Introduction
Gastric cancer (GC) is one of the leading causes of
cancer-related death worldwide (1). Although the exact mechanism of
gastric carcinogenesis is not fully known, several associated
environmental factors have been identified, such as Helicobacter
pylori infection (2). GC has
been classified histologically into either intestinal or diffuse
types by the Lauren classification system (3). Intestinal-type cancer is thought to
be related to environmental factors and is considered to evolve
through well-characterized sequential stages that progress through
chronic gastritis, atrophy, intestinal metaplasia, and dysplasia
(4). The intestine-specific
expression of the homeoprotein, Cdx2, makes it one of the most
likely candidates to be involved in inducing intestinal meta-plasia
of the stomach (5,6).
The Cdx2 gene encodes for a Drosophila
caudal-related homeobox transcription factor that regulates the
proliferation and differentiation of intestinal cells and maintains
the intestinal phenotype (7).
While Cdx2 protein is not expressed in the normal stomach; it is
highly expressed in the remainder of the normal intestine, in
intestinal metaplasia of the stomach, and in a subset of GC
(8). Ectopic expression of Cdx2 in
the stomach of transgenic mice has shown that Cdx2 is involved in
the initiation of intestinal metaplasia formation (9). Mutoh et al(10), further observed that
intestinal-type GC developed from intestinal metaplasia in
Cdx2-transgenic mice. Cdx2 has also been adequately
validated for the diagnosis of colorectal cancer. The studies that
have investigated the relationship between Cdx2 expression and GC
prognosis have failed to result in unanimous agreement (8,11–13).
Roessler et al failed to observe any significant correlation
between levels of Cdx2 and survival probability (14).
Phosphatase and tensin homolog deleted on chromosome
10 (PTEN) is an important tumor suppression gene and is
widely expressed in normal human tissues. Together with the
activity of lipid phosphatase and protein phosphatase, it is
involved in the regulation of cell growth, proliferation, migration
and apoptosis (15). PTEN
is mutated in a wide range of human cancers and is associated with
the apoptosis, proliferation and metastasis of tumor cells
(16,17). It is well known that PTEN is
a tumor suppressor gene that localizes to the cytoplasm and the
patients with cytoplasmic expression of PTEN protein have a more
favorable prognosis. Recent studies have also reported the
involvement of nuclear PTEN involvement in chromosomal stability
and GC prognosis (18,19).
A study by Kim et al has suggested that Cdx-2
was a target of both PTEN/ phosphatidylinositol 3-kinase (PI3K)
signaling and tumor necrosis factor α (TNF-α) signaling via nuclear
factor κB-dependent pathways (20). However, the relationship between
Cdx2 and PTEN has not been established. Cdx2 protein has an
important role in the differentiation of GC, whilst PTEN is closely
related to tumor invasion and metastasis. The codetection of these
two proteins could provide improved specificity and sensitivity in
determining the prognosis of GC (19).
This study aimed to determine the expression
patterns of Cdx2 and nuclear PTEN in GC tissue and in different
cell lines in order to discover their relationship to clinical and
pathological features. The tumorigenic potential of the BGC823 cell
line was observed after high expression of Cdx2 and PTEN was
established through the exogenous expression of Cdx2. The protein
expression of Cdx2, PTEN, PI3K and pAkt was observed after
transfecting Cdx2 into BGC823 cells.
Materials and methods
Patients and follow-up
A group of 228 consecutive patients who presented
with GC to Beijing’s People’s Hospital, Beijing Friendship Hospital
and the Beijing Cancer Hospital between 1999 and June 2003 were
included in this study. All patients were treated by radical D2
gastrostomy. There were 170 males and 58 females with a mean age of
60.72±12.95 years (range 19–93 years). All patients were followed
by clinical evaluation or phone interview until either June 2007 or
their death, which provided a minimum of 5 years of follow-up. This
study was approved by the Ethics Committee of Peking University
People’s Hospital, China.
Tissue microarray and
immunohistochemistry
Tissue arrays and immunohistochemical analyses were
undertaken in accordance with previously published research
(19). Briefly, the slides were
deparaffinized, and then rehydrated and treated with a 3% hydrogen
peroxide solution. After antigen retrieval, the sections were
incubated with primary antibodies overnight at 4°C. Primary
antibodies were detected using the Powervision two-step
histostaining reagent (Zhongshan, Beijing, China) with PV-6001 and
the secondary antibody detection was performed with the use of the
diaminobenzidine (DAB) chromogenic reaction. Tissues were
counterstained, dehydrated and mounted. Positive and negative
controls were included. Two experienced pathologists independently
examined the patterns of protein staining in a blinded manner
relating to the clinical information. The intensity rating used
was: 0, no staining of tumor cells; ±, <10% of cells stained
(weak); 1+, 10–50% of cells stained yellow in color (moderate); and
2+, >50% of cells stained deep brown in color (strong). For the
purpose of statistical analysis, groups that scored 0, ± and 1+
were combined into a weaker staining group and compared with the
group that scored 2+ (strong staining).
RT-PCR
Total RNA from tissue samples and cells were
prepared by TRizol reagent (Invitrogen) and cDNA libraries were
generated by reverse transcription using Moloney murine leukemia
virus reverse transcriptase (MMLV) and oligo(dT) primers. The
primers used for Cdx2 amplification were 5′-AGC CAA GTG AAA
ACC AGG AC-3′ (forward) and 5′-TTT CCT CTC CTT TGC TCT GC-3′
(reverse). The primers used for amplification of the PTEN
gene were 5′-ACC AGG ACC AGA GGA AAC CT-3′ (forward) and 5′-GCT AGC
CTC TGG ATT TGA CG-3′ (reverse). The β-actin gene was adopted as an
internal control for all RT-PCR reactions.
Western blotting
Equal amounts of protein from different samples were
electrophoresed on 12% SDS-PAGE and electro-transferred onto
polyvinylidene fluoride (PVDF) membranes using Mini PROTEAN 3
system (Bio-Rad). PVDF membranes were blocked with
phosphate-buffered saline (PBS) containing 5% fat-free milk powder
for 2 h and incubated with primary antibodies (Cdx2, PI3KCA,
Abgent; PTEN, pAkt, Santa Cruz; GAPDH, Shanghai Kangchen) at 4°C
overnight. Anti-mouse or anti-rabbit antibodies against IgG
conjugated with horseradish peroxidase (HRP) were adopted as the
secondary antibodies. Peroxidase activity was visualized with ECL
kit (GE Healthcare).
