Introduction
The induction of cancer is a multistage process and
its stages have been defined experimentally as initiation,
promotion, and progression. Carcinogenesis depends on inherited and
acquired susceptibility factors, such as mutations in oncogenes and
tumor suppressor genes (1,2), on exposure to initiation factors,
i.e. exogenous and endogenous carcinogens and on promotion and
progression factors. It is well known that a variety of chemical
and physical agents can cause skin cancer in rodents and man.
Repetitive treatment with known skin carcinogens will lead to skin
damage followed by inflammation and regenerative hyperplasia,
dysplasia, papillomas, basal and/or squamous cell carcinomas
(3–5).
Papilloma formation can be chemically induced by the
two-stage carcinogenesis protocol (reviewed in ref. 6). Application of the carcinogen
7,12-dimethylbenz[a]anthracene (DMBA) to the skin surface
results in Ras mutations in long-lived keratinocytes,
probably stem cells (7,8). In response to applications of the
tumor promoter 12-O-tetradecanoylphorbol-13-acetate (TPA),
oncogenic transcription factors NFκB and AP-1 are activated
(9–11), leading to increased inflammation,
proliferation of initiated cells and finally to clonal expansion
and papilloma formation. The mouse skin carcinogenesis model has
provided great understanding of the important cellular and
molecular events involved in tumor initiation, promotion and
progression (3,4).
The mouse skin cancer model has provided an
important system not only for studying mechanisms involved in the
various stages of carcinogenesis and for bioassay of
tumor-promoting and carcinogenetic agents, but also for the study
of inhibitors of tumor formation and malignant conversion (5). This model has been used to show that
a variety of naturally occurring phytochemicals may be very useful
for the prevention of skin cancer as well as for the prevention of
other epithelial cancers in humans (12). The mouse skin cancer model relates
very well to other models where squamous cell carcinomas are
induced. The phytochemicals may modify carcinogen activation,
enhance phase II enzymes detoxification, modify antioxidant
enzymes, prevent oxidative damage to DNA bases and mutations,
decrease inflammation and proliferation, modulate the immune
response and induce apoptosis. Because of their diverse mechanisms
of action, many combinations of phytochemicals can interact in a
synergistic fashion. One phytochemical may impact the metabolism of
the other (13,14), or change its ability to enter or
leave the cell (15).
Phytochemicals may synergize by acting along different points on
cell regulatory systems (16,17).
Synergistic interactions between phytochemicals allow for a
decreased concentration of each drug to achieve the same effect.
This may reduce cost of the therapy as well as reduce potential
side effects.
The phytochemicals selected for this study occur in
many medicinal herbs and plants. Resveratrol (RES) is a naturally
occurring phytoalexin associated with many health benefits, most
notably the mitigation of age-related diseases, including
neurodegeneration, carcinogenesis and atherosclerosis (18–20).
RES has also shown promise as an antidiabetic (21) and anticancer (22,23)
agent in recent clinical trials. Calcium D-glucarate (CG) is the
salt and the commercial form of D-glucaric acid, which occurs
naturally in a variety of foods, including broccoli, oranges and
apples. Following oral administration, CG is converted to
D-glucaro-1,4-lactone, which inhibits the enzyme β-glucuronidase
and enhances phase II detoxification (24). Some in vitro and animal data
suggest that inhibition of β-glucuronidase may suppress
carcinogenesis (25), as well as
inhibit the initiation and promotion/progression stages of
tumorigenesis (12,26,27).
Ursolic acid (UA) is a pentacyclic triterpenoid which has been
shown to suppress skin (28) and
breast (29) tumorigenesis. UA has
also been found to induce apoptosis in a wide variety of cancer
cells (30–32).
The overall goal of the present study was to
determine the effect of combined action of phytochemicals on early
stages of skin tumorigenesis, i.e. initiation and promotion. Our
hypothesis was that concurrent topical and dietary treatment with
selected compounds would lead to more efficient synergistic
prevention of chemically-induced murine skin tumorigenesis. Tumors
per mouse, tumor incidence, epidermal thickness, epidermal
proliferation, and a number of inflammatory biomarkers were
measured to determine the effects of these combinations of
phytochemicals on the initiation and promotion stages of
tumorigenesis.
Materials and methods
Scheme of DMBA-initiated, TPA-promoted
skin carcinogenesis
Female SENCAR mice, 5 weeks old, were purchased from
the National Cancer Institute, Frederick Cancer Research and
Development Center (Frederick, MD, USA). At 6–7 weeks of age the
backs of mice were shaved and 20 nmol of DMBA in 0.2 ml acetone
(ACT) was applied topically, then after one month, two weekly doses
of 2 μg TPA in 0.2 ml of ACT were applied for up to 14 weeks until
sacrifice. The phytochemicals RES (2.5 μmol in 0.2 ml ACT) or UA (1
μmol in 0.2 ml DMSO) were applied topically to the dorsal surface
of mice 20 min prior to DMBA or TPA treatment and 2% dietary CG was
given in the AIN-93G diet from 2 weeks prior until 2 weeks after
the DMBA dose or continually beginning 2 weeks prior to the first
dose of TPA. The compounds were used in these same concentrations
for the combination studies. After the 14th week of treatment, mice
were euthanized, tumors were removed and two 1-cm2 skin
samples were removed for histology and RNA extraction.
Tumor counting
Upon the appearance of papillomas (7th week of TPA
treatment) tumors on the backs of each mouse were counted weekly.
Tumor multiplicity and tumor incidence were calculated for each
group.
Histological evaluation
The tissues were prepared for histological
evaluation by using conventional paraffin sections and
hematoxylin-eosin staining. The average epithelial thickness was
determined from at least 12 randomly selected sites in
formalin-fixed skin samples, with 3 measurements per image.
For proliferative analysis, mice were given an i.p.
injection of BrdU (Sigma Chemical Co., St. Louis, MO, USA) (1.5 mg
in saline per mouse) 60 min prior to sacrifice. The tissue sections
were immunostained with anti-BrdU antibody (Lab Vision Corp.,
Fremont, CA, USA). The percentage of stained cells in the basal
layer of the epidermis of 12 randomly selected sites was
determined. For analysis of NFκB and AP-1 activities, slides were
stained with anti-p50 (Lab Vision Co.) or anti-c-jun antibodies (BD
Biosciences, Franklin Lakes, NJ, USA). Positive cells were counted
as with BrdU samples, however, 3 mice per group were used in the
analyses.
Real-time PCR analysis
Total RNA was extracted using TRI reagent (MRC Inc.,
Cincinnati, OH, USA). RNA (1 mg) was reverse transcribed with
oligo(dT), using cMaster RT kit (Eppendorf North America, Westbury,
NY, USA) according to the manufacturer’s protocol. Primers used
were: 5′-ATCCTGC CAGCTCCACCG-3′, 5′-TGGTCAAATCCTGTGCTCATA CAT-3′
for COX-2, 5′-GATGTGGAGCAACTTGGAAT-3′, 5′-AGCTCTCCACTTGCAGAAAA-3′
for IL-6 and 5′-CAT CCTGGCCTCGCTGTC-3′, 5′-CTCGTCGTACTCCTGC
TTGGT-3′ for β-actin. Standard quantitative RT-PCR was performed in
triplicate using SYBR Green RealMasterMix (Eppendorf North America)
on the Realplex MasterCycler (Eppendorf). RT-PCR cycle thresholds
(Ct) of candidate genes were normalized to control gene β-actin.
The formula 2Ct(Candidate)/2Ct(Control) was used to
calculate the normalized ratios.
