Introduction
Lung cancer is the leading cause of cancer-related
deaths worldwide. Despite advances in early detection and
chemotherapy, its prognosis is generally poor, with a 5-year
survival of approximately 15%. Given a lack of effective
therapeutics, the use of chemopreventive agents that abrogate lung
carcinogenesis represents a promising approach for controlling this
disease.
Compelling evidence has emerged that non-steroidal
anti-inflammatory drugs (NSAIDs) can reduce the incidence of
various cancers and limit metastatic disease (1–3).
However, the chronic use of NSAIDs is associated with significant
gastrointestinal and renal toxicities. To reduce toxicity and
enhance the efficacy of conventional NSAIDs, our group has
developed novel phospho-derivatives of NSAIDs. One such derivative
is phospho-sulindac (PS, OXT-328), which is efficacious in the
prevention and treatment of colon and breast cancer in preclinical
models (4–7) and shows a favorable safety profile
(5). In contrast, PS as a single
agent was ineffective in the treatment of human lung cancer
xenografts (8), which prompted us
to develop more potent PS-based therapy incorporating other
anticancer agents. Here, we describe the combination of PS with
curcumin for the prevention of lung cancer.
Curcumin, the principal bioactive component in
turmeric, exhibits antitumorigenic activities (9–11).
In pre-clinical models of lung cancer, however, curcumin as a
single agent has demonstrated poor efficacy (<30%) (12); and according to one report, it may
even promote Kras-driven lung tumorigenesis in mice
(13). On the other hand, curcumin
significantly potentiates the antitumor activity of sulindac,
docetaxel and gefitinib in animal models (14–16).
Curcumin can also overcome resistance to anticancer drugs such as
paclitaxel, thalidomide and bortezomib (17,18).
Thus, curcumin is a versatile chemosensitizer for mechanistically
diverse anticancer agents.
Here, we demonstrate that curcumin potentiates the
anticancer efficacy of PS in human non-small cell lung cancer
(NSCLC) cells and that such a combination synergistically inhibits
the growth of A549 xenografts in mice. These findings suggest that
PS plus curcumin is a promising combination for the prevention of
NSCLC.
Materials and methods
Reagents
Phospho-sulindac (OXT-328) was a gift from Medicon
Pharmaceuticals, Inc., Setauket, NY, USA. Cell culture reagents
were purchased from Cellgro (Herndon, VA, USA). Other reagents,
unless otherwise stated, were obtained from Sigma-Aldrich (St.
Louis, MO, USA).
Cell culture
Human NSCLC (A549), breast (MCF-7 and MDA-MB-231),
colon (SW480) and pancreatic (MIAPaCa-2) cancer cell lines were
obtained from American Type Culture Collection (ATCC) and
maintained in the recommended culture media containing 10% fetal
bovine serum and penicillin/streptomycin. All experiments were
performed with cells between passages 1 and 10.
Curcumin formulation
Polymeric nanoparticles of poly(ɛ-caprolactone)
(11,000)-polyethylene glycol (5,000) with entrapped curcumin were
prepared according to the nanoprecipitation-solvent displacement
method (19,20). Briefly, 50 mg of polymer and 5 mg
of curcumin were dissolved in 1 ml of acetone and the solution was
added dropwise to 2 ml of water under constant stirring. The
organic solvent was allowed to slowly evaporate under reduced
pressure and the resulting suspension was centrifuged to remove
aggregates and drug precipitates. Curcumin concentration was
determined using HPLC. Ten minutes after diluting the suspension in
water, we determined the size and zeta potential of the
nanoparticles using Dynamic Light Scattering (Zeta-Plus Brookhaven
instrument, Holtsville, NY, USA). Particle size was also determined
using transmission electron microscopy. Curcumin loading in the
nanoparticles was 8±0.3%. The mean nanoparticle size was 45.2 nm
and their polydispersity index was 0.271±0.005.
Cell growth inhibition assays
Cell viability was determined by a modified
colorimetric assay using
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT).
Briefly, A549 cells seeded in 96-well plates were treated with
different concentrations of PS for 24 h with or without
pretreatment with curcumin for 3 h. The culture medium was removed
and replaced with 100 μl complete medium containing 0.5 mg/ml MTT.
Following 4-h incubation at 37°C, 100 μl of a solution containing
10% SDS and 0.01 N HCl was added. The plate was incubated and
gently mixed until MTT formazan crystals were dissolved. Absorbance
at 570 nm was measured on a microplate reader and IC50
was calculated after subtraction of blank values.
Apoptotic cell death analysis
After drug treatment, cells were trypsinized, washed
once with PBS and stained with Annexin V/propidium iodide.
Percentage of apoptotic cells was determined by flow cytometry on a
FACSCalibur.
Cellular uptake assay
For the uptake study, cells were seeded into 12-well
plates at a cell density of 2.5×105 per well. After
overnight incubation, uptake experiments were carried out using
serum-free media. After washing the monolayer twice, the cells were
pre-incubated for 15 min with serum-free media with or without
transporter inhibitors. PS was then added and incubated for 1 h. At
the end of the incubation, the medium was quickly aspirated. The
cells were washed twice, each with 2 ml of ice-cold transport
buffer containing 0.2% bovine serum albumin (BSA). Finally, the
cells were rinsed with 0.5 ml of ice-cold transport buffer without
BSA. Cells were collected with 0.5 ml of 50% methanol. Extraction
was performed by sonication for 5 min followed by the addition of
0.5 ml of ice-cold methanol and centrifuged at 17,000 × g for 12
min. The supernatant was collected and analyzed by HPLC. The
protein pellet was re-dissolved in 0.1 N NaOH and the protein
content was determined by the Bradford assay. All the uptake values
were corrected against protein content.
