Introduction
Mesenchymal stem cells (MSCs) are a subgroup of stem
cells with the capacity of osteogenesis, chondrogenesis,
adipogenesis, and myogenesis in adult organs and tissues (1–5).
Although MSCs show promising potential in the stem-cell based
therapies for wound repairing and tissue engineering (6,7), the
application is hindered due to our limited understanding on the
detailed regulation of MSCs. Currently a combination of
cell-surface markers has to be employed for routine identification
of MSCs from other cell types (8).
Previous studies indicate that Trop2, a 36-kDa
glycoprotein overexpressed in variety of epithelial cancers with
unknown functions (9–14), could potentially serve as a
stem-cell marker. Expressions of Trop2 were observed in
progenitor/stem-cell-like prostate basal and hepatic oval cells,
and only those with a higher level of the expression were
associated with a higher capacity of proliferation and
differentiation (15,16). These findings imply that Trop2 may
be associated with certain functions of the stem-like cells,
including self renewal of the cells.
To investigate the potential association between
Trop2 and MSCs, we constructed a Trop2 knockout mouse by
homologous recombination. We report that Trop2 is exclusively
expressed on the MSCs membrane and is involved in regulation of
proliferation and differentiation of MSCs. Trop2 deficiency
impaired the differentiation of MSCs by significant reduction of
adipogenesis and osteogenesis. AKT activation was impaired and
cyclin D1 expression was downregulated in the
Trop2-deficient MSCs, resulting in significant delay in cell
cycle progression and inhibition of MSC self-renewal. These studies
presented first-line evidence of close association between Trop2
and MSC self-renewal, providing a novel platform for further
investigation of Trop2 on other types of stem cells.
Materials and methods
Trop2 gene knockout mice
Trop2 KO mice were generated by targeted gene
interruption via homologous recombination (Fig. 1) at the Institute of Genetics and
Developmental Biology, Chinese Academy of Sciences, following the
modified protocol of Hall et al(17). Briefly, the full-length genomic
sequence of Trop2 was obtained by screening a mouse 129/Sv
bacterial artificial chromosome (BAC) library constructed in the
laboratory of Dr R.Z. Ma. ES cells of 129/Sv origin were a gift
from Professor R. Xu at the Fudan University.
Trop2+/− heterozygous mice were used for breeding
homozygous KO mice. All the animals were maintained in certified
SPF facilities and the experiments were approved by the ethics
committees for animal use and care at the Institute of Genetics and
Developmental Biology, Chinese Academy of Sciences, and Tongji
Medical College of Huazhong University of Science and Technology.
The Trop2 KO mice (Trop2−/−) show
no obvious phenotypes differences with the WT under normal
conditions.
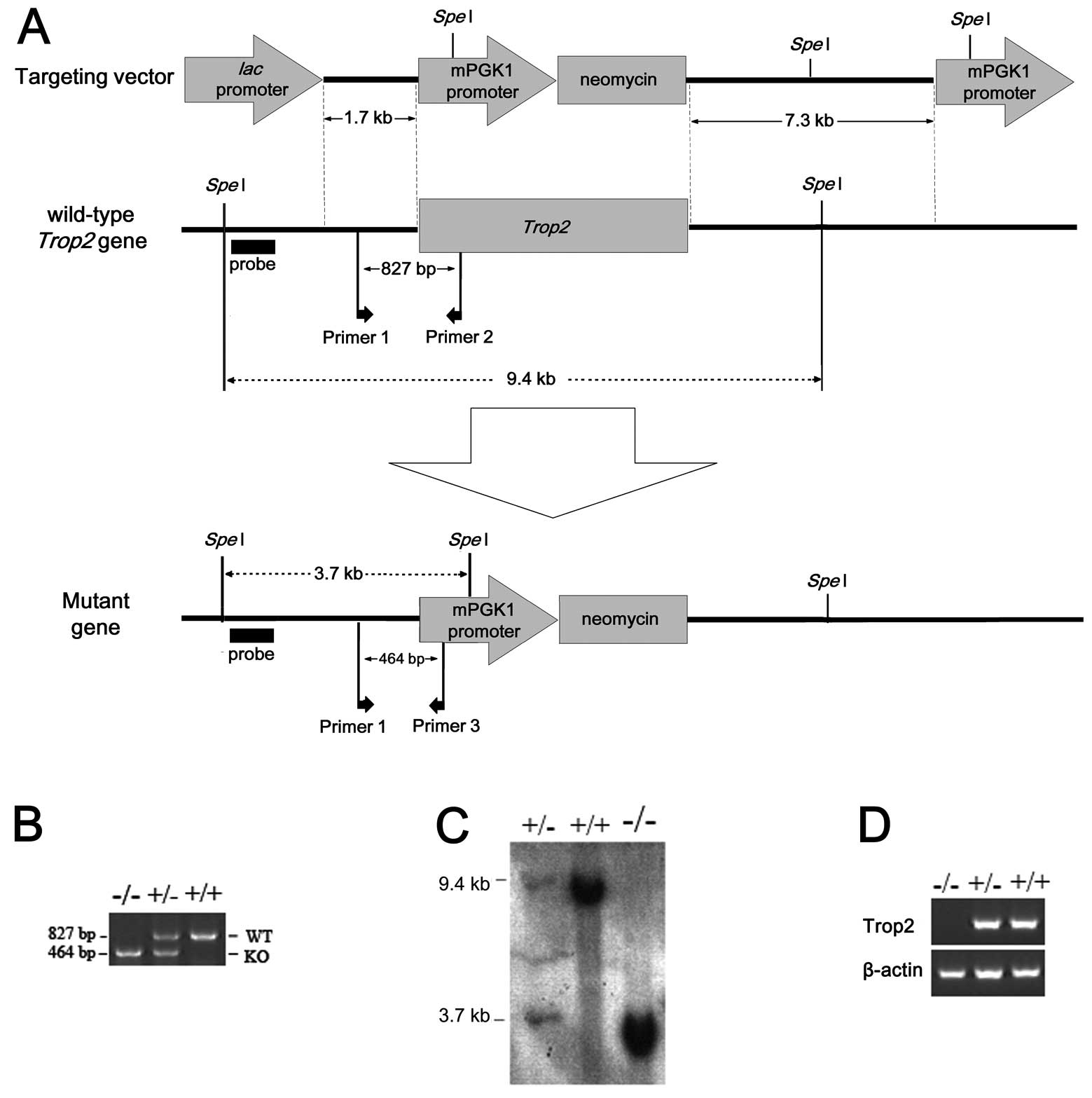 | Figure 1Construction of Trop2 knockout
mice by the targeted gene interruption. The whole ORF of
Trop2 was replaced by a piece of DNA fragment coding for
mPGK1 promoter-Neomycin via homologous recombination. (A)
Experimental design strategy for the homologous recombination, with
locations of target and replacement fragments, SpeI
restriction sites, probes, primers, short arms and long arms marked
as shown. (B) Genotype identification of Trop2 homozygous,
heterozygous, and wild-type mice by SP-PCR. Genomic DNA isolated
from the individual mouse tail was used as templates for routine
mouse colony screening. WT: Wild-type, KO: Knockout. (C) Southern
blot analysis of mice genomic DNA digested with SpeI
restriction enzyme, showing the expected 32P-labeled
Trop2 band in wild-type, heterozygous, and homozygous mouse.