Statistical analysis
The results for Figs.
2–8 are expressed as means ±
standard deviation (SD). For visual clarity, SD is not shown in
Fig. 1. For comparison of the
differences between the groups, a two-tailed, unpaired, Student’s
t-test was used. p<0.05 was considered to be statistically
significant.
Results
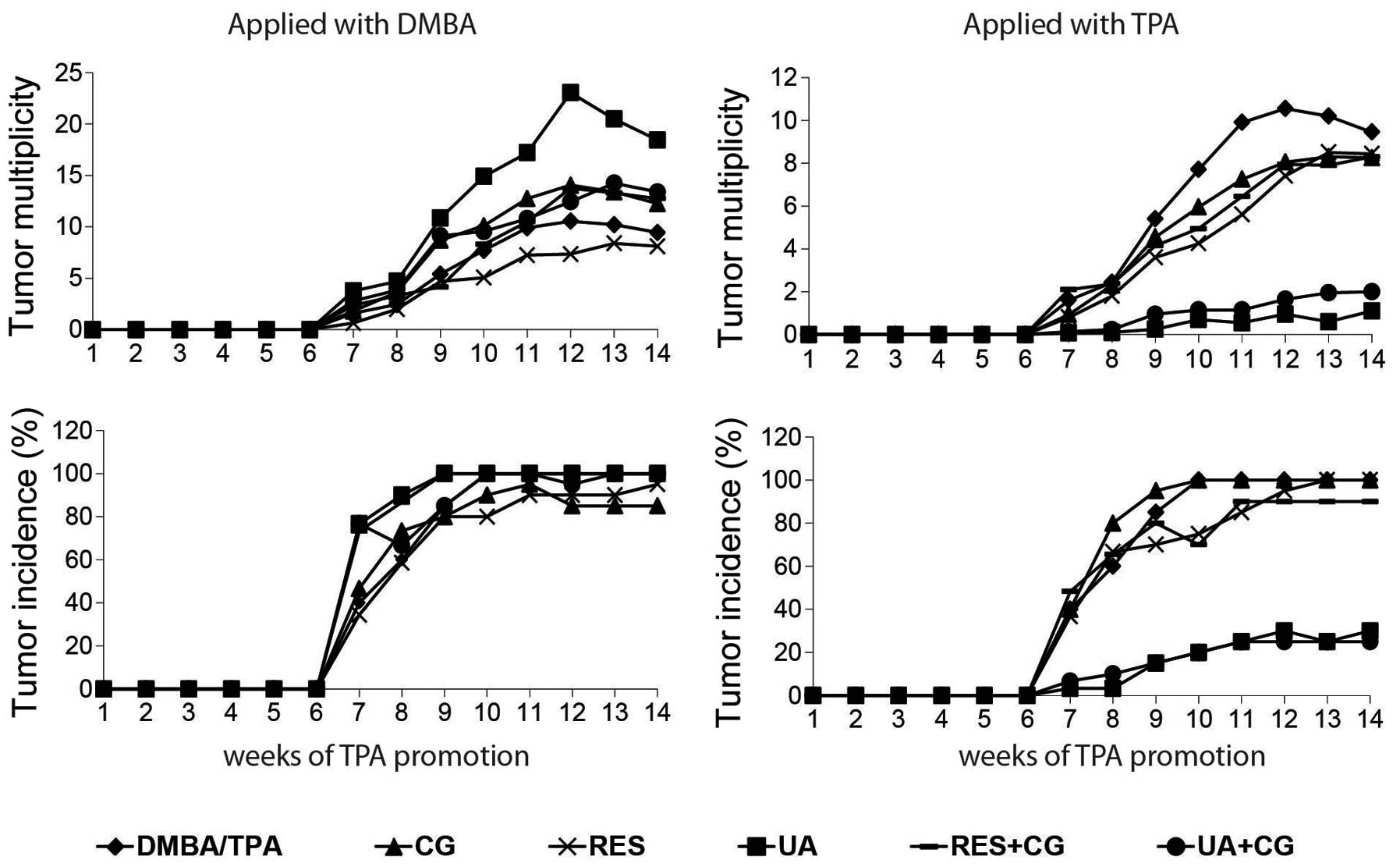
Effect of phytochemicals on tumor
multiplicity and tumor incidence
Tumor multiplicity (average papillomas per mouse)
and tumor incidence (% of mice with at least one papilloma) for
each treatment group were determined by counting papillomas on the
backs of mice once per week for the 7th through the 14th week of
TPA treatment. No tested compound or combination inhibited
carcinogenesis when applied during the initiation (DMBA) phase. In
fact, UA significantly increased DMBA/TPA-mediated tumor formation
when applied with DMBA in weeks 7 and 9–14 of TPA treatment
(p<0.05). However, when applied with TPA, UA inhibited tumor
multiplicity by 90% by itself and by 78.9% in CG-fed mice, and both
treatments inhibited tumor incidence by 75% at the 14th week of TPA
treatment. UA and UA+CG significantly p<0.05 inhibited tumor
multiplicity when applied with TPA in each of weeks 7–14 of TPA
treatment (Fig. 1).
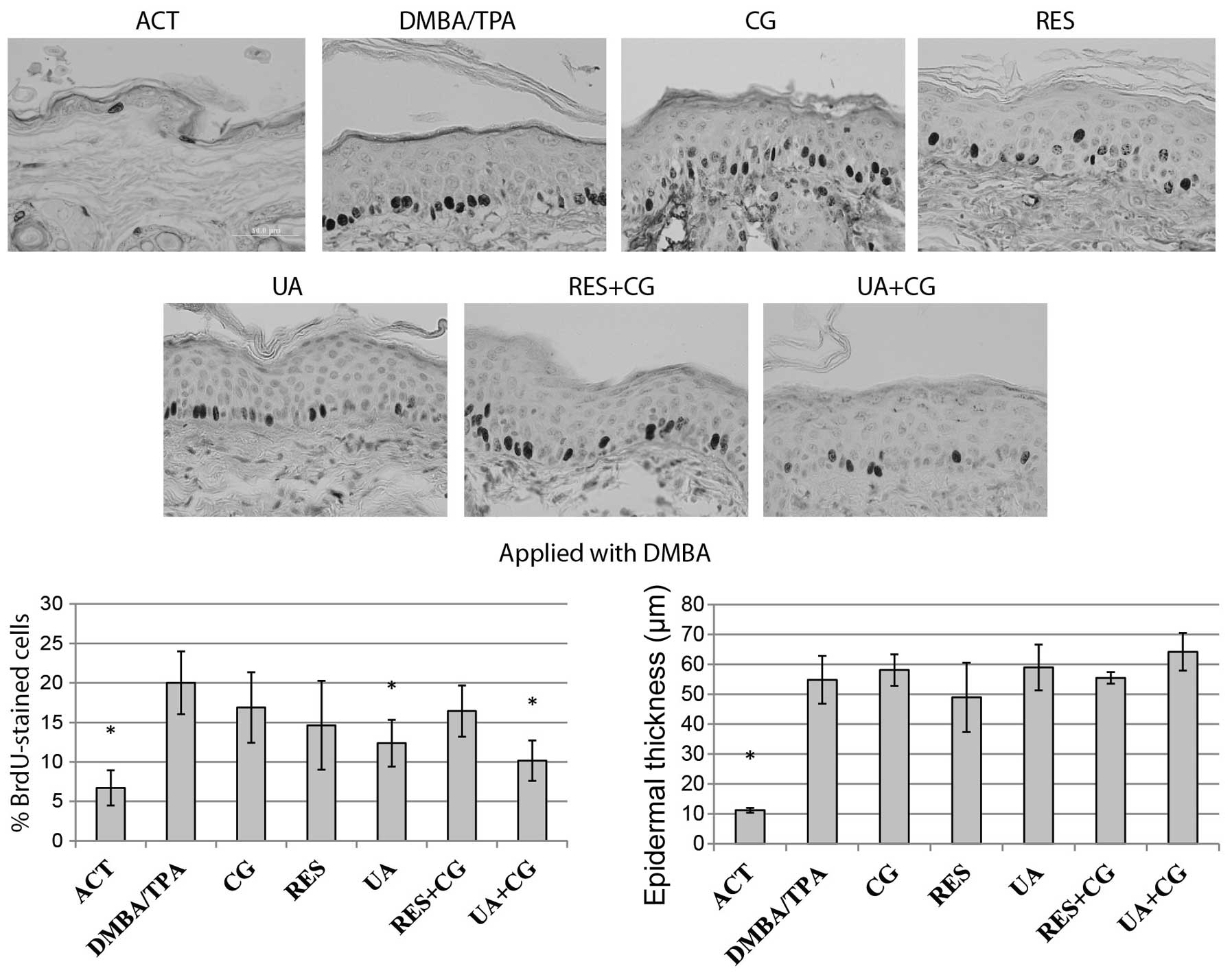
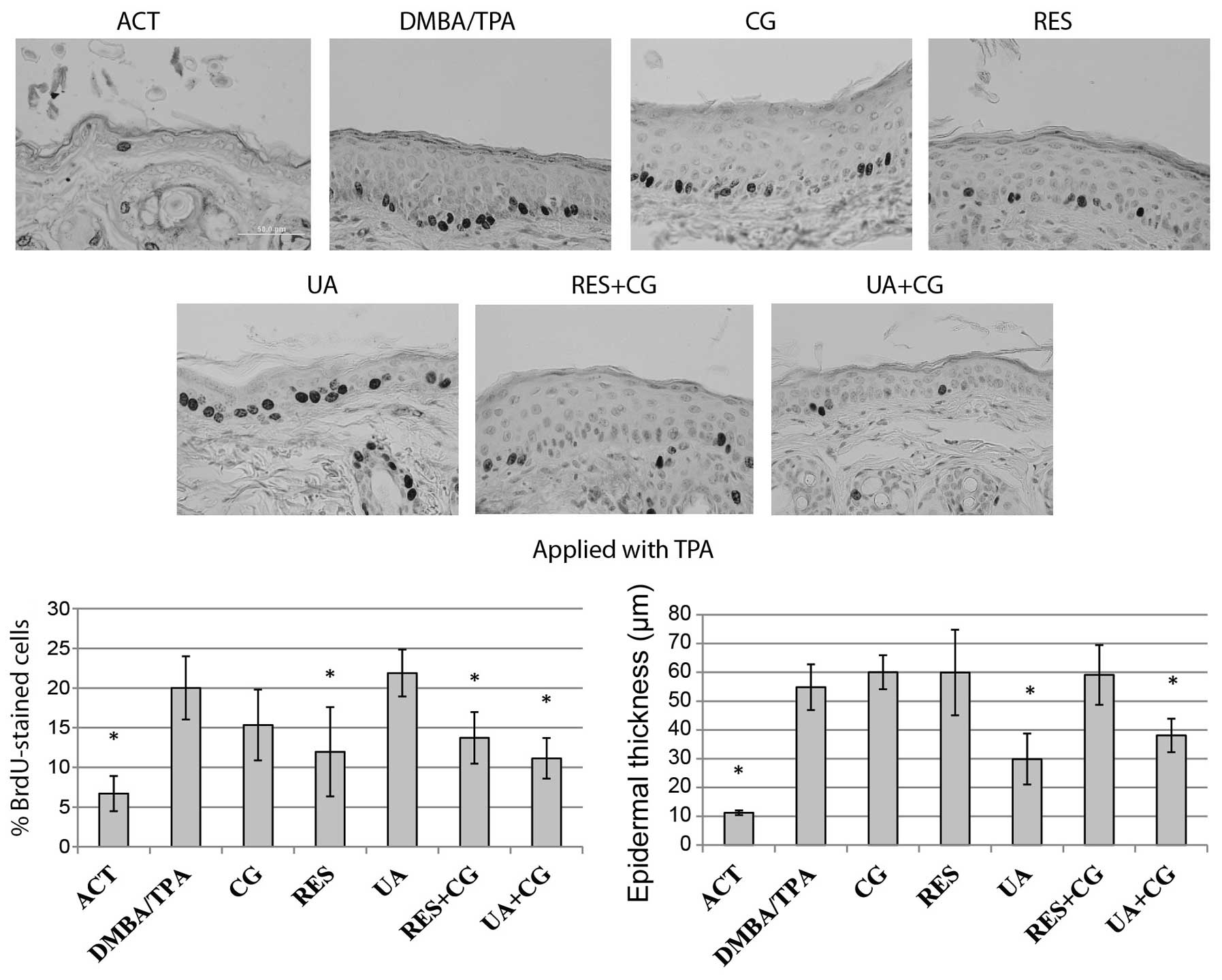
Effect of phytochemicals on epidermal
hyperplasia and cell proliferation determined by incorporation of
BrdU
The level of epidermal proliferation for each
treatment group was determined by counting BrdU-positive cells at
12 random locations per sample, then expressing the data as a
percentage of BrdU-positive cells in the basal layer. The
proliferation rate of keratinocytes located in the basal layer of
epidermis and overall epidermal thickness were greatly increased in
the DMBA/TPA treated mice compared to negative control. Despite not
having an effect on tumor formation when applied with DMBA, UA
alone and in combination with CG was able to inhibit proliferation
when applied during the initiation phase, while no compound
significantly reduced epidermal hyperplasia when applied with DMBA
(Fig. 2). All treatments except CG
or UA alone had a statistically significant impact on proliferation
when applied during promotion, however, only UA and UA+CG
significantly affected epidermal hyperplasia when applied with TPA
(Fig. 3). In similar studies,
there is typically a strong concordance between levels of epidermal
proliferation (measured by BrdU staining) and epidermal
hyperplasia. However, in our experiments, we observed some
disagreement between these two metrics of tumor promotion. For
example, we observed that UA was the strongest inhibitor of
epidermal hyperplasia when applied with TPA, however, it had no
effect on epidermal proliferation (Fig. 3). This effect may be mediated by a
UA-induced upregulation of apoptosis, which would decrease
epidermal thickness without specifically affecting
proliferation.
