Introduction
Human ovarian carcinoma remains a major cause of
mortality and morbidity in the United States (1). Roughly 80% of patients will present
with advanced-stage disease, but current chemotherapy is able to
produce significant response rates and even long-term remission in
only 20% of these women. cis-Diamminedichloroplatinum (II)
(cisplatin) is one of the most effective anticancer drugs in the
treatment of human ovarian cancer and other tumors (2–4).
However, the efficacy of cisplatin is hampered because cancer cells
acquire resistance to its cytotoxicity. Although the mechanism of
cisplatin resistance in vivo is not clearly defined,
laboratory studies with tumor tissues and cell lines suggest that
enhanced nucleotide excision repair (NER) of cisplatin-caused DNA
damage and impaired cisplatin-induced apoptosis play crucial roles
in the development of the cisplatin-resistance phenotype (4–6). The
expression of DNA repair genes such as excision repair
cross-complementation group-1 (ERCC-1) and xeroderma
pigmentosum complementation group A (XPA) is reported to be
strongly associated with a poor prognosis in ovarian carcinoma and
other tumors (7,8). Therefore, compounds that can
circumvent cisplatin resistance and augment the effects of
chemotherapy are needed.
One candidate drug to fulfill this role is β-elemene
(β-1-methyl-1-vinyl-2,4-di-isopropenyl-cyclohexane). β-Elemene
(Fig. 1), a natural anticancer
plant-derived drug, was approved by the Chinese Food and Drug
Administration for the treatment of human cancers. The major
advantages of β-elemene as an anticancer drug are: i) it has
broad-spectrum antitumor effects in many types of cancer, including
drug-resistant tumors; ii) it does not direct multidrug resistance
and can reverse the resistance to other drugs; and iii) it has low
toxicity and is therefore well tolerated and accepted by patients
with cancer. The effect of β-elemene on other drug sensitivity in
human tumors is unknown. We have recently reported that β-elemene
increases sensitivity to cisplatin and augments cisplatin-induced
apoptosis in chemoresistant human ovarian cancer cells and other
tumor cells (9–20). These novel findings indicate that
β-elemene may be efficacious in the treatment of
cisplatin-resistant tumors.
We hypothesized that β-elemene enhancement of
cisplatin sensitivity in human chemoresistant ovarian cancer cells
is mediated at least in part through the regulation of DNA repair
activity and apoptotic death signaling. To test this hypothesis and
elucidate the mechanism underlying the effect of β-elemene on
cisplatin cytotoxicity, we investigated whether β-elemene promotes
apoptotic cell death in cisplatin-treated resistant ovarian cancer
cells by downregulating the expression of ERCC-1 and X-linked
inhibitor of apoptosis protein (XIAP) and inactivating c-Jun
NH2-terminal kinase (JNK). This research is significant
because β-elemene may be useful therapeutically as a modulator of
platinum drug resistance in human malignancies. Our results provide
a framework for exploiting the combination of β-elemene and
cisplatin as a potentially effective chemotherapy regimen to
overcome cisplatin drug resistance in ovarian cancer and other
tumors.
Materials and methods
Chemicals and immunoreagents
The (−)-β-elemene (98% purity) was obtained from
Yuanda Pharmaceuticals Ltd. Inc. (Dalian, China). Cisplatin,
dimethylsufoxide and other chemicals were purchased from
Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Antibodies against
XIAP and β-actin, peroxidase-labeled anti-rabbit immunoglobulin G
(IgG), Blotto B, and the ECL western blot analysis system were
purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA).
Anti-phospho-JNK1 (Thr183/Tyr185) antibody was purchased from Cell
Signaling Technology (Beverly, MA, USA), as described previously
(21,22).
Cells and cell culture conditions
The human cisplatin-resistant ovarian cancer cell
lines A2780/CP70 and MCAS and the parental sensitive ovarian cancer
cell line A2780 have been studied extensively by our laboratory
(15,17,18).
The cells were cultured in monolayers using RPMI-1640 medium
(Invitrogen, Life Technologies, Gaithersburg, MD, USA) supplemented
with 10% (v/v) fetal bovine serum, 50 U/ml penicillin, and 50 μg/ml
streptomycin (Invitrogen), and were grown in logarithmic growth at
37°C in a humidified atmosphere of 5% CO2 and 95% air.
The cells were routinely tested for mycoplasma infection using a
commercial assay system (MytoTect; Invitrogen), and new cultures
were established monthly from frozen stocks. All media and reagents
contained <0.1 ng/ml endotoxin as determined by a Limulus
polyphemus amebocyte lysate assay (Whittaker Bioproducts,
Walkersville, MD, USA). Before starting the experiments, the cells
were sub-cultured and grown to 70–80% confluence. Cisplatin was
initially dissolved at 5 mM in phosphate-buffered saline (PBS)
without Ca2+ or Mg2+. Cisplatin and β-elemene
were serially diluted, respectively, in culture medium to obtain
the desired concentrations.
Cell growth inhibition assay
The antiproliferative effects of β-elemene alone,
cisplatin alone, and cisplatin plus β-elemene were assessed using
the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) assay (Promega Corp., Madison, WI, USA) according to the
manufacturer’s instructions. In brief, the cells were evenly
distributed in 96-well plates (5×103 cells/well), grown
overnight and then treated for 24, 48, 72 and 96 h with β-elemene
alone (0, 20, 40, 60, 80, 100, 120, 140, 160, 180 and 200 μg/ml),
cisplatin alone (0, 1.0, 2.0, 4.0, 8.0, 16.0, 32.0, 64.0, 128.0,
256.0 and 512.0 μM), or a combination of cisplatin (at the above
concentrations) plus β-elemene (40 μg/ml). After incubation, 20 μl
of CellTiter 96 Aqueous One Solution reagent were added to each
well of the assay plates containing treated and untreated cells in
100 μl of culture medium, and the plates were incubated at 37°C and
5% CO2 for 1–4 h. The optical density at 590 nm was
determined using a 96-well Opsys MR™ microplate reader (Thermo
Labsystems, Chantilly, VA, USA). Proliferation rates were
calculated from the optical density of drug-treated cells relative
to that of cells with no added drug (control value, 100%), as
follows: percentage cell viability = [(OD with drug - blank) ÷ (OD
without drug - blank)] × 100. The half-maximal inhibitory
concentration (IC50) was determined from the
dose-response curves. The dose-modifying factor (DMF) was
calculated as the IC50 for cisplatin without β-elemene
divided by the IC50 for cisplatin with β-elemene: DMF =
IC50 (cisplatin) ÷ IC50 (cisplatin +
β-elemene).
Generation of ERCC-1 antiserum
Polyclonal anti-peptide antiserum was generated by
Bio-Synthesis Inc. (Lewisville, TX, USA). A synthetic peptide
containing the carboxy-terminus of ERCC-1 was coupled to keyhole
limpet hemocyanin using m-maleimidobenzoyl-N-hydroxysuccinimide
ester as a cross-linker. This was used to immunize New Zealand
white female rabbits, which were bled at regular intervals to
obtain serum containing the antibodies. The undiluted antiserum was
used in western blot analyses, as described previously (23,24).
