Introduction
Oral squamous cell carcinoma (OSCC), the 8th most
common cancer worldwide, is a common cancer of the head and neck
region (1). Despite advances in
diagnostic methods and treatment strategies in recent years, the
overall survival rate for patients with OSCC has not significantly
improved. The poor prognosis of OSCC patients, with the 5-year
mortality rate currently remaining at 53%, is due to its local
invasion and distant metastasis (2). Tumor cell invasion and metastasis are
regarded as multi-step phenomena involving the proteolytic
degradation of the basement membrane (BM) and extracellular matrix
(ECM), altered cell adhesion, and physical movement of tumor cells.
Invasion and metastasis are important factors that impact OSCC
patient outcomes and prognosis (1), and complicate cancer treatment.
Among the many steps involved in invasion and
metastasis, excessive degradation of the ECM is a hallmark of this
process (3). Many proteases can
degrade ECM components, matrix metalloproteinases (MMPs),
particularly the combination of MMP-2 and -9, are the main enzymes
involved in degradation of type IV collagen (4). Studies have shown that MMP-2 and -9
are overexpressed in OSCC (5).
Overexpression of the genes encoding MMP-2 and -9 is closely
related to OSCC tumor angiogenesis, local tumor invasion, and lymph
node metastasis, and indicates a poor prognosis (4,6,7).
Therefore, blocking the expression of MMP-2 and -9 can inhibit
tumor cell degradation of collagen IV in the ECM and BM, thereby
suppressing tumor development, invasion, and metastasis.
Integrins are a family of heterodimeric cell surface
receptors and are the major extracellular matrix receptors.
Integrins regulate diverse processes, including proliferation,
tumor invasion, and apoptosis (8).
Unlike most epithelial integrins, integrin αvβ6 is not expressed in
healthy adult epithelia, but is upregulated during wound healing
and in cancer (9). Integrin αvβ6
may have multiple regulatory functions in oncogenesis. Integrin
αvβ6, via its extracellular and transmembrane domains, activates
TGF-β, leading to adhesion and epithelial-mesenchymal transition,
while its cytoplasmic domain affects proliferation, generation of
MMPs, and cell migration and survival (10,11).
Studies have shown that integrin αvβ6 induces epithelial to
mesenchymal transition in oral cancer (12), promotes invasion of squamous
carcinoma cells (13), and is
closely linked to liver metastasis of colon cancer cells (14). Therefore, targeted inhibition of
integrin αvβ6 expression should reduce the malignant potential of
tumors, and inhibit the ability of tumor cells to invade and
metastasize.
Collagen fibers, the main components of the ECM, are
widely distributed in different layers of the oral mucosa. The ECM
not only provides a protective screen for the tissues, organs, or
cells it supports, but also participates in cell proliferation,
survival, and apoptosis (15,16).
Clinically, many diseases of the oral mucosa are related to the
remodeling of collagen fibers. Therefore, pathological changes of
the oral mucosa fiber structure and the relationship of oral cancer
have recently gained attention (17). Recent studies have focused on the
relationship between collagen fibers and carcinogenesis, but few
reports have investigated the change in collagen fibers during the
course of treatment.
The activator protein-1 (AP-1) participates in
multiple aspects of tumor metastasis. AP-1 can induce cell
proliferation and cancer, and can promote degradation of the
tumor-surrounding matrix, changes in cell adhesion, and enhanced
cell motility; it can promote angiogenesis and lymphangiogenesis
through the regulation of downstream target genes, thus promoting
tumor invasion and metastasis (18). AP-1 cis-regulatory elements exist
in multiple members of the integrin family, and AP-1 influences the
effect of tumor cells on ECM through integrins (19). Many metastasis-promoting factors
can enhance the expression of MMP-2 and -9 through AP-1 signaling
pathways, thus contributing to degradation of the basement membrane
(20,21).
OSCC with local invasion and successive transfer of
biological characteristics are the main factors affecting success
of treatment and prognosis (1).
Therefore, identifying new drugs that inhibit invasion and
metastasis has become critical in the cancer treatment process. Our
previous studies have shown that scutellarin can inhibit the
proliferation and migration of tongue cancer cells in vitro
and has the ability to regulate cell adhesion (22). To further explore the anticancer
effects of scutellarin, we studied the impact of scutellarin on the
growth and invasion of tongue cancer cells (SAS) xenografted in
nude mice, and evaluated the effect of scutellarin on the
expression of MMP-2, -9, integrin αvβ6, and c-JUN, as well as on
changes in collagen fibers, to explore the possible mechanisms
underlying the anti-tumor effect of scutellarin.
Materials and methods
Reagents and cells
Scutellarin (purity 99%, HPLC) was purchased from
Beidouxing Pharmaceutical Co. Ltd. (Tianjin, P.R. China). SAS cells
were cultured as previously described (22). Briefly, SAS cells were cultured in
Roswell Park Memorial Institute 1640 (RPMI-1640) medium
supplemented with 10% (v/v) heat-inactivated fetal bovine serum
(FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin, and
maintained at 37°C in a humidified incubator (Hanau, Germany)
containing 5% CO2.
Animal treatment with scutellarin
Athymic Balb/ca nude mice (4-week-old males) were
obtained from Shanghai Laboratory Animal Center (Shanghai, P.R.
China). The care and treatment of the experimental animals complied
with the Harbin Medical University guidelines for animal
experiments. To create an animal xenograft model of human tongue
squamous carcinoma, a 0.2-ml suspension of SAS cells
(1×105 cells/ml) in a serum-free medium was
subcutaneously injected into the left dorsal flank of each mouse.
Seven days after implantation, induced tumor dimensions were
recorded. Six mice in each group received oral gavage with
scutellarin (5, 10, 20 mg/kg body weight) or with 0.9% normal
saline solution, every other day. Length and width of each tumor
were measured every other day. Following 3 weeks of administration
of scutellarin or normal saline, the mice were sacrificed and the
tumors removed for hematoxylin and eosin staining,
immunohistochemical staining, Masson’s trichrome staining, and
transmission electron microscope observation. Tumor volume was
determined by direct measurement with calipers and calculated by
the formula: π/6 × (large diameter) × (small diameter)2.
Inhibition rate (IR) of the xenograft tumor was calculated as
follows: IR (%) = [1 − (tumor volume of scutellarin-treated
group/tumor volume of normal saline-treated group)] × 100%.
Masson’s trichrome staining
Formalin-fixed, paraffin-embedded tissues were
sectioned (5 μm thick), deparaffinized, and rehydrated.
Masson’s trichrome staining (23)
was used to observe the content and distribution of collagen
fibers.
TUNEL assay for apoptotic cells in
vivo
Apoptosis was assessed in xenograft tumors using the
terminal deoxyribonucleotide transferase-mediated nick-end labeling
(TUNEL) method in combination with an in situ apoptotic cell
detection kit (Roche, Basel, Switzerland) according to the
manufacturer’s instructions. The extent of apoptosis was evaluated
by counting the number of TUNEL-positive (brown-stained) cells. The
apoptotic index was calculated as the number of TUNEL-positive
cells divided by the total number of cells in 10 randomly selected
high-power fields.
