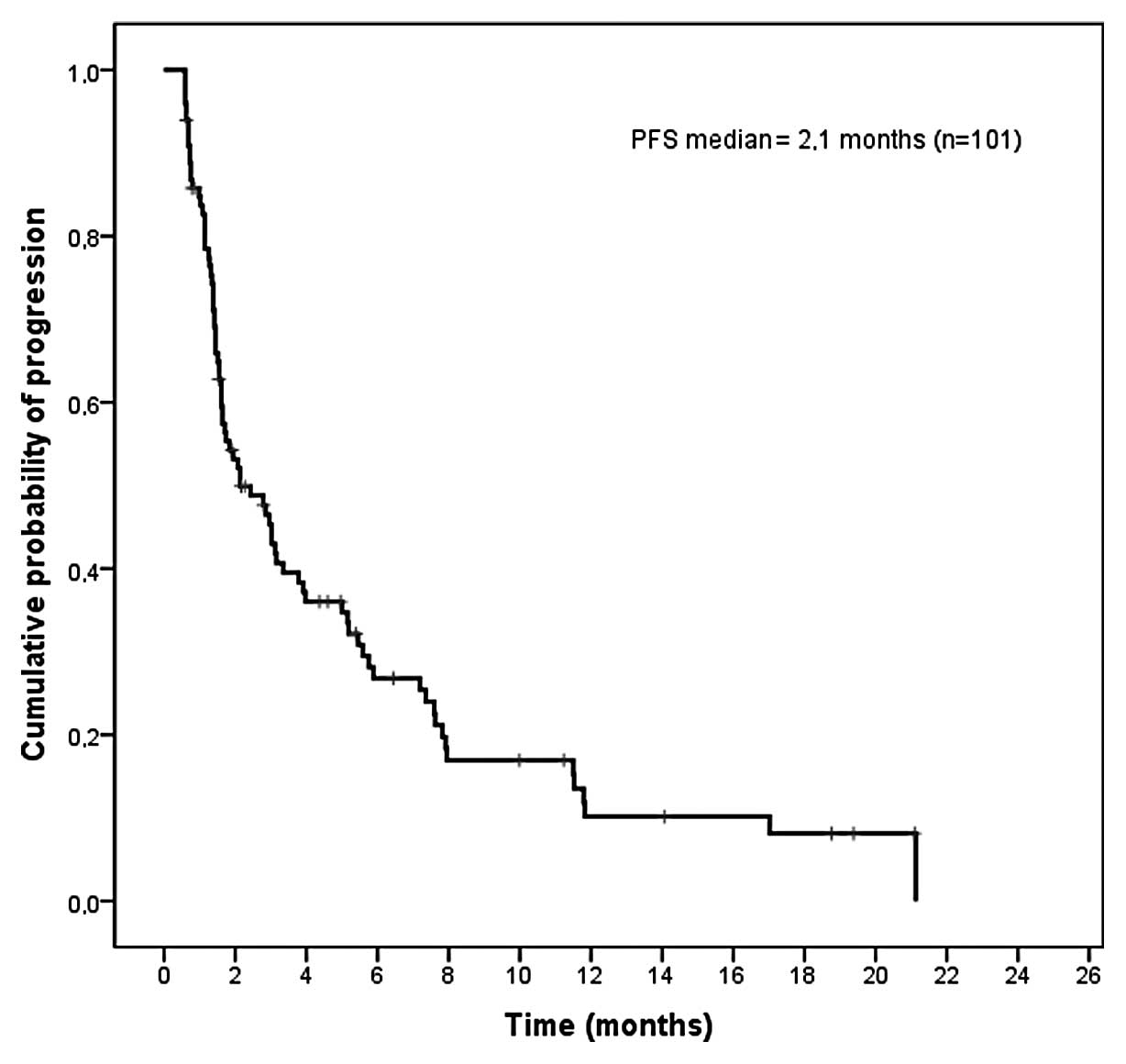
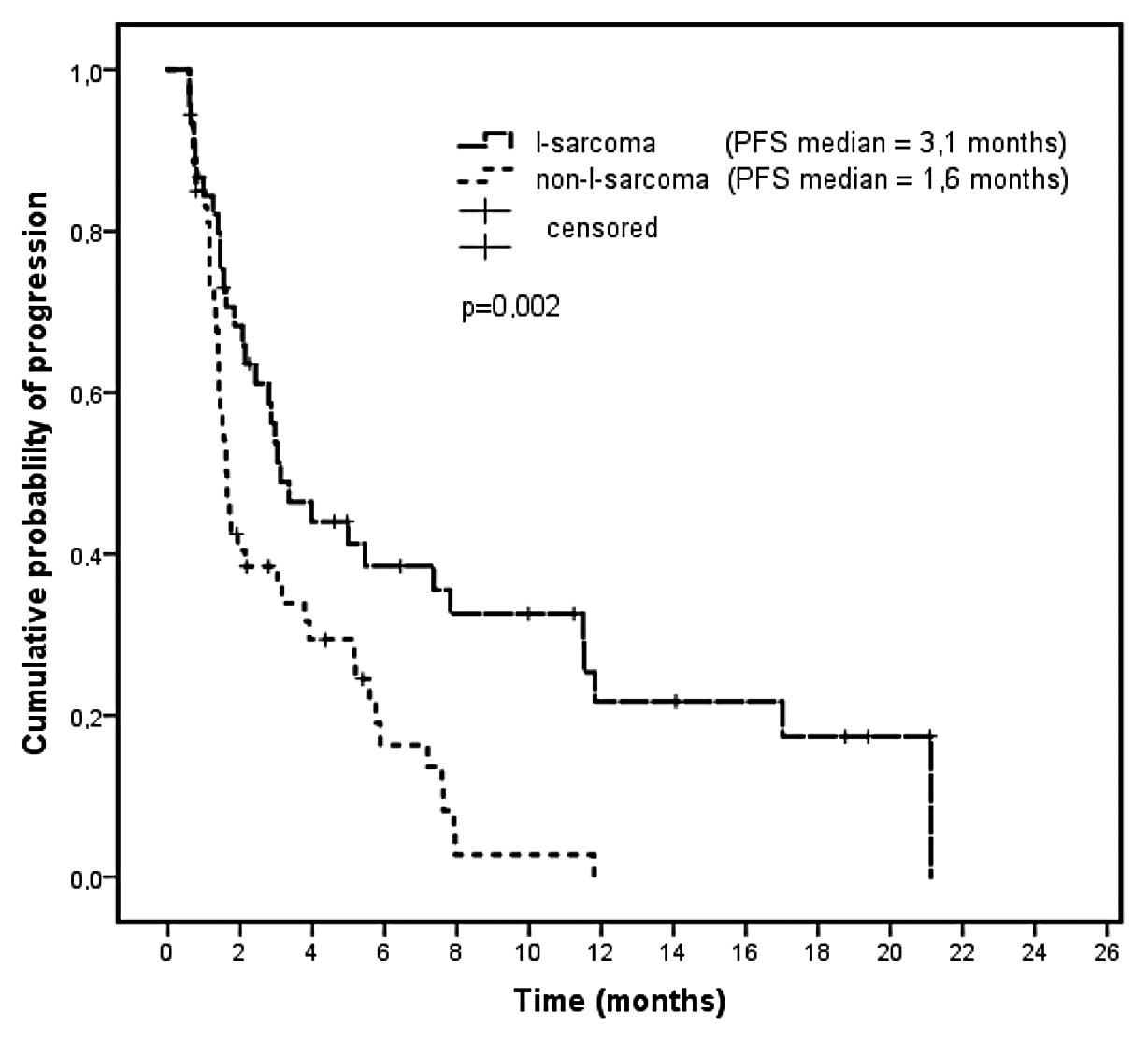
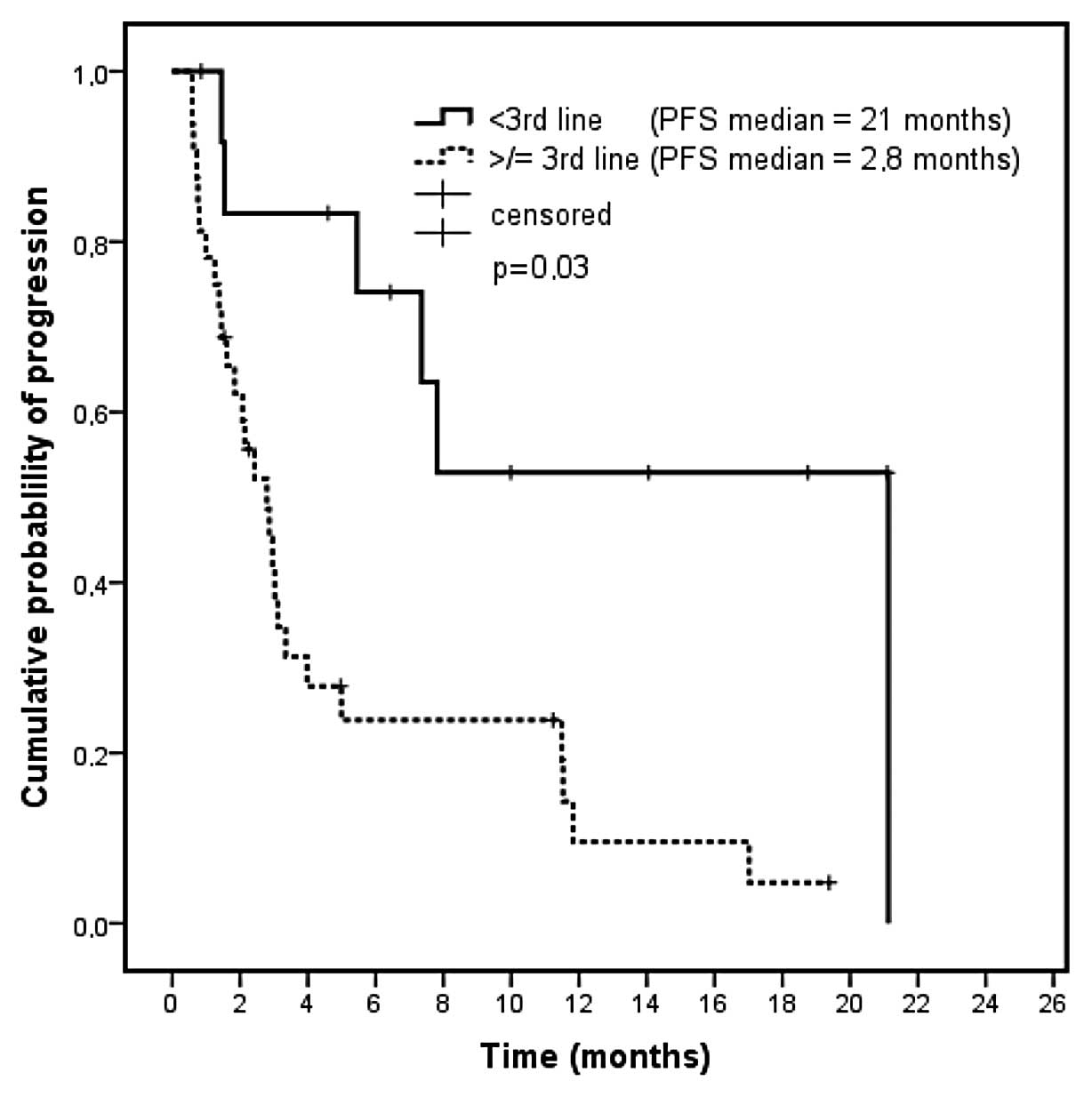
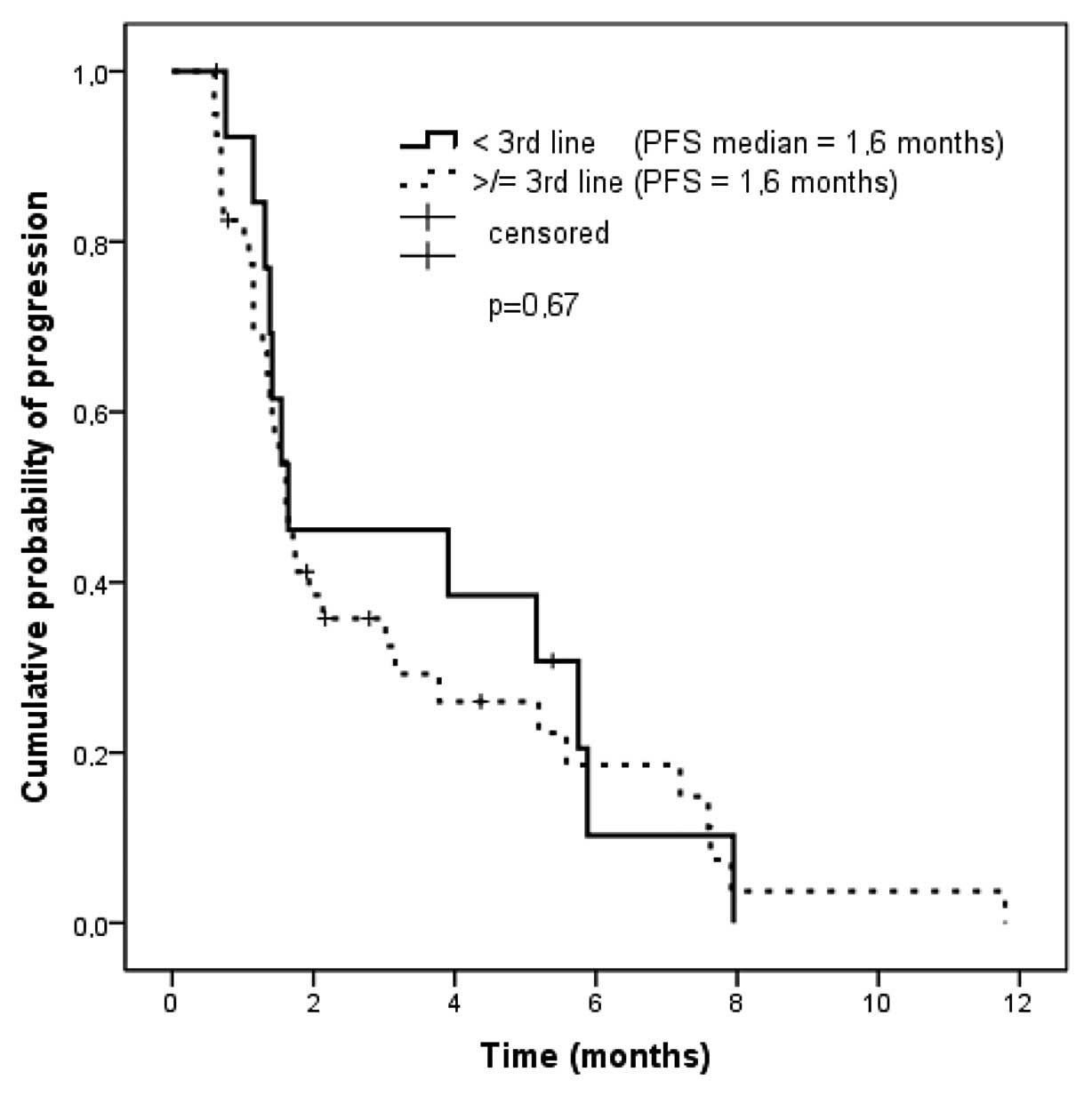
|
1.
|
Sleijfer S, Ouali M, van Glabbeke M, et
al: Prognostic and predictive factors for outcome to first-line
ifosfamide-containing chemotherapy for adult patients with advanced
soft tissue sarcomas: an exploratory, retrospective analysis on
large series from the European Organization for Research and
Treatment of Cancer-Soft Tissue and Bone Sarcoma Group
(EORTC-STBSG). Eur J Cancer. 46:72–83. 2010.
|
|
2.
|
Reichardt P: High-dose chemotherapy in
adult soft tissue sarcoma. Crit Rev Oncol Hematol. 41:157–167.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Antman K, Crowley J, Balcerzak SP, et al:
An intergroup phase III randomized study of doxorubicin and
dacarbazine with or without ifosfamide and mesna in advanced soft
tissue and bone sarcomas. J Clin Oncol. 11:1276–1285.
1993.PubMed/NCBI
|
|
4.
|
Zewail-Foote M and Hurley LH:
Ecteinascidin 743: a minor groove alkylator that bends DNA toward
the major groove. J Med Chem. 42:2493–2497. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Minuzzo M, Marchini S, Broggini M, et al:
Interference of transcriptional activation by the antineoplastic
drug ecteinascidin-743. Proc Natl Acad Sci USA. 97:6780–6784. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Takebayashi Y, Pourquier P, Zimonjic DB,
et al: Antiproliferative activity of ecteinascidin 743 is dependent
upon transcription-coupled nucleotide-excision repair. Nat Med.