(D) PCR amplification of Trop2 cDNA reverse transcribed from
mRNA of WT, heterozygous, or homozygous. No Trop2 mRNA was
detected in homozygous mice, indicating successful knockout of the
target gene. |
MSCs isolation and culture
Procedures of Zhu et al(18) were followed in isolation of the
MSCs from the murine compact bone. Briefly, 2–3-week old WT and KO
mice were sacrificed by cervical dislocation and all tissues on
femur, humerus and tibia were removed. The epiphyseal end was cut
and 20-gauge syringe was used to flush the bone marrow cavity with
α-MEM (Hyclone Laboratories, Logan, UT, USA). The bone fragments
(cut in 1 mm3) were digested in 1 mg/ml type II Collagen
(Gibco BRL, Grand Island, NY, USA) at 37°C for 90 min and then
washed for 3–5 times in α-MEM before seeding into a
25-cm2 flask for cell culture in complete medium with 5%
CO2. The medium contains α-MEM (Hyclone Laboratories),
10% MSC-qualified FBS (Gibco BRL), 2 mM L-glutamine (Sigma-Aldrich,
St. Louis, MO, USA), 100 U/ml penicillin (Sigma), and 100 μg/ml
streptomycin (Sigma). Cells were digested for 2–3 min with 0.025%
trypsin/0.02% EDTA (Sigma) when reached 80% confluences for
propagation. The passage was performed at a ratio of 1:3 or
1:4.
Flow cytometry
Adherent cells from WT mice or Trop2 KO mice
were digested and harvested for in vitro experiments. Cells
were incubated with the anti-mouse monoclonal antibodies FITC-CD45,
FITC-Sca-1, PE-CD34, PE-CD44, and PE-CD117 (all from eBioscience,
San Diego, CA, USA) for 30 min at the room temperature. FITC or PE
conjugated IgG was used in the control group. Cellular phenotypes
were assessed by a FACSCalibur CellSorting System (BD Biosciences,
Mountain View, CA, USA). For cell cycle analyses, the cells were
fixed in pre-chilled 70% alcohol (−20°C) for 24 h and then
incubated with RNase A (0.1 mg/ml, Roche Applied Science, Basel,
Switzerland) and propidium iodide (20 μg/ml, Gibco) at 37°C for 25
min. Detection channel FL1 or FL2 was used for the signal
collection. CellQuest software (BD Biosciences) was utilized to
analyze data.
Immunofluorescence
MSCs from WT and Trop2 KO mice were cultured
on Polysine™ Microscopy Slides (Menzel-Gläser, Braunschweig,
Germany) and fixed with 4% paraformaldehyde, then blocked with
serum from non-immunized goats containing 0.3% Triton X-100. The
cells were then incubated with diluted primary antibodies overnight
at 4°C. The antibodies used were Sca-1 (1:200; ab25195; Abcam,
Cambridge, UK) and mTrop2 (1:40; AF1122; R&D Systems,
Minneapolis, MN, USA). The cells were washed and incubated with
FITC- or PE-conjugated secondary antibodies for 60 min. DAPI
(4′,6-diamidino-2-phenylindole, Invitrogen) was used to stain the
nucleus. The slides were visualized using a Nikon A1R laser
scanning confocal microscopy (Digital Eclipse C1si; Nikon Corp.,
Tokyo, Japan).
Cell viability and CFSE assays
Colorimetric CCK-8 assays (Dojindo Laboratories,
Kumamoto, Japan) were used to measure cell viability. Briefly, MSCs
at a designated passage were seeded into 96-well plates at 2000
cells/100 μl for culturing. At 0, 24, 48, 72, or 96 h of incubation
time point, 10 μl of CCK-8 solution was added followed by
incubation in the dark for 2–3 h. The optical density (OD) was
measured at 450 nm on a microplate spectrophotometer (Multscan;
Thermo Scientific, Rockford, IL, USA).
For CFSE assay, cells (2×106) at passage
5 were harvested and mixed in 2.5 mM CFSE (Dojindo Laboratories),
followed by incubation at 37°C in the dark for 5–10 min. The
reaction was terminated with 10% FBS and seeded into 60-mm flasks
after washing. Flow cytometry was performed at 0, 24, 48, 72, or 96
h of incubation, and ModFit software (Mac3.1 SP2; Verity Software
House, Toshan, ME, USA) was used for proliferation analyses.
Differentiation assays
MSCs at passage 5 from WT mice and Trop2 KO
mice were harvested and seeded into a 24-well plate at a density of
5×103/cm2 and then cultured in condition
medium for adipogenesis or osteogenesis induction ~2–3 weeks,
respectively. For adipogenesis induction the medium contains α-MEM,
10% FBS, 10−3 mM dexamethasone, 0.5 mM IBMX (Sigma), and
10 ng/ml insulin (Sigma); while for osteogenesis the medium
contains α-MEM, 10% FBS, 10−4 mM dexamethasone (Sigma),
10 mM β-glycerol phosphate (Sigma), and 50 μM ascorbic acid
(Sigma). For adipogenesis induction the cells were fixed with 4%
paraformaldehyde (4°C) for 1 h and stained with oil red O for 30
min; for osteogenesis induction the cells were fixed in pre-chilled
70% alcohol (4°C) for 1 h and then stained with alizarin red at
room temperature for 15 min. After removing the staining solutions,
and rinsing, representative images were captured.
Real-time RT-PCR
Following induction of osteogenesis or adipogenesis,
MSCs from WT and Trop2 KO mice were harvested and total RNA
was extracted using an RNeasy RNA isolation kit (Qiagen, Hilden,
Germany). DNase-treated total RNA (2 μg) underwent reverse
transcription using oligo (dT)12–18 as a primer and
Superscript II RNase reverse transcriptase. cDNA was used as a
template for the amplification of target genes by PCR using La-Taq
and GC buffer I (Takara Bio, Shiga, Japan). The primers for
PPAR-γ2, Osteocalcin, Osteopontin, Adipsin and GAPDH
(glyceraldehyde-3-phosphate dehydrogenase) are shown in Table I and were designed with Primer
Premier Software (Premier Biosoft International, Palo Alto, CA,
USA). Statistical analysis was performed with a Student’s
two-tailed test. These experiments were performed in
triplicate.
 | Table ISpecific primers for RT-PCR
amplification. |
Table I
Specific primers for RT-PCR
amplification.
