Introduction
Ovarian cancer remains the leading cause of death in
women with gynecological malignancies, despite significant
improvements in surgical cytoreduction and chemotherapeutic
treatment (1). One of the major
obstacles to curing ovarian cancer is the cancer exerts a high rate
of drug-resistance either inherent or acquired to chemotherapy
(2). It is thus imperative to
develop more effective therapeutic strategies that circumvent the
resistance mechanism of cancer cells.
Autophagy is a process of bulk degeneration of
damaged organelles, protein aggregates, and other macromolecules in
the cytoplasm (3). It is
traditionally regarded as a cellular response to nutrient
deprivation or starvation, whereby cells digest a portion of
cytoplasm to recycle nutrients for survival (3,4).
Previous studies have suggested that autophagy is a general
response mechanism to a wide range of cellular stresses (4,5).
Autophagy either constitutes stress adaptation machinery for cell
survival in certain cellular scenarios, or in other settings,
activates an alternative pathway leading to cellular demise. The
latter is referred as autophagic cell death (or type II cell
death), although the physiological significance of this type of
cell death remains controversial (6,7).
Notably, radiation and chemotherapeutic drugs that kill cancer
cells through apoptosis were often observed to induce autophagy in
certain human cancer cells (8).
However, the functional relationship between apoptosis and
autophagy and the potential cross-regulation between these two
processes in cancer chemotherapy are still largely unknown.
FTY720
(2-amino-2-[2-(4-octylphenyl)-1,3-propanediol-hydrochloride], also
known as Fingolimod, is a synthetic analog of spingosine (9). The drug was originally developed as
an immunosuppressive agent (10),
which is currently undergoing multiple clinical trials, including
prevention of kidney graft rejection (11) and treatment of relapsing multiple
sclerosis (12). In addition,
FTY720 has also shown preclinical antitumor efficacy in various
cancer models, including those of breast, bladder, prostate, lung,
liver and hematopoietic malignancies (reviewed in ref. 13). More recently, we have reported that
FTY720 exhibited potent cytotoxic effects in ovarian cancer cells
through a mechanism differing from that of cisplatin (CDDP), the
most commonly used drug in ovarian cancer treatment (14). Combination therapy with different
anticancer drugs has been proven to be an effective strategy for
overcoming drug resistance and achieving better outcomes in
chemotherapy of ovarian cancer. The present study was designed to
investigate the effect of FTY720 combing with CDDP in ovarian
cancer cells. Surprisingly, the combination yields a wide rang of
antagonism towards the cytotoxicity of CDDP in a variety of ovarian
cancer cell lines, including CDDP-sensitive and -resistant cells.
This suggests that combination of FTY720 with CDDP is not an ideal
combinational regime, although they exert their own anticancer
efficacy through distinct killing mechanisms.
Materials and methods
Cell lines and reagents
Human ovarian carcinoma cell lines A2780, 2008,
SKOV-3 and IGROV-1 were cultured in DMEM medium supplemented with
10% heat-inactivated fetal calf serum, penicillin (100 U/ml) and
streptomycin (100 μg/ml) at 37°C in a humidified atmosphere
of 5% CO2. FTY720 and SKI-II
(4-[4-(chlorophenyl)-2-thiazolyl]aminophenol) were purchased from
Cayman (Ann Arbor, MI, USA). Cis-diaminedichloroplatinum (CDDP),
3-methyladenine (3-MA) and all other chemicals were purchased from
Sigma-Aldrich (St. Louis, MO, USA).
Cell viability assay
Cells (3,000 cell/well) were seeded in three to four
replication into 96-well plates and incubated with or without
FTY720 at the indicated concentration for a desired time period.
After the indicated treatments, cell viability was assessed using
the MTS (3-(4,
5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium,
inner salt; Sigma) assay as described previously (15). The absorbance intensity of the MTS
product is directly proportional to the number of viable cells in
culture when cell number is between 2,000 and 200,000; otherwise
the exponential dependence was determined. The cell viability was
expressed as a percentage of absorbance in cells with indicated
treatments to that in cells with vehicle control treatment.
Clonogenic assay
Cells were treated for 24 h with the indicated
drugs, washed and incubated in drug-free media for 14 days. Cells
were fixed with 70% methanol and stained with crystal violet.
Colonies, defined as clusters of ≥50 cells, were scored manually
with the aid of a Nikon TS100 inverted microscope. Clonogenic
survival was expressed as the percentage of colonies formed in
drug-treated wells with respect to vehicle alone. Under standard
conditions, >100 colonies were formed in untreated control cells
following 14 days of incubation.
Combined drug effect analysis
The multiple drug effect analysis of Chou and
Talalay was used to evaluate combined drug effects, which is based
on the median-effect principle (16). The combination index (CI) and
dose-reduction index (DRI) were calculated for determining the mode
of interaction (synergism, antagonism and additive effect) between
FTY720 and CDDP as described by Chou and Talalay (16,17).
CompuSyn software was applied for above drug combination analysis
as described previously (18).
Immunoblot analysis
Equal amounts of proteins collected from cell
lysates were resolved by 8–15% SDS-PAGE using a NuPAGE system
(Invitrogen, Carlsbad, CA, USA) and then transferred onto PVDF
membranes according to the manufacturer’s instructions. After
blocking with 5% non-fat milk in PBS containing 0.1% Tween-20, the
PVDF membranes were probed with primary antibodies against
Beclin-1, LC-3 (Novus Biologicals, Littleton, CO, USA) and β-actin
(Sigma) at 4°C overnight, followed by horseradish
peroxidase-conjugated proper second antibodies. The
immunoreactivities were detected with enhanced chemiluminescence
Plus kit (GE Healthcare, Piscataway, NJ, USA). Densitometry was
performed on a Kodak Imager.