7:961–966. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Damia G, Silvestri S, Carrassa L, et al: A
unique pattern of ET-743 activity in different cellular systems
with defined deficiencies in DNA-repair pathways. Int J Cancer.
92:583–588. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kanzaki A, Takebayashi Y, Ren XQ, et al:
Overcoming multidrug drug resistance in
P-glycoprotein/MDR1-overexpressing cell lines by ecteinascidin 743.
Mol Cancer Ther. 1:1327–1334. 2002.PubMed/NCBI
|
|
9.
|
Delaloge S, Yovine A, Taamma A, Riofrio M,
Brain E, Raymond E, Cottu P, Goldwasser F, Jimeno J, Misset JL,
Marty M and Cvitkovic E: Ecteinascidin-743: a marine-derived
compound in advanced, pretreated sarcoma patients - preliminary
evidence of activity. J Clin Oncol. 19:1248–1255. 2001.PubMed/NCBI
|
|
10.
|
Yovine A, Riofrio M, Blay JY, et al: Phase
II study of ecteinascidin-743 in advanced pretreated soft tissue
sarcoma patients. J Clin Oncol. 22:890–899. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Le Cesne A, Blay JY, Judson I, Van
Oosterom A, Verweij J, Radford J, Lorigan P, Rodenhuis S,
Ray-Coquard I, Bonvalot S, Collin F, Jimeno J, Di Paola E, Van
Glabbeke M and Nielsen OS: Phase II study of ET-743 in advanced
soft tissue sarcomas: a European Organisation for the Research and
Treatment of Cancer (EORTC) soft tissue and bone sarcoma group
trial. J Clin Oncol. 23:576–584. 2005.
|
|
12.
|
Garcia-Carbonero R, Supko JG, Manola J,
Seiden MV, Harmon D, Ryan DP, Quigley MT, Merriam P, Canniff J,
Goss G, Matulonis U, Maki RG, Lopez T, Puchalski TA, Sancho MA,
Gomez J, Guzman C, Jimeno J and Demetri GD: Phase II and
pharmacokinetic study of ecteinascidin 743 in patients with
progressive sarcomas of soft tissues refractory to chemotherapy. J
Clin Oncol. 22:1480–1490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Le Cesne A, Judson I, Crowther D, et al:
Randomized phase III study comparing conventional-dose doxorubicin
plus ifosfamide versus high-dose doxorubicin plus ifosfamide plus
recombinant human granulocyte-macrophage colony-stimulating factor
in advanced soft tissue sarcomas: a trial of the European
Organisation for Research and Treatment of Cancer/Soft Tissue and
Bone Sarcoma Group. J Clin Oncol. 18:2676–2684. 2002.
|
|
14.
|
Demetri GD, Chawla SP, von Mehren M, Ritch
P, Baker LH, Blay JY, Hande KR, Keohan ML, Samuels BL, Schuetze S,
Lebedinsky C, Elsayed YA, Izquierdo MA, Gómez J, Park YC and Le
Cesne A: Efficacy and safety of trabectedin in patients with
advanced or metastatic liposarcoma or leiomyosarcoma after failure
of prior anthracyclines and ifosfamide: results of a randomized
phase II study of two different schedules. J Clin Oncol.
27:4188–4196. 2009. View Article : Google Scholar
|
|
15.
|
Baruchel S, Pappo A, Krailo M, Baker KS,
Wu B, Villaluna D, Lee-Scott M, Adamson PC and Blaney SM: A phase 2
trial of trabectedin in children with recurrent rhabdomyosarcoma,
Ewing sarcoma and non-rhabdomyosarcoma soft tissue sarcomas: a
report from the Children’s Oncology Group. Eur J Cancer.
48:579–585. 2012.PubMed/NCBI
|
|
16.
|
Grosso F, Jones RL, Demetri GD, Judson IR,
Blay JY, et al: Efficacy of trabectedin (ecteinascidin-743) in
advanced pretreated myxoid liposarcomas: a retrospective study.