| Marker | Forward
(5′→3′) | Reverse
(5′→3′) | Length (bp) |
|---|
| PPAR-γ2 |
ccgtgatggaagaccactcg |
tcgcactttggtattcttggag | 165 |
| Osteocalcin |
ctgctcactctgctgaccctg |
tcactaccttattgccctcctg | 118 |
| Osteopontin |
tcaccattcggatgagtctg |
acttgtggctctgatgttcc | 222 |
| Adipsin |
tgggagcggctgtatgtg |
agtcgtcatccgtcactccat | 189 |
| GAPDH |
aactttggcattgtggaagg |
ggatgcagggatgatgttct | 132 |
Western blotting
MSCs in the logarithmic phase at the designated
passage were collected and lysed on ice in PhosphoSafe Extraction
Reagent (Merck KGaA, Darmstadt, Germany). Proteins in the lysate
were resolved on 8–10% SDS-polyacrylamide gels, transferred to PVDF
membranes, and subjected for western blot analyses using the
primary antibodies for Trop2 (1:600; AF1122; R&D Systems), Akt
(1:500; MAB2055; R&D Systems), Phospho-Akt (Ser473, 1:1000;
#9271; Cell Signaling Technology, Beverly, MA, USA), and cyclin D1
(1:500; sc-56302; Santa Cruz Biotechnology, Santa Cruz, CA, USA).
The blots were incubated with HRP-conjugated secondary IgG
antibodies (Bio-Rad Laboratories, Hercules, CA, USA) and the images
were captured and analyzed using a UVP AutoChem Image System (UVP,
Upland, CA, USA).
Results
Breeding of the Trop2 KO mice
We successfully generated the Trop2 knockout mice
(Fig. 1B–D). Initial analysis
showed no significant differences in growth, development and
reproduction between WT and Trop2 homozygous
(Trop2−/−) mice under normal SPF condition.
However, we noted that when both WT and KO mice were placed under
heat, humidity, and other stress conditions, the life expectancy of
Trop2 KO mice was significantly reduced (data not
shown).
Trop2 deficiency suppresses the total MSC
number in the compact bone
Phenotype analysis of the compact-bone derived MSCs
with 5 cell surface markers by flow cytometry showed the successful
isolation of the compact bone-derived stem cells from both of the
WT and Trop2 KO mice (Fig.
2). The MSC-positive rate for FITC-CD45 and PE-CD34 was
significantly higher in WT (CD45=37.28%, CD34=6.30%) than in
Trop2 KO (CD45=26.99%, CD34=1.64%) for passage 1,
demonstrating Trop2 deficiency significantly inhibited the
total MSC number in the compact bone (Fig. 2). With progression of the primary
cell cultures to passage 5 and higher, the differences between WT
and KO MSCs become non-significant, due to the continuous
