Introduction
Cholangiocellular carcinoma (CCA) is the second most
common hepatobiliary malignancy after hepatocellular carcinoma
(HCC). With a growing incidence (2–4/100,000 per year), it accounts
for approximately 15% of liver cancer cases worldwide (1,2).
CCAs grow as highly malignant adenocarcinomas, arising from the
epithelial cells of intrahepatic bile ducts. Based on their
anatomical origin, CCAs are classified into peripheral intrahepatic
cholangiocarcinomas, originating from interlobular bile ducts,
extrahepatic hilar (Klatskin) tumors and distal tumors, arising
from the main hepatic ducts or the bifurcation of the common
hepatic duct.
The only curative therapy for CCA is surgical
resection; however, due to its non-specific symptoms, the tumor is
usually diagnosed in its advanced stages, by which time it is
non-resectable. The prognosis and overall survival for CCA patients
is very poor. Even in resectable tumors, 5-year survival rates are
<15%, with a 30% tumor recurrence rate (2,3).
Chemotherapeutic options for CCA are rather limited and the median
survival with palliative chemotherapy is very low (4.6–15.4 months)
(4). Therefore, novel molecular
markers that may help predict clinical outcome and therapeutic
response are urgently required.
The 3 organic cation transporters, OCT1
(SLC22A1), OCT2 (SLC22A2) and OCT3 [synonym:
extraneuronal monoamine transporter (EMT); SLC22A3], belong
to the amphiphilic solute facilitator (ASF) family of integral
transmembrane proteins. They mediate the interactions between cells
and their environment and are involved in numerous metabolic
processes and detoxification. OCTs bidirectionally transport a
broad spectrum of endogenous substrates (e.g., catecholamines),
toxins and anticancer drugs (5).
OCTs are determinants of the cytotoxicity of platinum derivatives
(6). Therefore, these transporters
are of pharmaceutical interest.
In humans, OCT1 is mainly expressed in the liver and
OCT2 in the kidney, whereas OCT3 is widely distributed in a number
of tissues. Nevertheless, OCTs exhibit overlapping substrate
specificities and tissue expression patterns. They functionally
substitute each other, ensuring that important metabolic pathways
are still conducted, even though one OCT may be defective or
deficient (7,8).
We recently demonstrated a significant
downregulation of OCT1 and OCT3 in human HCCs (9). We reported that the downregulation of
OCT1 mRNA in HCC is associated with advanced tumor stages and worse
overall patient survival rates.
Thus far, there are no studies available on OCT
expression in CCA. To our knowledge, our study is the first to
address this issue by investigating the effect of OCT expression on
patient survival and tumor characteristics in CCA. Expression
levels were measured in tumorous and corresponding non-neoplastic
tumor-surrounding tissue (TST). The expression patterns were
correlated with clinicopathological parameters and outcomes.
Materials and methods
Patient tissue samples
CCA and corresponding TST samples were obtained from
27 patients undergoing tumor resection between 2006 and 2011 at the
Department of Hepatobiliary and Transplantation Surgery of the
Johannes Gutenberg University Mainz, Germany. All CCA samples
analyzed were classified as intrahepatic cholangiocarcinoma.
Extrahepatic hilar (Klatskin) tumors or distal tumors were not
included in this study. All CCAs analyzed were classified as M0 (no
distant metastasis) and N0 (no regional lymph node metastasis),
according to the WHO TNM classification of liver tumors. The
patients did not receive any chemotherapy prior to resection.
Detailed patient and tumor characteristics are listed in Table I. Informed consent was obtained
from each patient. The study was conducted in accordance with the
ethical guidelines of the declaration of Helsinki and was approved
by the local ethics committee. Liver tissues were immediately
shock-frozen following resection and stored in liquid nitrogen
prior to analysis. All CCAs were diagnosed or confirmed by
histological examination.
 | Table IPatient and tumor
characteristics. |
Table I
Patient and tumor
characteristics.
|
Characteristics | |
|---|
| Number of
patients | n=27 |
| Median follow-up in
days (range) | 461 (111–1363) |
| Median
recurrence-free survival in days (range) | 161 (0–1110) |
| Gender
(male/female; n) | 16/11 |
| Median age in years
(range) | 66.5
(32.4–83.7) |
| Nodules
(1–3/multiple; n) | 19/8 |
| Tumor diameter
(<3 cm/>3 cm; n) | 4/23 |
| Median tumor
diameter in cm (range) | 7.5 (1.3–19.5) |
| T-classification
(T1/T2/T3; n) | 7/12/8 |
| Grading
(G1/G2/G3/Gxa;
n) | 1/21/4/1 |
| CEA (ng/ml)
(>5/<5; n)b | 5/20 |
| CA 19–9 (ng/ml)
(>37/<37; n)b | 13/12 |
RNA isolation, reverse transcription
real-time PCR (qRT-PCR)
RNA was extracted from the tissue samples by the
High Pure RNA Tissue kit (Roche Diagnostics, Mannheim, Germany).