Transmission electron microscopy
(TEM)
Xenografts were dissected and fixed with 2.5%
glutaraldehyde for 2 h, post-fixed in 1% osmium tetroxide
(OsO4) at 4°C for 2 h, and embedded with Epon-812 (EM
Sciences, USA) for 72 h at 60°C. Ultrathin sections (50 nm thick)
were cut and stained with uranium acetate, followed by lead
citrate, and then observed under a transmission electron microscope
(JEOL Ltd., Tokyo, Japan).
Immunohistochemical staining
Deparaffinized sections taken from each tumor were
incubated with 1% BSA for 30 min and then stained with mouse
monoclonal anti-MMP-2 (Santa Cruz Biotechnology, Inc., CA, USA;
sc-13595, 1:200 dilution), goat polyclonal anti-MMP-9 (Santa Cruz
Biotechnology; sc-6840, 1:200 dilution), mouse monoclonal anti-PCNA
(Santa Cruz Biotechnology; sc-25280, 1:200 dilution), or rabbit
polyclonal anti-integrin αvβ6 (Beijing Biosynthesis Biotechnology
Co., Ltd., Beijing, P.R. China; bs-5791R, 1:1000 dilution). The
extent of cell proliferation was evaluated by counting the number
of PCNA-positive cells in 10 randomly selected high-power fields
and the proliferation index was calculated as the number of
PCNA-positive cells divided by the total number of cells in the
field. For MMP-2, -9, and integrin αvβ6 analysis, 10 areas were
randomly selected under a microscope at a magnification of x200.
Image Pro Plus 6.0 (Media Cybernetics, Inc., Bethesda, MD, USA) was
used to quantify the extent of immunopositive expression in cells
with integrated optical density (IOD) values.
Western blot analysis
The effect of scutellarin on proteins was assessed
by western blotting. Cells were cultured to at least 80%
confluence, and then exposed to different concentrations (0, 3, 15,
75 nM) of scutellarin for 24 h. Collected cells were lysed with
RIPA buffer (Beyotime, Nantong, P.R. China) on ice. Protein
concentration in conditioned media samples was determined
(Beyotime). Cell lysates (50 μg total protein) were
separated by 10% SDS-PAGE and electrophoretically transferred onto
Hybond PVDF membranes. After blocking in TBS-T containing 5%
non-fat dry milk, the membranes were incubated overnight at 4°C
with primary antibodies against the target proteins. After washing
twice with 0.01 M PBS, the membranes were incubated with secondary
anti-rabbit IgG antibody linked to horseradish peroxidase, and
protein levels were detected using an ECL detection system
(Amersham, Uppsala, Sweden).
RT-PCR analysis
Total RNA was isolated from experimental cells using
TRIzol reagent (Invitrogen, CA, USA) according to the
manufacturer’s protocol. Total RNA (1 μg) was reverse
transcribed into cDNA and amplified using an RT-PCR system
(Promega, Madison, WI, USA). The primer sequences and product sizes
are listed in Table I. Amplified
products were fractionated using 1% agarose gel electrophoresis.
GAPDH was used as an internal control.
 | Table IPrimers used for RT-PCR analysis. |
Table I
Primers used for RT-PCR analysis.
| Sequence name | Primers |
|---|
| MMP-2 | |
| Forward |
5′-GTGGATGATGCCTTTGCTCG-3′ |
| Reverse |
5′-CCATCGGCGTTCCCATACTT-3′ |
| MMP-9 | |
| Forward |
5′-GCTTGCCCTGGTGCAGTAC-3′ |
| Reverse |
5′-ATTGCCGTCCTGGGTGTAG-3′ |
| αvβ6 | |
| Forward |
5′-AGAGAAGAAGCAGGCACATTATC-3′ |
| Reverse |
5′-AGGTAGGACATCGTTCACAGG-3′ |
| GAPDH | |
| Forward |
5′-AACGGATTTGGTCGTATTGG-3′ |
| Reverse |
5′-TGGAAGATGGTGATGGGATT-3′ |
Statistical analysis
Experiments were independently performed at least 3
times. Data are presented as the mean ± SEM (standard error of the
mean). Data were analyzed using one-way ANOVA and Student’s t-test.
Statistical evaluation was performed using SPSS 16.0 software.
Statistical significance was established at P<0.05.
Results
Scutellarin inhibited SAS xenograft tumor
growth in vivo
Our previous studies show that scutellarin can
inhibit the proliferation of tongue carcinoma SAS in vitro.
This study investigated the effects of scutellarin on SAS xenograft
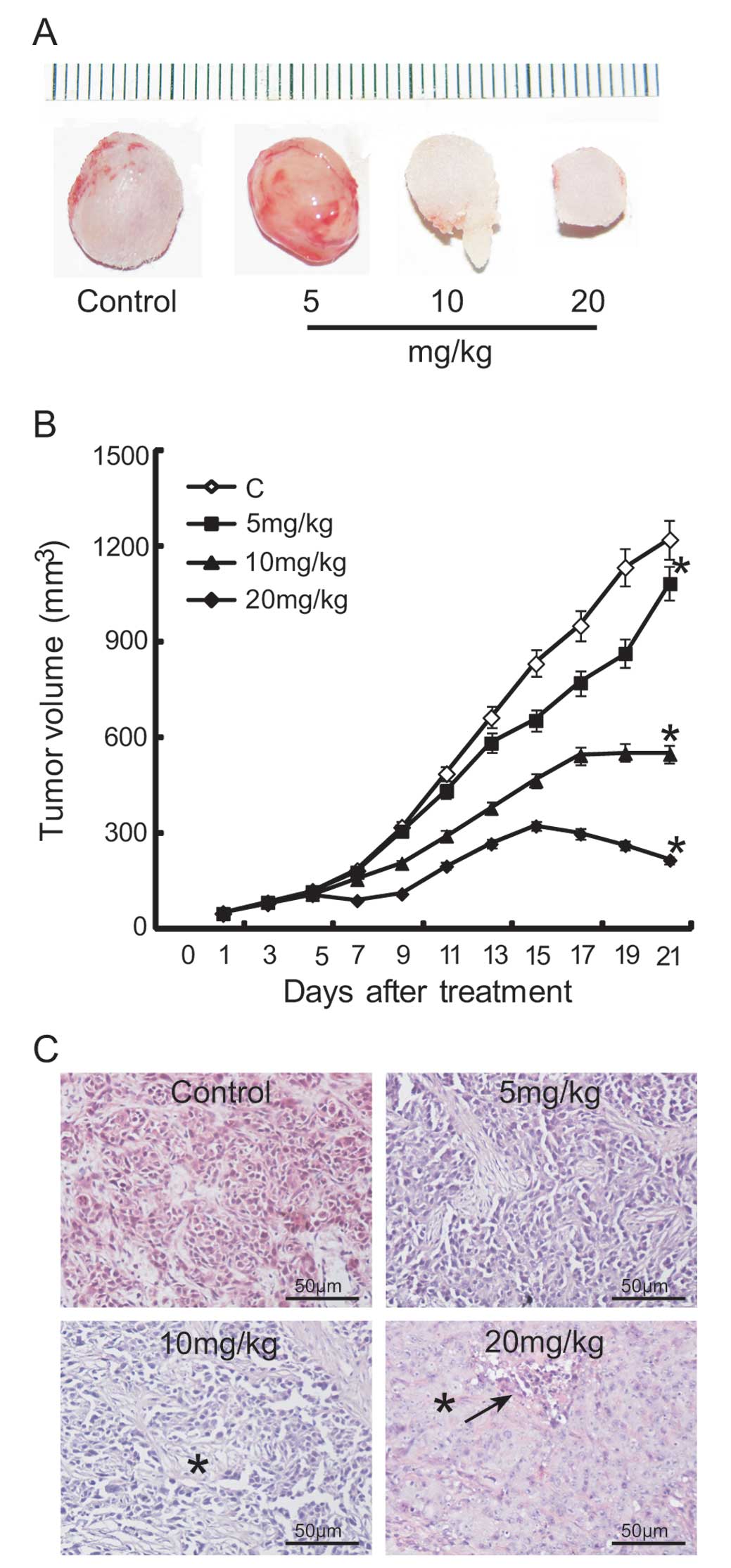
tumor growth. Fig. 1 shows the
growth of nude xenografts in different treatment groups.
