Introduction
Oral cancer is one of the common cancers and is the
fourth leading cause of cancer death in Taiwan male population but
the 16th in females (1–3). Based on the 2008 report from the
Department of Health, R.O.C. (Taiwan) indicated that 7.9
individuals per 100,000 die annually from oral cancer (1). It has been demonstrated that about
95% of these oral cavity cancers are squamous cell carcinomas and
human oral cancer is largely associated with the risk factors of
chronic smoking or alcohol consumption (4–6).
Furthermore, the susceptibility of an individual to oral cancer is
associated with the individual genetic factors and
carcinogen-exposure behavior (7–9). In
Taiwan, it was suggested that smoking and betel nut chewing are the
two most important risk factors (1,10).
Based on the above report the government indicated that
approximately 85% of the oral cancer patients are regular users of
betel quid (areca nut). The current treatments for oral cancer are
inadequate and a significant proportion of oral cancer patients may
develop local invasion and metastases (11). Numerous studies have been performed
to find novel agents (especial from natural plant) which can
trigger programmed cell death (apoptosis) in tumor cells and
hopefully to provide a new therapeutic approach for anticancer
design (12–14).
It is well documented that numerous natural
compounds present anticancer effects and some of them play
important roles in cancer treatment clinically. Tetrandrine
(C38H42O8N2), a
bisbenzylisoquinoline alkaloid, was isolated from the root of
Stephania tetrandra S. Moore, has been used as an
anti-inflammatory, antipyretic and analgesic herb in Chinese
medicine (15–18), furthermore, in myocyte and vascular
smooth muscle cells, tetrandrine inhibits the
ICa-L and reduces Ca2+ flows into the
cytosol (19,20). Tetrandrine has been found to induce
cytotoxic effect such as the inhibiting proliferation and inducing
apoptosis of various cancer cells such as breast cancer, lung
cancer, neuroblastoma, Burkitt’s lymphoma, hepatoma, glioma,
leukemia and colon cancer (21–25).
Tetrandrine induced G0/G1 phase arrest in Neuro 2a mouse
neuroblastoma cells (26) and
human Hep G2 cells (27).
Tetrandrine have been demonstrated to inhibit lipid peroxidation
and platelet aggregation and reduces ischemia/reperfusion injury
(28–30) and tetrandrine suppressed T and B
cells and inhibited the production of cytokines (29). It was reported that tetrandrine is
cytotoxic to RT-2 glioma cells and it has antitumor effects on
subcutaneous and intra-cerebral gliomas, and inhibits angiogenesis
in subcutaneous gliomas (31–34).
Tetradine induced apoptosis by activating reactive oxygen species
and repressing Akt activity in human hepatocellular carcinoma HCC
cells (35,36). Recently, it was reported that
tetrandrine induced apoptosis and triggers caspase cascade in human
bladder cancer cells (37).
There is no previous report on studies investigating
the effects of tetrandrine on the cytotoxic on human oral cancer
cells. In the present study, we investigated the antitumor effect
of tetrandrine against human oral squamous carcinoma cells and the
mechanism by which the association occurs. Using human oral cancer
SAS cells, we demonstrated that tetrandrine exhibits antitumor
activity on the cells by inhibiting the activity of the caspase
pathway and inducing autophagy and apoptosis. Our study suggests
that tetrandrine may be a potential agent to combat oral cancer
cells.
Materials and methods
Cell culture and chemicals
Tetrandrine, dimethyl sulfoxide (DMSO), propidium
iodide (PI), potassium phosphates, ribonuclease-A, Triton X-100,
Tris-HCl and trypan blue were obtained from Sigma Chemical Co. (St.
Louis, MO, USA). Dulbecco’s Modified Eagle’s medium (DMEM),
L-glutamine, fetal bovine serum (FBS), penicillin, streptomycin and
trypsin-EDTA were obtained from Gibco-BRL/Invitrogen Corp (Grand
Island, NY, USA). The SAS cell line (human oral squamous cell
carcinoma) was obtained from Dr Pei-Jung Lu (Graduate Institute of
Clinical Medicine, National Cheng Kung University, Tainan, Taiwan).
Cells were cultured in DMEM containing 10% FBS, 2 mM L-glutamine,
100 U/ml penicillin and 100 μg/ml streptomycin in 75
cm2 tissue culture flasks at 37°C under a humidified 5%
CO2 and 95% air atmosphere.
Assessment of cell morphology and
viability
Tetrandrine was prepared and dissolved in DMSO. SAS
cells (2.5×105) were plated in 24-well plates in DMEM
and incubated at 37°C for 24 h. Cells were then treated with 0, 1,
5, 10, 15, 20, 25, 30, 35 and 40 μM tetrandrine for 24 and
48 h. DMSO was used as a vehicle control. For inhibitor treatment,
cells were pre-treated with autophagic inhibitor (bafilomycin A1,
3-MA, chloroquine), caspase inhibitor (pan-caspase, caspase-3,
caspase-8 and caspase-9 inhibitor) or NAC (Sigma Chemical Co) and
then treated with 0, 5, 10, 15, 20, 25, 30, 35 and 40 μM
tetrandrine for 24 and 48 h. At the end of the incubation period,
cells were photographed with a phase-contrast microscope. They were
then harvested, stained with PI (5 μg/ml) and analyzed by
flow cytometry (Becton-Dickinson, San Jose, CA, USA) (38–40).
DAPI staining
SAS cells were treated with or without 0, 1, 10, 20,
25 and 30 μM tetrandrine for 24 h. They were then isolated,
stained with DAPI and photographed using a fluorescence microscope
(38–40).
Annexin V and PI staining assay
SAS cells were treated with or without 0 and 25
μM tetrandrine for 24 h then were re-suspended in Annexin
V-FITC (Becton-Dickinson, San Jose, CA, USA) alone or in
combination with 10 ml of PI (50 mg/ml) and were incubated at room
temperature for 15 min. The staining was analysed by flow cytometry
(38–40).
Electron microscopy
SAS cells were treated with or without (0 and 25
μM) tetrandrine for 24 h then were fixed with a solution
containing 2.5% glutaraldehyde and 2% paraformaldehyde (in 0.1 M
cacodylate buffer, pH 7.3) for 1 h. After fixation, the samples
were postfixed for 30 min in the same buffer containing 1%
OsO4. Ultra-thin sections were observed under a
transmission electron microscope (JEM-1200EX, JEOL Ltd., Tokyo,
Japan) at 100 kV (41).
Acridine orange staining
SAS cells were treated with 25 μM tetrandrine
for 0, 2, 4 and 6 h and then cells were harvested and stained with
1 mg/ml acridine orange for a period of 20 min and analysed by a
fluorescence microscope (41).
Monodansyl cadaverine MDC staining
SAS cells were treated with 0, 10, 20 and 30
μM tetrandrine for 6 h and then cells were harvested and
stained with 1 mg/ml MDC for a period of 20 min and analysed by a
fluorescence microscope (42).