Protein extraction and western blot
analysis
Ovarian tumor cells treated with β-elemene,
cisplatin or their combination were harvested by trypsinization,
washed with ice-cold PBS, and lysed on ice for 30 min in mammalian
cell lysis buffer (Quality Biological Inc., Gaithersburg, MD, USA)
containing 10 μl/ml 200 mM phenylmethylsulfonyl fluoride, 10 μl/ml
100 mM sodium orthovanadate and 10 μg/ml aprotinin. Lysates were
clarified by centrifugation at 13,000 × g for 30 min at 4°C, and
the protein concentrations in the supernatants were determined by
Bradford assay (Bio-Rad, Richmond, CA, USA). Proteins (40 to 60 μg)
from whole-cell lysates were mixed 1:1 with 2X sodium dodecyl
sulfate (SDS) gel solution (Quality Biological Inc.), heated for 5
min at 95°C, separated by 10% SDS-polyacrylamide gel
electrophoresis, and transferred to nitrocellulose membranes
(Schleicher & Schuell BioScience Inc., Keene, NH, USA). After
blocking in Blotto B for 1 h at room temperature, the membranes
were incubated overnight at 4°C with specific primary antibodies
(diluted 1:100–1:300). The membranes were washed with TBS/0.1%
Tween-20 solution, incubated with anti-rabbit peroxidase-conjugated
secondary antibody (diluted 1:10,000), and washed again.
Immunoreactive bands were detected with enhanced chemiluminescence
substrate according to the manufacturer’s instructions and
visualized using X-ray film (Eastman Kodak, Rochester, NY, USA).
All blots shown are representative of three independent
experiments.
Statistical analysis
All quantitative values are presented as means ± SD.
Data were analyzed using two-way analysis of variance (ANOVA) for
comparison among groups. Student’s t-test was used to
analyze the significance of differences between untreated and
treated groups. All p-values were determined using a two-sided
t-test, and p-values <0.05 were considered to indicate
significance.
Results
β-Elemene suppresses cell proliferation
and augments cisplatin-induced cytotoxicity in resistant and
sensitive human ovarian cancer cells
We first examined the in vitro antitumor
activity of β-elemene in human ovarian carcinoma cells, as
determined by the MTT assay. β-Elemene at concentrations of 20–200
μg/ml dose-dependently inhibited the growth and proliferation of
both A2780 and A2780/CP70 cells at 24, 48, 72 and 96 h, with
IC50 values at 24, 48, 72 and 96 h ranging between 60
and 65 μg/ml for A2780 cells and between 65 and 80 μg/ml for
A2780/CP70 cells (Table I).
Similarly, the IC50 values of β-elemene for MCAS cells
were between 60 and 78 μg/ml. The IC50 values were not
significantly different between the cisplatin-sensitive (A2780) and
cisplatin-resistant (A2780/CP70 and MCAS) cell lines (p>0.05),
indicating that β-elemene has a similar antitumor activity toward
both sensitive and resistant ovarian cancer cells in vitro.
Thus, cisplatin-resistant ovarian tumor cells are still sensitive
to β-elemene.
 | Table Iβ-Elemene increases cisplatin
cytotoxicity and enhances cisplatin sensitivity in human ovarian
carcinoma cells, as determined by MTT assay. |
Table I
β-Elemene increases cisplatin
cytotoxicity and enhances cisplatin sensitivity in human ovarian
carcinoma cells, as determined by MTT assay.
|
IC50 |
|---|
|
|
|---|
| Drug | 24 h | 48 h | 72 h | 96 h |
|---|
| A2780 cells |
| β-Elemene
(μg/ml) | 65 | 65 | 65 | 60 |
| Cisplatin (μM) | 6.2 | 1.75 | 1.6 | 1.5 |
| β-Elemene +
cisplatin (μM) | 3.8 | 0.8 | 0.75 | 0.6 |
| Dose-modifying
factor | 1.6 | 2.2 | 2.1 | 2.5 |
| A2780/CP70
cells |
| β-Elemene
(μg/ml) | 80 | 70 | 68 | 65 |
| Cisplatin (μM) | 95 | 66 | 65 | 60 |
| β-Elemene +
cisplatin (μM) | 2.5 | 1.9 | 1.85 | 1.0 |
| Dose-modifying
factor | 38 | 34.7 | 35.1 | 60 |
Next, we assessed the enhancing effect of β-elemene
on cisplatin cytotoxicity in human ovarian tumor cells using the
MTT assay. A2780 and A2780/CP70 cells were exposed to cisplatin
alone or in combination with β-elemene (40 μl/ml) for 24, 48, 72
and 96 h, and the inhibition of cell growth was measured in
vitro. At concentrations of 0 to 512.0 μM, cisplatin caused a
dose-dependent inhibition of A2780 and A2780/CP70 cell
proliferation at all four time points. The IC50 values
of cisplatin alone for chemoresistant A2780/CP70 cells at 24, 48,
72 and 96 h were 95.0, 66.0, 65.0, and 60.0 μM, respectively, and
decreased strikingly to 2.5, 1.9, 1.85 and 1.0 μM, respectively,
when cisplatin was combined with β-elemene (Table I; p<0.01); the dose-modifying
factors (DMFs) for cisplatin in A2780/CP70 cells ranged from 35 to
60. Similarly, the IC50 of cisplatin alone for MCAS
cells was 38.0 μM and was reduced markedly to 6.5 μM when cisplatin
was combined with β-elemene (p<0.01); the DMF in this cell line
was 5.85. Although the IC50 values of cisplatin alone
for chemosensitive A2780 cells (6.2, 1.75, 1.6 and 1.5 μM at 24,
48, 72 and 96 h, respectively) decreased significantly when
cisplatin was combined with β-elemene (3.8, 0.8, 0.75 and 0.6 μM,
respectively) (Table I;
p<0.05), the DMFs for cisplatin in A2780 cells, which ranged
from 1.6 to 2.5, were significantly lower than those in A2780/CP70
and MCAS cells (p<0.05). These results suggest that β-elemene
and cisplatin may act synergistically to enhance cytotoxicity in
both chemoresistant and chemosensitive ovarian carcinoma cells, but
that β-elemene has a greater effect on cisplatin sensitivity in
chemoresistant ovarian tumor cells.