Constructed plasmids
The CDS (coding sequence) region of the human
Cdx2 gene was cloned by polymerase chain reaction (PCR).
Sequence analysis was performed to confirm the nucleotide
sequences. The following sequences of oligonucleotides were used as
primers that contained a linker recognizable by 5′-XhoI and
3′-EcoRI (underlined): 5′-CGC CTC GAG ATG TAC GTG AGC TAC
CTC CT-3′ (forward); 5′-TGG AAT TCC TGG GTG ACG GTG GGG TT-3′
(reverse). Amplified 942-bp fragments that contained the human
Cdx2 CDS were ligated into the XhoI and EcoRI
sites of pcDNA3.1. According to the previous method, the human
Cdx2 CDS and PTEN CDS were ligated into the
XhoI and BamHI sites of pEGFP-C3 (Clontech).
siRNA-mediated downregulation of gene
expression
The target sequence for Cdx2-specific siRNA duplex
(Cdx2 siRNA) was derived from an mRNA sequence (5′-AAC CAG GAC GAA
AGA CAA AUA-3′) of human Cdx2 and chemically synthesized
(Invitrogen) (21). A chemically
synthesized mock siRNA (fluorescein-labeled, non-silencing) was
also purchased from Invitrogen. Transfection of these oligos (50
nM) was performed using Lipofectamine 2000 (Invitrogen). For RNA
extraction, cells were harvested 48 h after transfection. To
measure drug cytotoxicity, cells were grown in 6-well plates then
subcultured into 96-well plates 24 h after transfection.
Cell lines and transfection
The GC cell lines BGC823 and SGC7901 were
established in the People’s Hospital, Peking University, China. AGS
and NCI-N87 were purchased from ATCC (American Type Culture
Collection). The cells were cultured in complete DMEM (Hyclone) at
37°C in a humidified atmosphere containing 5% CO2.
Cells were plated and grown to confluence of 70–90%
without antibiotics. Transfections were performed with
Lipofectamine 2000 (Invitrogen), according to the manufacturer’s
instructions. For stable cell expression, cells transfected with
Cdx2-expressing vectors (pCDNA-Cdx2) were selected
with 400 μg/ml G418 (Sigma) for 28 days. Clones were picked
and expanded for an additional 2 months. Transient transfection was
performed in a similar manner, but using PEGFP-Cdx2 and
PEGFP-PTEN vectors. Experiments that used transiently
transfected cells were undertaken 72 h later. Changes in the size,
shape and nuclear morphology of transfected cells were observed
with the use of phase-contrast microscopy and compared to cells
that were transfected with empty vectors and non-transfected
cells.
MTT assay
Monolayer culture growth rate was determined by the
conversion of 3-(4, 5-dimethylthiazol-2-yl)-2,
5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich China Inc.) to a
water-insoluble form. Three thousand cells in 200 μl of
medium were plated into 96-well plates and grown under standard
conditions. Cultures were assayed on days 0–5. Absorbance values
were determined on an enzyme-linked immunosorbent assay reader
(ELISA; Bio-Rad) at 570 nm.
Colony formation assays
Five hundred cells were plated into 35-mm plates and
allowed to grow for 2 weeks to assess colony formation on the
culture plates. Cell colonies were stained with 0.1% crystal violet
and the total number of colonies per well was counted.
Flow cytometry
Cells (5×105) were harvested and washed
with PBS and fixed in cold 75% ethanol at 4°C overnight. After
staining with propidium iodide (PI) solution for 30 min, cells were
collected on a FACScan flow cytometer equipped with a 488-nm argon
laser and analyzed using the CellQuest software
(Becton-Dickinson).
Tumorigenic assay in nude mice
To analyze the tumorigenicity of GC cells in
vivo, 5×105 cells were injected subcutaneously into
athymic nude mice. The diameters of the tumors were recorded from
the day of tumor cell injection.
Statistical analysis
All data were analyzed using SPSS11.0 software. The
association of Cdx2 and nuclear PTEN expression and
the associated clinical and pathological features was analyzed
using the χ2 test. P-values of <0.05 were considered
to be statistically significant.
Results
Expression of Cdx2 and nuclear PTEN
correlated with the clinicopathological features of GC
A favorable prognosis was associated with the
following: location in the gastric antrum (p=0.013); intestinal
type (p=0.002); well-differentiated (p=0.027); earlier pTNM stage
(p=0.001); without lymph node metastasis (p=0.032); and without
distant metastasis (p=0.018).
Cdx2 protein was detected in the nuclei of 43.4% (99
out of 228) gastric tumors and nuclear PTEN was highly expressed in
36.4% (83 out of 228). It was found that nuclear PTEN expression is
present in normal gastric mucosa, intestinal metaplasia, dysplasia
and GC with well-differentiated samples. Cdx2 expression was
detected in the nuclei of cells with intestinal metaplasia (33 out
of 38), dysplasia (14 out of 17) and well-differentiated GC (45 out
of 85), but not in normal gastric mucosa (Fig. 1).