Macroscopic appearance of xenografted tumors revealed a smaller
tumor mass in the groups treated with 10 or 20 mg/kg scutellarin
than in the normal saline-treated group (Fig. 1A). Our results showed that the
growth of SAS xenografts was significantly affected by scutellarin
treatment. After treatment for 3 weeks, the average tumor size in
the 5, 10, and 20 mg/kg scutellarin-treated groups was 1085, 550,
and 385 mm3, respectively; however, that in the normal
saline-treated group was 1223 mm3 (Fig. 1B). The rate of inhibition by
scutellarin treatment, as assessed by tumor volume, was determined
to be 11.3, 55.0, and 68.5% in the 5, 10, and 20 mg/kg
scutellarin-treated groups, respectively.
Histologically, moderately differentiated SAS cells
were noted in tumors from the normal saline-treated group and in
tumor remnants of the scutellarin-treated groups (Fig. 1C). Tumor cells were sparsely
distributed in the 10 mg/kg treatment group, displaying
degenerative changes with vacuoles within the cytoplasm, while the
visible cell mass was surrounded by a large amount of extracellular
matrix. Furthermore, tumor necrosis was observed in the 20 mg/kg
scutellarin-treated group.
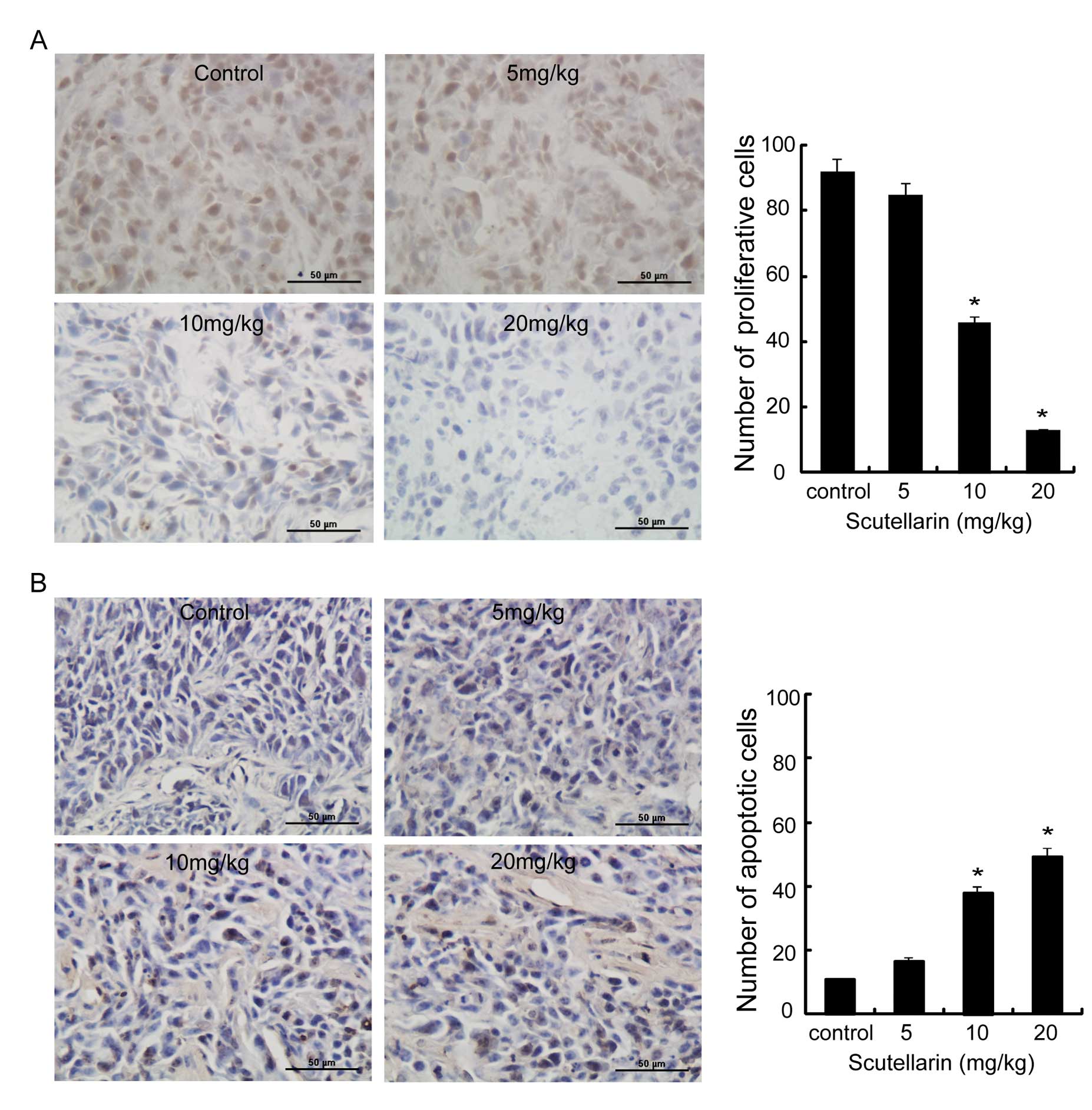
Effects of scutellarin on cell
proliferation and apoptosis in vivo
Histological sections of SAS tumors grown in nude
mice for 3 weeks were analyzed for proliferation and apoptosis.
Immunohistochemical analysis of cell proliferation (PCNA) showed no
difference between the tumors from control and those from the 5
mg/kg scutellarin-treated mice; however, those from the 10 and 20
mg/kg scutellarin-treated mice demonstrated reduced proliferative
ability of the transplanted tumor tissue; moreover, the positive
cell number and intensity of PCNA expression was reduced with
increasing drug concentration (Fig.
2A).
