Introduction
Kaempferol (3,5,7,4-tetrahydroxyflavone) is a
phytoestrogen found in a variety of vegetables and fruits, such as
tomatoes, hops, red grapes and strawberries. Kaempferol has been
used in traditional medicine and is believed to have various
biological functions, including inhibition of inflammation
(1). Kaempferol is a member of the
flavonoid compounds, which are known for defensive properties
against micro-organisms or pests and are a part of the oxidative
stress protection pathway (2). It
has been shown that kaempferol suppresses oxidative stress in
animal cells by inhibiting the induction of nitric oxide synthase
(iNOS) mRNA expression (3,4) and prostaglandin E2 production. In
addition, kaempferol has been reported in treatment of various
types of tumors, including breast cancer (5,6),
lung cancer (7), colon cancer
(8,9) and leukemia (10). Kaempferol has been shown to have a
protective effect in CCl4-induced liver damage. These studies
suggest that kaempferol possesses beneficial activities as an
inherent antioxidant and may be an attractive anticancer agent, but
the underlying mechanisms remain to be elucidated.
Autophagy is a conserved catabolic process used by
all eukaryotic cells to degrade long-lived proteins and damaged
organelles. The disintegration of vesicles is a hallmark
morphological characteristic of autophagy (11). Double-membrane vesicles transport
contents, such as cytoplasmic proteins, to lysosomes and then form
autophagosomes (12), where
degradative enzymes break the engulfed materials; the resulting
macromolecules are recycled (12–14).
In addition, autophagy allows cells to adapt and survive under
adverse conditions, such as nutrient deficiency. Moreover,
autophagy has been implicated in development and pathophysiology
(15). Potential links between
autophagy and a number of human diseases have been proposed. For
instance, cancer is associated with increased autophagic activity
(16). Autophagy can protect from
cell death in times of stress (12). Therefore, the impact of autophagy
induction on the efficacy of chemotherapeutic drugs can be highly
variable and depends on both the cell type and treatment. However,
the precise role that autophagy plays in cancer development is very
controversial (17,18).
Although kaempferol has been reported to have
antifungal, antitumor, anti-inflammatory, anti-oxidative,
anti-carcinogenic and anti-mutagenic abilities (2,19–21),
the underlying mechanisms of kaempferol-mediated autophagy remain
to be elucidated. In this study, we aimed to elucidate the role of
kaempferol-mediated autophagy. Furthermore, the study of
kaempferol-mediated autophagy may shed light on long-term cancer
prevention.
Materials and methods
Chemicals and reagents
Acridine orange (AO), anti-actin primary antibody,
kaempferol, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT), dimethyl sulfoxide (DMSO) and Triton X-100 were
purchased from Sigma-Aldrich Corporation (St. Louis, MO, USA).
Fetal bovine serum (FBS), L-glutamine, LysoTracker Red,
penicillin/streptomycin, propidium iodide (PI), Premo™ Autophagy
Sensor LC3B-GFP Kit and trypsin-EDTA were obtained from Invitrogen
Life Technologies (Carlsbad, CA, USA). The caspase-3 activity assay
kit was purchased from R&D Systems Incorporated (Minneapolis,
MN, USA). The anti-Atg 5, Atg 7, Atg 12, AKT, p-AKT (Ser473),
AMPKα, p-AMPKα (Thr172), beclin, LC3, mTOR and p-mTOR primary
antibodies were purchased from Cell Signaling Technology (Beverly,
MA, USA). The anti-CDK1, cyclin B and peroxidase-conjugated
secondary antibodies were obtained from Santa Cruz Biotechnology
Incorporated (Santa Cruz, CA, USA). The enhanced chemiluminescence
(ECL) detection kit was obtained from Pierce Chemical (Rockford,
IL, USA).
Cell culture
The SK-HEP-1 human hepatic cancer cell line was
purchased from the Food Industry Research and Development Institute
(Hsinchu, Taiwan). The cells were grown in Eagle’s Minimum
Essential medium fortified with 10% FBS, 2 mM L-glutamine and
penicillin/streptomycin and incubated at 37°C under a humidified 5%
CO2 atmosphere (11).
Cell viability and morphology
Cell viability was assessed by MTT assays. Briefly,
SK-HEP-1 cells were cultured in a 96-well plate at a density of
2.5×104 cells/per well and incubated with 0, 25, 50, 75
or 100 μM of kaempferol for 24 h. At the end of the
kaempferol treatment, culture medium containing 0.5 mg/ml MTT was
added to each well. The cells were then incubated at 37°C for 4 h,
and the blue formazan crystal products were dissolved with
isopropanol, 0.04 N HCl. The absorbance of each well was measured
at 570 nm with an ELISA reader using a reference wavelength of 620
nm. The cell viability after each treatment was defined as the
percentage of the control. Kaempferol-treated cells were
morphologically examined for autophagic vacuoles under a
phase-contrast microscope (11).
Cell cycle analysis
The cell cycle distribution was analyzed by flow
cytometric analysis using DNA staining with PI. SK-HEP-1 cells were
cultured in a 24-well plate at a density of 2.5×105
cells/per well and incubated with 0, 25, 50, 75 or 100 μM
kaempferol for 24 h. After treatment, the cells were collected,
washed with phosphate-buffered saline (PBS) and fixed in 70%
ethanol at −20°C. The cells were collected by centrifugation and
re-suspended in PBS containing 50 mg/ml PI at room temperature.
Stained cells were analyzed by flow cytometry on a FACSCalibur™
flow cytometer (Becton-Dickinson, Franklin Lakes, NJ, USA) as
previously described. The percentage of cells in the different
phases of the cell cycle was determined using the CellQuest
software program (22).
DNA fragmentation assay
SK-HEP-1 cells were seeded into 75-T flasks
(1×107 cells/flask). Kaempferol (0, 50, 75 or 100
μM) was added, and the cells were incubated for 24 h. At the
end of the incubation, the cells were harvested and washed with
ice-cold PBS. The cells were re-suspended in lysis buffer (20 mM
Tris-HCl, pH 8.0, 10 mM EDTA, 0.2% Triton X-100) containing 0.1
mg/ml proteinase K and 50 μg/ml RNase A, and the samples
were incubated at 37°C overnight. The DNA was subjected to
electrophoresis using a 1.5% agarose gel (Sigma-Aldrich). The
agarose gel was stained with 1 μg/ml ethidium bromide
(Invitrogen Life Technologies) in 0.5X TBE buffer to visualize the
DNA fragmentation, and the gel was examined using photographs taken
under UV light as previously described (23,24).
DAPI stain
DNA condensation was detected using the DAPI
staining method as previously described. SK-HEP-1 cells were seeded
in 24-well plates at 2.5×107 per well and followed by
treatment with 0 or 100 nM kaempferol. The cells were fixed gently
by the addition of 70% ethanol, stained with DAPI and then
photographed using a fluorescence microscope (25,26).