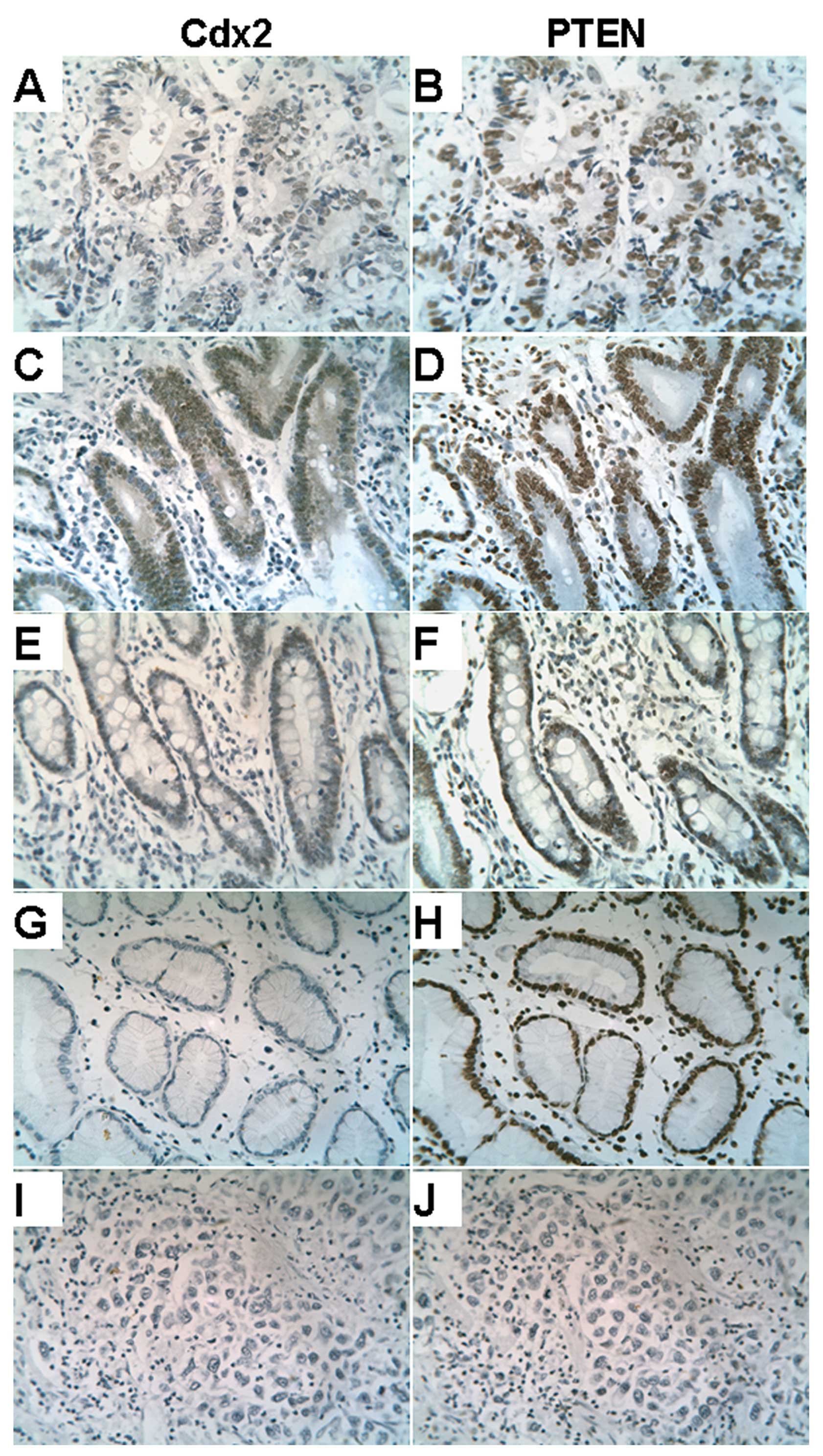 | Figure 1Expression patterns of Cdx2 (A, C, E,
G and I) and PTEN (B, D, F, H and J) in gastric cancer and other
gastric tissue (brown chromogenic reaction). (A and B)
Intestinal-type gastric cancer. (C and D) Dysplasia. (E and F)
Intestinal metaplasia. (G and H) Normal gastric mucosa. (I and J)
Diffuse-type gastric cancer. Cdx2 and nuclear PTEN have similar
expression patterns and highly expressed levels in A, C, E (Cdx2)
and B, D, F (PTEN); there is low expression in (I) (Cdx2) and (J)
(PTEN). Original magnification, ×200. |
The expression of Cdx2 in the nuclei was
significantly higher in stages I and II (59.4%), than in stages III
and IV (37.0%, p=0.001). A higher level of expression of Cdx2 was
found in intestinal-type cancer (47.6%) compared with diffuse-type
GC (19.4%, p=0.002). There was a negative correlation between Cdx2
expression and histological grade (poorly differentiated vs.
moderate and well-differentiated tumors; p=0.027). The expression
of Cdx2 was found to correlate with tumor location (p=0.013), depth
of wall invasion (p=0.002), lymph node metastasis (p=0.032), or
distant metastasis (p=0.018, Table
I).
 | Table IRelationship between Cdx2 or nuclear
PTEN expression and clinicopathological features. |
Table I
Relationship between Cdx2 or nuclear
PTEN expression and clinicopathological features.
| | Cdx2 expression
| | PTEN expression
| |
|---|
| Case | Negative | Positive | P-value | Lower | Higher | P-value |
|---|
| Sex | | | | | | | |
| Female | 58 | 39 | 19 | 0.066 | 41 | 17 | 0.210 |
| Male | 170 | 90 | 80 | | 104 | 66 | |
| Age (years) | | | | | | | |
| 19–55 | 76 | 46 | 30 | 0.479 | 58 | 18 | 0.005 |
| 56–90 | 152 | 83 | 69 | | 87 | 65 | |
| Location | | | | | | | |
| Corpus or
fundus | 71 | 49 | 22 | 0.013 | 48 | 23 | 0.371 |
| Antrum | 146 | 74 | 72 | | 89 | 57 | |
| Lauren
classification | | | | | | | |
| Intestinal
type | 191 | 100 | 91 | 0.002 | 114 | 77 | 0.002 |
| Mixed or diffuse
type | 36 | 29 | 7 | | 31 | 5 | |
|
Differentiation | | | | | | | |
| Poor or
undifferentiated | 142 | 89 | 53 | 0.027 | 99 | 43 | 0.022 |
| Moderate or
well-differentiated | 85 | 40 | 45 | | 46 | 39 | |
| TNM
classification | | | | | | | |
| I | 30 | 8 | 22 | 0.001 | 13 | 17 | 0.003 |
| II | 34 | 18 | 16 | | 19 | 15 | |
| III | 119 | 70 | 49 | | 76 | 43 | |
| IV | 43 | 32 | 11 | | 36 | 7 | |
| Depth of wall
invasion | | | | | | | |
| T1 | 16 | 6 | 10 | 0.002 | 10 | 6 | 0.017 |
| T2 | 26 | 7 | 19 | | 10 | 16 | |
| T3 | 148 | 90 | 58 | | 96 | 52 | |
| T4 | 36 | 25 | 11 | | 28 | 8 | |
| Lymph node
metastasis | | | | | | | |
| No | 59 | 26 | 33 | 0.032 | 32 | 27 | 0.087 |
| Yes | 169 | 103 | 66 | | 113 | 56 | |
| Distant
metastasis | | | | | | | |
| M0 | 195 | 104 | 91 | 0.018 | 118 | 77 | 0.015 |
| M1 | 31 | 24 | 7 | | 26 | 5 | |
A negative correlation existed between high
expression of nuclear PTEN and age (p=0.005), differentiation
(p=0.022), TNM classification (p=0.003), depth of wall invasion
(p=0.017) and distant metastasis (p= 0.015). There was a
significantly smaller amount of nuclear PTEN expression in the
diffuse-type cancer (13.9%) than in the intestinal-type cancer
(40.3%, p=0.002). The expression of nuclear PTEN did not correlate
with other clinicopathological features (Table I).