Pharmacokinetic studies in mice
This and subsequent animal studies were approved by
the Institutional Animal Care and Use Committee (IACUC) of Stony
Brook University. Mice (n=2) were given a single dose of the
following treatments: i) PS 200 mg/kg; ii) PS 200 mg/kg plus
curcumin 500 mg/kg, in 10% Tween-80; and iii) PS 200 mg/kg plus
micellar curcumin 500 mg/kg. At designated time-points, mice were
euthanized by CO2 inhalation and blood was collected and
immediately centrifuged. The resulting plasma was deproteinized by
immediately mixing it with 2.5× volumes of acetonitrile. The
deproteinized samples were analysed by HPLC as described below.
A549 xenografts
Female nude mice 6–7 weeks old were purchased from
Harlan Sprague-Dawley, Indianapolis, IN, USA. At 7–8 weeks of age,
four groups of mice (n=6 per group) were pre-treated for 3 days
with: i) vehicle; ii) PS 200 mg/kg/d; iii) curcumin 500 mg/kg/d;
and iv) PS 200 mg/kg/d plus curcumin 500 mg/kg/d. Then, the mice
were inoculated subcutaneously into both flanks with A459 cells
(2×106 each) suspended in 100 μl complete F12K medium:
Matrigel Matrix gel (BD Biosciences, San Jose, CA, USA) (1:1, v/v).
The treatment was resumed one day after tumor implantation and
continued daily until the end of the study. The tumors were
measured twice a week with a digital microcaliper and tumor volumes
were calculated using the formula: tumor volume = [length × width ×
(length + width/2) × 0.56]. At the end of the experiment, the
animals were sacrificed and their tumors were removed. The levels
of PS and its metabolites in the tumors were determined by HPLC
(21).
HPLC analysis
The HPLC system consisted of a Waters Alliance 2695
Separations Module equipped with a Waters 2998 photo-diode array
detector (Waters, Milford, MA, USA) and a Thermo BDS Hypersil C18
column (150×4.6 mm, particle size 3 μm) (Thermo Fisher Scientific,
Waltham, MA, USA). The mobile phase consisted of a gradient between
solvent A [(trifluoroacetic acid, acetonitrile, H2O
(0.1:4.9:95, v/v/v)] and 100% acetonitrile.
Statistical analyses
Data are expressed as mean ± SEM. Statistical
analyses were performed by ANOVA. P-values <0.05 were considered
statistically significant.
Results
Curcumin synergizes with PS in inhibiting
the growth of lung cancer cells in vitro
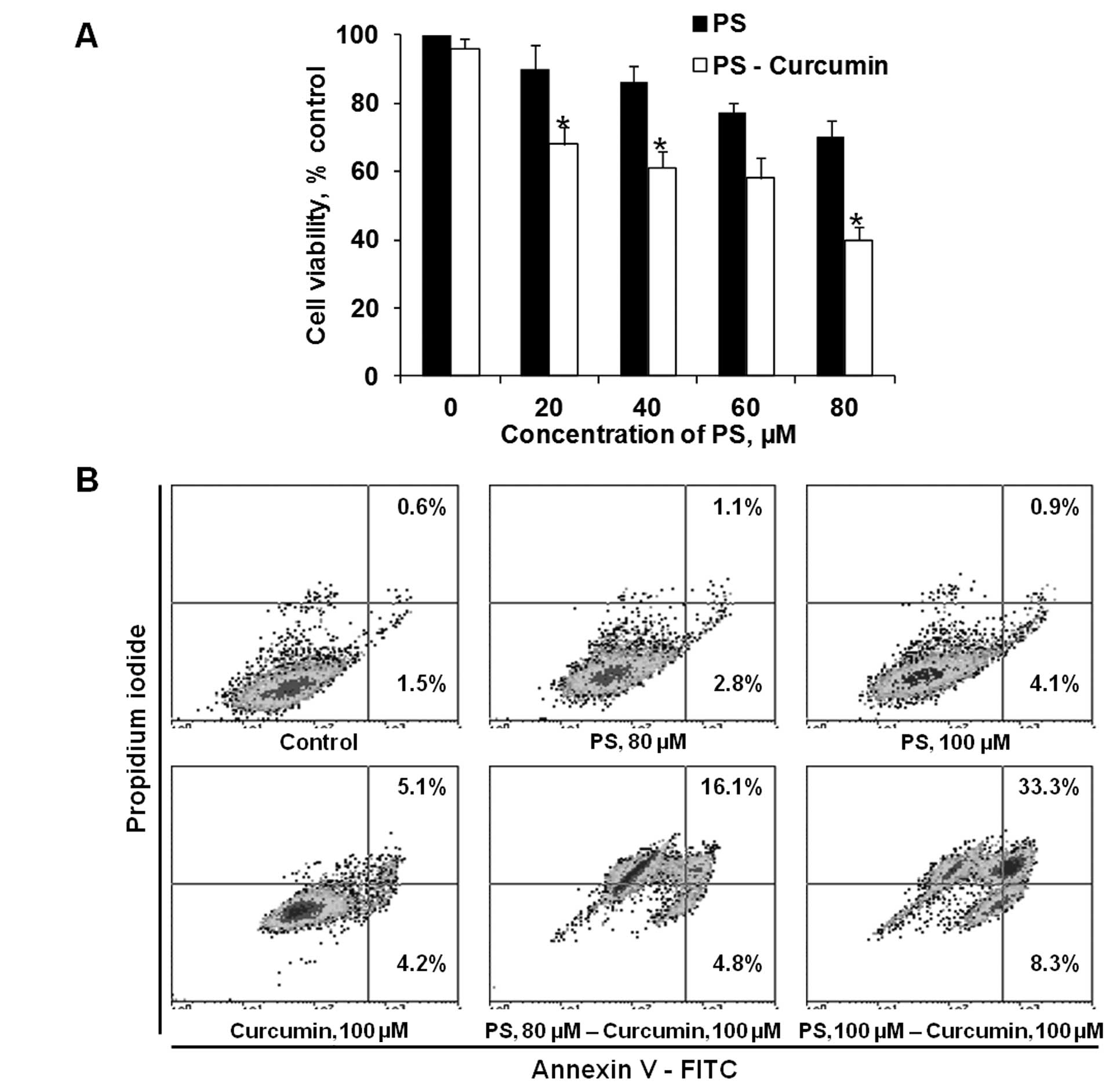
Pretreatment with curcumin sensitizes A549 lung
cancer cells to the cytotoxic effect of PS. As shown in Fig. 1A, pretreatment of A549 cells with
non-cytotoxic levels of curcumin 100 μM enhanced the cytotoxicity
of PS. Following treatment with 100 μM curcumin, reductions of cell
viability were as follows: PS 80 μM alone, 30%; curcumin alone, 4%;
and PS plus curcumin, 60%. A similar synergistic effect was also
observed in the induction of apoptosis. After 20-h incubation, the
percentage of apoptotic cells treated with 80 and 100 μM PS with
curcumin 100 μM was 20.9 and 41.6%, respectively, compared to 3.9
and 5.0% with 80 and 100 μM PS alone. These findings establish that
curcumin potentiates the cytotoxic activity of PS in lung cancer
cells.