Caspase-3 activity assays
The caspase-3 activity was determined using the
caspase-3 colorimetric assay kit as described by the manufacturer
(Caspase Colorimetric Kit; R&D Systems Inc.). Briefly, after 24
h of incubation with 0, 25, 50, 75 or 100 μM kaempferol, the
SK-HEP-1 cells were harvested. The collected cells were then lysed
in a lysis buffer containing 50 mM Tris-HCl (pH 7.4), 1 mM EDTA, 10
mM EGTA, 10 mM digitonin and 2 mM DTT. The cell lysates were used
to determine caspase-3 activity by adding the peptide substrate
acetyl-Asp-Glu-Val-Asp p-nitroanilide (Ac-DEVD-pNA). Cleavage of
the peptide by the caspase releases pNA, which has a high
absorbance at 405 nm. The concentration of pNA released from the
substrate was calculated using a calibration curve prepared from
defined pNA solutions. The experiments were performed in triplicate
(27).
Transmission electron microscopy
(TEM)
SK-HEP-1 cells were seeded in 6-well plates at
1×106 per well and treated with kaempferol (100
μM) for 24 h. The cells were washed with PBS and then fixed
in 2% paraformaldehyde and 2.5% glutaraldehyde in PBS buffer. The
cells were rinsed twice in the same buffer and subsequently
post-fixed in 1% osmium tetraoxide. After rinsing and dehydration
in a graded alcohol series, the cells were embedded in LR White
resin and polymerized at 70°C overnight. Ultrathin sections were
then cut with a diamond knife and loaded onto TEM grids. The
sections were examined by a Philips CM10 electron microscope at an
accelerating voltage of 120 kV and micrographs were taken (11).
LysoTracker red staining
SK-HEP-1 cells were seeded in 6-well plates at
1×106 per well and treated with kaempferol (100
μM) for 24 h. After treatment, the cells were stained with
LysoTracker Red (50 nM) at 37°C for 15 min. After washing three
times with PBS, the cells were immediately visualized by
fluorescence microscopy (Nikon, Melville, NY, USA) for the
detection of acidic lysosome compartments (11).
LC3B protein aggregation
Autophagy was detected by measuring the aggregation
of LC3B protein coupled to green fluorescence protein (GFP) using
Premo Autophagy Sensor kits (Invitrogen Life Technologies).
Briefly, SK-HEP-1 cells were seeded in 12-well plates at
5×105 per well. Then, the cells were transduced with
non-replicating baculoviral vectors expressing LC3B-GFP (component
A) or LC3BΔ(G120A)-GFP (component B), which encodes a point
mutation in the LC3B gene which prevents cleavage. Twenty-four
hours after transduction, the SK-HEP-1 cells were treated with
kaempferol (100 μM) for 24 h. The appearance of LC3B-GFP
aggregates was observed and photographed using a fluorescence
microscope (11).
Acridine orange (AO) stain
SK-HEP-1 cells were seeded in 6-well plates at
1×106 per well and treated with kaempferol (100
μM) for 24 h. After treatment, the cells were stained with
acridine orange (AO) at 37°C for 15 min. After washing three times
with PBS, the cells were immediately visualized by fluorescence
microscopy (Nikon) for the detection of acidic vesicular organelles
(AVO) (11).
Western blot analysis
SK-HEP-1 cells were seeded in 75-T flasks at
1×107 per flask and treated with 0, 50, 75 or 100
μM kaempferol for 6 or 24 h. Then, the cells were harvested
and lysed; the total proteins were collected. Thirty micrograms of
protein from each treatment were separated using 10 to 12%
SDS-polyacrylamide gel electrophoresis and electro-transferred to a
nitrocellulose membrane using the iBot Dry Blotting System
(Invitrogen Life Technologies). The transferred membranes were
blocked in 5% non-fat dry milk in 20 mM Tris-buffered saline/0.05%
Tween-20 for 1 h at room temperature followed by incubation with
primary antibodies against Atg 5, Atg 7, Atg 12, AKT, p-AKT
(Ser473), AMPKα, p-AMPKα (Thr172), beclin, LC3, mTOR, p-mTOR, CDK1
or cyclin B at 4°C overnight. At the end of the incubation, the
membranes were incubated with secondary antibodies conjugated with
horseradish peroxidase (HRP). The blots were developed using the
chemiluminescence kit (Millipore, Bedford, MA, USA) and the bands
were captured with X-ray film. Each membrane was stripped and
re-probed with anti-actin antibody to ensure equal protein loading
during the experiment (11,28).
Statistical analysis
All the statistical results are presented as the
mean ± SEM for the indicated numbers of separate experiments.
Statistical analyses of data were performed using a one-way ANOVA
followed by Student’s t-test, and P<0.001 was considered
significant (11).
Results
The effects of kaempferol on cell
viability in SK-HEP-1 cells
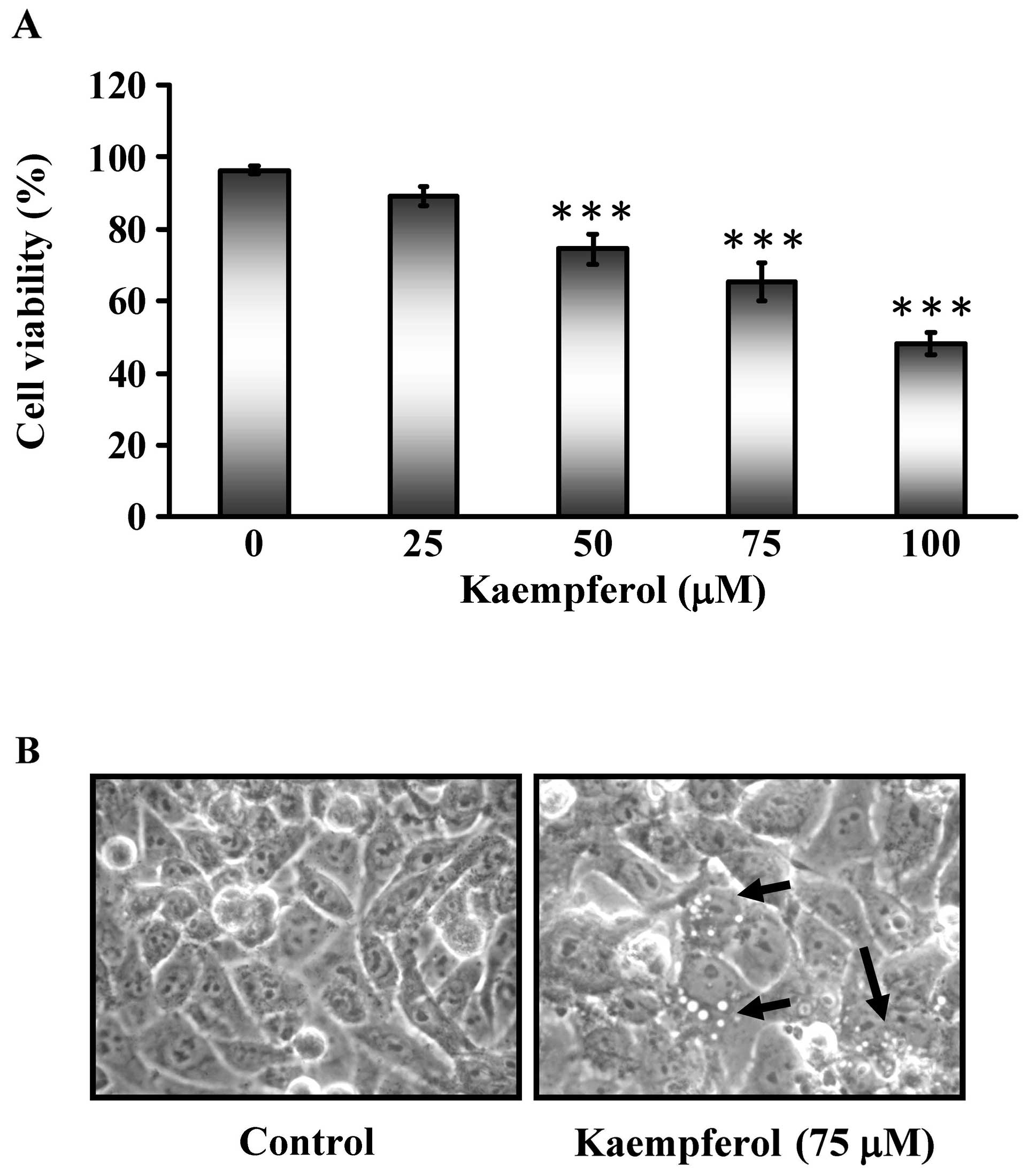
In this study, we assessed the cytotoxic effects of
kaempferol on SK-HEP-1 cells. Various doses of kaempferol (25, 50,
75 and 100 μM) were applied for 24 h and the cell viability
was analyzed by MTT assays as shown in Fig. 1A. The cell viability of SK-HEP-1
cells decreased in a dose-dependent manner. Morphological changes
of SK-HEP-1 cells were visualized by optic microscopy after
exposure to 75 μM kaempferol for 24 h (Fig. 1B). This finding suggested that
kaempferol might have chemotherapeutic potential in SK-HEP-1
cells.
