Introduction
Ameloblastoma, the most common benign tumor of the
jaws, is characterized by slow growth and local invasion with
potentially destructive behavior. It is thought to arise from the
odontogenic epithelium because of a histological resemblance to the
enamel organ of the developing tooth germ. However, the detailed
mechanisms of its proliferation and invasion are not
understood.
Sonic hedgehog (SHH) signaling pathway is crucial to
growth and patterning during organogenesis including the limb bud,
hair, glands, gut and gonads (1–3). SHH
is a secreted protein that activates a membrane-receptor complex
formed by patched (PTCH) and smoothend (SMO) (1,4).
PTCH and SMO are membrane-bound proteins with seven transmembrane
domains, respectively (5,6). In the absence of SHH, PTCH inhibits
SMO, whereas the binding of SHH to PTCH suspends this inhibition,
thereby activating zinc finger DNA-binding proteins GLI1, GLI2 and
GLI3 (1). The GLI proteins mediate
SHH signaling by translocating from the cytoplasm to the nucleus to
act as transcription factors to activate target genes (1,7,8).
Recent studies have implicated inherited or sporadic alterations in
SHH signaling pathway genes in a number of developmental defects
and aberrant activation of the SHH signaling pathway can result in
tumor formation (9–16). In the developing tooth germ, SHH is
expressed in the epithelial component and regulates the
proliferation and differentiation of ameloblasts (17,18).
Furthermore, alterations in PTCH expression have been demonstrated
in keratocystic odontogenic tumors, which are characterized by
cystic structures with proliferation of the odontogenic epithelium
within the jaw (19,20). These studies suggest that the SHH
signaling pathway is closely associated with proliferation of
odontogenic epithelial cells. In this study, we examined the
expression of SHH, PTCH and GLI proteins and elucidated the
functional roles of the SHH signaling pathway, in the proliferation
of ameloblastoma.
Materials and methods
Patients
The subjects gave informed consent before enrolment.
Specimens were surgically removed from 29 patients with primary
ameloblastoma (male, 22 and female, 7 cases, mean age, 37.8±19.4
years; age range, 14–80 years) at the Department of Oral and
Maxillofacial Surgery, Kyushu University Hospital. Following the
initial biopsy, all specimens were fixed in 4% buffered formalin
solution and embedded in paraffin blocks. Subsequently, the
specimens were processed into 5-μm thick sections and stained with
hematoxylin and eosin. The tumors were further classified as 17
follicular and 12 plexiform types according to the World Health
Organization guidelines for histologic typing of odontogenic tumors
(21).
Immunohistochemistry
The sections were deparaffinized in xylene and
hydrated in graded ethanol. For antigen retrieval, the sections
were immersed in Target Retrieval Solution (Dako, Denmark) and
autoclaved at 121°C for 5 min. After elimination of endogenous
peroxide activity and blocking by incubation with 10% normal goat
serum (Nichirei Bioscience, Japan), the sections were incubated
with primary antibody overnight. The following antibodies were
used: anti-human SHH monoclonal (Abcam, UK; diluted 1:100),
anti-human PTCH polyclonal (Santa Cruz, USA; diluted 1:100),
anti-human GLI1 polyclonal (Abcam; diluted 1:80), anti-human GLI2
polyclonal (Abcam; 1:200) and anti-human GLI3 polyclonal (Novus
Biological, USA; 1:100) antibodies. The sections were then
incubated with horseradish peroxidase-conjugated secondary
antibodies for 1 h. The immunoreactivities were visualized by
immersing the sections in 3,3′-diaminobenzidine (Nichirei
Bioscience). Subsequently, the sections were dehydrated, cleared
with xylene and finally mounted. Negative controls were prepared by
substituting phosphate-buffered saline for primary antibody.
Cell culture and immunocytochemistry
The human ameloblastoma cell line AM-1, which was
established from human ameloblastoma tissue and immortalized by the
transfection of human papillomavirus type 16 DNA, was maintained in
defined keratinocyte-serum-free medium supplemented with adjunctive
growth supplement (22). The
immortalized human keratinocyte cell line (HaCat) was cultured in
Dulbecco’s modified Eagle’s medium/F-12 (Sigma-Aldrich, USA)
supplemented with 10% fetal bovine serum. All cell lines were
maintained with 100 U/ml penicillin/streptomycin in a humidified
atmosphere of 5% CO2 at 37°C.
For immunocytochemistry, cultured cells were fixed
in 75% methanol and then incubated with primary antibodies as
described above and the anti-human BCL-2 polyclonal (Ana Spec, USA;
1:500) and anti-human BAX polyclonal (R&D Systems, USA; 1:500).
Subsequently, the cells were incubated with Alexa Fluor®
488- or 546-conjugated secondary antibodies (Molecular Probes, USA;
diluted 1:400). The cells were counterstained with 1 μg/ml Hoechst
33342 (Molecular Probes) and observed under a fluorescence
microscope (BZ-8000; Keyence, Japan).
RNA extraction and cDNA synthesis
Total RNA was extracted from cultured cells using a
PureLink™ RNA Mini kit (Invitrogen, USA). The amount of RNA
extracted from each sample was measured spectrophotometrically on a
NanoDrop 1000 (Thermo Scientific, USA). Of the total RNA
preparation 2 μg was used for cDNA synthesis. Briefly, RNA was
incubated for 15 min at 42°C with 25 U/μl of recombinant RNase
inhibitor (Nacalai Tesque, Japan), 1.0 μl of 50 μM random hexamers
(Applied Biosystems), 2.0 μl of each 2.0 μM dNTP (Toyobo, Japan)
and 50 U/μl of Moloney murine leukemia virus reverse transcriptase
(Roche Diagnostics, Switzerland).
Reverse transcription-PCR (RT-PCR)
For RT-PCR, 100 ng of template DNA, 0.5 μl of 20 pM
sense and antisense primers, 1.0 μl of 25 mM MgCl2, 1.25
μl of 10X Taq DNA polymerase buffer (Bio Basic, Canada), 5 U/μl of
Taq DNA polymerase (Bio Basic), 0.5 μl of 2.0 mM dNTP mix (Toyobo)
and 9.65 μl of sterilized water were used in a total volume of 13.5
μl. The PCR conditions were: 25 cycles of denaturing at 94°C for 30
sec, annealing at 60°C for 30 sec and elongation at 72°C for 15
sec. For amplification of specific regions of target genes, the
primers used were: SHH, forward 5′-GATGACTCAGAG
GTGTAAGGACAA-3′ and reverse 5′-CCACCGAGTTCTCT GCTTTCA-3′;
PTCH, forward 5′-GGATCATTGTGATGGTC CTG-3′ and reverse
5′-GTCAGAAAGGCCAAAGCAAC-3′; GLI1, forward
5′-CACCACATCAACAGCGAGCA-3′ and reverse 5′-TTCCGGCACCCTTCAAACG-3′;
GLI2, forward 5′-AGCAGCAGCAACTGTCTGAGTGA-3′ and reverse
5′-GAC CTTGCTGCGCTTGTGAA-3′; GLI3, forward 5′-TCCAAC
ACAGAGGCCTATTCCAG-3′ and reverse 5′-CTCTTGTTGT GCATCGGGTCA-3′;
glyceraldehyde 3-phosphate dehydrogenase (GAPDH), forward
5′-ATCAGCAATGCCTCCT GCA-3′ and reverse 5′-ATGGCATGGACTGTGGTCAT-3′.