Coexpression of Cdx2 and PTEN is
correlated with prognosis in GC patients
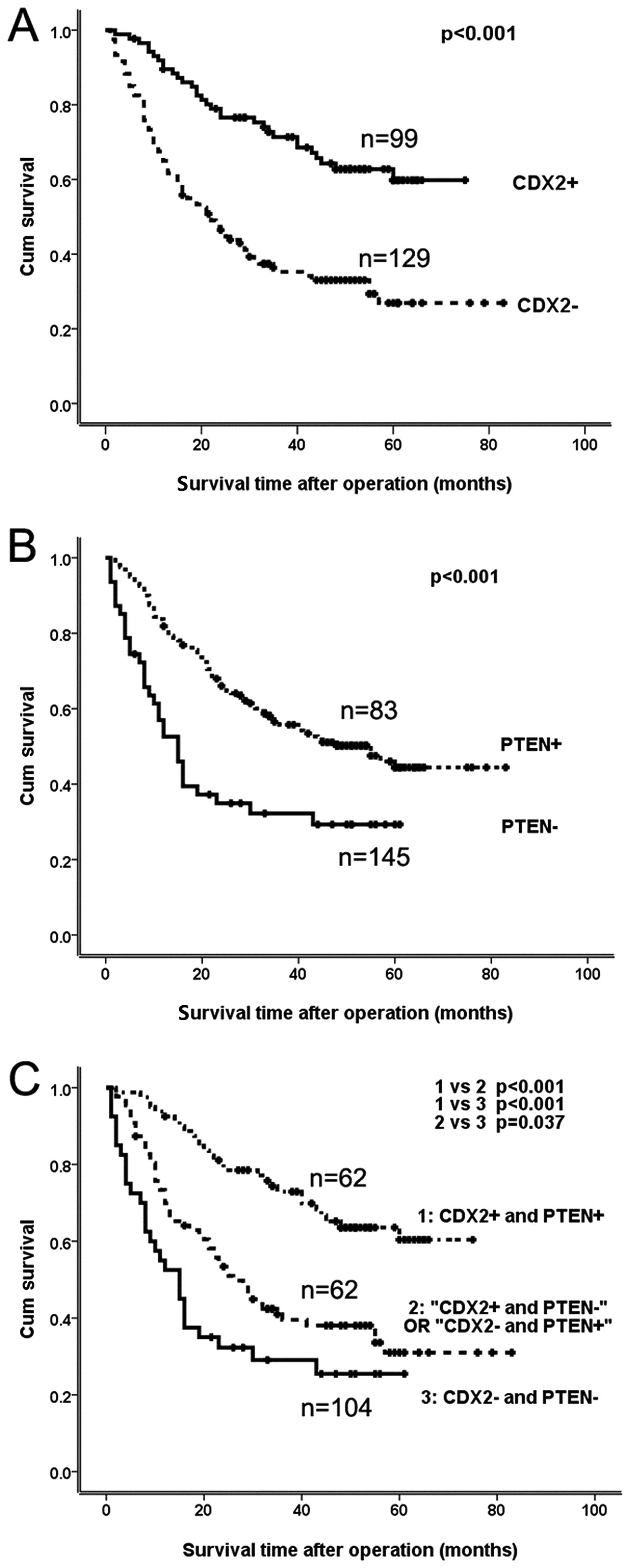
Tissue biopsies with Cdx2-positive cells had a
significantly higher postoperative 5-year survival rate (64.4%)
than Cdx2-negative patients (32.5%; p<0.001; Fig. 2A). The nuclear PTEN-positive group
had significantly higher 5-year survival rates (56.4%) than the
nuclear PTEN-negative group (39.5%; p=0.003; Fig. 2B). Using a Cox regression analysis
for the 228 patient samples, Cdx2 nuclear expression (p=0.004), TNM
stage (p<0.001), and the Lauren classification score (p= 0.001)
appear to be independent prognostic indicators (Table II).
 | Table IIMultivariate analysis of the
prognostic factors in 228 cases using the Cox proportional hazard
model. |
Table II
Multivariate analysis of the
prognostic factors in 228 cases using the Cox proportional hazard
model.
| | | | | | | 95.0% CI for exp
(B)
|
|---|
| B | SE | Wald | df | P-value | Exp (B) | Lower | Upper |
|---|
| Lauren
classification | −0.882 | 0.265 | 11.062 | 1 | 0.001 | 0.414 | 0.246 | 0.696 |
| TNM
classification | | | 29.260 | 3 | <0.001 | | | |
| TNM I vs. IV | −2.543 | 0.626 | 16.530 | 1 | <0.001 | 0.079 | 0.023 | 0.268 |
| TNM II vs.
IV | −1.694 | 0.395 | 18.371 | 1 | <0.001 | 0.184 | 0.085 | 0.399 |
| TNM III vs.
IV | −0.607 | 0.248 | 5.991 | 1 | 0.014 | 0.545 | 0.335 | 0.886 |
| Cdx2
expression | 0.666 | 0.229 | 8.460 | 1 | 0.004 | 1.946 | 1.243 | 3.049 |
Based on the expression profiles of Cdx2 and nuclear
PTEN, the 228 patients were categorized into the following: group
A, Cdx2+/nuclear PTEN+; group
B, Cdx2+/nuclear PTEN− or
Cdx2−/nuclear PTEN+; and group C,
Cdx2−/nuclear PTEN−. There were significant
differences in the survival rates between group A and one of the
other groups (p<0.001; Fig.
2C). The survival rate of group A was significantly higher than
that of groups B and C (p=0.001 and p<0.001; Fig. 2C), respectively.
