COM1
overexpression. The role of p21 has been well studied in mediating
the G1/S checkpoint in response to treatment with agents the induce
genotoxic stress (20,21). It has been suggested that as a
negative regulator of cell cycle progression, P21 has a certain
role to play in the inhibitory effect on growth by COM1. Recently,
it has been shown that COM1 is one of the key modulators in the
antitumour (pro-apoptotic) effects of the cannabinoids, by
mediating its apoptotic effect via upregulation of the endoplasmic
reticulum stress related genes ATF-4, CHOP and TRB3 (22). COM1 may also exert its inhibitory
effect on prostate cancer via the peroxisome proliferator activated
receptor γ pathway (23). Together
with the recent report that COM1 interacts with p53, a well
established tumour suppressor in certain tumour types (15), the present study indicates that
regulation of cell cycle and tumour suppressors is a key mechanism
in its role in cancer progression. Further investigations would be
required to elucidate the molecular mechanisms underlying these
impacts on bladder cancer cells by COM1, including the candidate
downstream responsive genes, MMP-9 and MMP-13 (24). However, one should bear in mind
that the tumour suppressive role of COM1 has only been confirmed in
certain tumours, whereas in other tumour types including thyroid
and pancreatic cancers, COM1 was found to be overexpressed in
tumours (3,4). In colorectal cancer, another
intriguing example, although COM1 was found to be lost/reduced in
tumour tissues compared with normal tissues (25), the molecule is anti-apoptotic and
increase the growth of colorectal cancer cell lines in
vitro(26). The tumour
suppressive or stimulating role of COM1 appears therefore to be
dependent upon the type of tumours, and the experimental
conditions. It also depends on the cellular location of the
protein, in that cytoplasmic or nuclear location of the protein may
govern how the COM1 protein acts, clearly an interesting area to
pursue in future studies. Finally, the link between COM1, cellular
invasion and apoptosis as seen in the present study and recent
studies indicates that COM1 may be a player in the regulation of
Anoikis (27,28) and that this interesting link
warrants further investigation.
In conclusion, the current study showed reduced
expression of COM1 in invasive human bladder cancer. Together with
our genetic manipulation study which demonstrated an inverse
relationship between COM1 expression and the in vitro
growth/invasion, it is concluded that COM1 is a negative regulator
of the growth and invasion in bladder cancer.
Acknowledgements
P.D. is a recipient of Cardiff
University China Medical Scholarship. The authors wish to thank the
Albert Hung Foundation and Cancer Research Wales for supporting the
study.
References
|
1
|
Ree AH, Tvermyr M, Engebraaten O, Rooman
M, Rosok O, Hovig E, Meza-Zepeda LA, Bruland OS and Fodstad O:
Expression of a novel factor in human breast cancer cells with
metastatic potential. Cancer Res. 59:4675–4680. 1999.
|
|
2
|
Mallo GV, Fiedler F, Calvo EL, Ortiz EM,
Vasseur S, Keim V, Morisset J and Iovanna JL: Cloning and
expression of the rat p8 cDNA, a new gene activated in pancreas
during the acute phase of pancreatitis, pancreatic development, and
regeneration, and which promotes cellular growth. J Biol Chem.
272:32360–32369. 1997. View Article : Google Scholar
|
|
3
|
Su SB, Motoo Y, Iovanna JL, Berthezene P,
Xie MJ, Mouri H, Ohtsubo K, Matsubara F and Sawabu N:
Overexpression of p8 is inversely correlated with apoptosis in
pancreatic cancer. Clin Cancer Res. 7:1320–1324. 2001.PubMed/NCBI
|
|
4
|
Ito Y, Yoshida H, Motoo Y, et al:
Expression and cellular localization of p8 protein in thyroid
neoplasms. Cancer Lett. 201:237–244. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang WG, Watkins G, Douglas-Jones A,
Mokbel K, Mansel RE and Fodstad O: Expression of COM-1/P8 in human
breast cancer and its relevance to clinical outcome and ER status.
Int J Cancer. 117:730–737. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang WG, Davies G and Fodstad O: COM-1/P8
in oestrogen regulated growth of breast cancer cells, the ER-beta
connection. Biochem Biophys Res Commun. 330:253–262. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang WG, Davies G, Martin TA, Kynaston H,
Mason MD and Fodstad O: COM-1/p8 acts as a putative tumour
suppressor in prostate cancer. Int J Mol Med. 18:981–986.
2006.PubMed/NCBI
|
|
8
|
Clark DW, Mitra A, Fillmore RA, Jiang WG,
Samant RS, Fodstad O and Shevde LA: NUPR1 interacts with p53,
transcriptionally regulates p21 and rescues breast epithelial cells
from doxorubicin-induced genotoxic stress. Curr Cancer Drug
Targets. 8:421–430. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Allred DC, Harvey JM, Berardo M and Clark
GM: Prognostic and predictive factors in breast cancer by
immunohistochemical analysis. Mod Pathol. 11:155–168.