differentiation of the MSCs under the culture conditions.
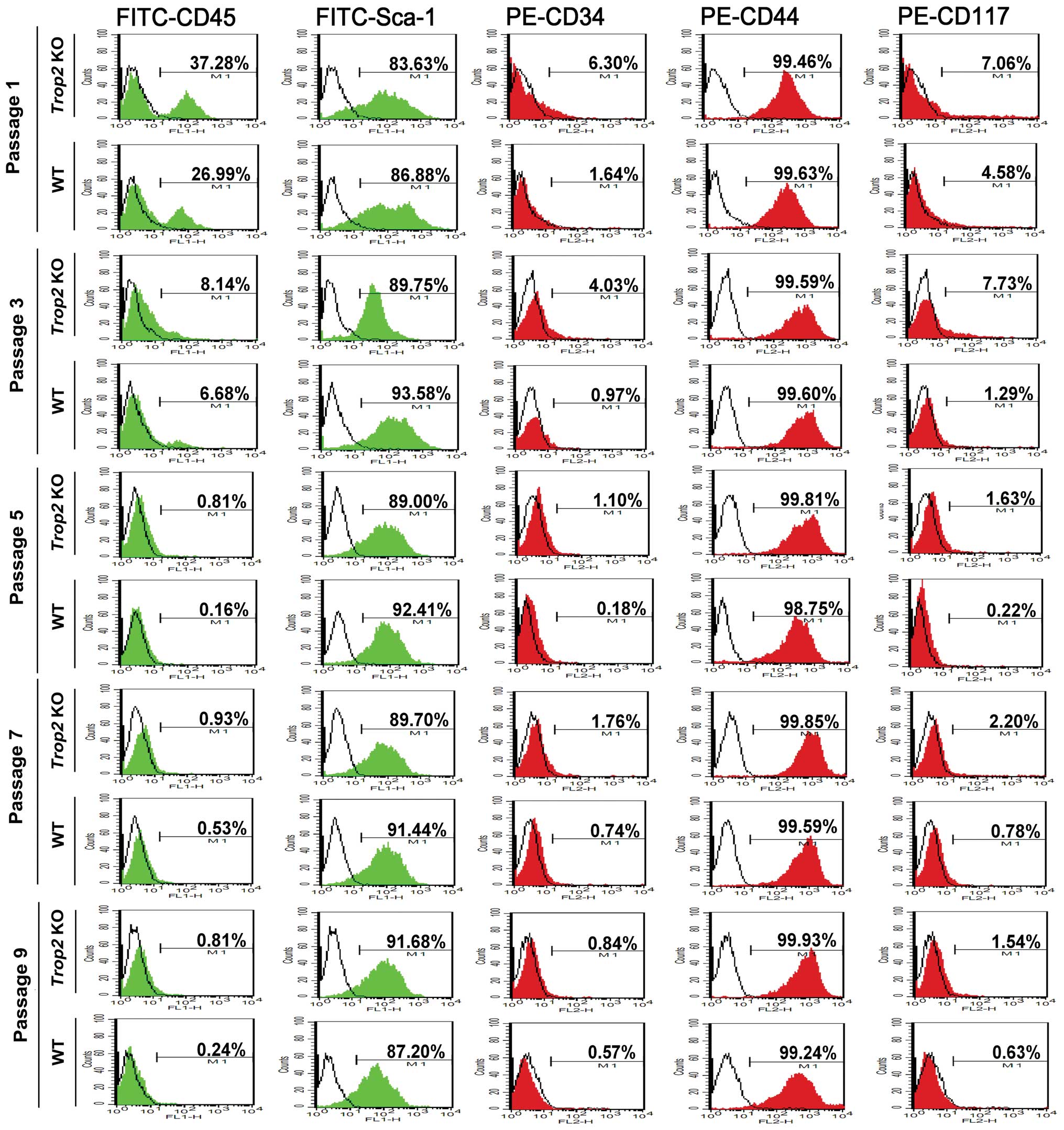 | Figure 2Phenotype analysis of compact-bone
derived MSCs from WT and KO mice. Cell surface markers FITC-CD45,
FITC-Sca-1, PE-CD34, PE-CD44, and PE-CD117 were selected to
characterize the phenotypes of compact-bone derived MSCs from
Trop2 KO and WT mice at the indicated passages by flow
cytometry. Compared to WT, MSCs from KO mice showed significantly
higher expression for CD45 (37.90±3.45% vs. 27.50±2.33%, n=3;
P<0.05) and CD34 (6.79±1.65% vs. 1.60±0.83%, n=3; P<0.05) at
passage 1, illustrating a significantly fewer compact-bone derived
MSCs from Trop2 KO mice. The levels of CD45 and CD34
expression decreased along with the progression of cell passages,
indicating an increasing purity of compact-bone derived MSCs over
passages due to gradual elimination of hematopoietic cells. The red
and green colored peak represents the specific PE-labeled
antibodies (CD34, CD44 and CD117) and FITC-labeled antibodies (CD45
and Sca-1), respectively. The blank peak represents the
corresponding isotope antibodies. |
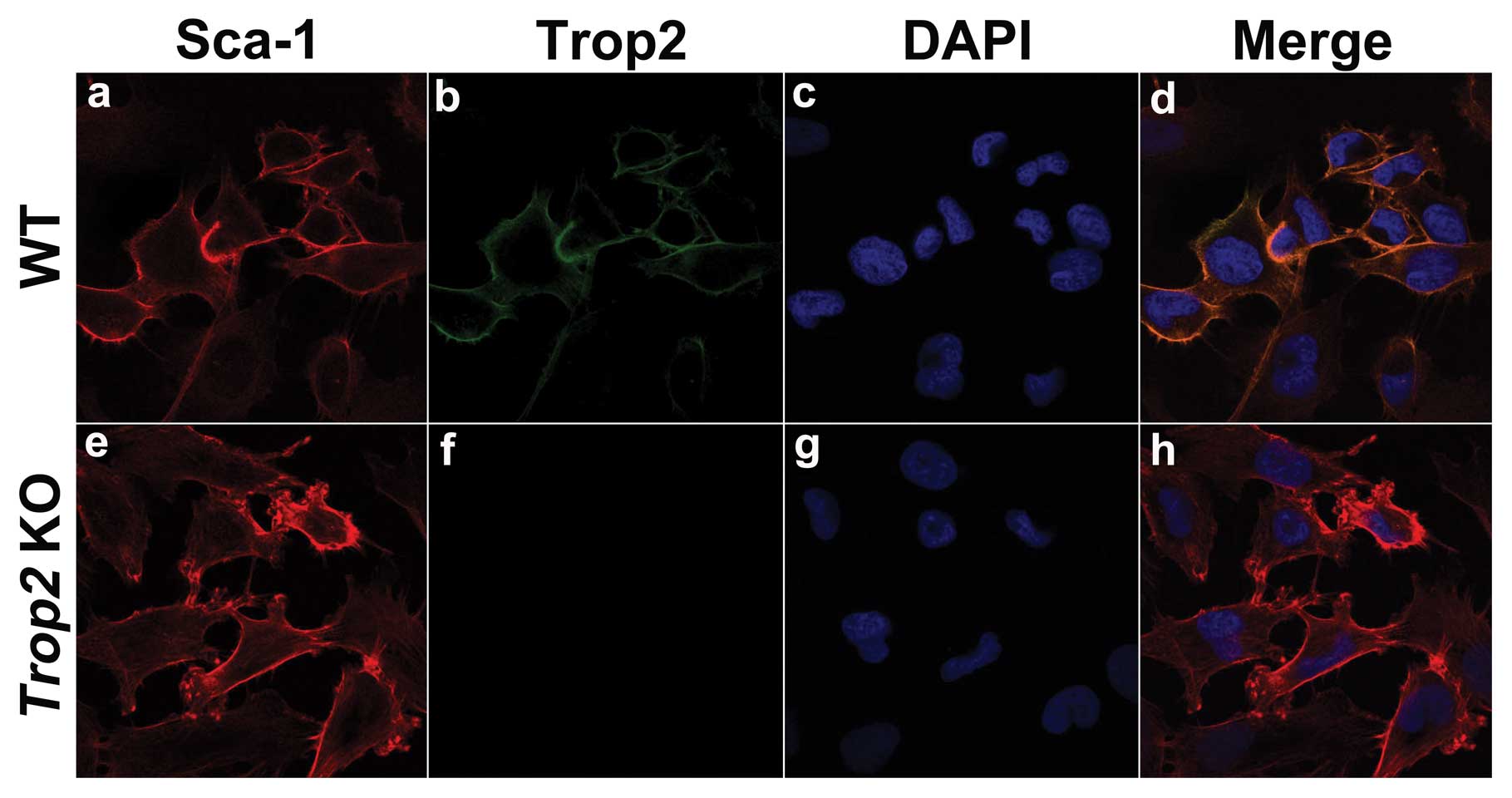
Trop2 is exclusively expressed on the MSC
membrane
Immunofluorescence staining of the WT and KO derived
MSCs using the specific anti-mouse Trop2 antibody illustrated that,
intensive Trop2 fluorescence was localized exclusively on the cell
membrane of MSCs from WT mice (Fig.
3), which was colocalized with Sca-1, a surface marker of MSCs
(Fig. 3b and a). Trop2
fluorescence was absent on MSCs derived from the Trop2 KO
mice, although Sca-1 fluorescence was present (Fig. 3e and f).
Absence of Trop2 impairs the MSC
proliferation
Measurement of the cell viability using CCK-8 assays
showed that, the MSCs isolated from Trop2 KO mice exhibited
a decreased viable cell number compared to WT, and the difference
was maintained significant (P<0.05) throughout the assays except
at passage 13 (Fig. 4A).
Determination of MSC doubling time using the CFSE labeling showed
that, Trop2 KO MSCs had a significantly prolonged cellular
doubling time after 48 h of culturing as compared to that of the WT
MSCs (Fig. 4B). These results
indicated that Trop2 deficiency leads to a reduction in MSC
growth rate or the cell proliferation, via reduced cell viability
and a prolonged cellular doubling time.