cDNA preparation from total RNA was performed with 500 ng RNA (20
μl total volume) using the iScript cDNA Synthesis kit (Bio-Rad,
Munich, Germany). All kits mentioned above were used according to
the manufacturer’s recommendations. Quantitative analyses of OCT1
(SLC22A1) and OCT3 (SLC22A3) transcripts were
performed by qRT-PCR. The QuantiTect SYBR-Green PCR kit (Qiagen,
Hilden, Germany) and validated primers of a QuantiTect Primer assay
with the primer sets, HS_SLC22A1_1_SG (OCT1; 120-bp fragment),
HS_SLC22A3_1_SG (OCT3; 115-bp fragment) and HS_GAPDH_2_SG (GAPDH;
119-bp fragment) (Qiagen), were used according to the
manufacturer’s instructions. For the amplification, an initial
denaturation at 95°C for 15 min, followed by 15 sec at 94°C, 30 sec
at 55°C and 30 sec at 72°C for 40 cycles was used. Samples were run
on a LightCycler® 480 real-time PCR system (Roche
Diagnostics). The relative expression levels of OCT1
(SLC22A1) and OCT 3 (SLC22A3) mRNA in CCA and TST
were calculated by normalization to GAPDH gene expression using the
LightCycler® 480 Software v1.5.0. For examination of
OCT1 (SLC22A1) and OCT3 (SLC22A3) mRNA regulation,
the relative mRNA expression of tumor tissue (CCA) was correlated
with the relative mRNA expression of the corresponding TST. The
median expression of this ratio was used to define a cut-off value
to subdivide the tumor tissues into significantly or moderately
downregulated CCAs.
Western blot analysis
Total protein extracts were prepared in sample
buffer (pH 8.0) containing 20 mM Tris, 5 mM EDTA, 0.5% Triton X-100
and cOmplete, Mini, EDTA-Free Protease Inhibitors (1:25; Roche
Diagnostics). For western blot analyses, 60 μg of protein
were loaded onto a 12% SDS-PAGE gel. The gel was transferred onto a
nitrocellulose membrane (Optitran BA-S85; Whatman) following
separation. Mouse anti-human OCT1 monoclonal antibody (1:1,000;
Novus Biologicals, Littleton, CO, USA), rabbit anti-human OCT3
(1:2,000; LifeSpan Biosciences, Seattle, WA, USA) or mouse
anti-human GAPDH polyclonal antiserum (1:5,000; EnoGene Biotech,
New York, NY, USA) were used as the primary antibodies. Horseradish
peroxidase (HRP)-conjugated anti-mouse or anti-rabbit IgG
(DakoCytomation, Hamburg, Germany) were used as the secondary
antibodies at 1:10,000 dilution. Protein bands were visualized
using Western Lightning® Plus-ECL Enhanced
Chemiluminescence Substrate (PerkinElmer, Waltham, MA, USA).
Immunohistochemistry
Immunohistochemical staining was performed on
formalin-fixed, paraffin-embedded 4-μM tissue sections.
Following deparaffinization and rehydration, the endogenous
peroxidase activity was inhibited with 4% hydrogen peroxide in
methanol. For antigen retrieval, tissue sections were treated for
20 min in a steamer in 10 mM citrate buffer (pH 6.0). Cells were
permeabilized with 2% saponin in PBS for 20 min at room
temperature. For blocking of non-specific antibody binding, tissues
were incubated for 30 min with protein blocking buffer (5% normal
serum, 0.2% Triton-X and 2% BSA). The following primary antibodies
were used for immunohistochemistry: mouse monoclonal anti-human
OCT1 antibody (Novus Biologicals) and rabbit monoclonal anti-human
OCT3 antibody (Epitomics, Burlingame, CA, USA) and incubated
overnight at 4°C in PBS (2% saponin, 5% BSA and 5% normal serum).
For the control sections, the specific primary antibody was
omitted. The following day, sections were washed 3 times and
incubated for 20 min with Pierce Peroxidase Suppressor (Thermo
Fisher Scientific, Epsom, UK). After washing, the sections were
incubated with a secondary biotinylated anti-rabbit or anti-mouse
antibody (DakoCytomation), then treated with the
avidin-biotin-peroxidase complex-based Vectastain Elite ABC kit
(Vector Laboratories, Burlingame, CA, USA). Following incubation
with HRP-conjugated streptavidin, tissues were dyed with
diaminobenzidine (DAB) substrate (Sigma-Aldrich, St. Louis, MO,
USA). Counterstaining was performed with Meyer’s hemalaun solution
and slides were mounted for examination under a light
microscope.
Statistical analysis
Data management and all statistical analyses were
performed with the SPSS program (IBM® SPSS®
v19.0). For categorical variables, between-group differences were
analyzed by the Chi-square or Fisher’s exact test. For quantitative
variables, data were expressed as median and range. If distribution
was normal and sample sizes tested sufficient, the equality of
variances was analyzed with the Levene’s test and samples were
compared using the paired t-test. If variables were not normally
distributed or sample size was inadequate, we applied the Wilcoxon
rank-sum non-parametric test. All tests were performed using a 5%
level of significance (two-sided). Overall survival rates were
calculated using the Kaplan-Meier method and compared using the
log-rank test.
Results
Expression of OCT1 (SLC22A1) and OCT3
(SLC22A3) mRNA in CCA.