The cell cycle distributions of
kaempferol-treated SK-HEP-1 cells
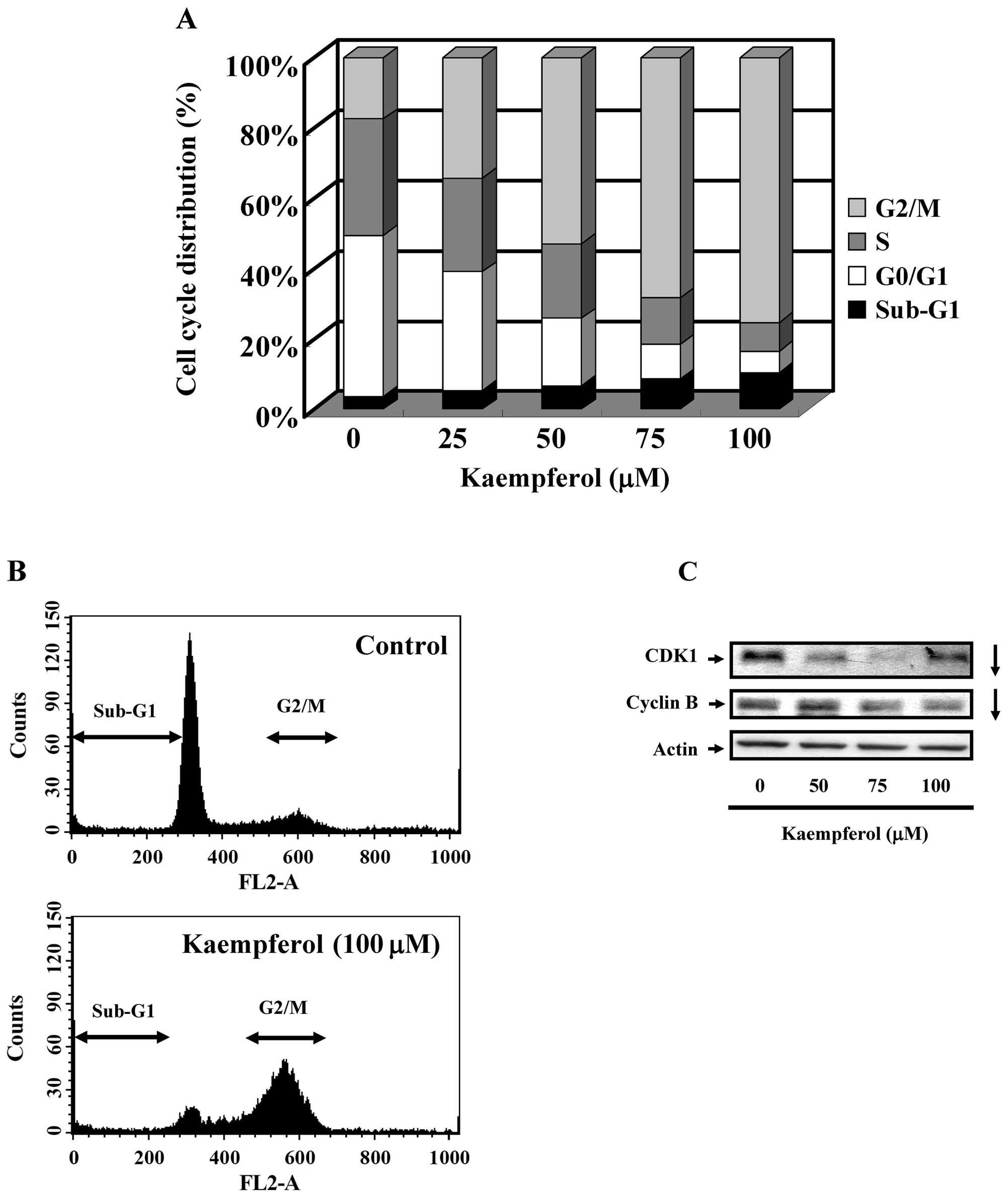
To characterize whether kaempferol caused SK-HEP-1
cells to arrest in a specific cell cycle phase, SK-HEP-1 cells were
treated with different doses of kaempferol (25, 50, 75 or 100
μM) for 24 h. As shown in Fig.
2A and B, the sub-G1, G1, and
G2 phases observed by PI staining and flow cytometry
were significantly different between the control and
kaempferol-treated groups. Kaempferol treatment resulted in
G2/M cell cycle arrest. As shown in Fig. 2C, the expression of CDK1 and cyclin
B decreased in kaempferol-treated SK-HEP-1 cells. The data were
consistent with the finding in Fig.
2A. These results indicated that kaempferol treatment resulted
in G2/M cell cycle arrest.