The effect of β-elemene on the protein
level of ERCC-1, phospho-JNK1 and XIAP in chemoresistant human
ovarian carcinoma cells
Enhanced DNA repair capacity contributes to
cisplatin resistance in human tumors, and NER is responsible for
the repair of platinum-DNA adducts in human cells. Furthermore,
high levels of ERCC-1 protein support the efficient DNA repair
capacity of resistant cancer cells and ERCC-1 is a marker
gene for the NER mechanism. Thus, to investigate whether the
mechanism by which β-elemene reverses drug resistance to cisplatin
involves, at least in part, the inhibition of DNA repair activity,
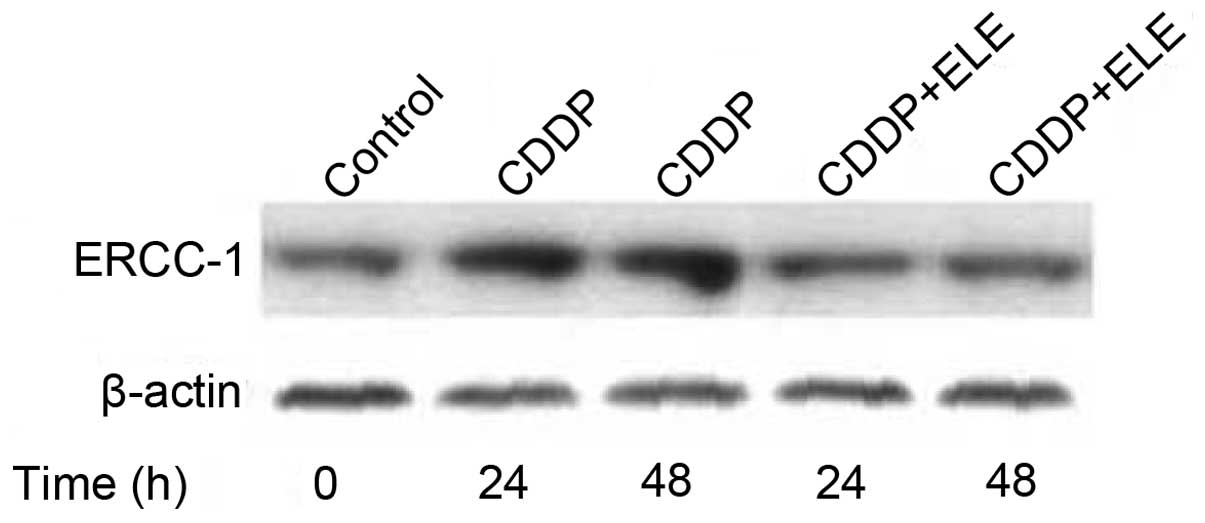
we tested the effect of β-elemene on cisplatin-upregulated ERCC-1
expression in resistant ovarian cancer cells. β-Elemene
significantly attenuated the cisplatin-induced increase in the
ERCC-1 protein level in A2780/CP70 ovarian cancer cells (Fig. 2), indicating that the reduction of
DNA repair activity by β-elemene is positively associated with
increased cisplatin cytotoxicity in resistant ovarian tumor
cells.
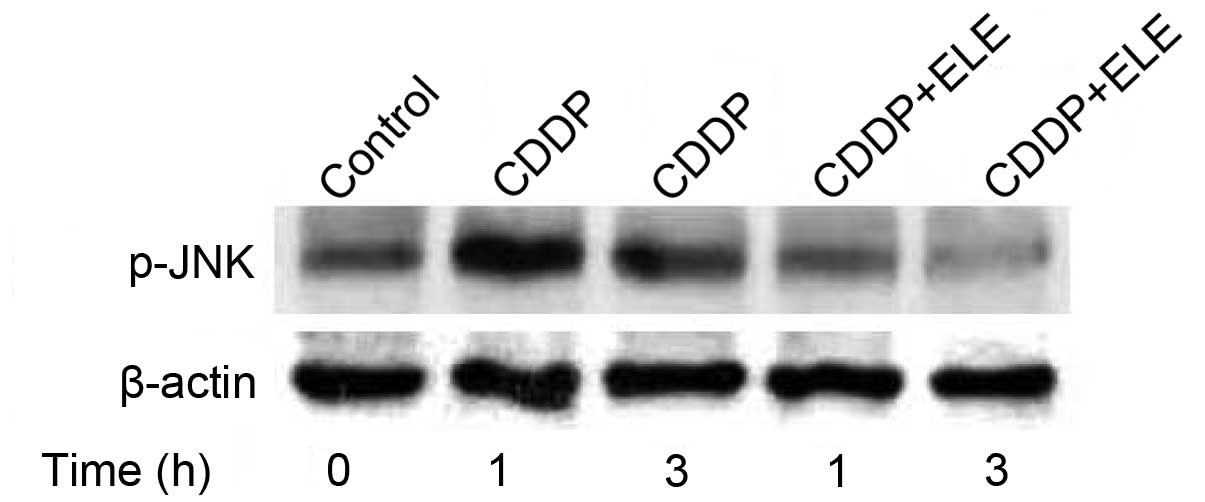
The upregulation of ERCC-1 gene expression is
mediated by activator protein 1 (AP-1) transcriptional activity,
which is activated by a JNK phosphorylation cascade. In A2780/CP70
cells, β-elemene inhibited the cisplatin-induced increase in JNK
phosphorylation (Fig. 3),
suggesting that β-elemene may suppress ERCC-1 expression via a
phosphatidylinositol 3-kinase (PI3K)/JNK signaling pathway.
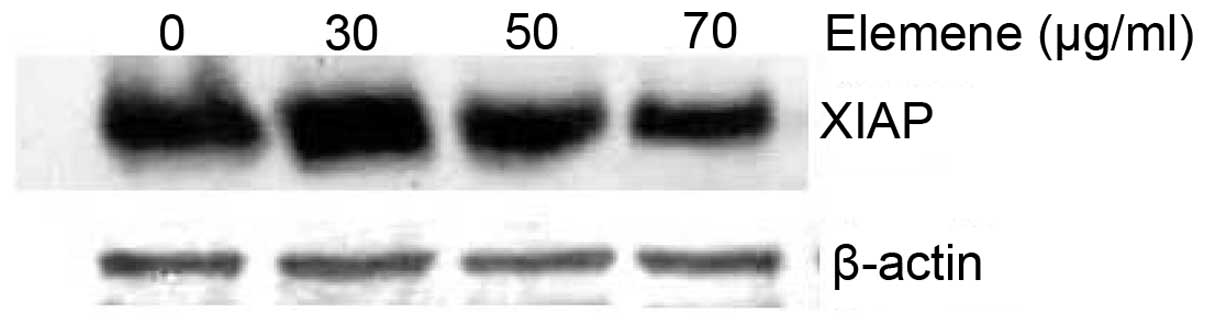
Activation of the PI3K/protein kinase B (Akt)
pathway leads to the upregulated expression of XIAP, which
modulates death signaling pathways and is a determinant of
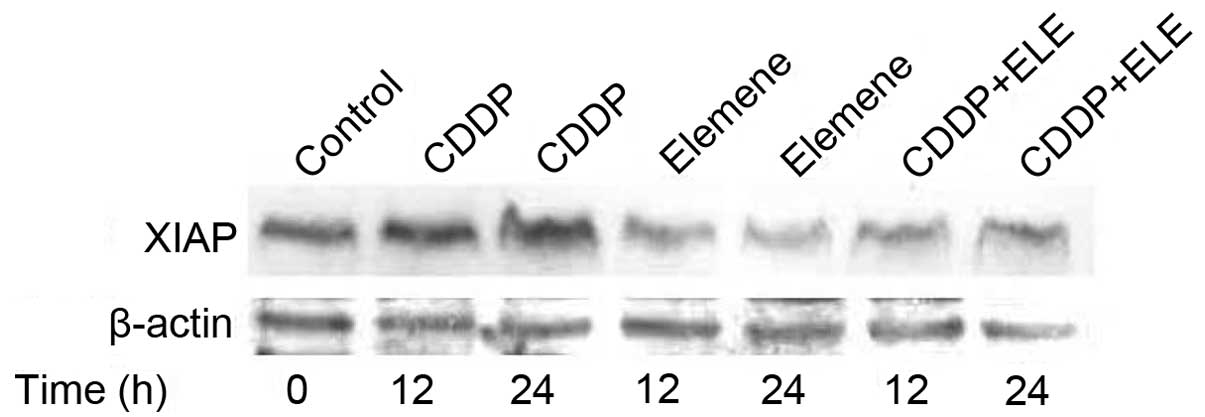
cisplatin resistance in ovarian cancer cells. β-Elemene not only
reduced the XIAP protein level (Fig.
4) but also abrogated cisplatin-induced XIAP expression in
resistant ovarian tumor cells (Fig.