To analyze the role of OCT1 and OCT3 in malignant
cholangiocarcinoma, we first investigated the mRNA expression of
SLC22A1 and SLC22A3 in CCA tissue and corresponding
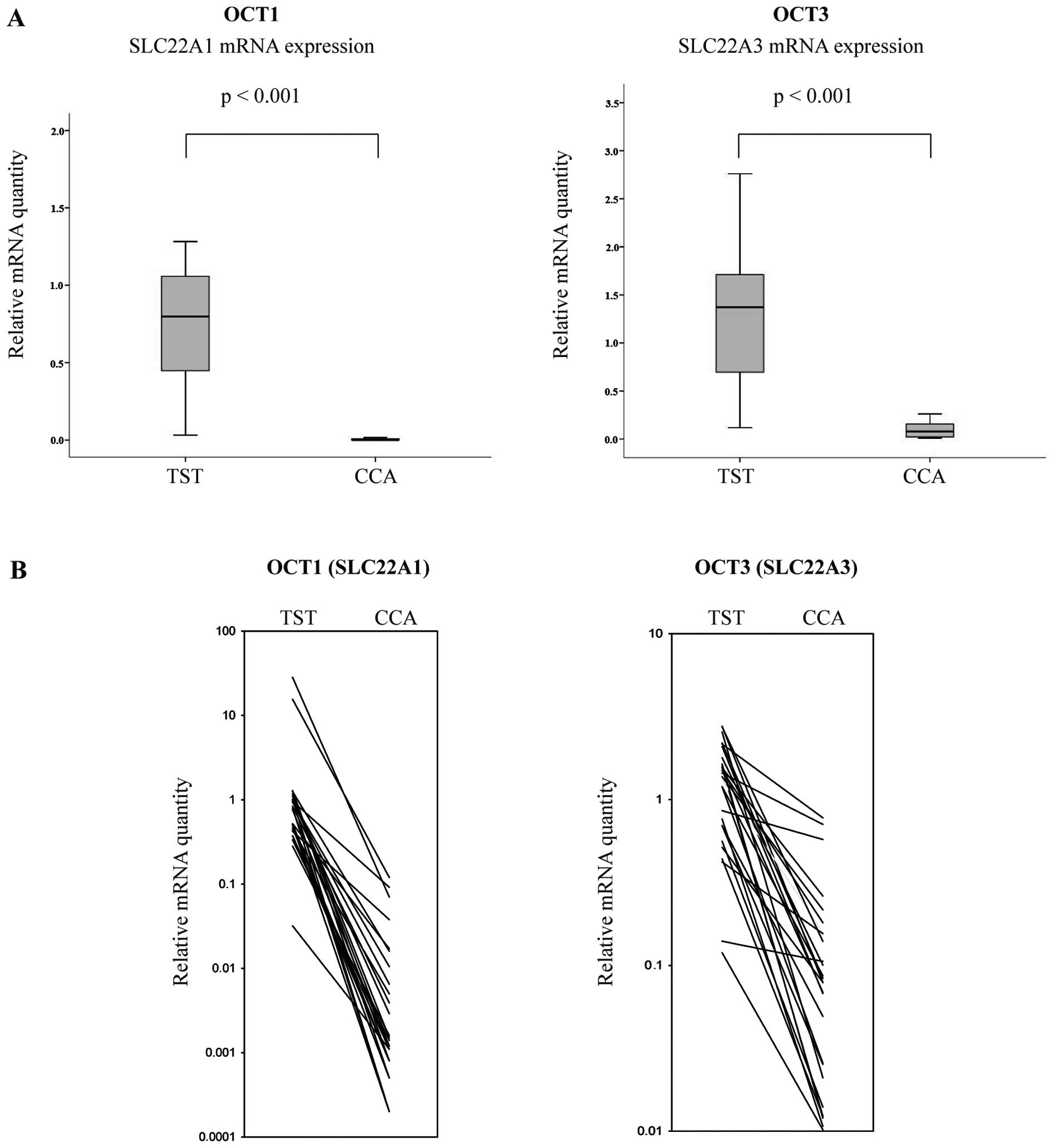
non-neoplastic TST (n=27). qRT-PCR results demonstrated significant
differences in the expression of SLC22A1 mRNA between TST
and CCA (Fig. 1A). SLC22A1
and SLC22A3 mRNA were highly expressed in TST and distinctly
downregulated in cancerous tissues (p<0.001; Fig. 1A).
Individual expression patterns in CCA and the
corresponding TST are presented in Fig. 1B. In CCA tissues, the median
SLC22A1 mRNA expression was reduced by 99.7% compared to
that in the corresponding TST (min, 90.5%; max, 99.9%). The median
SLC22A3 mRNA expression of CCA tissues was also lower by
94.4% (min, 24.5%; max, 99.3%) than that in TST (Fig. 1A and B). The details of patient and
tumor clinicopathological characteristics are summarized in
Table I, according to WHO
specifications.