Kaempferol had no effect on apoptosis in
SK-HEP-1 cells
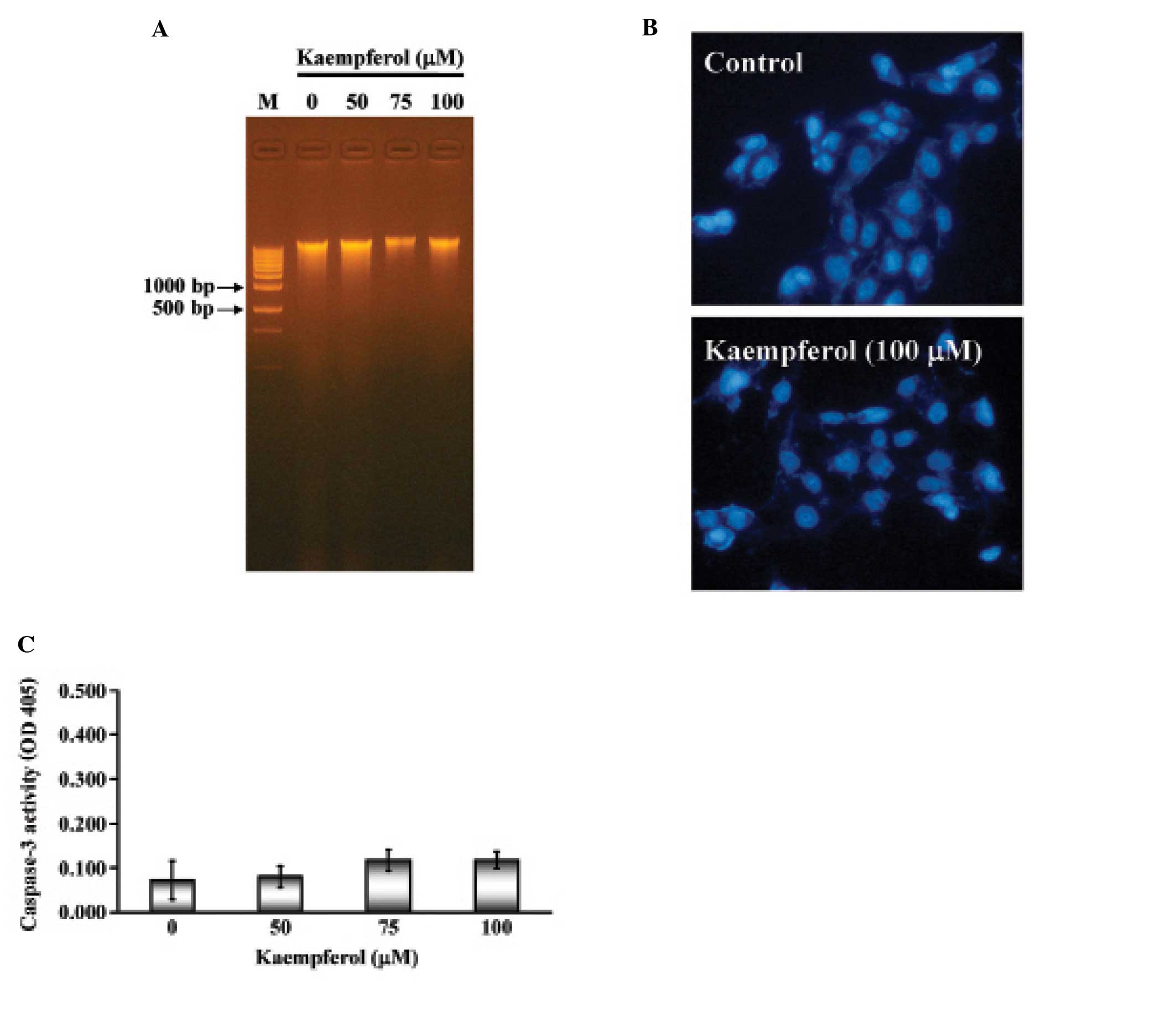
Apoptosis is characterized by DNA fragmentation,
chromatin condensation and caspase activation. We next examined
whether kaempferol induced apoptotic cell death in SK-HEP-1 cells.
SK-HEP-1 cells were treated with various concentrations of
kaempferol (25, 50, 75 or 100 μM) for 24 h. The
characteristic apoptotic laddering pattern of DNA fragments with
multiple bands indicated the induction of apoptosis. As shown in
Fig. 3A, not all of the samples
showed the DNA fragment band. Next, SK-HEP-1 nuclei were stained by
DAPI and observed by fluorescence microscope. There was no
significant difference between the control and kaempferol-treated
cells (Fig. 3B). Furthermore,
caspase-3 activity remained unchanged in both the control and
kaempferol-treated cells (Fig.
3C). These results indicated that kaempferol treatment did not
induce a typical apoptotic response in SK-HEP-1 cells.
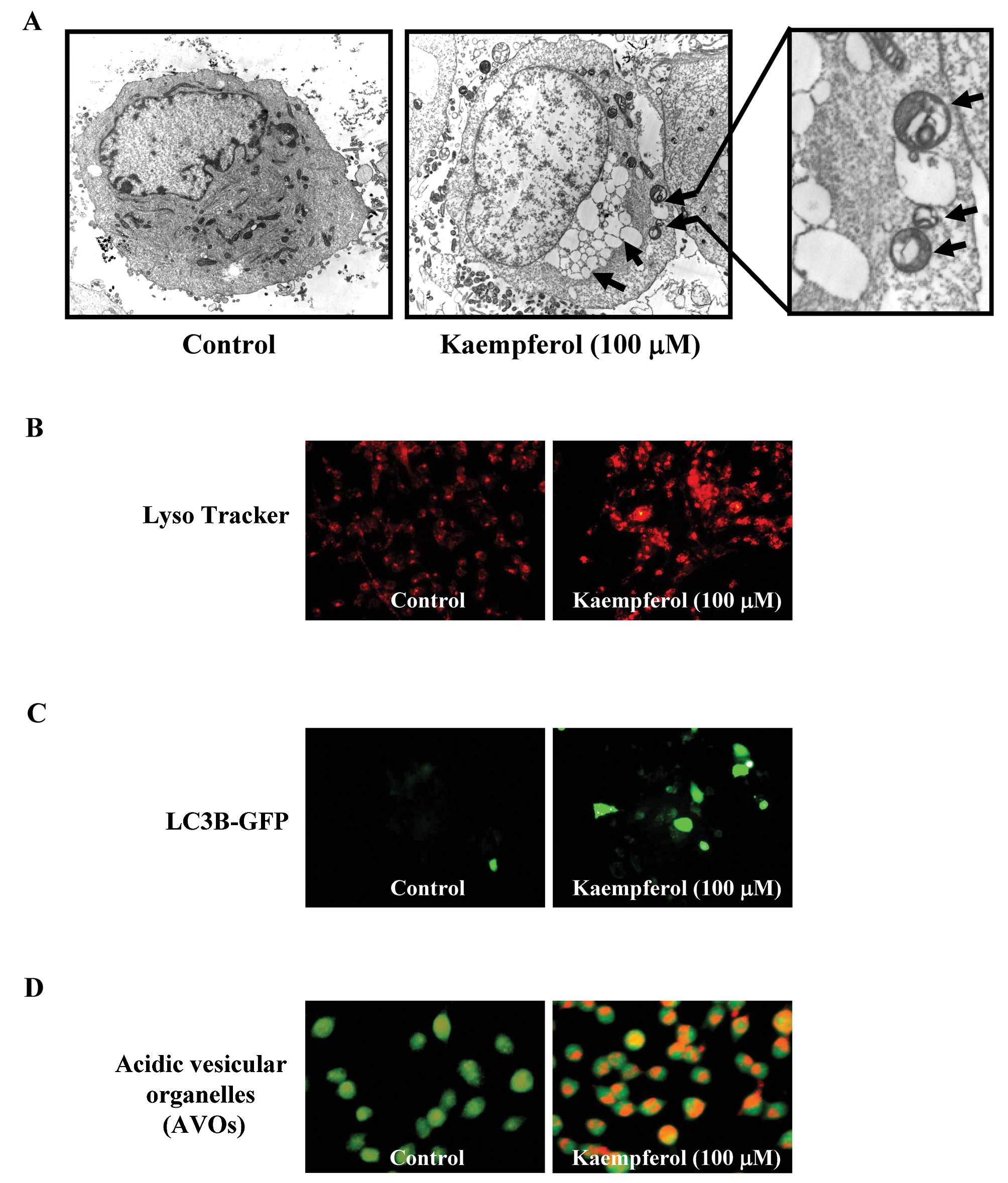
Kaempferol induced autophagy in SK-HEP-1
cells
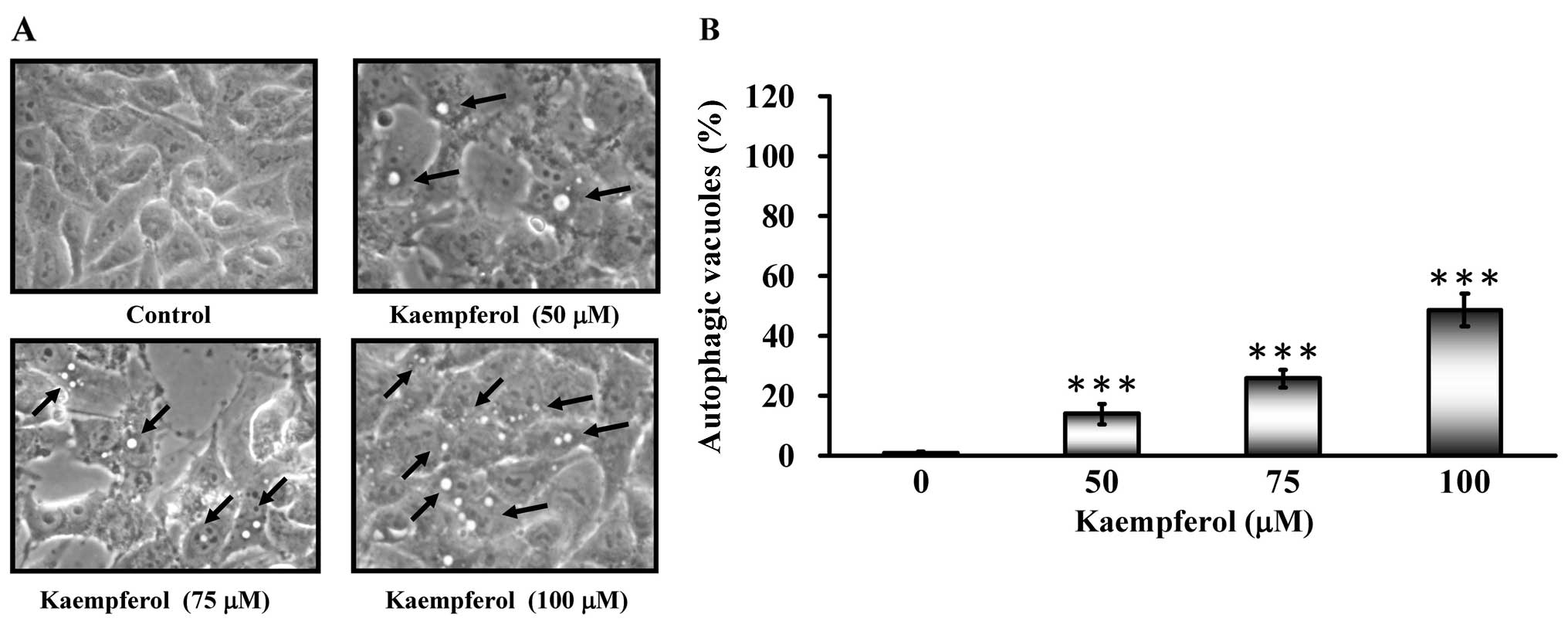
Autophagic vacuoles appeared when SK-HEP-1 cells
were treated with kaempferol (Fig.
4A). To determine whether kaempferol treatment induced
autophagy, cells were stained with MDC to detect AVO formation. The
AVO positive cells increased after kaempferol treatment in a
concentration-dependent manner compared to the control (Fig. 4B). Based on these data, we
concluded that kaempferol induced autophagy.
Autophagy is a series of biochemical steps through