Effect of phytochemicals on COX-2 and
IL-6 expression
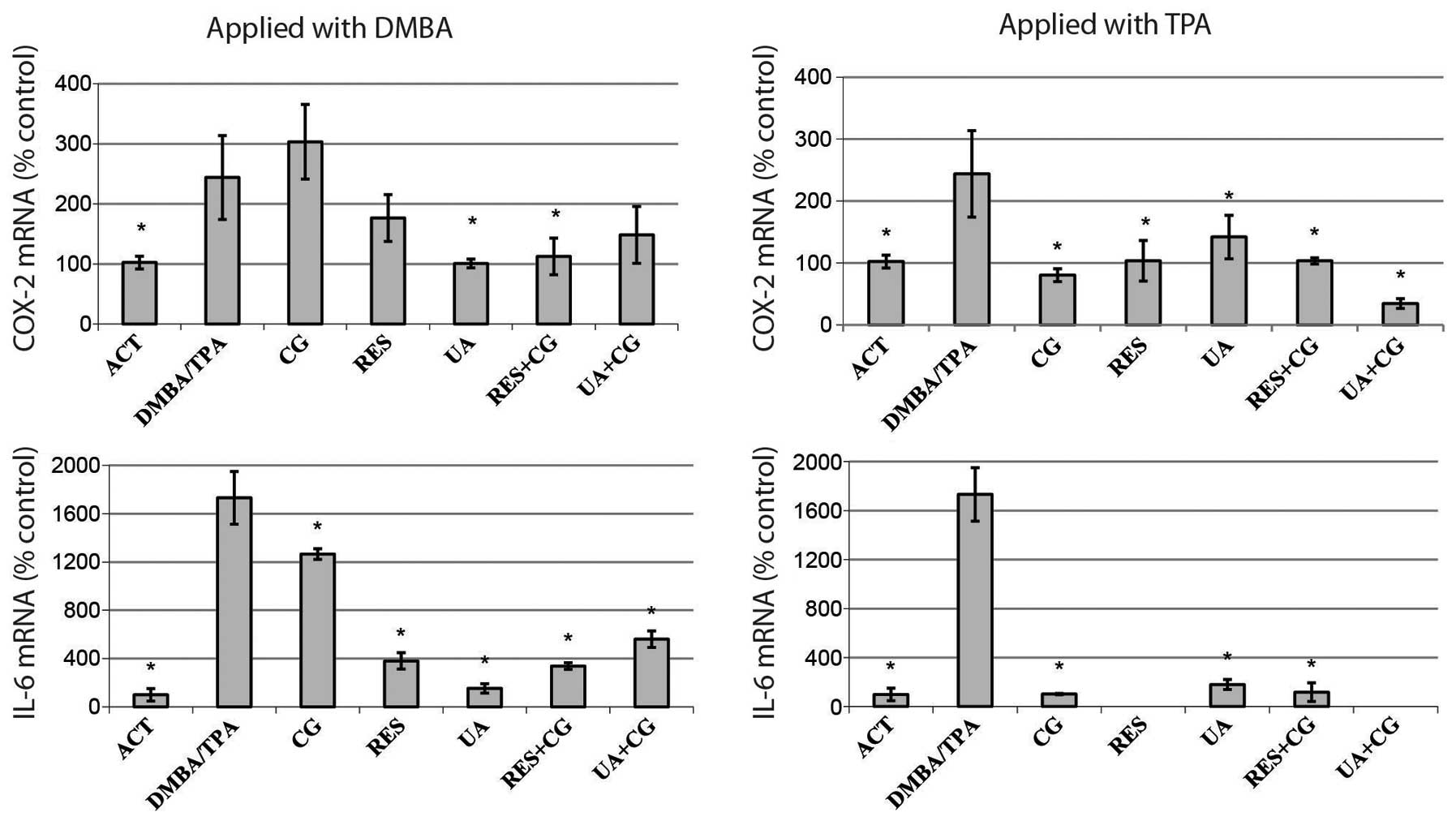
DMBA/TPA treatments resulted in increased expression
of the inflammation markers COX-2 and IL-6, compared to control.
These genes play important roles in such inflammatory responses as
edema formation and hyperplasia, as well as in papilloma
development in mouse skin (33,34).
While only UA and RES+CG significantly reversed COX-2 mRNA levels
when applied with DMBA in the two-stage model, every compound or
combination decreased COX-2 mRNA levels when applied alongside TPA
during the promotion stage. All treatments strongly decreased IL-6
expression; moreover, anti-promotion treatments reduced IL-6
expression to the negative control level (Fig. 4).
Effect of phytochemicals on
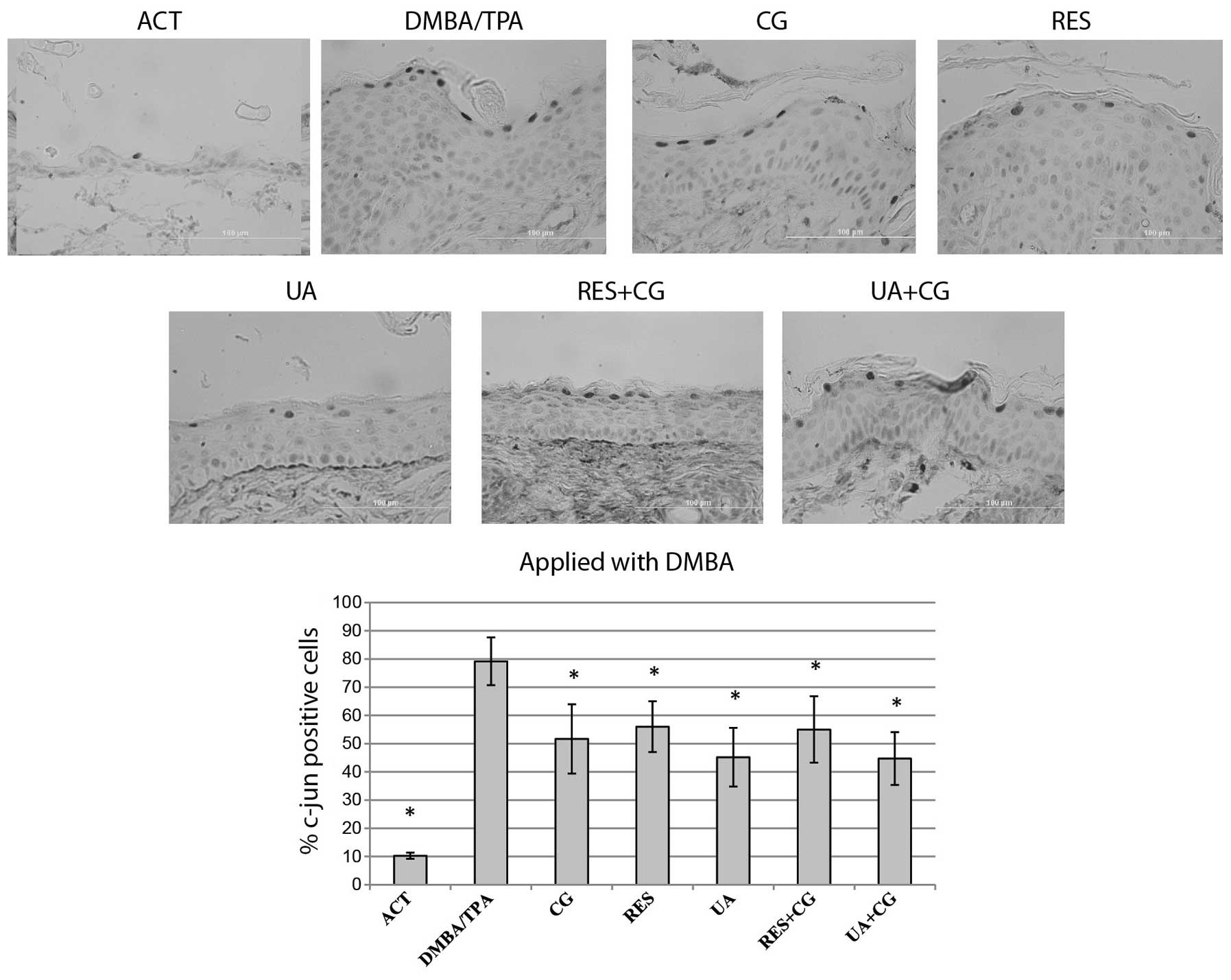
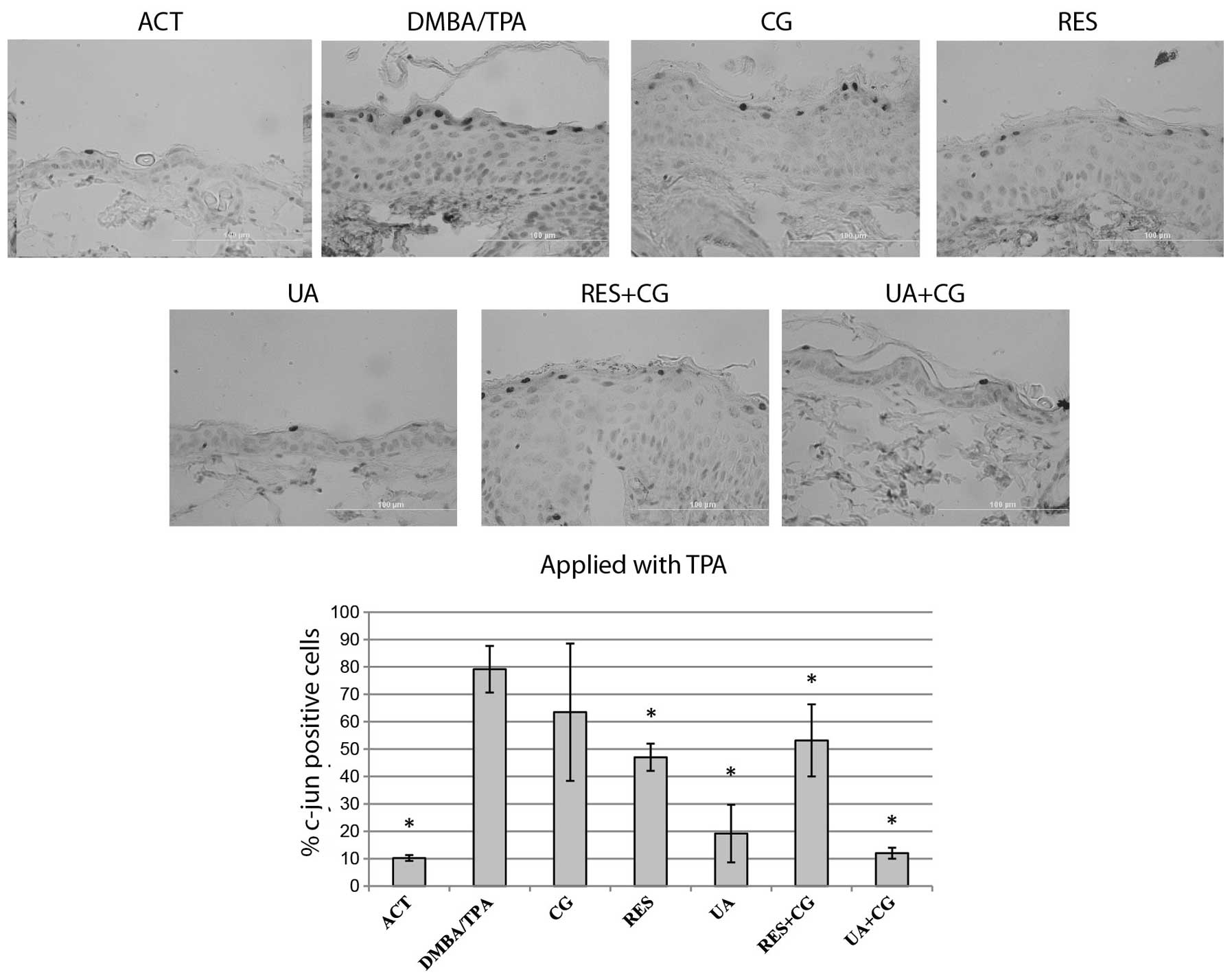
c-jun-positive cells
AP-1 activation levels were measured by
immunostaining for c-jun-positive cells within the interfollicular
epidermis. An average of 200 cells per tissue section were counted
at 12 random locations per sample, with the data expressed as the
mean ± SD of three animals per treatment group. As expected,
DMBA/TPA treatment strongly increased the number of c-jun-positive
cells. All treatments resulted in a slight decrease of
c-jun-positive cells when applied with DMBA (Fig. 5). All treatments except for CG
alone resulted in a significant decrease in c-jun-positive cells
when applied alongside TPA. In case of mice treated with UA+CG
during the TPA stage, the percentage of c-jun-positive cells was
decreased to the negative control level revealing at least an