Fluorescence microscope
The subcellular localization of LC3 was examined in
cells overexpressing GFP-LC3 (gift from Dr M.J. Zhao, Shanghai
Institutes for Biological Science, China) by fluorescence
microscopic analysis. Cells displaying a diffuse distribution of
GFP-LC3 in the cytoplasm and nucleus were considered
non-autophagic, whereas cells presenting intense GFP-LC3
punctations with no nuclear GFP-LC were classified as autophagic
formation. Autophagy was quantified by counting the percentage of
GFP-LC3-transfected cells showing accumulation of GFP-LC3 in puncta
(≥100 cells were counted for each experiment).
RNA interference
Chemically synthesized scrambled RNAi
oligonucleotides and siRNA targeting human Beclin 1 and LC3 were
purchased from GenePharma (Shanghai, China). The siRNA
corresponding to human cDNA sequence are: Beclin 1, GAA TGT CAG AAC
TAC AAA CGC TGT T; LC3, GAA GGC GCU UAC AGC UCA A. The siRNA
transfection was performed using Hiperfect reagent (Qiagen,
Germantown, MD, USA) according to the manufacturer’s protocol. The
expression levels of targeted genes were detected by western blot
assays after 36–48-h transfection.
Statistical analysis
SPSS11.0 software package was used to perform
statistical analysis. Unpaired Student’s t-tests were used for
comparison between two groups. For multiple comparisons, results
were analyzed by ANOVA followed by the Dunnet’s test. A p<0.05
was considered statistically significant.
Results
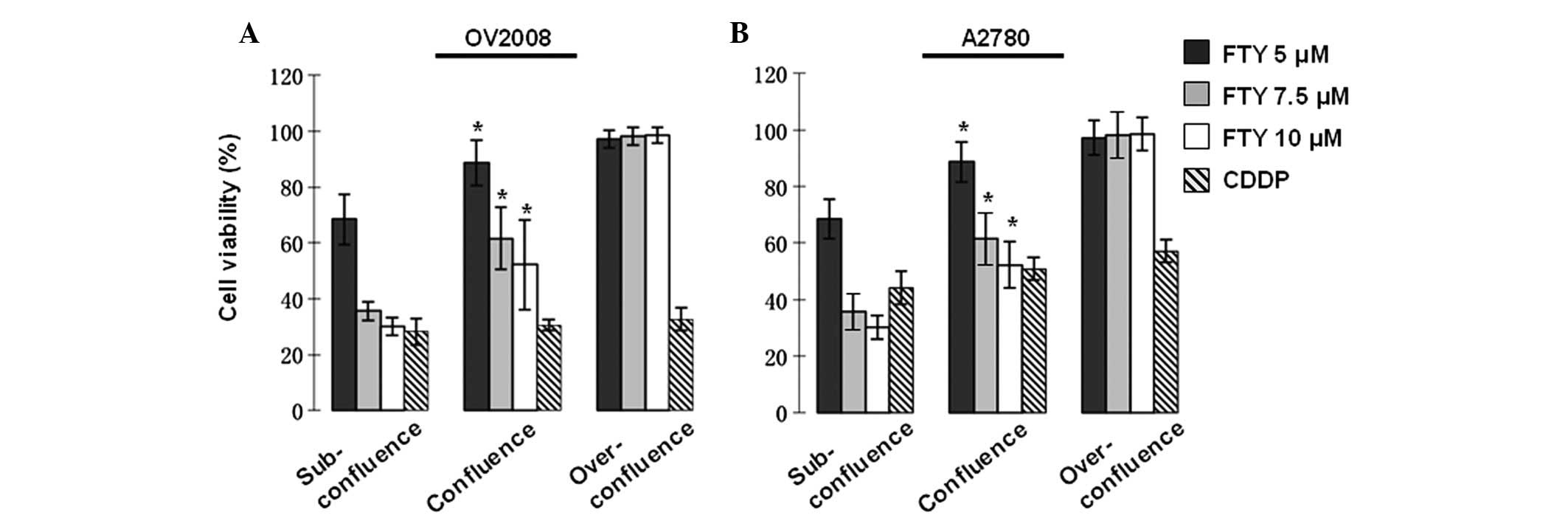
FTY720-induced cytotoxicity in ovarian
cancer cells is cell density-dependent
Our previous study reported that FTY720 exhibits a
potent cytotoxic effect in ovarian cancer cells via non-apoptotic
programmed cell death pathways, which is distinct from the
CDDP-mediated apoptotic cell death (14). In an attempt to further
characterize the killing mechanism of FTY720, we investigated the
influence of cell density on the cytotoxicity of FTY720 in ovarian
cancer cells. Consistent with our previous report, FTY720 induced
cell death in subconfluent cultures in a dose-dependent manner in
ovarian cancer cells (Fig. 1).
However, the sensitivity to FTY720 was significantly reduced when
the treatment was conducted on cells reaching confluence. Moreover,
almost no cell death was observed in over-confluent cultures
treated with FTY720 (Fig. 1). In
contrast, CDDP induced cell death was independent of the cell
density, irrespective of cellular sensitivity to CDDP (Fig. 1A). These observations further
strengthen the notion that FTY720 functions through a distinct
pathway to CDDP in killing of ovarian cancer cells.
Combination of FTY720 with CDDP exhibits
an antagonistic effect on the cytotoxicity in ovarian cancer
cells
Combination of CDDP with other therapeutic agents
that exert different killing mechanisms has becoming the
predominate trend for better clinical outcomes of platinum-based
chemotherapy in ovarian cancer patients (19,20).
Thus, we evaluated the effect of FTY720 in combination with CDDP in
both CDDP-sensitive (OV2008 and IGROV-1) and -resistant (A2780 and
SKOV-3) ovarian cancer cells. As reported previously (14), treatment with either FTY720 or CDDP
resulted in a dose-dependent cytotoxicity in these ovarian cancer
cell lines, although A2780 and SKOV-3 cells exhibited resistance to
CDDP to a certain degree (Fig.