GFP-LC3 expression
SAS cells were transfected with pEGFP-LC3 for 16 h
and then were treated with 0, 10, 20 and 30 μM tetrandrine
for 6 h. The cells were harvested, fixed with 4% paraformaldehyde
for 10 min at room temperature. Cells were stained with LC-3-FITC
for a period of 20 min and analysed by a fluorescence microscope
(41).
Western blot analysis
SAS cells were pre-treated with Atg-5 or beclin-1
siRNA bafilomycin A1 then treated with 0, 10, 15, 20, 25 and 30
μM tetrandrine and then harvested by scraping into RIPA
buffer and sonicated for 15 min with 30 sec pulses. The protein
concentration was measured using the BCA assay (Thermo Scientific)
following the instructions of the manufacturer. Incubation with
primary antibodies was done overnight at 4°C. Immunoreactive
proteins were visualised with the ECL chemiluminescent detection
system (Perkin Elmer Life Sciences, MA, USA) and BioMax LightFilm
(Eastman Kodak, New Heaven, CT, USA) according to the
manufacturer’s instructions (43).
Statistical analysis
Data of control and experimental groups were
expressed as mean ± standard deviation for at least three separate
experiments. Statistical analyses of the data were performed using
Student’s t-test and one-way analysis of variance (ANOVA).
Statistical significance was set at P<0.05 (43).
Results
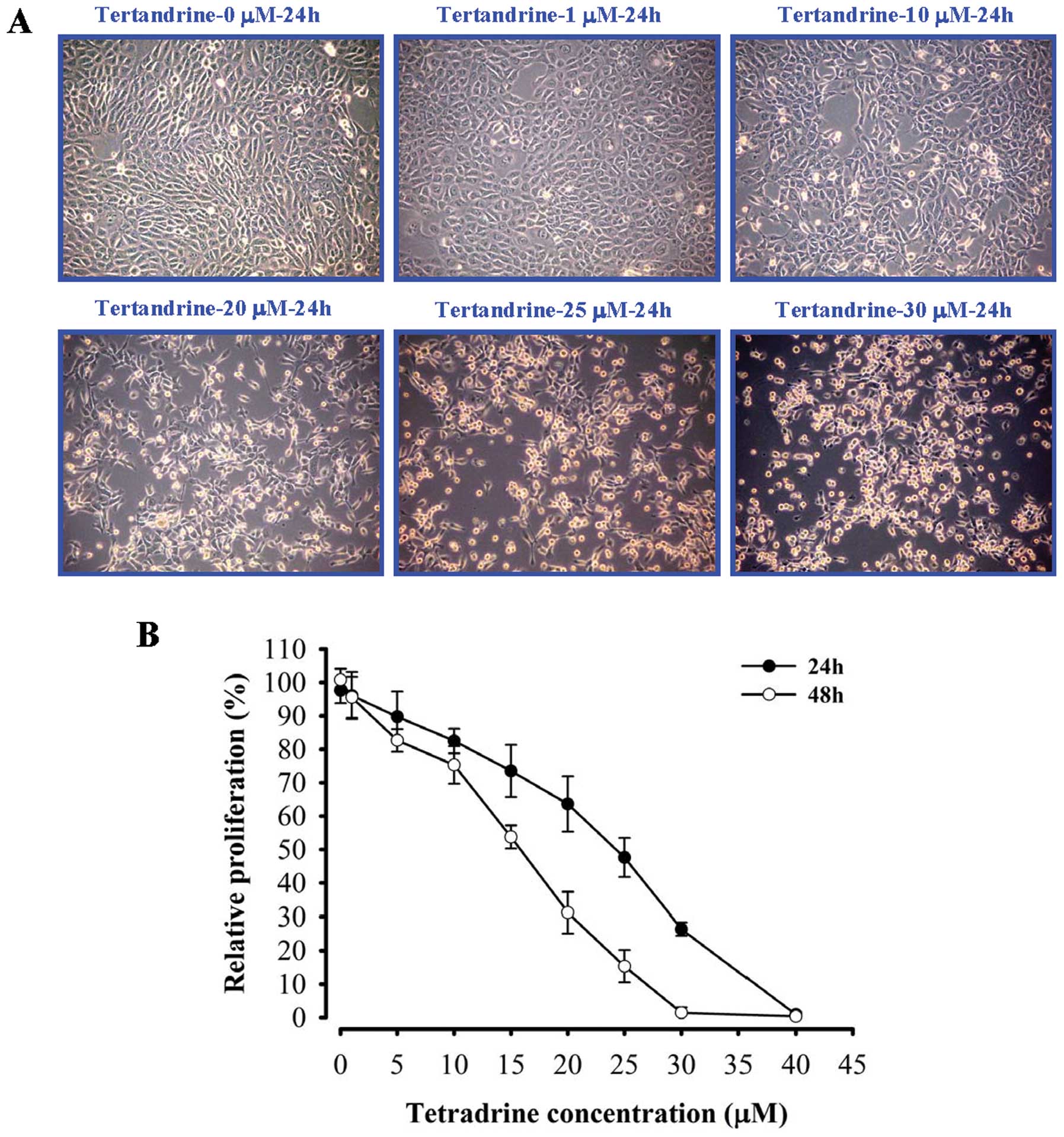
Effects of tetrandrine on cell morphology
and viability in SAS cells
Cells were morphologically altered by tetrandrine
treatment as shown in Fig. 1A. We
tested the cell viability of tetrandrine in SAS cell lines. SAS
cells were treated with 0, 5, 10, 15, 20, 25, 30, 35 and 40
μM tetrandrine for 24 and 48 h. Tetrandrine significantly
decreased the growth of cells in a concentration- and
time-dependent manner (Fig.
1B).