The expression trends of Cdx2 and PTEN were similar
following immunoassay analysis in well-differentiated GC, dysplasia
and intestinal metaplasia (Table
I). Statistical analysis confirmed a significant correlation
and trend between the expression of Cdx2 and PTEN in the group of
GC patients (Table III,
p<0.001). These data suggest that the combined analysis of Cdx2
and nuclear PTEN expression offer significant value in
distinguishing between the histological types of GC as well as in
assessing the associated prognosis in patients.
 | Table IIIComparative analysis of Cdx2 and PTEN
expression in GC. |
Table III
Comparative analysis of Cdx2 and PTEN
expression in GC.
| Nuclear PTEN
| |
|---|
| Weak
| Strong
| Total
|
|---|
| n (%) | n (%) | 228 |
|---|
| Cdx2 | | | |
| Negative | 105 (81.4) | 24 (18.6) | 129 |
| Positive | 40 (40.4) | 59 (59.6) | 99 |
Simultaneous expression of Cdx2 and PTEN
in different GC cell lines
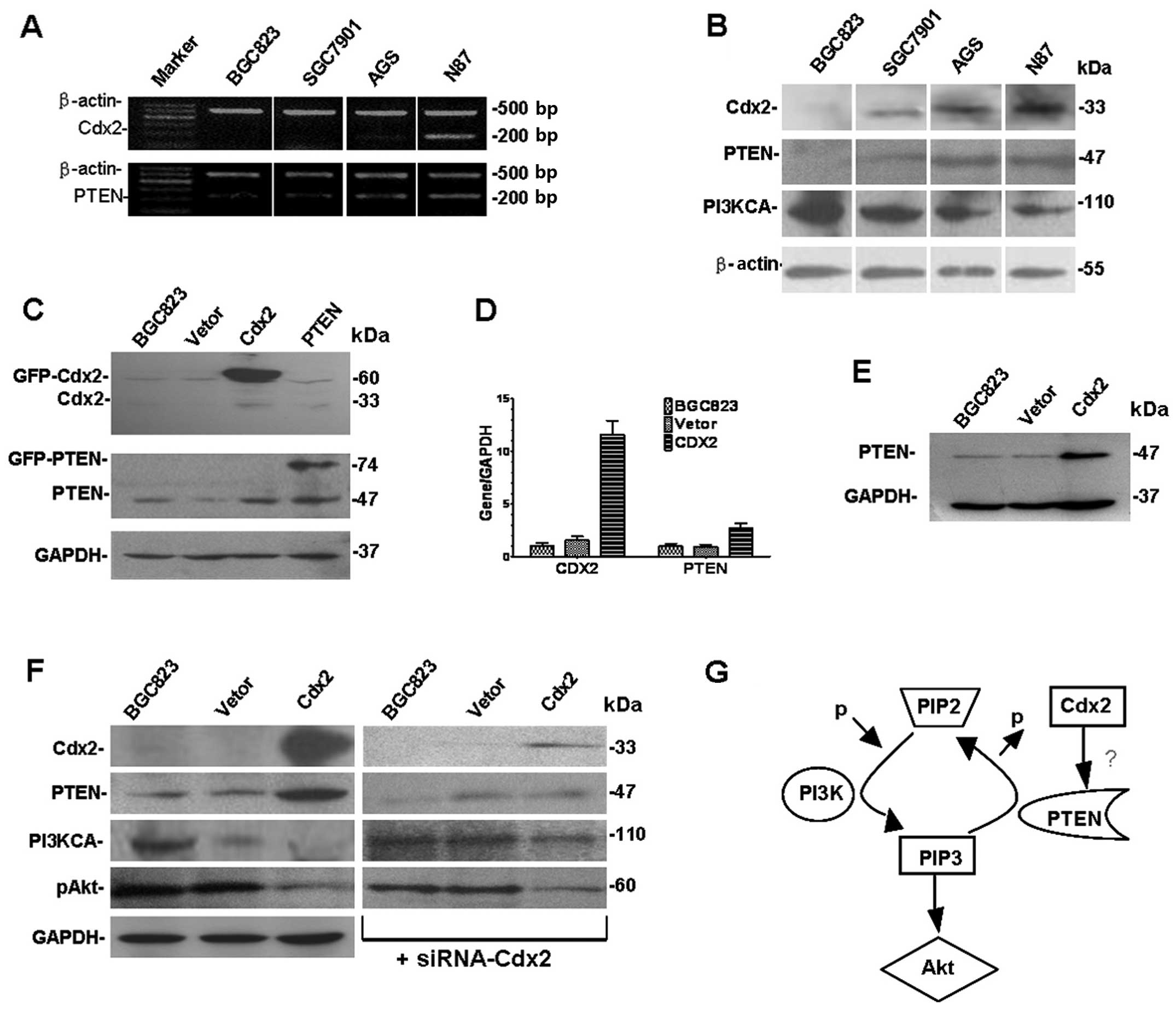
Expression levels of Cdx2 and PTEN were examined and
compared by RT-PCR and western blot analyses in all four GC cell
lines. Higher levels of Cdx2 and PTEN mRNA and protein were found
in the AGS and N87 cell lines than in BGC823 and SGC7901. Notably,
there were low expression levels for Cdx2 and PTEN proteins in
BGC823, and higher levels of expression for PI3K (Fig. 3). The data suggests that there was
simultaneous expression of both Cdx2 and PTEN in the different GC
cell lines and PI3K were activated in BGC823 cells.
Inactivation of PI3KCA and Akt in BGC823
cells with over-expressed exogenous Cdx2, and vice versa
Transfection of PTEN and Cdx2 genes revealed that
Cdx2 and PTEN were overexpressed in Cdx2-BGC823 cells when compared
with the control groups. There were no 33-kDa bands in BGC823,
BGC823-EGFP, Cdx2-BGC823 and PTEN-BGC823 cells. A 60-kDa band was
seen in Cdx2-BGC823 cells in accordance with the molecular weight
of the Cdx2-GFP fusion protein. Compared with the control group,
PTEN expression was significantly increased in PTEN-BGC823, but
expression changes of Cdx2 did not appear (Fig. 3C and E). GAPDH was used as the
internal control. After the transfection of Cdx2 real-time PCR
assay demonstrated that the mRNA expression level of both Cdx2 and
PTEN increased compared with the control group (Fig. 3D).