The housekeeping gene GAPDH was used as the internal
control.
Water-soluble terazolium (WST)-8 cell
proliferation assay
Cell proliferation assays were performed using the
WST-8 Cell Counting kit (Dojin, Japan), according to the
manufacturer’s instructions. Briefly, 3.0×103 cells/well
were seeded into 96-well microtiter plates. After 24-h incubation,
an inhibitor of sonic hedgehog signaling-SHH neutralizing antibody
(1 ng/ml; StemRD, USA) or cyclopamine (1 mM; Enzo Life Science,
USA) was added to each well and the absorbance at 450 nm was
measured using a microplate reader (Multiskan FC, Thermo
Scientific).
Apoptosis assay
The Annexin V assay was performed to detect
apoptotic cells. Briefly, 3.0×104 AM-1 cells were seeded
into culture plates, after 24-h incubation, 1 ng/ml SHH
neutralizing antibody was added to each well and further incubated
for 48 h. Apoptotic cells were stained by Annexin V conjugated with
fluorescein isothiocyanate (MBL, Japan) and counted under a
fluorescence microscope.
Statistical analyses
All statistical analyses were performed using JMP
software version 8 (SAS Institute, Japan).
Results
Expression of SHH molecules in
ameloblastoma and normal gingiva
In the normal gingiva, immunoreactivity for SHH,
PTCH, GLI1, GLI2 and GLI3 was more evident in the epithelial cells