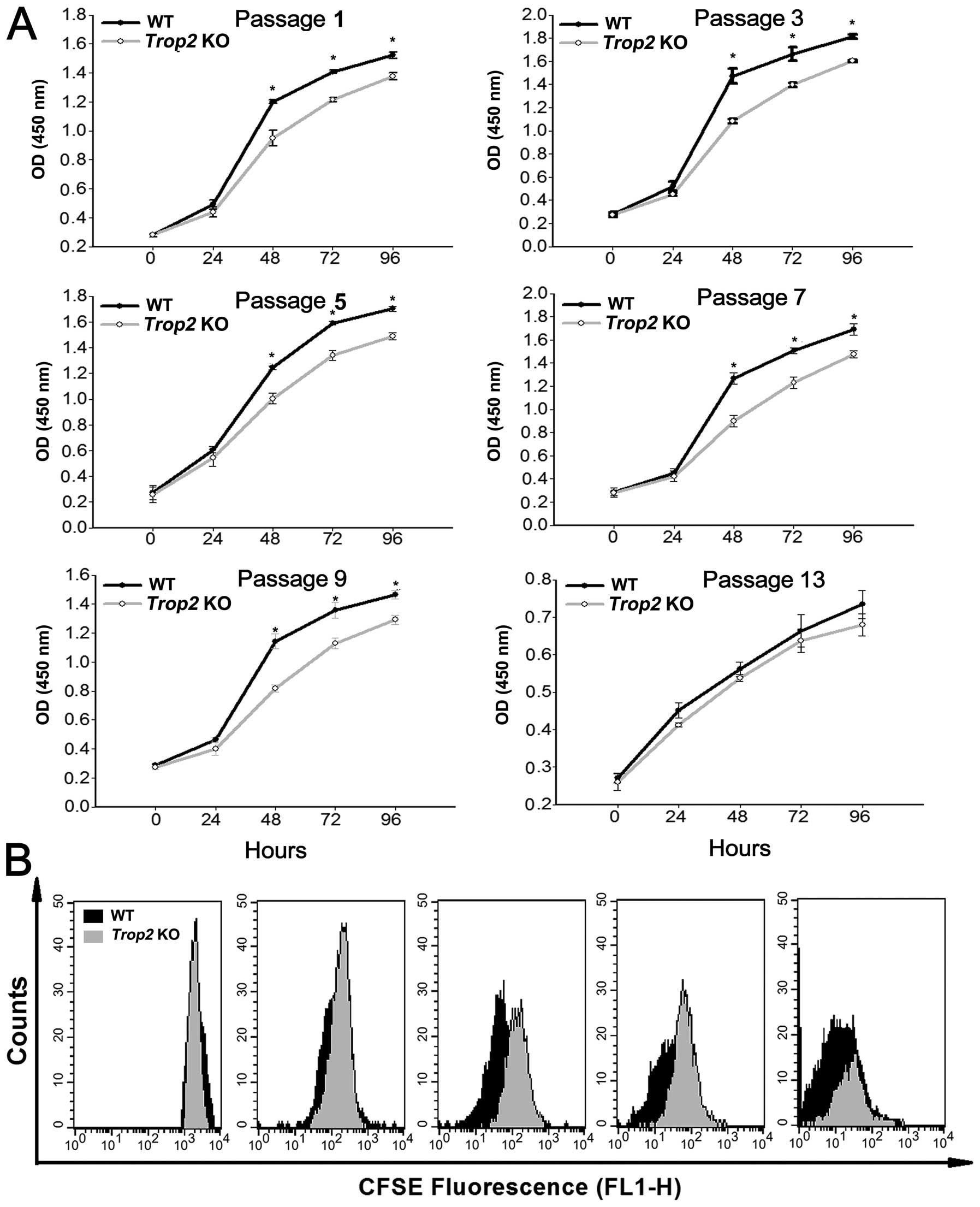 | Figure 4Absence of Trop2 impairs
proliferation of compact-bone derived MSCs. (A) CCK-8 assay of MSCs
after different passages of the cultures. For both KO and WT
derived MSCs at the designated passages, equal amounts of the cells
(2000 cells/100 μl) were seeded in flat-bottomed 96-well plates at
a final volume of 100 μl. The cell proliferation (total viable cell
numbers) in each well was estimated by optical density (450 nm)
using a Multiscan microplate spectrophotometer (Thermo Scientific,
Rockford, IL, USA) at 0, 24, 48, 72, and 96 h, after initial
incubation for 3 h. Except for the passage 13 (the last passage),
MSCs from Trop2 KO mice showed a significantly decreased
viable cell number than that of WT for the assays after 48 h of
culture (n=3, *P<0.01). (B) CFSE cellular doubling
time analyses. MSCs premixed with CFSE from both of the KO and WT
mice at the passage 5 were harvested and sorted by flow cytometry
for CFSE decay at 0, 24, 48, 72, and 96 h of culturing. The
detected fluorescence between KO and WT MSCs at each time point was
compared and composed using ModFit software (Mac 3.1 SP2; Verity
Software House, Toshan, ME, USA). Compared with WT (black peak
area), Trop2 KO MSCs have a significantly longer cellular
doubling time (grey peak area) after 48 h of culturing. |
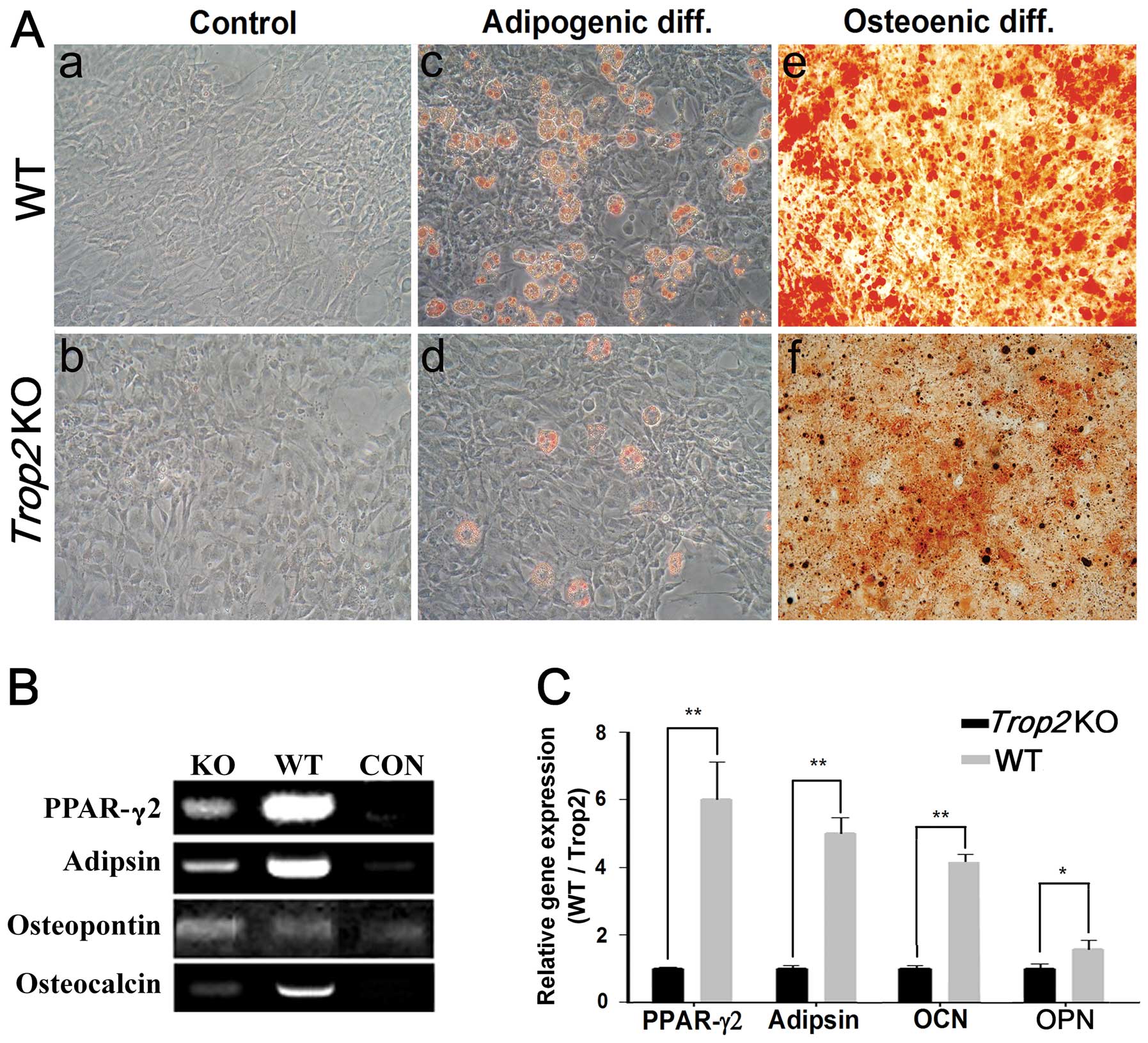
Cell differentiation is inhibited in the
Trop2-deficient MSCs
Induction of cell differentiation showed that,
compared to the MSCs from the WT mice, the process of adipogenesis
and osteogenesis in the Trop2-deficient MSCs was
significantly hindered, exhibited considerably less aggregation of
lipid drops and mineral deposition for the MSCs at passage 5
(Fig. 5). In contrast, a
significantly higher percentage of adipogenesis was observed in
MSCs derived from the WT mice (Fig.