Fig. 2B shows that
scutellarin significantly induced apoptosis. Statistical analysis
of the cell proliferation index demonstrated that the cell
proliferation index was significantly lower with increasing drug
concentration (Fig. 2A;
P<0.01); the proliferation index in the 10 mg/kg-treatment group
was only half of that of the control group. Furthermore, the
apoptotic index increased with the concentration of the drug used
in treatment (Fig. 2B; P<0.01);
in particular, the apoptotic index of the 10 mg/kg-treated group
was 4-fold higher than that of the control group, indicating a
direct killing effect of scutellarin on transplanted tumors.
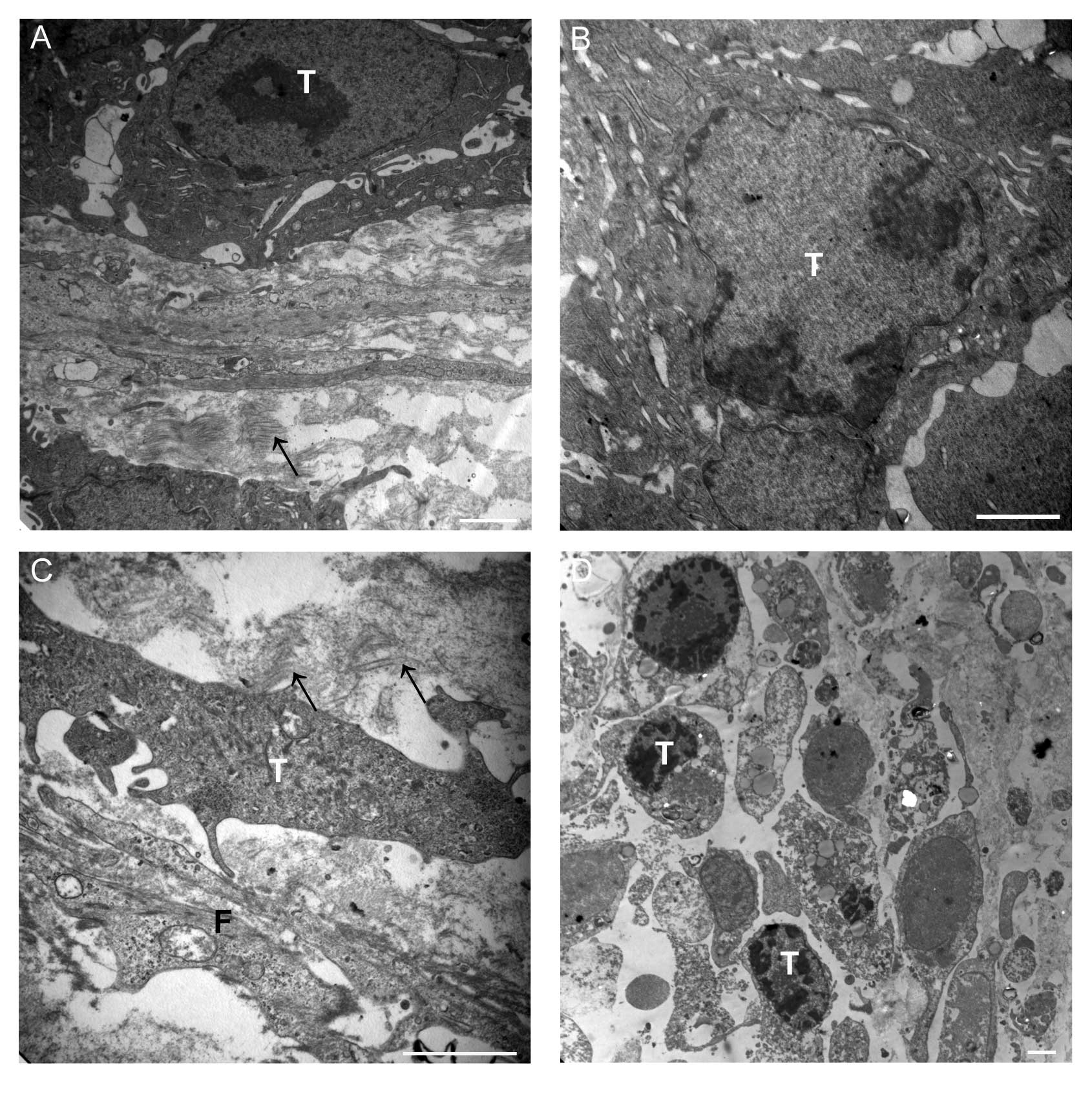
In addition, we observed the ultrastructural changes
of transplanted tumor tissue after scutellarin treatment using TEM.
The results revealed tumor cell membrane integrity, normal
cytoplasm, and organelles without enlargement, large nuclei, and
uniform chromatin distribution in the control group; in addition
inactive fibroblasts, and fewer fibers with a sparse distribution
was seen in this group (Fig. 3A).
This was compared to the transplanted tumor tissue in the
scutellarin-treated group. In the latter group, cell membranes
demonstrated integrity, but the nuclei showed aggregation,
mitochondria and endoplasmic reticulum demonstrated mild swelling
in the 5 mg/kg scutellarin-treated group (Fig. 3B). Moreover, apoptosis was
significant, and the numbers of fibers were increased and
surrounded the tumor cells in the 10 mg/kg scutellarin-treated
group (Fig. 3C). We also observed
that many cell nuclei showed more aggregation, and tumor cell
apoptosis and necrotic cells were frequently observed in the 20
mg/kg scutellarin-treated group (Fig.
3D); however, fibers were not significantly increased compared
with control group.