2A). However, combination of FTY720 and CDDP did not yield any
additive cytotoxic effects on these ovarian cancer cells (Fig. 2A). For detailed analysis of the
combinational drug effect, we determined the mass-action law
parameters, including Dm, m and r values, which represent the
potency, shape of dose-effect curve and the conformity to the
mass-action law, respectively, for each drug and their combinations
(Table I). These Dm and m values
were then used to calculate the combination index (CI) values based
on the Chou-Talalay method, where CI <1, =1 and >1, indicate
synergism, additive effect and antagonism, respectively (16,17).
The CI-fa (fraction affected) curves of FTY720/CDDP combination
show the CI values ranging from 0.97 to 1.51 at different effect
levels from IC50 to IC90 (Fig. 2B), indicating an extensive range of
antagonism in the combinational treatment.
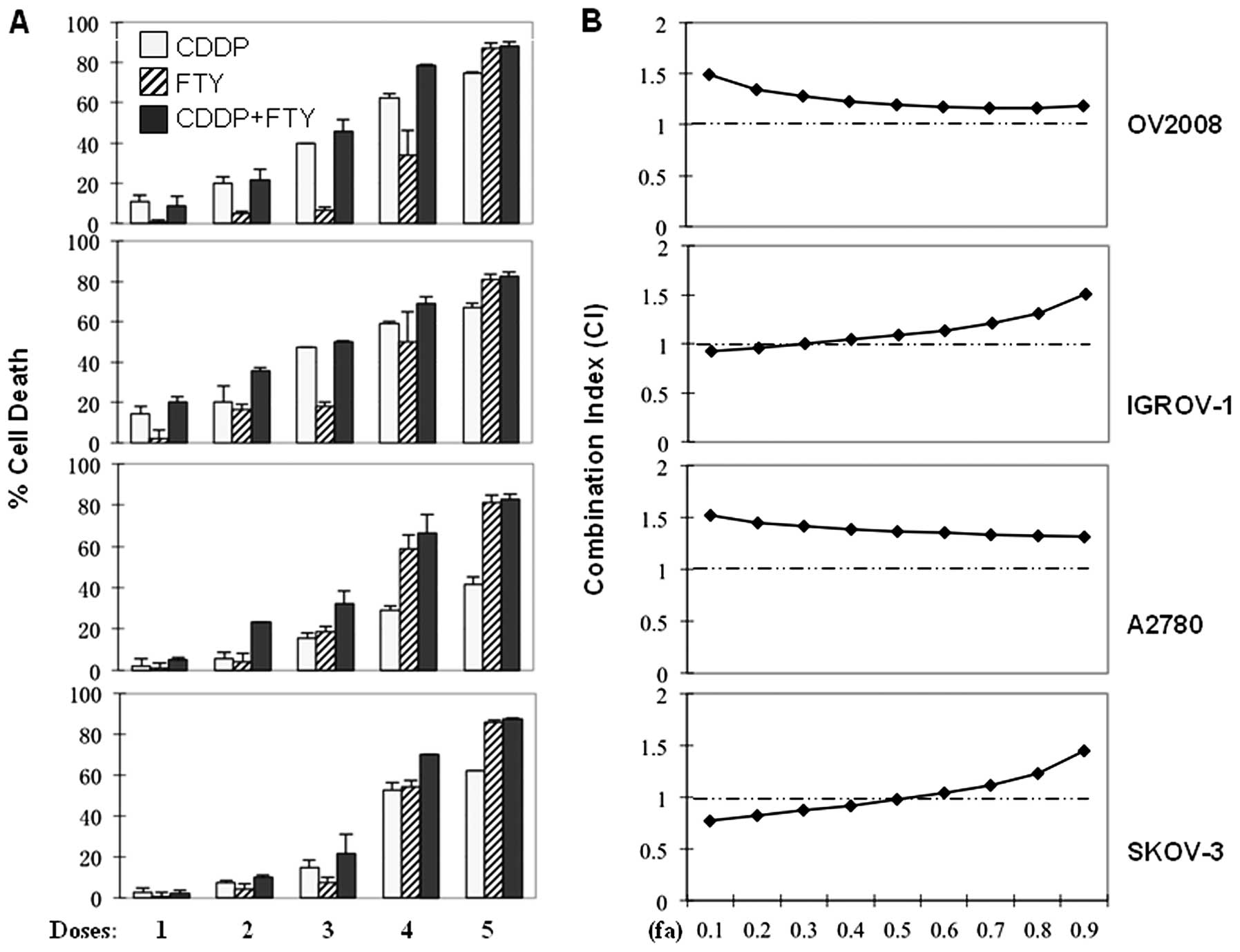 | Figure 2Combined effect of FTY720 with CDDP in
ovarian cancer cells. (A) OV2008, IGROV-1, A2780 and SKOV-3 cells
were cultured reaching confluence and treated for 24 h with
increasing concentrations of CDDP (2.5, 5.0, 10, 20 and 40
μM, respectively for D1–D5) and/or FTY720 (1.25, 2.5, 5, 10
and 20 μM, respectively for D1–D5). The percentages of cell
death were measured by MTS assay. All values represent the means
with bars as SD of three samples in an independent experiment,
which were repeated three times with similar results. (B)
Combination index (CI) of CDDP and FTY720 on various ovarian cancer
cells was calculated as described (16,17).