5A–c). Similarly, osteogenesis differentiation in MSCs of WT
(Fig. 5A–e) was significantly
higher than that of KO (Fig.
5A–f). Accordingly, mRNA transcription of the marker genes for
the adipogenesis (PPAR-γ2 and Adipson) and
osteogenesis (Osteocalcin and Osteopontin) was dramatically
decreased in the MSCs of Trop2 KO mice. (n=3; P<0.05;
P<0.01) (Fig. 5B and C).
Trop2 deficiency impairs the AKT
activation, Cyclin D1 expression, and cell cycle progression
MSCs from the WT and Trop2 KO mice were
stained with propidium iodide at different time points, and their
cell cycle distribution was measured by flow cytometry for the
designated cell passages. Compared with the MSCs of the WT, the
proportion of MSCs entering the S phase from the Trop2 KO
mouse was significantly reduced at each passage (P<0.05,
Fig. 6A).
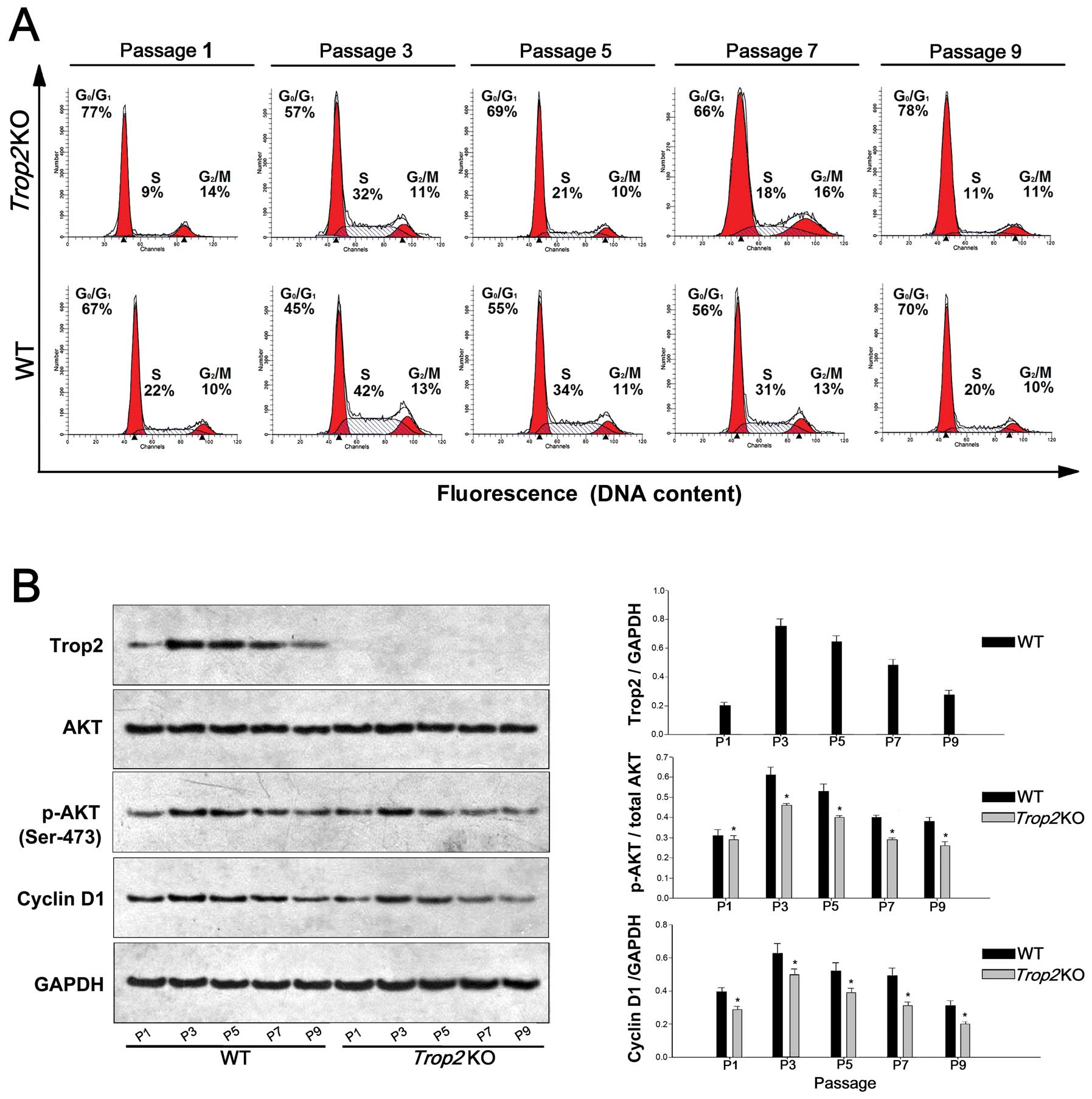 | Figure 6Trop2 deficiency impairs AKT
activation, Cyclin D1 expression, and cell cycle progression. (A)
Cell cycle analysis of the MSCs cultures at different passages. WT
and Trop2 KO mouse compact bone-derived MSCs after different
passages of cultures were collected, fixed, stained with propidium
iodide and analyzed for cell cycle phase distribution. Compared
with WT, the proportion of MSCs entering the S phase from
Trop2 KO mice was significantly reduced compared to that of
WT at the same passage (n=3, *P<0.01), indicating
that the cell cycle progression was hindered in the MSCs from KO
mice. (B) Western blot analysis of the cells at different passages.