5), indicating the involvement of XIAP in the mechanism of
β-elemene action in resistant ovarian cancer cells.
Discussion
The greatest limitation to the successful treatment
of ovarian cancer is the development of clinical resistance to
cisplatin. Although the mechanism of cisplatin resistance in
vivo is not clearly understood, laboratory studies on tumor
tissues and cell lines suggest that resistance to cisplatin is
multifactorial (25,26). These factors include impaired
cellular uptake of cisplatin (27), increased intracellular
detoxification by glutathione and metallothionein systems (28), altered patterns of DNA platination,
impaired cisplatin-induced apoptosis and enhanced repair of
platinum-damaged DNA (4–8,26,27,29,30).
We have been investigating the mechanisms of
cisplatin drug resistance, focusing on the relationship between DNA
repair and cisplatin resistance in ovarian cancer and other tumors
(21–24,31–36).
Increasing evidence indicates that NER is responsible for the
repair of platinum-caused DNA damage (4–8,37).
Repair-defective cells are hypersensitive to cisplatin (38,39),
and enhanced DNA repair has been implicated in the
cisplatin-resistance phenotype (27,30,40).
Furthermore, increased repair of cisplatin-caused interstrand
cross-links and intrastrand adducts is associated with resistance
in human ovarian cancer cells (41) and in laboratory-derived
cisplatin-resistant lines (27).
ERCC-1 is a key DNA repair protein in the NER process and a useful
biomarker for NER activity in human cells. The overexpression of
ERCC-1 and other NER genes has been associated with the
repair of cisplatin-induced DNA damage (30,37,41)
and clinical resistance to cisplatin (42,43).
The expression levels of ERCC-1 in cisplatin hypersensitive,
repair-deficient cells are 50- to 30-fold lower than those in
resistant cells. These observations indicate that enhanced DNA
repair capacity contributes to the development of cisplatin
resistance in human cancers. In the present study, we showed that
β-elemene increased the sensitivity to cisplatin and blocked
cisplatin-induced ERCC-1 protein expression in resistant human
ovarian cancer cells, suggesting that β-elemene enhances cisplatin
sensitivity in resistant ovarian cancer cells by decreasing the
proficiency of repair of cisplatin-induced DNA damage.
A number of studies have reported that NER gene
expression is regulated by the JNK/AP-1 pathway in response to
cisplatin in vitro. The AP-1 family of transcription factors
is a heterodimeric protein composed of proteins belonging to the
c-Fos, c-Jun, ATF and JDP families, and is responsible for the
activation of a wide variety of genes in different cell types and
tissues. AP-1 binding sites (5′-TGAG/CTCA-3′) are frequently found
in promoters or enhancers of genes that are inducible by cisplatin.
Evidence has shown that cisplatin induces the expression of
c-fos/c-jun(21,22,32)
and activates JNK (21,22,32,44,45)
in ovarian cancer cells. Therefore, the activation of AP-1 and
subsequent overexpression of AP-1-regulated NER genes may enhance
DNA repair capacity in affected cells and contribute to decreased
chemosensitivity in human ovarian cancer cells (37,41).
This hypothesis is supported by several lines of
evidence. We have previously demonstrated that cisplatin exposure
activates an AP-1-mediated increase in ERCC-1 expression in
human ovarian tumor cells (23,32,33).
Treatment with phorbol ester, an AP-1 agonist, also induced
increases in ERCC-1 mRNA and protein levels in human ovarian
carcinoma cells in vitro(23,24,34).
AP-1 may be a common activator of NER genes (46). The overexpression of wild-type
c-Jun is associated with cisplatin resistance (44). In contrast, the inhibition of AP-1
activity in cells modified by inhibition of Gli1 with a specific
short-hairpin RNA downregulates c-Jun activity and NER gene
(ERCC-1 and XPD) expression, blocks platinum-DNA
adduct repair, and results in supra-additive cell killing with
cisplatin (44,47). Furthermore, cells in which AP-1 has
been genetically inactivated are hypersensitive to genotoxic
insults, including anticancer agents. These findings suggest that
AP-1 may play a prominent role in modulating DNA repair processes
in both physiological and pathophysiological conditions.
AP-1-dependent DNA repair activities may provide a
stress-protective function in cells by effectively reducing the
cytotoxic, mutagenic, and carcinogenic consequences of DNA damage.
This may also explain, at least in part, the observed increase in
mRNA levels of ERCC-1 and other NER genes in clinical
platinum-resistant specimens (6,42,43,48).
Given that the promoters of ERCC-1 and other
NER repair genes contain AP-1 binding sites, signal transduction
pathways that modulate AP-1 may be important in the regulation of
DNA repair. JNK, a member of the MAP kinase family and Ras pathway,
is responsible for the phosphorylation of c-Jun protein at serine
residues 63 and 73 in the NH2-terminal domain, which
results in greatly enhanced AP-1 binding to regulated genes and
subsequent transcriptional regulation (49). Recent work has shown that cellular
damage induced by DNA damaging agents, including cisplatin
(44,50), activates the JNK pathway involving
AP-1. This response has been reported to protect against
cisplatin-induced DNA damage by allowing DNA repair and survival
following cisplatin treatment (44). Inhibition of this pathway in cells
modified by the overexpression of a dominant-negative mutant of
c-Jun blocks DNA repair and leads to decreased viability following
treatment with cisplatin (44).
These observations suggest that the Ras/JNK pathway may mediate a
physiological response to DNA damage. We have previously shown that
upon stimulation with cisplatin, JNK may directly phosphorylate
c-Jun at serine residues 63 and 73 to activate ERCC-1
transcription via AP-1 in A2780/CP70 ovarian cancer cells (32), which would place ERCC-1
under the influence of the JNK/Ras/AP-1 signal transduction
pathway. However, the upstream signaling cascade leading to the
activation of JNK in response to cisplatin-induced DNA damage
remains to be further elucidated.
PI3K is a heterodimer composed of one regulatory
subunit (p85) and one catalytic subunit (p110) (51). The catalytic subunit of PI3K
phosphorylates phosphatidylinositol (PI) at the 3′ position of the
inositol sugar ring, generating PI 3-phosphate, PI
3,4-bisphosphate, and PI 3,4,5-trisphosphate (51). Experiments with PI3K inhibitors,
constitutively active PI3K mutants, and dominant-negative PI3K
mutants have established an important role for PI3K in apoptosis
(51,52). The best-known downstream target of
PI3K is the serine-threonine kinase Akt, which transmits survival
signals from growth factors (53).
However, PI3K also has many other targets, including NF-κB
(54), BAD (55) and JNK (56), and is involved in cell growth,
proliferation, differentiation and survival. Therefore, β-elemene
may affect DNA repair activity in these cells by regulating the
PI3K/JNK/AP-1 signaling pathway, leading to the downregulated
expression of ERCC-1 and other NER genes, and cell
death.