which eukaryotic cells commit suicide by degrading their own
cytoplasm and organelles. In this process, these components are
engulfed and then digested in double membrane-bound vacuoles called
autophagosomes (15). To verify
whether the kaempferol treatment induced autophagy, SK-HEP-1 cells
were treated with kaempferol (0 or 100 μM) for 24 h and
examined by fluorescence microscopy. Fig. 5A shows the formation of
autolysosomes in SK-HEP-1 cells treated with 100 μM of
kaempferol observed using electron microscopy. Moreover, an
increase in autophagosomes was visualized and analyzed by
LysoTracker Red staining, LC3B-GFP staining and acidic vesicular
organ-elle formation in kaempferol-treated SK-HEP-1 cells compared
to control SK-HEP-1 cells (Fig.
5B–D). Based on these data, we concluded that kaempferol could
induce autophagic cell death in SK-HEP-1 cells.
The effects of kaempferol on the
expression of autophagy-related proteins in SK-HEP-1 cells
We next examined the protein level of LC3, which is
a protein marker of autophagy. Fig.
6A shows that the LC-3 level increased with kaempferol
treatment in a dose-dependent manner. In addition, the levels of
p-AKT and p-mTOR (a negative regulator of autophagy and apoptosis)
decreased in a dose-dependent manner after kaempferol treatment for
24 h. In contrast, p-AMPK, which is a positive regulator of
autophagy, increased significantly. These results suggested the
kaempferol induced autophagy in SK-HEP-1 cells through the
downregulation of p-AKT or the upregulation of p-AMPK.