MSCs cultures at different passages were used to prepare cell
lysates and the total cellular proteins were used for
immunoblotting by antibodies against Trop2 (1:600; AF1122;
R&D Systems, Minneapolis, MN, USA), phosphorylated AKT (Ser473,
1:1000; #9271; Cell Signaling Technology, Beverly, MA, USA), total
AKT (1:500; MAB2055; R&D Systems) and Cyclin D1 (1:500;
sc-56302; Santa Cruz Biotechnology, Santa Cruz, CA, USA). No
Trop2 expression was detected in MSCs from KO mice at any
passage. Compared with WT, MSCs from Trop2 KO mice have
markedly lower expression for both activated AKT and its downstream
target cyclin D1 at same passages of cultures (n=3,
*P<0.01), indicating that AKT/Cyclin D1 pathway of
MSCs from KO mice was impaired. |
MSCs of the Trop2 KO mice have markedly lower
expression for both of activated AKT and its downstream target
Cyclin D1 at the same passages (n=3, P<0.01, Fig. 6B), indicating the AKT/Cyclin D1
pathway was impaired. In addition, activated AKT (p-AKT) expression
in MSCs from both WT and Trop2 KO mice had a decreasing
trend with the increase of passage, and the proportion of p-AKT in
MSCs from WT mice was positively correlated with the reduction of
Trop2 with the increase of passage.
These results imply that Trop2 deficiency
impaired AKT phosphorylation and may have induced a cascade
reaction, leading to downregulation of cyclin D1, and thus
resulting in hindering cell cycle progression, which ultimately
impaired proliferation and differentiation of the MSCs in
vitro.
Discussion
In this study, we constructed the Trop2 KO
mice and investigated the roles of Trop2 on the proliferation and
differentiation of murine compact bone-derived MSCs in
vitro. We showed that Trop2 was exclusively localized on the
MSC cell membrane; Trop2 deficiency impaired the
proliferation, differentiation, cell cycle progression of the
compact-bone derived MSCs, probably via partial inhibition of
AKT/Cyclin D1 pathway. These results demonstrated the importance of
Trop2 for the normal function of MSCs. Although the molecular
details on how exactly the Trop2 interacts with the AKT/Cyclin D1
signal pathway remains unknown, our results may help to establish a
novel platform for the future investigation.
Successful isolation and expansion of the primary
MSCs from the compact-bone of both WT and KO mice enabled us to
carry out the subsequent investigation. Previous studies showed
that MSCs isolated from compact-bone were less contaminated with
hematopoietic cells and had a better homogeneity compared to those
isolated from bone marrow (18–20).
Our results demonstrated that MSCs prior to the first 3 passages
were inevitably contaminated by hematopoietic cells, but the cells
after passage 4 reached much higher homogeneity with typical
phenotypes of MSCs.
The result that Trop2 deficiency impaired the
adipogenesis and osteogenesis of the MSCs have profound
significance. Our data showed that the inhibition was due to the
downregulation of the marker genes involved in both processes
(Fig. 5). This fact indicates that
one of the biological functions of Trop2 is to mediate an unknown
signal pathway to maintain the proper function of the MSCs. Since
it is critical for MSCs to maintain the differentiation potential
for wound repairing and tissue regeneration (21,22),
any future clinical application of MSCs for wound repairing or
tissue engineering would require the presence of Trop2 as a factor
for the proper function of the stem cells. Further investigation of
the mechanism of the downregulation would help to resolve the
puzzle.
The specific ligand of Trop2 has not been
identified, and thus the specific pathway in which Trop2 functions
remains unclear (23). Trop2 is
involved in signal transduction in that it possesses the
PIP2 binding motif and tyrosine/serine phosphorylation
sites (24–26). The presence of a PIP2
motif may promote the aggregation of PIP2 on both sides of the cell
membrane. This in turn could increase the probability of
PIP2 hydrolysis, leading to an increased PIP3 level
(23). PIP3 is involved in the
activation of the PI3K/AKT signaling pathway (27,28),
which is a pivotal signaling pathway involved in the regulation of
cell survival (29–31). We thus speculate that Trop2 may be
helpful in the maintenance of AKT phosphorylation and is likely
involved in AKT activation, which stabilizes the dephosphorylation
of cyclin D1, resulting in promoting cell cycle progression and
maintaining self-renewal potential.
The result that the proportion of active AKT in MSCs
from Trop2 KO mice was significantly lower than that from WT
mice suggested that Trop2 deficiency impaired the activation
of AKT. However, we noted that the ratio of p-AKT/AKT and
expression of cyclin D1 showed a tread of decline with the
progression of the MSC culture passage, and the same was true for
Trop2 expression except in passage 1, which was affected by
hematopoietic cell contamination. This may be attributed to the
fact of no addition of cytokines for both promoting proliferation
and maintaining of undifferentiated state of MSCs during our
subculture (32,33). Without addition of the cytokines,
MSCs became senescent and lost the potential of self-renewal,
accompanied by gradually reduced expression of Trop2 during the
passage. Thus, Trop2 may protect MSCs from aging during the
passage, while the absence of Trop2 may promote MSCs
senescence, which would subsequently impair the self-renewal
potential of MSCs. However, Trop2 loss may not completely block AKT
activation. On one hand, Trop2 deficiency cannot completely abolish
AKT activation by some unknown cytokines in the MSC-qualified fetal
bovine serum, or AKT may be activated in multiple pathways through
complex signal networks (34). On
the other hand, Trop2 may have pleiotropic functions as well
as EpCAM, which regulates both adhesion (35), and intracellular signaling via
cleavage products (36). Latest
study revealed that Trop2 loss promoted the tumor aggressiveness
and epithelial-mesenchymal trans-differentiation (37); while previous studies emphasized
that Trop2 expression was positively related to the tumor
aggressiveness and metastasis. Hence, further studies are required
to elucidate the relationships between Trop2 and related signaling
pathways in MSCs or other types of stem cells.
Acknowledgements
We thank Rene Xu and Yuan Zhuang for assistance in
construction of the Trop2 KO mice. This study was supported
by grants from National Natural Science Foundation of China
(30571053; 30571840; 30771148), Specialized Research Fund for the
Doctoral Program of Higher Education (20110142110009), and Chinese
Academy of Sciences (KSCX1-YW-R-45; KSCX2-EW-R-05).
References
|
1
|
Caplan AI: Mesenchymal stem cells. J
Orthop Res. 9:641–650. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Friedenstein AJ, Gorskaja JF and Kulagina
NN: Fibroblast precursors in normal and irradiated mouse
hematopoietic organs. Exp Hematol. 4:267–274. 1976.PubMed/NCBI
|
|
3
|
Pittenger MF, Mackay AM, Beck SC, et al:
Multilineage potential of adult human mesenchymal stem cells.
Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang Y, Jahagirdar BN, Reinhardt RL, et
al: Pluripotency of mesenchymal stem cells derived from adult
marrow. Nature. 418:41–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL
and Chen TH: Isolation of multipotent mesenchymal stem cells from
umbilical cord blood. Blood. 103:1669–1675. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Song H, Song BW, Cha MJ, Choi IG and Hwang
KC: Modification of mesenchymal stem cells for cardiac
regeneration. Expert Opin Biol Ther. 10:309–319. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mizuno H: Adipose-derived stem cells for
tissue repair and regeneration: ten years of research and a
literature review. J Nippon Med Sch. 76:56–66. 2009.PubMed/NCBI
|
|
8
|
Bianco P, Robey PG and Simmons PJ:
Mesenchymal stem cells: revisiting history, concepts, and assays.
Cell Stem Cell. 2:313–319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fong D, Moser P, Krammel C, et al: High
expression of TROP2 correlates with poor prognosis in pancreatic
cancer. Br J Cancer. 99:1290–1295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fong D, Spizzo G, Gostner JM, et al:
TROP2: a novel prognostic marker in squamous cell carcinoma of the
oral cavity. Mod Pathol. 21:186–191. 2008.PubMed/NCBI
|
|
11
|
Muhlmann G, Spizzo G, Gostner J, et al:
TROP2 expression as prognostic marker for gastric carcinoma. J Clin
Pathol. 62:152–158. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakashima K, Shimada H, Ochiai T, et al:
Serological identification of TROP2 by recombinant cDNA expression
cloning using sera of patients with esophageal squamous cell
carcinoma. Int J Cancer. 112:1029–1035. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ohmachi T, Tanaka F, Mimori K, Inoue H,
Yanaga K and Mori M: Clinical significance of TROP2 expression in
colorectal cancer. Clin Cancer Res. 12:3057–3063. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang J, Day R, Dong Y, Weintraub SJ and
Michel L: Identification of Trop-2 as an oncogene and an attractive
therapeutic target in colon cancers. Mol Cancer Ther. 7:280–285.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goldstein AS, Lawson DA, Cheng D, Sun W,
Garraway IP and Witte ON: Trop2 identifies a subpopulation of
murine and human prostate basal cells with stem cell
characteristics. Proc Natl Acad Sci USA. 105:20882–20887. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Okabe M, Tsukahara Y, Tanaka M, et al:
Potential hepatic stem cells reside in EpCAM+ cells of
normal and injured mouse liver. Development. 136:1951–1960. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hall B, Limaye A and Kulkarni AB:
Overview: generation of gene knockout mice. Curr Protoc Cell Biol.
Chapter 19(Unit 19.12): 19.12.1–17. 2009. View Article : Google Scholar
|
|
18
|
Zhu H, Guo ZK, Jiang XX, et al: A protocol
for isolation and culture of mesenchymal stem cells from mouse
compact bone. Nat Protoc. 5:550–560. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun S, Guo Z, Xiao X, et al: Isolation of
mouse marrow mesenchymal progenitors by a novel and reliable
method. Stem Cells. 21:527–535. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo Z, Li H, Li X, et al: In vitro
characteristics and in vivo immunosuppressive activity of compact
bone-derived murine mesenchymal progenitor cells. Stem Cells.
24:992–1000. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maxson S, Lopez EA, Yoo D,
Danilkovitch-Miagkova A and Leroux MA: Concise review: role of
mesenchymal stem cells in wound repair. Stem Cells Transl Med.
1:142–149. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu Y, Chen L, Scott PG and Tredget EE:
Mesenchymal stem cells enhance wound healing through
differentiation and angiogenesis. Stem Cells. 25:2648–2659. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cubas R, Li M, Chen C and Yao Q: Trop2: a
possible therapeutic target for late stage epithelial carcinomas.
Biochim Biophys Acta. 1796:309–314. 2009.PubMed/NCBI
|
|
24
|
Yu FX, Sun HQ, Janmey PA and Yin HL:
Identification of a polyphosphoinositide-binding sequence in an
actin monomer-binding domain of gelsolin. J Biol Chem.
267:14616–14621. 1992.PubMed/NCBI
|
|
25
|
Linnenbach AJ, Seng BA, Wu S, et al:
Retroposition in a family of carcinoma-associated antigen genes.
Mol Cell Biol. 13:1507–1515. 1993.PubMed/NCBI
|
|
26
|
El Sewedy T, Fornaro M and Alberti S:
Cloning of the murine TROP2 gene: conservation of a PIP2-binding
sequence in the cytoplasmic domain of TROP-2. Int J Cancer.
75:324–330. 1998.PubMed/NCBI
|
|
27
|
Taniguchi CM, Tran TT, Kondo T, et al:
Phosphoinositide 3-kinase regulatory subunit p85alpha suppresses
insulin action via positive regulation of PTEN. Proc Natl Acad Sci
USA. 103:12093–12097. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wan X, Dennis AT, Obejero-Paz C, et al:
Oxidative inactivation of the lipid phosphatase and tensin homolog
on chromosome ten (PTEN) as a novel mechanism of acquired long QT
syndrome. J Biol Chem. 286:2843–2852. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Datta SR, Dudek H, Tao X, et al: Akt
phosphorylation of BAD couples survival signals to the
cell-intrinsic death machinery. Cell. 91:231–241. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brunet A, Bonni A, Zigmond MJ, et al: Akt
promotes cell survival by phosphorylating and inhibiting a Forkhead
transcription factor. Cell. 96:857–868. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Song G, Ouyang G and Bao S: The activation
of Akt/PKB signaling pathway and cell survival. J Cell Mol Med.
9:59–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Re RN and Cook JL: Senescence, apoptosis,
and stem cell biology: the rationale for an expanded view of
intracrine action. Am J Physiol Heart Circ Physiol. 297:H893–H901.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sethe S, Scutt A and Stolzing A: Aging of
mesenchymal stem cells. Ageing Res Rev. 5:91–116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lindsley CW: The Akt/PKB family of protein
kinases: a review of small molecule inhibitors and progress towards
target validation: a 2009 update. Curr Top Med Chem. 10:458–477.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Litvinov SV, Velders MP, Bakker HA,
Fleuren GJ and Warnaar SO: Ep-CAM: a human epithelial antigen is a
homophilic cell-cell adhesion molecule. J Cell Biol. 125:437–446.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Maetzel D, Denzel S, Mack B, et al:
Nuclear signalling by tumour-associated antigen EpCAM. Nat Cell
Biol. 11:162–171. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang J, Zhang K, Grabowska D, et al: Loss
of Trop2 promotes carcinogenesis and features of epithelial to
mesenchymal transition in squamous cell carcinoma. Mol Cancer Res.
9:1686–1695. 2011. View Article : Google Scholar : PubMed/NCBI
|