Patient survival and tumor recurrence in
patients with intratumoral OCT1 (SLC22A1) or OCT3 (SLC22A3)
downregulation
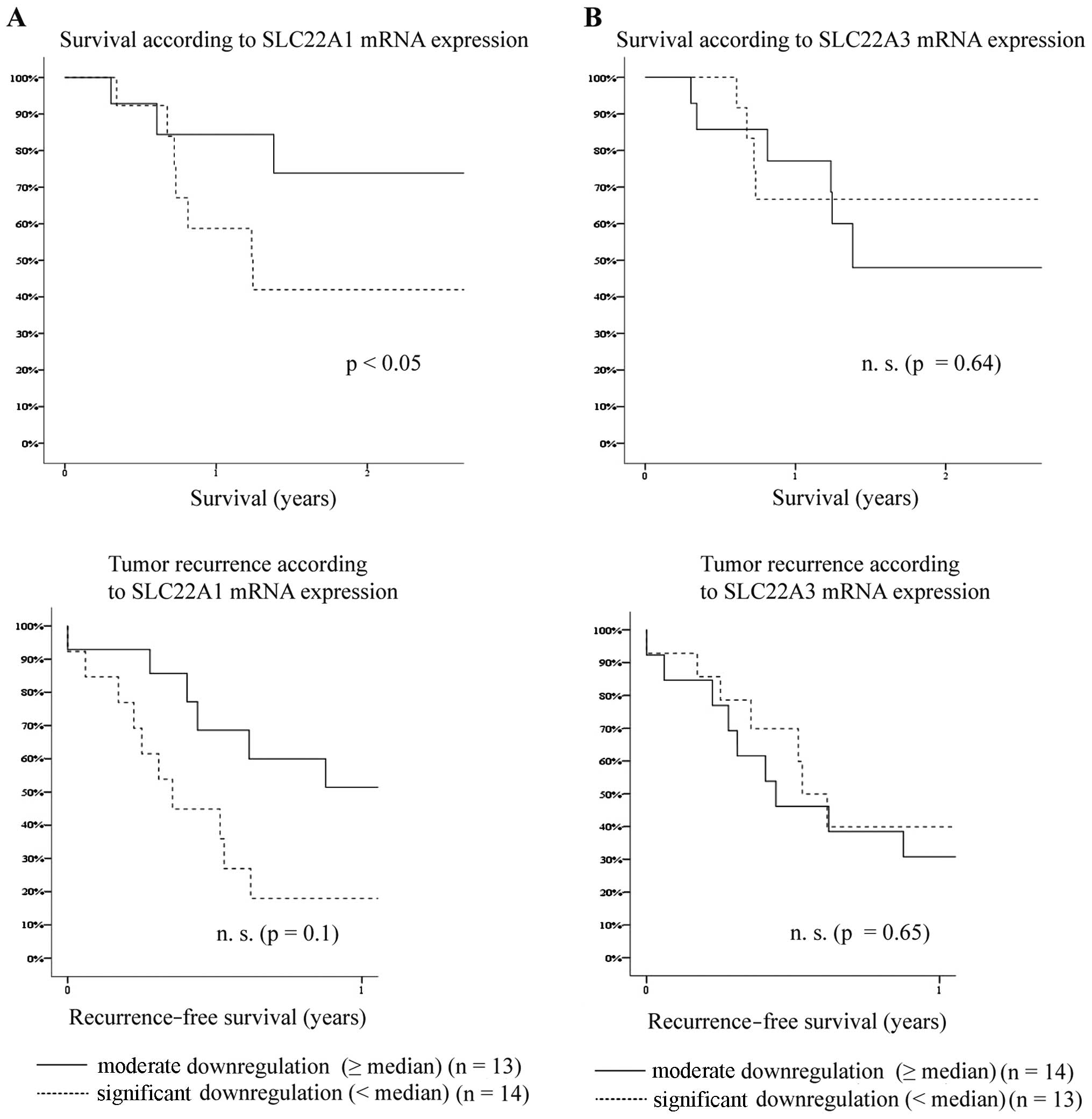
According to the qRT-PCR results, we divided the
patients into 2 groups: a significantly downregulated (CCA/TST
expression level < median) and a moderately downregulated group
(CCA/TST expression level ≥ median). As regards the SLC22A1
mRNA expression, overall patient survival was significantly reduced
in patients with a significant downregulation of SLC22A1
mRNA expression (p<0.05) (Fig.
2A). The significant downregulation of SLC22A1 did not
markedly affect the risk of early tumor recurrence within the first
year following resection (p=0.11) (Fig. 2A).
Although OCT3 (SLC22A3) mRNA expression was
downregulated as well, the overall survival and tumor recurrence
rates of CCA patients with a significant downregulation of
SLC22A3 mRNA were not markedly different from those of
patients with a moderate downregulation of SLC22A3 mRNA
expression (overall survival, p=0.64; tumor recurrence, p=0.65)
(Fig. 2B).
Patient and tumor characteristics in
relation to OCT1 (SLC22A1) and OCT3 (SLC22A3) mRNA expression
Low SLC22A1 mRNA expression levels were
associated with advanced CCA stages, since CCAs with a low
SLC22A1 mRNA expression presented with larger tumor
diameters (p=0.02) (Table II).
SLC22A1 mRNA expression did not correlate with other tumor
characteristics (Table II). There
was no significant difference in tumor characteristics in relation
to SLC22A3 mRNA expression (Table III).
 | Table IIPatient and tumor characteristics in
relation to the intratumoral OCT1 (SLC22A1) mRNA
expression. |
Table II
Patient and tumor characteristics in
relation to the intratumoral OCT1 (SLC22A1) mRNA
expression.
|
Characteristics | OCT1
(SLC22A1)
| p-value |
|---|
| Moderate
downregulation (≥ median) | Significant
downregulation (< median) |
|---|
| Number of
patients | 14 | 13 | |
| Median follow-up in
days (range) | 533 (111–1363) | 451 (125–1177) | 0.30 (n.s.) |
| Median
recurrence-free survival in days (range) | 273 (0–1110) | 125 (0–833) | 0.12 (n.s.) |
| Male/female
(n) | 8/6 | 8/5 | 1.00 (n.s.) |
| Median age in years
(range) | 64 (32–84) | 69 (44–82) | 0.43 (n.s.) |
| 1–3
Nodules/multiple nodules (specimen) | 10/4 | 9/4 | 1.00 (n.s.) |
| Tumor diameter
<3 cm/≥3 cm (n) | 3/11 | 1/12 | 0.60 (n.s.) |
| Median tumor
diameter in cm (range) | 4.8 (1.3–12.0) | 12.8
(1.7–19.5) | 0.02 |
| T-classification
(T1/T2/T3/T4) (n) | 3/8/2/1 | 4/4/4/1 | 0.55 (n.s.) |
| Grading
(G1/G2/G3/Gxa)
(n) | 1/10/3/0 | 0/11/1/1 | 0.39 (n.s.) |
| CEA (ng/ml)
>5/<5 (n)b | 3/10 | 2/10 | 1.00 (n.s.) |
| CA 19–9 (ng/ml)
>37/<37 (n)b | 7/6 | 6/6 | 1.00 (n.s.) |
| SLC22A3 mRNA
expression ratio CCA/TST (range) | 0.072
(0.028–0.755) | 0.043
(0.073–0.670) | 0.24 (n.s.) |
 | Table IIIPatient and tumor characteristics in
relation to the intratumoral OCT3 (SLC22A3) mRNA
expression. |
Table III
Patient and tumor characteristics in
relation to the intratumoral OCT3 (SLC22A3) mRNA
expression.