Curcumin enhances the cellular uptake of
PS in cancer cells
Since curcumin is known to synergize with other
compounds and to also inhibit cellular efflux transporters, we
reasoned that curcumin might have synergistic activity with PS
through an effect on ATP-binding cassette (ABC) transporters which
are implicated in the cellular efflux of xenobiotics, such as
anticancer drugs. Isoform-specific inhibitors of ABC transporters
enable better understanding of the role of individual ABC
transporter(s) in the efflux of a drug (22). To identify specific ATP
transporters involved in the efflux of PS, changes in the cellular
levels of PS were evaluated in the presence of isoform-specific
inhibitors. In this study, the involvement of efflux transporters
was studied using inhibitors of multidrug resistance proteins
(MRPs; MK571), breast cancer resistance protein (BCRP; Ko143) and
P-glycoprotein (P-gp; verapamil).
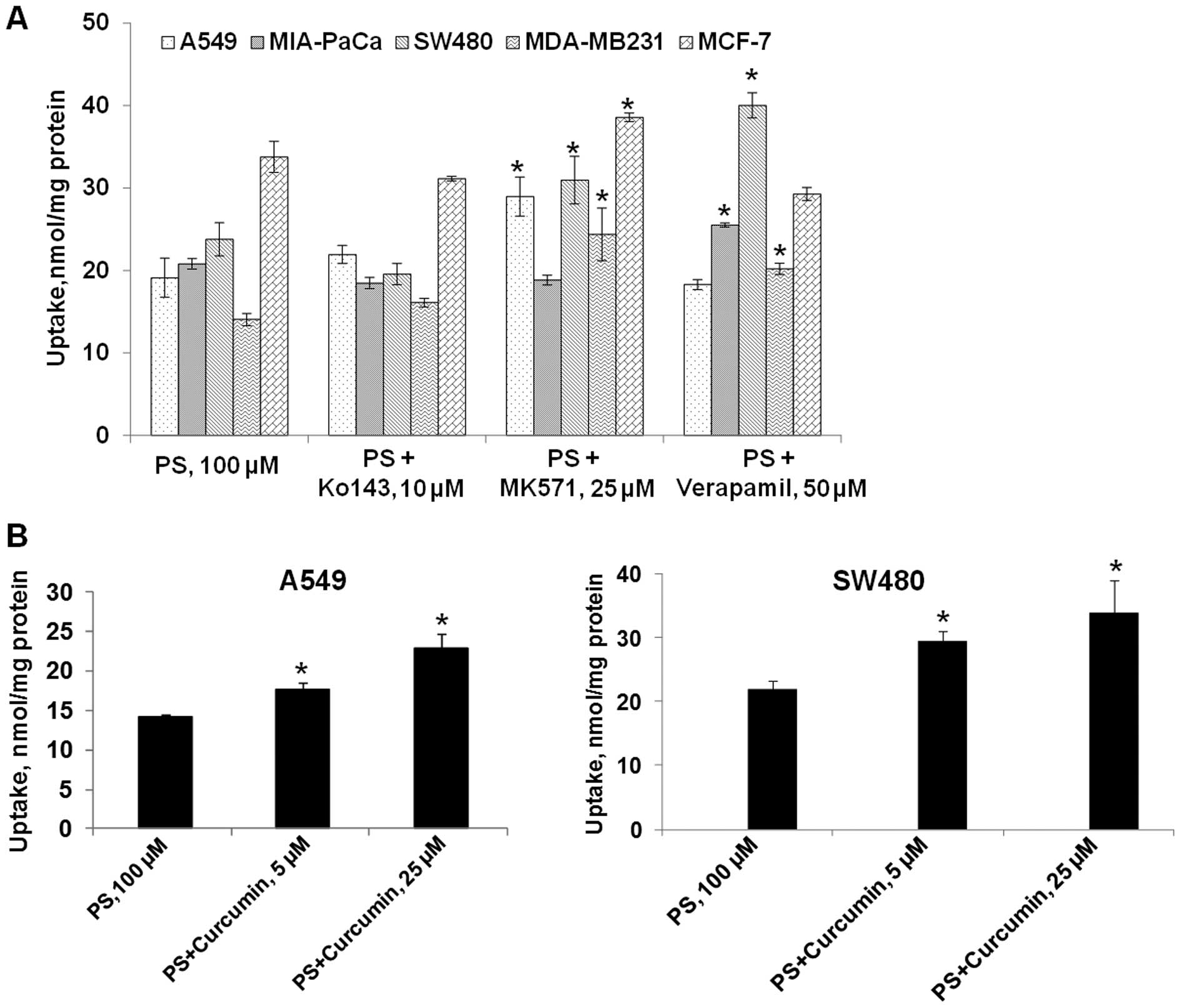
In A549 cells, co-incubation of PS with MK571
resulted in a 51% increase in the intracellular accumulation of PS
(Fig. 2A). Ko143 and verapamil had
no effect on the uptake of PS in A549 cells. In SW480 cells, MK571
and verapamil, but not Ko143, enhanced the cellular accumulation of
PS by 30 and 67%, respectively (Fig.