It is known that PTEN is a tumor suppressor gene,
and that it executes its biological functions through multiple
biological mechanisms. Expression of PTEN was obviously induced by
Cdx2-BGC823 compared with the control group. The expression of
PI3KCA and pAkt was reduced by the transfection of Cdx2 compared
with the controls (Fig. 3F). The
exogenous expression of Cdx2 induced PTEN expression and PI3K
inactivation in BGC823 cells. Similarly, gene knockdown experiments
showed the corresponding experimental results (Fig. 3F). After transfection, the
exogenous expression of PTEN could not induce Cdx2 expression in
the BGC823 cell line, which was inconsistent with previous studies
on colorectal cancer cell lines (20).
Increased PTEN expression and suppressed
proliferation in BGC823 cells with overexpressed exogenous
Cdx2
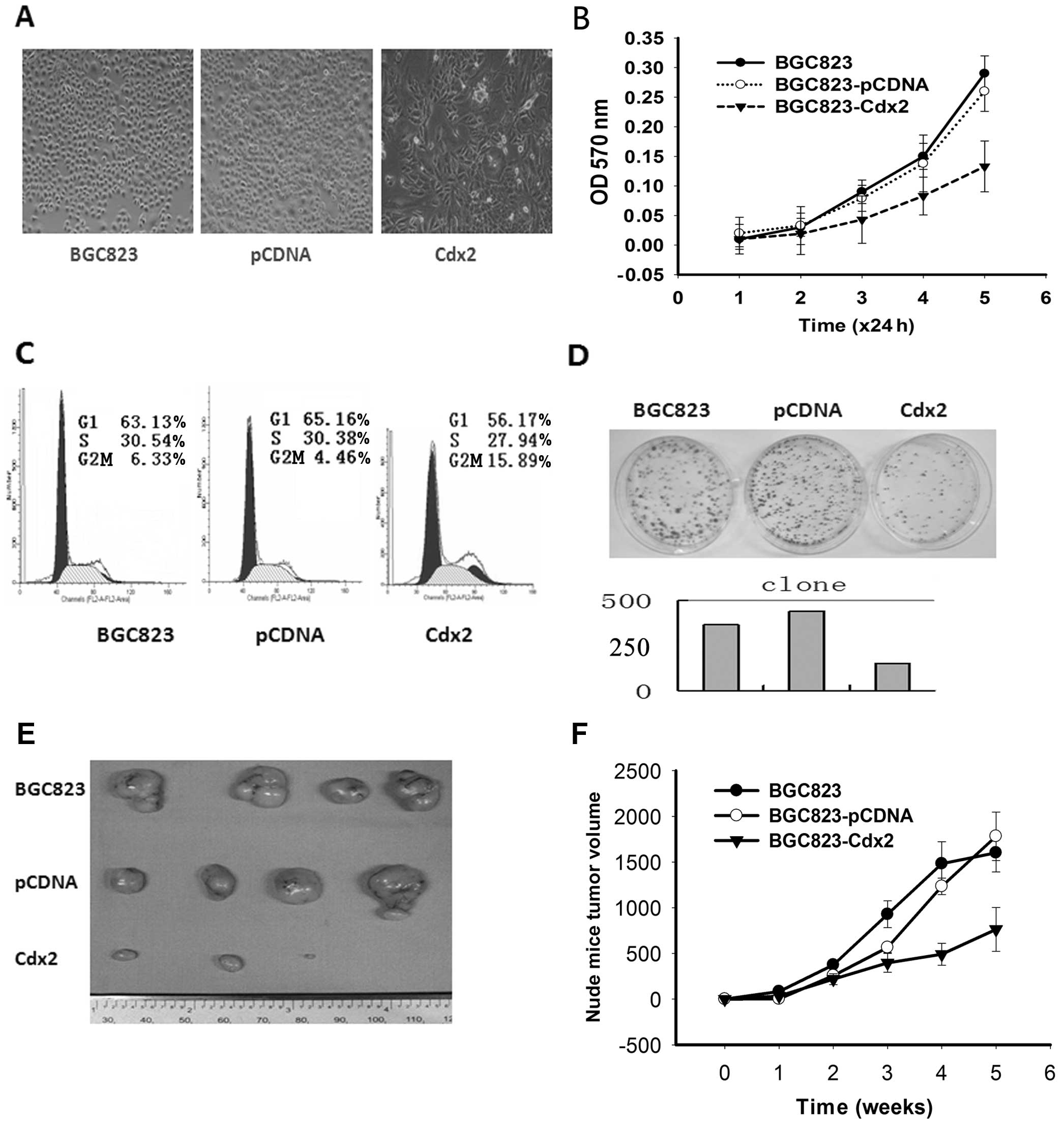
Cdx2 was transfected into the BGC823 cell line with
an efficiency of ∼30%. The total quantity of intracellular protein
was extracted. A western blot assay was used to observe the
increased expression of Cdx2 and PTEN proteins. Consequently, a
cell model with high expression of both Cdx2 and PTEN was
established (Fig. 3); the BGC823
cell line that was transfected with Cdx2 grew slowly and became
larger and more irregular (Fig.
4A).
Since the Cdx2 gene is known to be involved in the
regulation of cell differentiation, the overexpression of Cdx2 may
result in suppressed cancer cell growth. We found that cells grew
significantly slower than the control cells with BGC823-pCDNA
(Fig. 4A, B and D). Cell cycle
analysis revealed that the population of cells in the G2/M phase
was increased by the exogenous expression of Cdx2: 15.89% of
Cdx2-BGC823 cells in the G2/M phase of the cell cycle compared with
4.46% of control cells (Fig. 4C);
this indicates that Cdx2 and PTEN play an important role in driving
cell cycle progression and the promotion of GC cell
proliferation.
To confirm the in vivo effect on tumors,
athymic nude mice were subcutaneously injected with either
Cdx2-BGC823 or control cells. The tumors appeared more slowly in
the mice injected with Cdx2-BGC823 when compared with the controls
(Fig. 4E and F). Furthermore, the
average size of the tumors was smaller in the Cdx2-BGC823 mice
compared with the controls. These results demonstrate that higher
than normal expression of Cdx2 and PTEN led to the inhibition of
gastric tumor cell growth in vivo.