than in the stromal cells. SHH was strongly expressed in the
cytoplasm of basal cells and weakly in the cells of the stratum
spinosum. The expression of PTCH was observed in the cell membrane
and cytoplasm of the epithelial cells. GLI1, GLI2 and GLI3 were
localized in the nucleus of the epithelial cells. GLI1 and GLI3
were mainly expressed in the basal layer, while GLI2 was strongly
expressed in the parabasal cells rather than basal cells. In
ameloblastoma, immunoreactivity for SHH, PTCH, GLI1, GLI2 and GLI3
was seen in almost all tumor cells, but not in the stromal cells.
SHH was expressed in the cytoplasm, PTCH in the cytoplasm and cell
membrane and the GLI proteins only in the nucleus. The reactivity
was stronger in the peripheral cuboidal and columnar cells than in
the central polyhedral cells of the tumor nests. There was no
difference in the expression pattern of these proteins between the
follicular and plexiform types (Fig.
1).
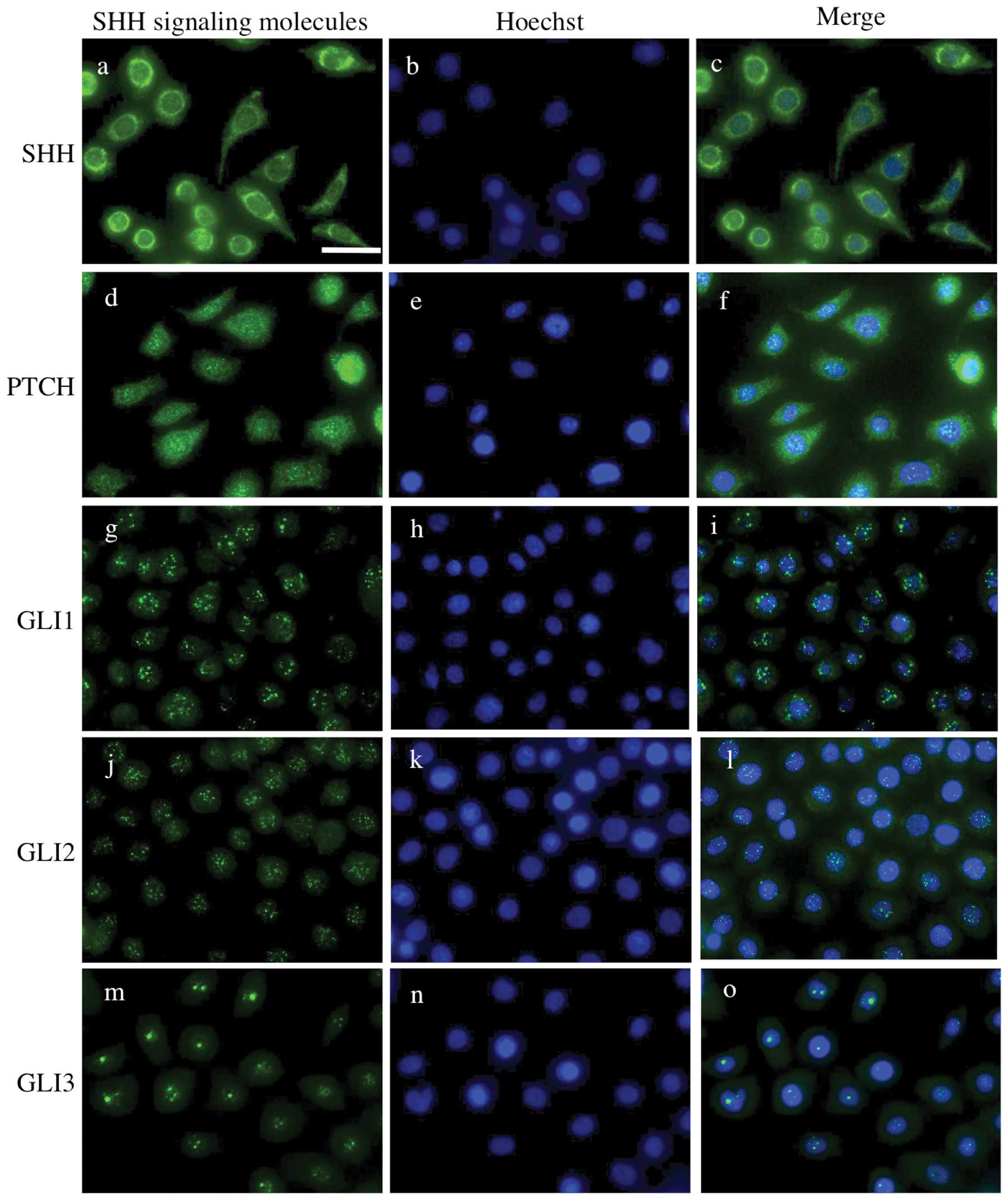
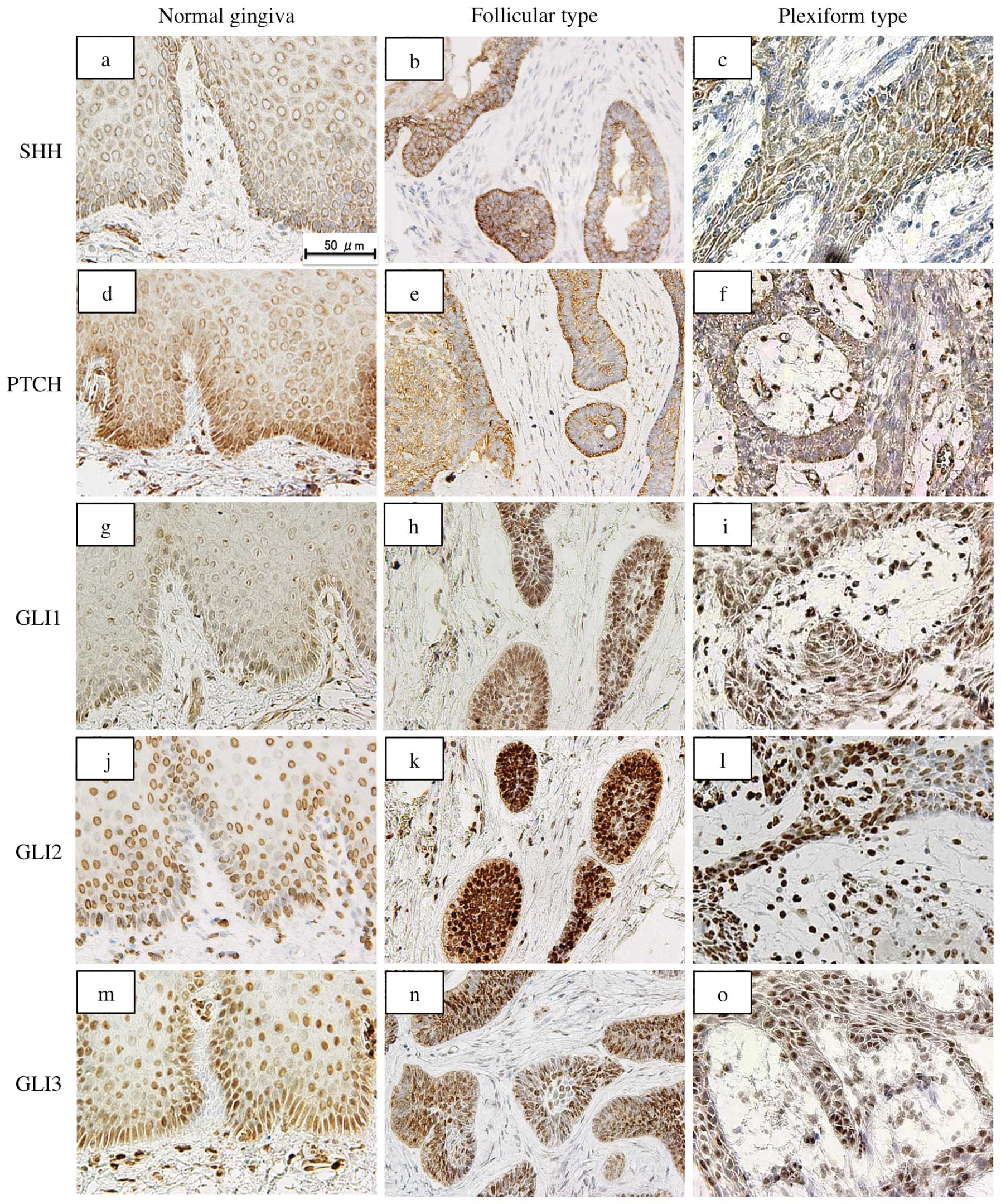 | Figure 1Immunohistochemical staining of SHH,
PTCH, GLI1, GLI2 and GLI3 in ameloblastoma specimens. In the normal
gingiva, SHH is expressed strongly in the cytoplasm of basal cells
(a). The expression of PTCH is observed in the cell membrane and