1998.PubMed/NCBI
|
|
10
|
Yu H, Ye L, Mansel RE, Zhang Y and Jiang
WG: Clinical implications of the influence of Ehm2 on the
aggressiveness of breast cancer cells through regulation of matrix
metalloproteinase-9 expression. Mol Cancer Res. 8:1501–1512. 2010.
View Article : Google Scholar
|
|
11
|
Ploeg M, Aben KK and Kiemeney LA: The
present and future burden of urinary bladder cancer in the world.
World J Urol. 27:289–293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Youssef RF and Lotan Y: Predictors of
outcome of non-muscle-invasive and muscle-invasive bladder cancer.
Scientific World J. 11:369–381. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ree AH, Pacheco MM, Tvermyr M, Fodstad O
and Brentani MM: Expression of a novel factor, com1, in early tumor
progression of breast cancer. Clin Cancer Res. 6:1778–1783.
2000.PubMed/NCBI
|
|
14
|
Ito Y, Yoshida H, Motoo Y, et al:
Expression of p8 protein in medullary thyroid carcinoma. Anticancer
Res. 25:3419–3423. 2005.PubMed/NCBI
|
|
15
|
Ree AH, Bratland A, Kroes RA, Aasheim HC,
Florenes VA, Moskal JR, Fodstad O, Bruland OS and Maelandsmo GM:
Clinical and cell line specific expression profiles of a human gene
identified in experimental central nervous system metastases.
Anticancer Res. 22:1949–1957. 2002.PubMed/NCBI
|
|
16
|
Giroux V, Malicet C, Barthet M, Gironella
M, Archange C, Dagorn JC, Vasseur S and Iovanna JL: p8 is a new
target of gemcitabine in pancreatic cancer cells. Clin Cancer Res.
12:235–241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Su SB, Motoo Y, Iovanna JL, et al:
Expression of p8 in human pancreatic cancer. Clin Cancer Res.
7:309–313. 2001.PubMed/NCBI
|
|
18
|
Bratland A, Risberg K, Maelandsmo GM, et
al: Expression of a novel factor, com1, is regulated by
1,25-dihydroxyvitamin D3 in breast cancer cells. Cancer Res.
60:5578–5583. 2000.PubMed/NCBI
|
|
19
|
Trump DL, Muindi J, Fakih M, Yu WD and
Johnson CS: Vitamin D compounds: clinical development as cancer
therapy and prevention agents. Anticancer Res. 26:2551–2556.
2006.PubMed/NCBI
|
|
20
|
Niculescu AB III, Chen X, Smeets M, Hengst
L, Prives C and Reed SI: Effects of p21(Cip1/Waf1) at both the G1/S
and the G2/M cell cycle transitions: pRb is a critical determinant
in blocking DNA replication and in preventing endoreduplication.
Mol Cell Biol. 18:629–643. 1998.
|
|
21
|
Smits VA and Medema RH: Checking out the
G(2)/M transition. Biochim Biophys Acta. 1519:1–12. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Malicet C, Giroux V, Vasseur S, Dagorn JC,
Neira JL and Iovanna JL: Regulation of apoptosis by the
p8/prothymosin alpha complex. Proc Natl Acad Sci USA.
103:2671–2676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang WG, Davies G, Kynaston H, Mason MD
and Fodstad O: Does the PGC-1/PPARgamma pathway play a role in
COM-1/p8 mediated cell growth inhibition in prostate cancer? Int J
Mol Med. 18:1169–1175. 2006.PubMed/NCBI
|
|
24
|
Goruppi S and Ionanna JL: Stress-inducible
protein p8 is involved in several physiological and pathological
processes. J Biol Chem. 285:1577–1581. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Davies ML, Parr C, Sanders AJ, Fodstad O
and Jiang WG: The transcript expression and protein distribution
pattern in human colorectal carcinoma reveal a pivotal role of
COM-1/p8 as a tumour suppressor. Cancer Genomics Proteomics.
7:75–80. 2010.
|
|
26
|
Li X, Martin TA and Jiang WG: COM-1/p8
acts as a tumour growth enhancer in colorectal cancer cell lines.
Anticancer Res. 32:1229–1237. 2012.PubMed/NCBI
|
|
27
|
Frisch SM and Francis HL: Disruption of
epithelial cell-matrix interactions induces apoptosis. J Cell Biol.
124:619–626. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shao J, Sheng H, DuBois RN and Beauchamp
RD: Oncogeneic Ras-medicated cell growth arrest and apoptosis are
associated with increased ubiquitin-dependent cyclin D1
degradation. J Biol Chem. 275:22916–22924. 2000. View Article : Google Scholar : PubMed/NCBI
|