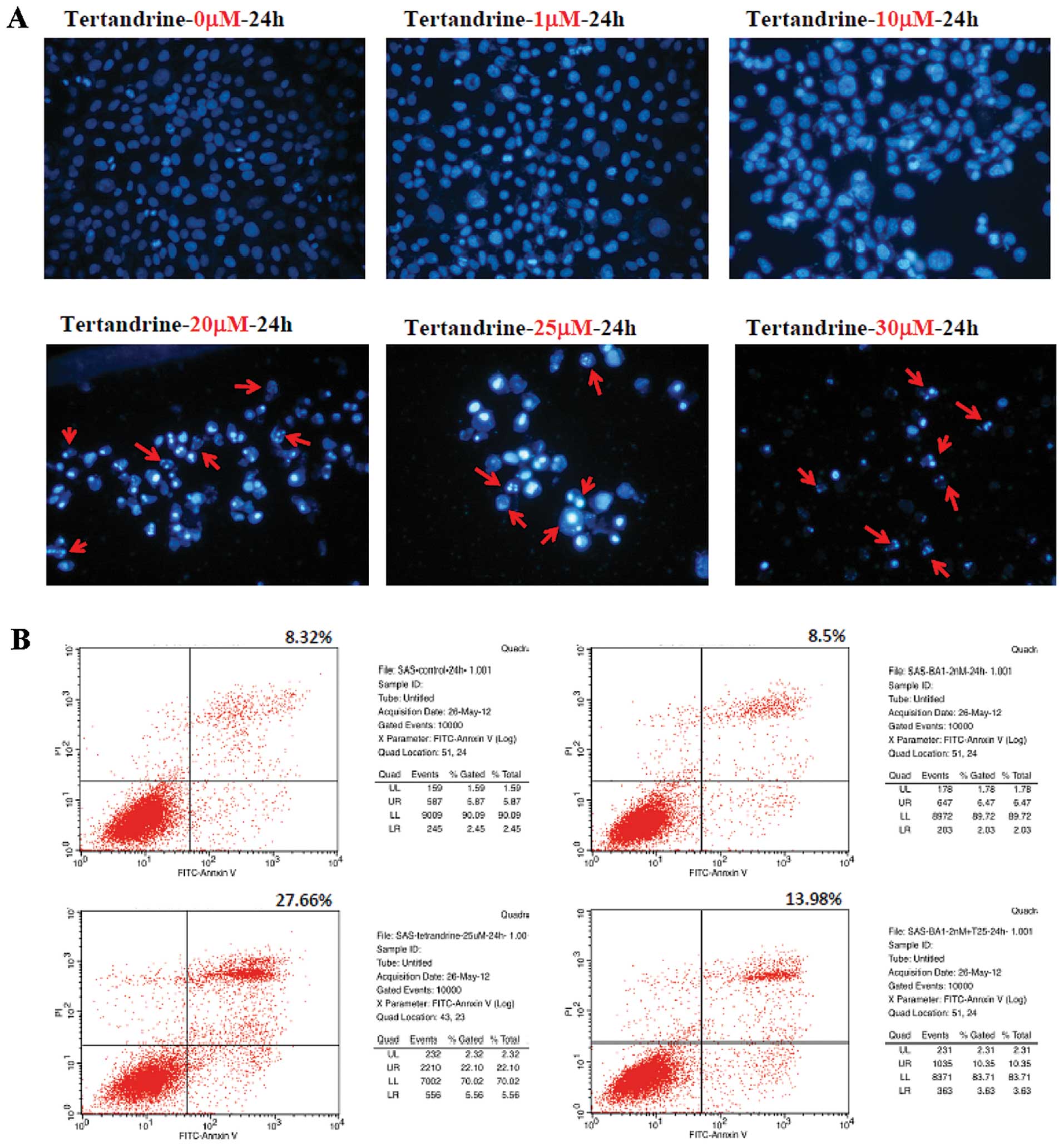
Tetrandrine-induced apoptosis in SAS
cells
Induction of apoptosis by tetrandrine in SAS cells
was confirmed by DAPI staining, as seen in Fig. 2B, which showed that tetrandrine
induced nuclei condensation. These effects were dose-dependent as
noted in Fig. 2B. Higher
concentrations of tetrandrine resulted in a greater number of
apoptotic cells being stained. We analysed surface exposure of
phosphatidylserine, an early event in apoptosis by Annexin V/PI
staining. The percentage of cells staining positive for Annexin V
was slightly increased in SAS cells types after 24 h of tetrandrine
treatment but was much lower following bafilomycin A1 of
pre-treatment.
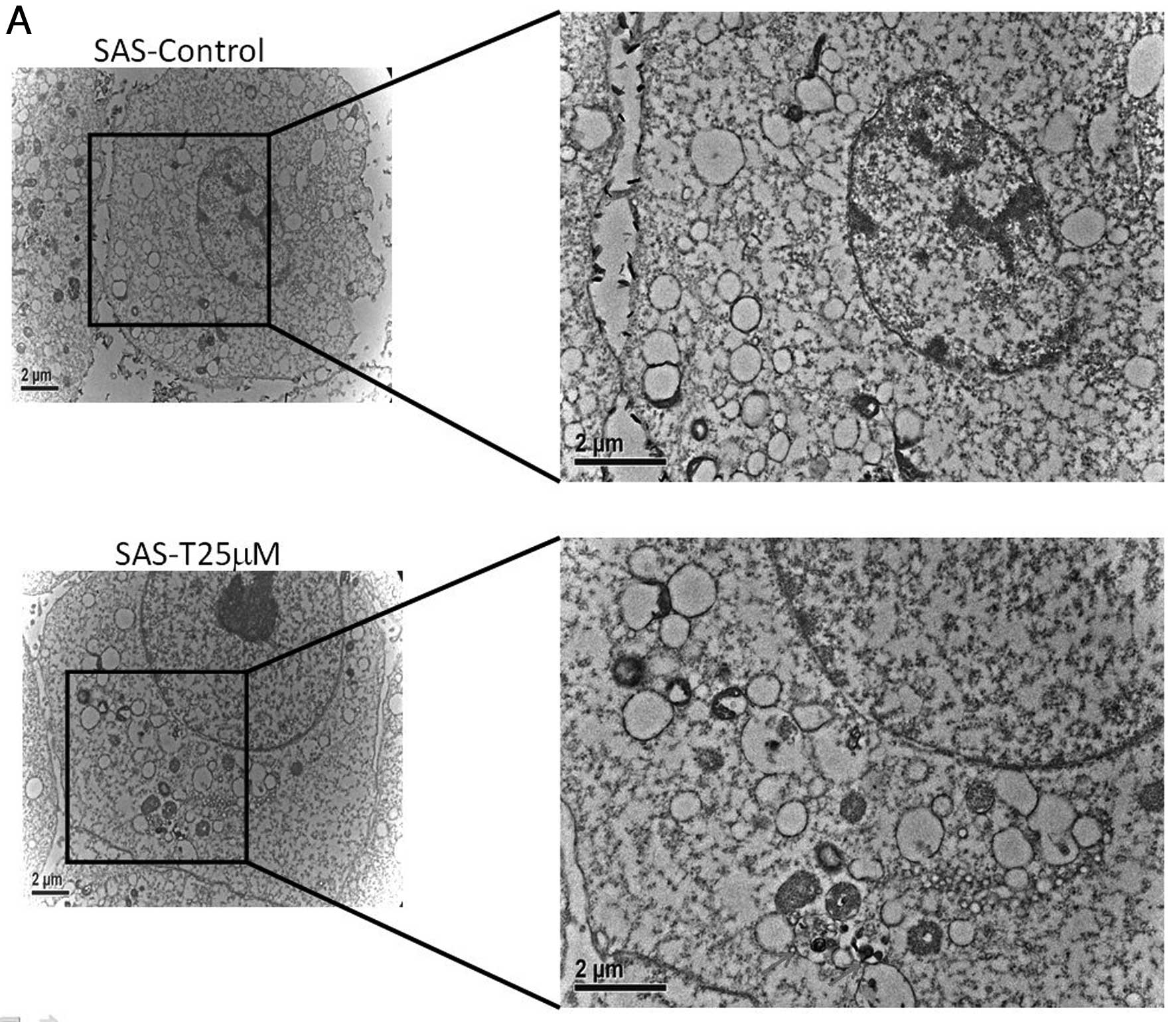
Tetrandrine induced autophagy in SAS
cells
Autophagic vacuoles containing cellular membranous
structures were increased in SAS-treated with tetrandrine for 24 h
compared with untreated cells, as determined by electron microscopy
(Fig. 3A). We used Acridine orange
staining to AVOs, including autophagic vacuoles and lysosomes.