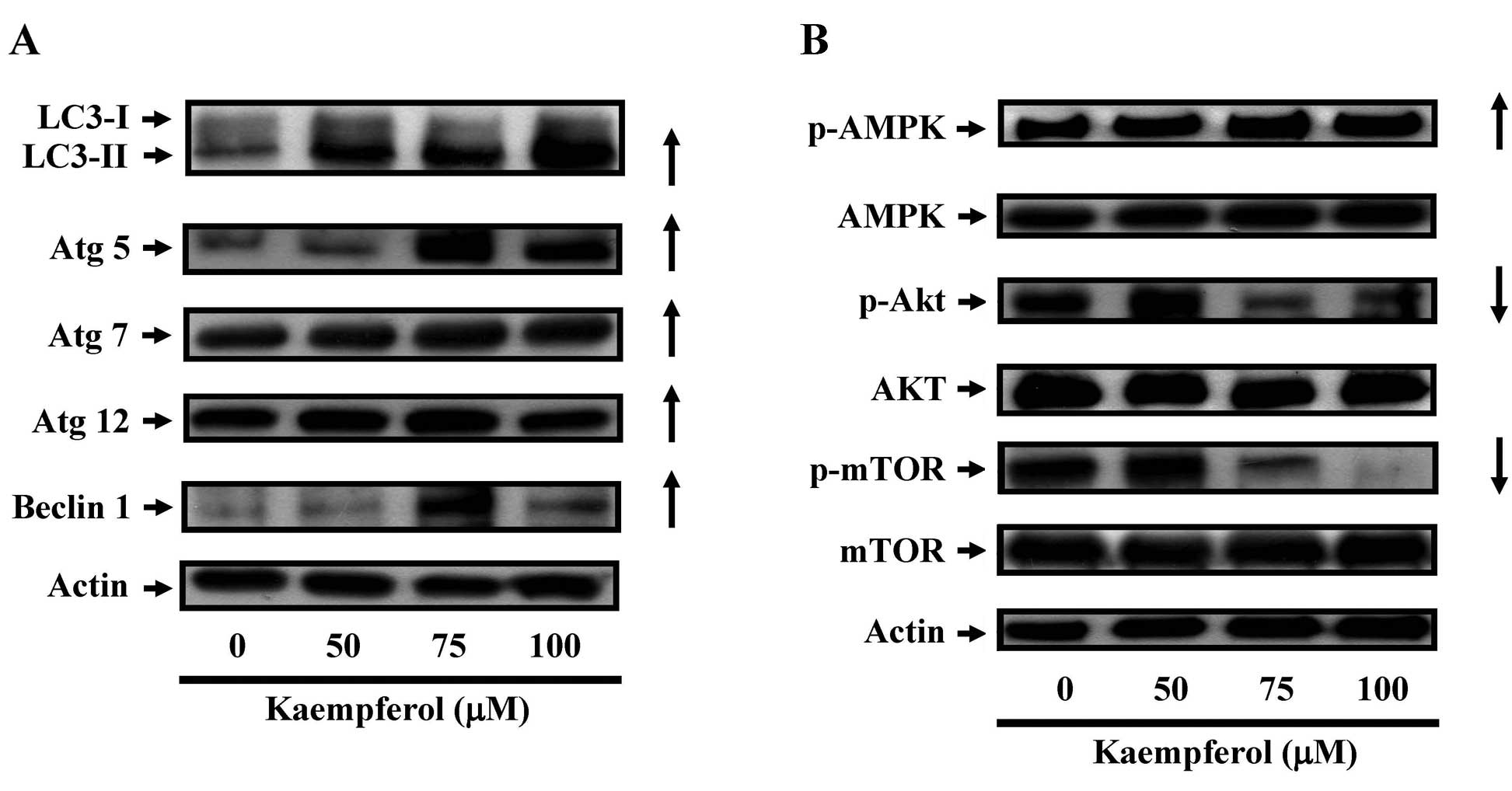 | Figure 6The effects of kaempferol on the
expression of autophagy-related proteins in SK-HEP-1 cells. Cells
were treated with kaempferol (0, 50, 75 or 100 μM) for 24 h
and then subjected to western blot analysis. (A) Western blot
analysis of LC3, Atg-5, Atg-7, Atg-12 and beclin 1 expression in
SK-HEP-1 cells. (B) Western blot analysis of p-AMPK, AMPK, p-AKT,
AKT, p-mTOR, p-AMPK and mTOR expression in SK-HEP-1 cells. β-actin
was detected for equivalent protein loading. |
Discussion
Kaempferol is a flavonoid that can be found in many
edible plants, including broccoli, cabbage, strawberries and
tomatoes (2,21). Kaempferol is also found in
traditional Chinese medicines, such us Acacia nilotica,
Aloe vera and Tilia spp. It was reported that
kaempferol has a broad spectrum of pharmacological activities,
including anti-inflammatory, anti-oxidant and cardio-protective
(2,19–21).
Many studies have demonstrated that kaempferol has anticancer
activity in various human cancer cell lines, including
osteosarcoma, breast cancer, lung cancer, colorectal cancer,
leukemia, oral cancer and ovarian cancer (2,5–10,29–31).
In contrast, kaempferol has low toxicity against normal cells.
Berger et al demonstrated that kaempferol reduced the cell
viability and proliferation rates of HepG2 and Hep3B hepatic cancer
cell lines. Kaempferol also induced cell apoptosis and inhibited
HIF-1 activity in Huh7 and H4IIE hepatic cancer cell lines. In this
study, we investigated the anti-hepatic effects of kaempferol on
SK-HEP-1 cells in vitro. In the present study, we focused on
cell cycle arrest and autophagy induction by kaempferol in SK-HEP-1
cells. Our results showed that kaempferol exerted a significant
antiproliferative effect in SK-HEP-1 cells (Fig. 1A). Kaempferol induced
G2/M phase cell cycle arrest in a
concentration-dependent manner in SK-HEP-1 cells (Fig. 2A and B). However, DNA fragmentation
was observed in SK-HEP-1 cells treated with certain doses of
kaempferol (Fig. 3A and B). The
caspase-3 activity did not change in kaempferol-treated SK-HEP-1
cells (Fig. 3C). Our results
suggested that kaempferol did not induce apoptosis after 24 h in
SK-HEP-1 cells, and another mechanism might be involved in
kaempferol-induced growth inhibition in SK-HEP-1 cells. Our results
demonstrated that kaempferol could induce growth inhibitory effects
through G2/M cell cycle arrest (Fig. 2A and B) and autophagy in SK-HEP-1
cells. In addition to the G2/M phase arrest, kaempferol
treatment decreased the cyclin B and CDK1 protein levels in a
concentration-dependent manner (Fig.
2C). Many studies have demonstrated that kaempferol induced
G2/M arrest in HL-60 leukemia cells, mouse T
lymphocytes, MDA-MB-453 breast cancer cells and OCM-1 melanoma
cells (5,6). Our results agree with previous
studies showing that kaempferol induces G2/M cell cycle
arrest.
There are three types of morphological processes
that lead to cell death: apoptosis, necrosis and autophagy
(32). Autophagy, or cellular
self-digestion, plays an important role in normal physiology in
animals. Many pharmacologic studies have suggested that autophagy
has an anticancer role. Kaempferol-induced autophagy in SK-HEP-1
cells was supported by several pieces of evidence, including the
formation of autophagic vesicles (Fig.
4B) double-membrane vacuoles (Fig.
5A), acidic lysosomal compartments (Fig. 5B), and acidic vesicular organelles
(Fig. 5D); the cleavage of
microtubule-associated protein 1 light chain 3 (LC3) (Figs. 5C and 6A); and elevated levels of autophagic
proteins, Atg complex (Atg 5, Atg 7 and Atg 12) and beclin-1
(Fig. 6A). Our results suggested
that kaempferol-induced cell death may involve autophagy in
SK-HEP-1 cells. This is the first study to present detailed
evidence for kaempferol-induced autophagy in SK-HEP-1 cells. Our
findings are in agreement with previous studies that demonstrated
kaempferol-induced autophagy in HeLa cervical cancer cells
(33).
Many protein kinases are involved in the cell
survival response, and previous studies suggested that AMPK and AKT
serine/threonine kinase [also called protein kinase B (PKB)] play
key roles and are involved in the induction of cell autophagy
(34,35). AKT activation was associated with
anti-apoptotic responses, cell proliferation and cellular energy
metabolism. Liu et al suggested that the AKT gene was
over-expressed in hepatic cancer and AKT activation participates in
the pathogenesis and progression of hepatic cancer (36). Therefore, regulation of the AKT
pathway may be essential for developing therapeutic inhibitors for
hepatic cancer. Park et al demonstrated that kaempferol was
able to reduce LPS-induced inflammatory mediators via MAPK and AKT
downregulation, suggesting that kaempferol has therapeutic
potential for the treatment of neuro-inflammatory diseases
(34). Luo et al reported
that kaempferol inhibited angiogenesis and VEGF expression through
repression of AKT phosphorylation in human ovarian cancer cells
(37). Nguyen et al also
showed that kaempferol-induced apoptosis in A549 lung cancer cells
is through AKT phosphorylation (7). In addition, Filomeni et al
presented that kaempferol-induced autophagy in HeLa cells are
mediated by the AMPK pathway (33). Our study demonstrated
kaempferol-induced autophagy was accompanied with the upregulation
of p-AMPKα (Thr172) and the downregulation of phospho-AKT (Ser473)
and phospho-mTOR protein levels (Fig.