Here fa in X-axis denotes fraction affected (e.g., fa of 0.5 is
equivalent to a 50% of cell death). |
 | Table IDose-effect relationship parameters of
FTY720 or/and CDDP against various ovarian cancer cell lines. |
Table I
Dose-effect relationship parameters of
FTY720 or/and CDDP against various ovarian cancer cell lines.
| Cell lines | Compound | m | Dm (μM) | r | n |
|---|
| OV2008 | FTY720 | 2.416 | 11.15 | 0.912 | 5 |
| CDDP | 1.193 | 14.83 | 0.988 | 5 |
| FTY720+CDDP | 1.627 | 16.02 | 0.984 | 5 |
| IGROV-1 | FTY720 | 1.555 | 9.26 | 0.917 | 5 |
| CDDP | 0.980 | 15.74 | 0.907 | 5 |
| FTY720+CDDP | 0.995 | 13.86 | 0.995 | 5 |
| A2780 | FTY720 | 2.486 | 10.10 | 0.951 | 5 |
| CDDP | 1.564 | 25.21 | 0.940 | 5 |
| FTY720+CDDP | 2.103 | 22.98 | 0.976 | 5 |
| SKOV-3 | FTY720 | 2.244 | 9.63 | 0.990 | 5 |
| CDDP | 1.164 | 48.10 | 0.979 | 5 |
| FTY720+CDDP | 1.405 | 20.07 | 0.966 | 5 |
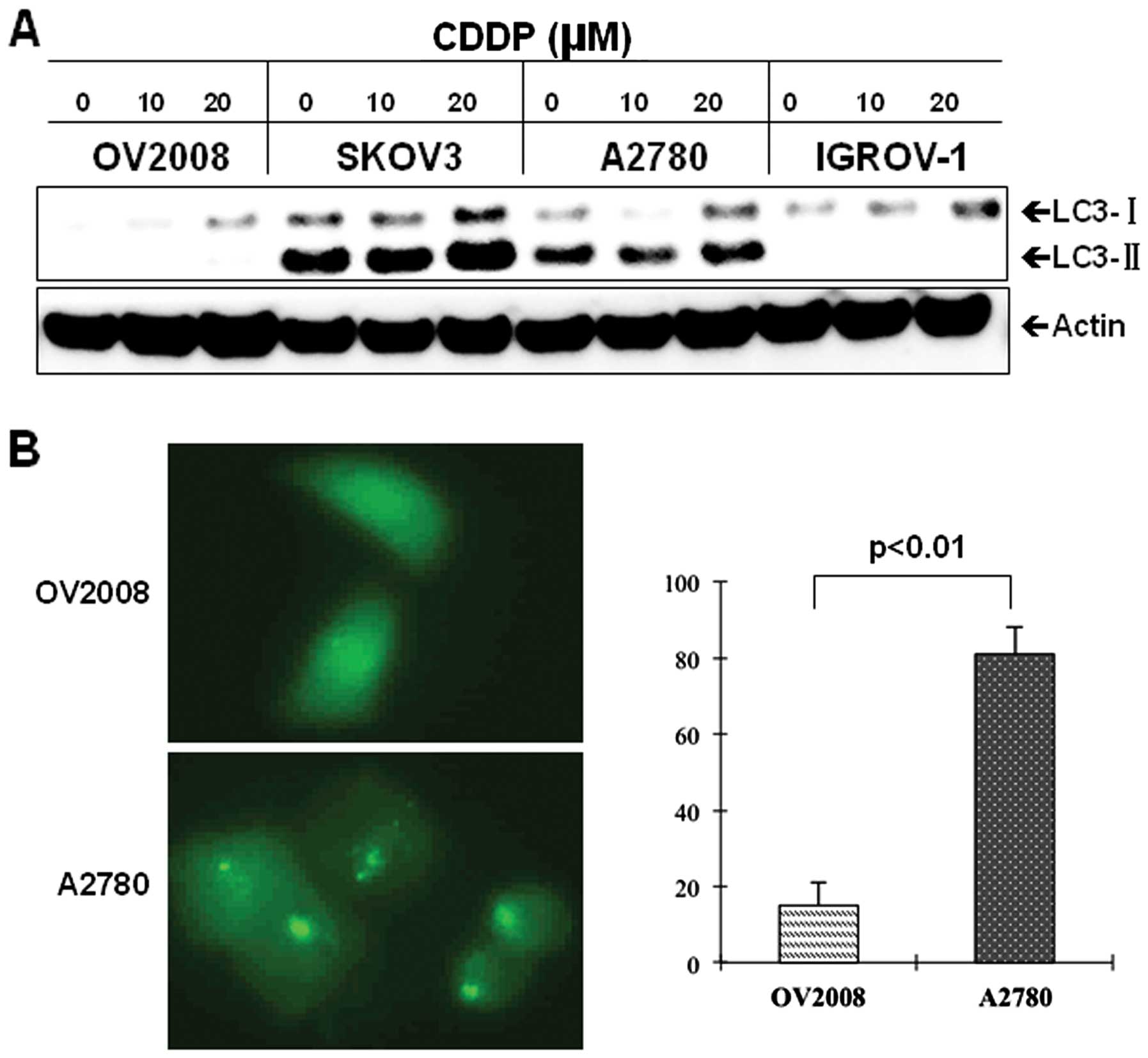
CDDP-resistant cells exhibit increased
levels of autophagy
We have previously reported that in addition to its
cytotoxic effect, FTY720 induces autophagy in ovarian cancer cells
(14). We thought that the
antagonistic effect of FTY720 in the combination with CDDP might be
attributed to autophagy formation. To test this hypothesis, we
firstly investigated the potential role of autophagy in ovarian
cancer cells response to CDDP. Interestingly, the CDDP-resistant
cells, SKOV3 and A2780, exhibited a significant increase in
baseline levels of autophagy, as determined by LC3 expression and
its lipidation status (LC3-II), compared with the sensitive OV2008
and IGROV-1 cells (Fig. 3A). The
increased levels of autophagy in the resistant cells remained
following CDDP treatment (Fig.