2A). It appeared that multidrug resistance proteins (MRPs) are
involved in PS efflux in both A549 and SW480 cells; while
P-glycoprotein (P-gp) is only involved in the efflux of PS in
SW480. BCRP, on the other hand, has little impact on the efflux of
PS. Thus, the specific ABC transporter(s) involved in the efflux of
PS is cell-line dependent.
Given that curcumin can inhibit efflux transporters
including MRPs and P-gp, we explored the effect of curcumin on the
uptake of PS in A549 and SW480 cells. The effect of curcumin (5 and
25 μM) on the accumulation of PS is shown in Fig. 1B. At 5 and 25 μM, curcumin
increased the intracellular levels of PS in A549 cells by 23 and
60%, respectively. Similarly, curcumin also increased the uptake of
PS in SW480 cells by 37% at 5 μM and 54% at 25 μM. Therefore,
co-incubation with curcumin recapitulated the effect of transporter
inhibitors. Since curcumin is an inhibitor of MRPs and P-gp, these
findings suggest that curcumin may enhance PS accumulation via
inhibition of efflux transports in these cancer cell lines.
PS and curcumin synergistically inhibit
the growth of A549 xenografts in mice
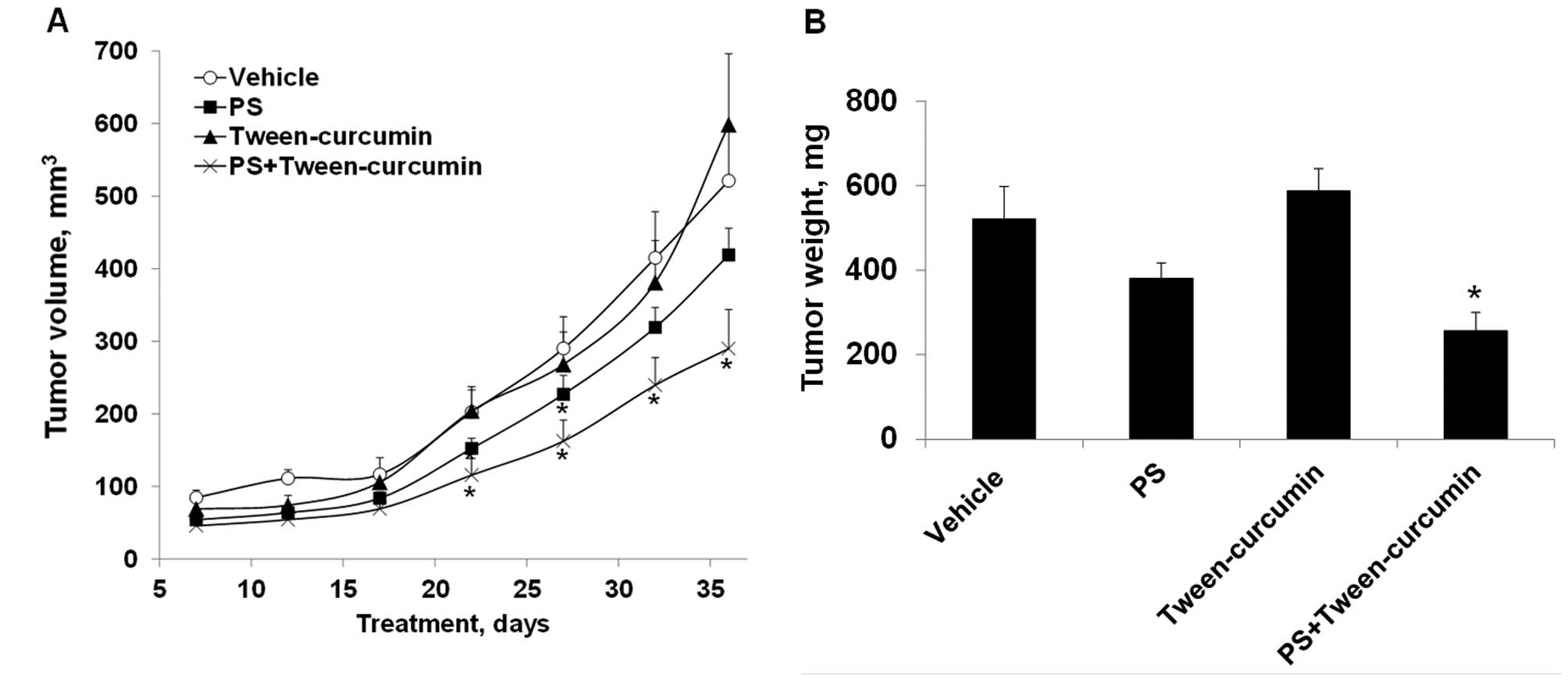
We investigated the antitumor efficacy of PS,
curcumin or their combination in subcutaneous xenografts of A549
human lung cancer cells in nude mice. PS and curcumin, when given
alone, did not significantly inhibit the growth of A549 xenografts
(Fig. 3A). PS alone produced a
small inhibition of tumor growth that was statistically significant
on days 17–27 after tumor implantation; whereas neither formulation
of curcumin was effective for the duration of the study. On the
other hand, PS in combination with curcumin suspended in 10%
Tween-80 synergistically inhibited the growth of A549 xenografts
and the effect was statistically significant (p<0.05) beginning
on day 12 until the end of the study (day 36). At the end of the
study, tumor volume of each group was as follows: control, 521±76
mm3; PS, 419±36 mm3; curcumin in 10%
Tween-80: 599±98 mm3; PS plus curcumin, 290±54
mm3. This corresponds to a reduction in tumor volume of
19.6 and 44.3% for PS and PS plus curcumin, respectively. In terms
of tumor weight (Fig. 3B), a
reduction was observed in the PS (27%, p=0.06) and the PS plus
curcumin (51%, p<0.01) groups, but not in the curcumin-treated
group. Of note, treatment with PS plus curcumin in 10% Tween-80 was
significantly more effective than PS or curcumin alone (p<0.05).