additive effect of this combination (Fig. 6).
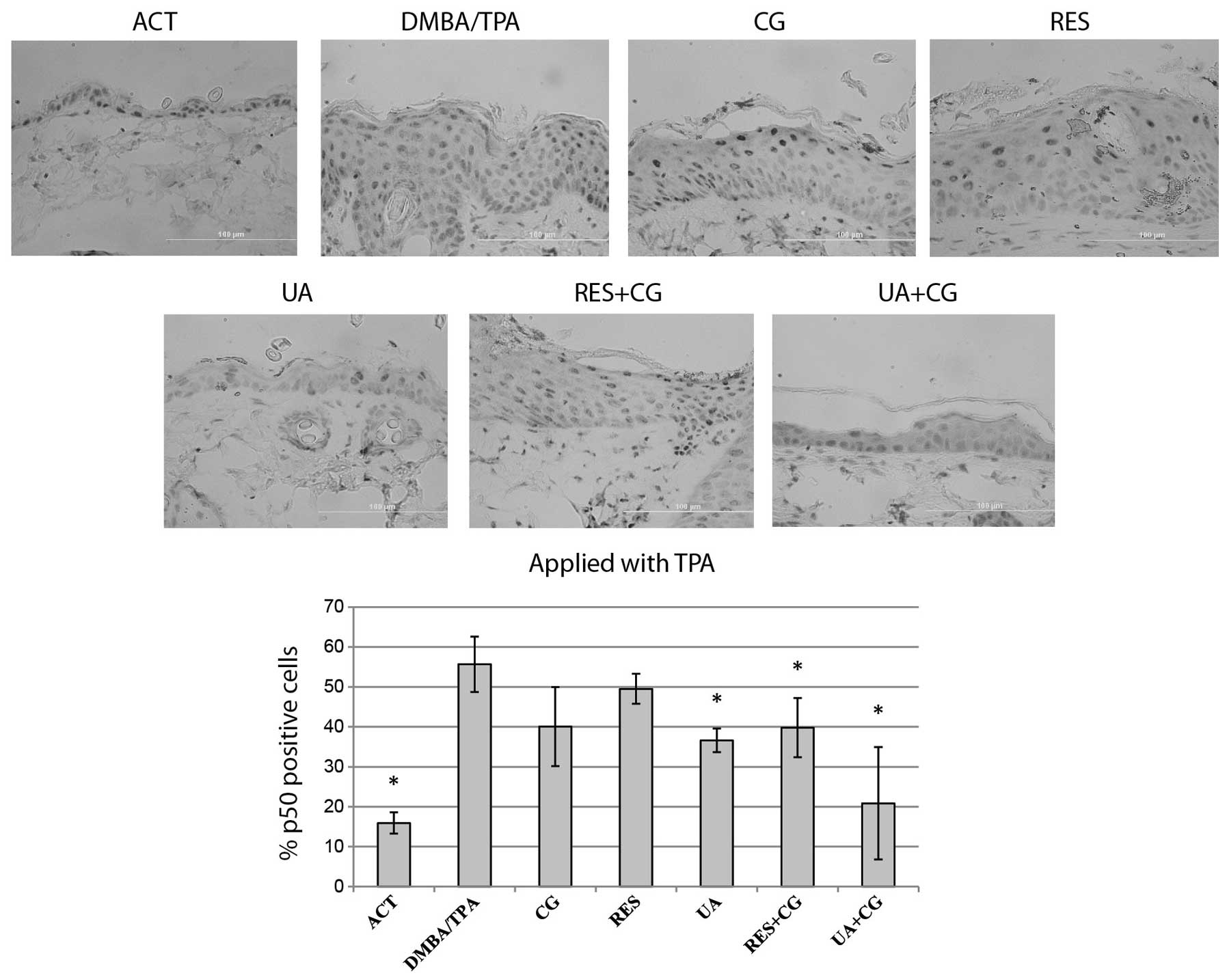
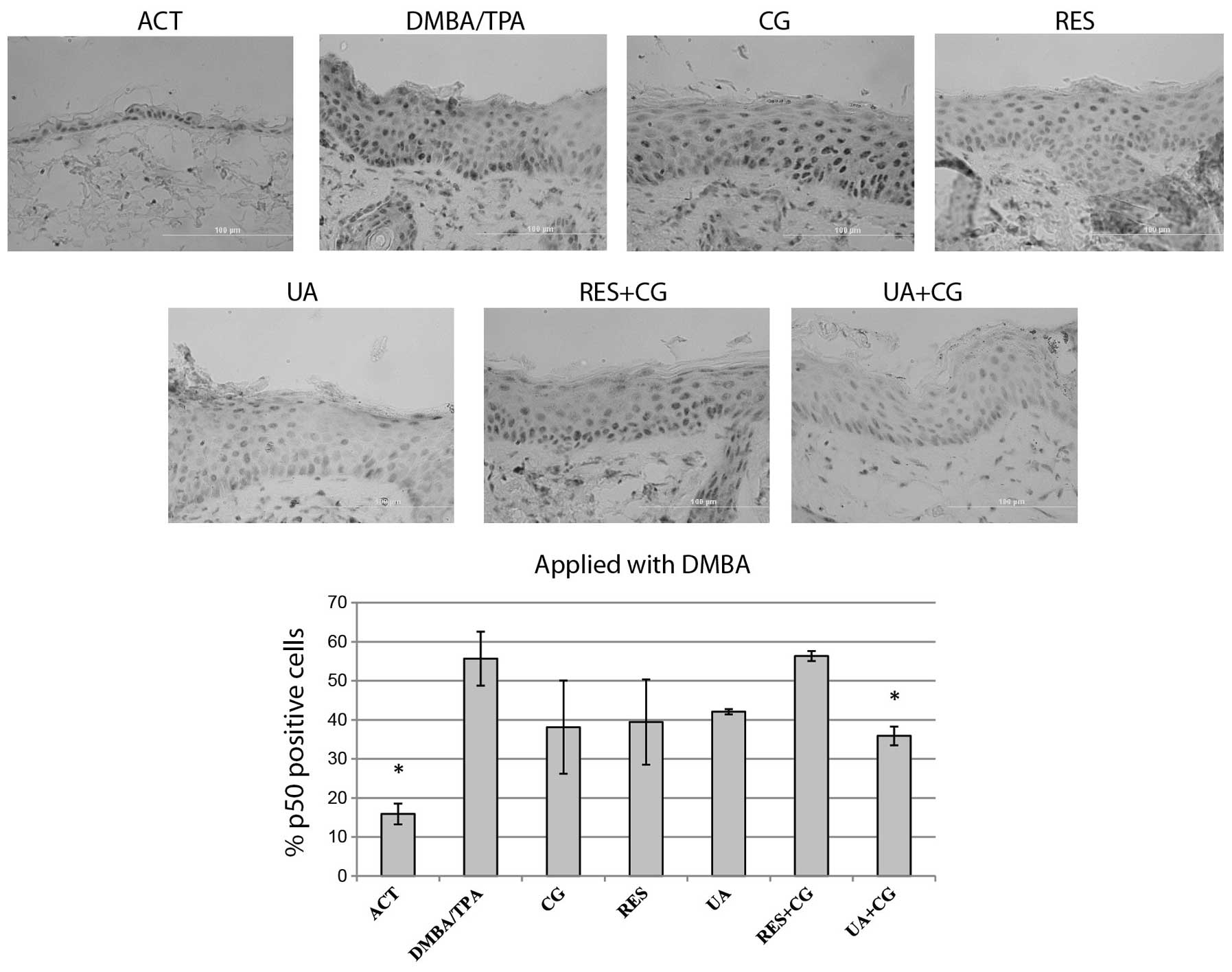
Effect of phytochemicals on p50-positive
cells
NFκB expression for each treatment group was
determined by counting p50 protein-positive cells at 12 random
locations per sample, then expressing the data as a percentage of
positive cells within the interfollicular epidermis. The
DMBA/TPA-treated skin presented strong nuclear staining. UA+CG
applied during the DMBA initiation stage was the only treatment to
significantly suppress the percentage of p50-positive cells
(Fig. 7). UA alone, its
combination with CG and additionally dietary CG with RES during the
promotion stage significantly reduced the percentage of p50-stained
cells. UA+CG treatment applied during the promotion phase was the
greatest inhibitor of the increase in p50-positive cells among used
treatments, showing some additive effect in comparison to single
treatments (Fig. 8).
Discussion
DMBA/TPA treatments resulted in epidermal
hyperplasia, characterized by a significant increase in cells
positive for AP-1 and NFκB components with a simultaneously
increased expression of the inflammation markers COX-2 and IL-6,
compared to untreated controls. Despite our initial hypothesis, we
observed no definitive synergistic effect between any of the tested
phytochemicals.
UA applied alone and in combination with CG during
the promotion stage was a strong inhibitor of tumor multiplicity
and tumor incidence. While different treatments reduced epidermal
proliferation when applied with either DMBA or TPA, only UA and the