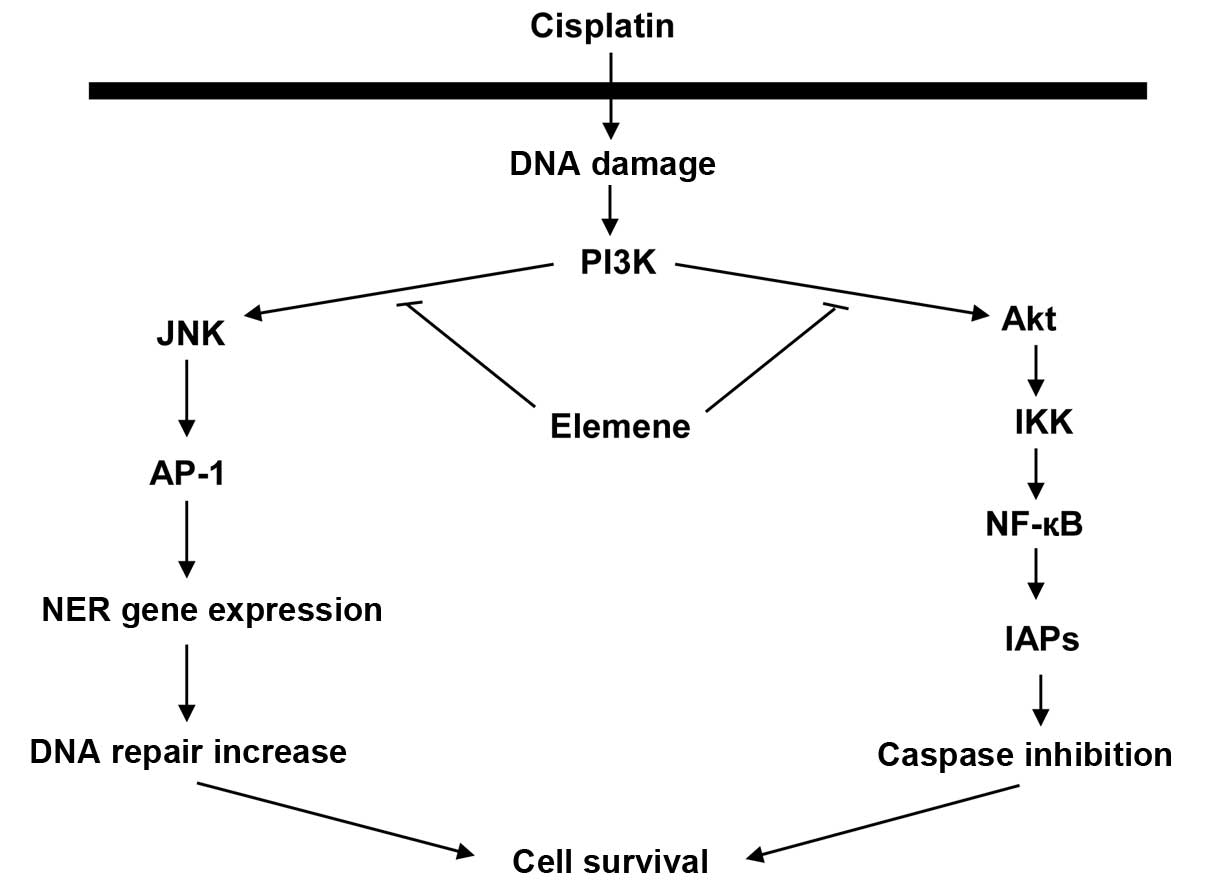
A proposed mechanism that is consistent with the
evidence is presented in Fig. 6.
In this model, cisplatin increases PI3K activity, which activates
JNK, and JNK activates AP-1. Activated AP-1 upregulates NER gene
expression, thereby increasing DNA repair activity and cell
survival. This mechanism may be responsible for reduced cellular
sensitivity to cisplatin. β-Elemene may sensitize resistant ovarian
cancer cells to cisplatin by blocking the activation of the
PI3K/JNK signaling pathway, which would reduce DNA repair activity
and enhance cisplatin cytotoxicity. In our previous study,
cisplatin increased Raf-1 and c-Fos expression in human ovarian
carcinoma cells (22). PI3K and
Akt regulate the effect of Raf on gene expression. Therefore, there
are also three alternative PI3K/Akt/Raf signaling pathways that may
link cisplatin to AP-1 activation. In all three pathways,
cisplatin-activated PI3K acts through a phosphorylation cascade to
activate Akt and Raf, which can then trigger three different
pathways to activate AP-1: i) Raf → MEKK1 → MKK4 → JNK → c-Jun; ii)
Raf → MEK1/2 → ERK1/2 → c-Jun; and iii) MEK1/2 → ERK1/2 →
p90RSK → CREB → c-Fos. Phosphorylated c-Jun and/or c-Fos
then augment AP-1 activity, which results in upregulated NER gene
expression, enhanced DNA repair capacity and increased cell
survival.
The major goal of cancer chemotherapy is to commit
tumor cells to death or apoptosis following exposure to anticancer
agents. Considerable evidence collected during the past decades
indicates that cisplatin kills cells through the induction of cell
apoptosis (57). The mechanisms of
cisplatin-induced apoptosis are complex and involve many regulators
(58). Caspase cascades are
activated in response to cisplatin exposure, and this activation
leads to an irreversible commitment to apoptotic cell death
(25,59,60).
Caspases are held in check, in part, by protein-protein
interactions with inhibitor of apoptosis proteins (IAPs). The IAPs
such as XIAP, cellular IAP-1, and cellular IAP-2 bind directly to
caspases such as caspase-3, -7 and -9, and inhibit their enzymatic
activities. In mammalian cells, two major regulatory pathways have
been proposed for caspase cascades, an extrinsic pathway and an
intrinsic pathway (61).
In the extrinsic pathway, the Fas receptor is
activated by the binding of an extracellular ligand such as Fas
ligand (FasL), and this induces the assembly of a death-inducing
signaling complex, which includes the Fas-associated death domain
protein as an adaptor and procaspase-8 (or procaspase-10). The
procaspase is activated and in turn initiates the activation of two
effector caspases, caspase-3 and -7 (62). The Fas/FasL-activated
caspase-8/caspase-3 pathway may be involved in tumor cell response
to cisplatin (58,63–65).
Alterations in this apoptotic signaling pathway, such as defects in
the expression of CD95L or CD95, or defects in caspase-8 or
caspase-3 activation, may contribute to cisplatin-resistance.
Conversely, increased caspase levels may restore sensitivity to
cisplatin chemotherapy in tumor cells (64,65).
The intrinsic pathway of apoptosis is mediated by members of the
Bcl-2 family, which destabilize the mitochondrial membrane, causing
the release of cytochrome c from mitochondria. In the
presence of ATP and cytochrome c, apoptotic
protease-activating factor-1 (Apaf-1) activates caspase-9, which in
turn activates caspase-3 (62,66).
Although the exact mechanism whereby Bcl-2 family members regulate
mitochondrial damage remains under debate, Bcl-2 and
Bcl-XL are thought to exert anti-apoptotic effects by
stabilizing the mitochondrial membrane potential and preventing the
release of apoptosis-inducing molecules such as cytochrome
c(66–68). Cisplatin may cause mitochondrial
release of cytochrome c and activation of caspase-3 by
acting through Bcl-2 family proteins (59,69).
Cisplatin has been shown to induce the expression of Bax and/or the
cleavage of Bcl-2 to increase the Bax:Bcl-2 ratio and activate the
apoptotic cascade (59,70). Moreover, antisense oligonucleotides
targeting Bcl-2 and Bcl-XL sensitized tumor cells to the
cytostatic effect of cisplatin (68).