cytoplasm of the epithelial cells (d). GLI1 (g), GLI2 (j) and GLI3
(m) localize in the nucleus of the epithelial cells. In
ameloblastoma, immunoreactivity for SHH, PTCH, GLI1, GLI2 and GLI3
is seen in almost all the tumor cells. SHH is expressed in the
cytoplasm (b and c), PTCH in the cytoplasm and cell membrane (e and
f) and GLI proteins only in the nucleus (h, i, k, l, n and o). The
reactivity is stronger in the peripheral cuboidal and columnar
cells than in the central polyhedral cells. Bar, 50 μm. |
Expression of SHH-related genes and gene
products in the ameloblastoma cell line AM-1
By immunocytochemistry, SHH was mainly expressed in
the cytoplasm. Immunoreactivity for PTCH was observed in the cell
membrane and cytoplasm. The expression of GLI1, GLI2 and GLI3 was
localized in the nucleus, but not in the cytoplasm or membrane
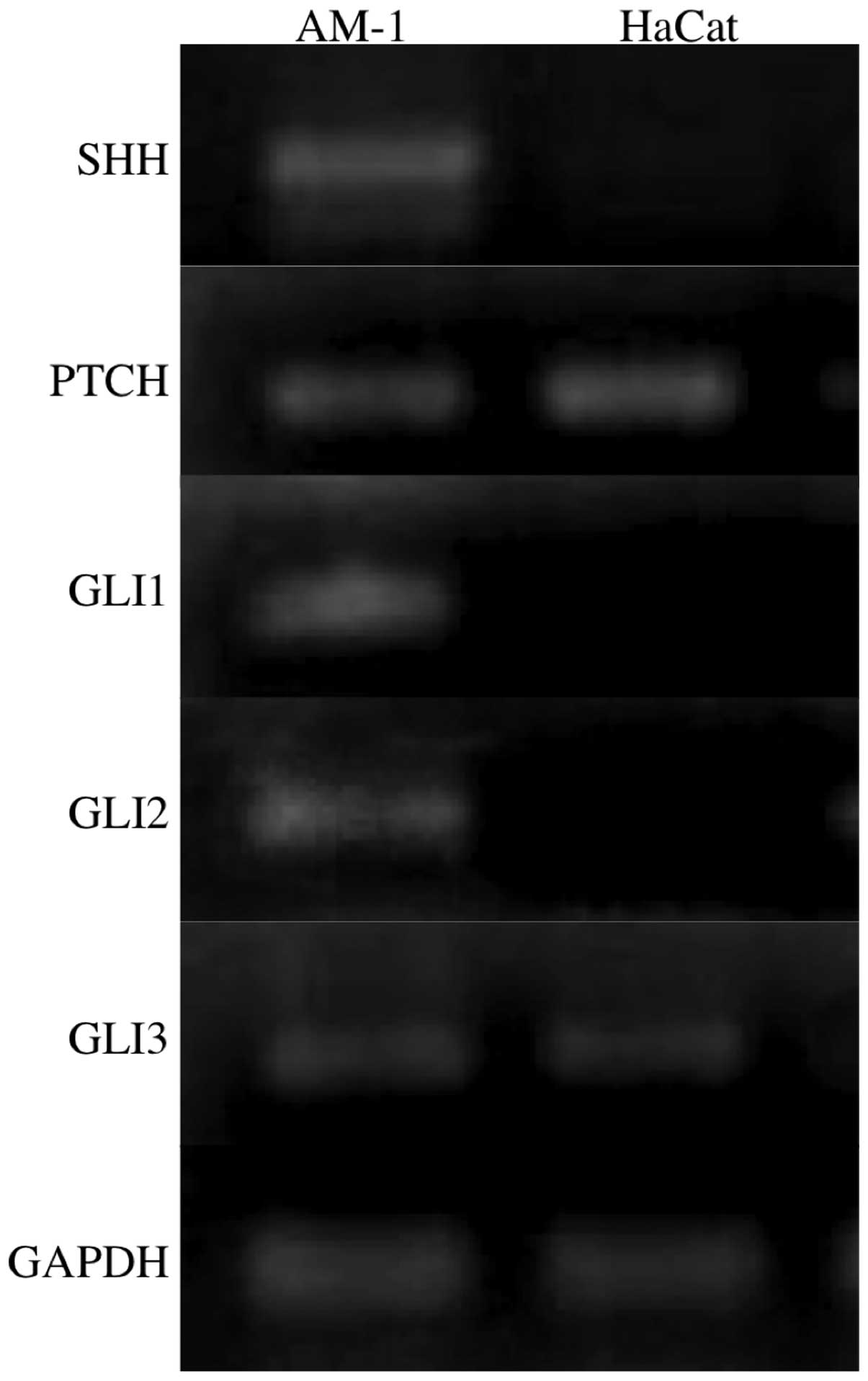
(Fig. 2). RT-PCR analyses revealed
that SHH, PTCH, GLI1, GLI2 and GLI3 were expressed in
the AM-1 cells, while PTCH and GLI3 were also
expressed in the HaCat cells (Fig.
3).
SHH neutralizing antibody and cyclopamine
suppress AM-1 cell proliferation
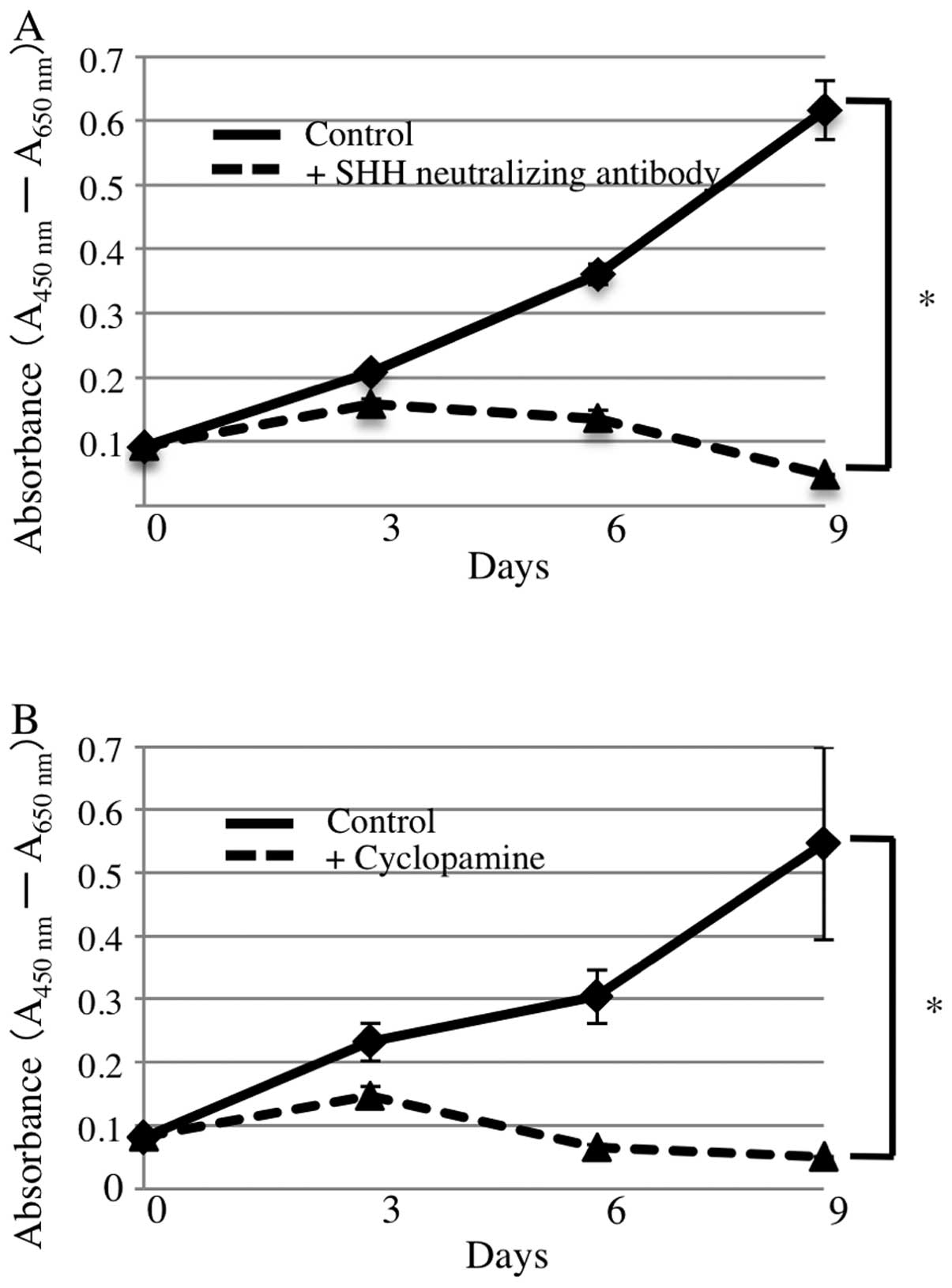
To examine the effects of SHH on the proliferation
of AM-1 cells, we added SHH neutralizing antibody or cyclopamine,
both inhibitors of SHH signaling, to the culture medium. In the