combination UA+CG applied during promotion significantly reduced
hyperplasia. All anti-promotion treatments (either single compounds
or their combinations), a majority of single treatments and all
combinations during anti-initiation stage caused a marked decrease
of inflammation related genes levels compared to the DMBA/TPA
control. On the other hand, only UA+CG significantly diminished
both c-jun-positive cells and p50-positive cells. These results
show that UA alone or in combination with CG is a potent inhibitor
of skin tumor promotion and it may be useful in the prevention of
skin cancer and other epithelial cancers in humans. In addition,
UA+CG is a strong inhibitor of AP-1 and NFκB, and may be useful for
the prevention or treatment of maladies associated with activation
of these factors.
In our study, we observed UA to be a much stronger
inhibitor of tumor formation than RES or CG. This may be because UA
and UA+CG were the most potent inhibitors of DMBA/TPA-induced c-jun
positive cells and p50-positive cells of the treatments tested.
This agrees with studies in other systems, which also show UA is a
more potent inhibitor of inflammation-mediated processes such as
monocyte recruitment than RES (35). Our other in vivo studies
have demonstrated that UA more strongly inhibits TPA-induced
phosphorylation and activation of NFκB subunit p65 in vivo
(unpublished data). As AP-1 (36,37)
and NFκB (9) activities have been
shown to be necessary for chemically-induced skin cancer formation,
we suggest that UA and UA+CG-mediated decreases in these
transcription factors resulted in the observed antitumor
effect.