3A). To further confirm the difference in autophagy levels
between CDDP-resistant and sensitive cells, we transfected GFP-LC3
into A2780 and OV2008 cells and monitored the accumulation of
GFP-LC3 puncta in the transfected cells. As seen in Fig. 3B, the resistant A2780 cells showed
a significant increase in the number of cells with puncta
fluorescence, whereas the majority of GFP-LC3 transfected OV2008
cells displayed a diffused distribution of fluorescence. Together,
these findings indicated that CDDP-resistant ovarian cancer cells
exhibit elevated levels of autophagy compared to CDDP-sensitive
cells.
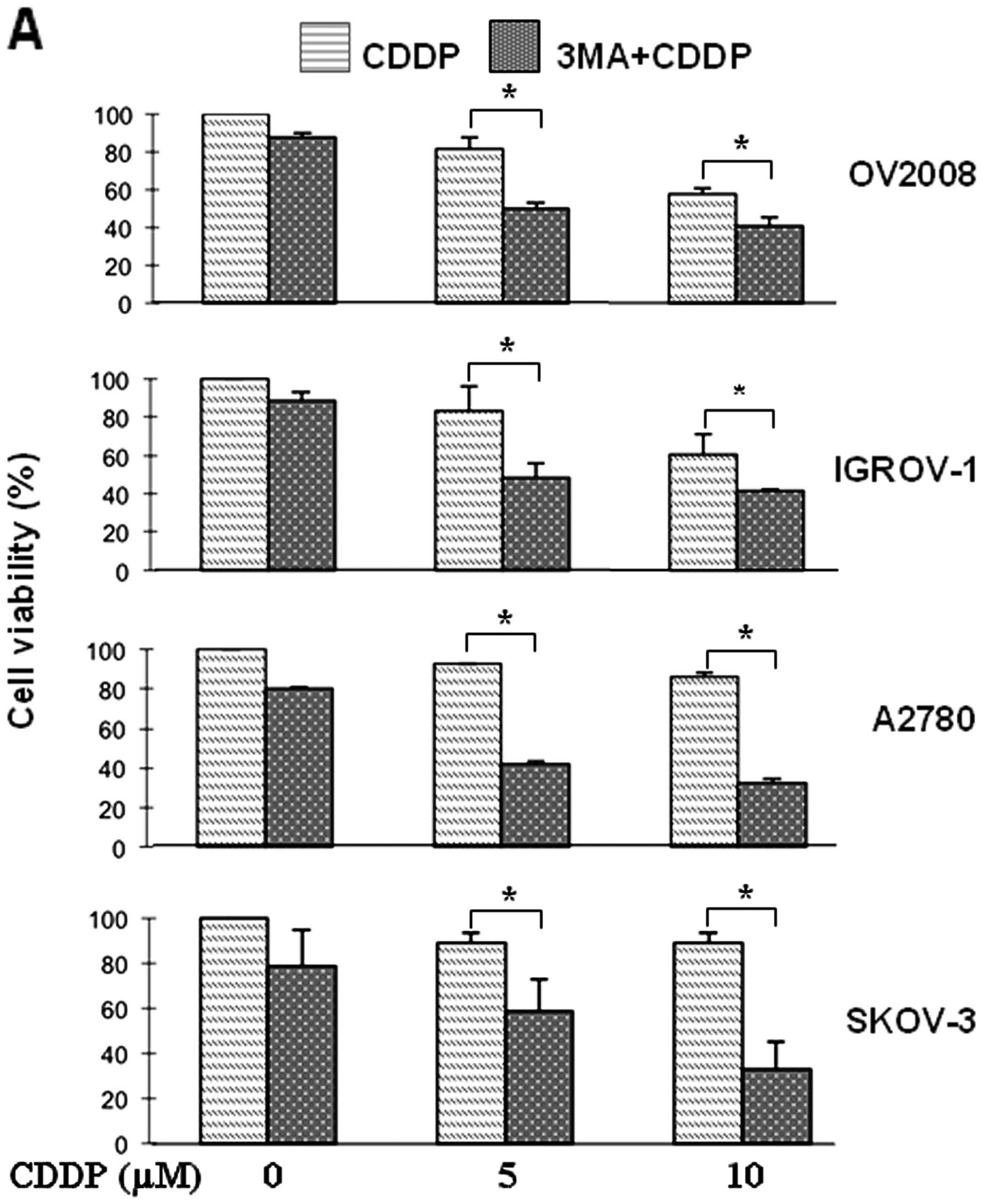
Blockage of autophagy augments
CDDP-induced cell death
To examine the role of autophagy in the cytotoxic
effect of CDDP in ovarian cancer cells, we employed the
pharmacological inhibitor, 3-methyladenine (3-MA), that has been
demonstrated to effectively block the formation of autophagosome
(21). Notably, treatment with
3-MA resulted in a significant increase in CDDP-induced cell death
in either CDDP-sensitive or -resistant cells (Fig. 4A). In order to exclude off-target
effects of the chemical inhibitor 3-MA, we used siRNA to
specifically knock-down the expression of LC3 and Beclin 1, known
to be crucial for autophagy formation and function (22). As shown in Fig. 4B, transfection of A2780 cells with
either Beclin 1- or LC3-siRNA significantly decreased the target
protein expression, compared with the control siRNA-transfected
cells. In line with the 3-MA experimental data, silencing of Beclin
1 or LC3 expression significantly enhanced cellular response to
CDDP killing effect (Fig. 4B,
bottom panels). Furthermore, inhibition of autophagy by 3-MA
reverses the antagonism of FTY720 into a significant additive
killing effect in its combination with CDDP in either the
CDDP-sensitive OV2008 cells or the resistant A2780 cells (Fig. 5A). This observation was further
confirmed by long-term colony formation assays (Fig. 5B). Collectively, these data suggest
that autophagy is capable of affecting cell response to CDDP
treatment and that blockade of autophagy renders ovarian cancer
cells susceptible to the cytotoxicity of either CDDP or FTY720 or
their combination.