6B). Our results suggested that the AMPK and AKT/mTOR pathways
are associated with the induction of autophagy in
kaempferol-treated SK-HEP-1 cells.
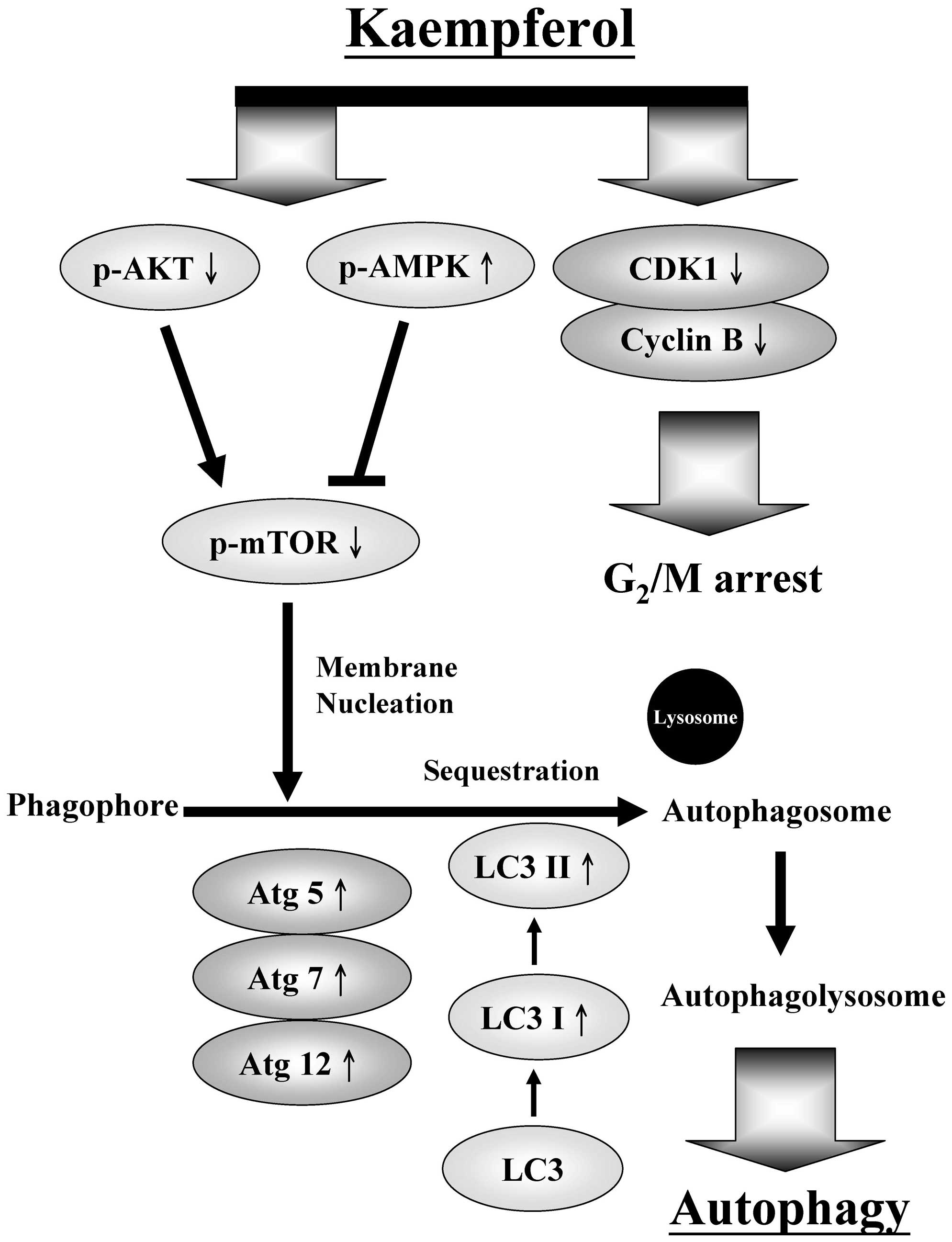
A schematic of the kaempferol-induced
G2/M arrest and autophagy pathways in SK-HEP-1 human
hepatic cancer cells is presented in Fig. 7. Our findings imply that kaempferol
may be used as a novel anticancer drug candidate for the treatment
of human hepatic cancer.
Acknowledgements
This study was supported in part by
research grants from the National Science Council of the Republic
of China (NSC 101-2313-B-039-008) awarded to J.-S.Y. and (NSC
101-2320-B-039-041) awarded to W.-W.H. This study was supported in
part by a research grants from China Medical University
(CMU100-TC-08) awarded to S.-C.T.
References
|
1
|
Gong JH, Shin D, Han SY, et al: Kaempferol
suppresses eosionphil infiltration and airway inflammation in
airway epithelial cells and in mice with allergic asthma. J Nutr.
142:47–56. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang WW, Chiu YJ, Fan MJ, et al:
Kaempferol induced apoptosis via endoplasmic reticulum stress and
mitochondria-dependent pathway in human osteosarcoma U-2 OS cells.
Mol Nutr Food Res. 54:1585–1595. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sharma V, Joseph C, Ghosh S, et al:
Kaempferol induces apoptosis in glioblastoma cells through
oxidative stress. Mol Cancer Ther. 6:2544–2553. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Samhan-Arias AK, Martin-Romero FJ and
Gutierrez-Merino C: Kaempferol blocks oxidative stress in
cerebellar granule cells and reveals a key role for reactive oxygen
species production at the plasma membrane in the commitment to
apoptosis. Free Radic Biol Med. 37:48–61. 2004. View Article : Google Scholar
|
|
5
|
Choi EJ and Ahn WS: Kaempferol induced the
apoptosis via cell cycle arrest in human breast cancer MDA-MB-453
cells. Nutr Res Pract. 2:322–325. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsiklauri L, An G, Ruszaj DM, et al:
Simultaneous determination of the flavonoids robinin and kaempferol
in human breast cancer cells by liquid chromatography-tandem mass
spectrometry. J Pharm Biomed Anal. 55:109–113. 2011. View Article : Google Scholar
|
|
7
|
Nguyen TT, Tran E, Ong CK, et al:
Kaempferol-induced growth inhibition and apoptosis in A549 lung
cancer cells is mediated by activation of MEK-MAPK. J Cell Physiol.
197:110–121. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li W, Du B, Wang T, et al: Kaempferol
induces apoptosis in human HCT116 colon cancer cells via the
Ataxia-telangiectasia mutated-p53 pathway with the involvement of
p53 upregulated modulator of apoptosis. Chem Biol Interact.
177:121–127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yoshida T, Konishi M, Horinaka M, et al:
Kaempferol sensitizes colon cancer cells to TRAIL-induced
apoptosis. Biochem Biophys Res Commun. 375:129–133. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Benyahia S, Benayache S, Benayache F, et
al: Isolation from Eucalyptus occidentalis and
identification of a new kaempferol derivative that induces
apoptosis in human myeloid leukemia cells. J Nat Prod. 67:527–531.
