Spandidos Publications style
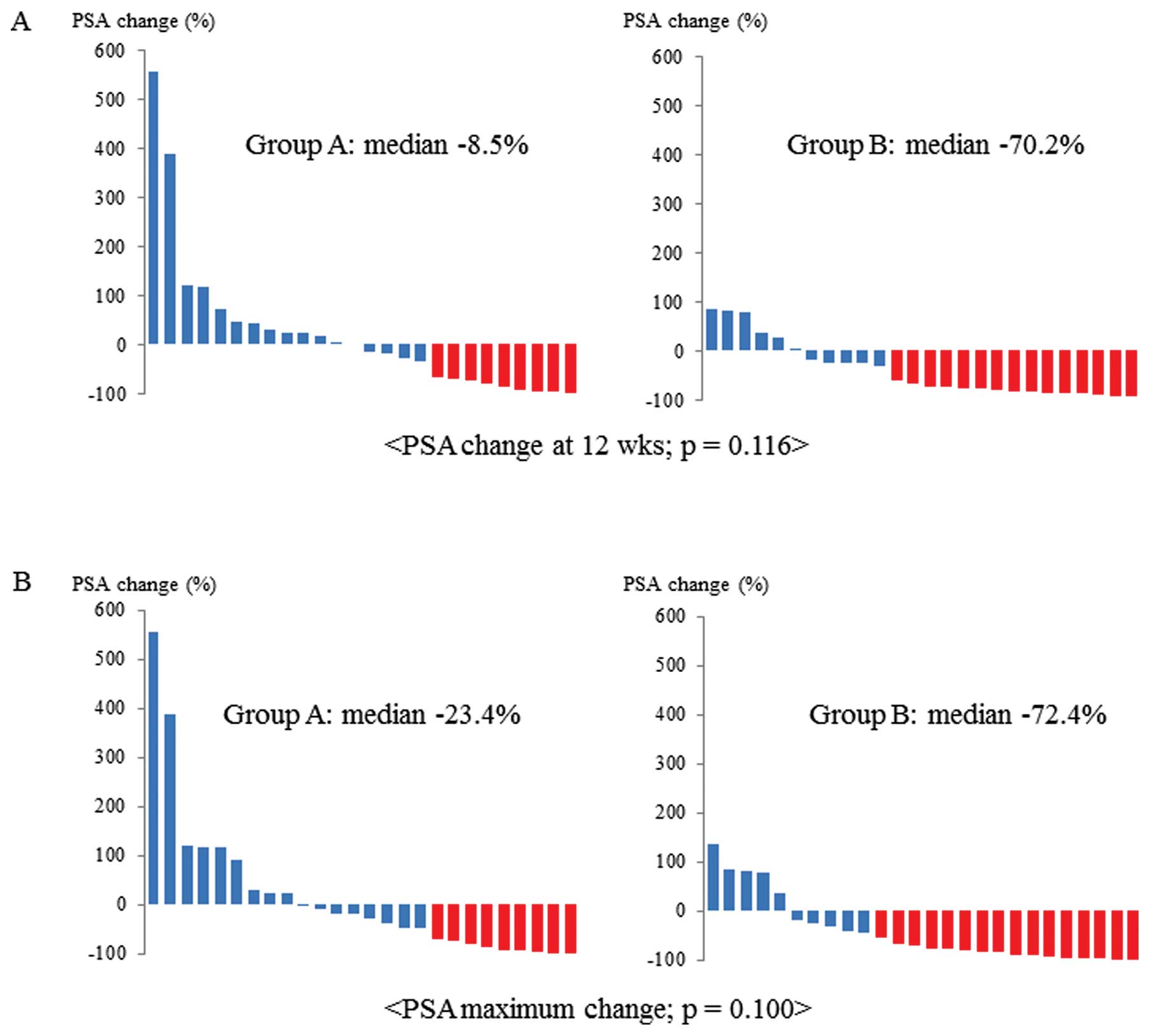
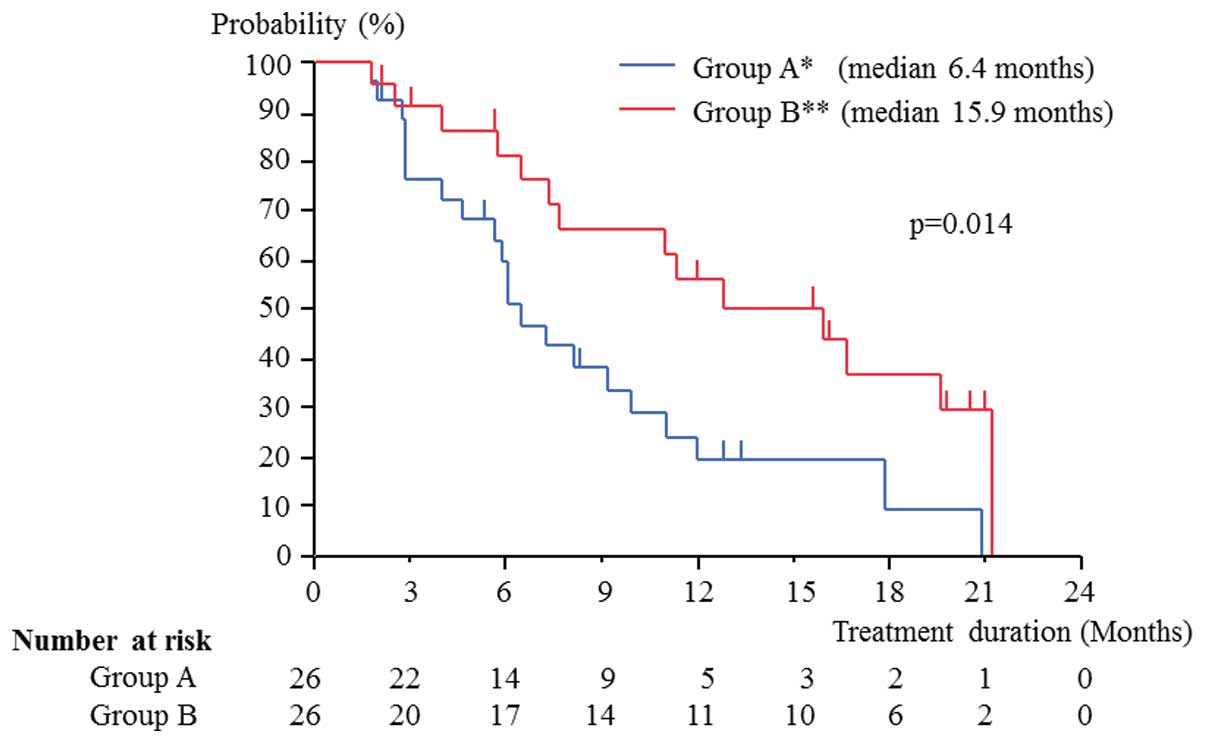
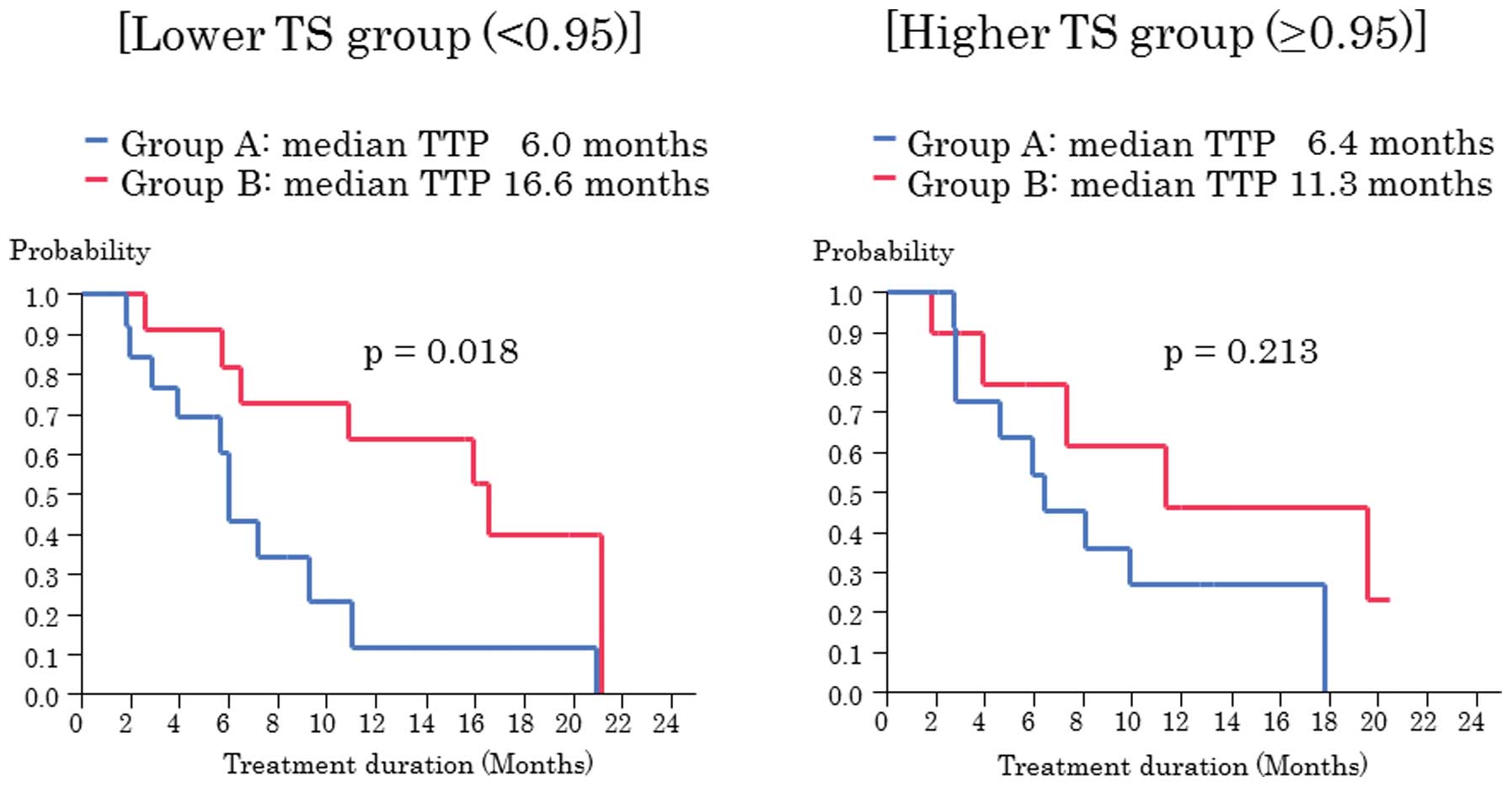
Takahashi M, Kawabata R, Kawano A, Murakami Y, Sutou Y, Inai T, Akazawa S, Hamao T, Hayashi H, Fukawa T, Fukawa T, et al: Substitution of anti-androgens and tegafur-uracil combination therapy for castration-resistant prostate cancer: Results of a multi-center randomized phase II study. Int J Oncol 43: 713-720, 2013.
APA
Takahashi, M., Kawabata, R., Kawano, A., Murakami, Y., Sutou, Y., Inai, T. ... Kanayama, H. (2013). Substitution