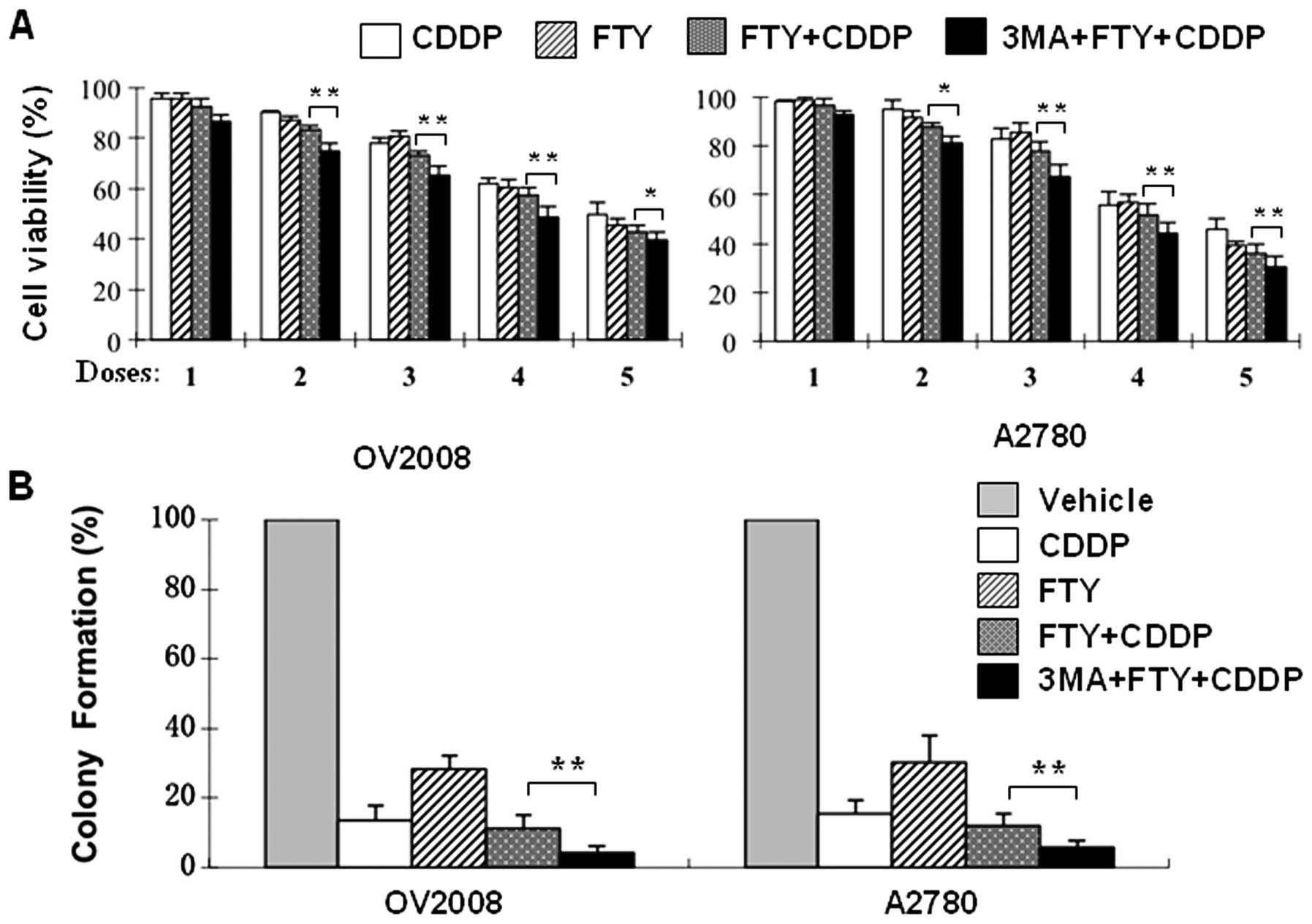 | Figure 5Autophagy inhibition modulates the
combined effect of FTY720 with CDDP in ovarian cancer cells. (A)
OV2008 and A2780 cells were exposed to increasing concentrations of
CDDP (2.5, 5.0, 10, 20 and 40 μM, respectively for D1–D5)
and/or FTY720 (1.25, 2.5, 5, 10 and 20 μM, respectively for
D1–D5) for 24 h in the absence or presence of 3-MA (5 mM) and then
cell viability was determined. (B) Clonogenic survival assays were
conducted in OV2008 and A2780 cells treated for 24 h with vehicle,
CDDP (5 μM), FTY720 (2.5 μM) or combination of CDDP
and FTY720 in the absence or presence of 3-MA (2.5 mM) as described
in Materals and methods. Data are means ± SD of triplicates.
*p<0.05, **p<0.01; presence vs absence
of 3-MA. |
Discussion
Drug resistance is still a major obstacle to
successful treatment of ovarian cancer. While platinum-based
chemotherapy has achieved a high response rate, the great majority
relapses and develops resistance to further chemotherapy (19,20).
As such, CDDP combination therapy with multiple drugs or multiple
modalities that overcome drug resistance is one of the major
strategies for achieving better therapeutic outcomes of ovarian
cancer (2).
FTY720, a synthetic analog of sphingosine, has been
shown to have a powerful therapeutic potential in the treatment of
various types of cancers (13). In
our previous studies, we found that FTY720 exerts a potent
cytotoxic effect against ovarian cancer cells via the
apoptosis-independent programmed cell death pathway accompanied
with autophagy formation (14).