Cells with AVOs had enhanced red fluorescence that was detected by
fluorescence microscopy (Fig. 3B).
Tetrandrine caused MDC induction in SAS cells in a
concentration-dependent manner (Fig.
3B). Tetrandrine also caused LC-3 expression in SAS cells in a
time-dependent manner.
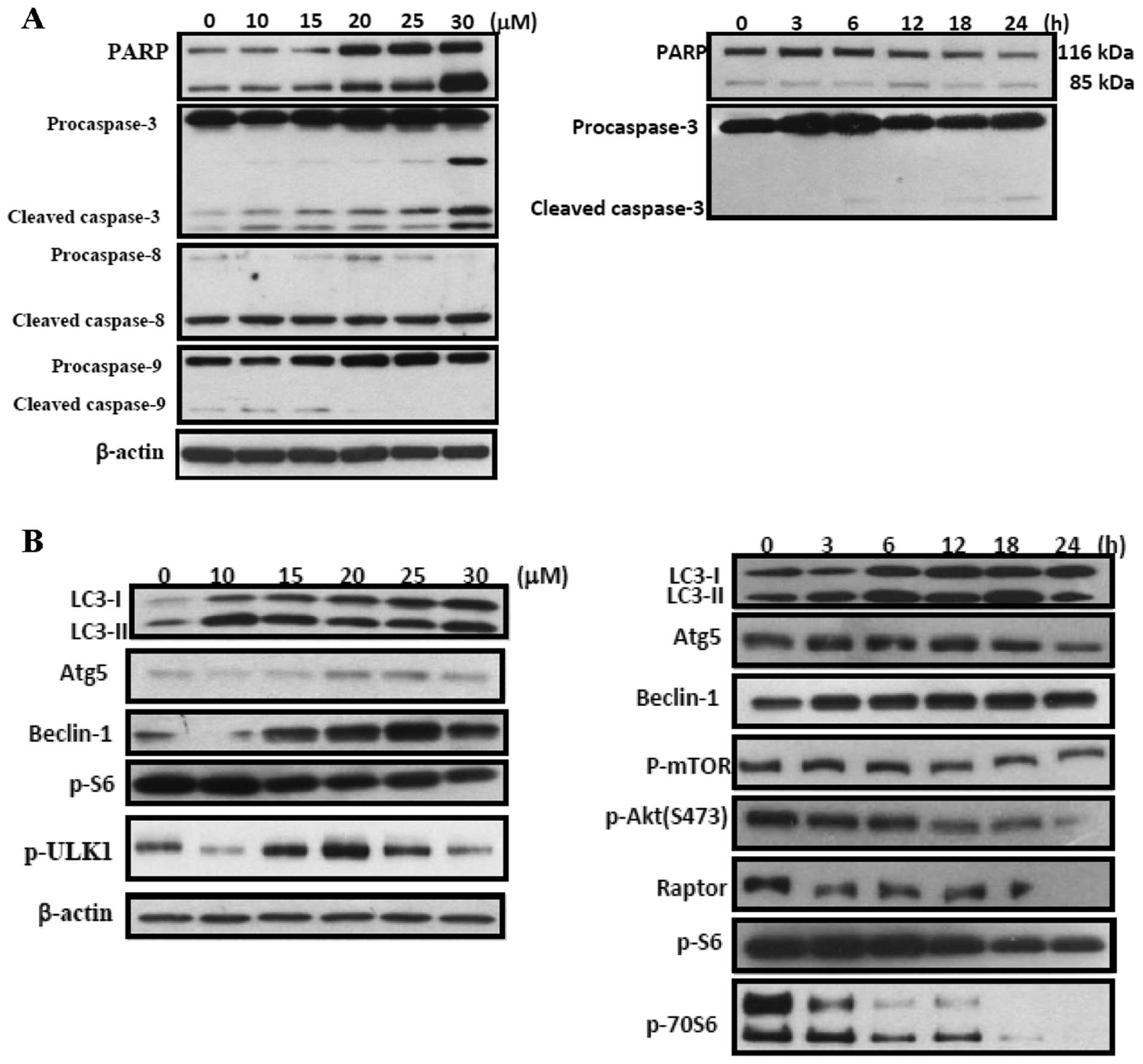
Effects of tetrandrine on levels of
proteins associated with apoptosis and autophagy
Results are presented in Fig. 4A, tetrandrine treatment induced the
levels of cleaved-caspase-3 in a concentration- and time-dependent
manner. Tetrandrine treatment induced the levels of LC-3 II, Atg-5,
beclin-1, p-S6, p-ULK, p-mTOR, p-Akt (S473), and raptor in a
concentration- and time-dependent manner (Fig. 4B).