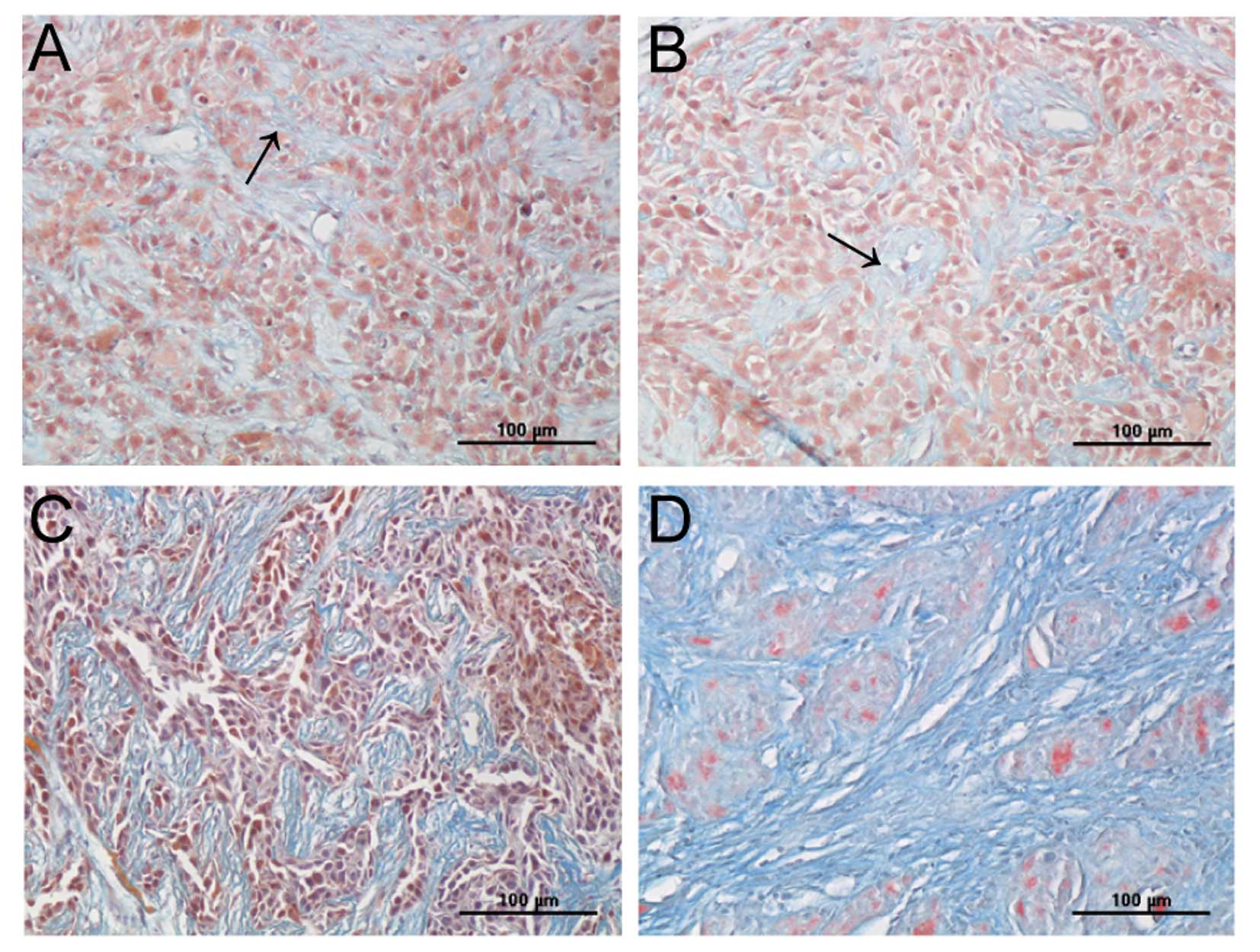
Effects of scutellarin on collagen
matrix
We observed a significant change in interstitial
collagen fibers in transplanted tumor tissue between control and
treated groups using TEM, as described above. To further observe
the change in collagen fibers, we assessed the collagen matrix
using Masson’s trichrome staining. We found that the collagen
fibers were sparsely dispersed in tumor cells in the control and 5
mg/kg scutellarin-treated group (Fig.
4A and B); in comparison, increased collagen fibers, and
bundles of fibers that separates the tumor cells were seen in the
10 mg/kg scutellarin-treated group (Fig. 4C), while the tumor was divided into
many small sections by collagen fibers in the 20 mg/kg
scutellarin-treated group (Fig.
4D).
Scutellarin suppresses molecular events
driving invasion and adhesion in xenografts
Furthermore, we studied the effect of scutellarin on
tumor invasion-related factors. As MMPs degrade the tissue matrix
and facilitate tumor invasion, we first analyzed their levels. We
found that MMP-2 and -9 levels were decreased in tumors by
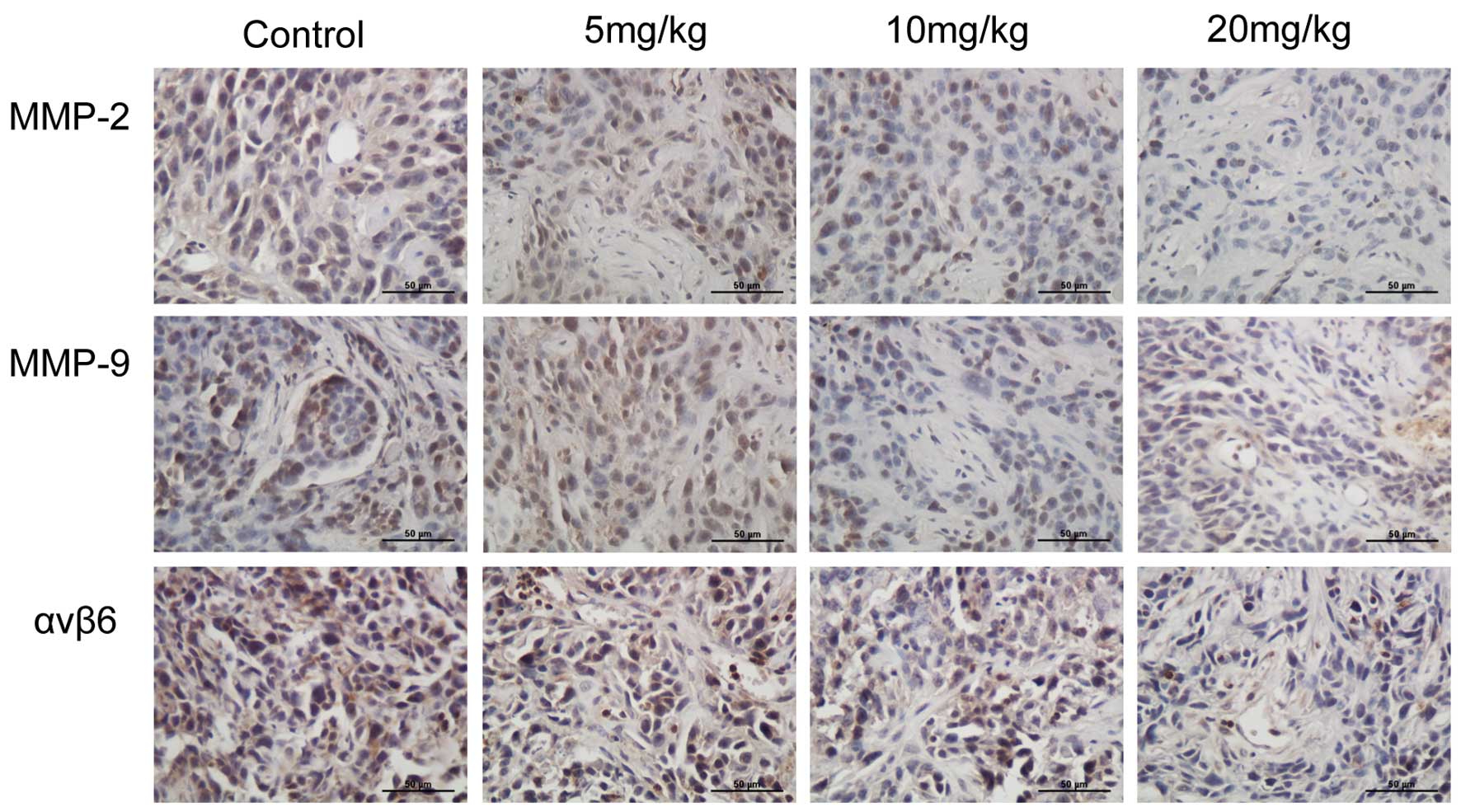
scutellarin treatments, specifically at higher doses (Fig. 5). Immunohistochemistry revealed
that the expression level of integrin αvβ6 was significantly
decreased with an increase in the concentration of scutellarin
(Fig. 5).