WST-8 assay, cell proliferation in the presence of 1 ng/ml SHH
neutralizing antibody was significantly inhibited compared with
that of the control (repeated measures analysis of variance
(ANOVA), p<0.05) (Fig. 4A). The
addition of 1 mM cyclopamine also suppressed proliferation of AM-1
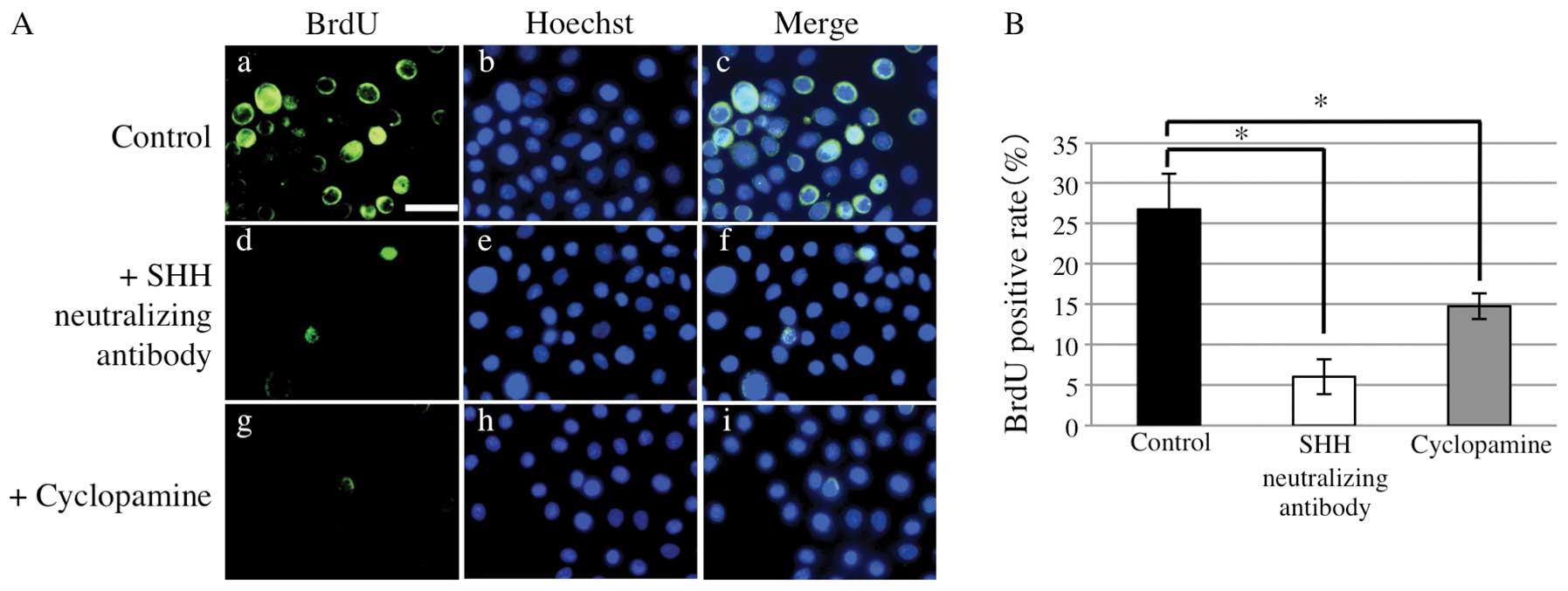
cells (repeated measures ANOVA, p<0.05) (Fig. 4B). BrdU incorporation assays
revealed that the BrdU positivity rate in the presence of Shh
neutralizing antibody or cyclopamine was significantly lower than
that in the controls (Mann-Whitney U test, p<0.05) (Fig. 5).
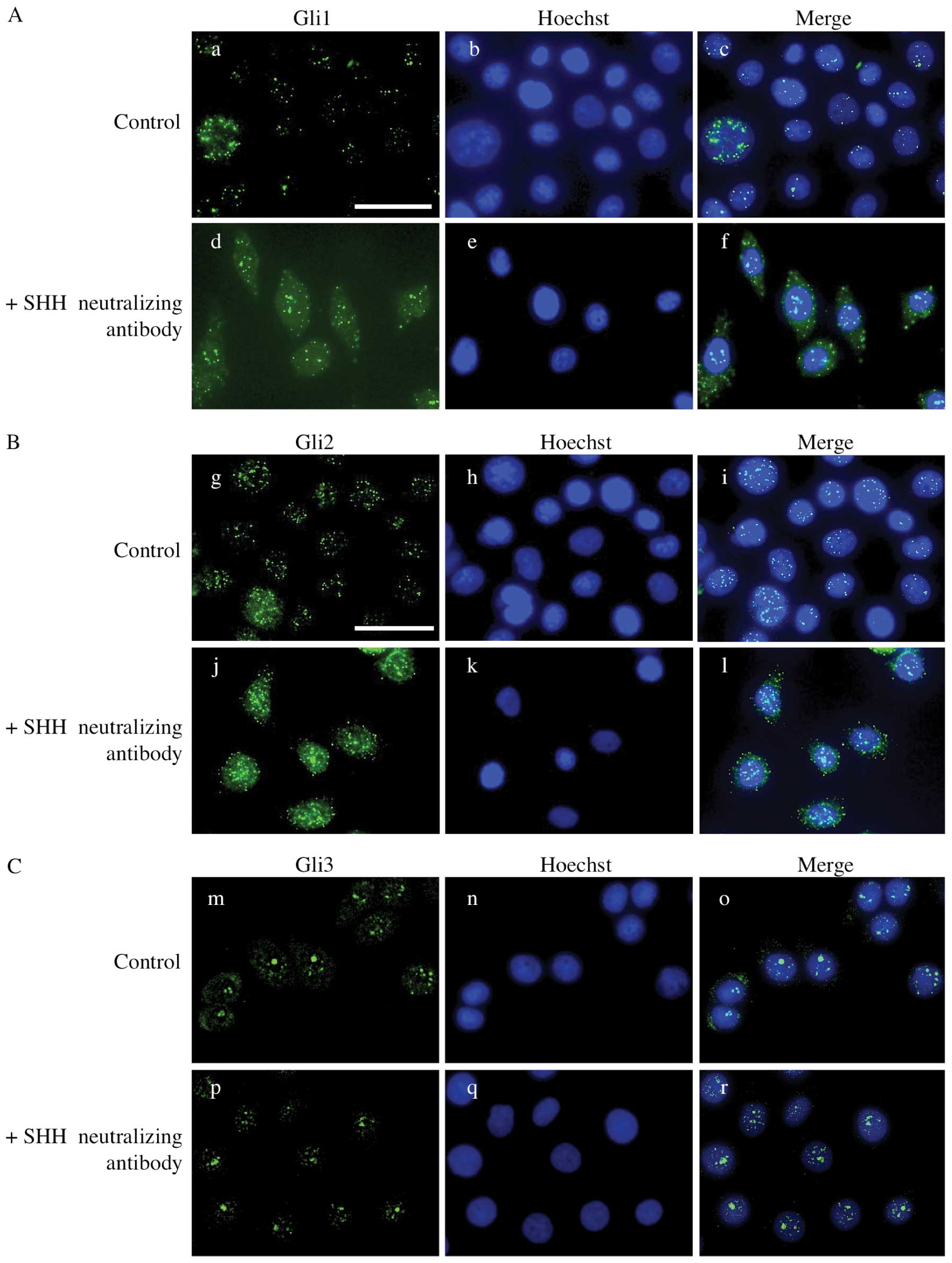
Nuclear translocation of Gli proteins is
abolished by SHH neutralizing antibody
To examine whether SHH signal transduction is
affected by SHH neutralizing antibody, we performed
immunocytochemical staining for GLI proteins. In the control
groups, immunoreactivity for GLI1, GLI2 and GLI3 was observed in
the nucleus of AM-1 cells. However, in the presence of SHH
neutralizing antibody, the expression of GLI1 and GLI2 was detected
in the cytoplasm rather than the nucleus suggesting that the
nuclear translocation of GLI proteins is abolished by SHH
neutralizing antibody. GLI3 remained in the nucleus after the
addition of SHH neutralizing antibody (Fig. 6).