Surprisingly, no synergistic antitumor activity was observed for
the combination between PS and nanoparticles-encapsulated curcumin
(data no shown). Taken also into account that PS plus curcumin in
10% Tween-80 generated a better PK profile for PS than did PS plus
curcumin in nanoparticles, the results of the xenograft study
likely suggest the existence of a threshold level for the
pharmacological effective dose of PS and/or its metabolites.
PS, curcumin or their combination produced no
apparent adverse effects on the mice during the whole duration of
the study; and the mean body weights of the treatment groups were
comparable to that of the control. At sacrifice, the body weight of
the 4 groups of mice was as follows: control, 23±2 g; PS, 23±1 g;
curcumin, 23±1 g; and PS plus curcumin, 24±3 g.
Curcumin improves the bioavailability of
PS in mice
Having shown that curcumin enhances the cellular
uptake of PS and synergises with PS in inhibiting the growth of
human lung cancer xenografts, we next examined the effect of
curcumin co-administration on the bioavailability of PS in mice.
Curcumin in two formulations, 10% Tween-80 or encapsulated in
nanoparticles, was given to the mice 30 min prior to PS
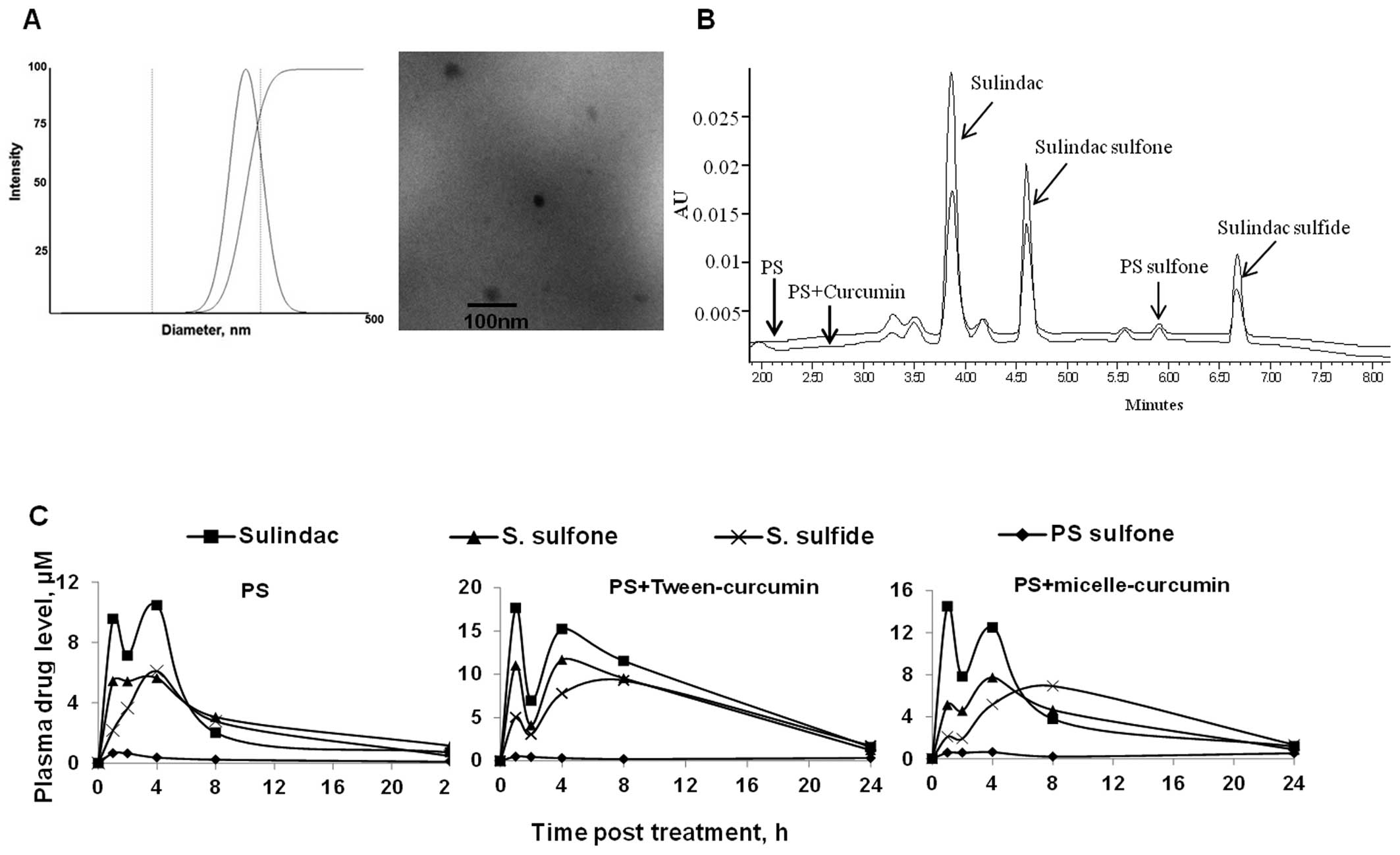
administration. As shown in Fig. 4
and Table I, curcumin in both
formulations increased the bioavailability of PS in vivo. PS
is rapidly metabolized in vivo into several metabolites, of
which quantitatively most important are sulindac, sulindac sulfide
and sulindac sulfone (23).