Effects of scutellarin on the expression
of MMP-2, -9, integrin αvβ6, and c-JUN in SAS cells
We next evaluated the mechanism underlying the
anti-tumor effect of scutellarin in vitro by measuring
expression of the related factors; in particular, we investigated
factors associated with cell adhesion and invasion. We examine the
effect of scutellarin on the expression of MMP-2, -9, integrin
αvβ6, and c-JUN in SAS cells treated with different concentrations
of scutellarin (0, 3, 15, 75 nM). Western blot analysis showed that
scutellarin reduced the expression of MMP-2, -9, integrin αvβ6, and
c-JUN in a concentration-dependent manner (Fig. 6A).
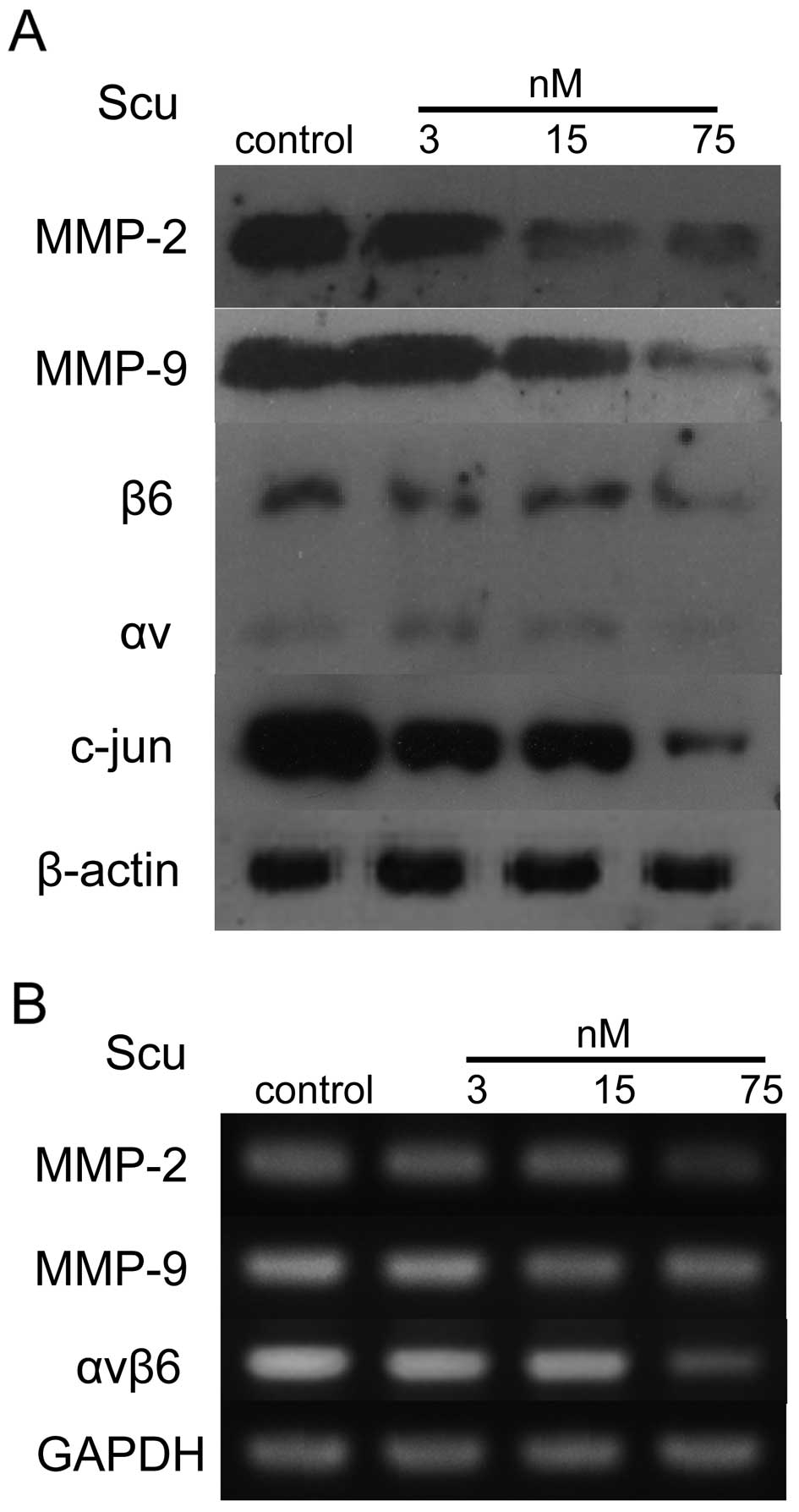 | Figure 6Scutellarin regulates the expression
of proteins and the production of mRNA associated with cell
adhesion and invasion in SAS cells. A, After incubation with
scutellarin for 24 h, total cell lysates were prepared and
subjected to SDS-PAGE, followed by western blotting. The membranes
were probed with specific primary antibodies against MMP-2, -9,
integrin αvβ6, c-JUN, and β-actin, followed by treatment with the
appropriate peroxidase-conjugated secondary antibodies. B, mRNA
expression related to genes encoding MMP-2, -9, integrin αvβ6, and
GAPDH was examined by RT-PCR. β-actin and GAPDH were used as
internal controls for western blot analysis and RT-PCR,
respectively. Scu, scutellarin. |
RT-PCR was used to evaluate the expression levels of
the relevant mRNAs after scutellarin treatment of SAS cells. As
shown in Fig. 6B, we found that,
compared to controls, mRNA levels corresponding to MMP-2, -9, and
integrin αvβ6 decreased in cells treated with scutellarin in a
dose-dependent manner. This demonstrated that scutellarin can
directly modulate the expression of these factors.
Discussion
Scutellarin, also known as scutellarin
7-O-β-D-glucuronide, is a known flavone glycoside. Studies have
shown that scutellarin has a variety of biological effects in
numerous mammalian systems (24,25).
Recently, studies reported that scutellarin inhibited the growth of
the human breast cancer cell line MCF-7 (26). Scutellarin can also inhibit cell
proliferation and induce cell apoptosis in cells of the human
Burkitt lymphoma Namalwa cell line (27). Scutellarin promoted the apoptosis
of cobalt chloride-mediated human prostate cancer cell line PC12
(28); in addition, scutellarin
sensitized 5-fluorouracil (5-FU)-induced apoptosis of colon cancer
cells (29). Previous studies by
our research group have shown that scutellarin inhibits the growth
of tongue cancer cells in vitro and has the ability to
regulate cell adhesion (22).
However, the effect of scutellarin on OSCC cells had not previously
been fully examined. In this study, we found that scutellarin
efficiently inhibited OSCC xenograft tumors in vivo in a
dose-dependent manner.