Discussion
As an intestine-specific transcription factor, Cdx2
has been shown to play a key role in regulating the proliferation
and differentiation of intestinal cells and in maintaining
intestinal phenotypes (5,22). Cdx2-positive patients in this study
had an improved survival rate compared with Cdx2-negative patients.
Multivariate analysis further revealed that the level of Cdx2
protein was an independent prognostic indicator. These results are
consistent with reports from earlier studies of Cdx2 expression
(8,11–13).
Levels of Cdx2 expression were high in the tumor cells of
well-differentiated cancer biopsies but were decreased in the
poorly-differentiated carcinomas. This study found that nuclear
PTEN expression had a negative relationship with distant
metastasis, suggesting that nuclear PTEN may inhibit the
progression of GC. Nuclear PTEN was associated with a good
prognosis. However, the full mechanism of nuclear PTEN expression
in intestinal metaplasia and GC requires further investigation.
Since Cdx2 and nuclear PTEN are both intestinal markers, and
because the initial data of the study found the level of Cdx2
protein to be an independent prognostic factor, the significance of
Cdx2 and nuclear PTEN coexpression in the prognosis of GC was
analyzed. Patients with positive expression of Cdx2 and nuclear
PTEN had the more favorable outcome in this assessment, which
suggests that the coexpression of Cdx2 and nuclear PTEN has the
potential to be powerful indicator of the prognosis in GC
patients.
Cdx2 and nuclear PTEN are both intestinal biomarkers
and tumor suppressor genes; their coexpression reduced the degree
of malignancy in the GC BGC823 cell line. The present study
indicates that the expression patterns of Cdx2 and nuclear PTEN are
significantly correlated. Therefore, it was proposed that the
expression of Cdx2 and nuclear PTEN may be significantly correlated
to GC; the level of nuclear PTEN reflected the potential for
metastasis in GC (p=0.015) and the level of the intestinal
transcription factor, Cdx2, reflected the differentiation of GC
(p=0.002). Preliminary data found that patients with GC who had
high expression levels of both Cdx2 and nuclear PTEN have a lower
likelihood of malignancy and better prognosis. A combined analysis
of Cdx2 and nuclear PTEN may be useful in predicting the prognosis
for GC (p<0.001).
Cdx2 promotes the occurrence of intestinal
metaplasia and has played an important role in the incidence of GC
(5). The level of Cdx2 expression
in the AGS and N87 GC cell lines was significantly higher than in
BGC823 and SGC7901 in this study. The degree of malignancy of the
four GC cell lines (BGC823, SGC7901, AGS and N87) was probably
related to the Cdx2 expression level. The BGC823 cell line has a
higher malignant potential and more potent tumor-forming potential,
but the expression level of Cdx2 is lower in this study (23,24).
It was therefore presumed that the malignant potential of the GC
cell was negatively correlated to the degree of expression of
Cdx2.
There was increased expression of PTEN when Cdx2 was
transfected into the BGC823 cell line. As a result, the cell model
with a high expression of both Cdx2 and PTEN was delineated and was
similar to that in GC patients with high expression of both Cdx2
and PTEN. In the cell model with a high expression of both Cdx2 and
PTEN, there was a significantly slower rate of growth in larger
cells, multinucleated cells, and cells that were oval in shape.
There was also reduced adhesion, reduced malignant change and
capacity for tumorigenicity. In vitro, the GC cells that
highly expressed Cdx2 and PTEN tended to reduce the degree of
malignancy (Fig. 4). This
demonstrated that Cdx2 may act as a tumor suppressor gene in the GC
BGC823 cell line. However, it has been reported that Cdx2 does not
suppress tumorigenicity in the human GC MKN45 cell line (25). Further investigations will be
required to clarify the mechanisms that are involved in the
regulation of Cdx2 expression.
The Cdx2 gene may be involved in the PI3K/Akt
pathway and in the regulation of PTEN expression. PTEN is a tumor
suppressor gene with phosphatase activity, in which mutations can
often promote tumorigenicity (26). PTEN can dephosphorylate the
3-phosphate of PI (3–5) P3, to reduce the level of
phosphorylation Akt-related apoptosis. These findings suggest that
the distribution of Cdx2 expression is consistent with that of PTEN
in the intestinal mucosa. In human colon cancer cell lines with
reduced PTEN expression, the expression of Cdx2 has been found to
be significantly decreased. Therefore, PTEN appears to improve the
activity of the Cdx2 gene promoter significantly, although
methylation of the Cdx2 promoter was not associated with mRNA
expression in GC cell lines (27).
Inhibiting the activity of PI3K resulted in increased expression of
Cdx2. Therefore, Cdx2 was demonstrated to be the target of
PTEN/PI3K pathway in a human colon cancer cell line (20). Our studies have found that in
BGC823 cell lines that were transfected with pcDNA3.1-Cdx2,
expression of PTEN and Cdx2 increased, while PI3KCA and pAkt
expression declined. Furthermore, in BGC823 cell lines that were
transfected with pcDNA3.1-PTEN, Cdx2 expression was not increased.
This suggested that Cdx2 upregulated the expression of PTEN through
the PI3K/Akt pathway in the BGC823 cell line. Cdx2 appears to be a
downstream target of PTEN regulation in colon cancer cell lines,
but this signaling pathway in GC has not been reported to date
(20).
There was no Cdx2 expression in normal adult gastric
mucosa but there was a high expression of PTEN. It is possible that
Cdx2 did not activate PTEN through the PI3K/Akt pathway in normal
gastric mucosa, but that PTEN was regulated by a variety of other
factors. High expression of Cdx2 in intestinal metaplasia and
intestinal-type GC may activate the PTEN/PI3K/Akt pathway but the
causal relationship between Cdx2 and PTEN expression is unclear.
The regulation of Cdx2 and PTEN through the PI3K/Akt pathway may be
via previously unknown branches of PTEN regulation network. The
precise mechanism through which the expression of Cdx2 and PTEN
proteins are regulated requires ongoing investigation to improve
our understanding of the pathogenesis of intestinal type GC.
In conclusion, the combined analysis of Cdx2 and
nuclear PTEN expression could enable clinicians to provide a more
accurate prognosis in patients with GC. The coexpression of Cdx2
and PTEN may reduce the degree of malignancy in GC cell line
BGC823, and Cdx2 might regulate PTEN through the PI3K/Akt
pathway.