Failure of the apoptotic pathways may lead to
cisplatin resistance. Recent evidence has demonstrated that the
failure to upregulate FasL in response to cisplatin exposure is
associated with chemoresistance in ovarian cancer cells (71). In one study, cisplatin decreases
the XIAP content in cisplatin-sensitive, but not
cisplatin-resistant, human ovarian cancer cells (72). Other researchers have confirmed
this finding, showing that the acquisition of cisplatin resistance
is associated with the ability of cisplatin-treated ovarian tumor
cells to upregulate XIAP expression (73). These observations indicate that
impaired cisplatin-induced apoptosis may account for the
chemoresistance to cisplatin therapy in ovarian tumors.
In the present study, β-elemene suppressed XIAP
expression and blocked cisplatin-induced XIAP upregulation in
resistant ovarian tumor cells. PI3K activates Akt and NF-κB, which
block apoptosis by upregulating the expression of IAP family of
proteins, thereby inhibiting the activities of caspase-3, -7 and
-9. Thus, β-elemene may enhance cisplatin sensitivity in resistant
ovarian cancer cells by abrogating cisplatin-induced PI3K activity
and the PI3K/Akt signaling pathway, resulting in decreased IAP
expression and increased apoptotic cell death. In a proposed
mechanism that is consistent with these findings, cisplatin-induced
activation of the PI3K/Akt pathway causes the phosphorylation of
IKKα and subsequent activation of the transcription factor NF-κB,
which upregulates IAP expression to inhibit caspase activation and
promote cell survival: cisplatin → PI3K → Akt → IKKα → NF-κB → IAP
expression → caspase inhibition → cell survival (Fig. 6). Our previous demonstration of
cisplatin-enhanced Raf-1 activity in human ovarian cancer cells
(22) suggests that IAP expression
may also be upregulated by two alternative signaling pathways
involving Raf: i) cisplatin → Ras → PI3K → PDK → Akt → Raf → MEKK1
→ IKKα → NF-κB → IAP expression; and ii) cisplatin → Ras → Raf →
MEKK1 → IKKα → NF-κB → IAP expression. This presents the
possibility of crosstalk between the cisplatin-activated
PI3K/PDK/Akt/IKK signal transduction pathway and the
cisplatin-activated Ras/Raf/MEKK1/IKK signaling pathway in the
activation of NF-κB and IAP expression, and the inhibition of
caspase activity. These pathways collaboratively lead to cell
survival in cisplatin-resistant ovarian cancer cells.
On the basis of the evidence obtained in our studies
and those of other groups, we propose that cisplatin increases PI3K
activity, which increases JNK and AP-1 activation; activated AP-1
upregulates NER gene expression and increases DNA repair activity.
This mechanism may reduce cellular sensitivity to cisplatin. In the
current study, β-elemene promoted cisplatin cytotoxicity and
apoptosis by blocking cisplatin-induced increases in the levels of
ERCC-1 and XIAP in resistant ovarian tumor cells. Based on these
observations, we propose that β-elemene alters DNA repair activity
and cisplatin sensitivity in human ovarian carcinoma cells by
preventing the cisplatin-induced activation of PI3K/JNK and
PI3K/Akt, consequently blocking the activation of the downstream
transcription factors AP-1 and NF-κB. This results in reduced DNA
repair activity, enhanced caspase activity, and increased apoptotic
cell death in cisplatin-resistant ovarian cancer cells. Fig. 6 illustrates the proposed signaling
pathways responsible for chemotherapeutic resistance to cisplatin
and a possible mechanism by which β-elemene may act as a
drug-resistance modulator to enhance the antitumor activity of
cisplatin in resistant human ovarian cancer cells.
Taken altogether, we showed in this study that
β-elemene increases sensitivity to cisplatin; decreases
cisplatin-induced expression of ERCC-1, a DNA repair gene,
through blocking a JNK/AP-1 pathway; and augments cisplatin-induced
cell death by downregulating XIAP expression in resistant ovarian
cancer cells. These results suggest that β-elemene enhances
susceptibility to cisplatin in resistant tumor cells through the
regulation of DNA repair activity and apoptotic death signaling in
human ovarian cancer. These novel findings indicate that β-elemene
may be efficacious as a drug-resistance modulator for
cisplatin-resistant carcinomas. This information provides a better
understanding of the mechanisms of modulation of cisplatin
sensitivity and assists in the design of potentially effective
β-elemene-based chemotherapy regimens to overcome cisplatin
resistance in human ovarian cancer and other tumor types.
Acknowledgements
This publication was made possible by grants from
the Natural Science Foundation of Science and Technology Department
of Guangxi Province (no. 0991294); the Guangxi Scientific Research
and Technological Development Program (no. 200901059); and by
grants from the National Institutes of Health (nos.
P20RR16440-010003, P20RR16440-020003, P20RR16440-030003,
P20RR16440-040003) and a West Virginia University School of
Medicine Research Grant (to Q.Q.L.).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
2
|
Reed E: Cisplatin, carboplatin, and
oxaliplatin. Cancer Chemotherapy and Biotherapy: Principles and
Practice. Chabner BA and Longo DL: 4th edition. Lippincott,
Williams and Wilkins; Philadelphia, PA: pp. 332–343. 2006
|
|
3
|
Reed E: Cisplatin and platinum analogs.
Cancer Principles and Practice of Oncology. DeVita VT, Rosenberg SA
and Lawrence TS: 8th edition. Lippincott, Williams and Wilkins;
Philadelphia, PA: pp. 419–426. 2008
|
|
4
|
Reed E: Platinum-DNA adduct, nucleotide
excision repair and platinum based anticancer chemotherapy. Cancer
Treatment Rev. 24:331–344. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reed E: DNA damage and repair in clinical
oncology: an overview. Clin Cancer Res. 16:4511–4516. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reed E: Nucleotide excision repair and
anticancer chemotherapy. Cytotechnol. 27:187–201. 1998. View Article : Google Scholar
|
|
7
|
Reed E: ERCC1 and clinical resistance to
platinum-based therapy. Clin Cancer Res. 11:6100–6102. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reed E: ERCC1 measurements in clinical
oncology. N Engl J Med. 355:1054–1055. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang G, Li X, Huang X, Zhao J, Ding H,
Cunningham C, Coad J, Flynn D, Reed E and Li QQ: Antitumor effect
of β-elemene in non-small cell lung cancer cells is mediated via
induction of cell cycle arrest and apoptotic cell death. Cell Mol
Life Sci. 62:881–893. 2005.
|
|
10
|
Li X, Wang G, Zhao J, Ding H, Cunningham
C, Chen F, Flynn DC, Reed E and Li QQ: Antiproliferative effect of
β-elemene in chemoresistant ovarian carcinoma cells is mediated
through arrest of the cell cycle at the G2-M phase. Cell Mol Life
Sci. 62:894–904. 2005.
|
|
11
|
Zhao J, Li QQ, Zou B, Wang G, Li X, Kim
JE, Cuff CF, Huang L, Reed E and Gardner K: In vitro
combination characterization of the new anticancer plant drug
β-elemene with taxanes against human lung carcinoma. Int J Oncol.
31:241–252. 2007.
|
|
12
|
Li QQ, Wang G, Zhang M, Cuff CF, Huang L
and Reed E: β-elemene, a novel plant-derived antineoplastic agent,
increases cisplatin chemosensitivity of lung tumor cells by
triggering apoptosis. Oncol Rep. 22:161–170. 2009.
|
|
13
|
Li QQ, Wang G, Reed E, Huang L and Cuff
CF: Evaluation of cisplatin in combination with beta-elemene as a
regimen for prostate cancer chemotherapy. Basic Clin Pharmacol
Toxicol. 107:868–876. 2010.PubMed/NCBI
|
|
14
|
Li QQ, Wang G, Huang F, Banda M and Reed
E: Antineoplastic effect of beta-elemene on prostate cancer cells
and other types of solid tumour cells. J Pharm Pharmacol.