The FTY720-induced cytotoxicity is also demonstrated to be clearly
distinct from CDDP-mediated apoptotic cell death in ovarian cancer
cells (14). Such a difference has
been further demonstrated by the present study, showing that
FTY720-induced cell death is cell density-dependent, whereas CDDP
killing effect is independent of cell density (Fig. 1). We expected that the distinct
killing mechanisms of FTY720 and CDDP might lead to a potent and
effective combination of these two agents for treatment of ovarian
cancer cells. Surprisingly, the combination index analysis revealed
that FTY720/CDDP combination exhibited a broad rang of antagonism
in both CDDP-sensitive (OV2008 and IGROV-1) and -resistant (A2780
and SKOV-3) ovarian cancer cells, with CI ranging from 0.97 to 1.51
for IC50 to IC90. This suggests that the
combination of FTY720 with CDDP does not produce a favorable
interaction for their cytotoxicity in ovarian cancer cells in
vitro or in vivo. Instead, the combination of FTY720 and
CDDP results in an antagonistic effect, although these two
individual drugs induce cell death through strikingly different
mechanisms.
In an attempt to investigate the possible mechanisms
by which FTY720 antagonizes the cytotoxicity of CDDP, we examined
the role of autophagy, as it has been suggested to be an important
protective mechanism against anticancer therapies (8). Indeed, the ability of FTY720 to
induce autophagy formation and enhance autophagic flux in ovarian
cancer cells has been demonstrated by our earlier studies (14). Therefore, we thought that the
antagonism resulted from the combination of FTY720 with CDDP may be
ascribable to the protective role of autophagy in refractory
response to CDDP treatment. This notion is supported by our
findings. The CDDP-resistant SKOV3 and A2780 cells exhibit a
significantly higher level of autophagy than that in the sensitive
OV2008 and IGROV-1 cells under both basal and drug-stimulated
conditions, as determined by the expression levels of LC3-II and
GFP-fussed LC3 translocation (Fig.
3). Notably, inhibition of autophagy by using small-molecule
inhibitor 3-MA strengthened the cytotoxicity of CDDP in all ovarian
cancer cells that we tested. The sensitizing effects of 3-MA appear
independent of cellular responsiveness to CDDP (Fig. 4). Blockage of autophagy formation
by the siRNA-mediated knockdown of LC3 and Beclin 1 expression
significantly enhanced the killing effect of CDDP. Moreover,
inhibition of autophagy by 3-MA converted the combination of FTY720
with CDDP from an antagonistic into an additive effect towards to
killing ovarian cancer cells (Fig.
5). These observations not only confirm the effect of FTY720 in
autophagy induction, but also suggest that autophagy is, at least
partially, responsible for the antagonistic of FTY720 in its
combination with CDDP. Interestingly, a recent study showed that
combination of FTY720 with milatuzumab, an anti-CD74 mAb, resulted
in a significant synergistic effect in treatment of mantle cell
lymphoma cells (23). The
synergistic effect of FTY720 was suggested to be ascribable to its
function in blockage of autophagic flux and disruption of the
autophagic-lysosomal degradation of CD74 (23). The inhibitory effect of FTY720 on
autophagy in the lymphoma cells strikingly conflicts with the
findings that FTY720 induces autophagy formation and promotes
autophagic flux in ovarian cancer cells and other neoplastic cells,
as reported from our studies and others (14,24–26).
This discrepancy may suggest that the effect of FTY720 on autophagy
is cell type-dependent. Notably, there is accumulating evidence
showing autophagy can function as either a pro-survival or a
pro-death mechanism in a highly context-dependent manner (6). As such, the actions of FTY720 on
autophagy should be taken into account in evaluation of the
anticancer efficacy of FTY720, especially in its combination with
other chemotherapeutic agents.
There is considerable evidence indicating that
autophagy is often induced in response to various stress
conditions, such as exposure to anticancer drugs or other cytotoxic
agents (8,27–29).
Under these conditions, autophagy has been suggested to play an
important cytoprotective role for cell survival (3,29,30).
Indeed, inhibition of autophagy has been shown to enhance the
anticancer efficacy of various anticancer therapies (8,31,32).
Along with these observations, our findings further support the
concept that inhibition of autophagy may represent a promising
strategy for the treatment of refractory cancers, including ovarian
cancer.
Acknowledgements
We are grateful to Dr M.J. Zhao for
providing GFP-LC3 constructs. This study was supported by grants
from Australian National Health and Medical Research Council
(Program 571408; P.X.) and Chinese National Natural Science
Foundation (81072137, W.D.), National Natural Science Foundation
(81101972, N.Z.) and Science and Technology Fund of Shanghai
Jiaotong University School of Medicine (11XJ21018, N.Z.), Ph.D.
Programs Foundation of Ministry of Education of China
(20100073110075, W.D.) and Shanghai Education Commission Key
Disciplines Foundation. P.X. is supported by Australian Cancer
Institute NSW Research Fellowship.
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar
|
|
2
|
Bast RC Jr, Hennessy B and Mills GB: The
biology of ovarian cancer: new opportunities for translation. Nat
Rev Cancer. 9:415–428. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shintani T and Klionsky DJ: Autophagy in
health and disease: a double-edged sword. Science. 306:990–995.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kroemer G and Levine B: Autophagic cell
death: the story of a misnomer. Nat Rev Mol Cell Biol. 9:1004–1010.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kondo Y, Kanzawa T, Sawaya R and Kondo S:
The role of autophagy in cancer development and response to
therapy. Nat Rev Cancer. 5:726–734. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Suzuki S, Enosawa S, Kakefuda T, Shinomiya
T, Amari M, Naoe S, Hoshino Y and Chiba K: A novel
immunosuppressant, FTY720, with a unique mechanism of action,
induces long-term graft acceptance in rat and dog
allotransplantation. Transplantation. 61:200–205. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suzuki S, Enosawa S, Kakefuda T, Li XK,
Mitsusada M, Takahara S and Amemiya H: Immunosuppressive effect of
a new drug, FTY720, on lymphocyte responses in vitro and cardiac
allograft survival in rats. Transpl Immunol. 4:252–455. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tedesco-Silva H, Mourad G, Kahan BD, Boira
JG, Weimar W, Mulgaonkar S, Nashan B, Madsen S, Charpentier B,
Pellet P and Vanrenterghem Y: FTY720, a novel immunomodulator:
efficacy and safety results from the first phase 2A study in de
novo renal transplantation. Transplantation. 77:1826–1833.