 | Table IPharmacokinetic parameters of major
metabolites of PS following administration of a single oral dose of
PS (200 mg/kg) alone or in combination with curcumin (500 mg/kg) in
10% Tween-80 (Tw-curcumin) and micelles (Mic-curcumin),
respectively. |
Table I
Pharmacokinetic parameters of major
metabolites of PS following administration of a single oral dose of
PS (200 mg/kg) alone or in combination with curcumin (500 mg/kg) in
10% Tween-80 (Tw-curcumin) and micelles (Mic-curcumin),
respectively.
| AUC0–24
h | Cmax,
μM | Tmax,
h |
|---|
|
|
|
|
|---|
| PS metabolite | PS | PS+Tw− curcumin | PS+Mic− curcumin | PS | PS+Tw− curcumin | PS+Mic− curcumin | PS | PS+Tw− curcumin | PS+Mic− curcumin |
|---|
| Sulindac | 77.5 | 201.9 | 110.8 | 10.4 | 17.7 | 14.5 | 4 | 1 | 1 |
| Sulindac sulfide | 57.3 | 137.6 | 100.3 | 6.1 | 9.3 | 6.9 | 4 | 8 | 8 |
| Sulindac sulfone | 70.3 | 153.6 | 88.5 | 5.6 | 11.7 | 7.7 | 4 | 4 | 4 |
| PS sulfone | 5.8 | 6.8 | 9.5 | 0.66 | 0.49 | 0.63 | 1 | 1 | 4 |
Peak plasma levels (Cmax) of sulindac,
sulindac sulfone and sulindac sulfide were much higher after the
co-administration of PS with curcumin. The Cmax of
sulindac, the main PS metabolite, was increased by 70% (17.7 μM)
and 40% (14.5 vs. 10.4 μM for PS alone) when co-administered with
curcumin in 10% Tween-80 or nanoparticles, respectively. As with
the case for sulindac, the Cmax of sulindac sulfone and
sulindac sulfide were also higher following administration of PS
with curcumin. Intact PS, however, was not detected in any of the
three treatments, presumably due to the high carboxylesterase
activity in the mouse blood (23).
In terms of total plasma AUC0–24 h, PS given with
curcumin in 10% Tween-80 (500 μM*h) or nanoparticles (309 μM*h)
increased the sum of AUC0–24 h of all metabolites by
2.4- and 1.5-fold, respectively, compared to PS alone (211 μM*h).
Interestingly, curcumin suspended in 10% Tween-80 proved to be more
effective than the nanoparticle formation in enhancing the
pharmacokinetics of PS, giving rise to much higher levels (30–50%)
of PS metabolites. On the other hand, curcumin had no apparent
effect on the metabolism of PS in mice, as indicated by their
similar metabolic profile (sulindac > sulindac sulfone =
sulindac sulfide > PS sulfone). Our results suggest that the
co-administration with curcumin enhances the bioavailability of PS
without affecting its metabolism in vivo (Fig. 4B and C).
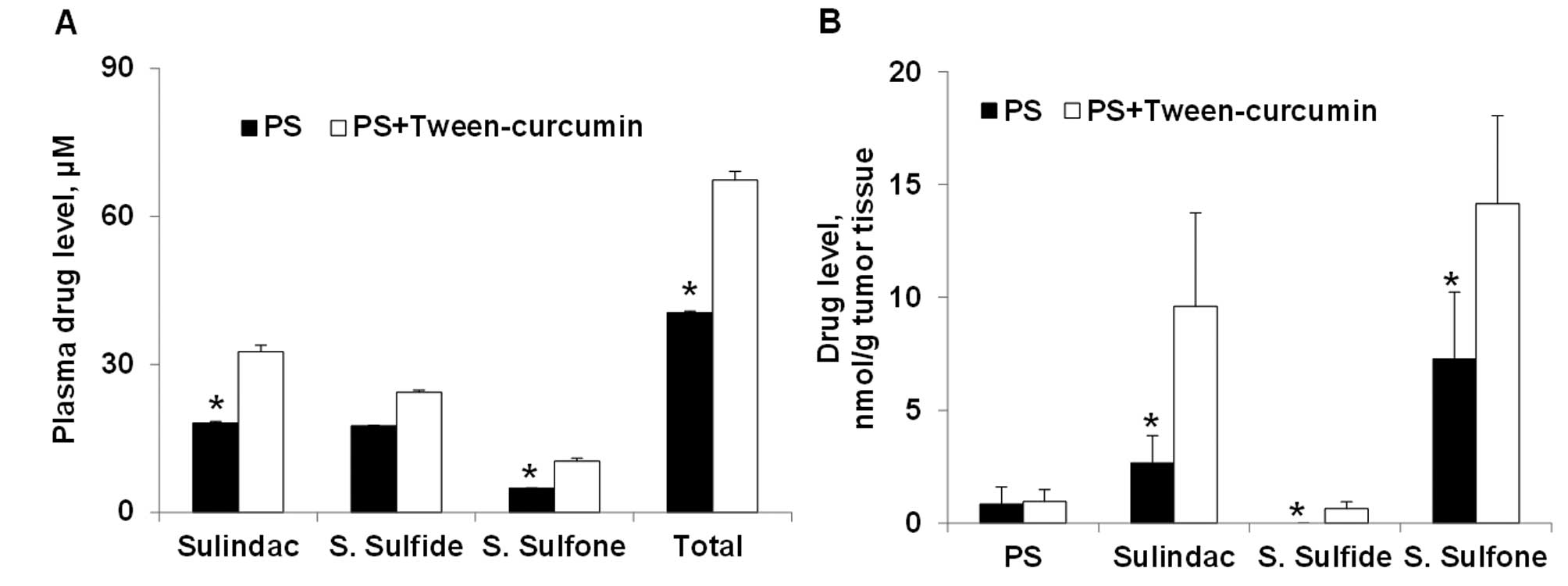
Curcumin enhances PS levels in A549
xenografts
Given the enhanced efficacy of the combined PS and
curcumin treatment, we assessed drug levels in the plasma and A549
xenografts from PS, curcumin and the combination treatment groups
(Fig. 5). Compared to PS alone, PS
plus curcumin generated higher levels of its three main metabolites
in the blood. In the A549 xenografts, the levels of sulindac,
sulindac sulfide and sulindac sulfone in the PS plus curcumin group
were 1-, 3- and 5-fold higher than those of the PS alone group. The
higher levels of PS metabolites are consistent with the higher
efficacy achieved with the PS and curcumin combination treatment.