62:1018–1027. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee RX, Li QQ and Reed E: β-elemene
effectively suppresses the growth and survival of both
platinum-sensitive and -resistant ovarian tumor cells. Anticancer
Res. 32:3103–3113. 2012.
|
|
16
|
Li QQ, Lee RX, Liang H and Zhong Y:
Anticancer activity of β-elemene and its synthetic analogs in human
malignant brain tumor cells. Anticancer Res. 33:65–76. 2013.
|
|
17
|
Li QQ, Lee RX, Liang H, Zhong Y and Reed
E: Enhancement of cisplatin-induced apoptosis by β-elemene in
resistant human ovarian cancer cells. Med Oncol. 30:424–436.
2013.
|
|
18
|
Zou B, Li QQ, Zhao J, Li JM, Cuff CF and
Reed E: β-elemene and taxanes synergistically induce cytotoxicity
and inhibit proliferation in ovarian cancer and other tumor cells.
Anticancer Res. 33:929–940. 2013.
|
|
19
|
Li QQ, Wang G, Huang F, Li JM, Cuff CF and
Reed E: Sensitization of lung cancer cells to cisplatin by
β-elemene is mediated through blockade of cell cycle progression:
antitumor efficacies of β-elemene and its synthetic analogs. Med
Oncol. 30:488–498. 2013.
|
|
20
|
Li QQ, Wang G, Liang H, Li JM, Huang F,
Agarwal PK, Zhong Y and Reed E: β-elemene promotes
cisplatin-induced cell death in human bladder cancer and other
carcinomas. Anticancer Res. 33:1421–1428. 2013.
|
|
21
|
Zhong X, Li QQ and Reed E: SU5416
sensitizes ovarian cancer cells to cisplatin through inhibition of
nucleotide excision repair. Cell Mol Life Sci. 60:794–802. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhong X, Li X, Wang G, Zhu Y, Hu G, Zhao
J, Neace C, Ding H, Reed E and Li QQ: Mechanisms underlying the
synergistic effect of SU5416 and cisplatin on cytotoxicity in human
ovarian tumor cells. Int J Oncol. 25:445–451. 2004.PubMed/NCBI
|
|
23
|
Li Q, Ding L, Yu JJ, Mu C, Tsang B,
Bostick-Bruton F and Reed E: Cisplatin and phorbol ester
independently induce ERCC-1 protein in human ovarian tumor cells.
Int J Oncol. 13:987–992. 1998.PubMed/NCBI
|
|
24
|
Li Q, Yu JJ, Mu C, Yunmbam MK, Slavsky D,
Cross CL, Bostick-Bruton F and Reed E: Association between the
level of ERCC-1 expression and the repair of cisplatin-induced DNA
damage in human ovarian cancer cells. Anticancer Res. 20:645–652.
2000.PubMed/NCBI
|
|
25
|
Wang G, Reed E and Li QQ: Molecular basis
of cellular response to cisplatin chemotherapy in non-small cell
lung cancer (Review). Oncol Rep. 12:955–965. 2004.PubMed/NCBI
|
|
26
|
Gosland M, Lum B, Schimmelpfennig J, Baker
J and Doukas M: Insights into mechanisms of cisplatin resistance
and potential for its clinical reversal. Pharmacotherapy. 16:16–39.
1996.PubMed/NCBI
|
|
27
|
Parker RJ, Eastman A, Bostick-Bruton F and
Reed E: Acquired cisplatin resistance in human ovarian cancer cells
is associated with enhanced DNA repair of cisplatin-DNA lesions and
reduced drug accumulation. J Clin Invest. 87:773–777. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Godwin A, Meister A, O’Dwyer P, Huang C,
Hamilton T and Anderson M: High resistance to cisplatin in human
ovarian cancer cell lines is associated with marked increase of
glutathione synthesis. Proc Natl Acad Sci USA. 89:3070–3074. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Masuda H, Ozols RF, Lai GM, Fojo A,
Rothenberg M and Hamilton TC: Increased DNA repair as a mechanism
of acquired resistance to cis-diamminedichloroplatinum (II) in
human ovarian cancer cell lines. Cancer Res. 48:5713–5716.
1988.PubMed/NCBI
|
|
30
|
Ferry KV, Hamilton TC and Johnson SW:
Increased nucleotide excision repair in cisplatin-resistant ovarian
cancer cells: role of ERCC1-XPF. Biochem Pharmacol. 60:1305–1313.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Q, Bostick-Bruton F and Reed E: Effect
of interleukin-1α and tumor necrosis factor-α on cisplatin-induced
ERCC-1 mRNA expression in a human ovarian carcinoma cell line.
Anticancer Res. 18:2283–2288. 1998.
|
|
32
|
Li Q, Gardner K, Zhang L, Tsang B,
Bostick-Bruton F and Reed E: Cisplatin induction of ERCC-1 mRNA
expression in A2780/CP70 human ovarian cancer cells. J Biol Chem.
273:23419–23425. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Q, Tsang B, Bostick-Bruton F and Reed
E: Modulation of excision repair cross complementation group 1
(ERCC-1) mRNA expression by pharmacological agents in human ovarian
carcinoma cells. Biochem Pharmacol. 57:347–353. 1999. View Article : Google Scholar
|
|
34
|
Li Q, Zhang L, Tsang B, Gardner K,
Bostick-Bruton F and Reed E: Phorbol ester exposure activates an
AP-1-mediated increase in ERCC-1 messenger RNA expression in human
ovarian tumor cells. Cell Mol Life Sci. 55:456–466. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li QQ, Ding L and Reed E: Proteasome
inhibition suppresses cisplatin-dependent ERCC-1 mRNA expression in
human ovarian tumor cells. Res Commun Mol Pathol Pharmacol.
107:387–396. 2000.PubMed/NCBI
|
|
36
|
Li QQ, Yunmbam MK, Zhong X, Yu JJ,
Mimnaugh EG, Neckers L and Reed E: Lactacystin enhances cisplatin
sensitivity in resistant human ovarian cancer cell lines via
inhibition of DNA repair and ERCC-1 expression. Cell Mol Biol.
47:61–72. 2001.PubMed/NCBI
|
|
37
|
Sancar A: Mechanisms of DNA excision
repair. Science. 266:1954–1956. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Calsou P, Barret JM, Cros S and Salles B:
DNA excision repair synthesis is enhanced in a murine leukemia
L1210 cell line resistant to cisplatin. Eur J Biochem. 211:403–409.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hill BT, Scanlon KJ and Hansson J:
Deficient repair of cisplatin-DNA adducts identified in human
testicular teratoma cell lines established from tumours from
untreated patients. Eur J Cancer. 30:832–837. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pooter CMD, Oosterom ATV, Scalliet PG,
Maes RA and Brujin EAD: Correlation of the response to cisplatin of
human ovarian cancer cell lines, originating from one tumor but
with different sensitivity, with the recovery of DNA adducts.
Biochem Pharmacol. 51:629–634. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhen W, Link CJ, O’Connor PM, Reed E,
Parker RJ, Howell SB and Bohr VA: Increased gene-specific repair of
cisplatin interstrand cross-links in cisplatin-resistant human
ovarian cancer cell lines. Mol Cell Biol. 12:3689–3698.