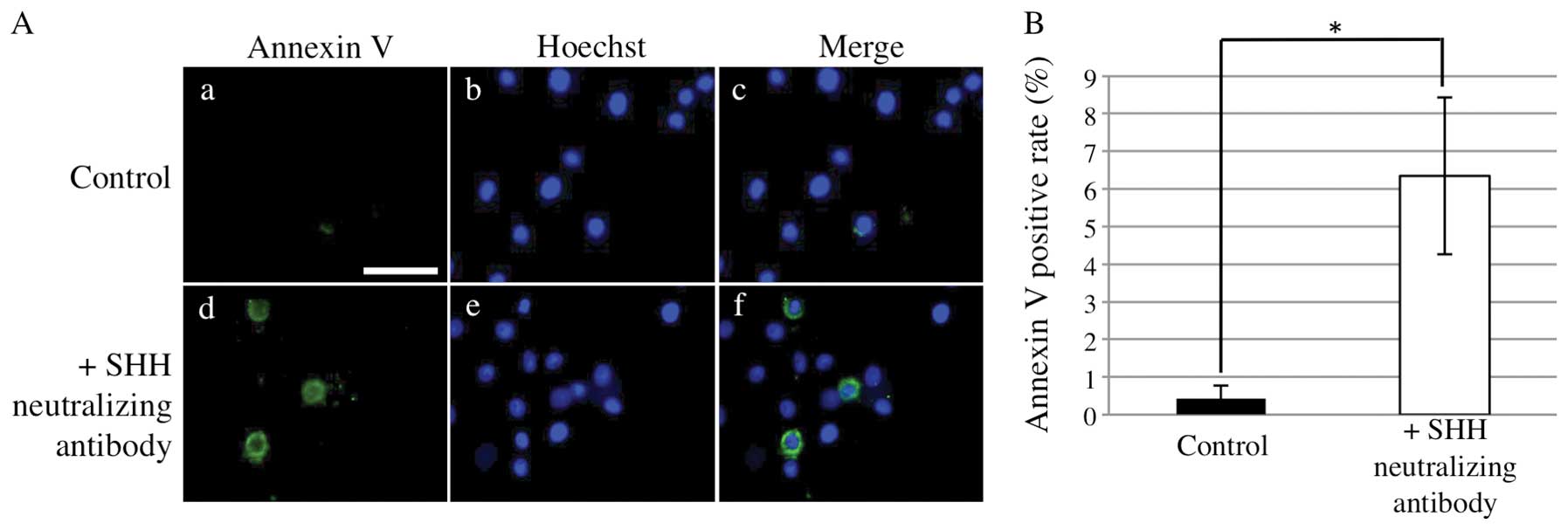
SHH neutralizing antibody induces
apoptosis of AM-1 cells
We next examined the influence of SHH neutralizing
antibody on the apoptosis of AM-1 cells. Annexin V-positive rates
were significantly higher in the presence of SHH neutralizing
antibody than those in its absence (Mann-Whitney U test, p<0.05)
(Fig. 7). To examine the
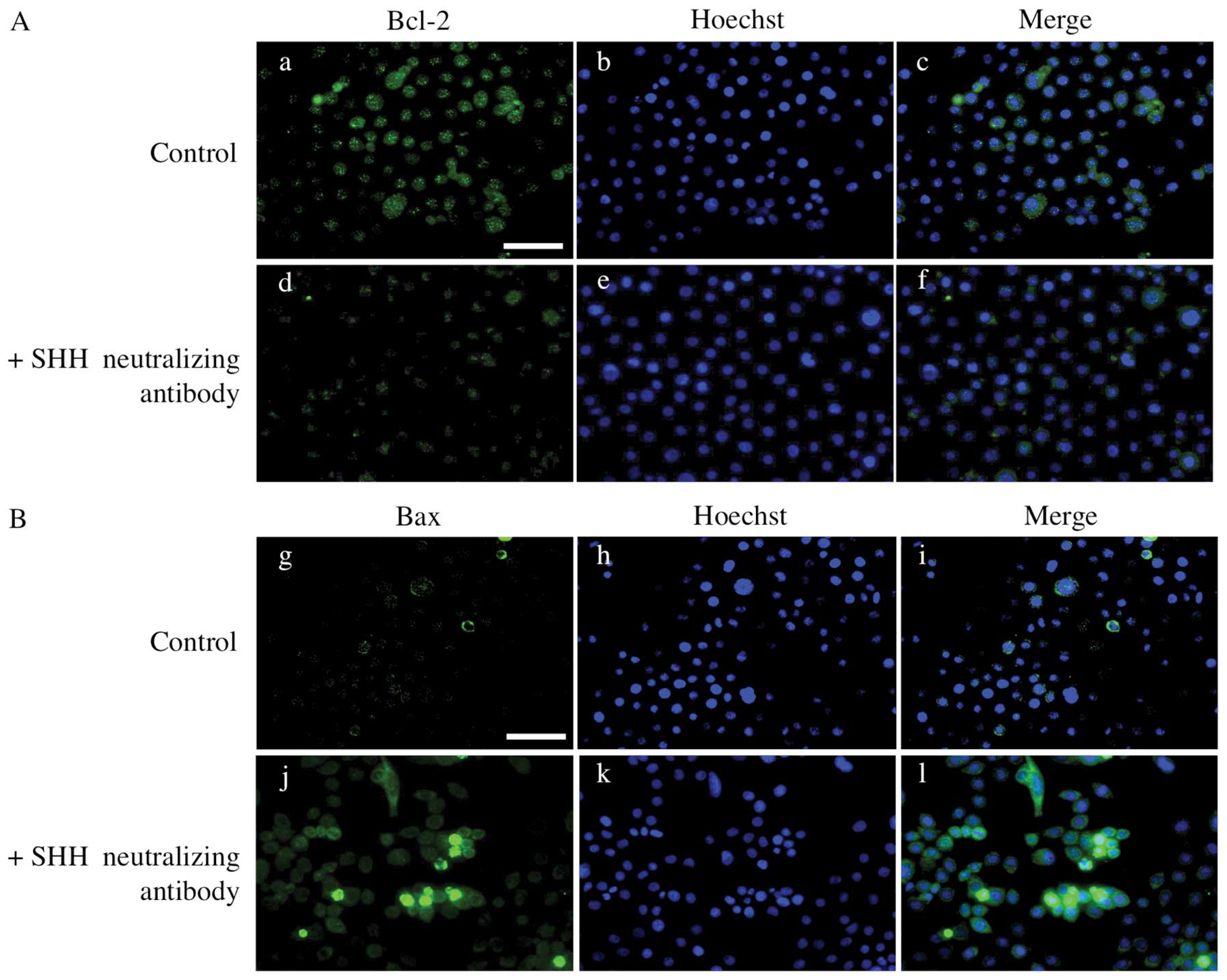
expression of apoptosis-associated proteins, we performed
immunocytochemistry in AM-1 cells for BCL-2, an anti-apoptotic
protein and BAX, a pro-apoptotic protein. The expression of BCL-2
decreased in the presence of SHH neutralizing antibody, while the
expression of BAX increased (Fig.
8).
Discussion
In the present study, we examined the expression of
SHH, PTCH, GLI1, GLI2, and GLI3 in ameloblastoma and investigated
their functions using an ameloblastoma cell line. Several studies
have revealed that SHH signaling-associated molecules are expressed
in odontogenic tumors including ameloblastoma (23–25).
Kumamoto et al and Zhang et al demonstrated by
immunohistochemistry that SHH, PTCH, SMO and GLI1 were expressed in
all cases of ameloblastoma and that reactivity was stronger in
peripheral cuboidal cells than in central polyhedral cells
(23,24). Our findings were almost identical
to these.
Little is known about the significance and function
of SHH signaling in ameloblastoma. In a previous study, we revealed
that Ki-67 and proliferating cell nuclear antigen were mainly
expressed in the outer layer of tumors strongly expressing SHH
signaling molecules (26). Here,
we examined the association of SHH signaling with the cell
proliferation of ameloblastoma using AM-1 cells. We found that the
AM-1 cells expressed SHH and PTCH and that proliferation was
suppressed by adding SHH neutralizing antibody or cyclopamine.
Furthermore, the nuclear translocation of GLI1 and GLI2 was
abolished by SHH neutralizing antibody. These results suggest that
the SHH signaling pathway is constitutively activated in
ameloblastoma cells and that AM-1 cells proliferate by autocrine
loop SHH stimulation. Constitutive activation of SHH signaling and
SHH-dependent proliferation have been found in a variety of cancers
including lung, esophagus, stomach and pancreas (27–30).
Ameloblastoma might now be added to this list. However, we found
that the nuclear translocation of GLI3 was not abolished by Shh
neutralizing antibody. It has been demonstrated that inhibition of