|
Characteristics | OCT3
(SLC22A3)
| p-value |
|---|
| Moderate
downregulation (≥ median) | Significant
downregulation (< median) |
|---|
| Number of
patients | 13 | 14 | |
| Median follow-up in
days (range) | 823 (161–1363) | 458 (111–1357) | 0.22 (n.s.) |
| Median
recurrence-free survival in days (range) | 161 (0–1110) | 165 (0–833) | 0.87 (n.s.) |
| Male/female
(n) | 9/4 | 7/7 | 0.44 (n.s.) |
| Median age in years
(range) | 63 (32–76) | 70 (34–84) | 0.14 (n.s.) |
| 1–3 Nodules /
multiple nodules (specimen) | 8/5 | 11/3 | 0.42 (n.s.) |
| Tumor diameter
<3 cm/≥3 cm (n) | 1/12 | 3/11 | 0.60 (n.s.) |
| Median tumor
diameter in cm (range) | 6.5 (2.5–18.3) | 8.4 (1.3–19.5) | 0.94 (n.s.) |
| T-classification
(T1/T2/T3/T4) (n) | 4/4/3/2 | 3/8/3/0 | 0.33 (n.s.) |
| Grading
(G1/G2/G3/Gxa)
(n) | 0/10/3/0 | 1/11/1/1 | 0.39 (n.s.) |
| CEA (ng/ml)
>5/<5 (n)b | 4/9 | 1/11 | 0.32 (n.s.) |
| CA 19–9 (ng/ml)
>37/<37 (n)b | 7/5 | 6/7 | 0.70 (n.s.) |
| SLC22A1 mRNA
expression ratio CCA/TST (range) | 0.004
(0.001–0.095) | 0.002
(0.0002–0.089) | 0.26 (n.s.) |
Protein expression of OCT1 and OCT3 in
human CCAs
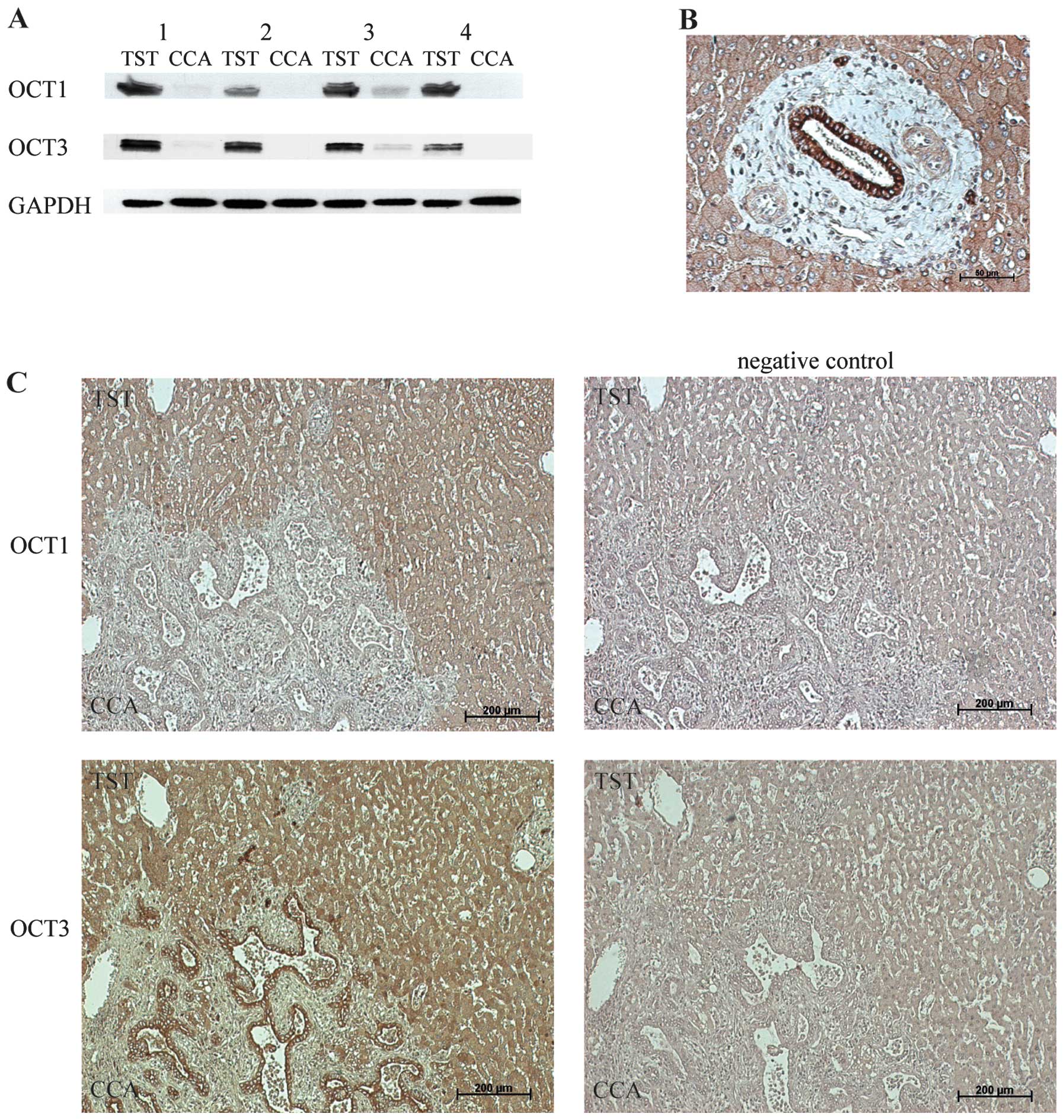
To examine the protein expression levels of the
transporters in CCA, we performed western blot analysis in CCA
tissue and corresponding TST. Fig.