We also detected minute levels of curcumin glucuronide in the
plasma of curcumin and PS plus curcumin groups, but there was no
significant difference in its levels between the two groups.
Neither curcumin, nor its glucuronide, was detected in A549
xenografts.
Discussion
Our study demonstrates that curcumin enhances the
efficacy of PS against human lung cancer in pre-clinical models. We
establish that curcumin: a) potentiates the cytotoxicity of PS
in vitro; b) increases the cellular uptake of PS in cancer
cells; c) improves the systemic bioavailability of PS and its
metabolites; and d) enhances the delivery of PS and its metabolites
to the A549 xenografts, leading to their synergistic growth
inhibition by the two agents.
The combination of PS and curcumin suspended in 10%
Tween-80 exerted a strong inhibitory effect on A549 xenografts in
nude mice, reducing tumor volume by 44% and tumor weight by 51%
(both p<0.05). In contrast, PS (20% inhibition) or curcumin (no
inhibition) alone did not produce a significant inhibitory effect
on A549 xenografts. A key finding of this study is that curcumin
co-administration improves the bioavailability and pharmacokinetic
properties of PS, as illustrated by higher peak levels
(Cmax) and a 2.4-fold increase in total AUC0–24
h of PS and its metabolites. Consequently, administration of
PS plus curcumin resulted in much greater accumulation of PS and
its metabolites in A549 xenografts compared to PS alone. The
pharmacokinetic profile of curcumin, however, was not affected when
combined with PS. Given that curcumin (and its metabolites) showed
no detectable accumulation in tumors and had no tumor inhibitory
effect, these data indicate that the synergistic effect of PS plus
curcumin in 10% Tween-80 is predominantly a consequence of the
enhanced delivery of PS and its metabolites to the tumors.
The chemo-sensitizing effect of curcumin is
dependent upon its ability to inhibit ATP-binding cassette (ABC)
proteins. ABC transporters are the primary active transporters that
mediate the efflux of xenobiotics such as anticancer drugs; and
their overexpression in cancer cells is associated with multidrug
resistance (24). Curcumin is a
promiscuous inhibitor of drug transporters from the ABC family,
including P-glycoprotein (MDR1/ABCB1) (25), BCRP (ABCG2) (26) and multiple MRPs [MRP1/ABCC1,
MRP2/ABCC2 (27,28), MRP5/ABCC5 (29)]. Our study revealed that curcumin
enhances the bioavailability of PS in vitro and in
vivo by improving the cellular uptake of PS. The uptake of PS
into lung and colon cancer cells in vitro is influenced by
drug efflux transporters, such as MRPs and P-glycoproteins, which
decrease the accumulation and cellular toxicity of PS. Curcumin, at
non-cytotoxic levels, antagonizes the effect of these transporters
and thus increases the cellular uptake of PS in cancer cells,
thereby potentiating its cytotoxic activity in vitro.
Curcumin may improve the bioavailability of PS in
two ways. First, curcumin may inhibit efflux transporters in the
intestinal barrier, thus enhancing the absorption of PS. Second,
curcumin may also inhibit drug efflux in tumor xenografts,
resulting in increased biodistribution of PS and its metabolites to
the target tissue. The higher levels of PS and its metabolites in
the A549 xenografts were consequential, as they correlated with
reduced tumor volume. On the other hand, curcumin did not affect
the metabolism of PS by carboxylesterases and cytochrome P450s. Our
findings support the idea that curcumin potentiates the antitumor
activity of PS through enhanced delivery of PS and its metabolites
to tumors. The three quantitatively important metabolites of PS
(sulindac, sulindac sulfide and sulindac sulfone) are known to have
anticancer properties (23) both
through COX-dependent and -independent pathway (30).
In conclusion, our data demonstrate that the
co-administration of PS and curcumin synergistically inhibits the
growth of human lung cancer xenografts in nude mice. The enhanced
efficacy is attributed to inhibition of efflux transporters by
curcumin, leading to improved PS bioavailability including the
target tumor. This promising drug combination merits further
evaluation.
Acknowledgements
This study was supported by National Institutes of
Health Grants HHSN261201000109C, R01 CA101019 and R01 CA139454 and
DOD Grants W81XWH 11-1-0799, W81XWH-0710171 and W81XWH1010873.
References
|
1
|
Elwood PC, Gallagher AM, Duthie GG, Mur LA
and Morgan G: Aspirin, salicylates and cancer. Lancet.
373:1301–1309. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rothwell PM, Price JF, Fowkes FG, et al:
Short-term effects of daily aspirin on cancer incidence, mortality
and non-vascular death: analysis of the time course of risks and
benefits in 51 randomised controlled trials. Lancet. 379:1602–1612.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rothwell PM, Wilson M, Price JF, et al:
Effect of daily aspirin on risk of cancer metastasis: a study of
incident cancers during randomised controlled trials. Lancet.
379:1591–1601. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng KW, Mattheolabakis G, Wong CC, et
al: Topical phospho-sulindac (OXT-328) is effective in the
treatment of non-melanoma skin cancer. Int J Oncol. 41:1199–1203.