of anti-androgens and tegafur-uracil combination therapy for castration-resistant prostate cancer: Results of a multi-center randomized phase II study. International Journal of Oncology, 43, 713-720. https://doi.org/10.3892/ijo.2013.1997
MLA
Takahashi, M., Kawabata, R., Kawano, A., Murakami, Y., Sutou, Y., Inai, T., Akazawa, S., Hamao, T., Hayashi, H., Fukawa, T., Takemura, M., Yamamoto, Y., Yamaguchi, K., Izaki, H., Fukumori, T., Kanayama, H."Substitution of anti-androgens and tegafur-uracil combination therapy for castration-resistant prostate cancer: Results of a multi-center randomized phase II study". International Journal of Oncology 43.3 (2013): 713-720.
Chicago
Takahashi, M., Kawabata, R., Kawano, A., Murakami, Y., Sutou, Y., Inai, T., Akazawa, S., Hamao, T., Hayashi, H., Fukawa, T., Takemura, M., Yamamoto, Y., Yamaguchi, K., Izaki, H., Fukumori, T., Kanayama, H."Substitution of anti-androgens and tegafur-uracil combination therapy for castration-resistant prostate cancer: Results of a multi-center randomized phase II study". International Journal of Oncology 43, no. 3 (2013): 713-720. https://doi.org/10.3892/ijo.2013.1997