Acknowledgments
This study was supported by National Bio-Tech 86-3
(2006AA02A402) and Beijing Natural Science Foundation
(Differentiation and regulation mechanism of nuclear PTEN on
gastric cancer, 7112034; study of gene therapy by nuclear PTEN and
Cdx2 on gastric cancer animal model, 7122052), we are grateful to
Tissue Bank of Beijing University People’s Hospital and Beijing
Friendship Hospital for specimens.
References
|
1.
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2.
|
Peek RM Jr and Blaser MJ: Helicobacter
pylori and gastrointestinal tract adenocarcinomas. Nature
reviews Cancer. 2:28–37. 2002. View
Article : Google Scholar
|
|
3.
|
Lauren P: The two histological main types
of gastric carcinoma: diffuse and so-called intestinal-type
carcinoma. An attempt at a histo-clinical classification Acta
Pathol Microbiol Scand. 64:31–49. 1965.PubMed/NCBI
|
|
4.
|
Correa P: Human gastric carcinogenesis: a
multistep and multifactorial process - First American Cancer
Society Award Lecture on Cancer Epidemiology and Prevention. Cancer
Res. 52:6735–6740. 1992.
|
|
5.
|
Barros R, Freund J-N, David L and Almeida
R: Gastric intestinal metaplasia revisited: function and regulation
of CDX2. Trends Mol Med. 18:555–563. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Barros R, Camilo V, Pereira B, Freund JN,
David L and Almeida R: Pathophysiology of intestinal metaplasia of
the stomach: emphasis on CDX2 regulation. Biochem Soc Trans.
38:358–363. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Stairs DB, Kong J and Lynch JP: Chapter 10
- Cdx genes, inflammation, and the pathogenesis of intestinal
metaplasia. In Progress in Molecular Biology and Translational
Science. 96. Klaus HK: Academic Press; Philadelphia: pp. 231–270.
2010
|
|
8.
|
Qin R, Wang NN, Chu J and Wang X:
Expression and significance of homeodomain protein Cdx2 in gastric
carcinoma and precancerous lesions. World J Gastroenterol.
18:3296–3302. 2012.PubMed/NCBI
|
|
9.
|
Silberg DG, Sullivan J, Kang E, et al:
Cdx2 ectopic expression induces gastric intestinal metaplasia in
transgenic mice. Gastroenterology. 122:689–696. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Mutoh H, Sakurai S, Satoh K, et al:
Development of gastric carcinoma from intestinal metaplasia in
Cdx2-transgenic mice. Cancer Res. 64:7740–7747. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Seno H, Oshima M, Taniguchi MA, et al:
CDX2 expression in the stomach with intestinal metaplasia and
intestinal-type cancer: Prognostic implications. Int J Oncol.
21:769–774. 2002.PubMed/NCBI
|
|
12.
|
Mizoshita T, Tsukamoto T, Nakanishi H, et
al: Expression of Cdx2 and the phenotype of advanced gastric
cancers: relationship with prognosis. J Cancer Res Clin Oncol.
129:727–734. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Fan Z, Li J, Dong B and Huang X:
Expression of Cdx2 and hepatocyte antigen in gastric carcinoma:
correlation with histologic type and implications for prognosis.
Clin Cancer. 11:6162–6170. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Roessler K, Mönig SP, Schneider PM, et al:
Co-expression of CDX2 and MUC2 in gastric carcinomas: correlations
with clinicopathological parameters and prognosis. World J
Gastroenterol. 11:3182–3188. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Gil A, Andrés-Pons A, Fernández E, et al:
Nuclear localization of PTEN by a Ran-dependent mechanism enhances
apoptosis: involvement of an N-terminal nuclear localization domain
and multiple nuclear exclusion motifs. Mol Biol Cell. 17:4002–4013.
2006. View Article : Google Scholar
|
|
16.
|
Sansal I and Sellers WR: The biology and
clinical relevance of the PTEN tumor suppressor pathway. J Clin
Oncol. 22:2954–2963. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Tamura M, Gu J, Matsumoto K, Aota S,
Parsons R and Yamada KM: Inhibition of cell migration, spreading,
and focal adhesions by tumor suppressor PTEN. Science.
280:1614–1617. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Shen WH, Balajee AS, Wang J, et al:
Essential role for nuclear PTEN in maintaining chromosomal
integrity. Cell. 128:157–170. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Bai Z, Ye Y, Chen D, et al: Homeoprotein
Cdx2 and nuclear PTEN expression profiles are related to gastric
cancer prognosis. APMIS. 115:1383–1390. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Kim S, Domon-Dell C, Wang Q, et al: PTEN
and TNF-alpha regulation of the intestinal-specific Cdx-2 homeobox
gene through a PI3K, PKB/Akt, and NF-kappaB-dependent pathway.
Gastroenterology. 123:1163–1178. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Hinoi T, Gesina G, Akyol A, et al:
CDX2-regulated expression of iron transport protein hephaestin in
intestinal and colonic epithelium. Gastroenterology. 128:946–961.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Yuasa Y: Control of gut differentiation
and intestinal-type gastric carcinogenesis. Nature reviews. Cancer.
3:592–600. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Xu Y, Zhang J, Liu QS and Dong WG:
Knockdown of liver-intestine cadherin decreases BGC823 cell
invasiveness and metastasis in vivo. World J Gastroenterol.
18:3129–3137. 2012.PubMed/NCBI
|
|
24.
|
Wu T, Li JY and Lu SX: Isolation and
characterization of side population cells in human gastric cancer
cell line BGC-823. Zhonghua Zhong Liu Za Zhi. 34:264–268. 2012.In
Chinese.
|
|
25.
|
Dang LH, Chen F, Knock SA, et al: CDX2
does not suppress tumorigenicity in the human gastric cancer cell
line MKN45. Oncogene. 25:2048–2059. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Wrighton KH: Tumour suppressors: role of
nuclear PTEN revealed. Nature reviews Cancer. 11:154. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Pereira B, Oliveira C, David L and Almeida
R: CDX2 promoter methylation is not associated with mRNA
expression. Int J Cancer. 125:1739–1742. 2009. View Article : Google Scholar : PubMed/NCBI
|