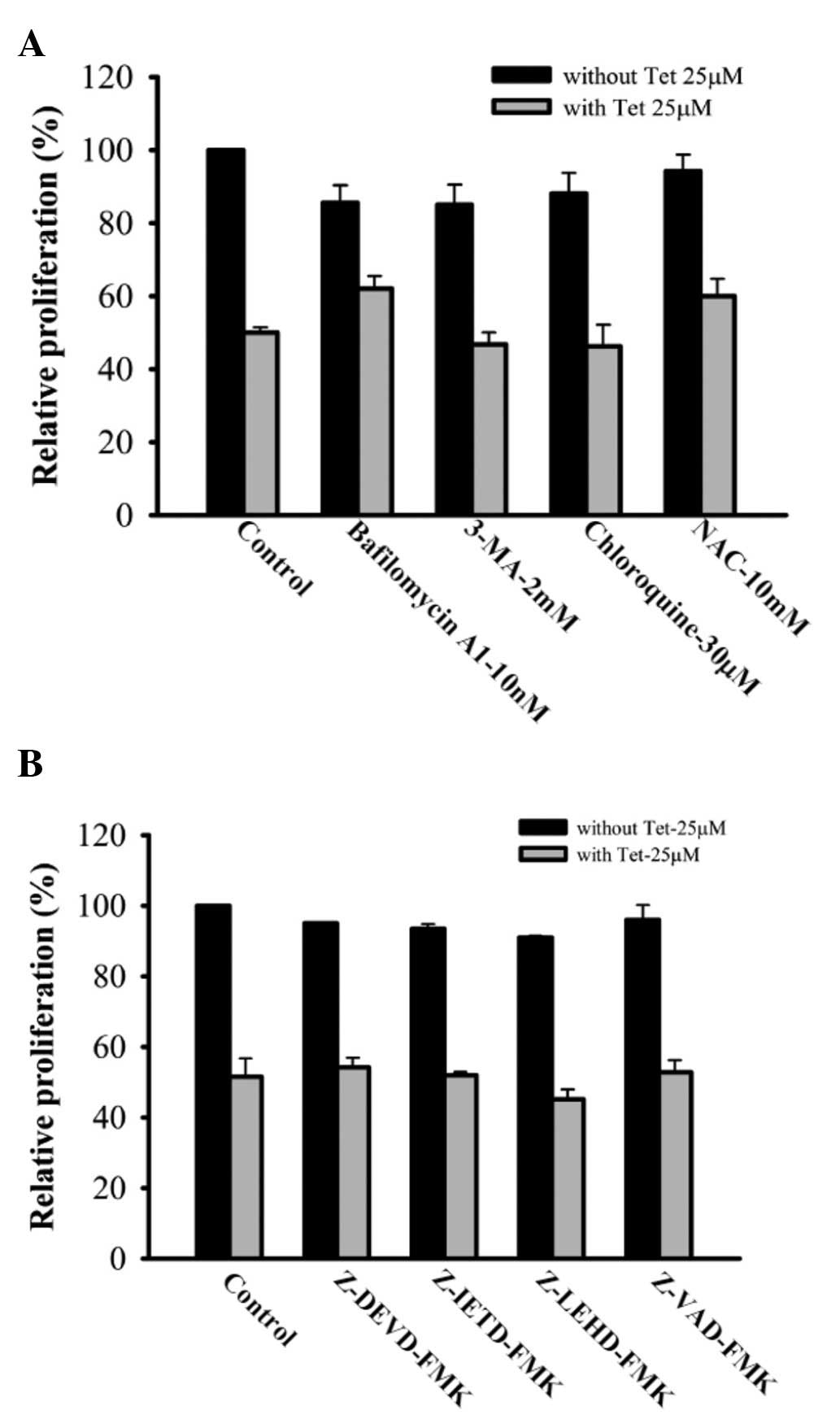
Autophagy inhibitors decreased
tetrandrine-induced apoptosis in SAS cells
Results are presented in Fig. 5A, tetrandrine decreased the cell
viability, but bafilomycin A1, 3-MA, chlorobafilomycin A1, 3-MA,
chloro-A1, 3-MA, chloroquine and NAC protected tetrandrine-treated
SAS cells against the decrease of cell viability. On the other
hand, pan-caspase, caspase-3, caspase-8 and caspase-9 inhibitor
also protected tetrandrine-treated SAS cells against the decrease
of cell viability (Fig. 5B).
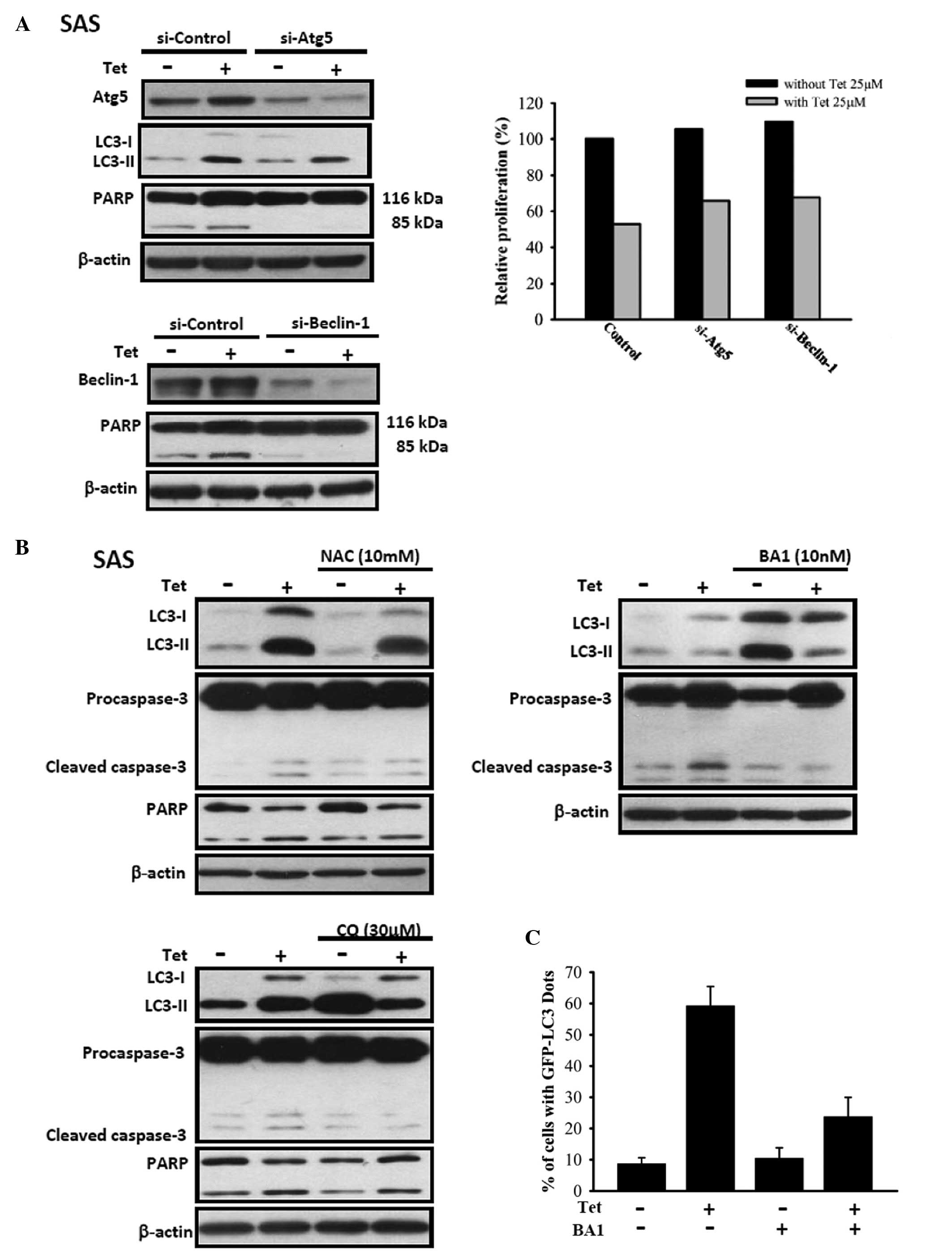
Atg-5, beclin-1 siRNA, bafilomycin A1 and
NAC decrease tetrandrine-induced cleaved caspase-3 and cleaved PARP
in SAS cells
Results are presented in Fig. 6A, tetrandrine treatment induced the
levels of cleaved caspase-3 and cleaved PARP, however, Atg-5,
beclin-1 siRNA decreased tetrandrine-induced cleaved caspase-3 and
cleaved PARP in SAS cells and protected tetrandrine-treated SAS
cells against decrease of cell viability. In Fig. 6B, chloroquine, NAC and bafilomycin
A1 also decreased tetrandrine-induced cleaved caspase-3 and cleaved
PARP in SAS cells. In Fig. 6C,
bafilomycin A1 decreased tetrandrine-induced LC3 expression in SAS
cells.