3A depicts the significant downregulation of OCT1 and OCT3 in
CCA tissue compared to that in the corresponding TST of 4 patients.
The downregulation of OCT1 and OCT3 proteins, as detected by
western blot analysis, correlated well with the corresponding mRNA
levels measured by qRT-PCR (Fig.
1A).
To identify the localization of OCT1 and OCT3
expression in tumor tissue, we subsequently assessed protein
expression by immunohistochemistry (Fig. 3B and C). Fig. 3B shows the strong membranous OCT3
staining of bile duct epithelial cells in a normal liver portal
field. Fig. 3C depicts OCT1 and
OCT3 staining at the tumor border. In detail, we detected a
predominantly membranous staining of OCT1 and OCT3 in TST
hepatocytes. OCT1 staining was not detected in carcinoma cells
(Fig. 3C). Positive staining of
OCT3 was observed in the membrane of bile duct epithelial cells in
carcinomatous (Fig. 3C) as well as
normal liver tissue.
Discussion
OCTs have been extensively investigated in several
types of tissue and tumor cell lines. However, data regarding their
presence and role in human malignancies are limited (10–12).
We recently demonstrated a significant downregulation of OCT1 and
OCT3 in human HCCs and reported that the downregulation of OCT1
mRNA in HCC is associated with advanced tumor stage and poor
patient survival (9). To date,
there are no data available regarding OCT expression in human CCA.
Therefore, to the best of our knowledge, this study is the first to
analyze the expression profiles of the OCTs in human CCA with
direct correlation to clinical and tumor-specific data.
The human CCA samples analyzed, revealed a
significant downregulation of OCT1 (SLC22A1) and OCT3
(SLC22A3) expression in cancerous tissue compared to the
corresponding non-neoplastic TST (p<0.001). Our results are the
first to provide evidence that the downregulation of SLC22A1
mRNA expression is associated with advanced tumor stage and worse
overall patient survival (Table II
and Fig. 2A). Median tumor
diameters of the CCAs with a significant downregulation of
SLC22A1 mRNA (CCA/TST expression level < median) were
significantly larger than those of the CCAs with a moderate
downregulation of SLC22A1 mRNA (CCA/TST expression level ≥
median) (p= 0.02, Table II). The
OCT1 and OCT3 proteins were also downregulated in the cancerous
tissues, as demonstrated by western blot analysis (Fig. 3A) and immunohistochemistry
(Fig. 3C). Whether the
intratumoral downregulation of the OCTs results in more aggressive
cancer growth remains to be elucidated. The results from qRT-PCR
and western blot analyses demonstrated a more significant
downregulation of OCT1 (SLC22A1) mRNA and protein, in
comparison to OCT3 (SLC22A3). This may be explained by the
positive OCT3 staining of bile duct epithelial cells in CCA tissue
and TST, which was distinctly demonstrated by immunohistochemistry
(Fig. 3B and C). At present, we
can only hypothesize that the expression of OCT3 in bile duct
epithelial cells plays an important role in transporting
physiological substrates to regulate bile flow or to protect the
cells from bile toxicity.
Although OCTs are functionally influenced by several
endogenous and exogenous substances, to date, data regarding the
pathways and regulatory mechanisms of OCT expression in cancer are
limited. The transporters are located on the chromosomal locus
6q26-27 in a conserved gene cluster and are influenced by genomic
imprinting mechanisms involving the insulin-like growth factor 2
receptor (IGF2R) tumor suppressor gene (13,14).
This may contribute to the complex genetic and epigenetic
regulations in the context of human malignancy. Recently,
Schaeffeler et al (15)
revealed that the DNA methylation of SLC22A1 was associated
with the downregulation of SLC22A1 in HCC and suggested that
the targeting of SLC22A1 methylation by demethylating agents
may provide a novel strategy for the treatment of HCC. Further
studies are required to elucidate the mechanism involved in the
downregulation of OCTs in CCA.
OCTs may play an important role in the therapy of
malignant tumors, since they are considered responsible for the
cytotoxicity of platin derivatives in colorectal cancer (6,16,17).
OCTs are major determinants of the anticancer activity of
oxaliplatin and it has been suggested by Zhang et al that
the expression of OCTs in tumors should be assessed as a marker for
selecting specific platinum-based therapies in individual patients
(6).
In general, bile duct tumors are known to be
relatively unresponsive to conventional chemotherapy. Gemcitabine
combined with platinum compounds (e.g., oxaliplatin) has been
recommended as palliative chemotherapy in advanced biliary tract
cancer (18,19). The chemotherapeutic outcome and
median survival times are still quite limited, with short life
expectancies (not exceeding 15.4 months). In a recent phase 2
study, Gruenberger et al achieved improved response rates
for CCA (63%) with a combination therapy of GEMOX (gemcitabine and
oxaliplatin) and cetuximab (monoclonal anti-EGFR antibody; EGFR
kinase inhibitor) (3). Due to the
limited number of patients included in our study and the short
survival times of the CCA patients following resection, it was not
possible to correlate OCT downregulation with the therapeutic
response to platinum derivates. Further studies are warranted to
evaluate the potential diagnostic and therapeutic consequences of
OCT regulation in CCA.