2004.PubMed/NCBI
|
|
12
|
Pelletier D and Hafler DA: Fingolimod for
multiple sclerosis. N Engl J Med. 366:339–347. 2012. View Article : Google Scholar
|
|
13
|
Vadas M, Xia P, McCaughan G and Gamble J:
The role of sphingosine kinase 1 in cancer: oncogene or
non-oncogene addiction? Biochim Biophys Acta. 1781:442–447. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang N, Qi Y, Wadham C, Wang L, Warren A,
Di W and Xia P: FTY720 induces necrotic cell death and autophagy in
ovarian cancer cells: a protective role of autophagy. Autophagy.
6:1157–1167. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sukocheva O, Wang L, Verrier E, Vadas MA
and Xia P: Restoring endocrine response in breast cancer cells by
inhibition of the sphingosine kinase-1 signaling pathway.
Endocrinology. 150:4484–4492. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: the combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chou TC: Theoretical basis, experimental
design, and computerized simulation of synergism and antagonism in
drug combination studies. Pharmacol Rev. 58:621–681. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang N, Wu ZM, McGowan E, Shi J, Hong ZB,
Ding CW, Xia P and Di W: Arsenic trioxide and cisplatin synergism
increase cytotoxicity in human ovarian cancer cells: therapeutic
potential for ovarian cancer. Cancer Sci. 100:2459–2464. 2009.
View Article : Google Scholar
|
|
19
|
Agarwal R and Kaye SB: Ovarian cancer:
strategies for overcoming resistance to chemotherapy. Nat Rev
Cancer. 3:502–516. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Muggia F: Platinum compounds 30 years
after the introduction of cisplatin: implications for the treatment
of ovarian cancer. Gynecol Oncol. 112:275–281. 2009.PubMed/NCBI
|
|
21
|
Seglen PO and Gordon PB: 3-Methyladenine:
specific inhibitor of autophagic/lysosomal protein degradation in
isolated rat hepatocytes. Proc Natl Acad Sci USA. 79:1889–1892.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tassa A, Roux MP, Attaix D and Bechet DM:
Class III phosphoinositide 3-kinase - Beclin1 complex mediates the
amino acid-dependent regulation of autophagy in C2C12 myotubes.
Biochem J. 376:577–586. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alinari L, Mahoney E, Patton J, Zhang X,
Huynh L, Earl CT, Mani R, Mao Y, Yu B, Quinion C, Towns WH, Chen
CS, Goldenberg DM, Blum KA, Byrd JC, Muthusamy N, Praetorius-Ibba M
and Baiocchi RA: FTY720 increases CD74 expression and sensitizes
mantle cell lymphoma cells to milatuzumab-mediated cell death.
Blood. 118:6893–6903. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Romero Rosales K, Singh G, Wu K, Chen J,
Janes MR, Lilly MB, Peralta ER, Siskind LJ, Bennett MJ, Fruman DA
and Edinger AL: Sphingolipid-based drugs selectively kill cancer
cells by down-regulating nutrient transporter proteins. Biochem J.
439:299–311. 2011.PubMed/NCBI
|
|
25
|
Wallington-Beddoe CT, Hewson J, Bradstock
KF and Bendall LJ: FTY720 produces caspase-independent cell death
of acute lymphoblastic leukemia cells. Autophagy. 7:707–715. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liao A, Hu R, Zhao Q, Li J, Li Y, Yao K,
Zhang R, Wang H, Yang W and Liu Z: Autophagy induced by FTY720
promotes apoptosis in U266 cells. Eur J Pharm Sci. 45:600–605.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Paglin S, Hollister T, Delohery T, Hackett
N, McMahill M, Sphicas E, Domingo D and Yahalom J: A novel response
of cancer cells to radiation involves autophagy and formation of
acidic vesicles. Cancer Res. 61:439–444. 2001.
|
|
28
|
Kanzawa T, Germano IM, Komata T, Ito H,
Kondo Y and Kondo S: Role of autophagy in temozolomide-induced
cytotoxicity for malignant glioma cells. Cell Death Differ.
11:448–457. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sato K, Tsuchihara K, Fujii S, Sugiyama M,
Goya T, Atomi Y, Ueno T, Ochiai A and Esumi H: Autophagy is
activated in colorectal cancer cells and contributes to the
tolerance to nutrient deprivation. Cancer Res. 67:9677–9684. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Amaravadi RK, Yu D, Lum JJ, Bui T,
Christophorou MA, Evan GI, Thomas-Tikhonenko A and Thompson CB:
Autophagy inhibition enhances therapy-induced apoptosis in a
Myc-induced model of lymphoma. J Clin Invest. 117:326–336. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Apel A, Herr I, Schwarz H, Rodemann HP and
Mayer A: Blocked autophagy sensitizes resistant carcinoma cells to
radiation therapy. Cancer Res. 68:1485–1494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bellodi C, Lidonnici MR, Hamilton A,
Helgason GV, Soliera AR, Ronchetti M, Galavotti S, Young KW, Selmi
T, Yacobi R, Van Etten RA, Donato N, Hunter A, Dinsdale D, Tirrò E,
Vigneri P, Nicotera P, Dyer MJ, Holyoake T, Salomoni P and
Calabretta B: Targeting autophagy potentiates tyrosine kinase
inhibitor-induced cell death in Philadelphia chromosome-positive
cells, including primary CML stem cells. J Clin Invest.
119:1109–1123. 2009. View
Article : Google Scholar
|