Discussion
Oral cancer is most frequent in men between 55 and
65 and in women between 50 and 75, and in men this disease is the
fourth most common cause of cancer death (44,45).
The treatment for oral cancer is still unsatisfactory. Thus, it is
necessary to identify and develop new anticancer agents that will
selectively target the tumor. It was reported that the induction of
apoptosis through the caspase cascade triggered by tetrandrine has
inhibitory effects to various tumor cells (20,21,27,37).
Many studies have demonstrated that tetrandrine has pharmacologic
potential in cancer therapy but to date the effects of tetrandrine
on human oral cancer is little known and have not been identified
and reported in oral cancer cells. In the present study, we
demonstrated the anticancer effect of tetrandrine on oral SAS
cancer and elucidate the underlying mechanisms of action.
Tetrandrine treatment showed growth inhibitory effect and cell
apoptosis and autophagy induction on oral cancer SAS cells, which
was associated with caspase activation (Fig. 4) and autophagic vesicle formation
(Fig. 3). To the best of our
knowledge, this is the first report of the effects of tetrandrine
on human oral cancer.
The current studies indicate that SAS cells are
sensitive to the cytotoxic actions of the tetrandrine and the
sensitivity was largely similar to that observed previously in the
liver and bladder tumor cell lines (27,31,35,37).
Based on the observation and results of Annexin V and
electro-microscope examination indicated that tetrandrine induced
cell death may be through the formation of autophagy and the
induction of apoptosis in SAS cells (Fig. 2). Autophagy from several
complementary assays provided evidence for the formation of
autophagic vesicles in SAS cells and LC3-I and II expression which
confirmed the occurrence of autophagic flux. This is in agreement
with a report on human hepatocellular carcinoma (HCC) cells after
exposure to tetrandrine inducing autophagy (17). Tetrandrine also promoted apoptosis
in SAS cells, although at low levels, however, both may lead to
cell death. In contrast, tetrandrine appears to preferentially
promote autophagy in SAS cells.
Based on other reports autophagy is quite
controversial, for example, substantial evidence shows autophagy
may occur through cytoprotective or through cytotoxic functions in
different cancer cells (46,47).
Herein, our results confirm that autophagy mediates the
cell-killing effects of tetrandrine on SAS cells. The main reason
is that the observations of autophagy from tetrandrine treated SAS
cells was blocked by using preatment of 3-MA, NAC, chloroquine or
bafilomycin that demonstrated only a modest degree of protection
from the tetrandrine (Fig. 5). It
may be due to autophagy being blocked thus leading to the
tetrandrine-treated cells to cause apoptosis. These reasons are
also seen from other studies (48,49).
These observations indicate that SAS cells exposed to tetrandrine
may be lethal to the tumor cells and that the cells may die by an
alternative pathway if the primary pathway is blocked. Thus,
further investigation is needed by in vivo experiments to
investigate whether or not tetrandrine can be used as a potential
anti-oral cancer agent. Other reports have demonstrated that if an
agent is capable of promoting autophagy it is likely to have
clinical utility (50–54).
We demonstrated that tetrandrine induced apoptosis
in SAS cells based on the results from flow cytometric assay
(Fig. 2) and this may involve the
activations of caspase-8, -9 and -3 (Fig. 4). Therefore, the possibility of
mitophagy exists, at the low dose of tetrandrine and then selective
removal of damaged mitochondria. However, other studies
demonstrated that tetrandrine induced the release of cytochrome
c but did not cause the activation of the caspases or
apoptosis in human hepatocellular carcinoma cells (27). It was reported that during
mitophagy (55), cell did not show
caspase activation in apoptotic cell death. Our results show that
tetrandrine promoted the expressions of LC3-I and -II (Fig. 4) and it is well documented that
LC3-I and -II are involved in the development of autophagy and both
proteins play an important role during autophagy (56–58).
Furthermore, other proteins such as Atg-5, Atg-7 and beclin-1 are
also involved in the development, especial Atg-5 is essential for
autophagy (59–62). In this study, we found that protein
levels of Atg-5 and beclin-1 (Fig.
4) were increased after tetrandrine treatment in SAS cells. As
shown in Fig. 6,
tetrandrine-induced autophagy in SAS cells is dependent on the
upregulation of Atg-5 and beclin-1. However, tetrandrine did not
affect the levels of Atg-7 in SAS cells, but other reports show
that tetrandrine promoted the expression of Atg-5 and Atg-7 in HCC
cells (17). The detailed
mechanism of Atg-5 and Atg-7 in tetrandrine treated SAS cells will
also require further investigation as other factors may be
involved. We also used siRNA to knock down Atg-5, beclin-1, and
LC3-1 and -II. The results show affect on Atg-5 and beclin-1 but
not on LC3-I and LC3-II in SAS cells. After the knockdown of Atg-5
and beclin-1 by siRNA the cell proliferation was decreased;
however, no significant reduction was observed in LC3-1 and LC3-II
siRNA treatment (Fig. 6).
Taken together, we found that tetrandrine may be a
promising chemotherapeutic agent with a variety of anticancer
effects such as induction of autophagy and apoptosis in human oral
cancer SAS cells in vitro. Tetrandrine treatment induced
cancer cells to undergo apoptosis and autophagy. The upregulation
of Atg-5, and beclin-1 was essential to the induction of
tetrandrine-induced autophagy in SAS cells (Fig. 4). These findings suggest that
tetrandrine may be a potential clinical candidate for the treatment
of SAS.
References
|
1.
|
Tseng CH: Oral cancer in Taiwan: is
diabetes a risk factor? Clin Oral Investig. Aug 16–2012.(Epub ahead
of print).
|
|
2.
|
Bau DT, Tseng HC, Wang CH, et al: Oral
cancer and genetic polymorphism of DNA double strand break gene
Ku70 in Taiwan. Oral Oncol. 44:1047–1051. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Chie WC, Li CY, Huang CS, Chang KJ, Yen ML
and Lin RS: Oral contraceptives and breast cancer risk in Taiwan, a
country of low incidence of breast cancer and low use of oral
contraceptives. Int J Cancer. 77:219–223. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Zavras AI, Douglass CW, Joshipura K, et
al: Smoking and alcohol in the etiology of oral cancer:
gender-specific risk profiles in the south of Greece. Oral Oncol.
37:28–35. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Moreno-Lopez LA, Esparza-Gomez GC,
Gonzalez-Navarro A, Cerero-Lapiedra R, Gonzalez-Hernandez MJ and
Dominguez-Rojas V: Risk of oral cancer associated with tobacco
smoking, alcohol consumption and oral hygiene: a case-control study
in Madrid, Spain. Oral Oncol. 36:170–174. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Kerawala CJ: Oral cancer, smoking and
alcohol: the patients’ perspective. Br J Oral Maxillofac Surg.