None of the tested treatment groups significantly
decreased tumor formation when applied during the DMBA stage. These
compounds may only affect traits associated with skin tumor
promotion, such as proliferation/clonal expansion, resistance to
apoptosis and inflammation. In fact, UA added during the DMBA stage
increased tumor formation. This may have been due to UA increasing
the reactive concentration of DMBA. UA shows relatively little
inhibition of CYP1A1 and CYP1B1, which metabolize polycyclic
aromatic hydrocarbons like DMBA to their more reactive form
(38). However, RES inhibits
CYP1A1 and CYP1B1, but still showed no decrease in tumor
multiplicity when applied with DMBA in our study. Another potential
explanation is that the glucocorticoid-like structure of UA may
cause an increase in nuclear envelope permeability (39), allowing for more DMBA intake and an
increase in tumor formation. However, the classical glucocorticoid
dexamethasone has been shown to inhibit skin tumor formation when
applied with different initiators, including DMBA, in the two-stage
skin carcinogenesis model (40).
Finally, in our study, UA was applied topically in DMSO, as we
could not solubilize the desired dose in ACT. Relative to ACT, DMSO
has been shown previously to enhance solute penetration in skin
explants of various animals (41)
and enhance penetration of the carcinogen benzidine into porcine
skin explants (42). The
penetration-enhancing effects of DMSO may be mediated by a number
of processes, including changing keratin structure and interactions
with lipid bilayers (43), as well
as the hygroscopic nature of DMSO (42). These effects of DMSO may have
allowed for more DMBA epidermal penetration in the UA-treated mice
in our study. However, we did not observe the same effect in the
UA+CG group, which was also treated with UA in DMSO 20 min prior to
DMBA application. Further studies are needed to determine how UA
may increase chemically-mediated skin tumor formation when applied
with DMBA.
Other studies have shown a potent antitumor effect
of RES (44–46), including in the DMBA/TPA-mediated
skin cancer model in Balb/c and ICR mouse strains (47,48).
However, in our studies we observed no antitumor effect of RES,
even at doses which significantly inhibited the transcription of
inflammatory genes and the levels of c-jun protein. This lack of
effect may be explained by the strain of mouse used. Our previous
studies revealed RES has limited effect on epidermal hyperplasia
and proliferation when applied with DMBA or TPA in short-term,
DMBA- or TPA-only models in SENCAR mice (49) (unpublished data). However, in the
same TPA-only model equimolar doses of UA reduced epidermal
hyperplasia to the level of negative control. The lack of
sensitivity to RES relative to UA observed in SENCAR mice may be
explained by different levels of metabolizing enzymes or RES
targets in the skin of different strains. Future studies, perhaps
in a simplified system with keratinocytes isolated from each mouse
strain, are needed to confirm this hypothesis.
These results indicate UA, alone or in combination
with CG, functions as a potent preventer of chemically-induced skin
cancer formation. Our previous experiments also identified
combinations of natural compounds which synergistically inhibited
DMBA-mediated mutation frequency as well as inflammation in the
epidermis (49). Future studies
may reveal these treatments to have an effect on full skin tumor
formation or on other cancers.
Acknowledgements
This study was supported by NIH grants R01 CA 102747
and P30 CA 54174-1651.
Abbreviations:
|
RES
|
resveratrol
|
|
GC
|
calcium D-glucarate
|
|
UA
|
ursolic acid
|
|
DMBA
|
7,12-dimethylbenz[a]anthracene
|
|
TPA
|
12-O-tetradecanoylphorbol-13-acetate
|
References
|
1
|
Bishop JM: Molecular themes in
oncogenesis. Cell. 64:235–248. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Knudson AG: Hereditary predisposition to
cancer. Ann NY Acad Sci. 833:58–67. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boutwell RK: Some biological aspects of
skin carcinogenisis. Prog Exp Tumor Res. 4:207–250. 1964.PubMed/NCBI
|
|
4
|
Slaga TJ: Cancer: etiology, mechanisms,
and prevention - a summary. Carcinog Compr Surv. 5:243–262.
1980.PubMed/NCBI
|
|
5
|
DiGiovanni J: Multistage carcinogenesis in
mouse skin. Pharmacol Ther. 54:63–128. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kemp CJ: Multistep skin cancer in mice as
a model to study the evolution of cancer cells. Semin Cancer Biol.
15:460–473. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Perez-Losada J and Balmain A: Stem-cell
hierarchy in skin cancer. Nat Rev Cancer. 3:434–443. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hanausek M, Ganesh P, Walaszek Z, Arntzen
CJ, Slaga TJ and Gutterman JU: Avicins, a family of triterpenoid
saponins from Acacia victoriae(Bentham), suppress H-ras
mutations and aneuploidy in a murine skin carcinogenesis model.
Proc Natl Acad Sci USA. 98:11551–11556. 2001.PubMed/NCBI
|
|
9
|
Kim EJ, Park H, Kim J and Park JH:
3,3′-diindolylmethane suppresses
12-O-tetradecanoylphorbol-13-acetate-induced inflammation
and tumor promotion in mouse skin via the downregulation of
inflammatory mediators. Mol Carcinog. 49:672–683. 2010.
|
|
10
|
Budunova IV, Perez P, Vaden VR, Spiegelman
VS, Slaga TJ and Jorcano JL: Increased expression of p50-NF-kappaB
and constitutive activation of NF-kappaB transcription factors
during mouse skin carcinogenesis. Oncogene. 18:7423–7431. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Przybyszewski J, Yaktine AL, Duysen E, et
al: Inhibition of phorbol ester-induced AP-1-DNA binding, c-Jun
protein and c-jun mRNA by dietary energy restriction is reversed by
adrenalectomy in SENCAR mouse epidermis. Carcinogenesis.
22:1421–1427. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Walaszek Z, Hanausek M and Slaga TJ:
Mechanisms of chemoprevention. Chest. 125:S128–S133. 2004.
View Article : Google Scholar
|
|
13
|
Taesotikul T, Dumrongsakulchai W,
Wattanachai N, Navinpipat V, Somanabandhu A and Tassaneeyakul W:
Inhibitory effects of Phyllanthus amarus and its major lignans on
human microsomal cytochrome P450 activities: evidence for CYP3A4
mechanism-based inhibition. Drug Metab Pharmacokinet. 26:154–161.
2011. View Article : Google Scholar
|
|
14
|
Kimura Y, Ito H, Ohnishi R and Hatano T:
Inhibitory effects of polyphenols on human cytochrome P450 3A4 and
2C9 activity. Food Chem Toxicol. 48:429–435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suganuma M, Okabe S, Kai Y, Sueoka N,
Sueoka E and Fujiki H: Synergistic effects of (−)-epigallocatechin
gallate with (−)-epicatechin, sulindac, or tamoxifen on
cancer-preventive activity in the human lung cancer cell line PC-9.
Cancer Res. 59:44–47. 1999.
|
|
16
|
Saw CL, Cintron M, Wu TY, et al:
Pharmacodynamics of dietary phytochemical indoles I3C and DIM:
induction of Nrf2-mediated phase II drug metabolizing and
antioxidant genes and synergism with isothiocyanates. Biopharm Drug
Dispos. 32:289–300. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khafif A, Schantz SP, Chou TC, Edelstein D
and Sacks PG: Quantitation of chemopreventive synergism between
(−)-epigallocatechin-3-gallate and curcumin in normal, premalignant
and malignant human oral epithelial cells. Carcinogenesis.