Lancet Oncol. 8:595–602. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Fayette J, Boyle H, Chabaud S, et al:
Efficacy of trabectedin for advanced sarcomas in clinical trials
versus compassionate use programs: analysis of 92 patients treated
in a single institution. Anticancer Drugs. 21:113–119. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
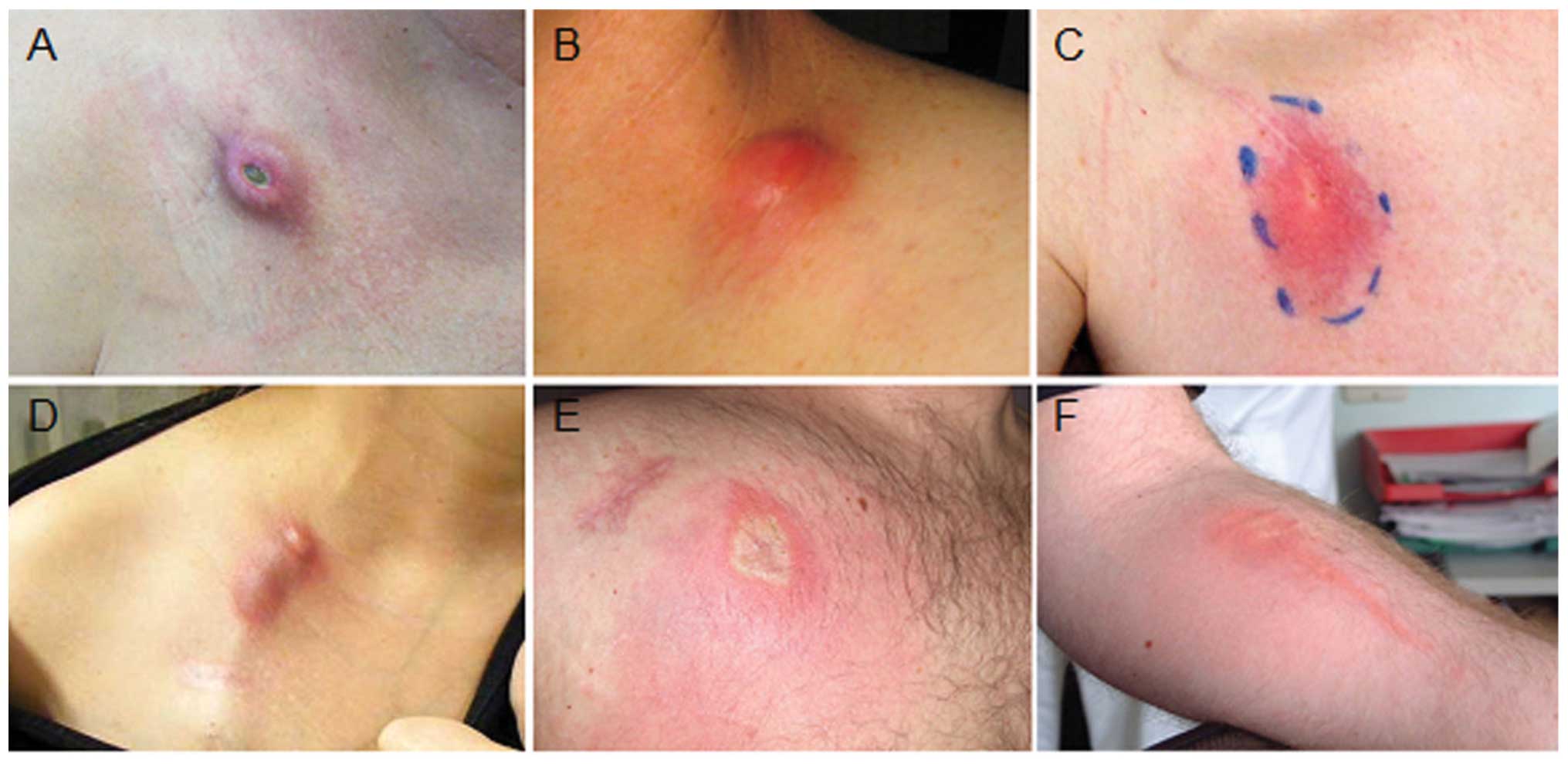
Theman TA, Hartzell TL, Sinha I, et al:
Recognition of a new chemotherapeutic vesicant: trabectedin
(Ecteinascidin-743) extravasation with skin and soft tissue damage.
J Clin Oncol. 27:198–200. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
van Glabbeke M, Verweij J, Judson I, et
al: EORTC Soft Tissue and Bone Sarcoma Group. Progression-free rate
as the principal end-point for phase II trials in soft-tissue
sarcomas. Eur J Cancer. 38:543–549. 2002.PubMed/NCBI
|