We applied 3 different concentrations of scutellarin
(5, 10, and 20 mg/kg) to treat SAS tumor-bearing mice, and found
that the growth inhibition was weak in the 5 mg/kg-treated group,
with an inhibition rate of only 11.3%. Inhibition of xenograft
tumor increased significantly with an increase in dose, up to 68.5%
in the 20 mg/kg-treatment group; however, we observed tumor
necrosis under both light and electron microscopy in 20 mg/kg
group. This shows that scutellarin can dose-dependently inhibit
tumor growth, but that excessive drug concentrations may directly
cause tumor cell necrosis, resulting in some side-effects. We
believe that in vivo administration of a 10 mg/kg dose is
the ideal concentration: 10 mg/kg scutellarin significantly
inhibited tumor growth, while the animals did not undergo
significantly abnormal deaths; deaths that occurred were mainly
procedurally-related deaths.
In order to observe the malignant state of cells in
the transplanted tumor tissue after scutellarin treatment, we
evaluated the proliferation and apoptosis of tumor cells. Our
results showed that with 10 mg/kg scutellarin-treatment, the
proliferation index was 50% of that of the control group, while the
apoptosis index was 3 times that of the control group. These
results confirmed that scutellarin inhibited SAS xenograft tumor
cell proliferation and induced apoptosis in vivo. Moreover,
we also observed scutellarin-medicated induction of tumor cell
apoptosis in a dose-dependent manner under TEM. Therefore,
scutellarin inhibited tumor cell proliferation and induced
apoptosis; these effects may play an important role in tumor growth
arrest.
Integrin αvβ6 is undetectable in normal oral tissues
but is upregulated in carcinomas of the colon, cholangiocarcinoma,
and ovarian, breast, endometrial, gastric, cervical, and oral
squamous cell carcinoma (10).
Expression of integrin αvβ6 promotes migration and invasion in SCC
(11,30). Therefore inhibition of integrin
αvβ6 expression may reduce cell invasion. Our results show that
scutellarin reduces integrin αvβ6 expression in tumor cells, which
is consistent with our in vitro results (22). Therefore, we speculate that
scutellarin reduces the connection of the tumor cells with the
extracellular matrix by inhibiting integrin αvβ6, thereby
regulating the different signal transduction pathways that mediate
tumor invasion and metastasis (8).
This phenomenon is worthy of further study, and indicated that the
effect of scutellarin is not confined to the induction of
differentiation and apoptosis, and that it has anti-metastatic
potential.
Invasion via degradation of the ECM and BM is one of
the critical steps in the cascade of metastasis (31). Gelatinase (MMP-2 and -9) is the key
regulatory enzyme in the degradation of the ECM and BM (4,32).
In a variety of malignant tumors, the expression of MMP-2 and -9 is
higher than in normal tissue (33). Some studies have shown that the
expression of MMP-2 and -9 is increased in OSCC tissues (5); high expression of MMP-2 and -9 is
closely related to the invasion and metastasis of OSCC (6,34).
Therefore, reduction in expression of these MMPs can theoretically
be used to treat this disease. We observed the expression of MMP-2
and -9 in scutellarin-treated transplanted tumor tissue by
immunohistochemistry. The results showed that scutellarin
significantly reduced expression of MMP-2 and -9. This indicated
that scutellarin directly inhibited tumor invasion by reducing
gelatinase activity.
To infiltrate into the surrounding tissue, it is
necessary for tumor cells to puncture the surrounding matrix
fibers. Collagen fiber is an important fiber component in the ECM.
Collagen fiber content increased in scutellarin-treated
transplanted tumor tissue; the collagen fibers showed great
morphological changes: scutellarin promoted envelopment and attack
of the tumor by collagen fibers. Thus, the content and distribution
of collagen fibers affected tumor cell invasion. Increases in
collagen content and reduced expression of MMPs are coherent
results. This suggests that scutellarin may be involved in
regulating collagen synthesis and distribution in the tumor
microenvironment.
By using different doses of scutellarin for
treatment of nude mice with SAS-transplanted tumors, we observed
that scutellarin inhibited cell proliferation and induced
apoptosis, and inhibited tumor invasion and metastasis by
regulating the expression of adhesion molecules and MMPs. To
further explore the mechanism of the anti-tumor effect of
scutellarin, we assessed the expression of related molecules in
vitro. The results showed that scutellarin reduced expression
of MMP-2, -9, integrin αvβ6 at protein and mRNA levels in a
dose-dependent manner.
Some studies have shown that the expression of
MMP-2, -9, and integrin αvβ6 are regulated by AP-1 (20,35,36).
Multiple members of the MMP family have promoter regions containing
1 or more AP-1 binding sites; AP-1 is a key factor regulating high
expression of the relevant genes (37). The AP-1 signaling pathway is
involved in tumor cells with high expression of MMP-2 and -9
(36). Moreover, there are AP-1
cis-regulatory elements in a the genes encoding a variety of
members of the integrins; the AP-1 signaling pathway is closely
related to the abnormal expression of these integrin molecules
(38). Therefore, we detected the
impact of scutellarin on the expression of c-JUN, and found that
scutellarin inhibited the expression of c-JUN. This indicated that
scutellarin may regulate the activity of c-JUN, and thereby the
expression of the downstream genes, thus exerting an anti-tumor
effect; however, the exact anti-tumor mechanisms require further
research.
Our previous studies have shown that scutellarin
does not exhibit any significant toxicity on human umbilical vein
endothelial cells (39). In the
xenograft model, we used the maximum dose of 20 mg/kg every other
day. In our experiment, neither weight-loss nor poor appetite was
noted in animals in the scutellarin-treated group. This showed that
a low concentration of scutellarin may have no effect on normal
cells and may target tumor cells (22,39).
In conclusion, our study showed that scutellarin
inhibited xenograft tumor growth in nude mice and down-regulated
expression of MMP-2, -9, and integrin αvβ6 in SAS cells. These data
suggest the suitability of scutellarin as an adjuvant anti-invasive
treatment for oral SCCs.
Acknowledgments
This work was supported by grants from Heilongjiang
Province Postdoctoral Science Foundation (No. LRB-05-153), Graduate
Innovation Foundation of Harbin Medical University (No.
HCXB2010006).
References
|
1.
|
Scully C and Bagan J: Oral squamous cell
carcinoma overview. Oral Oncol. 45:301–308. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Gustafsson E and Fassler R: Insights into
extracellular matrix functions from mutant mouse models. Exp Cell
Res. 261:52–68. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Thomas GT, Lewis MP and Speight PM: Matrix
metalloproteinases and oral cancer. Oral Oncol. 35:227–233. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Singh RD, Haridas N, Patel JB, et al:
Matrix metalloproteinases and their inhibitors: correlation with
invasion and metastasis in oral cancer. Indian J Clin Biochem.
25:250–259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Fan HX, Li HX, Chen D, Gao ZX and Zheng
JH: Changes in the expression of MMP2, MMP9, and ColIV in stromal
cells in oral squamous tongue cell carcinoma: relationships and
prognostic implications. J Exp Clin Cancer Res. 31:902012.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
De Vicente JC, Fresno MF, Villalain L,
Vega JA and Hernandez Vallejo G: Expression and clinical
significance of matrix metalloproteinase-2 and matrix
metalloproteinase-9 in oral squamous cell carcinoma. Oral Oncol.