19:419–424. 1998.
|
|
18
|
Vingtdeux V, Dreses-Werringloer U, Zhao H,
Davies P and Marambaud P: Therapeutic potential of resveratrol in
Alzheimer’s disease. BMC Neurosci. 9(Suppl 2): S62008.
|
|
19
|
Bishayee A: Cancer prevention and
treatment with resveratrol: from rodent studies to clinical trials.
Cancer Prev Res. 2:409–418. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Das M and Das DK: Resveratrol and
cardiovascular health. Mol Aspects Med. 31:503–512. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brasnyo P, Molnar GA, Mohas M, et al:
Resveratrol improves insulin sensitivity, reduces oxidative stress
and activates the Akt pathway in type 2 diabetic patients. Br J
Nutr. 106:383–389. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Howells LM, Berry DP, Elliott PJ, et al:
Phase I randomized, double-blind pilot study of micronized
resveratrol (SRT501) in patients with hepatic metastases - safety,
pharmacokinetics, and pharmacodynamics. Cancer Prev Res.
4:1419–1425. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Patel KR, Brown VA, Jones DJ, et al:
Clinical pharmacology of resveratrol and its metabolites in
colorectal cancer patients. Cancer Res. 70:7392–7399. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Walaszek Z, Szemraj J, Narog M, et al:
Metabolism, uptake, and excretion of a D-glucaric acid salt and its
potential use in cancer prevention. Cancer Detect Prev. 21:178–190.
1997.PubMed/NCBI
|
|
25
|
Yoshimi N, Walaszek Z, Mori H, Hanausek M,
Szemraj J and Slaga TJ: Inhibition of azoxymethane-induced rat
colon carcinogenesis by potassium hydrogen D-glucarate. Int J
Oncol. 16:43–48. 2000.PubMed/NCBI
|
|
26
|
Hanausek M, Walaszek Z and Slaga TJ:
Detoxifying cancer causing agents to prevent cancer. Integr Cancer
Ther. 2:139–144. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Abou-Issa H, Moeschberger M, el-Masry W,
Tejwani S, Curley RW Jr and Webb TE: Relative efficacy of glucarate
on the initiation and promotion phases of rat mammary
carcinogenesis. Anticancer Res. 15:805–810. 1995.PubMed/NCBI
|
|
28
|
Huang MT, Ho CT, Wang ZY, et al:
Inhibition of skin tumorigenesis by rosemary and its constituents
carnosol and ursolic acid. Cancer Res. 54:701–708. 1994.PubMed/NCBI
|
|
29
|
De Angel RE, Smith SM, Glickman RD,
Perkins SN and Hursting SD: Antitumor effects of ursolic acid in a
mouse model of postmenopausal breast cancer. Nutr Cancer.
62:1074–1086. 2010.PubMed/NCBI
|
|
30
|
Shan JZ, Xuan YY, Zheng S, Dong Q and
Zhang SZ: Ursolic acid inhibits proliferation and induces apoptosis
of HT-29 colon cancer cells by inhibiting the EGFR/MAPK pathway. J
Zhejiang Univ Sci B. 10:668–674. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kassi E, Papoutsi Z, Pratsinis H,
Aligiannis N, Manoussakis M and Moutsatsou P: Ursolic acid, a
naturally occurring triterpenoid, demonstrates anticancer activity
on human prostate cancer cells. J Cancer Res Clin Oncol.
133:493–500. 2007. View Article : Google Scholar
|
|
32
|
Kassi E, Sourlingas TG, Spiliotaki M, et
al: Ursolic acid triggers apoptosis and Bcl-2 downregulation in
MCF-7 breast cancer cells. Cancer Invest. 27:723–733. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fischer SM, Pavone A, Mikulec C,
Langenbach R and Rundhaug JE: Cyclooxygenase-2 expression is
critical for chronic UV-induced murine skin carcinogenesis. Mol
Carcinog. 46:363–371. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lederle W, Depner S, Schnur S, et al: IL-6
promotes malignant growth of skin SCCs by regulating a network of
autocrine and paracrine cytokines. Int J Cancer. 128:2803–2814.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ullevig SL, Zhao Q, Zamora D and Asmis R:
Ursolic acid protects diabetic mice against monocyte dysfunction
and accelerated atherosclerosis. Atherosclerosis. 219:409–416.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Young MR, Li JJ, Rincon M, et al:
Transgenic mice demonstrate AP-1 (activator protein-1)
transactivation is required for tumor promotion. Proc Natl Acad Sci
USA. 96:9827–9832. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Thompson EJ, MacGowan J, Young MR, Colburn
N and Bowden GT: A dominant negative c-jun specifically blocks
okadaic acid-induced skin tumor promotion. Cancer Res.
62:3044–3047. 2002.PubMed/NCBI
|
|
38
|
Kowalczyk MC, Walaszek Z, Kowalczyk P,
Kinjo T, Hanausek M and Slaga TJ: Differential effects of several
phytochemicals and their derivatives on murine keratinocytes in
vitro and in vivo: implications for skin cancer prevention.
Carcinogenesis. 30:1008–1015. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shahin V, Ludwig Y, Schafer C, Nikova D
and Oberleithner H: Glucocorticoids remodel nuclear envelope
structure and permeability. J Cell Sci. 118:2881–2889. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Thompson S and Slaga TJ: The effects of
dexamethasone on mouse skin initiation and aryl hydrocarbon
hydroxylase. Eur J Cancer. 12:363–370. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Baynes RE, Halling KB and Riviere JE: The
influence of diethyl-m-toluamide (DEET) on the percutaneous
absorption of permethrin and carbaryl. Toxicol Appl Pharmacol.
144:332–339. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Baynes RE, Brownie C, Freeman H and
Riviere JE: In vitro percutaneous absorption of benzidine in
complex mechanistically defined chemical mixtures. Toxicol Appl
Pharmacol. 141:497–506. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Williams AC and Barry BW: Penetration
enhancers. Adv Drug Deliv Rev. 56:603–618. 2004. View Article : Google Scholar
|
|
44
|
Banerjee S, Bueso-Ramos C and Aggarwal BB:
Suppression of 7,12-dimethylbenz(a)anthracene-induced
mammary carcinogenesis in rats by resveratrol: role of nuclear
factor-kappaB, cyclooxygenase 2, and matrix metalloprotease 9.
Cancer Res. 62:4945–4954. 2002.PubMed/NCBI
|
|
45
|
Cui X, Jin Y, Hofseth AB, et al:
Resveratrol suppresses colitis and colon cancer associated with
colitis. Cancer Prev Res. 3:549–559. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Harper CE, Patel BB, Wang J, Arabshahi A,
Eltoum IA and Lamartiniere CA: Resveratrol suppresses prostate
cancer progression in transgenic mice. Carcinogenesis.
28:1946–1953. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
George J, Singh M, Srivastava AK, et al:
Resveratrol and black tea polyphenol combination synergistically
suppress mouse skin tumors growth by inhibition of activated MAPKs
and p53. PLoS One. 6:e233952011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kapadia GJ, Azuine MA, Tokuda H, et al:
Chemopreventive effect of resveratrol, sesamol, sesame oil and
sunflower oil in the Epstein-Barr virus early antigen activation
assay and the mouse skin two-stage carcinogenesis. Pharmacol Res.
45:499–505. 2002. View Article : Google Scholar
|
|
49
|
Kowalczyk MC, Kowalczyk P, Tolstykh O,
Hanausek M, Walaszek Z and Slaga TJ: Synergistic effects of
combined phytochemicals and skin cancer prevention in SENCAR mice.
Cancer Prev Res. 3:170–178. 2010. View Article : Google Scholar : PubMed/NCBI
|