37:374–376. 1999.
|
|
7.
|
Shukla D, Dinesh Kale A, Hallikerimath S,
Vivekanandhan S and Venkatakanthaiah Y: Genetic polymorphism of
drug metabolizing enzymes (GSTM1 and CYP1A1) as risk factors for
oral premalignant lesions and oral cancer. Biomed Pap Med Fac Univ
Palacky Olomouc Czech Repub. 156:253–259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Plu-Bureau G, Bossard N and Thalabard JC:
Oral contraception and genetic factors in breast cancer:
characteristics and limits of case-only studies. Rev Epidemiol
Sante Publique. 48:294–303. 2000.(In French).
|
|
9.
|
Hung HC, Chuang J, Chien YC, et al:
Genetic polymorphisms of CYP2E1, GSTM1, and GSTT1; environmental
factors and risk of oral cancer. Cancer Epidemiol Biomarkers Prev.
6:901–905. 1997.PubMed/NCBI
|
|
10.
|
Yang YY, Koh LW, Tsai JH, et al:
Involvement of viral and chemical factors with oral cancer in
Taiwan. Jpn J Clin Oncol. 34:176–183. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Jung J, Cho NH, Kim J, et al: Significant
invasion depth of early oral tongue cancer originated from the
lateral border to predict regional metastases and prognosis. Int J
Oral Maxillofac Surg. 38:653–660. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Hour MJ, Lee KT, Wu YC, et al: A novel
antitubulin agent, DPQZ, induces cell apoptosis in human oral
cancer cells through Ras/Raf inhibition and MAP kinases activation.
Arch Toxicol. Dec 5–2012.(Epub ahead of print).
|
|
13.
|
Shin JA, Kim JS, Hong IS and Cho SD: Bak
is a key molecule in apoptosis induced by methanol extracts of
Codonopsis lanceolata and Tricholoma matsutake in
HSC-2 human oral cancer cells. Oncol Lett. 4:1379–1383.
2012.PubMed/NCBI
|
|
14.
|
Tsai SC, Lu CC, Lee CY, et al: AKT
serine/threonine protein kinase modulates bufalin-triggered
intrinsic pathway of apoptosis in CAL 27 human oral cancer cells.
Int J Oncol. 41:1683–1692. 2012.PubMed/NCBI
|
|
15.
|
Xu XH, Gan YC, Xu GB, et al: Tetrandrine
citrate eliminates imatinib-resistant chronic myeloid leukemia
cells in vitro and in vivo by inhibiting Bcr-Abl/β-catenin axis. J
Zhejiang Univ Sci B. 13:867–874. 2012.PubMed/NCBI
|
|
16.
|
Gao S, Cui YL, Yu CQ, Wang QS and Zhang Y:
Tetrandrine exerts antidepressant-like effects in animal models:
role of brain-derived neurotrophic factor. Behav Brain Res.
238:79–85. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Gong K, Chen C, Zhan Y, Chen Y, Huang Z
and Li W: Autophagy-related gene 7 (ATG7) and reactive oxygen
species/extracellular signal-regulated kinase regulate
tetrandrine-induced autophagy in human hepatocellular carcinoma. J
Biol Chem. 287:35576–35588. 2012. View Article : Google Scholar
|
|
18.
|
Shan QQ, Gong YP, Guo Y, Lin J, Zhou RQ
and Yang X: Anti-tumor effect of tanshinone II A, tetrandrine,
honokiol, curcumin, oridonin and paeonol on leukemia cell lines.
Sichuan Da Xue Xue Bao Yi Xue Ban. 43:362–366. 2012.(In
Chinese).
|
|
19.
|
Wang G, Lemos JR and Iadecola C: Herbal
alkaloid tetrandrine: fron an ion channel blocker to inhibitor of
tumor proliferation. Trends Pharmacol Sci. 25:120–123. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Rao MR: Effects of tetrandrine on cardiac
and vascular remodeling. Acta Pharmacol Sin. 23:1075–1085.
2002.PubMed/NCBI
|
|
21.
|
Li X, Lu X, Xu H, et al:
Paclitaxel/tetrandrine coloaded nanoparticles effectively promote
the apoptosis of gastric cancer cells based on ‘oxidation therapy’.
Mol Pharm. 9:222–229. 2012.PubMed/NCBI
|
|
22.
|
Wu JM, Chen Y, Chen JC, Lin TY and Tseng
SH: Tetrandrine induces apoptosis and growth suppression of colon
cancer cells in mice. Cancer Lett. 287:187–195. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Liu B, Wang T, Qian X, Liu G, Yu L and
Ding Y: Anticancer effect of tetrandrine on primary cancer cells
isolated from ascites and pleural fluids. Cancer Lett. 268:166–175.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Chen YJ: Potential role of tetrandrine in
cancer therapy. Acta Pharmacol Sin. 23:1102–1106. 2002.PubMed/NCBI
|
|
25.
|
Ye Z, Sun A, Li L, Cao X and Ye W:
Reversal of adriamycin or vincristine resistance by tetrandrine in
human cancer cells in vitro. Zhongguo Zhong Yao Za Zhi. 21:369–371.
3841996.(In Chinese).
|
|
26.
|
Jin Q, Kang C, Soh Y, et al: Tetrandrine
cytotoxicity and its dual effect on oxidative stress-induced
apoptosis through modulating cellular redox states in Neuro 2a
mouse neuroblastoma cells. Life Sci. 71:2053–2066. 2002. View Article : Google Scholar
|
|
27.
|
Kuo PL and Lin CC: Tetrandrine-induced
cell cycle arrest and apoptosis in Hep G2 cells. Life Sci.
73:243–252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Cheng F, Li Y, Feng L and Li S: Effects of
tetrandrine on ischemia/reperfusion injury in mouse liver.
Transplant Proc. 40:2163–2166. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Zhang P, Tang Iu N and Zhao YC: The
effects of Tetrandrine on the changes of IL-1beta, TNF-alpha and
IL-8 after cerebral ischemia/reperfusion in rats. Zhongguo Ying
Yong Sheng Li Xue Za Zhi. 22:443–444. 4782006.(In Chinese).
|
|
30.
|
Liu Z, Xu Z, Shen W, Li Y, Zhang J and Ye
X: Effect of pharmacologic preconditioning with tetrandrine on
subsequent ischemia/ reperfusion injury in rat liver. World J Surg.
28:620–624. 2004.PubMed/NCBI
|
|
31.
|
Chen Y, Chen JC and Tseng SH: Tetrandrine
suppresses tumor growth and angiogenesis of gliomas in rats. Int J
Cancer. 124:2260–2269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Qian XP, Liu BR, Hu J, et al: Inhibitory
effect of tetrandrine on angiogenesis. Ai Zheng. 27:1050–1055.