SHH signaling by cyclopamine promotes the processing of GLI3 into a
shortened form that can act as a transcription factor in cultured
cells and limb explant cultures (31,32).
These results suggest that a dynamic interplay between the GLI
signals occurs in the proliferation of ameloblastoma, although the
molecular mechanisms that control such interactions are largely
undefined.
We found here that SHH neutralizing antibody induced
apoptosis of AM-1 cells and demonstrated decreased BCL-2 and
increased BAX expression. In a previous study, we demonstrated that
BCL-2, which prevents apoptosis, was mainly expressed in the outer
layer of ameloblastoma cells, whereas the inner cells (stellate
reticulum-like cells and squamoid cells) did not express this
protein (33). This expression
pattern of BCL-2 was similar to that of SHH in ameloblastoma.
Furthermore, it has been demonstrated that hedgehog signaling
induced apoptosis of colorectal cancer cells in the presence of
cyclopamine (34). Taken together,
it seems that SHH plays an anti-apoptotic role in the proliferation
of ameloblastoma cells.
Recent studies have reported SHH overexpression in
basal cell carcinoma and lung squamous cell carcinoma (15,35).
Furthermore, transgenic mice overexpressing SHH develop various
tumors, such as basal cell carcinoma, medulloblastoma and breast
carcinoma (36). These results
suggest a role for SHH in tumorigenesis. Our present results
suggest that inhibition of SHH signaling might be a good target for
a molecular treatment for ameloblastoma, although further studies
are needed to understand the precise role of the SHH signaling
pathway in tumor progression.
Acknowledgements
This study was supported by a Grant-in-Aid (no.
23792358) from the Japanese Ministry of Education, Culture, Sports,
Science and Technology of Japan.
References
|
1
|
Ingham PW and McMahon AP: Hedgehog
signaling in animal development: paradigms and principals. Gens
Dev. 15:3059–3087. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Echelard Y, Epstein DJ, St-Jacques B, Shen
L, Mohler J, McMahon JA, et al: Sonic hedgehog, a member of a
family of putative signaling molecules, is implicated in the
regulation of CNS polarity. Cell. 75:1417–1430. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bitgood MJ and McMahon AP: Hedgehog and
Bmp genes are coexpressed at many diverse sites of cell-cell
interaction in the mouse embryo. Dev Biol. 172:126–138. 1995.