41:283–293. 2005.PubMed/NCBI
|
|
8.
|
Hynes RO: Integrins: versatility,
modulation, and signaling in cell adhesion. Cell. 69:11–25. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Breuss JM, Gallo J, De Lisser HM, et al:
Expression of the beta 6 integrin subunit in development, neoplasia
and tissue repair suggests a role in epithelial remodeling. J Cell
Sci. 108:2241–2251. 1995.PubMed/NCBI
|
|
10.
|
Bandyopadhyay A and Raghavan S: Defining
the role of integrin alphavbeta6 in cancer. Curr Drug Targets.
10:645–652. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Thomas GJ, Lewis MP, Whawell SA, et al:
Expression of the alphavbeta6 integrin promotes migration and
invasion in squamous carcinoma cells. J Invest Dermatol. 117:67–73.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Ramos DM, Dang D and Sadler S: The role of
the integrin alpha v beta6 in regulating the epithelial to
mesenchymal transition in oral cancer. Anticancer Res. 29:125–130.
2009.PubMed/NCBI
|
|
13.
|
Thomas GJ, Lewis MP, Hart IR, Marshall JF
and Speight PM: AlphaVbeta6 integrin promotes invasion of squamous
carcinoma cells through up-regulation of matrix
metalloproteinase-9. Int J Cancer. 92:641–650. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Yang GY, Xu KS, Pan ZQ, et al: Integrin
alpha v beta 6 mediates the potential for colon cancer cells to
colonize in and metastasize to the liver. Cancer Sci. 99:879–887.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Souza LF, Souza VF, Silva LD, Santos JN
and Reis SR: Expression of basement membrane laminin in oral
squamous cell carcinomas. Braz J Otorhinolaryngol. 73:768–774.
2007.PubMed/NCBI
|
|
16.
|
Sakamoto S and Kyprianou N: Targeting
anoikis resistance in prostate cancer metastasis. Mol Aspects Med.
31:205–214. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Rajalalitha P and Vali S: Molecular
pathogenesis of oral submucous fibrosis - a collagen metabolic
disorder. J Oral Pathol Med. 34:321–328. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Verde P, Casalino L, Talotta F, Yaniv M
and Weitzman JB: Deciphering AP-1 function in tumorigenesis:
fraternizing on target promoters. Cell Cycle. 6:2633–2639. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Cowles EA, Brailey LL and Gronowicz GA:
Integrin-mediated signaling regulates AP-1 transcription factors
and proliferation in osteoblasts. J Biomed Mater Res. 52:725–737.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Jin YJ, Park I, Hong IK, et al:
Fibronectin and vitronectin induce AP-1-mediated matrix
metalloproteinase-9 expression through integrin
alpha(5)beta(1)/alpha(v)beta(3)-dependent Akt, ERK and JNK
signaling pathways in human umbilical vein endothelial cells. Cell
Signal. 23:125–134. 2011. View Article : Google Scholar
|
|
21.
|
Singh NK, Quyen DV, Kundumani-Sridharan V,
Brooks PC and Rao GN: AP-1 (Fra-1/c-Jun)-mediated induction of
expression of matrix metalloproteinase-2 is required for
15S-hydroxyeicosate traenoic acid-induced angiogenesis. J Biol
Chem. 285:16830–16843. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Li H, Huang D, Gao Z, et al: Scutellarin
inhibits cell migration by regulating production of alphavbeta6
integrin and E-cadherin in human tongue cancer cells. Oncol Rep.
24:1153–1160. 2010.PubMed/NCBI
|
|
23.
|
Laitakari J and Stenback F: Collagen
matrix in development and progression of experimentally induced
respiratory neoplasms in the hamster. Toxicol Pathol. 29:514–527.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Suh SJ, Yoon JW, Lee TK, et al:
Chemoprevention of Scutellaria bardata on human cancer cells and
tumorigenesis in skin cancer. Phytother Res. 21:135–141. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Goh D, Lee YH and Ong ES: Inhibitory
effects of a chemically standardized extract from Scutellaria
barbata in human colon cancer cell lines, LoVo. J Agric Food Chem.
53:8197–8204. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Wang CZ, Li XL, Wang QF, Mehendale SR and
Yuan CS: Selective fraction of Scutellaria baicalensis and its
chemopreventive effects on MCF-7 human breast cancer cells.
Phytomedicine. 17:63–68. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Feng Y, Zhang S, Tu J, et al: Novel
function of scutellarin in inhibiting cell proliferation and
inducing cell apoptosis of human Burkitt lymphoma Namalwa cells.
Leuk Lymphoma. 53:2456–2464. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Wang LX, Zeng JP, Wei XB, Wang FW, Liu ZP
and Zhang XM: Effects of scutellarin on apoptosis induced by cobalt
chloride in PC12 cells. Chin J Physiol. 50:301–307. 2007.PubMed/NCBI
|
|
29.
|
Chan JY, Tan BK and Lee SC: Scutellarin
sensitizes drug-evoked colon cancer cell apoptosis through enhanced
caspase-6 activation. Anticancer Res. 29:3043–3047. 2009.PubMed/NCBI
|
|
30.
|
Ramos DM, But M, Regezi J, et al:
Expression of integrin beta 6 enhances invasive behavior in oral
squamous cell carcinoma. Matrix Biol. 21:297–307. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Mignatti P and Rifkin DB: Biology and
biochemistry of proteinases in tumor invasion. Physiol Rev.
73:161–195. 1993.PubMed/NCBI
|
|
32.
|
Vargova V, Pytliak M and Mechirova V:
Matrix metalloproteinases. EXS. 103:1–33. 2012.
|
|
33.
|
Itoh Y and Nagase H: Matrix
metalloproteinases in cancer. Essays Biochem. 38:21–36.
2002.PubMed/NCBI
|
|
34.
|
Patel BP, Shah SV, Shukla SN, Shah PM and
Patel PS: Clinical significance of MMP-2 and MMP-9 in patients with
oral cancer. Head Neck. 29:564–572. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Sullivan BP, Kassel KM, Manley S, Baker AK
and Luyendyk JP: Regulation of transforming growth
factor-beta1-dependent integrin beta6 expression by p38
mitogen-activated protein kinase in bile duct epithelial cells. J
Pharmacol Exp Ther. 337:471–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Hong IK, Kim YM, Jeoung DI, Kim KC and Lee
H: Tetraspanin CD9 induces MMP-2 expression by activating p38 MAPK,
JNK and c-Jun pathways in human melanoma cells. Exp Mol Med.
37:230–239. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Chakraborti S, Mandal M, Das S, Mandal A
and Chakraborti T: Regulation of matrix metalloproteinases: an
overview. Mol Cell Biochem. 253:269–285. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Corbi AL, Jensen UB and Watt FM: The
alpha2 and alpha5 integrin genes: identification of transcription
factors that regulate promoter activity in epidermal keratinocytes.
FEBS Lett. 474:201–207. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Gao ZX, Huang DY, Li HX, et al:
Scutellarin promotes in vitro angiogenesis in human umbilical vein
endothelial cells. Biochem Biophys Res Commun. 400:151–156. 2010.
View Article : Google Scholar : PubMed/NCBI
|