2008.(In Chinese).
|
|
33.
|
Kobayashi S, Kimura I, Fukuta M, et al:
Inhibitory effects of tetrandrine and related synthetic compounds
on angiogenesis in streptozotocin-diabetic rodents. Biol Pharm
Bull. 22:360–365. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Kobayashi S, Inaba K, Kimura I and Kimura
M: Inhibitory effects of tetrandrine on angiogenesis in
adjuvant-induced chronic inflammation and tube formation of
vascular endothelial cells. Biol Pharm Bull. 21:346–349. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Liu C, Gong K, Mao X and Li W: Tetrandrine
induces apoptosis by activating reactive oxygen species and
repressing Akt activity in human hepatocellular carcinoma. Int J
Cancer. 129:1519–1531. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Chen XL, Ren KH, He HW and Shao RG:
Involvement of PI3K/ AKT/GSK3beta pathway in tetrandrine-induced G1
arrest and apoptosis. Cancer Biol Ther. 7:1073–1078. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Li X, Su B, Liu R, Wu D and He D:
Tetrandrine induces apoptosis and triggers caspase cascade in human
bladder cancer cells. J Surg Res. 166:e45–e51. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Lu KW, Chen JC, Lai TY, et al: Gypenosides
suppress growth of human oral cancer SAS cells in vitro and in a
murine xenograft model: the role of apoptosis mediated by
caspase-dependent and caspase-independent pathways. Integr Cancer
Ther. 11:129–140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Lu KW, Chen JC, Lai TY, et al: Gypenosides
causes DNA damage and inhibits expression of DNA repair genes of
human oral cancer SAS cells. In Vivo. 24:287–291. 2010.PubMed/NCBI
|
|
40.
|
Lu KW, Chen JC, Lai TY, et al: Gypenosides
inhibits migration and invasion of human oral cancer SAS cells
through the inhibition of matrix metalloproteinase-2 -9 and
urokinaseplasminogen by ERK1/2 and NF-kappa B signaling pathways.
Hum Exp Toxicol. 30:406–415. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Xie CM, Chan WY, Yu S, Zhao J and Cheng
CH: Bufalin induces autophagy-mediated cell death in human colon
cancer cells through reactive oxygen species generation and JNK
activation. Free Radic Biol Med. 51:1365–1375. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Vazquez CL and Colombo MI: Assays to
assess autophagy induction and fusion of autophagic vacuoles with a
degradative compartment, using monodansylcadaverine (MDC) and
DQ-BSA. Methods Enzymol. 452:85–95. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Chueh FS, Chen YL, Hsu SC, et al:
Triptolide induced DNA damage in A375.S2 human malignant melanoma
cells is mediated via reduction of DNA repair genes. Oncol Rep.
29:613–618. 2013.PubMed/NCBI
|
|
44.
|
Revonta M, Raitanen J, Sihvo S, et al:
Health and life style among infertile men and women. Sex Reprod
Healthc. 1:91–98. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Palefsky JM, Gillison ML and Strickler HD:
Chapter 16: HPV vaccines in immunocompromised women and men.
Vaccine. 24(Suppl 3): S3/140–146. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Gondi CS and Rao JS: Cathepsin B as a
cancer target. Expert Opin Ther Targets. 17:281–291. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Tekpli X, Holme JA, Sergent O and
Lagadic-Gossmann D: Role for membrane remodeling in cell death:
Implication for health and disease. Toxicology. 304:141–157. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Zhang XY, Xi XY and Zhao ZD: Autophagy
inhibitor 3-MA decreases the production and release of infectious
enterovirus 71 particles. Zhonghua Shi Yan He Lin Chuang Bing Du
Xue Za Zhi. 25:176–178. 2011.(In Chinese).
|
|
49.
|
Liu D, Yang Y, Liu Q and Wang J:
Inhibition of autophagy by 3-MA potentiates cisplatin-induced
apoptosis in esophageal squamous cell carcinoma cells. Med Oncol.
28:105–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
He Y, Zhao X, Gao J, et al: Quantum
dots-based immuno-fluorescent imaging of stromal fibroblasts
caveolin-1 and light chain 3b expression and identification of
their clinical significance in human gastric cancer. Int J Mol Sci.
13:13764–13780. 2012. View Article : Google Scholar
|
|
51.
|
Liu GH, Zhong Q, Ye YL, et al: Expression
of beclin 1 in bladder cancer and its clinical significance. Int J
Biol Markers. Nov 5–2012.(Epub ahead of print).
|
|
52.
|
Levy JM and Thorburn A: Targeting
autophagy during cancer therapy to improve clinical outcomes.
Pharmacol Ther. 131:130–141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Schrump DS: Cytotoxicity mediated by
histone deacetylase inhibitors in cancer cells: mechanisms and
potential clinical implications. Clin Cancer Res. 15:3947–3957.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
54.
|
Shen Y, Liang LZ, Hong MH, Xiong Y, Wei M
and Zhu XF: Expression and clinical significance of
microtubule-associated protein 1 light chain 3 (LC3) and Beclin1 in
epithelial ovarian cancer. Ai Zheng. 27:595–599. 2008.(In
Chinese).
|
|
55.
|
Pavlides S, Vera I, Gandara R, et al:
Warburg meets autophagy: cancer-associated fibroblasts accelerate
tumor growth and metastasis via oxidative stress, mitophagy, and
aerobic glycolysis. Antioxid Redox Signal. 16:1264–1284. 2012.
View Article : Google Scholar
|
|
56.
|
McLeland CB, Rodriguez J and Stern ST:
Autophagy monitoring assay: qualitative analysis of MAP LC3-I to II
conversion by immunoblot. Methods Mol Biol. 697:199–206. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
57.
|
Reggiori F, Monastyrska I, Verheije MH, et
al: Coronaviruses Hijack the LC3-I-positive EDEMosomes, ER-derived
vesicles exporting short-lived ERAD regulators, for replication.
Cell Host Microbe. 7:500–508. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58.
|
Gimenez-Xavier P, Francisco R, Platini F,
Perez R and Ambrosio S: LC3-I conversion to LC3-II does not
necessarily result in complete autophagy. Int J Mol Med.
22:781–785. 2008.PubMed/NCBI
|
|
59.
|
Cao Y, Nair U, Yasumura-Yorimitsu K and
Klionsky DJ: A multiple ATG gene knockout strain for yeast
two-hybrid analysis. Autophagy. 5:699–705. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
60.
|
Noda NN, Ohsumi Y and Inagaki F: ATG
systems from the protein structural point of view. Chem Rev.
109:1587–1598. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61.
|
Stasyk OV, Nazarko TY and Sibirny AA:
Methods of plate pexophagy monitoring and positive selection for
ATG gene cloning in yeasts. Methods Enzymol. 451:229–239. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
62.
|
Chung T, Suttangkakul A and Vierstra RD:
The ATG autophagic conjugation system in maize: ATG transcripts and
abundance of the ATG8-lipid adduct are regulated by development and
nutrient availability. Plant Physiol. 149:220–234. 2009. View Article : Google Scholar : PubMed/NCBI
|