View Article : Google Scholar
|
|
4
|
NuÈsslein-Vohhard C and Wieschaus E:
Mutations affecting segment number and polarity in
Drosophila. Nature. 287:795–801. 1980.PubMed/NCBI
|
|
5
|
Stone DM, Hynes M, Armanini M, et al: The
tumour-suppressor gene patched encodes a candidate receptor for
Sonic hedgehog. Nature. 384:129–134. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alcedo J, Ayzenzon M, Von Ohlen T, Noll M
and Hooper JE: The Drosophila smoothened gene encodes a
seven-pass membrane protein, a putative receptor for the Hedgehog
signal. Cell. 86:221–232. 1996.
|
|
7
|
Kalderon D: Similarities between the
Hedgehog and Wnt signaling pathways. Trends Cell Biol. 12:523–531.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ruel L, Rodriguez R, Gallet A,
Lavenant-Staccini L and Thérond PP: Stability and association of
Smoothened, Costal2 and fused with Cubitus interruptus are
regulated by Hedgehog. Nat Cell Biol. 5:907–913. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hahn H, Wojnowski L, Miller G and Zimmer
A: The patched signaling pathway in tumorigenesis and development:
lessons from animal models. J Mol Med. 77:459–468. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Altaba AR, Sánchez P and Dahmane N: Gli
and Hedgehog in cancer: tumours, embryos and stem cells. Nat Rev
Cancer. 2:361–372. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gailani MR, Ståhle-Bäckdahl M, Leffell DJ,
et al: The role of the human homologue of Drosophila patched
in sporadic basal cell carcinomas. Nat Genet. 14:78–81.
1996.PubMed/NCBI
|
|
12
|
Hahn H, Wicking C, Zaphiropoulos PG, et
al: Mutations of the human homolog of Drosophila patched in
the nevoid basal cell carcinoma syndrome. Cell. 85:841–851.
1996.PubMed/NCBI
|
|
13
|
Johnson RL, Rothman AL, Xie J, et al:
Human homolog of patched, a candidate gene for the basal cell nevus
syndrome. Science. 272:1668–1671. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Roessler E, Belloni E, Gaudenz K, et al:
Mutations human Sonic hedgehog gene cause holoprosencephaly. Nat
Genet. 14:357–360. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dahmane N, Lee J, Robins P, Heller P and
Altaba AR: Activation of the transcription factor Gli1 and the
Sonic hedgehog signaling pathway in skin tumours. Nature.
389:876–881. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reifenberger J, Wolter M, Weber RG, et al:
Missense mutations in SMOH in sporadic basal cell carcinomas of the
skin and primitive neuroectodermal tumors of the central nervous
system. Cancer Res. 58:1798–1803. 1998.PubMed/NCBI
|
|
17
|
Dassule HR and McMahon AP: Analysis of
epithelial-mesenchymal interactions in the initial morphogenesis of
the mammalian tooth. Dev Biol. 202:215–227. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hardcastle Z, Mo R, Hui C and Sharpe PT:
The Shh signaling pathway in tooth development. defects in Gli2 and
Gli3 mutants. Development. 125:2803–2811. 1998.PubMed/NCBI
|
|
19
|
Barreto DC, Gomez RS, Bale AE, Boson WL
and De Marco L: PTCH gene mutations in odontogenic keratocysts. J
Dent Res. 79:1418–1422. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Diniz MG, Borges ER, Guimarães AL, Moreira
PR, Brito JA, Gomez MV, et al: PTCH1 isoforms in odontogenic
keratocysts. Oral Oncol. 45:291–295. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sciubba JJ, Fantasia JE and Kahn LB:
Tumors and Cysts of the Jaw. Armed Forces Institute of Pathology;
Washington, DC: pp. 71–99. 2001
|
|
22
|
Harada H, Mitsuyasu T, Nakamura N, Higuchi
Y, Toyoshima K, Taniguchi A, et al: Establishment of ameloblastoma
cell line, AM-1. J Oral Pathol Med. 27:207–212. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kumamoto H, Ohki K and Ooya K: Expression
of sonic hedgehog (SHH) signaling molecules in ameloblastomas. J
Oral Pathol Med. 33:185–190. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang L, Chen XM, Sun ZJ, Bian Z, Fan MW
and Chen Z: Epithelial expression of SHH signaling pathway in
odontogenic tumors. Oral Oncol. 42:398–408. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vered M, Peleg O, Taicher S and Buchner A:
The immunoprofile of odontogenic keratocyst (keratocystic
odontogenic tumor) that includes expression of PTCH, SMO, GLI-1 and
bcl-2 is similar to ameloblastoma but different from odontogenic
cysts. J Oral Pathol Med. 38:597–604. 2009. View Article : Google Scholar
|
|
26
|
Sandra F, Mitsuyasu T, Nakamura N,
Shiratsuchi Y and Ohishi M: Immunohistochemical evaluation of PCNA
and Ki-67 in ameloblastoma. Oral Oncol. 37:193–198. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Watkins DN, Berman DM, Burkholder SG, Wang
B, Beachy PA and Baylin SB: Hedgehog signalling within airway
epithelial progenitors and in small-cell lung cancer. Nature.
422:313–317. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thayer SP, di Magliano MP, Heiser PW,
Nielsen CM, Roberts DJ, Lauwers GY, et al: Hedgehog is an early and
late mediator of pancreatic cancer tumorigenesis. Nature.
425:851–856. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Berman DM, Karhadkar SS, Maitra A, Montes
De Oca R, Gerstenblith MR, Briggs K, et al: Widespread requirement
for Hedgehog ligand stimulation in growth of digestive tract
tumours. Nature. 425:846–851. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yanai K, Nagai S, Wada J, Yamanaka N,
Nakamura M, Torata N, et al: Hedgehog signaling pathway is a
possible therapeutic target for gastric cancer. J Surg Oncol.
95:55–62. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Litingtung Y, Dahn RD, Li Y, Fallon JF and
Chiang C: Shh and Gli3 are dispensable for limb skeleton formation
but regulate digit number and identity. Nature. 418:979–983. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang B, Fallon JF and Beachy PA:
Hedgehog-regulated processing of Gli3 produces an
anterior/posterior repressor gradient in the developing vertebrate
limb. Cell. 100:423–434. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mitsuyasu T, Harada H, Higuchi Y, Kimura
K, Nakamura N, Katsuki T, et al: Immunohistochemical demonstration
of bcl-2 protein in ameloblastoma. J Oral Pathol Med. 26:345–348.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qualtrough D, Buda A, Gaffield W, Williams
AC and Paraskeva C: Hedgehog signaling in colorectal tumor cells:
induction of apoptosis with cyclopamine treatment. Int J Cancer.
110:831–837. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fujita E, Khoroku Y, Urase K, Tsukahara T,
Momoi MY, Kumagai H, et al: Involvement of Sonic hedgehog in the
cell growth of LK-2 cells, human lung squamous carcinoma cells.
Biochem Biophys Res Commun. 238:658–664. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Oro AE, Higgins KM, Hu Z, Bonifas JM,
Epstein EH Jr and Scott MP: Basal cell carcinomas in mice
overexpressing Sonic hedgehog. Science. 276:817–821. 1997.
View Article : Google Scholar : PubMed/NCBI
|