Furthermore, it is unclear whether the transporters
are functional in CCA tissue and corresponding non-neoplastic TST.
To date, several genetic variations of SLC22A1(20,21)
and SLC22A3(22–24) have been identified. There are no
data available concerning the genetic variations of the OCT genes
in CCA. Further investigation of non-functional or reduced-function
polymorphisms of OCTs in CCA is required, since genetic
polymorphisms of these genes may significantly influence the
transport and action of drugs (25,26).
Furthermore, in colorectal cancer, a common mutation in the
SLC22A3 gene was identified and associated with the
increased risk of distal colon cancer in an Asian population
(27). Another single nucleotide
polymorphism (SNP) in the SLC22A3 gene has been shown to be
associated with prostate cancer in Caucasian populations (28). Thus, SLC22A3 is likely
associated with multiple types of cancer.
In conclusion, this study demonstrates that OCTs are
downregulated in CCA and, to our knowledge, we are the first to
provide evidence that the downregulation of OCT1 is associated with
a worse overall patient survival. Further studies are required to
determine the role of OCTs in CCA development and treatment. The
downregulation of OCT1 (SLC22A1) expression in CCA is
associated with larger tumor diameters and a worse overall patient
survival. These findings may prove to be significant for the
treatment of CCA.
Acknowledgements
We would like to thank Larissa Herbel,
First Department of Internal Medicine, University of Mainz and
Ulrike Suessdorf, Department of Hepatobiliary and Transplantation
Surgery, University of Mainz, for their excellent technical
assistance with the patient samples. The present study was
supported by an intramural fund of the University of Mainz (MAIFOR
grant) to Tim Zimmermann.
References
|
1
|
Khan SA, Toledano MB and Taylor-Robinson
SD: Epidemiology, risk factors, and pathogenesis of
cholangiocarcinoma. HPB (Oxford). 10:77–82. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Seol MA, Chu IS, Lee MJ, Yu GR, Cui XD,
Cho BH, Ahn EK, Leem SH, Kim IH and Kim DG: Genome-wide expression
patterns associated with oncogenesis and sarcomatous
transdifferentation of cholangiocarcinoma. BMC Cancer. 11:782011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gruenberger B, Schueller J, Heubrandtner
U, Wrba F, Tamandl D, Kaczirek K, Roka R, Freimann-Pircher S and
Gruenberger T: Cetuximab, gemcitabine, and oxaliplatin in patients
with unresectable advanced or metastatic biliary tract cancer: a
phase 2 study. Lancet Oncol. 11:1142–1148. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu L, Hausmann M, Dietmaier W, Kellermeier
S, Pesch T, Stieber-Gunckel M, Lippert E, Klebl F and Rogler G:
Expression of growth factor receptors and targeting of EGFR in
cholangiocarcinoma cell lines. BMC Cancer. 10:3022010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
White DL, Radich J, Soverini S, Saunders
VA, Frede AK, Dang P, Cilloni D, Lin P, Mongay L, Woodman R, Manley
P, Slader C, Kim DW, Pane F, Martinelli G, Saglio G and Hughes TP:
Chronic phase chronic myeloid leukemia patients with low OCT-1
activity randomized to high-dose imatinib achieve better responses
and have lower failure rates than those randomized to standard-dose
imatinib. Haematologica. 97:907–914. 2012. View Article : Google Scholar
|
|
6
|
Zhang S, Lovejoy KS, Shima JE, Lagpacan
LL, Shu Y, Lapuk A, Chen Y, Komori T, Gray JW, Chen X, Lippard SJ
and Giacomini KM: Organic cation transporters are determinants of
oxaliplatin cytotoxicity. Cancer Res. 66:8847–8857. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jonker JW, Wagenaar E, Van Eijl S and
Schinkel AH: Deficiency in the organic cation transporters 1 and 2
(Oct1/Oct2 [Slc22a1/Slc22a2]) in mice abolishes renal secretion of
organic cations. Mol Cell Biol. 23:7902–7908. 2003.PubMed/NCBI
|
|
8
|
Zwart R, Verhaagh S, Buitelaar M,
Popp-Snijders C and Barlow DP: Impaired activity of the
extraneuronal monoamine transporter system known as uptake-2 in
Orct3/Slc22a3-deficient mice. Mol Cell Biol. 21:4188–4196. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Heise M, Lautem A, Knapstein J,
Schattenberg JM, Hoppe-Lotichius M, Foltys D, Weiler N, Zimmermann
A, Schad A, Gründemann D, Otto G, Galle PR, Schuchmann M and
Zimmermann T: Downregulation of organic cation transporters OCT1
(SLC22A1) and OCT3 (SLC22A3) in human hepatocellular carcinoma and
their prognostic significance. BMC Cancer. 12:1092012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang Q, Chen J, Beezhold KJ, Castranova
V, Shi X and Chen F: JNK1 activation predicts the prognostic
outcome of the human hepatocellular carcinoma. Mol Cancer.
8:642009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okabe H, Satoh S, Kato T, Kitahara O,
Yanagawa R, Yamaoka Y, Tsunoda T, Furukawa Y and Nakamura Y:
Genome-wide analysis of gene expression in human hepatocellular
carcinomas using cDNA microarray: identification of genes involved
in viral carcinogenesis and tumor progression. Cancer Res.