2012.PubMed/NCBI
|
|
5
|
Huang L, Mackenzie GG, Sun Y, et al:
Chemotherapeutic properties of phospho-nonsteroidal
anti-inflammatory drugs, a new class of anticancer compounds.
Cancer Res. 71:7617–7627. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mackenzie GG, Ouyang N, Xie G, et al:
Phospho-sulindac (OXT-328) combined with difluoromethylornithine
prevents colon cancer in mice. Cancer Prev Res. 4:1052–1060. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu C, Cheng KW, Ouyang N, et al:
Phosphosulindac (OXT-328) selectively targets breast cancer stem
cells in vitro and in human breast cancer xenografts. Stem Cells.
30:2065–2075. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu R, Cheng KW, Mackenzie G, et al:
Phospho-sulindac (OXT-328) inhibits the growth of human lung cancer
xenografts in mice: enhanced efficacy and mitochondria targeting by
its formulation in solid lipid nanoparticles. Pharm Res.
29:3090–3101. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hanif R, Qiao L, Shiff SJ and Rigas B:
Curcumin, a natural plant phenolic food additive, inhibits cell
proliferation and induces cell cycle changes in colon
adenocarcinoma cell lines by a prostaglandin-independent pathway. J
Lab Clin Med. 130:576–584. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kawamori T, Lubet R, Steele VE, et al:
Chemopreventive effect of curcumin, a naturally occurring
anti-inflammatory agent, during the promotion/progression stages of
colon cancer. Cancer Res. 59:597–601. 1999.PubMed/NCBI
|
|
11
|
Villegas I, Sanchez-Fidalgo S and de la
Lastra CA: Chemopreventive effect of dietary curcumin on
inflammation-induced colorectal carcinogenesis in mice. Mol Nutr
Food Res. 55:259–267. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Su CC, Yang JS, Lu CC, et al: Curcumin
inhibits human lung large cell carcinoma cancer tumour growth in a
murine xenograft model. Phytother Res. 24:189–192. 2010.PubMed/NCBI
|
|
13
|
Dance-Barnes ST, Kock ND, Moore JE, et al:
Lung tumor promotion by curcumin. Carcinogenesis. 30:1016–1023.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giladi N, Kazanov D, Shpitz B, Aroch I,
Kraus S and Arber N: Curcumin potentiates the pro-apoptotic effects
of sulindac sulfone in colorectal cancer. Expert Opin Investig
Drugs. 19:S117–S124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee JY, Lee YM, Chang GC, et al: Curcumin
induces EGFR degradation in lung adenocarcinoma and modulates p38
activation in intestine: the versatile adjuvant for gefitinib
therapy. PloS One. 6:e237562011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yin H, Guo R, Xu Y, et al: Synergistic
antitumor efficiency of docetaxel and curcumin against lung cancer.
Acta Biochim Biophys Sin. 44:147–153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ganta S and Amiji M: Coadministration of
Paclitaxel and curcumin in nanoemulsion formulations to overcome
multidrug resistance in tumor cells. Mol Pharm. 6:928–939. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sung B, Kunnumakkara AB, Sethi G, et al:
Curcumin circumvents chemoresistance in vitro and potentiates the
effect of thalidomide and bortezomib against human multiple myeloma
in nude mice model. Mol Cancer Ther. 8:959–970. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Francois G and Katz JL: Nanoparticles and
nanocapsules created using the Ouzo effect: spontaneous
emulisification as an alternative to ultrasonic and high-shear
devices. Chemphyschem. 6:209–216. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Galindo-Rodriguez S, Allemann E, Fessi H
and Doelker E: Physicochemical parameters associated with
nanoparticle formation in the salting-out, emulsification-diffusion
and nanoprecipitation methods. Pharm Res. 21:1428–1439. 2004.
View Article : Google Scholar
|
|
21
|
Wong CC, Cheng KW, Xie G, et al:
Carboxylesterases 1 and 2 hydrolyze phospho-nonsteroidal
anti-inflammatory drugs: relevance to their pharmacological
activity. J Pharmacol Exp Ther. 340:422–432. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Balimane PV, Han YH and Chong S: Current
industrial practices of assessing permeability and P-glycoprotein
interaction. AAPS J. 8:E1–E13. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xie G, Nie T, Mackenzie GG, et al: The
metabolism and pharmacokinetics of phospho-sulindac (OXT-328) and
the effect of difluoromethylornithine. Br J Pharmacol.
165:2152–2166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chearwae W, Anuchapreeda S, Nandigama K,
Ambudkar SV and Limtrakul P: Biochemical mechanism of modulation of
human P-glycoprotein (ABCB1) by curcumin I, II and III purified
from Turmeric powder. Biochem Pharmacol. 68:2043–2052. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shukla S, Zaher H, Hartz A, et al:
Curcumin inhibits the activity of ABCG2/BCRP1, a multidrug
resistance-linked ABC drug transporter in mice. Pharm Res.
26:480–487. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wortelboer HM, Usta M, van der Velde AE,
et al: Interplay between MRP inhibition and metabolism of MRP
inhibitors: the case of curcumin. Chem Res Toxicol. 16:1642–1651.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wortelboer HM, Usta M, van Zanden JJ, et
al: Inhibition of multidrug resistance proteins MRP1 and MRP2 by a
series of alpha,beta-unsaturated carbonyl compounds. Biochem
Pharmacol. 69:1879–1890. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Y, Revalde JL, Reid G and Paxton JW:
Modulatory effects of curcumin on multi-drug resistance-associated
protein 5 in pancreatic cancer cells. Cancer Chemother Pharmacol.
68:603–610. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rigas B and Shiff SJ: Nonsteroidal
anti-inflammatory drugs (NSAIDs), cyclooxygenases and the cell
cycle. Their interactions in colon cancer. Adv Exp Med Biol.
470:119–126. 1999. View Article : Google Scholar : PubMed/NCBI
|