1992.PubMed/NCBI
|
|
42
|
Dabholkar M, Vionnet J, Bostick-Bruton F,
Yu JJ and Reed E: Messenger RNA levels of XPA and ERCC1 in ovarian
cancer tissue correlate with response to platinum-based
chemotherapy. J Clin Invest. 94:703–708. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dabholkar M, Bostick-Bruton F, Weber C,
Bohr V, Egwuagu C and Reed E: ERCC1 and ERCC2 expression in
malignant tissues from ovarian cancer patients. J Natl Cancer Inst.
84:1512–1517. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Potapova O, Haghighi A, Bost F, Liu C,
Birrer MJ, Gjerset R and Mercola D: The Jun kinase/stress-activated
protein kinase pathway functions to regulate DNA repair and
inhibition of the pathway sensitizes tumor cells to cisplatin. J
Biol Chem. 272:14041–14044. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Potapova O, Gorospe M, Bost F, Dean NM,
Gaarde WA, Mercola D and Holbrook NJ: c-Jun N-terminal kinase is
essential for growth of human T98G glioblastoma cells. J Biol Chem.
275:24767–24775. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhong X, Thornton K and Reed E: Computer
based analyses of the 5′-flanking regions of selected genes
involved in the nucleotide excision repair complex. Int J Oncol.
17:375–380. 2000.
|
|
47
|
Kudo K, Gavin E, Das S, Amable L, Shevde
LA and Reed E: Inhibition of Gli1 results in altered c-Jun
activation, inhibition of cisplatin-induced upregulation of ERCC1,
XPD and XRCC1, and inhibition of platinum-DNA adduct repair.
Oncogene. 31:4718–4724. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Britten RA, Liu D, Tessier A, Hutchison MJ
and Murray D: ERCC-1 expression as a molecular marker of cisplatin
resistance in human cervical tumor cells. Int J Cancer. 89:453–457.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Davis RJ: Signal transduction by the JNK
group of MAP kinases. Cell. 103:239–252. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu ZG, Lea-Chou ET, Wood LD, Chen Y,
Karin M and Wang JY: Three distinct signalling responses by murine
fibroblasts to genotoxic stress. Nature. 384:273–276. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Carpenter CL and Cantley LC:
Phosphoinositide 3-kinase and the regulation of cell growth.
Biochim Biophys Acta. 1288:M11–M16. 1996.PubMed/NCBI
|
|
52
|
Burgering BMT and Coffer PJ: Protein
kinase B (c-Akt) in phosphatidylinositol 3-OH kinase signal
transduction. Nature. 376:599–602. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chan TO, Rittenhouse SE and Tsichlis PN:
AKT/PKB and other D3 phosphoinositide-regulated kinase: kinase
activation by phosphoinositide-dependent phosphorylation. Annu Rev
Biochem. 68:965–1014. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Romashkova JA and Makarov SS: NF-κB is a
target of Akt in anti-apoptotic PDGF signalling. Nature. 401:86–90.
1999.
|
|
55
|
Del Peso L, Page C, Herrera R and Nunez G:
Interleukin-3-induced phosphorylation of BAD through the protein
kinase Akt. Science. 278:687–689. 1997.PubMed/NCBI
|
|
56
|
Klippel A, Reinhard C, Apell G, Escobedo
MA and Williams LT: Membrane localization of phosphatidylinositol
3-kinase is sufficient to activate multiple signal-transducing
kinase pathways. Mol Cell Biol. 16:4117–4127. 1996.PubMed/NCBI
|
|
57
|
Eastman A: The mechanism of action of
cisplatin: from adducts to apoptosis. Cisplatin, Chemistry and
Biochemistry of a Leading Anticancer Drug. Lippert E: Wiley-VCH;
Basel: pp. 111–134. 1999
|
|
58
|
Johnstone R, Ruefli A and Lowe S:
Apoptosis: a link between cancer genetics and chemotherapy. Cell.
108:153–164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang X, Martindale JL and Holbrook NJ:
Requirement for ERK activation in cisplatin-induced apoptosis. J
Biol Chem. 275:39435–39443. 2000. View Article : Google Scholar
|
|
60
|
Makin G, Corfe B, Griffiths G,
Thistlethwaite A, Hickman J and Dive C: Damage-induced Bax
N-terminal change, translocation to mitochondria and formation of
Bax dimers/complexes occur regardless of cell fate. EMBO J.
20:6306–6315. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang G, Reed E and Li QQ: Apoptosis in
prostate cancer: progressive and therapeutic implications (Review).
Int J Mol Med. 14:23–34. 2004.PubMed/NCBI
|
|
62
|
Cryns V and Yuan J: Proteases to die for.
Genes Dev. 12:1551–1570. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Okouoyo S, Herzer K, Ucur E, Mattern J,
Krammer P, Debatin K and Herr I: Rescue of death receptor and
mitochondrial apoptosis signaling in resistant human NSCLC in vivo.
Int J Cancer. 108:580–587. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Fulda S, Los M, Friesen C and Debatin K:
Chemosensitivity of solid tumor cells in vitro is related to
activation of the CD95 system. Int J Cancer. 76:105–114. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Spierings D, de Vries E, Vellenga E and de
Jong S: Loss of drug-induced activation of the CD95 apoptotic
pathway in a cisplatin-resistant testicular germ cell tumor cell
line. Cell Death Differ. 10:808–822. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cory S, Huang D and Adams J: The Bcl-2
family: roles in cell survival and oncogenesis. Oncogene.
22:8590–8607. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Gross A, McDonnell J and Korsmeyer S:
Bcl-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Hopkins-Donaldson S, Cathomas R,
Simoes-Wust A, Kurtz S, Belyanskaya L, Stahel R and
Zangemeister-Wittke U: Induction of apoptosis and
chemosensitization of mesothelioma cells by Bcl-2 and Bcl-xL
antisense treatment. Int J Cancer. 106:160–166. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kojima H, Endo K, Moriyama H, Tanaka Y,
Alnemri E, Slapak C, Teicher B, Kufe D and Datta R: Abrogation of
mitochondrial cytochrome c release and caspase-3 activation in
acquired multidrug resistance. J Biol Chem. 273:16647–16650. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Del Bello B, Valentini M, Zunino F,
Comporti M and Maellaro E: Cleavage of Bcl-2 in oxidant- and
cisplatin-induced apoptosis of human melanoma cells. Oncogene.
20:4591–4595. 2001.PubMed/NCBI
|
|
71
|
Mansouri A, Ridgway LD, Zhang Q, Tian L,
Wang Y and Claret FX: Sustained activation of JNK/p38 MAPK pathways
in response to cisplatin leads to Fas ligand induction and cell
death in ovarian carcinoma cells. J Biol Chem. 278:19245–19256.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Li J, Feng Q, Kim J, Schneiderman D,
Liston P, Li M, Vanderhyden B, Faught W, Fung M, Senterman M,
Korneluk R and Tsang B: Human ovarian cancer and cisplatin
resistance: possible role of inhibitor of apoptosis proteins.
Endocrinol. 142:370–380. 2001.PubMed/NCBI
|
|
73
|
Mansouri A, Zhang Q, Ridgway LD, Tian L
and Claret FX: Cisplatin resistance in an ovarian carcinoma is
associated with a defect in programmed cell death control through
XIAP regulation. Oncol Res. 13:399–404. 2003.PubMed/NCBI
|