2004.
|
|
11
|
Tsai SC, Yang JS, Peng SF, et al: Bufalin
increases sensitivity to AKT/mTOR-induced autophagic cell death in
SK-HEP-1 human hepatocellular carcinoma cells. Int J Oncol.
41:1431–1442. 2012.PubMed/NCBI
|
|
12
|
Jiang W and Ogretmen B: Ceramide stress in
survival versus lethal autophagy paradox: ceramide targets
autophagosomes to mitochondria and induces lethal mitophagy.
Autophagy. 9:258–259. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tumbarello DA, Waxse BJ, Arden SD, et al:
Autophagy receptors link myosin VI to autophagosomes to mediate
Tom1-dependent autophagosome maturation and fusion with the
lysosome. Nat Cell Biol. 14:1024–1035. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Orsi A, Razi M, Dooley HC, et al: Dynamic
and transient interactions of Atg9 with autophagosomes, but not
membrane integration, are required for autophagy. Mol Biol Cell.
23:1860–1873. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Son SM, Song H, Byun J, et al:
Accumulation of autophagosomes contributes to enhanced
amyloidogenic APP processing under insulin-resistant conditions.
Autophagy. 8:1842–1844. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pan L, Li Y, Jia L, et al: Cathepsin S
deficiency results in abnormal accumulation of autophagosomes in
macrophages and enhances Ang II-induced cardiac inflammation. PLoS
One. 7:e353152012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Griffiths RE, Kupzig S, Cogan N, et al:
Maturing reticulocytes internalize plasma membrane in glycophorin
A-containing vesicles that fuse with autophagosomes before
exocytosis. Blood. 119:6296–6306. 2012. View Article : Google Scholar
|
|
18
|
Mijaljica D, Prescott M and Devenish RJ:
The intriguing life of autophagosomes. Int J Mol Sci. 13:3618–3635.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gabrielska J, Soczynska-Kordala M and
Przestalski S: Antioxidative effect of kaempferol and its equimolar
mixture with phenyltin compounds on UV-irradiated liposome
membranes. J Agric Food Chem. 53:76–83. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hamalainen M, Nieminen R, Vuorela P,
Heinonen M and Moilanen E: Anti-inflammatory effects of flavonoids:
genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and
NF-kappaB activations, whereas flavone, isorhamnetin, naringenin,
and pelargonidin inhibit only NF-kappaB activation along with their
inhibitory effect on iNOS expression and NO production in activated
macrophages. Mediators Inflamm. 2007:456732007.
|
|
21
|
Garcia-Mediavilla V, Crespo I, Collado PS,
et al: The anti-inflammatory flavones quercetin and kaempferol
cause inhibition of inducible nitric oxide synthase,
cyclooxygenase-2 and reactive C-protein, and down-regulation of the
nuclear factor kappaB pathway in Chang Liver cells. Eur J
Pharmacol. 557:221–229. 2007. View Article : Google Scholar
|
|
22
|
Lan YH, Chiang JH, Huang WW, et al:
Activations of both extrinsic and intrinsic pathways in HCT 116
human colorectal cancer cells contribute to apoptosis through
p53-mediated ATM/Fas signaling by Emilia sonchifolia
extract, a folklore medicinal plant. Evid Based Complement Alternat
Med. 2012:1781782012.PubMed/NCBI
|
|
23
|
Tsai SC, Huang WW, Huang WC, et al:
ERK-modulated intrinsic signaling and G(2)/M phase arrest
contribute to the induction of apoptotic death by allyl
isothiocyanate in MDA-MB-468 human breast adenocarcinoma cells. Int
J Oncol. 41:2065–2072. 2012.
|
|
24
|
Chiang JH, Yang JS, Ma CY, et al:
Danthron, an anthraquinone derivative, induces DNA damage and
caspase cascades-mediated apoptosis in SNU-1 human gastric cancer
cells through mitochondrial permeability transition pores and
Bax-triggered pathways. Chem Res Toxicol. 24:20–29. 2011.
View Article : Google Scholar
|
|
25
|
Huang WW, Ko SW, Tsai HY, et al:
Cantharidin induces G2/M phase arrest and apoptosis in human
colorectal cancer colo 205 cells through inhibition of CDK1
activity and caspase-dependent signaling pathways. Int J Oncol.
38:1067–1073. 2011.
|
|
26
|
Lu CC, Yang JS, Huang AC, et al:
Chrysophanol induces necrosis through the production of ROS and
alteration of ATP levels in J5 human liver cancer cells. Mol Nutr
Food Res. 54:967–976. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chiu YJ, Hour MJ, Lu CC, et al: Novel
quinazoline HMJ-30 induces U-2 OS human osteogenic sarcoma cell
apoptosis through induction of oxidative stress and up-regulation
of ATM/p53 signaling pathway. J Orthop Res. 29:1448–1456. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lu CC, Yang JS, Chiang JH, et al: Novel
quinazolinone MJ-29 triggers endoplasmic reticulum stress and
intrinsic apoptosis in murine leukemia WEHI-3 cells and inhibits
leukemic mice. PLoS One. 7:e368312012. View Article : Google Scholar
|
|
29
|
Luo H, Rankin GO, Li Z, et al: Kaempferol
induces apoptosis in ovarian cancer cells through activating p53 in
the intrinsic pathway. Food Chem. 128:513–519. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Luo H, Jiang B, Li B, et al: Kaempferol
nanoparticles achieve strong and selective inhibition of ovarian
cancer cell viability. Int J Nanomed. 7:3951–3959. 2012.PubMed/NCBI
|
|
31
|
Bandyopadhyay S, Romero JR and
Chattopadhyay N: Kaempferol and quercetin stimulate
granulocyte-macrophage colony-stimulating factor secretion in human
prostate cancer cells. Mol Cell Endocrinol. 287:57–64. 2008.
View Article : Google Scholar
|
|
32
|
Edinger AL and Thompson CB: Death by
design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol.
16:663–669. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Filomeni G, Desideri E, Cardaci S, et al:
Carcinoma cells activate AMP-activated protein kinase-dependent
autophagy as survival response to kaempferol-mediated energetic
impairment. Autophagy. 6:202–216. 2010. View Article : Google Scholar
|
|
34
|
Park SE, Sapkota K, Kim S, et al:
Kaempferol acts through mitogen-activated protein kinases and
protein kinase B/AKT to elicit protection in a model of
neuroinflammation in BV2 microglial cells. Br J of Pharmacol.
164:1008–1025. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vucicevic L, Misirkic M, Janjetovic K, et
al: Compound C induces protective autophagy in cancer cells through
AMPK inhibition-independent blockade of Akt/mTOR pathway.
Autophagy. 7:40–50. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu H, Xu L, He H, et al: Hepatitis B
virus X protein promotes hepatoma cell invasion and metastasis by
stabilizing Snail protein. Cancer Sci. 103:2072–2081. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Luo H, Rankin GO, Juliano N, et al:
Kaempferol inhibits VEGF expression and in vitro angiogenesis
through a novel ERK-NFκB-cMyc-p21 pathway. Food Chem. 130:321–328.
2012.PubMed/NCBI
|