61:2129–2137. 2001.
|
|
12
|
Park T, Yi SG, Shin YK and Lee S:
Combining multiple microarrays in the presence of controlling
variables. Bioinformatics. 22:1682–1689. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Verhaagh S, Schweifer N, Barlow DP and
Zwart R: Cloning of the mouse and human solute carrier 22a3
(Slc22a3/SLC22A3) identifies a conserved cluster of three organic
cation transporters on mouse chromosome 17 and human 6q26-q27.
Genomics. 55:209–218. 1999. View Article : Google Scholar
|
|
14
|
Zwart R, Sleutels F, Wutz A, Schinkel AH
and Barlow DP: Bidirectional action of the Igf2r imprint control
element on upstream and downstream imprinted genes. Genes Dev.
15:2361–2366. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schaeffeler E, Hellerbrand C, Nies AT,
Winter S, Kruck S, Hofmann U, van der Kuip H, Zanger UM, Koepsell H
and Schwab M: DNA methylation is associated with downregulation of
the organic cation transporter OCT1 (SLC22A1) in human
hepatocellular carcinoma. Genome Med. 3:822011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kitada N, Takara K, Minegaki T, Itoh C,
Tsujimoto M, Sakaeda T and Yokoyama T: Factors affecting
sensitivity to antitumor platinum derivatives of human colorectal
tumor cell lines. Cancer Chemother Pharmacol. 62:577–584. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yokoo S, Masuda S, Yonezawa A, Terada T,
Katsura T and Inui K: Significance of organic cation transporter 3
(SLC22A3) expression for the cytotoxic effect of oxaliplatin in
colorectal cancer. Drug Metab Dispos. 36:2299–2306. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eckel F and Schmid RM: Chemotherapy in
advanced biliary tract carcinoma: a pooled analysis of clinical
trials. Br J Cancer. 96:896–902. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Valle J, Wasan H, Palmer DH, Cunningham D,
Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira
SP, Roughton M and Bridgewater J: Cisplatin plus gemcitabine versus
gemcitabine for biliary tract cancer. N Engl J Med. 362:1273–1281.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kerb R, Brinkmann U, Chatskaia N, Gorbunov
D, Gorboulev V, Mornhinweg E, Keil A, Eichelbaum M and Koepsell H:
Identification of genetic variations of the human organic cation
transporter hOCT1 and their functional consequences.
Pharmacogenetics. 12:591–595. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sakata T, Anzai N, Shin HJ, Noshiro R,
Hirata T, Yokoyama H, Kanai Y and Endou H: Novel single nucleotide
polymorphisms of organic cation transporter 1 (SLC22A1) affecting
transport functions. Biochem Biophys Res Commun. 313:789–793. 2004.
View Article : Google Scholar
|
|
22
|
Chen L, Pawlikowski B, Schlessinger A,
More SS, Stryke D, Johns SJ, Portman MA, Chen E, Ferrin TE, Sali A
and Giacomini KM: Role of organic cation transporter 3 (SLC22A3)
and its missense variants in the pharmacologic action of metformin.
Pharmacogenet Genomics. 20:687–699. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lazar A, Gründemann D, Berkels R, Taubert
D, Zimmermann T and Schömig E: Genetic variability of the
extraneuronal monoamine transporter EMT (SLC22A3). J Hum Genet.
48:226–230. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sakata T, Anzai N, Kimura T, Miura D,
Fukutomi T, Takeda M, Sakurai H and Endou H: Functional analysis of
human organic cation transporter OCT3 (SLC22A3) polymorphisms. J
Pharmacol Sci. 113:263–266. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shu Y, Sheardown SA, Brown C, Owen RP,
Zhang S, Castro RA, Ianculescu AG, Yue L, Lo JC, Burchard EG, Brett
CM and Giacomini KM: Effect of genetic variation in the organic
cation transporter 1 (OCT1) on metformin action. J Clin Invest.
117:1422–1431. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song IS, Shin HJ and Shin JG: Genetic
variants of organic cation transporter 2 (OCT2) significantly
reduce metformin uptake in oocytes. Xenobiotica. 38:1252–1262.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cui R, Okada Y, Jang SG, Ku JL, Park JG,
Kamatani Y, Hosono N, Tsunoda T, Kumar V, Tanikawa C, Kamatani N,
Yamada R, Kubo M, Nakamura Y and Matsuda K: Common variant in
6q26-q27 is associated with distal colon cancer in an Asian
population. Gut. 60:799–805. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Eeles RA, Kote-Jarai Z, Giles GG, Olama
AA, Guy M, Jugurnauth SK, Mulholland S, Leongamornlert DA, Edwards
SM, Morrison J, Field HI, Southey MC, Severi G, Donovan JL, Hamdy
FC, Dearnaley DP, Muir KR, Smith C, Bagnato M, Ardern-Jones AT,
Hall AL, O’Brien LT, Gehr-Swain BN, Wilkinson RA, Cox A, Lewis S,
Brown PM, Jhavar SG, Tymrakiewicz M, Lophatananon A, Bryant SL; UK
Genetic Prostate Cancer Study Collaborators; British Association of
Urological Surgeons' Section of Oncology; UK ProtecT Study
Collaborators; Horwich A, Huddart RA, Khoo VS, Parker CC, Woodhouse
CJ, Thompson A, Christmas T, Ogden C, Fisher C, Jamieson C, Cooper
CS, English DR, Hopper JL, Neal DE and Easton DF: Multiple newly
identified loci associated with prostate cancer susceptibility. Nat
Genet. 40:316–321. 2008. View
Article : Google Scholar : PubMed/NCBI
|